Abstract
Objective
To evaluate prognostic factors in women with invasive VSCC at Sun Yat-sen University Cancer Center (SYSUCC).
Methods
137 patients with VSCC at SYSUCC were retrospectively analyzed. The Kaplan-Meier method assessed the overall survival (OS) and progression-free survival (PFS) time. Prognostic factors were identified using univariable and multivariable Cox regression analysis.
Results
Only 2 out of 137 patients had positive postoperative margins after intraoperative supplemental excision. The international federation of gynecology and obstetrics (FIGO) Stage III-IV (HR: 4.67, 95 % confidence intervals (CI): 2.48–8.79) and BMI ≥25 kg/m2 (HR: 1.86, 95 % CI: 1.08–3.23) were independent risk factors for OS. The independent risk factors affecting PFS included FIGO stage III-IV (HR: 3.72, 95 % CI: 2.10–6.60), BMI ≥25 kg/m2 (HR: 2.15, 95 % CI: 1.28–3.64), and squamous cell carcinoma antigen (SCC-Ag) > 1.5 ng/ml (HR: 2.06, 95 % CI: 1.23–3.47). The survival of 12 individuals with perineural invasion (PNI) was extremely poor, with a median OS of 37 months and a median PFS of 22 months.
Conclusion
The surgical margin should be at least 1.0 cm away from the tumor edge. When the surgeons cannot ensure the negative margins, detecting surgical margins with rapid pathological examination may reduce the incidence of postoperative positive margins. FIGO stage III-IV, ILN metastases, and BMI ≥25 kg/m2 are important adverse prognostic factors in VSCC patients. Cases with PNI may have poor prognosis. SCC-Ag might be a useful marker for predicting relapse.
Keywords: Vulvar squamous cell carcinoma (VSCC), Surgical margins, Intraoperative pathological examination, Inguinal lymph nodes (ILN) status, Body mass index (BMI)
1. Introduction
Vulvar cancer (VC) is a rare malignancy, accounting for 0.3 % of all new cancer cases and about 4–5% of female genital system cancers. About 90 % of VC cases are squamous cell carcinoma [1]. Treatments of vulvar squamous cell carcinoma (VSCC) include surgery, radiotherapy, chemoradiation, and targeted therapies. Patients of VSCC with stage I to stage II can be effectively treated with surgery [2]. For advanced VSCC, neoadjuvant chemotherapy with cisplatin or carboplatin in combination with paclitaxel is an option [3]. However, partially advanced VSCC shows poor sensitivity to adjuvant chemoradiotherapy, and the efficacy of targeted therapies in VSCC lacks substantial evidence [4].
Advanced stages and inguinal lymph node (ILN) metastases are poor prognostic factors for VSCC [5,6]. Additionally, lymph vascular space invasion, large tumor size, positive pathological margins, insufficient surgical margins, pathological grade, perineural invasion (PNI), depth of stromal invasion, and abnormal squamous cell carcinoma antigen (SCC-Ag) serum levels may increase the risk of recurrence [[7], [8], [9], [10]]. Tumor-free margin distance is an important element for locoregional control [11]. Most guidelines recommend a gross surgical margin of at least 1 cm and a pathologic margin of at least 8 mm. However, there is still controversy regarding the optimal tumor-free margin.
However, the impact of these pathological factors on the prognosis of patients with VSCC remains controversial. High body mass index (BMI) is a known risk factor for various types of cancer [12], but its relationship with VSCC is unclear. Due to the rarity and controversies of VSCC, we analyzed the prognostic factors for VSCC at Sun Yat-sen University Cancer Center (SYSUCC), one of the largest cancer centers in China, to provide treatment insights for this rare disease.
2. Method
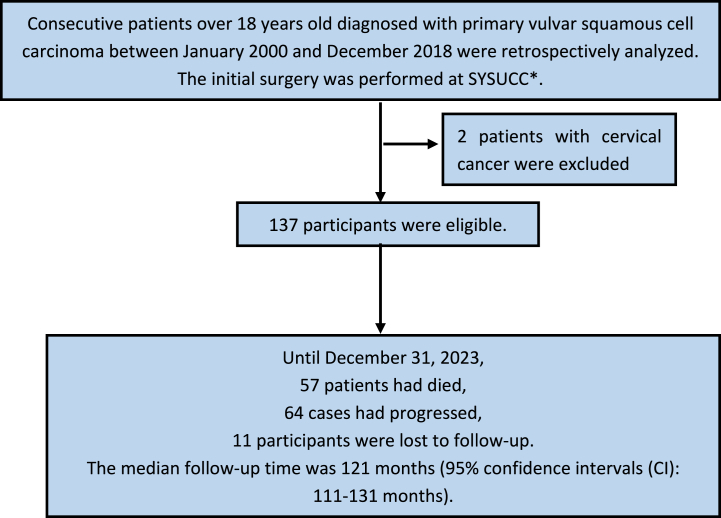
Consecutive patients diagnosed with primary VSCC between January 2000 and December 2018 were retrospectively analyzed(Fig. 1). The initial surgery was performed at SYSUCC. Patients with other malignancies and life-threatening concomitant diseases were excluded. The follow-up period was extended until December 31, 2023. Follow-up was conducted every 3 months within the previous two years, every 6 months in the third to fifth years, and once a year after 5 years. Gynecological examination, tumor markers, HPV testing, ultrasonography and other imaging examinations will be inspected. If the patient did not follow up at the scheduled time, we will send emails to the patients. A total of 137 participants were eligible. This study was approved by the Ethics Committee of SYSUCC (approval number B2023-068-01).
