Abstract
Leontiasis ossea, also known as craniofacial fibrous dysplasia, is a rare form of osseous hypertrophy of the facial bones associated with chronic kidney disease and secondary hyperparathyroidism. As the condition progresses, changes in bone structure can lead to severe facial disfigurement, respiratory difficulties, dysarthria, and dysphagia. We describe the case of an approximately 30-year-old male with a long-standing history of end-stage renal disease who experienced rapidly progressing facial swelling and underlying bone changes following a period of poor access to dialysis. Despite brief symptomatic improvement following parathyroidectomy, the patient's hyperparathyroidism ultimately persisted. Subsequent immunomodulator therapy again offered subjective improvements but was discontinued in the setting of adverse effects. We provide a brief overview of the pathophysiology of leontiasis ossea, review imaging findings pertinent to the case presentation, and discuss relevant implications in the diagnosis and management of this disease.
Keywords: Uremic leontiasis ossea, Renal osteodystrophy, Hyperparathyroidism, Craniofacial fibrous dysplasia
Introduction/Background
Leontiasis ossea is a particularly drastic complication of chronic kidney disease (CKD) characterized by significant hypertrophy of the facial bones, most prominently affecting the maxilla and mandible. The pathogenesis is closely tied to the metabolic disturbances associated with CKD: phosphate retention, inadequate activation of vitamin D, and consequent hypocalcemia. These imbalances stimulate excessive parathyroid hormone (PTH) production, with a resulting secondary hyperparathyroidism that continues to progress as renal function declines. Although this hyperparathyroidism is a compensatory mechanism, it ultimately produces abnormal bone resorption and mineralization, leading to pathological skeletal remodeling [1]. Beyond facial disfigurement, leontiasis ossea can result in significant psychosocial and medical complications, including compressive cranial neuropathies. In cases of respiratory complications, the condition can be life-threatening [2].
Case presentation
The patient, an approximately 30-year-old male, had a prolonged history of end-stage renal disease (ESRD), diagnosed at approximately 12 years old following an acute febrile illness in Central America. The patient initially received regular nephrology care and dialysis, which were maintained after immigrating to the United States as a teenager. However, upon reaching adulthood, barriers arose that limited his continued access to care. Notably, due to lack of health insurance, the patient relied on emergency room visits for hemodialysis and management of the ESRD.
Consequently, the patient's adherence to treatment became suboptimal, presenting for dialysis only when symptoms became significant. His treatment team emphasized the importance of routine dialysis, implemented phosphate binders, and prescribed dietary supplements including iron, calcium, and vitamin D. However, the patient's adherence to the proposed therapy over the course of his early twenties was unclear.
The patient's renal disease was further complicated by osteoporosis, with resulting atraumatic fractures of the femurs in separate incidents. Around this time, the patient also reported notable swelling and changes to his facial structure, as well as progressive difficulties with speaking and mouth closure, posing severe detriments to his quality of life. Imaging studies subsequently revealed severe interval enlargement of the facial bones, as well as additional findings consistent with leontiasis ossea, as demonstrated in Fig. 1, Fig. 2 below. The patient's medical condition continued to deteriorate with persistent hypertension and frequent hospitalizations for shortness of breath and heart failure. It was recommended that the patient receive 3-gland parathyroidectomy to alleviate his hyperparathyroidism, which he underwent in his late 20s.
