Highlights
-
•
Microalgae's multifaceted role in agroecosystems explored.
-
•
Interdisciplinary insights integrated for comprehensive understanding.
-
•
Emerging trends in microalgae technology identified and analyzed.
-
•
Challenges and opportunities critically assessed for practical implications.
Keywords: Agroecosystems, Biofertilizer, Microalgae, Phycoremediation, wastewater treatment
Abstract
The increasing need for sustainable agricultural practices due to the overuse of chemical fertilizers has prompted interest in microalgae as biofertilizers. This review investigates the potential of microalgae as biofertilizers and phycoremediators within sustainable agroecosystems, addressing both soil fertility and wastewater management. Microalgae provide a dual benefit by absorbing excess nutrients and contaminants from wastewater, generating nutrient-rich biomass that can replace chemical fertilizers and support plant growth. Implementation strategies include cultivating microalgae in wastewater to offset production costs, using closed photobioreactor systems to enhance growth efficiency, and applying microalgal biomass directly to soil or crops. Additionally, microalgae extracts provide essential bioactive compounds, such as phytohormones and amino acids, that enhance plant growth and resilience. While microalgae offer an eco-friendly solution for nutrient recycling and crop productivity, challenges in scalability, production cost, and regulatory frameworks hinder widespread adoption. This review highlights the potential pathways and technological advancements necessary for integrating microalgae into sustainable agriculture, emphasizing the need for interdisciplinary collaboration and innovative approaches to overcome these barriers. Ultimately, microalgae biofertilizers represent a promising approach to reducing environmental impact and advancing sustainable farming practices.
1. Introduction
The surging global demand for food presents formidable challenges to agricultural systems worldwide, urging a transition towards sustainable methodologies to curb environmental degradation and uphold enduring food security [1,2]. Conventional agricultural practices, marked by excessive use of chemical inputs, monocropping, and deforestation, exacerbate soil erosion, loss of biodiversity, and contamination of water bodies [3,4]. The extensive application of synthetic fertilizers and pesticides compounds these environmental woes, with fertilizer runoff causing eutrophication and consequent harm to aquatic habitats [5,6]. In addressing these pressing concerns, sustainable agricultural techniques emerge as indispensable for combating water pollution, rectifying ecosystem imbalances, and mitigating biodiversity decline [7].
Microalgae have emerged as a promising solution for sustainable agriculture, offering unique advantages such as efficient nutrient extraction from wastewater and potential as a biofertilizer [1,5]. Harnessing the properties of microalgae, particularly their ability to thrive on wastewater and extract nutrients, presents a dual solution for wastewater treatment and soil fertility enhancement in agricultural ecosystems. The exploration of microalgae in agriculture dates back to the 1960s, with research indicating their potential to enhance soil fertility through the activity of micronutrients and metabolites [8,9]. Microalgae-derived materials contribute to improved soil structure and fertility, containing a rich source of macronutrients and biologically active compounds [10,11]. Such advancements underscore the significance of microalgae as a sustainable resource in the quest for eco-friendly agricultural practices. Furthermore, microalgae-based biofertilizers or biostimulants offer promising avenues for sustainable agriculture by promoting plant growth and enhancing soil fertility [12,13]. Specific strains, such as Chlorella vulgaris, Scenedesmus quadricauda, Desmodesmus subspicatus and Spirulina platensis, are particularly effective in absorbing nitrogen, phosphorus, and trace minerals from various wastewater sources, including agricultural runoff and municipal wastewater, transforming pollutants into a nutrient-rich biomass [5]. This biomass can then be applied to soils, enhancing fertility, and supporting plant growth, as it contains essential nutrients along with bioactive compounds like amino acids, phytohormones, and vitamins [1]. For instance, applying Scenedesmus quadricauda, Spirulina platensis and Chlorella vulgaris to beetroot resulted in beneficial effects on root architecture, such as increases in root length and lateral root number, which in turn increased the root surface area available for nutrient uptake [14]. In a greenhouse, Desmodesmus subspicatus aqueous extracts and lyophilized biomass boosted germination in vitro and sped up development during the transplanting and acclimatization phase [15]. The exopolysaccharides that Spirulina platensis releases into the soil also serve the crucial purpose of sequestering sodium and metal ions, which lowers their uptake by maize plants and promotes their growth in saline or contaminated soils [16].
As part of the Sustainable Development Goals (SDGs), sustainable agriculture plays a crucial role in addressing goals related to food security, terrestrial ecosystem preservation, and water quality [17,18]. However, meeting the growing global food demand while upholding sustainability goals remains a complex challenge, necessitating innovative solutions like microalgae-based agriculture [19]. The integration of microalgae into agricultural practices holds promise not only for increasing crop productivity but also for mitigating environmental impacts, thus aligning with the overarching objectives of sustainable development. The existence of microalgae as a biofertilizer can be observed in Fig. 1, illustrating the documents compiled from the Scopus database. Employing a meticulous search criterion focusing on documents featuring the terms "microalgae" and "biofertilizer" in "all fields," and refining the search to review papers and articles, the research team systematically collected data. This rigorous selection process aimed to provide a targeted and comprehensive analysis of the literature, offering insights into the relationship between microalgae and biofertilizers within the academic discourse.
Fig. 1.
Documents contained keywords “microalgae” and “biofertilizer” collected from scopus database.
The compiled data, spanning from 2000 to 2023, showcases a progressive increase in research output, with the number of documents rising from 2 in 2000 to a peak of 466 in 2023. This upward trajectory reflects the growing scholarly interest in the topic, indicating sustained and heightened attention to the exploration of microalgae as a biofertilizer. With a total of 1,834 documents found, the thorough analysis of this data, grounded in the specificity of search terms and document types, adds credibility and rigor to the meta-analysis, establishing a robust foundation for understanding the evolving landscape of microalgae-based biofertilizer research within the academic domain.
Despite their potential, challenges such as the high cost of nutrient provision for microalgae cultivation hinder their widespread adoption in agriculture [20,21]. Addressing these challenges and exploring cost-effective strategies for integrating microalgae into agricultural practices are crucial steps towards realizing their full potential as sustainable and resilient components of agricultural ecosystems. This review is dedicated to evaluating the opportunities and challenges inherent in leveraging microalgae as a biofertilizer in agroecosystems. With the rising global food demand and the environmental degradation caused by the overuse of chemical fertilizers, there is a pressing need for sustainable alternatives. Current agricultural practices contribute to soil depletion, eutrophication, and biodiversity loss, challenging long-term food security. Despite its potential as a biofertilizer, the widespread use of microalgae in agriculture faces significant hurdles such as high production costs and scalability issues. This review aims to explore the potential of microalgae as a biofertilizer and its role in phycoremediation for wastewater treatment in agroecosystems. It evaluates both the opportunities and the challenges that hinder large-scale implementation, providing a comprehensive assessment of the current landscape and potential solutions to promote sustainability in agriculture.
2. Chemical fertilizer and its implications for environmental
In the field of soil fertility management, both macronutrients and micronutrients play indispensable roles in sustaining plant growth and ensuring optimal crop yield. Macronutrients, including nitrogen, phosphorus, and potassium, are essential elements required by plants in relatively large quantities. Nitrogen facilitates chlorophyll synthesis and promotes vigorous vegetative growth, phosphorus aids in root development and energy transfer, while potassium regulates water uptake and enhances disease resistance [22]. Alongside macronutrients, micronutrients such as iron, zinc, and manganese are equally vital, albeit needed in smaller quantities, for catalyzing enzymatic reactions and maintaining overall plant health (Table 1). These micronutrients serve as cofactors in various metabolic processes, influencing nutrient uptake, photosynthesis, and plant defence mechanisms [23,24].
Table 1.
Soil nutrient requirements and critical concentrations.
| Nutrient | Critical Concentration | Reference |
|---|---|---|
| Primary nutrients | ||
| Nitrogen (N) | 25–50 mg/kg | [25] |
| Phosphorus (P) | 1 µM * | [26] |
| Potassium (K) | 141–370 mg/kg | [27] |
| Secondary nutrients | ||
| Calcium (Ca) | 6–778 mg/kg * | [28] |
| Sulfide (S) | >15 mg/kg | [29] |
| Magnesium (Mg) | 0.05–0.5 % * | [29] |
| Micronutrients | ||
| Cobalt (Co) | 15–25 mg/kg * | [30] |
| Copper (Cu) | >0.04 mg/kg | [29] |
| Boron (B) | >0.75 mg/kg | [31] |
| Chlorine (Cl) | 100 mg/kg * | [32] |
| Iron (Fe) | >7.5 mg/kg | [33] |
| Zinc (Zn) | >1.5 mg/kg | [33] |
| Manganese (Mn) | >4mg/kg | [34] |
| Molybdenum (Mo) | >0.2 mg/kg | [35] |
However, the overutilization of chemical fertilizers in agriculture presents several disadvantages that have significant environmental and ecological implications [11,36,37]. Firstly, the runoff of excess chemical fertilizers into water bodies can lead to the acidification of water sources, contributing to the phenomenon known as acid rain. Acid rain not only affects aquatic ecosystems but also damages soil fertility and vegetation, posing a threat to biodiversity. Furthermore, prolonged use of chemical fertilizers can result in soil denaturation, depleting essential organic matter and beneficial microorganisms crucial for soil health and productivity. This degradation of soil quality diminishes its ability to support plant growth and can lead to long-term environmental degradation.
Another adverse consequence of chemical fertilizers is the promotion of algae bloom in water bodies. Excessive nutrients, particularly nitrogen and phosphorus from fertilizers, fuel the rapid growth of algae, resulting in algal blooms [38,39]. These blooms disrupt aquatic ecosystems, depleting oxygen levels in water bodies and causing harm to aquatic life. Moreover, nutrient runoff from chemical fertilizers contributes to eutrophication, where an excess of nutrients in water bodies leads to the proliferation of algae and aquatic plant growth, ultimately causing oxygen depletion and ecological imbalance. From a sustainability standpoint, the reliance on chemical fertilizers is not viable in the long term, as it contributes to soil degradation, water pollution, and ecosystem disruption. Transitioning towards more sustainable agricultural practices, such as organic farming and the use of alternative fertilizers, is essential for mitigating these adverse impacts and ensuring the health and resilience of agricultural ecosystems.
3. The role of microalgae as biofertilizer in agriculture ecosystems
A biofertilizer is a substance consisting of living microorganisms, such as bacteria, fungi, or algae, which enhance plant growth and nutrient availability in the soil [40,41]. Unlike traditional chemical fertilizers, biofertilizers promote sustainable agriculture by fostering natural processes that contribute to soil fertility. These microorganisms form symbiotic relationships with plants or stimulate plant growth through various mechanisms, such as nitrogen fixation, phosphorus solubilization, and the production of plant growth-promoting substances [42]. Biofertilizers play a crucial role in improving soil health, nutrient cycling, and overall agricultural sustainability.
Agricultural ecosystems, often referred to as agroecosystems, constitute intricate environments where human cultivation practices intersect with natural elements and ecological processes [43]. These systems encompass cultivated fields, crops, livestock, and the surrounding environment where farming activities unfold. Within agricultural ecosystems, a dynamic interplay occurs among living organisms—such as plants, animals, and microorganisms—and non-living elements, including soil, water, and air [43,44]. Shaped by human interventions, such as crop choices, cultivation methods, and the application of inputs like fertilizers and pesticides, these ecosystems significantly impact soil health, biodiversity, water quality, and overall ecological resilience. Sustainable agricultural practices strive to optimize these interactions, seeking a balance that enhances productivity while mitigating negative environmental consequences [45]. Central components include crops, livestock, soil health, water management, biodiversity, and the intricate role of human activities, collectively shaping the intricate tapestry of agricultural ecosystems. In navigating the complexities of agricultural systems, the goal is to foster sustainable practices that not only meet the demands of food production but also prioritize environmental conservation and long-term ecosystem vitality.
Microalgae stand as versatile and promising agents in addressing critical challenges within agricultural ecosystems. As the global demand for food surges, the quest for sustainable solutions becomes paramount. Microalgae exhibit multifaceted potency that directly addresses key concerns in agriculture. Their biofertilizing capabilities offer an eco-friendly alternative to traditional fertilizers, presenting a sustainable approach to enhance soil fertility and meet the increasing demand for food production [21]. Additionally, microalgae's ability to biostimulate plant growth not only improves crop yields but also contributes to reducing reliance on excessive chemical fertilizers, mitigating the associated risks of soil denaturation [46,47].
Within the intricate tapestry of agricultural ecosystems, the incorporation of microalgae stands poised as a transformative force across diverse phases of cultivation. In the initial cultivation phase, microalgae, employed as biofertilizers, infuse the soil with essential nutrients, cultivating a foundation for robust plant growth while mitigating the environmental impact associated with traditional chemical fertilizers. In the context of pest management, microalgae's role as a biopesticide introduces a natural, environmentally friendly alternative, safeguarding crops while circumventing the pitfalls of conventional pesticide usage [48]. Beyond the harvest, microalgae extend their influence to the waste and residue management phase, offering a potential avenue for sustainable disposal and recycling [10]. As we delve into each facet of the agricultural continuum, the integration of microalgae emerges as a holistic and promising approach, aligning with the imperatives of sustainable agriculture, and fostering resilience within the complex web of agricultural ecosystems.
