Summary
Background
Lung metastasis is a critical and often fatal progression in cancer patients, with monocyte-derived macrophages (Mo-macs) playing multifaceted roles in this process. Despite the recognized importance of Mac-macs, most studies focus on these cells themselves, while the precise mechanisms through which tumor cells manipulate Mo-macs to promote metastasis remain poorly understood.
Methods
We developed an in vivo CRISPR screening system to identify genes involved in macrophage-dependent metastasis by depleting Mo-macs. Osteoprotegerin (OPG) was identified as the factor significantly enhances lung metastasis. We validated its function in lung metastasis by modulating the expression of OPG in an array of cell lines and performed spontaneous and experimental lung metastasis assays. Genetically engineered mice were utilized to confirm the role of RANKL-RANK signaling in OPG-mediated metastasis. Additionally, we employed different neutralizing antibodies to elucidate the roles of Mo-macs and NK cells and inhibitor to clarify the role of CXCL10 signaling.
Findings
Employing in vivo screening techniques, we elucidate the role of OPG, a protein secreted by cancer cells, in driving lung metastasis, contingent upon regulating Mo-mac activity. OPG blocks the signaling cascade between receptor activator of nuclear factor kappa-B ligand (RANKL) and its receptor RANK on Mo-macs, thereby hindering Mo-macs from secreting CXCL10, a chemokine crucial for recruiting natural killer (NK) cells that help control lung metastasis. Moreover, we observe an enrichment of OPG amplifications in metastatic cancer patients, and elevated levels of OPG expression in lung metastatic sites compared to paired primary breast cancer samples.
Interpretation
Our work revealed that OPG works as a lung metastasis promoting factor by blocking the RANKL-RANK-CXCL10 axis to drive the paucity of NK cells, which could be a therapeutic target for lung metastatic cancer patients.
Funding
The full list of funding supporting this study can be found in the Acknowledgements section.
Keywords: Lung metastasis, OPG, Macrophages, RANKL, CXCL10, NK cells
Research in context.
Evidence before this study
OPG has been shown to inhibit osteoclast maturation by blocking RANKL-RANK signaling, thereby reducing bone metastases in cancer patients. Beyond its role in bone development and osteoclastogenesis, RANKL-RANK signaling is also involved in regulating the tumor immune microenvironment. Furthermore, research in immunodeficient mice suggests that OPG secreted by endothelial cells helps tumor cells evade TRAIL-induced apoptosis, promoting lung metastasis. However, the specific role of tumor-derived OPG in lung metastasis, particularly its influence on the immune microenvironment at metastatic sites, remains largely unexplored. Additionally, monocyte-derived macrophages (Mo-macs) can either promote or inhibit lung metastasis through various mechanisms, but how tumor cells interact with Mo-macs to drive metastasis progression is still unclear.
Added value of this study
RANKL-RANK signaling is crucial for osteoclast differentiation, osteolytic bone metastasis, and promoting primary tumor initiation. OPG, acting as a decoy receptor for RANKL, inhibits this signaling and is widely considered a tumor suppressor. However, our findings reveal that OPG gene amplification is frequently observed in cancer patients, suggesting it may play a tumor-promoting role rather than a suppressive one. Our functional and mechanistic studies confirm that OPG enhances lung metastasis by modulating Mo-mac-mediated signaling and inhibiting NK cell recruitment.
Implications of all the available evidence
Our results reveal an unexpected role of OPG in lung metastasis. While previous studies suggest that OPG suppresses bone metastasis progression, our findings indicate that OPG promotes lung metastasis by inhibiting RANKL-RANK signaling in Mo-macs. This inhibition reshapes the macrophage-dependent immune microenvironment, highlighting the potential of activating RANKL signaling or targeting OPG as therapeutic strategies for patients with lung metastasis. Given these new insights, it becomes crucial to stratify cancer patients based on their risk of developing lung or bone metastases, enabling the tailored selection of anti-OPG or anti-RANKL treatments accordingly.
Introduction
Metastasis is the leading cause of death in cancer patients.1 Immune cells are in charge of mass control in many steps of cancer metastasis. Among tumor-infiltrating immune cells, macrophages are a major immune cell type in many solid human cancers.2 Like the CCL2-CCR2 axis, tumor-associated macrophages (TAMs) can also be recruited by chemokines to metastatic sites.3 TAMs can either be tumor-promoting or tumor-inhibiting during metastasis. For example, TAMs promote tumor growth and metastasis by enhancing the epithelial–mesenchymal transition (EMT) of cancer cells4,5 and by secreting VEGF6 to induce angiogenesis or inhibiting other immunologic effector cells.7,8 In addition to their prometastatic function, TAMs are reported to directly engulf cancer cells to inhibit metastasis, through a process known as phagocytosis. To resist macrophage-mediated phagocytosis, cancer cells have evolved to express phagocytosis checkpoint proteins, including CD479 and STC1.10 In the lung tissue, TAMs are mostly derived from tissue-resident alveolar macrophages (AMs) and monocyte-derived macrophages (Mo-macs), which potentially play different functional roles in metastasis.11 AMs can promote EMT and inhibit dendritic cell and T-cell function,5,12 but influenza-trained AMs also exhibit long-term antitumour immunity.13 Mo-macs are also suggested to have dual functions in lung metastasis. As a subpopulation of Mo-macs, TREM2+ Mo-macs promote lung metastasis,14 while LAIR1+ Mo-macs suppress lung metastasis.15 Mo-macs trained by whole beta-glucan particles also inhibit metastasis by augmenting phagocytosis.16 Previous studies focused on the function of Mo-macs in metastasis, but how cancer cells interact with Mo-macs to affect lung metastasis remains unclear.
Osteoprotegerin (OPG), a protein encoded by TNFRSF11B, belongs to the TNF receptor superfamily. OPG acts by competing for binding with RANK to RANKL, thereby inhibiting RANKL-RANK signalling. This mechanism effectively prevents the stimulation of osteoclast maturation and hinders cancer bone metastasis.17,18 RANKL was first identified in CD4+ T cells and is important for dendritic cell stimulation.19 In addition to its well-known role in bone development and osteoclastogenesis, RANKL-RANK signalling is suggested to be involved in regulating the tumor immune microenvironment (TiME).20 In a murine mammary tumor model, MMTV-Erbb2, RANKL+ tumor-infiltrating regulatory T cells were shown to stimulate breast cancer lung metastasis through RANKL-RANK signalling.21 In another murine mammary tumor model, MMTV-PyMT, RANK knockout in breast cancer cells induced an antitumour immune response mediated by CD8+ T cells.22 Blockade of RANKL-RANK signalling by pharmacologic inhibitors reduces the recurrence of breast cancer cells based on its ability to induce tumor cell differentiation.23 Given OPG's antagonistic role against RANKL signalling, it may influence tumor growth and metastasis by regulating the tumor immune microenvironment. Recent findings suggest that endothelial cell-derived OPG could facilitate lung metastasis by inhibiting TRAIL-induced apoptosis in immunodeficient mice.24 Notably, increased TNFRSF11B copy number amplification is observed specifically within lung metastases compared to primary tumors in triple-negative breast cancer patients, indicating a potential role for tumor-derived OPG in promoting lung metastasis progression.25 However, direct studies examining tumor-derived OPG in lung metastasis progression, particularly its impact on the regulation of the lung metastatic tumor immune microenvironment, are lacking.
Natural killer (NK) cells are major immune-killing cells and are innate lymphoid cells that can quickly destroy virally infected or malignant cells without antigen priming or sensitization. The balance between NK cell inhibitory receptors (NKIRs) and NK cell activating receptors (NKARs) regulates NK cell function by interacting with corresponding ligands on target cells.26 NK cells are reportedly pivotal immune cells that control metastasis.27 NK cells can eliminate circulating tumor cells28 and inhibit the reactivation of dormant metastases.29 Tumor cells develop several mechanisms to evade attack by NK cells, such as decreasing the expression of NKARs,30 encasing tumor cells in thrombi generated by platelets31 and increasing the expression of immune checkpoints such as HLA-E:CD94-NKG2A.32 Tumor cells also secrete other proteins, including DKK1, which protects dormant metastatic cells from NK cell-mediated death, to inhibit NK cell function.33 In addition to intrinsic tumor factors, the activity of NK cells is also regulated by other tumor-infiltrating immune cells in the TiME. For instance, through the expression of MS4A4A, macrophages promote evasion of metastatic cancer cells from NK cell-mediated killing.34 Patrolling monocytes recruit NK cells via CCL3/4/5,35 and TREM2+ Mo-macs secrete IL18BP to inhibit NK cell activation.14 In our current study, we performed an in vivo CRISPR knockout screen to uncover lung metastatic drivers that are dependent on Mo-macs. We report that cancer cells secrete OPG to promote lung metastasis, which drives the paucity of NK cells by blocking the RANKL-RANK-CXCL10 axis on Mo-macs.
Methods
Animal studies
C57BL/6J and BALB/c mice were obtained from Vital River (Beijing, China) or GemPharmatech (Nanjing, China). Six-to eight-week-old female mice were used for these studies. Rankf/f mice and Rankl−/− mice were kindly provided by Dr. Xiaohuan Guo (Tsinghua University). Rankl−/− mice were fed a liquid diet. Lyz2-iCre mice were kindly provided by Dr. Peng Jiang (Tsinghua University). All mice were generated on a C57BL/6J genetic background. Mice were maintained under specific pathogen-free (SPF) conditions, with regulated temperature and humidity, autoclaved food and water and a 12-h light/dark cycle.
Cell lines
HEK-293T (CRL-11268), 4T1 (CRL-2539), 4T07 and B16–F10 (CRL-6475) cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin‒streptomycin (P/S). Yumm1.7 (CRL-3362) cells were cultured in DMEM:F12 medium supplemented with 10% FBS and 1% P/S. The cells were cultured at 37 °C in a humidified atmosphere with 5% CO2.
Experimental mouse model
For lung metastasis assays, 2 105 cells (B16F10, Yumm1.7 and 4T07) or 1 105 cells (4T1) were resuspended in 100 μL of PBS and injected into the lateral tail vein of the mice. Mice will be euthanized, and their lungs will be harvested approximately 2–3 weeks after injection. Lung colonization will be quantified, and tissues will be fixed for H&E staining.
To induce spontaneous metastasis, mice were anesthetized and 1 105 4T1 cells were inoculated into the fourth mammary glands of mice. Tumor volume will be measured, and lung colonization will be assessed when the tumor reaches a size of 15 mm.