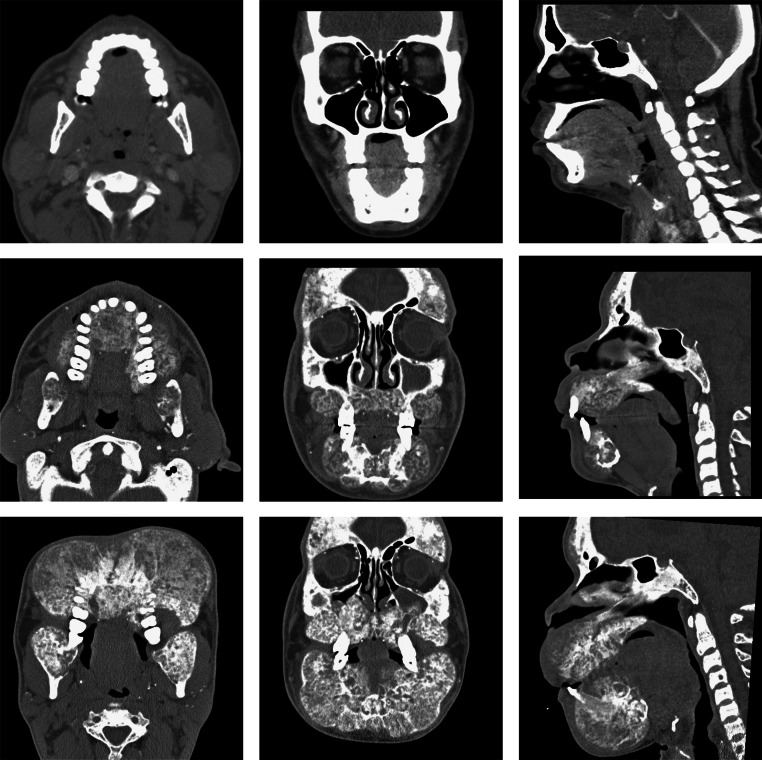
Fig. 1.
Evolution of uremic leontiasis ossea on CT imaging. Representative CT imaging of the patient's facial bones at baseline (top row), and approximately 3 years later (middle row) with interval development of several features characteristic of leontiasis ossea, including osseous overgrowth with a “salt and pepper” appearance and serpiginous tunneling, as well as loss of cortico-medullary differentiation. Further progression of the disease process is evident on follow-up images 5 years postbaseline (bottom row).
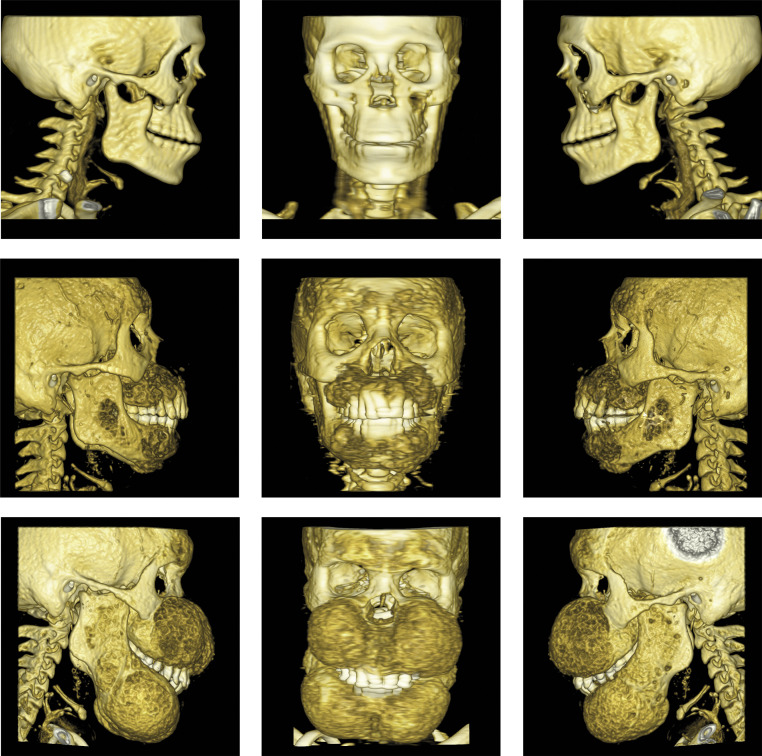
Fig. 2.
Representative facial bone surface renderings from CT scans taken at baseline (top row), 3-year (middle row), and 5-year (bottom row) timepoints.
Despite initial postoperative improvements, including reduced facial swelling that allowed him to close his mouth and eat without difficulty, the patient's PTH levels fluctuated and began to increase again several months following the procedure. The postoperative period was also complicated by “hungry bone syndrome” manifesting with hypocalcemia, a feature that has previously been reported following parathyroidectomy for leontiasis ossea [3]. In this case, the patient's calcium levels rapidly decreased from a preoperative baseline of 8.5 mg/dL to 6.1 mg/dL on the second postoperative day. Months after parathyroidectomy, the patient's facial symptoms recurred and continued to deteriorate. Soon after, treatment with imatinib was initiated. Despite initial patient-reported improvement in his facial symptoms, the patient reported persistent weakness associated with the medication, necessitating discontinuation.