Fig. 1.
Procedures for patients selection and follow-up.
∗SYSUCC: Sun Yat-sen University Cancer Center.
Overall survival (OS) and progression-free survival (PFS) time were analyzed by the Kaplan-Meier method. The correlation of clinical and histopathological factors with OS and PFS was assessed by a standard log-rank test. Prognostic factors were identified with univariate and multivariate Cox regression analyses. Factors with P < 0.05 in univariate COX regression analysis were included in multivariate COX regression analysis. The effects were expressed as hazard ratio (HR) and 95 % CI. A P-value less than 0.05 was considered statistically significant. All data analyses were performed using SPSS software, version 25.
3. Results
3.1. Clinical and histological characteristics
According to the inclusion and exclusion criteria, 137 patients were eligible. Of the 137 patients, 57 had died and 64 had progressed. The median follow-up time was 121 months (95 % confidence intervals (CI): 111–131 months). Eleven participants (8.0 %) were lost to follow-up. The median OS was 160 months, with a 5-year OS rate of 66.0 %. The median PFS was 129 months, with a 5-year PFS rate of 59.5 %.
The clinicopathological characteristics are presented in Table 1. Overweight was defined as a BMI of 25–30 kg/m2. Participants were divided into two groups based on a BMI cut-off point of 25 kg/m2. The incidence of ILN metastases was low for tumors with a diameter of less than 4 cm, and sentinel ILN biopsy was used instead of ILN dissection [13,14]. Patients were divided into two groups using a tumor diameter cut-off point of 4 cm. Pathological results regarding tumor invasion depth were not recorded before 2008, so invasion depth was re-evaluated. For 38 of the 137 patients, tumor invasion data were unavailable due to the partial loss of pathological slides.
Table 1.
Clinical and histological characteristics.
| Characteristics | Frequency | Proportion of patients |
|---|---|---|
| Age (median: 58, range: 29–89) | ||
| <60 years | 75 | 54.74 % |
| ≥60 years | 62 | 45.26 % |
| BMI (median: 23.5, range: 16.6–35.3) | ||
| <25 kg/m2 | 86 | 62.77 % |
| ≥25 kg/m2 | 51 | 37.23 % |
| SCC-Ag (median: 0.9, range: 0.0–13.6) | ||
| ≤1.5 ng/ml | 89 | 64.96 % |
| >1.5 ng/ml | 48 | 35.04 % |
| FIGO (2021)a | ||
| I | 65 | 47.45 % |
| II | 11 | 8.03 % |
| III | 55 | 40.15 % |
| IV | 6 | 4.38 % |
| ILN status | ||
| Positive | 61 | 44.53 % |
| Negative | 61 | 44.53 % |
| Unknown | 15 | 10.94 % |
| Tumor size (median: 3.0, range: 0.5–12.0) | ||
| <4 cm | 87 | 63.50 % |
| ≥4 cm | 50 | 36.50 % |
| Pathological grade | ||
| G1 | 70 | 51.09 % |
| G2 | 52 | 37.96 % |
| G3 | 15 | 10.95 % |
| Infiltration depth (median: 7, range: 1.0–23.0) | ||
| ≤7 mm | 50 | 36.50 % |
| >7 mm | 49 | 35.77 % |
| Unknown | 38 | 27.74 % |
| Margin status | ||
| Positive | 2 | 1.46 % |
| Negative | 135 | 98.54 % |
| LVSI | ||
| Yes | 3 | 2.19 % |
| No | 134 | 97.81 % |
| PNI | ||
| Yes | 12 | 8.76 % |
| No | 125 | 91.24 % |
All enrolled patients were re-staged according to the 2021 FIGO staging.
Only 2 cases had positive postoperative margins. The range of minimal surgical margins was 0.5 cm–2.0 cm, with a median of 1.5 cm. 10 patients had the smallest surgical margin of less than 1.0 cm, with a range from 0.5 cm to 0.8 cm, mainly in tumors near the urethra or anus. Seven of the ten cases were sent for rapid pathologic examination. Two of the seven patients had positive margins. The margins were negative after a supplemental 1.0 cm urethrotomy. Three of the ten patients were not sent for intraoperative examination; one case had a positive margin postoperatively. Thirty percent of cases were positive with minimal surgical margins of less than 1.0 cm. One hundred and twenty-seven patients had minimal surgical margins of at least 1.0 cm, ranging from 1.0 cm to 2.0 cm. Twenty-two cases were sent for intraoperative pathologic examination, and one case had a positive margin and underwent supplemental resection. One hundred and five patients were not sent for rapid pathologic examination; one case had a positive basal margin postoperatively. Only 1.6 % of cases were positive with minimal surgical margins of at least 1.0 cm. One patient with positive postoperative margins progressed at 4 months after surgery and died at 26 months, while the other had an OS of 108 months.
