Highlights
-
•
UGCG is elevated in chemoresistant breast cancer tissues and cells, serving as an independent predictor of chemotherapy efficacy.
-
•
UGCG enhances the activation of NF-κB and Wnt/β-catenin signaling pathways, contributing to chemoresistance and cancer progression.
-
•
Targeting UGCG could be a promising strategy to overcome chemoresistance in breast cancer.
Keywords: Breast cancer, Chemoresistance, UGCG, NF-κB, Wnt/β-catenin
Abstract
Background
Taxane-based chemotherapy is the primary treatment for triple-negative breast cancer (TNBC), yet clinical outcomes remain unsatisfactory due to the persistence of chemoresistance. Identifying key factors that contribute to chemoresistance and understanding the associated molecular mechanisms is therefore essential.
Method
The GEO databases were utilized to pinpoint factors related to chemoresistance, which were subsequently validated using clinical tissue samples. The role of UGCG in the malignant progression and chemoresistance of TNBC was assessed through various functional assays. Western blotting, qRT-PCR, and immunohistochemistry were employed to investigate the signaling pathways associated with UGCG in TNBC.
Results
UGCG expression was notably elevated in chemoresistant breast cancer tissues and cells, as identified in GEO databases and confirmed through immunohistochemistry. Additionally, findings from our cohorts indicated that higher levels of UGCG expression correlated with a lower rate of pathological complete response (pCR), suggesting it could serve as an independent predictor of chemotherapy effectiveness. Gain- and loss-of-function experiments demonstrated that UGCG enhanced the proliferation, metastasis, and stemness of breast cancer cells. Furthermore, treatment with paclitaxel or docetaxel resulted in increased UGCG expression, which in turn reduced chemotherapy-induced cell apoptosis and improved drug resistance and metastatic capabilities. Mechanistically, UGCG was found to amplify the activation of NF-κB and Wnt/β-catenin pathways, and the use of inhibitors targeting these pathways diminished the UGCG-induced malignant effects.
Conclusion
Our findings underscore the significant role of UGCG in the chemoresistance and progression of breast cancer, suggesting it as a predictive biomarker and potential therapeutic target to combat chemoresistance in this disease.
Introduction
Breast cancer (BC) is the prevailing form of cancer worldwide and ranks as the second leading cause of cancer-related deaths among women, resulting in significant social and economic repercussions [1]. BC is a very heterogeneous disease with a wide range of morphologies and clinical characteristics, and can be categorized into different subtypes based on the levels of expression of the human epidermal growth factor receptor 2 (HER2), estrogen receptor (ER), and progesterone receptor (PR) [2]. About 15% of all breast tumors are triple-negative breast cancers (TNBCs), a subtype that does not express ER, PR, or HER2 [3]. More than 30% of individuals with TNBC would develop distant metastases, seriously impacting their prognosis [4,5]. Due to the unique receptor expression patterns, TNBC patients lack effective systemic treatment options except adjuvant chemotherapy [6]. However, the effectiveness of chemotherapy is severely undermined by the presence of intrinsic or acquired chemoresistance, leading to dismal prognosis and challenging clinical issue [[7], [8], [9]]. Thus, it is imperative to clarify the molecular basis of chemoresistance in TNBC in order to identify new treatment targets and enhance patient prognosis.
Chemoresistance, a significant obstacle in cancer therapy, arises from various mechanisms such as enhanced drug efflux, cell death inhibition, and upregulation of pro-survival pathways. P-glycoprotein (P-gp), also known as or multidrug resistance protein 1 (MDR1), is a crucial drug transporter in the process of chemoresistance [10]. The dysregulation of P-gp is related to altered drug efflux, which could be regulated by many pathways [11], including phosphoinositide 3-kinase (PI3K)-AKT, nuclear factor-κB (NF-κB), phosphatase and tensin homolog (PTEN), and non-coding RNAs (ncRNAs). Recent researches highlight the potential of natural compounds and nanotherapeutic approaches in improving treatment outcomes. Zhang et al. reported a cell membrane-camouflaged and bufalin-loaded polylactic-co-glycolic acid nanoparticle (CBAP), which could enhance the sensitivity of cancer cells to several drugs through modulating NF-κB/ABC transporter pathway [12]. Additionally, previous studies have shown that the dysregulation of autophagy plays a crucial role in chemoresistance. For instance, the activation of protective autophagy can promote drug resistance, while its suppression can enhance the efficacy of chemotherapy [13,14]. Targeting the pathways involved in autophagy, such as PI3K-AKT-mTOR, NF-κB, and Nrf2 signaling, might offer novel strategies to overcome chemoresistance. However, in breast cancer, many mysteries about the mechanisms of chemoresistance remain unsolved.
The use of taxanes-based regimens is widely recommended by clinical guidelines as the first-line chemotherapy for patients with breast cancer [15,16]. The main taxanes currently used in TNBC chemotherapy include paclitaxel (PTX) and docetaxel (DOC) [17]. Docetaxel and paclitaxel both could bind with β-tubulin, hence stabilizing microtubules and preventing the formation of mitotic spindles [18], which further caused mitotic arrest and cell death [19,20]. Furthermore, recent studies reported that taxanes were able to strongly induce the production of TNF-α, subsequently promoting apoptosis through binding with its receptor TNFR1 [21]. Unfortunately, around 30% of patients would become resistant to taxanes, which resulted in the ineffectiveness of treatment and advancement of cancer [22]. There are still many unanswered questions regarding the mechanism of chemoresistance in BC, despite the fact that several mechanisms also contributed to the resistance of taxanes, including overexpression of drug efflux proteins within the ATP-binding cassette (ABC) superfamily [23], altered expression of microtubule-associated protein (MAP) [24], and induced protective autophagy [25].
The UDP-glucose ceramide glucosyltransferase (UGCG), a critical enzyme responsible for the production of glucosylceramide (GlcCer), was first cloned by Ichikawa et al. in 1996 [26]. UGCG mainly localizes to the Golgi apparatus, catalyzing the GlcCer synthesis using UDP-glucose and ceramide [27], which is the precursor of all glycosphingolipids (GSLs) and essential for various cellular processes. Previous studies reported that UGCG knockout in mice led to embryonic lethality, occurring during gastrulation. Moreover, disrupting UGCG expression could affect the growth of cancer cells in vitro and in vivo [28,29]. Interestingly, accumulating evidence has implied that UGCG was upregulated in various cancers and correlated with poor patient survival [30], modulating various malignant behaviors of cancers through regulating several signaling pathway [[31], [32], [33]], such as AKT and ERK1/2. However, the functional roles and specific mechanisms of UGCG in breast cancer is unclear.
In this study, we found that chemoresistant BC tissues or cells had significantly higher levels of UGCG expression based on the application of GEO databases. Additionally, elevated UGCG expression was correlated with poorer response to chemotherapy in TNBC. Moreover, Wnt/β-catenin and NF-κB pathways mediated the UGCG-regulated chemotherapeutic sensitivity and metastatic potential of BC cells. The present investigation provides compelling evidence that UGCG is a crucial regulator of BC progression and chemoresistance, and targeting UGCG may represent a viable therapeutic approach.
Materials and methods
Clinical samples
Breast cancer tissues as well as the neighboring normal tissues were collected from patients with BC who were having surgery at Qilu Hospital of Shandong University.
Fifty-two patients with BC were administered neoadjuvant chemotherapy, which included either taxane or anthracycline and did not receive targeted therapy. The clinicopathological information of BC patients is presented in Table S1. Based on MP classification, the patients were categorized into groups that were chemosensitive and chemoresistant. Chemosensitive group: G4 and G5. Chemoresistant group: G1, G2, and G3. The Ethical Committee of Qilu Hospital of Shandong University approved the study, and each participant provided written informed permission.
Cell lines and cultures
The non-tumorigenic epithelial cell line MCF10A and the human BC cell lines (MDA-MB-468, MDA-MB-231, ZR75–1, T47D, MCF-7, MDA-MB-453, HS578T) were acquired from ATCC (Manassas, VA, USA). The BC cells were cultured in DMEM supplemented with 1% penicillin-streptomycin (Invitrogen, USA) and 10% fetal bovine serum (FBS, Gibco, USA) at 37 °C in a humidified atmosphere with 5% CO2. Short tandem repeat (STR) DNA profiling was used to validate the cell lines, and all of the tests for mycoplasma contamination were negative.
