Abstract
Previous observational studies have reported inconsistent associations between nut consumption and cardiovascular diseases (CVD). This study aims to identify the causal relationship between different types of nuts consumption and CVD, and to quantify the potential mediating effects of cardiometabolic factors. We utilized Genome-Wide Association Study (GWAS) data to assess the causal effects of nut consumption on CVD using two-sample Mendelian randomization (MR) and a two-step MR analysis. The inverse variance weighted (IVW) method indicated that processed (salted or roasted) peanuts were potentially and positively associated with ischaemic heart disease (IHD) (OR 1.4866; 95%CI 1.0491-2.1065). No causal relationships were found between nuts consumption and other CVD outcomes, including atrial fibrillation, angina, coronary atherosclerosis, coronary heart disease, IHD, myocardial infarction, subarachnoid hemorrhage, intracerebral haemorrhage and stroke. Both MR-Egger and median-based methods yielded similar results to IVW. Furthermore, in the two-step MR analysis, fasting insulin, low-density lipoprotein cholesterol and fasting blood glucose were identified as mediators in the potential causal relationship between processed peanuts and IHD, explaining 16.98%, 6.38% and 4.91% of the mediation, respectively. In total, these mediators accounted for 28.27% of the association between salted or roasted peanuts and IHD.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-85070-z.
Keywords: Nut, Peanut, Cardiovascular diseases, Mendelian randomization analysis, Mediation
Subject terms: Cardiovascular diseases, Nutrition, Public health, Genetics research
Introduction
Cardiovascular diseases (CVD) remain the leading cause of global disease burden, with coronary heart disease (CHD), stroke and atrial fibrillation (AF) being the most prevalent conditions1. According to the latest statistics, the prevalence of CVD is nearly 127 million, affecting approximately 48.6% of adults, with the mortality rate of CVD continuing to rise in recent years, leading to 19 million global deaths in 2020 2. Therefore, it is essential to identify potential risk and protective factors to develop effective preventive measures, ultimately reducing the burden of CVD and improving global health3.
The potential impact of dietary intake on CVD varies, and nuts consumption, as an important dietary habit, has been recommended in daily life guidelines due to its association with a reduced risk of CVD4. Previous studies have suggested that nut consumption may be beneficial in reducing the risk of total CVD, CHD5, cardiovascular mortality6, AF, non-fatal myocardial infarction (MI)7 and hemorrhagic stroke8. Some observational studies and umbrella reviews have indicated that no significant association between nut consumption and fatal MI, ischaemic or hemorrhagic stroke7–9 and AF10, with the strength of evidence being limited for MI, AF, heart failure and stroke. Conclusions regarding different nut types, gender, and geographic region remain inconsistent11,12. Previous studies have also investigated the relationship between nut consumption and cardiovascular risk factors, including blood lipids, blood pressure, anthropometric factors and glycemic factors. A pooled analysis indicated that nuts have beneficial effects on total cholesterol (TC) and low-density lipoprotein cholesterol (LDL)13. Studies have shown that nuts do not lead to an increase in body weight, body mass index (BMI), or waist circumference (WC), but appear to improve glycemic control12, which contrasts with findings from a random trial conducted on adults with Type 2 diabetes in America14. However, after adjusting for various confounding variables, no statistically significant association was found for other cardiovascular factors, except for obesity15. The China and SUN cohort study16,17 found no association between nut and blood pressure, fasting blood glucose (FG), blood lipids and Framingham score. Two randomized trials18,19 investigating the effects of various hazelnuts and peanuts flavors on blood pressure and lipids levels suggested no significant negative association. Approximately one-third of nuts and 68% of peanuts are processed, including salting and roasting18. The flavor of processed nuts is more palatable than that of raw nuts20. Therefore, the findings regarding the correlation between nut consumption and CVD in observational studies remain contradictory, with potential confounders, recalling bias, reverse causality, and limited data on nut processing contributing to these inconsistencies. It is of vital importance to assess the uncertain impact of various types of nut exposure on CVD and explore the mediating role of risk factors in this association.
Similar to the natural randomized trials, Mendelian randomization (MR) analysis is widely used as causal inference method for assessing the effects of specific exposures on disease outcomes21. Using genetic variants as instrumental variables (IVs) of dietary exposure, MR can overcome the risks of reverse causation and the distortion of confounding in conventional observational studies22. With key assumptions, MR analysis can estimate the causal relationship without the bias of unmeasured confounding providing more robust causal evidence. Recent MR studies have estimated the causal relationship between independent dietary habits and CVD, including the association between coffee consumption23–25, tea consumption26,27, raw and cooked vegetables intake28, dried fruits intake29 and the risk of stroke, AF, ischaemic cardio-cerebral vascular diseases and other CVD. Two-step MR can be used to estimate the mediating proportion of potential factors between exposure and diseases outcomes30. Currently, MR analysis has not been applied to investigate the relationship and mediating factors between nut consumption and CVD. Using two-sample MR and two-step MR analysis, this study aims to explore the causal relationship between nuts consumption and CVD (including AF, angina, coronary atherosclerosis, CHD, ischaemic heart disease (IHD), MI, subarachnoid hemorrhage (SAH), intracerebral haemorrhage (ICH) and stroke) and to quantify the potential mediating effects of cardiometabolic factors, including glycemic factors, lipids and anthropometric factors.