Three patients had LVSI, and 12 cases had PNI. Three cases with positive LVSI had an OS of 84, 116, and 146 months, respectively. Twelve individuals with PNI had a median OS of 37 months (95 % CI: 20–54 months), and a median PFS of 22 months (95 % CI: 0–80 months). Both OS and PFS were poorer in this group. Due to the small number of positive patients for these three pathologic factors, they were not included in the statistical analysis.
3.2. Univariate analysis
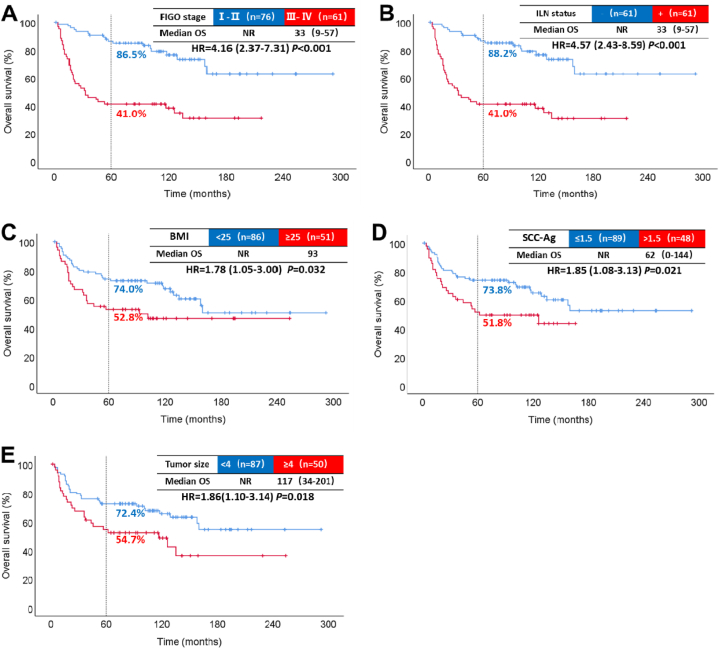
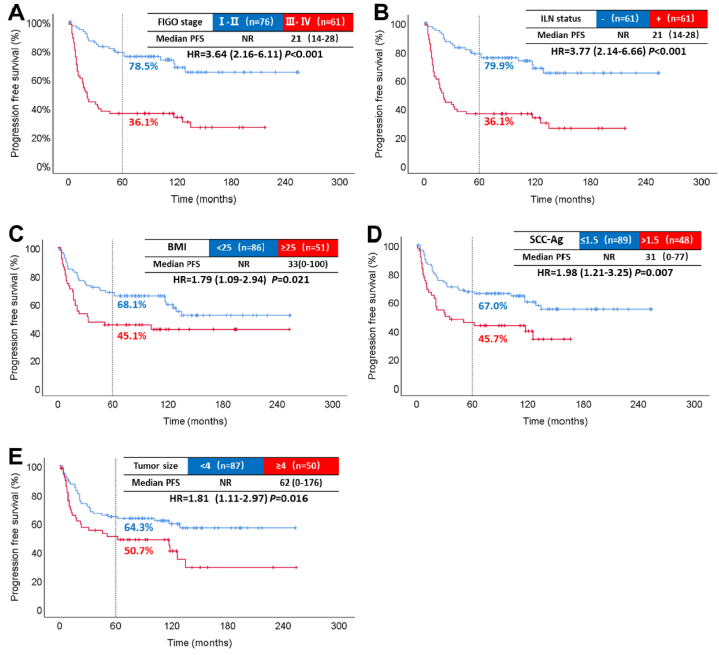
Patients with the international federation of gynecology and obstetrics (FIGO) stage III-IV, ILN metastases, BMI ≥25 kg/m2, SCC-Ag > 1.5 ng/mL, and tumor diameter ≥4 cm had significantly shortened OS and PFS. There was no significant survival correlation between pathological grade, depth of invasion, and age. The results of the univariate analysis are shown in Table 2. The median OS for stage III-IV was 33 months. The OS HR compared to stage I-II was 4.16, with a 95 % CI of 2.37–7.31 (P < 0.001, Fig. 2 A). The 5-year PFS rate was 78.5 % for stage I-II and 36.1 % for stage III-IV (P < 0.001, Fig. 3. A). Furthermore, the 5-year OS rate for patients with metastatic ILN (41.0 %) was lower than for those without metastasis (88.2 %) (P < 0.001, Fig. 2 B).
Table 2.
