Abstract
Male tephritid fruit flies typically emit pheromones from rectal glands to attract mates. Consistent with this, virgin females of the cucumber fruit fly, Zeugodacus cucumis (French), were found to be attracted to volatiles emitted by crushed male rectal glands in Y-tube olfactometer bioassays. Electrophysiological studies identified several male rectal gland compounds that triggered responses in female antennae. In other studied tephritids, the proportion of each compound is similar in excised rectal glands and headspace of calling intact flies, but our initial investigations revealed substantial discrepancies in the abundance of aliphatic amides, suggesting additional sources of these compounds. To address the discrepancies, we examined the volatile chemistry of headspace, rectal glands, tergal glands, and cuticles from both sexes using gas chromatography-mass spectrometry (GC-MS). Our analyses confirmed previously identified compounds and also detected several previously unreported compounds. Notably, the aliphatic amides were found to be more abundant in both tergal glands and cuticle than in rectal glands in both sexes, suggesting glands associated with these sites as additional sources of these compounds in headspace. Most studies of tephritid sex pheromones have focused on rectal gland extracts, but insights of the present study indicate that headspace volatiles of live flies can also reflect contributions from other glands.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84356-6.
Keywords: Cucumber fly; rectal gland; headspace, Cuticular hydrocarbons, Tergal land, GC-MS
Subject terms: Ecology, Zoology, Chemistry
Introduction
The cucumber fruit fly, Zeugodacus cucumis(French), is distributed along the north-eastern coast of Australia and New Guinea1. Among the 44 known hosts of this species, cucurbits are the most preferred1. Notably, Z. cucumismales do not respond to any known standard male attractants of dacine fruit flies2,3. As a result, the available monitoring options for assessing the prevalence of this pest are limited to less effective lures. Available options include orange ammonia lure4 and a cucumber volatile lure initially developed for attracting ovipositing females in another cucurbit-infesting fruit fly, the melon fruit fly, Z. cucurbitae5. While the cucumber volatile lure has been found to successfully attract Z. cucumisand surpass the efficacy of both orange ammonia lure and Cera Trap6, it remains unavailable in the commercial market. This gap necessitates ongoing exploration of alternative options, such as pheromone components, as prospective attractants.
Rectal glands are the most common source of mate-attracting pheromones in tephritid flies. The rectal gland components of Z. cucumismales have been described, resolving the absolute configuration of their stereoisomers7–10. A total of fourteen volatile compounds, including the three diastereomers of 2,8-dimethyl-1,7-dioxaspiro5undecane were reported as the most prominent molecules, as well as eight other spiroacetals, two pyrazines, and a diol in the male rectal glands8. Previous studies have provided particular insights into the pathways involved in the syntheses of the spiroacetals found in the male rectal glands, but the function of the identified male rectal gland components remains unknown. Further, the rectal gland chemistry of Z. cucumis females has not been described.
Recent studies on headspace volatiles and rectal glands in other fruit fly species have consistently shown a high degree of similarity in chemical composition of headspace of live flies and rectal gland extracts11–15. However, we found a clear discrepancy in Z. cucumis, suggesting additional sources of headspace volatiles in this species, and we here investigate cuticle and tergal glands as potential sources of headspace volatiles.
Cuticular chemistry plays a pivotal role in intra- and interspecies communication in insects16–18. For example, many studies of Drosophilahave shown the involvement of cuticular hydrocarbons in chemical communication19. In tephritids, however, studies of cuticular chemistry20–22and its role in communication23have been relatively scarce, despite intriguing observations of sexual dimorphism in cuticular chemistry that have prompted speculation about its relevance to sexual communication21,22. Rather than distributing secretory glands across the body, tephritids commonly distribute compounds originating in their rectal glands over the cuticle. Tergal glands are an additional source of cuticular compounds in tephritids24. While cuticular hydrocarbons are produced in the cell membrane of oenocytes in the cuticle of insects18, the presence of more numerous oenocytes beneath the tergal glands, located in the fifth segment of the abdomen of dacine fruit flies25, is notable. The tergal glands of B. tryoniproduce secretory droplets, consisting of aqueous inner material coated by a waxy film, that is distributed over the body when grooming26. Tergal gland secretions are important in protecting newly emerged B. tryoniagainst desiccation but their role in sexually mature adults has not been investigated24. The involvement of the tergal glands in sexual communication has been suggested in B. oleae, which transfers (Z)−9-tricosene from the rectal glands to the tergal glands to facilitate its release27. While this study confirms the presence of (Z)−9-tricosene on tergal glands, the quantity trend in individual flies indicates the rectal glands as the primary production site. As such, despite the potential significance of cuticular and tergal glands chemistry in communication, there have been no prior investigations into these aspects in Z. cucumis.
To gain a more complete understanding of chemical production and distribution in Z. cucumis, the present study conducted detailed analyses of headspace, rectal glands, tergal glands, and cuticle of male and female Z. cucumis. This study (1) confirms attractive pheromonal function of rectal gland secretions of male Z. cucumis, (2) identifies electrophysiologically active compounds as putative pheromone components, and (3) investigates the chemistry of headspace, rectal glands, tergal glands, and cuticle of Z. cucumis males and females to determine the sources of headspace and cuticular chemistry.
Results
Behavioral assays
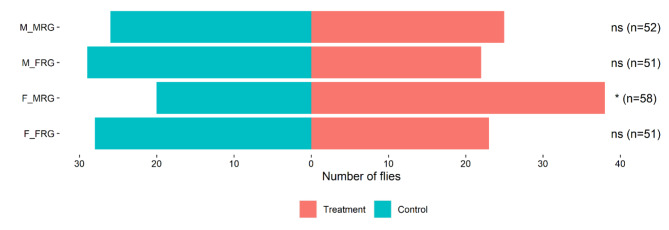
In Y-tube olfactometer bioassays, sexually mature virgin females significantly preferred the upwind arm containing male rectal glands volatiles over the control upwind arm (df = 1, p = 0.0247) (Fig. 1). In contrast, males did not show any preference for male rectal glands volatiles over the control (p = 0.890). Neither females nor males exhibited any preference for the female rectal gland volatiles (p = 0.576 and p = 0.401, respectively) (Fig. 1).
Fig. 1.
Y-tube olfactometer behavioral assay. M_MRG: response of males to male rectal gland; M_FRG: response of males to female rectal gland; F_MRG: response of females to male rectal gland; F_FRG: response of females to female rectal gland; * denotes a significance at α = 0.05 level.
Electrophysiology
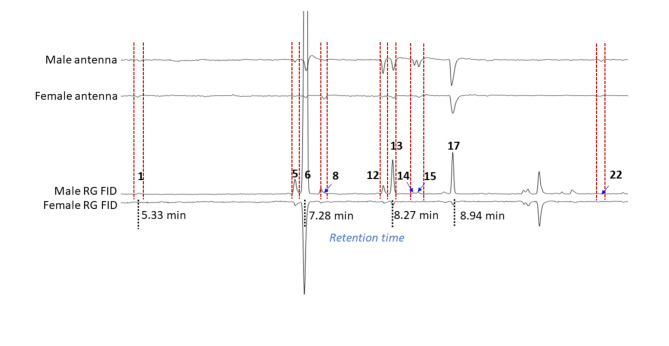
A summary of gas chromatography-electroantennogram detection (GC-EAD) and gas chromatography-electropalpogram detection (GC-EPD) responses of males and females to rectal gland compounds from both sexes is presented in Fig. 2. Antennae of both sexes responded to 2,3,5-trimethylpyrazine 1, (E,E)−2,8-dimethyl-1,7-dioxaspiro[5.5]undecane 6, the isomers of 2,8-dimethyl-1,7-dioxaspiro[5.5]undecane 13 and 17, 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane isomer 15, and 2-(hydroxymethyl)−8-methyl-1,7-dioxaspiro[5.5]undecane isomer 22 (Fig. 2). Male antennae responded to N-(3-methylbutyl)acetamide 5, N-(3-methylbutyl)propanamide 12 and N-(3-methylbutyl)isobutyramide 14, but female antennae did not. Female antennae responded to 3,5-dimethyl-2-porpylpyrazine 8, but male antennae did not. Maxillary palps of both sexes responded only to (E,E)−2,8-dimethyl-1,7-dioxaspiro[5.5]undecane 6 and 2,8-dimethyl-1,7-dioxaspiro[5.5]undecan-3-ol 20 (Fig. 2).
Fig. 2.
Gas chromatography electroantennogram detection (GC-EAD, top) and gas chromatography electropalpogram detection (GC-EPD, bottom) of Z. cucumis males and females. Compound numbers indicate the compounds that triggered responses in the antenna and paps. 1: 2,3,5-trimethylpyrazine ; 5: N-(3-methylbutyl)acetamide; 6: (E,E)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane; 8: 3,5-dimethyl-2-propylpyrazine; 12: N-(3-methylbutyl)propanamide; 13: 2,8-dimethyl-1,7-dioxaspiro[5.5]undecane isomer1; 14: N-(3-methylbutyl)isobutyramide; 15: 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane isomer3; 17: 2,8-dimethyl-1,7-dioxaspiro[5.5]undecane isomer2; 20: 2,8-dimethyl-l,7-dioxaspiro[5.5]undecan-3-ol isomer2; 22: 1,3-nonanediol.
