Abstract
Primary and secondary alkyl iodides and primary alkyl bromides were quickly and conveniently converted into their corresponding alkyl chlorides via SN2 halide-halide substitution. The resultant alkyl chlorides simultaneously demonstrated increased volatility and stability paired with standard headspace GC-FID methodology. The derivatization was performed on both standard and sample alike and occurred during the headspace oven equilibration phase, eliminating the extra reaction step traditionally performed during many derivatization analyses. Reaction times, temperatures, and completeness of conversion were studied as well as response from common headspace solvents and application of various chloride sources. Recovery of iodomethane from four challenging substrates was studied from the trace level to approximately 1000 ppm. Recovery ranged 94–110 % from verapamil hydrochloride (2.5–1007 ppm), 95–102 % from methylnaltrexone bromide (26–1054 ppm), and 92–106 % from (S)-laudanosine (49–942 ppm). Using hydrogen chloride as the chloride source, a method for determination of residual iodomethane in (S)-N-methyl-laudanosine iodide was validated over a 0.5–24.9 μg/mL range with a 0.1 μg/mL detection limit, 91–103 % accuracy, and 3.2 % relative standard deviation.
Keywords: Alkyl halide, Iodomethane, Drug substance, Derivatization, Headspace-GC, Methyl iodide
Highlights
-
•
1° and 2° alkyl iodides and 1° bromides can be converted to alkyl chlorides using HCl.
-
•
Resultant alkyl chlorides exhibit increased stability and volatility for HS-GC.
-
•
105 % GC-FID recovery of MeI as MeCl at 2.5 ppm in verapamil hydrochloride.
-
•
MeI to MeCl conversion with HCl outcompetes amine alkylation and Br−/I− competition.
1. Introduction
There are two primary methods for determination of residual alkyl halides in an active pharmaceutical ingredient (API) manufacturing environment: gas chromatography (GC) and liquid chromatography (LC) [[1], [2], [3]]. For GC, volatile analytes are preferably introduced via headspace autosamplers and commonly paired with a flame ionization detector (FID), an electron capture detector (ECD), or mass spectrometry (MS) [[1], [2], [3]]. Notably, ECD has demonstrated capability for nanogram and lower detection limits [4,5] and has recently been paired with headspace autosamplers using deep eutectic solvents offering further enhanced sensitivity by affording higher headspace oven temperatures [6]. As target analytes lose volatility LC techniques become more applicable, commonly using ultraviolet, charged aerosol, or MS detectors with the latter being the preferred option for trace level due to sensitivity and selectivity advantages [1,2]. However, the determination of alkyl halides is often complicated by either sample matrices or instrumental conditions that contribute to the reaction or degradation of the alkyl halide, particularly alkyl iodides, leading to failure of accuracy and stability validation criteria [5,[7], [8], [9]].
As is studied in this paper, the reactivity of alkyl halides is increasingly exploited. A novel class of derivatization reagents such as 4-(dimethylamino)pyridine (4-DMAP) and butyl 1-(pyridine-4yl) piperidine 4-carboxylate (BPPC) have been recently developed by applying amine alkylation reactions [9,10]. The use of these reagents has proven effective in screening methods for alkyl halides and other alkylation compounds, featuring enhanced selectivity via LC separation and MS fragmentation. Similarly, piperidine has been used to derivatize 2-chloroacetyl chloride and related compounds [11], clearly emphasizing the reactivity of alkyl halides with amines. However, each of these methods requires a relatively inconvenient derivatization step prior to analysis, and may find limitations with incomplete conversion and sample stability [[9], [10], [11]].
Using common knowledge of periodic trends and leaving group stability, it is known that iodide is a better leaving group than bromide, which is a better leaving group than chloride. When combined with the knowledge learned by Finkelstein that primary alkyl halides exist in equilibrium with other halides present in polar solutions [12], it is immediately understood that chloride can favorably replace bromide or iodide. This paper empirically demonstrates that the chloro-product can be favored at effectively 100 % under a wide range of conditions.
Thus, the presence of free or labile chloride in sample matrices can be detrimental to direct determination of alkyl iodides and bromides, especially when samples are exposed to heat. It is shown herein that the presence of chloride in solution with primary and secondary alkyl iodides and primary alkyl bromides, even at room temperature, can potentially lead to substitution of the iodide or bromide. Exploitation of this fact, particularly for volatile alkyl bromides and iodides, discovers that the purposeful conversion to the chloro-product simultaneously affords increased volatility and sample stability when analyzed using standard headspace GC methodology. Because many APIs are hydrochloride salts [13] the applicability of this technique is broad, and the relative instability of alkyl bromides and iodides to free chloride makes the necessity of this technique increasingly likely.
This article presents a novel methodology focusing on the determination of residual iodomethane as chloromethane via conversion using hydrogen chloride (HCl). Stoichiometries, reaction times and temperatures, various chloride sources, and chloromethane response differences from common headspace diluents are presented as necessary studies toward development of method robustness. The applicability of the methodology for other primary and secondary alkyl iodides and bromides is studied. Recovery is demonstrated from a variety of challenging substrates (see Fig. 1) offering competition from other halide ions, amine alkylation side reactions, and interferences from the degradation of quaternary amines. Finally, a validation dataset is presented demonstrating the efficacy of the method in a practical setting by determining iodomethane in (S)-N-methyl-laudanosine iodide which was prepared via Menschutkin [14] alkylation of laudanosine with iodomethane. In accordance with ICH M7(R2) guidelines [15], iodomethane is a class 5 mutagenic impurity possessing a permitted daily exposure (PDE) limit of 375 μg/day [16]. Based on a default daily dosage of 1 g, this corresponds to a 375 ppm limit in the drug substance. The ranges of all experiments described herein encompass this limit.