Materials and methods
Study design
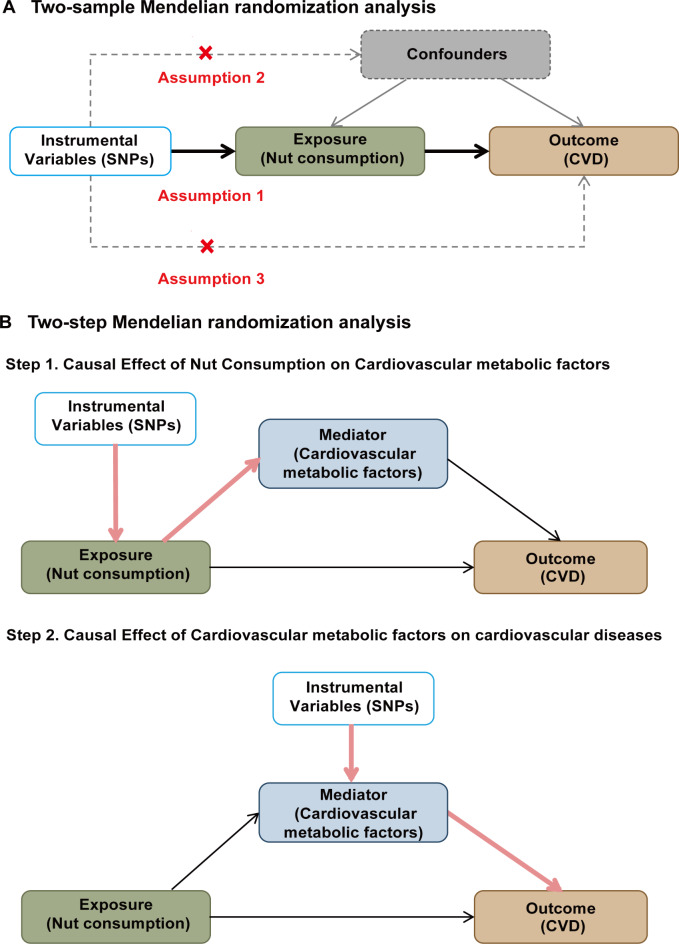
In this study, a two-sample MR analysis (Fig. 1) was performed to investigate the causal relationship between four different types of nut consumption and various CVD. A two-step MR analysis was conducted to investigate the mediating pathways from nut consumption to CVD through 14 cardiometabolic factors. Single nucleotide polymorphisms (SNPs) were used as IVs to assess causality. Three key assumptions must be satisfied in MR analyses: (i) the IVs are strongly associated with the exposure, (ii) the IVs are independent of confounders, and (iii) the IVs influence the outcome only through the exposure.
Fig. 1.
MR study design for causal relationship between nuts consumption and CVD. (A) design of two-sample MR analysis. (B) design of two-step MR analysis. CVD, cardiovascular diseases; MR, Mendelian randomization; SNPs, single nucleotide polymorphisms.
Data sources
The exposure data for MR analysis were obtained from the UK Biobank, which is a large cohort study involving more than 500,000 participants. Participants were asked to report their nuts consumption (salted or roasted, unsalted) and peanuts consumption (salted or roasted, unsalted (monkey nuts)) based on 24-hour dietary recall questionnaires completed on the previous day. These questionnaires were administered during the assessment center on a touch-screen device, along with four additional online rounds at three to four monthly intervals. The Genome-Wide Association Study (GWAS) summary data for the exposure can be accessed from the MRC IEU Open GWAS project database (https://gwas.mrcieu.ac.uk/). The summary statistics for CVD outcomes were extracted from the FinnGen consortium, which conducted a study in Finland involving a cohort of 500,000 participants from the Finnish biobank31. The R9 data were released in May 2023, with a total sample size of 377,277 (https://www.finngen.fi/en). Detailed information on the exposures and outcomes from the European population is provided in Supplemental Table 1.
Fourteen cardiovascular metabolic factors were identified as potential mediators, which were categorized into three groups: glycemic factors, lipids and anthropometric factors. Genetic variations in anthropometric factors were extracted from the Genetic Investigation of Anthropometric Traits (GIANT)32,33 consortium, including BMI, hip circumference (HIP), BMI-adjusted HIP (HIPadjBMI), waist circumference, BMI-adjusted WC (WCadjBMI), waist-to-hip ratio (WHR) and BMI-adjusted WHR (WHRadjBMI). Summary statistics for lipemic factors were obtained from the Global Lipid Genetics Consortium (GLGC)34, which included total cholesterol, triglycerides (TG), high-density lipoprotein cholesterol (HDL), and LDL. For blood glucose factors, data were obtained from the Meta-Analysis of Glucose and Insulin Correlated Traits Consortium (MAGIC)35, which included glycated hemoglobin levels (HbA1c), fasting insulin (FI), and FG. Detailed information was provided in Supplemental Table 2.
Selection of genetic instrumental variants
SNPs associated with different types of nuts consumption were extracted from the GWAS datasets as significant IVs, with a threshold of p < 5 × 10-636. In the two-step MR, SNPs that might serve as mediators were extracted at a threshold of p < 5 × 10-8. SNPs in linkage disequilibrium (LD) were excluded to ensure the independence of each IV within a window size of 10,000 kb and R2 < 0.001. SNPs that were closely associated with the outcomes were selected, and missing SNPs were replaced by highly linkage proxies (r2 > 0.8, MAF > 0.01). Potential confounders such as LDL, HDL, TC, FG, FI, type 2 diabetes, BMI, hypertension, insomnia and smoking were checked to see whether SNPs associated with the exposure were also linked to them (p < 5 × 10-8), by extracting data from GIANT, GLGC, DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) (https://diagram-consortium.org/about.html), MAGIC and UK Biobank. Similarly, mediators were examined to check if SNPs of mediators were associated with confounders, including BMI, hypertension, smoking, insomnia and type 2 diabetes (p < 5 × 10-8). Furthermore, all IVs related to potential confounding factors or directly associated with outcomes were assessed using PhenoScanner V2 37.
To avoid weak instruments bias, F statistic (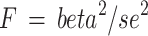 ) was calculated for each single IV38. For each exposure (nuts and peanuts), a general F was calculated using the equation:
) was calculated for each single IV38. For each exposure (nuts and peanuts), a general F was calculated using the equation: 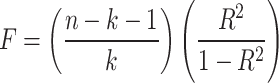 , which is related to the proportion of variance in the exposure explained by the genetic variants (R2), sample size (n) and the number of SNPs (k)39. R2 for each exposure was calculated with the equation 40:
, which is related to the proportion of variance in the exposure explained by the genetic variants (R2), sample size (n) and the number of SNPs (k)39. R2 for each exposure was calculated with the equation 40: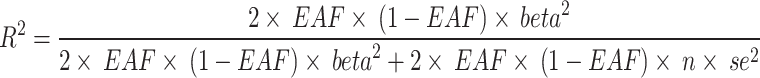 , where EAF is the effect allele frequency, beta is the estimated genetic effect and se is the standard error of the genetic effect. If F-statistic was higher than 10, the correlation between the IV and exposure was considered sufficiently strong39.
, where EAF is the effect allele frequency, beta is the estimated genetic effect and se is the standard error of the genetic effect. If F-statistic was higher than 10, the correlation between the IV and exposure was considered sufficiently strong39.
