Abstract
Luteolin is a kind of natural flavonoid, widely existing in a variety of plants, has been revealed to have a wide range of biological activities. In recent years, the research results of luteolin are abundant. Here we review the latest research results of luteolin in order to provide new ideas for further research and development of luteolin. In this paper, the focus of the search was published between 2010 and 2024 on the extraction and determination of luteolin, biological activities, and the development and application of luteolin products. A comprehensive search using the keyword "luteolin" was conducted in the PubMed, Web of Science and WIPO databases. Through the collection of related literature, this paper summarized a variety of extraction techniques of luteolin, including immersion extraction, solvent extraction, ultrasonic-assisted extraction, supercritical fluid extraction and so on. The determination methods include: thin layer chromatography (TLC), high performance liquid chromatography (HPLC), capillary electrophoresis (CE), electrochemical method (ED) and so on. In addition, the biological activities of luteolin, including antioxidant, anti-inflammatory, anti-tumor, antibacterial, analgesic and so on, were described. And luteolin as the main component of the product is being gradually developed, and has been used in the field of food, medicine and cosmetics. This paper provides a reference for further study of luteolin.
Keywords: Extraction techniques, Bioactivity, Detection methods, Luteolin, Product development
Graphical abstract
1. Introduction
Luteolin is a natural flavonoid compound with the chemical name of 3′, 4′, 5,7-tetrahydroxyflavone, also known as yellow flavin and yellow shiling. It is a yellow needle shaped crystal with weak water solubility and can be dissolved in organic solvents such as ethanol, ether, methanol, and alkaline solutions. The molecular formula is C15H10O6 and the molecular weight is 286.24. It is a secondary metabolite produced by plants through the phenylpropanoid pathway, and its chemical structure is shown in Fig. 1. Luteolin is originally isolated from the leaves, stems and branches of Reseda odorata L., a herbaceous plant of the Resedaceae family. According to research, now luteolin can be isolated from more than 300 plants [1], such as Cichorium intybus L., Raw Chinese celery, Thyme fresh, Cucumber and other crops. In addition, natural medicinal plants such as honeysuckle, Perilla, olive leaf and Origanum vulgare L also contain abundant luteolin [2]. Modern research has shown that luteolin has various biological activities such as antioxidant, antibacterial, anti-inflammatory, anti-tumor, and neuroprotective effects [3].
Fig. 1.
Chemical structure of luteolin.
This review mainly summarizes and understands the extraction process, detection methods, biological activities and product development patents of luteolin, in order to fully tap the research potential of luteolin and further develop high value-added products. The aim is to provide a scientific basis and theoretical basis for promoting the sustainable and healthy development of luteolin production industry.
2. Extraction process of luteolin
The extraction process of luteolin includes impregnation extraction, solvent extraction, ultrasonic assisted extraction, supercritical fluid extraction, etc., as shown in Table 1.
Table 1.
Extraction method of luteolin.
| Abstraction technics | Raw material | Extraction time | Conventional process temperature (°C) | Extraction efficiency(%)/content(mg/g) | Process characteristics | Reference |
|---|---|---|---|---|---|---|
| Impregnation extraction method | Viticis leaves | >72 h | 40 | 9.4 % | Mild, low cost; low extraction efficiency and long extraction time. | [4] |
| Solvent extraction- Soxhlet extraction | Viticis leaves | 2 h | 50 | 14.5 % | Automatic continuous process, less solvent consumption | [4] |
| ultrasonic assisted method | Lobelia chinensis | 30 min | 50 | 0.323 ± 0.014 mg/g | The operation is simple and convenient; the extraction time, temperature and solvent consumption were reduced. | [11] |
| Peanut shell | 15 min | 60 | 1409 mg/g | [12] | ||
| supercritical fluid extraction | – | – | – | 6.56 % | High efficiency, not easy to oxidize, pure natural, no chemical pollution. | [14] |
| microwave-assisted method | Peony pod | >4 h | 66 | 0.151 mg/g | High repeatability, simplified operation, reduced solvent consumption, reduced energy input | [18] |
| Alkali destruction technology (natural deep eutectic solvent) | Peanut shell | 105 min | 80 | 23.33 mg Rutin equivalent/g | It is helpful for flavonoid recovery, high extraction efficiency, and maintaining the antioxidant activity of the extract. | [168] |
| ultrasound-assisted enzymatic extraction method | celery | 30 min | 25∼30 | 9.31 mg/g | The extraction efficiency is high and meets the high performance and economic requirements of the extraction process. | [22] |
2.1. Macerated extraction
Maceration extraction (ME) is an ancient and gentle extraction technique, which achieves physical extraction by immersing solid powders in a solution of soluble compounds containing the active component and adhering the active ingredient to the solid, and is commonly used in pharmaceutical preparations [2]. Abidin et al. [4] used methanol, ethanol and chloroform as solvents to extract luteolin from Vitex negundo leaves by impregnation method, and determined the content of luteolin in the extract by high performance liquid chromatography. The results showed that the extraction efficiency of luteolin was up to 9.4 % when methanol was used as solvent, and the lowest was 5.2 % when chloroform was used as solvent.
2.2. Solvent extraction
Solvent extraction is a method of transferring a substance from one solvent to another by utilizing the property that the solubility or partition coefficient of the substance is different in two immiscible solvents. Solvent extraction is the most commonly used method for extracting flavonoids [5]. Hot water bath extraction and Soxhlet extraction (SE) are the most commonly used methods for extracting bioactive compounds including flavonoids [2]. SE technology combines the advantages of extraction and reflux extraction. The principle of siphon reflux is adopted to realize automatic continuous extraction and reduce solvent consumption [6]. Abidin et al. [4] described that the highest luteolin yield was observed by SE technique when methanol was used as the extraction solvent compared to ethanol, chloroform and dichloromethane.
2.3. Ultrasonic assisted method
Ultrasound-assisted extraction technology is mainly based on the existence of active ingredients in the material state in the role of ultrasound quickly into the solvent to get a multi-component mixture of extracts, and then the extracts with appropriate methods of separation, separation, purification, and ultimately obtain the required monomer chemical composition of a new technology [7], especially in food and pharmaceutical industries. Its main advantage is to reduce the extraction time, processing time and energy consumption, and improve the extraction yield [8,9]. Giacometti et al. [10] optimized the ultrasonic-assisted extraction process of luteolin-4 ' -O-glucoside and several other components in olive leaves by response surface methodology. The results showed that the ultrasonic-assisted method significantly improved the yield of luteolin-4 ' -O-glucoside. Wei et al. [11] established a combined procedure of thermal reflux and ultrasonic-assisted extraction, and an accurate high performance liquid chromatography to determine the content of apigenin, baicalin and luteolin in Scutellaria barbata. It was found that this method reduced the extraction time, extraction temperature and solvent consumption compared with traditional thermal reflux extraction. The study of Imran et al. [12] also proved that the effect of ultrasonic extraction was significantly better than that of conventional extraction. According to the determination of luteolin content by high performance liquid chromatography, the conventional extraction was 1187 mg/g, and the ultrasonic extraction was 1409 mg/g.
2.4. Supercritical fluid extraction
The basic principle of this technology is to increase the temperature and pressure of the target molecule above its critical value, and to extract the soluble components from the solid or liquid with supercritical fluid as solvent [13]. Wang et al. reported that the extraction rate of luteolin by supercritical CO2 extraction was 6.56 %, and the content of luteolin was 0.656 % by HPLC analysis [14]. Devequi et al. [15] studied the chemical properties and biological activities of six different extracts of propolis by conventional methods and supercritical extraction. The results showed that supercritical fluid extraction was fast and solvent-free for obtaining the highest content of antioxidants (including luteolin).
2.5. Microwave-assisted method
Microwave-assisted extraction (MAE) is a new type of green extraction technology that utilizes microwave energy for substance extraction [16], which can provide high repeatability, simplified operation, reduced solvent consumption and reduced energy input in a short time without reducing the extraction rate of the target material [17].
According to the research, when the irradiation power increased to 230 W, the extraction rate of luteolin increased significantly, but with the increase of irradiation power, the extraction rate of luteolin decreased continuously, which may be due to the oxidation or thermal degradation of luteolin caused by excessive irradiation [18]. Studies had also shown that the extraction rate of luteolin was significantly reduced in the order of acetone, methanol, water and ethyl acetate/water. In MAE with ethanol as solvent, the total yield of luteolin was up to 56–60 % [2]. Wang et al. [18] used effective MAE to extract luteolin and apigenin from peony pods at the same time, and used the response surface method (RSM) to optimize the MAE program. The results showed that 151 μg/g luteolin was extracted from peony pods under the optimal conditions. Darvish and Orsat proposed that MAE was the best method to extract three therapeutic flavonoids, isorhamnetin, luteolin and rutin, from the leaves and flowers of Russian olive [19].
2.6. Other approaches
On the basis of natural deep eutectic solvent (NADES) and alkali damage, Deng et al. used a green and efficient natural extraction process to extract flavonoids (including luteolin) from peanut shells [20]. The results showed that NADES had good antioxidant activity to maintain flavonoids, and the maximum extraction efficiency was 23.33 mg rutin equivalent/g. Hang et al. [21] prepared 34 kinds of deep eutectic solvents of choline and betaine. The study showed that the mixture of betaine hydrochloride and propylene glycol with a ratio of 1:8 had the best ability to extract apigenin and luteolin from celery seeds. Zhang et al. [22] applied ultrasound-assisted enzymatic hydrolysis (USAEH) technology to the extraction of luteolin and apigenin for the first time. Under the optimized conditions, the yield of luteolin increased to 42.5 %, which was 26.1 times and 32.2 times higher than that of the control model without enzyme extraction and ultrasonic treatment, respectively.
3. Method for the determination of luteolin
Suitable detection methods are of great significance for the quality control of plants containing luteolin and for products prepared with luteolin as the main ingredient. The commonly used methods for the determination of luteolin include thin layer chromatography (TLC), high performance liquid chromatography (HPLC), capillary electrophoresis (CE), electrochemical method (EC), and other methods. The methods and results for determining luteolin are shown in Table 2.
Table 2.
Determination methods and results of luteolin.
| Method | Object | Condition |
Outcome |
References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mobile phase/buffer | Temperature(°C) | Scan wavelength | Time | Linear range/concentration range | Limit of detection (LOD) | Correlation coefficient | Recovery | Reproducibility (RSD) | |||
| TLC | Artemisia rupestris L. | Chloroform: methanol: formic acid: water = 6.35:0.63:0.17:0.07 | 28–32 | 250、352 nm | – | 0.0172–0.0976 μg | – | 0.9934 | 99.9 % | – | [169] |
| Hygrophila spinosa T.Anders | Toluene: ethyl acetate: formic acid = 6:4:1 | – | 349 nm | – | 40–280 ng/mL | 2.36 ng | 0.998 | 99.48–100.82 % | – | [170] | |
| HPLC | Aster tataricus | 0.1 % formic acid acetonitrile solution and water velocity of flow:0.3 mL/min | 40 | – | – | – | – | >0.9970 | 97.32–102.0 % | – | [171] |
| Propolis | Phosphate-buffered saline(pH = 4.5)and methanol(40/60, v/v) velocity of flow:0.8 mL/min | 25 | 260 nm | 50 min | – | 0.06 mg/mL | >0.9977 | 95.71–104.26 % | 1.58 % (n = 5) | [172] | |
| Vernonia amygdalina Del. | 2 % formic acid acetonitrile solution and water | 25 | – | – | – | 0.5 μg/mL | 0.998 | 2–10 % | [173] | ||
| Honeys | Formic acid solution(pH = 2.8) and acetonitrile velocity of flow:0.2 mL/min | – | – | – | – | 0.5 ng/mL | – | 95.2 % | >5 % | [174] | |
| UPLC | Compositae Species | Water with acetonitrile and 0.1 % formic acid velocity of flow:0.44 mL/min | 45 | 254、280、360 nm | 14 min | – | 0.26 μg/mL | 0.999 | 95.49 ± 0.23 % | 146–2.23 % | [175] |
| RP-HPLC | abnormal savda munziq decoction | 0.3 % formic acid in water and 0.3 % methanol formic acid velocity of flow:1.0 mL/min | 25、30、35 | 290 nm | 97.678 min | 0.2300–1.3800 μg | 0.022 μg /mL |
0.9991 | 99.39–104.85 % | 00.66 % | [171] |
| CE | Red Wine Samples |
40 mM borate pH = 8.9 voltage:26 kV |
18–25 | 215、280、320 nm | 15 min | – | 0.16 μM | >0.999 | 98.1 % | 0.358 % | [176] |
| Flos Chrysanthemi and Flos Chrysanthemi Indici | 20 mmol/LSodium borate-50 mmol/LSodium phosphate pH = 9.6 voltage:15 kV |
30 | 330 nm | 30 min | – | – | – | 95.1–110.7 % | – | [177] | |
| Herbs | 20 mmol/L borax buffer and 10 % methanol pH = 10.0 voltage:23 kV |
23 | 210 nm | 30 s | – | 1.05 μg/mL | – | 97.29–104.88 % | – | [178] | |
| ED | Peanut shells | Graphene quantum dots (GQDs)、Gold nanoparticles (GNPs) | – | – | – | 1 × 10−8-1 × 10−5 M | 1.0 nm | 0.997 | 98.8–101.4 % | 0.94–1.27 % | [179] |
| Peanut shells | Multi-walled carbon nanotubes (MWCNT) | – | – | 20 min | 2.4 × 10−3-1.75 μmol/L | 5.0 × 10−10 mol/L | 0.9964 | – | 7.3 % | [180] | |
| Luteolin | β-Cyclodextrin(β-CD), Indium tin oxide (ITO) pH = 6.0 | – | – | – | 5.0 × 10−8 mol/L-3.0 × 10−5 mol/L | 2.4 × 10−8 mol/L | 0.9981 | 96.0–105.2 % | 5.1 % | [181,182] | |
3.1. Thin layer chromatography (TLC)
TLC utilizes the different adsorption capacities of each component on the same adsorbent to generate a continuous process of adsorption, desorption, re-adsorption, and re-desorption when the component flows through the stationary phase with the mobile phase, thereby achieving the goal of separating each component from each other [23]. As a chromatographic separation method with simple equipment, convenient operation, and low cost, TLC can quantitatively analyze target substances in a short time, and is often used for the content determination of many chemical components [24].
Swaha et al. established and validated an efficient TLC method for the quantitative determination of luteolin, and this method was validated by the International Conference on Harmonization (ICH) guidelines [25]. However, TLC needs to be compared with the control substance, with poor accuracy. With the continuous deepening of research, TLC is more often used for the identification of chemical components and less used for the determination of the content of chemical components in practical applications.
3.2. High performance liquid chromatography (HPLC)
Compared with TLC, which is simple and fast but with poor accuracy, HPLC is more widely used. It is mainly used to analyze the specimen by pumping mobile phases such as single solvents with different polarities or mixed solvents with different ratios into a column equipped with a stationary phase. When the components in the column are separated, they subsequently enter the detector for detection [26].
Lee et al. used gradient HPLC to simultaneously determine the content of luteolin and luteolin in dandelion, and ultimately determined the maximum content of luteolin in dandelion to be 5.817 ± 0.030 mg/g. This gradient HPLC can be used as a reference for quality control of dandelion pharmaceutical preparations [27]. HPLC has the advantages of simple, reliable, sensitive, easy to perform, short time, good resolution, and accurate determination results. It can be used as one of the quality control methods for pharmaceutical preparations.
3.3. Capillary electrophoresis (CE)
CE is a new type of liquid-phase separation technology that uses a capillary as the separation channel and a high-voltage DC electric field as the driving force to realize separation based on the differences in mobility and distribution behaviors among the components in the sample [28]. Wang et al. used CE to separate and detect flavonoids in traditional Chinese medicine, and the results showed that the detection range of flavonoids was 0.28~0.85 μM. The recovery rate is 84.7~113 %, indicating that CE has the advantages of simple operation, low cost, fast analysis speed, and wide application range [29].
3.4. Electrochemical method (EC)
Since lignocaine has a hydroxyl structure and is an electroactive compound, the content of lignocaine can be determined by EC. Compared with the traditional chromatographic analysis, EC can be an instrumental analytical method for qualitative and quantitative analysis of components based on the electrochemical properties of solutes and their patterns of change on the basis of the stoichiometric relationship between the electrical quantities and the quantities of the substances being measured, which has the advantages of high sensitivity, easy operation. It has the advantages of high sensitivity, easy operation, low cost, miniaturization, etc. [30].
With the deepening of research on luteolin, more and more scholars prefer to use electrochemical methods to determine luteolin. Liu et al. combined biomass porous carbon (BPC) and platinum (Pt) nanoparticles to form a synergistic composite material, which was further applied to the surface of carbon ionic liquid electrodes (CILE) to obtain an electrochemical sensing platform for rapid and accurate determination of luteolin [31].
3.5. Other methods
In addition to the commonly used methods mentioned above, many scholars have also explored other rapid and accurate methods for determining the content of luteolin. Shui et al. used multispectral imaging (MSI) to identify chrysanthemum varieties and determine the content of luteolin [32]. They combined MSI with Principal Component Analysis (PCA), Least Squares Support Vector Machine (LS-SVM), and Partial Least Squares (PLS) to classify 23 chrysanthemum varieties and determine the content of luteolin.
4. Bioactivities of luteolin
4.1. Antioxidation
There are many ways to produce free radicals in organisms. Under physiological conditions, there is a balance between the production and scavenging of free radicals. Once the production of free radicals becomes greater than the body's ability to scavenge them, oxidative stress occurs, which is an imbalance between reactive oxygen species and antioxidant defense systems. Oxidative stress can lead to the accumulation of reactive oxygen species (ROS). It is known that excessive ROS can lead to mitochondrial oxidative stress and irreparable damage to DNA, proteins and lipids, eventually leading to diseases including cancer [33,34]. The antioxidant mechanism of luteolin is mainly reflected in the following three aspects: ①scavenging free radicals by natural molecular structure; ②Activating the antioxidant signaling pathway, regulating the expression level of related genes, and enhancing the antioxidant capacity; ③ Regulating the activity of endogenous oxidase system and related proteins. The antioxidant mechanism is shown in Fig. 2.
Fig. 2.
The main antioxidant mechanism of luteolin.
4.1.1. Free radical scavenging by natural molecular structure
The natural antioxidant properties of luteolin depend on its unique molecular structure. Its structural feature is that two benzene rings (A and B) are connected to an oxygen-containing pyran ring (C), which contains four hydroxyl groups. In the hydrogen atom transfer (HAT) mechanism, the phenolic hydroxyl group mainly stabilizes peroxyl, superoxide and hydroxyl radicals by contributing a hydrogen atom, and scavenges free radicals by reducing oxidation mechanism, showing strong antioxidant effect. Researchers measured the ability of luteolin to scavenge free radicals by homolytic cleavage of the O-H bond. It was found that the 4' − OH on the B ring of the four hydroxyl groups had the lowest bond dissociation enthalpy (BDE) value.4' − OH was the strongest antioxidant hydroxyl group in luteolin, which was more involved in the free radical scavenging process of luteolin than other OH groups. Under the same conditions, the 5' − OH group had the worst ability to scavenge free radicals [35].