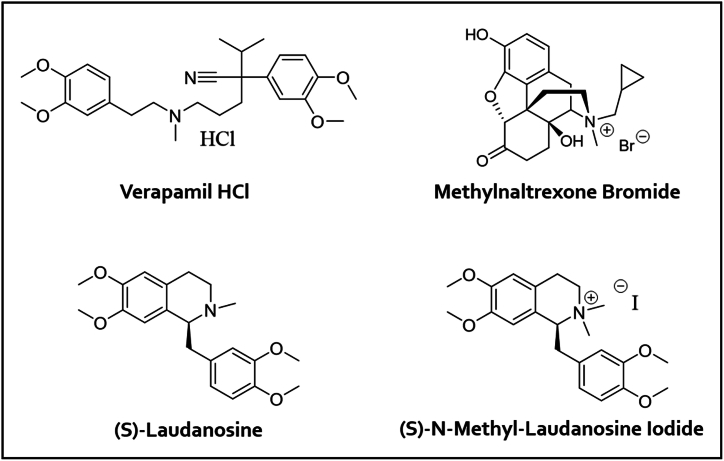
Fig. 1.
Structures of the substrates used during recovery experiments.
To the knowledge of the author, this article is the first example discussing the application of halide-halide substitutions in the context of residual alkyl halide determinations.
2. Materials and methods
2.1. Materials
Iodomethane (ReagentPlus, 99 % and 99.5 %, contains copper as stabilizer), 1-iodopropane (99 %), 1-bromopropane (99 %), 2-iodopropane (contains copper as stabilizer, 99 %), 2-bromopropane (99 %), 1-chloropropane (98 %), 2-chloropropane (≥99 %), 4.0M hydrogen chloride in dioxane, sodium chloride (ACS grade), tetrabutylammonium chloride (≥97.0 % NT), ammonium chloride (for molecular biology, ≥99.5 %), N,N-dimethylformamide (DMF, suitable for HPLC, ≥99.9 %), 1-methyl-2-pyrrolidinone (NMP, suitable for HPLC, ≥99.9 %), 1,3-dimethyl-2-imidazolidinone (DMI, ≥99.0 % GC), Cyrene® (dihydrolevoglucosenone, BioRenewable), and verapamil hydrochloride (≥99 % titration, powder, lot: MKBV4993V) were sourced from MilliporeSigma. N,N-dimethylacetamide (DMAc, headspace GC) was sourced from both Supelco and Fisher Scientific. Hydrochloric acid (ACS reagent, 36.5–38 %) was sourced from JT Baker. Methylnaltrexone bromide (lot: C0038822, purity 99.8 %), (S)-laudanosine (lot: 927-88, purity 99 %), and (S)-N-methyl-laudanosine iodide (lot: 4266-34, purity 98.8 %, contains 0.85 % laudanosine by HPLC area %) were synthesized by Curia Wisconsin, Inc. Water was purified in-house using an Integral 5 Milli-Q system.
2.2. Instrumentation
The GC systems were Agilent 7890A and 6890 GC systems, each paired with PerkinElmer Turbomatrix Headspace 40 headspace autosamplers. Helium was the carrier gas. Flame ionization detectors were used. The chromatography data system used was Chromeleon 7.2 SR5. The column used was an Agilent DB-1, 60m × 0.32 mm I.D., 3.0 μm film thickness (P/N: 123–1064).
2.3. Nominal instrument parameters
The nominal GC and headspace conditions used for all data presented are given in Table 1. Experiments are presented where the headspace oven temperature, length of time in the oven, and GC cycle time are varied. Similarly, the run time of the GC method was extended at the high temperature portion of the oven program as needed to ensure all high-boiling components were eluted. In these instances, the modified conditions are indicated where appropriate.
Table 1.
Nominal instrument parameters.
| GC Parameter | Setting |
|---|---|
| GC System | Agilent 7890A GC/Agilent 6890 GC (Section 3.7, analyst 2 only) |
| Column | Agilent DB-1, 60m × 0.32 mm, 3.0 μm film thickness, P/N: 123-1064 |
| Carrier Flow | 18 psi (constant pressure) |
| Carrier Gas | Helium |
| Inlet Temperature | 180 °C |
| Split Ratio | 5:1 |
| Oven Program | 5 °C hold for 6 min, 25 °C/min to 250 °C, hold 5.6 min (variable) |
| Detector | FID |
| Detector Temperature | 300 °C |
| Detector Gasses | Hydrogen: 40 mL/min. |
| Air: 400 mL/min. | |
| Make up (He): 30 mL/min (Constant) | |
| Analysis Time | 20 min (variable) |
| Data Collection Rate | 10 Hz |
| HS Parameter | Setting |
| Headspace Autosampler | Perkin Elmer Turbomatrix 40 |
| Headspace Oven Temperature | 110 °C (variable) |
| Needle Temperature | 130 °C |
| Transfer Line Temperature | 140 °C |
| GC Cycle Time | 30 min |
| Vial Equilibration Time | 10 min (variable) |
| Pressurization Time | 2.0 min |
| Inject Time | 0.05 min |
| Withdraw Time | 0.2 min |
| Shaker | On |
| Vial Vent | On |
| Track Oven | Off |
| Carrier Pressure | 24 psi |
2.4. Headspace vial and diluent preparations
Unless otherwise stated, all headspace vial preparations were prepared by transferring 1.0 mL of appropriate solution into a 20-mL headspace vial. To prepare unspiked and spiked samples material was weighed into a 20-mL headspace vial and dissolved with 1.0 mL of the appropriate solution. Unless otherwise specified, HCl diluent solutions were prepared from 4.0M HCl in dioxane.