Comparisons of GC intensities between male rectal glands and headspace.
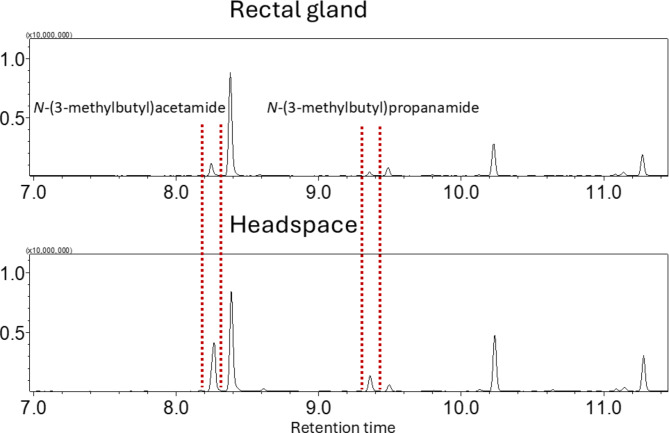
In our initial studies of rectal gland and headspace volatiles of male Z. cucumis, the GC intensities of N-(3-methylbutyl)acetamide and N-(3-methylbutyl)propanamide were substantially higher in the headspace than in rectal glands (Fig. 3). The relative proportions of N-(3-methylbutyl)acetamide and N-(3-methylbutyl)propenamide were significantly higher in headspace than rectal gland (two sample t-test, p = 1.154 × 10−7 (df = 10) and p = 1.236 × 10−4 (df = 10), respectively). This discrepancy strongly indicates the presence of additional sources for these compounds in the headspace.
Fig. 3.
Comparison of the GC intensities between rectal gland and headspace.
Comparisons of the chemical profiles of rectal gland, headspace, tergal gland, and cuticle
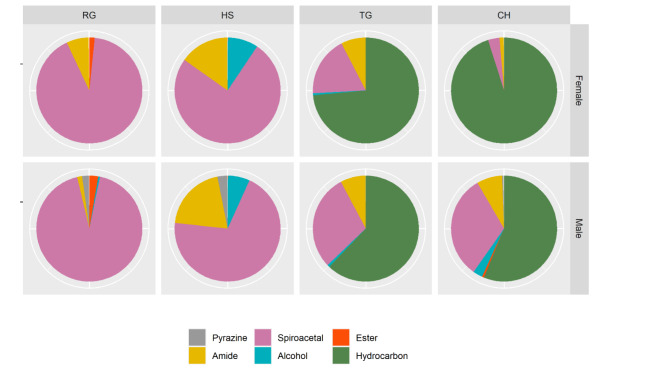
Table 1 provides a list of the identified volatile compounds (non-hydrocarbons) in extracts of rectal gland, headspace, tergal gland, and cuticle of both male and female Q-flies. Hydrocarbons were exclusively found in the tergal gland and cuticle extracts (Table 2). The compounds were categorized into six classes, including pyrazine, spiroacetal, ester, amide, alcohol, and hydrocarbon. The proportions of these compound classes are visualized in Fig. 4.
Table 1.
Identified non-hydrocarbon compounds in the headspace, rectal glands, tergal glands, and cuticle of Z. cucumis. - denotes a compound not detected in extraction types, and numerical values represent the compound percentages. Note that the percentages of compounds from the tergal gland and cuticle presented in this table are calculated using the sum of both volatile compounds and hydrocarbons. RI: Kovats retention index calculated by using n-alkane series; RI (ref): RI from the literature; MRG: male rectal gland; FRG: female rectal gland; MC: male cuticle; FC: female cuticle; MTG: male tergal gland; FTG: female tergal gland; MHS: male headspace; FHS: female headspace.
| No | Compound name | RI | RI (Ref) | Diagnostic ions | MRG/% | FRG/% | MHS/% | FHS/% | MTG/% | FTG/% | MC/% | FC/% | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2,3,5-trimethylpyrazine | 1006 | 1002 | 122 (100) | 0.37 | - | 3.56 | - | - | - | 0.41 | - | 55 |
| 2 | 2,7-dimethyl-1,6-dioxaspiro[4,5]decane | 1070 | 170 (5.9), 126 (18.2 ), 101 (100), 98 (91), 83 (34), 69 (24), 57 (28), 55 (44), 43 (43), 41 (26) | 0.02 | 0.17 | 0.04 | - | - | - | < 0.01 | - | ||
| 3 | 2,3,5,6-tetramethylpyrazine | 1091 | 1089 | 136 (100) | < 0.01 | - | 0.08 | - | - | - | 0.00 | - | 56 |
| 4 | N-(2-methylbutyl)acetamide | 1127 | 129 (6), 114 (14), 100 (52), 72 (100), 60 (70) | 0.01 | < 0.01 | 0.11 | 0.16 | 0.02 | 0.11 | 0.12 | 0.01 | 15 | |
| 5 | N-(3-methylbutyl)acetamide | 1135 | 1150 | 129 (6), 114 (20), 86 (31), 73 (100), 72 (77), 60 (29) | 0.15 | 0.74 | 10.93 | 10.22 | 4.93 | 4.15 | 6.81 | 0.96 | 15 |
| 6 | E, E-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane | 1144 | 184 (13), 140 (21), 115 (100), 112 (100), 97 (80) | 70.13 | 56.39 | 66.66 | 64.56 | 13.29 | 7.05 | 11.02 | 1.82 | 8 | |
| 7 | 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane isomer 1 | 1160 | 184 (4.0), 155 (33), 140 (12), 115 (100), 112 (61), 97 (70) | 1.16 | 0.53 | 1.37 | 0.73 | 0.46 | 0.47 | 0.29 | 0.01 | 8 | |
| 8 | 3,5-dimethyl-2-propylpyrazine | 1164 | 150 (10.2), 135 (18.9), 122 (100) | 0.24 | - | 0.12 | - | 0.01 | < 0.01 | < 0.01 | - | ||
| 9 | 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane isomer 2 | 1172 | 184 (5.0), 155 (43.7), 140 (10.4), 115 (100), 112 (55.0), 97 (72.9) | 0.07 | 0.08 | 0.11 | 0.13 | 0.05 | 0.07 | 0.02 | < 0.01 | 28 | |
| 10 | 2,7-dimethyl-1,6-dioxaspiro[4.6]undecane | 1188 | 169 (7.8), 140 (20.2), 111 (100), 101 (89.5), 98 (74.9), 83 (48.9), 55 (55) | 0.05 | < 0.01 | 0.09 | < 0.01 | 0.04 | 0.06 | 0.09 | 0.01 | 28 | |
| 11 | N-(2-methylbutyl)propanamide | 1206 | 142 (9), 114 (59), 87 (49), 86 (100), 74 (76), 57 (89) | 0.07 | 0.38 | 0.02 | 0.03 | 1.51 | 0.57 | 0.02 | < 0.01 | 15 | |
| 12 | N-(3-methylbutyl)propanamide | 1211 | 143 (5), 128 (13), 114 (20), 87 (100), 86 (64), 72 (13), 71(12), 57 (63) | 0.23 | 0.81 | 1.69 | 4.44 | 0.79 | 1.25 | 0.82 | 0.29 | 15 | |
| 13 | 2,8-dimethyl-1,7-dioxaspiro[5.5]undecane isomer1 | 1220 | 184 (5), 115 (100), 112 (42), 97 (75) | 6.40 | 3.52 | 7.37 | 4.50 | 0.06 | 0.14 | 2.94 | 0.20 | 8 | |
| 14 | N-(3-methylbutyl)isobutyramide | 1237 | 157 (16), 142 (23), 114 (30),101 (95), 100 (26), 88 (29), 71 (100) | 0.01 | 0.02 | 0.09 | 0.12 | 0.44 | 0.57 | 0.04 | 0.01 | 15 | |
| 15 | 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane isomer 3 | 1242 | 184 (4), 155 (28), 140 (7), 115 (100), 112 (45), 97 (79) | 0.44 | 0.02 | 0.08 | 0.01 | 0.07 | 0.02 | 0.18 | < 0.01 | 8 | |
| 16 | 2-methyl-1,7-dioxaspiro[5.6]dodecane | 1264 | 184 (5), 125 (29), 115 (100), 112 (89), 97 (72) | 0.29 | 2.74 | 0.16 | 0.03 | 4.37 | 3.99 | 0.42 | 0.03 | 8 | |
| 17 | 2,8-dimethyl-1,7-dioxaspiro[5.5]undecane isomer2 | 1272 | 184 (4), 115 (100), 112 (30), 97 (81) | 10.52 | 0.95 | 0.38 | 0.14 | 0.78 | 0.08 | 10.93 | 0.20 | 8 | |
| 18 | 2-(1’-hydroxyethyl)−7-methyl-1,6-dioxaspiro[4,5]decane isomer1 | 1333 | 200 (0.2), 169 (1), 155 (100), 131 (21), 128 (31), 113 (36), 95 (24), 85 (98) | 0.34 | 0.31 | 1.20 | 1.71 | 1.15 | 1.20 | 0.38 | 0.06 | 8 | |
| 19 | 2,8-dimethyl-l,7-dioxaspiro[5.5]undecan-3-ol isomer1 | 1337 | 200 (0.3), 156 (36), 128 (21), 115 (7), 113 (35), 112 (100), 85 (34) | 1.09 | 4.57 | 0.74 | 2.80 | 5.02 | 3.09 | 0.56 | 0.06 | 8 | |
| 20 | 2,8-dimethyl-l,7-dioxaspiro[5.5]undecan-3-ol isomer2 | 1347 | 200 (0.1), 156 (32), 128 (65), 115 (4), 113 (19), 112 (100), 97 (46), 81 (53) | 7.34 | 28.67 | 5.06 | 10.30 | 5.43 | 1.82 | 4.05 | 1.10 | 8 | |
| 21 | 1,3-nonanediol | 1361 | 159 ( 0.2), 113 (20.1), 97 (22.8), 75 ( 100) | 0.03 | < 0.01 | < 0.01 | < 0.01 | 0.72 | 0.59 | 2.97 | 0.01 | ||
| 22 | 2-(hydroxymethyl)−8-methyl-1,7-dioxaspiro[5.5]undecane isomer | 1379 | 200 (2), 184 (5), 169 (61), 115 (100), 112 (69), 97 (100) | 0.14 | < 0.01 | 0.07 | 0.02 | 0.05 | 0.03 | 0.08 | < 0.01 | 8 | |
| 23 | 2-(1’-hydroxyethyl)−7-methyl-1,6-dioxaspiro[4,5]decane isomer2 | 1424 | 200 (0.3), 155 (86), 131 (22), 128 (32), (113 (65), 95 (50), 85 (100) | 0.11 | 0.07 | 0.08 | 0.12 | 0.07 | 0.03 | 0.12 | 0.01 | 8 | |
| 24 | Octadecyl acetate | 2192 | 2211 | 312 (0.1), 252 (5), 224 (4), 125 (28), 111 (56), 97 (96), 83 (100), 61 (50) | 0.80 | 0.02 | - | - | 0.03 | < 0.01 | 0.62 | < 0.01 | 57 |
Table 2.