Cell transfection
UGCG was subcloned in its entirety into the pcDNA3.1 vector to produce constructs that overexpressed UGCG. Following transfection with either the UGCG-overexpressing or empty vectors, cells were subjected to selection using G418 for a period exceeding 2 months in order to obtain stable transfectants. The siRNAs were acquired from GenePharma (Shanghai, China), and the lipofectamine 2000 reagent (Invitrogen, USA) was utilized for the transfection of plasmids or siRNAs, as per the provided protocol.
Real-time quantitative polymerase chain reaction (qRT-PCR) analysis
Cells or tissues were subjected to RNA isolation with TRIzol Regent, followed by cDNA synthesis through the PrimeScript reverse transcriptase (RT) reagent kit (Takara, Japan). The qRT-PCR assay was performed using the LightCycler480 Detection System (Roche, Germany) and SYBR Green (Takara, Japan). The 2-ΔΔCt technique was employed to calculate the relative gene expression levels. The following are the UGCG primers: F: 5′-GGTTCACGGGCTGCCTTAC-3′, R: 5′-CCAGTTACATTGGCAGAGAT-3′. The endogenous control used was β-actin.
Western blot analysis and protein extraction
The cells were lysed by RIPA lysis buffer (Beyotime, China), which was supplemented with PMSF and phosphatase inhibitor (NaF), then centrifuge at 12,000 g for 20 min at 4 °C. Using a BCA kit (Beyotime, China), the protein concentration was determined. SDS-PAGE gels were utilized to separate the protein samples, which were then transferred to a PVDF membrane (Millipore, USA). Using 5% skim milk, the membranes were blocked for an hour at room temperature. Then, they were treated with primary antibodies at 4 °C overnight and inoculated with appropriate secondary antibodies (Table S2). The outcomes were viewed using an enhanced chemiluminescence (ECL) kit (Vazyme, China).
Cell proliferation assays
A 96-well plate was seeded with 1.5 × 103 transfected cells that had been resuspended in 100 μl of complete medium. Following the specified culture time, each well was added with 20 μl of 5 mg/ml MTT following a 4h-incubation. Following the removal of the culture supernatant, dimethyl sulfoxide (DMSO, 100 μl) was added. The absorbance at 490 nm was measured using a Microplate Reader from Bio-Rad, USA. In order to determine the IC50 or cytotoxicity of paclitaxel and docetaxel, a total of 3 × 103 transfected cells were placed in wells of a 96-well plate and subjected to several concentrations of paclitaxel or docetaxel for 48 hours. The vitality of the cells was assessed using the MTT test. In the colony formation assay, a total of 1 × 103 transfected cells were placed in 6 cm plates and incubated for 2 weeks. Following the washing step with PBS, methanol fixation, and the staining with 0.2% crystal violet, the colonies containing more than 50 cells were enumerated and photographed.
Sphere formation assay
Ultra-low attachment 96-well plates were seeded with 1 × 103 transfected cells. The culture media used for cell stem maintenance was DMEM/F12, which contained 1% penicillin-streptomycin, 1 x B27 supplement, 5 μg/mL insulin, and 20 ng/mL FGF and EGF. The spheres were photographed with a light microscope after ten days.
ALDEFLUOR assay
The ALDEFLOUR kit (StemCell Technologies) was employed for the determination of the aldehyde dehydrogenase (ALDH) activity of BC cells, following the guidelines provided by the manufacturer. The ALDH-positive cell population was identified by utilizing a FACSCalibur flow cytometer (BD Biosciences, USA).
Culture of patient-derived organoid (PDO) and subsequent treatment
Chopped tissues were incubated at 37 °C for 2 h with the addition of 1% BSA, ITS-G (BasalMedia Technologies Company, China), Primocin (Invitrogen, USA), 5 μM Y-27,632 (MCE, USA), 10 mM HEPES (Thermo, USA), collagenase I and III (Worthington, Italy), and 1000 U/mL hyaluronidase (Sigma-Aldrich, USA). After passing through a 100 μm filter strainer, the cell pellets were collected and resuspended in a digestion termination solution. After lysing the erythrocytes with the TAC buffer, the cell pellets were reconstituted using growth factor-reduced matrigel that had been diluted 1:3 in an organoid culture medium. The DMEM/F12 medium was combined with particular amounts of several growth agents and components to create the organoid culture medium, including FGF7 (5 ng/ml), EGF (5 ng/ml), R-spondin-1 (0.5 µg/ml), Neuregulin 1 (0.5 µg/ml), FGF10 (20 ng/ml), 0.1 µg/mL Noggin, 500 nM A83–01, 5 µM Y-27632, 500 nM SB202190, HEPES, 1.25 mM N-acetyl-L-cysteine, 5 mM Nicotinamide, Primocin, GlutaMax, and B27. The suspension of organoids was placed into a 48-well plate and kept at 37 °C for 60 minutes to encourage the formation of a solid structure. Each well was supplemented with 300 μl of organoid culture media, and the medium was refreshed every three days. Lentiviral vectors containing UGCU-overexpressing or control genes were utilized for transfecting the organoids. Light microscopy was used to observe organoid size and take images.
Wound healing assay
After being seeded onto 24-well plates, the transfected cells were cultured until the cell reached 90% density. Using a 10 µL pipette tip, the cell monolayer was scraped in a straight line, and detached cells were removed by washing with PBS. Photographs were taken using a light microscope at the specified time.
Cell migration and invasion assays
Transwell chambers with an 8 μm chamber size (Corning) and a 24-well plate were utilized for the cell migration test. Upper chambers were seeded with 1 × 105 transfected cells in serum-free DMEM (200 μL). The lower chamber was added with 700 μL of DMEM containing 20% FBS. For the invasion assay, each chamber received 50 μL of diluted Matrigel (BD Biosciences, USA) that had been diluted six times with serum-free DMEM. Methanol was used to fix the invading or migrating cells, followed by crystal violet staining. An Olympus microscope was employed to observe the cells, which were then counted for statistics.
Apoptosis analysis
The cells were exposed to PTX, DOC, or DMSO for one day. The PE Annexin V Apoptosis Detection Kit (BD Biosciences, USA) was utilized to evaluate the apoptosis of the cells. Each sample was treated with 5 μL of Annexin V-FITC and 10 μL of propidium iodide (PI), and it was then incubated at room temperature in the dark for 15 minutes. The stained cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences, USA) following the addition of 300 μL binding buffer. FlowJo software was used for data processing.
TUNEL assay
The TUNEL experiment was conducted using a one-step TUNEL apoptosis assay kit manufactured by Beyotime in China. The cells were rinsed with PBS, treated with 4% PFA for fixation, and made permeable with 0.3% Triton X-100. Following incubation with TUNEL detection solution at a temperature of 37 °C in the dark for 60 minutes, the cell nuclei were subsequently stained with DAPI. A fluorescent microscope (ZEISS, Germany) was used to observe and take photos.
Animal experiments
GemPharmatech Co., Ltd. (Nanjing, China) provided female nude mice that were 4–5 weeks old. In the subcutaneous tumor model, mice were given subcutaneous injections in the dorsal flanks with either control cells (5 × 106) or UGCG-stable overexpressed MDA-MB-231 cells. Every five days, tumors were measured, and the tumor volumes were calculated by employing the formula: tumor volume (mm3) = 0.5 × Width2 × Length. The tumors were removed and weighed when the trial concluded. To create the lung metastasis model, 200 μl PBS was used to resuspend MDA-MB-231 cells (at 5 × 105 density) that were either stably expressing UGCG or control vectors, and intravenously injected into the tail veins of nude mice. After four weeks, the mice were sacrificed and their lungs were removed and examined. The Shandong University Animal Care and Use Committee approved the animal-related experiments.