In addition, the antioxidant activity of luteolin also depends on the 3′, 4' -dihydroxy structure (catechol group) on the phenolic B ring. The double bond at the C2-C3 position is linked to the carbonyl group at the C4 position, allowing unpaired electron delocalization on the B ring, significantly improving stability, thereby enhancing the activity of scavenging free radicals [[36], [37], [38]]. It was reported that the coordination of luteolin with Cu (II) significantly inhibited the formation of hydroxyl and superoxide radicals in the Cu-Fenton system (80 %), and had a dose-dependent protective effect on ROS-induced DNA damage [39]. The scavenging kinetics of semi-stable free radical ABTS was studied by 734 nm absorption spectroscopy, and the scavenging activity of luteolin and its Cu (II) complex was quantitatively determined. The results showed that luteolin had different binding sites with Cu (II) under different pH conditions. The 3 ′, 4 ' -dihydroxy structure could not only change the coordination of Cu (II), but also control the free radical scavenging efficiency. At low pH, Cu (II) further promoted the free radical scavenging activity of active 3′, 4' -catechol by coordinating with weaker antioxidant groups 5-phenol and 4-carbonyl. At neutral pH, Cu (II) coordination occupied the antioxidant active group 3′, 4' -catechol group, but the activity of the 5-phenol group with poor antioxidant activity was increased [40].
4.1.2. Activation of antioxidant signaling pathways and regulation of related gene expression levels
Luteolin directly or indirectly promotes the expression and activation of antioxidant signaling pathways, thereby exerting antioxidant effects. In the study of luteolin on aflatoxin B1 (AFB1) -induced oxidative stress in mouse liver, it was found that luteolin scavenged ROS and malondialdehyde (MDA) accumulation and increased antioxidant enzymes (catalase (CAT), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px) and total antioxidant capacity (T-AOC)). Compared with AFB1 treatment group, ROS and MDA levels in luteolin treatment group were reduced by 38 % and 20 %, respectively. The activities of CAT, T-SOD, GSH-Px and T-AOC antioxidant enzymes were increased by 36.85, 30.27, 27.26 and 40.00 %, respectively. At the same time, the nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant pathway was activated, and the expression of NADH Quinone Oxidoreductase 1 (NQO1), HO-1 and glutamate cysteine ligase catalytic subunit proteins (GCLC) was significantly up-regulated [41]. Nrf2 is considered to be the most important endogenous factor associated with cellular response to oxidative stress. It plays a central role in the activation of cell protection genes by binding to antioxidant response elements (ARE) to cope with oxidative stress. The activation of Nrf2 promotes the expression of downstream proteins heme oxygenase (HO-1), NQO1, SOD, and GCLC, reducing the production of ROS to alleviate oxidative stress [34,42,43]. Other studies have shown that the protective effect of luteolin on the heart of middle-stage diabetic rats is related to the antioxidant signaling pathway eNOS-Keap1-Nrf2. In some animal models of ischemia-reperfusion (I/R) injury, activation of endothelial NO synthase (eNOS) has been shown to reduce cardiac oxidative stress injury. Luteolin pretreatment can trigger Nrf2-dependent antioxidant response by activating eNOS and promoting the s-nitrosylation of Keap1, consistent with the results of the above antioxidant pathway, thereby reducing cardiac I/R injury in diabetic rats [44]. In addition, luteolin also has the effect of restoring Nrf2 pathway and enhancing antioxidant response in polycystic ovary syndrome (PCOS) rats [45]. It is worth noting that low levels of HO-1 expression (less than 5-fold) have protective activity against cells, while high levels of HO-1 expression (more than 15-fold) cause heme degradation to produce hydroxyl radicals, showing significant oxygen cytotoxicity. In the oxidative stress experiment of NRK-52E rat kidney cells induced by ochratoxin A (OTA), compared with the control, luteolin inhibited the expression of HO-1mRNA within 3 h, and significantly promoted the transcription of HO-1 within 24 h, indicating that luteolin may immediately inhibit the free radical reaction at the beginning and promote Nrf2 translocation at the later stage. Although different mechanisms may be involved, luteolin can produce preventive effects in any case [46].
4.1.3. Regulating the activity of endogenous oxidase system and related proteins
Luteolin can regulate the activity of antioxidant enzymes or change the activity level of related proteins to exert antioxidant effects. GSH and SOD themselves are key factors in maintaining cellular redox balance and are also involved in regulating cellular signaling and repair pathways. Researchers used dexamethasone (DXM) to establish an in vivo and in vitro model of glucocorticoid-induced osteoporosis (GIO). It was found that luteolin treatment could increase the SOD activity and glutathione level of GIO, reduce ROS level and lactate dehydrogenase release, inhibit oxidative damage, promote osteoblast proliferation and enhance osteoblast activity to maintain bone mass in GIO [47]. In irinotecan (CPT-11) -induced intestinal mucositis in the duodenum, luteolin showed a similar effect thereby reducing oxidative damage to cell membranes [48]. It has been reported that in the experiment of liver and kidney dysfunction in rats, doxorubicin (DOX) alone caused a significant decrease in liver and kidney antioxidant enzyme activity, GSH and TSH levels, as well as an increase in lipid peroxidation (LPO), reactive oxygen species and nitrogen (RONS) levels and xanthine oxidase (XO) activity. The combination of luteolin and DOX reversed these reactions and alleviated DOX-induced oxidative stress caused by liver and kidney system damage [49]. These facts suggest that luteolin has the ability to regulate antioxidant enzyme activity or change the activity level of related proteins. Chicken ileum inflammation and oxidative stress caused by Avian pathogenic E. coli (APEC) are one of the main causes of animal death and egg production decline, causing economic losses to the poultry industry. Chickens were fed with luteolin for 13 days, and the differences with or without luteolin were compared. It was found that the levels of HO-1, SOD1, SOD2, CAT, NQO1, glutamate cysteine ligase modifier subunit (GCLM) and GPX1 mRNA in 10 mg/kg luteolin group were significantly increased (p < 0.05). But there was no significant difference in the expression levels of CAT, NQO1 and HO-1 mRNA between the 20 mg/kg luteolin group and the E. coli group (p > 0.05). The results showed that luteolin could increase the mRNA expression levels of antioxidant genes (GCLM, GPX1, SOD1 and SOD2) and alleviate APEC-induced oxidative stress to a certain extent [50].
4.1.4. Other
Fipronil (FPN) is used to control pests and increase food production, and it can also cause toxicity to various cells and tissues of non-target organisms through peroxide stress. It was found that luteolin can reduce the elevated levels of oxidative stress biomarkers caused by FPN, and increase mitochondrial antioxidant levels to alleviate ROS mediated oxidative stress and mitochondrial damage, and play a protective role in FPN-induced neurotoxicity [51]. Studies have also shown that under oxidative stress conditions, luteolin acts on NADPH oxidase complexes to reduce ROS production. In endothelial cells, the NADPH oxidase complex in the mitochondrial membrane is an effective mechanism for ROS release. The NADPH oxidase complex consists of a membrane-bound heterodimer, including a catalytic NOX2 (gp91phox) and p22phox subunits, and several cytoplasmic subunits assembled in activating enzymes, such as p47phox, p67phox, p40phox and Rac. In the in vitro model of human HUVECs, luteolin treatment inhibited the oxidative effects of membrane proteins gp91 and p22 phox, and reduced H2O2-induced ROS production. At the same time, luteolin treatment enhanced the expression of p-AMPK protein in a dose-dependent manner, significantly down-regulated the p-PKC subtype to prevent the excessive activity of NAD (P) H oxidase, thereby inhibiting H2O2-induced oxidative stress in HUVECs [52]. In addition, the protective effect of luteolin on oxidative stress in endothelial cells was also achieved by down-regulating ROS-mediated P38MAPK/NF-κB. H2O2-induced phosphorylation of P38MAPK and nuclear translocation of NF-κB were inhibited in luteolin-treated cells, and similar inhibition was observed after pretreatment with ROS inhibitor DPI, suggesting that luteolin has antioxidant effects [53].
4.2. Anti-inflammatory
Inflammation is a complex biochemical reaction between immune cells and non-immune cells when the body is stimulated by external stimuli to maintain homeostasis. Appropriate inflammatory response is beneficial to the body to resist external invasion, but excessive inflammatory response can cause serious diseases, such as rheumatoid arthritis, asthma, and even cancer [54]. In the process of inflammatory reaction, the body secretes a large number of inflammatory factors to react with each other or trigger subsequent reactions. Inflammatory factors are divided into two types: pro-inflammatory factors and anti-inflammatory factors. Common chemokines, eicosane, Interleukin (IL) −1, IL-6, Tumor necrosis factor (TNF), Nitric Oxide (NO), Adrenaline (NA), IL-10, etc. IL-10 is an effective anti-inflammatory factor [55]. These inflammatory factors are closely related to fever, pain, tissue damage and increased vascular permeability caused by inflammatory response [56]. Aziz et al. [57] summarized the reports on the anti-inflammatory activity of luteolin published from 2009 to 2018. It was pointed out that luteolin could inhibit IL-1β, IL-2, IL-6, IL-8, IL-12, IL-17, TNF-α, Interferon (IFN)-β and granulocyte-macrophage colony stimulating factor (GM-CSF) (stimulating the proliferation of granulocytes, monocytes and T cells), and increase the level of anti-inflammatory cytokine IL-10. Luteolin could also reduce the permeability of leukocytes and other inflammatory factors [58].
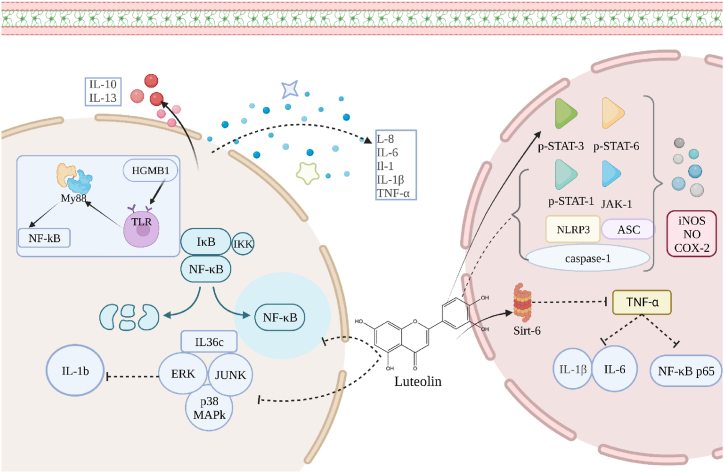
The Anti-inflammatory mechanism of luteolin mainly includes: ① regulating NF-κB pathway; ②regulating MAPK signaling pathway; ③regulating JAK-STAT signaling pathway.The core idea of the luteolin anti-inflammatory pathway is to regulate the inflammatory factors in the body to a balanced level. The main anti-inflammatory mechanism is shown in Fig. 3.
Fig. 3.
The main anti-inflammatory mechanism of luteolin.
4.2.1. Luteolin down-regulates NF-κB pathway
Nuclear factor kappa-B (NF-κB) is a highly conserved family of multifunctional transcription factors. Activated NF-κB induces transcription of inflammation-related genes and produces a large number of inflammatory factors. It is considered to be related to many diseases and has been used for targeted therapy of many inflammatory diseases [59,60]. Studies had confirmed that luteolin could reduce norepinephrine (NE), NA and NF-κB levels, reduce sympathetic nerve activity, reduce the number of helper T cells, and treat hypertension (a chronic inflammation) [61]. Haidy Abbas et al. [62] established an animal model of sporadic Alzheimer 's disease (SAD), and confirmed that luteolin could improve the antioxidant level and reduce the levels of pro-inflammatory factors nitric oxide synthase (NOS), cyclooxygenase-2 (COX-2), and TNF-α. It was also observed that the NF-κB signaling pathway was inhibited by 0.6 times, with significant anti-inflammatory activity. Luteolin could inhibit NF-κB activation by targeting inhibitor of kappa B kinase (IKK) activation in mouse bone marrow [63].
High Mobility Group Box 1 (HMGB1) is a nuclear protein present in all cells. HMGB1 is linked to Toll-like receptors (TLRs) on target cells to induce inflammatory responses and is used as a key protein in the study of targeted therapy for inflammatory diseases [64]. Toll-like receptor 4(TLR4) can recognize and bind extracellular HMGB1, activate the myeloid differentiation primary response protein 88 (MYD88) pathway and NF-κB signaling pathway. Cao et al. [50] found that the mRNA expression levels of HMGB1, MYD88 and NF-κB in the ileum of chickens treated with E. coli were significantly lower than those in the E.coli group. The mRNA expression level of TLR4 was significantly decreased, and the mRNA levels of pro-inflammatory cytokines (IL-1β, IL-6, IL-8 and TNF-α) and anti-inflammatory cytokines (IL-13 and IL-10) were down-regulated, which reduced inflammation and intestinal injury. Luteolin mainly exists in the blood in the form of luteolin glucuronide. Kure, A. et al. [65] found that luteolin glucuronic acid treatment reduced the expression levels of IL-6, IL-1β, NF-κB1 and recombinant Jun B proto-oncogene (JunB) in LPS-treated RAW264.7 cells after luteolin treatment for 24 h. Sirtuin silent information regulator sirtuin (Sirt) was a post-translational regulator that was closely related to inflammation [66]. Luteolin was believed to activate Sirt6 by binding to Sirt6 (a member of the Sirt family) -specific acyl-binding channel, reduce TNF-α-induced pro-inflammatory factors IL-1β and IL-6 levels, and inhibit the phosphorylation of NF-κB phosphorylation [67]. In addition, luteolin could also be combined with curcumin to treat cell inflammation. Curcumin (1 μm) and luteolin (0.5 μm) synergistically inhibited the protein expression of vascular cell adhesion molecule-1 (VCAM-1) by TNF-α, and synergistically reduced monocyte chemoattractant protein-1 (MCP-1) and NF-κB translocation in EA.hy 926 cells [68].
4.2.2. Inhibition of IL-36 activity regulates MAPK pathway
Mitogen-activated protein kinase (MAPK) includes c-Jun N-terminal kinase (JNK), p38MAPK and extracellular regulated protein kinases (ERK). IL-36 is activated by MAPK and NF-κB pathways and receptor IL-36R. Studies had shown that luteolin reduced the expression of IL-36c. Reducing the protein phosphorylation levels of p38MAPK, ERK and JNK proved that luteolin could treat neutrophilic asthma by inhibiting IL-36c (belonging to the IL-1 cytokine family) in the MAPK pathway to secrete pro-inflammatory factor 1L-1b, improving inflammation and reducing the number of neutrophils [69].
4.2.2.1. Down-regulation of JAK-STAT signaling pathway inhibits the secretion of pro-inflammatory factors
The JAK-STAT signaling pathway consists of the non-receptor tyrosine protein kinase JAK family and the 750–900 amino acid STAT family. There was increasing evidence that JAK-STAT signaling pathway disorders were associated with various cancers and autoimmune diseases [70]. Luteolin had anti-inflammatory effects by down-regulating the phosphorylation signal transducer, activating the transcription of p-STAT3 (mainly involved in the negative feedback of immune response) and up-regulating p-STAT6 (mainly involved in the transduction of anti-inflammatory factors IL-4 and IL-13 signals) [71,72]. Luteolin at 14.3 μg/mL and 28.6 μg/mL reduced the total levels of phosphorylated JAK-1 and phosphorylated STAT-1 in cytokine-stimulated HT-29 intestinal cells, thereby significantly inhibiting IL-8 production, as well as iNOS, NO and COX-2 and overexpression [73]. The luteolin rich portion of Perilla seed meal significantly inhibited the expression of NLRP3, ASC, proproteinase-1 (p50), cleavage of caspase-2 (p20) and down-regulation of phosphorylation of JAK1 and STAT3 proteins in a dose-dependent manner. NLRP3 inflammasome was an intracellular complex composed of NLRP3, ASC and proproteinase-1 (p50). The receptor protein of NLRP3 was stimulated to activate caspase-1 and induce its cleavage of caspase-1 (p20). Activated caspase-1 promoted the hydrolysis of pre-IL1β and pre-IL-18 proteins into IL-1β and IL-18 and releases them outside the cell [74].
4.3. Antitumor
Cancer is a major health problem worldwide, referring to a group of diseases caused by abnormal cell growth with invasive potential [75]. Oxidative stress plays an important role in the pathophysiology of different types of cancer. Therefore, antioxidants have received extensive attention as a new therapeutic strategy for cancer [76]. In vitro and in vivo data showed that luteolin can inhibit the growth of malignant tumor cells, such as human liver cancer cells, lung cancer cells, gastric cancer cells, breast cancer cells and colon cancer cells [77,78]. It had been found that the anti-tumor activity of luteolin mainly promoted the induction of apoptosis, cell cycle arrest, inhibition of cell proliferation or migration, or angiogenesis associated with increased invasiveness and tumor development [79]. Its main anti-tumor mechanism was shown in Fig. 4.
Fig. 4.
The main anti-tumor mechanism of luteolin.
4.3.1. Liver cancer
Recent studies had shown that luteolin combined with oncolytic virus (VV) carrying interleukin 24 (IL-24) could synergistically increase the apoptosis of hepatocellular carcinoma cells [80]. Luteolin could increase VV-mediated IL-24 gene expression in hepatocellular carcinoma cells in vitro and in vivo. IL-24 could activate apoptosis by making cancer cells sensitive to chemotherapy [81], thereby improving anti-tumor effects. Cao et al. [82] found that luteolin increased the expression of lactate dehydrogenase (LDH), thereby limiting the growth of liver cancer cells by interfering with the mitochondrial pathway of tumor cells. Targeted modification of luteolin nanoliposomes more effectively enhanced the function of luteolin. Uddin et al. [83] had revealed the mechanism of synergistic anticancer effects of luteolin and tumor necrosis factor related apoptosis inducing ligand (TRAIL) in liver cancer cells. Luteolin promoted the expression of death receptor (DR)5 by inducing the activation of JNK, thereby promoting autophagy flux. Luteolin mediated elevated levels of LC3-I-phospholipid binding compound LC3-II also participate in the upregulation of DR5, and TRAIL triggers the trimerization of DR5 and the aggregation of intracellular adaptive death domains. A death inducing signaling complex is produced, which in turn incites downstream apoptotic executor caspase proteins to promote apoptosis, including in liver cancer cells.