Discussion
Leontiasis ossea is diagnosed via physical exam findings, patient history, lab values, and imaging. CT is the preferred imaging modality [4]. Imaging is particularly useful in distinguishing the condition from diseases with similar features, which include Paget's disease of bone, malignancy, and fibrous dysplasia. Characteristic radiologic features include a “salt and pepper” appearance of the craniofacial bones caused by the mixed lytic/sclerotic lesions of hyperparathyroidism, as well as serpiginous tunneling and/or reduced cortical definition [5]. It is believed that the predilection of leontiasis ossea to the craniofacial bones may be due to their formation by membranous ossification [6]. MR imaging is warranted in cases of soft tissue complications, including suspected cranial nerve impingement [7]. Beyond diagnostic utility, imaging can also be valuable in monitoring for disease progression and treatment response.
Management of leontiasis ossea to date is predominantly based upon correction of the underlying electrolyte disturbances associated with CKD. Mainstays of treatment for CKD-related secondary hyperparathyroidism are frequently used, including phosphate-binding agents, calcium supplementation, and supplementation with Vitamin D analogues [4,8]. Access and adherence to dialysis also likely play a pivotal role in preventing further disease progression, while monitoring of serum calcium, phosphorus, and PTH levels may provide additional opportunities for intervention.
Tyrosine kinase inhibitors, such as imatinib, have previously been shown to impact bone metabolism, osteoclast activity and bone density [9,10]. Accordingly, such therapies may be of use in the management of leontiasis ossea. In this case, initiation of imatinib coincided with patient-reported improvements in facial deformities and associated symptoms. However, the generalizability of this finding is limited by the absence of definitive imaging or laboratory support and a paucity of literature pertaining to the use of tyrosine kinase inhibitors for this indication. Adverse effects, such as our patient's persistent muscle weakness prompting discontinuation of imatinib therapy, also emphasize the importance of weighing risks and benefits for individual patients. The impacts of imatinib therapy on PTH levels raise further considerations regarding the use of tyrosine kinase inhibitors in hyperparathyroidism-mediated renal osteodystrophy [11]. Nonetheless, the patient-reported improvements in this case, and documented reductions on bone fibrosis in animal models suggest that further research into tyrosine kinase inhibitors may reveal novel therapeutic treatments for leontiasis ossea and other osteodystrophies [12].
In patients with refractory hyperparathyroidism despite adherence to CKD management, invasive treatment options such as parathyroidectomy may be necessary. However, as demonstrated in this case and others, hyperparathyroidism may recur or persist despite surgical intervention, and the risk of postoperative complications must be considered [13]. In this case, the patient experienced “hungry bone syndrome,” a phenomenon in which a sudden decrease in PTH levels following parathyroidectomy induces a period of unbalanced osteoblastic activity without subsequent osteoclast activation [14]. This relative increase in osteoblast activity drives circulating calcium and phosphate into the bone, rapidly resulting in severe hypocalcemia and hypophosphatemia. Furthermore, while parathyroidectomy may address underlying mechanisms and can theoretically prevent progression, they are not capable of restoring a patient's baseline facial structure. Oromaxillofacial surgical interventions are thought to provide the best chances for facilitating a cosmetic and functional return to normal facial structure, but recurrence and/or further progression remain possible, particularly if the underlying renal or parathyroid disturbances are not adequately addressed.
In highlighting the drastic deterioration and systemic sequelae (including leontiasis ossea) that this patient experienced after a loss of regular dialysis access, our case report further contributes to the existing abundance of evidence that health disparities and consistent, reliable access to medical care play pivotal roles in the outcomes of patients with CKD [[15], [16], [17]]. Given the lack of nonsurgical options for the correction of leontiasis ossea and its associated complications, early recognition and management are crucial. Further research into therapeutic options is needed.
Conclusion
Leontiasis ossea presents unique challenges and opportunities for clinical practice. The distinctive radiologic findings, driven by underlying metabolic disturbances, emphasize the role of imaging in clarifying diagnosis. While medical and surgical interventions exist, the disease is difficult to treat, and further progression and/or recurrence may occur. This case emphasizes the need for careful assessment and individualized management while highlighting the necessity for further research into management options.