Statistical analysis
The fixed-effect inverse variance weighted (IVW-FE) method was used as the main analysis method for two-sample MR, supplemented by several other methods, including MR-Egger regression, median-based methods (simple median, weighted median and penalized weighted median) and random-effect inverse variance weighted (IVW-RE) method. The inverse variance weighted (IVW) method assumes that all the genetic variants are valid IVs and that there are no pleiotropic effects41. The IVW-RE is robust even in the presence of heterogeneity42. MR-Egger, using weighted linear regression with an intercept regression, was employed to obtain valid causal effect estimates in the presence of horizontal pleiotropy, assuming the independence of the associations between IVs and exposures43,44. Median-based methods assess the effect of the majority (or weighted majority) of IVs on the outcomes43. The weighted median provides a consistent estimate of the effect, even when half of the genetic variants are invalid41.
Cochrane’s Q statistic was computed to assess heterogeneity among different genetic variants45. The MR-Egger intercept was tested to detect horizontal pleiotropy44. If the intercept was not significantly different from zero with p > 0.05, no pleiotropic effects were assumed. Additionally, Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) was used to obtain unbiased causal estimates by detecting and removing potential outliers46. Leave-one-out analyses were conducted to assess the robustness of the results with respect to single SNPs.
In the two-step MR analysis, SNPs associated with nuts consumption were used to estimate the causal effects of nuts consumption on various cardiometabolic factors, and the effects of these mediators on the corresponding diseases using the IVW method. The indirect effect of nuts consumption on CVD through specific mediators was estimated using the product of coefficients method47. To quantify the proportion of the effect mediated by each mediator, the indirect effect was divided by the total effect obtained from the two-sample MR, and the standard error was calculated using the delta method48.
Statistical analyses were performed using R software (version 4.3.0) with the ‘TwoSampleMR’ (version 0.5.7) R package. Two-sided p-values < 0.05 were considered significant, while the Bonferroni-corrected threshold for statistical significance was set at p < 0.0056 (0.05/9). P-values between 0.05 and 0.0056 were considered suggestive of significance.
Results
After checking for the IVs closely associated with confounding factors, the SNP of rs3815692 and rs13284665 were removed, as both were closely related to the LDL and TC. 45, 16, 22 and 14 independent SNPs associated with unsalted peanuts, unsalted nuts, salted or roasted nuts and salted or roasted peanuts, respectively, were identified. Details of the exposure SNPs were presented in Supplemental Tables 3-6. All the F statistics of genetic variants were larger than 10.
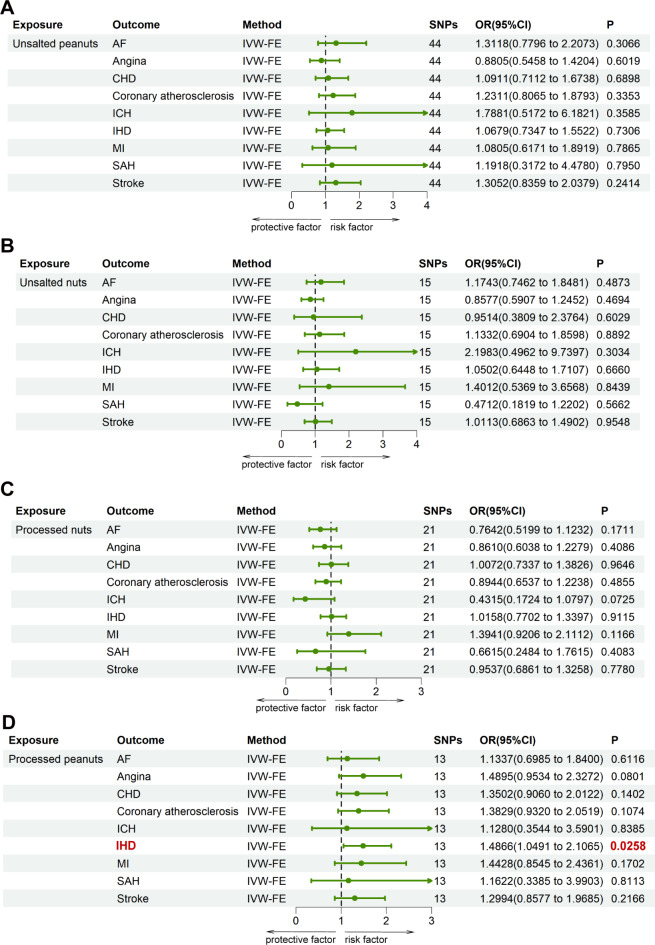
The results of the causal relationship between four types of nuts consumption and CVD were analyzed by MR methods (IVW-FE) (Fig. 2). There was a suggestive significant causal relationship of salted or roasted peanuts consumption and IHD (OR: 1.4866; 95%CI: 1.0491-2.1065; p = 0.0258), based on the method of IVW-FE, which was considered suggestive of significance. There was no significant evidence of causal relationship between nuts consumption and other CVD outcomes, including AF, angina, coronary atherosclerosis, CHD, MI, SAH, ICH and stroke. Since the statistical power of other methods, such as MR-Egger, weighted median, simple median and penalized weighted median, was lower than IVW, some of their results were found insignificant based on the p-value threshold. Without heterogeneity and pleiotropy was identified, these results were used to confirm consistent estimates in the same direction, demonstrating the robustness of IVW as complementary approaches42,49, as presented in Supplemental Tables 7-10.
Fig. 2.
MR assessment for causal relationship between different types of nuts consumption and CVD. All the SNPs associated with exposure were extracted as significant instrumental variants at a level of p < 5 × 10-6. (A) The exposure factor is unsalted peanuts consumption. (B) The exposure factor is unsalted nuts consumption. (C) The exposure factor is processed nuts consumption. (D) The exposure factor is processed peanuts consumption. AF, atrial fibrillation; CHD, coronary heart disease; ICH, intracerebral haemorrhage; IHD, ischaemic heart disease; MI, myocardial infarction; MR, Mendelian randomization; SAH, subarachnoid hemorrhage; IVW-FE, fixed-effect inverse variance weighted; SNPs, single nucleotide polymorphisms; OR, odds ratio.