2.5. Concentration of hydrogen chloride, derivatization times and temperatures
Three HCl containing diluents were prepared in DMAc at three different concentrations: 0.1 mM, 0.5 mM, and 1 mM. Using these diluents, three separate solutions of iodomethane were prepared, each at approximately 50 μg/mL. The HCl solutions afforded 0.3, 1.4, and 2.8 molar equivalents of HCl to iodomethane, respectively. The solutions were analyzed after 20 min in the headspace oven. Additionally, times and temperatures in the headspace oven ranging 1–20 min and 35–110 °C using 1 mM HCl in DMAc were studied.
2.6. Completeness of conversion
A certified analytical standard of chloromethane (b.p. −23.7 °C [17]) in methanol (200 μg/mL) was commercially sourced from Supelco in 1-mL ampules. A 1:20 dilution of this standard solution was made into 1 mM HCl in DMAc. An iodomethane test solution was prepared in the same diluent at a molarity closely matching the diluted chloromethane standard. Methanol was included in the iodomethane preparation to match matrices between standard and test solutions. Solutions were analyzed under headspace conditions spanning 50–110 °C for 5 min.
The conversion experiment was repeated using 25 μg/mL 1-chloropropane and 50 μg/mL 1-iodopropane, this time excluding methanol from the sample matrix.
2.7. Alternative chloride sources
A solution of iodomethane was prepared at 50 μg/mL in 50/50 (Water/DMAc) containing 5 mM NaCl. Vial preparations were exposed to a 100 °C headspace oven over 10–200 min. Additionally, 50 μg/mL solutions of iodomethane were analyzed using the following diluents: 10 mM tetrabutylammonium chloride in DMAc, 10 mM tetrabutylammonium chloride in 50/50 (DMAc/water), 10 mM ammonium chloride in 50/50 (DMAc/water), 8 mM HCl in DMAc prepared from aqueous hydrochloric acid, and 1 mM HCl in 50/50 (DMAc/water) diluent prepared from aqueous hydrochloric acid.
2.8. Chloromethane response from various diluents
The response of the chloromethane peak was assessed from a variety of chloride containing diluents. The various high-boiling solvents used to prepare the diluents were DMAc, DMF, NMP, DMI, and Cyrene®. All experiments used an HCl concentration of 1 mM except one which used 10 mM tetrabutylammonium chloride in DMAc. For each experiment, system suitability was established by monitoring replicate 25 μg/mL iodomethane solutions for injection precision. Within each run six replicate injections were made of a 0.5 μg/mL quantitation limit (QL) solution, and linearity was studied from 0.5 to 50 μg/mL. An additional 6 min at the upper 250 °C GC oven temperature was given to the NMP, DMI, and Cyrene® experiments to ensure elution of the solvent peak. Typical calculations for linearity data and injection precision were made. The same instrument, column, and jet capillary were used throughout this study.
2.9. Application to other alkyl halides
Using both 1 mM and 10 mM HCl in DMAc, 50 μg/mL preparations of 2-iodopropane, 1-bromopropane, and 2-bromopropane were exposed to a 110 °C headspace oven for 20 and/or 40 min. Retention time markers were injected that contained no HCl for each of the above alkyl halides as well as 1-chloropropane and 2-chloropropane.
2.10. Recovery of iodomethane from various substrates
Iodomethane recovery studies were performed on three substrates: verapamil hydrochloride, methylnaltrexone bromide (MNTX-Br), and (S)-laudanosine (see Fig. 1). Throughout all experimentation, nominal substrate concentrations ranged 10–100 mg/mL, the concentration of HCl in DMAc diluent ranged 1–140 mM, and times and temperatures in the headspace oven were varied as necessary. For verapamil hydrochloride, recovery was studied across 2.5–1000 ppm, and an additional experiment studied the extent of iodomethane conversion in the absence of additional HCl in the diluent. For MNTX-Br and (S)-laudanosine, recovery was studied across 10–1000 ppm. For (S)-laudanosine, another critical set of experiments studied recovery when the order of addition of HCl and iodomethane was varied. In each experiment system suitability was established by monitoring injection precision from replicate injections of an iodomethane standard prepared at a concentration near the middle of the studied range.
2.11. Stability of the N-methyl-laudanosine quaternary amine
(S)-N-methyl-laudanosine iodide (see Fig. 1) was synthesized via direct alkylation of (S)-laudanosine with iodomethane. The details of its preparation and isolation can be found in the supporting information. Ranging experiments were performed to assess the temperature at which the quaternary amine would degrade and verify the robustness of the derivatization conditions. Sample preparations ranged 10–50 mg/mL, HCl concentrations ranged 5–10 mM, and headspace oven temperatures ranged 40–100 °C.
3. Results and discussion
3.1. HCl concentration, derivatization times, and temperatures
No iodomethane or any other evidence of incomplete conversion was observed at any length of time above 1 min in the headspace oven or at any headspace oven temperature greater than 40 °C. Complete conversion was visually observed with use of the 0.5 mM and 1 mM HCl diluents. Chloromethane peak area increased as the temperature increased. No difference in chloromethane peak area was observed between one and 20 min in a 40° headspace or between 10 and 20 min using a 110 °C oven. The liberation of iodide is noticed almost immediately as solutions begin turning yellow before the instrumental analysis is initiated.
3.2. Completeness of conversion
The first attempt to confirm the completeness of the conversion after 5 min in a 50 °C oven resulted in 128 % recovery. Repeat analysis after 5 min in a 110 °C oven also resulted in 128 % recovery. The experiment was repeated again using 60 °C and 5 min resulting in 126 % recovery. Because no chloromethane was observed in any of the diluent blank injections, the volatility of chloromethane in the room temperature standard ampules was suspected.
