Summary
Background
Co-existence of efficient transportation networks and geographic imbalance of medical resources greatly facilitated inter-city migration of patients of infectious diseases in China.
Methods
To characterize the migration patterns of major notifiable infectious diseases (NIDs) during 2016–2020 in China, we collected migratory cases, who had illness onset in one city but were diagnosed and reported in another, from the National Notifiable Infectious Disease Reporting System, and conducted a nationwide network analysis of migratory cases of major NIDs at the city (prefecture) level.
Findings
In total, 2,674,892 migratory cases of NIDs were reported in China during 2016–2020. The top five diseases with the most migratory cases were hepatitis B, tuberculosis, hand, foot and mouth disease (HFMD), syphilis, and influenza, accounting for 79% of all migratory cases. The top five diseases with the highest proportions of migratory cases were all zoonotic or vector-borne (37.89%‒99.98%). The network analysis on 14 major diseases identified three distinct migration patterns, where provincial capitals acted as key node cities: short distance (e.g., pertussis), long distance (e.g., HIV/AIDS), and mixed (e.g., HFMD). Strong drivers for patient migration include population mobility and labor flow intensities between cities as well as the economic development level of the destination city.
Interpretation
Collaborative prevention and control strategies should target cities experiencing frequent patient migration and cater to unique migration patterns of each disease. Addressing disparity in healthcare accessibility can also help alleviate case migration and thereby reduce cross-regional transmission.
Funding
National Key Research and Development Program of China.
Keywords: Human mobility, Network analysis, Disease migration
Research in context.
Evidence before this study
We searched PubMed database on June 14th, 2024 by terms “((Communicable Diseases [MeSH Major Topic]) AND ((Human Migration [MeSH Major Topic]) OR (human mobility))) AND (China [Title/Abstract])”, without language restrictions. Among 81 articles found, 64 related articles revealed the associations between human mobility and infectious diseases, mainly focusing the sexually transmitted diseases represented by HIV/AIDS and pandemic infectious diseases such as COVID-19. Many studies identified significant associations between human mobility and the spread of infectious diseases, e.g., migrant workers and cross-border travelers served as drivers while travel restrictions served as an inhibitor for disease transmission. Most of these studies focused on individual infectious diseases. Only one study examined the effect of human mobility changes on multiple NIDs during the COVID-19 pandemic, but the study was limited to a single city. In addition, these studies focused on either long-distance (international or inter-provincial) and short-distance travels (between cities within a province or between areas within a city), but not both. There lacks a comprehensive comparison of inter-city migration patterns and network structures across different infectious diseases at the national scale and a thorough investigation on potential socioeconomic and geographical drivers for the migration of NID cases.
Added value of this study
In this study, we conducted a nationwide epidemiological study on the migration patterns of major NIDs during 2016–2020 in China. We characterized the similarities and differences of the number and proportion of migratory cases among NIDs and mapped city-specific inflow and outflow proportions of the NIDs. We constructed city-level networks to study the inter-city migration of 14 major NIDs and developed machine learning models to associate the flow of migratory cases with socio-economic and geographic variables for the 14 major NIDs. We found zoonotic and vector-borne diseases were associated with higher proportions of migratory cases. We identified three typical migration modes of infectious disease patients: short distance between adjacent cities represented by pertussis, brucellosis, mumps, hepatitis E, hepatitis A and Shigellosis; long distance for HIV/AIDS, syphilis, gonorrhea, and tuberculosis; and mixed for HFMD, influenza, hepatitis B and hepatitis C. We found strong connections between several developing inland cities in southwestern China and developed coastal cities in the Yangtze River Delta and the Pearl River Delta in the long-distance mode. We demonstrated that the intensity of population mobility and labor flow between cities were the most important driving factors and provincial capitals acted as key node cities.
Implications of all the available evidence
Migratory NID cases mainly flow from under-developed areas towards developed provincial capitals or coastal cities, suggesting geographic disparity in healthcare resources as an underlying driver for the migration. The similarity between the migration network structure of sexually transmitted diseases and the labor force flow implies the role of geographic disparity in economic development as another driver. Collaborative prevention and control strategies between cities bearing frequent patient migration and policies reducing disparity in healthcare accessibility across regions are needed.
Introduction
A key component in preventing and controlling infectious diseases is accurate characterization of the role of human mobility in disease spread, especially in densely populated countries like China.1, 2, 3, 4, 5, 6, 7 Many studies have demonstrated the impact of human mobility on the spatial spread of a variety of infectious diseases, including but not limited to COVID-19, influenza, measles, hand-foot-and-mouth disease (HFMD), dengue and HIV/AIDS.8, 9, 10, 11, 12, 13 While network analysis has been widely and successfully applied to fields like child trafficking, illegal wildlife trade, and human mobility patterns, few studies have examined the role of human mobility in disease transmission from a network perspective, possibly due to the challenge of tracking the travel history of individual patients.14, 15, 16, 17, 18 The spectrum of diseases spreading via short- and long-distance migration remains unclear, and less is known about the relative importance between short-distance vs. long-distance travel. While long-distance traveling of patients or carriers of infectious pathogens is less frequent, it could help seed the pathogens in previously less exposed, and thus more susceptible, populations. This may help to sustain endemicity even if the pathogens are not highly transmissible locally.19 The relative importance of short-vs. long-distance traveling on the transmission dynamics of infectious diseases may be context-dependent, driven by demographic, environmental and socioeconomic features of the population as well as biological characteristics of the underlying pathogens.13,20
Since its reopening in the early 1980s, China has undergone rapid urbanization, resulting in a highly mobile population,3,4 facilitating inter-regional spread of infectious diseases. During its modernization, China established a real-time internet-based infectious disease surveillance system, the National Notifiable Infectious Disease Reporting System (NNIDRS),21 which has covered over 168,000 various medical and health institutions across China; the timely reporting rate for notifiable infectious diseases nationwide has reached over 99%, with the average time from diagnosis to reporting being approximately 4 h.22 The NNIDRS captures comprehensive data on notifiable infectious diseases (NID), including demographic, clinical and diagnostic information for every individual case. In particular, the availability of addresses at the times of disease onset and reporting for each case makes it possible to characterize domestic inter-regional migration patterns for a variety of infectious diseases. Here we perform a nationwide network analysis of migratory cases of major notifiable infectious disease at the city (prefecture) level in China.
Methods
Data collection
Data on NIDs reported from 1 January 2016 to 31 December 2020 was collected from the NNIDRS. According to the Law on the Prevention and Control of Infectious Diseases in the People’s Republic of China, 39 types of notifiable infectious diseases were reported in NNIDRS. We further divided three primary disease groups into finer disease types: the viral hepatitis cases were separated into hepatitis A, hepatitis B, hepatitis C, hepatitis D, and hepatitis E, the dysentery cases into shigellosis and amoebic dysentery, and the typhoid/paratyphoid cases into typhoid and paratyphoid. We excluded 2 diseases (untyped viral hepatitis; infectious diarrhea other than cholera, dysentery and typhoid) due to unclear pathogenesis. In total, we assessed 44 infectious diseases in this study. Similar to previous studies, a migratory case is defined as a patient whose residential city on the date of reporting differs from the residential address of the case at disease onset (referred to as current address in the NNIDRS), regardless of their motivation of movement (business, tourism, medical consultation, etc.). According to the National Guidelines of Infectious Diseases Report (http://cdcp.gd.gov.cn/zwgk/jsbzywj/content/post_3437495.html), the current address of an individual case in the NNIDRS refers to his/her residential place at the disease onset. The detailed definition of current address of an individual case is given in Appendix (p 2); otherwise, the patient is considered a local case.17,18,23 Data on inter-city human mobility among the 337 prefectural cities in China were obtained by web crawling from AMAP (https://trp.autonavi.com/migrate/page.do), which is the biggest location-based data provider in China (Appendix p 35). Inter-city labor flow intensity was calculated using the Microdata of 2015 (1% Population Survey of China) provided by the National Bureau of Statistics (https://microdata.stats.gov.cn/#/). Specifically, the calculation was based on the geographical information of the migrant workers who had left their hometowns and worked in other cities for more than six months (Appendix p 36). The maps depicting the distributions of infectious diseases were based on the map of China (reference number: GS (2022) 1873) obtained from the official website of the Ministry of Civil Affairs (http://xzqh.mca.gov.cn/map). Other demographic and socioeconomic data about the cities involved in the analysis were also collected. Details of data collection and processing are given in the Appendix pp 2 and 3.