CRISPR screen
B16F10 cells were infected with Cas9-blast lentivirus to generate pooled B16F10-Cas9 cells. To obtain clones with high Cas9 activity, B16F10-Cas9 cells were sorted into 96-well plates to obtain single clones through FACS. Multiple clones were then infected with the Cd274 sgRNA lentivirus. Five days after infection, each single clone was stimulated with IFN-γ (10 ng/mL) for 24 h, and the expression of Cd274 was assessed through FACS to evaluate Cas9 activity. Clone 12, which exhibited an editing efficiency of more than 85%, was selected for subsequent screening.
To enhance the therapeutic and clinical relevance of our screening results, we selected a druggable library for the screening. This library consists of 1217 genes, all of which are either established drug targets or associated with drug mechanisms of action36 (Table S1). Each gene is represented by four sgRNAs, along with 100 off-target and intergenic control sequences.
After packaging the sgRNA library into lentivirus, we first tested the virus titer. We plated 1 105 B16F10-Cas9 clone 12 cells per well in a 6-well plate and infected with different amounts of lentivirus for 24 h in the presence of 8 μg/mL polybrene. The next day, puromycin (1.5 μg/mL) was added to eliminate uninfected cells over 48 h. Infected cells were then counted in each well to calculated the multiplicity of infection (MOI).37 For the screening, we ultimately chose an MOI of 0.1 to ensure that each cell is infected by a single virus, carrying only one sgRNA. Additionally, we maintained a coverage of 300 cells per sgRNA to guarantee that each perturbation will be adequately represented in the final screening readout.
IgG and α-Csf1R antibodies were administered to the control and experimental groups two weeks before inoculation of the library-infected cells via tail vein. Two weeks after tumor cell innoculation, the lungs were harvested from the euthanized mice. Lung metastases were isolated for genomic DNA extraction. Then, the amplification of the gRNA cassette was performed by PCR system for high-throughput sequencing.
For the purpose of candidate gene discovery, the normalized gRNA count table was analysed using MAGeCK.38,39 This analysis involved a comparison between IgG and α-CSF1R samples. Our focus lies in the genes represented by gRNAs that are lost in the IgG group but enriched in the α-CSF1R group. The identification of the top genes was predicated upon a fold change greater than 3, accompanied by a p value less than 0.01. CRISPR screen sequencing datasets have been deposited in the Gene Expression Omnibus under accession number GSE260469.
Knockdown, knockout and overexpression constructs
shRNA-mediated knockdown was achieved by cloning shRNAs into the pLKO.1-puro vector (Addgene, #8453). The sequences of the shRNAs are listed in Table S5. For Tgfbr2 knockout, CRISPR sgRNAs were cloned and inserted into the lentiCRISPR v2 backbone (Addgene, #42230). To overexpress OPG, RANKL and CXCL10, cDNAs were cloned and inserted into either the retroviral expression vector pMSCV-puro or the lentiviral expression vector pLVX-blast, depending on the different cell lines and experimental requirements.
Lentiviruses were packaged using pMD2. G and psPAX2 plasmids, while retroviruses were packaged with pCMV-Gag/Pol and pCMV-VSVG packing plasmids in HEK293T cells. Conditional media containing virus from these packaging cells was collected and purified through a 0.22 μm filter at 48 and 72 h post transfection. Recipient cell lines were infected with the virus supplemented with 8 μg/mL polybrene for 24 h. Puromycin or blasticidin was used to select stably expressing cell lines.
Flow cytometry and fluorescence-activated cell sorting
For analysis of immunocytes in the lung, the lungs were perfused with at least 20 mL of PBS through the right ventricle until they were cleared of blood. Then, the tissues will be minced and digested with collagenase I (1 mg/mL; Sigma #C0130) and DNase I (0.1 mg/mL; Sigma #260913) at 37 °C for 1 h with agitation (100 RPM) followed by passage through a 70-μm cell strainer. Red blood cells will be depleted through red blood cell (RBC) lysis buffer (Thermo Fisher #00-4333-57) for 5 min at room temperature. For flow cytometry, cells were stained in FACS buffer (PBS supplemented with 2% fetal bovine serum (FBS)) with different combinations of the following monoclonal antibodies (1:200 dilution): CD45 (BD #557659), CD3 (BD #555275), NK1.1 (Biolegend #108706), CD4 (Biolegend #100451), CD8 (Biolegend #126610), CD19 (Biolegend #115511), CD11b (Thermo Fisher #12-0112-81), Ly6G (BD #551460), Siglec F (Thermo Fisher #46-1702-82), F4/80 (Thermo Fisher #63-4801-82), RANK (Biolegend #119805), CD11b (Thermo Fisher #25-0112-82), and Ly6C (Biolegend #128016). To calculate the absolute number of NK cells in the lungs with metastases, we used CountBright absolut counting beads (Thermo Fisher #C36950), following the manufacturer's protocol. To exclude dead cells, the cells were stained with DAPI (Solarbio #C0060) after antibody staining. Flow cytometry was performed on a BD LSRFortessa, and the data were analysed using FlowJo software. BD FACSAria was used for cell sorting.
Generation of bone marrow-derived macrophages
Bone marrow cells were isolated from 6- to 8-week-old C57BL/6 mice by flushing murine femurs, tibias and humeri with cold PBS. The cells were strained through a 70-μm filter and centrifuged before being resuspended in 1 × RBC lysis buffer for 5 min at room temperature.
Total bone marrow cells were plated in treated dishes with DMEM (10% FBS and 1% P/S) overnight culture. Then, nonadherent cells were plated in ultralow-attachment dishes with DMEM supplemented with 10% FBS, 1% P/S and 50 ng/mL murine recombinant colony stimulating factor (M-CSF) (PeproTech #315-02). The medium was changed every 2 days. After 6 days of differentiation, bone marrow-derived macrophages (BMDMs) were prepared for further experiments.
To test the MHC expression and polarization of macrophages in vitro, BMDMs were collected and plated in 12-well plates. Then, BMDMs were stimulated with IL-4 (10 ng/mL), IFN-γ (10 ng/mL), IFN-γ + LPS and OPG (10 ng/mL) for 24 h. The expression of markers, including CD86 (Biolegend #105125), CD206 (Biolegend #141708), I-A/I-E (Biolegend #107619) and H-2Kb (Biolegend #116517), was tested by FACS.
Flow cytometry-based phagocytosis assay
To boost macrophage phagocytic activity and effectively target tumor cells, we aim to express a fusion protein, HER2-mCherry, on the tumor cell surface. This fusion protein enhances antibody-dependent cellular phagocytosis (ADCP) by macrophages. To circumvent interference from HER2 signaling, we cloned the cDNA encoding the extracellular region of HER2 and fused it with the mCherry sequence at the C-terminus. Subsequently, we employed a lentiviral system to express this construct in B16F10 cells, followed by flow sorting to isolate mCherry-positive cells.
In vitro phagocytosis assays were performed by coculturing target cells and bone marrow-derived macrophages at a ratio of 200,000 target cells to 100,000 macrophages for 4 h in a humidified 5% CO2 incubator at 37 °C in 24-well plates. The macrophages were plated overnight before coculture, and the medium was replaced with serum-free DMEM for 2 h. Then, B16F10 cells with the HER2-mCherry fusion protein were cocultured with complete DMEM. IgG or an α-HER2 antibody was added to different plates according to the experimental requirements. After coculture, all cells were collected from the plates and stained with a FITC-labelled α-CD11b antibody (BioLegend #101206) to identify macrophages. Phagocytosis was measured as the number of CD11b+ mCherry+ macrophages and quantified as a percentage of the total number of CD11b+ macrophages.
In vivo treatment schedule and dosing
To deplete macrophages, an α-CSF1R (AFS98) antibody was administered by intraperitoneal injection two weeks before tumor cell injection, starting with an initial injection of 1 mg/mouse, followed by a subsequent injection of 0.5 mg/mouse every 5 days. The depletion of CD4+ T cells and NK cells was achieved by administering α-CD4 (GK1.5) and α-NK1.1 (PK136) antibodies through injection at a dosage of 0.2 mg/mouse every 3 days.
Recombinant mouse RANKL protein was administered to mice via tai vein injection, starting 5 days prior to tumor injection, at a dosage of 0.1 mg per mouse every 3 days.
The CXCR3 antagonist AMG-487 was dissolved in vehicle (10% DMSO + 40% PEG300 + 5% Tween 80 + 45% saline) and administered at a dose of 5 mg/kg to each mouse via intraperitoneal injection beginning three days prior to tumor inoculation, with subsequent injections every 2 days. The control groups received the corresponding solvent injection.
Western blot
For western blotting, cultured cells were lysed with lysis buffer (50 mM Tris–HCl pH 7.4 + 150 mM NaCl + 1 mM EDTA + 1% NP-40 + protease inhibitors) on ice for 10 min. Then, whole-cell lysates were collected, mixed with loading buffer, and heated at 95 °C for 10 min. Denatured protein was loaded on SDS‒PAGE gels and subsequently transferred to PVDF membranes. The membranes were blocked in 5% milk for 1 h at room temperature and incubated at 4 °C overnight with primary antibodies. For immunoblotting of secreted protein, cancer cells were cultured in serum-free medium at 37 °C for 24 h. The media were collected and concentrated with a centrifugal concentrator (3 kDa MWCO, 0.5 mL sample volume, Sigma #UFC5003). Then, the proteins were concentrated following the above standard procedures.
RNA extraction and qPCR analysis
For cultured cell lines, total RNA was isolated using RNAiso Plus Regent (Takara #9108) following the manufacturer's instructions. For RNA extraction, harvested tissues were crushed in RNAiso Plus Regent through a Bullent Blender following the standard protocol. cDNA was generated using ReverTra Ace qPCR RT Master Mix (TOYOBO #FSQ-201). Relative gene expression was determined using SYBR Green assays (GDSBIO #P2105).
For isolated lung macrophages, RNA was extracted using an RNeasy Plus Micro Kit (QIAGEN #74034). Then, cDNA was synthesized using the LunaScript RT SuperMix Kit (NEB #E3010S), and the expression of the tested gene was subsequently analysed using THUNDERBIRD Next SYBR qPCR Mix (TOYOBO #QPX-201). Quantitative PCR was performed on a QuantStudio 3 System (Thermo Fisher). Relative gene expression was normalized to Gapdh expression. The primers used for qPCR are listed in Table S6.
RNA sequencing
For ultralow-input bulk RNA sequencing, 104–105 Mo-Macs were sorted from digested lungs via FACS. Mo-Macs were cultured in a 96-well plate in 1640 medium supplemented with M-CSF (50 ng/mL). RANKL (BioLegend #769402, 100 ng/mL) was added to the medium 24 h after isolation, followed by a 24-h stimulation period. RNA was then extracted using an RNeasy Plus Micro Kit (QIAGEN) according to the manufacturer's instructions. RNA was retrotranscribed and processed to complementary DNA libraries using the Smart-Seq RNA kit for sequencing. Library construction and sequencing were performed by GENEWIZ (Suzhou, China). RNA-seq data are accessible under the accession number GSE259293.