IHC staining
Samples embedded in paraffin were sectioned at a thickness of 4 μm, deparaffinized, and then rehydrated. Following antigen retrieval in citrate buffer and endogenous peroxidase blocking with 3% H2O2, the slices were incubated for 1 h at room temperature in the blocking buffer. The tissue slices were incubated with primary antibodies at 4 °C overnight. Afterwards, corresponding HRP-conjugated secondary antibodies were applied. Following this, the slices were observed using a DAB solution and counterstained with hematoxylin. To capture pictures, an Olympus light microscope was employed. The IHC score of UGCG was measured by calculating the degree of intensity and the proportion of positively stained tumor cells as follows: IHC score = percentage score × intensity score. The percentage scores were defined as follows: 0, < 10%; 1, 10–25%; 2, 25–50%; 3, 50–75%; 4, > 75%. The definition of the intensity scores was as follows: 1, light brown; 2, brown; 3, dark brown; 0, no staining. Four grades were assigned to the UGCG expression: negative (IHC score ≤ 3), weak (IHC score > 3 and ≤ 6), moderate (IHC score > 6 and ≤ 9), and strong (IHC score > 9). A receiver operating characteristic (ROC) curve was constructed based on the IHC scores and response to chemotherapy of BC patients. The cut-off values were determined by calculating the sensitivity and specificity of the ROC curves, with a threshold set at 5. The BC patients were categorized into two groups based on their UGCG expression levels: UGCG-high (IHC score > 5) and UGCG-low (IHC score < 5).
Statistical analysis
All data comes from a minimum of three distinct studies and is represented as mean ± SD. For statistical analysis, SPSS software and GraphPad Prism 8.0 were utilized. Student's t-test was used to assess statistical significance when comparing two groups, and one-way ANOVA was used when comparing multiple samples. The association between clinicopathological traits and neoadjuvant chemotherapy response was evaluated using the chi-square test. While univariate and multivariate logistic regression analyses were utilized to investigate potential variables for chemosensitivity, Spearman regression analysis was employed to determine the association between UGCG and chemosensitivity. The statistical significance was deemed when P < 0.05.
Results
UGCG is highly expressed in chemoresistant BC cells and tumor tissues
We first examined the gene expression profile data from GEO databases (GSE21974 and GSE28784) to identify the differently expressed genes (DEGs) to determine the fundamental mechanism of BC chemoresistance. The genes with |log2FC| ≥ 1 and P value < 0.05 were selected (Figs. 1A and S1A). After intersecting the results, we identified 21 upregulated and 23 downregulated DEGs in chemoresistant BC tissues or cells (Fig. 1B, Table S3). We mainly focused on the elevated DEGs due to their potential as therapeutic targets. Among these, we were particularly interested in UGCG, which, according to multiple public databases, was substantially elevated in BC tissues and chemoresistant BC tissues (Fig. S1B and C). Furthermore, elevated RNA expression of UGCG was also seen in several different types of cancer (Fig. S1D). The analysis of IHC tests utilizing tissues from our cohort and the Protein Atlas database revealed that the protein expression of UGCG was increased in BC tissues in comparison to normal tissues (Figs. 1C and S1E). The expression of UGCG was observed in 52 TNBC tissues that underwent neoadjuvant chemotherapy in order to assess the importance of UGCG in chemosensitivity. The findings demonstrated that the protein expression of UGCG was considerably enhanced in chemoresistant BC tissues than the chemosensitive tissues (Figs. 1D and S1F). The area under the curve (AUC) of the ROC analysis was 0.8911, suggesting that UGCG may be used to predict chemosensitivity in TNBC (Fig. 1E). The cut-off value for UGCG expression was established at 5 based on the sensitivity and specificity of ROC curves, which categorized these patients into two groups. The pCR rate of the high UGCG-expressing group (42.31 %) was a mere 4.55 %. In comparison, the low UGCG-expressing group (57.69% of the total patients) exhibited a notably higher pCR rate of 66.67% (Fig. 1F, P < 0.0001). Furthermore, poor neoadjuvant chemotherapy results in TNBC patients were positively correlated with UGCG expression, according to the correlation analysis (Fig. 1G). In addition, both univariate and multivariate logistic regression analyses were conducted, revealing that high expression of UGCG independently predicted the response to neoadjuvant chemotherapy (P < 0.05, Tables S4 and S5). We detected more abundant expression of UGCG in BC cells compared to normal cells (MCF-10A), especially TNBC cells (Fig. 1H). Consequently, MDA-MB-468 and MDA-MB-231, the two TNBC cells, were utilized in additional studies. The findings mentioned above demonstrated the possible impact of UGCG on the development and chemosensitivity of BC.
Fig. 1.
UGCG expression is correlated with chemotherapy efficacy in breast cancer. (A) Heat maps show the differentially expressed genes (DEGs) between chemoresistant and chemosensitive breast cancer tissues (GSE21974) or cells (GSE28784) based on GEO cohorts. (B) Venn diagrams display DEGs that are commonly upregulated or downregulated in GES21974 and GSE28784 cohorts. (C) Representative images of IHC staining of UGCG in normal and breast cancer tissue samples. Scale bar, 100 μm. (D) The IHC staining score of UGCG in chemosensitive and chemoresistant breast cancer tissues was analyzed, and the representative images of IHC staining of UGCG in two groups were shown. (E) ROC analysis of sensitivity and resistance to chemotherapy between breast cancer patients with high and low UGCG expression. (F) The four-fold table shows the expression of UGCG in chemosensitive and chemoresistant breast cancer tissues. (G) The association between IHC score of UGCG staining and MP classification was evaluated through spearman regression analysis. (H) The protein levels of UGCG in normal breast epithelial cell line (MCF10A) and breast cancer cell lines were examined by western blot.
UGCG promotes malignant behaviors of BC cells
Initially, the impact of UGCG on the biological traits of BC cells was studied. The upregulation efficacy of UGCG was validated using qRT-PCR and western blot analysis (Fig. 2A). The impact of UGCG on the proliferation of organoids obtained from BC patients was evaluated. The findings demonstrated that the overexpression of UGCG resulted in an augmented size of organoids (Fig. 2B). Furthermore, the outcomes from MTT, colony formation, and EdU assays suggested that the overexpression of UGCG increased the proliferative capacity of MDA-MB-231 and MDA-MB-468 cells (Fig. S2). The sphere formation assay demonstrated that UGCG enhanced the stem-like properties of BC cells (Fig. 2C). The flow cytometry assay also found an elevated percentage of ALDH+ cells after UGCG overexpression (Fig. 2D). Furthermore, the wound healing test and transwell assay demonstrated that the overexpression of UGCG greatly enhanced the migration and invasion of BC cells (Fig. 2E and F). Moreover, the CM derived from BC cells with UGCG overexpression resulted in enhanced tube-forming capabilities of HUVECs compared to control cells (Fig. 2G). The western blot experiment revealed increased levels of Vimentin, N-cadherin, and Fibronectin following UGCG overexpression. Conversely, the expression of E-cadherin reduced (Fig. 2H), suggesting an augmented epithelial-mesenchymal transition. UGCG knockdown consistently reduced BC cell migration, invasion, stemness, and proliferation (Figs. S3 and S4). Together, all of these investigations indicated that UGCG functions as a facilitator of tumor progression in BC.
Fig. 2.
UGCG overexpression induced malignant behaviors of breast cancer cells. (A) The overexpression efficiency of UGCG was validated by qRT-PCR and western blot assays. (B) The effect of UGCG on the growth of patient-derived organoid was analyzed. (C) Sphere formation assay was used to evaluate the influence of UGCG on tumor stemness. (D) Flow cytometry assay was performed to detect the percentage of ALDH+ breast cancer cells. (E) The migratory and invasive capacities of breast cancer cells with or without UGCG overexpression were evaluated using transwell assay. (F) Wound healing assay was used to measure the effect of UGCG overexpression on the migratory capacity of breast cancer cells. (G) Tube formation assay was performed to analyze the angiogenesis-promoting effect of UGCG overexpression on HUVEC cells. (H) The expression of EMT-related proteins in breast cancer cells transfected with control or UGCG-overexpressing vectors was detected by western blot. (*P < 0.05, **P < 0.01, ***P < 0.001).
