Highlights
-
•
This study utilizes multigenerational data to assess the relationship between cardiovascular risk factors and Parkinson’s disease.
-
•
In this retrospective cohort study, obesity was found to potentially be protective against the development of Parkinson’s disease.
-
•
The present study prospectively assesses the relationship between cardiovascular risk factors and the development of Parkinson’s disease.
-
•
This study is one of the largest cohort studies which assesses the relationship between midlife CV risk factors and the development of PD.
Keywords: Parkinson’s disease, Epidemiology, Risk factors, Neuroepidemiology
Abstract
Objective
To determine the role of obesity in the development of Parkinson’s disease (PD).
Background
Obesity has been reported to be both a risk factor for PD, as well as potentially protective. The Framingham Heart Study (FHS) is a multigenerational longitudinal cohort study that was started in 1948, which is well-known for its cardiovascular health studies. In this study, we utilized the extensive cardiovascular and neurological data to determine if obesity contributes to the risk of the development of PD.
Methods
Participants in the FHS Original and Offspring cohorts were included in this study. Controls were selected based on sex and age at baseline examination, 1:10. Cox proportional hazard regression models were used, adjusting for age and sex. PD case status was determined utilizing prior medical and neurological examination data, Framingham Heart Study examinations, and self-report data by a panel of movement disorders neurologists using the UK Brain Bank Criteria (UKBB) and other supporting clinical details after being flagged for review by FHS neurologists. We used p < 0.05 for significance.
Results
Accounting for missing covariate data, this study included 117 participants with PD, with 1170 controls. We found that higher BMI was associated with lower PD risk, with participants with BMI 25 kg/m2 to 30 kg/m2 having HR of 0.66 (CI 0.44–0.98; p = 0.04) and BMI >= 30 kg/m2 having HR 0.47 (CI 0.27–0.84; p = 0.01). When the overweight and obese BMI groups were combined, we noted a more robust association, with combined HR of 0.67 (0.41–0.86; p = 0.01).
Conclusions
Obesity during mid-life potentially reduces the risk of developing PD; however, additional studies are needed to further explore this association.
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder in the world. PD affects 1 % of the world population over the age of 60, and it is estimated that by 2030, there will by 8.7 to 9.3 million peopled living with PD. Approximately 10–15 % PD cases are associated with genetic mutations; however, the etiology of other cases is considered to be multifactorial [1].
Cardiovascular (CV) risk factors are modifiable risk factors and are known to be associated with the development of PD; however, studies have demonstrated conflicting results [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]. For example, researchers have suggested that increased pack years of cigarette smoking is associated with a reduced risk of developing PD, however, there is no clear consensus regarding the relationship between other CV risk factors, such as diabetes, hyperlipidemia, and hypertension, and obesity and the risk of developing PD [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]. In this study, we assessed the relationship between CV risk factors and the development of PD, with particular focus on the relationship between body mass index (BMI) and the development of PD in the Framingham Heart Study using a retrospective cohort design.
2. Methods
2.1. The Framingham Heart Study
The Framingham Heart Study (FHS) is a multigenerational longitudinal cohort study that was started in 1948 and is well-known for CV health studies [11]. FHS participants are evaluated during core FHS examinations every 2–4 years. During each FHS core examination, clinical history, anthropometric measures, vital signs, blood samples, and imaging are collected. These core examinations are conducted either in the FHS offices, in the participant’s home, or clinical facility. Inclusion criteria for FHS are adult men and women who are at least 20 years old and have completed an informed consent [11], [14].
The FHS has evolved over time, and currently imaging, genetic studies, and wearable device data are collected during these examinations, however, during early core examinations (i.e. Generation 1 Examination 1, 1948–1953), only vital signs, physical examination, smoking status, and neurocognitive assessments were obtained. Although blood samples were collected during these early assessments, some CV risk factor measures were not collected. For example, cholesterol levels were first checked in FHS in 1957 [15].
2.2. PD case surveillance
FHS has screened for PD since 1988 using diagnostic criteria through medical records (including general and neurological examinations, medication records, and hospitalizations), participant self-report, hospital surveillance and core examinations conducted by FHS clinicians. If there is concern for PD during review of FHS data collected during the core examination, the participant is then flagged for review by a group of FHS neurologists who are not sub-specialized in movement disorders. Similarly, if deceased patients are suspected to have PD, the potential case is referred to FHS neurologists for review. During the initial review by FHS neurologists, if participant examination findings include tremor, rigidity, or bradykinesia, participants are flagged by FHS movement disorders neurologists for additional evaluation—“PD Review”. Similarly, if a historical diagnosis of PD is mentioned in either their FHS history intake or in outside medical records, they are also flagged for PD Review.
