Abstract
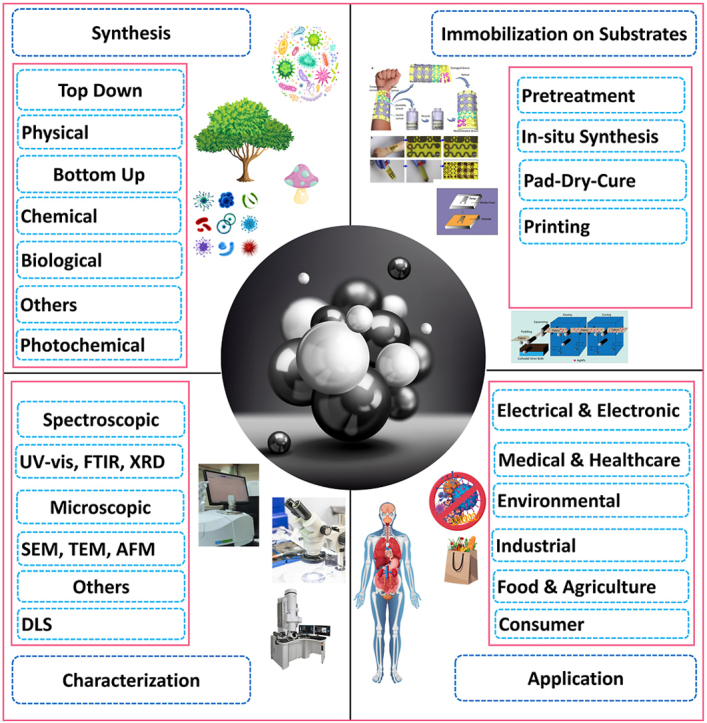
Silver nanoparticles (AgNPs) have attracted significant interest in recent years owing to their unique physicochemical properties, including antimicrobial reduction capabilities, photocatalytic activity, self-cleaning features, superhydrophobicity, and electrical conductivity. Their characteristics render them highly advantageous for various textile, electronics, food and agriculture, water treatment, and biomedical applications. This detailed analysis explores the recent benefits and drawbacks of various synthesis methods, immobilization techniques, and characterization of AgNPs while emphasizing novel strategies that improve their functionality across different substrates. A comprehensive analysis is conducted on various synthesis methods, including physical, chemical, and biological approaches. Additionally, immobilization techniques such as in-situ synthesis, pad-dry-cure, and printing on diverse substrates are thoroughly examined for their role in enhancing the functionality of textile substrates. Advanced characterization techniques, encompassing spectroscopic and microscopic methods, have been reviewed to provide a comprehensive understanding of AgNPs' structural and functional properties. This review highlights the progress made in synthesizing AgNPs, focusing on the ability to control their size and shape for targeted applications. Improved immobilization methods have significantly enhanced the stability of AgNPs in intricate environments. In contrast, advanced characterization techniques facilitate a more accurate control and assessment of the properties of AgNPs. The utilization of AgNPs as an antimicrobial agent for surface and food protection, medical devices, antiviral agents, and therapeutic tools showcases their extensive influence across the field. The cytotoxic effects of AgNPs on the human body have been thoroughly examined. This review examines recent advancements in AgNPs to encourage additional research and the development of innovative formulations. It also highlights future perspectives and research directions to effectively and sustainably utilize the potential of AgNPs.
Keywords: Silver nanoparticle, Green synthesis, Advanced characterization techniques, Functional nanomaterials, Antimicrobial, Cytotoxicity and biocompatibility
Graphical abstract
Highlights
-
•
AgNPs are synthesized via eco-friendly green methods, alongside physical, chemical, and biological approaches.
-
•
Microscopy and spectroscopy techniques reveal AgNPs' shape, size, and surface properties, influencing their applications.
-
•
AgNPs are integrated into textiles and substrates using methods like pad-dry-cure, in-situ synthesis, and printing.
-
•
AgNPs are used in antimicrobial applications in healthcare, agriculture, water treatment, sensors, and solar cells.
-
•
The cytotoxicity of AgNPs on humans and the environment raises concerns, emphasizing the need for further study.
1. Introduction
The particles whose diameter is in the nanoscale range of 1–100 nm are called nanoparticles [1]. Due to the high surface-to-volume ratio and nanoscale range, the particle shows significant changes in physical, chemical, optical, and biological properties. Consequently, researchers and industries increasingly acknowledge the immense potential of silver nanoparticles (AgNPs). There are various metal nanoparticle types, including iron, gold, silver, titanium, cerium, platinum, thallium, copper, zinc, and so on [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11]]. Among these, AgNPs are drawing much interest from the scientific community and business sector due to their wide range of biological and physicochemical applications. These properties include a large surface area-to-volume ratio, impressive surface plasmonic resonance, and the ability to easily customize their properties by attaching different ligands. AgNPs also show promising activity in their toxicity against pathogens, effectiveness against cancer cells, and various catalytic applications [12].
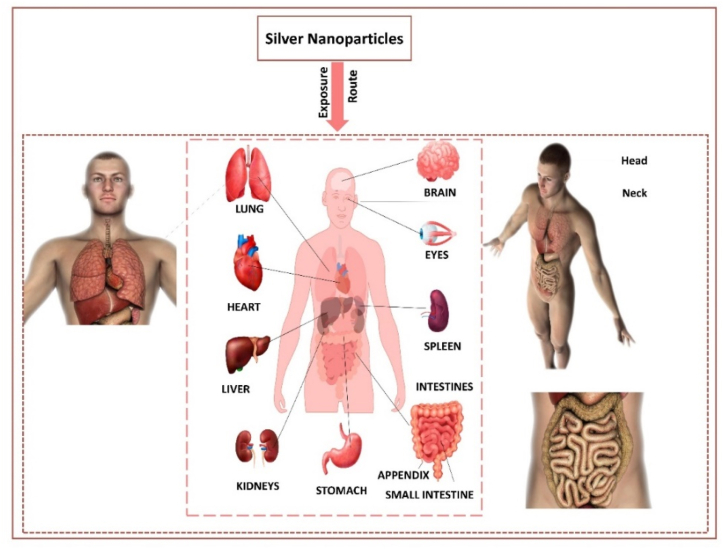
AgNPs are synthesized using different methods, including physical [7], chemical [13], and biological methods [[14], [15], [16]]. The green synthesized method has gained popularity recently due to its low toxicity and chemical content. The distinctiveness of AgNPs can be analyzed using microscopic and spectroscopic techniques to analyze the particle size, shape, and surface plasmon resonance [17,18]. AgNPs have become a subject of significant research interest. Their unique properties, such as surface plasma resonance (SPR), biocompatibility, electrical conductivity, antioxidant properties, and chemical stability, have led to a multitude of applications, including combating viruses, bacteria [19], fungi [20], and cancer [21]. They are also utilized in anti-corrosive [22], biosensors [23], drug delivery [24], solar cells [25], water treatment [26], agriculture [27], consumer [28] and catalysis [29]. Different immobilization techniques include pad-dry-cure [30], in-situ synthesis [31], printing [32], layer-by-layer deposition [33], solution immersion [34], and sonochemical method [35]. Different types of textiles like cotton, PC blend, wool, silk, polyester, regenerated cellulose, and polyamide are treated with AgNPs [36]. AgNPs can pass through biological membranes, directly enter cells, and accumulate in the brain, heart, liver, spleen, lungs, kidneys, etc., which may affect physiology [37].
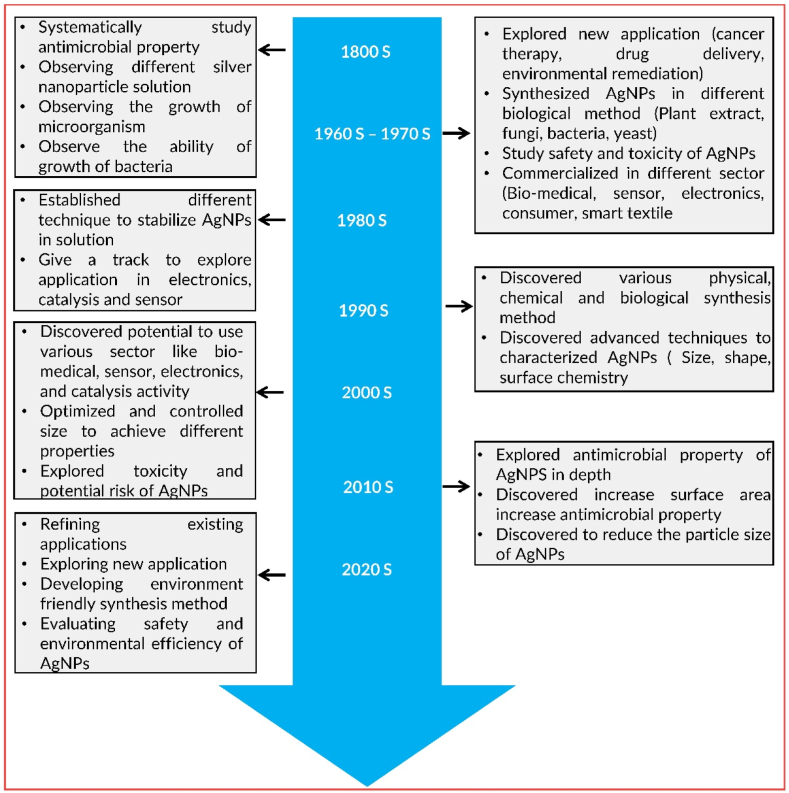
Fig. 1 provides a comprehensive timeline of the discovery and research journey of AgNPs. It showcases the progression from studying their properties to refining existing applications, exploring new uses, and assessing their environmental safety. This review aims to provide an in-depth analysis of the current synthesis, characterization, and applications of AgNPs. This comprehensive review offers valuable insights into various synthesis methods and an overview of the diverse applications of AgNPs across different fields.
Fig. 1.
Timeline for discovery and research history of AgNPs.
2. Synthesis of AgNPs
AgNPs are broadly synthesized using two distinct methods: the top-down and the bottom-up approach (Fig. 2). The top-down approach involves reducing bulk materials to fine particles in the nanoscale range. In contrast, the bottom-up approach resembles a self-assembly process where nanoparticles are formed from molecular precursors at the atomic level [38]. AgNPs can be synthesized through physical, chemical, and biological approaches [39]. Due to low productivity [40], physical methods, such as laser ablation and thermal evaporation/condensation, are environmentally friendly and scalable for industrial use. Chemical synthesis using water or an organic solvent with reducing and stabilizing agents is versatile and cost-effective, allowing control over nanoparticle properties. However, due to the use of solvents and reducing agents, it is less environmentally friendly [41]. The biological method, utilizing organisms like plants, algae, and microbes, is the most suitable as it is non-toxic, safe, and minimizes chemical usage, ensuring pollution-free AgNPs [42]. Fig. 2 provides an overview of the different synthesis approaches for AgNPs.
Fig. 2.
An overview of various approaches used in the synthesis of AgNPs.
2.1. Physical method for synthesis AgNPs
2.1.1. Synthesis of AgNPs using the ball milling method
AgNPs can be synthesized via ball milling, a physical technique involving high-energy collisions of a powder mixture with milling balls in a chamber containing a silver precursor, milling media, and stabilizing agent [43]. This method is highly promising as it deforms the bulk crystal with minimal chemical reagents, producing pure colloids or nanoparticles without byproducts or impurities. The high-energy ball milling introduces the elastic strain energy into the bulk crystal through ball-powder collisions, producing atomic vacancies, dislocations, and chemical disorders [44]. Advantages of this method include scalability for large-scale production, dry environment conductance, and simplicity without solvents. However, it can lead to nanoparticles with a random size range and potential contamination from milling media [45]. Monika et al. developed an efficient technique (Fig. 3 A-E) for creating antibacterial filters by milling Ag (I) salts without solvents, using a lignin-PAM support polymer and lignin as a reducing agent, resulting in well-incorporated AgNPs with particle sizes of 1–30 nm [46]. A. Pragatheeswaran et al. demonstrated microstructural and morphological changes in Fig. 3 F-H during ball milling of Copper-Silver-Graphite flake mixtures, showing a decrease in crystalline size with increasing milling time [47]. Takuya Tsuzuki explained mechanochemical processing to produce nanoparticles via a solid-state displacement reaction in Fig. 3 I-N. The repeated fracture and welding of raw materials during ball milling leads to the formation of a nanocomposite, triggering a chemical reaction to produce nanoparticles in a solid matrix. Post-milling heat treatment may finalize the reaction or control nanoparticle characteristics.
Fig. 3.
(A) Solvent-free mechanochemical synthesis of AgNP@TMPLig/PAM, Transmission electron microscopy (TEM) images of (B) AgNP@KLig, (C) AgNP@ KLig/PAM, (D) AgNP@TMPLig and (E) AgNP@TMPLig/PAM. Reproduced with permission. Copyright 2024, Royal Society (adapted) of Chemistry [46], (F) Scanning electron microscopy (SEM) image of AgNPs, Backscattered scanning electron microscopy (SEM) images of Cu-Ag powder milled for 5 h along with the energy dispersive spectroscopy (EDS) elemental distribution map, (G) After 40 h of milling(H). Reproduced (adapted) with permission. Copyright 2024, Elsevier. [47], (I) Dry raw reactant materials are placed in a milling container along with milling balls. (J) Repeated fracture and welding of the raw materials during ball milling form a nanocomposite of the reactants. (K) Mechanical energy input into the reactant nanocomposite induces a chemical reaction to produce nanoparticles in a solid matrix48 (reproduced with permission from Springer). (L) If necessary, post-milling heat treatment completes the chemical reaction or controls nanoparticle size, shape, or crystallinity. (M) The Soluble by-product matrix phase is removed. (N) Final nanoparticle products. Reproduced (adapted) with permission. Copyright 2024, Springer Nature [48].
2.1.2. Synthesis of AgNPs using laser ablation method
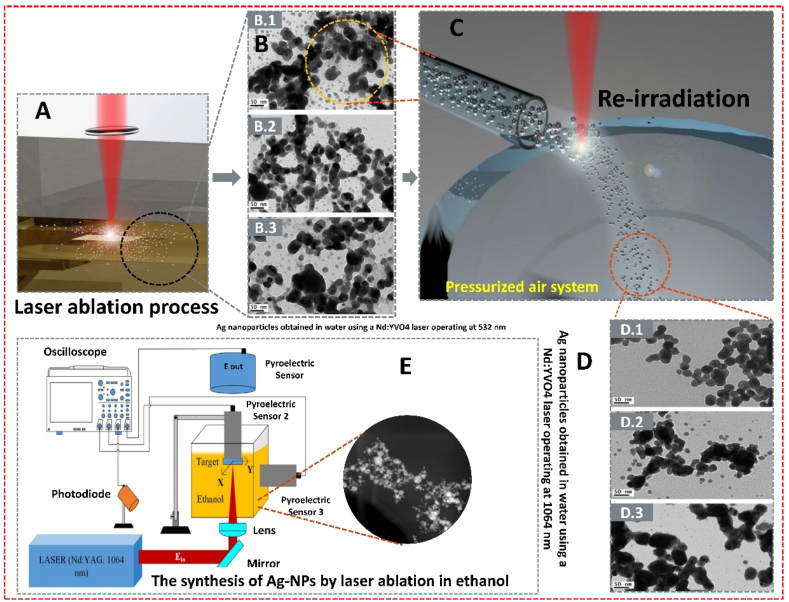
Laser ablation is a standard physical method for synthesizing AgNPs, known for its environmental friendliness as it requires no chemical reagents. This technique involves applying intense energy through pulse irradiation to a solid target, causing the material to be removed from its surface. The process generates a plasma plume that later transforms into nanoparticles [49]. Several factors affect the ablation rate, including transmission, liquid composition, light wavelength, fluence, pulse duration, repetition rate, and light absorption efficacy of the intended material. M. Fernández-Arias et al. demonstrated re-sizing AgNPs produced through laser ablation in an aqueous medium by subjecting them to re-irradiation. AgNPs were generated by ablating a silver target immersed in pure de-ionized water using 2 ns Nd: YVO4 lasers operating at 1064 and 532 nm, respectively (Fig. 4 A-D). A compressed air system injected the colloidal solution for further resizing through one and three additional rounds of irradiation with the same laser, resulting in uniformity and a 40 % reduction in average size [50]. M.A. Valverde-Alva et al. showed the laser ablation process for producing AgNPs (Fig. 4 E) in an ethanol medium using a pulsed Nd laser with a wavelength of 1064 nm and pulse duration of 7 ns (YAG laser) at an energy range of 10–100 mJ [51]. While laser ablation is an effective physical method, it requires high temperatures and substantial energy, making it less productive and economical [52].
Fig. 4.
(A) Laser ablation process (B) TEM image and size distribution of Ag nanoparticles obtained in water using a Nd: YVO4 laser operating at 532 nm. B.1) after laser ablation of Ag foil, B.2) after one re-irradiation, B.3) after 3 re-irradiations. (C) Pressurized air system scheme (D) TEM image and size distribution of Ag nanoparticles obtained in water using an Nd: YVO4 laser operating at 1064 nm. D.1) after laser ablation of Ag foil, D.2) after one re-irradiation, D.3) after 3 re-irradiations. Reproduced (adapted) with permission. Copyright 2024, Elsevier [50], (E) The synthesis of Ag-NPs by laser ablation in ethanol, as well as to perform PA and transmittance measurements. Reproduced (adapted) with permission. Copyright 2024, Elsevier [51].
2.1.3. Synthesis of AgNPs using gamma irradiation method
Gamma irradiation (60Co-gamma rays) is an eco-friendly method for synthesizing AgNPs [53]. Precise control of nanoparticle quantity, size, and shape is achieved by adjusting the irradiation dose. Stabilizers like Poly (N-vinylpyrrolidone) (PVP) [54], poly(N-vinylcarbazole) (PVK) [55], and polyaniline (PANI) [56] affect particle growth and promote the formation of various nanomaterials [57]. Gamma irradiation in water radiolysis generates solvated electrons, which interact with solvated metal ions, reducing them to Ag atoms and forming Ag nanoparticles [58]. This process also produces oxidizing and reducing species, including electrons and radicals like H• and OH• [59]. To mitigate OH• radicals, scavengers like secondary alcohols or formate anions (HCOO−) are introduced [60]. In Fig. 5 a-c, Islam Ali demonstrated the physicochemical characteristics of nanocomposites made of silver, polystyrene, and polyvinylpyrrolidone using different doses of γ-ray irradiation [61]. Li H et al. demonstrated the fabrication of AgNPs via gamma irradiation within metal-organic structure templates, with nanoparticles exhibiting strong surface plasmon resonance, high durability, and efficient catalytic properties in Fig. 5 e-m [62]. γ-irradiation-induced synthesis of AgNPs in aqueous poly(N-vinylpyrrolidone) solution (Fig. 5 n) has been shown to favor the formation of Ag nanoplates under specific conditions at pH 5, silver perchlorate concentration 3.0 × 10−2 M in the presence of 0.01 % PVP.
Fig. 5.
AgNPs nanoparticles TEM image of (a) 40 kGy, (b) 80 kGy and (c) 120 kGy irradiated silver/polystyrene/polyvinylpyrrolidone (Ag/PS/PVP) (1 × 10−1 M). Reproduced (adapted) with permission. Copyright 2024, Elsevier [61] (d) Schematic of Ag@HKUST-1 crystals preparation, (e) Rietveld refinement results: the red cycle point indicates the calculated peak position, the blue line indicates the HKUST-1 powder samples peak position; optical images of HKUST-1 crystals (f) HKUST-1 (g) 1 kGy Ag@HKUST-1. SEMs and EDX spectra of Ag@HKUST-1 crystals under different irradiation doses (h) HKUST-1 (i) 1 kGy (j) 10 kGy (k) 100 kGy (l) 200 kGy (m) EDX spectra. Reproduced (adapted) with permission. Copyright 2024, Royal Society of Chemistry [62] (n) γ-Radiation-Induced Synthesis of AgNPs in Aqueous Poly (N-vinylpyrrolidone) Solution. Reproduced (adapted) with permission. Copyright 2024, Elsevier [63].
2.1.4. Synthesis of AgNPs using evaporation condensation method
Evaporation-condensation is a highly efficient physical method for synthesizing AgNPs requiring an atmospheric pressure tube furnace or a small ceramic heater. The process involves three main steps: converting the material into a vapor phase, transporting it to the substrate, and forming particles or films through nucleation and growth. The vapor quickly cools, producing numerous small AgNPs [64]. This method requires precise power input and duration to maintain a consistent temperature. Radiation is used as a reducing agent instead of harmful chemicals, though the process is lengthy and energy-intensive. In one study, a mixture of AgNO3 and sodium acetate was heated in a tube furnace, converting it into gas, which then condensed into AgNPs [65]. Another study using inert gas helium (He) within the process chamber found that lower evaporation temperatures and gas pressure produced smaller, less agglomerated AgNPs, sized 9–32 nm [66]. In an independent experiment, a ceramic heater at 1500 °C produced polydisperse AgNPs, sized 6.2–21.5 nm, with a spherical shape and no clumping [67]. A furnace study at 1300–1400 °C, with vapor diluted by nitrogen (N2) gas, yielded AgNPs of 50, 90, and 130 nm at different temperatures. M. Raffi et al. synthesized AgNPs using an inert gas condensation method with flowing helium, producing 9–32 nm particles characterized by X-ray diffraction (XRD) and transmission electron microscopy (TEM). Juha Harra et al. demonstrated precise control over AgNP sizes, producing nanoparticles with 50, 90, and 130 nm diameters [68]. Table 1 summarizes the different physical methods used for synthesizing AgNPs.
Table 1.
Summary of different physical synthesis methods used in synthesized AgNPs.
| Physical Method | Summary | Nanoparticle Size (nm), Shape | Ref. |
|---|---|---|---|
| Ball Milling |
|
10,50 Round, Spherical |
[69] |
|
6–8 Spherical |
[70] | |
|
1, 30 Spherical |
[71] | |
|
15 Spherical |
[72] | |
|
9 Spherical |
[73] | |
|
9–15 Spherical |
[74] | |
|
20–25, 48–51 Spherical |
[75] | |
|
10 Spherical |
[76] | |
|
10 Spherical |
[77] | |
|
1–3 Spherical |
[78] | |
| Gamma Irradiation |
|
4.3, 6.1, 7.6, 10.2 | [79] |
|
25 Spherical |
[80] | |
|
1.4–3 Spherical |
[81] | |
|
15 ± 3 Spherical |
[82] | |
|
80, 100 Spherical |
[83] | |
|
15–45 Spherical |
[84] | |
|
– | [85] | |
| Evaporation Condensation |
|
6.2–21.5; 1.231, 88 Spherical |
[67] |
|
9–32 Spherical |
[66] | |
|
3–50 Spherical |
[65] | |
|
70, 150, 220 | [68] |
2.2. Chemical method for the synthesis of AgNPs
2.2.1. Chemical reduction method for synthesis AgNPs
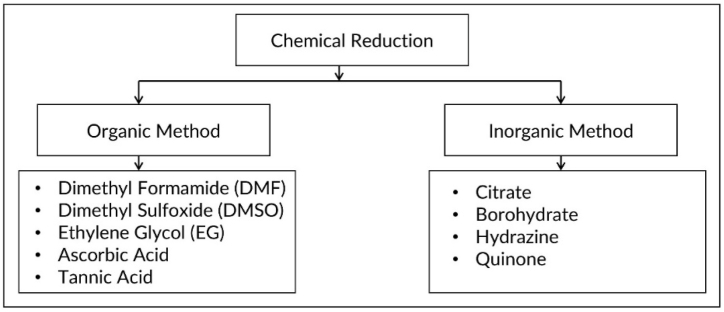
The typical synthesis of AgNPs involves chemical techniques using three types of precursors: (i) reducing agents such as metal borohydrides and metal salts, (ii) stabilizers, and (iii) surfactants such as organic acids [86]. The synthesis process is carried out in an aqueous solution at elevated temperatures using silver salts such as silver nitrate, silver acetate, and silver sulfate, where organic solvents enhance the reductants' ability to dissolve in water. The precursor selection significantly impacts the characteristics of the AgNPs, allowing customization of their shape and sizes. AgNPs are usually synthesized from AgNO3 salt using reducing agents like sodium citrate and sodium borohydride [87,88] and capping agents such as polyvinyl alcohol [89], trisodium citrate [90], and sodium borohydride [91]. Typical solvents include water, ethanol, dimethylformamide, amine, and ethylene glycol [92]. Fig. 6 illustrates the organic and inorganic methods in the chemical reduction process for AgNP production. Fig. 7 illustrates the general reaction scheme for the synthesis of AgNPs. Table 2 summarizes the synthesis of AgNPs synthesis via chemical reduction.
Fig. 6.
Chemical reducing agent to synthesize AgNPs.
Fig. 7.
General reaction scheme for synthesis of AgNPs.
Table 2.
Chemical reduction method for synthesizing AgNPs: a brief overview.
| Solvent | Size(nm) | Reducing Agent | Capping Agent | Ref. |
|---|---|---|---|---|
| Water | 35 | Ascorbic acid | Cetyltrimethylammonium bromide | [93] |
| Water | – | ã- irradiation | Polyvinyl pyrrolidone (PVP) | [90] |
| Water | – | Sonochemical irradiation | Fluff pulp | [94] |
| Water/Chloroform | – | Hexadecyl amine | [95] | |
| Ethanol/Toluene | 7 | Trisodium Citrate + Dodecyl amine | [96] | |
| 50 | Bacillus licheniformis | Bacillus licheniformis | [97] | |
| 15 | Sodium carboxymethyl cellulose | Sodium carboxymethyl cellulose | [98] | |
| Water/Cyclohexane | 4 | Dodecyl amin& Formaldehyde | Dodecyl amin& Formaldehyde | [99] |
| Octadecyl amine | 4.7 | Octadecyl amine | Cetyltrimethylammonium bromide | [100] |
| Ethanol | 15 | Polyvinylpyrrolidone (PVP)with HAS-7% | [101] | |
| Water | 12.28–38.45 | Glucose | Polyvinyl Alcohol | [102] |
| Water | – | Sodium borohydride | Sodium Citrate | [103] |
| Water | 13–15 | Sodium borohydride, Sodium hypophosphite, Sodium hexametaphosphate |
Trisodium citrate dihydrate | [104] |
| Water | 20–60 | Ethylene glycol | Polyvinylpyrrolidone | [105] |
| Water | 5–25 | Trisodium citrate | [106] | |
| Water | 14.6 | Alkali lignin (low sulfonate) | Alkali lignin (low sulfonate) | [107] |
| Water | 4–16 | Sodium borohydride | Polyvinylpyrrolidone | [108] |
2.2.2. Spray pyrolysis method for synthesis AgNPs
Spray pyrolysis (SP) is a versatile process for synthesizing thin films or powder particles with various topologies. It allows for the formation of a metastable phase that controls the morphology of nanomaterials, unaffected by thermodynamics [109]. SP-produced powders retain the stoichiometric ratio of the precursor solution without impurities or by-products. Thus, this method can produce mixed metal oxides, metal powders, composite particles, and semiconductor thin films. Table 3 and Fig. 8 represent the three SP methods and their applications in creating different nanostructures.
Table 3.
Features of the three spray pyrolysis methods and how they can be used to make different nanostructures.
| SP Techniques | Advantages | Disadvantages | Nano-Structures | Ref. |
|---|---|---|---|---|
| Spray Pyrolysis |
|
|
|
[[109], [110], [111]] |
| Spray Pyrolysis Deposition |
|
|
|
[[112], [113], [114]] |
| Flame Spray Pyrolysis |
|
|
|
[112,113] |
Fig. 8.
Schematic diagram of (A) Flame spray pyrolysis (B) Spray pyrolysis (C) Spray pyrolysis deposition. Reproduced (adapted) with permission. Copyright 2024, Elsevier [115].
Besides spray pyrolysis, the ultrasonic spray pyrolysis (USP) technique is promising for synthesizing pure, spherical, and fine nanoparticles in a one-step process. It can synthesize metal/metal oxides with precise control over chemical composition, particle size, and morphology by manipulating solution concentration, temperature, gas-flow rate, and ultrasound [[116], [117], [118], [119]]. The experimental setup comprised several components, including an atomizer, a heating section consisting of a mullite tube and two electric furnaces (300 °C for solvent evaporation and 850 °C for pyrolysis), a test-tube filter for powder collection, and a controller. A droplet introduction method involved the utilization of an aspirator, which operated at a suction rate of 1.5 dm3 min−1, to introduce droplets into the heating section [120]. The process involves generating droplets with ultrasonic irradiation, removing the solvent, precipitating microcrystals, and growing the precipitates. Fig. 9 depicts a representation of the ultrasonic spray-pyrolysis equipment, and the production yields of specific aerosol pathways for particle formation USP can produce a composite powder consisting of hydroxyapatite (HAp) and AgNPs [121]. This simple, inexpensive method allows controllable, reproducible particle sizes. Controlling and defining nanoparticles' dimensions, distribution, shape, and chemical makeup is essential for achieving AgNPs with specific characteristics. The USP technique can theoretically calculate the mean diameter of NPs with a spherical shape. However, it cannot provide information about the particle-size distribution [122]. Table 4 illustrates the synthesis of silver nanoparticles (AgNPs) using the ultrasonic spray-pyrolysis (USP) method.
Fig. 9.
Schematic diagram of ultrasonic spray-pyrolysis apparatus. Reproduced (adapted) with permission. Copyright 2024, Elsevier [115].
Table 4.
Size and shape of synthesized AgNPs by ultrasonic spray-pyrolysis (USP) method.
| Particle Size of AgNPs (nm) | Nanoparticle Shape | Ref. |
|---|---|---|
| Size can be regulated | – | [123] |
| 20–80 | Spherical, Cylindrical | [124] |
| 20–40 | Spherical | [117] |
| Depending on the AgNO3 Concentration | Spherical Metallic | [125] |
| 1–2 | – | [126] |
| 18.9–24.6 | Spherical | [127] |
| 40–80 | – | [128] |
| 95-94 (400 °C) 124–119 (600 °C) 328–79 (800 °C) | Solid Spherical, Nano-Convex | [129] |
| 12–32 | Spherical | [130] |
| 200–300 | Spherical | [131] |
2.2.3. Sol-gel method for synthesis of AgNPs
The sol-gel method is widely used in many technical and technological fields as a bottom-up nanoparticle synthesis approach. It is especially prominent in the fabrication of metal oxide nanostructured materials. The primary objective of employing this technique is to attain meticulous regulation over the shape and size of nanoparticles [132]. Sol-gel technology presents numerous inherent benefits in comparison to traditional methods. These advantages include utilizing low-temperature synthesis, which improves multicomponent systems' homogeneity. Additionally, sol-gel processes require less chemical usage and are insensitive to atmospheric conditions, ensuring the durability of properties. Moreover, sol-gel techniques offer high yield while reducing machinery and production costs. Lastly, this technology has the advantage of minimizing the adverse environmental effects. The utilization of technology offers a promising avenue for exerting efficient control over the physicochemical parameters of synthesized products by manipulating the kinetic and mechanism of chemical processes and precisely adjusting the technological conditions at various stages of the ongoing reactions within the system [133]. The unique properties exhibited by sols and gels enable the production of diverse fibers and thin-film coatings through various techniques. Spinning (spin coating) and dip-coating are common in research and industry [134]. Spin coating involves depositing a liquid onto a spinning substrate. The sol-gel method uses a homogeneous solution from dissolving a precursor in a solvent, such as H2O or an organic solvent. This stage is crucial, regardless of whether the initial precursors are inorganic salts or different metal alkoxides [135]. The sol forms a gel, which can be made from metal alkoxides or chlorides, producing particles or polymers. This gel can create materials like nanoparticles (NPs), xerogel, glass, or ceramics. The sol-gel process provides a high degree of precision in manipulating the phase, shapes, and sizes of the materials it generates [136]. Sol-gel allows precise control over material properties through hydrolysis and polycondensation of precursors like tetraethoxysilane, mercaptopropyltrimethoxysilane, and polymethyl hydrosiloxane [[137], [138], [139]]. These compounds have been found to exhibit properties that make them suitable for use as framework constructors, complexing agents for metal ions, and in situ reducing agents for Ag+ [140]. Table 5 summarizes the synthesis of AgNPs using the sol-gel technique.
