Abstract
Tendon injuries present significant medical, social, and economic challenges globally. Despite advancements in tendon injury repair techniques, outcomes remain suboptimal due to inferior tissue quality and functionality. Tissue engineering offers a promising avenue for tendon regeneration, with biocompatible scaffolds playing a crucial role. Ostrich eggshell membrane (ESM), characterized by a strong preferential orientation of calcite crystals, forms a semipermeable polymer network with excellent mechanical properties compared to membranes from other bird species, emerging as a potential natural scaffold candidate. Coupled with platelet-rich plasma (PRP), known for its regenerative properties, ESM holds promise for improving tendon repair. This study aims to evaluate the biocompatibility and efficacy of an ESM-PRP scaffold in treating Achilles tendon ruptures, employing in vitro and in vivo assessments to gauge its potential in tendon regeneration in living organisms. Ostrich ESM was prepared from pathogen-free ostrich eggs, sterilized with UV radiation and prepared in desired dimensions before implantation (1.5 × 1 cm). High-resolution scanning electron microscopy (HRSEM) was utilized to visualize the sample morphology and fiber bonding. In vitro biocompatibility was assessed using the MTT assay and DAPI staining, while in vivo biocompatibility was evaluated in a rat model. For the in vivo Achilles tendinopathy assay, rats were divided into groups and subjected to AT rupture followed by treatment with ESM, PRP, or a combination. SEM was employed to evaluate tendon morphology, and real-time PCR was conducted to analyze gene expression levels. The in vivo assay indicated that the ESM scaffold was safe for an extended period of 8 weeks, showing no signs of inflammation based on histopathological analysis. In the Achilles tendon rupture model, combining ESM with PRP enhanced tendon healing after 14 weeks post-surgery. This finding was supported by histopathological, morphological, and mechanical evaluations of tendon tissues compared to normal tendons, untreated tendinopathy, and injured tendons treated with the ESM scaffold. Gene expression analysis revealed significantly increased expression of Col1a1, Col3a1, bFGF, Scleraxis (Scx), and tenomodulin in the ESM-PRP groups. The findings of our study demonstrate that the combination of Ostrich ESM with PRP significantly enhances AT repair and is a biocompatible scaffold for the application in living organisms.
Keywords: Achilles tendon ruptures, Biocompatibility, Ostrich eggshell membrane, Platelet-rich plasma, Tendon injuries, Tissue engineering
Subject terms: Musculoskeletal models, Biomaterials, Genetics research, Preclinical research, Translational research, Cells, Musculoskeletal system, Trauma, Electron microscopy
Introduction
Tendon, a structurally complex tissue, serves the essential role of translating muscular contractions into joint movement by transmitting forces from muscle to bone1. Each year, approximately 60 thousand patients in the United States experience tendon injuries, posing significant medical, social, and economic challenges globally2. The Achilles tendon (AT) stands as the body’s thickest and strongest tendon, forming from the merging of the gastrocnemius and soleus muscles3,4. Injuries to the AT are common among individuals engaging in vigorous physical activities like running and jumping, prevalent in sports such as volleyball, tennis, soccer, and badminton5–10. Sports-related Achilles tendinopathy often manifests as a complete rupture of the tendon11, characterized by hypoxic and mucoid degeneration, tissue necrosis, limited vascular supply, cell necrosis, calcification, and irregular collagen fibers12–14.
Despite advancements in tendon injury repair techniques, clinical outcomes remain suboptimal due to the inferior quality and functionality of the repaired tissue15,16. The process of tendon regeneration, such as following rotator cuff tears, is recognized as intricate and gradual, presenting a clinical challenge in achieving effective tendon repair. Factors such as patient age, tendon condition, and tear size contribute to varying re-rupture rates17,18. Throughout the healing process, excessive disorganized collagen deposition occurs, leading to the formation of scar tissue and adhesions between newly formed and adjacent tissues19. Moreover, there is an increase in collagen type III at the injury site during the healing phase20. These factors collectively diminish the mechanical properties of the repaired tendon, thereby elevating the risk of re-rupture later in life. The treatment of ruptured tendons has transitioned from nonsurgical techniques to open surgeries and now to minimally invasive methods. Despite lower re-rupture rates associated with surgical treatment, this approach still presents complications such as scar formation, infection risk, and prolonged recovery21–23. Currently, allograft and autograft reconstruction techniques are utilized for treating AT ruptures. However, allograft procedures may lead to rejection or disease transmission, while autografts are constrained by limited availability24. Consequently, there is an urgent and unmet need to address how to effectively and biologically repair injured tendons, including Achilles tendinopathy.
Tissue engineering and regenerative medicine, which involve the use of biocompatible scaffolds, offer new options for reconstructing damaged tendon tissues25–27. A wide range of natural and synthetic polymeric scaffolds have been utilized in regenerative medicine. However, these biomedical applications and tissue engineering regeneration strategies often lack cellular recognition28,29. A suitable scaffold must possess several essential properties, including biocompatibility, biodegradability, high mechanical strength, and non-toxicity30–34. Previous studies have explored the use of the amniotic membrane as a potential scaffold in tissue engineering; however, its limitations necessitate the search for a more suitable and cost-effective alternative35.
Natural eggshell membrane (ESM) has emerged as a promising and valuable bioresource in tissue regeneration, attributed to its availability, biocompatibility, and low toxicity36. Proteins constitute the primary components of the inner ESM fibers, accounting for 80–85% of its composition. Each fiber comprises a core rich in collagen (types I, V, and X) and a cortex abundant in glycoproteins37–44. Additionally, previous studies have identified the presence of other proteins such as lysozyme45, ovotransferrin46, ovalbumin47, ovocalyxin-3648,49, desmozine42, isodesmozine50, osteopontin, sialoprotein, and keratin51 within the ESM structure. Amino acids also contribute to the chemical composition of ESM, with proline, glutamic acid, glycine, serine, hydroxyproline, aspartic acid, valine, threonine, arginine, leucine, alanine, histidine, and lysine being among the amino acids found in ESM52. These components have been proven to assist in the healing of wound and tendons in previous studies53–56. Studies have also demonstrated anti-microbial activities of these components, such as ovocalyxin-36, lysozyme, and ovotransferrin48,57. Ostrich eggshell membrane represents an abundant industrial and household waste characterized by a strong preferential orientation of calcite crystals. Its structure forms a semipermeable polymer network with excellent mechanical properties compared to membranes from other bird species58.
One popular strategy to stimulate regeneration in poorly healing tissues like tendons is the use of platelet-rich plasma (PRP)59. PRP is derived from centrifuging whole blood, resulting in a concentration of platelets rich in various growth factors such as transforming growth factor-b1, insulin-like growth factors 1 and 2, vascular endothelial growth factor (VEGF), basic fibroblast growth factor, and hepatocyte growth factor (HGF)60–62. These growth factors play crucial roles in platelet adhesion and activation, fibrin formation, and the release of intracellular stores, predominantly a-granules (50–80 a-granules per platelet), dense granules (3–5 granules per platelet), and lysosomes63. Previous studies have indicated that PRP initially inhibits excessive inflammation and subsequently promotes proliferation and maturation, while also enhancing fibroblast production and collagen synthesis in the early stages of treatment64–66. Over the long term, PRP leads to increased collagen and glycosaminoglycans content, enhanced collagen organization, metabolic activity, improved load to failure, and elastic modulus62. While several studies have reported the benefits of PRP injection for the treatment of AT injuries, few have investigated its effects in combination with a scaffold.
This study introduces a novel, completely natural scaffold composed of Ostrich ESM and PRP, designed to address AT rupture. The innovative use of ESM leverages its unique biological properties, offering a promising alternative for developing advanced tendon repair materials. The biocompatibility of ESM was rigorously evaluated through in vitro assays (MTT and DAPI staining) and in vivo implantation, confirming its safety and non-toxicity. Furthermore, the study investigated the scaffold’s therapeutic potential in an in vivo AT rupture model, where the combination of ESM and PRP demonstrated significant regenerative effects. Comprehensive evaluations, including histopathological analysis, Scanning Electron Microscopy (SEM), and mechanical property assessments, underscored the scaffold’s capacity to support tendon healing. These findings highlight the potential of ESM as a groundbreaking approach in tendon tissue engineering.
Materials and methods
Ostrich ECM
Preparation
Pathogen-free ostrich eggs were obtained from a local market. The eggs were rinsed with sterile distilled water and disinfected with ethanol. After removal of the albumin and yolk from eggs, the eggshells were washed several times with normal saline and the ESM were carefully peeled off from eggshells with sterile forceps. The detached ESM was then placed in normal saline for 1 to 2 h before being kept in a falcon for 24 h at −70 °C. Following this, the ECM were subjected to freeze-drying for 48 h until completely dried. Samples of membranes were sterilized with UV radiation and prepared in desired dimensions before implantation (1.5 × 1 cm).