4.3.2. Breast cancer
Breast cancer is one of the three most common gynecological cancers and the leading cause of cancer death in women under the age of 40 [84]. According to 2017 data, there are 255,180 new cases and 41,070 deaths of breast cancer patients in the United States [85]. Among a large number of phytochemicals, polyphenols showed excellent anti-breast cancer activity [86]. MicroRNAs(miRNAs) are small non-coding RNAs involved in the occurrence and development of breast cancer. MiR-203 is a carcinogenic miRNA located on chromosome 14q32.33. However, studies had shown that miR-203 was involved in the proliferation and migration of breast cancer cells as an anti-tumor factor [87]. Gao et al. [88] treated human breast cancer cells (MCF-7 and MDA-MB-453 cells) with luteolin. After treatment with luteolin, the apoptosis of MCF-7 and MDA-MB-453 cells increased significantly. The balance between Bax and Bcl-2 was broken, and Caspase-3 was clearly cut. Epithelial-mesenchymal transition (EMT) was an important process for cancer cells to achieve metastasis by reducing the specific protein expression of epithelial cells and the specific protein expression of mesenchymal cells. It was also one of the most important causes of poor tumor growth [89]. In the process of EMT, the transformation of E-cadherin into N-cadherin was considered to be a landmark event. In addition, increased expression of vimentin and Zeb1 was another feature that enhanced the EMT process [90]. In this study, it was found that luteolin inhibited the progression of EMT because the expression of N-cadherin, vimentin and Zeb1 was decreased, while the expression of E-cadherin was increased. In addition, the Ras/Raf/MEK/ERK signaling pathway plays a leading role in driving the physiological effects of breast cancer [91]. Ras was a proto-oncogene, and Raf was one of the downstream genes of Ras. Ras strongly activated Raf and the subsequent MEK/ERK kinase cascade, which in turn induced the metastasis and invasion of breast cancer cells. The current data found that luteolin could significantly inhibit the Ras/Raf/MEK/ERK signaling pathway, which may be achieved by up-regulating miR-203 [88].
4.3.3. Lung cancer
Fine particulate matter (PM2.5) is a toxic air pollutant that significantly increases the incidence of asthma, chronic obstructive pulmonary disease, cardiovascular disease, and cancer progression by inducing intracellular oxidative stress, mutagenicity/genotoxicity, and inflammatory responses [92]. It had been reported that PM2.5 exposure promoted the expression of intercellular adhesion molecule (ICAM)-1 in lung epithelial A549 cells and rat plasma [93,94]. The expression of EMT markers such as matrix metalloproteinase (MMP)-2 and MMP-9, which degrade the extracellular matrix, was also increased in PM2.5-exposed cells. These studies showed that PM2.5 exacerbated the migration and invasion of cancer cells. Lin et al. [95] found that the treatment of luteolin inhibited the epidermal growth factor receptor (EGFR)-phosphatidylinositol 3-kinase (PI3K)-protein kinase B(AKT)cascade signal transduction induced by PM2.5 exposure. EGFR was highly expressed in non-small cell lung cancer patients, driving the activation of downstream PI3K-AKT signaling and promoting lung cancer angiogenesis, invasion, survival and metastasis. In addition, luteolin reduced the expression of MMP-2 and ICAM-1 in PM2.5-stimulated H460 lung cancer cells.
Activated Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations are often observed in non-small cell lung cancer. More and more evidence showed that the expression of PD-L1(immunosuppressive ligand) in lung cancer was related to the KRAS signaling pathway [96]. Interferon-γ (IFN-γ) was an important cytokine, and activation of IFN-γ could down-regulate the downstream JAK-STAT3 pathway, resulting in STAT3 phosphorylation on tyrosine 705, initiating the PD-1/PD-L1 axis and helping tumor cells escape immune surveillance and immune killing [97]. In T cell-mediated cell killing experiments, luteolin increased the sensitivity of T cells to non-small cell carcinoma H460 and H358 cells. Luteolin showed a good inhibitory effect on IFN-γ-induced up-regulation of PD-L1 mRNA and protein expression in mouse and human KRAS mutant lung cancer [98]. Zhang et al. [99] mentioned that luteolin inhibited the migration and invasion of lung cancer cells by targeting the LIMK/cofilin signaling pathway. LIM domain kinase (LIMK)1 was a member of the serine/threonine kinase family and is highly expressed in various cancers [100]. LIMK1 was activated by upstream kinases such as Rho-associated coiled-coil-containing protein kinase (ROCK) and transmits signals to downstream effectors to regulate actin/filament dynamics through phosphorylation to promote cancer cell growth [99].
4.3.4. Gastric cancer
Zang et al. [101] reported that after luteolin treatment of gastric cancer cells, the cytoskeleton was reduced and the epithelial biomarker E-cadherin increased. In contrast, mesenchymal biomarkers N-cadherin, vimentin and Snail were reduced in a dose-dependent manner, indicating that luteolin reversed the progression of EMT. At the same time, luteolin inhibited Akt, β-catenin and Notch signaling pathways. The Notch signaling pathway was associated with most cancers and promotes malignant changes in tumors by inducing cell proliferation, metastasis, drug resistance and reversing apoptosis [102]. It had been reported that luteolin could also play an anti-gastric cancer role by enhancing the sensitivity of gastric adenocarcinoma cells (SGC-7901) to oxaliplatin (OXA). The combination of the two drugs arrested the cell cycle in G0/G1 phase, significantly increased the expression of cyclin D1, and further enhanced the inhibitory effect on the proliferation of SGC-7901 cells. In addition, this combination also activated the Cyt c/caspase signaling in SGC-7901 cells. By releasing Cyt c, Cyt c formed a complex with Apaf1 and procaspase-9, which induced caspase-9 activation, further activated cleaved-caspases-3, and reduced the Bcl-2/Bax ratio, eventually leading to apoptosis of cancer cells [103]. Wu et al. [104] showed that the inhibition of Bcl-2 expression by luteolin may be based on the miR-34a-mediated pathway.
4.3.5. Colorectal cancer
Epidemiological and experimental studies had shown that colon cancer was strongly influenced by nutritional factors, including the number and composition of dietary fat, and lipid peroxidation (LPO) was a free radical-mediated process. It was involved in the formation of lipid free radicals. It was the rearrangement of unsaturated lipids, leading to various degradation products, such as alkanes, MDA, conjugated diene and lipid hydrogen peroxide, and which ultimately damaged cells. Research reports had shown that luteolin could reduce the number of tumors, inhibit lipid peroxidation and restore the role of antioxidant enzymes in colon cancer rat models induced by 1,2-dimethylhydroxy compounds, which may be due to the strong antioxidant properties of luteolin [105]. Some studies had reported that luteolin could inhibit the migration and invasion of colorectal cancer cells in vitro and in vivo, but does not affect the proliferation of colorectal cancer cells. Pleiotropic protein (PTN) was a small heparin-binding cytokine. It had been found that the expression level of PTN was positively correlated with the occurrence of several cancer cell lines and primary tumors [106]. In colorectal cancer cells, PTN was a direct target of miR-384, and inhibiting the expression of miR-384 reverses the inhibitory effect induced by luteolin, indicating that the anti-colorectal cancer mechanism of luteolin may be mediated by the miR-384/PTN axis. Therefore, miR-384 or PTN could be used as a therapeutic target for colorectal cancer treatment [107].
It had been reported that cAMP response element binding protein 1 (CREB1) was also a promising target for cancer treatment. CREB1 was a nuclear transcription factor and a proto-oncogene that was activated by Ser/Thr kinase phosphorylation at Ser133 [108]. Overexpression of CREB1 had been detected in various cancer patients, including rectal cancer. Liu et al. [109] reported that luteolin inhibited CREB1 expression at the transcriptional level and prevents EMT in colorectal cancer cells. In HCT-116 and LoVo colorectal cancer cells without luteolin treatment, overexpression of CREB1 restored mesenchymal phenotype, migration ability and expression of mesenchymal markers, whereas mRNA levels of CREB1 and its downstream target genes, including Cyclin A1, Cyclin D1, Bcl-2, VEGF and MMP- 2, were significantly decreased. It had also been found that luteolin showed its anticancer effect by increasing p53 phosphorylation and p53 target gene expression, leading to apoptosis and cell cycle arrest in HCT116 human colon cancer cells with wild-type p53 [110].
4.3.6. Bladder cancer
Bladder cancer is the tenth most common cancer in the world, with an estimated 549,000 new cases and 200,000 deaths each year [111]. Smoking is the largest risk factor for bladder cancer death and the largest risk factor for its incidence. Excessive ROS produced by cigarette smoke, as an oxidative stress, can induce genomic instability and promote tumorigenesis [112]. Thioredoxin-1 (TRX1) was a 12-kDa thiol redox-active protein that was thought to protect individuals from oxidative stress-induced damage by scavenging ROS [113]. Mammalian target of rapamycin (mTOR) was a highly conserved serine-threonine kinase that acted as an anticancer agent to inhibit cell growth or proliferation and as an immunosuppressant for a variety of cancers, including bladder cancer cells [114]. Studies had shown that luteolin could up-regulate TRX1 and down-regulate mTOR signaling in human bladder cancer, and the use of TRX1 inhibitors would reverse the inhibitory effect of luteolin, indicating that TRX1 was a tumor suppressor gene in bladder cancer. In the study, it was also found that luteolin up-regulated the expression of cell cycle inhibitory protein p21 protein and induced G2/M phase arrest in human bladder cancer cells. In addition, the concentration of luteolin-3′-O-glucuronic acid, a metabolite of luteolin in plasma and urine of experimental rats, was closely related to the inhibition of cell proliferation and mTOR signaling. With the increase of luteolin-3′-O-glucuronic acid concentration, the squamous differentiation of bladder cancer decreased significantly. These results provided a theoretical basis for the treatment of bladder cancer with luteolin and its derivatives [115].
4.3.7. Pancreatic cancer
Induction of cancer cell apoptosis is an effective method for cancer treatment. It is regulated by members of the B-cell lymphoma 2 (BCL-2) protein family on the mitochondrial outer membrane, which is called the intrinsic pathway regulation of apoptosis [116]. Li et al. [117] found that the apoptosis rate of SW1990 pancreatic cancer cells increased from 13.6 % to 31.48 % and 88.38 % after exposure to 50 μM and 100 μM luteolin for 24 h, and SW1990 cells with higher BCL-2 expression (IC50 = 35.67 ± 0.40 μM) were more sensitive to luteolin than other cells (IC50 = 112.3 ± 19.66 μM). Compared with the control cells, the thermal stability of BCL-2 in luteolin-pretreated cells was better. At the same time, it was revealed that luteolin induced the death of pancreatic cancer cells by stimulating BAX release from BCL-2 to activate the mitochondrial apoptosis pathway. These results indicated that the specific binding of luteolin to BCL-2 in SW1990 cells could be used for the treatment of pancreatic cancer.
4.4. The effect of luteolin on microorganisms
Luteolin has a broad effect on microorganisms, which is mainly divided into three aspects: ① The antibacterial effect of luteolin; ② The antiviral effect of luteolin; ③ Regulation of luteolin on intestinal flora. The main mechanism was shown in Fig. 5.
Fig. 5.
The main mechanism of luteolin on microorganisms.
4.4.1. Antibacterial effect of luteolin
Luteolin not only has a wide antibacterial spectrum and strong antibacterial activity, but also can eliminate bacterial resistance, and has a good effect on the treatment of bacterial infections, especially drug-resistant bacterial infections. The antibacterial mechanism of luteolin mainly includes three parts: ① Affect the formation of biofilm, destroy the integrity of cell wall and biofilm; ② Affect the secretion of toxic factors and reduce the cytotoxicity of pathogenic bacteria; ③ Inhibiting enzyme activity in pathogenic bacteria and affecting related metabolic pathways.
4.4.1.1. Affect the formation of biofilm, destroy the integrity of cell wall and biofilm
The integrity of bacterial biofilm is of great significance to maintain the normal life activities of cells. If the integrity of cell membrane and cell wall is damaged, the growth, development and reproduction of bacteria will be affected, leading to bacterial death [118]. Trueperella pyogenes(T. pyogenes) is often found on the surface and mucosa of the upper respiratory tract and genitourinary tract of healthy animals. It can develop into pathogenic bacteria in a certain state, infect animals and humans, and cause purulent infection of tissue and organ mucosa [119]. Zhang et al. [120] and Guo et al. [121] showed that luteolin could inhibit the formation ability of suppurative T. pyogenes biofilms, destroy cell walls, eliminate mature biofilms, result in intracellular component spill and rough cell edges. In addition, Zhang et al. [120] evaluated the effect of luteolin on biofilm-related genes of T. pyogenes isolated by real-time quantitative PCR detection. The results showed that luteolin could significantly inhibit the relative expressions of biofilm-related genes LuxS, plo, RbsB and LsrB.
Methicillin-resistant S. aureus (MRSA) is a clinically common and highly toxic bacterium. Sun et al. [122] found that MRSA N315 bacteria treated with luteolin (16 μg/mL) had sparse cell walls, light color, blurred cell membrane boundaries, and the synthesis of d-hemolysin, which played an important role in crossing the plasma membrane, was blocked compared with untreated cells. These results indicated that luteolin could regulate the synthesis or depolymerization of MRSA N315 bacterial wall, inhibit the ability of biofilm formation, and promote the morphological changes of MRSA. Escherichia coli, Enterobacter cloacae, Staphylococcus aureus and Listeria monocytogenes are common foodborne pathogens associated with foodborne diseases. Qian et al. [123,124] found that after luteolin treatment of the above foodborne pathogens, the number of damaged cells increased significantly, the integrity of the cell membrane was destroyed, and the cell morphology changed significantly. In addition, luteolin also had a strong inhibitory effect on the formation of biofilms. It could promote the diffusion of antibiotics in biofilms and effectively kill single and double biofilm cells. Pseudomonas aeruginosa is an important opportunistic pathogen that can cause life-threatening infections in immunocompromised cystic fibrosis and burn patients. Extracellular polysaccharides are the main components of extracellular polymeric substances (EPS) in bacterial biofilms and play an important role in biofilm adhesion and provide a protective barrier for bacterial cells [125]. Geng et al. [125] found that luteolin could significantly reduce the production of EPS during the initial biofilm formation of Pseudomonas aeruginosa, making the biofilm to be formed thinner.
4.4.1.2. Affect the secretion of toxic factors and reduce the cytotoxicity of pathogenic bacteria
The pathogenicity of bacteria depends on the pathogenic factors they secrete, which can cause direct damage to host tissues or assist in invading the body and evade the immune response of the body [118]. Group A streptococcus (GAS, Streptococcus pyogenes) is a common pathogen that can cause a variety of human diseases. Streptolysin O (SLO) is an exotoxin produced by GAS, which allows GAS to evade phagocytosis and clearance of neutrophils, induce eukaryotic cell lysis, and activate inflammasomes [126]. Guo et al. [127] showed that luteolin could bind SLO with high affinity, inhibit its dissolution of red blood cells, affect its conformational stability and inhibit the formation of oligomers, thus inhibiting the toxicity of SLO. Pyocyanin was a special virulence factor of Pseudomonas aeruginosa, which has a variety of toxic effects by promoting systemic oxidative stress and inflammatory response [128]. The results of Geng et al. [125] showed that 200 μM luteolin could significantly inhibit the production of pyocyanin. A-hemolysin may cause tissue damage and it is a key component of Staphylococcus aureus products. Hla-A is a virulence-encoding gene of Staphylococcus aureus [122]. Sun et al. [122] found that luteolin inhibited the cytotoxicity of MRSA by reducing the level of Hla-A and blocking the synthesis of bacterial toxins. The α-toxin was a toxic factor encoded by the Hla gene and secreted by most pathogenic Staphylococcus aureus strains. This toxin was a 33.2 kDa cytolytic protein that dissolves red blood cells and some white blood cells [129]. δ-toxin, a member of the phenol-soluble modulins (PSMs) secreted peptide family, was encoded by the Hld gene and had lytic activity on human neutrophils, contributing to synergistic hemolysis and PSM-mediated phenotype and also playing an important role in the pathogenesis of Staphylococcus aureus [130]. Yuan et al. [131] showed that luteolin could reduce the pathogenicity of Staphylococcus aureus by inhibiting the expression of Hla and Hld genes and inhibiting the production of α-toxin and δ-toxin. Shiga toxin-producing Escherichia coli (STEC) is a food-poisoning bacterium that grows in the intestine to produce Shiga toxin (Stx), which is the main virulence factor leading to many symptoms. Yuan et al. [132] showed that luteolin might inhibit the cytotoxicity of Stx1 and Stx2 by inhibiting the incorporation of Stxs into cells.
4.4.1.3. Inhibiting enzyme activity in pathogenic bacteria and affecting related metabolic pathways
DNA topoisomerase is a key enzyme in the regulation of nucleic acid metabolism, which can catalyze the expansion and breakage of DNA strands and complete the process of DNA replication and transcription [133]. Guo et al. [121] found that luteolin could inhibit the activity of the key enzymes topoisomerase I and II in the nucleic acid metabolism of T. pyogenes, resulting in a decrease in nucleic acid content. Under aerobic conditions, pyruvate was completely oxidized to carbon dioxide and water through the tricarboxylic acid cycle, thus becoming the main energy source for bacterial life activities. ATP is the direct energy source of metabolism and plays an important role in the energy metabolism of organisms. Under normal physiological conditions, the intracellular ATP content is always in a dynamic equilibrium state. Succinate dehydrogenase (SDH) is a key enzyme in the tricarboxylic acid cycle and is one of the centers linking oxidative phosphorylation with electron transport. The results of Guo et al. [121] showed that luteolin could inhibit the SDH activity of T. pyogenes and reduce the intracellular ATP content, thus interfering with energy metabolism. TatD DNase was a DNA enzyme that can be synthesized in a variety of organisms. It not only participated in the immune evasion process of pathogens and affected the pathogenicity of pathogens, but also has been proved to be closely related to the biofilm formation of T. pyogenes [134]. Zhang et al. [134] showed that luteolin might inhibit the binding of TatD DNase to DNA, resulting in a decrease or complete loss of TatD DNase activity, thereby reducing T. pyogenes biofilm and virulence.
4.4.2. Antiviral effect of luteolin
After virus infects specific living cells, viral nucleic acids and proteins can be synthesized by using the energy system, tRNA, ribosomes of host cells under the control of viral nucleic acids (genomes), and finally assembled into mature viral particles with complete structure and infectivity. At present, luteolin has been found to have an effective antiviral effect on influenza A virus (IAV), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), respiratory syncytial virus (RSV), dengue virus (DENV), coxsackie virus B3 (CVB3), hepatitis B virus (HBV), etc. Inhibition of viral replication is the main mechanism for its antiviral effect.
IAV is the main pathogen of influenza, and influenza caused by IAV is one of the major public health problems facing mankind [135]. The COPI complex is involved in the content transport between the Golgi apparatus and the endoplasmic reticulum, mediating the entry and endocytosis pathways of influenza viruses [136]. Yan et al. [136] found that luteolin could not only inhibit the replication of IAV virus in the early stage, but also exert antiviral activity by inhibiting the expression of coat protein I (COPI) and affecting the entry and endocytosis of IAV. SARS-CoV-2 had rapidly become a global health pandemic. Among viral proteins, RNA-dependent RNA polymerase (RdRp) was responsible for viral genome replication. Munafò et al. [137] found that luteolin showed stronger anti-RdRp polymerase activity against SARS-CoV-2. RSV was the main cause of acute lower respiratory tract infection in infants, children, immunocompromised adults and the elderly. MiR-155 and its target gene cytokine signaling pathway gene 1 (SOCS1) were key regulators of effector CD8 (+) T cells, which affect cytokine signaling pathway through STAT5 [138]. Wang et al. [138] showed that luteolin could inhibit the replication of RSV in vitro and in vivo by inducing miR-155 targeting SOCS1, thereby enhancing the activation of STAT1 phosphorylation and the expression of IFN-stimulated gene (ISG). Dengue fever is a mosquito-borne viral disease caused by DENV. The host enzyme furin plays a key role in activating a variety of viruses. Peng et al. [139] found that luteolin disrupted the late life cycle of intracellular dengue virus by inhibiting furin in a non-competitive mode, resulting in inefficient cleavage of precursor membrane (prM) proteins and production of less mature viral particles, thereby effectively reducing or preventing subsequent viral infections. CVB3 infection can cause many inflammation-related diseases, such as viral myocarditis and aseptic meningitis [140]. Wu et al. [140] found that luteolin could effectively inhibit the replication of CVB3, and its antiviral mechanism may be through inhibiting the phosphorylation of p38 MAPK and JNK MAPK, inhibiting the nuclear translocation of NF-κB, and then weakening the expression of inflammatory cytokines such as IL-8, IL-6, IL-1β and TNF-α in CVB3 infected cells. HBV can cause transient and chronic liver infections, and chronic hepatitis B (CHB) is a major public health problem worldwide. Cui et al. [141] showed that luteolin-7-O-glucoside might inhibit the expression of hepatitis B virus antigen and viral replication by reducing HBV-induced mitochondrial ROS production and preventing the continuous activation of related signaling pathways.