Immunohistochemical (IHC) staining
For staining of 5 μm sections, murine metastatic tissue samples were fixed in 4% paraformaldehyde for 24 h prior to paraffin embedding. Sections were deparaffinized using xylene, rehydrated, and incubated with 3% hydrogen peroxide to inhibit endogenous peroxidase activity, followed by antigen retrieval in sodium citrate (pH = 6.0) in a steamer for 30 min. For OPG immunohistochemistry, after antigen retrieval, sections were incubated with 1 × Animal-Free Blocking Solution (CST #15019) and then α-OPG antibody (R&D #AF459, 5 μg/mL) overnight at 4 °C. SignalStain Boost IHC Detection Reagent (HRP, Goat, CST #63707) and a SignalStain DAB Substrate Kit (CST #8059) were used for detection.
Luciferase assay
To test the survival rate of B16F10 cells treated with TRAIL, a luciferase assay was performed. Luciferase-transfected B16F10-TR cells were lysed using luciferase lysis buffer (2 mM EDTA + 20 mM DTT + 10% glycerol + 1% Triton X-100 + 25 mM Tris base, pH 7.8, adjusted with H3PO4) on a shaker for 1 h at room temperature. Then, 25 μL of lysate and 75 μL of luciferase substrate solution (2 mM ATP + 10 mM DTT + 1 mM D-luciferin +15 mM MgSO4) were added to each well of a 96-well white plate (with at least 3 replicates per sample). Luciferase activity was assayed using a multiple microplate luminometer (EnVision).
NK cell killing assay
Spleens were taken from 6-week-old C57BL/6 mice and filtered to prepare a single-cell splenocyte suspension through 70-μm cell strainer. Red blood cells were depleted through red blood cell (RBC) lysis buffer. Primary NK cells were isolated using Mojosort Mouse NK Cell lsolation Kit (Biolegend #480050) and cultured in murine NK cell complete medium (RPMI1640 + 10% FBS +1% P/S + 1 × GlutaMax (Gibco #35050061) + 1 × MEM NEAA (Gibco #11140050) + 1 × 2-Mercaptoethanol (Gibco #21985023) + 50 ng/mL IL-2 (PeproTech #212-12) + 50 ng/mL lL-15 (PeproTech #210-15)).
Luciferase-labeled B16F10-TR cells were applied to flat-bottom 96-well plates (1 × 104 cells/well) one day before adding primary NK cells (1 × 104 cells/well, E:T ration = 1:1). After 6 h of co-incubation, 50 μL of luciferase lysis buffer was added to each well, followed by a 1-h shake. The cell mixture was then lysed for a luciferase assay.
In vitro proliferation and apoptosis assays
To test the proliferation of tumor cells in vitro, a CCK-8 assay was performed. A total of 1000 control and OPG knockdown cells were seeded into 96-well plates. The data were collected by a multiple microplate luminometer at 405 nm every 24 h. Fresh medium supplemented with 10% CCK-8 solution (Yeasen #40203ES76) was added to the cell plates and incubated at 37 °C for 4 h before the data were collected.
Apoptosis was tested with an APC Annexin V Apoptosis Detection Kit with 7-AAD (BioLegend, #640930) following the manufacturer's protocol. The ratio of APC+ 7-AAD- apoptotic cells was analysed by FACS.
Bioinformatic analysis of RNA-seq and clinical data
Differential gene expression in lung Mo-Macs stimulated with PBS and RANKL was analysed by using the R package edgeR40 (3.40.2) with a bcv value of 0.2 and FDR was calculated using Benjamini-Hochberg method. Volcano plots were drawn by using the R package ggplot2 (3.4.4).
Mutation of TNFRSF11B were analysed by cBioPortal. Overall survival and relapse-free survival data from the METABRIC dataset were downloaded from cBioPortal and analysed by using the R packages survminer (3.5–7) and survival (0.4.9). The TCGA-SKCM data were downloaded and analysed using the R package TCGAbiolinks41 (2.25.3). For public RNA-seq data analysis, raw count data of paired lung metastasis and primary breast cancer samples from the AURORA cohort (GSE209998) and RAP cohort (GSE193103) were downloaded from GEO and analysed using the R package DESeq242 (1.38.3) to compare the expression levels of TNFRSF11B in lung metastasis and primary tumors. The raw counts of all lung metastases in the AURORA and RAP cohorts were downloaded and converted to TPM by using the R package biomaRt43 (2.54.1) for the calculation of gene length. Standard CIBERSORT44 (0.1.0) was utilized to calculate NK infiltration in lung metastases. Those samples without activated NK infiltration were removed, and in the remaining samples, the correlation of log2 (TPM +1) of TNFRSF11B and activated NK cells was plotted by using the R package ggstatsplot (0.12.1). NKI-295 data was required through R package breastCancerNKI. CIBERSORT was used to calculate the NK cell infiltration and survminer (3.5–7) and survival (0.4.9) were used to analyze the overall survival and distant metastasis-free survival in TCGA-SKCM and NKI-295 dataset.
Quantification and statistical analysis
All statistical analyses and graphs were calculated and created using Graphpad Prism 10.0 software. Statistical analyses are described in detail for each figure panel. Mann–Whitney U test, unpaired student's t-test, wilcoxon matched-pairs signed-rank test, one-way ANOVA or two-way ANOVA were used to calculate the statistical significance of differences in a particular measurement between groups. Pearson's correlation coefficient was used to assess correlations, and statistical significance was evaluated using student's t-test. Survival data was analyzed by utilizing Kaplan–Meier survival analysis, with significance tested via the log-rank test. The number of samples (n) is indicated for each figure panel. All experiments were reproducible.
Ethics statement
All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Tsinghua University. The approval number is 16-ZHQ-1 and 16-ZHQ.G23-1. There were no human participants involved in this study. Race or ethnicity data were not collected in this study because it is a preclinical research study.
Role of funders
The funding organisations did not take part in the study including the experiment design, data collection, analysis, interpretation of results, or the preparartion, revision and decision to submit the manuscript for publication.
Results
Genetic screening reveals potential cancer cell-Mo-mac interaction mediators involved in lung metastasis
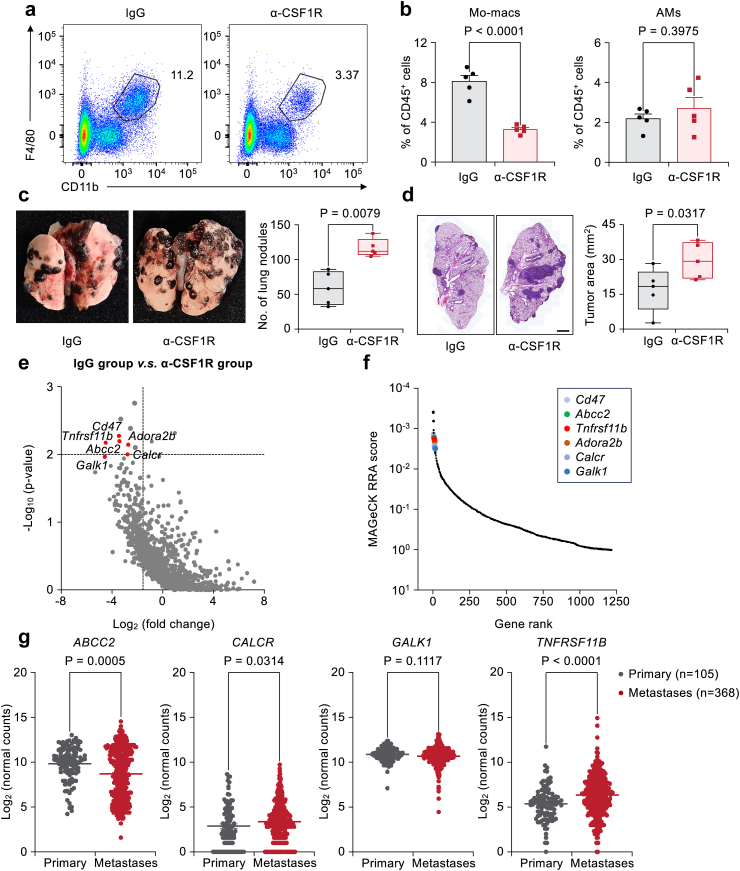
To understand how lung metastasis progression is affected by interactions between cancer cells and Mo-macs, it is essential to first confirm the function of Mo-macs in lung metastasis. We pretreated mice with an α-CSF1R neutralizing antibody. This antibody treatment only specifically depleted Mo-macs and had no apparent effect on AMs (Fig. 1a and b and Fig. S1a). We then injected murine melanoma B16F10 cells into the tail vein of mice pretreated with IgG control or a-CSF1R neutralizing antibody to deplete Mo-macs. Mo-mac depletion led to an increased number of metastatic tumor nodules in the lung and larger metastatic areas in hematoxylin and eosin (H&E)-stained lung tissues (Fig. 1c and d). This result suggested that Mo-mac cells could suppress lung metastasis progression.
Fig. 1.
Genetic screening identified OPG as a lung metastasis promoter through Mo-macs. (a, b) Flow cytometry graphs (a) and quantification (b) showing the percentages of CD11b+F4/80+ Mo-macs (b, left) and AMs (b, right) among CD45+ immune cells in the lung after antibody injection. n = 5 mice per group. The data is presented as the mean ± s.e.m., Student's two-sided unpaired t test. (c, d) Female B6 mice were first treated with either IgG or α-CSF1R antibody and continued until the experimental endpoint. Cells were then tail vein injected with 2 105 B16F10 cells. The whole-lung images show the lung surface nodules (c), and H&E staining (d) shows the tumor areas. n = 5 mice per group. Scale bar: 1 mm. Boxplots showed the minimum, first quartile, median, third quartile, and maximum of the data points. P values were obtained by Mann–Whitney U test. (e) Genes encoded by corresponding depleted sgRNAs in the IgG v.s. α-CSF1R group from the screening were identified. Candidate genes were plotted based on the mean log2 (fold change) of sgRNA counts, and p values were computed using MAGeCK. A horizontal line was drawn at a p value of 0.01, and a vertical line represented a fold change of 3. (f) MAGeCK RRA ranking of the top depleted genes compared with the IgG group v.s. the α-CSF1R group from the screening. (g) Candidate genes (ABCC2, CALCR, GALK1 andTNFRSF11B) mRNA levels in primary SKCM tumors and metastases in the TCGA cohort. The lings representing the mean values of all candidate genes. P values were calculated by Mann–Whitney U test.