Tentatively identified hydrocarbons (H1 to H25) in the cuticle and tergal glands of Z. cucumis. Note that the percentages of hydrocarbons in this table are calculated using the sum of both volatile compounds and hydrocarbons. MM: molar mass; RI: Kovats retention index calculated by using n-alkane series; RI (ref): RI from the literature; MC: male cuticle; FC: female cuticle; MTG: male tergal gland; FTG: female tergal gland.
| No | Hydrocarbons | MM | RI | RI (Ref) | Diagnostic ions | MC/% | FC/% | MTG/% | FTG/% | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| H1 | 10-MeC28 | 408.8 | 2830 | 280/282, 154/155 | 0.28 | 0.15 | 0.20 | 0.24 | ||
| H2 | 2-MeC28 | 408.8 | 2860 | 2860 | 364/365 | 1.45 | 1.52 | 3.28 | 4.81 | |
| H3 | 6,10-DiMeC28 | 422.8 | 2870 | 322/323, 98/99, 252/253, 168/169 | 0.31 | 0.23 | 0.25 | 0.33 | ||
| H4 | 2,10-dimethylC28 | 422.8 | 2894 | 406/407, 280/281, 168/169 | 6.57 | 6.90 | 7.53 | 10.18 | ||
| H5 | 2,6-dimethylC28 | 422.8 | 2901 | 406/407, 336/337, 112/113 | 2.51 | 4.03 | 3.48 | 5.44 | ||
| H6 | 11-MeC29 | 422.8 | 2927 | 2931 | 280/281, 168/169 | 12.22 | 12.48 | 11.28 | 15.03 | 21 |
| H7 | 7-MeC29 | 422.8 | 2940 | 2945 | 336/337, 112/113 | 2.45 | 2.84 | 2.39 | 4.01 | 21 |
| H8 | 5-MeC29 | 422.8 | 2948 | 2952 | 364/365, 84/85 | 0.73 | 0.85 | 2.71 | 2.89 | 21 |
| H9 | 9,13-DiMeC29 | 436.9 | 2958 | 2963 | 322/323, 140/141, 252/253, 210/211 | 1.91 | 1.87 | 0.24 | 0.52 | 58 |
| H10 | 7,11-DiMeC29 | 436.9 | 2964 | 2965 | 350/351, 112/113, 280/281, 182/183 | 2.87 | 2.84 | 1.11 | 0.76 | 21 |
| H11 | 3-MeC29 | 422.8 | 2974 | 2974 | 392/393, 56/57 | 0.13 | 0.18 | 1.96 | 3.34 | 58 |
| H12 | 5,11-DiMeC29 | 436.9 | 2980 | 2983 | 378/379, 84/85, 280/281, 182/183 | 1.56 | 1.63 | 0.79 | 1.75 | 58 |
| H13 | 3,11-DiMeC29 | 436.9 | 3004 | 406/407, 56/57, 280/281, 182/183 | 2.43 | 2.82 | 0.73 | 1.65 | ||
| H14 | 6-MeC30 | 436.9 | 3041 | 3044 | 364/365, 98/99 | 2.06 | 2.49 | 4.07 | 8.83 | 21 |
| H15 | 4-MeC30 | 436.9 | 3060 | 3058 | 392/393, 70/71 | 0.23 | 0.37 | 10.26 | 19.98 | 21 |
| H16 | 2,12-; 2,14-DiMeC30 | 450.9 | 3094 | 435, 280/281, 196/197; 435, 252/253, 224/224 | 3.26 | 5.41 | 2.28 | 4.21 | ||
| H17 | 2,6-DiMeC30 | 450.9 | 3201 | 406/407, 70/71, 364/365, 112/113 | 8.38 | 12.83 | 3.77 | 9.01 | ||
| H18 | 13-MeC31; 15-MeC31 | 450.9 | 3127 | 3130 | 280/281, 196/197; 224/225, 252/253 | 3.01 | 4.71 | < 0.01 | < 0.01 | 21 |
| H19 | 7-MeC31 | 450.9 | 3137 | 3140 | 364/365, 112/113 | 4.67 | 6.25 | < 0.01 | < 0.01 | 21 |
| H20 | 9,13-DiMeC31 | 464.9 | 3156 | 3162 | 352/353, 140/141, 280/281, 210/211 | 1.12 | 1.06 | 0.34 | 0.88 | 58 |
| H21 | 7,13-DiMeC31 | 464.9 | 3163 | 378/379, 112/113, 280/281, 210/211 | 0.30 | 0.25 | 0.39 | 1.36 | ||
| H22 | 3- Me-C31 | 450.9 | 3174 | 3174 | 420/421 | 0.32 | 0.31 | 0.03 | < 0.01 | 21 |
| H23 | 3,13-DiMeC31 | 464.9 | 3202 | 434/435, 280/281, 210/211 | 0.60 | 0.82 | 0.02 | < 0.01 | ||
| H24 | 3,7-DiMeC31 | 464.9 | 3204 | 434/435, 364/365, 126/127 | 0.57 | 1.09 | < 0.01 | < 0.01 | ||
| H25 | 4,12-DiMeC32 | 478.9 | 3290 | 70/71, 308/309, 196/197; | 0.55 | 0.44 | < 0.01 | < 0.01 |
Fig. 4.
Proportions of the six classes of the compounds found in the headspace (HS), rectal glands (RG), tergal glands (TG), and cuticular hydrocarbon (CH) extracts of males and females.
The chemical profile of the male rectal glands includes a diverse suite of 24 volatile compounds, including three pyrazines, 14 spiroacetals, five aliphatic amides, an alcohol, and an ester. Female rectal glands and headspace exhibit similar chemical profiles but are distinguished from that of males by the absence of the three pyrazines. The headspace of both sexes contains fewer spiroacetals than the female rectal glands (Table 1). Males and females share similar chemical profiles in their tergal glands and cuticles, with most volatile compounds paralleling those in the rectal glands, along with 25 methyl-branched alkanes of C28 to C32 carbon chains. The detected hydrocarbons are mono- and dimethyl-branched alkanes. There were no aromatic compounds or unsaturated hydrocarbons detected in any of the extraction origins in both sexes.
This study confirms the previously reported spiroacetals in the male rectal glands8. Additionally, we report 2,7-dimethyl-1,6-dioxaspiro[4.5]undecane 2, 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane 9, and 2,7-dimethyl-1,6-dioxaspiro[4.6]undecane 10, N-(2-methylbutyl)acetamide 4, and N-(2-methylbutyl)propanamide 11, 3,5-dimethyl-2-propylpyrazine 8, and octadecyl acetate 24,. These compounds have not been previously reported in the male rectal glands of Z. cucumis8,28.