4.4.3. Regulation of luteolin on intestinal flora
Intestinal microflora is an important part of the intestinal microecosystem, which plays a key role in the physiological functions and processes of nutrition absorption, growth and development, biological barrier, immune regulation, fat metabolism and anti-tumor activities in the host [142]. At present, many studies had found that luteolin had the function of regulating the composition and diversity of intestinal flora and improving nonalcoholic fatty liver disease, ulcerative colitis, diabetes and other diseases.
In the development of simple steatosis (SS) to nonalcoholic steatohepatitis (NASH), enhancing intestinal barrier function is one of the basic methods to inhibit inflammation [143]. Sun et al. [143] found that supplementation of luteolin could enrich more than 10 % of bacterial species, reduce intestinal permeability, plasma endotoxin, and inhibit TLR4/TLR/NF-κB pathway to reduce liver inflammatory factors TNF-α and IL-6, and prevent the progression from SS to NASH. Liu et al. [144] found that luteolin could play a therapeutic role in nonalcoholic fatty liver disease (NAFLD) through the intestinal-hepatic axis. It could not only actively up-regulate the expression of intestinal tight junction proteins ZO-1, occludin and claudin-1 to help protect and maintain the integrity of the intestinal mucosal barrier, but also inhibit the TLR4 signaling pathway in the liver, thereby reducing the secretion of pro-inflammatory factors IL-1β, IL-6 and TNF-α, and effectively restoring the symbiotic ecosystem of the intestinal microflora. Ulcerative colitis (UC) is a chronic inflammatory bowel disease associated with intestinal biological disorders. Li et al. [145] found that luteolin treatment could change the diversity and composition of intestinal microflora in UC rats, reduce the levels of NF-κB, IL-17 and IL-23 in UC rats, increase the level of PPAR-γ, reduce colon injury in UC rats, and inhibit colon inflammation. Ge et al. [146] found that the modified 6, 8-(1,3-diaminoguanidine) luteolin (DAGL) and its Cr complex (DAGL·Cr) could increase the relative abundance of beneficial microorganisms such as Alistipes and Ruminiclostridium in the cecum, improve islet function indicators, regulate serum and liver biochemical indicators, repair damaged tissues, and regulate PI3K/AKT-1 to improve hyperglycemia in T2DM mice. In addition, they also found that luteolin combined with metformin hydrochloride (MH) could regulate SREBP-1c/FAS and SREBP-1c/ACC/Cpt-1 signaling pathways, reduce the ratio of Firmicutes to Bacteroidetes (F/B), and increase the relative abundance of some microbiota to alleviate lipid metabolism disorders in HFD-fed mice [147].
4.5. Other biological activities of luteolin
4.5.1. Neuroprotective effect
Alzheimer's disease (AD)is a progressive neurodegenerative disease characterized by cognitive impairment and behavioral changes caused by synaptic damage and neuronal loss. It is the most common cause of dementia in the elderly (accounting for 50 %-70 %of all dementias). More than 50 million patients worldwide are affected by AD, and this number is expected to double by 2050.The endoplasmic reticulum stress response induced by overactive astrocytes is considered to be involved in the development of AD. Kou et al. [148] studied the protective effect of luteolin on AD. The AD mouse model was administered with 20 and 40 mg/kg luteolin for three weeks. From the molecular level, it was observed that luteolin not only inhibited the excessive activation of astrocytes and the secretion of neuroinflammatory cells, but also reduced the overexpression of endoplasmic reticulum stress markers glucose-regulated protein 78 (GRP78) and inositol enzyme 1α(IRE1α) in brain tissue. In LPS-induced rat C6 glioma cells, similar results were observed after luteolin treatment, which indicated that luteolin had the potential to improve AD and laid a certain experimental foundation for the future development of luteolin as a therapeutic agent for AD.
Ischemic stroke refers to hemiplegia and disturbance of consciousness caused by cerebral infarction and cerebral artery occlusion on the basis of cerebral thrombosis or cerebral thrombosis. The resulting cell stress can also cause mitochondrial disorders, oxidative stress and a series of nerve damage [149]. Dong linked the neuroprotective effect of luteolin to neuroinflammation, endothelial cell injury, blood-brain barrier rupture, apoptosis, oxidative stress, thrombosis, and reduction in infarct volume. PC-12 cells were pretreated with 5,10 and 20 μM luteolin for 24 h, followed by 6 h of OGD treatment. It was not only observed that luteolin maintained the viability of PC-12 cells after oxygen and glucose deprivation (OGD)-induced injury in a concentration-dependent manner, but also down-regulated the expression of inflammatory factors IL-1β and TNF, MAPK signaling pathway, cyclooxygenase- 2(COX-2), MMP-9 and JNK signaling pathways to maintain brain tissue viability after ischemic brain injury [150].
4.5.2. Analgesic effect
Bone cancer pain (BCP)is common in patients with advanced breast cancer, prostate cancer and lung cancer because the skeletal system is the most common site of metastasis in these cancers. The mechanism of BCP is complex, involving the communication between osteocytes, cancer cells and bone nerve fibers and neurons. Lung cancer is the most common cause of cancer-related death worldwide. It is estimated that 34.3 % of cancer cells are transferred to bone. Zhou et al. [151] use Lewis's lung cancer cells to establish a BCP mouse model. The experimental results showed that luteolin effectively alleviated bone pain in mice caused by lung cancer, and inhibited neuroinflammation by regulating phosphorylated p-38 mitogen-activated protein kinase (MAPK) in the spinal dorsal horn (SDH)and blocking the activation of glial cells and NOD-like receptor protein 3 (NLRP3) inflammasome, which played an important role in cancer-induced bone pain.
5. Product development
Based on the excellent antioxidant, anti-inflammatory, anti-tumor functions and the characteristics of microorganisms, the application potential of luteolin in the food field has also been explored. Luteolin can be applied to the preservation of fruits and other agricultural products. The quality deterioration of fresh fruits after harvest is mainly caused by the imbalance of cell redox homeostasis and fungal infection caused by changes in environmental conditions. Liu et al. [152] studied the maintenance and disease resistance of sweet cherry quality by exogenous application of luteolin. The results showed that luteolin could not only improve the antioxidant capacity, but also inhibit the mycelial growth of fungal pathogens. Luteolin treatment significantly reduced the incidence of membrane lipid peroxidation in sweet cherries and significantly increased enzyme activities (SOD, POD, and GR), thereby maintaining the balance of cell redox state. At the same time, luteolin had different degrees of inhibitory effect on the mycelial growth and pathogenicity of the main fungal pathogens (B. cinerea and P. expansum) that caused postharvest decay of sweet cherries. It could better maintain the sensory quality of sweet cherries and reduce the incidence of diseases during storage.
As the main food and feed crop planted worldwide, corn is highly susceptible to Aspergillus flavus infection and aflatoxin contamination. Among them, aflatoxin is extremely toxic and classified as a class of carcinogens by the World Health Organization and is highly regulated by the United States Food and Drug Administration (FDA). The production of flavonoids is related to the resistance of maize to aflatoxin accumulation. Corn has been shown to produce O-methyl flavonoids to deal with fungal infections and exhibit antifungal activity against several fungi. Castano et al. [153] showed that flavonoids such as luteolin regulated fungal proliferation and aflatoxin production in corn in a dose-dependent manner. High concentrations of flavonoids increased fungal proliferation and aflatoxin concentration, while low concentrations of flavonoids inhibited fungal proliferation. In addition, it was observed by scanning electron microscopy that the integrity of the fungal cell wall was destroyed after the treatment of flavonoids, and luteolin and apigenin may localize in the vesicle-like structure, suggesting that flavonoids can be used as potential signal molecules at low concentrations. The vesicle-like structure enters the fungal cells and changes the oxidation state of the microenvironment, which may lead to changes in proliferation, development and aflatoxin production.
In addition, flavonoids, as a chain-breaking antioxidant, supply hydrogen to replace unsaturated fatty acids (UFA) to peroxyl radicals during lipid oxidation, thereby delaying the oxidation rate of peroxy-induced UFA. Tsimogiannis et al. [154] studied the antioxidant structure-activity relationship of flavonoids in the process of cottonseed oil autoxidation. It was found that the rate constants of luteolin and rutin in the initial rapid stage of the reaction in the free radical 2,2-Diphenyl-1-picrylhydrazyl (DPPH) experiment were much higher than the rate constants of taxifolin, iodine diol, etc., which may be related to the presence of 2,3-double bonds leading to the increase of resonance structure, allowing electrons to delocalize from the aromatic oxygen group on the B-ring to the C-ring, thereby increasing the reaction rate with the peroxide group. The antioxidant activity of luteolin has been confirmed and can be used to develop natural antioxidants for oil, fat and fat-containing foods.
Based on the excellent antioxidant, anti-inflammatory and anti-tumor functions of luteolin and its effects on microorganisms, researchers in the fields of food, medicine and cosmetics have developed products with luteolin as the main ingredient.
Among them, in the food sector, Sung has developed a health functional food composition for improving joint health with luteolin as the main active ingredient that can be used to alleviate or treat autoimmune diseases. KIM's invention could be used to prevent or treat liver disease.
In the medical field, Hofleitner et al. developed a composition for the treatment of viral infections using luteolin, quercetin, kaempferol, and vitamin C, as well as therapeutically acceptable vectors. Li et al. developed a drug based on luteolin that could be used to prevent and control dengue virus. Shang et al. found a composition with luteolin as the main active ingredient that could be used to combat myocardial ischemia-reperfusion injury (MIRI).
In the field of cosmetics, with luteolin as the main active ingredient, Il et al. invented an anti-itch composition that was very effective in inhibiting the production of itch-related cytokines without causing toxicity or side effects. Chool et al. developed a skin whitening composition with luteolin -7-sulfate as the main active ingredient, which could inhibit the effect of melanin production by inhibiting tyrosinase activity with little side effects. In addition, in view of the good biological activity of luteolin, its application in various industries is more and more extensive. Table 3 shows the patents for some of the products.
Table 3.
Patent table of luteolin related products.
| Application type | Product name | Essential component | Manufacturing enterprise | Function | Website |
|---|---|---|---|---|---|
| Medicine field | Compositions for the treatment of viral infections | Luteolin, quercetin, kaempferol, vitamin C | Hofleitner, Peter[Us/Us] | Prevention or treatment of viral infection | https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2021257252 |
| Medicine field | Compositions containing antioxidants for the treatment of pain and inflammation | Oleuropein, hydroxytyrosol (3 ′ 4 ' -DHPEA) and tyrosol (p-DHPEA) and flavonoids (rutin, quercetin, luteolin and apigenin). | Atlas Olive Oils Sarl.[Ma/Ma] | In vitro inhibition of PGE2, LTB4, TNF-a, IL-6, IL-1 and high-sensitivity c-reactive protein (hs-CRP). | https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2020095236 |
| Medicine field | Use of luteolin in preparation of medicament for preventing and treating dengue virus infection | Luteolin | Dongguan Mathematical Engineering Academy Of Chinese Medicine, Guanzhou University Of Chinese Medicine[Cn/Cn] | Anti-dengue fever virus, prevention and treatment of dengue fever infection | https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2018107614 |
| Medicine field | Composition containing active components of dracocephalum moldavica L. Against myocardial ischemia-reperfusion injury | Luteolin, kaempferol and luteolin-7-0 glucoside | Nanjing Road Yinglunze Biopharmaceutical Technology Co., Ltd.,Nanjing, Jiangsu (CN) | Reduce hypoxia/reoxygenation-induced rat primary myocardial cell injury and apoptosis rate; reduce cell LDH leakage; improve cell glycolysis, improve cell energy metabolism disorders | https://patentscope2.wipo.int/search/en/detail.jsf?docId=US204140502 |
| drugs & medical technology | Use of luteolin-7-diglucuronide in preparation of drug for preventing cardiac damage or fibrosis | Luteolin-7-diglucuronide | Yueyang Hospital of Integrated Traditional Chinese and Western Medicine Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai Institute of Materia Medica, Chinese Academy of Sciences | Inhibition of myocardial cell necrosis, granulation tissue formation, inflammatory cell invasion, myocardial fibrosis formation | https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2017148338 |
| Nanomedicine and pharmaceutical nanotechnology | Development of nanomaterial for controlled release of luteolin in the treatment of neurodegenerative diseases | Luteolin-p (HEMA-MATrp) nanopolymer | EGE Universltesi[TR/TR] | Increasing oral bioavailability, increasing plasma half-life, reducing dose requirements and intake frequency play a neuroprotective and neuroregulatory role in neurodegeneration. | https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2022139732 |
| The field of nutritional supplements | Nutraceutical composition for pde4 inhibition, enhanced dopamine metabolism and long term potentiation | Luteolin, quercetin, hesperidin, oleanthin A, luteolin, xanthone or resveratrol and cyclic adenosine monophosphate (cAMP) enhancer. | Justin Sher, San Mateo, CA(US) | Increase cognitive function | https://patentscope2.wipo.int/search/en/detail.jsf?docId=US294690857 |
| Drugs, health products | composition for preventing or treating attention deficit hyperactivity disorder comprising luteolin | Luteolin, artichoke extract or part thereof | Fuqing University Industry-University Cooperation Group, Quanbei University Industry-University Cooperation Group | It has antagonist effect on dopamine Da receptor (DR). | https://patentscope2.wipo.int/search/en/detail.jsf?docId=KR335127762 |
| Pharmaceuticals, health functional foods | composition for preventing or treating arthritis and autoimmune diseases comprising luteolin 7-o-(6 prime; -malonylglucoside) derived from leaf of anthriscus sylvestris as active component | Luteolin 7-0- (6 ' -malonylglucoside) | Korea University Industry-University Cooperation Group | Inhibition of NF-KB nuclear translocation; inhibition of MMP-3 or MMP-13 production; for the prevention or treatment of arthritis | https://patentscope2.wipo.int/search/en/detail.jsf?docId=KR248945790 |
| Pharmaceuticals, health food | pharmaceutical composition for preventing or treating liver disease containing luteolin as active component | luteolin | Daegu Korean Medical University Industry-University Cooperation Group | Inhibition of hepatocyte CHOP protein expression; inhibit the activity of endoplasmic reticulum stress in hepatocytes | https://patentscope2.wipo.int/search/en/detail.jsf?docId=KR237670820 |
| Pharmaceutical composition or health care composition | composition for inhibiting skin cell proliferation and/or anti-inflammation method for inhibiting skin cell proliferation and/or anti-inflammation and method for treating skin diseases and/or inflammatory diseases | Apigenin, luteolin | Industrial Technology Research Institute, Hsinchu(TW) | Inhibit skin cell proliferation and/or anti-inflammation | https://patentscope2.wipo.int/search/en/detail.jsf?docId=US313640107 |
| Pharmaceutical products, functional foods | pharmaceutical composition for preventing or treating endometriosis comprising quercetin, luteolin, delphinidin or mixture thereof | Quercetin, luteolin, delphinidin or their mixture | Koryo University Industry-University Cooperation Group | Inhibit the phosphorylation of lower signal transduction substances in PI3K/AKT signaling pathway; inhibition of endometriosis cell proliferation; prevention and treatment of endometriosis | https://patentscope2.wipo.int/search/en/detail.jsf?docId=KR323639814 |
| Pharmaceuticals, health products, functional foods | use of luteolin and derivatives thereof in the prevention and treatment of heart failure | Luteolin or its derivatives | The University Of Tokyo, Jp; Theravalues Corporation, Jp |
Inhibition of cardiac fibrosis, ventricular wall thickening, large heart, heart failure and aneurysm, prevention and treatment of heart disease. | https://patentscope2.wipo.int/search/en/detail.jsf?docId=CA96757129 |
| Health functional food field | composition for preventing or treating diabetes | More than two compounds composed of glyceollin, luteolin, genistein and Daidzein. | Qingbei University Industry-University Cooperation Group | Inhibit α-glucosidase activity, prevent or improve diabetes | https://patentscope2.wipo.int/search/en/detail.jsf?docId=KR332966388 |
| Cosmetics, pharmaceutical field | antipruritic composition containing mixture of luteolin and apigenin as active ingredient | Luteolin, apigenin | Atq & A Technology Co., LTD | Inhibit the production of IL-31 and IL33 in vivo, prevent or improve pruritus. | https://patentscope2.wipo.int/search/en/detail.jsf?docId=KR339300676 |
| Cosmetics, drugs, food | composition for skin whitening containing ruteolin-7-sulfate as active ingredient | Luteolin-7-sulfate | Qingbei University Industry-University Cooperation Group | Inhibition of tyrosinase activity, inhibition of melanin production | https://patentscope2.wipo.int/search/en/detail.jsf?docId=KR203611707 |
| Pharmaceuticals, cosmetics, food | extracellular atp concentration inhibitor | Rosmarinic acid, luteolin glucan | Ichimarupharcos Co., Ltd.[Jp/Jp] | Inhibit the increase of extracellular ATP concentration and improve skin viscoelasticity. | https://patentscope2.wipo.int/search/en/detail.jsf?docId=JP311618549 |
| Medical products, pharmaceuticals, health functional food, cosmetics, animal feed | antibacterial composition for inhibiting antibiotic-resistant staphylococcus aureus containing luteolin | luteolin | Republic of Korea (Director of Rural Revitalization) | Inhibition of drug-resistant Staphylococcus aureus | https://patentscope2.wipo.int/search/en/detail.jsf?docId=KR200974894 |
| Food, cosmetics and pharmaceuticals | Composition containing luteolin and its manufacturing method | Water-dispersible powder compositions of luteolin and quinoa saponin | Petroeuroasia Co., Ltd. Shizuoka 411–0903(JP) |
Increased water solubility of luteolin | https://patentscope2.wipo.int/search/en/detail.jsf?docId=JP300867650 |
| Soil regulating material technology field | A soil conditioner which can reduce the sodium ion content in rhizosphere soil and improve the salt resistance of crops. | Quercetin, luteolin, naringenin | China agricultural university | Stimulate soil microbial activity, improve rhizosphere soil biological fertility, significantly reduce rhizosphere soil sodium ion content, reduce salt damage, and enhance crop salt resistance and increase yield. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN394457854 |