As shown in Supplemental Table 11, no evidence of heterogeneity was observed between the unsalted and salted or roasted nuts for CVD outcomes. Under the circumstances that some results for unsalted or salted or roasted peanuts of Cochrane’s Q statistics showed heterogeneity (unsalted peanuts for angina, Q = 65.9822; unsalted peanuts for CHD, Q = 61.0486; unsalted peanuts for IHD, Q = 60.2355; salted or roasted peanuts for MI, Q = 32.2798), MR-PRESSO method with significant outliers removed was conducted and showed the same no-causal effect as IVW (removal of rs28868570, salted or roasted peanuts for MI, OR: 2.0880, 95%CI: 0.9734-4.4789, p = 0.0853). Besides, for the situation that no significant outliers were identified and removed, the result of IVW-RE method provided consistent non-causal estimations (unsalted peanuts for angina, OR: 0.8805, 95%CI: 0.4869-1.5922, p = 0.6737; unsalted peanuts for coronary atherosclerosis, OR: 1.2311, 95%CI: 0.7438-2.0379, p = 0.4187; unsalted peanuts for IHD, OR:1.0679, 95%CI: 0.6860-1.6625, p = 0.7711). The result of the MR-Egger intercepts suggested no significant evidence of horizontal pleiotropy with all p-values > 0.05. The scatter plots and Funnel plots were showed in Supplemental Figs. 1-8. The result of the leave-one-out method were showed in Supplemental Figs. 9-12, and Supplemental Fig. 12 suggested that the association for salted or roasted peanuts on IHD was not influenced by a single SNP.
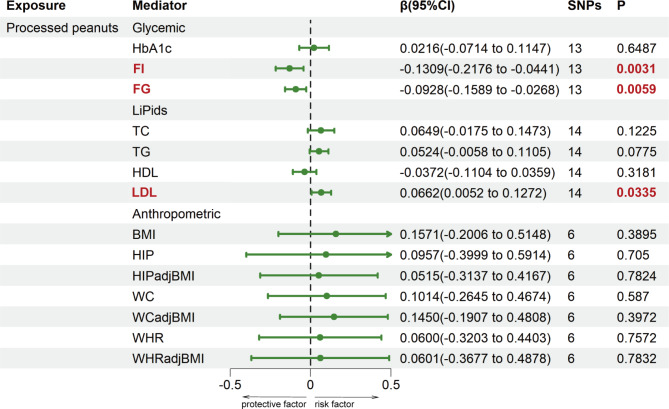
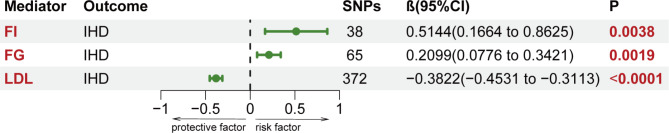
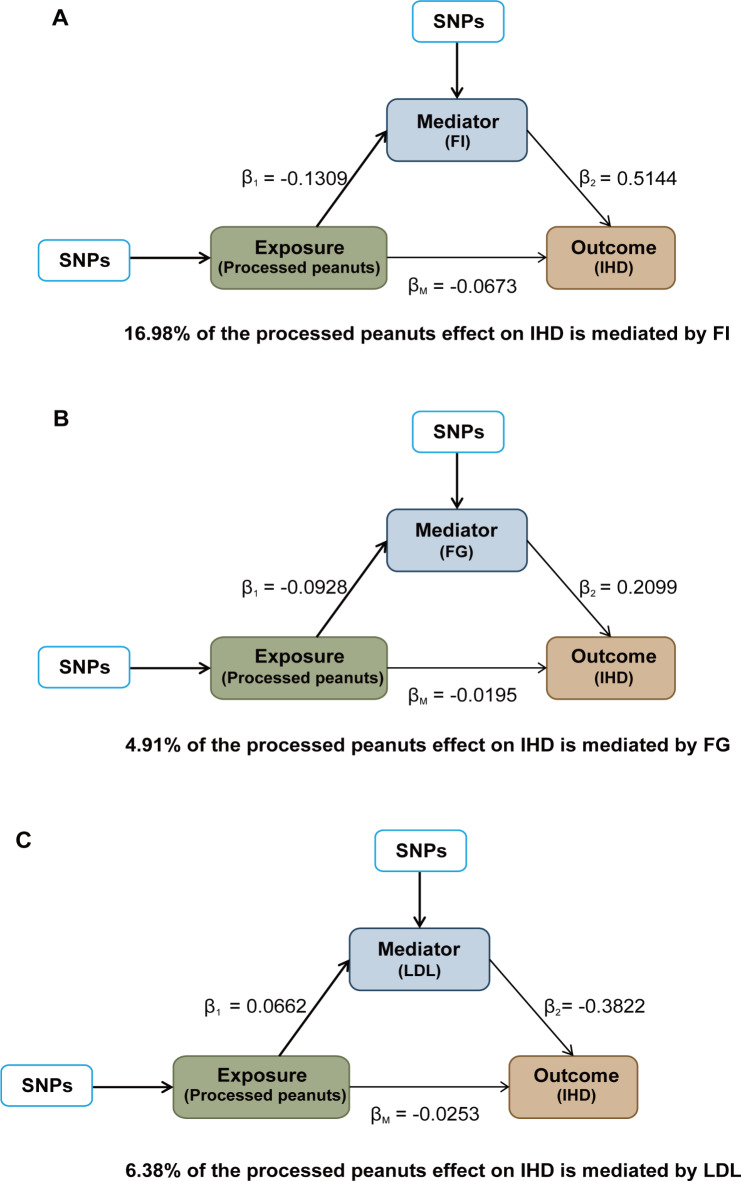
The two-step MR analysis was utilized to assess the mediation effects of 14 potential mediators in causal relationships that showed causal significance. The information of selected SNPs related to mediators was shown in Supplemental Tables 12-14. Figure 3 and Supplemental Table 15 showed the result of the causal effects of salted or roasted peanut consumption on 14 cardiometabolic factors estimated using the IVW method in the first step MR analysis. Furthermore, three mediators were identified in the association between salted or roasted peanuts and IHD. Salted or roasted peanut consumption was negatively correlated with FI (β1: -0.1309; 95%CI: -0.2176 to -0.0441) and FG (β1: -0.0928; 95%CI: -0.1589 to -0.0268), and positively associated with LDL (β1: 0.0662; 95%CI: -0.0052 to -0.1272). No causal effect of salted peanut consumption on BMI, HIP, HIPadjBMI, WC, WCadjBMI, WHR, WHRadjBMI, TC, TG, HDL and HbA1c was found. Therefore, FI, FG and LDL may be mediators of the pathogenic pathways of peanuts and IHD and were included in the second step of the two-step MR analysis. In addition, FI (β2: 0.5144; 95%CI: 0.1664 to 0.8625) and FG (β2: 0.2099; 95%CI: 0.0776 to 0.3421) were positively related with IHD, and LDL (β2: -0.3822; 95%CI: -0.4531 to -0.3113) was negatively associated with IHD (Fig. 4, Supplemental Table 16). Finally, the mediating role of three mediators in the association between salted or roasted peanut consumption and IHD was assessed, including the largest mediator, FI, explaining 16.98% of the mediation (95%CI: 0.90-33.06%), and LDL and fasting glucose, accounting for 6.38% (95%CI: 0.38-12.38%) and 4.91% (95% CI: 0.24-9.58%) of the mediation, respectively. Together, the mediators explained 28.27% of the association between salted or roasted peanuts and IHD, as presented in the Fig. 5, the detailed information was shown in Supplemental Table 17.