To identify candidate genes that mediate the interaction between cancer cells and Mo-macs responsible for this potential metastasis suppression effect, we designed an in vivo screen, as summarized in Fig. S1b. We first generated B16F10-Cas9 single-cell clones and selected a clone based on its consistent high gene editing efficiency when cells were transduced with test gRNAs (representative PD-L1 expression after cells were transduced with gRNA targeting Cd274 and Fig. S1c and Table S1). To enhance the potential clinical translational value of the identified candidate targets, we utilized a druggable gRNA library based on identified candidate druggable gene candidates.36 This gRNA library was used to transduce stable Cas9-expressing single-cell B16F10 clones (B16F10-Cas9). Since we hypothesize that certain pro-metastatic genes are Mo-mac-dependent, we predict that gRNAs targeting these genes will make tumor cells more susceptible to Mo-macs. Therefore, we expect these sgRNAs to be depleted in the control group but retained in the α-CSF1R antibody treatment group, as a result of Mo-mac depletion.The B16F10-Cas9 clone 12 with the highest efficiency was selected for transduction with a druggable gRNA library (Table S1) for further screening.
The screening results were analysed by Model-based Analysis of Genome-wide CRISPR-Cas9 Knockout (MAGeCK),38,39 and candidate genes were selected based on their fold changes greater than 3 and p values less than 0.01 (Fig. 1e and Table S2). Cd47, a well-known gene that induces cancer cell resistance to phagocytosis by macrophages, and Adora2b, a gene that has been reported to promote metastasis,45 were among our top candidate genes, demonstrating the efficacy of our screening approach. In addition to these known genes, several top candidates, including Abcc2, Calcr, Galk1, and Tnfrsf11b (encoding the OPG protein), were among the candidate genes and have not been well studied for their roles in lung metastasis (Fig. 1f).
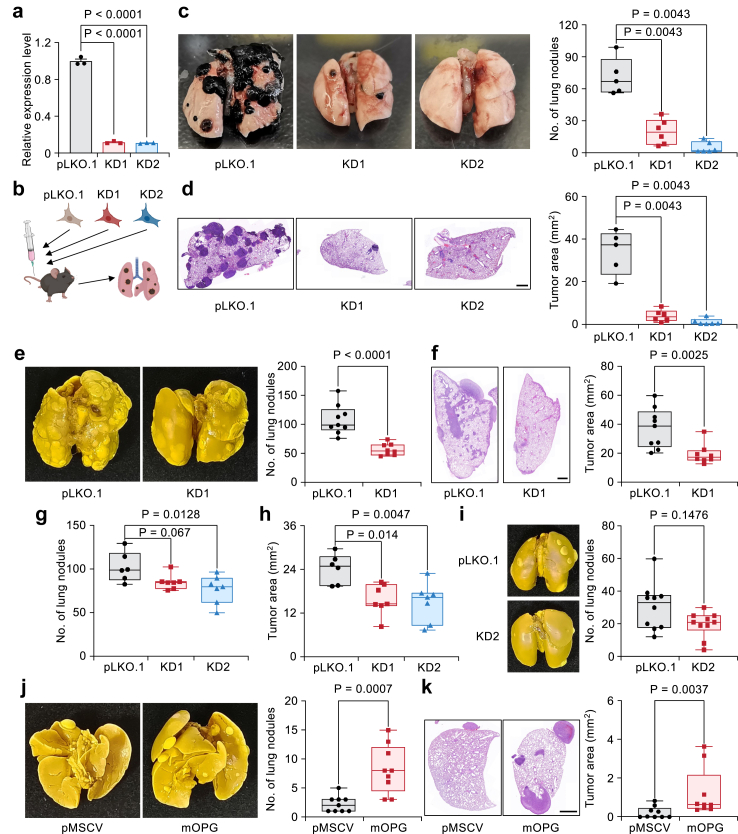
To identify the potential mediators which was responsible for the cancer cell-Mo-mac interaction in lung metastasis, we first analyzed the expression of candidate genes in primary skin cutaneous melanoma (SKCM) tumors and metastases in the TCGA cohort. Our analysis revealed that among the four candidate genes, TNFRSF11B and CALCR mRNA exhibited significantly higher expression levels in metastatic tumors compared to primary tumors (Fig. 1g). We also used short hairpin RNAs (shRNAs) to knock down individual candidate genes in B16F10 cells and confirmed their knockdown (KD) efficiencies by quantitative PCR (qPCR) analysis (Fig. S2a). These cells were injected via the tail vein into 6- to 8-week-old female C57BL/6 mice to generate experimental lung metastases. The KD of these genes decreased the lung metastasis burden in the mice (Fig. S2b and c), with the KD of OPG displaying the most striking reduction in lung metastasis burden, as demonstrated by the decreased number of lung metastatic nodules and smaller metastatic areas on H&E-stained lung images (quantified in Fig. S2d and e). Thus, our further work focused on the role of OPG in promoting lung metastasis.
OPG promotes lung metastasis
We first excluded the influence of OPG on cell proliferation and apoptosis, as the KD of OPG by two independent shRNAs had little effect on cell proliferation and cell apoptosis (Fig. 2a, Fig. S3a and b). Next, we tail-vein injected these two independentent OPG-KD B16F10 cells into B6 mice (Fig. 2b) and again confirmed consistently reduced lung metastasis nodules (Fig. 2c) and decreased tumor areas (Fig. 2d). Similarly, the KD of OPG significantly attenuated lung metastasis progression in the Yumm1.7 murine melanoma cell line (Fig. 2e and f and Fig. S3c). Additionally, we examined the role of OPG in 4T1 and 4T07 murine breast cancer lines with strong and weak lung metastasis potential, respectively.46 Silencing OPG expression in 4T1 cells reduced lung metastasis in both the experimental lung metastasis assay by tail vein injection and the spontaneous metastasis assay by mammary fat pad injection (Fig. 2g–i, Fig. S3d–f and h). Notably, the KD of OPG had no effect on primary tumor growth (Fig. S3g). We then overexpressed OPG in 4T07 cells (Fig. S3i), and its expression significantly increased the metastasis burden when the cells were tail-vein injected into syngeneic female BALB/c mice (Fig. 2j and k). In summary, these findings suggest a prometastatic function of OPG across different types of cancers.
Fig. 2.
OPG promotes lung metastasis. (a) Relative expression levels of Tnfrsf11b mRNA in B16F10 cells by shRNAs (n = 3 per group); the data is presented as the mean ± s.e.m., one-way ANOVA followed by Dunnett's multiple comparisons test. (b) Cartoon showing that OPG-KD cells were injected into mice through the tail vein to generate lung metastasis. (c, d) Analysis of lung metastasis in OPG-KD B16F10 cells. Whole-lung images showing lung surface nodules (c), and H&E staining (d) showing the metastatic tumor areas. n = 5–6 mice per group. (e, f) Pulmonary surface nodules (e) and tumor areas (f) of Yumm1.7 cells with OPG-KD. n = 9 (pLKO.1) or 8 (OPG-KD1) mice per group. (g, h) 4T1 cells with OPG-KD were injected into BALB/c mice through the tail vein. (g) Statistical analysis of lung surface nodules. (h) Pulmonary metastatic tumor area. n = 6 (pLKO.1) or 7 (KD1 and KD2) mice per group. (i) Representative whole-lung images and statistics of spontaneous lung metastasis of 4T1 cells with OPG-KD. n = 10 mice per group. (j, k) Whole-lung images (j) and H&E staining images (k) showing the representative lung metastasis samples generated by 4T07 cells overexpressing OPG. n = 9 mice per group. All H&E staining scale bars: 1 mm. (c–k) Statistics are shown by boxplots, including the minimum, first quartile, median, third quartile, and maximum of the data points. Mann–Whitney U test was used for statistical analysis.
The amplification of TNFRSF11B correlates with shortened patient survival in cancer patients
To assess the clinical relevance of OPG in cancer patients, we analysed three different public cancer patient datasets. Approximately 20–30% of melanoma patients and breast cancer patients with metastasis exhibit TNFRSF11B mutations (Fig. 3a).47,48 Specifically, the major mutation of TNFRSF11B is gene amplification, with more than 70% of patients with TNFRSF11B mutations exhibiting TNFRSF11B gene amplification (Fig. 3a and b). In the breast cancer METABRIC dataset,49,50 amplification of TNFRSF11B was significantly associated with shorter overall survival (OS) and relapse-free survival (RFS) (Fig. 3c and d). We also analyzed the data from the TCGA PanCancer Atlas and found that amplification of TNFRSF11B is the predominant mutation across various cancer types (Fig. S4a). Meantwhile, TNFRSF11B amplification was correlated with poor progression-free survival (PFS) and disease-free survival (DFS) (Fig. S4b and c). Since the analysis of the TCGA dataset revealed elevated the expression of TNFRSF11B in metastatic lesions of skin cancer patients (Fig. 1g), we sought to investigate whether the expression of TNFRSF11B is also induced in metastasis lesions of breast cancer patients. We analysed published data51 to compare the expression level of TNFRSF11B between primary breast tumors and matched lung metastatic tissue samples. In the AURORA US Metastatic Project cohort, most lung metastases (4/6) exhibited increased TNFRSF11B expression compared to paired primary tumor samples (Fig. 3e). In another cohort from the Rapid Autopsy Program (RAP), upregulated expression of TNFRSF11B was also observed in most lung metastasis samples compared to paired primary breast tumor samples, except for one of the paired samples (7 out of 8 pairs) (Fig. 3f). These data suggest that OPG is correlated with increased lung metastatic potential and worse prognosis in cancer patients.
Fig. 3.
Clinical correlation of OPG amplification with lung metastasis in cancer patients. (a, b) The types and proportions of mutations in TNFRSF11B in the metastatic patient cohorts. The datasets and pictures (a) were obtained from cBioPortal. Pie charts (b) showed the statistics of mutations in the metastatic melanoma patient cohort, metastatic breast cancer patient cohort, and another metastatic breast cancer patient cohort from MBCProject. (c, d) Kaplan–Meier analysis showing overall survival (c) and relapse-free survival (d) of patients with breast cancer in the METABRIC dataset stratified into those with TNFRSF11B amplification (red line) and those with WT TNFRSF11B (gray line). P-values are obtained from log-rank test. (e) The mRNA expression level of TNFRSF11B in lung metastases compared with that in matched primary breast tumors from the human breast cancer AURORA cohort. n = 6. Wilcoxon matched-pairs signed-rank test. (f) The mRNA expression level of TNFRSF11B in lung metastases compared with that in matched primary breast tumors from the RAP cancer patient cohort. n = 8. Wilcoxon matched-pairs signed-rank test.