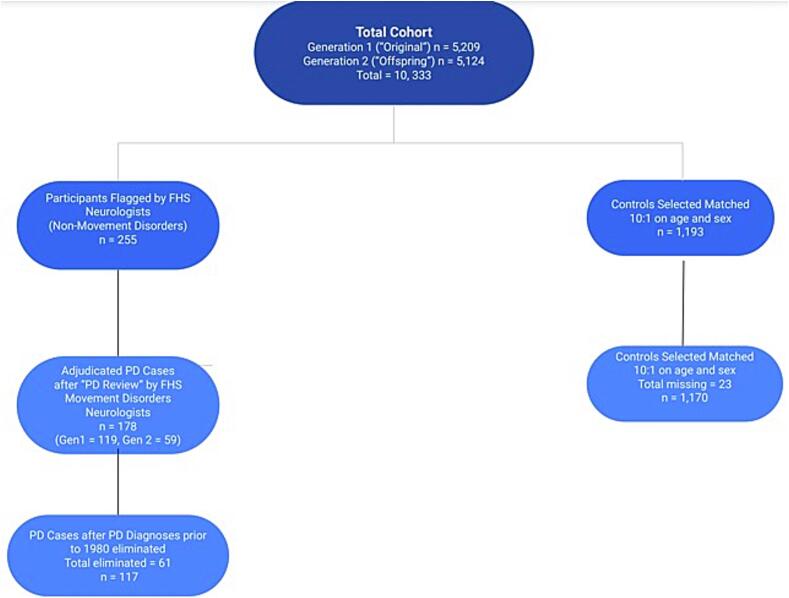
During “PD Review”, flagged participant charts and examinations are reviewed by a consensus panel of three FHS movement disorders neurologists [SAF, SAO, LCS]. The diagnostic criteria used by the movement disorders neurologists are the validated and widely accepted UK Brain Bank Criteria, which includes bradykinesia, in addition to 1.) muscular rigidity, 2.) rest tremor, or postural instability. Prior to the development of the UK Brain Bank Criteria in 2001, the Boston University Criteria were utilized, which reflects the criteria from the Deprenyl and Tocopherol Anti-Oxidative Therapy of Parkinsonism. Participant charts are then reviewed for PD exclusion criteria and “red flag” symptoms that suggest that the participant may have or had atypical parkinsonism or other non-PD neurological disease [13], [14]. We excluded 61 potential PD cases diagnosed prior to 1980 due to incomplete data in diagnosis. Please see Fig. 2 for a summary of these procedures.
Fig. 2.
Description of PD case and control ascertainment.
2.3. Study cohort
To ensure we included participants in our study who had data collected during and after the average age of PD onset in the US (approximately age 60 [1]), we included only Generation 1 and Generation 2 in our analyses. Since data collection was not consistently performed during early core examinations of the Original Cohort, participants with a PD diagnosis prior to 1980 were not included to avoid missing data (61 cases total were excluded for this reason). The total number of incident PD cases within the cohort was 117 (total confirmed in Generation 1 and Generation 2) (Fig. 2). Only participants with complete datasets were included in this study (i.e. all CV risk factor covariates, neuropsychological testing, and neurological evaluations available for analysis). Given the need for complete datasets, some PD participant data was collected as late as four years prior to PD motor symptom onset. PD participants were excluded if “baseline” FHS core exams were not performed at least four years prior to PD onset (Supplemental Table 4). Please note that “baseline” does not refer to the initial FHS examination in this study, rather the examination time point in which CV risk factors were assessed prior to PD onset.
2.4. Controls
In order to increase our sample size as much as possible and reduce the amount of missing data, we included participants from the Original Cohort (“Generation 1”) (n = 5209) and Offspring Cohort (“Generation 2”) (n = 5,124), who have completed up to nine examinations approximately every two years. The Original Cohort of participants were recruited into the multigenerational longitudinal cohort from the Framingham community. In 1971, children of the Original Cohort and their spouses were recruited into the Offspring Cohort [11], [12], [14].
Controls (1:10) were selected and matched on age (within two years) and self-reported sex at baseline examination to those with PD. Controls missing anthropomorphic measurements and CV datapoints were not included in our analyses. Another condition for control matching was survival of at least 15 years after the core examination in which CV risk factor data was initially obtained for the analyses. For example, if a female participant were diagnosed with PD in 2000, the core examination with her BMI and other CV risk factor assessed would be obtained from the 1990 core examination, ten years prior to diagnosis. If she were 62 years old during that baseline core examination, then ten female controls aged 62 ± 2 would be selected and would be included in the analyses if they lived 15 years beyond the FHS core examination from which the covariate (i.e. CV risk factor) data was obtained (“baseline examination”). It should be noted, however, that we chose 15 years of follow-up because the mean time to death after PD diagnosis is 15.8 years and we wanted to ensure that potential PD cases survived to that point, as more rapidly progressive disease is associated with atypical parkinsonian diseases [34].
2.5. CV risk factors
Medical history (diabetes, hypertension, hypercholesterolemia, and history of smoking) data was collected by participant self-report and medical records data. CV data was collected prior to PD onset during a core examination. We used covariates, including age at baseline, sex, BMI, diabetes, hypertension, total cholesterol, and current smoker. Please see Supplemental Table 1 for variable definitions.