OS and PFS Results of univariate analysis.
| Factors | mOS (95 % CI) | 5-y OS | HR (95 % CI) | P-value | mPFS (95 % CI) | 5-y PFS | HR (95 % CI) | P-value |
|---|---|---|---|---|---|---|---|---|
| FIGO stagea | ||||||||
| I-IIa | NR | 86.5 % | NR | 78.5 % | ||||
| III-IVa | 33 (9–57) | 41.0 % | 4.16 (2.37–7.31) | <0.001 | 21 (14–28) | 36.1 % | 3.64 (2.16–6.11) | <0.001 |
| ILN status | ||||||||
| Negative | NR | 88.2 % | NR | 79.9 % | ||||
| Positive | 33 (9–57) | 41.0 % | 4.57 (2.43–8.59) | <0.001 | 21 (14–28) | 36.1 % | 3.77 (2.14–6.66) | <0.001 |
| BMI | ||||||||
| <25 kg/m2 | NR | 74.0 % | NR | 68.1 % | ||||
| ≥25 kg/m2 | 93 | 54.8 % | 1.78 (1.05–3.00) | 0.029 | 33 (0–100) | 45.1 % | 1.79 (1.09–2.94) | 0.018 |
| SCC-Ag | ||||||||
| ≤1.5 ng/ml | NR | 73.8 % | NR | 67.0 % | ||||
| >1.5 ng/ml | 62 (0–144) | 51.8 % | 1.85 (1.08–3.13) | 0.021 | 31 (0–77) | 45.7 % | 1.98 (1.21–3.25) | 0.005 |
| Tumor size | ||||||||
| <4 cm | NR | 72.4 % | NR | 64.3 % | ||||
| ≥4 cm | 117 (34–201) | 54.7 % | 1.86 (1.10–3.14) | 0.018 | 62 (0–176) | 50.7 % | 1.81 (1.11–2.97) | 0.016 |
| Grade | ||||||||
| G1 | NR | 67.1 % | 117 | 55.7 % | ||||
| G2-G3 | NR | 64.8 % | 1.02 (0.60–1.71) | 0.954 | 135 | 63.5 % | 0.85 (0.52–1.39) | 0.513 |
| Infiltration depth | ||||||||
| ≤7 mm | NR | 70.0 % | NR | 62.0 % | ||||
| >7 mm | 158 (30–217) | 62.5 % | 1.03 (0.75–1.43) | 0.747 | 117 (54–222) | 56.3 % | 1.04 (0.76–1.40) | 0.577 |
| Age | ||||||||
| <60 years | NR | 70.6 % | NR | 64.0 % | ||||
| ≥60 years | 126 (74–178) | 60.4 % | 1.49 (0.88–2.50) | 0.132 | 102 (37–174) | 53.9 % | 1.38 (0.85–2.26) | 0.192 |
mOS: median OS; mPFS: median PFS; 5-y OS: 5-year OS rate; 5-y PFS: 5-year PFS rate; NR: not reached.
Due to the small number of patients in Stage II and Stage IV, these patients are divided into Stages I-II and III-IV.
Fig. 2.
Results of single-factor analysis affecting OS. Overall survival by FIGO stage (A). Overall survival by inguinal lymph node (B). Overall survival by BMI (C). Overall survival by SCC-Ag (D). Overall survival by tumor size (E).
NR: not reached; -: negative; +: positive; ILN: inguinal lymph node.
Fig. 3.
Results of single-factor analysis affecting PFS. Progression-free survival by FIGO stage (A). Progression-free survival by inguinal lymph node status (B). Progression-free survival by BMI (C). Progression-free survival by SCC-Ag (D). Progression-free survival by tumor size (E).
NR: not reached; -: negative; +: positive; ILN: inguinal lymph node.
Similarly, when the ILN was positive, the 5-year PFS rate decreased to 36.1 % (P < 0.001, Fig. 3. B). Moreover, patients with BMI ≥25 kg/m2 had worse OS (P = 0.029, Fig. 2 C) and shorter PFS (P = 0.016, Fig. 3. C) compared to those with BMI <25 kg/m2. Additionally, the SCC-Ag > 1.5 ng/mL group had a lower 5-year OS rate (51.8 %) than the normal SCC-Ag group (73.8 %) (P = 0.021, Fig. 2 D). The median PFS in the SCC-Ag abnormal group was 31 months (Fig. 3. D). Furthermore, patients with tumor diameter ≥4 cm had a lower 5-year OS rate (P = 0.018, Fig. 2 E) and poorer PFS rate (P = 0.016, Fig. 3. E).
Grade (G) 2–3 might be a high-risk factor affecting VSCC [15]. Due to the small number of patients with G3 VSCC, both G2 and G3 were combined for analysis. There was no significant difference between OS (P = 0.954) and PFS (P = 0.513) in the G1 and G2-3 groups. Similarly, no statistical significance was found with regards to infiltration depth and age.