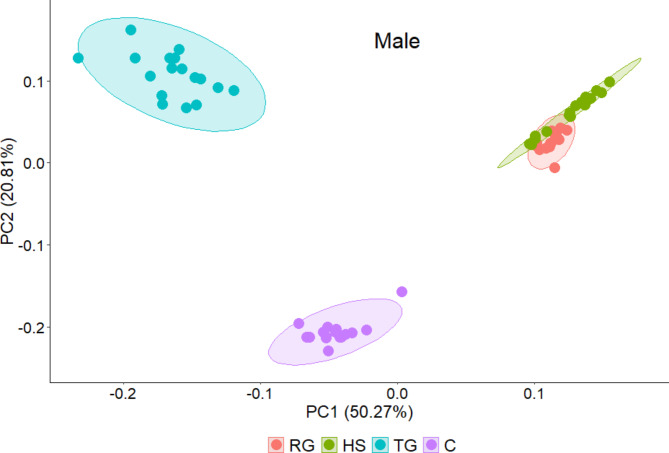
Principal component analysis (PCA) on the relative abundance of the individual compounds was employed to visualize differences in the chemical profiles of rectal gland, headspace, tergal gland, and cuticle (Fig. 5). The results showed that the first two principal components explained 64.45% and 71.08% of variances in the female and male data, respectively. The chemical profiles of the rectal gland and headspace for both sexes are generally similar, as they contain the same compounds with most, but not all, compounds in comparable proportions (e.g., compound 5, 6, or 12). In contrast, the profiles of the cuticles and tergal gland are distinct from each other as well as from those of the rectal gland and headspace. While both tergal glands and cuticles exhibit qualitatively similar profiles including hydrocarbons, the abundances of their compounds differ. For example, compounds 6, 17, and 20 show significant variations in abundance across each extraction origin.
Fig. 5.
Principal component analysis (PCA) on the proportions of the individual compounds in the four extraction origins. RG: rectal gland; HS: headspace; TG: tergal glands; C: cuticles.
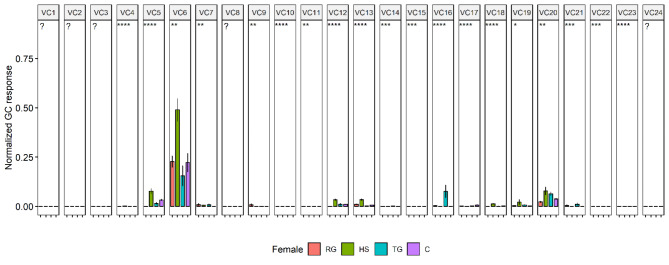
Furthermore, the absolute abundances of the volatile compounds, excluding hydrocarbons, were compared using Kruskal-Willis test. Significant differences were observed in the abundances of 23 volatile compounds in males and 19 volatile compounds in females. Figure 6 illustrates the comparisons of the abundances of the compounds that were significantly different between the rectal gland, headspace, tergal gland, and cuticle. The statistical results of the Kruskal-Wallis test, as a supplement to Fig. 6, are presented in Table S1 in the Supplementary Information.
Fig. 6.
Comparing absolute abundances of volatile compounds that were significantly different between the rectal gland, headspace, tergal gland, and cuticle extracts. The Kruskal-Wallis test was used, with detailed statistical results provided in Table S1of the Supplementary Information. RG: rectal gland; HS: headspace; TG: tergal gland; C: cuticle; VC1: 2,3,5-trimethylpyrazine, VC2: 2,7-dimethyl-1,6-dioxaspiro4,5decane; VC3: 2,3,5,6-tetramethylpyrazine; VC4: N-(2-methylbutyl)acetamide; VC5: N-(3-methylbutyl)acetamide; VC6: (E,E)−2,8-dimethyl-1,7-dioxaspiro[5.5]undecane; VC7: 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane isomer1; VC8: 3,5-dimethyl-2-propylpyrazine; VC9: 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane isomer 2; VC10: 2,7-dimethyl-1,6-dioxaspiro[4.6]undecane; VC11: N-(2-methylbutyl)propenamide; VC12: N-(3-methylbutyl)propanamide, VC13: 2,8-dimethyl-1,7-dioxaspiro[5.5]undecane isomer 1; VC14: N-(3-methylbutyl)isobutyramide, VC15: 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane isomer 2, VC16: 2-methyl-1,7-dioxaspiro[5.6]dodecane; VC17: 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane isomer3; VC18: 2-(1’-hydroxyethyl)−7-methyl-1,6-dioxaspiro4,5decane, VC19: 2,8-dimethyl-l,7-dioxaspiro[5.5]undecan-3-ol isomer1; VC20: 2,8-dimethyl-1,7-dioxaspiro[5.5]undecan-3-ol isomer; VC21: 3-nonanediol; VC22: 2-(hydroxymethyl)−8-methyl-1,7-dioxaspiro[5.5]undecane isomer; VC23: 2-(1’-hydroxyethyl)−7-methyl-1,6-dioxaspiro4,5decane isomer; VC24: octadecyl acetate. In females, VC1, 2, 3, 8, and 24 with were not analysed as they were not detected, and this is denoted by the question mark (?).
Discussion
The present study provides a detailed description and comparison of the chemical profiles of the rectal glands, headspace, tergal glands, and cuticle of Zeugodacus cucumis. Several previously unidentified compounds are reported in male rectal gland extracts and the first analysis of compounds produced by females is presented. This study not only demonstrates the attraction of virgin females to volatiles emitted from male rectal glands but also identifies electrophysiologically active compounds as putative components of a pheromone blend. Our findings suggests the tergal glands may also serve as a source of N-(2-methylbutyl)acetamide, N-(3-methylbutyl)acetamide, N-(3-methylbutyl)propenamide, and N-(3-methylbutyl)isobutyramide in headspace. The high abundance of aliphatic amides in the tergal glands provides an explanation for higher abundance of these aliphatic amides in the headspace compared to what would be anticipated from the composition of rectal gland extracts.
Additional compounds in male rectal gland extracts were identified by examining mass fragmentation patterns. The mass spectrum of 2,7-dimethyl-1,6-dioxaspiro[4.5]decane 2 showed a molecular ion at m/z 170 and predominant fragment ions at m/z 101 (base peak) and 98, indicative of a methyl substituted five-membered ring. In contrast, the fragment ions of six-membered ring counterpart, i.e., m/z115 and 112, appeared as very minor in the mass spectrum of this molecule. These observations align with those of a previous study in which the three stereo isomers of 2,7-dimethyl-1,6-dioxaspiro4,5decane were identified in Bactrocera tryoni29. The additional pyrazine 8 was characterized by confirming molecular ions at m/z 150, 149, a product at m/z 135, and a base peak at m/z122, which are consistent with the proposed structure. These observations are consistent with the previously reported structure30. While a previous study of Z. cucumis reported only the (E, E)- and (Z, Z)-isomer of 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane8, our study detected another peak with very similar mass fragmentations but at a later retention time than the (E, E)-isomer, suggesting the presence of another diastereomer 9, likely (E, Z)-/(Z, E)-isomer. The spiroacetal 2,7-dimethyl-1,6-dioxaspiro[4.6]undecane 10 appeared as very minor but showed distinct mass fragmentations. Although no molecular ion was observed, the ions at m/z169 indicated a molecular mass of 184. Prominent ions at m/z 111, 101, and 98 corresponded to products from the five-membered ring, while ions at m/z 126 and 129, from the seven-membered ring, were minor. These observations are consistent with the previously documented spectrum of a sample31. Additionally, it is noteworthy that the two additional amides 4 and 11are known to occur in other tephritids11,12,32, this study presents the first report of their presence in Z. cucumis. Many of the mono and dimethyl-branched C28 to C32 carbon chain alkanes identified in the present study are also known to occur in other tephritids. For example, nine of the methyl-branched hydrocarbons also found in B. tryoni21, and five of the hydrocarbons are found in B. dorsalis20. Hydrocarbons commonly function in water proofing, and in some insects also have a role in communication33. Given that these hydrocarbons are not recognized by the antennae or palps of Z. cucumis, they may be relevant to communication via contact through other body parts, such as tarsi. For instance, Rhagoletis pomonellaextends its proboscis to feed sugar when its tarsi touch a sugar solution, a process mediated by contact chemosensory sensilla34–36. The major spiroacetal (E, E)−2,8-dimethyl-1,7-dioxaspiro[5.5]undecane, found in Z. cucumisrectal glands, is a common constituent in other tephritids10–13,37–41. However, electrophysiological studies confirming the detection of this compound by conspecifics have only been conducted in Bactrocera bryoniae and B. frauenfeldi12,41. Wild Z. cucumis rectal glands have been reported to contain N-(3-methylbutyl)acetamide, N-(3-methylbutyl)propenamide and N-(3-methylbutyl)isobutyramide28. Notably, N-(3-methylbutyl)acetamide is widely spread across tephritids, including B. tryoni32, Z. cucurbitae42, B. cacuminata43, B. carambolae44, B. dorsalis45, B. tau, B. facialis and B. passiflorae10, B. musae14, B. frauenfeldi15, B. kraussi13, and B. bryoniae12.
Our electrophysiology study highlights the importance of exploring roles for individual and minor compounds. Female antennae responded to the two pyrazines 1 and 8, of which 8 has not been reported in Z. cucumis previously. Although the spiroacetal 7 and pyrazine 8 exhibited similar interactions with the GC column, EAD signals did not exactly match with the GC peak of 7. This discrepancy prompted a closer examination of several trace peaks, leading to the subsequent characterization of the corresponding compounds. Despite GC peak of 1 having higher intensity than 8, the female EAD signal of 8 is stronger. This highlights the sensitivity of the electrophysiology technique employed in the present study. Pyrazine 8 may be a significant mediator of female behavior, as this compound only elicited antennal responses in females. conversely, amides 5, 12 and 14 may be important mediators of male behavior because these compounds only elicited antennal responses in males. These electrophysiologically active compounds may affect fly behavior alone or in combination.