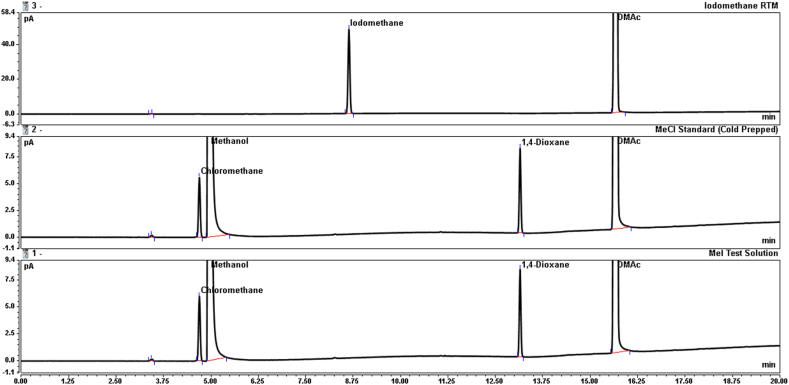
The completeness of the iodomethane conversion was finally demonstrated by cooling the chloromethane standard ampules in a freezer and performing the dilution using chilled glassware. Here, the percent recovery of iodomethane against the chloromethane standard was found to be 105 %. Fig. 2 shows example chromatography of the iodomethane conversion. The completeness of the conversion is also supported by GC-MS experiments that show no iodomethane present in the samples. Once the chloromethane was dissolved in DMAc the volatility/loss to the atmosphere was greatly reduced.
Fig. 2.
Overlays Showing Recovery of Iodomethane, (Top) – Iodomethane RTM (No HCl), (Middle) – Cold prepared chloromethane standard (10.0 μg/mL, 1 mM HCl, 5 % v/v methanol), (Bottom) – Iodomethane test solution (28.9 μg/mL, 1 mM HCl).
The experiment studying 1-iodopropane conversion removed the need for refrigeration and demonstrated a percent recovery of 101 % against the 1-chloropropane standard.
3.3. Alternative chloride sources
With use of sodium chloride, complete conversion to chloromethane was not achieved after 200 min in the headspace oven. Use of 10 mM tetrabutylammonium chloride in DMAc afforded complete conversion to chloromethane, and GC-MS also detected 1-chlorobutane in trace amounts. Use of 8 mM HCl in DMAc prepared from aqueous HCl also showed complete conversion. The 10 mM ammonium chloride in 50/50 (DMAc/water), 1 mM HCl in 50/50 (DMAc/water), and 10 mM tetrabutylammonium chloride in 50/50 (DMAc/water) diluents each resulted in ≤35 % (by area) conversion.
The ineffectiveness of sodium chloride where tetrabutylammonium chloride was successful suggests the extent of salt dissociation and/or solvation of analytes can significantly affect conversion. In all instances where chloride sources failed to achieve complete conversion water was significantly present. However, diluents prepared from aqueous HCl with lower water content (<0.5 % v/v) showed complete conversion. An inverse consideration might view water as a stabilizer of the alkyl iodide, inhibiting reaction in the presence of chloride. This was demonstrated by Wang [18] whose reported diluent was 100 % water.
3.4. Chloromethane response from various diluents
Linearity and QL were successfully established for all studied solvents except Cyrene®. Table 2 summarizes all data from the linearity and QL experiments. Relative parity in response of chloromethane was observed across all solvents except Cyrene®. The greatest response of chloromethane was observed with use of DMF.
Table 2.
Injection precision, linearity, and QL data from various solvent systems.
| Solvent System | Injection Precision (% RSDall of Standard Peak Area) | Correlation of Determination (R) | Slope (pA∗min.∗mL/mg) | Y-intercept (pA∗min.) | Std. Dev. of Residuals | Residual Sum of Squares | % RSD of RFs Across the Range | QL S/N at 0.5 μg/mL (Each Injection) | QL Injection Precision (% RSD6 of Peak Area) |
|---|---|---|---|---|---|---|---|---|---|
| 1 mM HCl in DMAc | 1.1 % | 1.0000 | 17.248 | −0.00047 | 0.00100 | 4.0 × 10−6 | 3.0 % | 17, 19, 19, 20, 19, 16 | 4.4 % |
| 1 mM HCL in DMF | 2.8 % | 1.0000 | 19.873 | −0.00097 | 0.00276 | 3.0 × 10−5 | 3.2 % | 19, 19, 21, 22, 22, 21 | 2.5 % |
| 1 mM HCl in DMI | 0.5 % | 0.9999 | 18.768 | 0.00132 | 0.00437 | 7.6 × 10−5 | 5.5 % | 19, 15, 20, 19, 20, 20 | 2.9 % |
| 1 mM HCl in NMP | 1.6 % | 1.0000 | 18.380 | 0.00010 | 0.00207 | 1.7 × 10−5 | 3.3 % | 15, 17, 12, 17, 14, 15 | 3.6 % |
| 1 mM HCl in Cyrene® | 5.7 % | 0.9988 | 9.611 | 0.01010 | 0.00889 | 3.2 × 10−4 | 22.7 % | 8, 13, 10, 10, 12, 12 |
5.8 % |
| 10 mM Tetrabutylammonium Chloride in DMAc | 0.8 % | 1.0000 | 17.294 | −0.00320 | 0.00241 | 2.3 × 10−5 | 1.0 % | 10, 11, 12, 11, 13, 11 | 5.8 % |
3.5. Application to other alkyl halides
Using 1 mM HCl in DMAc, 2-iodopropane demonstrated a 98 % (by area) conversion to 2-chloropropane after 20 min in a 110 °C oven. Doubling the time in the headspace oven afforded complete conversion. Use of 10 mM HCl in DMAc resulted in complete conversion of 2-iodopropane after 20 min at 110 °C. No degradation or other impurity peaks were detected.