3.3. Multivariate analysis
In univariate COX regression analysis, factors with P < 0.05 include FIGO stage, ILN status, BMI, SCC-Ag, and tumor size. These factors were included in multivariate COX regression analysis. The main difference between stage I and stage III of VSCC is whether there is ILN metastasis. The variables of the FIGO stage and ILN status exhibited multicollinearity. Therefore, the ILN status was automatically excluded from the analysis.
As a result, FIGO III-IV stage(HR: 4.67, 95 % CI: 2.48–8.79, P < 0.001) and BMI ≥25 kg/m2 (HR: 1.86, 95 % CI: 1.08–3.23, P = 0.027) were independent risk factors for OS. The independent risk factors affecting PFS included FIGO stage III-IV (HR: 3.72, 95 % CI: 2.10–6.60, P < 0.001), BMI ≥25 kg/m2 (HR: 2.15, 95 % CI: 1.28–3.64, P = 0.004), and SCC-Ag > 1.5 ng/ml (HR: 2.06, 95 % CI: 1.23–3.47 P = 0.006).
3.4. Further analysis of BMI
To exclude confounding factors, we compared baseline and adjuvant radiotherapy in different BMI groups (Table 3). Sixty-one patients were diagnosed with stage III-IV and needed postoperative radiotherapy. Four cases received adjuvant chemotherapy and did not undergo adjuvant radiotherapy. Three cases did not have postoperative radiotherapy because of the full dose of preoperative radiotherapy. Nine patients refused radiotherapy because of old age and poor basal status. Forty-five patients received adjuvant radiotherapy. In the low BMI group, the median interval between operation and adjuvant radiotherapy was 52 days. The median interval was 67 days in the high BMI group (P = 0.169).
Table 3.
Baseline and adjuvant radiotherapy in patients with different BMI.
| Factors | BMI <25 kg/m2 | BMI ≥25 kg/m2 | P-value |
|---|---|---|---|
| Age | |||
| <60 years | 48 (55.8 %) | 27 (52.9 %) | |
| ≥60 years | 38 (44.2 %) | 24 (47.1 %) | 0.744 |
| FIGO stage | |||
| I-II | 48 (55.8 %) | 28 (54.9 %) | |
| III-IV | 38 (44.2 %) | 23 (45.1 %) | 0.917 |
| ILN status | |||
| Negative | 38 (44.2 %) | 23 (45.1 %) | |
| Positive | 38 (44.2 %) | 23 (45.1 %) | |
| Unknown | 10 (11.6 %) | 5 (9.8 %) | 0.947 |
| SCC-Ag | |||
| ≤1.5 ng/ml | 53 (61.6 %) | 36 (70.6 %) | |
| >1.5 ng/ml | 33 (38.4 %) | 15 (29.4 %) | 0.288 |
| Grade | |||
| G1 | 44 (51.2 %) | 26 (51.0 %) | |
| G2-G3 | 42 (48.8 %) | 25 (49.0 %) | 0.984 |
| Tumor size | |||
| <4 cm | 31 (36.0 %) | 19 (37.3 %) | |
| ≥4 cm | 55 (64.0 %) | 32 (62.7 %) | 0.887 |
| Infiltration depth | |||
| ≤7 mm | 29 (33.7 %) | 20 (39.2 %) | |
| >7 mm | 30 (34.9 %) | 20 (39.2 %) | |
| unknown | 27 (31.4 %) | 11 (21.6 %) | 0.461 |
| Adjuvant radiotherapy rates | |||
| No | 56 (65.1 %) | 36 (70.6 %) | |
| Yes | 30 (34.9 %) | 15 (29.4 %) | 0.510 |
| Median interval between surgery and adjuvant radiotherapy | 52 days | 67 days | 0.169 |
4. Discussion
4.1. Summary of main results
Only 2 of 137 patients had positive postoperative margins after intraoperative supplemental excision. FIGO stage III-IV and BMI ≥25 kg/m2 were independent risk factors for OS. The independent risk factors affecting PFS included FIGO stage III-IV, BMI ≥25 kg/m2, and SCC-Ag > 1.5 ng/ml.
4.2. Results in the context of published literature
Tumor-positive margin was the independent risk factor for local recurrence. A positive postoperative margin is an independent risk factor for local recurrence. In a cohort study, 30 of 148 patients had positive tumor margins, with a probability of about 20 % [16]. In our study, of the 137 patients enrolled, only 2 cases had positive postoperative margins, undetected by intraoperative pathology. The incidence of positive margins was very low and was reconfirmed by the pathologist in our study. Most guidelines recommend a gross surgical margin of at least 1 cm and a pathologic margin of at least 8 mm. However, there is still controversy regarding the optimal tumor-free margin. The tumor-free margin of <8 mm is associated with a higher risk of local recurrence, while some studies suggested that a recurrence rate of pathologic margins less than 8 mm is acceptable [17]. More than 2 mm tumor-free margins are associated with better local control [18]. 3.6 % (5/137) of people had positive margins if without additional incision in our research. Only 1.6 % (2/127) of cases were positive with minimal surgical margins of at least 1 cm. However, the positive rate of surgical margins <1 cm is 30 % (3/10) without supplemental resection. Rapid pathology and sufficient tumor-free margins are the important reasons for the low positive margins after surgery.