Antennae and maxillary palps of males and females responded differently to the extracted compounds. Similar differences in responses of antennae and palps to compounds extracted from conspecifics are known in other tephritids, such as B. frauenfeldi15, B. bryoniae12, and B. kraussi13. Differences in the responses of antennae and maxillary palps to phytochemicals are also known in some tephritids. For example, males of some dacine species detect cuelure a naturally occurring raspberry ketone analog46,47, a male-specific lure, mainly through the chemosensory neurons that are more abundant in the maxillary palps48–50. Furthermore, the present study illustrates sex differences in the responses of antennae to compounds extracted from conspecifics. These sex-specific variations in electrophysiological responses suggest differences in the functional significance of these compounds, aligning with previous reports in other tephritids12,13,15.
The results of Y-tube olfactometer behavioral assays highlighted the importance of pyrazine 8 in male rectal glands (Fig. 1). While the compounds in male rectal glands, except pyrazine 8, are also present in female rectal glands (Table 1; Fig. 2), it is notable that if male rectal gland volatiles lacking pyrazine 8were capable of triggering the behavioral response, females should have been equally attracted to female rectal gland volatiles. Alternatively, the proportions of the compounds may be critical in female attraction. Although such examples are not documented in tephritids, the proportions of pheromone components have been found to be critical in attracting Lepidopterans51,52. Consequently, it is conceivable that pyrazine 8 or a combination of the compounds including 8 may be responsible for triggering the attraction of females when exposed to male rectal gland volatiles. Interestingly, while males exhibited electrophysiological antennal response to male rectal gland volatiles, they were not attracted to these odors at dusk, the time when Z. cucumis is sexually active. Most studies on fruit fly responses to conspecific-produced compounds have focused on mating contexts and times when mating occurs. However, fruit flies may also use chemical cues to gather information about conspecifics at other times of the day.
The current study revealed differences in the chemical profiles of the extraction origins. PCA analysis indicated that the chemical profiles of rectal gland and headspace appear are quite similar, albeit with some exceptions, suggesting that rectal glands are a major contributor to headspace volatiles. In contrast, the chemical profiles of tergal gland and cuticle appear to be distinct due to differences in relative abundances, despite their qualitatively similar profiles. This suggests that tergal glands serve as production sites for certain compounds found in headspace. While hydrocarbons are typically produced in the insect cuticle, other volatile compounds found on the cuticle likely are transferred from rectal glands or tergal glands through grooming. Compounds abundant in tergal glands are likely produced independently. Given the matching abundances of the major compounds including (E, E)−2,8-dimehtyl-1,7-dioxapsiro[5.5]undecane 6, its isomers 13, 17, and 3,5-dimehtyl-2-propylpyrazine 8 in the male rectal glands and headspace suggest that these compounds in the cuticle and headspace extracts likely originate from the rectal glands. In females, only the major spiroacetal 6 shows high abundance in the female rectal gland and headspace. Conversely, the higher abundances of the amides, 4, 5, 12, and 14 in the headspace and tergal glands of both sexes (Fig. 5) indicate that the amides may be primarily produced in the tergal glands. It is likely that amides are transferred to the cuticle by grooming, eventually contributing to the high quantities in both cuticles and headspace. This accounts for the observed discrepancies in the proportions of the amides between the headspace and rectal glands. While rectal gland chemistry has been extensively studied as a presumptive pheromone in many tephritids10,12–15,32,37–40, the chemistry of tergal glands has received very little attention. It has been suggested that tergal gland secretions are not involved in the mating activity of B. tryoni24. However, in Lutzomyia longipalpis, the tergal glands secret 9S-methylgermacrene-B, the male sex pheromone53; females of L. longipalpisare highly attracted to a chromatographic fraction of the male tergal gland extracts54. In tephritids, the involvement of tergal glands in pheromonal activity has only been studied in Bactrocera oleae27. The present study indicates that tergal glands can indeed contribute to headspace composition and may even be part of the pheromone profile.
Materials and methods
Insects
Pupae of Zeugodacus cucumis were obtained from a colony that had been established and maintained for at least eight generations at Department of Agriculture and Fisheries (Cairns, QLD, Australia). Approximately 1000 pupae were allowed to emerge into a mesh cage (47.5 × 47.5 × 47.5 cm) (Megaview Bugdorm 4S4545, Taiwan). Adult flies were provided with ad libitum access to a standard diet (sucrose and hydrolysed yeast) and water. Flies used in the experiments were reared from the colony. Eggs were collected by placing lengthwise halved zucchinis in the cage for 3 h. All zucchinis used in this study were sourced from a local supermarket in North Ryde, Australia. The infested zucchinis were cut into 3 pieces. One piece of cut zucchini was placed on top of several freshly halved zucchinis in a 900 mL plastic container, which has many holes (1 mm diameter) at the bottom. The container with holes was stacked on top of another container, which had no holes. This prevented the accumulation of excessive liquid in the top container. The stacked containers were placed on top of vermiculite (1 cm) in a secondary container (12.5 L). The third instar larvae exited zucchinis on the fifth and sixth day and pupated in vermiculite. Pupae were separated by gently sieving the vermiculite 2–3 days before the expected emergence date. Approximately 1000 pupae were allowed to emerge into a mesh cage (47.5 × 47.5 × 47.5 cm) (Megaview Bugdorm 4S4545, Taiwan). Flies were separated by sex within three days after emergence and maintained in 12.5 L plastic cages that had two 10 cm diameter mesh screens for air flow. Flies were provided with dry sugar and hydrolyzed yeast, and water through a soaked sponge ad libitum. All the experimental flies were 12 to 20-day old (sexually mature). All cages were maintained at 25 ± 1 °C and 65 ± 5% relative humidity on a photoperiod of 11.5: 0.5: 11.5: 0.5; dark: dawn: light: dusk.
Behavioral assays using Y-tube olfactometers
The attraction of sexually mature (12–20 days old) Z. cucumis males and females toward the volatiles of the rectal glands of the same or opposite sex were evaluated using acrylic Y-tube olfactometers. Y-tube olfactometers comprised of one central arm (inner dimensions: 6.5 × 4.2 × 4.7 cm) to which the release chamber (inner dimensions: 5 × 4.2 × 4.7 cm) was attached at the bottom and two upwind lateral arms (inner dimensions: 12.5 × 4.2 × 4.7 cm) to which rectangular chambers (inner dimensions: 7.5 × 4.2 × 4.7 cm) were attached. The Y-tube olfactometer was positioned horizontally on a white table and a charcoal-filtered and humidified air stream was passed through the tube at a flow rate of 140 ± 5 mL/min. The stimulus cartridge was prepared by crushing 15 rectal glands of males or females on a filter paper (1.5 × 1.5 cm) (Advantec, Japan) inserted to the neck of a glass Pasteur pipette (14.5 cm long). The number of rectal glands used was determined through testing 5, 10 or 15 glands, with 15 being necessary to elicit a meaningful response. The control cartridge was prepared using a filter paper (1.5 × 1.5 cm) without crushed rectal glands inserted in the same type of glass Pasteur pipette. One cartridge of each type was fitted to one of the Y-tube upwind arms using Tygon tubing (Tygon® formula E-3603, Sigma-Aldrich). An individual fly was placed in the release chamber 30 min before experiments commenced to settle. Once the stimulus and control cartridges were connected to the upwind arms, the air flow was allowed to equilibrate the system for one minute. The barriers of the release chamber were removed. A choice was recorded when the fly reached one of the two upwind arms and stayed there for one minute and longer. Those flies that did not make any choice, i.e., remained in the release chamber, did not reach one of the two upwind arms or did not stay in one arm for one minute and longer, were not counted. Every trial lasted 30 min from the start of dusk to dark of the photocycle. For each treatment, at least 51 replicates of responsive flies were carried out. The positions of stimulus and control were alternated every trial to minimize positional effects. Each fly was tested once, and fresh rectal glands were used in each experiment. Before each experiment, the Y-tube olfactometer was washed with 5% Extran aqueous solution, rinsed with hot tap water and air-dried.
Electrophysiological analysis
Gas chromatography-electroantennogram detection (GC-EAD) and Gas chromatography-electropalpogram detection (GC-EPD) recordings were conducted by a coupled system of Agilent GC 7890 (Agilent, CA, US) and Syntech electrophysiological recording equipment (Syntech, Hilversum, The Netherlands). GC system was equipped with a split/splitless injector, SH Rtx-5MS (30 m × 0.25 mm, 0.25 μm film) fused silica capillary column (Shimadzu Corporation, Kyoto, Japan) and a flame ionization detector (FID). The carrier gas was hydrogen (99.999%) (BOC, North Ryde, NSW, Australia) at a flow rate of 2.5 mL/min. An aliquot of 1 µL of a rectal gland sample was injected in splitless mode where the injector temperature was 270 °C. The oven temperature program as follows: 50 °C (1 min), increased to 260 °C at 15 °C/min and held for 5 min. Effluent from GC was split, and the split ratio directed to FID and electrophysiological detector was 1: 1.5. For GC-EAD/EPD analysis, the rectal gland samples of both sexes were prepared by extracting 30 rectal glands in 200 µL hexane using the same extraction method as described in the Rectal gland extraction section. The male rectal gland samples were selected for GC-EAD/EPD studies because they contain all the volatile compounds found in the four extraction origins. Hydrocarbons in cuticle extracts did not elicit any responses in preliminary studies, leading to their exclusion from EAD and EPD analyses. The identities of FID peaks were confirmed by GC-MS.