Using 1 mM HCl in DMAc, 1-bromopropane showed 94 % (by area) conversion to 1-chloropropane after 20 min in a 110 °C oven. Doubling time to 40 min found ∼1 % 1-bromopropane remaining. Increasing the HCl concentration to 10 mM in conjunction with 20 min afforded complete conversion.
Using 1 mM HCl in DMAc, 2-bromopropane showed ∼30 % (by area) conversion to 2-chloropropane after 20 min in a 110 °C oven. Using 10 mM HCl in DMAc in conjunction with 40 min in a 110 °C oven produced 83 % (by area) conversion. The relative stability observed for 2-bromopropane suggests that this species could be stable for direct determination.
3.6. Recovery of iodomethane from various substrates
Table 3 provides a summary of the recovery experiments. All recovery experiments for verapamil HCl demonstrated satisfactory recovery, including 105 % recovery at the 2.5 ppm level. Trace levels of chloromethane were discovered in the diluent blank and determined to be from the 4.0M HCl in dioxane reagent used to prepare the diluent. The % recovery of iodomethane from the spiked verapamil sample that contained no HCl demonstrated 102 % recovery.
Table 3.
Recovery of Iodomethane from Various Substrates. Spiked ppm and % recoveries are correlated from left to right. All samples were spiked with diluents containing HCl and iodomethane except the bottom two (S)-laudanosine experiments.
| Substrate | Diluent | Nominal Sample Concentration | Critical Headspace Conditions | Spiked ppm | Recovery |
|---|---|---|---|---|---|
| Verapamil Hydrochloride | 1 mM HCl in DMAc | 50 mg/mL (102 mM) | 110 °C, 10 min | 10, 500, 1007 | 110 %, 101 %, 100 % |
| Verapamil Hydrochloride | 1 mM HCl in DMAc | 100 mg/mL (204 mM) | 120 °C, 10 min | 2.5, 5, 247, 500 | 105 %, 94 %, 98 %, 100 % |
| MNTX-Br | 1 mM HCl in DMAc | 50 mg/mL (115 mM) | 110 °C, 10 min | 10, 513, 1044 | 45 %, 36 %, 33 % |
| MNTX-Br | 1 mM HCl in DMAc | 25 mg/mL (57 mM) | 50 °C, 5 min | 23, 471, 928 | 73 %, 73 %,72 % |
| MNTX-Br | 10 mM HCl in DMAc | 25 mg/mL (57 mM) | 50 °C, 10 min | 26, 540, 1054 | 102 %, 95 %, 95 % |
| (S)-Laudanosine | 1 mM HCl in DMAc | 50 mg/mL (140 mM) | 110 °C, 10 min | 10, 498, 1032 | 84 %, 70 %, 68 % |
| (S)-Laudanosine | 80 mM HCl in DMAc | 25 mg/mL (70 mM) | 50 °C, 10 min | 50, 485, 988 | 75 %, 98 %, 100 % |
| (S)-Laudanosine | 40 mM HCl in DMAc | 10 mg/mL (28 mM) | 90 °C, 10 min | 49, 100, 507, 942 | 92 %, 106 %, 99 %, 102 % |
| (S)-Laudanosine1 | 150 mM HCl in DMAc | 50 mg/mL (140 mM) | 110 °C, 10 min | 9, 441, 902 | 59 %, 35 %, 39 % |
| (S)-Laudanosine2 | 40 mM HCl in DMAc | 10 mg/mL (28 mM) | 90 °C, 10 min | 49, 93, 464, 915 | 103 %, 102 %, 100 %, 101 % |
1 – Substrate spiked by dissolving in DMAc containing iodomethane followed by HCl once dissolved.
2 – Substrate first dissolved in DMAc, then separate and simultaneous addition of HCl and iodomethane.
Recovery of iodomethane at the 0.25 μg/mL (2.5 ppm) level demonstrates that the FID can potentially remain a viable option for class 3 mutagenic alkyl halides whose daily exposure limits are often defaulted to 1.5 μg/day in accordance with ICH M7(R2) guidance [15,19]. The nominal substrate concentration in this experiment was 100 mg/mL. Concentrations at least double this amount are commonplace in headspace GC analysis [20,21].
Recovery of iodomethane from MNTX-Br prepared at 50 mg/mL was initially complicated by competition from bromide ions and dealkylation of the quaternary amine. With use of 1 mM HCl in DMAc in conjunction with 10 min in a 110 °C headspace oven, iodomethane was calculated to be present at 120 ppm in the unspiked sample, and recovery of iodomethane ranged from 33 to 45 % across the 10–1000 ppm range. GC-MS also detected presence of (bromomethyl)cyclopropane and confirmed significant presence of bromomethane with a greater FID peak area than chloromethane. At 25 mg/mL with a 50 °C headspace oven, recovery across the 25–1000 ppm range was found to be 72–73 %. Bromomethane was still observed with ∼15 % relative peak area to the chloromethane peak. However, no chloromethane was observed in the unspiked sample. After increasing the HCl concentration to 10 mM with use of a 50 °C headspace oven, the recovery of iodomethane across a 10–1000 ppm range was found to be 95–102 %. Trace amounts of bromomethane were detected. These experiments demonstrate both that bromide can substitute iodide in the same manner as chloride and that chloride can outcompete bromide for the final conversion.
Satisfactory recovery of iodomethane from (S)-laudanosine was not observed until the nominal sample size was reduced to 10 mg/mL (28 mM) in conjunction with 40 mM HCl in DMAc. After 10 min in a 90 °C headspace oven, 92–106 % recovery was observed across the studied 50–1000 ppm range. Here, protonation of the laudanosine amine was critical to stabilize the sample matrix as previously suggested by Sun et al. [7].