ESM characterization
High-resolution scanning electron microscopy (TESCAN-vega3, TESCAN Czech Republic) was employed to visualize the sample morphology and fiber bonding. ESM scaffolds (1 cm2) were sputter-coated with gold for 120 s before observation to reduce the charging effect. Images with different magnifications were captured to provide a better view of the fibers’ morphology. The HRSEM micrographs were taken using a voltage of 5 kV67,68.
In vitro biocompatibility
MTT assay
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay was used to investigate cell viability six days after seeding. 5000 seeded cells were used for each assay. Cell-seeded scaffolds were rinsed with PBS, and MTT solution at 0.5 mg/ml was added to each well and incubated for 4 h. The yellow MTT dye was reduced by the mitochondrial reductase enzyme in living cells to purple Formosan after the incubation period; the Formosan was then dissolved with 400 µl of DMSO, and its absorbance was measured with a spectrophotometer at 590 nm. The cell survival assessment was conducted on days 3 and 6 following cell culture as per previous studies69,70.
DAPI staining
ESM samples were placed in a 24-well plate and treated with cells for 72 h. The procedure involved adding 2 ml of 4% paraformaldehyde to each well to fix the scaffolds at room temperature for 30 min. The paraformaldehyde was then removed, and 2 ml of 0.01 mol/l Phosphate Buffered Saline (PBS) was added to each well for rinsing for 5 min. The PBS was discarded and the process repeated. Subsequently, 5 µg/ml DAPI (Sigma) was added to each well, and the plate was covered and incubated in the dark for 5 min at room temperature. After incubation, DAPI was removed, and 2 ml of 0.01 mol/l PBS was added to each well for rinsing. The plate was rinsed for 10 min, and this step was repeated until no more dye could be removed. Cell residue was observed in random fields of view using a Leica laser confocal microscope (Leica Microsystems GmbH) and a normal light microscope. The present evaluation verified whether there was cell residue inside the scaffold. Additionally, HRSEM micrographs were taken using a voltage of 5 kV for a better view of the cells.
After being cultured for a predetermined period, the specimens with attached cells were gently rinsed three times in PBS and then immersed in PBS containing 2.0% glutaraldehyde for 4 h to fix the cells. Following fixation, they were rinsed three times with PBS for 10 min each. Subsequently, they underwent dehydration through a graded series of ethanol solutions (30%, 50%, 70%, 90%, and 95%) for 10 min each, followed by immersion in 100% ethanol for an additional 30 min. Afterward, the samples were air-dried for at least 10 h. The dry cell-seeded scaffolds were then mounted on aluminum stubs, sputter-coated with gold–palladium, and examined under a scanning electron microscope.
In vivo biocompatibility assay
Animals
For in vivo assessment, a total of 18 adult male Wistar rats weighing 180 ± 5 g were used in this experiment. The animals were housed in a controlled environment with a 12-hour light/12-hour dark cycle at 21 ± 1 °C and a humidity of 55 ± 5%. Prior to the experiments, a seven-day acclimatization period was provided. The rats were granted ad libitum access to food and water throughout the study period. All experimental procedures were ethically approved by the Ethics Committee of Shiraz Medical University under permission number M1310.
In vivo model
To assess the in vivo biocompatibility of the scaffolds, the prepared specimens were subcutaneously implanted. Eighteen male rats were evenly distributed into three groups for the experiment, which were sacrificed after the first, fourth, and eight week of scaffold implantation. Anesthesia was induced via intramuscular injection of 90 mg/kg ketamine HCl and 8 mg/kg xylazine. Sterile surgical technique was applied throughout the experiment. Prior to implantation, all scaffolds were sterilized using gamma radiation. The implantation site was shaved and sterilized with Betadine. A transverse incision of approximately 2 cm was made on the dorsal side of each rat using a sterile surgical blade, followed by dissection of the underlying connective tissue. The prepared ECM scaffolds were then implanted into the subcutaneous tissue, and the incision was closed using 5.0 sutures (Fig. 1). Each rat was housed under standard conditions for a duration of eight weeks post-surgery and monitored for any clinical signs or symptoms. At weeks 1, 4, and 8 post-implantation, the rats were euthanized, and the implanted samples along with surrounding tissues were harvested and fixed in neutral buffered formalin 10% for subsequent histological studies.
Fig. 1.
Implantation and grafting of the scaffold onto the ruptured tendon.
Histopathologic assay
For histological analysis, the skin region implanted with scaffolds and its surrounding tissues were immersed in a 10% formalin solution and fixed for 48 h prior to routine processing. Subsequently, they underwent processing and embedding in paraffin blocks. Thick layers (5 mm longitudinal sections) were prepared using a microtome and stained with hematoxylin and eosin (H&E). Microscopic images were then captured using an Olympus microscope (model BX53F, Japan). The specimens were evaluated by a blind pathologist for inflammation, cell infiltration, scar tissue formation, and neovascularization. The average of these values was determined for each animal, and a median score for each group and organ was assessed71.
In vivo Achilles tendinopathy assay
Animal
Fifty adult male Wistar rats (180 ± 5 g body weight) were used for this experiment. Similar to our previous assessment, the animals were housed in a controlled environment with a 12-hour light/12-hour dark cycle at 21 ± 1 °C and a humidity of 55 ± 5%. Prior to the experiments, a seven-day acclimatization period was provided, during which the rats had ad libitum access to food and water.
In vivo model
The fifty rats were divided into five groups:
Control Positive (CP) Group: Normal control group where ten rats were kept without injuries.
Control Negative (CN) Group: Negative control group where AT rupture was induced, but no graft was applied. The AT was removed, and the sides of the rupture were sutured together.
Scaffold (SC) Group: Treatment group where double-layered ESM was grafted onto the ruptured tendon by suturing on both sides.
Scaffold PRP (SP) Group (SP): Treatment group where double-layered ESM with PRP was grafted onto the injured site by suturing on both sides.
Scaffold Self-derived PRP (SS) Group: Treatment group where double-layered ESM with self-derived PRP was grafted onto the ruptured AT by suturing on both sides.
To induce AT rupture, the rats were anesthetized with an intramuscular injection of ketamine (90 mg/kg) and xylazine (8 mg/kg). The area around the ankle was shaved and sterilized with Betadine. A small incision was made in the skin above the AT, and the tendon was sliced using a scalpel (1.5 cm). The incision was closed with 3.0 mm propylene suture, and each rat was housed under standard conditions for fourteen weeks post-surgery. The general condition of the rats was evaluated weekly post-surgery. After 14 weeks, the gait of the rats was evaluated, followed by euthanasia using CO2 (70%) and the tendons were extracted for further evaluation. Our model was adapted from previous evidence and studies72–74.
Preparation of PRP
To obtain PRP from the rats, they were anesthetized using an intramuscular injection of xylazine (8 mg/kg) and ketamine HCl (90 mg/kg). Subsequently, the rats underwent jugular vein cannulation. A volume of 3.15 ml of blood was collected from each animal using a 5 ml syringe containing 0.35 ml of 10% sodium citrate. The collected blood was transferred to 5 ml silicone vacuum tubes. Following blood collection, an equal volume of sterile saline was injected into the animals. The blood from each rat was centrifuged at 22 °C for 20 min. The upper fraction, corresponding to the plasma, was then transferred to separate 5 ml silicone vacuum tubes. After further centrifugation of the plasma for 15 min, the lower fraction, containing the PRP, was obtained. The PRP profile included the following parameters: WBC: 0.5 × 103/µL, RBC: 0.04 × 106/µL, Hemoglobin: 0.2 g/dL, platelet: 1180 × 103/µL.
SEM assay
To evaluate the morphology of the repaired AT, the tendons were observed using SEM (TESCAN-Vega 3, Czech Republic). To prepare the samples, they were rinsed in distilled water three times, fixed with 2.5% EM-grade glutaraldehyde for one hour, and triple-rinsed in distilled water. The samples were bisected and dehydrated using graded ethanol. To minimize charging effects, the AT samples were sputter-coated with gold palladium for 120 s. Subsequently, they were examined under SEM at various magnifications.
Histopathologic assay
For histological analysis, the animals were euthanized using CO2 (75%). The ATs were excised and placed in 10% formalin solution for 72 h. After dehydration with ethanol, the specimens were embedded in paraffin and longitudinally bisected. The sections were stained with hematoxylin–eosin (H&E), and microscopic images were captured using an Olympus microscope (model BX53F, Japan). The specimens were evaluated for inflammatory responses, edema or calcification in the paratendon, collagen fibrils, and neovascularization based on the Backman method75.