UGCG enhances chemoresistance of BC cells
Due to the elevated expression of UGCG in paclitaxel-resistant cells and docetaxel-resistant cells compared with control cells based on the GSE28784 database (Fig. S5), we wonder whether UGCG could regulate the chemosensitivity of BC cells. Remarkably, the qRT-PCR and western blot experiments demonstrated that the mRNA and protein levels of UGCG were significantly increased in a time-dependent manner following PTX and DOC therapy (Fig. 3A). PTX or DOC treatment also effectively increased UGCG expression in a dose-dependent manner (Fig. 3B). The results demonstrated that PTX and DOC therapy may stimulate the expression of UGCG, hence uncovering the association between UGCG and chemoresistance. In order to investigate the impact of UGCG on the resistance of BC cells to chemotherapy, we introduced plasmids that overexpress UGCG into MDA-MB-231 and MDA-MB-468 cells. Our findings indicate that increasing the expression of UGCG resulted in greater resistance to PTX. In MDA-MB-468 or MDA-MB-231 cells, PTX's IC50 values increased by approximately three and four times, respectively (Fig. 3C). Additionally, overexpression of UGCG led to strengthened resistance to DOC. The IC50 values of DOC were substantially elevated in MDA-MB-468 or MDA-MB-231 cells exhibiting UGCG overexpression (Fig. 3D). The reduced inhibition ratio of cell viability in cells treated with PTX or DOC (Fig. 3E and F) indicated that UGCG overexpression could alleviate the inhibitory effect of chemotherapy on colony-forming ability of BC cells. After treatment with PTX or DOC, the growth state of transfected organoids was evaluated. The results found that the overall structure of UGCG-overexpressing organoids was almost impervious to chemotherapeutic treatment. In contrast, the control organoids tended to decompose and die, further providing that UGCG could enhance the resistance of the organoids to PTX and DOC (Fig. 3G). In accordance with previous findings, when PTX or DOC was present, knockdown of UGCG reduced the viability and clonogenicity of BC cells (Fig. S6). Collectively, the present study revealed that the expression of UGCG is directly correlated with the chemoresistance in BC cells.
Fig. 3.
UGCG overexpression contributes to chemoresistance of breast cancer cells. (A) The RNA and protein expression levels of UGCG were detected utilizing qRT-PCR and western blot respectively after paclitaxel or docetaxel treatment for indicated time. (B) The RNA and protein expression levels of UGCG were detected utilizing qRT-PCR and western blot respectively after 48 h of paclitaxel or docetaxel treatment with increasing concentrations. (C-D) MTT assay shows that UGCG overexpression leads to increased resistance to paclitaxel (C) and docetaxel (D) of breast cancer cells. (E-F) The effect of UGCG overexpression on the cell viabilities of breast cancer cells after treatment with paclitaxel or docetaxel were analyzed by colony formation assay. (G) The patient-derived organoids infected using UGCG-overexpressing or control retroviruses were treated with paclitaxel or docetaxel, and the representative images were shown. (*P < 0.05, **P < 0.01, ***P < 0.001).
UGCG promotes the chemoresistance and survival of BC cells via repressing apoptosis
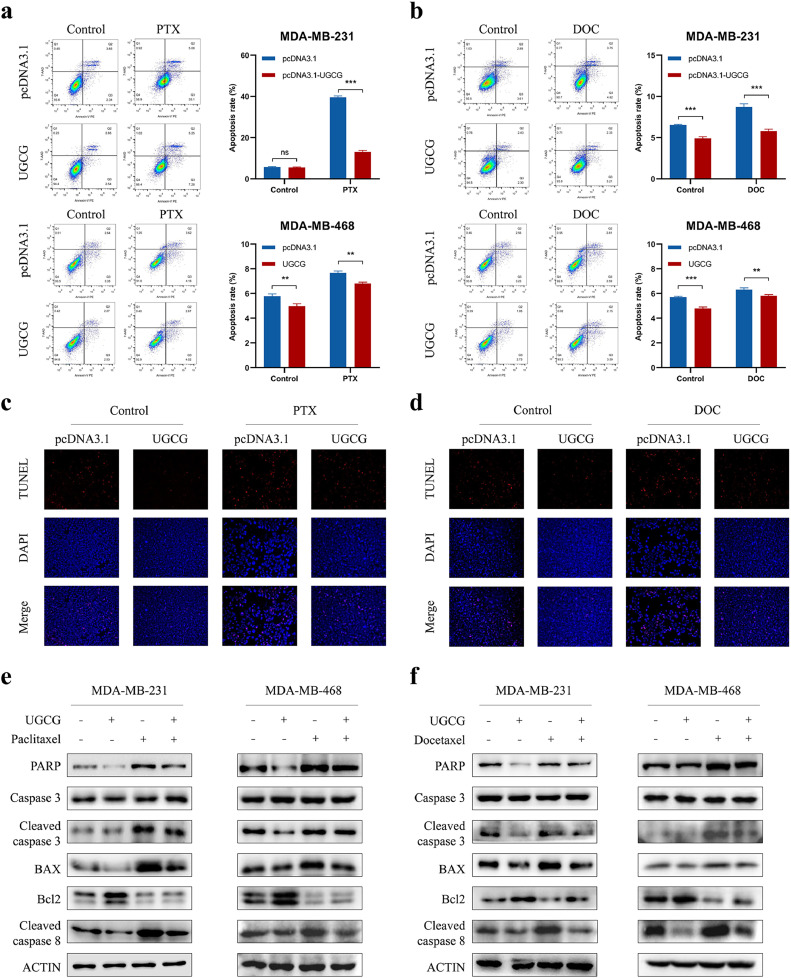
To further explore the role of UGCG in the resistance of BC to chemotherapy, we examined the impact of UGCG on apoptosis, a well-known process by which chemotherapy induces cell death. Flow cytometry indicated that the paclitaxel or docetaxel treatment resulted in an enhanced apoptotic rate of BC cells, and UGCG overexpression could significantly attenuate chemotherapy-induced cell apoptosis (Fig. 4A and B). In addition, the increased number of TUNEL-positive cells after paclitaxel or docetaxel treatment could be reduced after UGCG overexpression (Fig. 4C and D). In order to ascertain the fundamental process, we conducted an additional investigation into the impact of UGCG on the expression of proteins associated with apoptosis. The western blot experiment demonstrated that the overexpression of UGCG led to decreased levels of apoptosis-related proteins in the presence of paclitaxel or docetaxel (Fig. 4E and F). Consistently, the reduction in UGCG increased cell death caused by chemotherapy. This was confirmed through flow cytometry, TUNEL, and western blot assays (Fig. S7). Our results demonstrated that UGCG could repress the apoptotic process and was responsible for the chemoresistance of BC cells.
Fig. 4.
UGCG overexpression attenuates chemotherapy drugs-induced apoptosis in breast cancer cells. (A-B) Flow cytometry assay was used to detect cell apoptotic levels in breast cancer cells transfected with control or UGCG-overexpressing vectors after paclitaxel (A) or docetaxel (B) treatment. (C-D) TUNEL assay was performed to evaluate the effect of paclitaxel (C) or docetaxel (D) treatment on cell apoptosis. (E-F) The expression of apoptosis-related proteins in control or UGCG-overexpressing cells treated with paclitaxel (E) or docetaxel (F) were analyzed by western blot. (ns, no significance, **P < 0.01, ***P < 0.001).