4. Microalgae for wastewater phycoremediation
In recent decades, significant water contamination has occurred due to factors such as population growth, rapid urbanization, and industrialization. In areas where wastewater treatment plant (WTP) facilities are not well established, it is often considered a quick and inexpensive solution to dispose of untreated wastewater (such as commercial, residential, and industrial effluent) directly into aquatic environments like rivers, lakes, reservoirs, and the sea. This practice impacts the water cycle, alters precipitation patterns, and contributes to water scarcity and depletion in some areas [49]. Moreover, the potential for substantial quantities of detrimental pollutants to infiltrate both the human and animal food chains could give rise to grave health risks [50]. Effluent is a complex mixture of manmade materials, as well as organic and inorganic natural components. According to [51], the main pollutants found in effluent include sugars, lipids, carbohydrates, and amino acids. Wastewaters also contain notable concentrations of inorganic substances such as ammonium salts, bicarbonate, sodium, calcium, potassium, magnesium, arsenic, sulphur, phosphorus, and heavy metals. The discharge of raw or treated water from cities and villages, industrial or processing (of which 70 % come from agriculture industries in developing countries), and farm runoff from disposal sites are some of the sources of pollution [52]. Therefore, investigating a workable wastewater treatment and resource regeneration is necessary to address the issues of inadequate water supply [53,54].
One major step toward human progress is wastewater treatment. As a result, many researchers have studied how to minimize pollution using natural processes involving algae, including Cyanobacteria, microalgae, and macroalgae [49,55,56]. Microalgae, comprising prokaryotic cyanobacteria and photoautotrophic eukaryotic microalgae, inhabit a wide range of environments, from fresh to marine water, displaying diverse habitat preferences and thallus organizations. Notably adaptable, microalgae thrive in various water settings, including brackish and saline water, as well as the complex environments of wastewater sources, facilitated by the presence of essential nutrients such as nitrate, ammonia, phosphate, and trace elements [57,58]. Their exceptional nutrient uptake capacity renders microalgae a cost-effective and efficient solution for removing contaminants in tertiary wastewater treatment, while simultaneously sequestering CO2 and generating potentially valuable biomass through photosynthesis [59]. Microalgae-based wastewater treatment offers sustainable advantages over conventional methods, especially in energy efficiency and nutrient recovery. While traditional treatments like activated sludge processes consume 0.5 to 2.5 kWh/m³ due to high energy needs for aeration, algae-based systems rely on photosynthesis, cutting energy use by up to 50 % and thus lowering operational costs [60]. As a consequence of photosynthesis, microalgae generate oxygen, raising the dissolved oxygen content in wastewater. Aerobic bacteria in the wastewater benefit from this oxygenation since they need oxygen to properly metabolize and break down organic contaminants [61,62]. From an economic perspective, the use of microalgae in wastewater treatment can lower the process's overall cost because oxygen aeration accounts for more than half of a wastewater treatment process’ energy requirements [63]. Additionally, microalgae assimilate excess nitrogen and phosphorus, reducing the need for costly chemical treatments and generating nutrient-rich biomass that can be repurposed as biofertilizer, creating added value. However, algae systems are generally slower, often taking several days to weeks compared to the hours or days required by conventional systems, as algae growth depends on environmental factors like light and temperature [63]. Despite these temporal limitations, microalgae systems provide a natural and sustainable treatment option that not only reduces costs but also aligns with environmental goals by reducing chemical dependency and promoting resource recovery. With further optimization and integration of photobioreactors, microalgae-based treatment could potentially rival conventional systems in efficiency while offering enhanced sustainability benefits.
While microalgae can thrive in wastewater, the nutrient levels often fall short of the ideal requirements for optimal algal growth in phycoremediation. To address this, various techniques are employed either individually or in combination. The first approach involves utilizing specific algae species trained to adapt to the characteristics of the wastewater, while the second method entails modifying the wastewater to create an environment conducive to algae growth [64]. Furthermore, controlling environmental variables can enhance wastewater treatment and promote algae growth. Algae typically employ three different modes of carbon assimilation for biomass synthesis: autotrophic, mixotrophic, and heterotrophic (Fig. 2). However, the low biomass concentration in photoautotrophic systems necessitates lengthy cultivation periods. Heterotrophic culture may face challenges in environments lacking organic carbon, as microalgae predominantly favor autotrophic nutrition, with organic compounds serving as the sole source of carbon and energy in dark conditions. Both light-dependent (photo-heterotrophic) and light-independent (heterotrophic) modes of nutrition can occur, leading to stagnant algae growth. Mixotrophic nutrition, a hybrid of autotrophic and heterotrophic systems where carbon dioxide and organic substances are assimilated simultaneously, exhibits lower photo-inhibition and higher growth rates compared to autotrophic and heterotrophic cultures, making it widely utilized in microalgae-based bioremediation [65].
Fig. 2.
Schematic of wastewater remedied by microalgae and its potential valorisation.
Two primary approaches are employed to cultivate and manage the factors influencing microalgae growth: the open approach, utilizing open ponds, and the closed approach. Open pond systems are commonly utilized for large-scale algae cultivation, utilizing either man-made ponds or natural basins such as lakes, ponds, and lagoons. Within open pond systems, there are distinctions between stirred and non-stirred ponds. While non-stirred ponds are cost-effective and simpler to maintain, they are more susceptible to issues like disease growth, mixed algal populations, and predation by zooplankton. Conversely, closed systems have been developed to enhance control over variables influencing algae growth efficiency, employing technologies such as tubular, flat panel, and plastic (polyethylene) bag photobioreactors. Both natural sunlight and artificial light can be utilized in photobioreactors, which are designed to overcome biological and practical limitations of open systems by employing transparent tubes, containers, or sleeves. Currently, the tubular system stands as the most commonly utilized method for wastewater treatment based on microalgae. While closed systems offer enhanced control over abiotic variables, thus optimizing the phycoremediation process, they are not well-suited for large-scale wastewater treatment due to their complex operation, high construction costs, and challenges with scalability.
In addition to carbon uptake and wastewater treatment systems, several variables significantly impact microalgae bioremediation. Light intensity plays a crucial role, as it affects algae growth and modifies nutrient utilization within the waterbody. Wastewater turbidity reduces light penetration, necessitating careful consideration of light irradiance. Temperature fluctuations influence algae growth, with the ideal temperature typically between 15 and 30°C; colder climates may induce photoinhibition due to cold stress, while hotter temperatures can lead to severe evaporation. pH levels rise during wastewater treatment due to photosynthetic CO2 buildup, potentially causing microalgae flocculation; optimal pH for algal development falls between 7 and 9. CO2 availability, primarily sourced from bacteria breaking down nutrients, enhances algae biomass; an extra CO2 supply in water, with an air mixture of 1–5 % CO2, increases algae biomass within the optimal concentration range. Nutrient availability is crucial; although wastewater contains organic carbon, nitrogen, phosphorus, and other components, it typically lacks sufficient CNP compared to the Redfield Ratio of 106:16:1, the molar ratio for C:N:P in microalgae's internal composition.
5. Dual role of algae as phycoremediator and biofertilizer
Phycoremediation, also known as algae treatment, is deemed to be more economical and highly efficient in reducing nutrients and heavy metals [66]. Utilizing photoautotrophic microorganisms is considered environmentally benign and preferred as long as the generated biomass is recycled [65]. Additionally, due to their capacity for rapid growth, ability to be cultivated on non-arable land, low water needs, and reduced land requirements, algae are among the most significant bioresources currently enjoying immense popularity.
Algae serve as significant carbon dioxide sinks, effectively reducing the carbon footprint, which encompasses pollution, global warming, and greenhouse gas impacts resulting from atmospheric carbon dioxide [67]. Due to their widespread distribution and remarkable adaptation to various environments, algae are broadly categorized as micro and macroalgae—all commonly referred to as seaweeds. Microalgae, boasting an estimated 200,000–800,000 species globally, exhibit abundant biodiversity and are exceptional candidates for wastewater treatment and CO2 sequestration due to their remarkable adaptogenic capacity, enabling them to thrive in diverse settings. Their basic cellular structure allows algae to utilize CO2, water, and nutrients more efficiently than terrestrial plants [68]. Acknowledgment of algal biomass culture in wastewater dates back to 1957, coinciding with the recognition of algae's potential to produce additional products such as biofuels, food, cosmetics, feed, and biofertilizer [69,70,71,72]. Species like Nannochloropsis, Dunaliella, Chlorella, Scenedesmus, Tetraselmis, and many others are utilized for phycoremediation integrated with CO2 capture [66,73,74]. Additionally, macroalgae such as Gracilaria lemaneiformis, Padina australis, Sargassum hemiphyllum, and Ulva lactuca have been investigated for remediation purposes [75,76,77,78]. Given their widespread presence in soil and water environments and their adaptable metabolism, algae have demonstrated exceptional capacities for bioaccumulation and biosorption [79]. Several species of macroalgae (Ascophyllum nodosum, Ulva lactuca, and Caulerpa sertularioides) and microalgae (Chlorella minutissima, Chlorella vulgaris, Scenedesmus obliquus, Isochrysis galbana, Nannocloropsis salina, and Spirulina major) growing in wastewater have been modified to produce significant amounts of biofertilizers [76]. Micronutrients, plant hormones (such as auxins, cytokinins, and gibberellins), amino acids, and polysaccharides are found in macroalgae, which can aid in promoting plant growth, enhancing resistance to stress, and enhancing soil quality [80,81]. As for microalgae, they can release exopolysaccharides (EPS), which act as a carbon source and carbon sequestrant, improving soil stabilization and aggregation [82].
The dual role of algae as a phycoremediator for wastewater and as a biofertilizer in agroecosystems offers a sustainable solution to environmental and agricultural challenges. Algae have shown remarkable efficiency in removing contaminants from wastewater, including heavy metals, excess nutrients (such as nitrogen and phosphorus), and organic pollutants [83]. This remediation process is highly beneficial in reducing water pollution and preventing eutrophication in natural water bodies. For instance, studies have found that specific strains, like Chlorella vulgaris, Spirulina platensis, Ulva lactuca, Sargassum fusiforme demonstrate substantial nutrient uptake abilities, particularly in nitrogen and phosphorus removal, making them effective in treating municipal and industrial wastewater [75,84,85]. By absorbing these nutrients, algae biomass not only purifies water but also accumulates essential elements that enhance its nutrient profile, which, in turn, can be applied as a biofertilizer in agriculture [77]. Beyond wastewater treatment, the nutrient-enriched biomass of algae has demonstrated potential as an eco-friendly biofertilizer. Rich in nitrogen, phosphorus, potassium, and trace minerals, this biomass can improve soil fertility, support plant growth, and enhance crop yield without the environmental drawbacks associated with synthetic fertilizers [86]. According to a study, fertilizing green beans with dried algae increased their overall biomass and chlorophyll content [80]. [87] mentioned that algae are known to possess betaine-like chemicals that can lessen the effects of drought and salt. Algae extract's hormone content, which includes gibberellins, auxins, betaine, and cytokinins, has additional positive effects on plant growth [88]. Moreover, algae biomass can improve soil structure and microbial activity, thereby promoting a healthy agroecosystem. Studies indicate that microalgae-based biofertilizers can enhance root biomass and plant growth due to their high content of bioactive compounds, including growth-promoting hormones like auxins and cytokinins [21]. Algae can release nitrate, ammonium, total nitrogen, and phosphorus into the soil while also generating Na+, Ca2+, Mg2+, and K+ cations to create richer nutrient aggregates, enhance soil structure, and eventually boost soil microorganism activity. According to earlier studies, applying 25 kg/ha of algae can raise the soil's nutritional content of N, P, K, Ca, Mg, S, and micronutrients [89].
The advancement of algae-based technologies holds significant importance for both wastewater treatment and the utilization of algae as a natural fertilizer in agriculture. The integration of algae offers numerous benefits, including: (i) the utilization of algae extracts as non-toxic, non-polluting, non-hazardous, and biologically degradable substances; (ii) the production of large quantities of secondary metabolites by algae, such as terpenes, lipid-, steroid-, and aromatic-like compounds, acetogenins, phlorotannin, amino acid-derived products, and other polymeric substances; (iii) the positive effects of algae on plant growth and development, including the promotion of seed germination, root development, increased nutrient uptake, and enhanced frost resistance in unfavorable conditions; (iv) the extensive use of algae to improve soil quality and provide minerals for plants; and (v) the high potential of algae for biosorption due to their composition, which facilitates the formation of biosorbent particles as heavy metal adsorbents. Phycoremediation enhances the avoidance of harmful byproducts, making it highly encouraging from an agricultural and industrial standpoint. This dual focus underscores the broad dedication of environmental science to resource optimization and sustainability. The applications of phycoremediation encompass the reduction of excess nutrients from effluents containing organic material, the removal of nutrients and xenobiotic substances through algal-biosorbent biosorption, the processing of effluents containing heavy metal ions, the mitigation of CO2, the monitoring of potentially toxic substances using algae as biosensors, and the production of high-added value products [90]. This dual application not only addresses the challenge of wastewater management but also creates a circular economy model by converting waste into value-added products for agriculture. Table 2 provides a comprehensive list of microalgae strains extensively utilized in waste bioremediation, capable of producing biofertilizers and other byproducts while effectively reducing pollutants across various wastewater types.
Table 2.