RNA isolation and real-time PCR analysis
Total RNA was isolated from the AT tissue using an RNA extraction kit (FavorPrep Tissue Total RNA Mini Kit, TAIWAN). The purity, integrity, and concentration of RNA were assessed by measuring the optical density at 260/280 nm using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). The isolated RNA was then stored at −80 °C until further processing for Complementary DNA (cDNA) synthesis and agarose gel (1%) electrophoresis. cDNA was synthesized from 1 µg of RNA using the RevertAid First Strand cDNA Synthesis Kit (Fermentas Inc.). Real-time PCR was conducted following the protocol of RealQ Plus 2× Master Mix Green (Ampliqon Inc.) in an Applied Biosystems StepOne Instrument (ABI, Step One, USA). For each reaction, 200 nM of specific primers targeting the sequences of interest were added (see Table 1 for primer details). The specific primers targeted genes including collagen type I alpha 1 chain (Col1a1), collagen type III alpha 1 chain (Col3a1), basic fibroblast growth factor (bFGF), Scleraxis (Scx), bHLH transcription factor, and tenomodulin. Additionally, the β2 microglobulin (B2M) housekeeping gene was used as an internal control for real-time PCR reactions. The real-time PCR conditions were set for 10 min at 94 °C, followed by 40 cycles of 15 s at 94 °C, 60 s at 60 °C and extension steps. Following each real-time PCR run, gel electrophoresis and melting curve analysis were performed to confirm the specific amplification of targets. The amplification signals of different samples were normalized to the cycle threshold (Ct) values of B2M, and the delta-delta Ct (2-ΔΔCT) method was employed to compare the mRNA levels of test samples versus controls. The results were expressed as fold changes in data analysis76.
Table 1.
List of utilized primers in the study.
| Genes | Primer sequences | Sizes (bp) |
|---|---|---|
| B2m | Forward: 5′-CGTGCTTGCCATTCAGAAA-3′ | 244 |
| Reverse: 5′-ATATACATCGGTCTCGGTGG-3′ | ||
| Col1a1 | Forward: 5′-CTGCCCTCCTGACGCATGG-3′ | 151 |
| Reverse: 5′-CATAGCACGCCATCGCACAC-3′ | ||
| Col3a1 | Forward: 5′-TGGTGGCTTTCAGTTCAGCTA-3′ | 157 |
| Reverse: 5′-ATTGCCATTGGCCTGATCCA-3′ | ||
| bFGF | Forward: 5′-GCGGCTCTACTGCAAGAA-3′ | 209 |
| Reverse: 5′-CTTCTGTAACACACTTAGAAGCC-3′ | ||
| Scleraxis (Scx) | Forward: 5′-CAACCAGAGAAAGTTGAGCAAAGA-3′ | 295 |
| Reverse: 5′-CATGGAAAGTTCCAGTGGGC-3′ | ||
| Tenomodulin | Forward: 5′-TCCCACTCTAATAGCAGTTTCAGA-3′ | 169 |
| Reverse: 5′-AAGGTCTTCCTCGCTTGCTT-3′ |
bp base pair, Col1a1 collagen type I alpha 1 chain, Col3a1 collagen type III alpha 1 chain, bFGF basic fibroblast growth factor, Scx Scleraxis bHLH transcription factor.
Statistical analysis
All data were recorded and prepared in Microsoft Excel and subsequently analyzed using the Statistical Package for the Social Sciences (SPSS) software, version 26. Student’s t-test analysis and analysis of variance (ANOVA) test were conducted to assess statistical significance. Additionally, the Mann–Whitney U test was employed to compare the group medians of histopathologic results. For the real-time PCR results, data were expressed as the mean ± standard error of the mean (SEM) and analyzed using GraphPad Prism 8 software (GraphPad Software, Inc., La Jolla, CA, USA). A statistically significant value was determined based on a significance level of P < 0.05.
Results
Morphology of ostrich ESM
The SEM images of the cross-section of the ESM were captured to assess the morphology of the ESM. As depicted in Fig. 2, the ESM fibers exhibited a random orientation and were not aligned in a specific direction. These fibers were observed to occupy different planes, forming a cross-weave pattern within the ESM. Analysis of the fibers’ diameter revealed an average diameter of 2.49 ± 0.89 μm. The distribution of fiber diameters, illustrated in Fig. 2D through a Box and Whisker plot, demonstrated variability in thickness among the fibers. This variability in thickness can be attributed to the tendency of the fibers to merge, as evidenced by the SEM images of the ESM surface (Fig. 2A–C).
Fig. 2.
Scanning electron microscopy images of the ostrich eggshell membrane. Yellow arrows show the scale bar. Magnification of images are (A) ×500; (B) ×1000; (C) ×5000; (D) Box and Whisker plot of the ESM fiber diameters, n = 100.
In vitro biocompatibility
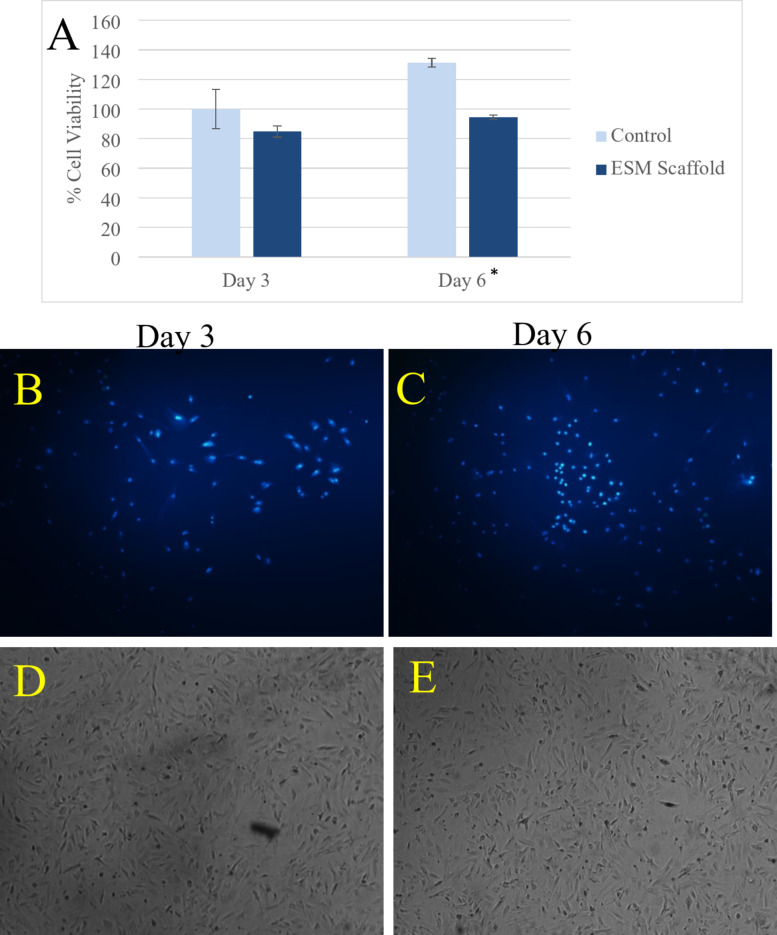
To evaluate the cell viability of the ESM, the MTT assay was employed to assess both the cytotoxicity of the ESM and its capacity to proliferate cell growth (Fig. 3A–E). As depicted in Fig. 3A, the cell survival assessment was conducted on days 3 and 6 following cell culture. The cell viability percentages of the ESM scaffold were determined to be 84.77 ± 3.74 and 94.51 ± 1.50 on days 3 and 6, respectively. According to the literature, cell viability exceeding 80% is considered indicative of biocompatibility71. Therefore, the MTT assay results indicated that the ESM scaffolds were non-toxic to the mesenchymal stem cell lines. Additionally, the ESM demonstrated a slight promotion of cell growth from the 3rd day to the 6th day.
Fig. 3.
The results of the cell viability assay of the eggshell membrane. (A) The cell viability percentages in days 3 and 6 after cell culture. * Indicates a P-value < 0.05; (B) The light microscopy images of the DAPI-stained viable cells in the 3rd day post-culture; (C) The light microscopy images of the DAPI-stained viable cells in the 6th day post-culture; (D) The SEM images of the DAPI-stained viable cells in the 3rd day post-culture; (E) The SEM images of the DAPI-stained viable cells in the 6th day post-culture.
In addition to the MTT assay, cellular growth and proliferation were observed using light microscopy and SEM with DAPI staining. As depicted in Fig. 3B, C, cells loaded onto the ESM scaffolds were viable after 3 and 6 days. These images also revealed a slight increase in cell proliferation facilitated by the scaffold.
In vivo biocompatibility
To better understand the biological response to the ESM membrane, an in vivo biocompatibility assay was conducted. Eighteen male Wistar rats were utilized to assess the biocompatibility of the ESM at 7-, 28-, and 56-days post-implantation of the scaffolds. Images representing the implantation site and histopathological assessment of tissues using H&E staining are presented in Fig. 4. Macroscopic evaluation of the implanted site revealed that during the initial week, chronic inflammation was observed at the site of implantation. However, this inflammatory response gradually diminished over time, with inflammation decreasing significantly four weeks post-surgery (Fig. 4B1). By the end of 8 weeks, there were no significant signs of inflammation in response to ESM implantation into the body (Fig. 4C1–C3). H&E staining of tissues corroborated these findings. As depicted in Fig. 4A1–A3, at 7 days post-surgery, the implantation site exhibited moderate chronic inflammatory cell infiltration and foreign body type giant cell reaction (*) around the membrane (arrow). By four weeks post-surgery, the tissue displayed mild chronic inflammatory cell infiltration without foreign body type giant cell reaction (Fig. 4B1,B2). Finally, at 56 days post-implantation, tissue sections showed no inflammatory response, neither chronic inflammation nor giant cell reaction (Fig. 4B1–B3).