TGF-β induces the expression of OPG to promote lung metastasis
The expression of prometastatic genes is often affected by other signaling cues in the metastatic microenvironment. For example, the pro-metastatic EMT gene TWIST1 is induced by the WNT singal.52,53 We sought to understand which microenvironmental signal regulates the expression of OPG during metastasis. Through immunohistochemical staining, we observed that OPG was highly expressed in metastatic tumors but not in paracancerous tissues (Fig. 4a and Fig. S4d). Compared to those in the parental cell lines, the B16F10 cell-generated metastases also exhibited increased OPG expression in the B16F10 model (Fig. 4b). OPG belongs to the TNF receptor superfamily, and it has been reported that TNF-α could induce the upregulation of TNFRSF11B in human melanoma cell lines54 and TGF-β could induce OPG protein expression in bone stromal cells in vitro.55 We stimulated B16F10 cells with several cytokines, including TNF-α, interferons and TGF-β, which are well known to be functionally important in metastasis.56, 57, 58 Only TGF-β, but not other cytokines, induced the upregulation of Tnfrsf11b in B16F10 cells (Fig. S4e). Similar results were observed in three additional cancer cell lines (Fig. 4c). Our screening data also revealed that among all genes in the tested gRNA library, Tgfbr2 (encoding TGF-β receptor 2) was among the top candidate genes when comparing the IgG group versus the cell line group (Fig. 4d and e and Table S3). These results suggest that TGF-β might control OPG expression in metastatic cancer cells to promote lung metastasis.
Fig. 4.
TGF-β increases OPG expression to promote lung metastasis. (a) IHC staining showed that OPG was expressed only within the metastatic colonies in the lungs of mice with B16F10 tumors. Scale bar: 200 μm. (b) The mRNA expression of Tnfrsf11b in the metastases compared to that in the parental cell line B16F10. n = 3 (cell line) or 4 (metastases) per group. The data is presented as the mean ± s.e.m., Student's two-sided unpaired t test was used for statistical analysis. (c) mRNA analyses showing the expression of OPG in the different cell lines after stimulation with TGF-β (5 ng/mL). n = 3 per group. The data is presented as the mean ± s.e.m., Student's two-sided unpaired t test was used. (d) Genes encoded by corresponding depleted sgRNAs in the IgG v.s. cell line group from the screening were identified. Candidate genes were plotted based on the mean log2 fold change in sgRNA counts, and p values were computed using MAGeCK. A horizontal line was drawn at a p value of 0.01, and a vertical line represented a fold change of 3. (e) MAGeCK RRA ranking of the top depleted genes in the IgG v.s. cell group from the screening. (f) The mRNA expression of Tnfrsf11b in B16F10 cells after stimulation with TGF-β (5 ng/mL) when Tgfbr2 was knocked out. n = 3 per group, data is presented as the mean ± s.e.m., Student's two-sided unpaired t test was used for statistical analysis. (g) Western blot showing the expression of TGFBR2 and OPG in B16F10 cells. (h, i) Presentative whole-lung images (h) and H&E staining images (i) showing the lung metastases of B16F10 cells with TGFBR2 knockout and OPG overexpression. n = 5 or 6 mice per group. Scale bar: 1 mm. Boxplots (h,i) showed the minimum, first quartile, median, third quartile, and maximum of the data points. P values were calculated by Mann–Whitney U test.
Next, we asked whether OPG is an essential downstream effector of the prometastatic signalling pathway mediated by TGF-β signalling during lung metastasis progression. In an in vitro experiment, we found that TGF-β treatment did not increase the expression of Tnfrsf11b mRNA when TGFBR2 was knocked out in B16F10 cells (Fig. 4f). Then, we expressed OPG in TGFBR2 knockout B16F10 cells (Fig. 4g) and injected these cells into syngeneic female mice. Our results demonstrated that the knockout of TGFBR2 significantly reduced the lung metastasis ability of B16F10 cells; moreover, a strong rescue effect on the ability of these cells to form lung metastases was observed when OPG was overexpressed in TGFBR2 knockout cells (Fig. 4h and i). Taken together, these findings indicate that stimulation with TGF-β leads to increased expression of OPG in cancer cells and that OPG promotes subsequent cancer metastasis to the lung.
OPG promotes lung metastasis by suppressing RANKL signalling
Next, we sought to determine the mechanism underlying the OPG-mediated lung metastasis progression. OPG was reported to promote cancer cell survival by binding with TRAIL to prevent induced apoptosis (hypothesis illustrated in Fig. S5a),24,59 which might help lung metastatic seeding. Our initial experiments aimed to test whether OPG inhibited TRAIL-induced apoptosis in our cells. However, TRAIL failed to induce apoptosis in B16F10 cells regardless of the concentration or stimulation time in vitro (Fig. S5b and c), which is consistent with the findings of a previous study on the B16F10 model.60 Furthermore, additional administration of OPG in B16F10 cells treated with TRAIL did not change the cell apoptosis status (Fig. S5d). These results suggest that the lung metastasis-promoting effect of OPG is not dependent on the resistance to TRAIL-induced cell apoptosis in these models and additional mechanisms exist.
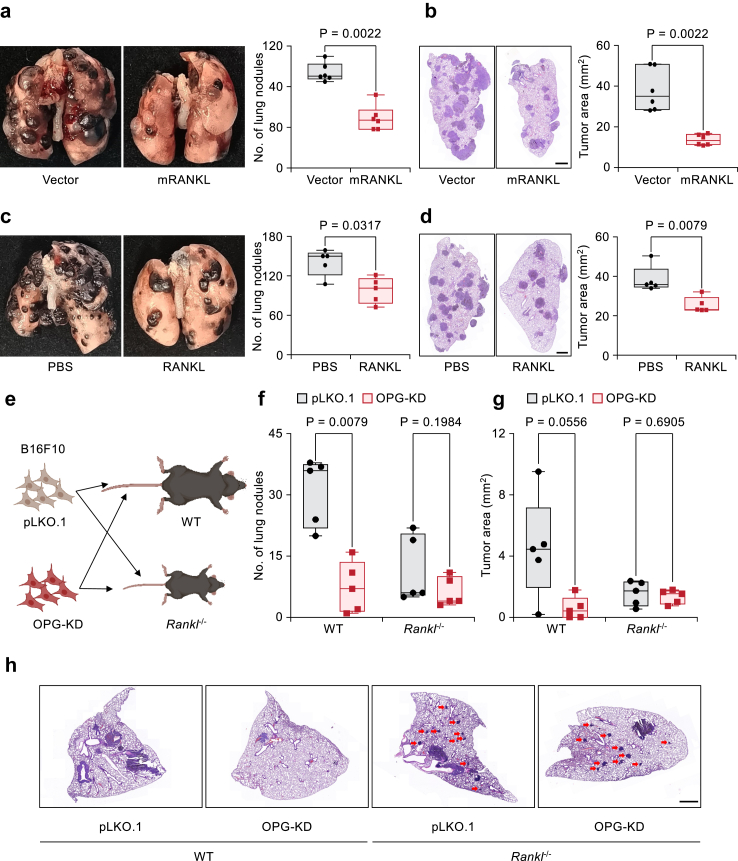
OPG functions as a decoy receptor to block RANKL signalling and inhibit osteoclastogenesis (Fig. S5a). We asked whether OPG exerts its prometastatic effect by blocking the RANKL-RANK axis within the lung microenvironment. We expressed RANKL in B16F10 cells and expect to neutralize the microenvironmental OPG, and tested its effect in lung metastasis assay. The expression of RANKL in B16F10 cells resulted in a significantly reduced burden of lung metastasis (Fig. 5a and b and the left panel of S5e). We then expressed RANKL in Yumm1.7 or 4T1 cells. The expression of RANKL in these two cell lines also significantly inhibited lung metastasis progression in the Yumm1.7 and 4T1 cell lines (Fig. S5f–i). Direct administration of recombinant RANKL protein through tail vein injection inhibited lung metastasis progression in the B16F10 model (Fig. 5c and d). Meanwhile, micro-computed tomography (μCT) revealed no obvious bone metastasis or degradation in mice similarly treated as in Fig. 5c (Fig. S5j). To directly test whether OPG promotes lung metastasis by blocking RANKL signalling, we injected B16F10 cells with OPG-KD via the tail vein into wild-type (WT) and Rankl−/− mice (Fig. 5e). While OPG-KD cells generated significantly less lung metastasis in WT mice, we observed no difference in the lung metastasis burden between the control and OPG-KD groups when cancer cells were injected into Rankl−/− mice (Fig. 5f and g). We noticed that Rankl−/− mice showed fewer lung surface nodules in all groups (Fig. 5f) but more metastatic colonies within the lung (Fig. 5h). It is possible that the tumor cells do not receive adequate nutrition or support from other stromal cells, limiting their ability to grow, due to the defects in their growth and immune system of Rankl−/− mice.61 Taken together, our data suggested that OPG promotes lung metastasis by blocking RANKL signalling.
Fig. 5.
OPG promotes lung metastasis in a RANKL-dependent manner. (a, b) Representative whole-lung images (a) and H&E staining images (b) showing the lung metastases of B16F10 cells overexpressing RANKL. n = 6 mice per group. (c, d) Analysis of the lung metastases of B16F10 cells after treatment with recombinant RANKL protein (100 μg/mouse every 3 days). n = 5 mice per group. (e) Cartoon showing the strategy of cancer cell injection of the WT and Rankl−/− mice. (f, g) Analysis of lung metastasis of B16F10 cells with OPG knockdown in WT and Rankl−/− mice, as shown in (e). (f) Statistics of pulmonary surface nodules. (g), Statistics of the metastatic tumor area. n = 5 mice per group. (h) H&E staining of (g). Red arrows indicate the metastatic clones within the lungs of the Rankl−/− mice. H&E staining scale bar: 1 mm. All boxplots showed the minimum, first quartile, median, third quartile, and maximum of the data points. P values were determined by Mann–Whitney U test.