| Medical health technology field | Application of luteolin in the preparation of drugs for the treatment or prevention of psoriasis | luteolin | Nanjing University (Suzhou) High-tech Research Institute | It is proved that luteolin exhibits good anti-inflammatory activity in the treatment of skin inflammation and can be used for anti-inflammatory treatment of psoriasis. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN383860716 |
| food field | An anti-allergic functional food based on egg albumin and luteolin and its application | Luteolin, egg white protein | Zhejiang Liziyuan Food Co., Ltd. | Using luteolin to form anti-allergic functional food | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN391948252 |
| Pharmaceutical preparation technology field | Luteolin green algae hydrogel preparation and its preparation method and application | Luteolin, green algae | Tibet university | It can be applied to dermatology, such as the treatment of diabetic refractory wounds, with high bioavailability; improving the storage stability of luteolin green algae hydrogel preparation | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN391138697 |
| Biological control technology field | A feeding inhibitor of soybean thrips and its screening method and application | Luteolin, coumaric acid, sinapic acid, phloridzin | China agricultural university | It is natural, green and safe, and the control effect on soybean thrips in the field can reach 82.6 %. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN376576852 |
| drugs & medical technology | Application of Luteolin in the Preparation of Atopic Dermatitis Lesions Treatment Drugs | Luteolin and its medicinal derivatives | Wuhan university | By inhibiting the increase of IL-4, IL-13, IL-6, TNF-α, IL-17, p-JAK2 and p-STAT3 levels to prevent, alleviate or treat atopic dermatitis lesions, it is more friendly to patients with spleen deficiency or poor thymus, and avoids the damage of glucocorticoid drugs to the spleen and thymus. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN379907896 |
| Emulsion preparation technology field | A Pickering emulsion stabilized by luteolin and its preparation method and application | Luteolin, deionized water, ethanol, specific edible oil | Dalian university of technology | The operation is simple, the light stability and viscosity of the prepared emulsion are improved, the retention rate of fat-soluble active substances is high, and the stability is good. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN382089055 |
| Emulsion preparation technology field | A fish skin gelatin emulsion stabilized by luteolin and its preparation method and application | Luteolin, ethanol, deionized water, corn oil, | Dalian university of technology | The emulsion prepared by the invention has small average particle size, uniform structure distribution, strong free radical scavenging ability and good stability, and can be used as an effective embedding system for fat-soluble active substances. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN380626173 |
| medicine field | The composition and application of luteolin and its derivatives as effective components to promote hair growth | Luteolin and its derivatives and preparation excipients | Zhejiang Xizhenglin Biotechnology Co., Ltd. | Promote hair growth in the hair removal area, increase growth rate and increase the number of hair follicles. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN376489988 |
| Drugs & medical technology | Application of luteolin combined with cichoric acid in the preparation of breast cancer therapeutic drugs | Luteolin, cichoric acid | China pharmaceutical university | It has a very good effect against triple negative breast cancer, and no side effects. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN376162969 |
| New drug use technology field | Application of a luteolin in the preparation of drugs for the treatment of gastric precancerous diseases | luteolin | Peking university third hospital | It significantly improved the state of gastric mucosal epithelial cells after bile acid intervention and down-regulated the expression of intestinal metaplasia-specific molecules CDX2 and KLF4. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN379067788 |
| Microneedle technology field | A luteolin soluble microneedle and its preparation method | luteolin | Zunyi medical university | Inhibit the expression of NLRP3 inflammasome in rheumatoid arthritis, reduce the expression of caspase-1, RANKL, VEGF and HIF-1α protein in toes, and break through the stratum corneum barrier of skin to effectively treat rheumatoid arthritis. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN377044029 |
| Food processing technology field | Simultaneous reduction of 4-methylimidazole and advanced glycation end products in baked foods based on natural active ingredients | Luteolin, rutin, naringenin | southern yangtze university | Reduce production costs and keep cake quality unaffected | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN371940248 |
| Biomedical technology field | A water celery composition for preventing toothache and its preparation method and application | Apigenin, luteolin, diosmetin, chlorogenic acid, caffeic acid and gallic acid | Chengdu Zhuxiang Biotechnology Co., Ltd. | Inhibition of Candida albicans, prevention and treatment of toothache | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN364708860 |
| medicine field | Application of luteolin in the preparation of drugs inhibiting the functional activity of Streptococcus pyogenes hemolysin | luteolin | Academy of Military Sciences, Chinese People 's Liberation Army | It can bind to SLO protein with high affinity to inhibit the functional activity of Streptococcus pyogenes hemolysin. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN362104413 |
| drugs & medical technology | A drug composition for the treatment of viral myocarditis and its preparation method | Luteolin, L-carnitine | Jiamusi university | Inhibit the inflammatory response of VMC myocardial tissue, reduce the apoptosis of myocardial tissue, and alleviate CVB3 virus-induced myocardial injury. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN360635831 |
| biosphere | An ES large biological fiber containing apigenin, luteolin and daidzein | Apigenin, luteolin, daidzein | Baicaobian Biotechnology (Qingdao) Co., Ltd. | It has excellent antibacterial properties, washing resistance and outstanding mechanical properties. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN364577449 |
| biotechnology field | Application of luteolin in the preparation of health care products or drugs to improve hypoxia tolerance | luteolin | Institute of Environmental Medicine and Occupational Medicine, Academy of Military Medical Sciences | It can significantly increase the activity of GSH-Px and T-SOD in serum, the activity of T-SOD in myocardial tissue and decrease the content of MDA in liver tissue under closed hypoxia. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN365364681 |
| Pharmaceutical cosmetics field | Application of luteolin and its pharmaceutically acceptable salts as dopamine receptor agonists in cosmetics | luteolin | Shanghai Chengmu Biotechnology Co., Ltd. | Activation of dopamine receptors, inhibition of adenylate cyclase activity and promotion of down-regulation of cyclic adenosine monophosphate | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN365362222 |
| biomedicine field | Targets of luteolin-autism related genes and their screening methods | luteolin | Air Force Medical University of Chinese PLA | Through 19 related disease targets such as TP53, AKT1, ERBB2, TNF, etc., we can regulate related inflammatory molecular signaling pathways. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN370573340 |
| drugs & medical technology | The use of luteolin in the preparation of drugs for the prevention and/or treatment of novel coronavirus infection | luteolin | Tianjin university of tcm | Inhibition of novel coronavirus S protein and ACE2 binding | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN351914442 |
| drugs & medical technology | Composition and its application in the preparation of drugs for the treatment of retinitis pigmentosa | Luteolin, Lycium barbarum glycopeptide | Aier Eye Hospital Group Co., Ltd. ; jinan University; ningxia Tianren Wolfberry Biotechnology Co., Ltd. | Significantly improve the survival rate of retinal photoreceptor cells in RP model mice, significantly improve the visual behavior of mice, and protect the retinal structure of RP model mice. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN342346824 |
| drugs & medical technology | New application of luteolin in enhancing the anti-Staphylococcus aureus effect of alkyl gallate compounds | luteolin | Taizhou university | Improve their inhibitory activity against Staphylococcus aureus, play a synergistic role | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN345642210 |
| Tumor drug research | Application of luteolin in the preparation of drugs targeting inhibition of ovarian cancer stem cells | luteolin | Chongqing University Affiliated Tumor Hospital | Inhibition of PPP2CA gene in Hippo/YPY pathway of ovarian cancer stem cells | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN341360642 |
| biomedicine | Application of luteolin in the preparation of drugs to increase the relative abundance of AKK bacteria in the intestine | luteolin | Shenzhen Xianhu Botanical Garden Management Office (Shenzhen Landscape Research Center) | Increase the relative abundance of AKK bacteria in the intestine and decrease the relative abundance of Firmicutes. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN353411664 |
| Disinfectant technology field | A medical antirust disinfectant and its preparation method | luteolin | Winnie Health (Shenzhen) Co., Ltd. | Improve the solubility of luteolin in aqueous solution and inhibit the oxidative corrosion of metals. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN334794875 |
| biomedicine | A drug composition for treating cerebral infarction and its application | Luteolin, kaempferol | Second Hospital of Hebei Medical University | Combined use can be prepared for the treatment of cardiovascular and cerebrovascular diseases, especially ischemic stroke. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN318561439 |
| Pesticide technology field | A class of flavonoid metal complexes inhibiting citrus canker pathogen | Luteolin, metal (tin, chromium, copper, iron, zinc) | Nankai university | It has a significant bacteriostatic effect in vitro, and has the potential to develop as a new pesticide to inhibit plant bacterial diseases. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN320295248 |
| drugs & medical technology | Application of luteolin and its drug combinations | luteolin | Hong kong university of science and technology | Improve the function of nerve growth factor, promote the growth of cell protrusions, activate the promoter of neurofilament coding gene, promote the up-regulation of neurofilament expression, and activate tyrosine receptors and their downstream pathways. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN357955599 |
| Ophthalmic disease treatment technology field | Application of luteolin-7-O-glucoside in the preparation of drugs for the treatment of diseases caused by retinal degeneration | luteolin-7-o-glucoside | Hubei Ming Molybdenum Health Technology Co., Ltd. | Luteolin-7-O-glucoside can effectively treat retinopathy caused by retinal degeneration. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN311262777 |
| Fruit and vegetable preservation technology field | Application of luteolin in delaying postharvest quality deterioration of fruits | luteolin | institute of botany | Significantly improve the sensory quality and antioxidant capacity of fruits, induce defense response and anthocyanin synthesis, thereby delaying senescence; inhibit the mycelial growth of Botrytis cinerea and Penicillium expansum and reduce the pathogenicity of Botrytis cinerea and Penicillium expansum. | https://patentscope.wipo.int/search/en/detail.jsf?docId=CN307162922 |
6. Discussion
Luteolin, a flavonoid compound, is widely found in many plants in nature. It has a variety of biological activities and biological activities, such as antioxidant, anti-inflammatory, anti-tumor and antibacterial, this paper reviews the source and synthesis, extraction and determination methods, biological activity and product development of luteolin. Although luteolin has a variety of biological activities that may play a variety of roles in health, future research on luteolin still faces many challenges. We believe that the following four aspects are the most noteworthy frontiers of luteolin:① The stability and bioavailability of luteolin; ② Issues related to the elimination of drug resistance by luteolin; ③ Toxicology and safety evaluation of luteolin; ④ The development and application of luteolin in food and drug.
The bioavailability, absorption and metabolism of luteolin are key to determining its health benefits. Because of its poor stability, low absorption rate and relatively low bioavailability in vivo, luteolin reduces its efficacy [155]. In order to overcome this problem, nanosystems are currently one of the main ways. Xiao et al. [156] prepared luteolin-loaded HER2 nanospheres (Her-2-NPs) by thin film ultrasonic method. The results showed that Her-2-NPs improved the absorption efficiency of luteolin, thereby improving the therapeutic effect of breast cancer. Shakeel et al. [157] prepared luteolin Self-Nano Emulsifying Drug Delivery Systems (LUT SNEDDS). The results showed that the optimized LUT SNEDDS had significant liver protection and could improve the dissolution rate and therapeutic effect of luteolin. Xiao and Shakeel's researches suggest that nano-delivery systems may be the main way to improve the bioavailability of luteolin and enhance its therapeutic targeting. In the future, the biological activity research and drug development of luteolin will not be limited to monomer luteolin, and the use of nano-delivery systems may promote the research and application of luteolin.
Luteolin has been found to have a strong inhibitory effect on many drug-resistant tumor cells and pathogenic bacteria. Drug-resistant tumor cells often cause recurrence and metastasis in cancer patients. Tamoxifen is one of the most commonly used hormone therapy drugs for the treatment of estrogen receptor-positive breast cancer. Wu et al. [158] found that luteolin could not only lead to cell cycle arrest in G2/M phase, but also inhibit Ras gene to up-regulate the expression of MLL3 and inactivate the PI3K/AKT/mTOR pathway, resulting in tamoxifen-resistant breast cancer cell apoptosis. Guo et al. [159] found that luteolin might increase the sensitivity of T. pyogenes to macrolides by inhibiting the macrolide resistance gene msrA and reducing the ATPase activity. Wu and Guo's study suggested that luteolin was a potential active substance to eliminate drug resistance of tumor cells and pathogenic bacteria and improve their sensitivity to drugs. More researches are needed on that tumor cells and pathogenic bacteria are affected by luteolin, and the related mechanism of action.
Studies had reported that luteolin had cytotoxicity [160], reproductive toxicity [161], prenatal developmental toxicity [162],and mutagenicity [163]. However, the toxicological data of luteolin still remain at the level of cells and rats, and there is a lack of animal toxicological data with high correlation with humans. Therefore, long-term consumption of high-dose flavonoids as dietary supplements, the use of appropriate concentrations and the mechanism of toxic effects should be the focus of research. In addition, Krewski [164] suggested that the toxicological genome was a key platform for toxicological research. At present, the toxicological genome data of luteolin is still lacking, which suggests that we can also conduct toxicological genomics research on luteolin.
At present, there are few products using luteolin as raw material on the market, presumably because of the low bioavailability of luteolin. If this problem can be solved, the application of luteolin will be greatly improved. We refer to the published patents on luteolin products. Luteolin is often used in medicine and health care products, daily chemicals and so on. In pharmaceuticals and health products, luteolin can be added to raw materials to prevent or treat myocardial ischemia-reperfusion or fibrosis, diabetes, pruritus, Staphylococcus aureus caused by a variety of diseases. In addition, Bi et al. found that luteolin could be encapsulated in nanoemulsions of oil-in-water system to develop a new type of active packaging material, which could effectively prevent the oxidation of substances in the packaging and the invasion of bacteria [165]. Luteolin nano emulsion (LUT NEs) prepared by Tu et al. could enhance oral absorption of lignocaine via the lymphatic transport pathway [166]. In cosmetic terms, luteolin can be used for skin whitening, or as a sunscreen product UV protection, and can be used to develop antibacterial products. Xi et al. developed an antibacterial hand sanitizer with luteolin. Because luteolin has a strong inhibitory effect on Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus), it can induce cell dysfunction in E. coli and S. aureus, change the permeability of cell membrane, and lead to the overflow of contents in cells, so it can clean the skin of the hand [167]. These studies provide new ideas for the development of luteolin products, which can increase the utilization rate of luteolin in various fields.
7. Conclusion
In this review, we summarized the extraction process, determination methods, biological activities, and the development and application of luteolin products, which will help us better understand the research status of luteolin and contribute to further targeted research. Luteolin is a very promising flavonoid compound, which has a wide range of biological activities and has great potential for development. However, due to its poor stability, low absorption rate in vivo, insufficient in vivo and in vitro experimental data and lack of clinical experimental data, a large number of molecular mechanism research data are still needed to better elucidate the benefits of luteolin. In addition, more attention needs to be paid to the toxicological properties of luteolin to verify its safety.
CRediT authorship contribution statement
Fajian Ren: Writing – review & editing, Writing – original draft. Ying Li: Writing – original draft. Hanyuan Luo: Writing – review & editing, Writing – original draft. Song Gao: Writing – review & editing, Writing – original draft. Shanshan Jiang: Writing – original draft, Visualization, Supervision. Jian Yang: Writing – original draft. Chaolong Rao: Writing – review & editing. Yan Chen: Writing – original draft, Visualization, Supervision, Resources. Cheng Peng: Supervision, Resources.
Ethics statement
This review article does not involve any experimental studies with human participants or animals conducted by the authors. Therefore, no ethics approval or informed consent was required.
Data availability statement
Data included in article is referenced in the article.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) by Projects (Nos. 81891012 and U19A2010); Sichuan TCM Science and Technology Industry Innovation Team (No.2022C001); Xinglin Scholar Research Promotion Project of Chengdu University of TCM (MPRC2022024); Xinglin Scholar Research Promotion Project of Chengdu University of TCM (202310633006).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We appreciate Figdraw for the help in drawing graphic summaries; We also thank BioRender for the assistance in drawing the diagrams.
Contributor Information
Chaolong Rao, Email: raocl@cdutcm.edu.cn.
Yan Chen, Email: danshuicao@cdutcm.edu.cn.
Cheng Peng, Email: cdtcmpengcheng@126.com.