Fig. 4.
Histopathological evaluation of bioavailability of ostrich eggshell membrane over time. (A: A1) Macroscopic image of the implanted site 7 days post-surgery; (A2, A3) Histopathological images of the implanted site 7 days after implantation, with magnifications of ×40 and ×200, respectively. These images depict moderate chronic inflammatory cell infiltration and foreign body type giant cell reaction (*) around the membrane (arrow). (B: B1) Macroscopic image of the implanted site 4 weeks post-surgery; (B2, B3) Histopathological images of the implanted site 4 weeks after implantation, with magnifications of ×40 and ×200, respectively. These sections show mild chronic inflammatory cell infiltration without foreign body type giant cell reaction. (C: C1) Macroscopic image of the implanted site 8 weeks post-surgery; (C2, C3) Histopathological images of the implanted site 8 weeks after implantation, with magnifications of ×40 and ×200, respectively. These sections demonstrate no inflammatory response, neither chronic inflammation nor giant cell reaction.
In vivo model of Achilles tendinopathy
Gait and posture evaluation
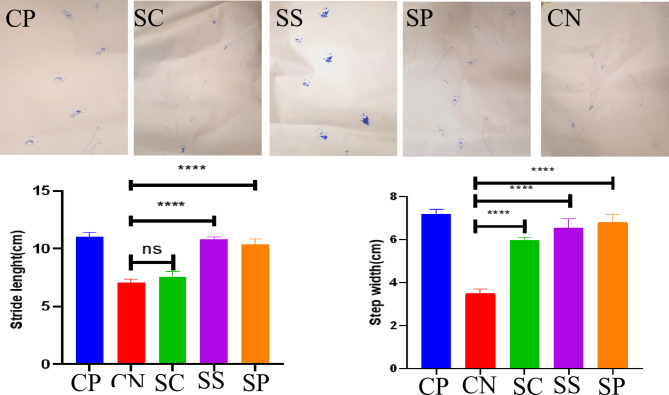
The ESM was grafted onto the ruptured AT of rats to assess its potential for tendon tissue recovery. Fourteen weeks post-surgery, the gait behavior of rats was examined to evaluate the recovered AT. The results of stride length and step length of the treated rats at 14 weeks post-surgery are presented in Fig. 5. It was observed that the stride length was higher in all treatment groups compared to the negative control group (CN). However, the SC treatment group showed no significant improvement in stride and step length (Fig. 5). Conversely, the treatment groups receiving scaffolds combined with PRP (either self (SS) or non-self (SP)) exhibited superior results compared to both the negative control group and the treatment group using only ESM scaffolds.
Fig. 5.
Gait analysis of rats 14 weeks post-surgery; CP Positive control group, where rats had normal tendons; CN Negative control group, where rats with Achilles tendinopathy received no treatment; SC Treatment group, where Ostrich eggshell membrane was grafted to the ruptured Achilles tendon; SP Treatment group, where Ostrich eggshell membrane combined with platelet-rich plasma was grafted to the ruptured Achilles tendon; ***P-value < 0.05; ns not statistically significant.
Morphology and histopathological analysis
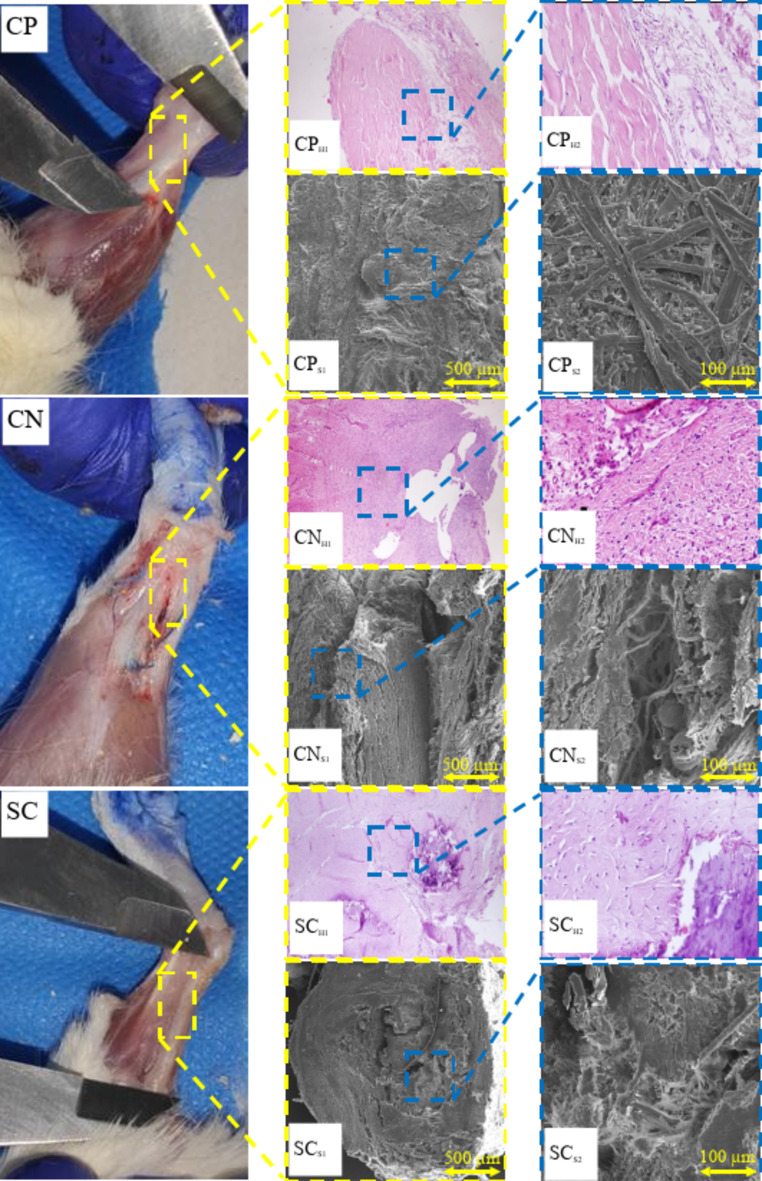
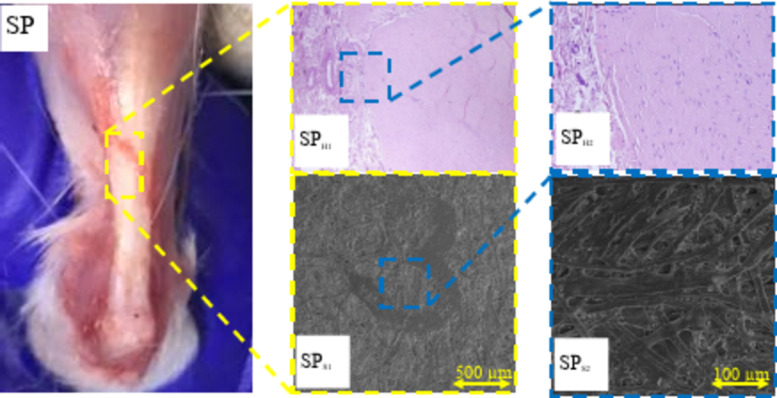
The H&E images of the tendons in the groups receiving the ESM scaffold (SC) and the ESM scaffold combined with PRP (SP) were captured and compared with those of the negative control group (CN) and the positive control group (CP). Additionally, SEM images of both treated and untreated groups were obtained to assess the effect of the ESM scaffold and ESM combined with PRP on tendon fiber formation (Fig. 6).
Fig. 6.
Histological and scanning electron microscopy (SEM) evaluation of tendon regeneration with Ostrich eggshell membrane (ESM) and platelet-rich plasma (PRP) treatment. H&E staining images of tendon tissue from the negative control (CN), positive control (CP), Ostrich ESM scaffold (SC), and Ostrich ESM scaffold combined with PRP (SP) groups are compared. SEM images depict the morphology of tendon fibers in each group. Normal tendons (CP) display organized fiber alignment and dense collagen fibrils (CPH1, CPH2). In contrast, CN group exhibits disorganized collagen bundles and signs of inflammation (CNH1, CNH2). SC group shows improved collagen fibril differentiation but disorganized fibers (SCS1, SCS2). SP group demonstrates enhanced collagen fibril differentiation and aligned fibers (SPS1, SPS2), with reduced inflammation and calcification (SPH1, SPH2).
The SEM images of the normal tendons (CP) revealed organized fiber alignment and a clear distinction between collagen fibrils and fibers (Fig. 6 CPS1, CPS2). As depicted in Fig. 6 CPS1 and CPS2, the fibers in the CP group formed a dense network through interweaving.