UGCG facilitates activation of NF-κB and Wnt/β-catenin pathways to enhance BC chemoresistance and metastasis
We further explored UGCG-regulated signaling pathways in BC. Notably, the western blot assay demonstrated that overexpression of UGCG increased the NF-κB, p-NF-κB, and p-IKβ-α expression levels, while decreasing the expression of IKβ-α, a major negative regulator of NF-κB, indicating the enhanced NF-κB activity activation, (Fig. 5A). Moreover, p-GSK-3β expression was downregulated, while β-catenin expression was upregulated after UGCG overexpression, revealing the role of UGCG in activating Wnt/β-catenin signaling (Fig. 5A). Accordingly, reduced activation of NF-κB and Wnt/β-catenin signaling pathways were detected in UGCG knockdown cells (Fig. 5B). To investigate whether UGCG might promote the chemoresistance and progression of BC through modulating Wnt/β-catenin and NF-κB signaling pathways, specific inhibitors were applied to perform rescue experiments (Fig. S8). BAY 11-7082, a potent inhibitor of the NF-κB pathway, could obviously reverse the UGCG-enhanced cell proliferation and migration (Figs. 5C and D and S9A and B). Furthermore, the administration of Xav-939, a potent inhibitor of the Wnt/β-catenin signaling pathway, could effectively eliminate the impact of UGCG on cell migration and proliferation (Figs. 5E and F and S9C and D). We further validated the enhanced PTX and DOC resistance due to UGCG is contingent upon activation of NF-κB or Wnt/β-catenin signaling pathway. BC cells overexpressing UGCG were treated with BAY 11–7082 or Xav-939, simultaneously administered with paclitaxel or docetaxel. The results showed that increased resistance to paclitaxel and docetaxel after UGCG overexpression could be reversed by the treatment of BAY 11–7082 (Fig. 6A and B). Moreover, we observed a conspicuous reduction in IC50 values of paclitaxel and docetaxel in UGCG-overexpressing BC cells upon BAY 11–7082 treatment (Fig. 6C and D). Similarly, the attenuated cell viability was found in UGCG-overexpressing BC cells after PTX and DOC treatment in the presence of Xav-939, concomitant with the decreased IC50 values of PTX and DOC (Fig. 6E–H). Together, these results suggested that UGCG contributed to PTX and DOC resistance predominantly through activating NF-κB and Wnt/β-catenin signaling pathways.
Fig. 5.
UGCG facilitates malignant behaviors of breast cancer cells through modulating NF-κB and β-catenin pathways. (A) Western blot was used to detect the effect of UGCG overexpression on the expression of NF-κB or β-catenin pathways-related proteins. (B) Western blot was used to evaluate the effect of UGCG knockdown on the expression of NF-κB or β-catenin pathways-related proteins. (C-D) Breast cancer cells were transfected with pcDNA3.1 or UGCG-overexpressing vectors and treated with BAY11–7082. The proliferative abilities and of treated cells were measured by colony formation assay (C). The migratory and invasive abilities of treated cells was evaluated by transwell assay (D). (E-F) Breast cancer cells were transfected with pcDNA3.1 or UGCG-overexpressing vectors and treated with XAV 939. The proliferative abilities and of treated cells were measured by colony formation assay (E), and the migratory and invasive abilities of treated cells was evaluated by transwell assay (F). (*P < 0.05, **P < 0.01, ***P < 0.001).
Fig. 6.
UGCG overexpression enhances resistance to chemotherapy drugs through modulating NF-κB and β-catenin pathways in breast cancer cells. (A-B) The cell viabilities of pcDNA3.1 or UGCG-overexpressing breast cancer cells treated with or without BAY11–7082 in the presence or absence of paclitaxel (A) or docetaxel (B) were evaluated by MTT assay. (C-D) The cell viabilities of pcDNA3.1 or UGCG-overexpressing breast cancer cells treated with or without BAY11–7082 in the presence of increasing concentrations of paclitaxel (C) or docetaxel (D) were measured by MTT assay. (E-F) The cell viabilities of pcDNA3.1 or UGCG-overexpressing breast cancer cells treated with or without XAV 939 in the presence or absence of paclitaxel (E) or docetaxel (F) were evaluated by MTT assay. (G-H) The cell viabilities of pcDNA3.1 or UGCG-overexpressing breast cancer cells treated with or without XAV 939 in the presence of increasing concentrations of paclitaxel (G) or docetaxel (H) were measured by MTT assay. (*P < 0.05, **P < 0.01, ***P < 0.001).
UGCG facilitates BC progression in vivo
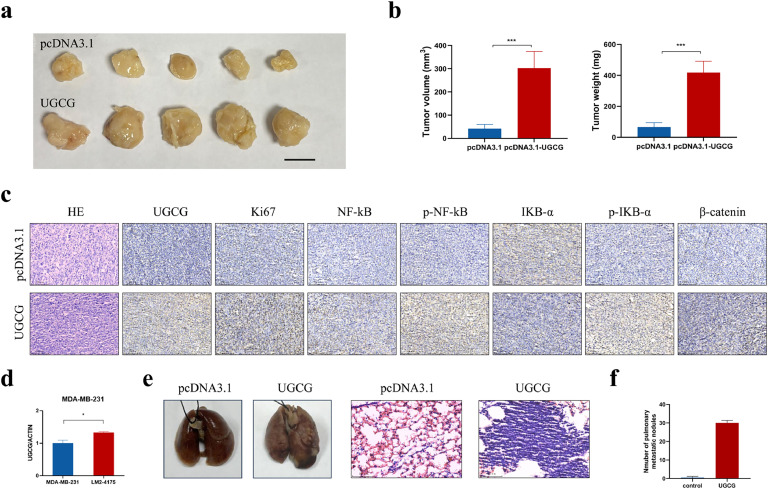
To determine the impact of UGCG on the progression of BC, a xenograft mouse model was utilized. The UGCG-overexpressing and control cells were injected subcutaneously into the nude mice. As anticipated, the xenograft tumors in the UGCG-overexpressing group demonstrated larger tumor volume and weight compared to the control group (Fig. 7A and B). IHC assay was conducted on subcutaneous tumor tissues after they were harvested. It was discovered that the overexpression of UGCG led to an increase in the expression of Ki67, which is a biomarker used to indicate cell proliferation (Fig. 7C). Moreover, xenograft tumors derived from cells with UGCG overexpression existed higher levels of p-NF-κB and β-catenin (Fig. 7C), which was accordant with the in vitro findings. According to the above findings, UGCG improved the activation of the β-catenin and NF-κB pathways, which in turn increased the growth of BC in vivo. We further explored the role of UGCG in mediating BC metastasis. The level of UGCG expression in BC cells with a potential for lung metastasis was considerably higher compared to their parental cells (Fig. 7D), demonstrating the metastasis-promoting potential of UGCG. Subsequently, a lung metastasis model was created by intravenously injecting UGCG-overexpressing or controlling MDA-MB-231 cells into BALB/c nude mice through their tail veins. The metastatic lesions were pathologically confirmed by HE staining (Fig. 7E). No mice injected with control cells exhibited lung metastasis. In contrast, all five mice in the UGCG-overexpressing group developed metastatic nodules in lung tissues. Notably, the UGCG-overexpressing group had more lung metastases both in terms of volume and count than the control group (Fig. 7E and F). In summary, these results indicated that increased UGCG aided the progression of BC in vivo.
Fig. 7.
UGCG promotes breast cancer progression in vivo. (A) Image of resected tumors from nude mice in different groups. Scale bar, 1 cm. (B) The volume and weight of xenograft tumors in different groups. (C) The expression levels of UGCG, Ki67, NF-κB, p-NF-κB, IKB-α, p-IKB-α, and β-catenin were determined by IHC staining in the tumor sections of different groups. Scale bars, 100 μm. (D) The RNA expression of UGCG in MDA-MB-231 cells with high lung metastatic potential and parental cells was evaluated. (E) The representative images of lung tissues and HE staining from nude mice in UGCG-overexpressing and control groups. (F) The number of metastatic nodules in lungs from nude mice in different groups. (*P < 0.05, ***P < 0.001).