Patient consent
Written consent from the patient was obtained for the publication of this case report. Patient information has been de-identified.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Slatopolsky E, Gonzalez E, Martin K. Pathogenesis and treatment of renal osteodystrophy. Blood Purif. 2003;21(4-5):318–326. doi: 10.1159/000072552. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Thuraisingham R, Yaqoob M. Big-head disease: uremic leontiasis ossea. Kidney Int. 2006;69(10):1709. doi: 10.1038/sj.ki.5001526. [DOI] [PubMed] [Google Scholar]
- 3.Gameiro J, Duarte I, Outerelo C, Lopes JA. Uremic lion face syndrome. J Bras Nefrol. 2019;41(2):304–305. doi: 10.1590/2175-8239-jbn-2018-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alamer A. Uremic leontiasis ossea: distinctive imaging features allow differentiation from other clinical causes of leontiasis ossea. Radiol Case Rep. 2022;17(3):553–557. doi: 10.1016/j.radcr.2021.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JI, Som PM, Lawson W. Unique imaging findings in the facial bones of renal osteodystrophy. AJNR Am J Neuroradiol. 2007;28(4):608–609. [PMC free article] [PubMed] [Google Scholar]
- 6.Lee VS, Webb MS, Jr., Martinez S, McKay CP, Leight GS., Jr Uremic leontiasis ossea: “bighead” disease in humans? Radiologic, clinical, and pathologic features. Radiology. 1996;199(1):233–240. doi: 10.1148/radiology.199.1.8633151. [DOI] [PubMed] [Google Scholar]
- 7.Romano N, Federici M, Castaldi A. Imaging of cranial nerves: a pictorial overview. Insights Imaging. 2019;10(1):33. doi: 10.1186/s13244-019-0719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan SY, Xing CY, Yang G, Wang NN, Zhang B. Dramatic alteration of the skull in a uremic patient with leontiasis ossea. Intern Med. 2014;53(17):1971–1976. doi: 10.2169/internalmedicine.53.2217. [DOI] [PubMed] [Google Scholar]
- 9.O’Sullivan S, Naot D, Callon K, Porteous F, Horne A, Wattie E, et al. Imatinib promotes osteoblast differentiation by inhibiting PDGFR signaling and inhibits osteoclastogenesis by both direct and stromal cell-dependent mechanisms. J Bone Miner Res. 2007;22(11):1679–1689. doi: 10.1359/jbmr.070719. [DOI] [PubMed] [Google Scholar]
- 10.Berman E, Nicolaides M, Maki RG, Fleisher M, Chanel S, Scheu K, et al. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med. 2006;354(19):2006–2013. doi: 10.1056/NEJMoa051140. [DOI] [PubMed] [Google Scholar]
- 11.O’Sullivan S, Horne AM, Wattie DJ, Porteous F, Gamble G, Browett P, et al. Bone metabolism during long-term treatment with imatinib. Leukemia & Lymphoma. 2013;54(8):1783–1785. doi: 10.3109/10428194.2012.760734. [DOI] [PubMed] [Google Scholar]
- 12.Turner RT, Iwaniec UT, Marley K, Sibonga JD. The role of mast cells in parathyroid bone disease. J Bone Miner Res. 2010;25(7):1637–1649. doi: 10.1002/jbmr.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biskobing DM. Significance of elevated parathyroid hormone after parathyroidectomy. Endocr Pract. 2010;16(1):112–117. doi: 10.4158/ep09122.Ra. [DOI] [PubMed] [Google Scholar]
- 14.Witteveen JE, van Thiel S, Romijn JA, Hamdy NA. Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. Eur J Endocrinol. 2013;168(3):R45–R53. doi: 10.1530/eje-12-0528. [DOI] [PubMed] [Google Scholar]
- 15.Crews DC, Liu Y, Boulware LE. Disparities in the burden, outcomes, and care of chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23(3):298–305. doi: 10.1097/01.mnh.0000444822.25991.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker CS, Gadegbeku CA. Addressing kidney health disparities with new national policy: the time is now. Cardiovasc Diagn Ther. 2023;13(1):115–121. doi: 10.21037/cdt-22-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes C, Dias FM, Franco L, Almeida M. Severe bone and mineral disease in an adolescent with chronic kidney disease: a case from the 70s? BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-211571. [DOI] [PMC free article] [PubMed] [Google Scholar]