In contrast, the untreated control group (CN) displayed not well-organized fibers with low collagen fibril to fiber differentiation (Fig. 6 CNS1, CNS2). Grafting the ESM onto the tendon site improved collagen fibril to fiber differentiation, although fiber organization remained suboptimal in this group (Fig. 6 SCS1, SCS2). Lastly, as shown in Fig. 6 SPS1, SPS2, the combination of ESM with PRP enhanced collagen fibril differentiation and promoted better fiber alignment along the tendon axis. Overall, SEM images indicated that tendon fibers in the SP group exhibited superior alignment and increased fiber differentiation compared to the CN and SC groups.
The H&E staining of the tendon tissue in the normal group (CP) revealed dense, regular, and organized collagen bundles within the connective tissue part of the tendon (Fig. 6 CPH1, CPH2). Notably, the tendon tissue exhibited a characteristic crimp pattern, where nuclei and cytoplasm formed a sinusoidal wave, as observed in Fig. 6 CPS1, CPS2. Minimal neutrophil presence in the histological images indicated an absence of inflammatory response in this group. Furthermore, there was no evidence of edema or calcification in the paratendon tissue.
In contrast, the negative control group (CN) exhibited disorganized collagen bundles within the tendon tissue, consistent with findings from SEM images. Moderate and chronic inflammation was highlighted by the presence of neutrophils in Fig. 6 CNH1, CNH2.
For the SC group, where the ESM was grafted onto the ruptured tendon, histopathological analysis revealed relatively disorganized collagen bundles at the tendon site. Additionally, extensive calcification deposition was observed in the paratendon site, accompanied by mild inflammation indicated by neutrophil presence (Fig. 6 SCH1, SCH2). However, the addition of PRP to the ESM in the SP treatment group inhibited calcium deposition within the tendon tissue. As depicted in Fig. 6 SPH1, SPH2, there was no evidence of calcification deposition, and collagen bundles appeared well-organized in this group.
Tensile strength
The mechanical strength of the treated tendons was evaluated using stress-strain assays performed 14 weeks after surgery. The results are presented in Table 2. Normal tendons exhibited a high Young’s modulus of 71.01 ± 10.75 MPa and an ultimate strength of 46.75 ± 4.59 N. In comparison, the SC group (Scaffold) showed a Young’s modulus of 15.27 ± 4.41 MPa and an ultimate strength of 41.42 ± 14.90 N. Meanwhile, the SP group (Scaffold + PRP) exhibited an ultimate strength of 48.91 ± 15.56 N and a Young’s modulus of 20.90 ± 7.53 MPa. However, the differences in the data were not statistically significant (P-Value > 0.05).
Table 2.
Mechanical strength evaluation of treated tendons 14 weeks post-surgery. Young’s modulus and ultimate strength are presented for the positive control group (normal tendons), the treatment group using scaffold only (SC), and the treatment group using scaffold combined with platelet-rich plasma (SP). Data are expressed as mean ± standard deviation.
| Group | Young’s modulus (MPa) | Ultimate strength (N) |
|---|---|---|
| Positive control | 71.01 ± 10.75 | 46.75 ± 4.59 |
| SC (Scaffold) | 15.27 ± 4.41 | 41.42 ± 14.90 |
| SP (Scaffold + Platelet-Rich Plasma) | 20.90 ± 7.53 | 48.91 ± 15.56 |
Gene expression
The gene expression analysis related to tendon regeneration and tenogenesis reveals that the surgical tissue exhibits high expression levels of Col1a1, Col3a1, bFGF, Scx, and tenomodulin, all crucial for the structural organization of the tendon. In comparison to the CP (control) group, the CN group exhibits a significant decrease in all genes (P < 0.05). However, both the SS (1.27-fold, P = 0.008) and SP (1.49-fold, P = 0.003) groups show a significant up-regulation in the expression of Col1a1 compared to the CN group (0.31-fold). Additionally, the Col3a1 gene expression is increased in the SC (0.78-fold, P = 0.019), SS (2.14-fold, P < 0.001), and SP (2.07-fold, P < 0.001) groups compared to the CN group (0.42-fold). Analysis of bFGF, Scx, and tenomodulin gene expression reveals a similar upward trend in the SS (1.17, 0.65, and 0.68, respectively) and SP (0.89, 0.53, and 0.82, respectively) groups compared to the CN group (0.21, 0.08, and 0.15, respectively) (P < 0.05) (Fig. 7).
Fig. 7.
Impact of double-layered Ostrich eggshell membrane (ESM) and platelet-rich plasma (PRP) on the gene expression related to tendon regeneration. The control negative (CN) group served as the control, where Achilles tendon rupture was induced without treatment. mRNA levels of Col1a1, Col3a1, bFGF, Scleraxis (Scx), and tenomodulin were quantified using real-time PCR in different groups; (A) The mRNA expression analysis for Col1a1 gene expression level; (B) Effect of ESM and PRP on Col3a1 expression in the groups; (C) bFGF mRNA expression levels in the groups; (D) Scleraxis (Scx) gene expression after the effect of ESM and PRP on tendon tissue; (E) The mRNA expression analysis for tenomodulin gene expression level. Expression data, relative to those of the reference gene, from at least three independent assays are presented as mean ± SEM. Statistical significance was tested using one-way ANOVA. * denotes P value < 0.05; ** denotes P value < 0.01.
Discussion
The study investigated Ostrich ESM as a scaffold for AT regeneration. Our findings demonstrate that the combination of Ostrich ESM with PRP significantly enhances AT repair and is a biocompatible scaffold for the application in living organisms. In vitro tests showed no toxicity to cells, and in vivo implantation in rats demonstrated safety over 8 weeks. Combining ESM with PRP in an Achilles tendinopathy model regenerated tendon tissue within 14 weeks, supported by various analyses. Previous research has predominantly employed synthetic scaffolds for tendon repair. Webb et al. utilized poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) combined with collagen and tenocytes to create a scaffold for Achilles tendinopathy treatment in an in vivo rat model, which highlighted collagen’s role in treating tendon injuries77. Sun et al. prepared a functional collagen scaffold tethered with recombinant SDF-1a (CBD-SDF-1a) for rat AT defect regeneration. The scaffold increased CXCR4-positive fibroblast-like cell recruitment and Tenascin-C deposition at 7 days. At 4 and 12 weeks, it enhanced type I collagen expression, collagen fibril diameter, and tendon mechanical properties. This scaffold facilitates tendon regeneration by regulating SDF-1a release, enhancing cell recruitment, and providing a supportive microenvironment78. Furthermore, Gabler et al. introduced a bovine cross-linked collagen scaffold for treating AT defects in an in vivo rat model; however, they encountered a high failure rate of 48%, primarily attributable to suture tear-out from the tendon79. Our study is the first to assess the biocompatibility of the Ostrich ESM scaffold in an in vivo AT model, yielding satisfactory in vivo and in vitro outcomes.
Regarding the morphology of Ostrich ESM, previous studies have also reported similar findings using different microscopy techniques. Richards et al. reported an average diameter of 2.8 ± 1 μm for ostrich ESM fibers using light microscopy. Furthermore, both this study and previous research have indicated that the ESM fibers are rich in type I collagen and contain minerals such as calcium, sulfur, and magnesium80,81.
We evaluated the in vitro biocompatibility of ostrich ESM for further application in living organism. By conducting the MTT assay and considering cell viability exceeding 80% as indicative of biocompatibility, we found that the ESM scaffolds did not exhibit toxicity towards the mesenchymal stem cell lines71. This observed cell growth can be attributed to the collagenous structure of the ESM fibers, which facilitated cellular proliferation82. However, the modest rate of cell proliferation may be attributed to insufficient time for extensive cell growth. This finding is consistent with a previous study conducted by Choi et al. in 202083, which evaluated the MTT assay of chicken ESM on days 1, 7, 14, and 28 post-cell culture. They reported that significant proliferation was not observed until the 7th day, after which the proliferation rate sharply increased83. Another study assessed the proliferation of human dermal fibroblast cells in the presence of chicken ESM and demonstrated that chicken ESM has the potential to promote cell growth84. Overall, it can be concluded that ostrich ESM exhibited no significant toxicity to mesenchymal stem cells and could support cellular proliferation.
In addition to the MTT assay, cellular growth and proliferation were observed using light microscopy and SEM with DAPI staining. As depicted in Fig. 3B, C, cells loaded onto the ESM scaffolds were viable after 3 and 6 days. These images also revealed a slight increase in cell proliferation facilitated by the scaffold. The interwoven and fibrous structure of the ESM can provide physical support for cell adhesion and growth85. Moreover, the high collagen content, presence of glycoproteins, and minerals in ostrich ESM could further enhance cellular proliferation86. In a study by Gharibi and Abdolmaleki, the MG-63 cell line was cultured on neat chicken ESM and chicken ESM modified with citric acid. They found that the modified ESM with citric acid exhibited superior results due to the more aligned fiber morphology86. Therefore, the relatively low proliferation rate of cells loaded onto the ESM in the early days after cell culture may be associated with the non-aligned fibrous structure of the ESM, as evidenced by SEM images of the ESM scaffolds.