3. Statistical analyses
Descriptive statistics were reported at baseline, including age (mean, standard deviation), using the t test and Chi square test. The baseline core examinations were performed every 2–4 years.
We carried out Cox proportional hazard regression models, with initial core examinations with complete datasets (i.e. all analyzed covariates available) serving as “baseline” datapoints. The exposures in our study are CV risk factors, including elevated BMI, blood pressure, serum cholesterol levels, glucose levels, and smoking status. All analyses were performed using SAS software (version 9.4).
3.1. Missing data
To maximize the sample size, we applied the carry-forward technique to fill in missing values. Specifically, if a participant had a missing value at baseline, we used the most recent non-missing value of that variable to fill the gap, provided that the observed value was within five years of the baseline examination (Supplemental Table 2). As previously noted, 61 PD participants were excluded given their diagnosis was made prior to 1980, otherwise all PD cases made during PD reviews (117 total) were included in our analyses.
4. Results
The study included 1,287 participants (56.4 % male; 43.5 % female) of the 10,333 total participants in the FHS study cohort (PD cases and 1:10 matched non-PD participants). The mean age was 68.0 years for controls and 69.0 years for PD cases (standard deviation 9.2 and 9.6, respectively). The median follow-up time for cases (i.e. time from FHS core examination to time of PD diagnosis) was 8.0 years, and 18.0 for controls (i.e. time from FHS core examination until death or March 1, 2021- date of last examination prior to this study) (Please see supplemental Table 3 for follow-up statistics). Accounting for missing covariate data, the total number of participants with PD was 117 and total number of controls was 1170 (Table 1, Fig. 2).
Table 1.
Distribution of cases and controls by sex. Controls were selected based on sex and age (within two years) at baseline exam and survived at least 15 years from the selected baseline.
| Frequency | Male | Female | Total |
|---|---|---|---|
| Controls | 660 | 510 | 1170 |
| PD Cases | 66 | 51 | 117 |
| Total | 726 | 561 | 1287 |
Cox proportional hazard regressions were performed, adjusted for age and gender, as well as the following covariates: total cholesterol, hypertension, smoking status, and diabetes. We did not find an association between total cholesterol, hypertension, smoking status, and diabetes history and PD (Table 2). Low and normal baseline BMI (BMI < 18.5 kg/m2 to <25 kg/m2), were not associated with incident PD. Overweight (BMI 25 kg/m2 to < 30 kg) was associated with lower PD risk, (HR = 0.66, 95 %CI, 0.44–0.98; p = 0.04) as was obesity (BMI >= 30 kg/m2) (HR = 0.47, 95 %CI, 0.26–0.82; p = 0.01). When combined, the overweight and obese BMI groups were combined, there was a protective effect of high BMI on the development of PD (HR = 0.67, 95 %CI, 0.41–0.86; p = 0.01), after adjustment for relevant covariates (Table 2; Fig. 1).
Table 2.
Cox proportional hazard regression outcomes.
| Characteristics |
Total |
PD cases |
Hazard ratio | 95 %CI | P-value | ||
|---|---|---|---|---|---|---|---|
| Number | % | Number | % | ||||
| Age group at baseline exam, years | 1287 | 100 | 117 | 9.09 | |||
| <55 | 103 | 8.00 | 8 | 7.77 | 1 | ||
| 55–64 | 265 | 20.59 | 23 | 8.68 | 1.14 | 0.51–2.55 | 0.75 |
| 65–74 | 572 | 44.44 | 49 | 8.57 | 1.15 | 0.54–2.45 | 0.71 |
| 75 or older | 347 | 26.96 | 37 | 10.66 | 1.47 | 0.67–3.20 | 0.34 |
| Gender | |||||||
| Male | 726 | 56.41 | 66 | 9.09 | 1 | ||
| Female | 561 | 43.59 | 51 | 9.09 | 0.94 | 0.64–1.37 | 0.75 |
| BMI | |||||||
| <25 | 406 | 31.55 | 51 | 12.56 | 1 | ||
| 25 − <30 | 593 | 46.08 | 49 | 8.26 | 0.66 | 0.44–0.98 | 0.04 |
| ≥ 30 | 275 | 21.37 | 16 | 5.82 | 0.46 | 0.26–0.82 | 0.01 |
| Total cholesterol | |||||||
| <200 | 461 | 35.82 | 46 | 9.98 | 1 | ||
| 200-<240 | 477 | 37.06 | 45 | 9.43 | 0.93 | 0.61–1.40 | 0.71 |
| ≥ 240 | 302 | 23.47 | 22 | 7.28 | 0.69 | 0.41–1.16 | 0.16 |
| Hypertension | |||||||
| No | 745 | 57.89 | 71 | 9.53 | 1 | ||
| Yes | 542 | 42.11 | 46 | 8.49 | 0.87 | 0.60–1.27 | 0.46 |
| Smoke | |||||||
| No | 1123 | 87.26 | 109 | 9.71 | 1 | ||
| Yes | 157 | 12.20 | 7 | 4.46 | 0.47 | 0.22–1.02 | 0.06 |
| Diabetes | |||||||
| No | 1135 | 88.19 | 108 | 9.52 | 1 | ||
| Yes | 131 | 10.18 | 8 | 6.11 | 0.62 | 0.30–1.28 | 0.20 |
Fig. 1.