Potency of microalgae as biofertilizer and the impact on cultures.
| Microalgae | Medium | Application | Culture | Wastewater | Results | Reference |
|---|---|---|---|---|---|---|
| Chlorella vulgaris UTEX-2714 | Swine farm wastewater | Liquid fertilizer mixed with broth and effluent | Arabidopsis thaliana | 13.8 mg/L.d of Total Nitrogen TN; 11.5 mg/L·d of Ammonia Nitrogen (-N); 24.8 mg/L·d of COD and 16.9 mg/L d of TP. | Arabidopsis roots and stems were 43.0 % and 55.0 % longer than the control group. Leaf count and maximum leaf length increased by 30.2 % and 39.7 %, while fresh and dry leaf weights rose by 44.0 % and 33.7 %. | [122] |
| Scenedesmus | Municipal wastewater | Microalgae biofertilizer mixed with a conventional mineral fertilizer | Lettuce (Lactuca sativa L. cv Maravilla) | The microalgal biomass met European regulations for fertilizing products regarding pathogens and heavy metal concentrations, except for cadmium | Microalgae can reduce the need for mineral nitrogen, as plants grown with various fertilizers showed similar fresh shoot weights. Lettuce samples from all treatments contained cadmium and CECs. | [123] |
| Chlorella sp., Scenedesmus sp. | NA | Dry biomass | Cauliflower (Brassica oleracea L. var. botrytis L.) | NA | Microalgae and compost mixture is an alternative fertilizer, equivalent to chemical fertilizers and manure, presenting the advantages of being more stable, sustainable, and environmentally friendly. | [124] |
| Mix culture (Aphanotece sp. and Aphanocapsa sp., Chlorella sp., Scenedesmus sp., Chlamydomonas sp.) | Sewage wastewater | Dry biomass | Amaranth (Amaranthus cruentus) | NA | Increased application of biosolid, wet, and dry microalgae significantly boosted the shoot biomass of amaranth, with dry microalgae being the most effective. | [125] |
| Chlorella sp., Spirulina sp. | Secondary treated wastewater effluent | Dry biomass | NA | NA | A mixture of 100 % Chlorella sp. combined with 50 % Spirulina sp., cultivated with a 70 % dilution of secondary treated wastewater effluent, yielded a biofertilizer containing 0.6 wt % phosphorus (P) and 0.3 wt % potassium (K) | [126] |
| Chlorella, Scenedesmus, Pediastrum | Prototype wastewater treatment plant | Dry biomass | Wheat | NA | Microalgae sourced from wastewater treatments serve as an appropriate organic fertilizer for wheat plants, with only moderate decreases in nitrogen use efficiency compared to mineral fertilizers. | [127] |
| Asterarcys quadricellulare (CCAP 294/1) | NA | Liquid fertilizer sprayed on plants | Potato | NA | Spraying microalgae biomass increased potato yield and induced biochemical changes that enhance chlorophyll, amino acid, sugar, and nitrate reductase enzyme activity. | [128] |
| Chlorella vulgaris | NA | Foliar spray and soil drench | Tomato | NA | C. vulgaris applied through soil drenching and combined with cow dung resulted in different fruit sizes and seed quantities. With soil drenching, fruit lengths were around 8.8 ± 0.36 cm, while combining it with cow dung increased lengths to about 10.5 ± 0.61 cm and 11.7 ± 0.35 cm. Fruit diameters ranged from approximately 13.3 ± 0.73 cm to 16.7 ± 0.31 cm. The number of seeds per fruit varied from about 89 ± 0.45 g to 153 ± 0.96 g, and seed weights per fruit ranged from approximately 4.5 ± 0.14 g to 6.5 ± 0.28 g. | [129] |
| Scenedesmus | Real domestic wastewater | Microalgae biomass application to the soil mixed with inorganic fertilizer | Basil (Ocimum basilicum L.) | 69, 91 COD and 81 % total inorganic nitrogen (TIN) and phosphates (PO43–-P) removal | Plant growth, including leaf count, shoot fresh and dry weight, and leaf fresh weight, significantly increased by 27 % in the microalgae fertilizer treatments. However, these treatments resulted in the lowest leaf content of chlorophyll, nitrogen (N), and potassium (K). | [130] |
| Acutodesmus obliquus | Poultry litter extract effluent | Lipid extracted biomass | Mung bean | NO3-N, NH3-N, and PO4-P removal of 79.51 %, 81.82 %, and 80.52 % | In comparison to the chemical fertilizer (CF) control, soil amended with LEA showed a significant increase in organic carbon (59.5 %) and dehydrogenase activity (130.8 %) after 30 days. | [131] |
| Chlorella vulgaris | Sewage wastewater | Dry biomass | Solanum lycopersicum (tomato) | COD and BOD were reduced up to 93 %, 95 % and 92 % respectively. | Tomato plants cultivated using treated sewage wastewater exhibited robust growth and productivity comparable to those aided by chemical fertilizer. | [132] |
| Chlorella minutissima | Sewage effluent | Dry biomass | Spinach and baby corn | >90 % TDS and EC removal efficiency | Adding microalgae biomass to the soil increased nitrogen and phosphorus levels by 75 % and 5 %, respectively, compared to the control. | [133] |
| Chlorella vulgaris, Scenedesmus obliquus, Isochrysis galbana, Nannocloropsis salina, and Spirulina major. | Aquaculture effluent | Biomass solution | Wheat and watercress | 100 % removal TN and TP for all microalgae. | The addition of C. vulgaris led to an increase in the germination index by up to 275 % for watercress and 185 % for wheat. | [134] |
| Chlorella protothecoides, Chlorella vulgaris, Scenedesmus obliquus | Cattle manure | Microalga suspensions and aqueous extracts (liquid) | Wheat and watercress | 94 % COD and N 97 % removal efficiency | Chlorella protothecoides resulted in a 177 % increase in the germination index for wheat, while Tetradesmus obliquus led to a 34 % increase for watercress. | [135] |
| Chlorella | Pre-treated municipal wastewater enriched with selenium | Microalga extract (liquid) Microalga biomass (solid) | Bean | 93 % NH4+, 77 % TP, and 70 % CODt removal efficiency | Lower selenium concentrations (0.5 to 5 %) favored germination rates, with higher rates observed in foliar spraying and soil irrigation using extracts enriched with 1 % selenium. Additionally, the application of biomass increased both the dry and fresh weight of bean plants. The biomass acted as a biofertilizer, slowly releasing nutrients such as nitrogen, phosphorus, and selenium. | [136] |
| Microalgae consortium | Primary effluent from meat processing industry treatment plant | Granules containing 12 % dry biomass incorporated into the chemical superphosphate triple fertilizer | Corn | NA | The shoot dry weight increased by 10.6 % compared to the control. | [137] |
| Chlorella, Scenedesmus, Spirulina, Synechocystis | Residual water from residential treatment plant enriched with human urine | Microalgae extracts (10 g of dried microalgal biomass dissolved in 100 ml deionized water) applied by spraying (liquid) | Tomato | NA | 46 % increase in plant height and chlorophyll content compared to control in 3 days | [119] |
| Desmodesmus, Pseudopediastrum, Tetradesmus, and Chlorella | NA | Supernatant from microalgae culture after centrifugation (liquid) | Soybean | NA | Microalga application outperformed the control. | [138] |
| Chorococcum sp. | NA | Dry microalgae powder mixed with distilled water (liquid) | Beans, tomato, cucumber, and pepper | NA | Compared to the control, root length increased by 78.9 % for beans, 150 % for cucumber, 195 % for pepper, and 56.3 % for tomato. Additionally, the number of roots increased by 52.5 % for beans, 103.3 % for cucumber, 121 % for pepper, and 181 % for tomato. | [139] | |
| Chlorella vulgaris | NA | Extracts of microalgal biomass (supernatant from centrifugation of microalga-melted slurry) applied by spraying (liquid) | Vigna mungo | NA | Compared to the control, plant height increased by up to 18.7 %, stem length by 34.5 %, root length by 33.3 %, fresh weight by 28.1 %, dry weight by 56.8 %, seeds per plant by 296 %, and root nodule by 127.9 %. | [140] |
| Scenedesmus | Domestic wastewater | Microalgae biomass application to the soil | Rice | NA | Increased soil availability of NPK up to 28 %, 39 %, and 33 %, respectively, compared to chemical fertilizer | [141] |
| Chlorella sp., Scenedesmus sp. | Anaerobic digestate derived from food waste that has been filtered | Dry biomass | Common pasture ryegrass | NA | Increase in shoot height (2.6 %), root height (20.9 %), root fresh weight (31.7 %), and root dry weight (52.4 %) occurred in relation to chemical fertilizer. Additionally, significant bacterial richness increase was observed in rhizospheric soil treated with dry biomass compared to the control. | [142] |
| Desmodesmus subspicatus | Wastewater from sugar cane processing | Dry compost application to the soil | NA | NA | Pectin with microalgae and vinasse increased 136 % nitrogen content compared to pectin alone | [143] |
| Chlorella, Scenedesmus, Tetraselmis, Nannochlorops | Primary wastewater from the municipal wastewater treatment plant; seawater | Dry biomass application in the soil (solid) | Wheat | >80 % TN recovered by Chlorella and Scenedesmus | Plant height increased by up to 101 %, number of leaves by up to 233 %, and leaf length by up to 142 % compared to the control. | [144] |
| Scenedesmus obliquus | Brewery wastewater or Bristol medium | Irrigation with medium plus microalgae | Wheat and barley | Removal of 88 % N, 30 % P and 71 % COD | 100 % increase in germination compared to control | [145] |
| Chlorella | Uninformed | Dilution of microalgae biomass with water (liquid) | | NA | NA | Increased soil aggregate stability compared to control | [146] |
| Chlorella vulgaris, Chlorophyceae, Oscillatoria sp. | Primary effluent from the meat processing industry | | Application of dry biomass to the soil (solid) | Millet | NA | 44 % increase in plant growth compared to control and 30 % increase in the number of sheets | [147] | |
| Ulothrix, Klebsormidium | Aquaculture wastewater | Application of dry biomass to the soil (solid) | | Tomato | NA | After 95 days, the nitrogen content ranged from 25 % to 31 %. Compared to inorganic fertilizer, leaf length increased by 5 %, sheet weight by 13 %, dry weight by 14 %, and the concentration of nitrogen and phosphorus in the plant increased by 78 % and 88 % respectively. | [148] |
| Chlorella, Scenedesmus, Chlorococcum, Chroococcus | Municipal wastewater | Application of dry biomass to the soil with vermiculite (solid) | Wheat | NA | Plant dry weight increased by 33 %, increases of 15 %, 122 %, and 23 % in NPK levels in the soil compared to chemical fertilizer. | [149] |
| Acutodesmus dimorphus | NA | Seeds soaked with extracts of microalgae diluted in water (liquid); leaf spraying microalgae extracts diluted in water (liquid); application of dry biomass to the soil (solid) | | Tomato | NA | Germination occurred up to 2 days earlier than the control with liquid biofertilizer. Increase of up to 390 % in flower buds compared to the foliar spray control, along with a 33 % increase in shoot length. | [150] |
6. Microalgae as biostimulant
The term biostimulant, commonly defined as a substance fostering plant development and growth, comprises four primary groups: humic substances, products containing amino acids, microbial inoculants, and microalgal extracts [91,92]. These biostimulants influence plant physiology, enhancing crop yield and vigor while bolstering resistance to both abiotic and biotic stresses. Microalgal extracts, a subset of biostimulants, are obtained by disrupting algal biomass cells, achievable through various techniques [93]. These disruption methods release bioactive compounds and biostimulants, including proteins, amino acids, plant hormones, and antimicrobial agents [94]. Physical, mechanical, chemical, or enzymatic techniques can all be used to cause this disruption [95]. The target molecules and the type of biomass mostly determine the extraction method to be used. For instance, the physical or mechanical techniques that are most frequently employed in research today, such as mechanically breaking down cell walls or using high pressure, high temperature, ultrasound, or a combination of these, cannot ensure high extraction yields for macro- and micro-algae, which may have thicker cell walls than cyanobacteria. Cell disruption may be followed by a phase of extraction using solvents or via chemical assistance to acquire particular fractions of the crude extract. For example, after the physical disintegration of cells, polysaccharides are typically precipitated with ethanol in the process of creating biostimulant polysaccharide extracts. Supercritical CO2 is a relatively new extraction method that preserves thermolabile bioactive compounds in biomass by using it as a solvent. This solvent has chemical and physical characteristics that are halfway between those of a liquid and a gas, and it is obtained at 50°C and 200–500 bars (Ronga et al. 2019). In the meanwhile, enzymatic techniques generate protein hydrolysates by using proteolytic enzymes that cleave peptide bonds and/or single enzymes that can break down cell walls producing products rich in free amino acids and soluble peptides [96].
Amino acids play crucial roles in plant physiology, contributing to processes such as chlorophyll synthesis, essential for promoting plant growth [94]. Additionally, amino acids contribute to the biosynthesis of other growth-promoting compounds like polyamines (PAs) and phenolic compounds, enhancing plant defense mechanisms [97]. Essential and non-essential amino acids extracted from microalgae such as Spirulina sp. encompass a wide range of compounds vital for plant metabolism and growth [98]. These amino acids undergo various reactions, including deamination and decarboxylation, to form ammonia, organic acids, and other compounds essential for plant nutrition. Overall, the utilization of microalgal-derived proteins and amino acids presents promising opportunities for enhancing plant productivity and sustainability in agriculture.