Discussion
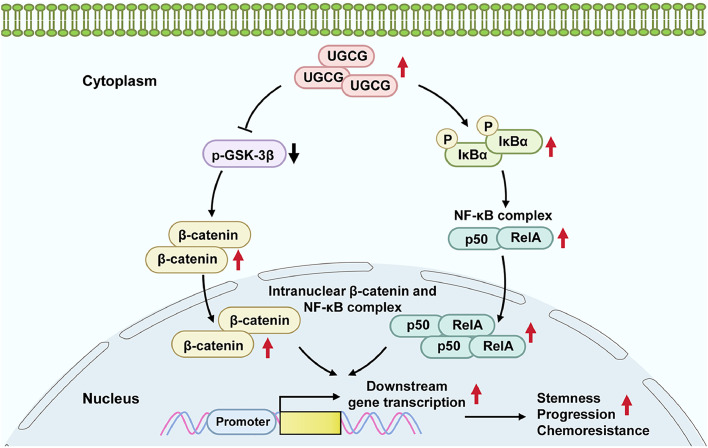
Due to the aggressive properties and lack of effective therapeutic targets of TNBC, the prognosis of TNBC patients remains poor and a challenging clinical problem. Chemotherapy is the major systemic treatment for TNBC patients, and taxanes, such as paclitaxel and docetaxel, are extremely important class of drugs in the treatment of TNBC [34]. Although many patients could benefit from treatment with taxanes, a significant portion of patients, particularly those with metastatic BC, exhibit inherent or acquired resistance to the drugs, ultimately resulting in cancer relapse or metastasis [35]. Therefore, exploring effective predictive biomarkers and potential therapeutic targets would be beneficial in enhancing the therapeutic outcome and prognosis of patients with TNBC. The current research utilized GEO databases to investigate the cross-resistance patterns of taxanes and explore potential resistance mechanisms. Based on bioinformatics analysis and our experimental results, we identified UGCG as a predictive biomarker for chemotherapy response, which was upregulated in chemoresistant TNBC tissues and cells. Furthermore, our data elucidated that UGCG contributed to progression and resistance to chemotherapy of TNBC cells via modulating NF-κB and Wnt/β-catenin signaling pathways (Fig. 8), serving as a potential therapeutic target to overcome chemoresistance.
Fig. 8.
Schematic diagram illustrating UGCG-mediated activation of NF-κB and β-catenin pathways to regulate chemoresistance and progression in breast cancer.
Previous studies have shown that UGCG served as a proto-oncogene and exhibited increased expression in different types of malignancies [30], which could modulate various malignant behaviors of cancers [27,[31], [32], [33]], such as proliferation, metabolism, and drug resistance. Similarly, we confirmed the higher UGCG expression in BC tissues, and UGCG overexpression promoted cell proliferation, migration, and invasion, while UGCG deficiency inhibited the malignant behaviors in TNBC cells. In consistent with the in vitro results, the animal experiments found that UGCG-overexpressing group exhibited increased tumor growth and lung metastatic capacities. Afterwards, the present research found a relationship between UGCG expression and resistance to chemotherapy in TNBC. According to our research, TNBC patients who expressed high levels of UGCG were found to respond less well to chemotherapy, suggesting that UGCG may be a useful predictor of efficacy. In line with this phenomenon, UGCG overexpression led to upregulated resistance to paclitaxel and docetaxel, whereas UGCG knockdown restored sensitivity to chemotherapeutics in TNBC cells.
The major anti-cancer mechanism of taxanes is binding with microtubules with high affinity to inhibit their dynamic changes and then interfering cell mitosis [36]. In addition, taxanes may be involved in the control of numerous additional biological processes in cancer cells, including apoptosis induction, angiogenesis regulation, and increased ROS production. Therefore, various mechanisms that disrupt the above-mentioned processes might contribute to the development of initial or acquired resistance to taxanes in TNBC, and a deeper understanding of these complex mechanisms would provide reliable ways to overcome drug resistance and improve therapeutic efficacy.
Cancer stem cells (CSCs), possessing the ability of self-renewal, differentiation, and tumor reconstruction, are the root cause of tumor formation and spread [37]. Due to the high cellular plasticity, DNA damage repair ability, and adaptability to the tumor microenvironment, CSCs had superior multidrug resistance with increased expression of multiple drug effector pumps [38]. While paclitaxel treatment may result in cell cycle arrest and death, some cancer cells manage to survive and change the way ALDH and stem cell markers are expressed, making them resistant to the drug [39]. In addition, epithelial-mesenchymal transition (EMT) is also essential for tumor migration and increased chemoresistance. There are various similarities between cells undergoing EMT and CSCs in drug resistance, both involving the regulation of Wnt, Notch, TGF-β, and Hedgehog pathways [40]. Significantly, a growing number of researches have unveiled the connection between chemoresistance and metastasis [41]. For instance, it appears possible that a number of EMT-inducing transcription factors, including Slug, Snail, and Twist, could be used as novel targets to reverse chemoresistance [42]. Moreover, EMT could confer CSC-like phenotypes to cancer cells, further leading to chemoresistance [43]. Following the observation of UGCG-induced chemoresistance in TNBC, our objective was to explore the impact of UGCG on cell stemness and mobility. These findings demonstrated that overexpression of UGCG improved CSC-like behaviors by increasing the fraction of ALDH+ BC cells and improving the ability to form spheres. Moreover, using transwell assay and detection of EMT-related markers, we identified that UGCG could promote mobility and induce the EMT process in BC cells.
Apoptosis is a crucial death pathway emerging under drug pressures, which is a significant strategy to combat cancer. In the treatment of breast cancer, cytotoxic drugs could induce cell death through activating key apoptosis-related proteins, such as p53, Pik1, and Bim. However, tumor cells have evolved several mechanisms to escape from apoptosis through regulating apoptosis-associated pathways [44], which is a major hallmark of cancers and associated with the resistance to various chemotherapy drugs. For example, the overexpression of BCL2 and BCLXL promotes chemoresistance [45,46], while decreased BAX expression attenuates chemotherapy-induced cell apoptosis [47]. Nevertheless, there is still a lack of specific drug resistance biomarkers related to apoptosis and the putative mechanisms of chemotherapy resistance. The present study discovered that the apoptotic effect and expression of apoptosis-related proteins induced by taxanes could be reduced by UGCG overexpression, providing specific drug resistance biomarkers and potential therapeutic strategies for TNBC chemotherapy.
Another significant mechanism mediating chemoresistance is drug efflux via ATP-dependent efflux pump superfamily, ATP-binding cassette (ABC) transporters, which consists of at least 48 genes and could be categorized into 7 subfamilies (ABCA to ABCG) [48,49]. ABCB1, also named as P-gp or MDR1, is one of the most critical ABC effervescent pumps. In ABCB1 knockout mice, the accumulation of cytotoxic drugs such as paclitaxel was increased, suggesting ABCB1 is a crucial regulator in the excretion process of paclitaxel [50]. Furthermore, the elevated ABCB1 expression was linked to resistance against many drugs and unfavorable prognosis in different types of cancers [[51], [52], [53]]. Interestingly, prior research has shown a strong relationship between the expression of UGCG and ABCB1. Additionally, targeting UGCG has the potential to make drug-resistant BC cells more responsive to anti-cancer therapies [54], further enriching the mechanisms of UGCG-mediated drug resistance.
The NF-κB and Wnt/β-catenin pathways have a role in various biological processes, such as cell proliferation, migration, and metabolism [55,56]. Several studies have also found a connection between these pathways and chemoresistance in various types of cancer [57,58]. In chemotherapy-resistant breast tumors, NF-κB and Wnt/β-catenin pathways were significant in controlling the expression of numerous anti-apoptotic proteins, including Lin28, BCL2, and BCLXL [59,60]. The expression of ABCB1 and β-tubulin can be enhanced by several transcription factors, including NF-κB and β-catenin, resulting in heightened resistance to paclitaxel [61,62]. Our findings demonstrate a notable increase in the activation of NF-κB and Wnt/β-catenin signaling pathways following UGCG overexpression, leading us to speculate that UGCG might facilitate progression and chemoresistance of breast cancer through regulating these pathways. BAY 11–7082 is a commonly used NF-κB inhibitor [63], which effectively prevents the phosphorylation and degradation of IκBα. As a result, it inhibits the nuclear transport and transcriptional activity of NF-κB. XAV-939, a cell-permeable Wnt/β-catenin signal transduction inhibitor, could stimulate β-catenin degradation by inhibiting tankyrase to stabilize the destructive complex component Axin [64]. In line with our hypothesis, inhibitors for NF-κB or Wnt/β-catenin have been shown to be effective in the suppression of UGCG-induced TNBC progression and chemoresistance. Therefore, targeting UGCG regulatory pathway to enhance the susceptibility of cancer cells to taxanes could potentially serve as a viable approach to overcome chemoresistance. However, it remains unclear whether alternative pathways contribute to UGCG-mediated progression and resistance to taxanes in TNBC, and whether the UGCG-regulated pathways identified in our study are also present in other types of cancers. More efforts are required to investigate the potential application of targeting UGCG regulatory pathways in different cancer types in the future.