Fig. 3.
First step of the causal relationship of processed peanuts with 14 Mediators. Mediators were classified into 3 categories: glycemic factors, lipids and anthropometric factors. BMI, body mass index; FG, fasting blood glucose; FI, fasting insulin; HbA1c, glycated hemoglobin levels; HDL, high-density lipoprotein cholesterol; HIP, hip circumference; HIPadjBMI, BMI-adjusted HIP; LDL, low-density lipoprotein cholesterol; SNPs, single nucleotide polymorphisms; TC, total cholesterol; TG, triglycerides; WC, waist circumference; WCadjBMI, BMI-adjusted WC; WHR, waist-to-hip ratio; WHRadjBMI, BMI-adjusted WHR.
Fig. 4.
Second step of the causal relationship of Mediators with IHD. All the SNPs associated with mediators were extracted as significant instrumental variants at a level of p < 5 × 10-8. FG, fasting blood glucose; FI, fasting insulin; IHD, ischaemic heart disease; LDL, low-density lipoprotein cholesterol; SNPs, single nucleotide polymorphisms.
Fig. 5.
The mediation effects of processed peanuts intake on IHD risks. (A) The mediating effect of FI. (B) The mediating effect of FG. (C) The mediating effect of LDL. FG, fasting blood glucose; FI, fasting insulin; IHD, ischaemic heart disease; LDL, low-density lipoprotein cholesterol; SNPs, single nucleotide polymorphisms.
Discussion
In this study, MR analysis was used to estimate the causal relationship between different types of nuts consumption and CVD and potential mediation factors. The results indicated that processed (salted or roasted) peanuts consumption may be a potential risk factor for IHD, suggesting a potential causal relationship after Bonferroni correction. No significant causal relationship was observed between the consumption of nuts or peanuts and other CVD outcomes such as AF, angina, CHD, myocardial infarction, SAH and stroke. The mediation analysis explored three cardio-metabolic factors - FI, FG and LDL - as mediators in the causal relationship between processed peanuts and IHD.
Nut consumption is recommended in dietary guidelines for reducing CVD risk4. However, previous observational studies on the effects of nut consumption on CVD outcomes have yielded inconsistent results. A prospective study found inverse associations between nut consumption and the risk of total and non-fatal MI and AF, but no significant associations with fatal MI, ischaemic stroke or ICH7. The inverse associations with AF remained after adjusting for multiple risk factors. The Physicians’ Health Study showed no association between nuts consumption and AF10, total stroke or ischaemic stroke8, but a suggestive J-shaped relationship between exposure and hemorrhagic stroke8. These findings only in male physicians may be related to the tendency of consuming more nuts in participants at risk of CVD due to medical knowledge. Cohort studies reported similar no significant relationship between total nuts and fatal or nonfatal stroke or ischaemic stroke9, but a negative relationship between peanuts and walnuts and total CVD, CHD and stroke5. A review50 of these studies found an inverse association between nut intake and CHD, but no such association with stroke risk. The relationships between nuts and stroke remain inconsistent. A review51 reported conflicting findings, with ten studies concluding no significant changes in vascular function, while others indicated improvements. This variation may be influenced by season and climates changes on the nutrient content of nuts. Umbrella reviews indicated that nut consumption reduces the risk of CHD and cardiovascular mortality, but evidence for MI, AF, heart failure and stroke was limited and varied based on nut types, gender, and geographic region, due to heterogeneity and potential confounders11,12. With limited information, these studies were not able to fully assess the influence of various nut processing methods, such as salted, roasted, or raw nuts. Our study suggested a potential relationship between long-term consumption of salted or roasted peanuts and the increased risk of IHD but no significant causal relationship was observed between consumption of nuts or peanuts and other CVD outcomes.
Nuts contain a variety of compounds, including macronutrients, micronutrients, unsaturated fatty acids such as monounsaturated fatty acids and polyunsaturated fatty acids, dietary fiber, water-soluble vitamins, non-sodium minerals and phenolic compounds, which have beneficial influences on antioxidant, anti-inflammatory and intermediate markers of cardiovascular risk such as blood cholesterol, glycaemia, blood pressure and vasomotion52,53. Due to the similarity of nutritional components, peanuts were also classified as nut foods52. Dose-response relationships suggested that the best nuts intake was 15-40 g/d, with limited benefits from intake beyond 28 g/d12. Recent studies have examined the effects of nut consumption on cardiovascular factors, yet the results remain inconclusive. Meta-analysis and reviews found that nuts were associated with improvements in blood lipids54, such as TC and LDL13, TG and ApoB55, but no significant effects were found for HDL55. Studies have shown that nuts do not increase body weight, BMI, WC, but they may improve glycemic factors12, such as FI, while no effect on FG or HbA1c was observed56. This may be due to the replacement of unsaturated fatty acids and carbohydrates with unsaturated fatty acids. The fatty acids and other bioactive constituents in nuts may act by increasing insulin sensitivity or though non-insulin mediated mechanisms to promote increased glucose uptake, influencing FG. Our MR analysis confirmed that the potential protective association between salted or roasted peanuts and IHD was mediated by FI, FG and LDL. The type of population is quite important. A random trial involving individuals with Type 2 diabetes in America found that increasing peanuts intake could reduce the weight, BMI, and WC14. Meta-analysis indicated that walnut intake did not result in changes to body weight, BMI, fat mass or WC57, which was not supportive for the improvement. In this MR analysis, genetic predictors linked to salted or roasted peanuts indicated no significant effect on anthropometric mediators, including BMI, WC, HIP and WHR. Besides, nuts may benefit to the patients with risk factors for CVD or some degree of impaired vascular function, while no benefit was observed in patients with healthy or poor vascular function. A study found women who consumed more nuts showed lower risk of left ventricular hypertrophy, which was also observed in women with or without hypertension and diabetes mellitus, but the cross-sectional design58 could not provide the causative relationship. An inverse association between nut consumption and obesity remained after adjusting for various potential confounders but no significant associations were observed for other cardiovascular factors15. Similarly, the China cohort16 and SUN prospective cohort17 found no associations between nut consumption and Framingham score, systolic and diastolic blood pressure, HDL-cholesterol, LDL-cholesterol or FG.