Kaplan-Meier Curve. Time-to-event (Parkinson’s disease diagnosis) plotted on x-axis, with probability of “survival” plotted on y-axis. Cox proportional hazard regressions were performed based on imputed data, adjusted for age and gender.
5. Discussion
Using a retrospective cohort design, we have demonstrated that higher BMI could potentially be protective against the development of PD in dose dependent manner. As this study is an observational study, we cannot conclude that obesity is protective. Additional studies are necessary to further explore the relationship between higher BMI and PD risk.
Several studies have demonstrated that higher BMI is a risk factor for the development of PD, whereas other studies demonstrate that higher BMI mitigates the risk of developing PD. As we have demonstrated in the present study, higher BMI could also confer protection against developing PD (Table 3, references 16–24). The disparate results amongst these studies could result from varying methodologies. Other factors that may contribute to the varying results amongst prior studies include sample size limitations. It has also been increasingly recognized that PD is a heterogenous disorder that is influenced by genetics and specific populations (i.e. diet variation across cultures, which could influence CV risk factors) [25], [26]. This heterogeneity could contribute to disparate results across PD and CV risk factor studies.
Table 3.
Summary of studies investigating BMI and PD.
| Study | Study Design | Cohort Population | Number of subjects | Follow-up | Risk | Notes |
|---|---|---|---|---|---|---|
| Abbott et al, 2002 [10] | Prospective Cohort | United States | 7,900 males | 30 years | Increased Risk of PD | Individuals with greater midlife triceps skin fold thickness had a threefold increase of risk for developing PD; Honolulu Aging Study. |
| Hu et al, 2006 [16] | Prospective Cohort | Finland | 22,367 Finnish males and 23,439 females | 18.8 years | Increase Risk of PD | Incremental increase in risk of developing PD with higher BMI |
| Wang et al, 2015 [17] | Metanalysis | Multiple | Variable 10 prospective studies | Variable | Increase Risk of PD | Findings did not support that higher BMI increases PD risk, however a week positive BMI-PD association was noted. |
| Ikeda et al, 2007 [18] | Prospective Cohort | Japan | 20,000 with 24 PD cases | 10 years | Increase Risk of PD | |
| Chen et al, 2004 [19] | Prospective Cohort | United States | 160,000 with 468 PD cases | Increase Risk of PD | No role for obesity in PD pathogenesis; however, central obesity may be associated with higher PD risk among never smokers; Nurses Health Study (NHS), Health Professionals Follow-up Study (HPFS). | |
| Savica et al, 2012 [20] | Case Control | United States | 196 with PD with 1:1 control match | 18 years | No Change in Risk of PD | |
| Ramhani et al, 2022 [21] | Systematic Review | Multiple | Multiple | Multiple | No Change in Risk of PD | |
| Logroscino et al, 2007 [22] | Prospective Cohort | United States | 10,812 males | 10 years | No Change in Risk of PD | |
| Jeong et al, 2020 [23] | Case Control | Korea | 33,443 with PD total of 6.8 million healthy controls | 7.3 years | Decreased Risk of PD | Being underweight and having diabetes mellitus were associated with an increased risk of PD incidence. |
| Ma et al, 2006 [24] | Nested Case Control | China | 16,488 (85 PD cases included in analyses | N/A | Decreased Risk of PD | Meat consumption and the body mass index (BMI) were inversely associated with PD. |
The present study is unique as it utilizes data obtained from two generations and over the course of nearly 75 years, with the initial examination of Generation 1 being in 1948 and the final examination of Generation 2 in 2022. The study is also unique because it demonstrates the challenges of utilizing historical epidemiological datasets and presents methods in which use of data could be maximized. Our study is one of the largest cohort studies which assesses the relationship between CV risk factors and the development of PD, specifically analyzing data from mid-life (age 40–60) within a decade of PD onset. The use of a retrospective cohort design is also unique and allowed us to prospectively assess the relationships between CV risk factors and the development of PD.