Abbreviations:
- ME
Maceration extraction
- SE
Soxhlet extraction
- HPLC
High Performance Liquid Chromatography
- MAE
Microwave-assisted extraction
- NADES
natural deep eutectic solvent
- USAEH
ultrasound-assisted enzymatic hydrolysis
- TLC
thin layer chromatography
- CE
capillary electrophoresis
- EC
electrochemical method
- ICH
International Conference on Harmonization
- BPC
biomass porous carbon
- Pt
platinum
- CILE
carbon ionic liquid electrodes
- MSI
multispectral imaging
- PCA
Principal Component Analysis
- LS-SVM
Least Squares Support Vector Machine
- PLS
Partial Least Squares
- ROS
reactive oxygen species
- HAT
hydrogen atom transfer
- BDE
bond dissociation enthalpy
- ABTS, 2
2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- AFB1
aflatoxin B1
- MDA
malondialdehyde
- CAT
catalase
- T-SOD
total superoxide dismutase
- GSH-Px
glutathione peroxidase
- T-AOC
total antioxidant capacity
- Nrf2
Nuclearfactor erythroidderived 2-like 2
- NQO1
NADH Quinone Oxidoreductase 1
- HO-1
Heme Oxygenase-1
- GCLC
glutamate cysteine ligase catalytic
- ARE
antioxidant response elements
- eNOS
endothelial NO synthase
- Keap1
Kelch-1ike ECH- associated protein l
- PCOS
polycystic ovary syndrome
- OTA
ochratoxin A
- DXM
dexamethasone
- GIO
glucocorticoid-induced osteoporosis
- DOX
doxorubicin
- TSH
Thyroid-stimulating hormone
- LPO
lipid peroxidation
- RONS
reactive oxygen species and nitrogen
- XO
xanthine oxidase
- APEC
Avian pathogenic E. coli
- GCLM
glutamate cysteine ligase modifier
- GPX1
Glutathione peroxidase 1
- FPN
Fipronil
- NADPH
Triphosphopyridine nucleotide
- NOX2
gp91phox
- TNF
Tumour necrosis factor
- NO
Nitric Oxide
- NA
Adrenaline
- IFN
Interferon
- GM-CSF
granulocyte-macrophage colony stimulating factor
- NF-κB
Nuclear factor kappa-B
- SAD
sporadic Alzheimer 's disease
- NOS
nitric oxide synthase
- COX-2
cyclooxygenase-2
- IKK
Inhibitor of kappa B kinase
- HMGB1
High Mobility Group Box 1
- TLRs
Toll-like receptors
- TLR4
Toll-like receptor 4
- MYD88
myeloid differentiation primary response protein 88
- IL-1β
Interleukin-1beta
- Sirt
Sirtuinsilent information regulator sirtuin
- VCAM-1
vascular cell adhesion molecule-1
- MCP-1
monocyte chemoattractant protein-1
- MAPK
Mitogen-activated protein kinase
- JNK
c-Jun N-terminal kinase
- ERK
extracellular regulated protein kinases
- STAT
Signal transducer and activator of transcription
- NLRP3
Nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 3
- ASC
Apoptosis-associated speck-like protein containing a CARD
- LDH
lactate dehydrogenase
- TRAIL
Tumor necrosis factor receptor superfamily member
- EMT
Epithelial-mesenchymal transition
- ICAM
intercellular adhesion molecule
- MMP
matrix metalloproteinase
- EGFR
epidermal growth factor receptor
- PI3K
phosphatidylinositol 3-kinase
- KRAS
Activated Kirsten rat sarcoma viral oncogene homolog
- PD-L1
Programmed cell death 1 ligand 1
- LIMK
LIM domain kinase
- ROCK
Rho-associated coiled-coil-containing protein kinase
- OXA
oxaliplatin
- Cyt c
Cytochrome Complex
- Apaf1
apoptotic protease activating factor-1
- LPO
lipid peroxidation
- PTN
Pleiotropic protein
- CREB1
cAMP response element binding protein 1
- TRX1
Thioredoxin-1
- mTOR
Mammalian target of rapamycin
- BCL-2
B-cell lymphoma 2
- MRSA
Methicillin-resistant S.aureus
- EPS
extracellular polymeric substances
- GAS
Group A streptococcus
- SLO
Streptolysin O
- PSMs
phenol-soluble modulins
- STEC
Shiga toxin-producing Escherichia coli
- Stx
Shiga toxin
- SDH
Succinate dehydrogenase
- IAV
influenza A virus
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- RSV
respiratory syncytial virus
- DENV
dengue virus
- CVB3
coxsackie virus B3
- HBV
hepatitis B virus
- COPI
coat protein I
- RdRp
RNA-dependent RNA polymerase
- SOCS1
Suppressor of cytokine signaling
- ISG
IFN-stimulated gene
- prM
precursor membrane
- CHB
chronic hepatitis B
- NASH
nonalcoholic steatohepatitis
- NAFLD
nonalcoholic fatty liver disease
- ZO-1
Zonula Occludens Protein 1
- UC
Ulcerative colitis
- DAGL
Diacylglycerol lipase
- MH
metformin hydrochloride
- F/B
Firmicutes to Bacteroidetes
- HFD
High-Fat Diet
- AD
Alzheimer's disease
- GRP78
glucose-regulated protein 78
- IRE1α
inositol enzyme 1α
- LPS
Lipopolysaccharide
- OGD
oxygen glucose deprivation
- BCP
Bone cancer pain
- SDH
spinal dorsal horn
- NLRP3
NOD-like receptor protein 3
- FDA
United States Food and Drug Administration
- UFA
unsaturated fatty acids
- DPPH
diphenyl picryl hydrazyl
- MIRI
myocardial ischemia-reperfusion injury
- Her-2-NPs
HER2 nanospheres
- LUT SNEDDS
luteolin Self-Nano Emulsifying Drug Delivery Systems
- LUT Nes
Luteolin nano emulsion
- GQDs
Graphene quantum dots
- GNPs
Gold nanoparticles
- MWCNT
Multi-walled carbon nanotubes
- β-CD
β-Cyclodextrin
- ITO
Indium tin oxide
- hs-CRP
high-sensitivity c-reactive protein
- cAMP
cyclic adenosine monophosphate
- RANKL
Receptor Activator for Nuclear Factor-κ B Ligand
References
- 1.Zhu M., Sun Y., Su Y., et al. Luteolin: a promising multifunctional natural flavonoid for human diseases. Phytother Res. 2024;38(7):3417–3443. doi: 10.1002/ptr.8217. [DOI] [PubMed] [Google Scholar]
- 2.Manzoor M.F., Ahmad N., Ahmed Z., et al. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019;43(9) doi: 10.1111/jfbc.12974. [DOI] [PubMed] [Google Scholar]
- 3.Almatroodi S.A., Almatroudi A., Alharbi H.O.A., et al. Effects and mechanisms of luteolin, a plant-based flavonoid, in the prevention of cancers via modulation of inflammation and cell signaling molecules. Molecules. 2024;29(5) doi: 10.3390/molecules29051093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abidin L., Mujeeb M., Mir S.R., et al. Comparative assessment of extraction methods and quantitative estimation of luteolin in the leaves of Vitex negundo Linn. by HPLC. Asian Pac J Trop Med. 2014;7s1:S289–S293. doi: 10.1016/s1995-7645(14)60248-0. [DOI] [PubMed] [Google Scholar]
- 5.Malićanin M., Karabegović I., Đorđević N., et al. Influence of the extraction method on the biological potential of solidago virgaurea L. Essential oil and hydrolates. Plants. 2024;13(16) doi: 10.3390/plants13162187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaves J.O., De Souza M.C., Da Silva L.C., et al. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020;8 doi: 10.3389/fchem.2020.507887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Zhang Q., Fang Q., et al. A novel ultrasound-assisted enzyme extraction method of total flavonoids from Viticis Fructus and processed Viticis Fructus: comparison of in vitro antioxidant activity. Ultrason. Sonochem. 2024;110 doi: 10.1016/j.ultsonch.2024.107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linares G., Rojas M.L. Ultrasound-assisted extraction of natural pigments from food processing by-products: a review. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.891462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali M.C., Chen J., Zhang H., et al. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta. 2019;203:16–22. doi: 10.1016/j.talanta.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Giacometti J., Žauhar G., Žuvić M. Optimization of ultrasonic-assisted extraction of major phenolic compounds from olive leaves (olea europaea L.) using response surface methodology. Foods. 2018;7(9) doi: 10.3390/foods7090149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei M.C., Yang Y.C., Chiu H.F., et al. Development of a hyphenated procedure of heat-reflux and ultrasound-assisted extraction followed by RP-HPLC separation for the determination of three flavonoids content in Scutellaria barbata D. Don. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;940:126–134. doi: 10.1016/j.jchromb.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Imran A., Humiyion M., Arshad M.U., et al. Extraction, amino acid estimation, and characterization of bioactive constituents from peanut shell through eco-innovative techniques for food application. Int. J. Food Prop. 2022;25(1):2055–2065. doi: 10.1080/10942912.2022.2119999. [DOI] [Google Scholar]
- 13.Yıldırım M., Erşatır M., Poyraz S., et al. Green extraction of plant materials using supercritical CO(2): insights into methods, analysis, and bioactivity. Plants. 2024;13(16) doi: 10.3390/plants13162295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L., Li W., Lin M., et al. Luteolin, ellagic acid and punicic acid are natural products that inhibit prostate cancer metastasis. Carcinogenesis. 2014;35(10):2321–2330. doi: 10.1093/carcin/bgu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devequi-Nunes D., Machado B.A.S., Barreto G.A., et al. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0207676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlucci V., Ponticelli M., Russo D., et al. Nutraceutical valorization of exhausted olive pomace from olea europaea L. Using advanced extraction techniques. Plants. 2024;13(16) doi: 10.3390/plants13162310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang G., Hu M., He L., et al. Optimization of microwave-assisted enzymatic extraction of polyphenols from waste peanut shells and evaluation of its antioxidant and antibacterial activities in vitro. Food Bioprod. Process. 2013;91(2):158–168. doi: 10.1016/j.fbp.2012.09.003. [DOI] [Google Scholar]
- 18.Wang H., Yang L., Zu Y., et al. Microwave-assisted simultaneous extraction of luteolin and apigenin from tree peony pod and evaluation of its antioxidant activity. Sci. World J. 2014;2014 doi: 10.1155/2014/506971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darvishzadeh P., Orsat V. Microwave-assisted extraction of antioxidant compounds from Russian olive leaves and flowers: optimization, HPLC characterization and comparison with other methods. Journal of Applied Research on Medicinal and Aromatic Plants. 2022;27 doi: 10.1016/j.jarmap.2021.100368. [DOI] [Google Scholar]
- 20.Deng M., Wang H., Geng S., et al. Application of an alkali destruction technique and natural deep eutectic solvent for greener extraction from peanut shells: optimization and extraction kinetics study. Anal. Methods. 2022;14(16):1594–1602. doi: 10.1039/D1AY02033A. [DOI] [PubMed] [Google Scholar]
- 21.Hang N.T., Thi Tu Uyen T., Van Phuong N. Green extraction of apigenin and luteolin from celery seed using deep eutectic solvent. J. Pharm. Biomed. Anal. 2022;207 doi: 10.1016/j.jpba.2021.114406. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q., Zhou M.M., Chen P.L., et al. Optimization of ultrasonic-assisted enzymatic hydrolysis for the extraction of luteolin and apigenin from celery. J. Food Sci. 2011;76(5):C680–C685. doi: 10.1111/j.1750-3841.2011.02174.x. [DOI] [PubMed] [Google Scholar]
- 23.Custodio-Mendoza J.A., Pokorski P., Aktaş H., et al. Advances in chromatographic analysis of phenolic phytochemicals in foods: bridging gaps and exploring new horizons. Foods. 2024;13(14) doi: 10.3390/foods13142268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhawani S.A., Mohamad Ibrahim M.N., Sulaiman O., et al. THIN-LAYER chromatography of amino acids: a review. J. Liq. Chromatogr. Relat. Technol. 2012;35(11):1497–1516. doi: 10.1080/10826076.2011.619039. [DOI] [Google Scholar]
- 25.Satpathy S., Patra A., Ahirwar B. Development and validation of a novel high-performance thin-layer chromatography method for the simultaneous determination of apigenin and luteolin in hygrophila spinosa T. Anders. JPC – Journal of Planar Chromatography – Modern TLC. 2018;31(6):437–443. doi: 10.1556/1006.2018.31.6.3. [DOI] [Google Scholar]
- 26.Halko R., Pavelek D., Kaykhaii M. High performance liquid chromatography - fourier transform infrared spectroscopy coupling: a comprehensive review. Crit. Rev. Anal. Chem. 2024:1–12. doi: 10.1080/10408347.2024.2391892. [DOI] [PubMed] [Google Scholar]
- 27.Lee S., Han S., Kim H.M., et al. Simultaneous determination of luteolin and luteoloside in dandelions using HPLC. Horticulture, Environment, and Biotechnology. 2011;52(5):536–540. doi: 10.1007/s13580-011-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jankech T., Gerhardtova I., Stefanik O., et al. Current green capillary electrophoresis and liquid chromatography methods for analysis of pharmaceutical and biomedical samples (2019-2023) - a review. Anal. Chim. Acta. 2024;1323 doi: 10.1016/j.aca.2024.342889. [DOI] [PubMed] [Google Scholar]
- 29.Wang W., Lin P., Ma L., et al. Separation and determination of flavonoids in three traditional Chinese medicines by capillary electrophoresis with amperometric detection. J Sep Sci. 2016;39(7):1357–1362. doi: 10.1002/jssc.201501287. [DOI] [PubMed] [Google Scholar]
- 30.Shafiee S., Dastmalchi S., Gharekhani A., et al. Analysis of indoxyl sulfate in biological fluids with emphasis on sample preparation techniques: a comprehensive analytical review. Heliyon. 2024;10(15) doi: 10.1016/j.heliyon.2024.e35032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J., Cheng H., Xie H., et al. Platinum nanoparticles decorating a biomass porous carbon nanocomposite-modified electrode for the electrocatalytic sensing of luteolin and application. RSC Adv. 2019;9(58):33607–33616. doi: 10.1039/C9RA06265C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shui S., Liu W., Liu C., et al. Discrimination of cultivars and determination of luteolin content of Chrysanthemum morifolium Ramat. using multispectral imaging system. Anal. Methods. 2018;10(14):1640–1646. doi: 10.1039/C7AY02721D. [DOI] [Google Scholar]
- 33.Prasher P., Sharma M., Singh S.K., et al. Luteolin: a flavonoid with a multifaceted anticancer potential. Cancer Cell Int. 2022;22(1):386. doi: 10.1186/s12935-022-02808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha J.K., Jorwal K., Singh K.K., et al. The potential of mitochondrial therapeutics in the treatment of oxidative stress and inflammation in aging. Mol. Neurobiol. 2024 doi: 10.1007/s12035-024-04474-0. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y.Z., Chen D.F., Deng G., et al. The surrounding environments on the structure and antioxidative activity of luteolin. J. Mol. Model. 2018;24(7):149. doi: 10.1007/s00894-018-3680-1. [DOI] [PubMed] [Google Scholar]
- 36.Sordon S., Popłoński J., Milczarek M., et al. Structure-antioxidant-antiproliferative activity relationships of natural C7 and C7-C8 hydroxylated flavones and flavanones. Antioxidants. 2019;8(7) doi: 10.3390/antiox8070210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmadi S.M., Farhoosh R., Sharif A., et al. Structure-antioxidant activity relationships of luteolin and catechin. J. Food Sci. 2020;85(2):298–305. doi: 10.1111/1750-3841.14994. [DOI] [PubMed] [Google Scholar]
- 38.Xu T., Wang C., Jiang S., et al. Glycosylation of luteolin in hydrophilic organic solvents and structure-antioxidant relationships of luteolin glycosides. RSC Adv. 2022;12(28):18232–18237. doi: 10.1039/d2ra03300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jomova K., Hudecova L., Lauro P., et al. The effect of Luteolin on DNA damage mediated by a copper catalyzed Fenton reaction. J. Inorg. Biochem. 2022;226 doi: 10.1016/j.jinorgbio.2021.111635. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y., Yang J., Lu Y., et al. Copper(II) coordination and translocation in luteolin and effect on radical scavenging. J. Phys. Chem. B. 2020;124(2):380–388. doi: 10.1021/acs.jpcb.9b10531. [DOI] [PubMed] [Google Scholar]
- 41.Rajput S.A., Shaukat A., Wu K., et al. Luteolin alleviates AflatoxinB(1)-induced apoptosis and oxidative stress in the liver of mice through activation of Nrf2 signaling pathway. Antioxidants. 2021;10(8) doi: 10.3390/antiox10081268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaukat A., Rajput S.A., Ali M., et al. Therapeutic administration of Luteolin protects against Escherichia coli-derived Lipopolysaccharide-triggered inflammatory response and oxidative injury. Acta Trop. 2024;255 doi: 10.1016/j.actatropica.2024.107236. [DOI] [PubMed] [Google Scholar]
- 43.Farkhondeh T., Amirabadizadeh A., Aramjoo H., Llorens S., Roshanravan B., Saeedi F., Talebi M., Shakibaei M., Samarghandian S. Impact of metformin on cancer biomarkers in non-diabetic cancer patients: a systematic review and meta-analysis of clinical trials. Curr. Oncol. 2021;28(2):1412–1423. doi: 10.3390/curroncol28020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao C., Xia M.L., Wang J., Zhou X.R., Lou Y.Y., Tang L.H., Zhang F.J., Yang J.T., Qian L.B. Luteolin attenuates cardiac ischemia/reperfusion injury in diabetic rats by modulating Nrf2 antioxidative function. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/2719252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Y., Zhang X. Luteolin alleviates polycystic ovary syndrome in rats by resolving insulin resistance and oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2021;320(6) doi: 10.1152/ajpendo.00034.2021. E1085-e92. [DOI] [PubMed] [Google Scholar]
- 46.Liu M., Cheng C., Li X., Zhou S., Hua J., Huang J., Li Y., Yang K., Zhang P., Zhang Y., Tian J. Luteolin alleviates ochratoxin A induced oxidative stress by regulating Nrf2 and HIF-1α pathways in NRK-52E rat kidney cells. Food Chem. Toxicol. 2020;141 doi: 10.1016/j.fct.2020.111436. [DOI] [PubMed] [Google Scholar]
- 47.Jing Z., Wang C., Yang Q., Wei X., Jin Y., Meng Q., Liu Q., Liu Z., Ma X., Liu K., Sun H., Liu M. Luteolin attenuates glucocorticoid-induced osteoporosis by regulating ERK/Lrp-5/GSK-3β signaling pathway in vivo and in vitro. J. Cell. Physiol. 2019;234(4):4472–4490. doi: 10.1002/jcp.27252. [DOI] [PubMed] [Google Scholar]
- 48.Boeing T., de Souza P., Speca S., Somensi L.B., Mariano L.N.B., Cury B.J., Ferreira Dos Anjos M., Quintão N.L.M., Dubuqoy L., Desreumax P., da Silva L.M., de Andrade S.F. Luteolin prevents irinotecan-induced intestinal mucositis in mice through antioxidant and anti-inflammatory properties. Br. J. Pharmacol. 2020;177(10):2393–2408. doi: 10.1111/bph.14987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Owumi S.E., Lewu D.O., Arunsi U.O., Oyelere A.K. Luteolin attenuates doxorubicin-induced derangements of liver and kidney by reducing oxidative and inflammatory stress to suppress apoptosis. Hum. Exp. Toxicol. 2021;40(10):1656–1672. doi: 10.1177/09603271211006171. [DOI] [PubMed] [Google Scholar]