When iodomethane was spiked into the sample matrix prior to addition of HCl, the recovery of iodomethane was found to be 35–59 % across a 10–1000 ppm range. When the substrate was first dissolved in DMAc prior to the separate and simultaneous addition of iodomethane and HCl, the recovery of iodomethane was found to be 101–103 % across a 50–1000 ppm range. This work demonstrates that laudanosine would sequester iodomethane in the absence of HCl, preventing conversion and detection. It was necessary to show that the HCl protonation of the amine was faster than the alkylation reaction, and thus conclude that the HCl derivatization reagent must be present at the time of dissolution. The efficacy of the derivatization, once confirmed with laudanosine, was inferred to N-methyl-laudanosine based on structural similarity.
Except for the inhibitory effects of water, three significant interferences with the derivatization were observed throughout the course of this study: alkylation of free amines, quaternary amine stability, and competition with bromide ions. In each case, issues were resolved by adjustment of temperatures and concentrations, demonstrating the robustness of the derivatization reaction and the wide applicability of this methodology.
3.7. Method optimization and validation for determination of iodomethane in N-methyl-laudanosine iodide
The above experimentation found three potential issues that N-methyl-laudanosine iodide could present: presence of 0.85 % laudanosine precursor, degradation of the quaternary amine, and iodide competition. The recovery experiments with laudanosine demonstrated that an approximate 10 mM excess of HCl to amine was successful in outcompeting the alkylation reaction. The temperature ranging experiments demonstrated that above 80 °C, the carbon atoms bound to the quaternary amine are susceptible to nucleophilic attack, and in this case result in excess chloromethane formation. Finally, iodide could potentially offer competition with chloride. Balancing stability data, solubility, and method sensitivity, a final nominal sample concentration of 25 mg/mL was selected to be used in conjunction with 10 mM HCl in DMAc and a 70 °C headspace oven temperature for method validation experiments. An HCl concentration of 10 mM keeps the iodide to chloride ratio at the at the same level that was successful with MNTX-Br where complete conversion was still favorable when the bromide to chloride ratio was 5.7. The 0.85 % content of laudanosine by HPLC equates to ∼0.44 mM in the sample matrix if present as a freebase.
Optimization of the GC method conditions required only the resolution of chloromethane and methanol. The choice of a 100 % dimethylpolysiloxane stationary phase was arbitrary. The GC oven program's initial 40 °C isothermal hold elutes both methanol and chloromethane with satisfactory resolution. A 60-m column with a 3.0 μm film thickness was selected to maximize this resolution. Once eluted, a 25 °C/min oven ramp was used to rapidly elute DMAc. The pressure of helium in the transfer line between the headspace autosampler and GC was set 8 psi higher than the GC flow/pressure to ensure rapid deposition of chloromethane onto the head of the column to prevent band broadening. Using these conditions, the determination of iodomethane in (S)-N-methyl-laudanosine iodide was successfully validated in accordance with ICH Q2(R2) guidance [22] for specificity, linearity, accuracy, precision, range, quantitation limit, detection limit, and solution stability.
System suitability for each run began by establishing a stable baseline using diluent blank injections. This was followed by six replicate injections of an iodomethane working standard nominally prepared at 12.5 μg/mL (500 ppm). Bracketing injections of the working standard were made throughout and at the end of each run.
Specificity was established by evaluation of diluent blank, working standard, and spiked sample injections for interferences with the elution of chloromethane and resolution to the nearest eluting peaks. Trace amounts of methanol were found in every lot of DMAc used in this study. The smallest observed resolution between chloromethane and methanol from any injection during the study was 2.5.
Linearity was validated at five points across the intended range. Iodomethane solutions were prepared at 0.5, 6.2, 12.4, 18.7, and 24.9 μg/mL, corresponding to 20 (target QL), 249, 498, 747, and 996 ppm of the nominal sample concentration. The linearity data produced a correlation coefficient (R) of 1.0000. The y-intercept was 1.8 × 10−4. The % y-intercept (relative to the 500-ppm level) was 0.1 %. The slope was 12.758 (mAU∗min.∗mL/mg). The standard deviation of the residuals was 3.5 × 10−4. The residual sum of squares was 4.8 × 10−7. The % relative standard deviation (RSD) of the response factors (peak area/concentration) across the range was 2.1 %.
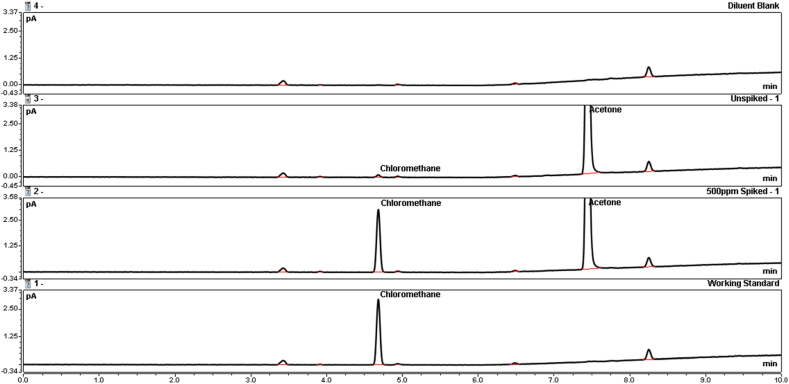
The accuracy and precision of the method were validated by using recovery studies. (S)-N-methyl-laudanosine iodide was spiked with iodomethane at three concentration levels: 20, 500, and 1000 ppm. Unspiked samples were prepared in duplicate. Each spiked sample was prepared in triplicate. Spiked samples were corrected for endogenous iodomethane content found in unspiked samples. The recovery experiments were conducted by two analysts on different days using different columns and instruments. The % recovery results ranged from 91 % to 103 %, and the % RSD18 of all recovery results from both analysts was 3.2 %. The results are given in Table 4. Fig. 3 shows a stacked overlay of example chromatography.
Table 4.