Written informed consent was waived by the National Health Commission of China for the surveillance of notifiable infectious disease. All identifiable personal information was removed from the data by China CDC before any analysis. This study was approved by the Institutional Review Boards of the Academy of Military Medical Science (IRB number: AF/SC-08/02.343).
Descriptive analysis
The overall proportion of migratory cases is defined as where is the number of migratory cases, and N is the total number of NID cases reported to the NNIDRS. Migratory proportions were calculated for different cities, time periods, age and sex groups, and specific types of infectious diseases, respectively. We also mapped city-specific inflow and outflow proportions of the NIDs. Inflow proportion of a given city is the ratio of the number of patients who were reported in the city but had onset elsewhere to the total number of patients who were reported in the city. Outflow proportion is the ratio of the number of patients who had symptom onset in the city but were reported elsewhere to the total number of patients who had symptom onset in the city. Specifically,
where is the number of cases who had symptom onset in city and were reported in city , and is the total number of cities. and represent the total number cases imported to and exported from city of , respectively.
In addition, we compared delays from disease onset to diagnosis and case fatality rates between migratory cases and local cases as a whole as well as between the following finer groups: (i) migratory cases from non-capital cities to capital cities, likely representing patients seeking better healthcare quality; (ii) all other migrant cases; (iii) local cases in capital cities; and (iv) local cases in non-capital cities.
Social network analysis
Out of the 44 NIDs, fourteen had >10,000 domestic migratory cases and accounted for 98.10% of all migratory cases reported to NNIDRS. We constructed city-level networks to study the inter-city migration of these 14 major NIDs, where cities align with prefectures (a prefecture has several counties) in the Chinese administrative division system. In each network, a node refers to a city, a directed edge indicates existence of migratory cases who had symptom onset in the origin city and were reported in the destination city, and the edge weight reflects the number of migratory cases. The following summary statistics and methods were used to characterize the features of infectious disease migration networks. A brief introduction of the social network analysis and the network statistics used were present in the Appendix pp 3, 4 and 37.
Node Strength reflects how active a node is in a weighted network in term of the weighted sum of edges connected to other nodes,24 and is defined as,
where is the number of nodes (cities) in the network, is the weight of the directed edge from node to node , and is the weight of the directed edge from node to node .
Node betweenness is the number of the shortest directed paths (i.e., the path between two nodes that minimizes the sum of the weights along the path) between all pairs of nodes that pass through an intermediary node,24 indicating how important a node is as a bridge for the paths the network. The betweenness centrality of the node is defined as,
where is the number of nodes in the network, is the number of shortest paths between node and node passing through node , and is the total number of shortest paths between node and node .
Community detection is a procedure to study the underlying structural patterns of a network. In this study we used the Louvain algorithm to detect the potential community pattern of the infectious disease migration network (IDMN), which is an unsupervised hierarchical clustering algorithm based on optimizing the increase in modularity (defined later) when a node is moved from its current community to a new community. This algorithm recursively merges detected communities into a single node and perform clustering on the condensed graphs.25 To avoid noise from weak links, we only included the edges with more than 10 migratory cases for community detection and performed a sensitivity analysis by changing this threshold to 20 (Appendix p 38). The modularity of a network is calculated as,
where is the sum of weights of edges among the internal nodes of community , is the sum of weights of all edges connecting to community with external nodes, and is the sum of weights of all edges in the graph. is the resolution parameter that affects the number of the communities, where a higher value leads to more communities. The main results of community detection in this study were produced with the default resolution at where modularity recovered to its original definition (if resolution is less than 1, the algorithm favors larger communities, and greater than 1 favors smaller communities). We provided a Shiny website to display community detection results at different resolutions and show graded risk levels of importing and exporting infectious diseases for each city (https://huashuiguaishou.shinyapps.io/mccx/).
Network backbone reflects the fundamental structure of a network. We applied the disparity filter algorithm and the force-directed edge bundling algorithm to visualize the backbone structure of each IDMN. Disparity filter is a network reduction algorithm that removes unimportant links at a significance level α (in this study α = 0.05) to identify the backbone structure.26 The force-directed edge bundling algorithm clusters edges with similar attributes (i.e., similarity in direction and proximity) together to facilitate visual interpretation.27 For better visualization, edge bundling was applied to backbone edges with more than 10 migratory cases for the top 5 diseases, more than 5 cases for the top 6 to 10, and more than one for the remaining diseases, where diseases were ranked according to the numbers of migratory cases. We used packages “backbone” and “edgebundle” in R 4.2.3 to perform the disparity filter and force-directed edge bundling in this study.
Network modeling
To identify potential driving factors for the spread of infectious diseases via human mobility, we used the eXtreme Gradient Boosting (XGBoost) model to associate the flow of migratory cases with socio-economic and geographic variables for the 14 major NIDs.28 We collected 12 variables to serve as the predictors, of which five are features of the edges (human mobility intensity, labor flow intensity, geographic distance, intra-province or not, and geographically adjacent or not), and six are socioeconomic features of the origin and destination nodes (per capita gross regional products [GRP], proportion of secondary and tertiary industry in local GRP, and whether the city is a provincial capital or not). The last one is the average annual disease incidence rate of the origin city. Metrics used to measure the performance of the models are common part of commuters (CPC), Pearson correlation coefficients (PCC) and root mean squared logarithmic error (RMSLE). We used the SHapley Additive exPlanations (SHAP) approach to quantify the relative importance of the potential socioeconomic and geographical factors in predicting the migratory flows of NIDs.29 It is a game theory method to explain the output of any machine learning model, where the core idea is to calculate the marginal contribution of features to the model's output, and then explain the “black box model” at both the global and local levels. SHAP builds an additive explanation model, where all features are considered “contributors”. For each predicted instance, the model produces a prediction value, and the Shapley value is the numerical value assigned to each feature in that instance, where a larger absolute Shapley value means higher importance. We calculated the SHAP importance by averaging the absolute Shapley values per feature across all pair of cities to estimate their contribution to the prediction of case flows:29,30
where is the Shapley value of feature j on the pair of cities, is the mean absolute Shapley value of feature j across all pair of cities, and is the normalized to reflect the relative contribution.
As an alternative, we also fitted an ordinary gravity model to the same data and compared its predictive performance with the XGBoost model. More details on the network modeling approach are given in the Appendix pp 4−6 and 8−13.
Role of the funding source
The funders of the study had no role in study design, data collection, data analyses, interpretation of the results, or the writing of the manuscript. The corresponding authors had full access to all the data and had final responsibility for the decision to submit for publication.
Results
Epidemiological characteristics
From 2016 to 2020, the NNIDRS reported 32,621,414 lab-confirmed or clinically diagnosed cases of the 39 types (44 subtypes) of NIDs in total across the mainland of China, of whom 2,674,892 (8.20%) cases were determined as inter-city migratory cases according to different addresses at the times of disease onset and reporting.