We recommend at least 1 cm of surgical margin to minimize the rate of positive margins. In addition, when the lesion is too large and near the urethra or anus, ensuring a negative margin is important, especially if the minimal surgical margin is less than 1 cm. It is important to send the margin for rapid pathologic examination to immediately supplement with resection which reduces the rate of positivity.
The 5-year OS rate is 85.6 % in the early stage, 47.5 % in locally advanced patients, and 23.3 % in distant metastasis according to the Surveillance, Epidemiology, and End Results database. A systematic review showed that the 5-year OS rates for FIGO stages I, II, III, and IV are 84.0 %, 74.6 %, 47.8 %, and 9.4 %, respectively [19]. In addition, the 5-year OS rate in patients with positive ILN is 57.2 % [6]. The HR for OS in positive patients is 5.6 compared to negative patients [20]. The prognostic results of the FIGO stage and ILN in our study are similar to the previous data.
Tumor diameter ≥4 cm affects OS and PFS in VSCC patients [21]. However, it is not an independent risk factor in our multifactor analysis. Factors like lymph node metastases and advanced staging may dilute the prognostic impact of tumor size. Elevated SCC-Ag levels are observed in various malignant tumors and correlate significantly with tumor stages in head and neck squamous cell carcinoma [22]. Similarly, elevated SCC-Ag may indicate the risk of cervical interstitial infiltration and pelvic lymph node metastases [23,24]. SCC-Ag is also used to monitor cervical squamous cell carcinoma recurrence [25]. In our study, increased SCC-Ag was an independent risk factor for PFS, suggesting that regular monitoring of SCC-Ag levels is valuable Radical surgery may reduce SCC-Ag to be normal for patients with baseline SCC-Ag elevation. If the SCC-Ag increased again during follow-up, an indication of recurrence might be possible. There is limited research on the relationship between BMI and VSCC. Increased BMI correlates with higher mortality rates in lung cancer, renal cell carcinoma, and other cancers [26]. In endometrial and breast cancer, BMI ≥25 kg/m2 is an adverse prognostic factor [27,28]. The mechanism may be that adipokines mediate chronic inflammation leading to genetic instability and DNA damage [29]. Research shows that adipose stromal cells can provide a nutritious tumor microenvironment for carcinoma [30]. BMI ≥25 kg/m2 is an independent risk factor for patients with VSCC in our research. High BMI is associated with poor incision healing, leading to delayed adjuvant radiotherapy by twelve days, though this difference is not statistically significant (P = 0.169). Only 45 patients received adjuvant radiotherapy after surgery. It may be that the sample size was too small to derive statistical significance.
Interestingly, high BMI has been associated with improved survival in non-small cell lung cancer patients receiving immune checkpoint inhibitor therapy [31] and better outcomes in platinum-resistant or refractory ovarian cancer patients receiving VEGF-targeted therapy [32]. Although the mechanism by which obesity affects drug efficacy is unclear, VSCC patients with high BMI may benefit from immunotherapy and VEGF-targeted therapy. Further research is needed to explore these mechanisms.
4.3. Strengths and weaknesses
VSCC is a rare malignancy with prognostic factors that are not yet fully understood, particularly in China. Single-center and retrospective studies might lead to limitations in our conclusion. As the largest cancer center in South China, the majority patients with vulvar cancers came to our center for treatment. Moreover, we involved a larger number of patients with a longer follow-up period. Therefore, we recognized that our study had some reference value for the treatment of the rare vulvar cancer. Compared with public databases, our data is more complete and accurate. Another strength of our study is adequate follow-up. The incidence of positive surgical margins was low at SYSUCC. Maintaining a surgical margin of at least 1 cm can minimize the rate of positive margins. When the lesion is too large and near the urethra or anus, to ensure a negative margin, especially if the minimal surgical margin is less than 1 cm, it is important to send the margin for rapid pathologic examination. In addition, high BMI was found to be associated with worse survival outcomes in VSCC patients for the first time in this study.
For 38 of the 137 patients, tumor invasion data were unavailable due to the partial loss of pathological slides. Therefore, it is difficult to understand the effect of infiltration on the prognosis of VSCC patients. In addition, only 3 patients with LVSI and 12 cases with PNI which were too few to be analyzed. We did not perform univariate and multivariate analyses of tumor invasion, LVSI, and PNI. This may have an impact on these three factors in our analysis. The main difference between stage I and stage III of VSCC is whether there is ILN metastasis. However, the majority of cases fell into stages I and III in our study. The variables of the FIGO stage and ILN status exhibited multicollinearity. Therefore, the ILN status was automatically excluded from the multivariate analysis. Of note, ILN status also plays an important role in the prognosis of VSCC patients.