The research identified UGCG as a novel factor contributing to chemoresistance and progression in breast cancer, serving as a novel therapeutic target and biomarker that help to improve the clinical outcome of breast cancer patients. Regarding limitations, although the laboratory results are promising, the sample size is relatively small, and more preclinical studies and larger-scale clinical trials are needed to validate these findings and translate these results from bench to bedside.
Conclusion
Our study reveals the role of UGCG in TNBC progression and its contribution to chemotherapy resistance, and UGCG overexpression conferred resistance to chemotherapy in TNBC cells through repressing apoptosis and was associated with EMT and CSC-like phenotypes. Additionally, our findings provided further evidence for the effectiveness of NF-κB or Wnt/β-catenin inhibitors in reversing chemoresistance generated by UGCG. These data revealed the potential of using UGCG as an as an independent predictor of chemotherapy efficacy and effective therapeutic target for treating chemoresistant TNBC. However, further studies are recommended to fully comprehend the regulatory mechanism and clinical importance of UGCG in cancer.
Ethics approval and consent to participate
The study was approved by the Ethical Committee of Qilu Hospital of Shandong University, and informed consents were obtained from all patients involved in this research.
Funding
This work was supported by Special Foundation for Taishan Scholars (no. ts20190971), Foundation from Clinical Research Center of Shandong University (no. 2020SDUCRCA015), Qilu Hospital Clinical New Technology Developing Foundation (no. 2019-3).
CRediT authorship contribution statement
Li Long: Writing – original draft, Methodology, Investigation, Conceptualization. Lei Wang: Investigation, Formal analysis. Yiran Liang: Methodology, Investigation. Fangzhou Ye: Investigation, Formal analysis. Yuhan Jin: Investigation, Formal analysis. Dan Luo: Methodology, Formal analysis. Xiaoyan Li: Supervision, Resources. Yajie Wang: Investigation, Formal analysis. Yaming Li: Methodology, Investigation. Dianwen Han: Visualization, Validation. Bing Chen: Supervision, Resources. Wenjing Zhao: Supervision, Data curation. Lijuan Wang: Supervision, Data curation. Qifeng Yang: Writing – review & editing, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
The authors declare no potential conflicts of interest.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.102241.
Appendix. Supplementary materials
Data availability
Data will be available from the corresponding author upon reasonable request.
References
- 1.Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA Cancer J. Clin. 2024;74(1):12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H., Zhang N., Moran M.S., Li Y., Liang Y., Su P., et al. Special subtypes with favorable prognosis in breast cancer: a registry-based cohort study and network meta-analysis. Cancer Treat. Rev. 2020;91 doi: 10.1016/j.ctrv.2020.102108. [DOI] [PubMed] [Google Scholar]
- 3.Smolarz B., Nowak A.Z., Romanowicz H. Breast cancer-epidemiology, classification, pathogenesis and treatment (review of literature) Cancer. (Basel) 2022;14(10) doi: 10.3390/cancers14102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y., Zhang N., Zhang H., Yang Q. Comparative prognostic analysis for triple-negative breast cancer with metaplastic and invasive ductal carcinoma. J. Clin. Pathol. 2019;72(6):418–424. doi: 10.1136/jclinpath-2018-205544. [DOI] [PubMed] [Google Scholar]
- 5.von Minckwitz G., Untch M., Blohmer J.U., Costa S.D., Eidtmann H., Fasching P.A., et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 6.Li Y., Zhang H., Merkher Y., Chen L., Liu N., Leonov S., et al. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022;15(1):121. doi: 10.1186/s13045-022-01341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longley D.B., Johnston P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005;205(2):275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 8.Nedeljkovic M., Damjanovic A. Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells. 2019;8(9) doi: 10.3390/cells8090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Reilly E.A., Gubbins L., Sharma S., Tully R., Guang M.H., Weiner-Gorzel K., et al. The fate of chemoresistance in triple negative breast cancer (TNBC) BBA Clin. 2015;3:257–275. doi: 10.1016/j.bbacli.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yano K., Tomono T., Ogihara T. Advances in studies of P-glycoprotein and its expression regulators. Biol. Pharm. Bull. 2018;41(1):11–19. doi: 10.1248/bpb.b17-00725. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y., Ashrafizadeh M., Tambuwala M.M., Ren J., Orive G., Yu G. P-glycoprotein (P-gp)-driven cancer drug resistance: biological profile, non-coding RNAs, drugs and nanomodulators. Drug. Discov. Today. 2024;29(11) doi: 10.1016/j.drudis.2024.104161. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W., Fan Y., Zhang J., Shi D., Yuan J., Ashrafizadeh M., et al. Cell membrane-camouflaged bufalin targets NOD2 and overcomes multidrug resistance in pancreatic cancer. Drug. Resist. Updat. 2023;71 doi: 10.1016/j.drup.2023.101005. [DOI] [PubMed] [Google Scholar]
- 13.Qin Y., Ashrafizadeh M., Mongiardini V., Grimaldi B., Crea F., Rietdorf K., et al. Autophagy and cancer drug resistance in dialogue: pre-clinical and clinical evidence. Cancer Lett. 2023;570 doi: 10.1016/j.canlet.2023.216307. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y., Liu L., Tian Y., Gu M., Wang Y., Ashrafizadeh M., et al. Autophagy-driven regulation of cisplatin response in human cancers: Exploring molecular and cell death dynamics. Cancer Lett. 2024;587 doi: 10.1016/j.canlet.2024.216659. [DOI] [PubMed] [Google Scholar]
- 15.Alqahtani F.Y., Aleanizy F.S., El Tahir E., Alkahtani H.M., AlQuadeib B.T. Paclitaxel. Profile. Drug. Subst. Excip. Relat. Methodol. 2019;44:205–238. doi: 10.1016/bs.podrm.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Tamirisa N., Hunt K.K. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. Ann. Surg. Oncol. 2022;29(3):1489–1492. doi: 10.1245/s10434-021-11223-3. [DOI] [PubMed] [Google Scholar]
- 17.Zelnak A. Overcoming taxane and anthracycline resistance. Breast J. 2010;16(3):309–312. doi: 10.1111/j.1524-4741.2010.00911.x. [DOI] [PubMed] [Google Scholar]
- 18.Jordan M.A., Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 2004;4(4):253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 19.Gascoigne K.E., Taylor S.S. How do anti-mitotic drugs kill cancer cells? J. Cell Sci. 2009;122(Pt 15):2579–2585. doi: 10.1242/jcs.039719. [DOI] [PubMed] [Google Scholar]
- 20.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer. 2010;10(3):194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 21.Sprowl J.A., Reed K., Armstrong S.R., Lanner C., Guo B., Kalatskaya I., et al. Alterations in tumor necrosis factor signaling pathways are associated with cytotoxicity and resistance to taxanes: a study in isogenic resistant tumor cells. Breast Cancer Res. 2012;14(1):R2. doi: 10.1186/bcr3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desmedt C., Fornili M., Clatot F., Demicheli R., De Bortoli D., Di Leo A., et al. Differential benefit of adjuvant docetaxel-based chemotherapy in patients with early breast cancer according to baseline body mass index. J. Clin. Oncol. 2020;38(25):2883–2891. doi: 10.1200/JCO.19.01771. [DOI] [PubMed] [Google Scholar]