Microelectrodes, Ag wire in a glass tube, were filled with electroconductive gel (Spectra 360, Parker Laboratories Inc., USA). The head of live male or female of Z. cucumis was separated from the body with micro scissors. The base of a head was affixed to the gel of the tip of the electrode. The tips of the insect antenna or maxillary palp were placed on the gel of the other recording electrode, in a way that the tips were slightly inserted into the gel. The signals were passed through a high impedance amplifier (IDAC4, Syntech, Hilversum, The Netherlands). The outputs from the electrophysiological amplifier and the FID were monitored simultaneously and analysed using GcEad 2014 (V1.2.5). At least six replicates with consistent results were collected for each combination.
Chemicals
Hexadecane, 2,3,5-tirmethylpyrazine, 2,3,5,6-tetramethylpyrazine, straight-chain C8 – C40 alkane standards were purchased from Merck Life Sciences (formerly Sigma-Aldrich) (Burlington, Massachusetts, US). All chemicals were analytical grade (> 98% purity), except for the alkane standards, which were at a concentration of 40 µg/mL of each alkane in DCM. N-(2-methylbutyl)acetamide, N-(3-methylbutyl)acetamide, N-(2-methylbutyl)propanamide, N-(3-methylbutyl)propanamide, N-(3-methylbutyl)isobutyramide were synthesized as described in Supporting Information. 2,8-Dimethyl-1,7- dioxaspiro[5.5]undecane isomers (EE and EZ/ZE) and 2-ethyl-7-methyl-1,6-dioxaspiro[4.5]decane was kindly provided by Dr Sally Noushini.
Gas Chromatography-Mass Spectrometry (GC-MS)
GC-MS analysis was carried out on a Shimadzu GCMS TQ8030 spectrometer equipped with a split/splitless injector, SH Rtx-5MS (30 m × 0.25 mm, 0.25 μm film) fused silica capillary column, and HS-20 headspace sampler. (Shimadzu Corporation, Kyoto, Japan). The carrier gas was helium (99.999%) (BOC, North Ryde, NSW, Australia) at a flow rate of 1.0 mL/min. For headspace analysis, the headspace sampler oven temperature was set to 60 °C with a 5-minute equilibration time. Headspace volatiles were trapped on Tenax TA within a built-in tube maintained at − 5 °C. The trapped volatiles were subsequently desorbed at 300 °C and injected onto the column. For liquid injection, an aliquot of 1 µL sample was injected at splitless mode where the injector temperature was 270 °C. The oven temperature program for the rectal gland and headspace samples as follows: 50 °C (1 min), increased to 300 °C at a rate of 10 °C/min and held for 4 min. The oven temperature program for cuticle and tergal gland extracts: 50 °C (1 min), increased to 280 °C at a rate of 10 °C/min and further increased to 302 °C at a rate of 2 °C/min. The ion source and transfer line temperatures were 200 and 290 °C, respectively. The ionization method was electron impact (70 eV). The spectra were obtained over a mass range of m/z 35–650 in scan mode.
The data were analyzed by Shimadzu GCMS Postrun program and compared with the mass fragmentation patterns in NIST libraries (NIST17-1, NIST17-2). Observed retention indices (RI) were compared with RI values published in the literature. The identities of compounds were confirmed by comparing retention time and fragmentation patterns of a compound with that of the authentic compound.
Rectal gland extraction
Flies were killed at – 79 °C by placing them in a 5 mL plastic vial on dry ice. Flies were then thawed at room temperature for three minutes. Rectal glands were excised under a stereomicroscope by pulling off the terminalia of the rectal ampulla with forceps and removing the attached track. A single gland was placed in a pre-cooled teardrop vial (Kinesis, Redland Bay, QLD, Australia) on dry ice. Once 15 replicates of each sex were collected, the vials were placed in a rack. An aliquot of 100 µL n-hexane (HPLC grade) was added to each vial. The vials were allowed to stand at room temperature for 30 min. Each of the n-hexane extracts was transferred to a glass insert (150 µL, Shimadzu Corporation, Kyoto, Japan) in a standard 1.5 mL GC vial (Shimadzu Corporation, Kyoto, Japan). The samples were stored at – 30 °C until analyzed. The n-hexane solvent contained an internal standard, n-hexadecane (1.6 µg/mL) that was used throughout the experiments to normalize GC-MS peaks for comparisons between the extraction origins. n-Hexadecane was selected as the internal standard because it was not present in B. cucumis rectal gland, headspace, cuticles, and tergal gland samples, and its retention time did not lead to co-elution with other compounds.
Extraction of cuticular compounds using n-hexane
Flies were killed and equilibrated by the same method as for rectal gland extraction. A single fly was introduced into a teardrop vial and 100 µL of n-hexane, containing the internal standard mentioned above, was pipetted into the vial. Cuticular compounds were extracted by standing the vial for three minutes at room temperature and the vial was vortexed for 5 s during this time. The n-hexane extract containing cuticular compounds was transferred to a glass insert (250 µL, Shimadzu Corporation, Kyoto, Japan) in a standard 1.5 mL GC vial (Shimadzu Corporation, Kyoto, Japan). The samples were stored at – 30 °C until analyzed. At least fifteen replicates were collected for each sex.
Tergal gland extraction using solid phase microextraction (SPME)
For qualitative analysis, SPME was used. A live male or female was inserted into a slit (5 mm) of synthetic sponge that allowed most of the insect body, including legs to be inside of the sponge (RS PRO, RS Components, NSW Australia) cut into a cube (2 × 2 × 2 cm) but the last segment of dorsal abdomen protruding. The immobilized fly was stood for 30 min to allow secretion from tergal gland while the fly could not use its hind legs to distribute secretions to the body. Secretions of tergal glands on the dorsal part of the last segment of abdomen, on which the tergal glands are clustered, was gently rubbed with polydimethylsiloxane (PDMS) fiber (100 μm, 100 μm, Supelco, Bellefonte, PA, USA) held in a manual holder (Supelco, Bellefonte, PA, USA) for 20 s. The fiber was retrieved and wrapped with aluminum foil until GC-MS analysis. Five replicates of each sex were collected.
For quantitative comparisons among the four extraction origins, tergal glands were also extracted with n-hexane containing the same internal standard. Flies were killed as the same method that was used for rectal gland extraction. Under a stereomicroscope, the dorsal sclerite of the fifth segment of the abdomen was removed with a pair of micro scissors and immediately placed in a pre-cooled teardrop vial on dry ice. The tergal glands of one fly constituted a replicate. Once 15 replicates of each sex were collected, the vials were placed in a rack. An aliquot of 100 µL n-hexane was added to each vial. The vials were allowed to stand at room temperature for 30 min. The supernatant of each n-hexane extract was pipetted and transferred to a glass insert (250 µL, Shimadzu Corporation, Kyoto, Japan) in a standard 1.5 mL GC vial (Shimadzu Corporation, Kyoto, Japan). The samples were stored at – 30 °C until analysed by GC-MS.
Collection of headspace from live insects
In the initial headspace analysis, a single male was placed inside a 20 mL screw-top headspace vial (Shimadzu Corporation, Kyoto, Japan) 30 min before dusk. The fly remained in the vial for an additional 30 min until complete darkness. The vial was then placed on dry ice to euthanize the fly and subsequently stored at − 80 °C until GC-MS analysis. A total of 15 replicates were collected.
For comparisons with rectal gland, tergal gland, and cuticle, groups of 10 sexually mature virgin males or females (12–20 days old) were placed in a cylindrical glass volatile collection chamber (120 mm long x 25 mm ID) 30 min before dusk, accompanied by an empty chamber in each collection as a control to identify any impurities in the analysis. A charcoal-filtered air stream was passed through the glass chamber at a flow rate of 0.2 L/min to collect the volatiles released by males during dusk for 30 min. Volatiles were adsorbed on 50 mg of Tenax-GR (60/80 mesh) (Scientific Instrument Services, Palmer, MA, US) traps fitted with glass wool plugs in a glass sorbent tube (6.4 mm OD, 60 mm length) attached to the outlet of the chamber. Volatile collection was carried out in the same room conditions under which the flies were maintained. Neither food nor water was provided during volatile collections. Volatiles were eluted with 1.0 mL of n-hexane into a standard GC vial (Shimadzu Corporation, Kyoto, Japan) and were stored at −30 °C until analyzed. Prior to each experiment, volatile collection chambers were washed with a solution of 5% Extran, rinsed with hot tap water, rinsed with acetone, and heated at 200 °C for at least 12 h. Activated charcoal filter was prepared by packing granular charcoal (20/40 mesh) Merck Life Sciences (formerly Sigma-Aldrich) (Burlington, Massachusetts, US) in custom blown glass tube. Activated charcoal filters were conditioned by heating at 200 °C for at least 12 h (El-Sayed et al., 2008), and Tenax was thermally conditioned by heating them under a nitrogen stream (75 mL/min) at 200 °C for at least three hours. Fifteen replicates were collected for each sex.