Validation accuracy and precision data.
| Analyst 1 | Analyst 2 | ||||
|---|---|---|---|---|---|
| Replicate | Spiked ppm | Recovery | Replicate | Spiked ppm | Recovery |
| 1 | 20 | 100% | 1 | 20 | 103% |
| 2 | 21 | 94% | 2 | 20 | 96% |
| 3 | 20 | 99% | 3 | 20 | 91% |
| 1 | 487 | 98% | 1 | 511 | 98% |
| 2 | 491 | 97% | 2 | 509 | 97% |
| 3 | 503 | 98% | 3 | 495 | 99% |
| 1 | 1010 | 98% | 1 | 1037 | 103% |
| 2 | 987 | 99% | 2 | 981 | 102% |
| 3 | 979 | 98% | 3 | 1004 | 102% |
| Std. Dev. (9) | 1.6% | Std. Dev. (9) | 4.2% | ||
| % RSD (9) | 1.7% | % RSD (9) | 4.2% | ||
| Std. Dev. (all) | 3.1% | ||||
| % RSD (all) | 3.2% |
Fig. 3.
Overlays showing recovery of iodomethane from (S)-N-methyl-laudanosine, 0–10 min, 70 °C headspace oven, (Top) – Diluent Blank (10 mM HCl in DMAc), (Middle-upper) – Unspiked (S)-N-methyl-laudanosine sample (25 mg/mL), (Middle-lower) – 500-ppm Spiked (S)-N-methyl-laudanosine sample (12.5 μg/mL, 25 mg/mL), (Bottom) – 500-ppm iodomethane working standard (12.5 μg/mL).
The quantitation limit of the method was determined by six replicate injections of a 0.498 μg/mL iodomethane solution. The % RSD of the chloromethane peak area from the six replicate injections was found to be 5.7 %. The signal-to-noise (S/N) ratio of the chloromethane peak in each of the six QL injections ranged 12–15. The results from this study are given in Table 5. The detection limit of the method was empirically established using a 0.100 μg/mL iodomethane solution and produced a S/N ratio of 5.
Table 5.
Validation quantitation limit data.
| Replicate Injection | pA∗min. | Chloromethane S/N |
|---|---|---|
| 1 | 0.00665 | 13 |
| 2 | 0.00608 | 12 |
| 3 | 0.00645 | 14 |
| 4 | 0.00670 | 12 |
| 5 | 0.00696 | 13 |
| 6 | 0.00716 | 15 |
| % RSD (6) | 5.7% | |
Finally, the percent recovery of iodomethane in aged vials of both a 500-ppm working standard and a 500-ppm spiked sample was assessed against a freshly prepared standard and found to be 94 % for both after 7 days at room temperature.
4. Conclusion
The provided analytical technique represents a robust and elegant approach for determination of residual primary and secondary alkyl iodides and primary alkyl bromides. Furthermore, any primary or secondary alkyl iodide and any primary alkyl bromide can theoretically be used via conversion to the corresponding alkyl chloride as a standard to quantify any other alkyl halide that shares the same alkyl group. The derivatization step occurs naturally as part of the headspace oven equilibration time, requiring only inclusion of an appropriate chloride source in the diluent. The alkyl chloride product simultaneously affords increased stability and volatility in the headspace vial, resulting in increased sensitivity. Paired with standard headspace GC-FID methodology, iodomethane was quantitatively recovered at the single ppm level. Interferences from halide competition, amine alkylation, and sample degradation were mitigated by adjustment of temperatures and concentrations. The determination of residual iodomethane in (S)-N-methyl-laudanosine iodide was validated for specificity, linearity, accuracy, precision, range quantitation limit, detection limit, and solution stability.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Brian Zielke reports administrative support was provided by Curia Global Inc. Brian Zielke reports financial support, administrative support, article publishing charges, and equipment, drugs, or supplies were provided by Curia Wisconsin, Inc. D/B/A Siegfried Acceleration Hub. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The author wishes to thank Dr. Lei Liu, Dr. Jonathan Pease, George Mack, Dr. Xiong Fu, and Dr. Jeff McGilvra for their technical consult, Roy Smith and Tyler Blanke for their assistance with GC-MS experiments, George Mack for assistance with validation experiments, Katelyn Melah for reviewing data, and Dr. R. Jason Herr and Dr. Rafail Usatinsky for guidance on writing the technical manuscript. The author would also like to thank Curia Wisconsin, Inc. D/B/A Siegfried Acceleration Hub for making this study possible.
Footnotes
All supporting data for this study can be found in the following Mendeley Data deposit: B.Zielke, Study of Residual Alkyl Halide Determinations via Conversion to Alkyl Chlorides [dataset], Mendeley Data, V3, 2024. https://doi.org/10.17632/6br2sg4mx4.3.