6.1. Microalgae as phytohormones
Microalgal biostimulants often contain phytohormones or exhibit similar hormonal activity. Phytohormones, such as gibberellins, auxins, and cytokinins, play crucial roles in various aspects of plant growth and development [99]. Microalgae have been found to produce a range of phytohormones, including cytokinins, gibberellins, abscisic acid, and jasmonic acid, alongside auxins like indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), and indole-3-propionic acid (IPA) [100]. These phytohormones can enhance plant morphological characteristics, chlorophyll levels, and protein content when introduced to plants via seed or root incubation, or through application to leaves or seeds [101].
Furthermore, microalgal extracts and biomass hydrolysates have been shown to contain various phytohormones, such as cytokinins, gibberellins, IAA, abscisic acid, salicylic acid, and jasmonic acid, which can stimulate plant growth [102]. Different species of microalgae may exhibit variations in their phytohormone content and effectiveness as biostimulants, influenced by factors like culture medium composition [101]. Additionally, microalgal biostimulants may contain PAs that promote plant growth by enhancing protein synthesis and enzymatic activities [103]. The presence of fatty acids in microalgae further contributes to biostimulation by serving as precursors for phytohormone synthesis and facilitating plant defence mechanisms [104]. These insights underscore the multifaceted potential of microalgal biostimulants in enhancing agricultural productivity and sustainability.
6.2. Microalgae as soil remediation
Microalgae-derived exopolysaccharides (EPSs) present promising prospects for soil remediation, offering multifaceted benefits. For instance, EPSs extracted from cyanobacteria exhibit potential in enhancing soil quality by stimulating plant growth and mitigating abiotic stress, particularly salt stress [64]. When applied foliarly, EPSs derived from microalgae effectively promote the growth of tomato plants, resulting in increased shoot height, dry weight, chlorophyll, and protein content, while simultaneously reducing stress indicators like proline and phenolic compounds under salt stress conditions [46]. Previous research also assessed the salt stress brought on by the presence of NaCl was lessened in tomato plants (Solanum lycopersicum) when EPS from Dunaliella salina was applied [105]. The decreased levels of salt stress markers, including proline, phenolic compounds, and antioxidant enzymes, were indicative of this decline. Additionally, the treatment boosted the dry weight and length of the shoots as well as the dry weight of the roots by improving soil quality.
The utilization of microalgal EPSs can impact enzymatic activity within plant leaves, including key enzymes associated with nitrogen metabolism and stress responses, thereby contributing to overall soil health and fertility [106]. Moreover, microalgal EPSs are instrumental in augmenting the profile of phytosterols and alkanes in plants, crucial components for various physiological processes such as cell division, elongation, and cuticle formation [107].
This diverse functionality underscores the potential of EPSs as eco-friendly agents for soil remediation and sustainable agricultural practices. Additionally, microalgal EPSs display notable antifungal activity against various bacterial and fungal strains, rendering them valuable assets for plant disease management, particularly when cultivated in environments like domestic wastewater [108,109]. In summary, the application of EPSs derived from microalgae presents a promising avenue for soil remediation, offering sustainable solutions for enhancing soil fertility, alleviating stress, and combating plant diseases.
6.3. Microalgae as biofertilizer
In modern agriculture, harnessing the biofertilizing potential of microalgae signifies a forward-thinking approach with profound implications. Biofertilizers derived from microalgae offer a sustainable alternative to conventional chemical fertilizers, addressing growing concerns regarding soil degradation and the overuse of synthetic nutrients. The mechanism through which microalgae, including cyanobacteria, act as biofertilizers involves a symbiotic relationship between these microscopic organisms and plants [110]. By processes such as nitrogen fixation and nutrient mobilization, microalgae enhance soil fertility and nutrient availability, fostering ideal conditions for plant growth. Previous research has demonstrated the efficacy of microalgae-derived biofertilizers in enhancing crop yields while simultaneously mitigating the environmental impact associated with traditional fertilization methods [111].
The adoption of microalgae as a biofertilizer in contemporary agriculture reveals compelling advantages over chemical fertilizers and traditional composting methods. Unlike chemical fertilizers, microalgae biofertilizers establish symbiotic relationships with plants, efficiently releasing nutrients and offering a more precise nutrient supply. This reduces the risk of nutrient runoff and its associated environmental consequences [112]. Moreover, microalgae biofertilizers boast a versatile nutrient composition, enabling a tailored approach to meet specific crop needs, a flexibility absent in conventional fertilizers. In contrast to compost, microalgae biofertilizers offer a quicker and customizable nutrient release, addressing the limitations of slower organic matter decomposition.
In addition to these benefits, the utilization of microalgae biofertilizers seamlessly aligns with sustainability principles. As previously demonstrated, the effective nutrient uptake capacity of microalgae, particularly from wastewater sources, underscores their role in nutrient recycling and mitigating environmental pollutants [113]. This capacity not only promotes sustainable agricultural practices but also aids in reducing the environmental impact of wastewater discharge. Furthermore, the ability of microalgae to capture carbon dioxide (CCUS) during cultivation further solidifies their sustainability credentials [114]. Through photosynthesis, microalgae sequester carbon dioxide, contributing to the mitigation of greenhouse gas emissions. This dual capability of nutrient recycling and carbon capture emphasizes the comprehensive sustainability of microalgae biofertilizers within the agricultural framework (Fig. 3).
Fig. 3.
Schematic integration of microalgae with carbon capture, wastewater phycoremediation, and biofertilizer utilization mechanism.
7. Microalgal biofertilizers/biostimulants: production and efficacy
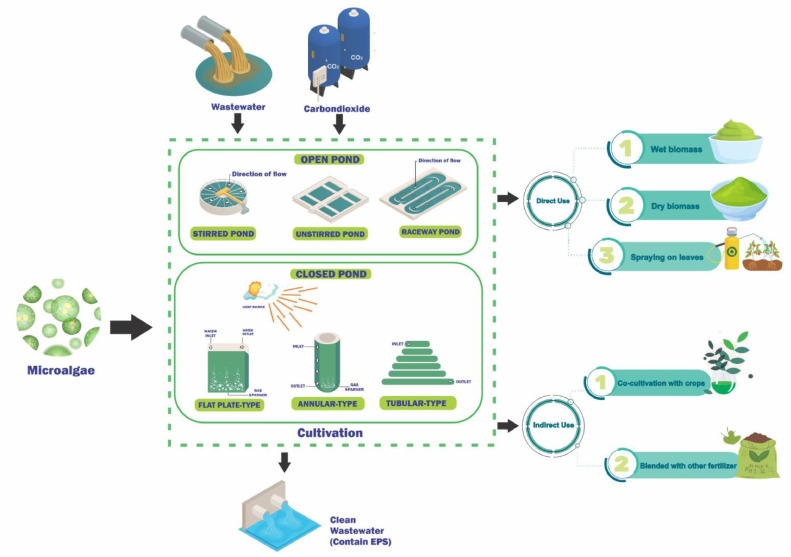
Microalgal biomass, celebrated for its rich content of proteins, carbohydrates, and lipids, emerges as a promising candidate for the next generation of biomass across various industries, offering not only waste utilization but also a spectrum of valuable bioproducts [115,116]. Beyond its role as biofertilizers, this biomass serves as a fundamental resource for producing biofuels, amino acids, alginate, pigments, and bioplastics. To illuminate the utilization of microalgal biomass in biofertilizer production and underscore prevalent methods, Table 2 provides a comprehensive overview of relevant studies, detailing microalgae types, cultivation techniques, production protocols, and their beneficial effects on plants. Encompassing the entire process, from microalgae cultivation to biofertilizer production, Fig. 3 offers comprehensive insight. The utilization of microalgae as biofertilizer encompasses various methodologies, such as direct application of wet biomass onto soil or crops, spraying extracts onto leaves, utilizing dry biomass, co-cultivation with crops like cyanobacteria on rice plants, and blending with conventional or other biofertilizers, each yielding diverse benefits including immediate nutrient supply, enhanced nutrient uptake, bolstered plant resilience, and minimized environmental impact. By harnessing the nutritional prowess of microalgae and their capacity to enhance soil health, these strategies present innovative solutions for sustainable agriculture.
As depicted in Table 2, an extensive range of microalgae species has been examined for their potential contribution to biofertilizer production. These investigations underscore the efficacy of microalgae biomass when applied to soil, effectively stimulating crop growth [[117], [118], [119]]. Primarily, freshwater microalgae like Chlorella, Scenedesmus, and Spirulina have been the focus of research, although some studies have also focused into marine microalgae such as Tetraselmis and Nannochloropsis [120,121]. The selection of microorganisms is influenced by diverse factors, including geographical considerations, with marine microalgae being less common in inland areas [119].
The cultivation of microalgae can make use of a wide range of media, with wastewater holding particular relevance due to its environmental implications [98]. Apart from its role in wastewater treatment, microalgae have the capacity to absorb the nutrients, rendering them valuable for biofertilizer production [115]. The production of biofertilizers includes the direct application of dry microalgal biomass onto soil or its dilution in water to create liquid fertilizers [[151], [152], [153]]. Dry biomass, especially, possesses desirable biofertilizer attributes, containing crucial proteins, amino acids, and phytohormones [154].
The utilization of microalgal extracts, whether applied through leaf spraying or seed treatment, has demonstrated positive effects on plant germination and growth, serving as a slow-release biofertilizer [155]. Moreover, incorporating microalgal biomass into a polymeric matrix can extend nutrient release, thereby reducing application rates. Microalgal biostimulants indirectly promote plant growth by stimulating soil biological activity and biochemical soil fertility indicators [106]. For instance, a study involving Scenedesmus subspicatus combined with humic acid resulted in increased onion yield by enhancing protein and sugar accumulation in bulbs [156]. Similarly, immersion in Spirulina platensis suspension affected radish plants, with outcomes varying based on immersion duration [157].
Alternative methods for applying biofertilizers include utilizing biomass biochar, hydroponic cultivation in microalgal growth medium, and applying microalgal hydrolysate. Promising results have been observed with foliar application of Scendesmus hydrolysates and Spirulina platensis hydrolysates, enhancing plant growth and mitigating soil salinity effects, respectively [158]. To enhance the production of microalgae biofertilizers/biostimulants, factors such as application rates and cultivar development requirements need to be considered [159].
8. Opportunities and challenges of microalgae as biofertilizer
Microalgae offer a promising pathway for sustainable agriculture, surpassing traditional chemical fertilizers and other biofertilizers with distinct advantages. Firstly, they provide a sustainable alternative to chemical fertilizers, addressing concerns about environmental degradation and ecosystem disruption. Harnessing the nutrient-rich biomass of microalgae facilitates a shift towards eco-friendly agricultural practices while enhancing crop productivity. Additionally, microalgae possess inherent biostimulant properties that extend beyond nutrient provision. These bioactive compounds play a multifaceted role in stimulating plant growth, improving stress tolerance, and enhancing overall crop resilience. Unlike conventional chemical fertilizers, which mainly focus on nutrient supplementation, microalgae biofertilizers offer holistic benefits, nurturing healthier plants and soil ecosystems. Moreover, the adaptable nature of microalgae cultivation allows for customized formulations tailored to specific crop and soil needs. By adjusting nutrient profiles and biostimulant blends, microalgae biofertilizers can optimize nutrient uptake, improve soil structure, and enhance agricultural sustainability. This versatility enables targeted solutions to diverse agricultural challenges across different regions and cropping systems. Lastly, microalgae exhibit promising traits like biological nitrogen fixation and climate resilience, making them well-suited for sustainable agriculture in various environmental conditions. Their ability to thrive in marginal lands, including arid and saline environments, opens new avenues for expanding agricultural production while minimizing environmental impacts.
The microalgae industry faces numerous hurdles that impede the widespread adoption of microalgae as biofertilizers. These challenges encompass high production costs, scalability limitations, and issues related to nutrient availability. Moreover, regulatory complexities and market acceptance barriers hinder the commercialization of microalgae-based products across many regions. Furthermore, the absence of standardized regulations and quality control measures for microalgae-based biofertilizers creates uncertainty and impedes market acceptance. Overcoming these obstacles necessitates ensuring a consistent and reliable supply of high-quality biomass, alongside addressing logistical hurdles in establishing a robust supply chain. Additionally, maintaining product quality and performance consistency remains a persistent challenge due to variations in biomass composition, nutrient content, and biostimulant activity, complicating farmers' reliance on these products for consistent crop yields.
One potential solution to tackle these challenges involves directly integrating microalgae cultivation with agricultural wastewater treatment processes. By leveraging microalgae for wastewater treatment, nutrients from the wastewater can be efficiently captured and converted into biomass, diminishing the need for external nutrient inputs and alleviating scalability constraints. Moreover, integrating microalgae cultivation with wastewater treatment can reduce the necessity for long-term storage and transportation of biomass, mitigating the risk of denaturation and ensuring product freshness and efficacy. This approach not only enhances the sustainability of microalgae production but also offers a direct and cost-effective means of supplying biofertilizers to agricultural systems. Furthermore, utilizing microalgae biomass directly as biofertilizer provides farmers with a continuous and readily available nutrient source, fostering soil health and enhancing crop productivity in a sustainable manner. In India, the use of cyanobacteria as biofertilizers in paddy fields has shown promising results, particularly in enhancing rice yield and reducing dependence on chemical fertilizers [160]. In a study conducted in an organic rice field in West Java, Indonesia, the application of nitrogen-fixing cyanobacteria, such as Anabaena and Nostoc species, contributed significantly to soil fertility by enhancing nitrogen availability [161].