The inconsistent results of previous studies may be related to the different processing of nuts. Roasting may enhance sensory characteristics, but it can also reduce antioxidant activity, nutritional content and total phenolic compounds due to blanching and peeling53. The skins of nuts, the main contributors to total phenolic content and antioxidant activity, are generally removed during the roasting process59. Studies found that the roasting affects lipids and other components such as linoleic acids and beneficial phytosterols. There is a significant loss of total phenolic compounds, especially tocopherols, and a decrease in thiamine in most types of nuts, while B vitamins showed high thermal resistance stability59–61. Nuts, especially walnuts and pistachios, were rich in alpha-linolenic acid (ALA), a shorter chain omega-3 fatty acid that can be converted to longer chain omega-3 fatty acids in body. Omega-3 fats were considered as protector for lowering blood pressure, modulating arterial lipoprotein lipase levels, producing anti-inflammatory and anti-arrhythmic effects. However, meta-analysis62 concluded that increasing ALA intake make little or no impact on the risk of CHD, and may modestly reduce the risk of cardiovascular events and arrhythmia, but the effects of ALA on stroke is unclear.
Furthermore, in many European and American countries, especially in Spain17, nuts and peanuts are often consumed as salted, grilled or fried ways63,which may counteract the beneficial effects of other nutrients in raw nuts. Randomized trial of 72 participants compared the dry roasting and lightly salting hazelnuts with raw hazelnuts, both of which could improve the HDL, apolipoprotein A1 and systolic blood pressure, but only dry roasting and lightly salting ones showed significant effect of reduction on diastolic blood pressure18. Another study19 of 151 participants reported no significance between four types of flavored peanuts and blood glucose and blood lipids. It was well established that excessive sodium intake is associated with an increased risk of CVD, especially hypertension64,65, with processed foods contributing the majority of daily sodium intake. The sodium content of roasted nuts (145 mg/100 g) is approximately 10-20 times that of raw nuts (13 mg/100 g), with salted nuts containing 568 mg/100 g18. Salted almonds may contain up to 700 mg/100 g, which would represent 10% of the recommended daily salt intake in a 30 g serving66. Sodium ions may inhibit protease activity and increase lipid peroxidation65. Research suggested that the process of soaking nuts, particularly in the presence of salt, may leach out minerals and water-soluble vitamins, without reducing phytates66. Phytates, a strong chelator, reduces the bioavailability of important micronutrients such as zinc, iron, and calcium by inhibiting their absorption67,68. In addition, the heat processing of peanuts, including boiling, roasting and frying, caused the loss of nutrients, such as amino acids, total reducing sugar, sucrose and unsaturated fatty acids69. Harmful compounds may be produced such as 5-hydroxymethylfurfural and furan69. A research18 suggested that it seemed no harmful impression when the sodium added in nuts ≤ 285 mg/d, which suggests the importance of proper sodium addition control. Due to limited availability for blood pressure data, this study was unable to incorporate relevant information into mediation analysis. Further exploration of potential mediators is necessary to explore whether the relationship between salt or roasted peanut and IHD through mediators can be altered by various processing methods. As an advantageous methodology, MR study could avoid bias from unobserved confounders of exposures and outcomes by using summary level data from GWAS. This approach provides more robust estimates of causality between salted or roasted or unsalted nuts and peanuts consumption and CVD outcomes compared to conventional observational epidemiology70, which has not been assessed previously. Because two-sample MR analyses were performed at each step, measurement error and reverse causal error relationships could be overcome to identify the mediating effects. Secondly, with LD removal and F-statistics above 10, the study ensured the independence and strong association of SNPs with exposures, thus avoiding weak instrument bias. Sensitivity analyses using MR-Egger regression and median-based methods confirmed the robustness of the results. The results of these approaches, albeit less statistically powerful as compared to the primary IVW method, remained consistent with the directions of estimates, demonstrating the robustness of IVW49.
The limitations were that summary statistics were extracted from European population, which may not apply to other ethnic groups. Additionally, the study was incapable of assessing the causal effects of specific nut types, including almonds, cashews and pistachios due to the insufficient data. The study could not test non-linear causal relationships between nut consumption and related outcomes. It is worth noting that there may be differences between genetic hypotheses, clinical trials and actual nuts consumption. The suggestive significant of the association should be interpreted carefully. Furthermore, due to the low number of SNPs (less than three) after LD on the p-value of 5 × 10-8 and 5 × 10-7, SNPs were extracted at a relaxed p-value threshold of 5 × 10-6. Further analyses with specific types of nuts, more relevant SNPs, larger sample sizes, and more comprehensive and longitudinal dietary assessment are needed to confirm these findings and more accurately capture usual or habitual nut consumption patterns.
In conclusion, this MR analysis suggested that the evidence that processed (salted or roasted) peanuts consumption was a potential risk factor for IHD, with the potential causal relationship mediated by three cardio-metabolic factors, FI, FG and LDL. Hence, it is necessary to evaluate the impact of nuts on health in further study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the following projects and institutions for making the summary data publicly available, including the IEU open GWAS project, the FinnGen consortium, GIANT, GLGC, MAGIC, DIAGRAM.