Studies have primarily focused on evaluating the in vitro biocompatibility of Avian ESM, with few reporting on in vivo biocompatibility. As demonstrated in the MTT assay, Ostrich ESM was found to be non-toxic to mesenchymal stem cells and promoted cellular growth and proliferation over time. Previous studies have also indicated that ESM can enhance cellular growth in various cell lines, including human dermal fibroblast cells, MG-63, and hemopoietin stem cells84,86,87. The in vivo biocompatibility assay in our study revealed that Ostrich ESM mitigated the inflammatory response over time. This finding aligns with a study by Vuong et al., where they investigated the anti-inflammatory effect of ESM on lipopolysaccharide-induced inflammatory U937–3xκB-LUC cells88. According to their study, ESM inhibited the activity of the transcription factor nuclear factor-κB and increased the secretion of the anti-inflammatory cytokine IL-10, thereby reducing inflammation88. Overall, it is concluded that ESM can attenuate the inflammatory response of the body and is safe for in vivo applications.
We further aimed to evaluate our finding in a in vivo model of Achilles Tendinopathy. The combination of Ostrich ESM with PRP showed promise in enhancing rat gait after 14 weeks, suggesting its potential efficacy in tendon recovery. This finding suggests that PRP played a significant role in the healing of the ruptured AT. Previous studies have also highlighted the significant role of PRP in AT treatment. For instance, Circi et al. evaluated the effect of PRP injection at different time intervals on Achilles tendinopathy treatment in a rat model. Their results demonstrated that immediate PRP injection at the injured site significantly enhanced tendon healing in rats89. Similarly, in a study by Parafirioti et al., eighty male rats were used to assess the impact of PRP injection on AT regeneration. The study concluded that a single PRP injection at the ruptured site facilitated injured tendon regeneration within the first week of treatment90.
Furthermore, the histopathological evaluation and SEM images demonstrated that the addition of PRP to the Ostrich ESM scaffold facilitated tendon tissue regeneration. The Ostrich ESM scaffold promoted fiber differentiation compared to the negative control (CN) group, albeit with signs of calcification in that group. The presence of calcium in the tendon tissue might be attributed to specific features of ESM, such as its morphology and structure. Previous studies have suggested that ESM is an appropriate site for the mammillary knobs where the nucleation of calcium carbonate crystals occur91,92. In contrast, the addition of PRP to the ESM scaffolds enhanced collagen fibril-to-fiber differentiation and resulted in more aligned fibers. PRP contains various growth factors like transforming growth factor-b1, insulin-like growth factors 1 and 2, vascular endothelial growth factor (VEGF), basic fibroblast growth factor, and hepatocyte growth factor (HGF), which promote cellular adhesion and induce fibroblast production, ultimately improving collagen synthesis and organization65,93. Overall, the combination of ESM and PRP facilitated better reformation of tendon fibers compared to the negative control group and the treatment group using only ESM scaffolds.
Regarding tensile strength, the treatment group receiving the ESM scaffold exhibited the lowest Young’s modulus. As previously mentioned, this treatment tended to result in the deposition of calcification in the tendon tissues. The low Young’s modulus observed in this group can be attributed to the accumulation of calcium, which reduces the elasticity of the tendons. Furthermore, the SP group demonstrated higher ultimate strength. The increased ultimate strength in tendons treated with the ESM scaffold combined with PRP is attributed to the well-organized and dense fiber structure of the tendon, as discussed in the Morphology and Histopathology Analysis section. Based on our findings, the elasticity modulus declined in the treatment groups compared to the normal tendons. This result aligns with the findings of a study by Webb et al., where various synthetic scaffolds of PHBHHx, collagen, and tenocytes were used77. In a study by Yu et al., a synthetic electrospun scaffold based on poly(lactic-co-glycolic acid) was developed to treat AT injuries. In their study, the ultimate strength obtained for normal tendons and treated tendons were 4.02 ± 4.74 N and 18.32 ± 2.69 N, respectively94. These data were significantly lower compared to our derived results. Another study utilized a bovine cross-linked collagen scaffold to regenerate defected AT in rat models. In this study, the ultimate strength of normal tendons was reported to be 76.6 ± 11.6 N, which was more similar to our findings95. However, differences in results could be attributed to various factors, such as variations in the average diameters of fibers in the tendon94.
Overall, the real-time PCR results emphasizes the significance of enhanced tendon formation in both ESM-PRP treatment groups. Gene expression shows a significant high expression of Col1a1, Col3a1, bFGF, Scx and tenomodulin both groups, which has also been reported in previous studies96,97. Sugimoto et al. employed a loss-of-function approach to demonstrate that Scx+/Sox9+ progenitors play a functional role in establishing the junction between hyaline cartilage and tendon/ligament tissues98. Scx expression is up-regulated during tendon formation, such as in Achilles and patella tendons, as well as in the cruciate ligaments of the knee joint. Conversely, its expression is down-regulated at sites undergoing chondrogenesis. These findings suggest that the coordinated expression of Scx is tightly regulated to specify and maintain tenocyte, chondrocyte, and osteocyte populations98,99.
Our study has its limitations. CCK-8 and Live-Dead assays are more commonly used to evaluated biocompatibility of a scaffold through in-vitro studies, which was not performed in our study. Although we’ve demonstrated that the ESM-PRP scaffold enhances fibroblast-like cell recruitment and fosters tendon regeneration, we couldn’t identify the proportion of each cell type—mesenchymal stem cells, dermal fibroblasts, and AT fibroblasts—due to the absence of specific markers. Our study also lacks blood and serum test evaluations. We also utilized the RT-qPCR method instead of COL-I and COL-III IHC staining, due to the wider dynamic range and higher sensitivity, and also based on our limited resources100. However, COL I/III ratio could have enhanced the validity of our technique, since successful remodeling is characterized by replacement of type III collagen with type I collagen, while the maintenance of high type ΙΙΙ collagen expression levels is reportedly associated with scar formation and adhesion rather than normal healing. Further research is necessary to determine which cell types contribute to tendon regeneration.
Conclusion
The present study explored the potential of Ostrich ESM as a natural scaffold for tissue regeneration. In vitro biocompatibility testing revealed no significant toxicity to mesenchymal stromal cells. Implantation of ESM in Wister rats demonstrated its safety for long-term use over 8 weeks. Consequently, ESM was deemed suitable for further investigation in tissue regeneration. In an in vivo Achilles tendinopathy model, combining ESM with PRP successfully regenerated tendon tissue within 14 weeks. This conclusion was supported by gait analysis, histopathology, and tensile strength assays, and gene expression of treated tendons. Future studies may explore combining ESM with other materials to enhance mechanical strength and biological efficiency.
Acknowledgements
None.
Author contributions
S.A.E, N.T., and O.K.H designed the study. O.K.H., R.S., A.D., L.A., S.B., A.D., O.A., S.P.K., S.H., A.A.M., R.K., collected the data and performed the laboratory evaluations. R.S. drafted the manuscript. S.A.E., N.T., and O.K.H revised the manuscript. All authors read and confirmed the final version of the manuscript.
Data availability
All data regarding this study has been reported in the manuscript. Please contact the corresponding author if interested in any further information.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All experimental protocols were approved by the Institutional Animal Care and the Ethics Committee of the Shiraz University of Medical Science. Also, the study was carried out in compliance in accordance with the relevant guidelines and regulations and the Declaration of Helsinki and also the ARRIVE guidelines.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Reza Shahriarirad, Email: R.shahriari1995@gmail.com.
Nader Tanideh, Email: tanidehn@sums.ac.ir, Email: tanidehn@gmail.com.
References
- 1.Voleti, P. B., Buckley, M. R. & Soslowsky, L. J. Tendon healing: repair and regeneration. Annu. Rev. Biomed. Eng.14, 47–71 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Raikin, S. M., Garras, D. N. & Krapchev, P. V. Achilles tendon injuries in a United States population. Foot Ankle Int. 34(4), 475–480 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Jarvinen, T. A., Kannus, P., Maffulli, N. & Khan, K. M. Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin.10(2), 255–266 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Arnoczky, S. P. & László, G. Józsa and Pekka Kannus. Champaign, Illinois, Human Kinetics, 1997. $79.00, 573 pp. J. Bone Joint Surg. 81(1), 148 (1999).