References
- 1.Elder D.P., Lipczynski A.M., Teasdale A. Control and analysis of alkyl and benzyl halides and other related reactive organohalides as potential genotoxic impurities in active pharmaceutical ingredients (APIs) J. Pharm. Biomed. Anal. 2008;48:497–507. doi: 10.1016/j.jpba.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Liu D.Q., Sun M.K., Kord A.S. Recent advances in trace analysis of pharmaceutical genotoxic impurities. J. Pharm. Biomed. Anal. 2010;51:999–1014. doi: 10.1016/j.jpba.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Lee K., Yoo W., Jeong J.H. Analytical method development for 19 alkyl halides as potential genotoxic impurities by analytical quality by design. Molecules. 2022;27:4437. doi: 10.3390/molecules27144437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho T.D., Yehl P.M., Chetwyn N.P., Wang J., Anderson J.L. Determination of trace level genotoxic impurities in small molecule drug substances using conventional headspace gas chromatography with contemporary ionic liquid diluents and electron capture detection. J. Chromatogr. A. 2014;1361:217–228. doi: 10.1016/j.chroma.2014.07.099. [DOI] [PubMed] [Google Scholar]
- 5.Poole C.F. Derivatization reactions for use with the electron-capture detector. J. Chromatogr. A. 2013;1296:15–24. doi: 10.1016/j.chroma.2013.01.108. [DOI] [PubMed] [Google Scholar]
- 6.Abbasi N.M., Anderson J.L., Pellett J.D., Yehl P.M., del Barrio M., Zhong Q. Deep eutectic solvents as green and sustainable diluents in headspace gas chromatography for the determination of trace level genotoxic impurities in pharmaceuticals. J. Pharm. Biomed. Anal. 2024;244 doi: 10.1016/j.jpba.2024.116128. [DOI] [PubMed] [Google Scholar]
- 7.Liu X.W., Zhang W.P., Han H.Y., Sun L., Chen D.Y. Trace determination of mutagenic alkyl toluenesulfonate impurities via derivatization headspace-GC/MS in an active pharmaceutical ingredient of a candidate drug. J. Pharm. Biomed. Anal. 2018;155:104–108. doi: 10.1016/j.jpba.2018.03.054. [DOI] [PubMed] [Google Scholar]
- 8.Sun M., Bai L., Terfloth G.J., Liu D.Q., Kord A.S. Matrix deactivation: a general approach to improve stability of unstable and reactive pharmaceutical genotoxic impurities for trace analysis. J. Pharm. Biomed. Anal. 2010;52:30–36. doi: 10.1016/j.jpba.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 9.van Wijk A.M., Niederländer H.A.G., Siebum A.H.G., Vervaart M.A.T., de Jong G.J. A new derivatization reagent for LC-MS/MS screening of potential genotoxic alkylation compounds. J. Pharm. Biomed. Anal. 2013;74:133–140. doi: 10.1016/j.jpba.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Yang E., Bowen C., Kratz J., Cyronak M.J., Dunbar J.R. Trapping 4-fluorobenzyl chloride in human plasma with chemical derivatization followed by quantitative bioanalysis using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19:759–766. doi: 10.1016/rcm.1849. [DOI] [PubMed] [Google Scholar]
- 11.Morissette M., Wigman L., Tso J. Trace level determination of chloroacetyl chloride and degradation products by derivatization gas chromatography. J. Pharm. Biomed. Anal. 2018;148:93–99. doi: 10.1016/j.jpbz.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein H. Darstellung organischer Jodide aus den entsprechenden Bromiden and Chloriden. Ber. Dtsch. Chem. Ges. 1910;43:1528–1532. doi: 10.1002/cber.19100430257. [DOI] [Google Scholar]
- 13.World Health Organization, WHO Model List of Essential Medicines – 23rd List, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02, 2023 (accessed 10 October 2024).
- 14.N. Menschutkin, Über die Affinitätskoeffizienten der Alkylhaloide und der Amine: Zweiter Teil. Über den Einfluss des chemisch indifferenten flüssigen Mediums auf die Geschwindigkeit der Verbindung des Triäthylamins mit den Alkyljodiden, Z. Phys. Chem. 6U (1890) 41-57. 10.1515/zpch-1890-0607. [DOI]
- 15.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Assessment and control of DNS reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk M7(R2) 2023. https://www.ich.org/page/multidisciplinary-guidelines
- 16.Bercu J.P., Galloway S.M., Parris P., Teasdale A., Masuda-Herrera M., Dobo K., Heard P., Kenyon M., Nicolette J., Vock E., Ku W., Harvey J., White A., Glowienke S., Martin E.A., Custer I., Jolly R.A., Thybaud V. Potential impurities in drug substances: compound-specific toxicology limits for 20 synthetic reagents and by-products, and a class-specific toxicology limit for alkyl bromides. Regul. Toxicol. Pharmacol. 2018;94:172–182. doi: 10.1016/j.yrtph.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Budavari S., O'Neil M.J., Smith A., Heckelman P.E., editors. The Merck Index - an Encyclopedia of Chemicals, Drugs, and Biologicals. eleventh ed. Merck &Co.; New Jersey: 1989. p. 952. [Google Scholar]
- 18.Wang Y. Determination of organic volatile impurities in azasetron hydrochloride. Yaowu Fenxi Zazhi. 2004;24:293–295. [Google Scholar]
- 19.Müller L., Mauthe R.J., Riley C.M., Andino M.M., Antonis D.D., Beels C., DeGeorge J., De Knaep A.G., Ellison D., Fagerland J.A., Frank R., Fritschel B., Galloway S., Harpur E., Humfrey C.D., Jacks A.S., Jagota N., Mackinnon J., Mohan G., Ness D.K., O'Donovan M.R., Smith M.D., Vudathala G., Yotti L. A rationale for determining, testing, and controlling specific impurities in pharmaceuticals that possess potential for genotoxicity. Regul. Toxicol. Pharmacol. 2006;44:198–211. doi: 10.1016/j.yrtph.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Wachełko O., Szpot P., Zawadzki M. A novel simple and precise method for the determination of azide impurity in sartans using headspace gas chromatography with two dissimilar capillary columns and two flame ionization detectors (HS-GC-FID/FID) J. Pharm. Biomed. Anal. 2010;52:30–36. doi: 10.1016/j.jpba.2020.113671. [DOI] [PubMed] [Google Scholar]
- 21.Liu H., Tang Q., Markovich R.J., Rustum A.M. A general static-headspace gas chromatographic method for determination of residual benzene in oral liquid pharmaceutical products. J. Pharm. Biomed. Anal. 2011;54:417–421. doi: 10.1016/j.jpba.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 22.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Validation of analytical procedures Q2(R2) 2023. https://www.ich.org/page/quality-guidelines