- 50.Cao Z., Xing C., Cheng X., Luo J., Hu R., Cao H., Guo X., Yang F., Zhuang Y., Hu G. Luteolin attenuates APEC-induced oxidative stress and inflammation via inhibiting the HMGB1/TLR4/NF-κB signal Axis in the ileum of chicks. Animals (Basel) 2022;13(1) doi: 10.3390/ani13010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seydi E., Mehrpouya L., Sadeghi H., Rahimi S., Pourahmad J. Luteolin attenuates Fipronil-induced neurotoxicity through reduction of the ROS-mediated oxidative stress in rat brain mitochondria. Pestic. Biochem. Physiol. 2021;173 doi: 10.1016/j.pestbp.2021.104785. [DOI] [PubMed] [Google Scholar]
- 52.Ou H.C., Pandey S., Hung M.Y., Huang S.H., Hsu P.T., Day C.H., Pai P., Viswanadha V.P., Kuo W.W., Huang C.Y. Luteolin: a natural flavonoid enhances the survival of HUVECs against oxidative stress by modulating ampk/PKC pathway. Am. J. Chin. Med. 2019;47(3):541–557. doi: 10.1142/s0192415x19500289. [DOI] [PubMed] [Google Scholar]
- 53.Chen H.I., Hu W.S., Hung M.Y., Ou H.C., Huang S.H., Hsu P.T., Day C.H., Lin K.H., Viswanadha V.P., Kuo W.W., Huang C.Y. Protective effects of luteolin against oxidative stress and mitochondrial dysfunction in endothelial cells. Nutr Metab Cardiovasc Dis. 2020;30(6):1032–1043. doi: 10.1016/j.numecd.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 54.Chen A., Huang H., Fang S., Hang Q. ROS: a "booster" for chronic inflammation and tumor metastasis. Biochim. Biophys. Acta Rev. Canc. 2024 doi: 10.1016/j.bbcan.2024.189175. [DOI] [PubMed] [Google Scholar]
- 55.Roe K. An inflammation classification system using cytokine parameters. Scand. J. Immunol. 2021;93(2) doi: 10.1111/sji.12970. [DOI] [PubMed] [Google Scholar]
- 56.Coperchini F., Greco A., Teliti M., Croce L., Chytiris S., Magri F., Gaetano C., Rotondi M. Inflamm-ageing: how cytokines and nutrition shape the trajectory of ageing. Cytokine Growth Factor Rev. 2024 doi: 10.1016/j.cytogfr.2024.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Aziz N., Kim M.Y., Cho J.Y. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018;225:342–358. doi: 10.1016/j.jep.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 58.Conti P., Caraffa A., Gallenga C.E., Ross R., Kritas S.K., Frydas I., Younes A., Di Emidio P., Ronconi G., Pandolfi F. Powerful anti-inflammatory action of luteolin: potential increase with IL-38. Biofactors. 2021;47(2):165–169. doi: 10.1002/biof.1718. [DOI] [PubMed] [Google Scholar]
- 59.Khan M.Z., Li L., Wang T., Liu X., Chen W., Ma Q., Zahoor M., Wang C. Bioactive compounds and probiotics mitigate mastitis by targeting NF-κB signaling pathway. Biomolecules. 2024;14(8) doi: 10.3390/biom14081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li H., Li Y., Luo S., Zhang Y., Feng Z., Li S. The roles and mechanisms of the NF-κB signaling pathway in tendon disorders. Front. Vet. Sci. 2024;11 doi: 10.3389/fvets.2024.1382239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao H.L., Yu X.J., Feng Y.Q., Yang Y., Hu H.B., Zhao Y.Y., Zhang J.H., Liu K.L., Zhang Y., Fu L.Y., Li Y., Qi J., Qiao J.A., Kang Y.M. Luteolin attenuates hypertension via inhibiting NF-κB-Mediated inflammation and PI3K/Akt signaling pathway in the hypothalamic paraventricular nucleus. Nutrients. 2023;15(3) doi: 10.3390/nu15030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abbas H., Sayed N.S.E., Youssef N., P M.E.G., Mousa M.R., Fayez A.M., Elsheikh M.A. Novel luteolin-loaded chitosan decorated nanoparticles for brain-targeting delivery in a sporadic alzheimer's disease mouse model: focus on antioxidant, anti-inflammatory, and amyloidogenic pathways. Pharmaceutics. 2022;14(5) doi: 10.3390/pharmaceutics14051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gendrisch F., Esser P.R., Schempp C.M., Wölfle U. Luteolin as a modulator of skin aging and inflammation. Biofactors. 2021;47(2):170–180. doi: 10.1002/biof.1699. [DOI] [PubMed] [Google Scholar]
- 64.Yuan J., Guo L., Ma J., Zhang H., Xiao M., Li N., Gong H., Yan M. HMGB1 as an extracellular pro-inflammatory cytokine: implications for drug-induced organic damage. Cell Biol. Toxicol. 2024;40(1):55. doi: 10.1007/s10565-024-09893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kure A., Nakagawa K., Kondo M., Kato S., Kimura F., Watanabe A., Shoji N., Hatanaka S., Tsushida T., Miyazawa T. Metabolic fate of luteolin in rats: its relationship to anti-inflammatory effect. J. Agric. Food Chem. 2016;64(21):4246–4254. doi: 10.1021/acs.jafc.6b00964. [DOI] [PubMed] [Google Scholar]
- 66.Chen H., Deng J., Gao H., Song Y., Zhang Y., Sun J., Zhai J. Involvement of the SIRT1-NLRP3 pathway in the inflammatory response. Cell Commun. Signal. 2023;21(1):185. doi: 10.1186/s12964-023-01177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie T., Yuan J., Mei L., Li P., Pan R. Luteolin suppresses TNF-α-induced inflammatory injury and senescence of nucleus pulposus cells via the Sirt6/NF-κB pathway. Exp. Ther. Med. 2022;24(1):469. doi: 10.3892/etm.2022.11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang L., Wang X., Zhang L., Virgous C., Si H. Combination of curcumin and luteolin synergistically inhibits TNF-α-induced vascular inflammation in human vascular cells and mice. J. Nutr. Biochem. 2019;73 doi: 10.1016/j.jnutbio.2019.108222. [DOI] [PubMed] [Google Scholar]
- 69.Qiao X.R., Feng T., Zhang D., Zhi L.L., Zhang J.T., Liu X.F., Pan Y., Xu J.W., Cui W.J., Dong L. Luteolin alleviated neutrophilic asthma by inhibiting IL-36γ secretion-mediated MAPK pathways. Pharm. Biol. 2023;61(1):165–176. doi: 10.1080/13880209.2022.2160770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lv Y., Qi J., Babon J.J., Cao L., Fan G., Lang J., Zhang J., Mi P., Kobe B., Wang F. The JAK-STAT pathway: from structural biology to cytokine engineering. Signal Transduct Target Ther. 2024;9(1):221. doi: 10.1038/s41392-024-01934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang S., Cao M., Xu S., Shi J., Mao X., Yao X., Liu C. Luteolin alters macrophage polarization to inhibit inflammation. Inflammation. 2020;43(1):95–108. doi: 10.1007/s10753-019-01099-7. [DOI] [PubMed] [Google Scholar]
- 72.Hu X., Li J., Fu M., Zhao X., Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6(1):402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nunes C., Almeida L., Barbosa R.M., Laranjinha J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct. 2017;8(1):387–396. doi: 10.1039/c6fo01529h. [DOI] [PubMed] [Google Scholar]
- 74.Dissook S., Umsumarng S., Mapoung S., Semmarath W., Arjsri P., Srisawad K., Dejkriengkraikul P. Luteolin-rich fraction from Perilla frutescens seed meal inhibits spike glycoprotein S1 of SARS-CoV-2-induced NLRP3 inflammasome lung cell inflammation via regulation of JAK1/STAT3 pathway: a potential anti-inflammatory compound against inflammation-induced long-COVID. Front. Med. 2022;9 doi: 10.3389/fmed.2022.1072056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Z, Kleis L, Depetris-Chauvin A, Jaskulski S, Damerell V, Michels KB, Gigic B, Nöthlings U, Panagiotou G. Beneficial microbiome and diet interplay in early-onset colorectal cancer. EMBO Mol Med. 9 Dec 2024 doi: 10.1038/s44321-024-00177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ashraf M.V., Khan S., Misri S., Gaira K.S., Rawat S., Rawat B., Khan M.A.H., Shah A.A., Asgher M., Ahmad S. High-altitude medicinal plants as promising source of phytochemical antioxidants to combat lifestyle-associated oxidative stress-induced disorders. Pharmaceuticals. 2024;17(8) doi: 10.3390/ph17080975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J., Ma Y. Luteolin as a potential therapeutic candidate for lung cancer: emerging preclinical evidence. Biomed. Pharmacother. 2024;176 doi: 10.1016/j.biopha.2024.116909. [DOI] [PubMed] [Google Scholar]
- 78.Rath P., Chauhan A., Ranjan A., Aggarwal D., Rani I., Choudhary R., Shahwan M., Ramniwas S., Joshi H., Haque S., Mathkor D.M., Tuli H.S. Luteolin: a promising modulator of apoptosis and survival signaling in liver cancer. Pathol. Res. Pract. 2024;260 doi: 10.1016/j.prp.2024.155430. [DOI] [PubMed] [Google Scholar]
- 79.Juszczak A.M., Wöelfle U., Končić M.Z., Tomczyk M. Skin cancer, including related pathways and therapy and the role of luteolin derivatives as potential therapeutics. Med. Res. Rev. 2022;42(4):1423–1462. doi: 10.1002/med.21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang C., Li Q., Xiao B., Fang H., Huang B., Huang F., Wang Y. Luteolin enhances the antitumor efficacy of oncolytic vaccinia virus that harbors IL-24 gene in liver cancer cells. J. Clin. Lab. Anal. 2021;35(3) doi: 10.1002/jcla.23677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Menezes M.E., Bhoopathi P., Pradhan A.K., Emdad L., Das S.K., Guo C., Wang X.Y., Sarkar D., Fisher P.B. Role of MDA-7/IL-24 a multifunction protein in human diseases. Adv. Cancer Res. 2018;138:143–182. doi: 10.1016/bs.acr.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao X., Wang B. Targeted PD-L1 PLGA/liposomes-mediated luteolin therapy for effective liver cancer cell treatment. J. Biomater. Appl. 2021;36(5):843–850. doi: 10.1177/08853282211017701. [DOI] [PubMed] [Google Scholar]
- 83.Nazim U.M., Park S.Y. Luteolin sensitizes human liver cancer cells to TRAIL-induced apoptosis via autophagy and JNK-mediated death receptor 5 upregulation. Int. J. Oncol. 2019;54(2):665–672. doi: 10.3892/ijo.2018.4633. [DOI] [PubMed] [Google Scholar]
- 84.Wen Q.E., Li L., Feng R.Q., Li D.H., Qiao C., Xu X.S., Zhang Y.J. Recent advances in immunotherapy for breast cancer: a review. Breast Cancer. 2024;16:497–516. doi: 10.2147/bctt.S482504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. Ca-Cancer J. Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 86.Zhang J., Wu Y., Li Y., Li S., Liu J., Yang X., Xia G., Wang G. Natural products and derivatives for breast cancer treatment: from drug discovery to molecular mechanism. Phytomedicine. 2024;129 doi: 10.1016/j.phymed.2024.155600. [DOI] [PubMed] [Google Scholar]
- 87.Wang C., Zheng X., Shen C., Shi Y. MicroRNA-203 suppresses cell proliferation and migration by targeting BIRC5 and LASP1 in human triple-negative breast cancer cells. J. Exp. Clin. Cancer Res. 2012;31(1):58. doi: 10.1186/1756-9966-31-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao G., Ge R., Li Y., Liu S. Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. Cells, Nanomed. Biotechnol. 2019;47(1):3265–3271. doi: 10.1080/21691401.2019.1646749. [DOI] [PubMed] [Google Scholar]
- 89.Hussain Y., Cui J.H., Khan H., Aschner M., Batiha G.E., Jeandet P. Luteolin and cancer metastasis suppression: focus on the role of epithelial to mesenchymal transition. Med. Oncol. 2021;38(6):66. doi: 10.1007/s12032-021-01508-8. [DOI] [PubMed] [Google Scholar]
- 90.Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 91.Saini K.S., Loi S., de Azambuja E., Metzger-Filho O., Saini M.L., Ignatiadis M., Dancey J.E., Piccart-Gebhart M.J. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013;39(8):935–946. doi: 10.1016/j.ctrv.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 92.Hu A., Li R., Chen G., Chen S. Impact of respiratory dust on health: a comparison based on the toxicity of PM2.5, silica, and nanosilica. Int. J. Mol. Sci. 2024;25(14) doi: 10.3390/ijms25147654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liang S., Zhao T., Hu H., Shi Y., Xu Q., Miller M.R., Duan J., Sun Z. Repeat dose exposure of PM(2.5) triggers the disseminated intravascular coagulation (DIC) in SD rats. Sci. Total Environ. 2019;663:245–253. doi: 10.1016/j.scitotenv.2019.01.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu C.W., Lee T.L., Chen Y.C., Liang C.J., Wang S.H., Lue J.H., Tsai J.S., Lee S.W., Chen S.H., Yang Y.F., Chuang T.Y., Chen Y.L. PM(2.5)-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-κB-dependent pathway. Part. Fibre Toxicol. 2018;15(1):4. doi: 10.1186/s12989-018-0240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin H.W., Shen T.J., Yang N.C., Wang M., Hsieh W.C., Chuang C.J., Lai C.Y., Chang Y.Y. Luteolin reduces aqueous extract pm2.5-induced metastatic activity in H460 lung cancer cells. Int. J. Med. Sci. 2022;19(10):1502–1509. doi: 10.7150/ijms.73947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu C., Zheng S., Jin R., Wang X., Wang F., Zang R., Xu H., Lu Z., Huang J., Lei Y., Mao S., Wang Y., Feng X., Sun N., Wang Y., He J. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020;470:95–105. doi: 10.1016/j.canlet.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 97.Jin W. Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial-mesenchymal transition. Cells. 2020;9(1) doi: 10.3390/cells9010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang Z.B., Wang W.J., Xu C., Xie Y.J., Wang X.R., Zhang Y.Z., Huang J.M., Huang M., Xie C., Liu P., Fan X.X., Ma Y.P., Yan P.Y., Liu L., Yao X.J., Wu Q.B., Lai-Han Leung E. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 2021;515:36–48. doi: 10.1016/j.canlet.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 99.Zhang M., Wang R., Tian J., Song M., Zhao R., Liu K., Zhu F., Shim J.H., Dong Z., Lee M.H. Targeting LIMK1 with luteolin inhibits the growth of lung cancer in vitro and in vivo. J. Cell Mol. Med. 2021;25(12):5560–5571. doi: 10.1111/jcmm.16568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee M.H., Kundu J.K., Chae J.I., Shim J.H. Targeting ROCK/LIMK/cofilin signaling pathway in cancer. Arch Pharm. Res. (Seoul) 2019;42(6):481–491. doi: 10.1007/s12272-019-01153-w. [DOI] [PubMed] [Google Scholar]
- 101.Zang M.D., Hu L., Fan Z.Y., Wang H.X., Zhu Z.L., Cao S., Wu X.Y., Li J.F., Su L.P., Li C., Zhu Z.G., Yan M., Liu B.Y. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the Notch signaling pathway. J. Transl. Med. 2017;15(1):52. doi: 10.1186/s12967-017-1151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kwon O.J., Zhang L., Wang J., Su Q., Feng Q., Zhang X.H., Mani S.A., Paulter R., Creighton C.J., Ittmann M.M., Xin L. Notch promotes tumor metastasis in a prostate-specific Pten-null mouse model. J. Clin. Invest. 2016;126(7):2626–2641. doi: 10.1172/jci84637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ren L.Q., Li Q., Zhang Y. Luteolin suppresses the proliferation of gastric cancer cells and acts in synergy with oxaliplatin. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/9396512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu H., Huang M., Liu Y., Shu Y., Liu P. Luteolin induces apoptosis by up-regulating miR-34a in human gastric cancer cells. Technol. Cancer Res. Treat. 2015;14(6):747–755. doi: 10.7785/tcrt.2012.500434. [DOI] [PubMed] [Google Scholar]
- 105.Pandurangan A.K., Esa N.M. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: a review. Asian Pac J Cancer Prev. 2014;15(14):5501–5508. doi: 10.7314/apjcp.2014.15.14.5501. [DOI] [PubMed] [Google Scholar]
- 106.Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J. Biochem. 2002;132(3):359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- 107.Yao Y., Rao C., Zheng G., Wang S. Luteolin suppresses colorectal cancer cell metastasis via regulation of the miR-384/pleiotrophin axis. Oncol. Rep. 2019;42(1):131–141. doi: 10.3892/or.2019.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Steven A., Seliger B. Control of CREB expression in tumors: from molecular mechanisms and signal transduction pathways to therapeutic target. Oncotarget. 2016;7(23):35454–35465. doi: 10.18632/oncotarget.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Y., Lang T., Jin B., Chen F., Zhang Y., Beuerman R.W., Zhou L., Zhang Z. Luteolin inhibits colorectal cancer cell epithelial-to-mesenchymal transition by suppressing CREB1 expression revealed by comparative proteomics study. J Proteomics. 2017;161:1–10. doi: 10.1016/j.jprot.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 110.Yoo H.S., Won S.B., Kwon Y.H. Luteolin induces apoptosis and autophagy in HCT116 colon cancer cells via p53-dependent pathway. Nutr. Cancer. 2022;74(2):677–686. doi: 10.1080/01635581.2021.1903947. [DOI] [PubMed] [Google Scholar]
- 111.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 112.Cumberbatch M.G., Rota M., Catto J.W., La Vecchia C., Reply to Wentao Liu, Xiaokun Zhao, Zhaohui Zhong's Letter to the Editor re The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur urol 2016;70:458-66. Cumberbatch Marcus G., Rota Matteo, Catto James W.F., Vecchia Carlo La., editors. Eur. Urol. 2016;70(4):e106–e107. doi: 10.1016/j.eururo.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 113.Samaranayake G.J., Troccoli C.I., Huynh M., Lyles R.D.Z., Kage K., Win A., Lakshmanan V., Kwon D., Ban Y., Chen S.X., Zarco E.R., Jorda M., Burnstein K.L., Rai P. Thioredoxin-1 protects against androgen receptor-induced redox vulnerability in castration-resistant prostate cancer. Nat. Commun. 2017;8(1):1204. doi: 10.1038/s41467-017-01269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chiong E., Lee I.L., Dadbin A., Sabichi A.L., Harris L., Urbauer D., McConkey D.J., Dickstein R.J., Cheng T., Grossman H.B. Effects of mTOR inhibitor everolimus (RAD001) on bladder cancer cells. Clin. Cancer Res. 2011;17(9):2863–2873. doi: 10.1158/1078-0432.Ccr-09-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iida K., Naiki T., Naiki-Ito A., Suzuki S., Kato H., Nozaki S., Nagai T., Etani T., Nagayasu Y., Ando R., Kawai N., Yasui T., Takahashi S. Luteolin suppresses bladder cancer growth via regulation of mechanistic target of rapamycin pathway. Cancer Sci. 2020;111(4):1165–1179. doi: 10.1111/cas.14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moldoveanu T., Follis A.V., Kriwacki R.W., Green D.R. Many players in BCL-2 family affairs. Trends Biochem. Sci. 2014;39(3):101–111. doi: 10.1016/j.tibs.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Z., Zhang Y., Chen L., Li H. The dietary compound luteolin inhibits pancreatic cancer growth by targeting BCL-2. Food Funct. 2018;9(5):3018–3027. doi: 10.1039/c8fo00033f. [DOI] [PubMed] [Google Scholar]
- 118.Liu B.G., Xie M., Dong Y., Wu H., He D.D., Hu G.Z., Xu E.P. Antimicrobial mechanisms of traditional Chinese medicine and reversal of drug resistance: a narrative review. Eur. Rev. Med. Pharmacol. Sci. 2022;26(15):5553–5561. doi: 10.26355/eurrev_202208_29426. [DOI] [PubMed] [Google Scholar]