The top five diseases with the largest number of migratory cases were hepatitis B (771,423 cases; accounting for 15.02% of all reported cases for hepatitis B), tuberculosis (469,204 cases; 11.73%), HFMD (349,092 cases; 3.71%), syphilis (310,715 cases; 12.69%) and influenza (218,749 cases; 3.51%), accounting for 79.23% of the overall migratory cases (Table 1 and Appendix pp 14 and 15). The top five diseases with the highest proportions of migratory cases were all zoonotic or vector-borne, including malaria (99.98%, 12,630/12,632), kala-azar (49.91%, 537/1076), plague (41.67%, 5/12), Japanese encephalitis (40.68%, 2004/4926) and hydatid disease (37.89%, 8934/23,578). The lowest proportions were found for acute hemorrhagic conjunctivitis (1.35%, 2396/177,229), mumps (2.39%, 26,739/1,117,231) and shigellosis (2.53%, 11,606/458,685).
Table 1.
Number and proportion of migratory cases of 44 notifiable infectious diseases in the mainland of China during 2016–2020.
| Ranka | Disease | Total number of cases | Migratory cases |
Type of migration |
|||
|---|---|---|---|---|---|---|---|
| Number | Proportionb | Intra-province | Inter-province | From abroad | |||
| 1 | Hepatitis B | 5,136,056 | 771,423 | 15.02% | 561,274 | 205,884 | 4265 |
| 2 | Tuberculosis | 3,999,387 | 469,204 | 11.73% | 342,723 | 123,171 | 3310 |
| 3 | Hand-foot-and-mouth disease | 9,409,017 | 349,092 | 3.71% | 245,308 | 97,264 | 6520 |
| 4 | Syphilis | 2,447,645 | 310,715 | 12.69% | 189,334 | 117,416 | 3965 |
| 5 | Influenza | 6,230,343 | 218,749 | 3.51% | 134,005 | 67,619 | 17,125 |
| 6 | Hepatitis C | 1,109,374 | 144,945 | 13.07% | 105,008 | 36,796 | 3141 |
| 7 | HIV/AIDS | 803,553 | 138,995 | 17.30% | 68,967 | 53,926 | 16,102 |
| 8 | Gonorrhea | 612,682 | 77,938 | 12.72% | 38,433 | 37,835 | 1670 |
| 9 | Brucellosis | 220,077 | 39,271 | 17.84% | 29,793 | 9405 | 73 |
| 10 | Mumps | 1,117,231 | 26,739 | 2.39% | 18,327 | 7477 | 935 |
| 11 | Hepatitis E | 133,935 | 22,049 | 16.46% | 16,221 | 5634 | 194 |
| 12 | Pertussis | 72,710 | 18,243 | 25.09% | 12,244 | 5956 | 43 |
| 13 | Malariad | 12,632 | 12,630 | 99.98% | 0 | 0 | 12,630 |
| 14 | Shigellosis | 458,685 | 11,606 | 2.53% | 6566 | 4542 | 498 |
| 15 | Hepatitis A | 91,265 | 10,893 | 11.94% | 8248 | 2460 | 185 |
| 16 | Hemorrhagic fever with renal syndrome | 49,844 | 9903 | 19.87% | 8306 | 1582 | 15 |
| 17 | Scarlet fever | 311,635 | 9260 | 2.97% | 5794 | 2976 | 490 |
| 18 | Hydatid disease | 23,578 | 8934 | 37.89% | 7567 | 1355 | 12 |
| 19 | Measles | 38,999 | 6250 | 16.03% | 4666 | 1340 | 244 |
| 20 | Dengue | 37,993 | 4251 | 11.19% | 1789 | 516 | 1946 |
| 21 | Typhoid | 37,969 | 3291 | 8.67% | 2428 | 770 | 93 |
| 22 | Acute hemorrhagic conjunctivitis | 177,229 | 2396 | 1.35% | 1491 | 798 | 107 |
| 23 | Japanese encephalitis | 4926 | 2004 | 40.68% | 1460 | 504 | 40 |
| 24 | Rubella | 44,820 | 1702 | 3.80% | 1190 | 470 | 42 |
| 25 | Paratyphoid | 11,062 | 963 | 8.71% | 737 | 202 | 24 |
| 26 | Kala-azar | 1076 | 537 | 49.91% | 407 | 129 | 1 |
| 27 | Rabies | 2084 | 484 | 23.22% | 364 | 117 | 3 |
| 28 | Amoebic dysentery | 4690 | 470 | 10.02% | 336 | 115 | 19 |
| 29 | Typhus | 5329 | 429 | 8.05% | 357 | 56 | 16 |
| 30 | Leprosy | 2201 | 404 | 18.36% | 304 | 97 | 3 |
| 31 | Schistosomiasis | 6958 | 202 | 2.90% | 169 | 30 | 3 |
| 32 | Anthrax | 1549 | 188 | 12.14% | 157 | 31 | 0 |
| 33 | Hepatitis D | 1734 | 188 | 10.84% | 146 | 40 | 2 |
| 34 | Avian flu H7N9 | 864 | 166 | 19.21% | 120 | 46 | 0 |
| 35 | Leptospirosis | 1225 | 160 | 13.06% | 133 | 27 | 0 |
| 36 | Epidemic cerebrospinal meningitis | 489 | 121 | 24.74% | 96 | 21 | 4 |
| 37 | Neonatal tetanus | 457 | 88 | 19.26% | 63 | 24 | 1 |
| 38 | Plaguec | 12 | 5 | 42% | 2 | 3 | 0 |
| 39 | Cholera | 96 | 4 | 4% | 1 | 3 | 0 |
| 40 | Diphtheriac | 2 | 0 | 0% | 0 | 0 | 0 |
| 41 | Avian flu H5N1c | 1 | 0 | 0% | 0 | 0 | 0 |
| 42 | Filariasis | 0 | 0 | – | 0 | 0 | 0 |
| 43 | Poliomyelitis | 0 | 0 | – | 0 | 0 | 0 |
| 44 | Severe acute respiratory syndrome | 0 | 0 | – | 0 | 0 | 0 |
| Total | 32,621,414 | 2,674,892 | 8.20% | 1,814,534 | 786,637 | 73,721 | |
Ranked by the disease specific number of migratory cases.
Proportion of migratory cases in all reported cases for each specific notifiable infectious disease.
The migratory proportion of diseases with few cases (≤20 cases) might not be reliable.
The last indigenous malaria case was reported on March 17, 2016 in Yingjiang County, Yunnan Province, China. “–” indicates that the total number of reported cases is 0.
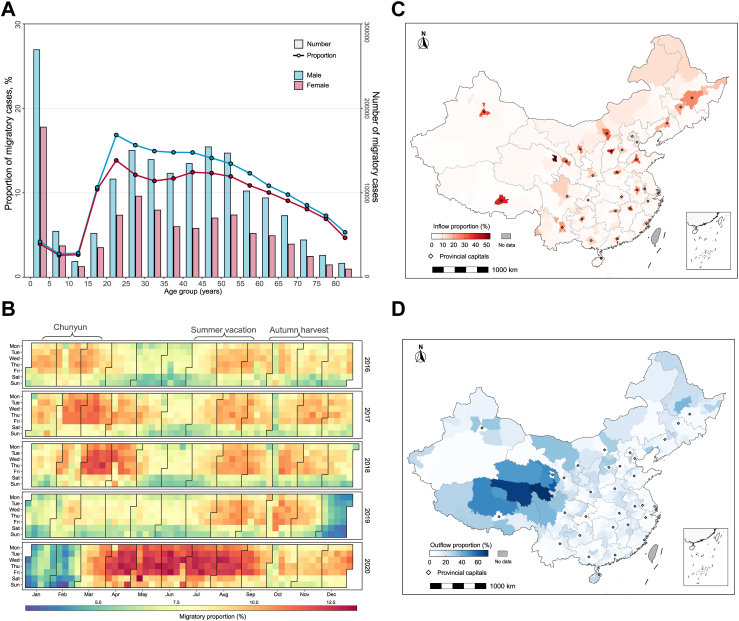
Combining all NIDs, children under five had the largest number of migratory cases among all five-year age groups, but working-age adults (16–60 years old) had the highest proportions (10.41%–16.84%) of migratory cases. Male patients were more likely to be migratory cases than female patients (Fig. 1A and Appendix p 16). For most diseases, migratory cases tended to be slightly younger than local cases; in particular, there were more children under 2 years old among the migratory cases for diseases dominated by pediatric infections such as influenza, HFMD and pertussis (Appendix p 39). The top 3 occupation categories associated with the highest number of cases exhibited a high degree of similarity between migratory cases and local cases, showing only minor variations in their respective rankings (Appendix pp 17−19). Farmer was the leading occupation for most diseases. In general, temporal peaks of proportions of migratory cases coincided with the travel rush periods associated with traditional holidays including lunar new year (also called Chunyun), summer vacation and the harvesting season in China in each year (Fig. 1B). The pattern is most obvious for acute infections like influenza, HFMD, mumps, and shigellosis (Appendix p 40). The proportions of migratory HIV/AIDS and pertussis cases declined from 2016 to 2019, while those of tuberculosis, gonorrhea, and hepatitis A increased (Appendix pp 20, 40, and 41). In 2020, the first year of the SARS-CoV-2 pandemic, the migratory proportions of many diseases (HFMD, brucellosis, mumps, pertussis, etc.) declined, likely a result of nonpharmaceutical interventions (Appendix p 41), but the overall migratory proportion increased notably (Fig. 1B).