4.4. Implications for practice and future research
Establishing sufficient surgical margins and rapid pathological examination may have contributed to the low incidence of positive surgical margins at SYSUCC. We think this might be an important experience for improving the prognosis for VSCC patients. In addition, we identified the negative prognostic factors such as FIGO stage III-IV, ILN metastases, and BMI ≥25 kg/m2. This is of great significance for identifying high-risk patients. However, more prospective studies on surgical margins are needed. We also encourage further research to explore the mechanisms between high BMI and VSCC.
5. Conclusions
In summary, detecting surgical margins with rapid pathological examination, especially if the minimal surgical margin is less than 1 cm, may reduce the incidence of postoperative positive margins. FIGO stage III-IV, ILN metastases, and BMI ≥25 kg/m2 are significant adverse prognostic factors in VSCC patients. Cases with PNI may have poor prognosis. SCC-Ag might be a useful marker for predicting relapse.
CRediT authorship contribution statement
Binghong Guo: Writing – review & editing, Writing – original draft, Formal analysis. Jiaqi Qiu: Visualization, Validation, Methodology. Yulin Wang: Formal analysis, Data curation. Nuerbiya Abula: Data curation. Longyi Chen: Methodology. Heqing Zhao: Data curation. Yongyi Zhu: Writing – review & editing. Min Zheng: Supervision. Zhimin Liu: Supervision. Yongwen Huang: Writing – review & editing, Supervision, Conceptualization.
Ethics statement
This study was approved by the Sun Yat-sen University Cancer Center Ethics Committee (approval number B2023-068-01). We certify that the study followed the 1964 Declaration of Helsinki and later amendments. All data have been anonymized to preserve the privacy of participants and in compliance with relevant ethical guidelines.
Data availability statement
The datasets are stored in Research Data Deposit with an access number RDDA2024527575. (https://www.researchdata.org.cn/UserHome/).
Funding
This work is supported by the Guangdong Provincial Technology Project for Xinjiang (KTPYJ2021020).
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Yongwen Huang reports financial support was provided by Guangdong Provincial Technology Project for Xinjiang (KTPYJ2021020). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Min Zheng, Email: zhengmin@sysucc.org.cn.
Zhimin Liu, Email: liuzhim@sysucc.org.cn.
Yongwen Huang, Email: huangyw@sysucc.org.cn.
References
- 1.Pedrao P.G., et al. Management of early-stage vulvar cancer. Cancers. 2022;14(17) doi: 10.3390/cancers14174184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellinger T.H., et al. Surgical management of vulvar cancer. J. Natl. Compr. Cancer Netw. 2017;15(1):121–128. doi: 10.6004/jnccn.2017.0009. [DOI] [PubMed] [Google Scholar]
- 3.Amant F., et al. Brief report on 3-weekly paclitaxel carboplatin efficacy in locally advanced or metastatic squamous vulvar cancer. Gynecol. Obstet. Invest. 2018;83(6):620–626. doi: 10.1159/000487435. [DOI] [PubMed] [Google Scholar]
- 4.Woelber L., et al. Targeted therapeutic approaches in vulvar squamous cell cancer (VSCC): case series and review of the literature. Oncol. Res. 2021;28(6):645–659. doi: 10.3727/096504020X16076861118243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hacker N.F., Barlow E.L. Staging for vulvar cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2015;29(6):802–811. doi: 10.1016/j.bpobgyn.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Homesley H.D., et al. Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group study) Am. J. Obstet. Gynecol. 1991;164(4):997–1003. doi: 10.1016/0002-9378(91)90573-a. ; discussion 1003-4. [DOI] [PubMed] [Google Scholar]
- 7.Maggino T., et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF Study. Cancer. 2000;89(1):116–122. doi: 10.1002/1097-0142(20000701)89:1<116::aid-cncr16>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Chan J.K., et al. Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: a multivariate analysis. Gynecol. Oncol. 2007;104(3):636–641. doi: 10.1016/j.ygyno.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Scampa M., Kalbermatten D.F., Oranges C.M. Squamous cell carcinoma of the vulva: a survival and epidemiologic study with focus on surgery and radiotherapy. J. Clin. Med. 2022;11(4) doi: 10.3390/jcm11041025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aragona A.M., et al. An analysis of reported independent prognostic factors for survival in squamous cell carcinoma of the vulva: is tumor size significance being underrated? Gynecol. Oncol. 2014;132(3):643–648. doi: 10.1016/j.ygyno.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Raimond E., et al. Surgical treatment of vulvar cancer: impact of tumor-free margin distance on recurrence and survival. A multicentre cohort analysis from the francogyn study group. Eur. J. Surg. Oncol. 2019;45(11):2109–2114. doi: 10.1016/j.ejso.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Tentolouris A., Ntanasis-Stathopoulos I., Terpos E. Obesity and multiple myeloma: emerging mechanisms and perspectives. Semin. Cancer Biol. 2023;92:45–60. doi: 10.1016/j.semcancer.2023.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Van der Zee A.G., et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J. Clin. Oncol. 2008;26(6):884–889. doi: 10.1200/JCO.2007.14.0566. [DOI] [PubMed] [Google Scholar]