Data analysis
One-sample binomial test using the binom.test() function in R (which performs a two-tailed test), with the probability level of P < 0.05, was used to compare the number of flies choosing the stimulus over the control for the Y-tube olfactometer assays. Relative abundances were calculated as percentages and are presented in the table. These percentages were also used in statistical analyses as applicable. In the initial analysis, relative abundances between male rectal glands and headspace were compared using a two sample t-test after log-transforming the data. These relative abundances were also used in principal component analysis (PCA) to visualize the extraction origins. PCA was conducted using a correlation matrix to standardize the variables due to their different scales. The analysis was performed disregarding group membership, treating all individuals as part of one dataset. For visualization, 95% confidence ellipses were added to illustrate differences between groups The hydrocarbons appeared exclusively in the tergal glands and cuticle. Therefore, the analysis focused on quantifying the differences in the volatile compounds within the rectal glands, headspace, tergal glands, and cuticle. To conduct this analysis, the abundances of the volatile compounds across the extraction origins were normalized by dividing the area of a peak by the area of the internal standard, of which the concentration was maintained throughout the experiments. The normality of the data was assessed using the Shapiro-Wilk test and visualized with Q-Q plots for each group. Since the data did not meet normality assumptions, appropriate transformations were applied using the bestNormalize() function in R. However, the transformed data did also not meet the normality assumption. Therefore, Kruskal-Wallis test with a significance level of α = 0.05 was used to assess the significant difference of each compound between the extraction origins. All statistical analyses and illustrations were conducted using R software (V4.3.2, R Core Team).
Data Availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information file).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Department of Agriculture and Fisheries, Queensland, particularly Peter Leach and Sybilla Oczkowicz, for providing insects.
Author contributions
Conceptualization - SJP, JP, and PT; methodology -SJP, JP, VM, and PT; software - SJP.; formal analysis - SJP.; investigation - SJP, JP, and VM.; resources - PT.; data curation - SJP.; writing—original draft preparation - SJP.; writing—review and editing -SJP, JP, VM, and PT; supervision - PT; funding acquisition - PT. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Australian Research Council Industrial Transformation Training Centre (ITTC) for Fruit Fly Biosecurity Innovation (Project IC50100026), funded by the Australian Government.
Declarations
Competing interests
The authors declare no conflict of interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dominiak, B. C. & Worsley, P. Review of cucumber fruit fly, Bactrocera cucumis (French) (Diptera: Tephritidae: Dacinae) in Australia: part 1, host range, surveillance and distribution. Crop Prot.106, 79–85. 10.1016/j.cropro.2017.11.015 (2018). [Google Scholar]
- 2.Drew, R. A. I. & Hooper, G. H. The responses of fruit fly species (Diptera: Tephritidae) in Australia to various attractants. Aust Entoml. 20, 201–205. 10.1111/j.1440-6055.1981.tb01032.x (1981). [Google Scholar]
- 3.Gillespie, P. Observations on fruit flies (Diptera: Tephritidae) in New South Wales. Gen. Appl. Entomol.10.3316/informit.233568425137268 (2003). [Google Scholar]
- 4.Bateman, M. A. Chemical Methods for suppression/eradication115–128 (Department of Primary Industries, 1982). [Google Scholar]
- 5.Siderhurst, M. S. & Jang, E. B. Cucumber volatile blend attractive to female melon fly, Bactrocera cucurbitae (Coquillett). J. Chem. Ecol.36, 699–708. 10.1007/s10886-010-9804-4 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Royer, J. E., De Faveri, S. G., Lowe, G. E. & Wright, C. L. Cucumber volatile blend, a promising female-biased lure for Bactrocera cucumisFrench (Diptera: Tephritidae: Dacinae), a pest fruit fly that does not respond to male attractants. Aust. Entoml. 53, 347–352, (1907). 10.1111/aen.12083 (2014).
- 7.Kitching, W. et al. Spiroacetals in rectal gland secretions of Australasian fruit fly species. J. Chem. Soc. Chem. Commun. 853–854. 10.1039/C39860000853 (1986).
- 8.Kitching, W. et al. Chemistry of fruit flies. Composition of the rectal gland secretion of (male) Dacus Cucumis (cucumber fly) and Dacus halfordiae. Characterization of (Z,Z)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane. J. Org. Chem.54, 3893–3902. 10.1021/jo00277a028 (1989). [Google Scholar]
- 9.Hayes, P., Fletcher, M. T., Moore, C. J. & Kitching, W. Synthesis and absolute stereochemistry of a constitutionally new spiroacetal from an insect. J. Org. Chem.66, 2530–2533. 10.1021/jo015502p (2001). [DOI] [PubMed] [Google Scholar]
- 10.Fletcher, M. T. & Kitching, W. Chemistry of fruit flies. Chem. Rev.95, 789–828. 10.1021/cr00036a001 (1995). [Google Scholar]
- 11.Noushini, S., Park, S. J., Jamie, I., Jamie, J. & Taylor, P. Sampling technique biases in the analysis of fruit fly volatiles: a case study of Queensland fruit fly. Sci. Rep.10, 19799. 10.1038/s41598-020-76622-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noushini, S., Park, S. J., Jamie, I., Jamie, J. & Taylor, P. Rectal gland exudates and emissions of Bactrocera bryoniae: chemical identification, electrophysiological and pheromonal functions. Chemoecology31, 137–148. 10.1007/s00049-020-00335-z (2021). [Google Scholar]
- 13.Noushini, S. et al. Electrophysiological responses of Bactrocera kraussi (hardy) (Tephritidae) to rectal gland secretions and headspace volatiles emitted by conspecific males and females. Molecules2610.3390/molecules26165024 (2021). [DOI] [PMC free article] [PubMed]
- 14.Noushini, S. et al. Rectal gland chemistry, volatile emissions, and antennal responses of male and female banana fruit fly, Bactrocera musae. Insects1110.3390/insects11010032 (2020). [DOI] [PMC free article] [PubMed]
- 15.Noushini, S. et al. Attraction and electrophysiological response to identified rectal gland volatiles in Bactrocera frauenfeldi (Schiner). Molecules25, 1275. 10.3390/molecules25061275 (2020). [DOI] [PMC free article] [PubMed]
- 16.Howard, R. W. & Blomquist, G. J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol.50, 371–393. 10.1146/annurev.ento.50.071803.130359 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Drijfhout, F., Kather, R. & Martin, S. The role of cuticular hydrocarbons in insects. Behav. Chem. Ecol., 91–114 (2013).
- 18.Blomquist, G. J. & Ginzel, M. D. Chemical ecology, biochemistry, and molecular biology of insect hydrocarbons. Annu. Rev. Entomol.66, 45–60. 10.1146/annurev-ento-031620-071754 (2021). [DOI] [PubMed]
- 19.Yew, J. Y. & Chung, H. Drosophila as a holistic model for insect pheromone signaling and processing. Curr. Opin. Insect Sci.24, 15–20. 10.1016/j.cois.2017.09.003 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Goh, S. H. et al. Cuticular hydrocarbons from two species of Malaysian Bactrocera fruit flies. Biochem. Syst. Ecol.21, 215–226. 10.1016/0305-1978(93)90039-T (1993). [Google Scholar]
- 21.Park, S. J. et al. Cuticular chemistry of the Queensland fruit fly Bactrocera tryoni (Froggatt). Molecules2510.3390/molecules25184185 (2020). [DOI] [PMC free article] [PubMed]
- 22.Vaníčková, L. et al. Cuticular hydrocarbons of the South American fruit fly Anastrepha fraterculus: variability with sex and age. J. Chem. Ecol.38, 1133–1142. 10.1007/s10886-012-0177-8 (2012). [DOI] [PubMed]
- 23.Shen, J. et al. Allyl-2,6-dimethoxyphenol, a female-biased compound, is robustly attractive to conspecific males of Bactrocera dorsalis at close range. Entomol. Exp. Appl.167, 811–819. 10.1111/eea.12833 (2019). [Google Scholar]
- 24.Evans, J. J. T. & Stanbury, P. J. The function of the tergal glands in the Queensland fruit fly, Dacus Tyroni. J. Insect Physiol.13, 1875–1883. 10.1016/0022-1910(67)90024-8 (1967). [Google Scholar]
- 25.Evans, J. J. T. Development and ultrastructure of the fat body cells and oenocytes of the Queensland fruit fly, Dacus tryoni (Frogg). Z. Zellforsch Mikrosk Anat.81, 49–61. 10.1007/BF00344551 (1967). [DOI] [PubMed] [Google Scholar]
- 26.Evans, J. J. T. The integument of the Queensland fruit fly, Dacus tryoni (Frogg). Z. Zellforsch Mikrosk Anat.81, 18–33. 10.1007/BF00344549 (1967). [DOI] [PubMed] [Google Scholar]
- 27.Canale, A. et al. Behavioural and electrophysiological responses of the olive fruit fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), to male- and female-borne sex attractants. Chemoecology23, 155–164. 10.1007/s00049-013-0131-4 (2013). [Google Scholar]
- 28.Perkins, M. V. Characterisation and synthesis of Bactrocera fruit fly pheromones Doctor of Philosophy thesis, University of Queensland, (1990).