- 5.Jarvinen, M. Epidemiology of tendon injuries in sports. Clin. Sports Med.11(3), 493–504 (1992). [PubMed] [Google Scholar]
- 6.Maffulli, N. & Kader, D. Tendinopathy of tendo achillis. J. Bone Joint Surg. Br.84(1), 1–8 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Paavola, M. et al. Achilles tendinopathy. J. Bone Joint Surg. Am.84(11), 2062–2076 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Johansson, C. Injuries in elite orienteers. Am. J. Sports Med.14(5), 410–415 (1986). [DOI] [PubMed] [Google Scholar]
- 9.Kvist, M. Achilles tendon injuries in athletes. Sports Med.18(3), 173–201 (1994). [DOI] [PubMed] [Google Scholar]
- 10.Leppilahti, J., Karpakka, J., Gorra, A., Puranen, J. & Orava, S. Surgical treatment of overuse injuries to the Achilles tendon. Clin. J. Sport Med.4(2), 100–107 (1994). [Google Scholar]
- 11.Schonbauer, H. R. Diseases of the Achilles tendon. Wien Klin. Wochenschr Suppl.168, 1–47 (1986). [PubMed] [Google Scholar]
- 12.Jarvinen, T. A., Jarvinen, T. L., Kannus, P., Jozsa, L. & Jarvinen, M. Collagen fibres of the spontaneously ruptured human tendons display decreased thickness and crimp angle. J. Orthop. Res.22(6), 1303–1309 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Kvist, M., Jozsa, L. & Jarvinen, M. Vascular changes in the ruptured Achilles tendon and paratenon. Int. Orthop.16(4), 377–382 (1992). [DOI] [PubMed] [Google Scholar]
- 14.Tallon, C., Maffulli, N. & Ewen, S. W. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med. Sci. Sports Exerc.33(12), 1983–1990 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Lonzarić, D., Kruščić, A., Dinevski, D., Povalej Bržan, P. & Jesenšek Papež, B. Primary surgical repair of acute Achilles tendon rupture: comparative results of three surgical techniques. Wien. Klin. Wochenschr.129, 176–185 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Sugimoto, K. et al. M9: recent developments in the treatment of ankle and subtalar instability. Open. Orthop. J.11, 687 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark, R. R. et al. Patch augmentation for rotator cuff repair: indications, techniques, and outcomes. Oper. Tech. Sports Med.20(3), 224–232 (2012). [Google Scholar]
- 18.Müller, A. M., Flury, M., Alsayed, H. N. & Audigé, L. Influence of patient and diagnostic parameters on reported retear rates after arthroscopic rotator cuff repair. Knee Surg. Sports Traumatol. Arthrosc.25, 2089–2099 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Galatz, L. M., Gerstenfeld, L., Heber-Katz, E. & Rodeo, S. A. Tendon regeneration and scar formation: the concept of scarless healing. J. Orthop. Res.33(6), 823–831 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eriksen, H. A., Pajala, A., Leppilahti, J. & Risteli, J. Increased content of type III collagen at the rupture site of human Achilles tendon. J. Orthop. Res.20(6), 1352–1357 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Ochen, Y. et al. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ364, k5120 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lantto, I. et al. A prospective randomized trial comparing surgical and nonsurgical treatments of acute Achilles tendon ruptures. Am. J. Sports Med.44(9), 2406–2414 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Wilkins, R. & Bisson, L. J. Operative versus nonoperative management of acute Achilles tendon ruptures: a quantitative systematic review of randomized controlled trials. Am. J. Sports Med.40(9), 2154–2160 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Aktas, E. et al. Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an Achilles tendon segmental defect. J. Orthop. Res.35(2), 269–280 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Liu, Y., Ramanath, H. S. & Wang, D. A. Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol.26(4), 201–209 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Wu, G., Deng, X., Song, J. & Chen, F. Enhanced biological properties of biomimetic apatite fabricated polycaprolactone/chitosan nanofibrous bio-composite for tendon and ligament regeneration. J. Photochem. Photobiol. B178, 27–32 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Zhang, C. et al. An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissue engineering. Acta Biomater.66, 141–156 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Langer, R. & Tirrell, D. A. Designing materials for biology and medicine. Nature428(6982), 487–492 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Place, E. S., Evans, N. D. & Stevens, M. M. Complexity in biomaterials for tissue engineering. Nat. Mater.8(6), 457–470 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Giannitelli, S. M., Accoto, D., Trombetta, M. & Rainer, A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater.10(2), 580–594 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Zhou, H. & Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater.7(7), 2769–2781 (2011). [DOI] [PubMed] [Google Scholar]
- 32.İlik, M. B., Durmuş, E. & Çelik, İ. Biocompatibility and osseointegration of the biomimetically coated and water-soluble eggshell membrane protein cross-linked Ti alloy screws. Iran. Biomed. J.28(1), 38 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durmuş, E., Celik, I., Ozturk, A., Ozkan, Y. & Aydin, M. Evaluation of the potential beneficial effects of ostrich eggshell combined with eggshell membranes in healing of cranial defects in rabbits. J. Int. Med. Res.31(3), 223–230 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Muhammed, M. R., Omer, O. A. & Ahmed, B. M. The use of Ostrich eggshell membrane as a barrier for guided bone regeneration in rabbit’s femoral condyle defects: histological study. Int. J. Pharm. Res.12(1), 963–969 (2020). [Google Scholar]
- 35.Leal-Marin, S. et al. Human amniotic membrane: a review on tissue engineering, application, and storage. J. Biomed. Mater. Res. Part. B Appl. Biomater.109(8), 1198–1215 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Park, S. et al. Eggshell membrane: review and impact on engineering. Biosyst Eng.151, 446–463 (2016). [Google Scholar]
- 37.Arias, J. L., Fernandez, M. S., Dennis, J. E. & Caplan, A. I. Collagens of the chicken eggshell membranes. Connect. Tissue Res.26(1–2), 37–45 (1991). [DOI] [PubMed] [Google Scholar]
- 38.Fernandez, M. S., Araya, M. & Arias, J. L. Eggshells are shaped by a precise spatio-temporal arrangement of sequentially deposited macromolecules. Matrix Biol.16(1), 13–20 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Harris, E. D., Blount, J. E. & Leach, R. M. Jr. Localization of lysyl oxidase in hen oviduct: implications in egg shell membrane formation and composition. Science208(4439), 55–56 (1980). [DOI] [PubMed] [Google Scholar]
- 40.Kawakami, H., Yoshida, K., Nishida, Y., Kikuchi, Y. & Sato, Y. Antibacterial properties of metallic elements for alloying evaluated with application of JIS Z 2801:2000. ISIJ Int.48(9), 1299–1304 (2008). [Google Scholar]
- 41.Leach, R. M. Biochemistry of the organic matrix of the eggshell. Poult. Sci.61(10), 2040–2047 (1982). [Google Scholar]
- 42.Nys, Y., Gautron, J., Garcia-Ruiz, J. M. & Hincke, M. T. Avian eggshell mineralization: biochemical and functional characterization of matrix proteins. C.R. Palevol. 3(6–7), 549–562 (2004). [Google Scholar]
- 43.Wong, M., Hendrix, M. J., von der Mark, K., Little, C. & Stern, R. Collagen in the egg shell membranes of the hen. Dev. Biol.104(1), 28–36 (1984). [DOI] [PubMed] [Google Scholar]
- 44.Zhao, Y-H. & Chi, Y-J. Characterization of collagen from eggshell membrane. Biotechnol. (Faisalabad). 8(2), 254–258 (2009). [Google Scholar]
- 45.Hincke, M. T. et al. Identification and localization of lysozyme as a component of eggshell membranes and eggshell matrix. Matrix Biol.19(5), 443–453 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Gautron, J. et al. Ovotransferrin is a matrix protein of the hen eggshell membranes and basal calcified layer. Connect. Tissue Res.42(4), 255–267 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Hincke, M. T. Ovalbumin is a component of the chicken eggshell matrix. Connect. Tissue Res.31(3), 227–233 (1995). [DOI] [PubMed] [Google Scholar]
- 48.Cordeiro, C. M., Esmaili, H., Ansah, G. & Hincke, M. T. Ovocalyxin-36 is a pattern recognition protein in chicken eggshell membranes. PLoS One. 8(12), e84112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gautron, J. et al. Cloning of ovocalyxin-36, a novel chicken eggshell protein related to lipopolysaccharide-binding proteins, bactericidal permeability-increasing proteins, and plunc family proteins. J. Biol. Chem.282(8), 5273–5286 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Starcher, B. C. & King, G. S. The presence of desmosine and isodesmosine in eggshell membrane protein. Connect. Tissue Res.8(1), 53–55 (1980). [DOI] [PubMed] [Google Scholar]
- 51.Nys, Y., Gautron, J., McKee, M. D., Garcia-Ruiz, J. M. & Hincke, M. T. Biochemical and functional characterisation of eggshell matrix proteins in hens. World’s Poult. Sci. J.57(4), 401–413 (2019). [Google Scholar]
- 52.Balaz, M. Eggshell membrane biomaterial as a platform for applications in materials science. Acta Biomater.10(9), 3827–3843 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Gong, W., He, W., Hou, Y., Li, Y. & Hu, J. Tendon-inspired hybrid hydrogel based on polyvinyl alcohol and gallic acid-lysozyme for promoting wound closure and healing. Int. J. Biol. Macromol.247, 125583 (2023). [DOI] [PubMed] [Google Scholar]