9. Future prospect
Looking ahead, microalgae emerge as promising biofertilizers, presenting sustainable solutions to pressing environmental concerns and advancing agricultural productivity. Embracing the potential of microalgae-based biofertilizers enables a transition toward a circular economy model, where waste materials are repurposed into valuable resources. Microalgae cultivation offers opportunities for wastewater remediation and carbon emissions mitigation through CCUS technologies. Integrating microalgae cultivation with wastewater treatment not only enhances water quality but also yields nutrient-rich biomass suitable for biofertilizers, closing nutrient cycles and reducing reliance on synthetic fertilizers. Amid escalating efforts to combat climate change, microalgae-based biofertilizers play a dual role in sequestering carbon dioxide from the atmosphere while enhancing soil health and bolstering crop resilience. Through interdisciplinary collaboration and strategic investment in research and infrastructure, we can unlock the full potential of microalgae as biofertilizers, charting a path towards a sustainable and resilient agricultural future.
Microalgae offer a unique opportunity with their dual role in enhancing soil fertility and wastewater treatment. These microorganisms are rich sources of antimicrobial agents, proteins, amino acids, and plant hormones, which play crucial roles in plant physiology, such as chlorophyll synthesis essential for promoting plant growth. Additionally, microalgae-derived amino acids can undergo various reactions, including deamination and decarboxylation, to produce organic acids and ammonia vital for plant nutrition. Alongside auxins like IAA, IBA, and IPA, microalgae have been reported to produce phytohormones like cytokinins, gibberellins, abscisic acid, and jasmonic acid, which, when applied to plants, can enhance morphological features, protein content, and chlorophyll levels. Furthermore, the composition of microalgae's EPSs plays a role in soil improvement and remediation, particularly when grown in nutrient-rich wastewater with absorbed CO2 gas, resulting in the production of natural fertilizers. Application of microalgae biomass as biofertilizers can be direct (by adding dry or wet biomass to soil or misting plants with microalgae liquid) or indirect (by growing plants in microalgae medium or combining microalgae with commercial fertilizer).
Integrating microalgae cultivation with wastewater treatment reduces the need for long-term storage and transportation of biomass, minimizing the risk of denaturation and ensuring product freshness and efficacy. Previous researchers demonstrated that Chlorella variabilis and Scenedesmus obliquus, grown on dairy wastewater, demonstrated high efficacy as biofertilizers for crops like corn and soybean. When applied in concentrations of 40 % and 60 %, these microalgal extracts significantly boosted plant growth, antioxidant activity, and mineral content compared to untreated controls, thus improving crop productivity without the need for additional storage or transport of the biomass. This approach not only enhances crop yields but also minimizes logistic constraints associated with biofertilizer production [162]. Similarly, a study on Chlorella vulgaris used for sewage wastewater treatment showed promising results in nutrient removal and biomass production. This microalga effectively reduced nitrates, chemical oxygen demand (COD), and biological oxygen demand (BOD) by over 90 %, generating biomass suitable for biofertilization. The treated wastewater, when applied to tomato plants, provided yields comparable to those achieved with chemical fertilizers, supporting the potential of microalgae-based biofertilizers as an effective and sustainable alternative to synthetic fertilizers [132]. This method eliminates the need for long-term biomass storage, as microalgae can be grown and applied directly, ensuring freshness and maximizing nutrient uptake by plants.
This approach offers a direct, cost-effective method of supplying biofertilizers to agricultural systems while enhancing the sustainability of microalgae production. However, realizing the full potential of microalgae as biofertilizers and advancing towards a more resilient and sustainable agricultural future requires interdisciplinary cooperation and investments in infrastructure and research.
Funding
This work was supported by RIIM LPDP with contract number 160/IV/KS/11/2023 and PKS/17/UN62.21/DT.07.00/2023. M.M. Azimatun Nur also thanks to LPPM UPN Veteran Yogyakarta year 2024 and World class university program, Indonesia endowment fund for education for the support.
Statement
During the preparation of this work the authors used ChatGPT 3.5 in order to improve English language and proofread the text. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
CRediT authorship contribution statement
Muhamad Maulana Azimatun Nur: Writing – original draft, Visualization, Conceptualization. Mahreni: Conceptualization. Sri Wahyu Murni: Writing – review & editing, Conceptualization. Tutik Muji Setyoningrum: Writing – review & editing, Conceptualization. Faizah Hadi: Formal analysis, Data curation, Conceptualization. Tunjung Wahyu Widayati: Writing – review & editing, Conceptualization. Danang Jaya: Conceptualization. Raden Roro Endang Sulistyawati: Data curation, Conceptualization. Dwi Aulia Puspitaningrum: Writing – original draft, Conceptualization. Resti Nurmala Dewi: Writing – original draft, Visualization, Conceptualization. Hadiyanto: Writing – review & editing. M. Hasanuzzaman: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Muhamad Maulana Azimatun Nur reports financial support was provided by Pembangunan National Veteran University Yogyakarta. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- 1.Pandey P.C., Pandey M. Highlighting the role of agriculture and geospatial technology in food security and sustainable development goals. Sustain. Dev. 2023;31:3175–3195. doi: 10.1002/sd.2600. [DOI] [Google Scholar]
- 2.Zhao H., Chang J., Havlik P., van Dijk M., Valin H., Janssens C., Ma L., Bai Z., Herrero M., Smith P., Obersteiner M. China's future food demand and its implications for trade and environment. Nat. Sustain. 2021;4:1042–1051. doi: 10.1038/s41893-021-00784-6. [DOI] [Google Scholar]
- 3.Grunert O., Hernandez-Sanbria E., Buysens S., Neve S.D., Labeke M.V., Reheul D., Boon N. In-depth observation on the microbial and fungal community structure of four contrasting tomato cultivation systems in soil based and soilless culture systems. Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.520834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang F.H.M., Maggi F. Pesticide mixtures in soil: a global outlook. Environ. Res. Lett. 2021 doi: 10.1088/1748-9326/abe5d6. [DOI] [Google Scholar]
- 5.Alam M., Rohani M.F., Hossain M.S. Heavy metals accumulation in some important fish species cultured in commercial fish farm of Natore, Bangladesh and possible health risk evaluation. Emerg. Contam. 2023;9 doi: 10.1016/j.emcon.2023.100254. [DOI] [Google Scholar]
- 6.Penuelas J., Coello F., Sardans J. A better use of fertilizers is needed for global food security and environmental sustainability. Agric. Food. Secur. 2023;12 doi: 10.1186/s40066-023-00409-5. [DOI] [Google Scholar]
- 7.Semenova E.I., Semenov A.V. Baking Business Sustainability Through Life Cycle Management. Springer International Publishing; Cham: 2023. Bread industry sustainability life cycle assessment (a proposal of analysis of sustainability assessment using environmental and social footprints) pp. 3–13. [DOI] [Google Scholar]
- 8.Dmytryk A., Chojnacka K. Algae Biomass: Characteristics and Applications. Springer International Publishing; Cham: 2018. Algae as fertilizers, biostimulants, and regulators of plant growth; pp. 115–122. [DOI] [Google Scholar]
- 9.Booth D. Taylor & Francis; 2016. The psychology of nutrition. [DOI] [Google Scholar]
- 10.Alvarez A.L., Weyers S.L., Goemann H.M., Peyton B.M., Gardner R.D. Microalgae, soil and plants: a critical review of microalgae as renewable resources for agriculture. Algal Res. 2021;54 doi: 10.1016/j.algal.2021.102200. [DOI] [Google Scholar]
- 11.Sharma N., Singhvi R. Effects of chemical fertilizers and pesticides on human health and environment: A review. International Journal of Agriculture, Environment and Biotechnology. 2017;10 doi: 10.5958/2230-732X.2017.00083.3. [DOI] [Google Scholar]
- 12.Mazhar S., Cohen J.D., Hasnain S. Auxin producing non-heterocystous Cyanobacteria and their impact on the growth and endogenous auxin homeostasis of wheat. J. Basic Microbiol. 2013;53:996–1003. doi: 10.1002/jobm.201100563. [DOI] [PubMed] [Google Scholar]
- 13.Gheda S.F., Ahmed D.A. Improved soil characteristics and wheat germination as influenced by inoculation of Nostoc kihlmani and Anabaena cylindrica. Rendiconti Lincei. 2015;26:121–131. doi: 10.1007/s12210-014-0351-8. [DOI] [Google Scholar]
- 14.Barone V., Baglieri A., Stevanato P., Broccanello C., Bertoldo G., Bertaggia M., Cagnin M., Pizzeghello D., Moliterni V.M.C., Mandolino G., Fornasier F., Squartini A., Nardi S., Concheri G. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.) J. Appl. Phycol. 2018;30:1061–1071. doi: 10.1007/s10811-017-1283-3. [DOI] [Google Scholar]
- 15.Navarro Q.R., Corrêa D.O, Behling A., Noseda M.D., Amano É., Suzuki R.M., Ribas L.L.F. Efficient use of biomass and extract of the microalga Desmodesmus subspicatus (Scenedesmaceae) in asymbiotic seed germination and seedling development of the orchid Cattleya warneri. J. Appl. Phycol. 2021;33:2189–2207. doi: 10.1007/s10811-021-02442-y. [DOI] [Google Scholar]
- 16.Seifikalhor M., Hassani S.B., Aliniaeifard S. Seed priming by cyanobacteria (Spirulina platensis) and salep gum enhances tolerance of maize plant against cadmium toxicity. J. Plant Growth Regul. 2020;39:1009–1021. doi: 10.1007/s00344-019-10038-7. [DOI] [Google Scholar]
- 17.Shahmohamadloo R.S., Febria C.M., Fraser E.D.G., Sibley P.K. The sustainable agriculture imperative: A perspective on the need for an agrosystem approach to meet the United Nations Sustainable Development Goals by 2030. Integr. Environ. Assess Manag. 2022;18:1199–1205. doi: 10.1002/ieam.4558. [DOI] [PubMed] [Google Scholar]
- 18.Ladha J.K., Peoples M.B., Reddy P.M., Biswas J.C., Bennett A., Jat M.L., Krupnik T.J. Biological nitrogen fixation and prospects for ecological intensification in cereal-based cropping systems. Field. Crops. Res. 2022;283 doi: 10.1016/j.fcr.2022.108541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bizikova L., Nkonya E., Minah M., Hanisch M., Turaga R.M.R., Speranza C.I., Karthikeyan M., Tang L., Ghezzi-Kopel K., Kelly J., Celestin A.C., Timmers B. A scoping review of the contributions of farmers’ organizations to smallholder agriculture. Nat. Food. 2020;1:620–630. doi: 10.1038/s43016-020-00164-x. [DOI] [PubMed] [Google Scholar]
- 20.Dagnaisser L.S., dos Santos M.G.B., Rita A.V.S., Chaves Cardoso J., de Carvalho D.F., de Mendonça H.V. Microalgae as bio-fertilizer: a new strategy for advancing modern agriculture, wastewater bioremediation, and atmospheric carbon mitigation. Water. Air. Soil Pollut. 2022;233:477. doi: 10.1007/s11270-022-05917-x. [DOI] [Google Scholar]
- 21.Renuka N., Guldhe A., Prasanna R., Singh P., Bux F. Microalgae as multi-functional options in modern agriculture: current trends, prospects and challenges. Biotechnol. Adv. 2018;36:1255–1273. doi: 10.1016/j.biotechadv.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 22.de Bang T.C., Husted S., Laursen K.H., Persson D.P., Schjoerring J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021;229:2446–2469. doi: 10.1111/nph.17074. [DOI] [PubMed] [Google Scholar]
- 23.Assunção A.G.L., Cakmak I., Clemens S., González-Guerrero M., Nawrocki A., Thomine S. Micronutrient homeostasis in plants for more sustainable agriculture and healthier human nutrition. J. Exp. Bot. 2022;73:1789–1799. doi: 10.1093/jxb/erac014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thapa S., Bhandari A., Ghimire R., Xue Q., Kidwaro F., Ghatrehsamani S., Maharjan B., Goodwin M. Managing micronutrients for improving soil fertility, health, and soybean yield. Sustainability. 2021;13:11766. doi: 10.3390/su132111766. Oct. 2021. [DOI] [Google Scholar]
- 25.J. Carson, L. Philips, Fact sheets soil nitrogen supply, available online: https://www.soilquality.org.au/factsheets/soil-nitrogen-supply (accessed on 20 January 2023).