- 29.Booth, Y. K. et al. Synthesis and absolute configuration of a constitutionally-new [5.6] spiroacetal from B. Tryoni (Queensland fruit fly). Org. Biomol. Chem.5, 1111–1117. 10.1039/B701833A (2007). [DOI] [PubMed] [Google Scholar]
- 30.Brophy, J. J. & Nelson, D. 2,5-Dimethyl-3-n-propylpyrazine from the head of the bull ant Myrmecia gulosa (Fabr). Insect Biochem.15, 363–365. 10.1016/0020-1790(85)90027-7 (1985). [Google Scholar]
- 31.Mori, K., Soga, H., Ikunaka, M. & Pheromone syntheses, L. X. X. V. I. I. I. Synthesis of the four thermodynamically stable stereoisomers of 2,7-dimethyl-1,6-dioxaspiro[4.6]undecane, a component of the volatile secretion from the mandibular glands of Andrena haemorrhoa F. Liebigs Ann. Chem. 2194–2205, (1985). 10.1002/jlac.198519851109 (1985).
- 32.Bellas, T. E. & Fletcher, B. S. Identification of the major components in the secretion from the rectal pheromone glands of the queensland fruit flies Dacus tryoni and Dacus neohumeralis (Diptera: Tephritidae). J. Chem. Ecol.5, 795–803. 10.1007/BF00986564 (1979). [Google Scholar]
- 33.Ginzel, M. D., Blomquist, G. J., Millar, J. G. & Hanks, L. M. Role of contact pheromones in mate recognition in Xylotrechus colonus. J. Chem. Ecol.29, 533–545. 10.1023/A:1022894419521 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Bowdan, E. Electrophysiological responses of tarsal contact chemoreceptors of the apple maggot fly Rhagoletis pomonella to salt, sucrose and oviposition-deterrent pheromone. J. Comp. Physiol. A. 154, 143–152. 10.1007/BF00605399 (1984). [Google Scholar]
- 35.Crnjar, R. M. & Prokopy, R. J. Morphological and electrophysiological mapping of tarsal chemoreceptors of oviposition-deterring pheromone in Rhagoletis pomonella flies. J. Insect Physiol.28, 393–400. 10.1016/0022-1910(82)90064-6 (1982).
- 36.Prokopy, R. J. & Spatcher, P. J. Location of receptors for oviposition-deterring pheromone in Rhagoletis pomonella flies 2. Ann. Entomol. Soc. Am.70, 960–962. 10.1093/aesa/70.6.960 (1977).
- 37.Baker, R. & Bacon, A. J. The identification of spiroacetals in the volatile secretions of two species of fruit fly (Dacus dorsalis, Dacus curcurbitae). Experientia41, 1484–1485. 10.1007/BF01950049 (1985). [Google Scholar]
- 38.Baker, R., Herbert, R. H. & Lomer, R. A. Chemical components of the rectal gland secretions of male Dacus cucurbitae, the melon fly. Experientia38, 232–233. 10.1007/BF01945082 (1982). [Google Scholar]
- 39.Booth, Y. K. et al. A diverse suite of spiroacetals, including a novel branched representative, is released by female Bactrocera tryoni (Queensland fruit fly). Chem. Commun.42, 3975–3977. 10.1039/B611953K (2006). [DOI] [PubMed] [Google Scholar]
- 40.Fletcher, M. T. et al. Chemistry of fruit-flies. Spiroacetal-rich secretions in several Bactrocera species from the South-West Pacific region. J. Chem. Soc. Perkin Trans.1, 2827–2831. 10.1039/P19920002827 (1992). [Google Scholar]
- 41.Noushini, S. et al. Attraction and electrophysiological response to identified rectal gland volatiles in Bactrocera frauenfeldi (Schiner). Molecules2510.3390/molecules25061275 (2020). [DOI] [PMC free article] [PubMed]
- 42.Nishida, R., Tan, K. H., Takahashi, S. & Fukami, H. Volatile components of male rectal glands of the melon fly Dacus Cucubitae Coquillett: Diptera: Tephritidae. Appl. Entomol. Zool.25, 105–112. 10.1303/aez.25.105 (1990).
- 43.Krohn, S. et al. Chemistry of fruit flies: nature of glandular secretion and volatile emission of Bactrocera (bactrocera) cacuminatus (Héring). J. Chem. Ecol.17, 485–495. 10.1007/BF00994347 (1991). [DOI] [PubMed] [Google Scholar]
- 44.Wee, S. L. & Tan, K. H. Female sexual response to male rectal volatile constituents in the fruit fly, Bactrocera carambolae (Diptera: Tephritidae). Appl. Entomol. Zool.40, 365–372. 10.1303/aez.2005.365 (2005). [Google Scholar]
- 45.Perkins, M. V., Kitching, W., Drew, R. A. I., Moore, C. J. & König, W. A. Chemistry of fruit flies: composition of the male rectal gland secretions of some species of South-East Asian Dacinae. Re-examination of Dacus cucurbitae (melon fly). J. Chem. Soc. Perkin Trans.1, 1111–1117. 10.1039/P19900001111 (1990). [Google Scholar]
- 46.Katte, T., Tan, K. H., Su, Z. H., Ono, H. & Nishida, R. Floral fragrances in two closely related fruit fly orchids, Bulbophyllum Hortorum and B. macranthoides (Orchidaceae): assortments of phenylbutanoids to attract tephritid fruit fly males. Appl. Entomol. Zool.55, 55–64. 10.1007/s13355-019-00653-x (2020). [Google Scholar]
- 47.Park, S. J. et al. Zingerone in the flower of Passiflora maliformis attracts an Australian fruit fly, Bactrocera jarvisi (Tryon). Molecules2510.3390/molecules25122877 (2020). [DOI] [PMC free article] [PubMed]
- 48.Oh, H. W., Jeong, S. A., Kim, J. & Park, K. C. Morphological and functional heterogeneity in olfactory perception between antennae and maxillary palps in the pumpkin fruit fly, Bactrocera depressa. Arch. Insect Biochem. Physiol.101, e21560. 10.1002/arch.21560 (2019). [DOI] [PubMed]
- 49.Verschut, T. A., Farnier, K., Cunningham, J. P. & Carlsson, M. A. Behavioral and physiological evidence for palp detection of the male-specific attractant cuelure in the Queensland fruit fly (Bactrocera tryoni). Front. Physiol.910.3389/fphys.2018.00990 (2018). [DOI] [PMC free article] [PubMed]
- 50.Park, K. C., Jeong, S. A., Kwon, G. & Oh, H. W. Olfactory attraction mediated by the maxillary palps in the striped fruit fly, Bactrocera scutellata: electrophysiological and behavioral study. Arch. Insect Biochem. Physiol.99, e21510. 10.1002/arch.21510 (2018). [DOI] [PubMed]
- 51.Andrade, R., Rodriguez, C. & Oehlschlager, A. C. Optimization of a pheromone lure for Spodoptera frugiperda (Smith) in Central America. J. Braz Chem. Soc.11, 609–613. 10.1590/S0103-50532000000600009 (2000).
- 52.Unbehend, M., Hänniger, S., Meagher, R. L., Heckel, D. G. & Groot, A. T. Pheromonal divergence between two strains of Spodoptera frugiperda. J. Chem. Ecol.39, 364–376. 10.1007/s10886-013-0263-6 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Hamilton, J. G. C., Ibbotson, H. C., Hooper, A. M. & Pickett, J. A. 9-Methylgermacrene-B is confirmed as the sex pheromone of the sandfly Lutzomyia Longipalpis from Lapinha, Brazil, and the absolute stereochemistry defined as S. Chem. Commun. 2335–2336. 10.1039/A907910F (1999).
- 54.Hamilton, J. G. C., Dougherty, M. J. & Ward, R. D. Sex pheromone activity in a single component of tergal gland extract of Lutzomyia Longipalpis (Diptera: Psychodidae) from Jacobina, Northeastern Brazil. J. Chem. Ecol.20, 141–151. 10.1007/BF02065997 (1994). [DOI] [PubMed] [Google Scholar]
- 55.Mebazaa, R. et al. Characterisation of volatile compounds in Tunisian fenugreek seeds. Food Chem.115, 1326–1336. 10.1016/j.foodchem.2009.01.066 (2009). [Google Scholar]
- 56.Fan, W. & Qian, M. C. Characterization of aroma compounds of Chinese Wuliangye and Jiannanchun liquors by aroma extract dilution analysis. J. Agric. Food Chem.54, 2695–2704. 10.1021/jf052635t (2006). [DOI] [PubMed] [Google Scholar]
- 57.Isidorov, V. A., Krajewska, U., Dubis, E. N. & Jdanova, M. A. Partition coefficients of alkyl aromatic hydrocarbons and esters in a hexane–acetonitrile system. J. Chromatogr. A. 923, 127–136. 10.1016/S0021-9673(01)00929-3 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Kenig, F. et al. Occurrence and origin of mono-, di-, and trimethylalkanes in modern and holocene cyanobacterial mats from Abu Dhabi, United Arab Emirates. Geochim. Cosmochim. Acta. 59, 2999–3015. 10.1016/0016-7037(95)00190-5 (1995). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information file).