- 54.Tack, C., Shorthouse, F. & Kass, L. The physiological mechanisms of effect of vitamins and amino acids on tendon and muscle healing: a systematic review. Int. J. Sport Nutr. Exerc. Metab.28(3), 294–311 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Farrar, G., Barone, J. & Morgan, A. Ovalbumin-based porous scaffolds for bone tissue regeneration. J. Tissue Eng.1(1), 209860 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marinovich, R. et al. The role of bone sialoprotein in the tendon–bone insertion. Matrix Biol.52, 325–338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cordeiro, C. M. & Hincke, M. T. Quantitative proteomics analysis of eggshell membrane proteins during chick embryonic development. J. Proteom.130, 11–25 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Daraei, H., Mittal, A., Mittal, J. & Kamali, H. Optimization of Cr(VI) removal onto biosorbent eggshell membrane: experimental and theoretical approaches. Desalin. Water Treat.52(7–9), 1307–1315 (2013). [Google Scholar]
- 59.Filardo, G., Di Matteo, B., Kon, E., Merli, G. & Marcacci, M. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg. Sports Traumatol. Arthrosc.26(7), 1984–1999 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Eppley, B. L., Woodell, J. E. & Higgins, J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast. Reconstr. Surg.114(6), 1502–1508 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Marx, R. E. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent.10(4), 225–228 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Paoloni, J., De Vos, R. J., Hamilton, B., Murrell, G. A. & Orchard, J. Platelet-rich plasma treatment for ligament and tendon injuries. Clin. J. Sport Med.21(1), 37–45 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Andia, I., Sanchez, M. & Maffulli, N. Tendon healing and platelet-rich plasma therapies. Expert Opin. Biol. Ther.10(10), 1415–1426 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Mishra, A., Woodall, J. Jr. & Vieira, A. Treatment of tendon and muscle using platelet-rich plasma. Clin. Sports Med.28(1), 113–125 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Kajikawa, Y. et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J. Cell. Physiol.215(3), 837–845 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Mahmoodabadi, R. A., Golafshan, H. A., Pezeshkian, F., Shahriarirad, R. & Namazi, M. Evaluation of the effect of platelet-rich fibrin matrix in the correction of periorbital wrinkles: an experimental clinical trial. Dermatol. Pract. Concept.13(1) (2023). [DOI] [PMC free article] [PubMed]
- 67.Elhambakhsh, A., Ghanaatian, A. & Keshavarz, P. Glutamine functionalized iron oxide nanoparticles for high-performance carbon dioxide absorption. J. Nat. Gas Sci. Eng.94, 104081 (2021). [Google Scholar]
- 68.Ghanaatian, A. et al. Coating SiO2 nanoparticles with polyvinyl alcohol for interfacial tension alteration in the system CO2 + polyethylen glycol + water. Surf. Interfaces32, 102164 (2022). [Google Scholar]
- 69.Rönicke, R. et al. Abeta mediated diminution of MTT reduction—an artefact of single cell culture? PLoS One3(9), e3236 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsiao, W. L., Mo, Z. Y., Fang, M., Shi, X. M. & Wang, F. Cytotoxicity of PM(2.5) and PM(2.5–10) ambient air pollutants assessed by the MTT and the Comet assays. Mutat. Res.471(1–2), 45–55 (2000). [DOI] [PubMed] [Google Scholar]
- 71.Zare, M. R. et al. Antimicrobial core-shell electrospun nanofibers containing Ajwain essential oil for accelerating infected wound healing. Int. J. Pharm.603, 120698 (2021). [DOI] [PubMed] [Google Scholar]
- 72.Locke, R. C., Ford, E. M., Silbernagel, K. G., Kloxin, A. M. & Killian, M. L. Success criteria for preclinical testing of cell-instructive hydrogels for tendon regeneration. Tissue Eng. Part. C Methods26:506–518 [DOI] [PMC free article] [PubMed]
- 73.Meimandi-Parizi, A., Oryan, A. & Moshiri, A. Tendon tissue engineering and its role on healing of the experimentally induced large tendon defect model in rabbits: a comprehensive in vivo study. PLoS One8(9), e73016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang, Z. et al. Effect of tendon stem cells in chitosan/β-glycerophosphate/collagen hydrogel on Achilles tendon healing in a rat model. Med. Sci. Monit.24, 4633 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Backman, C., Boquist, L., Fridén, J., Lorentzon, R. & Toolanen, G. Chronic achilles paratenonitis with tendinosis: an experimental model in the rabbit. J. Orthop. Res.8(4), 541–547 (1990). [DOI] [PubMed] [Google Scholar]
- 76.Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods25(4), 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- 77.Webb, W. R. et al. The application of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds for tendon repair in the rat model. Biomaterials34(28), 6683–6694 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Sun, J. et al. Controlled release of collagen-binding SDF-1α from the collagen scaffold promoted tendon regeneration in a rat Achilles tendon defect model. Biomaterials162, 22–33 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Gabler, C. et al. In vivo evaluation of different collagen scaffolds in an Achilles tendon defect model. Biomed Res. Int. 2018 (2018). [DOI] [PMC free article] [PubMed]
- 80.Panheleux, M. et al. Organic matrix composition and ultrastructure of eggshell: a comparative study. Br. Poult. Sci.40(2), 240–252 (1999). [DOI] [PubMed] [Google Scholar]
- 81.Richards, P. D., Botha, A. & Richards, P. A. Morphological and histochemical observations of the organic components of ostrich eggshell. J. S Afr. Vet. Assoc.73(1), 13–22 (2002). [DOI] [PubMed] [Google Scholar]
- 82.Torres-Mansilla, A. et al. Eggshell membrane as a biomaterial for bone regeneration. Polymers (Basel)15(6), 1342 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi, J. et al. Eggshell membrane/gellan gum composite hydrogels with increased degradability, biocompatibility, and anti-swelling properties for effective regeneration of retinal pigment epithelium. Polymers (Basel)12(12), 2941 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi, H. J., Kim, Y. M., Suh, J. Y. & Han, J. Y. Beneficial effect on rapid skin wound healing through carboxylic acid-treated chicken eggshell membrane. Mater. Sci. Eng. C Mater. Biol. Appl.128, 112350 (2021). [DOI] [PubMed] [Google Scholar]
- 85.Mensah, R. A. et al. The eggshell membrane: a potential biomaterial for corneal wound healing. J. Biomater. Appl.36(5), 912–929 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gharibi, H. & Abdolmaleki, A. Thermo-chemical modification of a natural biomembrane to induce mucoadhesion, pH sensitivity and anisotropic mechanical properties. J. Mech. Behav. Biomed. Mater.87, 50–58 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Tavassoli, M. Effect of the substratum on the growth of CFU-c in continuous marrow culture. Experientia39(4), 411–412 (1983). [DOI] [PubMed] [Google Scholar]
- 88.Vuong, T. T. et al. The extracellular matrix of eggshell displays anti-inflammatory activities through NF-kappaB in LPS-triggered human immune cells. J. Inflamm. Res.10, 83–96 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Circi, E. et al. Impact of platelet-rich plasma injection timing on healing of Achilles tendon injury in a rat model. Acta Orthop. Traumatol. Turc.50(3), 366–372 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Parafioriti, A. et al. Single injection of platelet-rich plasma in a rat Achilles tendon tear model. Muscles Ligaments Tendons J.1(2), 41–47 (2011). [PMC free article] [PubMed] [Google Scholar]
- 91.Gautron, J. et al. Avian eggshell biomineralization: an update on its structure, mineralogy and protein tool kit. BMC Mol. Cell. Biol.22(1), 11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Proudfoot, D. Calcium signaling and tissue calcification. Cold Spring Harb Perspect. Biol.11(10), a035303 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molloy, T., Wang, Y. & Murrell, G. The roles of growth factors in tendon and ligament healing. Sports Med.33(5), 381–394 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Yu, Y. H. et al. Tri-layered doxycycline-, collagen- and bupivacaine-loaded poly(lactic-co-glycolic acid) nanofibrous scaffolds for tendon rupture repair. Polymers (Basel)14(13), 2659 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gabler, C. et al. In vivo evaluation of different collagen scaffolds in an Achilles tendon defect model. Biomed. Res. Int.2018, 6432742 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eliasson, P., Andersson, T. & Aspenberg, P. Influence of a single loading episode on gene expression in healing rat Achilles tendons. J. Appl. Physiol.112(2), 279–288 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Xu, Y. et al. Cyclic tensile strain induces tenogenic differentiation of tendon-derived stem cells in bioreactor culture. Biomed. Res. Int.2015, 790804 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sugimoto, Y. et al. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development140(11), 2280–2288 (2013). [DOI] [PubMed] [Google Scholar]
- 99.Subramanian, A. & Schilling, T. F. Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development142(24), 4191–4204 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sinn, H. P. et al. Comparison of immunohistochemistry with PCR for assessment of ER, PR, and Ki-67 and prediction of pathological complete response in breast cancer. BMC Cancer17(1), 124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data regarding this study has been reported in the manuscript. Please contact the corresponding author if interested in any further information.