- 14.Oonk M.H.M., et al. Radiotherapy versus inguinofemoral lymphadenectomy as treatment for vulvar cancer patients with micrometastases in the sentinel node: results of GROINSS-V II. J. Clin. Oncol. 2021;39(32):3623–3632. doi: 10.1200/JCO.21.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Te Grootenhuis N.C., et al. Prognostic factors for local recurrence of squamous cell carcinoma of the vulva: a systematic review. Gynecol. Oncol. 2018;148(3):622–631. doi: 10.1016/j.ygyno.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Nooij L.S., et al. Tumour-free margins in vulvar squamous cell carcinoma: does distance really matter? Eur. J. Cancer. 2016;65:139–149. doi: 10.1016/j.ejca.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Woelber L., et al. Role of tumour-free margin distance for loco-regional control in vulvar cancer-a subset analysis of the Arbeitsgemeinschaft Gynäkologische Onkologie CaRE-1 multicenter study. Eur. J. Cancer. 2016;69:180–188. doi: 10.1016/j.ejca.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Arvas M., et al. The role of pathological margin distance and prognostic factors after primary surgery in squamous cell carcinoma of the vulva. Int. J. Gynecol. Cancer. 2018;28(3):623–631. doi: 10.1097/IGC.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J., Shan G. The prognostic role of FIGO stage in patients with vulvar cancer: a systematic review and meta-analysis. Curr. Med. Res. Opin. 2016;32(6):1121–1130. doi: 10.1185/03007995.2016.1162147. [DOI] [PubMed] [Google Scholar]
- 20.Papadia A., et al. Unilateral versus bilateral lymph-nodal metastases and oncologic outcome in vulvar cancer patients. J. Cancer Res. Clin. Oncol. 2020;146(7):1877–1881. doi: 10.1007/s00432-020-03196-9. [DOI] [PubMed] [Google Scholar]
- 21.Laliscia C., et al. Definitive radiotherapy for recurrent vulvar carcinoma after primary surgery: a two-institutional Italian experience. Tumori. 2019;105(3):225–230. doi: 10.1177/0300891618811279. [DOI] [PubMed] [Google Scholar]
- 22.Travassos D.C., et al. Squamous cell carcinoma antigen as a prognostic marker and its correlation with clinicopathological features in head and neck squamous cell carcinoma: systematic review and meta-analysis. J. Oral Pathol. Med. 2018;47(1):3–10. doi: 10.1111/jop.12600. [DOI] [PubMed] [Google Scholar]
- 23.Qin L. Study on the preoperative value of serum SCC-Ag in predicting the stromal invasion of cervical squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2023;149(11):9167–9171. doi: 10.1007/s00432-023-04836-6. [DOI] [PubMed] [Google Scholar]
- 24.van Schaik J.E., et al. SCC antigen concentrations in fine-needle aspiration samples to detect cervical lymph node metastases: a prospective analysis. Otolaryngol. Head Neck Surg. 2023;168(3):407–412. doi: 10.1177/01945998221102870. [DOI] [PubMed] [Google Scholar]
- 25.Fu J., et al. The role of squamous cell carcinoma antigen (SCC Ag) in outcome prediction after concurrent chemoradiotherapy and treatment decisions for patients with cervical cancer. Radiat. Oncol. 2019;14(1):146. doi: 10.1186/s13014-019-1355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrelli F., et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw. Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crosbie E.J., et al. Endometrial cancer. Lancet. 2022;399(10333):1412–1428. doi: 10.1016/S0140-6736(22)00323-3. [DOI] [PubMed] [Google Scholar]
- 28.Lee J., et al. Association of body mass index with 21-gene recurrence score among women with estrogen receptor-positive, ERBB2-negative breast cancer. JAMA Netw. Open. 2022;5(11) doi: 10.1001/jamanetworkopen.2022.43935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onstad M.A., Schmandt R.E., Lu K.H. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J. Clin. Oncol. 2016;34(35):4225–4230. doi: 10.1200/JCO.2016.69.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M., et al. Spontaneous formation of tumorigenic hybrids between human omental adipose-derived stromal cells and endometrial cancer cells increased motility and heterogeneity of cancer cells. Cell Cycle. 2019;18(3):320–332. doi: 10.1080/15384101.2019.1568743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kichenadasse G., et al. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2020;6(4):512–518. doi: 10.1001/jamaoncol.2019.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X., et al. Adipose tissue area as a predictor for the efficacy of apatinib in platinum-resistant ovarian cancer: an exploratory imaging biomarker analysis of the AEROC trial. BMC Med. 2020;18(1):267. doi: 10.1186/s12916-020-01733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are stored in Research Data Deposit with an access number RDDA2024527575. (https://www.researchdata.org.cn/UserHome/).