Abbreviations
- AF
Atrial fibrillation
- BMI
Body mass index
- CHD
Coronary heart disease
- CVD
Cardiovascular diseases
- DIAGRAM
DIAbetes genetics replication and meta-analysis
- FG
Fasting blood glucose
- FI
Fasting insulin
- GIANT
Genetic Investigation of Anthropometric Traits
- GLGC
Global Lipid Genetics Consortium
- GWAS
Genome-Wide Association Study
- HbA1c
Glycated hemoglobin levels
- HDL
High-density lipoprotein cholesterol
- HIP
Hip circumference
- HIPadjBMI
BMI-adjusted HIP
- ICH
Intracerebral haemorrhage
- IHD
Ischaemic heart disease
- IVs
Instrumental variables
- IVW
Inverse variance weighted
- IVW-FE
Fixed-effect inverse variance weighted
- IVW-RE
Random-effect inverse variance weighted
- LD
Linkage disequilibrium
- LDL
Low-density lipoprotein cholesterol
- MAGIC
Meta-Analysis of Glucose and Insulin Correlated Traits Consortium
- MI
Myocardial infarction
- MR
Mendelian randomization
- MR-PRESSO
Mendelian randomization pleiotropy residual sum and outlier
- SAH
Subarachnoid hemorrhage
- SNPs
Single nucleotide polymorphisms
- TC
Total cholesterol
- TG
Triglycerides
- WC
Waist circumference
- WCadjBMI
BMI-adjusted WC
- WHR
Waist-to-hip ratio
- WHRadjBMI
BMI-adjusted WHR
Author contributions
RW, XY conceived the ideas and designed the research; RW conducted the research and analyzed the data; and RW wrote the original draft. JS and XY reviewed and revised the manuscript critically. XY had primary responsibility for final content. All authors have read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data is provided within the manuscript or supplementary information files. The GWAS summary data related to exposure are available at https://gwas.mrcieu.ac.uk/. The GWAS summary data of FinnGen consortium are available at https://www.finngen.fi/en. The GWAS data of mediating factors are available at https://magicinvestigators.org/, http://csg.sph.umich.edu/willer/public/glgc-lipids2021/ and https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval and participant agreement are not necessary because the public summary data has already been approved. All original studies had been authorized by corresponding ethical standards committee.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vaduganathan, M., Mensah, G. A., Turco, J. V., Fuster, V. & Roth, G. A. The global burden of cardiovascular diseases and risk: A compass for future health. J. Am. Coll. Cardiol.80, 2361–2371 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Tsao, C. W. et al. Heart Disease and Stroke Statistics—2023 update: A report from the American Heart Association. Circulation147, e93–e621 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roger, V. L. et al. Recommendations for Cardiovascular Health and Disease Surveillance for 2030 and Beyond: A Policy Statement from the American Heart Association. Circulation141, e104–e119 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Snetselaar, L. G., de Jesus, J. M., DeSilva, D. M. & Stoody, E. E. Dietary guidelines for americans, 2020–2025. Nutr. Today. 56, 287–295 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guasch-Ferré, M. et al. Nut consumption and risk of cardiovascular disease. J. Am. Coll. Cardiol.70, 2519–2532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imran, T. F. et al. Nut consumption, risk of cardiovascular mortality, and potential mediating mechanisms: The women’s Health Study. J. Clin. Lipidol.15, 266–274 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson, S. C., Drca, N., Björck, M., Bäck, M. & Wolk, A. Nut consumption and incidence of seven cardiovascular diseases. Heart104, 1615–1620 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djoussé, L., Gaziano, J. M., Kase, C. S. & Kurth, T. Nut consumption and risk of stroke in US male physicians. Clin. Nutr.29, 605–609 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.di Giuseppe, R. et al. The association between nut consumption and the risk of total and ischemic stroke in a German cohort study. Eur. J. Clin. Nutr.69, 431–435 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Khawaja, O., Gaziano, J. & Djousse, L. Nut consumption and risk of atrial fibrillation in the Physicians’ Health Study. Nutr. J.11, 17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martini, D. et al. Nut and legume consumption and human health: An umbrella review of observational studies. Int. J. Food Sci. Nutr.72, 871–878 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Balakrishna, R., Bjørnerud, T., Bemanian, M., Aune, D. & Fadnes, L. T. Consumption of nuts and seeds and Health outcomes Including Cardiovascular Disease, Diabetes and Metabolic Disease, Cancer, and mortality: An Umbrella Review. Adv. Nutr.13, 2136–2148 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabaté, J., Oda, K. & Ros, E. Nut consumption and blood lipid levels: A pooled analysis of 25 intervention trials. Arch. Intern. Med.170, 821–827 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Wien, M., Oda, K. & Sabaté, J. A randomized controlled trial to evaluate the effect of incorporating peanuts into an American Diabetes Association meal plan on the nutrient profile of the total diet and cardiometabolic parameters of adults with type 2 diabetes. Nutr. J.13, 10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammadifard, N. et al. Long-term association of nut consumption and cardiometabolic risk factors. Nutr. Metabolism Cardiovasc. Dis.29, 972–982 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Sun, Y. et al. Nut Consumption and Cardiovascular Risk in Older Chinese: The Guangzhou Biobank Cohort Study. PLOS ONE. 10, e0137178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Lapiscina, E. H. et al. Nut consumption and incidence of hypertension: The SUN prospective cohort. Nutr. Metabolism Cardiovasc. Dis.20, 359–365 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Tey, S. L., Robinson, T., Gray, A. R., Chisholm, A. W. & Brown, R. C. Do dry roasting, lightly salting nuts affect their cardioprotective properties and acceptability? Eur. J. Nutr.56, 1025–1036 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Jones, J. B. et al. A randomized trial on the effects of flavorings on the health benefits of daily peanut consumption123. Am. J. Clin. Nutr.99, 490–496 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Brown, R. C. et al. Patterns and predictors of nut consumption: Results from the 2008/09 New Zealand Adult Nutrition Survey. Br. J. Nutr.112, 2028–2040 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Davey Smith, G. & Ebrahim, S. Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease?*. Int. J. Epidemiol.32, 1–22 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Burgess, S., Foley, C. N. & Zuber, V. Inferring causal relationships between risk factors and outcomes from genome-wide association study Data. Annu. Rev. Genomics Hum. Genet.19, 303–327 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan, S., Carter, P., Mason, A. M., Burgess, S. & Larsson, S. C. Coffee consumption and cardiovascular diseases: A mendelian randomization study. Nutrients13, 2218 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan, S. & Larsson, S. C. No association between coffee consumption and risk of atrial fibrillation: A mendelian randomization study. Nutr. Metabolism Cardiovasc. Dis.29, 1185–1188 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Qian, Y. et al. Coffee consumption and risk of stroke: A mendelian randomization study. Ann. Neurol.87, 525–532 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Wang, M. et al. Higher tea consumption is associated with decreased risk of small vessel stroke. Clin. Nutr.40, 1430–1435 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Gao, N. et al. Causal relationship between tea intake and cardiovascular diseases: A mendelian randomization study. Front. Nutr.9, 938201 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, J., He, Z., Xu, M., Du, J. & Zhao, Y. Socioeconomic status may affect association of vegetable intake with risk of ischemic cardio-cerebral vascular disease: A mendelian randomization