Some studies have suggested that adiposity can potentially contribute to the risk of developing PD, although the pathobiological mechanism has not been determined. There have been several hypotheses developed which aim to explain this association. Dopamine is known to play a role in energy intake regulation, and therefore it is thought that with loss of dopaminergic neurons in PD, dopamine activity in the hypothalamus is also affected, leading to obesity. Obese individuals are also noted to have depletion of their dopamine receptor activity. Other hypotheses include that adiposity acts as a source of lipid-soluble dopaminergic neurotoxins and increased adiposity acts as a metabolic mediator to increase neuronal susceptibility to damage from neurotoxins. It has also been shown that obese transgenic mouse models have increased vulnerability to neurotoxins within the striatal dopaminergic neurons [27], [28]. Lastly, researchers have also suggested that adiposity can result from environmental obesogens which disrupt estrogen-regulated metabolic pathways, noting that a reduction in endogenous estrogen can be associated with increased PD risk [29].
The pathobiological mechanism by which increased adiposity could potentially confer protection against the development of PD is also unknown. One theory is that higher BMIs affect levels of circulating and central insulin, which may play a beneficial role in neurodegeneration, as circulating insulin is known to be beneficial in conferring protection against neurodegenerative diseases, although it has also been hypothesized that impaired glucose homeostasis can indirectly play a role in the development of PD [28], [29], [30]. Lipids are necessary for normal neurodevelopment and central nervous function and cholesterol metabolism and reduced biosynthesis of cholesterol within the brain has been associated with the development of PD [26]. Prior studies have suggested that elevated triglycerides, LDL, and total cholesterol can be protective in the development of PD and another study has suggested patients with PD have lower triglycerides compared to controls [31], [32], [33].
The findings in the present study suggest that further investigations into the relationship between BMI and PD risk are necessary, as currently there is not a consensus regarding the relationship between increased adiposity and neurodegenerative disease. The possibility that obesity is protective against the development of PD suggests that leptin and insulin regulation may play a neuroprotective role in the development of neurodegenerative disease, highlighting the potential for metabolic and neuroendocrine interventions. Additional translational research investigating the molecular pathways that integrate leptin regulation and neural plasticity are necessary before a distinct pathobiological process can be determined and potential treatments are investigated.
Our study has limitations, particularly regarding PD case ascertainment and sample size. PD cases have historically been identified in FHS by assessing medical records, including clinic examinations conducted by FHS doctors, subject medical charts, and hospital surveillance, with the diagnosis being based on the UK Brain Bank Criteria (and previously the Boston University Criteria, prior to establishment of the UK Brain Bank Criteria). A movement disorders neurologist did not directly examine these individuals to determine the diagnosis, which can lead to case ascertainment bias, as cases can be missed if records alone are assessed. It should be noted, however, that it has previously been reported than PD diagnoses within the FHS cohort using self-report and use of antiparkinsonian medications could identify PD cases with good accuracy (with a positive predictive value of 100 %) compared to PD cases ascertained via formal clinical diagnostic criteria [14].
Excluding PD cases could also introduce bias to our study, specifically exclusion bias. Please note that of the cases flagged for potential PD in initial FHS review (i.e. 255 potential PD flags made by non-movement disorders neurologists), most were noted to have signs of atypical parkinsonism (i.e. dementia per neuropsychological assessments, severe orthostatic hypotension and other signs of dysautonomia, or other examination or imaging findings suggestive of atypical parkinsonism or normal pressure hydrocephalus) were excluded, with a total of 178 cases being marked as “PD cases”. Given that cases made prior to 1980 were excluded to ensure complete data sets were utilized, exclusion bias could occur, potentially reduce sample size and making a statistically significant result less likely (i.e. bias towards the null).
Another potential source of selection bias in our study is the fact that we required 15 years of follow-up after the “baseline” FHS core examination for controls. It is possible that individuals with higher BMI and co-morbidities associated with obesity were excluded from our analyses if they were not able to attend FHS core examinations due to mobility limitations or passed away due to these co-morbidities prior to 15 years. As previously noted, a 15-year follow-up was chosen to account for the mean time to death after PD diagnosis of 15.8 years [34].
FHS is also not a racially and ethnically diverse cohort. The FHS cohort is comprised of predominantly White participants of European descent. FHS has recruited more diverse participants in recent years, by developing the Omni1 and Omni2 cohorts, in 1994 and 2003, respectively. Participants within these cohorts were also recruited from Framingham, MA and reflect a more ethnically and racially and ethnically diverse population [11]. Data from these newer cohorts was not incorporated into this study, as these participants have not yet undergone PD review. Investigating the incidence of PD within these more diverse FHS cohorts is of the utmost importance in the future.
It should also be noted that weight loss can occur early in the PD disease course, which could contribute to the presence of lower BMI within this cohort; however, it has been reported that little weight loss in the setting of PD occurs during the first six years of the disease. It has also been noted in other studies that BMI does not change prior to PD symptom onset [35], [36].
Although BMI and other CV risk factor data was collected up to 12 years prior to PD onset, the prodromal period of Parkinson’s disease can be 5 to over 20 years in duration [37]. Given that weight loss is also known to be associated with the development of PD, it is possible that our results are influenced by the prodromal PD period and that lower BMI is associated with prodromal PD, rather than higher BMI being protective against the development of PD.
The relationship between diet and BMI may also have an impact on our findings. Diet is known to play a significant role in the development of PD, as higher Mediterranean-type diet adherence was associated with reduced odds for development of PD, with lower Mediterranean-type diet adherence being associated with earlier PD onset [38], therefore excluding this data may also impact our results. Mediterranean-type diets are also associated with lower BMI [39], so this would contradict our findings and highlights the importance of adjusting for diet in this study.
Similarly, physical activity is known to reduce the risk of PD [40]. A dose–response relationship has been noted between moderate to vigorous exercise and PD risk reduction [41]. Nutritional information and measures of physical activity were not collected in all FHS examinations, particularly early core examinations, so these measures could not be added to our models. It is possible that diet and activity could be potential confounders in our study.
In conclusion, our findings support prior non-U.S. studies that suggest increased adiposity may confer protection against the development of PD, although as previously noted, causation cannot be concluded in an observational study. Translational studies further assessing the relationship between BMI and neurodegenerative disease are needed, as elucidating this relationship can potentially steer the future of PD interventions and preventative therapies.
CRediT authorship contribution statement
Sarah O’Shea: . Yuilin Liu: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Chunyu Liu: Writing – review & editing, Formal analysis, Data curation. Samuel A. Frank: Writing – review & editing. Ludy C. Shih: Writing – review & editing. Rhoda Au: Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Rhoda Au reports financial support was provided by National Institutes of Health. Rhoda Au reports financial support was provided by National Institutes of Health. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
There are no acknowledgements.
Disclosures
S.A.O., Y.L., C.L., S.A.F., L.C.S., and R.A. have no financial disclosures. The authors declare that there are no conflicts of interest relevant to this work.
Ethical compliance statement
The protocol was reviewed and approved by the Boston University institutional review board as well as the Framingham Heart Study Executive Committee. Written consent was obtained for all participants. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding statement
This study was funded by Framingham Heart Study’s National Heart, Lung, and Blood Institute contract (N01-HC-25195), the National Institute on Aging (AG016495, AG008122, AG068753).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prdoa.2024.100291.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol, 2018. 17(11): p. 939–53. [DOI] [PMC free article] [PubMed]
- 2.Ritz B., Rhodes S.L. After half a century of research on smoking and PD, where do we go now? Neurology. 2010;74(11):870–871. doi: 10.1212/WNL.0b013e3181d63aa8. [DOI] [PubMed] [Google Scholar]
- 3.De Lau L.C.L., Koudstaal P.J., Hofman A., Breteler M.M.B. Serum cholesterol levels and the risk of Parkinson’s disease. Am. J. Epidemiol. 2006;164:998–1002. doi: 10.1093/aje/kwj283. [DOI] [PubMed] [Google Scholar]
- 4.De Pablo-Fernandez E., Goldcare R., Pakpoor J., Noyce A.J., Warner T.T. Association between diabetes and subsequent Parkinson disease: a record-linkage cohort study. Neurology. 2018;91:e139–e142. doi: 10.1212/WNL.0000000000005771. [DOI] [PubMed] [Google Scholar]
- 5.Vikdahl M., Bäckman L., Johansson I., et al. Cardiovascular risk factors and the risk of Parkinson’s disease. Eur. J. Clin. Nutr. 2015;69:729–733. doi: 10.1038/ejcn.2014.259. [DOI] [PubMed] [Google Scholar]
- 6.Fu X., Wang Y., He X., et al. A systematic review and meta-analysis of serum cholesterol and triglyceride levels in patients with Parkinson’s disease. Lipids Health Dis. 2020;19:97. doi: 10.1186/s12944-020-01284-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Pablo-Fernandez E., Goldacre R., Pakpoor J., Noyce A.J., Warner T.T. Association between diabetes and subsequent Parkinson disease: a record-linkage cohort study. Neurology. 2018;91(2):e139–e142. doi: 10.1212/WNL.0000000000005771. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y.W., Hsieh T.F., Li C.I., et al. Increased risk of Parkinson disease with diabetes mellitus in a population-based study. Medicine (Baltimore) 2017;96(3) doi: 10.1097/MD.0000000000005921. e5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou L., Li Q., Jiang L., et al. Hypertension and diagnosis of Parkinson’s Disease: a meta-analysis of cohort studies. Front. Neurol. 2018;9:162. doi: 10.3389/fneur.2018.00162. Published 2018 Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbott R.D., Ross G.W., White L.R., et al. Midlife adiposity and the future risk of Parkinson’s disease. Neurology. 2002;59(7):1051–1057. doi: 10.1212/wnl.59.7.1051. [DOI] [PubMed] [Google Scholar]
- 11.Tsao C.W., Vasan R.S. Cohort Profile: The Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int. J. Epidemiol. 2015;44(6):1800–1813. doi: 10.1093/ije/dyv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson C., Johnson A.D., Benjamin E.J., Levy D., Vasan R.S. 70-year legacy of the Framingham Heart Study. Nat. Rev. Cardiol. 2019;16(11):687–698. doi: 10.1038/s41569-019-0202-5. [DOI] [PubMed] [Google Scholar]
- 13.DATATOP: a multicenter controlled clinical trial in early Parkinson’s disease. Parkinson Study Group. Arch. Neurol. 1989;46 (10):1052-1060. [DOI] [PubMed]
- 14.Jain S, Himali J, Beiser A, Ton TG, Kelly-Hayes M, Biggs ML, Delaney JA, Rosano C, Seshadri S, Frank SA. Validation of secondary data sources to identify Parkinson disease against clinical diagnostic criteria. Am J Epidemiol. 2015;181(3):185-90. doi: 10.1093/aje/kwu326. Epub 2014 Dec 29. PMID: 25550359; PMCID: PMC4312428. [DOI] [PMC free article] [PubMed]
- 15.Li J.J. Oxford University Press; Oxford, New York: 2009. Triumph of the heart: the story of statins. [Google Scholar]
- 16.Hu G., Jousilahti P., Nissinen A., Antikainen R., Kivipelto M., Tuomilehto J. Body mass index and the risk of Parkinson disease. Neurology. 2006;67(11):1955–1959. doi: 10.1212/01.wnl.0000247052.18422.e5. [DOI] [PubMed] [Google Scholar]
- 17.Wang YL, Wang YT, Li JF, Zhang YZ, Yin HL, Han B. Body mass index and risk of Parkinson’s disease: a dose-response meta-analysis of prospective studies. PloS One. 2015;10(6):e0131778. Doi:10.1371/journal.pone.0131778. [DOI] [PMC free article] [PubMed]
- 18.Ikeda K., Kashihara H., Tamura M., Kano O., Iwamoto K., Iwasaki Y. Body mass index and the risk of Parkinson disease. Neurology. 2007;68(24):2156–2157. doi: 10.1212/01.wnl.0000269477.49238.ec. [DOI] [PubMed] [Google Scholar]
- 19.Chen H., Zhang S.M., Schwarzschild M.A., Hernan M.A., Willett W.C., Ascherio A. Obesity and the risk of Parkinson’s disease. Am. J. Epidemiol. 2004;159(6):547–555. doi: 10.1093/aje/kwh059. Epub 2004/03/09. [DOI] [PubMed] [Google Scholar]
- 20.Savica R., Grossardt B.R., Ahlskog J.E., Rocca W.A. Metabolic markers or conditions preceding Parkinson’s disease: a case-control study. Mov. Disord. 2012;27(8):974–979. doi: 10.1002/mds.25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahmani J., Roudsari A.H., Bawadi H., et al. Body mass index and risk of Parkinson, Alzheimer, Dementia, and Dementia mortality: a systematic review and dose-response meta-analysis of cohort studies among 5 million participants. Nutr. Neurosci. 2022;25(3):423–431. doi: 10.1080/1028415X.2020.1758888. [DOI] [PubMed] [Google Scholar]
- 22.Logroscino G., Sesso H.D., Paffenbarger R.S., Jr., Lee I.M. Body mass index and risk of Parkinson’s disease: a prospective cohort study. Am. J. Epidemiol. 2007;166:1186–1190. doi: 10.1093/aje/kwm211. [DOI] [PubMed] [Google Scholar]
- 23.Jeong S.M., Han K., Kim D., Rhee S.Y., Jang W., Shin D.W. Body mass index, diabetes, and the risk of Parkinson’s disease. Mov. Disord. 2020;35(2):236–244. doi: 10.1002/mds.27922. [DOI] [PubMed] [Google Scholar]
- 24.Ma L., Zhang L., Gao X.H., et al. Dietary factors and smoking as risk factors for PD in a rural population in China: a nested case-control study. Acta Neurol. Scand. 2006;113(4):278–281. doi: 10.1111/j.1600-0404.2005.00571. [DOI] [PubMed] [Google Scholar]
- 25.Marras C., Fereshtehnejad S.M., Berg D., Bohnen N.I., Dujardin K., Erro R., Espay A.J., Halliday G., Van Hilten J.J., Hu M.T., Jeon B., Klein C., Leentjens A.F.G., Mollenhauer B., Postuma R.B., Rodríguez-Violante M., Simuni T., Weintraub D., Lawton M., Mestre T.A. Transitioning from subtyping to precision medicine in Parkinson's Disease: A purpose-driven approach. Mov. Disord. 2024;39(3):462–471. doi: 10.1002/mds.29708. Epub 2024 Jan 20 PMID: 38243775. [DOI] [PubMed] [Google Scholar]
- 26.Marsili L, Mahajan A. Clinical milestones in Parkinson's disease: Past, present, and future. J Neurol Sci. 2022;432:120082. doi: 10.1016/j.jns.2021.120082. Epub 2021 Dec 14. PMID: 34923333. [DOI] [PubMed]
- 27.Sriram K., Benkovic S.A., Miller D.B., O’Callaghan J.P. Obesity exacerbates chemically induced neurodegeneration. Neuroscience. 2002;115(4):1335–1346. doi: 10.1016/s0306-4522(02)00306-8. [DOI] [PubMed] [Google Scholar]
- 28.Chen J.Q., Brown T.R., Russo J. Regulation of energy metabolism pathways by estrogens and estrogenic chemicals and potential implications in obesity associated with increased exposure to endocrine disruptors. Biochim. Biophys. Acta. 2009;1793(7):1128–1143. doi: 10.1016/j.bbamcr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craft S. Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis. Assoc. Disord. 2006;20(4):298–301. doi: 10.1097/01.wad.0000213866.86934.7e. [DOI] [PubMed] [Google Scholar]
- 30.Ekraminasab S., Dolatshahi M., Sabahi M., Mardani M., Rashedi S. The interactions between adipose tissue secretions and Parkinson’s disease: the role of leptin. Eur. J. Neurosci. 2022;55(3):873–891. doi: 10.1111/ejn.15594. [DOI] [PubMed] [Google Scholar]
- 31.Jin U., Park S.J., Park S.M. Cholesterol metabolism in the brain and its association with Parkinson's disease. Exp. Neurobiol. 2019;28(5):554–567. doi: 10.5607/en.2019.28.5.554. PMID: 31698548; PMCID: PMC6844833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu X., Wang Y., He X., Li H., Liu H., Zhang X. A systematic review and meta-analysis of serum cholesterol and triglyceride levels in patients with Parkinson's disease. Lipids Health Dis. 2020;19(1):97. doi: 10.1186/s12944-020-01284-w. PMID: 32430016; PMCID: PMC7236933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Y, Jin X, Zhao P. Serum lipids and the pathogenesis of Parkinson's disease: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021 Apr;75(4):e13865. doi: 10.1111/ijcp.13865. Epub 2020 Dec 8. PMID: 33244851. [DOI] [PubMed]
- 34.Forsaa E.B., Larsen J.P., Wentzel-Larsen T., Alves G. What predicts mortality in Parkinson disease?: a prospective population-based long-term study. Neurology. 2010;75(14):1270–1276. doi: 10.1212/WNL.0b013e3181f61311. PMID: 20921512. [DOI] [PubMed] [Google Scholar]
- 35.Willis AM, Li R, Pérez A, Ren X, Boyd J; NINDS NET-PD Investigators. Predictors of weight loss in early treated Parkinson’s disease from the NET-PD LS-1 cohort. J Neurol. 2017 Aug;264(8):1746-1753. Doi: 10.1007/s00415-017-8562-4. Epub 2017 Jul 15. PMID: 28712000; PMCID: PMC5789795. [DOI] [PMC free article] [PubMed]
- 36.Ragonese P., D’Amelio M., Callari G., Di Benedetto N., Palmeri B., Mazzola M.A., Terruso V., Salemi G., Savettieri G., Aridon P. Body mass index does not change before Parkinson’s disease onset. Eur. J. Neurol. 2008;15(9):965–968. doi: 10.1111/j.1468-1331.2008.02236.x. Epub 2008 Jul 14 PMID: 18637822. [DOI] [PubMed] [Google Scholar]
- 37.Roos D.S., Klein M., Deeg D.J.H., Doty R.L., Berendse H.W. Prevalence of prodromal symptoms of parkinson's disease in the late middle-aged population. J. Parkinsons. Dis. 2022;12(3):967–974. doi: 10.3233/JPD-213007. PMID: 35180132; PMCID: PMC9108586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alcalay RN, Gu Y, Mejia-Santana H, Cote L, Marder KS, Scarmeas N. The association between Mediterranean diet adherence and Parkinson's disease. Mov Disord. 2012 May;27(6):771-4. doi: 10.1002/mds.24918. Epub 2012 Feb 7. PMID: 22314772; PMCID: PMC3349773. [DOI] [PMC free article] [PubMed]
- 39.Dominguez L.J., Veronese N., Di Bella G., Cusumano C., Parisi A., Tagliaferri F., Ciriminna S., Barbagallo M. Mediterranean diet in the management and prevention of obesity. Exp. Gerontol. 2023;174 doi: 10.1016/j.exger.2023.112121. Epub 2023 Feb 17 PMID: 36792040. [DOI] [PubMed] [Google Scholar]
- 40.Nelson L.M. Physical activity and Parkinson disease risk: an intriguing link. JAMA Netw. Open. 2018;1(5) doi: 10.1001/jamanetworkopen.2018.2633. e182633. [DOI] [PubMed] [Google Scholar]
- 41.Fang X., Han D., Cheng Q., et al. Association of levels of physical activity with risk of Parkinson disease: a systematic review and meta-analysis. JAMA Open Netw. 2018;1(5) doi: 10.1001/jamanetworkopen.2018.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.