Fig. 1.
Characteristics of migratory cases of notifiable infectious diseases during 2016–2020 in China. (A) Numbers (connected dots) and proportions (bars) of migratory cases by sex and age group; (B) Temporal distribution of proportions of migratory cases by day of the week; (C) Spatial distribution of proportion of inflow cases among all reported cases at the city level; and (D) Spatial distribution of proportion of outflow cases among all reported cases at the city level. Centers of provincial capital cities are marked by dots in (C) and (D).
We mapped the proportions of inflow and outflow cases among all reported cases in each city. Provincial capital cities had higher inflow proportions than other cities (Fig. 1C), likely due to their better socioeconomic development and access to medical resources. In contrast, less developed cities, such as those in western China, had higher outflow proportions than the rest of the country (Fig. 1D). The spatial imbalance in the inflow and outflow of migratory cases is similar across diseases, though the exact cities with high outflow proportions may differ (Appendix pp 42−45). Cities with high outflow proportions for brucellosis are mainly in the north, while those for pertussis are in central and eastern China (Appendix pp 42−45). Compared to locally reported cases, migratory cases had longer delays from symptom onset to diagnosis for pertussis and hepatitis C, and shorter delays for tuberculosis, brucellosis and HIV/AIDS (Appendix p 21). They also had higher case fatality ratios (CFR) for influenza, pertussis and shigellosis, and lower CFRs for tuberculosis and HIV/AIDS (Appendix p 22). A look into finer grouping of migratory and local cases by city type (provincial capital vs. noncapital) revealed that the longer delays in the diagnosis of pertussis and hepatitis C for migratory cases mainly occurred in those traveling from noncapital cities to capital cities, and the longer delays in the diagnosis of tuberculosis and HIV/AIDS for local cases mainly occurred in those living in noncapital cities (Appendix p 23). The higher CFR of influenza among migratory patients was driven by those traveling from noncapital cities to capital cities, but this is not the case for pertussis and shigellosis (Appendix p 24). While migratory HIV/AIDS patients showed an overall lower CFR than local patients, the CFR was actually as high among migratory patients traveling from noncapital to capital cities as among local patients in noncapital cities. For hepatitis B, hepatitis C and hepatitis E, the CFRs were clearly led by local patients in capital cities (Appendix p 24), despite the similarity in the overall CFR of between migratory and local patients (Appendix p 22). For hepatitis E, the CFR was also relatively high among migratory patients from noncapital cities to capital cities.
Network patterns
During 2016–2020, 97.24% (2,601,171/2,674,892) of migratory cases were domestic, of which 69.76% (1,814,534/2,601,171) were intra-provincial migrants. We conducted a social network analysis on fourteen infectious diseases that accounted for 98.10% (2,551,836/2,601,171) of all the domestic migratory cases (Table 1). For each of the 14 diseases, the associated IDMN covered almost all of the 337 major Chinese cities (Table 2). The network density was positively associated with the number of migratory cases across the 14 IDMNs (Pearson r = 0.870, P < 0.001). The highest network density of 0.279 was found for hepatitis B, i.e., migration of hepatitis B cases was observed in 27.9% of the 337 × 336 directional city pairs), followed by syphilis (0.236) and tuberculosis (0.196; Table 2). Regardless of disease type, the distributions of the node degree (total number of directed edges) and node strength (total number of migratory cases, inflow and outflow) were highly skewed to the right in all the IDMNs, suggesting a large proportion of migratory cases were associated with only a few cities (Appendix p 46). In terms of node strength and node betweenness, provincial capital cities showed much higher influence than other cities for all the 14 major IDMNs (Appendix p 25). The top 20 cities in terms of node strength and node betweenness for each disease are listed in Appendix pp 26−32. By node strength, Chengdu, Changsha, Zhengzhou, Guangzhou, and Chongqing were among the top 20 for most (11−12 out of 14) major NIDs. By node betweenness, Chongqing, Shenzhen, Chengdu, Beijing, and Xi’An were ranked among the top 20 for most (11−14) major NIDs. Of these highly ranked cities, Beijing and Chongqing are administratively parallel with provinces, and the rest are provincial capitals except for Shenzhen which is one of the most economically developed cities in China.
Table 2.
Characteristics of migration networks for 14 major notifiable infectious diseases in the mainland of China during 2016–2020.
| Disease | Domestic migratory cases | Nodes | Edges | Density | City clustersa | Modularity |
|---|---|---|---|---|---|---|
| Hepatitis B | 767,158 | 337 | 31,561 | 0.279 | 15 | 0.769 |
| Tuberculosis | 465,894 | 337 | 22,230 | 0.196 | 17 | 0.806 |
| HFMD | 342,572 | 337 | 14,931 | 0.132 | 19 | 0.838 |
| Syphilis | 306,750 | 337 | 26,683 | 0.236 | 15 | 0.752 |
| Influenza | 201,624 | 337 | 14,285 | 0.126 | 14 | 0.790 |
| Hepatitis C | 141,804 | 337 | 13,970 | 0.123 | 16 | 0.831 |
| HIV/AIDS | 122,893 | 337 | 18,214 | 0.161 | 15 | 0.718 |
| Gonorrhea | 76,268 | 337 | 15,010 | 0.133 | 18 | 0.703 |
| Brucellosis | 39,198 | 330 | 2664 | 0.025 | 18 | 0.805 |
| Mumps | 25,804 | 337 | 5099 | 0.045 | 23 | 0.894 |
| Hepatitis E | 21,855 | 335 | 3294 | 0.029 | 20 | 0.867 |
| Pertussis | 18,200 | 315 | 1127 | 0.011 | 18 | 0.814 |
| Shigellosis | 11,108 | 337 | 3412 | 0.030 | 22 | 0.906 |
| Hepatitis A | 10,708 | 337 | 2374 | 0.021 | 21 | 0.885 |
The number of city clusters, where each cluster contains ≥3 cities.