- 26.Bhatla S.C., Lal M.A. Springer Singapore; Singapore: 2018. Plant Physiology, Development and Metabolism. [DOI] [Google Scholar]
- 27.Akbas F., Gunal H., Acir N. Spatial variability of soil potassium and its relationship to land use and parent material. Soil. Water Res. 2017;12:202–211. doi: 10.17221/32/2016-SWR. [DOI] [Google Scholar]
- 28.Prasad R., Shivay Y.S. Calcium as a plant nutrient. International Journal of Bio-resource and Stress Management. 2020;5:i–iii. [Google Scholar]
- 29.Fagnano M., Agrelli D., Pascale A., Adamo P., Fiorentino N., Rocco C., Pepe O., Ventorino V. Copper accumulation in agricultural soils: Risks for the food chain and soil microbial populations. Sci. Total Environ. 2020;734 doi: 10.1016/j.scitotenv.2020.139434. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee A., Roychoudhury A. Frontiers in Plant-Soil Interaction. Elsevier; 2021. Beneficial aspects of cobalt uptake in plants exposed to abiotic stresses; pp. 523–529. [DOI] [Google Scholar]
- 31.Mehboob N., Hussain M., Minhas W.A., Yasir T.A., Naveed M., Farooq S., Alfarraj S., Zuan A.T.K. Soil-applied boron combined with boron-tolerant bacteria (Bacillus sp. MN54) improve root proliferation and nodulation, yield and agronomic grain biofortification of chickpea (Cicer arietinum L.) Sustainability. 2021;13:9811. doi: 10.3390/su13179811. [DOI] [Google Scholar]
- 32.Redon P.-O., Abdelouas A., Bastviken D., Cecchini S., Nicolas M., Thiry Y. Chloride and organic chlorine in forest soils: storage, residence times, and influence of ecological conditions. Environ. Sci. Technol. 2011;45:7202–7208. doi: 10.1021/es2011918. [DOI] [PubMed] [Google Scholar]
- 33.Ishfaq M., Wakeel A., Shahzad M.N., Kiran A., Li X. Severity of zinc and iron malnutrition linked to low intake through a staple crop: a case study in east-central Pakistan. Environ. Geochem. Health. 2021;43:4219–4233. doi: 10.1007/s10653-021-00912-3. Oct. 2021. [DOI] [PubMed] [Google Scholar]
- 34.Fageria N.K. Adequate and toxic levels of copper and manganese in upland rice, common bean, corn, soybean, and wheat grown on an oxisol. Commun. Soil Sci. Plant Anal. 2001;32:1659–1676. doi: 10.1081/CSS-100104220. Jun. 2001. [DOI] [Google Scholar]
- 35.Srinivasan S., Selvi R., Ramesh S., Pandiyan M., Kannan R., Marimuthu R. International Conference on Indigenous Vegetables and Legumes. Prospectus for Fighting Poverty, Hunger and Malnutrition. 2006. Response of mungbean to different methods and levels of molybdenum application under acid soil conditions; pp. 473–476. [Google Scholar]
- 36.Chandini R.K., Kumar R., Om P. Research Trends in Environmental Sciences. 2nd ed. 2019. The impact of chemical fertilizers on our environment and ecosystem; pp. 71–86. [Google Scholar]
- 37.Savci S. Investigation of effect of chemical fertilizers on environment. APCBEE Procedia. 2012;1:287–292. [Google Scholar]
- 38.Sunil J.W. Agrochemicals and chronic kidney disease of multi-factorial origin: environmentally induced occupational exposure an occupational exposure disease. Int. J. Nephrol. Kidney Failure. 2015;1 doi: 10.16966/2380-5498.111. [DOI] [Google Scholar]
- 39.Chakraborty S., Tiwari P.K., Sasmal S.K., Misra A.K., Chattopadhyay J. Effects of fertilizers used in agricultural fields on algal blooms. Eur. Phys. J .Spec. Top. 2017;226:2119–2133. doi: 10.1140/epjst/e2017-70031-7. [DOI] [Google Scholar]
- 40.Chakraborty T., Akhtar N. Biofertilizers. Wiley; 2021. Biofertilizers: prospects and challenges for future; pp. 575–590. [DOI] [Google Scholar]
- 41.Mahanty T., Bhattacharjee S., Goswami M., Bhattacharyya P., Das B., Ghosh A., Tribedi P. Biofertilizers: a potential approach for sustainable agriculture development. Environ. Sci. Pollution Res. 2017;24:3315–3335. doi: 10.1007/s11356-016-8104-0. [DOI] [PubMed] [Google Scholar]
- 42.Nosheen S., Ajmal I., Song Y. Microbes as biofertilizers, a potential approach for sustainable crop production. Sustainability. 2021;13:1868. doi: 10.3390/su13041868. [DOI] [Google Scholar]
- 43.Alhameid A., Tobin C., Maiga A., Kumar S., Osborne S., Schumacher T. Soil Health and Intensification of Agroecosytems. Elsevier; 2017. Intensified agroecosystems and changes in soil carbon dynamics; pp. 195–214. [DOI] [Google Scholar]
- 44.McPhee C., Bancerz M., Mambrini-Doudet M., Chrétien F., Huyghe C., Gracia-Garza J. The defining characteristics of agroecosystem living labs. Sustainability. 2021;13:1718. doi: 10.3390/su13041718. [DOI] [Google Scholar]
- 45.Moonen A.-C., Bàrberi P. Functional biodiversity: An agroecosystem approach. Agric. Ecosyst. Environ. 2008;127:7–21. doi: 10.1016/j.agee.2008.02.013. [DOI] [Google Scholar]
- 46.Mutale-Joan C., Sbabou L., Hicham E.A. Microalgae and cyanobacteria: how exploiting these microbial resources can address the underlying challenges related to food sources and sustainable agriculture: a review. J. Plant Growth Regul. 2023;42:1–20. doi: 10.1007/s00344-021-10534-9. [DOI] [Google Scholar]
- 47.Popa D.G., Lupu C., Constantinescu-Aruxandei D., Oancea F. Humic substances as microalgal biostimulants—implications for microalgal biotechnology. Mar. Drugs. 2022;20:327. doi: 10.3390/md20050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaïb S., Pistevos J.C.A., Bertrand C., Bonnard I. Allelopathy and allelochemicals from microalgae: An innovative source for bio-herbicidal compounds and biocontrol research. Algal Res. 2021;54 doi: 10.1016/j.algal.2021.102213. [DOI] [Google Scholar]
- 49.Bella E.L., Occhipinti P.S., Puglisi I., Fragalà F., Saccone R., Russo N., Randazzo C.L., Caggia C., Baglieri A. Comparative phycoremediation performance of three microalgae species in two different magnitude of pollutants in wastewater from farmhouse. Sustainability. 2023;15:11644. doi: 10.3390/su151511644. [DOI] [Google Scholar]
- 50.Hena S., Gutierrez L., Croué J.-P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.124041. [DOI] [PubMed] [Google Scholar]
- 51.Guleri S., Singh K., Kaushik R., Dhankar R., Tiwari A. Phycoremediation: A novel and synergistic approach in wastewater remediation. J. Microbiol. Biotechnol. Food Sci. 2020;10:98–106. doi: 10.15414/jmbfs.2020.10.1.98-106. [DOI] [Google Scholar]
- 52.Apandi N., Radin Mohamed R.M.S., Al-Gheethi A., Latiffi A., Hidayah Arifin S.N., Gani P. IOP Conf Ser Earth Environ Sci. Vol. 140. 2018. Phycoremediation of heavy metals in wet market wastewater. [DOI] [Google Scholar]
- 53.Joon N.K., Barnsley J.E., Ding R., Lee S., Latonen R., Bobacka J., Gordon K.C., Lisak T.O.G. Silver(I)-selective electrodes based on rare earth element double-decker porphyrins. Sens. Actuators B Chem. 2020;305 doi: 10.1016/j.snb.2019.127311. [DOI] [Google Scholar]
- 54.Nagarajan D., Lee D.-J., Chang J.-S. Integration of anaerobic digestion and microalgal cultivation for digestate bioremediation and biogas upgrading. Bioresour. Technol. 2019;290 doi: 10.1016/j.biortech.2019.121804. Oct. [DOI] [PubMed] [Google Scholar]
- 55.Putri N.A., Dewi R.N., Lestari R., Yuniar R.A., Ma'arif L.M., Erianto R. Microalgae as a bioremediation agent for palm oil mill effluent: production of biomass and high added value compounds. Jurnal Rekayasa Kimia & Lingkungan. 2023;18:149–161. doi: 10.23955/rkl.v18i2.34018. [DOI] [Google Scholar]
- 56.Dewi R.N., Nur M.M.A., Astuti R.P., Andriyanto W., Panjaitan F.C.A., Febrianti D., Budiadnyani I.G.A., Utari S.P.S.D, Samanta P.N., Perceka M.L. Bioremediation of seafood processing wastewater by microalgae: Nutrient removal, and biomass, lipid and protein enhancement. Environ. Eng. Res. 2024;29 doi: 10.4491/eer.2023.673. –0. [DOI] [Google Scholar]
- 57.Salama E.-S., Roh H., Dev S., Khan M.A., Abou-Shanab R.A.I., Chang S.W., Jeon B. Algae as a green technology for heavy metals removal from various wastewater. World J. Microbiol. Biotechnol. 2019;35:75. doi: 10.1007/s11274-019-2648-3. [DOI] [PubMed] [Google Scholar]
- 58.Dewi R.N., Panjaitan F.C.A., Febriyanti D., Perceka M.L., Khairunnisa A., Farida1 I., Budiadnyani I.G.A., Utari S.P.S.D., Samanta1 P.N., Astiana I., Cesrany M. Potential of Spirulina sp. for remediating pollutants in aquaculture wastewater and producing phycocyanin. Indones Fish Res J. 2024;30:27–35. doi: 10.15578/ifrj.30.1.2024.27-35. [DOI] [Google Scholar]
- 59.Kaloudas D., Pavlova N., Penchovsky R. Phycoremediation of wastewater by microalgae: a review. Environ. Chem. Lett. 2021;19:2905–2920. doi: 10.1007/s10311-021-01203-0. [DOI] [Google Scholar]
- 60.Chaudry S. Integrating microalgae cultivation with wastewater treatment: a peek into economics. Appl. Biochem. Biotechnol. 2021;193:3395–3406. doi: 10.1007/s12010-021-03612-x. [DOI] [PubMed] [Google Scholar]
- 61.Raychaudhuric S., Simsekb H., Kumar S. Vol. 373. Elsevier; Amsterdam, The Netherlands: 2020. Algae-and bacteria-driven technologies for pharmaceutical remediation in wastewater; pp. 373–500. (Removal of Toxic Pollutants Through Microbiological and Tertiary Treatment; New Perspect). [Google Scholar]
- 62.Mujtaba G., Lee K. Advanced treatment of wastewater using symbiotic co-culture of microalgae and bacteria. Applied Chemistry for Engineering. 2016;27:1–9. doi: 10.14478/ace.2016.1002. [DOI] [Google Scholar]
- 63.Liberti D., Pinheiro F., Simões B., Varela J., Barreira L. Beyond bioremediation: the untapped potential of microalgae in wastewater treatment. Water (Basel) 2024;16:2710. doi: 10.3390/w16192710. [DOI] [Google Scholar]
- 64.Li K., Liu Q., Fang F., Luo R., Lu Q., Zhou W., Huo S., Cheng P., Liu J., Addy M., Chen P., Chen D., Ruan R. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019;291 doi: 10.1016/j.biortech.2019.121934. [DOI] [PubMed] [Google Scholar]
- 65.Brar A., Kumar M., Vivekanand V., Pareek N. Photoautotrophic microorganisms and bioremediation of industrial effluents: current status and future prospects. 3 Biotech. 2017;7 doi: 10.1007/s13205-017-0600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dewi R.N., Mahreni M.M.A.Nur, Siahaan A.A., Ardhi A.C. Enhancing the biomass production of microalgae by mixotrophic cultivation using virgin coconut oil mill effluent. Environ. Eng. Res. 2022;28 doi: 10.4491/eer.2022.059. –0. [DOI] [Google Scholar]
- 67.Ding Y., Wang Y., Liu X., Song X. Improving nutrient and organic matter removal by novel integration of a high-rate algal pond and submerged macrophyte pond. Pol J Environ Stud. 2019;29:997–1001. doi: 10.15244/pjoes/99824. [DOI] [Google Scholar]
- 68.Pachecoa M.M., Hoeltza M., Moraesa M.S.A., Schneiderab R.C.S. Microalgae: cultivation techniques and wastewater phycoremediation. J. Environ. Sci. Health. 2015;50:585–601. doi: 10.1080/10934529.2015.994951. [DOI] [PubMed] [Google Scholar]
- 69.Emparan Q., Harun R., Danquah M.K. Role of phycoremediation for nutrient removal from wastewaters: a review. Appl. Ecol. Environ. Res. 2019;17:889–915. doi: 10.15666/aeer/1701_889915. [DOI] [Google Scholar]
- 70.Huang W., Liu D., Huang W., Cai W., Zhang Z., Lei Z. Achieving partial nitrification and high lipid production in an algal-bacterial granule system when treating low COD/NH4–N wastewater. Chemosphere. 2020;248 doi: 10.1016/j.chemosphere.2020.126106. [DOI] [PubMed] [Google Scholar]
- 71.Al Azad S., Estim A., Mustafa S., Sumbing M.V. Assessment of nutrients in seaweed tank from land based integrated multitrophic aquaculture module. J. Geosci. Environ. Protect. 2017;05:137–147. doi: 10.4236/gep.2017.58012. [DOI] [Google Scholar]
- 72.Pradana Y.S., Dewi R.N., Livia K.D., Arisa F., Rochmadi R.B.Cahyono, Budiman A. Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction-transesterification process using palm oil as a co-solvent of methanol. Open Chem. 2020;18:833–842. doi: 10.1515/chem-2020-0133. [DOI] [Google Scholar]
- 73.Barman S., Khatoon H., Rahman M., Mazumder S., Hasan S. Effects of sodium nitrate on the growth and proximate composition of the indigenous marine microalgae Tetraselmis chuii (Butcher, 1959) Aquatic Sci. Eng. 2021;37:46–52. doi: 10.26650/ASE2021972678. [DOI] [Google Scholar]
- 74.Gorzelnik S.A., Zhu X., Angelidaki I., Koski M., Valverde-Pérez B. Daphnia magna as biological harvesters for green microalgae grown on recirculated aquaculture system effluents. Sci. Total Environ. 2023;873 doi: 10.1016/j.scitotenv.2023.162247. [DOI] [PubMed] [Google Scholar]
- 75.Meirinawati H., Wahyudi A.J. Seaweed as bioadsorbent for nitrogen and phosphorus removal. J. Environ. Sci. Sustain. Dev. 2023;6 doi: 10.7454/jessd.v6i1.1159. [DOI] [Google Scholar]
- 76.Patel R., Pandya K., Jasrai R., Brahmbhatt N. A review: scope of utilizing seaweed as a biofertilizer in agriculture. Int. J. Adv. Res. (Indore) 2017;5:2046–2054. doi: 10.21474/IJAR01/4941. [DOI] [Google Scholar]
- 77.Tanaka Y., Ashaari A., Mohamad F.S., Lamit N. Bioremediation potential of tropical seaweeds in aquaculture: low-salinity tolerance, phosphorus content, and production of UV-absorbing compounds. Aquaculture. 2020;518 doi: 10.1016/j.aquaculture.2019.734853. [DOI] [Google Scholar]
- 78.Zafar A., Ali I., Rahayu F. Marine seaweeds (biofertilizer) significance in sustainable agricultural activities: A review. IOP Conf. Ser. Earth. Environ. Sci. 2022;974 doi: 10.1088/1755-1315/974/1/012080. [DOI] [Google Scholar]
- 79.Zinicovscaia I., Cepoi L. Springer; Berlin: 2016. Cyanobacteria for bioremediation of wastewaters. [Google Scholar]
- 80.Breure M.S. Wageningen University; Wageningen: 2014. Exploring the potential for using seaweed (Ulva lactuca) as organic fertiliser. [Google Scholar]
- 81.Adderley A., Wallace S., Stubbs D., Bowen-O'Connor C., Ferguson J., Watson C., Gustave W. Sargassum sp. as a biofertilizer: is it really a key towards sustainable agriculture for The Bahamas? Bull Natl Res Cent. 2023;47 doi: 10.1186/s42269-023-01087-w. [DOI] [Google Scholar]
- 82.Costa O.Y.A., Raaijmakers J.M., Kuramae E.E. Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christophoridis C., Bourliva A., Evgenakis E., Papadopoulou L., Fytianos K. Effects of anthropogenic activities on the levels of heavy metals in marine surface sediments of the Thessaloniki Bay, Northern Greece: Spatial distribution, sources and contamination assessment. Microchem. J. 2019;149 doi: 10.1016/j.microc.2019.104001. [DOI] [Google Scholar]
- 84.Abdel-Raouf N., Al-Homaidan A.A., Ibraheem I.B.M. Microalgae and wastewater treatment. Saudi. J. Biol. Sci. 2012;19:257–275. doi: 10.1016/j.sjbs.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dominguez H., Loret E.P. Ulva lactuca, a source of troubles and potential riches. Mar. Drugs. 2019;17:357. doi: 10.3390/md17060357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aquilino F., Paradiso A., Trani R., Longo C., Pierri C., Corriero G., Pinto M.C. Chaetomorpha linum in the bioremediation of aquaculture wastewater: Optimization of nutrient removal efficiency at the laboratory scale. Aquaculture. 2020;523 doi: 10.1016/j.aquaculture.2020.735133. [DOI] [Google Scholar]
- 87.Hernández-Herrera R.M., Santacruz-Ruvalcaba F., Ruiz-López M.A., Norrie J., Hernández-Carmona G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.) J. Appl. Phycol. 2014;26:619–628. doi: 10.1007/s10811-013-0078-4. [DOI] [Google Scholar]
- 88.Ammaturo C., Pacheco D., Cotas J., Formisano L., Ciriello M., Pereira L., Bahcevandziev K. Use of Chlorella vulgaris and Ulva lactuca as biostimulant on lettuce. Applied Sciences. 2023;13:9046. doi: 10.3390/app13169046. [DOI] [Google Scholar]
- 89.F. Dean, S. Miller, A. Holmes, An investigation into the potential use of sea lettuce (Ulva lactuca) as a soil amendment in vegetable gardens and orchards, (2013), New Zealand.
- 90.Yadavalli R., Heggers G.R.V.N. Two stage treatment of dairy effluent using immobilized Chlorella pyrenoidosa. J. Environ. Health. Sci. Eng. 2013;11:36. doi: 10.1186/2052-336X-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oancea F., Velea S., Fătu V., Mincea C., Ilie L. Micro-algae based plant biostimulant and its effect on water stressed tomato plants. Romanian J. Plant Protect. 2013;VI:104–117. [Google Scholar]
- 92.González-Pérez B.K., Rivas-Castillo A.M., Valdez-Calderón A., Gayosso-Morales M.A. Microalgae as biostimulants: a new approach in agriculture. World J. Microbiol. Biotechnol. 2022;38:4. doi: 10.1007/s11274-021-03192-2. [DOI] [PubMed] [Google Scholar]
- 93.Chabili A., Minaoui F., Hakkoum Z., Douma M., Meddich A., Loudiki M. Effects of extraction methods on the plant biostimulant activity of the soil microalga Chlorella vulgaris. J. Appl. Phycol. 2024 doi: 10.1007/s10811-024-03328-5. [DOI] [Google Scholar]
- 94.Braun J.C.A., Colla L.M. Use of microalgae for the development of biofertilizers and biostimulants. Bioenergy Res. 2023;16:289–310. doi: 10.1007/s12155-022-10456-8. [DOI] [Google Scholar]
- 95.Ronga D., Biazzi E., Parati K., Carminati D., Carminati E., Tava A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy. 2019;9:192. doi: 10.3390/agronomy9040192. [DOI] [Google Scholar]
- 96.Sánchez-Quintero Á., Fernandes S.C.M., Beigbeder J.-B. Overview of microalgae and cyanobacteria-based biostimulants produced from wastewater and CO2 streams towards sustainable agriculture: a review. Microbiol. Res. 2023;277 doi: 10.1016/j.micres.2023.127505. [DOI] [PubMed] [Google Scholar]
- 97.Zeiss D.R., Piater L.A., Dubery I.A. Hydroxycinnamate amides: intriguing conjugates of plant protective metabolites. Trends Plant. Sci.. 2021;26:184–195. doi: 10.1016/j.tplants.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 98.Khalid S., Chaudhary K., Aziz H., Amin S., Sipra H.M., Ansar S., Rasheed M. Naeem H., Onyeaka H. Trends in extracting protein from microalgae Spirulina platensis, using innovative extraction techniques: mechanisms, potentials, and limitations. Crit. Rev. Food Sci. Nutr. 2024:1–17. doi: 10.1080/10408398.2024.2386448. [DOI] [PubMed] [Google Scholar]
- 99.Y. Lu, J. Xu, Phytohormones in microalgae: A new opportunity for microalgal biotechnology?, (2015), 10.1016/j.tplants.2015.01.006. [DOI] [PubMed]
- 100.S. Shah, X. Li, Z. Jiang, S. Fahad, S. Hassan, Exploration of the phytohormone regulation of energy storage compound accumulation in microalgae, (2022), 10.1002/fes3.418. [DOI]
- 101.Ronga D., Biazzi E., Parati K., Carminati D., Carminati E., Tava A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy. 2019 doi: 10.3390/AGRONOMY9040192. [DOI] [Google Scholar]
- 102.Han X., Zeng H., Bartocci P., Fantozzi F., Yan Y. Phytohormones and effects on growth and metabolites of microalgae: a review. Fermentation. 2018;4 doi: 10.3390/fermentation4020025. [DOI] [Google Scholar]
- 103.González-Pérez B.K., Rivas-Castillo A., Valdez-Calderón A., Gayosso-Morales M.A. Microalgae as biostimulants: a new approach in agriculture. World J. Microbiol. Biotechnol. 2021;38 doi: 10.1007/s11274-021-03192-2. [DOI] [PubMed] [Google Scholar]
- 104.Stirk W.A., van Staden J. Potential of phytohormones as a strategy to improve microalgae productivity for biotechnological applications. Biotechnol. Adv. 2020;4 doi: 10.1016/j.biotechadv.2020.107612. [DOI] [PubMed] [Google Scholar]
- 105.El Arroussi H., Benhima R., Elbaouchi A., Sijilmassi B., EL Mernissi N., Aafsar A., Meftah-Kadmiri I., Bendaou N., Smouni A. Dunaliella salina exopolysaccharides: a promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum) J. Appl. Phycol. 2018;30:2929–2941. doi: 10.1007/s10811-017-1382-1. [DOI] [Google Scholar]
- 106.Barone V., Puglisi I., Fragalà F., Lo Piero A.R., Giuffrida F., Baglieri A. Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J. Appl. Phycol. 2019;31:465–470. doi: 10.1007/s10811-018-1518-y. [DOI] [Google Scholar]
- 107.Rachidi F., Benhima R., Sbabou L., El Arroussi H. Microalgae polysaccharides bio-stimulating effect on tomato plants: growth and metabolic distribution. Biotechnol. Reports. 2020;25:e00426. doi: 10.1016/j.btre.2020.e00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Najdenski H.M., Gigova L., Iliev I., Pilarski P., Lukavsky J., Tsvetkova I., Ninova M.S., Kussovski V. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int. J. Food Sci. Technol. 2013;48:1533–1540. doi: 10.1111/ijfs.12122. [DOI] [Google Scholar]
- 109.Xiao R., Zheng Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016;34:1225–1244. doi: 10.1016/j.biotechadv.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 110.Gonçalves J., Freitas J., Fernandes I., Silva P. Microalgae as biofertilizers: a sustainable way to improve soil fertility and plant growth. Sustainability. 2023;15:12413. doi: 10.3390/su151612413. [DOI] [Google Scholar]
- 111.Khan S.A., Sharma G.K., Malla F.A., Kumar A., Rashmi N.Gupta. Microalgae based biofertilizers: A biorefinery approach to phycoremediate wastewater and harvest biodiesel and manure. J. Clean. Prod. 2019;211:1412–1419. doi: 10.1016/j.jclepro.2018.11.281. [DOI] [Google Scholar]
- 112.Osorio-Reyes J.G., Valenzuela-Amaro H.M., Pizaña-Aranda J.J.P., Ramírez-Gamboa D., Meléndez-Sánchez E.R., López-Arellanes M.E, Castañeda-Antonio M.D, Coronado-Apodaca K.G., Araújo R.G., Sosa-Hernández J.E., Melchor-Martínez E.M., Iqbal H.M.N., Parra-Saldivar R., Martínez-Ruiz M. Microalgae-based biotechnology as alternative biofertilizers for soil enhancement and carbon footprint reduction: advantages and implications. Mar. Drugs. 2023;21:93. doi: 10.3390/md21020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Silambarasan S., Logeswari P., Sivaramakrishnan R., Incharoensakdi A., Cornejo P., Kamaraj B., Chi N.T.L. Removal of nutrients from domestic wastewater by microalgae coupled to lipid augmentation for biodiesel production and influence of deoiled algal biomass as biofertilizer for Solanum lycopersicum cultivation. Chemosphere. 2021;268 doi: 10.1016/j.chemosphere.2020.129323. [DOI] [PubMed] [Google Scholar]
- 114.Balan V., Pierson J., Husain H., Kumar S., Saffron C., Kumar V. Potential of using microalgae to sequester carbon dioxide and processing to bioproducts. Green Chem. 2023;25:7934–7951. doi: 10.1039/D3GC02286B. 2023. [DOI] [Google Scholar]
- 115.Nur M.M.A., Buma A.G.J. Opportunities and challenges of microalgal cultivation on wastewater, with special focus on palm oil mill effluent and the production of high value compounds. Waste Biomass Valorization. 2019;10:2079–2097. doi: 10.1007/s12649-018-0256-3. [DOI] [Google Scholar]
- 116.Liang M.-H., Wang L., Wang Q., Zhu J., Jiang J.-G. High-value bioproducts from microalgae: Strategies and progress. Crit. Rev. Food Sci. Nutr. 2019;59:2423–2441. doi: 10.1080/10408398.2018.1455030. [DOI] [PubMed] [Google Scholar]
- 117.Castro R., Piazzon M.C., Zarra I., Leiro J., Noya M., Lamas J. Stimulation of turbot phagocytes by Ulva rigida C. Agardh polysaccharides. Aquaculture. 2006;254:9–20. doi: 10.1016/j.aquaculture.2005.10.012. [DOI] [Google Scholar]
- 118.Kumar S.D., Santhanam P., Ananth S., Kaviyarasan M., Nithya P., Dhanalakshmi B., Park Min S., Kim Mi-Kyung. Evaluation of suitability of wastewater-grown microalgae (Picochlorum maculatum) and copepod (Oithona rigida) as live feed for white leg shrimp Litopenaeus vannamei post-larvae. Aquac. Int. 2017;25:393–411. doi: 10.1007/s10499-016-0037-6. [DOI] [Google Scholar]
- 119.Supraja K.V., Behera B., Balasubramanian P. Efficacy of microalgal extracts as biostimulants through seed treatment and foliar spray for tomato cultivation. Ind. Crops Prod. 2020;151 doi: 10.1016/j.indcrop.2020.112453. [DOI] [Google Scholar]
- 120.Jochum M., Moncayo L.P., Jo Y.-K. Microalgal cultivation for biofertilization in rice plants using a vertical semi-closed airlift photobioreactor. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saadaoui I., Sedky R., Rasheed R., Bounnit T., Almahmoud A., Elshekh A., Dalgamouni T., Jmal K., Das P., Jabri H.A. Assessment of the algae-based biofertilizer influence on date palm (Phoenix dactylifera L.) cultivation. J. Appl. Phycol. 2019;31:457–463. doi: 10.1007/s10811-018-1539-6. [DOI] [Google Scholar]
- 122.Zhen X., Li Y., Qiang J., Tang M., Hua W., Wang W., Wu S., Perkins P., Ruan R., Cheng Y. Design, application and verification of a novel system utilizing bacteria and microalgae to treat swine farm wastewater and produce value-added biomass. Int. J. Agric. Biolog. Eng. 2023;16:222–230. doi: 10.25165/j.ijabe.20231604.8250. [DOI] [Google Scholar]
- 123.Álvarez-González A., Uggetti E., Serrano L., Gorchs G., Casas M.E., Matamoros V., Gonzalez-Flo E., Díez-Montero R. The potential of wastewater grown microalgae for agricultural purposes: Contaminants of emerging concern, heavy metals and pathogens assessment. Environ. Pollut. 2023;324 doi: 10.1016/j.envpol.2023.121399. [DOI] [PubMed] [Google Scholar]
- 124.Díaz-Pérez F.J., Díaz R., Valdés G., Valdebenito-Rolack E., Hansen F. Effects of microalgae and compost on the yield of cauliflower grown in low nutrient soil. Chil. J. Agric. Res. 2023;83:181–194. doi: 10.4067/s0718-58392023000200181. [DOI] [Google Scholar]
- 125.Ferreira E.T., Barrochelo S.C., Melo S.P., Araujo T., Xavier A.C.C., Cechin I., Silva G.H.R. Biofertilizers from wastewater treatment as a potential source of mineral nutrients for growth of amaranth plants. PLoS One. 2023;18 doi: 10.1371/journal.pone.0295624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rassman N.H.K., Abd Razak N.A.E., Mohamed R.M.S.R., Al-Gheethi A. Optimization of Microalgae Biomass Yield using Chlorella Sp. and Spirulina Sp. Cultivation in secondary treated wastewater effluent. IOP Conf. Ser. Earth. Environ. Sci. 2023;1205 doi: 10.1088/1755-1315/1205/1/012005. [DOI] [Google Scholar]
- 127.Mückschel F., Ollo E., Glaeser S.P., Düring R., Yan F., Velten H., Theilen U., Frei M. Nitrogen use efficiency of microalgae application in wheat compared to mineral fertilizer. J. Plant Nutr. Soil Sci. 2023;186:522–531. doi: 10.1002/jpln.202300125. [DOI] [Google Scholar]
- 128.Cordeiro E.C.N., Mógor Á.F., Amatussi J.O., Mógor G., Marques H.M.C., de Lara G.B. Microalga biofertilizer improves potato growth and yield, stimulating amino acid metabolism. J. Appl. Phycol. 2022;34:385–394. doi: 10.1007/s10811-021-02656-0. [DOI] [Google Scholar]
- 129.Suchithra M.R., Muniswami D.M., Sri M.S., Usha R., Rasheeq A.A., Preethi B.A., Dineshkumar R. Effectiveness of green microalgae as biostimulants and biofertilizer through foliar spray and soil drench method for tomato cultivation. S. Afr. J. Bot. 2022;146:740–750. doi: 10.1016/j.sajb.2021.12.022. [DOI] [Google Scholar]
- 130.Álvarez-González A., Uggetti E., Serrano L., Gorchs G., Ferrer I., Díez-Montero R. Can microalgae grown in wastewater reduce the use of inorganic fertilizers? J. Environ. Manage. 2022;323 doi: 10.1016/j.jenvman.2022.116224. [DOI] [PubMed] [Google Scholar]
- 131.Musetsho P. Durban University of Technology; Durban: 2022. An integrated approach for biofuel and fertilizer production using microalgae grown in wastewater. [Google Scholar]
- 132.Pooja K., Priyanka V., Rao B.C.S., Raghavender V. Cost-effective treatment of sewage wastewater using microalgae Chlorella vulgaris and its application as bio-fertilizer. Energy Nexus. 2022;7 doi: 10.1016/j.nexus.2022.100122. [DOI] [Google Scholar]
- 133.Sharma G.K., Khan S.A., Shrivastava M., Bhattacharyya R., Sharma A., Gupta D.K., Kishore P., Gupta N. Circular economy fertilization: Phycoremediated algal biomass as biofertilizers for sustainable crop production. J. Environ. Manage. 2021;287 doi: 10.1016/j.jenvman.2021.112295. [DOI] [PubMed] [Google Scholar]
- 134.Viegas C., Gouveia L., Gonçalves M. Aquaculture wastewater treatment through microalgal. Biomass potential applications on animal feed, agriculture, and energy. J. Environ. Manage. 2021;286 doi: 10.1016/j.jenvman.2021.112187. [DOI] [PubMed] [Google Scholar]
- 135.Viegas C., Gouveia L., Gonçalves M. Bioremediation of cattle manure using microalgae after pre-treatment with biomass ash. Bioresour Technol. Rep. 2021;14 doi: 10.1016/j.biteb.2021.100681. [DOI] [Google Scholar]
- 136.Li J., Lens P.N.L., Ferrer I., Laing G.Du. Evaluation of selenium-enriched microalgae produced on domestic wastewater as biostimulant and biofertilizer for growth of selenium-enriched crops. J. Appl. Phycol. 2021;33 doi: 10.1007/s10811-021-02523-y. [DOI] [Google Scholar]
- 137.de S. Castro J., Calijuri M.L., Mattiello E.M., Ribeiro V.J., Assemany P.P. Algal biomass from wastewater: soil phosphorus bioavailability and plants productivity. Sci. Total Environ. 2020;711 doi: 10.1016/j.scitotenv.2019.135088. [DOI] [PubMed] [Google Scholar]
- 138.Jo S.W., Do J.M., Na H., Hong J.W., Kim I.S., Yoon H.S. Assessment of biomass potentials of microalgal communities in open pond raceways using mass cultivation. PeerJ. 2020;7 doi: 10.7717/peerj.9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Deepika P., MubarakAli D. Production and assessment of microalgal liquid fertilizer for the enhanced growth of four crop plants. Biocatal. Agric. Biotechnol. 2020;28 doi: 10.1016/j.bcab.2020.101701. [DOI] [Google Scholar]
- 140.Dineshkumar R., Subramanian J., Sampathkumar P. Prospective of Chlorella vulgaris to augment growth and yield parameters along with superior seed qualities in black gram, Vigna mungo (L.) Waste Biomass Valorization. 2020;11 doi: 10.1007/s12649-018-0465-9. [DOI] [Google Scholar]
- 141.Nayak M., Swain D.K., Sen R. Strategic valorization of de-oiled microalgal biomass waste as biofertilizer for sustainable and improved agriculture of rice (Oryza sativa L.) crop. Sci. Total Environ. 2019;682 doi: 10.1016/j.scitotenv.2019.05.123. [DOI] [PubMed] [Google Scholar]
- 142.Zarezadeh S., Moheimani N.R., Jenkins S.N., Hülsen T., Riahi H., Mickan B.S. Microalgae and phototrophic purple bacteria for nutrient recovery from agri-industrial effluents: influences on plant growth, rhizosphere bacteria, and putative carbon- and nitrogen-cycling genes. Front. Plant Sci. 2019;10 doi: 10.3389/fpls.2019.01193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bettani S.R., Ragazzo G.O., Santos N.L., Kieckbusch T.G., Bastos R.G., Soares M.R., Silva M.A. Sugarcane vinasse and microalgal biomass in the production of pectin particles as an alternative soil fertilizer. Carbohydr. Polym. 2019;203:322–330. doi: 10.1016/j.carbpol.2018.09.041. [DOI] [PubMed] [Google Scholar]
- 144.Das P., Quadir M.A., Thaher M.I., Alghasal G.S.H.S., Aljabri H.M.S.J. Microalgal nutrients recycling from the primary effluent of municipal wastewater and use of the produced biomass as bio-fertilizer. Int. J. Environ. Sci. Technol. 2019;16:3355–3364. doi: 10.1007/s13762-018-1867-8. [DOI] [Google Scholar]
- 145.Ferreira A., Ribeiro B., Ferreira A.F., Tavares M.L.A., Vladic J., Vidović S., Cvetkovic D., Melkonyan L., Avetisova G., Goginyan V., Gouveia L. Scenedesmus obliquus microalga-based biorefinery – from brewery effluent to bioactive compounds, biofuels and biofertilizers – aiming at a circular bioeconomy. Biofuels Bioprod. Biorefin. 2019;13:1169–1186. doi: 10.1002/bbb.2032. 2019. [DOI] [Google Scholar]
- 146.Yilmaz E., Sönmez M. The role of organic/bio–fertilizer amendment on aggregate stability and organic carbon content in different aggregate scales. Soil Tillage Res. 2017;168:118–124. doi: 10.1016/j.still.2017.01.003. [DOI] [Google Scholar]
- 147.de S. Castro J., Calijuri M.L., Assemany P.P., Cecon P.R., de Assis I.R., Ribeiro V.J. Microalgae biofilm in soil: Greenhouse gas emissions, ammonia volatilization and plant growth. Sci. Total Environ. 2017;574 doi: 10.1016/j.scitotenv.2016.08.205. [DOI] [PubMed] [Google Scholar]
- 148.Coppens J., Grunert O., Hende S.V.D., Vanhoutte I., Boon N., Haesaert G., Gelder L.D. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016;28:2367–2377. doi: 10.1007/s10811-015-0775-2. 2016. [DOI] [Google Scholar]
- 149.Renuka N., Prasanna R., Sood A., Ahluwalia A.S., Bansal R., Babu S., Singh R., Shivay Y.S., Nain L. Exploring the efficacy of wastewater-grown microalgal biomass as a biofertilizer for wheat. Environ. Sci. Pollution Res. 2016;23:6608–6620. doi: 10.1007/s11356-015-5884-6. [DOI] [PubMed] [Google Scholar]
- 150.Garcia-Gonzalez J., Sommerfeld M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016;28:1051–1061. doi: 10.1007/s10811-015-0625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.de S. Castro J., Calijuri M.L., Mattiello E.M., Ribeiro V.J., Assemany P.P. Algal biomass from wastewater: soil phosphorus bioavailability and plants productivity. Sci. Total Environ. 2020;711 doi: 10.1016/j.scitotenv.2019.135088. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Z., Xu M., Fan Y., Zhang L., Wang H. Using microalgae to reduce the use of conventional fertilizers in hydroponics and soil-based cultivation. Sci. Total Environ. 2024;912 doi: 10.1016/j.scitotenv.2023.169424. nd. [DOI] [PubMed] [Google Scholar]
- 153.Nayak M., Swain D.K., Sen R. Strategic valorization of de-oiled microalgal biomass waste as biofertilizer for sustainable and improved agriculture of rice (Oryza sativa L.) crop. Sci. Total Environ. 2019;682:475–484. doi: 10.1016/j.scitotenv.2019.05.123. [DOI] [PubMed] [Google Scholar]
- 154.Arashiro L.T., Montero N., Ferrer I., Acién F.G., Gómez C., Garfí M. Life cycle assessment of high rate algal ponds for wastewater treatment and resource recovery. Sci. Total Environ. 2018;622–623:1118–1130. doi: 10.1016/j.scitotenv.2017.12.051. [DOI] [PubMed] [Google Scholar]
- 155.Jimenez R., Markou G., Tayibi S., Barakat A., Chapsal C., Monlau F. Production of microalgal slow-release fertilizer by valorizing liquid agricultural digestate: growth experiments with tomatoes. Appl. Sci. 2020;10:3890. doi: 10.3390/app10113890. [DOI] [Google Scholar]
- 156.Gemin L.G., Mógor Á.F., De Oliveira Amatussi J., Mógor G. Microalgae associated to humic acid as a novel biostimulant improving onion growth and yield. Sci. Hortic. 2019;256 doi: 10.1016/j.scienta.2019.108560. [DOI] [Google Scholar]
- 157.Godlewska K., Michalak I., Pacyga P., Baśladyńska S., Chojnacka K. Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol. 2019;35:80. doi: 10.1007/s11274-019-2653-6. Jun. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bayona-Morcillo P.J., Plaza B.M., Gómez-Serrano C., Rojas E., Jiménez-Becker S. Effect of the foliar application of cyanobacterial hydrolysate (Arthrospira platensis) on the growth of Petunia x hybrida under salinity conditions. J. Appl. Phycol. 2020;32:4003–4011. doi: 10.1007/s10811-020-02192-3. [DOI] [Google Scholar]
- 159.Michalak I., Chojnacka K., Dmytryk A., Wilk R., Gramza M., Rój E. Evaluation of Supercritical extracts of algae as biostimulants of plant growth in field trials. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.01591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sinha R.K. Embarking on the second green revolution for sustainable agriculture in India: A judicious mix of traditional wisdom and modern knowledge in ecological farming. J Agric Environ Ethics. 1997;10:183–197. doi: 10.1023/A:1007796609378. [DOI] [Google Scholar]
- 161.Purwanti D.P. IPB University; Bogor: 2019. Estimasi Produksi Padi Berbasis Model Simulasi Tanaman (Studi Kasus : Kabupaten Subang) [Google Scholar]
- 162.Gatamaneni L.B., Orsat V., Lefsrud M. Utilizing the microalgal biomass of Chlorella variabilis and Scenedesmus obliquus produced from the treatment of synthetic dairy wastewater as a biofertilizer. J. Plant Nutr. 2021;44:1486–1497. doi: 10.1080/01904167.2020.1862191. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.