Table 5.
Summary of AgNPs synthesis by sol-gel technique.
| Agent Use | Nanoparticle Size (nm) | Nanoparticle Shape | Ref. |
|---|---|---|---|
| Reducing Agent-N2H4.H2O Stabilizing Agent-CH3COONa |
6,9,22 | Spherical | [141] |
| Tetraethyl orthosilicate (TEOS) + CH3CH2OH | 8–25 | Spherical | [142] |
| Precursor-AgNO3, Organosiloxane | 10–100 | Spherical | [143] |
| Precursor-AgNO3, CH3CH2OH | 20–100 | – | [144] |
| Precursor-AgNO3 Reducing Agent: Citric Acid, Ascorbic Acid |
34–64 | Spherical | [145] |
| Co-precursor: Polymethylhydrosiloxane (PMHS), Tetraethyl orthosilicate (TEOS), 3-mercaptopropyltrimethoxysilane (MPTMPS) | 3.2 | – | [140] |
| Tetraethyl orthosilicate (TEOS), triethoxy(octyl)silane (TEOCS) | – | – | [146] |
| Precursor-Tetraethyl orthosilicate (TEOS), CH3CH2OH, AgNO3 | 18–20 | – | [147] |
| Precursor-Tetraethyl orthosilicate (TEOS), CH3CH2OH | 50–100 | – | [148] |
2.2.4. Hydrothermal method for synthesis of AgNPs
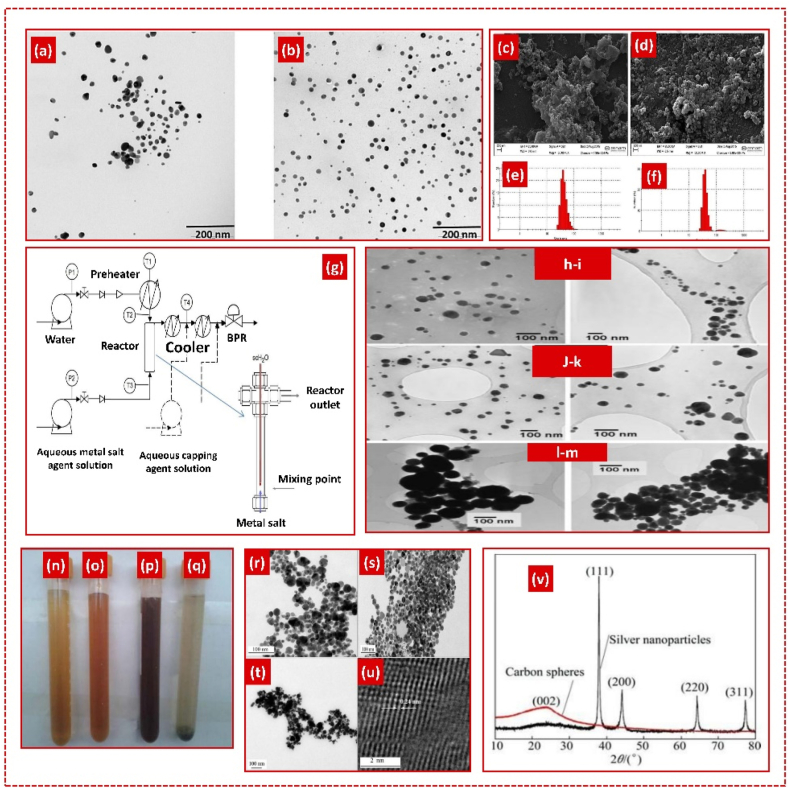
Hydrothermal synthesis involves chemical reactions within a solution, producing materials across a wide temperature range by controlling pressure based on the vapor pressure of the reactants [149]. It is known for generating nanomaterials with high vapor pressure and minimal material loss. Ismail Ocsoy et al. synthesized AgNPs (Fig. 10 a-f) with the highest inhibitory activity in E. coli (at 9.37 ppm), S. aureus (at 4.69 ppm), and C. albicans (at 4.69 ppm). The particle size decreased from 100 nm to 65 nm with increased incubation time. Combining green synthesis with hydrothermal approaches can enhance the antimicrobial activity of AgNPs against bacterial and fungal pathogens [150]. Gabriele Aksomaityte et al. used a hydrothermal reactor to continuously generate AgNPs, finding that temperature-controlled particle size, with TEM studies showing 30–40 nm (Fig. 10 g-m) average sizes at 415 °C. Incorporating polyvinyl pyrrolidone (PVP) decreased the particle size distribution. Increasing the temperature from 380 °C to 430 °C reduced particle size, while lower flow rates at 380 °C resulted in larger particles [151]. Ying-fen LI et al. produced well-dispersed quasispherical AgNPs using the hydrothermal method with Arabic gum, achieving optimal results at 160 °C with 10 mmol/L AgNO3, m(Ag)/m(AgNO3) = 1:1 (Fig. 10 n-v) [152]. Table 6 summarizes the synthesis of AgNPs using the hydrothermal method.
Fig. 10.
Scanning transmission electron microscopy (STEM) images of AgNPs where the volume of extracts are (a) 1 mL, (b) 5 mL, Scanning electron microscope (SEM) images and dynamic size measurements of AgNPs formed at 50 °C and 150 °C. c) SEM image at 50 °C, d) SEM image at 150 °C, e) dynamic size at 50 °C and f) dynamic size at 150 °C. Reproduced (adapted) with permission. Copyright 2024, Elsevier [150], (g) Continuous hydrothermal flow apparatus for synthesis of AgNPs, TEM images of AgNPs which are stabilized at (h) 0.0025M and (i) 0.01M silver acetate at 380 °C in PVP, (j) 0.0025M at 415 °C, (k) 0.03M at 430 °C, (l) 0.01M silver acetate at 380 °C without PVP, (m) 0.01M silver acetate at 415 °C without PVP. Reproduced (adapted) with permission. Copyright 2024, Elsevier [151], Solutions of AgNPs at different temperatures (c(Ag+) = 10 mmol/L, R = 1:1, t = 3 h): (n) 120 °C; (o) 140 °C; (p) 160 °C; (q) 180 °C, TEM images of AgNPs at different temperatures (c(Ag+) = 10 mmol/L, R = 1:1, t = 3 h): (r) 140 °C; (s) 160 °C; (t) 180 °C; (u) High-resolution transmission electron microscopy (HRTEM) image of AgNPs shown in (b) (v) XRD patterns of AgNPs and carbon spheres. Reproduced (adapted) with permission. Copyright 2024, Elsevier [152].
Table 6.
Summary of AgNPs synthesis by hydrothermal method.
| Material | Solvent | Temperature (°C) | Size (nm) | Shape | Ref. |
|---|---|---|---|---|---|
| H2O | 400 | Spherical | [151] | ||
| Supercritical methanol (scMeOH) | 400 | 30 | Spherical | [153] | |
| Supercritical ethanol (EtOH) | 400 | 320 | Spherical | [154] | |
| CH3COOAg/PVP | 380 3 −430 °C/23 MPa | 5–70 | Spherical | [151] | |
| AgNO3 and NaAlg | 100 | 15 | Spherical | [155] | |
| Chitosan and AgNO3 | 180 | 17–20 | Spherical | [156] | |
| Arabic gum aqueous solutions | 140–180 | 18–24 | Quasispherical | [152] | |
| AgNO3 and PQ11 | 100 | 5–45 | Spherical | [157] | |
| AgNO3 | 100 | 65–80 | Spherical | [150] |
2.3. Biological method for synthesis of AgNPs
2.3.1. Green synthesis of AgNPs
2.3.1.1. Microbiologically synthesized AgNPs
2.3.1.1.1. Green synthesis of Ag-NPs using bacteria
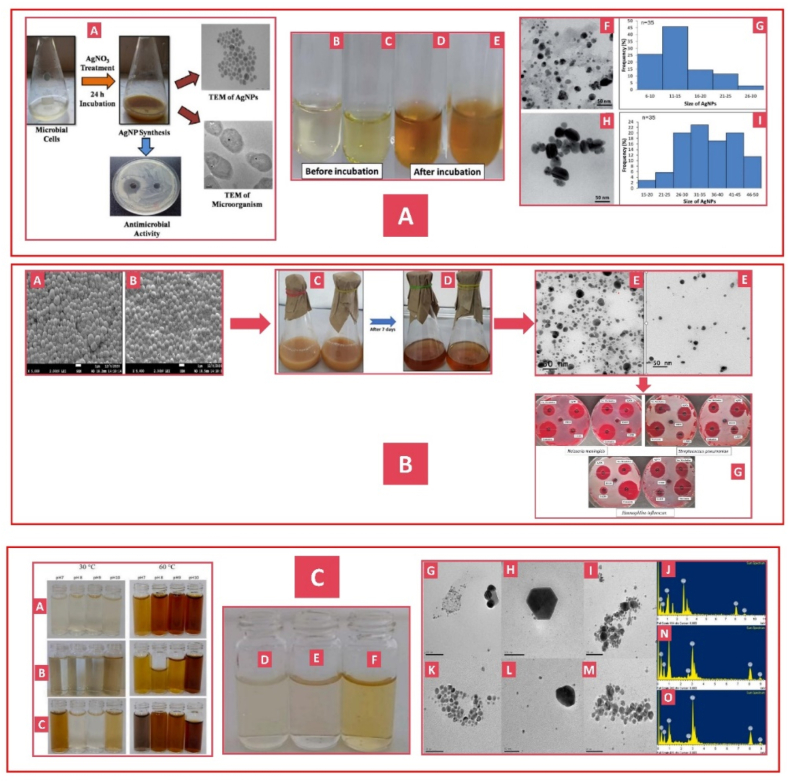
Microorganisms can convert metallic ions into nanoparticles, making them ideal for nanoparticle synthesis due to their easy handling and rapid growth. Bacteria can undergo genetic manipulation to aid biomineralization of metal ions and have developed defense mechanisms, including intracellular sequestration, efflux pumps, alterations in metal ion levels, and extracellular precipitation to thrive in environments with heavy metal ions [158]. The bacteria can effectively employ these defense systems to produce nanoparticles for many applications [159]. Using Rhodococcus spp., S.V. Otari demonstrated the antibacterial properties of AgNPs with sizes ranging from 5 to 50 nm, showing bactericidal and bacteriostatic efficacy against harmful microorganisms (Fig. 11 A, A) [160]. In a recent study, A'liyatur Rosyidah et al. demonstrated the remarkable ability of Streptomyces chiangmaiensis SSUT88A to facilitate the eco-friendly production of AgNPs with TEM analysis revealing spherical NPs with an average diameter of 13.57 nm for IS-AgNPs) 30.47 nm for ES-AgNPs. No antimicrobial activity was observed in the RS-AgNPs (Fig. 11 A, B-I) [161]. The antimicrobial activity of the synthesized AgNPs against Mycobacterium proteolytic LA2 and Streptomyces rochei LA2 (O) was highly intense, as demonstrated by Naushin Bano et al. Through TEM analysis, the average size of secondary metabolite-mediated AgNPs isolated by LA2(R) and LA2(O) was 27 ± 1 and 29 ± 2 nm, respectively. These nanoparticles demonstrated significant efficacy in combating bacterial pathogens, reducing S. pneumoniae, H. influenza, and N. meningitidis by 73.14 %, 71.89 %, and 64.81 %, respectively (Fig. 11 B, A-G) [162]. Ivan ´ Solís-Sandí demonstrated the efficient production of AgNPs through bacterial extracts, highlighting their powerful antimicrobial properties. AgNPs typically range in size from 20.8 to 118.4 nm, exhibiting a spherical shape. The antimicrobial activity of AgNPs synthesized using supernatant is exceptionally high (Fig. 11C, A-O) [163]. Fig. 12 illustrates the green synthesis of AgNPs from various bacterial species, and Table 7 summarizes the synthesized AgNPs from bacteria.
Fig. 11.
Green synthesis of AgNPs from bacteria. Reproduced (adapted) with permission. Copyright 2024, Elsevier. [159], Reproduced (adapted) with permission. Copyright 2024, Royal Society of Chemistry [160], Reproduced (adapted) with permission. Copyright 2024, Springer Nature [161], Reproduced (adapted) with permission. Copyright 2024, Elsevier [162].
Fig. 12.
Green synthesis of AgNPs from fungi. Reproduced with permission. Copyright 2024, Springer Nature. [164], Reproduced with permission. Copyright 2024, Springer Nature [165], Reproduced (adapted) with permission. Copyright 2024, Elsevier [166], Reproduced (adapted) with permission. Copyright 2024, Springer Nature [167].
Table 7.
Summary of green synthesis of silver nanoparticles using bacteria.
| Bacteria | Precursor | Size (nm) | Shape | Ref. |
|---|---|---|---|---|
| Streptomyces laurentii | AgNO3 | 7 to 15 | Spherical | [168] |
| Leuconostoc lactis | 35 | Spherical | [158] | |
| Novosphingobium sp. HG-C3 | 8–25 | Spherical and crystalline | [169] | |
| Kinneretia THG-SQ14 | 15–20 | Mono-disperse, FCC, spherical | [170] | |
| Rhodococcus spp. | 5–50 | Spherical | [160] | |
| Endosymbiotic Bacterium | 10–60 | Cubic, spherical, hexagonal, crystalline, oval | [171] | |
| Aeromonas sp. THG-FG1.2 | 8–16 | Face Centered Cubic (FCC), spherical | [172] | |
| Bacillus strain CS 11 | 45 ± 0.15 | FCC, spherical | [173] |
2.3.1.1.2. Green synthesis of Ag-NPs using fungi
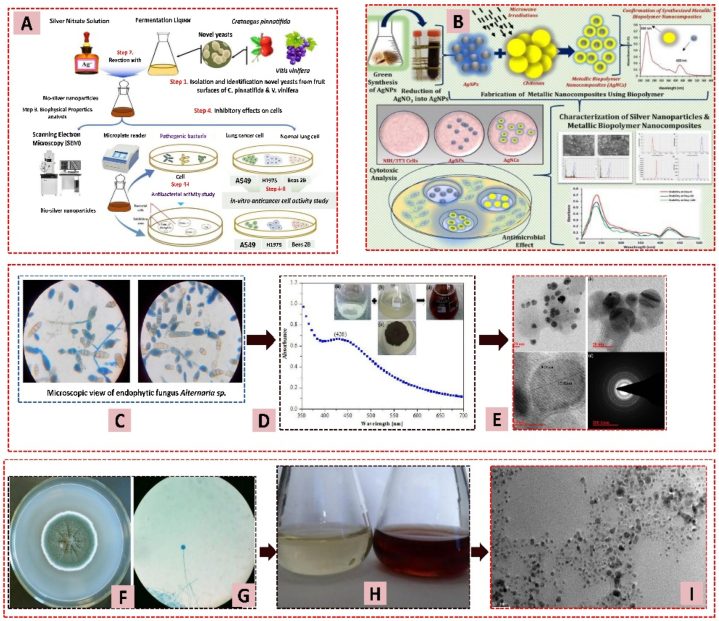
Fungi can synthesize metal nanoparticles due to their ability to secrete large amounts of enzymes and their capacity to accumulate and tolerate metals. Additionally, enzymes from fungi reduce AgNO3 and synthesize nanoparticles [164]. All these make fungi more manageable in research compared to bacteria. Xin Liu et al. used yeast strains HX-YS and LPP-12Y to create AgNPs with antibacterial and anticancer properties, showing minimal toxicity to normal lung cells (Fig. 12 A) [165]. Wang et al. demonstrated the use of Aspergillus sydowii to synthesize polydisperse spherical AgNPs ranging in size from 1 to 24 nm with antifungal and antiproliferative properties. The researchers optimized three key synthesis parameters for this process: temperature: 50 °C, pH: 8.0, and substrate concentration: 1.5 mM (Fig. 12 B) [166]. Tej Singh et al. used an endophytic fungal supernatant to produce antibacterial AgNPs from Alternaria sp., with particles ranging from 4 to 30 nm (Fig. 12C–E). There is hope for using AgNPs as an effective antibacterial agent because they poison human pathogenic bacteria [167]. Sadaf Raza et al. utilized Aspergillus fumigatus, a fungal biomass, to create AgNPs and coated them with chitosan, resulting in nanoparticles with antibacterial solid properties (Fig. 12F–I) [174]. Fig. 12 shows the green synthesis of AgNPs from different types of fungi, along with their particle size and antimicrobial activity. Table 8 shows the green synthesis of AgNPs from fungi using AgNO3 as precursor.
Table 8.
Summary of green synthesis of AgNPs using fungi.
| Fungi | Precursor | Size (nm) | Shape | Ref. |
|---|---|---|---|---|
| Humicola sp. | AgNO3 | 5–25 | Spherical | [175] |
| Trichoderma reesei | 5–50 | – | [176] | |
| Aspergillus fumigatus | 5–25 | Spherical and triangular | [177] | |
| Aspergillus flavus | 8.92 ± 1.61 | – | [178] | |
| Fusarium semitectum | 10–60 | Spherical | [179] | |
| Alternaria alternata | 32.5 | Spherical | [180] | |
| Rhizopus stolonifer | 3, 20 | Spherical | [181] | |
| Phanerochaete chrysosporium | 34–90 | Spherical | [166] |
2.3.1.1.3. Use of plants or plant extracts to synthesize AgNPs
After cleansing with distilled water, various plant components (leaves, roots, fruits, flowers, and seeds) are utilized to create AgNPs (Fig. 13) [[182], [183], [184]]. The plant extract contains biomaterials that serve as both a reducing agent and stabilizing agent, eliminating the need for any chemical stabilizers [[185], [186], [187], [188], [189], [190]]. Table 9 represents the synthesis of AgNPs using different plants. Francisco Rodríguez-Felix et al. conducted a sustainable-green synthesis of AgNPs using safflower waste extract from Carthamus tinctorius L. (Fig. 14 A-B). The reaction occurred in the dark for 12 h. TEM and SEM analyses showed spherical AgNPs with an average diameter of 8.67 ± 4.7 nm [191]. Md. Mahidul et al. synthesized AgNPs using Phyllanthus emblica fruit extract (Fig. 14C), resulting in spherical particles ranging from 19.8 to 92.8 nm, averaging 39 nm. At 20 μg/mL, these nanoparticles significantly reduced Acidovorax oryzae strain RS-2 growth by 62.41 % (Fig. 14 D) [192]. Khursheed Ali et al. used Eucalyptus globulus leaf extract with microwave acceleration to synthesize AgNPs (Fig. 14 E). The ELE and AgNO3 solutions, combined in a 1:4 ratio and irradiated at 2450 MHz for 30 s, produced nanoparticles ranging from 1.9 to 4.3 nm (with microwave) and 5–25 nm (without microwave) [193]. Tanmoy Dutta et al. produced AgNPs using Citrus limetta peel extract (Fig. 14 F), yielding nanoparticles with an average size of 18 nm, stable for 120 days [194]. Paulkumar Kanniah et al. synthesized cytotoxic and antibacterial AgNPs using Piper nigrum seed extract. TEM analysis showed spherical AgNPs between 15 and 38 nm, which exhibited significant antibacterial properties against both Escherichia coli and Bacillus subtilis and cytotoxic effects on perilous human cancer cell lines, including MDA-MB-231, PANC-1, SKOV-3, PC-3, and HeLa [195].
Fig. 13.
Green synthesis of AgNPs.
Table 9.
Summary of synthesis of AgNPs using plant.
| Plants/plant extracts | Precursor | Part | Size (nm) | Structure/shape | Ref. |
|---|---|---|---|---|---|
| Conocarpus Lancifolius | AgNO3 | Fruit extract | 5, 30 | Spherical | [196] |
| Phoenix dactylifera | AgNO3 | Seed extract | 28.72 | Spherical | [197] |
| Theobroma cacao linneu | AgNO3 | Aqueous extract of the leaves | 10.3 ± 1.7 | Quasi-spherical | [198] |
| Couroupita guianensis | AgNO3 | Flower petal extract | 34 | Spherical | [199] |
| Echinophora platyloba | AgNO3 | – | 15 ± 3, 52 ± 4 | Spherical | [200] |
| Potato, Garlic, White onion, Radish, Red pepper, Orange, Apple | AgNO3 | Fruit, Peel, Peel, Fruit, Fruit, Fruit extract | 9, 11, 10, 15, 19, 25, 30 | – | [201] |
| Commelina forskaolii | AgNO3 | Plant extract | 18–27 | Spherical | [202] |
| Carrot extract | AgNO3 | Fruit extract | 22, 17, 10 | Spherical | [188] |
| Solanum melongena | AgNO3 | Aqueous leaf extracts | 75.14 | Spherical | [203] |
| Argyreia nervosa | AgNO3 | Leaf extracts | 1–10 | Spherical | [204] |
| Camellia sinensis | Two silver rods with a purity of 99.9 % | Leaves Extract | 8–26 | Spherical | [205] |
| Nicotiana tabacum | AgNO3 | Leaves Extract | 45.81, 55.43, 61.1 | Spherical | [206] |
| Bixa orellana | AgNO3 | Seed extract | 20–40 | Quasi-spherical | [187] |
| Syzygium aromaticum | AgNO3 | Clove flower buds | 7–15 | Spherical | [207] |
| Aqueous curcumin | AgNO3 | Root extract | 30.99–68.20 | Spherical | [185] |
| Hibiscus cannabinus | AgNO3 | Seed extract | 15.06 | – | [208] |
| Cordia sebestena | AgNO3 | Leaves Extract | 20–50 | Spherical | [209] |
| Acacia ehrenbergiana | AgNO3 | Cortex Extract | 1–40 | Spherical | [210] |
| Capparis spinosa | AgNO3 | Fruit extract | 25–30 | Spherical | [186] |
| Crataegus microphylla | AgNO3 | Fruit extract | 30–50 | Spherical | [211] |
| Gomphrena globosa | AgNO3 | Leaves Extract | 15.64, 19.44, 22.16 | Spherical | [212] |
| Gymnema sylvestre | AgNO3 | Leaves Extract | 20–30 | Spherical | [213] |
| Citrus medica, Tagetes lemmonii, Tarenna asiatica | Leaves Extract | 60–350, 40–220, 30–120 | Rod, Spherical, Spherical | [214] | |
| Helicteres isora | AgNO3 | Root extract | 30–40 | Crystalline and spherical | [215] |
| Banana | AgNO3 | Peel extract | 23.7 | Crystalline and spherical | [216] |
| Calliandra haematocephala | AgNO3 | Leaves extract | 70 | Spherical and FCC | [217] |
| Calotropis procera | AgNO3 | Leaves extract | 29–46 | Spherical-to-cubic shape | [218] |
| Securidaca inappendiculata | AgNO3 | Steam extract | 10–15 | Spherical | [219] |
Fig. 14.
Green synthesis of AgNPs from plant extracts. Reproduced (adapted) with permission. Copyright 2024, Elsevier [219], Reproduced (adapted) with permission. Copyright 2024, Frontiers [220], Reproduced (adapted) with permission. Copyright 2024, PLOS ONE [221], Reproduced (adapted) with permission. Copyright 2024, Elsevier [222], Reproduced (adapted) with permission. Copyright 2024, Elsevier [223].
2.4. Silver nanoparticle synthesis using the photochemical method
Utilizing plants or plant extracts to create AgNP, photochemical synthesis presents a favorable method for producing AgNPs specifically for textiles. This process uses light sources such as UV irradiation or visible light to reduce Ag+ to AgNPs, eliminating the need for harsh chemicals or toxic reducing agents and supporting sustainable textile processing [224,225]. It allows precise control over reaction parameters such as light source, wavelength, and irradiation time, tailoring AgNPs for specific properties like optimized antimicrobial activity and natural appearance [226]. Additionally, photochemical synthesis is scalable, enabling the production of large quantities of AgNPs for industrial use. Photochemical synthesis begins with a photosensitizer molecule absorbing light of a specific wavelength, exciting it to a higher energy state [227]. In the excited state, the photosensitizer can transfer an electron to a silver ion (Ag+), reducing them to neutral silver atoms (Ag⁰). The silver atoms act as nucleation sites, attracting more silver ions that are reduced by electrons from the photosensitizer or a semiconductor's conduction band, leading to the growth of AgNPs. Stabilizing agents prevent excessive agglomeration by creating a repulsive force on the AgNP surface. Factors affecting this process include light wavelength, silver ion concentration, photosensitizer, stabilizing agents, and reaction time [228]. Photochemical synthesis is environmentally friendly, avoids harsh chemicals, and offers precise control and scalability for large-scale production. Challenges include selecting suitable photosensitizers, optimizing reaction conditions, and integrating the process with textile substrates for practical. Table 10 summarizes silver nanoparticle synthesis using a photochemical method.
Table 10.
Summary of photochemical synthesis of AgNPs.
| Nanoparticle | Irradiation Source, Wavelength, and Power | Exposure Time | Size (nm) | Shape | Ref. |
|---|---|---|---|---|---|
| AgNPs | UV-LED (365 nm) | 15 min | 30 | Spherical | [229] |
| AgNPs | UV Light 0.362 mW/cm2 | 3 h | 60, 100 | Nano prisms, Pentagons | [230] |
| AgNPs | UV reactor (UV-A, 6 W) | 24 h | 19 | – | [231] |
| AgNPs | UV light (λ = 365 nm) | 10 min | 11.7 ± 7.2 | Cubes, rods, and spheres | [232] |
| AgNPs | 30 W UV-C bactericidal lamp | 45 min | 50 | Spherical | [233] |
| Ag/Au/AgCl | UVC light 4.26 mW/cm2 | 50 min | 96 | Nanorod, Spherical | [234] |
| ZnO/Ag | UVC light with a wavelength of 254 nm | 30 min | 100 | Hexagonal nanorods | [235] |
| AgNPs | Ultraviolet–visible light (EXFO Acticure A4000, 85 mW/cm2) | 1 h | 3.3 ± 0.7 | Spherical | [236] |
| AgNPs | 365 nm LEDs (LZ4-V4UV0R, Mouser) | – | – | – | [237] |
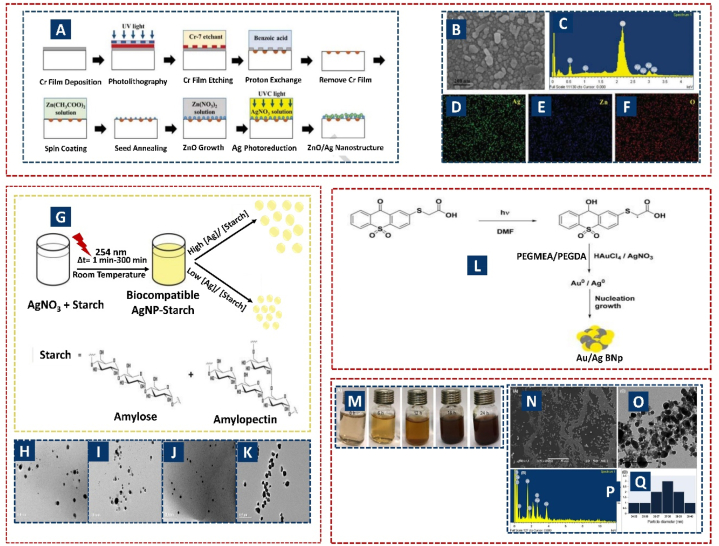
Wang et al. demonstrated the photochemical production of a ZnO/Ag heterogeneous nanostructure in Fig. 15 A-F on ferroelectric crystals for high-performance surface-enhanced raman spectroscopy (SERS) detection under UVC illumination, leveraging electrostatic fields for silver photo-reduction. The resulting SERS substrate exhibited superior detection limit [235]. Elif Ozcelik Kazancioglu and co-authors produced bimetallic Au/Ag NPs within a polymer matrix, achieving adjustable absorption characteristics and enhanced photocatalytic efficiency for methylene blue decomposition (Fig. 15G–K) [236]. Michele Avila dos Santos et al. facilitated AgNP production using potato starch, showing nearly spherical AgNPs formed under UV irradiation, with sizes ranging from 8 to 13 nm (Fig. 15 L) [238]. Smitha Chandrasekharan et al. used Gmelina arborea logging residue to sustainably produce AgNPs with antibacterial properties (Fig. 15M–Q). GC-MS analysis of plant parts identified bioactive chemicals optimal for AgNP phyto-synthesis, resulting in stable, spherical, crystalline GA-AgNPs with 34–40 nm diameters [239].
Fig. 15.
Photochemical synthesis of AgNPs. Reproduced (adapted) with permission. Copyright 2024, Elsevier [229], Reproduced (adapted) with permission. Copyright 2024, Springer Nature [230], Reproduced (adapted) with permission. Copyright 2024, Elsevier [232], Reproduced (adapted) with permission. Copyright 2024, Springer Nature [233].
2.5. Advantages and disadvantages of different methods for AgNP synthesis
Table 11 provides a comprehensive overview of the advantages and disadvantages of various synthesizing methods. Each synthesis technique has unique benefits and limitations that make it suitable for specific applications. Understanding these factors is crucial for selecting the appropriate method based on the desired properties and applications of AgNPs.
Table 11.
Summary of advantages and disadvantages of different synthesized methods of AgNPs.
| Method | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|
| Physical approach | Conventional ball milling |
|
|
[240,241] |
| Laser ablation |
|
|
[242,243] | |
| Electrospinning |
|
|
[244,245] | |
| Chemical Approach | Pyrolysis |
|
|
[246,247] |
| Sol-gel process |
|
|
[248] | |
| Hydrothermal |
|
|
[247] | |
| Biological Approach | Bacteriogenic synthesis |
|
|
[248,249] |
| Fungi-mediated synthesis |
|
|
[250,251] | |
| Plant/plant extract-mediated synthesis |
|
|
[249,250,252] | |
| Algae-mediated synthesis |
|
|
[251,253] | |
3. Immobilization techniques of AgNPs
3.1. Immobilization techniques of AgNPs in textiles
3.1.1. Pretreatment
Pretreatment is mandatory for coating fabric with AgNPs, requiring controlled alkaline solutions, temperature, and time. Mohammad Shateri-Khalilabad et al. used an aqueous KOH solution at room temperature for multifunctional coated fabric for 5 min [254]. L. Peng used acetone, ethanol, and deionized water for 15 min each [255]. Roya Ashayer-Soltani et al. used 1 wt % aqueous solution at room temperature for 30 min to make conductive, stretchable fabric [256]. Zulfiqar Ali Raza et al. used enzymatic desizing, bio-scouring, and bleaching for antibacterial cellulose fabric [257]. Hanadi Katouah et al. used supercritical CO2 pretreatment with 3-aminopropyltrimethoxysilane. After each treatment, the fabric was rinsed with distilled water [258].
3.1.2. Immobilization of AgNPs via pad-dry-cure method
The pad-dry-cure method (Fig. 16) is widely used in textile technology for various treatments, including flame retardants and water-repellent coatings [259,260]. It creates multifunctional textiles with improved flame resistance, thermal stability, and superhydrophobicity [261]. This continuous, efficient process suits commercial and industrial applications, producing composite materials like chitosan-cotton composites with better dye affinity and adsorption capabilities [262]. It also fabricates drug-incorporated textiles for medical applications. The process involves submerging fabric in a solution, padding under pressure, curing at high temperature, rinsing with distilled water, and drying to remove impurities and restore the fabric's texture [263]. The pad-dry-cure process enhances textile properties effectively. Xu et al. developed cotton fabric with long-lasting antimicrobial properties by esterifying carboxymethyl chitosan (CMC) onto cotton fibers and attaching AgNPs via coordination bonds with the amino group of CMCS. Jing et al. used L-methionine to immobilize AgNPs on cotton, resulting in fabric with excellent antibacterial properties and high laundering resistance, maintaining over 97 % bacterial reduction rates (BR) against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) 90 washes The modifications did not compromise cotton's tensile strength, water absorption, and vapor permeability [259]. Hebeish et al. created antibacterial textiles using green-synthesized AgNPs, achieving significant antibacterial efficacy with 50 ppm AgNPs against E. coli and S. aureus, which remained effective after 20 washing cycles [261].
Fig. 16.
The schematic diagrams of modification of cotton fabric via the pad-dry-cure method.
QingBo Xu et al. demonstrated a one-pot fabrication of durable antibacterial cotton fabric coated with AgNPs using carboxymethyl chitosan as a binder and stabilizer. The process, involving the pad-dry-cure method (wet pick-up of 80 ± 2 %, curing at 180 °C for 5 min, rinsing with distilled water, and drying at 100 °C), resulted in uniformly dispersed AgNPs (10–80 nm) on the fabric (Fig. 17 A) [264]. This provided remarkable antibacterial activity against S. aureus and E. coli, maintaining over 94 % bacterial reduction after 50 laundering cycles. Another study by Xu et al. used similar surface modification with carboxymethyl chitosan and the pad-dry-cure method (padding for 30 min, same conditions) (Fig. 17 B), showing over 94 % bacterial reduction after 50 laundering cycles [263].
Fig. 17.
Synthesis of AgNPs using pad-dry-cure method. Reproduced (adapted) with permission. Copyright 2024, Elsevier [257], Reproduced (adapted) with permission. Copyright 2024, Elsevier [258].
3.2. Immobilization of AgNPs via in-situ synthesis method
The in-situ synthesis method is widely used to incorporate nanoparticles into different types of fabric. Table 12 summarizes the in-situ synthesis of AgNPs. In Fig. 18 A, Zhengxin Ma et al. synthesized AgNPs on microfibrillated cellulose fabric, resulting in 19.1 nm AgNPs with a face-centered cubic structure, providing long-term antibacterial performance [265]. Yannan Liu et al. demonstrated AgNP-enhanced metal-organic matrices for efficient photocatalytic hydrogen evolution in (Fig. 18 B) [266]. Zhisong Lu et al. used UV-assisted in situ synthesis of AgNPs on silk fibers, resulting in excellent antimicrobial properties (Fig. 18C) [267]. Qiaoyi Wang et al. demonstrated in-situ synthesis of silver or selenium nanoparticles on cationized cellulose fabrics, achieving excellent antimicrobial properties with 100 nm particles (Fig. 18 D) [268]. In Fig. 18 E, Krishanu Ghosal et al. produced AgNPs using carbon dots from natural polysaccharides, showing significant antibacterial activity against E. coli and selective cytotoxicity against MCF7 breast cancer cells [269].
Table 12.
Summary of in-situ synthesis of AgNPs.
| Precursor | Reaction Time | Size (nm) | Shape | Ref. |
|---|---|---|---|---|
| AgNO3 | 40 min | 9.9–103.6 | Spherical | [270] |
| AgNO3 | 1–40 s | Up to 5 | Spherical | [271] |
| AgNO3, Tri-sodium Citrate | 400 s | 10–180 | Nano spherical | [272] |
| AgNO3, Ascorbic Acid, Cetyltriammonium bromide (CTAB) | 19 min | 84–178 | Spherical, plates, polygonal, wires | [273] |
| AgNO3, 3-Aminopropyl triethoxysilane (APTES), PhNHNH2 | 30 min | 100 | Irregular | [266] |
| AgNO3, Starch | 45 min | 25.71 ± 3.53 | Spherical | [270] |
| AgNO3, Dopamine hydrochloride | 124 h | 19.1 ± 8.5 | Spherical | [265] |
| AgNO3, Polyvinylpyrrolidone, Sodium Hypophosphite | 1 h | 15–35 | Spherical | [274] |
| AgNO3 | 35 min | 130 | Spherical | [275] |
| AgNO3 | 1–5 h | – | Spherical | [267] |
| AgNO3, Ascorbic Acid | 35 min | 14.1 | Spherical | [276] |
| AgNO3, Poly (diallyldimethylammonium chloride) (PDDA) | 1 h | 10 | Spherical | [277] |
| AgNO3, NaH2PO4 | 28 h | – | Spherical | [278] |
| AgNO3, Potato-dextrose-agar (PDA) | 72 h | 5–20 | Spherical | [279] |
| AgNO3 | – | 60 | Spherical | [280] |
| AgNO3, Ethylene diamine (EDA) | 24 h | 34.4 | Spherical | [281] |
Fig. 18.
In-situ synthesis of AgNPs. Reproduced with permission. Copyright 2024, Springer Nature [259], Reproduced with permission. Copyright 2024, Springer Nature [260], Reproduced with permission. Copyright 2024, Elsevier [261], Reproduced with permission. Copyright 2024, Elsevier [262], Reproduced (adapted) with permission. Copyright 2024, Elsevier [263].
3.3. Immobilization of AgNPs via printing
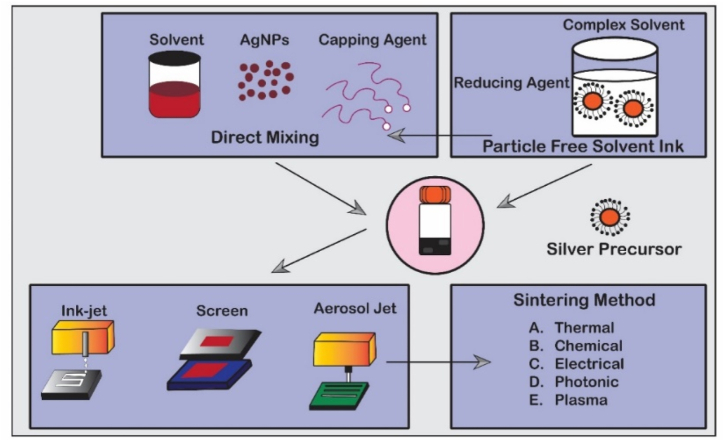
The immobilization of AgNPs through printing has been investigated in numerous research studies. Chen et al. introduced a novel methodology for producing silver micro/nanostructures through laser trapping. This innovative approach enabled the creation of silver wires and intricate three-dimensional structures [282]. Yamada et al. presented a novel printing technique that takes advantage of the chemisorption phenomenon exhibited by weakly encapsulated AgNPs on a photoactivated surface. This innovative approach allows for the production of ultrafine conductive patterns [283]. The micro- or nano-electronic industries utilize silver-based inks and printing processes for various applications. These applications include sensors [284], solar cells [285], thin-film transistors [286], and supercapacitors [287]. Diverse printing techniques for AgNP ink are detailed in Table 13. Several methods have been used to successfully immobilize AgNPs in the fabric treatment industry, including inkjet printing, screen printing, and aerosol jet printing (Fig. 19). Developing a silver ink formulation is essential for the printing process. Sintering procedures are often required after applying conductive silver inks, especially for metal-based nanoparticle inks, to establish efficient conductivity pathways. AgNPs are usually distributed in water and organic compound solutions, coated with organic stabilizers that act as insulating agents, affecting conductivity. Sintering removes these stabilizers through controlled heating, enhancing electrical conductivity. Sintering methods include conventional thermal, chemical, electrical, photonic, and plasma sintering. Table 14 summarizes the various methods and characteristics of immobilizing AgNPs in printing.
Table 13.
Summary of different printing techniques used to formulate AgNP ink.
Fig. 19.
Preparation of silver inks, different printing techniques, and sintering method. Reproduced (adapted) with permission. Copyright 2024, MDPI [288].
Table 14.
Summary of immobilizing AgNPs in printing.
| Printing Method | Size | Shape | Summary | Ref. |
|---|---|---|---|---|
| Inkjet printing | The thickness of the coating is 850 ± 150 nm | Spherical |
|
[289] |
| Inkjet printing | TA-AgNPs average diameter: 15 nm | Spherical |
|
[290] |
| Inkjet printing | AgNPs Diameter: 81.3 ± 34.1 nm | Spherical |
|
[291] |
| Inkjet printing | AgNPs Particle size:84 nm | Spherical |
|
[292] |
| Screen printing | AgNPs Particle size:5, 10, 15, 20, 25 nm | Spherical |
|
[293] |
| Screen printing | Average Diameter of AgNPs: 60 ± 8 nm | Spherical |
|
[294] |
| Inkjet printing | – | Spherical |
|
[295] |
| Direct Printing | – | Spherical |
|
[296] |
| Screen Printing | AgNPs Particle size: 29.185 nm | Spherical |
|
[297] |
| Aerosol Jet Printing | – | Spherical |
|
[298] |
4. Characterization and properties of AgNPs
Fig. 20 illustrates the different characterization techniques of synthesized AgNPs.
Fig. 20.
Characterization of AgNPs.
4.1. Microscopic techniques for characterization of AgNPS
4.1.1. Scanning electron microscopy (SEM) for characterization of AgNPs
Nanomaterials are increasingly investigated using high-resolution microscopy techniques, like SEM, which employs a very intense electron beam to probe objects at a microscopic level. SEM accurately determines particle sizes, size distributions, forms, and outer morphology of synthesized particles. Particle morphology can be analyzed by manual measurement and counting or using specialized software [299]. A synergistic technique integrating SEM and energy-dispersive X-ray spectroscopy (EDX) can investigate a sample's chemical composition analysis and morphology, offering valuable insights into particle aggregation. Field emission scanning electron microscopy (FESEM) provides valuable insights into surface morphology using a high-energy electron beam scanned across the sample's surface. The interplay between electrons and atoms at the sample's surface produces signals revealing the sample's topography and composition. The main difference between SEM and FESEM is the emitter type: SEM uses a thermionic emitter, which has lower brightness and issues with cathode material evaporation and thermal drift, while FESEM uses a field emitter, avoiding these problems. Energy-dispersive X-ray spectroscopy (EDX), integrated with SEM, determines the elemental composition of materials. When atoms in the specimen interact with the electron beam, they emit X-rays with distinct energy levels specific to the elements, creating identifiable peaks in the spectrum. Some components may have multiple peaks or overlapping peaks. Fig. 21 A-N displays various scanning electron micrographs and distinct morphologies of AgNPs.
Fig. 21.
SEM Images of AgNPs. Reproduced (adapted) with permission. Copyright 2024, Royal Society of Chemistry [300].
4.1.2. Transmission electron microscopy for characterization of AgNPs
TEM imaging allows observation of nanoparticle morphology, requiring the hydrogel sample to be semi-transparent to transmit electron beams effectively. Sample preparation is labor-intensive and time-consuming. In a typical TEM setup, a thin sample is exposed to a consistent electron beam, and the electron intensity distribution at the specimen's rear is amplified using a multi-stage lens system, observed on a fluorescent screen. Image capture methods include direct exposure of photographic emulsion, image plates, or digital recording with a CCD camera. TEM offers distinct advantages over SEM, including superior spatial resolution for detailed visualization of nanoscale structures and additional analytical measurements, such as elemental composition, crystal structure, and chemical bonding. These advantages make TEM a valuable tool in microscopy, allowing comprehensive material analysis. However, TEM requires a high vacuum environment and thin sample sections [301]. Fig. 22 A-N depicts the various morphologies of AgNPs using TEM.
Fig. 22.
TEM images of AgNPs. Reproduced (adapted) with permission. Copyright 2024, Elsevier [301], Reproduced (adapted) with permission. Copyright 2024, Elsevier [302].
4.1.3. Atomic force microscopy for characterization of AgNPs
Atomic force microscopy (AFM) is frequently used to examine nanomaterials' distribution, clustering, dimensions, morphology, absorption, and arrangement. AFM enables direct visualization without an incident beam unlike electron or light microscopy. It involves moving a pointed tip across a sample's surface and measuring the interaction force between the tip and the material, achieving resolution below one billionth of a meter and functioning under normal living conditions. The force applied to the tip is measured with pico-newton sensitivity (10−12 N) as it approaches and retracts from the sample. AFM operates in three modes: contact, non-contact, and intermittent contact [303,304]. It allows real-time examination of the dynamic interactions between nanomaterials and supported lipid bilayers, a capability impossible with electron microscopy. AFM can measure surfaces that are not oxide-free or electrically conducting and are non-destructive to native surfaces, providing sub-nanometer-level accuracy in aqueous solutions [305]. However, the cantilever size can lead to overestimating the sample's lateral [306]. The selection of the operating mode, either non-contact or contact, is a crucial determinant in sample analysis.
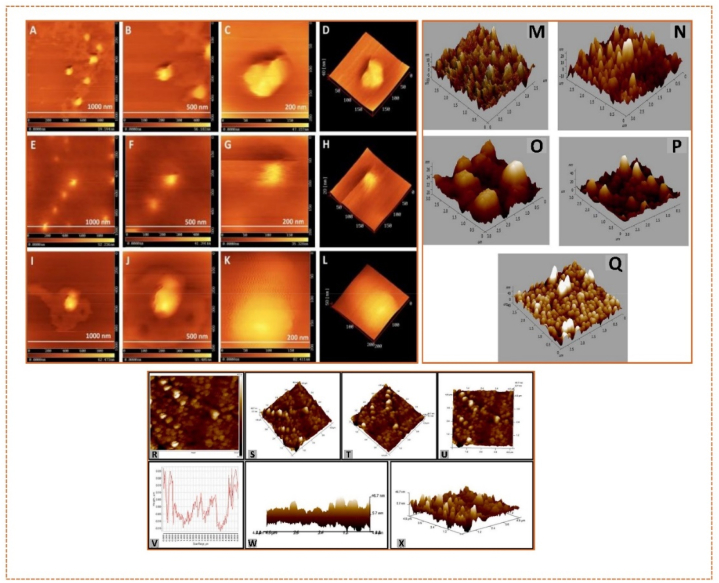
The size of biosynthesized AgNP-W, AgNP-F, and AgNP-S was determined with AFM. Fig. 23 (A-L) shows that the sizes: AgNP-W (70 nm), AgNP-F (33 nm), and AgNP-S (131 nm) with two-dimensional images (Fig. 23 A-C, E–G, and I-K), and three-dimensional image (Fig. 24 D, H, and L) [307]. AFM images also reveal changes in the surface morphology of poly (3,4-ethylene dioxythiophene) (PEDOT) films embedded with AgNPs/CuPc/C60, displaying irregular nano-holes and varied roughness (Fig. 23 M-Q). Particles of different shapes and sizes (41–54 nm) indicate AgNP presence in the poly(3,4-ethylene dioxythiophene):polystyrene sulfonate (PEDOT: PSS) layer [308]. Fig. 23 (R-U) shows the surface topology and roughness of AgNPs, mostly round with some aggregation, with sizes ranging from 5.6 to 47.2 nm, consistent with TEM results. Fig. 23 (V-X) presents height profiling of the nanoparticles, showing sizes below 50 nm [309].
Fig. 23.
AFM images of AgNPs at different synthesis methods. Reproduced (adapted) with permission. Copyright 2024, Springer Nature [302], Reproduced (adapted) with permission. Copyright 2024, Elsevier [310], Reproduced (adapted) with permission. Copyright 2024, Elsevier [303].
Fig. 24.
UV–visible absorbance spectra of AgNPs by different synthesis methods. Reproduced (adapted) with permission. Copyright 2024, Elsevier. [307], Reproduced (adapted) with permission. Copyright 2024, Springer Nature [308].
4.2. Spectroscopic techniques for characterization of AgNPs
4.2.1. UV–visible spectroscopy for characterization of AgNPs
UV–visible spectroscopy is a highly reliable method for initially assessing newly produced nanomaterials and monitoring the production and durability of AgNPs. This approach is fast, simple, user-friendly, and highly sensitive, with broad specificity for various nanoparticles. It does not require calibration to characterize colloidal suspensions. AgNPs exhibit remarkable optical properties due to their close conduction and valence band, allowing unlimited electron mobility and leading to surface plasmon resonance (SPR) absorption, often called Mie resonance. This resonance occurs when the frequency of free electrons near the nanoparticle surface matches the incident light frequency. Silver exhibits the highest SPR intensity, influenced by particle size, dielectric medium, and chemical environment [311]. SPR peaks in the visible spectrum confirm the presence of nanoparticles, with metallic NPs showing peaks for diameters from 2 to 100 nm [312].
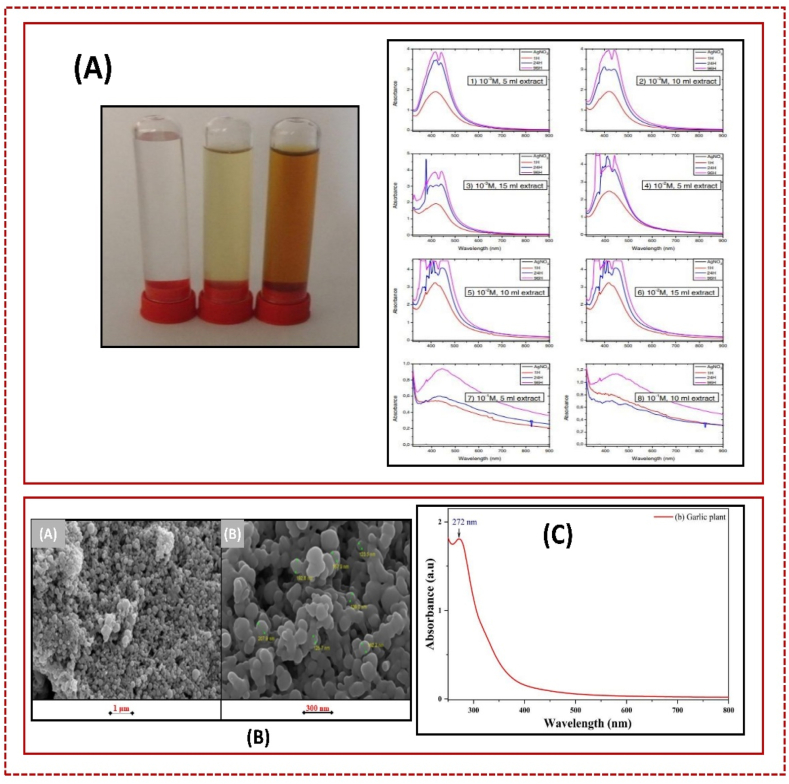
The reaction between Ag+ and the reducing material in the extract was monitored for 96 h, with UV–visible measurements taken at 1 h, 24 h, and 96 h. Fig. 24 A shows the UV–visible spectra of Ag-nanoparticles over time with varying amounts of M. macrostachyum leaf extract. The reduction, nucleation, and growth of nanoparticles increase from 24 h to 96 h, leading to polydispersity. Absorbance bands gradually increased, and the solution's color changed from pale yellow to dark brown due to AgNP accumulation and aggregation [313]. Fig. 24 B (A-C) shows the absorption spectrum of the garlic plant extract (250–800 nm), with a peak at 272 nm [314].
4.2.2. X-ray diffraction for characterization of AgNPs
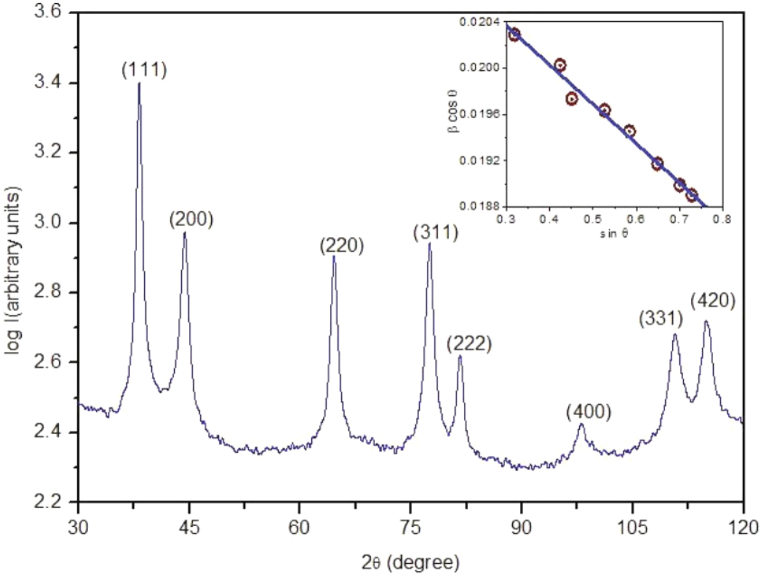
X-ray diffraction (XRD) is a non-destructive technique used to study materials' molecular and crystal structures. It quantitatively identifies chemicals, determines crystallinity levels, analyzes isomorphous substitutions, and estimates particle sizes. The evaluation of materials heavily depends on the interpretation of a diffraction pattern. Based on Bragg's law, XRD interprets diffraction patterns from wide-angle elastic scattering of X-rays. These patterns reflect the physicochemical attributes of the crystal structures. For powders, the diffracted beams reveal structural and physicochemical characteristics [315]. Each material has a distinct diffraction pattern compared with the Joint Committee on Powder Diffraction Standards (JCPDS) library for identification. XRD is widely used for characterizing bulk and nanomaterials, forensic specimens, industrial samples, and geochemical materials. Difficulties in growing crystals and the limited ability to acquire specific results for a single conformation or binding state. Moreover, the magnitude of diffracted X-rays is somewhat lower than electron diffractions. The crystalline nature of AgNPs is assessed by conducting XRD analysis within the 2θ range of 20°–80°, which offers morphological verification. Typically, it indicates the presence of face-centered cubic (FCC) structures in metallic Ag. The peaks of highest prominence are detected at 2θ angles of 38.19°, 44.46°, 64.63°, and 77.34°. These peaks correspond to the crystal lattice planes of (111), (200), (220), and (311), respectively. Khan et al. synthesized AgNPs using the wet chemical solution method and found an FCC structure with the reflex of crystalline planes (111), (200), (220), (311), (222), (400), (331) and (420) at 2θ values 38.1°,44.09°, 64.36°, 77.29°, 81.31, 97.92°, 110.81° and 114.61° as shown in Fig. 25 [316]. These are closely matched with the reported reference value of JCPDS, file No. 4–0783.
Fig. 25.
XRD Pattern of AgNPs. Reproduced with permission. Copyright 2024, Springer Nature [316].
Hetta et al. synthesized AgNPs via chemical reduction of Ag salts, characterized by TEM, showing spherical particles ranging from 10 to 50 nm (Fig. 26 A). XRD confirmed their crystalline structure (Fig. 26 B) [317]. Using E. roxburghii leaf extract, AgNPs were 3.5 nm in size, as analyzed by XRD (Fig. 26C–E) [309]. Muhammad Zahir Shah et al. produced AgNPs with Plantago lanceolata extract, producing spherical particles averaging 30 ± 4 nm (Fig. 26F–L). XRD revealed a non-uniform distribution and aggregation over time [318].
Fig. 26.
XRD of AgNPs. Reproduced (adapted) with permission. Copyright 2024, Springer Nature [304], Reproduced (adapted) with permission. Copyright 2024, Springer Nature [311], Reproduced (adapted) with permission. Copyright 2024, Springer Nature [312].
4.2.3. Fourier transform infrared spectroscopy for characterization of AgNPs
Infrared spectroscopy (IR) identifies significant functional groups and characterizes biomolecules attached to AgNPs. These biomolecules, containing amine groups, cysteine residues, or carboxyl groups, are drawn together by electrostatic interactions. The IR spectrum depicts absorption and transmission, creating a unique molecular fingerprint. Peak amplitude indicates the amount of the substance present, with spectral variations reflecting the organism or species used in AgNP synthesis. The advantages of Fourier Transform Infrared Spectroscopy (FTIR) encompass accuracy, reproducibility, and a good signal-to-noise ratio due to quick data acquisition and strong signal intensity [319]. It detects small absorbance variations, typically 10−3, distinguishing faint absorption bands of active residues from the background protein absorption [320,321]. FTIR is used to verify functional molecules attached to nanoparticles and examine enzyme-substrate interactions during the catalytic process [319,322]. The technique is non-destructive, using a Michaelson interferometer. Attenuated Total Reflection (ATR)-FTIR simplifies sample preparation and determines the chemical characteristics of polymer surfaces. Unlike traditional FTIR, sample preparation is a breeze [323]. FTIR is a non-invasive, inexpensive method for identifying biological molecules that reduce AgNO3 to Ag [324].
Fig. 27A shows the transmittance peaks for Pbet leaf extract and Pbet-AgNPs. The 3448.83 cm−1 and 3595.43 cm−1 bands correspond to O–H stretching vibrations of alcohols/phenols. The bands 3340.82 and 3458.48 cm−1 are attributed to the N–H stretching of primary amines. The 2893.48 and 2945 cm−1 bands correspond to C–H stretching vibration characteristics. The characteristic bands at 1647 and 1653 cm−1 are attributed to the amide I due to protein carbonyl stretching vibrations. The band at 750 cm−1 and 678 cm−1 represents the ortho-substituted and mono-substituted aromatic stretching (Fig. 27 A). This analysis suggests proteins and amino acids may adsorb on AgNP surfaces through possible interactions [325]. The FTIR spectral analysis in Fig. 27 B shows the leaf extract, containing phenolic compounds, acts as both a reducing and stabilizing agent in AgNP biosynthesis [326]. Fig. 27C and 27 D also confirm the presence of AgNPs [327,328].
Fig. 27.
FTIR analysis of AgNPs by different synthesis methods. Reproduced (adapted) with permission. Copyright 2024, Springer Nature [319], Reproduced (adapted) with permission. Copyright 2024, Elsevier [320], Reproduced (adapted) with permission. Copyright 2024, Springer Nature [321], Reproduced (adapted) with permission. Copyright 2024, Springer Nature [322].
4.2.4. Dynamic light scattering for characterization AgNPs
Dynamic Light Scattering (DLS) is prevalent in the non-destructive examination of particle dimensions and size distributions within physiological or aqueous solutions [329]. When it interacts with particles, the behaviour of light is a determining factor. The precise particle size distributions can be assessed within the 2–500 nm range. The device quantifies the amount of light diffused by a laser beam as it traverses a colloid. This measurement relies on the phenomenon of Rayleigh scattering caused by the suspended nanoparticles [299]. The analysis examines the temporal variation in the intensity of scattered light and determines the particles' hydrodynamic size [330,331]. Brownian motion significantly impacts the measured size in the DLS context more than TEM. Simultaneous probing of a substantial number of particles is feasible, yet it is subject to several restrictions on the sample [332]. A nanoparticle's size is determined by examining its metallic core and any surface-adsorbed chemicals, such as stabilizers. DLS measures the hydrodynamic size, including the NP diameter and the surrounding electrostatic potential. Comprehensive analysis requires data from XRD, TEM, and SEM techniques.
5. Applications of AgNPs
5.1. Fabrication of AgNPs for different properties
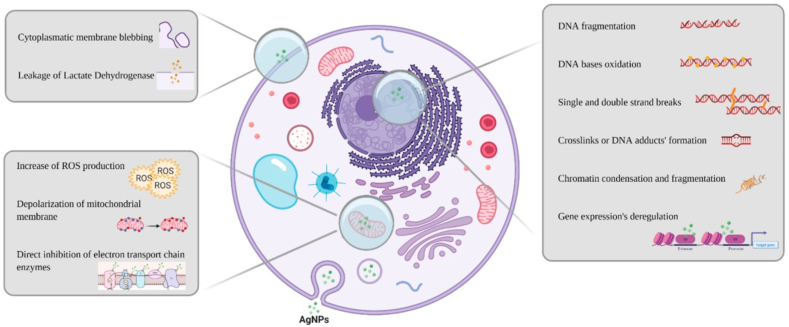
AgNPs show antimicrobial properties. The generation of silver ions depends on the surface area of AgNP, where a larger surface area increases the production of silver ions, increasing antimicrobial activity. AgNPs act as a “bank” of ions once inside the cell, continuously releasing them and prolonging or strengthening the antibacterial effect. Ag+ can uncouple the respiratory electron transport chain from oxidative phosphorylation and interfere with the penetration of H+ and phosphate into membranes. Within the bacterial cell, metal cations can form complexes with nuclear material by intercalation between base pairs, disrupting hydrogen bonds and ultimately preventing effective cell division. The reactive oxygen species (ROS) production, either by disrupting the thioredoxin system or by interacting with the respiratory chain and interrupting intracellular O2 reduction, is a major ion-mediated killing mechanism, shown in Fig. 28 [333]. AgNPs also show superhydrophobicity, anti-stain, self-cleaning, and photocatalysis activity. Javad Seyf et al. developed antibacterial superhydrophobic coatings based on polydimethylsiloxane/silver phosphate nanocomposites where the water contact angle of the nanocomposite is 152°, protein adsorption reduction is ∼83 % and antibacterial activity (>99 %) against gram-positive and gram-negative bacteria [334]. Hao Wen et al. created superhydrophobic cotton fabrics functionalized with Ag and PDMS, with a water contact angle of 155° ± 1.5° and oil-water separation efficiency >97 %, showing excellent antimicrobial resistance [335]. Fangfei Zhang et al. designed a high-breathable antimicrobial face mask with a water contact angle of 135°, enhancing air permeability and antimicrobial activity [336]. Ya-Nan Gao et al. developed multifunctional cotton non-woven fabrics coated with AgNPs and polymers for antibacterial, superhydrophobic, and high performance. The composite fabrics exhibited a high conductivity of ∼1000 S/cm, and their EMI shielding effectiveness increased to ∼110 dB. It also showed excellent antimicrobial properties, acid-alkali corrosion, and mechanical resistance [337].
Fig. 28.
Antimicrobial mechanisms of AgNPs. Reproduced with permission. Copyright 2024, American Chemical Society [333].
Using AgNP coatings on fibers, Chao-Hua Xue et al. created superhydrophobic conductive fabrics with antimicrobial characteristics. It was found that the water contact angle maintained to be 151.5° ± 1.4° for a 5 μL water droplet. The resistance of the textiles did not show significant change, maintaining to be 39.2 Ω ± 1.5 Ω. The reduction of bacterial growth of all the silver-modified samples was maintained at 99.99 % after ten times water washing [337]. Hakan et al. showed a superhydrophobic titanium dioxide (TiO2)-poly (dimethyl siloxane) (PDMS)-AgNPs (Ag NPs) coating fabricated on fabrics via a simple dipping method with versatile properties. The water contact angle (WCA) value was estimated at 153° with a sliding angle 15° for the superhydrophobic coating. AgNPs demonstrated antibacterial activity against Escherichia coli and Staphylococcus aureus and showed excellent photocatalysis activity [338].
Jagdeep Singh et al. demonstrated the photocatalytic degradation of Methylene Blue dye using green synthesized AgNPs, achieving 82.8 % degradation in light and 61.25 % in dark conditions [339]. Swati Jaast showed complete degradation of Malachite Green dye in sunlight within 120 h using green synthesized AgNPs for water purification [340]. Renganathan Rajkumar et al. synthesized AgNPs using Chlorella vulgaris, achieving 96.51 % decolorization of Methylene Blue dye (100 ppm) within 31 h [341]. Maryamosadat Mavaei et al. synthesized AgNPs with isoimperatorin, showing photocatalytic degradation of 96.5 % for Methylene Blue, 96.0 % for New Fuchsine, 92 % for Erythrosine B, and 95 % for 4-Chlorophenol under sunlight [342]. Tong-Huai Cheng et al. functionalized silk with AgNPs using tea stem waste extract, achieving 99 % antibacterial reduction against gram-positive and 92 % against gram-negative bacteria, with good UV protection even after 20 washing cycles [343].
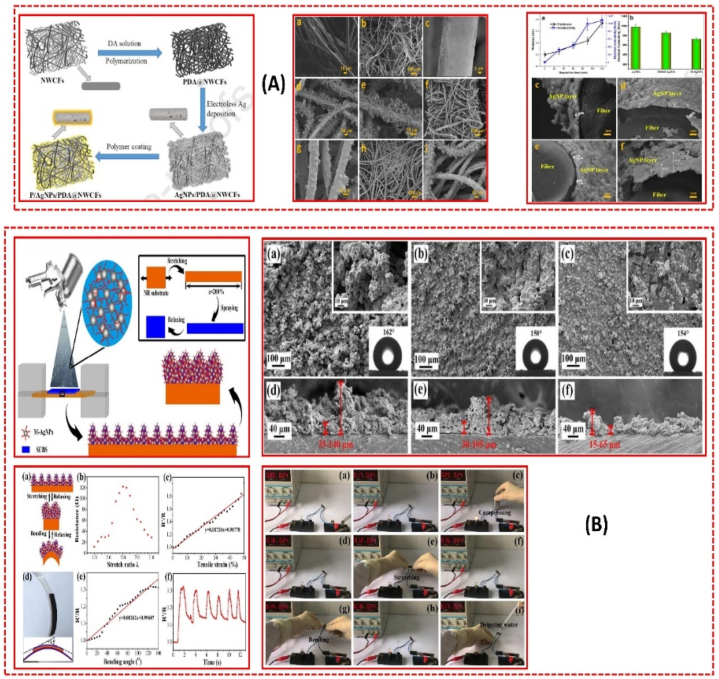
Ya-Nan Gao et al. presented multifunctional cotton non-woven fabrics coated with AgNPs and polymers for antibacterial, superhydrophobic, and high-performance microwave shielding, as shown in Fig. 29 A. Initially, cotton fabrics were chemically coated with AgNPs using polydopamine as the adhesive, followed by hydrophobic polydimethylsiloxane or polyimide. The introduction of polydopamine significantly enhanced the bond between AgNPs and cotton fibers, preventing AgNPs from detaching. The composite fabrics exhibited a high conductivity of approximately 1000 S/cm, with their EMI shielding effectiveness increasing to around 110 dB. Additionally, the composite fabrics demonstrated excellent self-cleaning performance and acid-alkali corrosion resistance due to their superhydrophobicity. Notably, the fabric composites exhibited significant antibacterial action against Staphylococcus aureus and Escherichia coli [344]. Xiaojing Su et al. developed a superhydrophobic, conductive, and highly elastic covering for flexible electronics, as shown in Fig. 29 B. The coating exhibits superhydrophobicity with a water contact angle exceeding 160° and high conductivity (resistance of about 10 Ω). It maintains superhydrophobicity at low/high stretch ratios, responding to stretching and bending with sensitivity, broad sensing range, and stable response cycles. The coating also shows excellent durability against heat, strong acid/alkali, and mechanical forces, including droplet impact, kneading, torsion, and repetitive stretching-relaxation [345]. Table 15 summarizes the fabrication of AgNPs for different properties.
Fig. 29.
(A) Schematic of processing procedure for fabricating P/AgNPs/PDA@ non-woven cotton fibers (NWCF) composite fabrics, SEM images of untreated NWCFs (a), PDA@NWCFs (b, c), AgNPs/PDA@ non-woven cotton fibers (NWCFs) (d, e), PDMS/AgNPs/PDA@ non-woven cotton fibers (NWCFs) (f, g), and PI/AgNPs/PDA@ non-woven cotton fibers (NWCFs) (h, i). Ag deposition time was 90 min for the samples with AgNPs. (a) Thickness and electrical conductivity of AgNPs/PDA@ non-woven cotton fibers (NWCFs) with different reaction times. (b) Electrical conductivity of AgNPs/PDA@NWCFs coated with polydimethylsiloxane (PDMS) and PI. The thickness of the silver layer in the samples with a different reaction time of (c) 30, (d) 60, (e) 90, and (f) 120 min. Reproduced (adapted) with permission. Copyright 2024, Elsevier [344] (B) Fabrication for highly stretchable and conductive superhydrophobic coating on NR strip. SEM images (inset on the top right is the higher magnification image, and the bottom right is the CA optical image) of the highly stretchable and conductive superhydrophobic coatings fabricated at different ε: (a) 200 %, (b) 100 %, and (c) 0 %. (d–f) The corresponding cross-section images. (a) Morphology changes under stretching and bending. (b) Resistance changes of the superhydrophobic coating with stretching to λ = 3 and then relaxing to λ = 1. (c) Relative resistance variation as a function of tensile strain and the linear fitting. (d) Optical photograph of the sensor (inset was the schematic of bending angle). (e) Relative resistance variation as a function of bending angle and the linear fitting. (f) Real-time response of the sensor's resistance variation to bending angle from 0o to 140o for repetitive cycles. The electric circuit with (a) switches off and (b) is on. The circuit is composed of a DC power supply, a switch, a tiny bulb, and a superhydrophobic coating. Electric circuit (c) with the superhydrophobic coating being compressed by a weight of 200 g and (d) after removing the weight. The electric circuit (e) with superhydrophobic coating is stretched, and (f) is stretched after relaxing the tensile strain. Electric circuit (g) with superhydrophobic coating bent and (h) after removing the bending force. (i) An electric circuit with continuous water droplets dripping from the superhydrophobic coating. Reproduced (adapted) with permission. Copyright 2024, American Chemical Society [345].
Table 15.
Summary of fabrication of AgNPs for different properties.
| Key Properties | Key Result | Ref. |
|---|---|---|
|
|
[334] |
|
|
[346] |
|
|
[347] |
|
|
[348] |
|
|
[349] |
|
|
[350] |
|
|
[344] |
|
|
[351] |
|
|
[352] |
|
|
[353] |
|
|
[354] |
|
|
[345] |
|
|
[355] |
|
|
[356] |
|
|
[357] |
|
|
[338] |
|
|
[358] |
|
|
[359] |
|
|
[360] |
|
|
[361] |
|
|
[343] |
|
|
[362] |
|
|
[363] |
|
|
[364] |
|
|
[341] |
|
|
[342] |
|
|
[365] |
5.2. Applications of AgNPs
Hyung Woo Choi demonstrated the Smart textile lighting/display system with multifunctional fiber devices for large-scale smart home and IoT applications via AgNPs. Fig. 30 A illustrates this intelligent textile system's fabrication protocol and mechanical stability integrated with six F-devices and a lighting/display apparatus (F-LED). The technology convergence between textile engineering and electronic science is depicted through schematic F-devices and corresponding fabricated F-devices.
-
•
Textile technology (weaving).
-
•
Smart textile system.
-
•
Mechanical stability of the innovative textile display system under folding, bending, and rolling conditions and a side view of the wall-mountable system [366].
Fig. 30.
Innovative applications of AgNPs in smart textiles and high-performance composites. Reproduced (adapted) with permission. Copyright 2024, Springer Nature [360], Reproduced (adapted) with permission. Copyright 2024, American Chemical Society [361], Reproduced (adapted) with permission. Copyright 2024, American Chemical Society [362], Reproduced (adapted) with permission. Copyright 2024, Elsevier [363].
Ling Wang et al. showed the bioinspired superhydrophobic and durable octadecanoic acid/Ag nanoparticle-decorated rubber composites for high-performance strain sensors. The CEC shows high conductivity (21.9 S/cm) and superhydrophobicity (with a contact angle of 152.6°) in Fig. 30. The superhydrophobic CEC with excellent corrosion resistance can be used as strain sensors with significant working strain, sensitivity, and excellent cycle stability [367]. Byungwoo Choi showed the highly conductive fiber with waterproof and self-cleaning properties for textile electronics in Fig. 30 B. They developed a highly conductive and waterproof fiber with excellent electrical conductivity (0.11 Ω/cm) and mechanical stability for advanced interconnector components in wearable textile electronics. The mechanical durability could withstand folding deformation 10,000 times in Fig. 30C [368]. Rui Xu et al. prepared the silver-plated Hollow Glass Microspheres and their application in infrared stealth coating fabrics in Fig. 30 D-F. The infrared stealth fabric was prepared by coating silver-plated HGMs doped aluminium powder on the cotton fabric. The optimized coating formula is 15 % silver-plated HGMs, 15 % aluminium powder, 10 % epoxy resin adhesive, 5 % thickener CN-WW, 5 % diffusing agent, and 50 % deionized water, resulting in low infrared radiation and good infrared stealth performance [369].
Table 16 describes the applications of AgNPs in different sectors, and Fig. 31 illustrates their biomedical applications in different sectors.
Table 16.
Summary of application of AgNPs.
| Applications | Case | Summary | Ref. |
|---|---|---|---|
| Electronic | Conductive Ink |
|
[370] |
|
[371] | ||
|
[372] | ||
|
[373] | ||
|
[374] | ||
|
[375] | ||
|
[376] | ||
|
[377] | ||
|
[378] | ||
|
[379] | ||
| Food Analysis |
|
[380] | |
| Solar Cell | Solar Cell |
|
[381] |
|
[382] | ||
|
[383] | ||
| Water Treatment | Water Treatment |
|
[384] |
| Food Preservation | Surface Protection and food preservation |
|
[385] |
| Painting | Outdoor Paint |
|
[386] |
| Medical Application | Dentistry |
|
[387] |
| Wound Dressing |
|
[388] | |
|
[389] | ||
|
[390] | ||
|
[391] | ||
|
[392] | ||
| Bone Cement |
|
[393] | |
| Antiviral activity |
|
[[394], [395], [396], [397], [398], [399], [400], [401], [402]] | |
| Medical Devices |
|
[403] | |
|
[404] | ||
| Face Mask |
|
[404,405] | |
| Antifungal activity |
|
[[406], [407], [408]] | |
| Implant |
|
[409,410] | |
| Anti-cancer |
|
[[411], [412], [413], [414], [415], [416]] | |
| Antibacterial activity |
|
[[417], [418], [419], [420], [421], [422], [423], [424], [425]] | |
| Agricultural Application | Nano fertilizer |
|
[426] |
| Consumer Applications | Consumer byproducts |
|
[408,427] |
|
[[428], [429], [430]] |
Fig. 31.
Biomedical application of AgNPs.
Morteza Abazari et al. showed the fabrication of AgNP-deposited fabrics as a potential candidate for developing reusable facemasks in Fig. 32 A-B. Coated fabrics showed enhanced filtration efficiency (against nanosized particles), desired pressure drops, and antibacterial activity without significant cytotoxicity toward HEK 293 cells and Artemia nauplii. As a result, the coated fabrics could find potential applications in developing facemasks for protection against different pathogenic entities [431]. Habeebur Rahman et al. showed the catalytic influence of AgNPs on the ZnO surface in the context of CO gas sensing applications in Fig. 32C. They observed that incorporating AgNPs on the ZnO surface led to enhancement in the surface resistance of active material due to the higher work function of AgNPs. At 150 °C, Ag nanoparticles they were effectively acted as catalysts, increasing the redox reaction rate between gas molecules and the sensor surface. Ag-loaded ZnO sensor exhibited remarkable improvement in the sensing response (∼40 %) compared to pure ZnO (sensing response %) for 10 ppm CO at 150 °C temperature [432]. Ashavani Kumar et al. demonstrated using vegetable oil as a foundation for antibacterial paints containing AgNPs in Fig. 32 D-G. They coated surfaces such as glass, polypropylene, and poly (methyl methacrylate) with metal ion-containing drying oils to investigate nanoparticle synthesis on surfaces. After about 6 h of drying at ambient conditions, the gold paint turned pink, and the silver paint turned slightly brownish yellow, indicating AuNPs and AgNPs forming in the coatings, respectively [433]. Ralf Kaegi et al. examine the emission of metallic AgNPs from paints utilized in outdoor settings in Fig. 33. They have provided the first direct evidence for releasing Ag-NP from a typical outdoor application to the aquatic environment. About 30 % of the Ag-NP initially contained in the paint was lost within one year of exposure, whereas the first few runoff events dominated the Ag mass flux. AgNPs were attached to an organic binder from the paint and released mainly as composite colloids [386]. Hiroyuki Yoshikawa et al. demonstrated the identification of H2O2 using an AgNPs grating device created using plasmonic plating in Fig. 32 H-I. They showed that the plasmonic plating technique combined with an interference laser exposure enables the high-throughput fabrication of sensor chips with silver grating structures [378]. Nan Zhang et al. showed the Tannic acid stabilized AgNPs for inkjet printing conductive flexible electronics in Fig. 32 J-K. The researchers produced highly stable and homogeneous inks of TA-Ag NPs by dispersing the TA-Ag NP powder in H2O. Various conductive silver patterns were deposited on photo paper by inkjet printing using a standard color printer with the help of silver conductive inks. The resistivity of the silver patterns could be decreased to 2 Ω/m with sintering at 200 °C for 100 min. A potential use of the silver patterns as conductive LED device circuits has been displayed. The printed silver pattern shows good adhesion toward the paper substrate under the tape test [434]. Yuesheng Huang et al. conducted a randomized comparative trial to assess the clinical efficacy and safety of Acticoat with nanocrystalline silver for external use in treating residual burn wounds in Fig. 32 L-O. The study included a safety analysis to investigate and evaluate the effectiveness and safety of Acticoat in managing postburn residual wounds. This study demonstrates the efficacy of Acticoat in enhancing the healing of residual wounds following burns and inhibiting bacterial growth in the wounds [435].
Fig. 32.
(A) Schematic illustration of the preparation of Sono chemically-coated fabrics to develop antibacterial and antiviral facemasks. (B) Uncoated and SNP-coated fabrics are coated using the Sono chemical coating procedure. Sample 0: Uncoated fabric, Samples 1–4 and 5–8: NS-coated fabrics at 2,10,20, 50 mM silver nitrate concentrations, and 25- and 50-min sonication time, respectively. Reproduced (adapted) with permission. Copyright 2024, Springer Nature [436] (C) Inside view of the gas-sensing chamber. Reproduced (adapted) with permission. Copyright 2024, Springer Nature [437] (D) Images of plain commercially available drying oil, silver benzoate, and chloroauric acid dissolved in drying oils (left to right). (E) Images of paint coatings without nanoparticles (left panels), with AgNPs (middle panels), and with AuNPs (right panels) on glass and (F) polymer surfaces. Reproduced (adapted) with permission. Copyright 2024, Springer Nature [438] (G) Model house. The panels are exposed to the west. The panel with the AgNPs paint was installed on the left side. Reproduced (adapted) with permission. Copyright 2024, Elsevier [439]. (H) Schematic and (I) photograph of an original portable device. Reproduced (adapted) with permission. Copyright 2024, American Chemical Society [440] (J) Digital photographs of LED test connected with printed silver patterns (K) Digital microscopy photographs of printed patterns. Reproduced (adapted) with permission. Copyright 2024, American Chemical Society [441] (L) Before treatment, 28 days post-burn, much excretion on the residual wounds. (M) Wounds completely healed after using Acticoat for 6 days (N) Before treatment, 50 days post-burn, not much excretion on the residual wounds (O) The residual wounds completely healed after using Acticoat for 5 days. Reproduced (adapted) with permission. Copyright 2024, Elsevier [442].
Fig. 33.
Mitochondria (increased ROS production), nucleus (DNA fragmentation), and cytoplasmic membrane (membrane blebbing) are the three areas where AgNPs cause cell damage, ultimately leading to cell cycle arrests and, consequently, apoptosis. Reproduced with permission. Copyright 2024, American Chemical Society [443].
5.3. Cytotoxic effect of AgNPs
AgNPs are extensively utilized in the medical and commercial industries. Due to their small size, they can easily pass through biological membranes and cause toxicity. This toxicity depends on the shape, size, concentration, coating process, synthesis method, and the level of targeted living organisms or test species [444]. AgNPs and Ag+ can damage cell membranes, generate reactive oxygen species (ROS), cause protein oxidation and denaturation, induce mitochondrial dysfunction, damage DNA, and inhibit cell proliferation. These findings indicate that the interaction of AgNPs with proteins or other sulfur-containing macromolecules is a significant toxicity mechanism attributable to the high affinity between silver and sulfur [444,445]. AgNPs can enter the human body and other organisms through different exposure routes. Upon contact, they damage the cell membrane, producing reactive oxygen species (ROS), oxidation and denaturation of proteins, and mitochondrial malfunction (Fig. 33). These processes can develop various types of cancer in humans and other animals [444,[446], [447], [448], [449]]. Table 17 summarizes the cytotoxicity effect of AgNPs in different tumor models and normal cell lines, whereas Fig. 34 illustrates their harmful impact on various bodily regions.
Table 17.
Cytotoxic effect of AgNPs.
| Shape of AgNPs | Size (nm) | Exposure Time (Hours) | Tumor Model | IC50/LD50(μg/mL) | Normal Cell line | Toxicity mg/L | Ref. |
|---|---|---|---|---|---|---|---|
| Spherical | 20.87 | 48 | Breast Cancer | 2.5 | 373-L1 | IC50 = 100 | [450] |
| Spherical | 8.19, 8.37 | 24 | Breast Cancer, Epithelioma | 49.94, 20.97 | NHDF | IC50 > 100 | [448] |
| Spherical | 20.44 | 24 | Breast Cancer | 65.27 | 3T3 | IC50 = 388.6 | [451] |
| Asymmetric nano dumbbell, Spherical | 20.87, 10-16 | 24, 48 | Lung Adenocarcinoma, Oral cancer | – | N1H3T3, HPBM, Rat Hepatocytes | High Toxicity, No toxicity | [452] |
| Spherical, circular, triangular | 5–20 | 48 | Pharynx Cancer | 47 | Vero and Hbl 1oo | Less toxic than cancer | [453] |
| Spherical | 30–40 | 24 | Ovarian Cancer | A 2780 | Human Fibroblast | IC50 = 100 μM | [446] |
| Spherical | 5–15 | 24 | Cervical Cancer | HELA | Normal Renal cell line | Non-toxic | [454] |
| Spherical | 80-98, 30–70, 34-70 | 48 | Prostate Cancer | 58.61, 28.42, 42.77 | Vero | IC50 = 96.41, 48.83, 48.86 | [455] |
| Spherical | 162.72 | 72, 120 | Glioblastoma, Myeloblastic leukemia | 6.053 | HUVEC, ECV-304 | Nontoxic | [456] |
| Spherical | 25 | – | Neuroblastoma | – | HVEC | Toxicity to cancer | [447] |
Fig. 34.
Exposure of AgNPs and cytotoxic effects on the human body.
6. Challenges, future perspectives
The past decade has seen significant advancements in the production of AgNPs, primarily through physical and chemical techniques. However, these methods are often expensive and involve hazardous substances, posing substantial environmental and health risks. These challenges underscore the need for more sustainable production methods. Green synthesis of AgNPs has emerged as a promising alternative, leveraging biological materials like plant extracts to produce nanoparticles. Despite its potential, green synthesis faces hurdles in achieving nanoparticle size, shape, and distribution consistency necessary for reliable industrial applications. Moreover, the scalability of these methods remains a significant challenge, necessitating further research to develop cost-effective, scalable processes. Integrating AgNPs into fabrics to enhance properties such as self-cleaning, UV protection, electrical conductivity, and anti-abrasion is another area ripe for innovation. Ensuring the durability of these properties, particularly their ability to withstand multiple wash cycles, is crucial. Current immobilization techniques must be refined to securely bind AgNPs to fabric surfaces without compromising functionality or increasing production costs excessively. Additionally, comprehensive studies are needed to understand the toxicity levels of AgNPs under various environmental conditions. Assessing the environmental impact of AgNPs, including their interaction with ecosystems and potential bioaccumulation, is essential to address safety concerns and ensure their sustainable use.
Looking forward, research should focus on optimizing green synthesis methods to enhance the consistency and scalability of AgNP production. Exploring a broader range of biological materials for synthesis could lead to more efficient and cost-effective processes. In fabric applications, novel immobilization techniques must be developed to create durable, multifunctional textiles. The potential of AgNPs in advanced wireless sensors and solar cells also presents exciting opportunities. Enhancing the performance and integration of AgNPs in these devices could lead to significant technological advancements. Furthermore, the biomedical, agricultural, and consumer sectors stand to benefit greatly from the unique properties of AgNPs, such as their antimicrobial activity. Finally, thorough environmental and safety assessments are crucial to evaluate the long-term effects of AgNPs on human health and the environment, ensuring their responsible and sustainable application across various industries.
7. Conclusion
The research has highlighted significant progress in the synthesis, immobilization, and characterization methods of AgNPs, underscoring the creation of controlled and efficient approaches that have significantly broadened the practical uses of AgNPs. AgNPs can be synthesized using various techniques, including physical, chemical, biological, and photochemical methods. Among these, biological approaches, particularly green synthesis, stand out as the most environmentally friendly, cost-effective, and health-preserving methods. The extensive versatility of AgNPs, ranging from their use as antimicrobial agents to their role in environmental remediation, highlights their significant potential to transform various sectors, providing sustainable and effective answers to pressing global issues. Nonetheless, in light of these advancements, challenges concerning the stability, environmental implications, and large-scale production of AgNPs continue to exist, highlighting the need for additional research to tackle these essential issues. Future investigations may concentrate on refining biocompatible, environmentally friendly synthesis techniques and examining novel immobilization approaches to improve the stability of AgNPs in intricate settings, thereby facilitating the development of more sophisticated and sustainable AgNP-based solutions. The long-term effects of AgNP exposure on animals, the environment, and humans require further investigation. Prioritizing in vivo testing of AgNPs is crucial to assess their toxicity and biocompatibility, which will be essential to advance these beneficial antibacterial treatments safely. In conclusion, while significant progress has been made in the synthesis and application of AgNPs, continued research is essential to address the challenges related to their production, scalability, and environmental impact. By optimizing green synthesis methods and conducting comprehensive safety assessments, the potential of AgNPs can be fully realized responsibly and sustainably across various industries.
CRediT authorship contribution statement
Md. Belal Uddin Rabbi: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Sadia Haque: Writing – review & editing. Sultana Bedoura: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Conceptualization.
Data statement
No data was generated in the preparation of this manuscript.
Declaration of generative AI in scientific writing
This manuscript was prepared with partial assistance from generative AI technology, specifically OpenAI's ChatGPT. The AI was utilized to enhance readability and language. All AI-generated content has been thoroughly reviewed and validated by the authors to ensure accuracy and originality. The authors take full responsibility for the integrity and scientific validity of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Khan I., Saeed K., Khan I. Nanoparticles: properties, applications and toxicities. Arab. J. Chem. Nov. 2019;12(7):908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 2.Fahmy H.M., et al. Review of green methods of iron nanoparticles synthesis and applications. BioNanoScience. Jun. 2018;8(2):491–503. doi: 10.1007/s12668-018-0516-5. [DOI] [Google Scholar]
- 3.Pattanayak D.S., Pal D., Thakur C., Kumar S., Devnani G.L. Bio-synthesis of iron nanoparticles for environmental remediation: status till date. Mater. Today Proc. 2021;44:3150–3155. doi: 10.1016/j.matpr.2021.02.821. [DOI] [Google Scholar]
- 4.Pal D., Pattanayak D.S., Mishra J., Sahoo N.K. In: NanoBioenergy: Application and Sustainability Assessment. Srivastava M., Mishra P.K., editors. Springer Nature Singapore; Singapore: 2023. Green route synthesized iron nanoparticles for biohydrogen production; pp. 109–134. Clean Energy Production Technologies. [DOI] [Google Scholar]
- 5.Leong G.J., et al. Shape‐directed platinum nanoparticle synthesis: nanoscale design of novel catalysts. Appl. Organomet. Chem. Jan. 2014;28(1):1–17. doi: 10.1002/aoc.3048. [DOI] [Google Scholar]
- 6.Zhao P., Li N., Astruc D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. Feb. 2013;257(3–4):638–665. doi: 10.1016/j.ccr.2012.09.002. [DOI] [Google Scholar]
- 7.Abou El-Nour K.M.M., Eftaiha A., Al-Warthan A., Ammar R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. Jul. 2010;3(3):135–140. doi: 10.1016/j.arabjc.2010.04.008. [DOI] [Google Scholar]
- 8.Rajeshkumar S., Naik P. Synthesis and biomedical applications of Cerium oxide nanoparticles – a Review. Biotechnol. Rep. Mar. 2018;17:1–5. doi: 10.1016/j.btre.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irshad M.A., et al. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: a review. Ecotoxicol. Environ. Saf. Apr. 2021;212 doi: 10.1016/j.ecoenv.2021.111978. [DOI] [PubMed] [Google Scholar]
- 10.Din M.I., Rehan R. Synthesis, characterization, and applications of copper nanoparticles. Anal. Lett. Jan. 2017;50(1):50–62. doi: 10.1080/00032719.2016.1172081. [DOI] [Google Scholar]
- 11.Moeinian M., Akhbari K. Various methods for synthesis of bulk and nano thallium(III) oxide. J. Inorg. Organomet. Polym. Mater. Jan. 2016;26(1):1–13. doi: 10.1007/s10904-015-0289-z. [DOI] [Google Scholar]
- 12.Akter M., et al. A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J. Adv. Res. Jan. 2018;9:1–16. doi: 10.1016/j.jare.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González E.A., et al. Sol–gel coatings doped with encapsulated silver nanoparticles: inhibition of biocorrosion on 2024-T3 aluminum alloy promoted by Pseudomonas aeruginosa. J. Mater. Res. Technol. Apr. 2019;8(2):1809–1818. doi: 10.1016/j.jmrt.2018.12.011. [DOI] [Google Scholar]
- 14.Rafique M., Sadaf I., Rafique M.S., Tahir M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells, Nanomed. Biotechnol. Oct. 2017;45(7):1272–1291. doi: 10.1080/21691401.2016.1241792. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad S., et al. Green nanotechnology: a review on green synthesis of silver nanoparticles — an ecofriendly approach. Int. J. Nanomed. Jul. 2019;14:5087–5107. doi: 10.2147/IJN.S200254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D. S. Pattanayak, N. Mallick, C. Thakur, and D. Pal, “Plant Mediated Green Synthesis of Silver Nanoparticles for Antimicrobial Application: Present Status”.
- 17.Chandrasekharan S., Chinnasamy G., Bhatnagar S. Sustainable phyto-fabrication of silver nanoparticles using Gmelina arborea exhibit antimicrobial and biofilm inhibition activity. Sci. Rep. Jan. 2022;12(1):156. doi: 10.1038/s41598-021-04025-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filippo E., Manno D., Buccolieri A., Di Giulio M., Serra A. Shape-dependent plasmon resonances of Ag nanostructures. Superlattice. Microst. Jan. 2010;47(1):66–71. doi: 10.1016/j.spmi.2009.07.036. [DOI] [Google Scholar]
- 19.Salar R.K., Sharma P., Kumar N. Enhanced antibacterial activity of streptomycin against some human pathogens using green synthesized silver nanoparticles. Resour.-Effic. Technol. Dec. 2015;1(2):106–115. doi: 10.1016/j.reffit.2015.11.004. [DOI] [Google Scholar]
- 20.Velmurugan P., et al. Synthesis and characterization comparison of peanut shell extract silver nanoparticles with commercial silver nanoparticles and their antifungal activity. J. Ind. Eng. Chem. Nov. 2015;31:51–54. doi: 10.1016/j.jiec.2015.06.031. [DOI] [Google Scholar]
- 21.Ghramh H.A., Ibrahim E.H., Kilany M. Study of anticancer, antimicrobial, immunomodulatory, and silver nanoparticles production by Sidr honey from three different sources. Food Sci. Nutr. Jan. 2020;8(1):445–455. doi: 10.1002/fsn3.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keast V.J., Myles T.A., Shahcheraghi N., Cortie M.B. Corrosion processes of triangular silver nanoparticles compared to bulk silver. J. Nanoparticle Res. Feb. 2016;18(2):45. doi: 10.1007/s11051-016-3354-9. [DOI] [Google Scholar]
- 23.Elhakim H.K.A., Azab S.M., Fekry A.M. A novel simple biosensor containing silver nanoparticles/propolis (bee glue) for microRNA let-7a determination. Mater. Sci. Eng. C. Nov. 2018;92:489–495. doi: 10.1016/j.msec.2018.06.063. [DOI] [PubMed] [Google Scholar]
- 24.Singh A., Dar M.Y., Joshi B., Sharma B., Shrivastava S., Shukla S. Phytofabrication of Silver nanoparticles: novel Drug to overcome hepatocellular ailments. Toxicol Rep. 2018;5:333–342. doi: 10.1016/j.toxrep.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sreeja S., Pesala B. Plasmonic enhancement of betanin-lawsone co-sensitized solar cells via tailored bimodal size distribution of silver nanoparticles. Sci. Rep. May 2020;10(1):8240. doi: 10.1038/s41598-020-65236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pant H.R., Pandeya D.R., Nam K.T., Baek W., Hong S.T., Kim H.Y. Photocatalytic and antibacterial properties of a TiO2/nylon-6 electrospun nanocomposite mat containing silver nanoparticles. J. Hazard Mater. May 2011;189(1–2):465–471. doi: 10.1016/j.jhazmat.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 27.Ram P., Vivek K., Kumar S.P. Nanotechnology in sustainable agriculture: present concerns and future aspects. Afr. J. Biotechnol. Feb. 2014;13(6):705–713. doi: 10.5897/AJBX2013.13554. [DOI] [Google Scholar]
- 28.Shahverdi A.R., Minaeian S., Shahverdi H.R., Jamalifar H., Nohi A.-A. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Process Biochem. May 2007;42(5):919–923. doi: 10.1016/j.procbio.2007.02.005. [DOI] [Google Scholar]
- 29.Choudhary M.K., Kataria J., Sharma S. Evaluation of the kinetic and catalytic properties of biogenically synthesized silver nanoparticles. J. Clean. Prod. Oct. 2018;198:882–890. doi: 10.1016/j.jclepro.2018.09.015. [DOI] [Google Scholar]
- 30.Xu Q., et al. Surface modification by carboxymethy chitosan via pad-dry-cure method for binding Ag NPs onto cotton fabric. Int. J. Biol. Macromol. May 2018;111:796–803. doi: 10.1016/j.ijbiomac.2018.01.091. [DOI] [PubMed] [Google Scholar]
- 31.Meng M., He H., Xiao J., Zhao P., Xie J., Lu Z. Controllable in situ synthesis of silver nanoparticles on multilayered film-coated silk fibers for antibacterial application. J. Colloid Interface Sci. Jan. 2016;461:369–375. doi: 10.1016/j.jcis.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes I.J., et al. Silver nanoparticle conductive inks: synthesis, characterization, and fabrication of inkjet-printed flexible electrodes. Sci. Rep. Jun. 2020;10(1):8878. doi: 10.1038/s41598-020-65698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu D.-G., Lin W.-C., Yang M.-C. Surface modification of poly(l -lactic acid) membrane via layer-by-layer assembly of silver nanoparticle-embedded polyelectrolyte multilayer. Bioconjugate Chem. Sep. 2007;18(5):1521–1529. doi: 10.1021/bc060098s. [DOI] [PubMed] [Google Scholar]
- 34.Shateri-Khalilabad M., Yazdanshenas M.E. Fabricating electroconductive cotton textiles using graphene. Carbohydr. Polym. Jul. 2013;96(1):190–195. doi: 10.1016/j.carbpol.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 35.Perkas N., Shuster M., Amirian G., Koltypin Y., Gedanken A. Sonochemical immobilization of silver nanoparticles on porous polypropylene. J. Polym. Sci. Part Polym. Chem. Mar. 2008;46(5):1719–1729. doi: 10.1002/pola.22513. [DOI] [Google Scholar]
- 36.Syafiuddin A. Toward a comprehensive understanding of textiles functionalized with silver nanoparticles. J. Chin. Chem. Soc. Aug. 2019;66(8):793–814. doi: 10.1002/jccs.201800474. [DOI] [Google Scholar]
- 37.Ema M., Okuda H., Gamo M., Honda K. A review of reproductive and developmental toxicity of silver nanoparticles in laboratory animals. Reprod. Toxicol. Jan. 2017;67:149–164. doi: 10.1016/j.reprotox.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Pryshchepa O., Pomastowski P., Buszewski B. Silver nanoparticles: synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. Oct. 2020;284 doi: 10.1016/j.cis.2020.102246. [DOI] [PubMed] [Google Scholar]
- 39.Khan S.A., et al. Leveraging the potential of silver nanoparticles-based materials towards sustainable water treatment. J. Environ. Manag. Oct. 2022;319 doi: 10.1016/j.jenvman.2022.115675. [DOI] [PubMed] [Google Scholar]
- 40.Sportelli M.C., et al. The pros and cons of the use of laser ablation synthesis for the production of silver nano-antimicrobials. Antibiotics. Jul. 2018;7(3):67. doi: 10.3390/antibiotics7030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gudikandula K., Charya Maringanti S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. Jun. 2016;11(9):714–721. doi: 10.1080/17458080.2016.1139196. [DOI] [Google Scholar]
- 42.Rafique M., Sadaf I., Rafique M.S., Tahir M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells, Nanomed. Biotechnol. Oct. 2017;45(7):1272–1291. doi: 10.1080/21691401.2016.1241792. [DOI] [PubMed] [Google Scholar]
- 43.Debnath D., Kim C., Kim S.H., Geckeler K.E. Solid‐state synthesis of silver nanoparticles at room temperature: poly(vinylpyrrolidone) as a tool. Macromol. Rapid Commun. Mar. 2010;31(6):549–553. doi: 10.1002/marc.200900656. [DOI] [PubMed] [Google Scholar]
- 44.Tri Handok C., Huda A., Gulo F. Synthesis pathway and powerful antimicrobial properties of silver nanoparticle: a critical review. Asian J. Sci. Res. Dec. 2018;12(1):1–17. doi: 10.3923/ajsr.2019.1.17. [DOI] [Google Scholar]
- 45.Jamkhande P.G., Ghule N.W., Bamer A.H., Kalaskar M.G. Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. Oct. 2019;53 doi: 10.1016/j.jddst.2019.101174. [DOI] [Google Scholar]
- 46.Rak M.J., Friščić T., Moores A. One-step, solvent-free mechanosynthesis of silver nanoparticle-infused lignin composites for use as highly active multidrug resistant antibacterial filters. RSC Adv. 2016;6(63):58365–58370. doi: 10.1039/C6RA03711A. [DOI] [Google Scholar]
- 47.Pragatheeswaran A., Ravi R., Bakshi S.R. Microstructural and morphological changes during ball milling of Copper-Silver-Graphite flake mixtures. Adv. Powder Technol. Nov. 2019;30(11):2759–2767. doi: 10.1016/j.apt.2019.08.023. [DOI] [Google Scholar]
- 48.Tsuzuki T. Mechanochemical synthesis of metal oxide nanoparticles. Commun. Chem. Oct. 2021;4(1):143. doi: 10.1038/s42004-021-00582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shih C.-Y., et al. Two mechanisms of nanoparticle generation in picosecond laser ablation in liquids: the origin of the bimodal size distribution. Nanoscale. 2018;10(15):6900–6910. doi: 10.1039/C7NR08614H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernández-Arias M., et al. RE-irradiation of silver nanoparticles obtained by laser ablation in water and assessment of their antibacterial effect. Appl. Surf. Sci. Apr. 2019;473:548–554. doi: 10.1016/j.apsusc.2018.12.182. [DOI] [Google Scholar]
- 51.Valverde-Alva M.A., et al. Synthesis of silver nanoparticles by laser ablation in ethanol: a pulsed photoacoustic study. Appl. Surf. Sci. Nov. 2015;355:341–349. doi: 10.1016/j.apsusc.2015.07.133. [DOI] [Google Scholar]
- 52.Sportelli M.C., et al. The pros and cons of the use of laser ablation synthesis for the production of silver nano-antimicrobials. Antibiotics. Jul. 2018;7(3):67. doi: 10.3390/antibiotics7030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Remita H., Remita S. Recent Trends in Radiation Chemistry. WORLD SCIENTIFIC; 2010. Metal clusters and nanomaterials: contribution of radiation chemistry; pp. 347–383. [DOI] [Google Scholar]
- 54.Choi S.-H., Lee S.-H., Hwang Y.-M., Lee K.-P., Kang H.-D. Interaction between the surface of the silver nanoparticles prepared by γ-irradiation and organic molecules containing thiol group. Radiat. Phys. Chem. Jun. 2003;67(3–4):517–521. doi: 10.1016/S0969-806X(03)00097-5. [DOI] [Google Scholar]
- 55.Kang Y., Choi S., Gopalan A., Lee K., Kang H., Song Y.S. One‐pot synthesis of a few nanocomposites with poly(N ‐vinylcarbazole) and CdS, Ag, Pd 50 –Ag 50 , and Pt 50 –Ru 50 nanoparticles with γ irradiation. J. Appl. Polym. Sci. May 2006;100(3):1809–1815. doi: 10.1002/app.23078. [DOI] [Google Scholar]
- 56.Kang Y.-O., Choi S.-H., Gopalan A., Lee K.-P., Kang H.-D., Song Y.S. Tuning of morphology of Ag nanoparticles in the Ag/polyaniline nanocomposites prepared by γ-ray irradiation. J. Non-Cryst. Solids. May 2006;352(5):463–468. doi: 10.1016/j.jnoncrysol.2006.01.043. [DOI] [Google Scholar]
- 57.Korolkov I.V., et al. Radiation induced deposition of copper nanoparticles inside the nanochannels of poly(acrylic acid)-grafted poly(ethylene terephthalate) track-etched membranes. Radiat. Phys. Chem. Jan. 2017;130:480–487. doi: 10.1016/j.radphyschem.2016.10.006. [DOI] [Google Scholar]
- 58.Flores-Rojas G.G., López-Saucedo F., López-Barriguete J.E., Isoshima T., Luna-Straffon M., Bucio E. Polypropylene films modified by grafting-from of ethylene glycol dimethacrylate/glycidyl methacrylate using γ-rays and antimicrobial biofunctionalization by Schiff bases. MRS Commun. Mar. 2018;8(1):168–177. doi: 10.1557/mrc.2018.14. [DOI] [Google Scholar]
- 59.Din M.I., Rehan R. Synthesis, characterization, and applications of copper nanoparticles. Anal. Lett. Jan. 2017;50(1):50–62. doi: 10.1080/00032719.2016.1172081. [DOI] [Google Scholar]
- 60.Belloni J. Nucleation, growth and properties of nanoclusters studied by radiation chemistry. Catal. Today. Apr. 2006;113(3–4):141–156. doi: 10.1016/j.cattod.2005.11.082. [DOI] [Google Scholar]
- 61.Ali I., Meligi G.A., Akl M.R., Saleh T.A. Influence of γ-ray irradiation doses on physicochemical properties of silver polystyrene polyvinyl pyrrolidone nanocomposites. Mater. Chem. Phys. Mar. 2019;226:250–256. doi: 10.1016/j.matchemphys.2018.12.084. [DOI] [Google Scholar]
- 62.He L., et al. Silver nanoparticles prepared by gamma irradiation across metal–organic framework templates. RSC Adv. 2015;5(14):10707–10715. doi: 10.1039/C4RA10260F. [DOI] [Google Scholar]
- 63.Dhayagude A.C., Das A., Joshi S.S., Kapoor S. γ-Radiation induced synthesis of silver nanoparticles in aqueous poly (N-vinylpyrrolidone) solution. Colloids Surf. A Physicochem. Eng. Asp. Nov. 2018;556:148–156. doi: 10.1016/j.colsurfa.2018.08.028. [DOI] [Google Scholar]
- 64.Iravani S., Korbekandi H., Mirmohammadi S.V., Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res. Pharm. Sci. 2014;9(6):385–406. [PMC free article] [PubMed] [Google Scholar]
- 65.Kruis F.E., Fissan H., Rellinghaus B. Sintering and evaporation characteristics of gas-phase synthesis of size-selected PbS nanoparticles. Mater. Sci. Eng. B. Jan. 2000;69(70):329–334. doi: 10.1016/S0921-5107(99)00298-6. [DOI] [Google Scholar]
- 66.Raffi M., Rumaiz A.K., Hasan M.M., Shah S.I. Studies of the growth parameters for silver nanoparticle synthesis by inert gas condensation. J. Mater. Res. Dec. 2007;22(12):3378–3384. doi: 10.1557/JMR.2007.0420. [DOI] [Google Scholar]
- 67.Jung J.H., Cheol Oh H., Soo Noh H., Ji J.H., Soo Kim S. Metal nanoparticle generation using a small ceramic heater with a local heating area. J. Aerosol Sci. Dec. 2006;37(12):1662–1670. doi: 10.1016/j.jaerosci.2006.09.002. [DOI] [Google Scholar]
- 68.Harra J., et al. Size-controlled aerosol synthesis of silver nanoparticles for plasmonic materials. J. Nanoparticle Res. Jun. 2012;14(6):870. doi: 10.1007/s11051-012-0870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khayati G.R., Janghorban K. The nanostructure evolution of Ag powder synthesized by high energy ball milling. Adv. Powder Technol. May 2012;23(3):393–397. doi: 10.1016/j.apt.2011.05.005. [DOI] [Google Scholar]
- 70.Kumar N., Biswas K., Gupta R.K. Green synthesis of Ag nanoparticles in large quantity by cryomilling. RSC Adv. 2016;6(112):111380–111388. doi: 10.1039/C6RA23120A. [DOI] [Google Scholar]
- 71.Rak M.J., Friščić T., Moores A. One-step, solvent-free mechanosynthesis of silver nanoparticle-infused lignin composites for use as highly active multidrug resistant antibacterial filters. RSC Adv. 2016;6(63):58365–58370. doi: 10.1039/C6RA03711A. [DOI] [Google Scholar]
- 72.Boutinguiza M., Comesaña R., Lusquiños F., Riveiro A., Del Val J., Pou J. Production of silver nanoparticles by laser ablation in open air. Appl. Surf. Sci. May 2015;336:108–111. doi: 10.1016/j.apsusc.2014.09.193. [DOI] [Google Scholar]
- 73.Rafique M., Rafique M.S., Kalsoom U., Afzal A., Butt S.H., Usman A. Laser ablation synthesis of silver nanoparticles in water and dependence on laser nature. Opt. Quant. Electron. Jun. 2019;51(6):179. doi: 10.1007/s11082-019-1902-0. [DOI] [Google Scholar]
- 74.Darroudi M., et al. Preparation and characterization of gelatin mediated silver nanoparticles by laser ablation. J. Alloys Compd. Jan. 2011;509(4):1301–1304. doi: 10.1016/j.jallcom.2010.10.018. [DOI] [Google Scholar]
- 75.Rhim J.-W., Wang L.-F., Lee Y., Hong S.-I. Preparation and characterization of bio-nanocomposite films of agar and silver nanoparticles: laser ablation method. Carbohydr. Polym. Mar. 2014;103:456–465. doi: 10.1016/j.carbpol.2013.12.075. [DOI] [PubMed] [Google Scholar]
- 76.Sportelli M.C., et al. Exceptionally stable silver nanoparticles synthesized by laser ablation in alcoholic organic solvent. Colloids Surf. A Physicochem. Eng. Asp. Dec. 2018;559:148–158. doi: 10.1016/j.colsurfa.2018.09.046. [DOI] [Google Scholar]
- 77.Fernández-Arias M., et al. RE-irradiation of silver nanoparticles obtained by laser ablation in water and assessment of their antibacterial effect. Appl. Surf. Sci. Apr. 2019;473:548–554. doi: 10.1016/j.apsusc.2018.12.182. [DOI] [Google Scholar]
- 78.Arce V.B., Santillán J.M.J., Muñetón Arboleda D., Muraca D., Scaffardi L.B., Schinca D.C. Characterization and stability of silver nanoparticles in starch solution obtained by femtosecond laser ablation and salt reduction. J. Phys. Chem. C. May 2017;121(19):10501–10513. doi: 10.1021/acs.jpcc.6b12384. [DOI] [Google Scholar]
- 79.Van Phu D., et al. Study on antibacterial activity of silver nanoparticles synthesized by gamma irradiation method using different stabilizers. Nanoscale Res. Lett. Dec. 2014;9(1):162. doi: 10.1186/1556-276X-9-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madhukumar R., Byrappa K., Wang Y., Sangappa Y. Effect of gamma irradiation on synthesis and characterization of bio-nanocomposite SF/Ag nanoparticles. Radiat. Eff. Defect Solid. Dec. 2017;172(11–12):915–921. doi: 10.1080/10420150.2017.1418873. [DOI] [Google Scholar]
- 81.He L., et al. Silver nanoparticles prepared by gamma irradiation across metal–organic framework templates. RSC Adv. 2015;5(14):10707–10715. doi: 10.1039/C4RA10260F. [DOI] [Google Scholar]
- 82.Dhayagude A.C., Das A., Joshi S.S., Kapoor S. γ-Radiation induced synthesis of silver nanoparticles in aqueous poly (N-vinylpyrrolidone) solution. Colloids Surf. A Physicochem. Eng. Asp. Nov. 2018;556:148–156. doi: 10.1016/j.colsurfa.2018.08.028. [DOI] [Google Scholar]
- 83.Eghbalifam N., Frounchi M., Dadbin S. Antibacterial silver nanoparticles in polyvinyl alcohol/sodium alginate blend produced by gamma irradiation. Int. J. Biol. Macromol. Sep. 2015;80:170–176. doi: 10.1016/j.ijbiomac.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 84.Ali I., Meligi G.A., Akl M.R., Saleh T.A. Influence of γ-ray irradiation doses on physicochemical properties of silver polystyrene polyvinyl pyrrolidone nanocomposites. Mater. Chem. Phys. Mar. 2019;226:250–256. doi: 10.1016/j.matchemphys.2018.12.084. [DOI] [Google Scholar]
- 85.Ardjoum N., Shankar S., Chibani N., Salmieri S., Lacroix M. In situ synthesis of silver nanoparticles in pectin matrix using gamma irradiation for the preparation of antibacterial pectin/silver nanoparticles composite films. Food Hydrocolloids. Dec. 2021;121 doi: 10.1016/j.foodhyd.2021.107000. [DOI] [Google Scholar]
- 86.Burda C., Chen X., Narayanan R., El-Sayed M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. Apr. 2005;105(4):1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 87.Li Q., et al. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res. Nov. 2008;42(18):4591–4602. doi: 10.1016/j.watres.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 88.Blaser S.A., Scheringer M., MacLeod M., Hungerbühler K. Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles. Sci. Total Environ. Feb. 2008;390(2–3):396–409. doi: 10.1016/j.scitotenv.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 89.Vigneshwaran N., Nachane R.P., Balasubramanya R.H., Varadarajan P.V. A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohydr. Res. Sep. 2006;341(12):2012–2018. doi: 10.1016/j.carres.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 90.Wang W., Efrima S., Regev O. Directing silver nanoparticles into Colloid−Surfactant lyotropic lamellar systems. J. Phys. Chem. B. Jul. 1999;103(27):5613–5621. doi: 10.1021/jp983125n. [DOI] [Google Scholar]
- 91.Mulfinger L., Solomon S.D., Bahadory M., Jeyarajasingam A.V., Rutkowsky S.A., Boritz C. Synthesis and study of silver nanoparticles. J. Chem. Educ. Feb. 2007;84(2):322. doi: 10.1021/ed084p322. [DOI] [Google Scholar]
- 92.Yang J., Lee J.Y., Too H.-P. Core−Shell Ag−Au nanoparticles from replacement reaction in organic medium. J. Phys. Chem. B. Oct. 2005;109(41):19208–19212. doi: 10.1021/jp052242x. [DOI] [PubMed] [Google Scholar]
- 93.Sławiński G.W., Zamborini F.P. Synthesis and alignment of silver nanorods and nanowires and the formation of Pt, Pd, and core/shell structures by galvanic exchange directly on surfaces. Langmuir. Sep. 2007;23(20):10357–10365. doi: 10.1021/la701606p. [DOI] [PubMed] [Google Scholar]
- 94.Navaladian S., Viswanathan B., Varadarajan T.K., Viswanath R.P. Microwave-assisted rapid synthesis of anisotropic Ag nanoparticles by solid state transformation. Nanotechnology. Jan. 2008;19(4) doi: 10.1088/0957-4484/19/04/045603. [DOI] [PubMed] [Google Scholar]
- 95.Kuila B.K., Garai A., Nandi A.K. Synthesis, optical, and electrical characterization of organically soluble silver nanoparticles and their poly(3-hexylthiophene) nanocomposites: enhanced luminescence property in the nanocomposite thin films. Chem. Mater. Oct. 2007;19(22):5443–5452. doi: 10.1021/cm7020214. [DOI] [Google Scholar]
- 96.Yang J., Lee J.Y., Too H.-P. A general phase transfer protocol for synthesizing alkylamine-stabilized nanoparticles of noble metals. Anal. Chim. Acta. Apr. 2007;588(1):34–41. doi: 10.1016/j.aca.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 97.Kalimuthu K., Suresh Babu R., Venkataraman D., Bilal Mohd., Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces. Aug. 2008;65(1):150–153. doi: 10.1016/j.colsurfb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 98.Chen J., Wang J., Zhang X., Jin Y. Microwave-assisted green synthesis of silver nanoparticles by carboxymethyl cellulose sodium and silver nitrate. Mater. Chem. Phys. Apr. 2008;108(2–3):421–424. doi: 10.1016/j.matchemphys.2007.10.019. [DOI] [Google Scholar]
- 99.Chen Y., Wang X. Novel phase-transfer preparation of monodisperse silver and gold nanoparticles at room temperature. Mater. Lett. May 2008;62(15):2215–2218. doi: 10.1016/j.matlet.2007.11.050. [DOI] [Google Scholar]
- 100.Wang D., Xie T., Peng Q., Li Y. Ag, Ag 2 S, and Ag 2 Se nanocrystals: synthesis, assembly, and construction of mesoporous structures. J. Am. Chem. Soc. Mar. 2008;130(12):4016–4022. doi: 10.1021/ja710004h. [DOI] [PubMed] [Google Scholar]
- 101.Najafi A., Khoeini M., Khalaj G., Sahebgharan A. Synthesis of silver nanoparticles from electronic scrap by chemical reduction. Mater. Res. Express. Dec. 2021;8(12) doi: 10.1088/2053-1591/ac406d. [DOI] [Google Scholar]
- 102.Eka Putri G., Rahayu Gusti F., Novita Sary A., Zainul R. Synthesis of silver nanoparticles used chemical reduction method by glucose as reducing agent. J. Phys. Conf. Ser. Oct. 2019;1317(1) doi: 10.1088/1742-6596/1317/1/012027. [DOI] [Google Scholar]
- 103.Quintero-Quiroz C., et al. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater. Res. Dec. 2019;23(1):27. doi: 10.1186/s40824-019-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oćwieja M., Adamczyk Z. Monolayers of silver nanoparticles obtained by chemical reduction methods. Surf. Innovations. Sep. 2014;2(3):160–172. doi: 10.1680/si.13.00042. [DOI] [Google Scholar]
- 105.Ahani M., Khatibzadeh M. Optimisation of significant parameters through response surface methodology in the synthesis of silver nanoparticles by chemical reduction method. Micro & Nano Lett. Sep. 2017;12(9):705–710. doi: 10.1049/mnl.2017.0118. [DOI] [Google Scholar]
- 106.Gudikandula K., Charya Maringanti S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. Jun. 2016;11(9):714–721. doi: 10.1080/17458080.2016.1139196. [DOI] [Google Scholar]
- 107.Hu S., Hsieh Y.-L. Silver nanoparticle synthesis using lignin as reducing and capping agents: a kinetic and mechanistic study. Int. J. Biol. Macromol. Jan. 2016;82:856–862. doi: 10.1016/j.ijbiomac.2015.09.066. [DOI] [PubMed] [Google Scholar]
- 108.Dang D.M.T., Dang C.M., Blanc E.F. Study of the formation of silver nanoparticles and silver nanoplates by chemical reduction method. Int. J. Nanotechnol. 2015;12(5/6/7):456. doi: 10.1504/IJNT.2015.067903. [DOI] [Google Scholar]
- 109.Jung D.S., Ko Y.N., Kang Y.C., Park S.B. Recent progress in electrode materials produced by spray pyrolysis for next-generation lithium ion batteries. Adv. Powder Technol. Jan. 2014;25(1):18–31. doi: 10.1016/j.apt.2014.01.012. [DOI] [Google Scholar]
- 110.Zhu Y., et al. Recent progress on spray pyrolysis for high performance electrode materials in lithium and sodium rechargeable batteries. Adv. Energy Mater. Apr. 2017;7(7) doi: 10.1002/aenm.201601578. [DOI] [Google Scholar]
- 111.Jung D.S., Koo H.Y., Wang S.E., Park S.B., Kang Y.C. Ultrasonic spray pyrolysis for air-stable copper particles and their conductive films. Acta Mater. Mar. 2021;206 doi: 10.1016/j.actamat.2020.116569. [DOI] [Google Scholar]
- 112.Teoh W.Y., Amal R., Mädler L. Flame spray pyrolysis: an enabling technology for nanoparticles design and fabrication. Nanoscale. 2010;2(8):1324. doi: 10.1039/c0nr00017e. [DOI] [PubMed] [Google Scholar]
- 113.Strobel R., Baiker A., Pratsinis S.E. Aerosol flame synthesis of catalysts. Adv. Powder Technol. 2006;17(5):457–480. doi: 10.1163/156855206778440525. [DOI] [Google Scholar]
- 114.Leng J., et al. Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 2019;48(11):3015–3072. doi: 10.1039/C8CS00904J. [DOI] [PubMed] [Google Scholar]
- 115.Leng J., et al. Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 2019;48(11):3015–3072. doi: 10.1039/C8CS00904J. [DOI] [PubMed] [Google Scholar]
- 116.Emil E., Gürmen S. Estimation of yttrium oxide microstructural parameters using the Williamson–Hall analysis. Mater. Sci. Technol. Sep. 2018;34(13):1549–1557. doi: 10.1080/02670836.2018.1490857. [DOI] [Google Scholar]
- 117.Alkan G., et al. Plasmon enhanced luminescence in hierarchically structured Ag@ (Y0.95Eu0.05)2O3 nanocomposites synthesized by ultrasonic spray pyrolysis. Adv. Powder Technol. Jul. 2019;30(7):1409–1418. doi: 10.1016/j.apt.2019.04.024. [DOI] [Google Scholar]
- 118.Toparli C., Ebin B., Gürmen S. Synthesis, structural and magnetic characterization of soft magnetic nanocrystalline ternary FeNiCo particles. J. Magn. Magn Mater. Feb. 2017;423:133–139. doi: 10.1016/j.jmmm.2016.09.005. [DOI] [Google Scholar]
- 119.Emil E., Alkan G., Gurmen S., Rudolf R., Jenko D., Friedrich B. Tuning the morphology of ZnO nanostructures with the ultrasonic spray pyrolysis process. Metals. Jul. 2018;8(8):569. doi: 10.3390/met8080569. [DOI] [Google Scholar]
- 120.Honda M., Kikushima K., Kawanobe Y., Konishi T., Mizumoto M., Aizawa M. Enhanced early osteogenic differentiation by silicon-substituted hydroxyapatite ceramics fabricated via ultrasonic spray pyrolysis route. J. Mater. Sci. Mater. Med. Dec. 2012;23(12):2923–2932. doi: 10.1007/s10856-012-4744-x. [DOI] [PubMed] [Google Scholar]
- 121.Aizawa M., Howell F.S., Itatani K. Characterization of strontiumapatite powders prepared by ultrasonic spray-pyrolysis technique. J. Ceram. Soc. Jpn. 1999;107(1251):1007–1011. doi: 10.2109/jcersj.107.1007. [DOI] [Google Scholar]
- 122.Gürmen S., Stopić S., Friedrich B. Synthesis of nanosized spherical cobalt powder by ultrasonic spray pyrolysis. Mater. Res. Bull. Oct. 2006;41(10):1882–1890. doi: 10.1016/j.materresbull.2006.03.006. [DOI] [Google Scholar]
- 123.Ueda M., et al. Regulating size of silver nanoparticles on calcium carbonate via ultrasonic spray for effective antibacterial efficacy and sustained release. Mater. Sci. Eng. C. Jun. 2021;125 doi: 10.1016/j.msec.2021.112083. [DOI] [PubMed] [Google Scholar]
- 124.Bogovic J., Rudolf R., Friedrich B. The controlled single-step synthesis of Ag/TiO2 and Au/TiO2 by ultrasonic spray pyrolysis (USP) JOM. Jan. 2016;68(1):330–335. doi: 10.1007/s11837-015-1417-5. [DOI] [Google Scholar]
- 125.Zhao C., Krall A., Zhao H., Zhang Q., Li Y. Ultrasonic spray pyrolysis synthesis of Ag/TiO2 nanocomposite photocatalysts for simultaneous H2 production and CO2 reduction. Int. J. Hydrogen Energy. Jul. 2012;37(13):9967–9976. doi: 10.1016/j.ijhydene.2012.04.003. [DOI] [Google Scholar]
- 126.Dermenci K.B., Genc B., Ebin B., Olmez-Hanci T., Gürmen S. Photocatalytic studies of Ag/ZnO nanocomposite particles produced via ultrasonic spray pyrolysis method. J. Alloys Compd. Feb. 2014;586:267–273. doi: 10.1016/j.jallcom.2013.10.004. [DOI] [Google Scholar]
- 127.Okuyama K., Wuled Lenggoro I. Preparation of nanoparticles via spray route. Chem. Eng. Sci. Feb. 2003;58(3–6):537–547. doi: 10.1016/S0009-2509(02)00578-X. [DOI] [Google Scholar]
- 128.E Al-Saedy M.A. Preparation method of silver nano particles. J. Adv. Sci. Eng. Technol. Oct. 2020;3(2):1–8. doi: 10.32441/jaset.03.02.01. [DOI] [Google Scholar]
- 129.Shih S.-J., Chien I.-C. Preparation and characterization of nanostructured silver particles by one-step spray pyrolysis. Powder Technol. Mar. 2013;237:436–441. doi: 10.1016/j.powtec.2012.12.032. [DOI] [Google Scholar]
- 130.Muñoz-Fernandez L., Alkan G., Milošević O., Rabanal M.E., Friedrich B. Synthesis and characterisation of spherical core-shell Ag/ZnO nanocomposites using single and two – steps ultrasonic spray pyrolysis (USP) Catal. Today. Feb. 2019;321–322:26–33. doi: 10.1016/j.cattod.2017.11.029. [DOI] [Google Scholar]
- 131.Emil Kaya E., Kaya O., Alkan G., Gürmen S., Stopic S., Friedrich B. New proposal for size and size-distribution evaluation of nanoparticles synthesized via ultrasonic spray pyrolysis using search algorithm based on image-processing technique. Materials. Dec. 2019;13(1):38. doi: 10.3390/ma13010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Danks A.E., Hall S.R., Schnepp Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016;3(2):91–112. doi: 10.1039/C5MH00260E. [DOI] [Google Scholar]
- 133.Esposito S. Traditional’ sol-gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials. Feb. 2019;12(4):668. doi: 10.3390/ma12040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hornbuckle K.C., Carlson D.L., Swackhamer D.L., Baker J.E., Eisenreich S.J. In: Persistent Organic Pollutants in the Great Lakes. Hites R.A., editor. 5N. Springer Berlin Heidelberg; 2006. Polychlorinated biphenyls in the Great Lakes; pp. 13–70. (The Handbook of Environmental Chemistry). 5N. [DOI] [Google Scholar]
- 135.Periyasamy A.P., Venkataraman M., Kremenakova D., Militky J., Zhou Y. Progress in sol-gel technology for the coatings of fabrics. Materials. Apr. 2020;13(8):1838. doi: 10.3390/ma13081838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thiagarajan S., Sanmugam A., Vikraman D. In: Recent Applications in Sol-Gel Synthesis. Chandra U., editor. InTech; 2017. Facile methodology of sol-gel synthesis for metal oxide nanostructures. [DOI] [Google Scholar]
- 137.Ali G.A.M., Fouad O.A., Makhlouf S.A. Structural, optical and electrical properties of sol–gel prepared mesoporous Co3O4/SiO2 nanocomposites. J. Alloys Compd. Dec. 2013;579:606–611. doi: 10.1016/j.jallcom.2013.07.095. [DOI] [Google Scholar]
- 138.Abdel Ghafar H.H., Ali G.A.M., Fouad O.A., Makhlouf S.A. Enhancement of adsorption efficiency of methylene blue on Co3O4/SiO2 nanocomposite. Desalination Water Treat. Mar. 2015;53(11):2980–2989. doi: 10.1080/19443994.2013.871343. [DOI] [Google Scholar]
- 139.Efficient and recyclable Cu incorporated TiO2 nanoparticle catalyst for organic dye photodegradation. Int. J. Thin Film Sci. Technol. Sep. 2021;10(3):169–182. doi: 10.18576/ijtfst/100306. [DOI] [Google Scholar]
- 140.Zhai S.-R., Shao X., Zhou D., Zhai B., An Q.-D. Sol–gel synthesis of nanosilver embedded hybrid materials using combined organosilica precursors. J. Sol. Gel Sci. Technol. Jun. 2012;62(3):281–286. doi: 10.1007/s10971-012-2713-y. [DOI] [Google Scholar]
- 141.Shahjahan M. Synthesis and characterization of silver nanoparticles by sol-gel technique. Nanosci. Nanometrol. 2017;3(1):34. doi: 10.11648/j.nsnm.20170301.16. [DOI] [Google Scholar]
- 142.Ahlawat D.S., Kumari R., Rachna, Yadav I. Synthesis and characterization of sol–gel prepared silver nanoparticles. Int. J. Nanosci. Feb. 2014;13(1) doi: 10.1142/S0219581X14500045. [DOI] [Google Scholar]
- 143.Maharjan S., et al. Sol-gel synthesis of stabilized silver nanoparticles in an organosiloxane matrix and its optical nonlinearity. Chem. Phys. Apr. 2020;532 doi: 10.1016/j.chemphys.2019.110610. [DOI] [Google Scholar]
- 144.Petit C.T.G., Alsulaiman M.S.A., Lan R., Mann G., Tao S. Preparation of silver nanoparticles by a non-aqueous sol–gel process. J. Nanosci. Nanotechnol. Aug. 2013;13(8):5445–5451. doi: 10.1166/jnn.2013.7446. [DOI] [PubMed] [Google Scholar]
- 145.Saraidarov T., Levchenko V., Reisfeld R. Synthesis of silver nanoparticles and their stabilization in different sol‐gel matrices: optical and structural characterization. Phys. Status Solidi C. Nov. 2010;7(11–12):2648–2651. doi: 10.1002/pssc.200983784. [DOI] [Google Scholar]
- 146.González E.A., et al. Sol–gel coatings doped with encapsulated silver nanoparticles: inhibition of biocorrosion on 2024-T3 aluminum alloy promoted by Pseudomonas aeruginosa. J. Mater. Res. Technol. Apr. 2019;8(2):1809–1818. doi: 10.1016/j.jmrt.2018.12.011. [DOI] [Google Scholar]
- 147.Arun Kumar K.V., John J., Sooraj T.R., Raj S.A., Unnikrishnan N.V., Selvaraj N.B. Surface plasmon response of silver nanoparticles doped silica synthesised via sol-gel route. Appl. Surf. Sci. Apr. 2019;472:40–45. doi: 10.1016/j.apsusc.2018.05.178. [DOI] [Google Scholar]
- 148.Li Q., Liu W.Y., Sun G.Y. Z-scan study on a silver nanoparticles embedded TeO2-SiO2 glass prepared by sol-gel method. Key Eng. Mater. Apr. 2018;768:239–245. doi: 10.4028/www.scientific.net/KEM.768.239. [DOI] [Google Scholar]
- 149.Thalji M.R., Ali G.A.M., Algarni H., Chong K.F. Al3+ ion intercalation pseudocapacitance study of W18O49 nanostructure. J. Power Sources. 2019;438(Oct) doi: 10.1016/j.jpowsour.2019.227028. [DOI] [Google Scholar]
- 150.Ocsoy I., Demirbas A., McLamore E.S., Altinsoy B., Ildiz N., Baldemir A. Green synthesis with incorporated hydrothermal approaches for silver nanoparticles formation and enhanced antimicrobial activity against bacterial and fungal pathogens. J. Mol. Liq. Jul. 2017;238:263–269. doi: 10.1016/j.molliq.2017.05.012. [DOI] [Google Scholar]
- 151.Aksomaityte G., Poliakoff M., Lester E. The production and formulation of silver nanoparticles using continuous hydrothermal synthesis. Chem. Eng. Sci. Jan. 2013;85:2–10. doi: 10.1016/j.ces.2012.05.035. [DOI] [Google Scholar]
- 152.Li Y., Gan W., Zhou J., Lu Z., Yang C., Ge T. Hydrothermal synthesis of silver nanoparticles in Arabic gum aqueous solutions. Trans. Nonferrous Metals Soc. China. Jun. 2015;25(6):2081–2086. doi: 10.1016/S1003-6326(15)63818-3. [DOI] [Google Scholar]
- 153.Choi H., Veriansyah B., Kim J., Kim J.-D., Kang J.W. Continuous synthesis of metal nanoparticles in supercritical methanol. J. Supercrit. Fluids. Apr. 2010;52(3):285–291. doi: 10.1016/j.supflu.2010.01.015. [DOI] [Google Scholar]
- 154.Kim J., Kim D., Veriansyah B., Won Kang J., Kim J.-D. Metal nanoparticle synthesis using supercritical alcohol. Mater. Lett. Aug. 2009;63(21):1880–1882. doi: 10.1016/j.matlet.2009.05.066. [DOI] [Google Scholar]
- 155.Yang J., Pan J. Hydrothermal synthesis of silver nanoparticles by sodium alginate and their applications in surface-enhanced Raman scattering and catalysis. Acta Mater. Jul. 2012;60(12):4753–4758. doi: 10.1016/j.actamat.2012.05.037. [DOI] [Google Scholar]
- 156.Wu Y., Wang Z., Chen S., Wu J., Guo X., Liu Z. One-step hydrothermal synthesis of silver nanoparticles loaded on N-doped carbon and application for catalytic reduction of 4-nitrophenol. RSC Adv. 2015;5(106):87151–87156. doi: 10.1039/C5RA07589K. [DOI] [Google Scholar]
- 157.Lu W., Liao F., Luo Y., Chang G., Sun X. Hydrothermal synthesis of well-stable silver nanoparticles and their application for enzymeless hydrogen peroxide detection. Electrochim. Acta. Feb. 2011;56(5):2295–2298. doi: 10.1016/j.electacta.2010.11.053. [DOI] [Google Scholar]
- 158.Faramarzi M.A., Sadighi A. Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Adv. Colloid Interface Sci. Mar. 2013;189–190:1–20. doi: 10.1016/j.cis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 159.Iravani S. Bacteria in nanoparticle synthesis: current status and future prospects. Int. Sch. Res. Notices. Oct. 2014;2014:1–18. doi: 10.1155/2014/359316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Otari S.V., Patil R.M., Ghosh S.J., Thorat N.D., Pawar S.H. Intracellular synthesis of silver nanoparticle by actinobacteria and its antimicrobial activity. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. Feb. 2015;136:1175–1180. doi: 10.1016/j.saa.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 161.Rosyidah A., Weeranantanapan O., Chudapongse N., Limphirat W., Nantapong N. Streptomyces chiangmaiensis SSUT88A mediated green synthesis of silver nanoparticles: characterization and evaluation of antibacterial action against clinical drug-resistant strains. RSC Adv. 2022;12(7):4336–4345. doi: 10.1039/D1RA08238H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bano N., et al. Antibacterial efficacy of synthesized silver nanoparticles of Microbacterium proteolyticum LA2(R) and Streptomyces rochei LA2(O) against biofilm forming meningitis causing microbes. Sci. Rep. Mar. 2023;13(1):4150. doi: 10.1038/s41598-023-30215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Solís-Sandí I., Cordero-Fuentes S., Pereira-Reyes R., Vega-Baudrit J.R., Batista-Menezes D., Montes De Oca-Vásquez G. Optimization of the biosynthesis of silver nanoparticles using bacterial extracts and their antimicrobial potential. Biotechnol. Rep. Dec. 2023;40 doi: 10.1016/j.btre.2023.e00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mandal D., Bolander M.E., Mukhopadhyay D., Sarkar G., Mukherjee P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. Jan. 2006;69(5):485–492. doi: 10.1007/s00253-005-0179-3. [DOI] [PubMed] [Google Scholar]
- 165.Liu X., et al. Biosynthesis of silver nanoparticles with antimicrobial and anticancer properties using two novel yeasts. Sci. Rep. Aug. 2021;11(1) doi: 10.1038/s41598-021-95262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang D., Xue B., Wang L., Zhang Y., Liu L., Zhou Y. Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Sci. Rep. May 2021;11(1) doi: 10.1038/s41598-021-89854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Singh T., Jyoti K., Patnaik A., Singh A., Chauhan R., Chandel S.S. Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J. Genet. Eng. Biotechnol. Jun. 2017;15(1):31–39. doi: 10.1016/j.jgeb.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Eid A.M., et al. Endophytic Streptomyces laurentii mediated green synthesis of Ag-NPs with antibacterial and anticancer properties for developing functional textile fabric properties. Antibiotics. Sep. 2020;9(10):641. doi: 10.3390/antibiotics9100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Du J., Singh H., Yi T.-H. Biosynthesis of silver nanoparticles by Novosphingobium sp. THG-C3 and their antimicrobial potential. Artif. Cells, Nanomed. Biotechnol. Feb. 2017;45(2):211–217. doi: 10.1080/21691401.2016.1178135. [DOI] [PubMed] [Google Scholar]
- 170.Singh H., Du J., Yi T.-H. Kinneretia THG-SQI4 mediated biosynthesis of silver nanoparticles and its antimicrobial efficacy. Artif. Cells, Nanomed. Biotechnol. Apr. 2017;45(3):602–608. doi: 10.3109/21691401.2016.1163718. [DOI] [PubMed] [Google Scholar]
- 171.Syed B., et al. Biomimetic synthesis of silver nanoparticles using endosymbiotic bacterium inhabiting Euphorbia hirta L. And their bactericidal potential. Scientifica. 2016;2016:1–7. doi: 10.1155/2016/9020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Singh H., Du J., Yi T.-H. Biosynthesis of silver nanoparticles using Aeromonas sp. THG-FG1.2 and its antibacterial activity against pathogenic microbes. Artif. Cells, Nanomed. Biotechnol. Apr. 2017;45(3):584–590. doi: 10.3109/21691401.2016.1163715. [DOI] [PubMed] [Google Scholar]
- 173.Das V.L., Thomas R., Varghese R.T., Soniya E.V., Mathew J., Radhakrishnan E.K. Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech. Apr. 2014;4(2):121–126. doi: 10.1007/s13205-013-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Raza S., Ansari A., Siddiqui N.N., Ibrahim F., Abro M.I., Aman A. Biosynthesis of silver nanoparticles for the fabrication of non cytotoxic and antibacterial metallic polymer based nanocomposite system. Sci. Rep. May 2021;11(1) doi: 10.1038/s41598-021-90016-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Syed A., Saraswati S., Kundu G.C., Ahmad A. Biological synthesis of silver nanoparticles using the fungus Humicola sp. and evaluation of their cytoxicity using normal and cancer cell lines. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. Oct. 2013;114:144–147. doi: 10.1016/j.saa.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 176.Vahabi K., Mansoori G.A., Karimi S. Biosynthesis of silver nanoparticles by fungus Trichoderma reesei (A route for large-scale production of AgNPs) Insci. J. Feb. 2011:65–79. doi: 10.5640/insc.010165. [DOI] [Google Scholar]
- 177.Bhainsa K.C., D'Souza S.F. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf. B Biointerfaces. Feb. 2006;47(2):160–164. doi: 10.1016/j.colsurfb.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 178.Vigneshwaran N., Ashtaputre N.M., Varadarajan P.V., Nachane R.P., Paralikar K.M., Balasubramanya R.H. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater. Lett. Mar. 2007;61(6):1413–1418. doi: 10.1016/j.matlet.2006.07.042. [DOI] [Google Scholar]
- 179.Basavaraja S., Balaji S.D., Lagashetty A., Rajasab A.H., Venkataraman A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater. Res. Bull. May 2008;43(5):1164–1170. doi: 10.1016/j.materresbull.2007.06.020. [DOI] [Google Scholar]
- 180.Gajbhiye M., Kesharwani J., Ingle A., Gade A., Rai M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed. Nanotechnol. Biol. Med. Dec. 2009;5(4):382–386. doi: 10.1016/j.nano.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 181.Banu A., Rathod V., Ranganath E. Silver nanoparticle production by Rhizopus stolonifer and its antibacterial activity against extended spectrum β-lactamase producing (ESBL) strains of Enterobacteriaceae. Mater. Res. Bull. Sep. 2011;46(9):1417–1423. doi: 10.1016/j.materresbull.2011.05.008. [DOI] [Google Scholar]
- 182.Vanaraj S., Keerthana B.B., Preethi K. Biosynthesis, characterization of silver nanoparticles using quercetin from Clitoria ternatea L to enhance toxicity against bacterial biofilm. J. Inorg. Organomet. Polym. Mater. Sep. 2017;27(5):1412–1422. doi: 10.1007/s10904-017-0595-8. [DOI] [Google Scholar]
- 183.Sumitha S., et al. Phyto-mediated photo catalysed green synthesis of silver nanoparticles using Durio zibethinus seed extract: antimicrobial and cytotoxic activity and photocatalytic applications. Molecules. Dec. 2018;23(12):3311. doi: 10.3390/molecules23123311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ahmad A., et al. The effects of bacteria-nanoparticles interface on the antibacterial activity of green synthesized silver nanoparticles. Microb. Pathog. Jan. 2017;102:133–142. doi: 10.1016/j.micpath.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 185.Khashan A.A., Dawood Y., Khalaf Y.H. Green chemistry and anti-inflammatory activity of silver nanoparticles using an aqueous curcumin extract. Results Chem. Jan. 2023;5 doi: 10.1016/j.rechem.2023.100913. [DOI] [Google Scholar]
- 186.Zarei M., Seyedi N., Maghsoudi S., Nejad M.S., Sheibani H. Green synthesis of Ag nanoparticles on the modified graphene oxide using Capparis spinosa fruit extract for catalytic reduction of organic dyes. Inorg. Chem. Commun. Jan. 2021;123 doi: 10.1016/j.inoche.2020.108327. [DOI] [Google Scholar]
- 187.Maitra B., et al. Biosynthesis of Bixa orellana seed extract mediated silver nanoparticles with moderate antioxidant, antibacterial and antiproliferative activity. Arab. J. Chem. May 2023;16(5) doi: 10.1016/j.arabjc.2023.104675. [DOI] [Google Scholar]
- 188.Fareed N., et al. Green synthesized silver nanoparticles using carrot extract exhibited strong antibacterial activity against multidrug resistant bacteria. J. King Saud Univ. Sci. Feb. 2023;35(2) doi: 10.1016/j.jksus.2022.102477. [DOI] [Google Scholar]
- 189.Jayeoye T.J., et al. Green synthesis of silver nanoparticles using cyto-compatible polymer derivative of tara gum for gold (III) ion detection in water samples. J. Polym. Environ. Sep. 2024 doi: 10.1007/s10924-024-03393-4. [DOI] [Google Scholar]
- 190.Kumar A., et al. Biogenic metallic nanoparticles: biomedical, analytical, food preservation, and applications in other consumable products. Front. Nanotechnol. May 2023;5 doi: 10.3389/fnano.2023.1175149. [DOI] [Google Scholar]
- 191.Rodríguez-Félix F., et al. Sustainable-green synthesis of silver nanoparticles using safflower (Carthamus tinctorius L.) waste extract and its antibacterial activity. Heliyon. Apr. 2021;7(4) doi: 10.1016/j.heliyon.2021.e06923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Masum Md.M.I., et al. Biogenic synthesis of silver nanoparticles using Phyllanthus emblica fruit extract and its inhibitory action against the pathogen Acidovorax oryzae strain RS-2 of rice bacterial Brown stripe. Front. Microbiol. Apr. 2019;10:820. doi: 10.3389/fmicb.2019.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ali K., Ahmed B., Dwivedi S., Saquib Q., Al-Khedhairy A.A., Musarrat J. Microwave accelerated green synthesis of stable silver nanoparticles with Eucalyptus globulus leaf extract and their antibacterial and antibiofilm activity on clinical isolates. PLoS One. Jul. 2015;10(7) doi: 10.1371/journal.pone.0131178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dutta T., Ghosh N.N., Das M., Adhikary R., Mandal V., Chattopadhyay A.P. Green synthesis of antibacterial and antifungal silver nanoparticles using Citrus limetta peel extract: experimental and theoretical studies. J. Environ. Chem. Eng. Aug. 2020;8(4) doi: 10.1016/j.jece.2020.104019. [DOI] [Google Scholar]
- 195.Kanniah P., Chelliah P., Thangapandi J.R., Gnanadhas G., Mahendran V., Robert M. Green synthesis of antibacterial and cytotoxic silver nanoparticles by Piper nigrum seed extract and development of antibacterial silver based chitosan nanocomposite. Int. J. Biol. Macromol. Oct. 2021;189:18–33. doi: 10.1016/j.ijbiomac.2021.08.056. [DOI] [PubMed] [Google Scholar]
- 196.Oves M., et al. Green synthesis of silver nanoparticles by Conocarpus Lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci. Jan. 2022;29(1):460–471. doi: 10.1016/j.sjbs.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ettadili F.E., et al. Green synthesis of silver nanoparticles using Phoenix dactylifera seed extract and their electrochemical activity in Ornidazole reduction. Food Chem. Adv. Oct. 2023;2 doi: 10.1016/j.focha.2022.100146. [DOI] [Google Scholar]
- 198.Herrera-Marín P., Fernández L., Pilaquinga F F., Debut A., Rodríguez A., Espinoza-Montero P. Green synthesis of silver nanoparticles using aqueous extract of the leaves of fine aroma cocoa Theobroma cacao linneu (Malvaceae): optimization by electrochemical techniques. Electrochim. Acta. Apr. 2023;447 doi: 10.1016/j.electacta.2023.142122. [DOI] [Google Scholar]
- 199.Logambal S., et al. Synthesis and antimicrobial activity of silver nanoparticles: incorporated couroupita guianensis flower petal extract for biomedical applications. J. King Saud Univ. Sci. Jan. 2023;35(1) doi: 10.1016/j.jksus.2022.102455. [DOI] [Google Scholar]
- 200.Rahmatian N., Abbasi S., Tavakkoli Yaraki M., Abbasi N. Echinophora platyloba extract-mediated green synthesis of silver nanoparticles: fine-tuning the size towards enhanced catalytic and antibacterial properties. J. Mol. Liq. Dec. 2023;391 doi: 10.1016/j.molliq.2023.123327. [DOI] [Google Scholar]
- 201.Wasilewska A., Klekotka U., Zambrzycka M., Zambrowski G., Święcicka I., Kalska-Szostko B. Physico-chemical properties and antimicrobial activity of silver nanoparticles fabricated by green synthesis. Food Chem. Jan. 2023;400 doi: 10.1016/j.foodchem.2022.133960. [DOI] [PubMed] [Google Scholar]
- 202.Vijay R., et al. Synthesis and characterization of silver nanomaterial from aqueous extract of Commelina forskaolii and its potential antimicrobial activity against Gram negative pathogens. J. King Saud Univ. Sci. Jan. 2023;35(1) doi: 10.1016/j.jksus.2022.102373. [DOI] [Google Scholar]
- 203.Pushparaj K., et al. Green synthesis, characterization of silver nanoparticles using aqueous leaf extracts of Solanum melongena and in vitro evaluation of antibacterial, pesticidal and anticancer activity in human MDA-MB-231 breast cancer cell lines. J. King Saud Univ. Sci. Jul. 2023;35(5) doi: 10.1016/j.jksus.2023.102663. [DOI] [Google Scholar]
- 204.Krishnamoorthy K., et al. Green synthesis and evaluation of anti-microbial, antioxidant, anti-inflammatory, and anti-diabetic activities of silver nanoparticles from Argyreia nervosa leaf extract: an invitro study. J. King Saud Univ. Sci. Dec. 2023;35(10) doi: 10.1016/j.jksus.2023.102955. [DOI] [Google Scholar]
- 205.Hermanto D., et al. Bio-mediated electrochemically synthesis of silver nanoparticles using green tea (Camellia sinensis) leaves extract and their antibacterial activity. South Afr. J. Chem. Eng. Jan. 2024;47:136–141. doi: 10.1016/j.sajce.2023.11.004. [DOI] [Google Scholar]
- 206.Al-Mhyawi S.R. Green synthesis of silver nanoparticles and their inhibitory efficacy on corrosion of carbon steel in hydrochloric acid solution. Int. J. Electrochem. Sci. Sep. 2023;18(9) doi: 10.1016/j.ijoes.2023.100210. [DOI] [Google Scholar]
- 207.Makky S., et al. Characterization of the biosynthesized Syzygium aromaticum-mediated silver nanoparticles and its antibacterial and antibiofilm activity in combination with bacteriophage. Results Chem. Jan. 2023;5 doi: 10.1016/j.rechem.2022.100686. [DOI] [Google Scholar]
- 208.Princy S.S.J., Hentry C., Alodaini H.A., Hatamleh A.A., Arokiyaraj S., Bindhu M.R. Hibiscus cannabinus seeds assisted spherical silver nanoparticles and its antibacterial and photocatalytic applications. Chem. Phys. Impact. Jun. 2023;6 doi: 10.1016/j.chphi.2023.100192. [DOI] [Google Scholar]
- 209.Sancheti R.S., et al. Cordia sebestena leaf extract mediated biosynthesis of silver nanoparticles, characterization, and screening of its antimicrobial activities. Green Anal. Chem. Sep. 2023;6 doi: 10.1016/j.greeac.2023.100075. [DOI] [Google Scholar]
- 210.Alamier W.M., D Y Oteef M., Bakry A.M., Hasan N., Ismail K.S., Awad F.S. Green synthesis of silver nanoparticles using Acacia ehrenbergiana plant cortex extract for efficient removal of Rhodamine B cationic dye from wastewater and the evaluation of antimicrobial activity. ACS Omega. May 2023;8(21):18901–18914. doi: 10.1021/acsomega.3c01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mortazavi-Derazkola S., et al. Facile green synthesis and characterization of Crataegus microphylla extract-capped silver nanoparticles (CME@Ag-NPs) and its potential antibacterial and anticancer activities against AGS and MCF-7 human cancer cells. J. Alloys Compd. Apr. 2020;820 doi: 10.1016/j.jallcom.2019.153186. [DOI] [Google Scholar]
- 212.Tamilarasi P., Meena P. Green synthesis of silver nanoparticles (Ag NPs) using Gomphrena globosa (Globe amaranth) leaf extract and their characterization. Mater. Today Proc. 2020;33:2209–2216. doi: 10.1016/j.matpr.2020.04.025. [DOI] [Google Scholar]
- 213.Gomathi M., Prakasam A., Rajkumar P.V., Rajeshkumar S., Chandrasekaran R., Anbarasan P.M. Green synthesis of silver nanoparticles using Gymnema sylvestre leaf extract and evaluation of its antibacterial activity. South Afr. J. Chem. Eng. Apr. 2020;32:1–4. doi: 10.1016/j.sajce.2019.11.005. [DOI] [Google Scholar]
- 214.Chandhirasekar K., et al. Plant-extract-assisted green synthesis and its larvicidal activities of silver nanoparticles using leaf extract of Citrus medica, Tagetes lemmonii, and Tarenna asiatica. Mater. Lett. Mar. 2021;287 doi: 10.1016/j.matlet.2020.129265. [DOI] [Google Scholar]
- 215.Bhakya S., Muthukrishnan S., Sukumaran M., Muthukumar M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. Jun. 2016;6(5):755–766. doi: 10.1007/s13204-015-0473-z. [DOI] [Google Scholar]
- 216.Ibrahim H.M.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. Jul. 2015;8(3):265–275. doi: 10.1016/j.jrras.2015.01.007. [DOI] [Google Scholar]
- 217.Raja S., Ramesh V., Thivaharan V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab. J. Chem. Feb. 2017;10(2):253–261. doi: 10.1016/j.arabjc.2015.06.023. [DOI] [Google Scholar]
- 218.Nagime P.V., et al. Facile synthesis of silver nanoparticles using Calotropis procera leaves: unraveling biological and electrochemical potentials. Discov. Nano. Sep. 2024;19(1):139. doi: 10.1186/s11671-024-04090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jayeoye T.J., Eze F.N., Olatunde O.O., Singh S., Zuo J., Olatunji O.J. Multifarious biological applications and toxic Hg2+ sensing potentiality of biogenic silver nanoparticles based on Securidaca inappendiculata Hassk stem extract. Int. J. Nanomed. Nov. 2021;16:7557–7574. doi: 10.2147/IJN.S325996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rodríguez-Félix F., et al. Sustainable-green synthesis of silver nanoparticles using safflower (Carthamus tinctorius L.) waste extract and its antibacterial activity. Heliyon. Apr. 2021;7(4) doi: 10.1016/j.heliyon.2021.e06923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Masum Md.M.I., et al. Biogenic synthesis of silver nanoparticles using Phyllanthus emblica fruit extract and its inhibitory action against the pathogen Acidovorax oryzae strain RS-2 of rice bacterial Brown stripe. Front. Microbiol. Apr. 2019;10:820. doi: 10.3389/fmicb.2019.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ali K., Ahmed B., Dwivedi S., Saquib Q., Al-Khedhairy A.A., Musarrat J. Microwave accelerated green synthesis of stable silver nanoparticles with Eucalyptus globulus leaf extract and their antibacterial and antibiofilm activity on clinical isolates. PLoS One. Jul. 2015;10(7) doi: 10.1371/journal.pone.0131178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dutta T., Ghosh N.N., Das M., Adhikary R., Mandal V., Chattopadhyay A.P. Green synthesis of antibacterial and antifungal silver nanoparticles using Citrus limetta peel extract: experimental and theoretical studies. J. Environ. Chem. Eng. Aug. 2020;8(4) doi: 10.1016/j.jece.2020.104019. [DOI] [Google Scholar]
- 224.Ahmed S.F., et al. Nanomaterials as a sustainable choice for treating wastewater. Environ. Res. Nov. 2022;214 doi: 10.1016/j.envres.2022.113807. [DOI] [PubMed] [Google Scholar]
- 225.Pattanayak D.S., Pal D., Thakur C., Raut P., Wasewar K.L. Sustainable Engineering, Energy, and the Environment. first ed. Apple Academic Press; Boca Raton: 2022. Catalytic potential of phyto-synthesized silver nanoparticles for the degradation of pollutants; pp. 465–481. [DOI] [Google Scholar]
- 226.Mota D.R., Lima G.A.S., Helene G.B., Pellosi D.S. Tailoring nanoparticle morphology to match application: growth under low-intensity polychromatic light irradiation governs the morphology and optical properties of silver nanoparticles. ACS Appl. Nano Mater. May 2020;3(5):4893–4903. doi: 10.1021/acsanm.0c01078. [DOI] [Google Scholar]
- 227.Gmurek M., Olak-Kucharczyk M., Ledakowicz S. Photochemical decomposition of endocrine disrupting compounds – a review. Chem. Eng. J. Feb. 2017;310:437–456. doi: 10.1016/j.cej.2016.05.014. [DOI] [Google Scholar]
- 228.Mukherji S., Bharti S., Shukla G., Mukherji S. Synthesis and characterization of size- and shape-controlled silver nanoparticles. Phys. Sci. Rev. Jan. 2019;4(1) doi: 10.1515/psr-2017-0082. [DOI] [Google Scholar]
- 229.Valandro S., Poli A., Neumann M., Schmitt C. Photochemical synthesis of Ag and Au nanoparticles using a thioxanthone substituted chitosan as simultaneous photoinitiator and stabilizer. J. Braz. Chem. Soc. 2019 doi: 10.21577/0103-5053.20190182. [DOI] [Google Scholar]
- 230.Huang H., Yang Y. Preparation of silver nanoparticles in inorganic clay suspensions. Compos. Sci. Technol. Nov. 2008;68(14):2948–2953. doi: 10.1016/j.compscitech.2007.10.003. [DOI] [Google Scholar]
- 231.Darroudi M., Ahmad M.B., Zak A.K., Zamiri R., Hakimi M. Fabrication and characterization of gelatin stabilized silver nanoparticles under UV-light. Int. J. Mol. Sci. Sep. 2011;12(9):6346–6356. doi: 10.3390/ijms12096346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Radoń A., Łukowiec D. Silver nanoparticles synthesized by UV-irradiation method using chloramine T as modifier: structure, formation mechanism and catalytic activity. CrystEngComm. 2018;20(44):7130–7136. doi: 10.1039/C8CE01379A. [DOI] [Google Scholar]
- 233.Babusca D., Popescu L., Sacarescu L., Dorohoi D.O., Creanga D., Oprica L.A. Two phase photochemical synthesis of silver nanoparticles and their impact on the chlorophylls. Mol. Cryst. Liq. Cryst. Feb. 2020;698(1):56–64. doi: 10.1080/15421406.2020.1731087. [DOI] [Google Scholar]
- 234.Riswana Barveen N., Wang T.-J., Chang Y.-H. Photochemical synthesis of Ag/Au/AgCl heterostructure from Ag nanowires as a reusable SERS substrate for ultrasensitive detection of analgesics and antibiotics. Chem. Eng. J. Nov. 2021;423 doi: 10.1016/j.cej.2021.130191. [DOI] [Google Scholar]
- 235.Wang T.-J., Huang Y.-T., Liu Z.-Y., Barveen N.R. Photochemical synthesis of ZnO/Ag heterogeneous nanostructure on chemically patterned ferroelectric crystals for high performance SERS detection. J. Alloys Compd. May 2021;864 doi: 10.1016/j.jallcom.2020.158120. [DOI] [Google Scholar]
- 236.Ozcelik Kazancioglu E., Aydin M., Arsu N. Photochemical synthesis of bimetallic gold/silver nanoparticles in polymer matrix with tunable absorption properties: superior photocatalytic activity for degradation of methylene blue. Mater. Chem. Phys. Sep. 2021;269 doi: 10.1016/j.matchemphys.2021.124734. [DOI] [Google Scholar]
- 237.Ross A., Muñoz M., Rotstein B.H., Suuronen E.J., Alarcon E.I. A low cost and open access system for rapid synthesis of large volumes of gold and silver nanoparticles. Sci. Rep. Mar. 2021;11(1):5420. doi: 10.1038/s41598-021-84896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dos Santos M.A., Paterno L.G., Moreira S.G.C., Sales M.J.A. Original photochemical synthesis of Ag nanoparticles mediated by potato starch. SN Appl. Sci. Jun. 2019;1(6):554. doi: 10.1007/s42452-019-0586-1. [DOI] [Google Scholar]
- 239.Chandrasekharan S., Chinnasamy G., Bhatnagar S. Sustainable phyto-fabrication of silver nanoparticles using Gmelina arborea exhibit antimicrobial and biofilm inhibition activity. Sci. Rep. Jan. 2022;12(1):156. doi: 10.1038/s41598-021-04025-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Amendola V., Meneghetti M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009;11(20):3805. doi: 10.1039/b900654k. [DOI] [PubMed] [Google Scholar]
- 241.Sportelli M.C., et al. The pros and cons of the use of laser ablation synthesis for the production of silver nano-antimicrobials. Antibiotics. Jul. 2018;7(3):67. doi: 10.3390/antibiotics7030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Howarth A.J., Wang T.C., Al-Juaid S.S., Aziz S.G., Hupp J.T., Farha O.K. Efficient extraction of sulfate from water using a Zr-metal–organic framework. Dalton Trans. 2016;45(1):93–97. doi: 10.1039/C5DT04163E. [DOI] [PubMed] [Google Scholar]
- 243.Keskar M., Sabatini C., Cheng C., Swihart M.T. Synthesis and characterization of silver nanoparticle-loaded amorphous calcium phosphate microspheres for dental applications. Nanoscale Adv. 2019;1(2):627–635. doi: 10.1039/C8NA00281A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pant B., Park M., Park S.-J. One-step synthesis of silver nanoparticles embedded polyurethane nano-fiber/net structured membrane as an effective antibacterial medium. Polymers. Jul. 2019;11(7):1185. doi: 10.3390/polym11071185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dahlin R.L., Kasper F.K., Mikos A.G. Polymeric nanofibers in tissue engineering. Tissue Eng. Part B Rev. Oct. 2011;17(5):349–364. doi: 10.1089/ten.teb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Muromachi T., Tsujino T., Kamitani K., Maeda K. Application of functional coatings by sol-gel method. J. Sol. Gel Sci. Technol. Dec. 2006;40(2–3):267–272. doi: 10.1007/s10971-006-8386-7. [DOI] [Google Scholar]
- 247.Asim N., Ahmadi S., Alghoul M.A., Hammadi F.Y., Saeedfar K., Sopian K. Research and development aspects on chemical preparation techniques of photoanodes for dye sensitized solar cells. Int. J. Photoenergy. 2014;2014:1–21. doi: 10.1155/2014/518156. [DOI] [Google Scholar]
- 248.Shivaji S., Madhu S., Singh S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem. Sep. 2011;46(9):1800–1807. doi: 10.1016/j.procbio.2011.06.008. [DOI] [Google Scholar]
- 249.Rafique M., Sadaf I., Rafique M.S., Tahir M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells, Nanomed. Biotechnol. Oct. 2017;45(7):1272–1291. doi: 10.1080/21691401.2016.1241792. [DOI] [PubMed] [Google Scholar]
- 250.Ranoszek-Soliwoda K., et al. The synthesis of monodisperse silver nanoparticles with plant extracts. Colloids Surf. B Biointerfaces. May 2019;177:19–24. doi: 10.1016/j.colsurfb.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 251.Sathishkumar R.S., et al. Green synthesis of silver nanoparticles by bloom forming marine microalgae Trichodesmium erythraeum and its applications in antioxidant, drug-resistant bacteria, and cytotoxicity activity. J. Saudi Chem. Soc. Dec. 2019;23(8):1180–1191. doi: 10.1016/j.jscs.2019.07.008. [DOI] [Google Scholar]
- 252.Behravan M., Hossein Panahi A., Naghizadeh A., Ziaee M., Mahdavi R., Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. Mar. 2019;124:148–154. doi: 10.1016/j.ijbiomac.2018.11.101. [DOI] [PubMed] [Google Scholar]
- 253.Dahoumane S.A., Wujcik E.K., Jeffryes C. Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzym. Microb. Technol. Dec. 2016;95:13–27. doi: 10.1016/j.enzmictec.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 254.Shateri-Khalilabad M., Yazdanshenas M.E., Etemadifar A. Fabricating multifunctional silver nanoparticles-coated cotton fabric. Arab. J. Chem. May 2017;10:S2355–S2362. doi: 10.1016/j.arabjc.2013.08.013. [DOI] [Google Scholar]
- 255.Peng L., Guo R., Lan J., Jiang S., Wang X. Silver nanoparticle coating on cotton fabric modified with poly(diallyldimethylammonium chloride) Mater. Technol. Jul. 2016;31(8):431–436. doi: 10.1179/1753555715Y.0000000074. [DOI] [Google Scholar]
- 256.Ashayer-Soltani R., Hunt C., Thomas O. Fabrication of highly conductive stretchable textile with silver nanoparticles. Textil. Res. J. Jun. 2016;86(10):1041–1049. doi: 10.1177/0040517515603813. [DOI] [Google Scholar]
- 257.Raza Z.A., et al. Development of antibacterial cellulosic fabric via clean impregnation of silver nanoparticles. J. Clean. Prod. Aug. 2015;101:377–386. doi: 10.1016/j.jclepro.2015.03.091. [DOI] [Google Scholar]
- 258.Katouah H., El-Metwaly N.M. Plasma treatment toward electrically conductive and superhydrophobic cotton fibers by in situ preparation of polypyrrole and silver nanoparticles. React. Funct. Polym. Feb. 2021;159 doi: 10.1016/j.reactfunctpolym.2021.104810. [DOI] [Google Scholar]
- 259.Chang S., Condon B., Nam S. Development of flame-resistant cotton fabrics with casein using pad-dry-cure and supercritical fluids methods. Int. J. Mater. Sci. Appl. 2020;9(4):53. doi: 10.11648/j.ijmsa.20200904.11. [DOI] [Google Scholar]
- 260.Al-Enizi A.M., Alothman A.A., Ubaidullah M., Nafady A. 2021. Production of Electroconductive, Superhydrphobic and Flame-Retardant Cotton Fibers via Pad-Dry-Cure Process Using Silicone Rubber and Ammonium Polyphosphate. Mar. 11. [DOI] [Google Scholar]
- 261.Hebeish A., El-Naggar M.E., Fouda M.M.G., Ramadan M.A., Al-Deyab S.S., El-Rafie M.H. Highly effective antibacterial textiles containing green synthesized silver nanoparticles. Carbohydr. Polym. Aug. 2011;86(2):936–940. doi: 10.1016/j.carbpol.2011.05.048. [DOI] [Google Scholar]
- 262.Kwon S., Singh R.K., Perez R.A., Abou Neel E.A., Kim H.-W., Chrzanowski W. Silica-based mesoporous nanoparticles for controlled drug delivery. J. Tissue Eng. Jan. 2013;4 doi: 10.1177/2041731413503357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Xu Q., et al. Surface modification by carboxymethy chitosan via pad-dry-cure method for binding Ag NPs onto cotton fabric. Int. J. Biol. Macromol. May 2018;111:796–803. doi: 10.1016/j.ijbiomac.2018.01.091. [DOI] [PubMed] [Google Scholar]
- 264.Xu Q., et al. One-pot fabrication of durable antibacterial cotton fabric coated with silver nanoparticles via carboxymethyl chitosan as a binder and stabilizer. Carbohydr. Polym. Jan. 2019;204:42–49. doi: 10.1016/j.carbpol.2018.09.089. [DOI] [PubMed] [Google Scholar]
- 265.Ma Z., Liu J., Shen G., Zheng X., Pei Y., Tang K. In-situ synthesis and immobilization of silver nanoparticles on microfibrillated cellulose for long-term antibacterial applications. Cellulose. Jul. 2021;28(10):6287–6303. doi: 10.1007/s10570-021-03941-4. [DOI] [Google Scholar]
- 266.Liu H., Lee Y.-Y., Norsten T.B., Chong K. In situ formation of anti-bacterial silver nanoparticles on cotton textiles. J. Ind. Text. Sep. 2014;44(2):198–210. doi: 10.1177/1528083713481833. [DOI] [Google Scholar]
- 267.Lu Z., Meng M., Jiang Y., Xie J. UV-assisted in situ synthesis of silver nanoparticles on silk fibers for antibacterial applications. Colloids Surf. A Physicochem. Eng. Asp. Apr. 2014;447:1–7. doi: 10.1016/j.colsurfa.2014.01.064. [DOI] [Google Scholar]
- 268.Wang Q., et al. In situ synthesis of silver or selenium nanoparticles on cationized cellulose fabrics for antimicrobial application. Mater. Sci. Eng. C. Feb. 2021;121 doi: 10.1016/j.msec.2020.111859. [DOI] [PubMed] [Google Scholar]
- 269.Ghosal K., Ghosh S., Ghosh D., Sarkar K. Natural polysaccharide derived carbon dot based in situ facile green synthesis of silver nanoparticles: synergistic effect on breast cancer. Int. J. Biol. Macromol. Nov. 2020;162:1605–1615. doi: 10.1016/j.ijbiomac.2020.07.315. [DOI] [PubMed] [Google Scholar]
- 270.Raza Z.A. In situ synthesis and immobilization of nanosilver on knitted cellulose fabric. J. Nat. Fibers. Mar. 2018;15(2):183–190. doi: 10.1080/15440478.2017.1321517. [DOI] [Google Scholar]
- 271.Loh N.D., et al. Multistep nucleation of nanocrystals in aqueous solution. Nat. Chem. Jan. 2017;9(1):77–82. doi: 10.1038/nchem.2618. [DOI] [PubMed] [Google Scholar]
- 272.Ge M., Lu M., Chu Y., Xin H. Anomalous growth rate of Ag nanocrystals revealed by in situ STEM. Sci. Rep. Nov. 2017;7(1) doi: 10.1038/s41598-017-15140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.V Kinhal K., Bhatt N., Subramaniam P. Transport and kinetic effects on the morphology of silver nanoparticles in a millifluidic system. Ind. Eng. Chem. Res. Apr. 2019;58(15):5820–5829. doi: 10.1021/acs.iecr.8b04156. [DOI] [Google Scholar]
- 274.Huang L., Zhao S., Wang Z., Wu J., Wang J., Wang S. In situ immobilization of silver nanoparticles for improving permeability, antifouling and anti-bacterial properties of ultrafiltration membrane. J. Membr. Sci. Feb. 2016;499:269–281. doi: 10.1016/j.memsci.2015.10.055. [DOI] [Google Scholar]
- 275.Ahmed H., Khattab T.A., Mashaly H.M., El-Halwagy A.A., Rehan M. Plasma activation toward multi-stimuli responsive cotton fabric via in situ development of polyaniline derivatives and silver nanoparticles. Cellulose. Mar. 2020;27(5):2913–2926. doi: 10.1007/s10570-020-02980-7. [DOI] [Google Scholar]
- 276.Tania I.S., Ali M., Azam Md.S. In-situ synthesis and characterization of silver nanoparticle decorated cotton knitted fabric for antibacterial activity and improved dyeing performance. SN Appl. Sci. Jan. 2019;1(1):64. doi: 10.1007/s42452-018-0068-x. [DOI] [Google Scholar]
- 277.Meng M., He H., Xiao J., Zhao P., Xie J., Lu Z. Controllable in situ synthesis of silver nanoparticles on multilayered film-coated silk fibers for antibacterial application. J. Colloid Interface Sci. Jan. 2016;461:369–375. doi: 10.1016/j.jcis.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 278.Gün Gök Z., Demiral A., Bozkaya O., Yiğitoğlu M. In situ synthesis of silver nanoparticles on modified poly(ethylene terephthalate) fibers by grafting for obtaining versatile antimicrobial materials. Polym. Bull. Dec. 2021;78(12):7241–7260. doi: 10.1007/s00289-020-03486-9. [DOI] [Google Scholar]
- 279.Shaheen Th.I., Abd El Aty A.A. In-situ green myco-synthesis of silver nanoparticles onto cotton fabrics for broad spectrum antimicrobial activity. Int. J. Biol. Macromol. Oct. 2018;118:2121–2130. doi: 10.1016/j.ijbiomac.2018.07.062. [DOI] [PubMed] [Google Scholar]
- 280.Kumar A., et al. In situ decoration of silver nanoparticles on single-walled carbon nanotubes by microwave irradiation for enhanced and durable anti-bacterial finishing on cotton fabric. Ceram. Int. Jan. 2019;45(1):1011–1019. doi: 10.1016/j.ceramint.2018.09.280. [DOI] [Google Scholar]
- 281.Rastegar L., Montazer M., Gaminian H. Clean low-temperature in situ synthesis of durable silver nanoparticles along with aminolysis of polyester fabric using dopamine hydrochloride. Clean Technol. Environ. Policy. Aug. 2016;18(6):2019–2026. doi: 10.1007/s10098-016-1127-x. [DOI] [Google Scholar]
- 282.Khan I., Saeed K., Khan I. Nanoparticles: properties, applications and toxicities. Arab. J. Chem. Nov. 2019;12(7):908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 283.Zhang X.-F., Liu Z.-G., Shen W., Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. Sep. 2016;17(9):1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.(Ross R., Salary, et al. Computational fluid dynamics modeling and online monitoring of aerosol jet printing process. J. Manuf. Sci. Eng. Feb. 2017;139(2) doi: 10.1115/1.4034591. [DOI] [Google Scholar]
- 285.Cano-Raya C., Denchev Z.Z., Cruz S.F., Viana J.C. Chemistry of solid metal-based inks and pastes for printed electronics – a review. Appl. Mater. Today. Jun. 2019;15:416–430. doi: 10.1016/j.apmt.2019.02.012. [DOI] [Google Scholar]
- 286.Chung S., Cho K., Lee T. Recent progress in inkjet‐printed thin‐film transistors. Adv. Sci. Mar. 2019;6(6) doi: 10.1002/advs.201801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sun J., et al. Printable nanomaterials for the fabrication of high-performance supercapacitors. Nanomaterials. Jul. 2018;8(7):528. doi: 10.3390/nano8070528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhang J., et al. Silver nanoparticles for conductive inks: from synthesis and ink formulation to their use in printing technologies. Metals. Jan. 2022;12(2):234. doi: 10.3390/met12020234. [DOI] [Google Scholar]
- 289.Balliu E., Andersson H., Engholm M., Öhlund T., Nilsson H.-E., Olin H. Selective laser sintering of inkjet-printed silver nanoparticle inks on paper substrates to achieve highly conductive patterns. Sci. Rep. Jul. 2018;8(1) doi: 10.1038/s41598-018-28684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhang N., Luo J., Liu R., Liu X. Tannic acid stabilized silver nanoparticles for inkjet printing of conductive flexible electronics. RSC Adv. 2016;6(87):83720–83729. doi: 10.1039/C6RA19800G. [DOI] [Google Scholar]
- 291.Zhuo L., et al. Cost-effective silver nano-ink for inkjet printing in application of flexible electronic devices. Chem. Phys. Lett. Oct. 2020;757 doi: 10.1016/j.cplett.2020.137904. [DOI] [Google Scholar]
- 292.Fernandes I.J., et al. Silver nanoparticle conductive inks: synthesis, characterization, and fabrication of inkjet-printed flexible electrodes. Sci. Rep. Jun. 2020;10(1):8878. doi: 10.1038/s41598-020-65698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Elmaaty T.A., El-Nagare Kh., Raouf S., Abdelfattah Kh., El-Kadi S., Abdelaziz E. One-step green approach for functional printing and finishing of textiles using silver and gold NPs. RSC Adv. 2018;8(45):25546–25557. doi: 10.1039/C8RA02573H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Li W., Yang S., Shamim A. Screen printing of silver nanowires: balancing conductivity with transparency while maintaining flexibility and stretchability. Npj Flex. Electron. Jul. 2019;3(1):13. doi: 10.1038/s41528-019-0057-1. [DOI] [Google Scholar]
- 295.Barmpakos D., Tsamis C., Kaltsas G. Multi-parameter paper sensor fabricated by inkjet-printed silver nanoparticle ink and PEDOT:PSS. Microelectron. Eng. Mar. 2020;225 doi: 10.1016/j.mee.2020.111266. [DOI] [Google Scholar]
- 296.Min S.-H., Lee G.-Y., Ahn S.-H. Direct printing of highly sensitive, stretchable, and durable strain sensor based on silver nanoparticles/multi-walled carbon nanotubes composites. Compos. Part B Eng. Mar. 2019;161:395–401. doi: 10.1016/j.compositesb.2018.12.107. [DOI] [Google Scholar]
- 297.Rabbi Md.B., Tania I.S., Sani A.A., Uddin Md.Z. Facile approach for preparing printed cotton fabric with antimicrobial activity by utilizing the functional characteristics of nano-silver. J. Inorg. Organomet. Polym. Mater. Aug. 2024;34(8):3675–3688. doi: 10.1007/s10904-024-03047-x. [DOI] [Google Scholar]
- 298.Skarżyński K., Krzemiński J., Jakubowska M., Słoma M. Highly conductive electronics circuits from aerosol jet printed silver inks. Sci. Rep. Sep. 2021;11(1) doi: 10.1038/s41598-021-97312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Fissan H., Ristig S., Kaminski H., Asbach C., Epple M. Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal. Methods. Jul. 2014;6(18):7324. doi: 10.1039/C4AY01203H. [DOI] [Google Scholar]
- 300.Tarannum N., Divya D., Gautam Y.K. Facile green synthesis and applications of silver nanoparticles: a state-of-the-art review. RSC Adv. 2019;9(60):34926–34948. doi: 10.1039/C9RA04164H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Mitić Ž., et al. Instrumental methods and techniques for structural and physicochemical characterization of biomaterials and bone tissue: a review. Mater. Sci. Eng. C. Oct. 2017;79:930–949. doi: 10.1016/j.msec.2017.05.127. [DOI] [PubMed] [Google Scholar]
- 302.Loiseau A., Asila V., Boitel-Aullen G., Lam M., Salmain M., Boujday S. Silver-based plasmonic nanoparticles for and their use in biosensing. Biosensors. Jun. 2019;9(2):78. doi: 10.3390/bios9020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lin P.-C., Lin S., Wang P.C., Sridhar R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. Jul. 2014;32(4):711–726. doi: 10.1016/j.biotechadv.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hinterdorfer P., Garcia-Parajo M.F., Dufrêne Y.F. Single-molecule imaging of cell surfaces using near-field nanoscopy. Acc. Chem. Res. Mar. 2012;45(3):327–336. doi: 10.1021/ar2001167. [DOI] [PubMed] [Google Scholar]
- 305.Yang L., Watts D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. Aug. 2005;158(2):122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 306.Gmoshinski I.V., et al. Nanomaterials and nanotechnologies: methods of analysis and control. Russ. Chem. Rev. Jan. 2013;82(1):48–76. doi: 10.1070/RC2013v082n01ABEH004329. [DOI] [Google Scholar]
- 307.Urnukhsaikhan E., Bold B.-E., Gunbileg A., Sukhbaatar N., Mishig-Ochir T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. Oct. 2021;11(1) doi: 10.1038/s41598-021-00520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ali A.M., Said D.A., Khayyat M., Boustimi M., Seoudi R. Improving the efficiency of the organic solar cell (CuPc/C60) via PEDOT: PSS as a photoconductor layer doped by silver nanoparticles. Results Phys. Mar. 2020;16 doi: 10.1016/j.rinp.2019.102819. [DOI] [Google Scholar]
- 309.Giri A.K., et al. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci. Rep. May 2022;12(1):8383. doi: 10.1038/s41598-022-12484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Khodashenas B., Ghorbani H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. Dec. 2019;12(8):1823–1838. doi: 10.1016/j.arabjc.2014.12.014. [DOI] [Google Scholar]
- 311.Patil R.B., Chougale A.D. Analytical methods for the identification and characterization of silver nanoparticles: a brief review. Mater. Today Proc. 2021;47:5520–5532. doi: 10.1016/j.matpr.2021.03.384. [DOI] [Google Scholar]
- 312.Sastry M., Patil V., Sainkar S.R. Electrostatically controlled diffusion of carboxylic acid derivatized silver colloidal particles in thermally evaporated fatty amine films. J. Phys. Chem. B. Feb. 1998;102(8):1404–1410. doi: 10.1021/jp9719873. [DOI] [Google Scholar]
- 313.Eya’ane Meva F., et al. Spectroscopic synthetic optimizations monitoring of silver nanoparticles formation from Megaphrynium macrostachyum leaf extract. Rev. Bras. Farmacogn. Sep. 2016;26(5):640–646. doi: 10.1016/j.bjp.2016.06.002. [DOI] [Google Scholar]
- 314.Ali I.A.M., Ahmed A.B., Al-Ahmed H.I. Green synthesis and characterization of silver nanoparticles for reducing the damage to sperm parameters in diabetic compared to metformin. Sci. Rep. Feb. 2023;13(1):2256. doi: 10.1038/s41598-023-29412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Chapman H.N., et al. Femtosecond X-ray protein nanocrystallography. Nature. Feb. 2011;470(7332):73–77. doi: 10.1038/nature09750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Majeed Khan M.A., Kumar S., Ahamed M., Alrokayan S.A., AlSalhi M.S. Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanoscale Res. Lett. Dec. 2011;6(1):434. doi: 10.1186/1556-276X-6-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Hetta H.F., et al. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. May 2021;11(1) doi: 10.1038/s41598-021-90208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Shah M.Z., et al. Synthesis of silver nanoparticles using Plantago lanceolata extract and assessing their antibacterial and antioxidant activities. Sci. Rep. Oct. 2021;11(1) doi: 10.1038/s41598-021-00296-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 319.Barth A., Zscherp C. What vibrations tell about proteins. Q. Rev. Biophys. Nov. 2002;35(4):369–430. doi: 10.1017/S0033583502003815. [DOI] [PubMed] [Google Scholar]
- 320.Vogel R., Siebert F. Vibrational spectroscopy as a tool for probing protein function. Curr. Opin. Chem. Biol. Oct. 2000;4(5):518–523. doi: 10.1016/S1367-5931(00)00125-3. [DOI] [PubMed] [Google Scholar]
- 321.Zscherp C., Barth A. Reaction-induced infrared difference spectroscopy for the study of protein reaction mechanisms. Biochemistry. Feb. 2001;40(7):1875–1883. doi: 10.1021/bi002567y. [DOI] [PubMed] [Google Scholar]
- 322.Baudot C., Tan C.M., Kong J.C. FTIR spectroscopy as a tool for nano-material characterization. Infrared Phys. Technol. Nov. 2010;53(6):434–438. doi: 10.1016/j.infrared.2010.09.002. [DOI] [Google Scholar]
- 323.Kazarian S.G., Chan K.L.A. Applications of ATR-FTIR spectroscopic imaging to biomedical samples. Biochim. Biophys. Acta BBA - Biomembr. Jul. 2006;1758(7):858–867. doi: 10.1016/j.bbamem.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 324.Zhang X.-F., Liu Z.-G., Shen W., Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. Sep. 2016;17(9):1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Khan S., Singh S., Gaikwad S., Nawani N., Junnarkar M., Pawar S.V. Optimization of process parameters for the synthesis of silver nanoparticles from Piper betle leaf aqueous extract, and evaluation of their antiphytofungal activity. Environ. Sci. Pollut. Res. Aug. 2020;27(22):27221–27233. doi: 10.1007/s11356-019-05239-2. [DOI] [PubMed] [Google Scholar]
- 326.Royji Albeladi S.S., Malik M.A., Al-thabaiti S.A. Facile biofabrication of silver nanoparticles using Salvia officinalis leaf extract and its catalytic activity towards Congo red dye degradation. J. Mater. Res. Technol. Sep. 2020;9(5):10031–10044. doi: 10.1016/j.jmrt.2020.06.074. [DOI] [Google Scholar]
- 327.Dua T.K., Giri S., Nandi G., Sahu R., Shaw T.K., Paul P. Green synthesis of silver nanoparticles using Eupatorium adenophorum leaf extract: characterizations, antioxidant, antibacterial and photocatalytic activities. Chem. Pap. Jun. 2023;77(6):2947–2956. doi: 10.1007/s11696-023-02676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Elsupikhe R.F., Shameli K., Ahmad M.B. Sonochemical method for the synthesis of silver nanoparticles in κ-carrageenan from silver salt at different concentrations. Res. Chem. Intermed. Nov. 2015;41(11):8515–8525. doi: 10.1007/s11164-014-1907-z. [DOI] [Google Scholar]
- 329.Murdock R.C., Braydich-Stolle L., Schrand A.M., Schlager J.J., Hussain S.M. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol. Sci. Feb. 2008;101(2):239–253. doi: 10.1093/toxsci/kfm240. [DOI] [PubMed] [Google Scholar]
- 330.Tomaszewska E., et al. Detection limits of DLS and UV‐vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. Jan. 2013;2013(1) doi: 10.1155/2013/313081. [DOI] [Google Scholar]
- 331.Koppel D.E. Analysis of macromolecular polydispersity in intensity correlation spectroscopy: the method of cumulants. J. Chem. Phys. Dec. 1972;57(11):4814–4820. doi: 10.1063/1.1678153. [DOI] [Google Scholar]
- 332.Kou T., Jin C., Zhang C., Sun J., Zhang Z. Nanoporous core–shell Cu@Cu2O nanocomposites with superior photocatalytic properties towards the degradation of methyl orange. RSC Adv. 2012;2(33) doi: 10.1039/c2ra21821f. [DOI] [Google Scholar]
- 333.Butler J., Handy R.D., Upton M., Besinis A. Review of antimicrobial nanocoatings in medicine and dentistry: mechanisms of action, biocompatibility performance, safety, and benefits compared to antibiotics. ACS Nano. Apr. 2023;17(8):7064–7092. doi: 10.1021/acsnano.2c12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Seyfi J., et al. Developing antibacterial superhydrophobic coatings based on polydimethylsiloxane/silver phosphate nanocomposites: assessment of surface morphology, roughness and chemistry. Prog. Org. Coat. Dec. 2020;149 doi: 10.1016/j.porgcoat.2020.105944. [DOI] [Google Scholar]
- 335.Wen H., et al. Robust super hydrophobic cotton fabrics functionalized with Ag and PDMS for effective antibacterial activity and efficient oil–water separation. J. Environ. Chem. Eng. Oct. 2021;9(5) doi: 10.1016/j.jece.2021.106083. [DOI] [Google Scholar]
- 336.Zhang F., et al. Durable and high-breathable antimicrobial face mask based on scalable superhydrophobic design. SSRN Electron. J. 2022 doi: 10.2139/ssrn.4128566. [DOI] [Google Scholar]
- 337.Xue C.-H., Chen J., Yin W., Jia S.-T., Ma J.-Z. Superhydrophobic conductive textiles with antibacterial property by coating fibers with silver nanoparticles. Appl. Surf. Sci. Jan. 2012;258(7):2468–2472. doi: 10.1016/j.apsusc.2011.10.074. [DOI] [Google Scholar]
- 338.Görgülüer H., Çakıroğlu B., Özacar M. Ag NPs deposited TiO2 coating material for superhydrophobic, antimicrobial and self-cleaning surface fabrication on fabric. J. Coating Technol. Res. Mar. 2021;18(2):569–579. doi: 10.1007/s11998-020-00412-6. [DOI] [Google Scholar]
- 339.Singh J., Dhaliwal A.S. Plasmon-induced photocatalytic degradation of methylene blue dye using biosynthesized silver nanoparticles as photocatalyst. Environ. Technol. May 2020;41(12):1520–1534. doi: 10.1080/09593330.2018.1540663. [DOI] [PubMed] [Google Scholar]
- 340.Jaast S., Grewal A. Green synthesis of silver nanoparticles, characterization and evaluation of their photocatalytic dye degradation activity. Curr. Res. Green Sustain. Chem. 2021;4 doi: 10.1016/j.crgsc.2021.100195. [DOI] [Google Scholar]
- 341.Rajkumar R., Ezhumalai G., Gnanadesigan M. A green approach for the synthesis of silver nanoparticles by Chlorella vulgaris and its application in photocatalytic dye degradation activity. Environ. Technol. Innov. Feb. 2021;21 doi: 10.1016/j.eti.2020.101282. [DOI] [Google Scholar]
- 342.Mavaei M., Chahardoli A., Shokoohinia Y., Khoshroo A., Fattahi A. One-step synthesized silver nanoparticles using isoimperatorin: evaluation of photocatalytic, and electrochemical activities. Sci. Rep. Feb. 2020;10(1):1762. doi: 10.1038/s41598-020-58697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Cheng T.-H., Yang Z.-Y., Tang R.-C., Zhai A.-D. Functionalization of silk by silver nanoparticles synthesized using the aqueous extract from tea stem waste. J. Mater. Res. Technol. May 2020;9(3):4538–4549. doi: 10.1016/j.jmrt.2020.02.081. [DOI] [Google Scholar]
- 344.Gao Y.-N., Wang Y., Yue T.-N., Weng Y.-X., Wang M. Multifunctional cotton non-woven fabrics coated with silver nanoparticles and polymers for antibacterial, superhydrophobic and high performance microwave shielding. J. Colloid Interface Sci. Jan. 2021;582:112–123. doi: 10.1016/j.jcis.2020.08.037. [DOI] [PubMed] [Google Scholar]
- 345.Su X., Li H., Lai X., Chen Z., Zeng X. Highly stretchable and conductive superhydrophobic coating for flexible electronics. ACS Appl. Mater. Interfaces. Mar. 2018;10(12):10587–10597. doi: 10.1021/acsami.8b01382. [DOI] [PubMed] [Google Scholar]
- 346.Wang L., et al. Bioinspired superhydrophobic and durable octadecanoic acid/Ag nanoparticle-decorated rubber composites for high-performance strain sensors. ACS Sustain. Chem. Eng. May 2021;9(21):7245–7254. doi: 10.1021/acssuschemeng.1c00917. [DOI] [Google Scholar]
- 347.Guo Z., et al. Multi-functional and water-resistant conductive silver nanoparticle-decorated cotton textiles with excellent joule heating performances and human motion monitoring. Cellulose. Jul. 2021;28(11):7483–7495. doi: 10.1007/s10570-021-03955-y. [DOI] [Google Scholar]
- 348.Wang Z., Zhu Q., Wang Y., Dou S., Chen Q., Lu N. Silver-nanoparticle-grafted silicon nanocones for reproducible Raman detection of trace contaminants in complex liquid environments. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. Apr. 2021;251 doi: 10.1016/j.saa.2021.119447. [DOI] [PubMed] [Google Scholar]
- 349.Li S., Liu Y., Tian Z., Liu X., Han Z., Ren L. Biomimetic superhydrophobic and antibacterial stainless-steel mesh via double-potentiostatic electrodeposition and modification. Surf. Coat. Technol. Dec. 2020;403 doi: 10.1016/j.surfcoat.2020.126355. [DOI] [Google Scholar]
- 350.Shen W., Zhang L., Li X., Yu H.-Z. Binary silanization and silver nanoparticle encapsulation to create superhydrophobic cotton fabrics with antimicrobial capability. Sci. Rep. Jun. 2019;9(1):9172. doi: 10.1038/s41598-019-45622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Wu M., Ma B., Pan T., Chen S., Sun J. Silver‐nanoparticle‐colored cotton fabrics with tunable colors and durable antibacterial and self‐healing superhydrophobic properties. Adv. Funct. Mater. Jan. 2016;26(4):569–576. doi: 10.1002/adfm.201504197. [DOI] [Google Scholar]
- 352.Bu Y., et al. Fabrication of durable antibacterial and superhydrophobic textiles via in situ synthesis of silver nanoparticle on tannic acid-coated viscose textiles. Cellulose. Feb. 2019;26(3):2109–2122. doi: 10.1007/s10570-018-2183-7. [DOI] [Google Scholar]
- 353.Hong H.R., Kim J., Park C.H. Facile fabrication of multifunctional fabrics: use of copper and silver nanoparticles for antibacterial, superhydrophobic, conductive fabrics. RSC Adv. 2018;8(73):41782–41794. doi: 10.1039/C8RA08310J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Agbe H., Sarkar D.K., Chen X.-G., Faucheux N., Soucy G., Bernier J.-L. Silver–polymethylhydrosiloxane nanocomposite coating on anodized aluminum with superhydrophobic and antibacterial properties. ACS Appl. Bio Mater. Jul. 2020;3(7):4062–4073. doi: 10.1021/acsabm.0c00159. [DOI] [PubMed] [Google Scholar]
- 355.Wang J., Yi G. Flexible and superhydrophobic silver nanoparticles decorated aligned silver nanowires films as surface-enhanced Raman scattering substrates. Nanoscale Res. Lett. Dec. 2019;14(1):292. doi: 10.1186/s11671-019-3117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Chobba M.B., et al. Ag-TiO2/PDMS nanocomposite protective coatings: synthesis, characterization, and use as a self-cleaning and antimicrobial agent. Prog. Org. Coat. Sep. 2021;158 doi: 10.1016/j.porgcoat.2021.106342. [DOI] [Google Scholar]
- 357.Abualnaja K.M., ElAassar M.R., Ghareeb R.Y., Ibrahim A.A., Abdelsalam N.R. Development of photo-induced Ag0/TiO2 nanocomposite coating for photocatalysis, self-cleaning and antimicrobial polyester fabric. J. Mater. Res. Technol. Nov. 2021;15:1513–1523. doi: 10.1016/j.jmrt.2021.08.127. [DOI] [Google Scholar]
- 358.Moridi Mahdieh Z., Shekarriz S., Afshar Taromi F., Montazer M. A new method for in situ synthesis of Ag–TiO2 nanocomposite particles on polyester/cellulose fabric by photoreduction and self-cleaning properties. Cellulose. Apr. 2018;25(4):2355–2366. doi: 10.1007/s10570-018-1694-6. [DOI] [Google Scholar]
- 359.Choi B., et al. Highly conductive fiber with waterproof and self-cleaning properties for textile electronics. ACS Appl. Mater. Interfaces. Oct. 2018;10(42):36094–36101. doi: 10.1021/acsami.8b10217. [DOI] [PubMed] [Google Scholar]
- 360.El-Naggar M.E., Khattab T.A., Abdelrahman M.S., Aldalbahi A., Hatshan M.R. Development of antimicrobial, UV blocked and photocatalytic self-cleanable cotton fibers decorated with silver nanoparticles using silver carbamate and plasma activation. Cellulose. Jan. 2021;28(2):1105–1121. doi: 10.1007/s10570-020-03537-4. [DOI] [Google Scholar]
- 361.Xu R., Wang W., Yu D. Preparation of silver-plated Hollow Glass Microspheres and its application in infrared stealth coating fabrics. Prog. Org. Coat. Jun. 2019;131:1–10. doi: 10.1016/j.porgcoat.2019.02.009. [DOI] [Google Scholar]
- 362.Taghavizadeh Yazdi M.E., Modarres M., Amiri M.S., Darroudi M. Phyto-synthesis of silver nanoparticles using aerial extract of Salvia leriifolia Benth and evaluation of their antibacterial and photo-catalytic properties. Res. Chem. Intermed. Mar. 2019;45(3):1105–1116. doi: 10.1007/s11164-018-3666-8. [DOI] [Google Scholar]
- 363.Ahmad T., et al. Biosynthesis, characterization and photo-catalytic degradation of methylene blue using silver nanoparticles. Mater. Today Proc. 2020;29:1039–1043. doi: 10.1016/j.matpr.2020.04.707. [DOI] [Google Scholar]
- 364.Mehwish H.M., et al. Green synthesis of a silver nanoparticle using Moringa oleifera seed and its applications for antimicrobial and sun-light mediated photocatalytic water detoxification. J. Environ. Chem. Eng. Aug. 2021;9(4) doi: 10.1016/j.jece.2021.105290. [DOI] [Google Scholar]
- 365.Viet P.V., et al. Silver nanoparticle loaded TiO 2 nanotubes with high photocatalytic and antibacterial activity synthesized by photoreduction method. J. Photochem. Photobiol. Chem. Feb. 2018;352:106–112. doi: 10.1016/j.jphotochem.2017.10.051. [DOI] [Google Scholar]
- 366.Choi H.W., et al. Smart textile lighting/display system with multifunctional fibre devices for large scale smart home and IoT applications. Nat. Commun. Feb. 2022;13(1):814. doi: 10.1038/s41467-022-28459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Wang L., et al. Bioinspired superhydrophobic and durable octadecanoic acid/Ag nanoparticle-decorated rubber composites for high-performance strain sensors. ACS Sustain. Chem. Eng. May 2021;9(21):7245–7254. doi: 10.1021/acssuschemeng.1c00917. [DOI] [Google Scholar]
- 368.Choi B., et al. Highly conductive fiber with waterproof and self-cleaning properties for textile electronics. ACS Appl. Mater. Interfaces. Oct. 2018;10(42):36094–36101. doi: 10.1021/acsami.8b10217. [DOI] [PubMed] [Google Scholar]
- 369.Xu R., Wang W., Yu D. Preparation of silver-plated Hollow Glass Microspheres and its application in infrared stealth coating fabrics. Prog. Org. Coat. Jun. 2019;131:1–10. doi: 10.1016/j.porgcoat.2019.02.009. [DOI] [Google Scholar]
- 370.Jung I., Jo Y.H., Kim I., Lee H.M. A simple process for synthesis of Ag nanoparticles and sintering of conductive ink for use in printed electronics. J. Electron. Mater. Jan. 2012;41(1):115–121. doi: 10.1007/s11664-011-1761-3. [DOI] [Google Scholar]
- 371.Shen W., Zhang X., Huang Q., Xu Q., Song W. Preparation of solid silver nanoparticles for inkjet printed flexible electronics with high conductivity. Nanoscale. 2014;6(3):1622–1628. doi: 10.1039/C3NR05479A. [DOI] [PubMed] [Google Scholar]
- 372.Doria G., et al. Noble metal nanoparticles for biosensing applications. Sensors. Feb. 2012;12(2):1657–1687. doi: 10.3390/s120201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Cannilla C., Bonura G., Frusteri F., Spadaro D., Trocino S., Neri G. Development of an ammonia sensor based on silver nanoparticles in a poly-methacrylic acid matrix. J. Mater. Chem. C. 2014;2(29):5778. doi: 10.1039/c4tc00515e. [DOI] [Google Scholar]
- 374.Rithesh Raj D., Prasanth S., Vineeshkumar T.V., Sudarsanakumar C. Ammonia sensing properties of tapered plastic optical fiber coated with silver nanoparticles/PVP/PVA hybrid. Opt Commun. Apr. 2015;340:86–92. doi: 10.1016/j.optcom.2014.11.092. [DOI] [Google Scholar]
- 375.Banihashemian S.M., Hajghassem H., Nikfarjam A., Azizi Jarmoshti J., Abdul Rahman S., Boon Tong G. Room temperature ethanol sensing by green synthesized silver nanoparticle decorated SWCNT. Mater. Res. Express. Mar. 2019;6(6) doi: 10.1088/2053-1591/ab07de. [DOI] [Google Scholar]
- 376.Zhang L., Li L. Colorimetric detection of hydrogen peroxide using silver nanoparticles with three different morphologies. Anal. Methods. 2016;8(37):6691–6695. doi: 10.1039/C6AY01108J. [DOI] [Google Scholar]
- 377.Srikhao N., Kasemsiri P., Lorwanishpaisarn N., Okhawilai M. Green synthesis of silver nanoparticles using sugarcane leaves extract for colorimetric detection of ammonia and hydrogen peroxide. Res. Chem. Intermed. Mar. 2021;47(3):1269–1283. doi: 10.1007/s11164-020-04354-x. [DOI] [Google Scholar]
- 378.Yoshikawa H., Hieda K., Ikeda K., Tamiya E. Hydrogen peroxide detection with a silver nanoparticle grating chip fabricated by plasmonic plating. Anal. Methods. 2019;11(23):2991–2995. doi: 10.1039/C9AY00576E. [DOI] [Google Scholar]
- 379.Zhan B., et al. A hydrogen peroxide electrochemical sensor based on silver nanoparticles decorated three-dimensional graphene. Appl. Phys. Lett. Jun. 2014;104(24) doi: 10.1063/1.4884418. [DOI] [Google Scholar]
- 380.Pérez-López B., Merkoçi A. Nanomaterials based biosensors for food analysis applications. Trends Food Sci. Technol. Nov. 2011;22(11):625–639. doi: 10.1016/j.tifs.2011.04.001. [DOI] [Google Scholar]
- 381.Photiphitak C., Rakkwamsuk P., Muthitamongkol P., Sae-Kung C., Thanachayanont C. Effect of silver nanoparticle size on efficiency enhancement of dye-sensitized solar cells. Int. J. Photoenergy. 2011;2011:1–8. doi: 10.1155/2011/258635. [DOI] [Google Scholar]
- 382.Kislov D.A. Effect of plasmonic silver nanoparticles оn the photovoltaic properties of Graetzel solar cells. Phys. Procedia. 2015;73:114–120. doi: 10.1016/j.phpro.2015.09.130. [DOI] [Google Scholar]
- 383.Sreeja S., Pesala B. Plasmonic enhancement of betanin-lawsone co-sensitized solar cells via tailored bimodal size distribution of silver nanoparticles. Sci. Rep. May 2020;10(1):8240. doi: 10.1038/s41598-020-65236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Pant H.R., Pandeya D.R., Nam K.T., Baek W., Hong S.T., Kim H.Y. Photocatalytic and antibacterial properties of a TiO2/nylon-6 electrospun nanocomposite mat containing silver nanoparticles. J. Hazard Mater. May 2011;189(1–2):465–471. doi: 10.1016/j.jhazmat.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 385.Pallavicini P., et al. A monolayer of a Cu2+-tetraazamacrocyclic complex on glass as the adhesive layer for silver nanoparticles grafting, in the preparation of surface-active antibacterial materials. New J. Chem. 2011;35(6):1198. doi: 10.1039/c0nj00829j. [DOI] [Google Scholar]
- 386.Kaegi R., et al. Release of silver nanoparticles from outdoor facades. Environ. Pollut. Sep. 2010;158(9):2900–2905. doi: 10.1016/j.envpol.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 387.Cheng L., et al. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. May 2012;28(5):561–572. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Maneerung T., Tokura S., Rujiravanit R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. Apr. 2008;72(1):43–51. doi: 10.1016/j.carbpol.2007.07.025. [DOI] [Google Scholar]
- 389.Chen J.-P., Chiang Y. Bioactive electrospun silver nanoparticles-containing polyurethane nanofibers as wound dressings. J. Nanosci. Nanotechnol. Nov. 2010;10(11):7560–7564. doi: 10.1166/jnn.2010.2829. [DOI] [PubMed] [Google Scholar]
- 390.Chaloupka K., Malam Y., Seifalian A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. Nov. 2010;28(11):580–588. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 391.Jacob J.M., et al. Bactericidal coating of paper towels via sustainable biosynthesis of silver nanoparticles using Ocimum sanctum leaf extract. Mater. Res. Express. Jan. 2019;6(4) doi: 10.1088/2053-1591/aafaed. [DOI] [Google Scholar]
- 392.Syukri D.M., et al. Prevention of post-operative bacterial colonization on mice buccal mucosa using biogenic silver nanoparticles-coated nylon sutures. Regen. Eng. Transl. Med. Jun. 2024;10(2):294–308. doi: 10.1007/s40883-024-00335-3. [DOI] [Google Scholar]
- 393.Ewald A., Hösel D., Patel S., Grover L.M., Barralet J.E., Gbureck U. Silver-doped calcium phosphate cements with antimicrobial activity. Acta Biomater. Nov. 2011;7(11):4064–4070. doi: 10.1016/j.actbio.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 394.Rogers J.V., Parkinson C.V., Choi Y.W., Speshock J.L., Hussain S.M. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. Apr. 2008;3(4):129–133. doi: 10.1007/s11671-008-9128-2. [DOI] [Google Scholar]
- 395.Lu S., Gao W., Gu H.Y. Construction, application and biosafety of silver nanocrystalline chitosan wound dressing. Burns. Aug. 2008;34(5):623–628. doi: 10.1016/j.burns.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 396.Ahmed S., Saifullah, Ahmad M., Swami B.L., Ikram S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. Jan. 2016;9(1):1–7. doi: 10.1016/j.jrras.2015.06.006. [DOI] [Google Scholar]
- 397.Baram-Pinto D., Shukla S., Perkas N., Gedanken A., Sarid R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjugate Chem. Aug. 2009;20(8):1497–1502. doi: 10.1021/bc900215b. [DOI] [PubMed] [Google Scholar]
- 398.Elechiguerra J.L., et al. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. Jun. 2005;3(1):6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Lara H.H., Ayala-Nuñez N.V., Ixtepan-Turrent L., Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010;8(1):1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Murugan K., et al. Nanoparticles in the fight against mosquito-borne diseases: bioactivity of Bruguiera cylindrica-synthesized nanoparticles against dengue virus DEN-2 (in vitro) and its mosquito vector Aedes aegypti (Diptera: Culicidae) Parasitol. Res. Dec. 2015;114(12):4349–4361. doi: 10.1007/s00436-015-4676-8. [DOI] [PubMed] [Google Scholar]
- 401.Aziz A.T., et al. Phytochemical analysis of Rhazya stricta extract and its use in fabrication of silver nanoparticles effective against mosquito vectors and microbial pathogens. Sci. Total Environ. Jan. 2020;700 doi: 10.1016/j.scitotenv.2019.134443. [DOI] [PubMed] [Google Scholar]
- 402.Zheng Y., Cloutier P., Hunting D.J., Sanche L. Radiosensitization by gold nanoparticles: comparison of DNA damage induced by low and high-energy electrons. J. Biomed. Nanotechnol. Dec. 2008;4(4):469–473. doi: 10.1166/jbn.2008.3282. [DOI] [Google Scholar]
- 403.Monteiro D.R., Gorup L.F., Takamiya A.S., Ruvollo-Filho A.C., Camargo E.R.D., Barbosa D.B. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Int. J. Antimicrob. Agents. Aug. 2009;34(2):103–110. doi: 10.1016/j.ijantimicag.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 404.Tran Q.H., Nguyen V.Q., Le A.-T. Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. May 2013;4(3) doi: 10.1088/2043-6262/4/3/033001. [DOI] [Google Scholar]
- 405.Morley K.S., et al. Synthesis and characterisation of advanced UHMWPE/silver nanocomposites for biomedical applications. Eur. Polym. J. Feb. 2007;43(2):307–314. doi: 10.1016/j.eurpolymj.2006.10.011. [DOI] [Google Scholar]
- 406.Coker R.J., Hunter B.M., Rudge J.W., Liverani M., Hanvoravongchai P. Emerging infectious diseases in southeast Asia: regional challenges to control. Lancet. Feb. 2011;377(9765):599–609. doi: 10.1016/S0140-6736(10)62004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Gajbhiye M., Kesharwani J., Ingle A., Gade A., Rai M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed. Nanotechnol. Biol. Med. Dec. 2009;5(4):382–386. doi: 10.1016/j.nano.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 408.Manjumeena R., Duraibabu D., Sudha J., Kalaichelvan P.T. Biogenic nanosilver incorporated reverse osmosis membrane for antibacterial and antifungal activities against selected pathogenic strains: an enhanced eco-friendly water disinfection approach. J. Environ. Sci. Health, Part A. Aug. 2014;49(10):1125–1133. doi: 10.1080/10934529.2014.897149. [DOI] [PubMed] [Google Scholar]
- 409.Belt H.V.D., Neut D., Schenk W., Horn J.R.V., Mei H.C.V.D., Busscher H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements: a review. Acta Orthop. Scand. Jan. 2001;72(6):557–571. doi: 10.1080/000164701317268978. [DOI] [PubMed] [Google Scholar]
- 410.Liu Y., et al. The antimicrobial and osteoinductive properties of silver nanoparticle/poly (dl-lactic-co-glycolic acid)-coated stainless steel. Biomaterials. Dec. 2012;33(34):8745–8756. doi: 10.1016/j.biomaterials.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 411.Oves M., et al. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater. Sci. Eng. C. Aug. 2018;89:429–443. doi: 10.1016/j.msec.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 412.Dadashpour M., et al. Biomimetic synthesis of silver nanoparticles using Matricaria chamomilla extract and their potential anticancer activity against human lung cancer cells. Mater. Sci. Eng. C. Nov. 2018;92:902–912. doi: 10.1016/j.msec.2018.07.053. [DOI] [PubMed] [Google Scholar]
- 413.Sánchez-Navarro M.D.C., et al. Cytotoxic and bactericidal effect of silver nanoparticles obtained by green synthesis method using Annona muricata aqueous extract and functionalized with 5-fluorouracil. Bioinorgan. Chem. Appl. Oct. 2018;2018:1–8. doi: 10.1155/2018/6506381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Abu Elella M.H., Mohamed R.R., Abdel-Aziz M.M., Sabaa M.W. Green synthesis of antimicrobial and antitumor N,N,N-trimethyl chitosan chloride/poly (acrylic acid)/silver nanocomposites. Int. J. Biol. Macromol. May 2018;111:706–716. doi: 10.1016/j.ijbiomac.2018.01.055. [DOI] [PubMed] [Google Scholar]
- 415.Palem R.R., Ganesh S.D., Kronekova Z., Sláviková M., Saha N., Saha P. Green synthesis of silver nanoparticles and biopolymer nanocomposites: a comparative study on physico-chemical, antimicrobial and anticancer activity. Bull. Mater. Sci. Apr. 2018;41(2):55. doi: 10.1007/s12034-018-1567-5. [DOI] [Google Scholar]
- 416.L G., S A., K. P T., M K. Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: assessment of their antibacterial and anticancer activity. Karbala Int. J. Mod. Sci. Mar. 2018;4(1):61–68. doi: 10.1016/j.kijoms.2017.10.007. [DOI] [Google Scholar]
- 417.Bhakya S., Muthukrishnan S., Sukumaran M., Muthukumar M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. Jun. 2016;6(5):755–766. doi: 10.1007/s13204-015-0473-z. [DOI] [Google Scholar]
- 418.Saxena A., Tripathi R.M., Zafar F., Singh P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater. Lett. Jan. 2012;67(1):91–94. doi: 10.1016/j.matlet.2011.09.038. [DOI] [Google Scholar]
- 419.Sinha S.N., Paul D., Halder N., Sengupta D., Patra S.K. Green synthesis of silver nanoparticles using fresh water green alga Pithophora oedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Appl. Nanosci. Aug. 2015;5(6):703–709. doi: 10.1007/s13204-014-0366-6. [DOI] [Google Scholar]
- 420.Govarthanan M., et al. Low-cost and eco-friendly synthesis of silver nanoparticles using coconut (Cocos nucifera) oil cake extract and its antibacterial activity. Artif. Cells, Nanomed. Biotechnol. Nov. 2016;44(8):1878–1882. doi: 10.3109/21691401.2015.1111230. [DOI] [PubMed] [Google Scholar]
- 421.Paulkumar K., et al. Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. Sci. World J. 2014;2014:1–9. doi: 10.1155/2014/829894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Fran Ccedil Ois E.R.A.M., et al. Unexplored vegetal green synthesis of silver nanoparticles: a preliminary study with Corchorus olitorus Linn and Ipomea batatas (L.) Lam. Afr. J. Biotechnol. Mar. 2016;15(10):341–349. doi: 10.5897/AJB2015.14962. [DOI] [Google Scholar]
- 423.Nwabor O.F., Singh S., Wunnoo S., Lerwittayanon K., Voravuthikunchai S.P. Facile deposition of biogenic silver nanoparticles on porous alumina discs, an efficient antimicrobial, antibiofilm, and antifouling strategy for functional contact surfaces. Biofouling. May 2021;37(5):538–554. doi: 10.1080/08927014.2021.1934457. [DOI] [PubMed] [Google Scholar]
- 424.Syukri D.M., Nwabor O.F., Singh S., Voravuthikunchai S.P. Antibacterial functionalization of nylon monofilament surgical sutures through in situ deposition of biogenic silver nanoparticles. Surf. Coat. Technol. May 2021;413 doi: 10.1016/j.surfcoat.2021.127090. [DOI] [Google Scholar]
- 425.Syukri D.M., et al. Antibacterial-coated silk surgical sutures by ex situ deposition of silver nanoparticles synthesized with Eucalyptus camaldulensis eradicates infections. J. Microbiol. Methods. Jul. 2020;174 doi: 10.1016/j.mimet.2020.105955. [DOI] [PubMed] [Google Scholar]
- 426.Ram P., Vivek K., Kumar S.P. Nanotechnology in sustainable agriculture: present concerns and future aspects. Afr. J. Biotechnol. Feb. 2014;13(6):705–713. doi: 10.5897/AJBX2013.13554. [DOI] [Google Scholar]
- 427.Yang N., Wei X.-F., Li W.-H. Sunlight irradiation induced green synthesis of silver nanoparticles using peach gum polysaccharide and colorimetric sensing of H2O2. Mater. Lett. Sep. 2015;154:21–24. doi: 10.1016/j.matlet.2015.03.034. [DOI] [Google Scholar]
- 428.Ahamed M., AlSalhi M.S., Siddiqui M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta. Dec. 2010;411(23–24):1841–1848. doi: 10.1016/j.cca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 429.Nwabor O.F., Singh S., Ontong J.C., Vongkamjan K., Voravuthikunchai S.P. Valorization of wastepaper through antimicrobial functionalization with biogenic silver nanoparticles, a sustainable packaging composite. Waste Biomass Valorization. Jun. 2021;12(6):3287–3301. doi: 10.1007/s12649-020-01237-5. [DOI] [Google Scholar]
- 430.Singh S., Chunglok W., Nwabor O.F., Ushir Y.V., Singh S., Panpipat W. Hydrophilic biopolymer matrix antibacterial peel-off facial mask functionalized with biogenic nanostructured material for cosmeceutical applications. J. Polym. Environ. Mar. 2022;30(3):938–953. doi: 10.1007/s10924-021-02249-5. [DOI] [Google Scholar]
- 431.Abazari M., Badeleh S.M., Khaleghi F., Saeedi M., Haghi F. Fabrication of silver nanoparticles-deposited fabrics as a potential candidate for the development of reusable facemasks and evaluation of their performance. Sci. Rep. Jan. 2023;13(1):1593. doi: 10.1038/s41598-023-28858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Rahman H., Kumar V., Singh P., Kumar A. Catalytic effect of silver nanoparticles on ZnO surface for CO gas-sensing applications. Appl. Nanosci. Nov. 2022;12(11):3517–3527. doi: 10.1007/s13204-022-02423-8. [DOI] [Google Scholar]
- 433.Kumar A., Vemula P.K., Ajayan P.M., John G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. Mar. 2008;7(3):236–241. doi: 10.1038/nmat2099. [DOI] [PubMed] [Google Scholar]
- 434.Zhang N., Luo J., Liu R., Liu X. Tannic acid stabilized silver nanoparticles for inkjet printing of conductive flexible electronics. RSC Adv. 2016;6(87):83720–83729. doi: 10.1039/C6RA19800G. [DOI] [Google Scholar]
- 435.Huang Y., et al. A randomized comparative trial between Acticoat and SD-Ag in the treatment of residual burn wounds, including safety analysis. Burns. Mar. 2007;33(2):161–166. doi: 10.1016/j.burns.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 436.Lekamge S., et al. The toxicity of silver nanoparticles (AgNPs) to three freshwater invertebrates with different life strategies: Hydra vulgaris, Daphnia carinata, and Paratya australiensis. Front. Environ. Sci. Dec. 2018;6:152. doi: 10.3389/fenvs.2018.00152. [DOI] [Google Scholar]
- 437.Tripathi D.K., et al. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: a concentric review. Front. Microbiol. 2017;8(Jan) doi: 10.3389/fmicb.2017.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Tortella G.R., et al. Silver nanoparticles: toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard Mater. May 2020;390 doi: 10.1016/j.jhazmat.2019.121974. [DOI] [PubMed] [Google Scholar]
- 439.Prasannaraj G., Sahi S.V., Ravikumar S., Venkatachalam P. Enhanced cytotoxicity of biomolecules loaded metallic silver nanoparticles against human liver (HepG2) and prostate (PC3) cancer cell lines. J. Nanosci. Nanotechnol. May 2016;16(5):4948–4959. doi: 10.1166/jnn.2016.12336. [DOI] [PubMed] [Google Scholar]
- 440.Ribeiro A.P.C., et al. Evaluation of cell toxicity and DNA and protein binding of green synthesized silver nanoparticles. Biomed. Pharmacother. May 2018;101:137–144. doi: 10.1016/j.biopha.2018.02.069. [DOI] [PubMed] [Google Scholar]
- 441.Qing W., Wang Y., Li X., Lu M., Liu X. Facile synthesis of mPEG-luteolin-capped silver nanoparticles with antimicrobial activity and cytotoxicity to neuroblastoma SK-N-SH cells. Colloids Surf. B Biointerfaces. Dec. 2017;160:390–394. doi: 10.1016/j.colsurfb.2017.09.048. [DOI] [PubMed] [Google Scholar]
- 442.Arumai Selvan D., Mahendiran D., Senthil Kumar R., Kalilur Rahiman A. Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: phytochemical, antioxidant and in vitro cytotoxicity studies. J. Photochem. Photobiol., B. Mar. 2018;180:243–252. doi: 10.1016/j.jphotobiol.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 443.Morais M., Teixeira A.L., Dias F., Machado V., Medeiros R., Prior J.A.V. Cytotoxic effect of silver nanoparticles synthesized by green methods in cancer. J. Med. Chem. Dec. 2020;63(23):14308–14335. doi: 10.1021/acs.jmedchem.0c01055. [DOI] [PubMed] [Google Scholar]
- 444.Jaswal T., Gupta J. A review on the toxicity of silver nanoparticles on human health. Mater. Today Proc. 2023;81:859–863. doi: 10.1016/j.matpr.2021.04.266. [DOI] [Google Scholar]
- 445.Tortella G.R., et al. Silver nanoparticles: toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard Mater. May 2020;390 doi: 10.1016/j.jhazmat.2019.121974. [DOI] [PubMed] [Google Scholar]
- 446.Ribeiro A.P.C., et al. Evaluation of cell toxicity and DNA and protein binding of green synthesized silver nanoparticles. Biomed. Pharmacother. May 2018;101:137–144. doi: 10.1016/j.biopha.2018.02.069. [DOI] [PubMed] [Google Scholar]
- 447.Qing W., Wang Y., Li X., Lu M., Liu X. Facile synthesis of mPEG-luteolin-capped silver nanoparticles with antimicrobial activity and cytotoxicity to neuroblastoma SK-N-SH cells. Colloids Surf. B Biointerfaces. Dec. 2017;160:390–394. doi: 10.1016/j.colsurfb.2017.09.048. [DOI] [PubMed] [Google Scholar]
- 448.Arumai Selvan D., Mahendiran D., Senthil Kumar R., Kalilur Rahiman A. Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: phytochemical, antioxidant and in vitro cytotoxicity studies. J. Photochem. Photobiol., B. Mar. 2018;180:243–252. doi: 10.1016/j.jphotobiol.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 449.Shah D.D., Chorawala M.R., Mansuri M.K.A., Parekh P.S., Singh S., Prajapati B.G. Biogenic metallic nanoparticles: from green synthesis to clinical translation. Naunyn-Schmiedeberg’s Arch. Pharmacol. Nov. 2024;397(11):8603–8631. doi: 10.1007/s00210-024-03236-y. [DOI] [PubMed] [Google Scholar]
- 450.Lee H.A., et al. Rhizome of Anemarrhena asphodeloides as mediators of the eco-friendly synthesis of silver and gold spherical, face-centred cubic nanocrystals and its anti-migratory and cytotoxic potential in normal and cancer cell lines. Artif. Cells, Nanomed. Biotechnol. Nov. 2018;46(sup2):285–294. doi: 10.1080/21691401.2018.1457038. [DOI] [PubMed] [Google Scholar]
- 451.Jinu U., Gomathi M., Saiqa I., Geetha N., Benelli G., Venkatachalam P. Green engineered biomolecule-capped silver and copper nanohybrids using Prosopis cineraria leaf extract: enhanced antibacterial activity against microbial pathogens of public health relevance and cytotoxicity on human breast cancer cells (MCF-7) Microb. Pathog. Apr. 2017;105:86–95. doi: 10.1016/j.micpath.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 452.Park J.S., Ahn E.-Y., Park Y. Asymmetric dumbbell-shaped silver nanoparticles and spherical gold nanoparticles green-synthesized by mangosteen (Garcinia mangostana) pericarp waste extracts. Int. J. Nanomed. Sep. 2017;12:6895–6908. doi: 10.2147/IJN.S140190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Venugopal K., et al. The impact of anticancer activity upon Beta vulgaris extract mediated biosynthesized silver nanoparticles (ag-NPs) against human breast (MCF-7), lung (A549) and pharynx (Hep-2) cancer cell lines. J. Photochem. Photobiol., B. Aug. 2017;173:99–107. doi: 10.1016/j.jphotobiol.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 454.Sreekanth T.V.M., et al. Ultra-sonication-assisted silver nanoparticles using Panax ginseng root extract and their anti-cancer and antiviral activities. J. Photochem. Photobiol., B. Nov. 2018;188:6–11. doi: 10.1016/j.jphotobiol.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 455.Prasannaraj G., Sahi S.V., Ravikumar S., Venkatachalam P. Enhanced cytotoxicity of biomolecules loaded metallic silver nanoparticles against human liver (HepG2) and prostate (PC3) cancer cell lines. J. Nanosci. Nanotechnol. May 2016;16(5):4948–4959. doi: 10.1166/jnn.2016.12336. [DOI] [PubMed] [Google Scholar]
- 456.Satpathy S., Patra A., Ahirwar B., Delwar Hussain M. Antioxidant and anticancer activities of green synthesized silver nanoparticles using aqueous extract of tubers of Pueraria tuberosa. Artif. Cells, Nanomed. Biotechnol. Nov. 2018;46(sup3):71–85. doi: 10.1080/21691401.2018.1489265. [DOI] [PubMed] [Google Scholar]