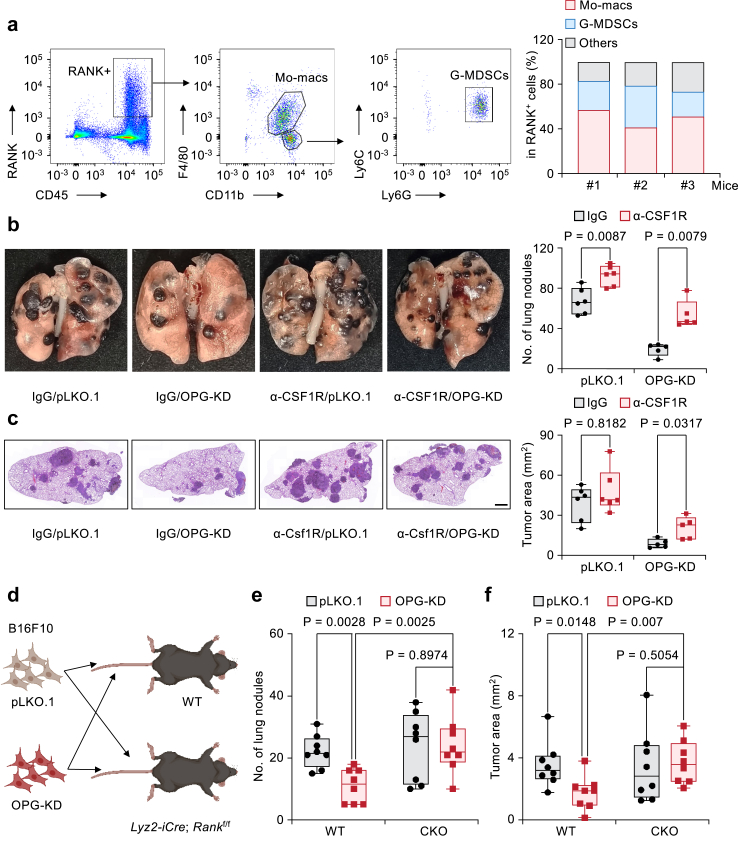
OPG promotes lung metastasis by blocking RANKL-RANK signalling on Mo-macs
Since the ability of tumor-derived OPG to promote lung metastasis is dependent on RANKL signalling, we examined the expression pattern of its receptor, RANK, on different tumor-infiltrated immune cells. Through FACS analysis, we determined that RANK is expressed mainly on Mo-macs, and a small population on Granulocytic-Myeloid-derived suppressor cells (G-MDSCs) (Fig. 6a). Considering that our initial in vivo screening was performed based on the depletion of Mo-macs by α-CSF1R antibody treatment, we asked whether OPG promotes lung metastasis by blocking RANKL-RANK on Mo-macs. Following the administration of the α-CSF1R antibody, OPG-KD B16F10 cells were injected via the tail vein into C57BL/6 mice. In macrophage-depleted mice, the KD of OPG no longer reduced the lung metastasis burden (Fig. 6b and c). To genetically test the function of RANK in Mo-macs, we constructed Lyz2-iCre; Rankf/f (conditional knockout, CKO) mice in which RANK was specifically knocked out in Mo-macs. OPG-KD B16F10 cells were injected into WT and CKO mice (Fig. 6d). As expected, OPG-KD B16F10 cells generated significantly fewer lung metastasis nodules or tumor areas than control cells in WT mice. In contrast, there was no significant difference in the number of lung metastasis nodules or tumor areas between OPG-KD B16F10 cells and control cells after tail vein injection into RANK CKO mice (Fig. 6e and f). Thus, OPG promotes lung metastasis by blocking RANKL-RANK signalling at Mo-macs.
Fig. 6.
OPG promotes lung metastasis by blocking RANKL-RANK signalling on Mo-macs. (a) Gating strategy for RANK-positive cells. Among the live and single cells, most RANK+ cells were CD45 positive. These cells were then subjected to CD11b+F4/80+ staining (Mo-Macs) and Ly6G+Ly6C+ staining (G-MDSCs). n = 3 mice per group. (b, c) Analysis of lung metastasis in B16F10 cells with OPG knockdown. Mice were treated with IgG or an α-CSF1R antibody to deplete Mo-macs in the lung. (b) Representative whole-lung images and statistics of surface metastatic nodules. (c) H&E staining and statistics of the metastatic area. n = 5–6 mice per group. All H&E staining scale bars: 1 mm. (d) Cartoon showing the cancer cell injection strategy used for the WT and Lyz2-iCre;Rankf/f (CKO) mice. (e, f) Analysis of lung metastasis of B16F10 cells with OPG knockdown in WT and CKO mice, as shown in (d). (e), Statistics of pulmonary surface nodules. (f), Statistics of the metastatic tumor area. n = 8 mice per group. All boxplots with all the data points show the minimum, first quartile, median, third quartile, and maximum values, as determined by Mann–Whitney U test.
We then tested whether OPG affects the functions of Mo-macs directly. Macrophages are a subset of antigen-presenting cells (APCs),11 and we first examined whether OPG attenuates the antigen-presenting capacity of macrophages. We treated bone marrow-derived macrophages (BMDMs) with recombinant OPG protein. IFN-γ, which can induce the upregulation of major histocompatibility complex I (MHC-I) and MHC-II, was used as a positive control. FACS analysis revealed that stimulation with the recombinant OPG protein did not alter the expression levels of the MHC-I (H-2Kb) and MHC-II (I-A/I-E) proteins (Fig. S6a and b), suggesting that OPG did not directly alter the antigen-presenting ability of Mo-macs. We also investigated whether OPG influences macrophage polarization.8,62,63 We stimulated BMDMs with the recombinant OPG protein. IFN-γ, LPS and IL-4 were used as positive controls. However, OPG had no significant effect on macrophage polarization (Fig. S6c and d). Next, we investigated whether cancer cells utilizing OPG function as a “don't eat me” signal to resist macrophage phagocytosis. We ectopically expressed the extracellular domain of HER2 fused with mCherry in B16F10 cells. Subsequently, these tumor cells were cocultured with macrophages in the presence of an α-HER2 antibody to enhance antibody-dependent cell phagocytosis (Fig. S6e). Cd47 was knocked down as a positive control9 (Fig. S6f). FACS analysis was performed to determine the percentage of mCherry + cells within the macrophage (CD11b+) cell population (Fig. S6g). In the α-HER2 antibody-treated group, KD of Cd47 significantly increased phagocytic activity. There were no significant differences between the control and OPG-KD groups (Fig. S6h). Taken together, our findings suggested that OPG does not affect antigen presentation, M1/M2 polarization, or macrophage phagocytosis directly.
The RANKL-RANK-CXCL10 axis recruits NK cells to inhibit lung metastasis
Given the role of OPG in promoting metastasis by inhibiting Mo-mac function and the importance of Mo-macs in mediating other immune effector cells, we further investigated whether OPG knockdown affects infiltrated immune lymphocytes in the lung (Fig. S7a). FACS analysis revealed no significant change on T-cell and B-cell infiltration (Fig. S7b). However, there was an increase in the number of NK cells in the lungs of mice injected with OPG-KD B16F10 cells compared to those injected with control cells (Fig. 7a and b). Through analyzing the TCGA-SKCM data and breast cancer dataset NKI-295,64 we found that higher NK cell infiltration was associated with better overall survival or distant metastasis-free survival in these patients (Fig. S7c and d). Combined analysis of the patients AURORA and RAP datasets revealed that, although the difference did not reach statistical significance due to the limited sample size, the expression of TNFRSF11B was showing a trend of negatively correlated with activated NK cells (Fig. 7c).To confirm the hypothesis that OPG promotes lung metastasis by driving the paucity of NK cells, we depleted NK cells using an α-NK1.1 antibody and then injected OPG-KD B16F10 cells. Depletion of NK cells abolished the difference in lung metastasis ability between control and OPG-KD cells, as demonstrated by the similar number of lung metastasis nodules and similar tumor areas between the two groups (Fig. 7d–g). Additionally, in vitro NK cell killing assay revealed that OPG did not directly affect the NK cell cytotoxicity (Fig. S7e). Taken together, these results suggest that OPG promotes lung metastasis by potentially reducing NK cell infiltration.
Fig. 7.
The RANKL-RANK-CXCL10 signalling axis recruits NK cells to inhibit lung metastasis. (a) Flow cytometry graphs and statistics showing the percentages of CD3-; NK1.1+ NK cells among CD45+ immune cells in the lungs with metastatic nodules generated by control or OPG-KD B16F10 cancer cells. n = 10 mice per group. (b) Number of NK cell in the lungs with metastatic nodules generated by control or OPG-KD B16F10 cancer cells. n = 10 mice per group. (c) Correlation analysis between TNFRSF11B mRNA expression and the activated NK cell signature in lung metastasis samples from the AURORA and RAP cohorts. Statistics was analysed by Pearson correlation analysis. (d–g) Analysis of lung metastases generated by control or OPG-KD B16F10 cells tail vein injected into female C57BL/6 mice. Mice were treated with IgG or an α-NK1.1 antibody. (d, e) Representative lung images and statistics of surface metastatic nodules. (f, g) H&E staining images and statistical analysis of the metastatic areas. n = 5 mice per group. Scale bar: 1 mm. (h) Top DEGs in lung Mo-Macs after treated with either PBS or recombinant RANKL protein (100 ng/mL) for 24 h. A horizontal line was drawn at a adj.p-value of 0.05, and vertical lines represented a fold change of 2. (i) Heat map of comparing chemokine gene expression levels of lung Mo-macs treated with either RANKL or PBS control. Data presented as relative log2 fold change (L2FC). (j, k) Representative whole-lung figures (j) and H&E staining images (k) showing the lung metastases of B16F10 cells overexpressing CXCL10. n = 6 mice per group. Scale bar: 1 mm. (l, m) Analysis of lung metastasis generated by B16F10 cells with OPG knockdown. Mice were treated with vehicle control or AMG-487 (5 mg/kg per mouse) every other day. (l) Statistical analysis of lung surface metastatic nodules and (m) statistical analysis of the metastatic tumor areas. n = 6 mice per group. All boxplots showed the minimum, first quartile, median, third quartile, and maximum of the data points. (a,b) Student's two-sided unpaired t test was used for statistical analysis. (e, g, j, k, l, m) Mann–Whitney U test was used for statistical analysis.
Our results showed that OPG promotes lung metastasis by inhibiting Mo-mac function by blockade of RANKL-RANK signaling and by reducing NK cell infiltration. Given that macrophages have been reported to secrete chemokines to recruit NK cells,65 we next examined whether RANKL signalling could stimulate Mo-macs to secrete chemokines to recruit NK cells. We isolated lung Mo-macs and stimulated them with RANKL for RNA-sequencing analysis. Our results revealed the upregulation of Cxcl10 upon RANKL stimulation (Fig. 7h and Table S4). Among the differentially regulated genes identified by comparing the RANKL-treated group to the control group, Cxcl10 showed the strongest upregulation among all chemokines (Fig. 7i). This result was further confirmed by qPCR (Fig. S7f) as OPG treatment blocked RANKL-induced Cxcl10 expression (Fig. S7g).
Considering that OPG might promote lung metastasis by decreasing CXCL10 availability in the lung metastatic microenvironment, we asked whether the direct expression of CXCL10 in cancer cells could reduce lung metastasis. We observed that the overexpression of CXCL10 in B16F10 cells led to decreased formation of metastatic nodules and metastatic tumor areas (Fig. 7j and k). Similar results were also obtained using Yumm1.7 and 4T1 cancer cell lines (Fig. S7h–k).
The chemokine CXCL10 exerts its biological functions through binding to CXCR3. We employed the CXCR3 antagonist AMG-487 for investigative purposes to substantiate the role of CXCL10-CXCR3 in OPG-mediated metastasis. We observed that AMG-487 effectively reduced lung metastasis in B16F10 control cells, consistent with findings from previous studies66 (Fig. 7l and m). Conversely, when CXCR3 was pharmacologically blocked, no changes were observed between the control group and the OPG-KD group (Fig. 7l and m). These results demonstrated that OPG promotes lung metastasis by blocking the RANKL-RANK-CXCL10 axis to drive the paucity of NK cells.
Lastly, we aimed to investigate whether OPG could serve as a biomarker in combination with other markers to improve the predictive value for clinical outcomes. In METABRIC breast cancer cohort, we observed that patients with amplification of TNFRSF11B and co-expression of PD-L1 or TIM3 expression which was important to immune checkpoint therapy,67,68 showed poor survival outcomes (Fig. S8a–d). These findings suggest that OPG may hold value as a predictive biomarker.
Discussion
In this study, we identified OPG as an important driver of tumor lung metastasis and elucidated a mechanism involving the lung Mo-macs and NK cells in regulating lung metastasis. Specifically, RANKL stimulation of RANK on Mo-macs leads to the secretion of CXCL10, which recruits NK cells via CXCR3 to inhibit metastasis. Conversely, OPG inhibits this axis by binding to RANKL, leading to NK cell paucity and promoting lung metastasis (Fig. 8).
Fig. 8.
Schematic illustration. Metastatic tumor cells in the lung are stimulated by TGF-β to increase the expression and secretion of OPG. OPG, in turn, suppresses the RANKL-RANK signals on Mo-macs, resulting in decreased CXCL10 production and diminished presence of NK cells in the lung microenvironment. Consequently, this cascade promotes the progression of lung metastasis. Schematic presentation was created with BioRender.com.
While RANKL is traditionally associated with bone development and osteoclastogenesis, our study reveals a previously unrecognized role in regulating lung metastasis. Most previous reports suggesting a pro-tumor function for RANKL in primary tumor progression and in bone metastasis,22,69,70 our findings demonstrate its inhibitory effect on lung metastasis through the recruitment of NK cells. We have identified myeloid cells, particularly Mo-macs, as the main cell population expressing RANK, receiving the RANKL signal and express CXCL10 to recruit tumor-killing NK cells in the lung microenvironment. These results underscore the importance of considering specific role of RANKL pathway in different metastatic microenvironment (i.e., lung v.s. bone metastasis) when targeting RANKL-RANK signalling pathway.
Based on our results, OPG appears to be a promising therapeutic target for lung metastasis. Firstly, OPG disrupts the anti-tumor immune response mediated by Mo-macs and NK cells, suggesting its potential as a therapeutic target. Secondly, analysis of published available cancer patient cohorts reveals TNFRSF11B amplification as a prevalent mutation in metastatic melanoma and breast cancer patients. The amplification of TNFRSF11B is correlates with worse clinical patient outcome in breast cancer and melanoma, an aspect that was previously overlooked. The amplification of TNFRSF11B and its association with shorter patient survival time strongly suggest the pro-tumor function of OPG in breast cancer and melanoma, in contrast to its previously postulated tumor inhibitory function. These clinical observations are also in line with our in vivo functional study, as the depletion of OPG expression significantly suppressed lung metastasis progression in multiple breast and melanoma cancer metastasis models. Additionally, compared to the primary tumors, metastatic tumor samples showed higher expression of TNFRSF11B in melanoma and breast cancer patients. Our experimental results suggest that lung environmental TGF-β induces the expression of OPG in metastatic tumor cells. Thus, OPG could be possible therapeutic target for lung metastatic cancer patients.
Given the role of the OPG-RANKL-RANK signaling pathway in lung metastasis, activating RANKL signaling using RANKL protein or analogs seems to be a promising strategy to inhibit lung metastasis. However, considering RANKL's potential effects on bone metastasis17 and its ability to stimulate RANK-positive tumor cells,22,69 localized administration of RANKL directly to the lung microenvironment might be a safer and more targeted approach. Innovative delivery methods, such as nebulized nanobodies71 or lung-specific delivery of mRNA therapeutics via lipid nanoparticles (LNPs),72 could enhance treatment efficacy. Although our experiments showed no significant bone metastasis or damage—likely due to the specific metastatic animal model used—the risk of RANKL-RANK signaling promoting or worsening bone metastasis in patients remains. Therefore, developing prognostic markers to differentiate patients at high risk for lung versus bone metastasis is critical. Such markers would help stratify patients who could benefit from RANKL administration while minimizing adverse effects.
TRAIL, known for its ability to induce apoptosis in various cancer cells,73 has been found to exhibit heightened expression in human breast cancer lung metastasis samples.74 Moreover, previous studies have implicated OPG in binding with TRAIL, thereby inhibiting apoptosis and promoting tumor growth and metastasis.59 Specifically, research suggests that endothelial cell-secreted OPG might confers resistance to TRAIL-induced apoptosis in tumor cells, facilitating metastasis in immunocompromised NSG mice.24 However, whether the KD of endogenous OPG in endothelial cells could reduce lung metastasis was not examined. Furthermore, the importance of the immune cells, especially the NK cells in mediating OPG function could not be determined in immunocompromised NSG mice. In our study, we demonstrated that knocking down endogenous OPG in tumor cells significantly decreased lung metastasis burden in immunocompetent, syngeneic mouse models. Importantly, depletion of NK cells in these mice by NK-depletion antibody diminished the lung metastasis difference between control and OPG-KD groups, suggesting the involvement of NK cells in OPG-mediated metastasis progression. In clinical patient dataset, the prevalence of OPG coding gene amplification and heightened metastatic expression in patient cancer tissues underscores tumor cells as a probable primary source of OPG. Nevertheless, it is plausible that resistance to TRAIL contributes to the mechanism leading to enhanced lung metastasis caused by OPG.
While this study primarily examines the role of OPG in lung metastasis using melanoma and breast cancer models, these cancers are also known to metastasize to other organs, such as the bone and liver. The role of OPG in promoting metastasis to these other sites remains unclear and requires further investigation. Additionally, given the significant tumor-immune cell interactions mediated by OPG, our study was limited to using mouse cancer cell lines. Future research should incorporate human cancer cell lines in humanized mouse models to better understand OPG's role and improve the translational relevance of these findings.
Contributors
H.H. and H.Z. designed and performed the experiments, and directly accessed and verified the underlying data. H.H. and X.L. performed the CRISPR screening and candidate gene verification. Z.X. analysed the published clinical data and performed the RNA-seq analysis. T.Z. analysed the screening sequencing data. Y.T. provided technical support for IHC staining and manuscript writing. H.Y. and Y.Z. provided the support for the screening. L.Z., H.Y. and G.X. provided help with the histopathology of murine lung tissues. G.X., H.F. and X.W. provided experimental assistance for the molecular and animal studies. W.C. provided help with the revision of manuscript. C.L. provided experimental assistance for the NK cell killing assay. H.Z. developed the concept, designed the experiments, and supervised the overall study. H.H. and H.Z. wrote and revised the manuscript.
Data sharing statement
This study generated multiple knockdown constructs that were made available through the standard Material Transfer Agreement (MTA). Further information and requests for resources and reagents should be directed to and will be fulfilled by lead contact Dr. Hanqiu Zheng (hanzheng@tsinghua.edu.cn).
Declaration of interests
The authors declare no conflicts of interest in this study.
Acknowledgements
We thank X. Guo and P. Jiang for sharing the mice. We appreciate Dr. Deng Pan (Tsinghua University) for sharing the sgRNA library. We thank all the members of the Zheng Laboratory for their helpful discussions and technical assistance. We thank the Technology Center for Protein Sciences at Tsinghua University for FACS support and the Laboratory Animal Research Center and the Center of Biomedical Analysis at Tsinghua University for animal research support. This study was partially supported by the National Key Research and Development Program of China (2020YFA0509400 to H.Z.), the National Science Foundation of China (82473018 and 81972462 to H.Z.), the Tsinghua University Initiative Scientific Research Program.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105503.
Appendix A. Supplementary data
References
- 1.Ganesh K., Massague J. Targeting metastatic cancer. Nat Med. 2021;27(1):34–44. doi: 10.1038/s41591-020-01195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gentles A.J., Newman A.M., Liu C.L., et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian B.Z., Li J.F., Zhang H., et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A., Allavena P., Marchesi F., Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21(11):799–820. doi: 10.1038/s41573-022-00520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casanova-Acebes M., Dalla E., Leader A.M., et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature. 2021;595(7868):578–584. doi: 10.1038/s41586-021-03651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin E.Y., Li J.F., Gnatovskiy L., et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66(23):11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 7.Kloosterman D.J., Akkari L. Macrophages at the interface of the co-evolving cancer ecosystem. Cell. 2023;186(8):1627–1651. doi: 10.1016/j.cell.2023.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Cassetta L., Pollard J.W. A timeline of tumour-associated macrophage biology. Nat Rev Cancer. 2023;23(4):238–257. doi: 10.1038/s41568-022-00547-1. [DOI] [PubMed] [Google Scholar]
- 9.Willingham S.B., Volkmer J.P., Gentles A.J., et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109(17):6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin H., Kryczek I., Li S., et al. Stanniocalcin 1 is a phagocytosis checkpoint driving tumor immune resistance. Cancer Cell. 2021;39(4):480–493.e6. doi: 10.1016/j.ccell.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christofides A., Strauss L., Yeo A., Cao C., Charest A., Boussiotis V.A. The complex role of tumor-infiltrating macrophages. Nat Immunol. 2022;23(8):1148–1156. doi: 10.1038/s41590-022-01267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma S.K., Chintala N.K., Vadrevu S.K., Patel J., Karbowniczek M., Markiewski M.M. Pulmonary alveolar macrophages contribute to the premetastatic niche by suppressing antitumor T cell responses in the lungs. J Immunol. 2015;194(11):5529–5538. doi: 10.4049/jimmunol.1403215. [DOI] [PubMed] [Google Scholar]
- 13.Wang T., Zhang J., Wang Y., et al. Influenza-trained mucosal-resident alveolar macrophages confer long-term antitumor immunity in the lungs. Nat Immunol. 2023;24(3):423–438. doi: 10.1038/s41590-023-01428-x. [DOI] [PubMed] [Google Scholar]
- 14.Park M.D., Reyes-Torres I., LeBerichel J., et al. TREM2 macrophages drive NK cell paucity and dysfunction in lung cancer. Nat Immunol. 2023;24(5):792–801. doi: 10.1038/s41590-023-01475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keerthivasan S., Senbabaoglu Y., Martinez-Martin N., et al. Homeostatic functions of monocytes and interstitial lung macrophages are regulated via collagen domain-binding receptor LAIR1. Immunity. 2021;54(7):1511–15126.e8. doi: 10.1016/j.immuni.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Ding C., Shrestha R., Zhu X., et al. Inducing trained immunity in pro-metastatic macrophages to control tumor metastasis. Nat Immunol. 2023;24(2):239–254. doi: 10.1038/s41590-022-01388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones D.H., Nakashima T., Sanchez O.H., et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440(7084):692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 18.Zheng H., Li W., Kang Y. Tumor-stroma interactions in bone metastasis: molecular mechanisms and therapeutic implications. Cold Spring Harb Symp Quant Biol. 2016;81:151–161. doi: 10.1101/sqb.2016.81.030775. [DOI] [PubMed] [Google Scholar]
- 19.Anderson D.M., Maraskovsky E., Billingsley W.L., et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390(6656):175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 20.Ahern E., Smyth M.J., Dougall W.C., Teng M.W.L. Roles of the RANKL–RANK axis in antitumour immunity — implications for therapy. Nat Rev Clin Oncol. 2018;15(11):676–693. doi: 10.1038/s41571-018-0095-y. [DOI] [PubMed] [Google Scholar]
- 21.Tan W., Zhang W., Strasner A., et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470(7335):548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Aleza C., Nguyen B., Yoldi G., et al. Inhibition of RANK signaling in breast cancer induces an anti-tumor immune response orchestrated by CD8+ T cells. Nat Commun. 2020;11(1):6335. doi: 10.1038/s41467-020-20138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoldi G., Pellegrini P., Trinidad E.M., et al. RANK signaling blockade reduces breast cancer recurrence by inducing tumor cell differentiation. Cancer Res. 2016;76(19):5857–5869. doi: 10.1158/0008-5472.CAN-15-2745. [DOI] [PubMed] [Google Scholar]
- 24.Hongu T., Pein M., Insua-Rodriguez J., et al. Perivascular tenascin C triggers sequential activation of macrophages and endothelial cells to generate a pro-metastatic vascular niche in the lungs. Nat Cancer. 2022;3(4):486–504. doi: 10.1038/s43018-022-00353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie G., Yang H., Ma D., et al. Integration of whole-genome sequencing and functional screening identifies a prognostic signature for lung metastasis in triple-negative breast cancer. Int J Cancer. 2019;145(10):2850–2860. doi: 10.1002/ijc.32329. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Soto A., Gonzalez S., Smyth M.J., Galluzzi L. Control of metastasis by NK cells. Cancer Cell. 2017;32(2):135–154. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Ma S.B., Caligiuri M.A., Yu J.H. Harnessing natural killer cells for lung cancer therapy. Cancer Res. 2023;83(20):3327–3339. doi: 10.1158/0008-5472.CAN-23-1097. [DOI] [PubMed] [Google Scholar]
- 28.Lo H.C., Xu Z., Kim I.S., et al. Resistance to natural killer cell immunosurveillance confers a selective advantage to polyclonal metastasis. Nature Cancer. 2020;1(7):709-+. doi: 10.1038/s43018-020-0068-9. [DOI] [PubMed] [Google Scholar]
- 29.Hu J., Sanchez-Rivera F.J., Wang Z., et al. STING inhibits the reactivation of dormant metastasis in lung adenocarcinoma. Nature. 2023;616(7958):806–813. doi: 10.1038/s41586-023-05880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlecker E., Fiegler N., Arnold A., et al. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res. 2014;74(13):3429–3440. doi: 10.1158/0008-5472.CAN-13-3017. [DOI] [PubMed] [Google Scholar]
- 31.Palumbo J.S., Talmage K.E., Massari J.V., et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 32.Liu X., Song J., Zhang H., et al. Immune checkpoint HLA-E:CD94-NKG2A mediates evasion of circulating tumor cells from NK cell surveillance. Cancer Cell. 2023;41(2):272–287.e9. doi: 10.1016/j.ccell.2023.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Malladi S., Macalinao D.G., Jin X., et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165(1):45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattiola I., Tomay F., De Pizzol M., et al. The macrophage tetraspan MS4A4A enhances dectin-1-dependent NK cell-mediated resistance to metastasis. Nat Immunol. 2019;20(8):1012–+. doi: 10.1038/s41590-019-0417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanna R.N., Cekic C., Sag D., et al. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350(6263):985–990. doi: 10.1126/science.aac9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finan C., Gaulton A., Kruger F.A., et al. The druggable genome and support for target identification and validation in drug development. Sci Transl Med. 2017;9(383) doi: 10.1126/scitranslmed.aag1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan D., Kobayashi A., Jiang P., et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science. 2018;359(6377):770–775. doi: 10.1126/science.aao1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W., Xu H., Xiao T., et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 2014;15(12):554. doi: 10.1186/s13059-014-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang B.B., Wang M., Zhang W.B., et al. Integrative analysis of pooled CRISPR genetic screens using MAGeCKFlute. Nat Protoc. 2019;14(3):756–780. doi: 10.1038/s41596-018-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colaprico A., Silva T.C., Olsen C., et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44(8) doi: 10.1093/nar/gkv1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durinck S., Moreau Y., Kasprzyk A., et al. BioMart and Bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics. 2005;21(16):3439–3440. doi: 10.1093/bioinformatics/bti525. [DOI] [PubMed] [Google Scholar]
- 44.Newman A.M., Liu C.L., Green M.R., et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mittal D., Sinha D., Barkauskas D., et al. Adenosine 2B receptor expression on cancer cells promotes metastasis. Cancer Res. 2016;76(15):4372–4382. doi: 10.1158/0008-5472.CAN-16-0544. [DOI] [PubMed] [Google Scholar]
- 46.Aslakson C.J., Miller F.R. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52(6):1399–1405. [PubMed] [Google Scholar]
- 47.Hugo W., Zaretsky J.M., Sun L., et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lefebvre C., Bachelot T., Filleron T., et al. Mutational profile of metastatic breast cancers: a retrospective analysis. PLoS Med. 2016;13(12) doi: 10.1371/journal.pmed.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curtis C., Shah S.P., Chin S.F., et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira B., Chin S.F., Rueda O.M., et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7 doi: 10.1038/ncomms11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Recio S., Hinoue T., Wheeler G.L., et al. Nature Cancer; 2022. Multiomics in primary and metastatic breast tumors from the AURORA US network finds microenvironment and epigenetic drivers of metastasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J., Mani S.A., Donaher J.L., et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Dongre A., Weinberg R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 54.Oliver J.L., Alexander M.P., Norrod A.G., Mullins I.M., Mullins D.W. Differential expression and tumor necrosis factor-mediated regulation of TNFRSF11b/osteoprotegerin production by human melanomas. Pigment Cell Melanoma Res. 2013;26(4) doi: 10.1111/pcmr.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thirunavukkarasu K., Miles R.R., Halladay D.L., et al. Stimulation of osteoprotegerin (OPG) gene expression by transforming growth factor-β (TGF-β) -: mapping of the OPG promoter region that mediates TGF-β effects. J Biol Chem. 2001;276(39):36241–36250. doi: 10.1074/jbc.M104319200. [DOI] [PubMed] [Google Scholar]
- 56.Batlle E., Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity. 2019;50(4):924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Derynck R., Turley S.J., Akhurst R.J. TGFbeta biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18(1):9–34. doi: 10.1038/s41571-020-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Propper D.J., Balkwill F.R. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19(4):237–253. doi: 10.1038/s41571-021-00588-9. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Liu Y., Huang Z., Chen X., Zhang B. The roles of osteoprotegerin in cancer, far beyond a bone player. Cell Death Discov. 2022;8(1):252. doi: 10.1038/s41420-022-01042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi K., Takeda K., Saiki I., Irimura T., Hayakawa Y. Functional roles of tumor necrosis factor-related apoptosis-inducing ligandDR5 interaction in B16F10 cells by activating the nuclear factor-B pathway to induce metastatic potential. Cancer Sci. 2013;104(5):558–562. doi: 10.1111/cas.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kong Y.Y., Yoshida H., Sarosi I., et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 62.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rolny C., Mazzone M., Tugues S., et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19(1):31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 64.van de Vijver M.J., He Y.D., van 't Veer L.J., et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 65.Ozga A.J., Chow M.T., Luster A.D. Chemokines and the immune response to cancer. Immunity. 2021;54(5):859–874. doi: 10.1016/j.immuni.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walser T.C., Rifat S., Ma X.R., et al. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res. 2006;66(15):7701–7707. doi: 10.1158/0008-5472.CAN-06-0709. [DOI] [PubMed] [Google Scholar]
- 67.Galluzzi L., Chan T.A., Kroemer G., Wolchok J.D., Lopez-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10(459) doi: 10.1126/scitranslmed.aat7807. [DOI] [PubMed] [Google Scholar]
- 68.Wei S.C., Duffy C.R., Allison J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 69.Ahern E., Harjunpaa H., Barkauskas D., et al. Co-Administration of RANKL and CTLA4 antibodies enhances lymphocyte-mediated antitumor immunity in mice. Clin Cancer Res. 2017;23(19):5789–5801. doi: 10.1158/1078-0432.CCR-17-0606. [DOI] [PubMed] [Google Scholar]
- 70.Dhuey E., Oldridge O., Ravishankar R., et al. Therapeutic interruption of T cell development generates high-affinity T cells that escape exhaustion and improve cancer immunotherapy. bioRxiv. 2022 doi: 10.1101/2022.01.19.476935. [DOI] [Google Scholar]
- 71.Li C., Zhan W., Yang Z., et al. Broad neutralization of SARS-CoV-2 variants by an inhalable bispecific single-domain antibody. Cell. 2022;185(8):1389–1401.e18. doi: 10.1016/j.cell.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiu M., Tang Y., Chen J., et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2022;119(8) doi: 10.1073/pnas.2116271119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnstone R.W., Frew A.J., Smyth M.J. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8(10):782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 74.Zhang X.H., Wang Q., Gerald W., et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16(1):67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.