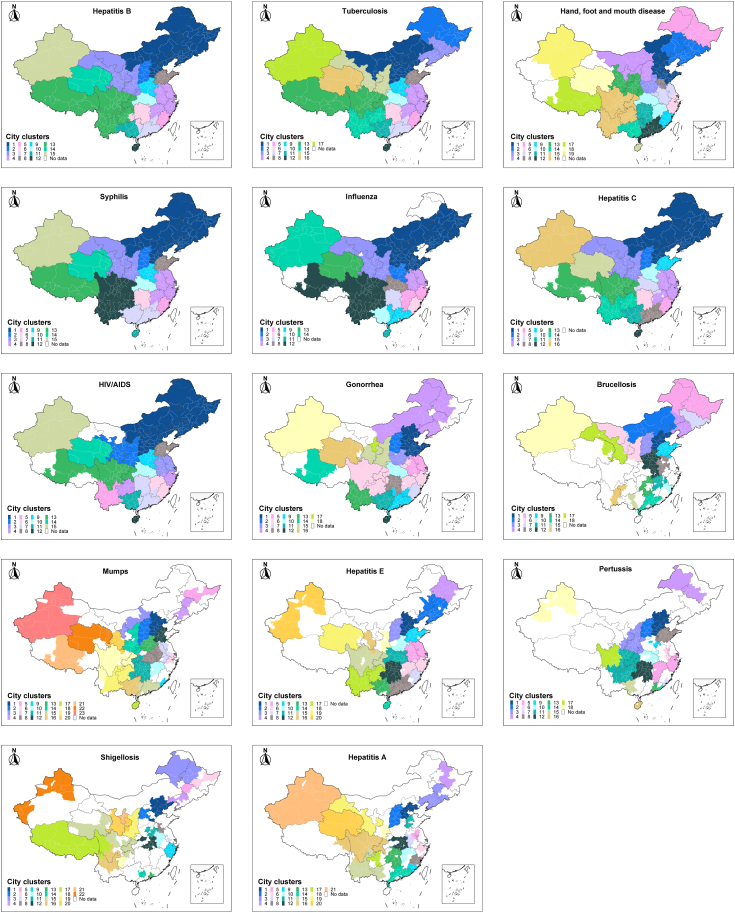
We further identified clustering patterns of the cities in these IDMNs. For most diseases, the community structure of the IDMNs largely coincided with the geographic structure of adjacent provinces (Fig. 2). The overall community structures of chronic diseases (Hepatitis B, Tuberculosis, syphilis, Hepatitis C, and HIV/AIDS) more or less resemble each other. For the two major sexually transmitted diseases, gonorrhea and HIV/AIDS, inland cities of Guizhou Province in Southwest China and coastal cities of Zhejiang Province in East China were clustered together. The clustering pattern of each city for each major infectious disease can be found on an interactive Shiny website (https://huashuiguaishou.shinyapps.io/mccx/).
Fig. 2.
Network community structure for fourteen major infectious diseases. Cities in the same community are colored the same, representing a sub-network.
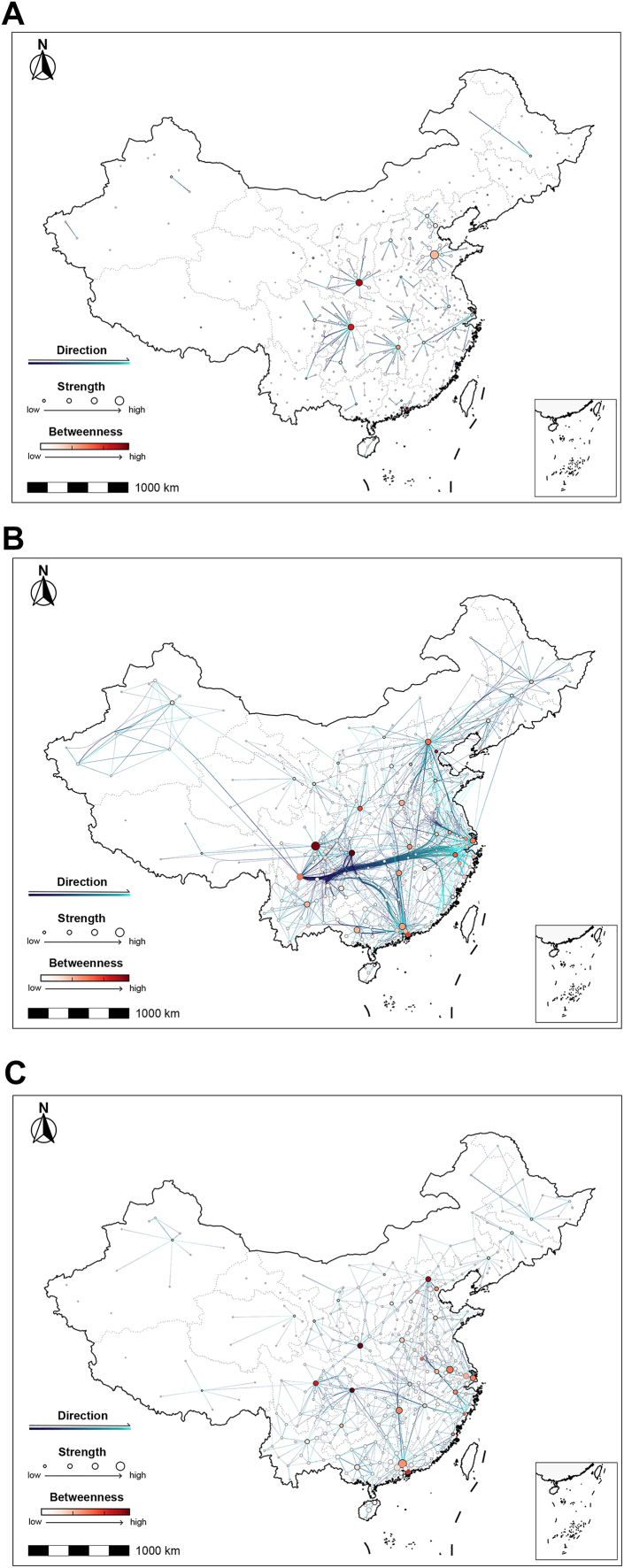
A visualization of the backbones of the IDMNs revealed three typical modes of major connections. (1) The short-distance mode represented by pertussis, brucellosis, mumps, hepatitis E, hepatitis A and Shigellosis (Fig. 3A and Appendix p 47), where backbone paths were mostly either between adjacent cities within a province or between capital cities of adjacent provinces. (2) The long-distance mode represented by HIV/AIDS, tuberculosis, syphilis, and gonorrhea (Fig. 3B and Appendix p 48), where bundled directed paths connected cities in Southwest and Central China to the coastal economically-developed urban agglomerations in the Yangtze River Delta near Shanghai and the Pearl River Delta around Guangzhou. (3) The mixed mode seen for HFMD, hepatitis B, influenza, and hepatitis C (Fig. 3C and Appendix p 49), where a mixture of short-distance and long-distance modes coexisted. Long-distance connections between Beijing and northeastern provincial capitals such as Harbin and Changchun are observed for tuberculosis, syphilis, hepatitis B, influenza, and hepatitis C.
Fig. 3.
Network backbones of migration networks for (A) pertussis, representing the short distance migration mode, (B) HIV/AIDS, representing the long distance migration mode, and (C) hand, foot and mouth disease, representing the mixed mode. The color of each directed edge changes from dark blue to bright blue to represent the direction of migration from the origin to the destination. The size of each node (city) represents the node strength of the city in the migration network. Each node is colored by the node betweenness in the network, with darker red indicating a larger value of node betweenness.
Socioeconomic and geographical drivers
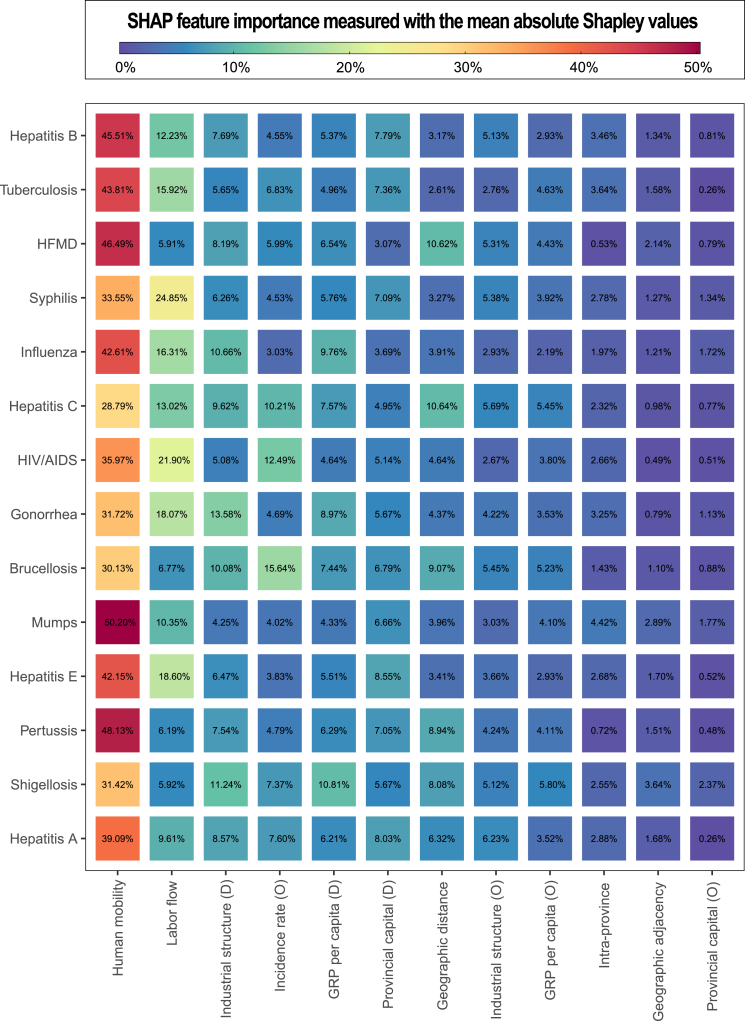
To identify potential drivers for the migration of NID cases, we further associated the structure of the IDMNs with urban indicators using regression models. The XGBoost model outperformed the ordinary gravity model for all the 14 major diseases as shown by multiple metrics based on the test data (Appendix p 33) and goodness of fit based on all data (Appendix pp 50−52). Here we describe important drivers for disease migration as factors with mean absolute Shapley values ≥ 10% based on the XGBoost models (Fig. 4). The exact association, particularly the direction of such association, of each risk factor with the SHAP values are shown in Appendix pp 53−61. The most relevant feature for the migration of human cases was human mobility intensity, contributing 29–50% to all the 14 diseases, which is not unexpected as large-scale between-city human mobility increased the chance of the migration of patients of infectious diseases. Labor flow intensity was another important driver, contributing 10−25% to the migration of sexually transmitted or bloodborne (HIV/AIDS, syphilis, gonorrhea, HBV and HCV), air-borne (TB, influenza, mumps), and fecal-oral (HEV) diseases. Geographical distance was negatively associated with the migration of HFMD and HCV. Incidence rate of the origin city was positively associated with the migration of HCV, HIV/AIDS and brucellosis. GRP per capita of the destination city was positively associated with the migration of shigellosis. Relative contributions of binary indicators are usually below 10%, but these indicators clearly differentiate the travel frequency of patients (Appendix p 61). Migration of cases was more frequent between pairs of cities with the following features: within the same province, spatially adjacent cities and either one being the provincial capital city.
Fig. 4.
The SHAP feature importance of predictors for infectious disease migration. The numbers in the grids represent the relative SHAP importance (measured as the mean absolute Shapley values) of the XGBoost models, scaled to sum up to 100% for each row. The grids are also colored in red (blue) for high (low) relative importance.
Discussion
Over four decades of modernization, China has experienced a significant increase in human movement, which has inevitably facilitated the spread of infectious diseases. In addition, the geographical imbalance in economy and accessibility to healthcare may have fueled the migration of patients. Utilizing the surveillance data from the NNIDRS, we conducted a comprehensive network-based analyses on the migration patterns of major notifiable infectious diseases and associated driving factors in China. The goal if our study was to inform programs and policies for preventing and controlling spread of infectious diseases and reducing geographic disparity in healthcare resources. Modern network analysis methodology has been applied in infectious diseases epidemiology including but not limited to monitoring key populations with HIV, tracking transmission of influenza, and detecting pre-outbreak signals of HFMD.31, 32, 33 Our study demonstrated its utility in modeling patient mobility when coupled with national surveillance data.
During 2016–2020, 8.20% of NID cases identified in China were inter-city migratory cases. The proportion of cases which were identified as migratory is exceptionally high for zoonotic and vector-borne diseases such as brucellosis, hydatid disease, kala-azar, and plague, which is closely related to inequality in local healthcare accessibility. These diseases had high incidences in under-developed areas where disease diagnosis and treatment capacities are inadequate, leading to a high frequency of cross-regional healthcare-seeking behavior.34, 35, 36 Key intervention measures should be focused on the radiation and sinking of quality healthcare resources, which resonates with the recent policy of the Chinese government on reducing geographical disparities in healthcare accessibility as parts of the efforts in advancing the nation’s modernization (https://www.gov.cn/zhengce/202407/content_6963770.htm). High proportions of migratory patients were also found for some vaccine-preventable pediatric diseases such as pertussis, measles, Japanese encephalitis, epidemic cerebrospinal meningitis, and neonatal tetanus. Routine vaccination of children is required for these infectious diseases, according to the Chinese Expanded Program of Immunization. However, the coverage of vaccination in migrant children was relatively low and often delayed.37
Our network analysis demonstrated strong connections between several developing inland cities in southwestern China and developed coastal cities in the Yangtze River Delta and the Pearl River Delta in the migration networks of TB, syphilis, HIV/AIDS, and gonorrhea (Appendix p 35). Additional connections with the Yangtze River Delta can be traced back to a cluster of cities near the border between Henan Province and Anhui Province, which are the two main providers of labor workers to Shanghai, the center of the Yangtze River Delta.38 Such disease flows are consistent with labor force flows driven by geographic disparity in economic development.39 Due to the synergy of relatively poor living conditions, lack of knowledge about STI prevention, and lack of STI clinics in their home villages, migrant labor workers are known to be at high risk of sexually transmitted diseases and tuberculosis and are often diagnosed and reported in cities where they work.40,41 Since 2016, China’s healthcare insurance system underwent a major reform, streamlining the reimbursement process for medical expenses across provinces. This reform has further facilitated inter-regional mobility of patients and improved access to healthcare services for migrant workers whose health insurances are usually provided by agencies at their hometown.42,43
Long-term temporal trends in the proportions of migratory cases of the NIDs reflect the impact of or need for control policies. From 2016 to 2019, migratory proportions of HIV/AIDS and pertussis decreased, likely benefitting from increased HIV screening and routine vaccination against Bordetella pertussis in the migrant workers and their families. On the other hand, growing proportions of migratory cases of tuberculosis, gonorrhea, and hepatitis A indicate the need for strengthening the surveillance and control of these diseases among the migrant population. Some temporal changes warrant careful interpretation. In 2020, a rise in the overall proportion of migratory cases among reported cases of NIDs was observed in the first half of the year. The most likely reason is that the intensive nonpharmaceutical interventions (NPI) for controlling the COVID-19 pandemic changed the spectrum of other diseases (Appendix p 62).44 Specifically, acute infectious diseases with low proportions of migratory cases, such as influenza and HFMD, had a sharp decrease under NPIs, whereas chronic infections with relatively high migration proportions, like hepatitis B and tuberculosis, were much less sensitive to the NPIs, leading to an overall increase in the proportion of migratory cases.
We observed that migratory cases exhibited notably higher CFRs for several acute NIDs including HFMD, influenza, pertussis, and shigellosis, but lower CFRs for two chronic NIDS, tuberculosis and HIV/AIDS. The higher CFR of the acute diseases in migratory patients could be partly associated with referral or transfer of severe patients from clinics in resource-limited regions to better equipped hospitals in metro cities. Given that HFMD, influenza and pertussis disproportionally affect children and are vaccine-preventable, a helpful addition to the general policies addressing geographic disparity in healthcare services would be to increase vaccine coverage among children in migrant families. The lower CFR of the chronic NIDs in migratory patients could be related to better quality of care and availability of more advanced treatments in large cities, e.g., treatments for multidrug-resistant TB are often lacking at county-level health facilities.45 In addition, a previous study found that a large proportion of inter-provincial migratory TB patients were working age males traveling from inland cities to developed coastal cities, suggesting that these patients were likely healthier than local TB patients.46 However, the lower CFR of migratory HIV/AIDS patients should be interpreted with caution, as we observed that the CFR of HIV/AIDS patients traveling from non-capital to capital cities was as high as local cases in noncapital cities (Appendix p 24), despite better health care in capital cities. Henceforth, these traveling patients, together with local patients in noncapital cities, might have been associated with more progressed AIDS stages and should be prioritized for antiretroviral treatments.
A few recent changes in health policies have affected the migration patterns of infectious disease patients in China. For example, the increasing availability and affordability of pre-surgical tests and public health screening campaigns for HBV, HCV, HIV, and Treponema pallidum have facilitated early detection and treatment of these bloodborne and sexually transmitted diseases, especially among migrants. Detections of latent syphilis via public health screening or routine pre-surgical tests accounted for 79.55% of all reported syphilis cases during 2016–2020.47, 48, 49 In addition, the Chinese government has been actively improving the capacity of primary healthcare services and building regional medical centers to alleviate the geographic disparity in medical resources.50,51 However, these fragmented and localized strategies may not be adequate to fully address the challenges raised by large-scale human movement. Given the infectious disease migration network we identified, we encourage policy-makers to foster close collaborations in prevention and control programs between major case-exporting and case-importing cities or provinces, for example, on top of streamlined health insurance payment systems across regions, to create channels for sharing medical and vaccination records to better serve migratory patients. For sexually transmitted diseases, educational programs on disease prevention and physical examination programs targeting migrant workers could be co-sponsored by labor-exporting and labor-importing cities.
A few limitations of our work should be acknowledged. First, current findings might have been biased by limitations in data quality. Recall bias is common for diseases with a long incubation period, e.g., TB and HIV, and self-reported residential addresses could be false for sensitive diseases such as STDs. Second, intermediate cities that each patient might have visited along the trip between the origin and the destination are unknown. However, such visits are primarily transient and thus unlikely to severely affect our findings about the key structural features of the IDMNs. Third, the surveillance database NNIDRS did not capture specific symptoms or disease stage of each patient, making it difficult to assess the risk of onward transmission during and after traveling. In addition, the flows for NID patients cannot be equated to inter-city transmission risks, as diseases are often transmitted by asymptomatic individuals or mild cases who do not seek healthcare and are thus not reported by the surveillance system; in particular, vector-borne diseases such as malaria and dengue would require established ecological niches of relevant mosquito species, which are absent in most northern cities of China. Moreover, although we explored a few potential driving factors for the migration of NID patients using an ecological modeling approach and SHAP importance, ecological fallacies are inevitable as the analysis was done at the city level, and no causal interpretation should be made. Finally, the inter-city labor flow intensity used in our analysis was based on survey data from 2015, which may not reflect changes from 2016 to 2020. More recent data from the seventh census in China (2020) has not yet been publicly released yet. The validity of our results is nevertheless supported by the fact that the travel patterns of migrant workers remained largely similar from 2015 to 2020, according to the summary statistics on inter-city labor flow provided in the from annual monitoring survey reports of the National Bureau of Statistics (Appendix p 35).
While human mobility is the primary driver for the spread of most infectious diseases, control strategies should factor in the differential migration patterns of patients closely related to the underlying transmission mechanisms and clinical features of the diseases. This study underscores the importance of ensuring equitable access to primary healthcare services and calls for collaborative efforts in screening and caring for migratory patients between cities bearing the heaviest patient flows.
Contributors
Conceptualization: LJY, YY, LQF, PSJ, WL, XR, SIH, LPW.
Methodology: LJY, TW, YHW, LCL, YT, YY, PSJ.
Data collection: LJY, XR, LCL, JJC, TT, YHW, YQS, YNL, MCL, TTL, YBQ, CXS, QX, SHL, LPW.
Visualization: LJY, TW, JJC, YT.
Supervision: YY, LQF, WL, LPW.
Writing—original draft: LJY.
Writing—review & editing: LQF, YY, WL, YHW, LPW, SIH, PSJ.
Data sharing statement
Raw data of reported cases for each notifiable infectious disease are not publicly available and are protected due to data privacy laws. De-identified and aggregated data may be requested from the corresponding author (Dr. Li-Qun Fang) with permission from the data provider (Li-Ping Wang). The authors do not have permission to share the inter-city human mobility data provided by AMAP, and data requests should be directed to the company (https://lbs.amap.com/).
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
Authors declare that they have no competing interests.
Acknowledgements
We would like to thank all healthcare and public health staff who contributed to National Notifiable Infectious Diseases Report System, and thank Dr. Jonathan E. Owen from School of Medicine, Emory University for his help in revising this paper. This work was financially supported by grants from National Key Research and Development Program of China grant 2022YFC2604000 (QX) and grant 2023YFC2605603 (LQF), Fund of State Key Laboratory of Pathogen and Biosecurity grant SKLPBS2205 (LCL), Natural Science Foundation of China grant 91846302 (LPW), and U.S. CDC U01CK000696 (YY).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2024.101261.
Contributor Information
Li-Ping Wang, Email: wanglp@chinacdc.cn.
Simon I. Hay, Email: sihay@uw.edu.
Wei Liu, Email: liuwei@bmi.ac.cn.
Yang Yang, Email: yy70876@uga.edu.
Li-Qun Fang, Email: fanglq@bmi.ac.cn.
Appendix ASupplementary data
References
- 1.Nájera J.A., González-Silva M., Alonso P.L. Some lessons for the future from the global malaria eradication programme (1955-1969) PLoS Med. 2011;8(1) doi: 10.1371/journal.pmed.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li B., National Health Commission of the PRC . 2013. Report on the prevention and control of infectious diseases and the implementation of the law on the prevention and control of infectious diseases. [Google Scholar]
- 3.Duan C., Lyu L., Wang H., Xie D. From rural China to migrating China: rethinking migration transition in China. Popul Res. 2020;44(1):19–25. [Google Scholar]
- 4.International Labour Organization . 2006. Internal labour migration in China: features and responses. [Google Scholar]
- 5.Xu Q., Li Z.W., Zhang X.A., et al. The imported infections among foreign travelers in China: an observational study. Global Health. 2022;18(1):97. doi: 10.1186/s12992-022-00893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y., Liu M.Y., Wang J.L., et al. Epidemiology of imported infectious diseases, China, 2014-18. J Travel Med. 2020;27(8) doi: 10.1093/jtm/taaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang L.Q., Sun Y., Zhao G.P., et al. Travel-related infections in mainland China, 2014-16: an active surveillance study. Lancet Public Health. 2018;3(8):e385–e394. doi: 10.1016/S2468-2667(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia J.S., Lu X., Yuan Y., Xu G., Jia J., Christakis N.A. Population flow drives spatio-temporal distribution of COVID-19 in China. Nature. 2020;582(7812):389–394. doi: 10.1038/s41586-020-2284-y. [DOI] [PubMed] [Google Scholar]
- 9.Su L., Liang S., Hou X., et al. Impact of worker emigration on HIV epidemics in labour export areas: a molecular epidemiology investigation in Guangyuan, China. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-33996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charu V., Zeger S., Gog J., et al. Human mobility and the spatial transmission of influenza in the United States. PLoS Comput Biol. 2017;13(2) doi: 10.1371/journal.pcbi.1005382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y., Wang H., Yi S., et al. Spatial and temporal characteristics of hand-foot-and-mouth disease and their influencing factors in Urumqi, China. Int J Environ Res Public Health. 2021;18(9):4919. doi: 10.3390/ijerph18094919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wesolowski A., Metcalf C.J., Eagle N., et al. Quantifying seasonal population fluxes driving rubella transmission dynamics using mobile phone data. Proc Natl Acad Sci USA. 2015;112(35):11114–11119. doi: 10.1073/pnas.1423542112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosetti P., Poletti P., Stella M., Lepri B., Merler S., De Domenico M. Heterogeneity in social and epidemiological factors determines the risk of measles outbreaks. Proc Natl Acad Sci USA. 2020;117(48):30118–30125. doi: 10.1073/pnas.1920986117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z., Wei L., Peng S., Deng L., Niu B. Child-trafficking networks of illegal adoption in China. Nat Sustain. 2018;1(5):254–260. [Google Scholar]
- 15.Patel N.G., Rorres C., Joly D.O., et al. Quantitative methods of identifying the key nodes in the illegal wildlife trade network. Proc Natl Acad Sci USA. 2015;112(26):7948–7953. doi: 10.1073/pnas.1500862112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan S., Lai S., Fang F., et al. Mobility in China, 2020: a tale of four phases. Natl Sci Rev. 2021;8(11) doi: 10.1093/nsr/nwab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yue Y., Liu Q. Exploring epidemiological characteristics of domestic imported dengue fever in mainland China, 2014–2018. Int J Environ Res Public Health. 2019;16(20):3901. doi: 10.3390/ijerph16203901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Q.N., Ma J.Q. Big data analysis of flow of tuberculosis cases in China, 2014. Chin J Epidemiol. 2016;37(5):668–672. doi: 10.3760/cma.j.issn.0254-6450.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Saita S., Pan-ngum W., Phuanukoonnon S., et al. Human population movement and behavioural patterns in malaria hotspots on the Thai–Myanmar border: implications for malaria elimination. Malar J. 2019;18(1):64. doi: 10.1186/s12936-019-2704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merrill R.D., Chabi A.I.B., McIntyre E., et al. An approach to integrate population mobility patterns and sociocultural factors in communicable disease preparedness and response. Humanit Soc Sci Commun. 2021;8(1):23. [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L., Wang Y., Jin S., et al. Emergence and control of infectious diseases in China. Lancet (London, England) 2008;372(9649):1598–1605. doi: 10.1016/S0140-6736(08)61365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The State Council of P.R.China . 2022. China has established the world's largest surveillance network for diseases and health risk factors.https://www.gov.cn/xinwen/2022-06/21/content_5696881.htm [Google Scholar]
- 23.Chinese Center for Disease Control and Prevention . 2016. Protocol of infectious disease reporting. [Google Scholar]
- 24.Opsahl T., Agneessens F., Skvoretz J. Node centrality in weighted networks: generalizing degree and shortest paths. Soc Networks. 2010;32:245–251. [Google Scholar]
- 25.Blondel V., Guillaume J.-L., Lambiotte R., Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech Theory Exp. 2008;10 [Google Scholar]
- 26.Serrano M.Á., Boguñá M., Vespignani A. Extracting the multiscale backbone of complex weighted networks. Proc Natl Acad Sci USA. 2009;106(16):6483–6488. doi: 10.1073/pnas.0808904106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holten D., van Wijk J. Force-directed edge bundling for graph visualization. Comput Graph Forum. 2009;28:983–990. [Google Scholar]
- 28.Chen T., Guestrin C. ACM; 2016. XGBoost: a scalable tree boosting system. [Google Scholar]
- 29.Lundberg S.M., Lee S.-I. NIPS 2017. Curran Associates Inc.; Long Beach, California, USA: 2017. A unified approach to interpreting model predictions; pp. 4768–4777. [Google Scholar]
- 30.Molnar C. 2nd ed. 2022. Interpretable machine learning: a guide for making black box models explainable. [Google Scholar]
- 31.Rwabiyago O.E., Katale A., Bingham T., et al. Social network strategy (SNS) for HIV testing: a new approach for identifying individuals with undiagnosed HIV infection in Tanzania. AIDS Care. 2024;36(sup1):201–210. doi: 10.1080/09540121.2024.2307383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan J., Holmes A., Rabadan R. Network analysis of global influenza spread. PLoS Comput Biol. 2010;6(11) doi: 10.1371/journal.pcbi.1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X., Xie R., Liu Z., Pan Y., Liu R., Chen P. Identifying pre-outbreak signals of hand, foot and mouth disease based on landscape dynamic network marker. BMC Infect Dis. 2021;21(Suppl 1):6. doi: 10.1186/s12879-020-05709-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong W., Li Y., Zhou H., Yin W. Internet-based real-time report quality and diagnosis of human brucellosis in China, 2004-2012. Dis Surveill. 2013;28(9):757. [Google Scholar]
- 35.Zhou H., Guo S. Two cases of imported pneumonic plague in Beijing, China. Medicine (Baltim) 2020;99(44) doi: 10.1097/MD.0000000000022932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L., Cao Y., Zeng L., Ren X., Li Z., Yu H. Diagnosis and reporting of communicable diseases in basic medical institutions in China. Dis Surveill. 2014;29(3):176. [Google Scholar]
- 37.Han K., Zheng H., Huang Z., et al. Vaccination coverage and its determinants among migrant children in Guangdong, China. BMC Publ Health. 2014;14(1):203. doi: 10.1186/1471-2458-14-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Statistical Bureau of Shanghai . 2021. Statistical yearbook of Shanghai 2021. [Google Scholar]
- 39.Statistic Bureau of Zhejiang Province . 2022. Analysis of the seventh population census series no.7: floating population. [Google Scholar]
- 40.Wang W., Jiang Q., Abdullah A.S., Xu B. Barriers in accessing to tuberculosis care among non-residents in Shanghai: a descriptive study of delays in diagnosis. Eur J Public Health. 2007;17(5):419–423. doi: 10.1093/eurpub/ckm029. [DOI] [PubMed] [Google Scholar]
- 41.Chen X.S., Peeling R.W., Yin Y.P., Mabey D.C. The epidemic of sexually transmitted infections in China: implications for control and future perspectives. BMC Med. 2011;9:111. doi: 10.1186/1741-7015-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie L.Q., Hu H.P. Evolution and trend of the cross-pooling healthcare policy of basic medical insurance in China: based on content analysis of policy document. Chinese J Health Policy. 2021;14(6):45–50. [Google Scholar]
- 43.Jiang K.J. Zhejiang University; 2014. A policy analysis of the effects on convenience of cross-district medical treatment in medical insurance system for urban employees: a study in city C, Zhejiang province. [Google Scholar]
- 44.Geng M.J., Zhang H.Y., Yu L.J., et al. Changes in notifiable infectious disease incidence in China during the COVID-19 pandemic. Nat Commun. 2021;12(1):6923. doi: 10.1038/s41467-021-27292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long Q., Guo L., Jiang W., Huan S., Tang S. Ending tuberculosis in China: health system challenges. Lancet Public Health. 2021;6(12):e948–e953. doi: 10.1016/S2468-2667(21)00203-6. [DOI] [PubMed] [Google Scholar]
- 46.Ni N., Huang F., Wang N., et al. Epidemic characteristics of pulmonary tuberculosis in migrants in China, 2016–2020. Dis Surveill. 2023;38(7):819. [Google Scholar]
- 47.National Health Commission of the PRC . 2018. Response to recommendation No. 4342 of the first session of the thirteenth national people's congress.http://www.nhc.gov.cn/wjw/jiany/201812/14726c604d3045469853ed5b12058b40.shtml [Google Scholar]
- 48.Tucker J.D., Cohen M.S. China’s syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis. 2011;24(1):50–55. doi: 10.1097/QCO.0b013e32834204bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu A.Y., Zang W.J., Yuan L.L., Chai Y.L., Wang S. Latent syphilis among inpatients in an urban area of China. Glob J Health Sci. 2014;7(3):249–253. doi: 10.5539/gjhs.v7n3p249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Health Commission of the PRC . 2019. National medical center and national regional medical center set up implementation plan. [Google Scholar]
- 51.The State Council of the PRC . 2023. Opinions on further improvement of the medical and health service system. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.