study. Front. Nutr.10, 1161175 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng, Y., Cao, S. & Yang, H. Causal associations between dried fruit intake and cardiovascular disease: A mendelian randomization study. Front. Cardiovasc. Med.10, 1080252 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richmond, R. C., Hemani, G., Tilling, K., Davey Smith, G. & Relton, C. L. Challenges and novel approaches for investigating molecular mediation. Hum. Mol. Genet.25, R149–R156 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature613, 508–518 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature518, 197–206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shungin, D. et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature518, 187–196 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham, S. E. et al. The power of genetic diversity in genome-wide association studies of lipids. Nature600, 675–679 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen, J. et al. The trans-ancestral genomic architecture of glycemic traits. Nat. Genet.53, 840–860 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Che, Y. et al. Dietary factors and the risk of atopic dermatitis: A mendelian randomisation study. Br. J. Nutr.131, 1873–1882 (2024). [DOI] [PubMed] [Google Scholar]
- 37.Kamat, M. A. et al. PhenoScanner V2: An expanded tool for searching human genotype–phenotype associations. Bioinformatics35, 4851–4853 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng, R. et al. Pulmonary embolism and 529 human blood metabolites: Genetic correlation and two-sample mendelian randomization study. BMC Genom Data. 23, 69 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgess, S., Thompson, S. G. & CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in mendelian randomization studies. Int. J. Epidemiol.40, 755–764 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Papadimitriou, N. et al. Physical activity and risks of breast and colorectal cancer: A mendelian randomisation analysis. Nat. Commun.11, 597 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in mendelian randomization with some Invalid instruments using a weighted median estimator. Genet. Epidemiol.40, 304–314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgess, S., Bowden, J., Fall, T., Ingelsson, E. & Thompson, S. G. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology28, 30 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burgess, S. & Thompson, S. G. Interpreting findings from mendelian randomization using the MR-Egger method. Eur. J. Epidemiol.32, 377–389 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol.44, 512–525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowden, J. et al. Improving the accuracy of two-sample summary-data mendelian randomization: Moving beyond the NOME assumption. Int. J. Epidemiol.48, 728–742 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat. Genet.50, 693–698 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.VanderWeele, T. J., Mediation Analysis: A practitioner’s guide. Annu. Rev. Public Health. 37, 17–32 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Carter, A. R. et al. Understanding the consequences of education inequality on cardiovascular disease: Mendelian randomisation study. BMJ365, l1855 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, X. et al. Genetic support of a causal relationship between Iron Status and Type 2 diabetes: A mendelian randomization study. J. Clin. Endocrinol. Metab.106, e4641–e4651 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bechthold, A. et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr.59, 1071–1090 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Morgillo, S., Hill, A. M. & Coates, A. M. The effects of nut consumption on vascular function. Nutrients11, 116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alasalvar, C., Salvadó, J. S. & Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem.314, 126192 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Gonçalves, B. et al. Composition of nuts and their potential health benefits-an overview. Foods12, 942 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guasch-Ferré, M., Li, J., Hu, F. B., Salas-Salvadó, J. & Tobias, D. K. Effects of Walnut consumption on blood lipids and other cardiovascular risk factors: An updated meta-analysis and systematic review of controlled trials. Am. J. Clin. Nutr.108, 174–187 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Del Gobbo, L. C., Falk, M. C., Feldman, R., Lewis, K. & Mozaffarian, D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analysis, and dose-response of 61 controlled intervention trials 123. Am. J. Clin. Nutr.102, 1347–1356 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tindall, A. M., Johnston, E. A., Kris-Etherton, P. M. & Petersen, K. The effect of nuts on markers of glycemic control: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr.109, 297–314 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang, Z. et al. Effects of walnut intake on anthropometric characteristics: A systematic review and dose-response meta-analysis of randomized controlled trials. Complement. Ther. Med.50, 102395 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Park, S. K., Oh, C. M., Kang, J. G., Seok, H. S. & Jung, J. Y. The association between left ventricular hypertrophy and consumption of nuts, including peanuts, pine nuts, and almonds. Nutr. Metabolism Cardiovasc. Dis.31, 76–84 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Taş, N. G. & Gökmen, V. Phenolic compounds in natural and roasted nuts and their skins: A brief review. Curr. Opin. Food Sci.14, 103–109 (2017). [Google Scholar]
- 60.Stuetz, W., Schlörmann, W. & Glei, M. B-vitamins, carotenoids and α-/γ-tocopherol in raw and roasted nuts. Food Chem.221, 222–227 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Perren, R. & Escher, F. E. 8 - Impact of roasting on nut quality. in Improving the Safety and Quality of Nuts (ed. Harris, L. J.) 173–197Woodhead Publishing, 80 High Street, Sawston, Cambridge CB22 3HJ, UK, (2013). 10.1533/9780857097484.2.173
- 62.Abdelhamid, A. S. et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev CD003177 (2018). (2018). [DOI] [PMC free article] [PubMed]
- 63.Lykomitros, D., Fogliano, V. & Capuano, E. Drivers of preference and perception of freshness in Roasted Peanuts (Arachis spp.) for European consumers. J. Food Sci.83, 1103–1115 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Sacks Frank, M. et al. Effects on blood pressure of reduced Dietary Sodium and the Dietary approaches to stop hypertension (DASH) diet. N. Engl. J. Med.344, 3–10 (2001). [DOI] [PubMed] [Google Scholar]
- 65.Albarracín, W., Sánchez, I. C., Grau, R. & Barat, J. M. Salt in food processing; usage and reduction: A review. Int. J. Food Sci. Technol.46, 1329–1336 (2011). [Google Scholar]
- 66.Kumari, S. et al. Does ‘activating’ nuts affect nutrient bioavailability? Food Chem.319, 126529 (2020). [DOI] [PubMed] [Google Scholar]
- 67.Hurrell, R. & Egli, I. Iron bioavailability and dietary reference values1234. Am. J. Clin. Nutr.91, 1461S–1467S (2010). [DOI] [PubMed] [Google Scholar]
- 68.Gibson, R. S., Bailey, K. B., Gibbs, M. & Ferguson, E. L. A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food Nutr. Bull.31, 134–146 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Guo, C. et al. Influence of different cooking methods on the nutritional and potentially harmful components of peanuts. Food Chem.316, 126269 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Sanderson, E. et al. Mendelian randomization. Nat. Rev. Methods Primers. 2, 6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files. The GWAS summary data related to exposure are available at https://gwas.mrcieu.ac.uk/. The GWAS summary data of FinnGen consortium are available at https://www.finngen.fi/en. The GWAS data of mediating factors are available at https://magicinvestigators.org/, http://csg.sph.umich.edu/willer/public/glgc-lipids2021/ and https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium.