Summary
Background
Re-irradiation of recurrent head and neck cancer (HNC) is often limited by tumour adherence to critical structures and/or radiation tolerance of critical normal tissues. Iopofosine I 131 (CLR 131) is a targeted small molecular phospholipid ether (PLE) drug conjugate that delivers iodine-131 selectively to tumour cells. We conducted a phase 1, single-centre, open-label study to determine whether CLR 131 given with reduced dose of external beam radiation therapy (EBRT) would be tolerable and feasible.
Methods
All participants received previous curative intent treatment with radiotherapy as primary or adjuvant treatment. Eligible participants demonstrated uptake of CLR 131 as indicated via single photon emission CT/CT (SPECT/CT) imaging following CLR 131 test dose. Participants received two therapeutic doses of CLR 131 (days 1 and 8) with SPECT/CT imaging performed to quantitate the biodistribution of CLR 131. Participants subsequently received EBRT to achieve the designated radiation dose (60–70 Gy). The primary endpoint was safety. This trial was registered with ClinicalTrials.gov, NCT04105543, and enrolment and follow-up are complete.
Findings
Twelve participants completed treatment with CLR 131 and EBRT. Eight participants experienced grade 4 non-DLT haematologic toxicities (2 anaemia, 8 leukopenia, 5 thrombocytopenia) at least probably attributed to CLR 131, consistent with the expected toxicity profile. Haematologic toxicities occurred during weeks 6–8 from the first dose of CLR 131 and resolved within three weeks without sequelae. There were no treatment-related grade 3–4 non-haematologic toxicities.
Interpretation
CLR 131 in combination with EBRT did not confer any safety concerns, and was tolerable in participants with recurrent/metastatic HNC. Myelosuppression was consistent with the known toxicity profile of CLR 131.
Funding
National Institutes of HealthP50 DE026787, National Cancer InstituteP30 CA014520, National Institutes of Health1UL1TR002373, Cellectar, NCT04105543.
Keywords: Head and neck cancer, Re-irradiation, Targeted radionuclide therapy, Radiopharmaceutical, Monte Carlo method
Research in context.
Evidence before this study
We searched PubMed for publications from the inception of the database until June 1, 2024, using the terms “head neck cancer” NOT “thyroid cancer” AND “re-irradiation” AND “targeted radionuclide therapy”. We found no previous studies investigating a targeted radionuclide therapy in patients with recurrent head and neck cancers.
Added value of this study
This study combines a radiotherapeutic with external beam radiation therapy in patients with recurrent head and neck cancers. Although radiopharmaceutical treatments are approved for patients with neuroendocrine and prostate cancer, treatment is currently reserved for patients with metastatic disease. This study showed meaningful activity of CLR 131 in patients with recurrent head and neck cancers who are able to undergo re-irradiation therapy. CLR 131 showed encouraging activity to decrease toxicities and improve the efficacy of re-irradiation for patients with recurrent head and neck cancer.
Implications of all the available evidence
These results support further clinical research to define which patients will derive most benefit from combining CLR 131 with external beam radiation therapy.
Introduction
Head and neck cancer (HNC) is a complex heterogeneous disease that arises from various sites including the oral cavity, pharynx, larynx, paranasal sinuses, and salivary glands. Over 90% of tumours that originate in the laryngopharyngeal axis are squamous cell carcinoma (SCC). Although many patients (particularly stage I and II) can be cured, patients with recurrent HNC following initial treatment experience poor survival.1 Overall survival for HNC has improved only marginally during the last 30 years.
Approximately 50% of patients with advanced stage HNC manifest recurrence following initial treatment, and the majority of these recurrences are locoregional (mouth, throat, neck).2,3 Although retreatment is warranted for selected patients, this is technically challenging and accompanied by a significant risk of irreversible damage to normal tissues such as hypothyroidism or osteoradionecrosis.4,5 Surgery is often limited by tumour adherence to critical structures (base of skull, neurovascular bundles, spinal column), whereas radiation is often limited by normal tissue tolerance (spinal cord, bone, cartilage). These retreatment modalities can result in profound adverse effects on patient health-related quality of life (QOL), and improved treatment approaches are needed.6, 7, 8
Tumour selectivity and dosing of CLR 131
CLR 131 [18-(p-[131I]-iodophenyl) octadecyl phosphocholine] is an investigational, radio-iodinated cancer therapy that exploits the tumour-targeting properties of phospholipid ethers (PLEs) and PLE analogs by malignant cells.9 The malignant cell selectivity of the alkyl-phospholipids appears to reflect association with lipid rafts, which are used as a portal of entry into the cell.10 The term “lipid rafts” refers to specialized plasma membrane microdomains, rich in cholesterol and sphingomyelin that spatially organize signalling pathways regulating cell proliferation and survival. Slow degradation of CLR 131 inside the cell results in prolonged tumour exposure.11 With β-emissions from 131I having a median range of several millimetres, radiation from CLR 131 can be sharply deposited within local tumours. CLR 131 antitumor activity has been documented in various tumour cell models, including glioma, multiple myeloma (MM), breast, prostate, colorectal, ovarian, renal, pancreatic, uterine, and lung cancers.11
CLR 131 has been investigated in a Phase 1a dosimetry study in eight participants with advanced solid tumours that demonstrated that a single 10 mCi infusion of CLR 131 was exceptionally well tolerated.12 In a phase 1b dose-escalation study, CLR 131 given as a single dose of 31.25 mCi/m2 was deemed to be dose limiting due to cytopenia.13 This study also documented selective tumour uptake quantified by single photon-emission CT/CT (SPECT/CT) imaging. Subsequent clinical trials using CLR 131 in MM, non-Hodgkin’s lymphoma, and Waldenstrom’s macroglobulinemia utilized a fractionated dosing of CLR 131, which was well tolerated with a more manageable adverse event profile than single doses of CLR 131. Thus, the fractionated schedule has been adopted as the standard for CLR 131 dosing in ongoing and future trials.14, 15, 16, 17
Rationale for combining CLR 131 plus external beam radiation therapy
Several studies examining the role of re-irradiation in HNC note considerable risk for high-grade toxicities (including death) with 46–50% patients experiencing grade 3 or greater toxicities.8 In the intensity-modulated radiotherapy (IMRT) era, re-irradiation potentially offers a 2-year overall survival in approximately 16–54% of patients.18 However, selection of patients who may benefit from re-irradiation remains an area of active investigation. Efforts to reduce the acute and long-term tissue injury profile by radiation with highly conformal techniques, including proton therapy, are important in the retreatment setting.
CLR 131 selectively delivers radiation to malignant tumour cells and minimizes radiation exposure to normal tissues, which is difficult to achieve even with highly conformal radiation techniques. The aim of this trial is to evaluate the use of CLR 131 as a modality to provide “internal radiation” combined with external beam radiation therapy (EBRT) for definitive treatment of recurrent HNC. We hypothesize that CLR 131 will reduce the overall dose of EBRT required for retreatment and thereby reduce the acute and long-term toxicities that are commonly associated with standard re-irradiation treatment.
Methods
Study design and participants
This was a phase 1, single-centre, open-label study evaluating patients with locoregionally recurrent HNC anticipated to undergo re-irradiation therapy. Participants were enrolled at the University of Wisconsin Carbone Cancer Center. The trial initially planned to enrol up to 12 participants in two dose escalation cohorts (15.6 mCi/m2 and 18.75 mCi/m2) using the mTPI-2 design followed by a dose expansion cohort (up to another 12 participants) at the dose based on the dose escalation schema (Supplementary Table S1).
Participants who had confirmed metastatic disease and recurred in the head and neck region were eligible to enrol on this trial provided that locoregional treatment with EBRT was deemed to be clinically beneficial. Participants must have demonstrated uptake of CLR 131 (after administration of the CLR 131 test dose) via SPECT/CT imaging in the specified site of recurrent disease that was to be treated with radiation therapy. Participants were excluded for any extradural disease in contact with the spinal cord where swelling in response to therapy may have impinged upon the spinal cord, or any condition requiring chronic immunosuppression equivalent to prednisone >10 mg daily.
Participants with no CLR 131 uptake were initially not allowed to participate in the treatment portion of the study. After evaluating CLR 131 uptake on SPECT/CT imaging for the first four consented participants, a protocol amendment increased the test dose of CLR 131 from 10 mCi to 15 mCi to assist in detection of CLR 131 uptake in small tumours, resulting in improved evaluation of CLR 131 uptake. In addition, a separate cohort for participants who did not uptake CLR 131 was added in order to allow for potential participants that may benefit from CLR 131 but did not uptake on SPECT/CT imaging.
Ethics
The study protocol was approved by the institutional review board at the University of Wisconsin (2019-0681). Assessments by the data safety and monitoring committee were performed biannually. The clinical trial registration number was NCT04105543. The trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All participants provided written informed consent.
Study procedures
All participants were required to have a pre-planned re-irradiation treatment dose that was designed based on the consensus opinion of the multidisciplinary head and neck oncology tumour board. The standard of care (SOC) radiation dose for this patient population was 60–70 Gy absorbed dose to the recurrent tumour as clinically evaluated by their treating radiation oncologist, irrespective of radiation delivery technique. Therefore, as long as the SOC radiation dose parameters were met, the EBRT was considered within SOC. All participants underwent a screening visit and received a dosimetry test dose of CLR 131 (10–15 mCi) to establish drug uptake by the tumour as determined by SPECT/CT imaging.
In the treatment portion of the trial, participants received two doses of CLR 131 intravenously with the first dose on day 1 followed by the second dose on day 8. This study planned for two dose levels with the starting dose level at 15.6 mCi/m2. The study used an mTPI-2 design, using cohorts of four participants. SPECT/CT imaging was performed on days 2, 3, 4–6, and 7–8 to visualize and quantify the biodistribution of CLR 131 in order to calculate the absorbed dose (AD) distribution in each participant. Participants were evaluated at 1, 3, 6, 12, and 24 months after CLR 131 and EBRT.
SPECT/CT imaging acquisition and Monte Carlo calculations
An in-house radiopharmaceutical dosimetry platform called RAPID (Radiopharmaceutical Assessment Platform for Internal Dosimetry) was used for the CLR 131 dosimetry.19,20 RAPID utilizes the radionuclide activity in each voxel measured by the SPECT images and the material composition and mass density of the patient geometry measured by the CT images to calculate the voxel AD rate distribution. The Monte Carlo simulations for the AD rate were conducted using GEANT4, a commonly utilized software package for medical applications requiring radiation transport calculations.21,22 Each AD rate was run with enough particles (8000 decays per voxel) to ensure Monte Carlo statistical uncertainty below 1·0% in the tumour region. The AD rate in each voxel was calculated for each time point and then the AD was calculated by utilizing trapezoidal-exponential integration across the time points. The EBRT tumour contours (gross tumour volume (GTV), clinical target volume (CTV), planning target volume (PTV)) were registered to the SPECT/CT images using MiM (MiM Software, Beachwood, OH) by a qualified medical physicist and the AD was calculated to those contours. Simulations were run both with and without partial volume corrections (PVC) to provide voxel level information and PVC-corrected tumour dosimetry.
AD colourwashes and dose volume histograms (DVHs) were created for the separate EBRT and CLR 131 treatments to visualize and evaluate the AD of each treatment (Fig. 1, representative participant). The colourwashes depict the distribution of the AD, and DVHs depict the proportion of a contour receiving a specific amount of AD; the PTV is depicted in Fig. 1. EBRT treatment plans were created using RayStation (Raysearch, Stockholm, Sweden) in accordance with SOC clinical practices. Based on the calculated mean AD of CLR 131 to the PTV, participants received EBRT to complete the designated radiation dose outlined in the re-irradiation plan.
Fig. 1.
Absorbed dose distributions superimposed upon sagittal, axial, and coronal CT slices reflecting a) CLR 131 treatment and b) external beam radiation therapy (EBRT) treatments for a representative study-enrolled subject and c) cumulative dose volume histogram (DVH) for each separate treatment to the planning target volume (PTV). The PTV is depicted in black on the CT images.
Participants received a cumulative tumour dose of 60–70 Gy using personalized dose calculation of CLR 131 (via Monte Carlo) combined with EBRT. If the Monte Carlo dose estimate for any participant was calculated greater than 60 Gy for the two doses of CLR 131 (highly unlikely based on preliminary data), then no additional EBRT would be administered.
Quality of life and swallowing function
At three and six months post-completion of EBRT, participants were assessed for changes from baseline in head and neck-specific subject health-related measures. Quality of life (QOL) was assessed using the MD Anderson Dysphagia Inventory (MDADI),23 the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30),24 and the Xerostomia-Related Quality of Life Scale (XeQoLS).25 Participant-reported mouth dryness was assessed by the Xerostomia Inventory,25 salivary flow rate was measured by calculating salivary output over a 5-min collection period under stimulated and unstimulated conditions, and swallow function was assessed with a modified barium swallow study (MBS). Each MBS was evaluated by a speech pathologic to provide a Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) score, and raters were blinded to participant details. DIGEST safety (airway invasion) and efficiency (oropharyngeal residue) grades were used to determine an overall pharyngeal dysphagia severity grade of 1 (mild), 2 (moderate), 3 (severe), and 4 (life threatening/profound).23
Assessment of outcomes
The primary endpoint was incidence of adverse events, assessed using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, with attributions to CLR 131, EBRT, disease, or other causes. Secondary endpoints included median radiation treatment time and median number of dose delays due to toxicity, CLR 131 tumour uptake as determined via SPECT/CT imaging and Monte Carlo calculations, and clinical response as measured by overall response rate (defined as the proportion of participants who experienced either a partial response or complete response within 6 months post completion of EBRT as measured by SOC imaging (e.g., CT, MR, PET-MR)), progression-free survival (PFS) (defined as the time from initiation of study treatment until progressive disease or death from any cause), and overall survival (OS) (defined as the time from initiation of study treatment until death from any cause). The trial involved visits for screening, CLR 131 treatments on days 1 and 8, SPECT/CT imaging visits (four visits), and follow up visits at 1, 3, 6, 12, and 24 months after completion of EBRT. Clinical response was assessed during treatment based on RECIST 1·1. Other secondary endpoints were changes from baseline in QOL measurements, salivary flow rate, and swallow function by the DIGEST score.
Statistics
This trial utilized the unique mTPI-2 design for the sample size consideration, which is an extension of the modified toxicity probability interval (mTPI-2) to identify the maximum tolerated dose using cohorts of four participants and up to three dose levels of CLR 131 (Supplementary Table S1).26 A toxicity monitoring was planned after accrual of the dose escalation cohorts to determine the dose for the expansion cohort. Adverse events and clinical endpoints are reported using frequency tables with confidence intervals (CI) constructed using exact binomial distribution. PFS and OS were analyzed using the Kaplan–Meier method. The number of participants at risk was 12, and one participant was censored for survival analysis. For QOL and swallowing function, tests were conducted based on Wilcoxon signed rank test. The enrolled population includes all 12 participants and the evaluable population includes 11 participants. All analyses were conducted using R package version 4.3.2.
Role of funders
Funding sources for the conduct of this clinical trial were from the University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520, the UW Head and Neck SPORE Grant P50 DE026787, the UW Institute for Clinical and Translational Research 1UL1TR002373. Cellectar Biosciences provided Iopofosine I 131. The funders of the study had no role in study design, study conduct, data collection, data analysis, data interpretation, and writing of the report. The authors were not paid to write this article by a pharmaceutical company or other agent. The authors were not precluded from accessing the data in the study. The authors accept responsibility to submit for publication.
Results
Participant demographics and accrual
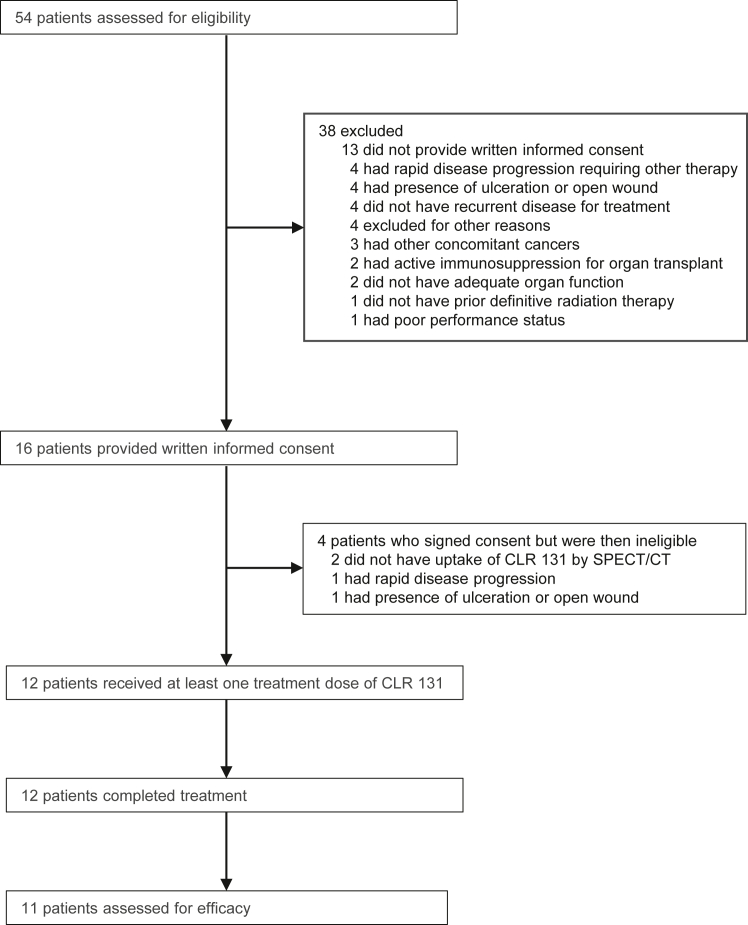
Between December 4, 2019 and February 16, 2022, 54 patients were assessed for eligibility of whom 16 patients met the eligibility criteria. Sixteen participants were consented to enrol in the study (Fig. 2). After consent, four participants were excluded from treatment; two did not have uptake of CLR 131 by SPECT/CT, one had rapid disease progression, and one had presence of an open wound or ulceration. After increasing the CLR 131 test dose to 15 mCi, all subsequent patients were noted to have CLR 131 uptake. Twelve participants were treated with CLR 131 (Table 1). An evaluable participant was defined as a participant who received all doses of CLR 131 in their entirety and at least one post-infusion efficacy assessment. Six participants were treated with CLR 131 at the first recurrence of their HNC; one participant had metastatic disease for which radiation therapy was clinically recommended for local treatment of progressive disease. Six participants had progressively recurrent disease. All twelve participants completed treatment with CLR 131 and EBRT.
Fig. 2.
Trial CONSORT diagram. ∗Other reasons for study exclusion included: transportation issues, insurance company approval to see Study Investigator for no more than one trial-related visit, and study accrual closure due to National Institute for Dental and Craniofacial Research requirements.
Table 1.
Participant characteristics at baseline for enrolled population (analysis population is all subjects).
| Characteristic | Total patients N = 12 | Dose escalation cohortc (15.6 mCi/m2) N = 8 | Dose expansion cohortd (15.6 mCi/m2) N = 4 |
|---|---|---|---|
| Sexa | n (%) | ||
| Female | 3 (25) | 3 | 0 |
| Male | 9 (75) | 5 | 4 |
| Age | |||
| Median age at baseline in years (range) | 65.5 (47–84) | 65.5 (55–75) | 64.5 (47–84) |
| Race | |||
| White | 12 | 8 | 4 |
| Other | 0 | 0 | 0 |
| Ethnicity | |||
| Hispanic | 0 | 0 | 0 |
| Non-Hispanic | 12 | 8 | 4 |
| ECOG performance status: | n (%) | ||
| ECOG 0 | 9 (75) | 7 | 2 |
| ECOG 1 | 3 (25) | 1 | 2 |
| Primary tumour site: | n (%) | ||
| Oropharynx | 5 (42) | 3 | 2 |
| p16 positive | 4 (80) | 2 | 2 |
| p16 negative | 1 (20) | 1 | 0 |
| Nasopharynx | 1 (8) | 0 | 1 |
| Larynx | 1 (8) | 1 | 0 |
| Oral cavity | 4 (33) | 3 | 1 |
| Salivary gland | 1 (8) | 1 | 0 |
| T stage, AJCC 8th ed. | |||
| T1–T2 | 6 (50) | 2 | 4 |
| T3–T4 | 6 (50) | 6 | 0 |
| N stage, AJCC 8th ed. | |||
| N0–1 | 6 (50) | 3 | 3 |
| N2–3 | 6 (50) | 5 | 1 |
| AJCC 8th ed. stage | |||
| I | 2 (17) | 0 | 2 |
| II | 3 (25) | 1 | 2 |
| III | 3 (25) | 3 | 0 |
| IV A | 2 (17) | 2 | 0 |
| IV B | 2 (17) | 2 | 0 |
| Initial treatment at diagnosis | |||
| Surgical resection | 7 (58) | 6 | 1 |
| No adjuvant treatment | 1 (8) | 1 | 0 |
| Adjuvant radiation | 4 (33) | 3 | 1 |
| Adjuvant chemoradiation | 2 (17) | 2 | 0 |
| Definitive chemoradiation | 5 (42) | 2 | 3 |
| Recurrence status at study entry | |||
| First Recurrenceb | 6 (50) | 4 | 2 |
| Multiply recurrent | 6 (50) | 4 | 2 |
| Metastatic diseaseb | 1 (8) | 1 | 0 |
| Quality of life and swallow function | |||
| MDADI | 10 (83) | 6 | 4 |
| EORTC QLQ-C30 | 9 (75) | 6 | 4 |
| XeQoLS | 10 (83) | 6 | 4 |
| Xerostomia inventory | 10 (83) | 6 | 4 |
| Salivary flow rate | 10 (83) | 6 | 4 |
| DIGEST | 10 (83) | 6 | 4 |
AJCC, American Joint Committee on Cancer; ECOG, Eastern Cooperative Oncology Group; MDADI, MD Anderson Dysphagia Inventory; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; XeQoLS, Xerostomia-Related Quality of Life Scale; DIGEST, Dynamic Imaging Grade of Swallowing Toxicity.
Participants were asked to report their biological sex (male/female) through a self-report questionnaire.
Participant developed metastatic disease at the first recurrence.
All participants in the dose escalation cohort received CLR 131 at a dose of 15.6 mCi/m2. CLR 131 dose was not escalated higher than the initial starting dose of 15.6 mCi/m2.
All participants in the dose expansion cohort received CLR 131 at a dose of 15.6 mCi/m2 as this dose was selected for the dose expansion portion of the study.
Of note, the trial was suspended for accrual for the COVID-19 pandemic from April 1, 2020 until July 23, 2020, which delayed accrual, and manufacturing issues of CLR 131 due to local COVID quarantine requirements impacted the treatment of two participants.
After completion of the dose escalation portion of the study, the protocol was later amended to reduce the sample size from 24 to 12 participants due to limitations of time for participant accrual, decision of the sponsor and study team, and DSMC approval. Furthermore, the protocol specified up to 12 participants to be evaluated at the MTD for statistical analysis for outcomes. Since the dose of CLR 131 was not escalated, all participants from the dose escalation cohort were treated at the MTD (eight participants), an additional four participants were accrued within the dose expansion cohort. The trial completed accrual with 12 enrolled participants at the MTD per protocol.
Adverse events during treatment
Eight participants experienced grade 4 non-DLT haematologic toxicities (2 anaemia, 8 leukopenia, 5 thrombocytopenia) at least probably attributed to CLR 131, which was consistent with the expected toxicity profile (Table 2, Supplementary Tables S2 and S3). The haematologic toxicities occurred during weeks 6–8 after the first dose of CLR 131, and in seven participants, the haematologic toxicities resolved within three weeks without sequelae (Supplementary Table S4). One participant was hospitalized during the prespecified DLT period and eventually died from aspiration pneumonia (grade 5) deemed unrelated to CLR 131 but possibly related to EBRT (Supplementary Tables S5 and S6). Because this participant did not complete the DLT period but developed expected grade 4 haematologic toxicities, the sponsor and study team mutually classified this as a DLT (grade 4 anaemia) probably related to CLR 131. This same participant did not complete additional imaging scans to evaluate the best response to treatment and was censored for response.
Table 2.
All Treatment-related adverse events at least possibly related to CLR 131a for all participants (for enrolled population, n = 12).
| Adverse event |
Grade |
||||
|---|---|---|---|---|---|
| At least possibly related to CLR 131 | 1 | 2 | 3 | 4 | 5 |
| Blood and lymphatic system disorders | 4 | 2 | 6 | 1 | |
| Anaemia | 4 | 2 | 4 | 1 | |
| Febrile neutropenia | 2 | ||||
| Gastrointestinal disorders | 4 | 4 | 1 | ||
| Dry mouth | 2 | 1 | |||
| Dysphagia | 2 | ||||
| Mucositis oral | 1 | 1 | |||
| Nausea | 1 | ||||
| Salivary duct inflammation | 1 | ||||
| General disorders and administration site conditions | 5 | 3 | |||
| Fatigue | 4 | 2 | |||
| Fever | 1 | ||||
| Pain | 1 | ||||
| Infections and infestations | 1 | ||||
| Thrush | 1 | ||||
| Injury, poisoning and procedural complications | 1 | ||||
| Dermatitis radiation | 1 | ||||
| Investigations | 2 | 6 | 11 | 24 | |
| Lymphocyte count decreased | 5 | 4 | |||
| Neutrophil count decreased | 1 | 1 | 7 | ||
| Platelet count decreased | 1 | 1 | 3 | 6 | |
| Thyroid stimulating hormone increased | 1 | ||||
| Weight loss | 2 | ||||
| White blood cell decreased | 2 | 2 | 7 | ||
| Metabolism and nutrition disorders | 1 | 1 | |||
| Anorexia | 1 | 1 | |||
| Musculoskeletal and connective tissue disorders | 1 | ||||
| Fibrosis deep connective tissue | 1 | ||||
| Nervous system disorders | 2 | ||||
| Dysgeusia | 1 | ||||
| Headache | 1 | ||||
| Respiratory, thoracic and mediastinal disorders | 2 | ||||
| Oropharyngeal pain | 2 | ||||
Defined and graded according to version 5.0 of the National Cancer Institute’s Common Terminology Criteria for Adverse Events.
For non-haematologic toxicities, one participant experienced grade 3 mucositis possibly related to CLR 131. Grade 1 and 2 adverse events possibly related to CLR 131 included anorexia, dry mouth, dysgeusia, dysphagia, fatigue, headache, nausea, salivary duct inflammation, thrush, increased thyroid-stimulating hormone levels, and weight loss. There were no other treatment-related grade 3–4 non-haematologic toxicities.
Because the above mentioned participant developed a DLT in the first dose level of 15.6 mCi/m2, a total of eight participants were treated at dose level 1 with a planned toxicity monitoring to determine the dose for the escalation portion of the trial. The safety and toxicity profile was consistent with data of CLR 131 in other global trials, which noted the MTD of CLR 131 was 40 mCi/m2 (fractionated into 20 mCi/m2 doses). However, given the addition of EBRT to CLR 131 in this study, the decision was made by the sponsor, study team, and DSMC not to escalate the CLR 131 dose. Thus, the CLR 131 dose of 15.6 mCi/m2 was selected for the dose expansion portion of the study.
Impact of CLR 131 on delivery of external beam radiation therapy
After completion of CLR 131 dosing, the median duration for delivery for EBRT was 43 days (range 36–44 days). No participants experienced toxicities associated with CLR 131 that delayed the start of EBRT. The median duration from the first dose of CLR 131 to the start of EBRT was 14 days (range 8–21 days). The larger range of duration for completion of CLR 131 treatment was primarily due to drug manufacturing issues. One participant experienced a 7-day delay in CLR 131 dose 2 due to not meeting the manufacturing quality control. A second participant experienced a delay in CLR 131 dose 2 due to manufacturing issues during the COVID-19 pandemic and ultimately the treating physician decided to forgo CLR 131 treatment and proceed with EBRT.
The median AD of CLR 131 to the PTV was 6·23 Gy (range 2·65–8·69 Gy). Due to the amount of AD imparted from the CLR 131 treatment, a range of one to four treatment sessions were removed across the participants enrolled. A supplemental table is included to denote the preplanned re-irradiation treatment dose, the amount of radiation delivered by CLR 131, and the amount of EBRT for each participant (Supplementary Table S7).
Clinical response
Three months after completion of EBRT, eleven participants underwent diagnostic cross-sectional imaging (any combination of CT, MR, or PET scans). The best overall response reported included seven participants with complete response (63.6%), one with a partial response (9%), one with stable disease (9%), and two with disease progression (18%) (Table 3). Of the participants with disease progression, one developed locoregional progression, and one developed distant metastatic disease. One participant did not complete the DLT period and was censored for response.
Table 3.
Overall response rate of CLR 131 with external beam radiation therapy for all participants with recurrent or metastatic head and neck cancer (for evaluable population, n = 11).
| Tumour Response by RECIST v1.1 | N = 11 |
|---|---|
| Best overall response | |
| CR, No. (%) | 7 (64; 30.8–89.1) |
| PR, No. (%) | 1 (9; 0.2–41.3) |
| SD, No. (%) | 1 (9; 0.2–41.3) |
| PD, No. (%) | 2 (18; 2.3–51.8) |
| Locoregional disease | 1 (9; 0.2–41.3) |
| Distant metastatic disease | 1 (9; 0.2–41.3) |
| ORR (%) | 8 (73; 39–94) |
| 1-year overall survival (%) | 8 (73; 39–94) |
| 1-year progression-free survival (%) | 4 (36; 10.9–69.2) |
CR, complete response; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease; RECIST v1.1, Response Evaluation Criteria in Solid Tumours version 1.1.
Twelve months after completion of treatment with CLR 131 plus EBRT, four participants (36.4%) were alive and free of disease. Of the four participants, one experienced a delay in receiving the second dose of CLR 131. Six participants (54%) developed disease progression. One participant had stable disease but died from complications from a tracheostomy and aspiration pneumonia. As of the data cut off on February 1, 2024, two participants remain alive and free of disease. For the nine participants who progressed and have imaging data after completion of treatment, the median PFS (defined from on treatment date to the date of disease progression) was 270 days (95% CI, 147 to NA; range, 73–513 days) (Supplementary Fig. S1). The 1-year PFS was 41·7% (95% CI, 0·213 to 0·814). The median OS was 536 days (95% CI, 395 to NA). The 1-year OS rate was 75% (95% CI, 0·541 to 1·0).
Quality of life and swallowing function
Ten out of 12 treated participants completed QOL, saliva, and swallowing assessments at baseline and 3 months, and five participants were seen at 6 months post-treatment (Table 4). At each time point, participants underwent an MBS, provided saliva samples, and completed the QOL questionnaires. While median salivary flow rates at each time point were lower than published normative data27 (males = 0·57 ml/min and females = 0·42 ml/min), flow rate did not change significantly following treatment at either time point. No significant change was observed in MDADI median composite scores at 3 months or 6 months compared with baseline; however, the decrease of 15·8 points from baseline to 6 months for five participants would be considered a clinically meaningful decline in dysphagia-related QOL.28 No significant changes were observed in EORTC QLQ-C30 median functional scores 3 months or at 6 months compared with baseline; nor in symptom scores, or in global scores. XeQoLS median scores and Xerostomia Inventory median scores also did not change significantly across treatment. Median DIGEST grades did not change significantly at 3 months. While scores increased at 6 months, this also did not reach statistical significance.
Table 4.
Quality of life, saliva, and swallowing outcomes.
| Quality of life assessment | Baseline | 3 months | 6 months | Baseline to 3 months | Baseline to 6 months |
|---|---|---|---|---|---|
| Median | Confidence intervals | ||||
| MDADI composite score | 71.6 (N = 10) | 62.1 (N = 9) | 55.8 (N = 5) | −12.6 to 4.2 (N = 9) | −28.4 to 7.4 (N = 5) |
| EORTC QLQ-C30 functional | 76.7 (N = 9) | 72.2 (N = 10) | 71.1 (N = 5) | −14.4 to 4.4 (N = 9) | −17.8 to 20 (N = 5) |
| EORTC QLQ-C30 symptom | 23.1 (N = 9) | 26.9 (N = 10) | 28.1 (N = 5) | −7.7 to 12.8 (N = 9) | −15.4 to 35.9 (N = 5) |
| EORTC QLQ-C30 global | 50 (N = 9) | 54.2 (N = 10) | 58.3 (N = 5) | −20.8 to 16.7 (N = 9) | −25 to 25 (N = 5) |
| XeQoLS | 15 (N = 10) | 16.5 (N = 10) | 18 (N = 5) | −2 to 9.5 (N = 9) | −10 to 16 (N = 5) |
| Xerostomia inventory | 34.5 (N = 10) | 34.5 (N = 10) | 36 (N = 5) | −3 to 5 (N = 5) | −6 to 6 (N = 5) |
| Saliva and swallowing outcomes | |||||
| Unstimulated salivary flow rate | 0.225 ml/min (N = 10) | 0.178 ml/min (N = 10) | 0.192 ml/min (N = 4) | −0.246 to 0.09 (N = 8) | −0.218 to 0.12 (N = 4) |
| DIGEST safety score | 1 (N = 10) | 1 (N = 9) | 2 (N = 5) | −0.5 to 0.5 (N = 9) | 0.0 to 2.0 (N = 5) |
| DIGEST efficiency score | 3 (N = 10) | 3 (N = 9) | 3 (N = 5) | −0.5 to 1.5 (N = 9) | 0.0 to 3.0 (N = 5) |
| DIGEST overall score | 2 (N = 10) | 2 (N = 9) | 3 (N = 5) | 0.0 to 0.5 (N = 9) | 0.0 to 3.0 (N = 5) |
MDADI, MD Anderson Dysphagia Inventory; EORTC, European Organization for Research and Treatment of Cancer; QLQ-C30, Quality of Life Questionnaire; XeQoLS, Xerostomia-Related Quality of Life Scale; DIGEST, Dynamic Imaging Grade of Swallowing Toxicity.
Discussion
Despite advances in techniques for the curative treatment of head and neck SCC, many patients unfortunately develop recurrent and/or distant metastatic disease. Selected patients with locoregional recurrence are potential candidates for salvage surgery or re-irradiation, but the long-term survival remains low.18
Radioligand therapies have demonstrated promise for the treatment of advanced malignancies with several agents approved for the treatment of neuroendocrine tumours and prostate cancer.29,30 CLR 131 is more selective and targeted to malignant tumour cells due to the higher lipid raft content in cancer cells, prompting selective uptake and retention. This trial combines a radiotherapeutic with EBRT to reduce the overall dose of EBRT delivered and severity of toxicities associated with traditional re-irradiation treatment.
Historically, the most common toxicities associated with re-irradiation include mucositis (grade 3, 32% and grade 4, 14%).8 In this clinical trial, no participants experienced grade 4 mucositis (0%), and one participant had grade 3 mucositis (8·3%). Overall grade 3 and higher treatment-related adverse events were primarily associated with myelosuppression with CLR 131 treatment, and these toxicities were recoverable. Utilizing CLR 131 affords a unique opportunity for EBRT dose reduction. The longitudinal evaluation of changes in our participants’ QOL, xerostomia, and swallowing measures highlight that the most severe dysphagia-related QOL occurred at six months, which may be considered clinically relevant, although this measure was only based on a small subset of patients. Having longitudinal evaluation in a larger population size warrants additional analysis.
Historically, the median PFS after re-irradiation of recurrent HNC typically is 10 months with significant toxicities.8 In the IMRT-based re-irradiation era,18 our clinical trial appears comparable to the currently published data with similar one-year OS and PFS. Our trial notes an improvement in the occurrence of mucositis, which has a profound negative impact on QOL.31,32 Myelosuppression is a known toxicity associated with CLR 131, but given that oncologists commonly incorporate concurrent cisplatin chemotherapy with re-irradiation, myelosuppression is an expected toxicity that many patients experience during treatment.
Limitations of this study include a small sample size, treatment performed in a single centre, and lack of diversity in the patient population. Interpretation of preliminary efficacy is limited in a single-arm study. Efficacy data was pooled for some analyses given that all treated participants received the same dose of CLR 131.
This clinical trial explores the unique strategy of combining tumour-selective radiotherapeutic agents with reduced-dose EBRT for re-irradiation of patients with recurrent HNC. This trial employed Monte Carlo methodology for calculating the therapeutic absorbed dose to tumour and normal tissue delivered by CLR 131 prior to EBRT, thereby enabling a personalized dosimetry profile for each participant. Although this phase 1 trial enrolled a small sample size (12 participants), the combination of CLR 131 plus EBRT did not demonstrate any safety concerns and was well tolerated. The rapidly expanding interest in tumour-selective radiopharmaceutical agents suggests that combinatorial treatment strategies using EBRT and/or chemotherapy/immunotherapy will expand significantly in the future.
Contributors
JYB, DA, MY, DT, KO, JL, NRP, BB, PMH had access to and verified the data. JYB, PMH, and BB conceived and designed the study. JYB, RK, AB, DA, SP, DT, TG, PH, GH, TM, AW, NRP, SC, BB, PMH were responsible for acquisition of the data. JYB, MY, DA, DT, NRP, SC, BB, PMH were responsible for analysis of the data. JYB, DA, MY, NRP, BB, PMH were responsible for interpretation of the results. JYB, DA, MY, BB, PMH were responsible for the writing of the manuscript. All authors had full access to the data. All authors were responsible for critically reviewing or revising the manuscript for important intellectual content and approved the final manuscript. JYB had the final responsibility to submit for publication.
Data sharing statement
Deidentified individual participant data that underlie the results reported here are available. Deidentified participant data can be made available upon request by qualified researchers to the corresponding author. Data transfer agreements and approval by the trial management team might be required. Enquiries should be made via email to the corresponding authors. The study protocol is available in Supplementary Materials.
Declaration of interests
JYB reports grants to her institution from National Institutes of Health, National Institute of Dental and Craniofacial Research, Merck, Kura Oncology, Astellas Pharma, Incyte, Cellectar Biosciences, SeaGen, and Lilly; participates on the data safety monitoring board for Kura Oncology. AB reports research support and consulting from GE Healthcare and Siemens. RK reports research funding from the National Institutes of Health, Cellectar, Bridge Bio, and Merck; royalty payments; and consulting fees from Mele Associates and HunaTek; participates in the editorial board of International Journal of Radiation Oncology Biology Physics. JL and KO are employees of Cellectar Biosciences. JL participates on the data safety monitoring board for Cellectar Biosciences. BB reports research support from the National Institutes of Health. DA and BB have financial interests with Voximetry, Inc. All other authors declare no competing interests.
Acknowledgements
The study team would like to acknowledge the support from the University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520, the UW Head and Neck SPORE Grant P50 DE026787, the UW Institute for Clinical and Translational Research 1UL1TR002373, and Cellectar for providing Iopofosine I 131. The study team would also like to thank the participants and their families for participating in this clinical trial. The study team thanks James P. Zacny, PhD for manuscript preparation and formatting assistance. The study team thanks the members of the National Institute for Dental and Craniofacial Research Data and Safety Monitoring Committee.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105496.
Appendix ASupplementary data
References
- 1.Pulte D., Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15(9):994–1001. doi: 10.1634/theoncologist.2009-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cognetti D.M., Weber R.S., Lai S.Y. Head and neck cancer: an evolving treatment paradigm. Cancer. 2008;113(7 Suppl):1911–1932. doi: 10.1002/cncr.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pignon J.P., le Maitre A., Maillard E., Bourhis J., Group M.-N.C. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 4.De Felice F., Thomas C., Patel V., et al. Osteoradionecrosis following treatment for head and neck cancer and the effect of radiotherapy dosimetry: the Guy's and St Thomas' Head and Neck Cancer Unit experience. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(1):28–34. doi: 10.1016/j.oooo.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Rooney M.K., Andring L.M., Corrigan K.L., et al. Hypothyroidism following radiotherapy for head and neck cancer: a systematic review of the literature and opportunities to improve the therapeutic ratio. Cancers. 2023;15(17) doi: 10.3390/cancers15174321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen A.M., Phillips T.L., Lee N.Y. Practical considerations in the re-irradiation of recurrent and second primary head-and-neck cancer: who, why, how, and how much? Int J Radiat Oncol Biol Phys. 2011;81(5):1211–1219. doi: 10.1016/j.ijrobp.2011.06.1998. [DOI] [PubMed] [Google Scholar]
- 7.Hoebers F., Heemsbergen W., Moor S., et al. Reirradiation for head-and-neck cancer: delicate balance between effectiveness and toxicity. Int J Radiat Oncol Biol Phys. 2011;81(3):e111–e118. doi: 10.1016/j.ijrobp.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Kasperts N., Slotman B., Leemans C.R., Langendijk J.A. A review on re-irradiation for recurrent and second primary head and neck cancer. Oral Oncol. 2005;41(3):225–243. doi: 10.1016/j.oraloncology.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Weichert J.P., Clark P.A., Kandela I.K., et al. Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Sci Transl Med. 2014;6(240):240ra75. doi: 10.1126/scitranslmed.3007646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mollinedo F. Antitumour ether liquids: proapoptotic agents with multiple therapeutic indications. Expert Opin Ther Pat. 2007;17(4):385–405. [Google Scholar]
- 11.Morris Z.S., Weichert J.P., Saker J., et al. Therapeutic combination of radiolabeled CLR1404 with external beam radiation in head and neck cancer model systems. Radiother Oncol. 2015;116(3):504–509. doi: 10.1016/j.radonc.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao J., Mortimer J.E., Cho S.Y., Marshall J., Wong T. A phase I, multicenter, open-label dosimetry study of I-131-CLR1404 in patients with relapsed or refractory advanced solid tumors who have failed standard therapy or for whom no standard therapy exists. J Clin Oncol. 2012;30(15) [Google Scholar]
- 13.Lubner S.J., Mullvain J., Perlman S., et al. A phase 1, multi-center, open-label, dose-escalation study of I-CLR1404 in subjects with relapsed or refractory advanced solid malignancies. Cancer Invest. 2015;33(10):483–489. doi: 10.3109/07357907.2015.1081691. [DOI] [PubMed] [Google Scholar]
- 14.Ailawadhi S., Stiff P.J., Ibrahim E., et al. Fractionated dosing of CLR 131 in patients with relapsed or refractory multiple myeloma (RRMM) Blood. 2019;134(Supplement_1):144. [Google Scholar]
- 15.Ailawadhi S., Chanan-Khan A., Peterson J.L., et al. Treatment free remission (TFR) and overall response rate (ORR) results in patients with relapsed/refractory Waldenstrom's macroglobulinemia (WM) treated with CLR 131. J Clin Oncol. 2021;39(15_suppl):7561. [Google Scholar]
- 16.Ailawadhi S., Stiff P.J., Ibrahim E., et al. CLR 131 (iopofosine I-131) treatment in triple class refractory and beyond multiple myeloma patients: preliminary efficacy and safety results from the phase 2 clover-1 trial. Blood. 2021;138(Supplement 1):1652. [Google Scholar]
- 17.Longcor J., Callander N., Oliver K., Chanan-Khan A., Ailawadhi S. Iopofosine I-131 treatment in late-line patients with relapsed/refractory multiple myeloma post anti-BCMA immunotherapy. Blood Cancer J. 2022;12(9):130. doi: 10.1038/s41408-022-00725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H.I., Kim J.H., Ahn S.H., et al. Re-irradiation for recurrent or second primary head and neck cancer. Radiat Oncol J. 2021;39(4):279–287. doi: 10.3857/roj.2021.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Besemer A.E., Yang Y.M., Grudzinski J.J., Hall L.T., Bednarz B.P. Development and validation of RAPID: a patient-specific Monte Carlo three-dimensional internal dosimetry platform. Cancer Biother Radiopharm. 2018;33(4):155–165. doi: 10.1089/cbr.2018.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adam D.P., Grudzinski J.J., Bormett I., et al. Validation of Monte Carlo (131) I radiopharmaceutical dosimetry workflow using a 3D-printed anthropomorphic head and neck phantom. Med Phys. 2022;49(8):5491–5503. doi: 10.1002/mp.15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrier J.F., Archambault L., Beaulieu L., Roy R. Validation of GEANT4, an object-oriented Monte Carlo toolkit, for simulations in medical physics. Med Phys. 2004;31(3):484–492. doi: 10.1118/1.1644532. [DOI] [PubMed] [Google Scholar]
- 22.Agostinelli S., Allison J., Amako K., et al. GEANT4-a simulation toolkit. Nucl Instrum Meth A. 2003;506(3):250–303. [Google Scholar]
- 23.Hutcheson K.A., Barbon C.E.A., Alvarez C.P., Warneke C.L. Refining measurement of swallowing safety in the dynamic imaging grade of swallowing toxicity (DIGEST) criteria: validation of DIGEST version 2. Cancer. 2022;128(7):1458–1466. doi: 10.1002/cncr.34079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 25.Rogers S.N., Johnson I.A., Lowe D. Xerostomia after treatment for oral and oropharyngeal cancer using the University of Washington saliva domain and a Xerostomia-Related Quality-of-Life Scale. Int J Radiat Oncol Biol Phys. 2010;77(1):16–23. doi: 10.1016/j.ijrobp.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Ji Y., Wang S.J. Modified toxicity probability interval design: a safer and more reliable method than the 3 + 3 design for practical phase I trials. J Clin Oncol. 2013;31(14):1785–1791. doi: 10.1200/JCO.2012.45.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenoll-Palomares C., Munoz Montagud J.V., Sanchiz V., et al. Unstimulated salivary flow rate, pH and buffer capacity of saliva in healthy volunteers. Rev Esp Enferm Dig. 2004;96(11):773–783. doi: 10.4321/s1130-01082004001100005. [DOI] [PubMed] [Google Scholar]
- 28.Hutcheson K.A., Barrow M.P., Lisec A., Barringer D.A., Gries K., Lewin J.S. What is a clinically relevant difference in MDADI scores between groups of head and neck cancer patients? Laryngoscope. 2016;126(5):1108–1113. doi: 10.1002/lary.25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strosberg J., El-Haddad G., Wolin E., et al. Phase 3 trial of (177)Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sartor O., de Bono J., Chi K.N., et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown T.J., Gupta A. Management of cancer therapy-associated oral mucositis. JCO Oncol Pract. 2020;16(3):103–109. doi: 10.1200/JOP.19.00652. [DOI] [PubMed] [Google Scholar]
- 32.Iovoli A.J., Turecki L., Qiu M.L., et al. Severe oral mucositis after intensity-modulated radiation therapy for head and neck cancer. JAMA Netw Open. 2023;6(10) doi: 10.1001/jamanetworkopen.2023.37265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.