- 119.Rzewuska M., Kwiecień E., Chrobak-Chmiel D., Kizerwetter-Świda M., Stefańska I., Gieryńska M. Pathogenicity and virulence of trueperella pyogenes: a review. Int. J. Mol. Sci. 2019;20(11) doi: 10.3390/ijms20112737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang L., Cai Y., Li L., Chen C., Zhao H., Zhang Z., Liu Y., Wang Y., Tian C., Liu M. Effects of luteolin on biofilm of trueperella pyogenes and its therapeutic effect on rat endometritis. Int. J. Mol. Sci. 2022;23(22) doi: 10.3390/ijms232214451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo Y., Liu Y., Zhang Z., Chen M., Zhang D., Tian C., Liu M., Jiang G. The antibacterial activity and mechanism of action of luteolin against trueperella pyogenes. Infect. Drug Resist. 2020;13:1697–1711. doi: 10.2147/idr.S253363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun Y., Sun F., Feng W., Wang Q., Liu F., Xia P., Qiu X. Luteolin inhibits the biofilm formation and cytotoxicity of methicillin-resistant Staphylococcus aureus via decreasing bacterial toxin synthesis. Evid Based Complement Alternat Med. 2022;2022 doi: 10.1155/2022/4476339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qian W., Fu Y., Liu M., Zhang J., Wang W., Li J., Zeng Q., Wang T., Li Y. Mechanisms of action of luteolin against single- and dual-species of Escherichia coli and Enterobacter cloacae and its antibiofilm activities. Appl. Biochem. Biotechnol. 2021;193(5):1397–1414. doi: 10.1007/s12010-020-03330-w. [DOI] [PubMed] [Google Scholar]
- 124.Qian W., Liu M., Fu Y., Zhang J., Liu W., Li J., Li X., Li Y., Wang T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020;142 doi: 10.1016/j.micpath.2020.104056. [DOI] [PubMed] [Google Scholar]
- 125.Geng Y.F., Yang C., Zhang Y., Tao S.N., Mei J., Zhang X.C., Sun Y.J., Zhao B.T. An innovative role for luteolin as a natural quorum sensing inhibitor in Pseudomonas aeruginosa. Life Sci. 2021;274 doi: 10.1016/j.lfs.2021.119325. [DOI] [PubMed] [Google Scholar]
- 126.Valfridsson C., Westerlund E., Hancz D., Persson J.J. Detection of inflammasome activation in murine bone marrow-derived macrophages infected with group A Streptococcus. Methods Mol. Biol. 2023;2674:261–282. doi: 10.1007/978-1-0716-3243-7_18. [DOI] [PubMed] [Google Scholar]
- 127.Guo T., Liu P., Wang Z., Zheng Y., Huang W., Kong D., Ding L., Lv Q., Wang Z., Jiang H., Jiang Y., Sun L. Luteolin binds streptolysin O toxin and inhibits its hemolytic effects and cytotoxicity. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.942180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arora D., Hall S., Anoopkumar-Dukie S., Morrison R., McFarland A., Perkins A.V., Davey A.K., Grant G.D. Pyocyanin induces systemic oxidative stress, inflammation and behavioral changes in vivo. Toxicol. Mech. Methods. 2018;28(6):410–414. doi: 10.1080/15376516.2018.1429038. [DOI] [PubMed] [Google Scholar]
- 129.Berube B.J., Bubeck Wardenburg J. Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins. 2013;5(6):1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Otto M. Phenol-soluble modulins. Int J Med Microbiol. 2014;304(2):164–169. doi: 10.1016/j.ijmm.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yuan Q., Feng W., Wang Y., Wang Q., Mou N., Xiong L., Wang X., Xia P., Sun F. Luteolin attenuates the pathogenesis of Staphylococcus aureus by interfering with the agr system. Microb. Pathog. 2022;165 doi: 10.1016/j.micpath.2022.105496. [DOI] [PubMed] [Google Scholar]
- 132.Yuan L., Nakamichi R., Hirata Y., Matsuda A., Shinohara Y., Yamada A., Masuda Y., Honjoh K.I., Miyamoto T. Mechanism for inhibition of cytotoxicity of Shiga toxin by luteolin. Toxicol. Vitro. 2023;87 doi: 10.1016/j.tiv.2022.105537. [DOI] [PubMed] [Google Scholar]
- 133.Matsumoto H., Yamashita M., Tahara T., Hayakawa S., Wada S.I., Tomioka K., Iida A. Design, synthesis, and evaluation of DNA topoisomerase II-targeted nucleosides. Bioorg. Med. Chem. 2017;25(15):4133–4144. doi: 10.1016/j.bmc.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Z., Guo Y., Guo Y., Zhang L., Niu S., Tian C., Han L., Zhang D., Liu M. Molecular basis for luteolin as a natural TatD DNase inhibitor in trueperella pyogenes. Int. J. Mol. Sci. 2022;23(15) doi: 10.3390/ijms23158374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee C.Y. Exploring potential intermediates in the cross-species transmission of influenza A virus to humans. Viruses. 2024;16(7) doi: 10.3390/v16071129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yan H., Ma L., Wang H., Wu S., Huang H., Gu Z., Jiang J., Li Y. Luteolin decreases the yield of influenza A virus in vitro by interfering with the coat protein I complex expression. J. Nat. Med. 2019;73(3):487–496. doi: 10.1007/s11418-019-01287-7. [DOI] [PubMed] [Google Scholar]
- 137.Munafò F., Donati E., Brindani N., Ottonello G., Armirotti A., De Vivo M. Quercetin and luteolin are single-digit micromolar inhibitors of the SARS-CoV-2 RNA-dependent RNA polymerase. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-14664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang S., Ling Y., Yao Y., Zheng G., Chen W. Luteolin inhibits respiratory syncytial virus replication by regulating the MiR-155/SOCS1/STAT1 signaling pathway. Virol. J. 2020;17(1):187. doi: 10.1186/s12985-020-01451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Peng M., Watanabe S., Chan K.W.K., He Q., Zhao Y., Zhang Z., Lai X., Luo D., Vasudevan S.G., Li G. Luteolin restricts dengue virus replication through inhibition of the proprotein convertase furin. Antiviral Res. 2017;143:176–185. doi: 10.1016/j.antiviral.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 140.Wu S., Wang H.Q., Guo T.T., Li Y.H. Luteolin inhibits CVB3 replication through inhibiting inflammation. J. Asian Nat. Prod. Res. 2020;22(8):762–773. doi: 10.1080/10286020.2019.1642329. [DOI] [PubMed] [Google Scholar]
- 141.Cui X.X., Yang X., Wang H.J., Rong X.Y., Jing S., Xie Y.H., Huang D.F., Zhao C. Luteolin-7-O-Glucoside present in lettuce extracts inhibits hepatitis B surface antigen production and viral replication by human hepatoma cells in vitro. Front. Microbiol. 2017;8:2425. doi: 10.3389/fmicb.2017.02425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gong X., Li X., Bo A., Shi R.Y., Li Q.Y., Lei L.J., Zhang L., Li M.H. The interactions between gut microbiota and bioactive ingredients of traditional Chinese medicines: a review. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104824. [DOI] [PubMed] [Google Scholar]
- 143.Sun W.L., Yang J.W., Dou H.Y., Li G.Q., Li X.Y., Shen L., Ji H.F. Anti-inflammatory effect of luteolin is related to the changes in the gut microbiota and contributes to preventing the progression from simple steatosis to nonalcoholic steatohepatitis. Bioorg. Chem. 2021;112 doi: 10.1016/j.bioorg.2021.104966. [DOI] [PubMed] [Google Scholar]
- 144.Liu X., Sun R., Li Z., Xiao R., Lv P., Sun X., Olson M.A., Gong Y. Luteolin alleviates non-alcoholic fatty liver disease in rats via restoration of intestinal mucosal barrier damage and microbiota imbalance involving in gut-liver axis. Arch. Biochem. Biophys. 2021;711 doi: 10.1016/j.abb.2021.109019. [DOI] [PubMed] [Google Scholar]
- 145.Li B., Du P., Du Y., Zhao D., Cai Y., Yang Q., Guo Z. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 2021;269 doi: 10.1016/j.lfs.2020.119008. [DOI] [PubMed] [Google Scholar]
- 146.Ge X., He X., Lin Z., Zhu Y., Jiang X., Zhao L., Zeng F., Chen L., Xu W., Liu T., Chen Z., Zhao C., Huang Y., Liu B. 6,8-(1,3-Diaminoguanidine) luteolin and its Cr complex show hypoglycemic activities and alter intestinal microbiota composition in type 2 diabetes mice. Food Funct. 2022;13(6):3572–3589. doi: 10.1039/d2fo00021k. [DOI] [PubMed] [Google Scholar]
- 147.Ge X., Wang C., Chen H., Liu T., Chen L., Huang Y., Zeng F., Liu B. Luteolin cooperated with metformin hydrochloride alleviates lipid metabolism disorders and optimizes intestinal flora compositions of high-fat diet mice. Food Funct. 2020;11(11):10033–10046. doi: 10.1039/d0fo01840f. [DOI] [PubMed] [Google Scholar]
- 148.Kou J.J., Shi J.Z., He Y.Y., Hao J.J., Zhang H.Y., Luo D.M., Song J.K., Yan Y., Xie X.M., Du G.H., Pang X.B. Luteolin alleviates cognitive impairment in Alzheimer's disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol. Sin. 2022;43(4):840–849. doi: 10.1038/s41401-021-00702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lian L., Zhang Y., Liu L., Yang L., Cai Y., Zhang J., Xu S. Neuroinflammation in ischemic stroke: focus on MicroRNA-mediated polarization of microglia. Front. Mol. Neurosci. 2020;13 doi: 10.3389/fnmol.2020.612439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dong R., Huang R., Shi X., Xu Z., Mang J. Exploration of the mechanism of luteolin against ischemic stroke based on network pharmacology, molecular docking and experimental verification. Bioengineered. 2021;12(2):12274–12293. doi: 10.1080/21655979.2021.2006966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou Y.S., Cui Y., Zheng J.X., Quan Y.Q., Wu S.X., Xu H., Han Y. Luteolin relieves lung cancer-induced bone pain by inhibiting NLRP3 inflammasomes and glial activation in the spinal dorsal horn in mice. Phytomedicine. 2022;96 doi: 10.1016/j.phymed.2021.153910. [DOI] [PubMed] [Google Scholar]
- 152.Liu X., Cui X., Ji D., Zhang Z., Li B., Xu Y., Chen T., Tian S. Luteolin-induced activation of the phenylpropanoid metabolic pathway contributes to quality maintenance and disease resistance of sweet cherry. Food Chem. 2021;342 doi: 10.1016/j.foodchem.2020.128309. [DOI] [PubMed] [Google Scholar]
- 153.Castano-Duque L., Lebar M.D., Carter-Wientjes C., Ambrogio D., Rajasekaran K. Flavonoids modulate Aspergillus flavus proliferation and aflatoxin production. J Fungi (Basel) 2022;8(11) doi: 10.3390/jof8111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tsimogiannis D., Oreopoulou V. Defining the role of flavonoid structure on cottonseed oil stabilization: study of A- and C-ring substitution. J. Am. Oil Chem. Soc. 2007;84(2):129–136. doi: 10.1007/s11746-006-1016-2. [DOI] [Google Scholar]
- 155.Wang Z., Zeng M., Wang Z., Qin F., Chen J., He Z. Dietary luteolin: a narrative review focusing on its pharmacokinetic properties and effects on glycolipid metabolism. J. Agric. Food Chem. 2021;69(5):1441–1454. doi: 10.1021/acs.jafc.0c08085. [DOI] [PubMed] [Google Scholar]
- 156.Xiao C., Wang X., Shen J., Xia Y., Qiu S., Liang X., Gu W. Luteolin-loading her-2 nanospheres enhances targeting and therapeutic effects of breast cancer. J. Biomed. Nanotechnol. 2021;17(8):1545–1553. doi: 10.1166/jbn.2021.3137. [DOI] [PubMed] [Google Scholar]
- 157.Shakeel F., Alamer M.M., Alam P., Alshetaili A., Haq N., Alanazi F.K., Alshehri S., Ghoneim M.M., Alsarra I.A. Hepatoprotective effects of bioflavonoid luteolin using self-nanoemulsifying drug delivery system. Molecules. 2021;26(24) doi: 10.3390/molecules26247497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu H.T., Liu Y.E., Hsu K.W., Wang Y.F., Chan Y.C., Chen Y., Chen D.R. MLL3 induced by luteolin causes apoptosis in tamoxifen-resistant breast cancer cells through H3K4 monomethylation and suppression of the PI3K/AKT/mTOR pathway. Am. J. Chin. Med. 2020;48(5):1221–1241. doi: 10.1142/s0192415x20500603. [DOI] [PubMed] [Google Scholar]
- 159.Guo Y., Huang C., Su H., Zhang Z., Chen M., Wang R., Zhang D., Zhang L., Liu M. Luteolin increases susceptibility to macrolides by inhibiting MsrA efflux pump in Trueperella pyogenes. Vet Res. 2022;53(1):3. doi: 10.1186/s13567-021-01021-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li X., He X., Chen S., Le Y., Bryant M.S., Guo L., Witt K.L., Mei N. The genotoxicity potential of luteolin is enhanced by CYP1A1 and CYP1A2 in human lymphoblastoid TK6 cells. Toxicol. Lett. 2021;344:58–68. doi: 10.1016/j.toxlet.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maciel M.D., Inocêncio L.C.L., Rechsteiner M.S., Jorge B.C., Balin P.D.S., Kassuya R.M., Heredia-Vieira S.C., Cardoso C.A.L., Vieira M.D.C., Kassuya C.A.L., Arena A.C. Effects of exposure to ethanolic extract from Achyrocline satureioides (Lam.) D.C. flowers on reproductive and developmental parameters in Wistar rats. J. Toxicol. Environ. Health. 2019;82(5):321–330. doi: 10.1080/15287394.2019.1593904. [DOI] [PubMed] [Google Scholar]
- 162.Fateh A.H., Mohamed Z., Chik Z., Alsalahi A., Md Zin S.R., Alshawsh M.A. Prenatal developmental toxicity evaluation of Verbena officinalis during gestation period in female Sprague-Dawley rats. Chem. Biol. Interact. 2019;304:28–42. doi: 10.1016/j.cbi.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 163.Zhang X., Wu C. In silico, in vitro, and in vivo evaluation of the developmental toxicity, estrogenic activity, and mutagenicity of four natural phenolic flavonoids at low exposure levels. ACS Omega. 2022;7(6):4757–4768. doi: 10.1021/acsomega.1c04239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Krewski D., Andersen M.E., Tyshenko M.G., Krishnan K., Hartung T., Boekelheide K., Wambaugh J.F., Jones D., Whelan M., Thomas R., Yauk C., Barton-Maclaren T., Cote I. Toxicity testing in the 21st century: progress in the past decade and future perspectives. Arch. Toxicol. 2020;94(1):1–58. doi: 10.1007/s00204-019-02613-4. [DOI] [PubMed] [Google Scholar]
- 165.Bi F.Y., Qin Y., Chen D., Kan J., Liu J. Development of active packaging films based on chitosan and nano-encapsulated luteolin. Int. J. Biol. Macromol. 2021;182:545–553. doi: 10.1016/j.ijbiomac.2021.04.063. [DOI] [PubMed] [Google Scholar]
- 166.Tu L., Wang J., Sun Y., Wan Y. Fabrication of luteolin nanoemulsion by box-behnken design to enhance its oral absorption via lymphatic transport. AAPS PharmSciTech. 2024;25(7):206. doi: 10.1208/s12249-024-02898-4. [DOI] [PubMed] [Google Scholar]
- 167.Xi M.H., Hou Y.J., Wang R.L., Ji M.H., Cai Y.Y., Ao J.F., Shen H.Y., Li M., Wang J., Luo A.W. Potential application of luteolin as an active antibacterial composition in the development of hand sanitizer products. Molecules. 2022;27(21) doi: 10.3390/molecules27217342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Deng M., Wang H., Geng S., Guan X., Liang N. Application of an alkali destruction technique and natural deep eutectic solvent for greener extraction from peanut shells: optimization and extraction kinetics study. Anal. Methods. 2022;14(16):1594–1602. doi: 10.1039/d1ay02033a. [DOI] [PubMed] [Google Scholar]
- 169.Lan D.D., Liu J.J., Guo X.F., Zhu G.Q., Tian S.G. Determining the contents of rupestonic acid, vitexicarpin, apigenin, and luteolin in artemisia rupestris L. In different growth stages by thin-layer chromatographic scanning. Jpc-Journal of Planar Chromatography-Modern Tlc. 2018;31(3):190–196. doi: 10.1556/1006.2018.31.3.2. [DOI] [Google Scholar]
- 170.Satpathy S., Patra A., Ahirwar B. Development and validation of a novel high-performance thin-layer chromatography method for the simultaneous determination of apigenin and luteolin in hygrophila spinosa T. Anders. Jpc-Journal of Planar Chromatography-Modern Tlc. 2018;31(6):437–443. doi: 10.1556/1006.2018.31.6.3. [DOI] [Google Scholar]
- 171.Yang H., Shi H., Zhang Q., Liu Y., Wan C., Zhang L. Simultaneous determination of five components in aster tataricus by ultra performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. Sci. 2016;54(4):500–506. doi: 10.1093/chromsci/bmv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang L., Yan Q.H., Ma J.Y., Wang Q., Zhang J.W., Xi G.X. High performance liquid chromatographic determination of phenolic compounds in propolis. Trop. J. Pharmaceut. Res. 2013;12(5):771–776. doi: 10.4314/tjpr.v12i5.17. [DOI] [Google Scholar]
- 173.Dagnon S., Novkova Z., Bojilov D., Nedialkov P., Kouassi C. Development of surrogate standards approach for the determination of polyphenols in Vernonia amygdalina Del. J. Food Compos. Anal. 2019;82 doi: 10.1016/j.jfca.2019.06.003. [DOI] [Google Scholar]
- 174.Sergiel I., Pohl P., Biesaga M. Characterisation of honeys according to their content of phenolic compounds using high performance liquid chromatography/tandem mass spectrometry. Food Chem. 2014;145:404–408. doi: 10.1016/j.foodchem.2013.08.068. [DOI] [PubMed] [Google Scholar]
- 175.Hwang S.H., Paek J.H., Lim S.S. Simultaneous ultra performance liquid chromatography determination and antioxidant activity of linarin, luteolin, chlorogenic acid and apigenin in different parts of compositae species. Molecules. 2016;21(11) doi: 10.3390/molecules21111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sanli S., Sanli N., Ozkan S.A., Lunte C. Development and validation of a green capillary electrophoretic method for determination of polyphenolic compounds in red wine samples. Chromatographia. 2016;79(19–20):1351–1358. doi: 10.1007/s10337-016-3147-4. [DOI] [Google Scholar]
- 177.Wu T.N., Yu C.Z., Li R. Determination of flavonoids in flos chrysanthemi and flos chrysanthemi indici by capillary electrophoresis. Instrum. Sci. Technol. 2017;45(4):412–422. doi: 10.1080/10739149.2016.1258572. [DOI] [Google Scholar]
- 178.Maher H.M., Al-Zoman N.Z., Al-Shehri M.M., Al-Showiman H., Al-Taweel A.M., Fawzy G.A., Perveen S. Determination of luteolin and apigenin in herbs by capillary electrophoresis with diode array detection. Instrum. Sci. Technol. 2015;43(6):611–625. doi: 10.1080/10739149.2015.1038560. [DOI] [Google Scholar]
- 179.Tang J., Huang R., Zheng S.B., Jiang S.X., Yu H., Li Z.R., Wang J.F. A sensitive and selective electrochemical sensor based on graphene quantum dots/gold nanoparticles nanocomposite modified electrode for the determination of luteolin in peanut hulls. Microchem. J. 2019;145:899–907. doi: 10.1016/j.microc.2018.12.006. [DOI] [Google Scholar]
- 180.Tesio A.Y., Granero A.M., Vettorazzi N.R., Ferreyra N.F., Rivas G.A., Fernandez H., Zon M.A. Development of an electrochemical sensor for the determination of the flavonoid luteolin in peanut hull samples. Microchem. J. 2014;115:100–105. doi: 10.1016/j.microc.2014.03.004. [DOI] [Google Scholar]
- 181.Wei M.T., Geng X., Liu Y.R., Long H.Y., Du J.Y. A novel electrochemical sensor based on electropolymerized molecularly imprinted polymer for determination of luteolin. J. Electroanal. Chem. 2019;842:184–192. doi: 10.1016/j.jelechem.2019.04.074. [DOI] [Google Scholar]
- 182.Prasher P., Sharma M., Singh S.K., Gulati M., Chellappan D.K., Zacconi F., De Rubis G., Gupta G., Sharifi-Rad J., Cho W.C., Dua K. Luteolin: a flavonoid with a multifaceted anticancer potential. Cancer Cell Int. 2022;22(1):386. doi: 10.1186/s12935-022-02808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article is referenced in the article.