- 23.Murray S., Briasoulis E., Linardou H., Bafaloukos D., Papadimitriou C. Taxane resistance in breast cancer: mechanisms, predictive biomarkers and circumvention strategies. Cancer Treat. Rev. 2012;38(7):890–903. doi: 10.1016/j.ctrv.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Feizabadi M.S., Castillon V.J. The effect of tau and taxol on polymerization of MCF7 microtubules in vitro. Int. J. Mol. Sci. 2022;23(2) doi: 10.3390/ijms23020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W., Tang X., Peng Y., Xu Y., Liu L., Liu S. GBP2 enhances paclitaxel sensitivity in triple‑negative breast cancer by promoting autophagy in combination with ATG2 and inhibiting the PI3K/AKT/mTOR pathway. Int. J. Oncol. 2024;64(4) doi: 10.3892/ijo.2024.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichikawa S., Sakiyama H., Suzuki G., Hidari K.I., Hirabayashi Y. Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc. Natl. Acad. Sci. U.S.A. 1996;93(22):12654. doi: 10.1073/pnas.93.22.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wegner M.S., Gruber L., Mattjus P., Geisslinger G., Grosch S. The UDP-glucose ceramide glycosyltransferase (UGCG) and the link to multidrug resistance protein 1 (MDR1) BMC Cancer. 2018;18(1):153. doi: 10.1186/s12885-018-4084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y., Zhang T., Gao P., Meng B., Gao Y., Wang X., et al. Targeting glucosylceramide synthase downregulates expression of the multidrug resistance gene MDR1 and sensitizes breast carcinoma cells to anticancer drugs. Breast Cancer Res. Treat. 2010;121(3):591–599. doi: 10.1007/s10549-009-0513-z. [DOI] [PubMed] [Google Scholar]
- 29.Wang T., Birsoy K., Hughes N.W., Krupczak K.M., Post Y., Wei J.J., et al. Identification and characterization of essential genes in the human genome. Science. 2015;350(6264):1096–1101. doi: 10.1126/science.aac7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jennemann R., Federico G., Mathow D., Rabionet M., Rampoldi F., Popovic Z.V., et al. Inhibition of hepatocellular carcinoma growth by blockade of glycosphingolipid synthesis. Oncotarget. 2017;8(65):109201–109216. doi: 10.18632/oncotarget.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wegner M.S., Schomel N., Gruber L., Ortel S.B., Kjellberg M.A., Mattjus P., et al. UDP-glucose ceramide glucosyltransferase activates AKT, promoted proliferation, and doxorubicin resistance in breast cancer cells. Cell Mol. Life. Sci. 2018;75(18):3393–3410. doi: 10.1007/s00018-018-2799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang F., Zhang H. UDP-glucose ceramide glycosyltransferase contributes to the proliferation and glycolysis of cervical cancer cells by regulating the PI3K/AKT pathway. Ann. Clin. Lab. Sci. 2021;51(5):663–669. [PubMed] [Google Scholar]
- 33.Schomel N., Gruber L., Alexopoulos S.J., Trautmann S., Olzomer E.M., Byrne F.L., et al. UGCG overexpression leads to increased glycolysis and increased oxidative phosphorylation of breast cancer cells. Sci. Rep. 2020;10(1):8182. doi: 10.1038/s41598-020-65182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obidiro O., Battogtokh G., Akala E.O. Triple negative breast cancer treatment options and limitations: future outlook. Pharmaceutics. 2023;15(7) doi: 10.3390/pharmaceutics15071796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., Zhan Z., Yin X., Fu S., Deng X. Targeted therapeutic strategies for triple-negative breast cancer. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.731535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh C.Y., Lin C.C., Chang W.C. Taxanes in the treatment of head and neck squamous cell carcinoma. Biomedicines. 2023;11(11) doi: 10.3390/biomedicines11112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta P.B., Pastushenko I., Skibinski A., Blanpain C., Kuperwasser C. Phenotypic plasticity: driver of cancer initiation, progression, and therapy resistance. Cell Stem. Cell. 2019;24(1):65–78. doi: 10.1016/j.stem.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turdo A., Veschi V., Gaggianesi M., Chinnici A., Bianca P., Todaro M., et al. Meeting the challenge of targeting cancer stem cells. Front. Cell. Dev. Biol. 2019;7:16. doi: 10.3389/fcell.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Khattouti A., Selimovic D., Haikel Y., Megahed M., Gomez C.R., Hassan M. Identification and analysis of CD133(+) melanoma stem-like cells conferring resistance to taxol: An insight into the mechanisms of their resistance and response. Cancer Lett. 2014;343(1):123–133. doi: 10.1016/j.canlet.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 40.Du B., Shim J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to overcome drug resistance in cancer. Molecules. 2016;21(7) doi: 10.3390/molecules21070965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iseri O.D., Kars M.D., Arpaci F., Atalay C., Pak I., Gunduz U. Drug resistant MCF-7 cells exhibit epithelial-mesenchymal transition gene expression pattern. Biomed. Pharmacother. 2011;65(1):40–45. doi: 10.1016/j.biopha.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 42.van Staalduinen J., Baker D., Ten Dijke P., van Dam H. Epithelial-mesenchymal-transition-inducing transcription factors: new targets for tackling chemoresistance in cancer? Oncogene. 2018;37(48):6195–6211. doi: 10.1038/s41388-018-0378-x. [DOI] [PubMed] [Google Scholar]
- 43.Singh A., Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inao T., Iida Y., Moritani T., Okimoto T., Tanino R., Kotani H., et al. Bcl-2 inhibition sensitizes triple-negative human breast cancer cells to doxorubicin. Oncotarget. 2018;9(39):25545–25556. doi: 10.18632/oncotarget.25370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi X., Dou Y., Zhou K., Huo J., Yang T., Qin T., et al. Targeting the Bcl-2 family and P-glycoprotein reverses paclitaxel resistance in human esophageal carcinoma cell line. Biomed. Pharmacother. 2017;90:897–905. doi: 10.1016/j.biopha.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 46.Aakko S., Straume A.H., Birkeland E.E., Chen P., Qiao X., Lonning P.E., et al. MYC-induced miR-203b-3p and miR-203a-3p control Bcl-xL expression and paclitaxel sensitivity in tumor cells. Transl. Oncol. 2019;12(1):170–179. doi: 10.1016/j.tranon.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tu Y.C., Yeh W.C., Yu H.H., Lee Y.C., Su B.C. Hedgehog suppresses paclitaxel sensitivity by regulating akt-mediated phosphorylation of bax in EGFR wild-type non-small cell lung cancer cells. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.815308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fletcher J.I., Williams R.T., Henderson M.J., Norris M.D., Haber M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug. Resist. Updat. 2016;26:1–9. doi: 10.1016/j.drup.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Li W., Zhang H., Assaraf Y.G., Zhao K., Xu X., Xie J., et al. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug. Resist. Updat. 2016;27:14–29. doi: 10.1016/j.drup.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Sparreboom A., van Asperen J., Mayer U., Schinkel A.H., Smit J.W., Meijer D.K., et al. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc. Natl. Acad. Sci. U.S.A. 1997;94(5):2031–2035. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergonzini C., Gregori A., Hagens T.M.S., van der Noord V.E., van de Water B., Zweemer A.J.M., et al. ABCB1 overexpression through locus amplification represents an actionable target to combat paclitaxel resistance in pancreatic cancer cells. J. Exp. Clin. Cancer. Res. 2024;43(1):4. doi: 10.1186/s13046-023-02879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aldonza M.B., Hong J.Y., Alinsug M.V., Song J., Lee S.K. Multiplicity of acquired cross-resistance in paclitaxel-resistant cancer cells is associated with feedback control of TUBB3 via FOXO3a-mediated ABCB1 regulation. Oncotarget. 2016;7(23):34395–34419. doi: 10.18632/oncotarget.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng Z., Li X., Yang B., Xu Q., Zhu X., Hu L., et al. SORL1 stabilizes ABCB1 to promote cisplatin resistance in ovarian cancer. Funct. Integr. Genom. 2023;23(2):147. doi: 10.1007/s10142-023-01075-3. [DOI] [PubMed] [Google Scholar]
- 54.Gouaze-Andersson V., Yu J.Y., Kreitenberg A.J., Bielawska A., Giuliano A.E., Cabot M.C. Ceramide and glucosylceramide upregulate expression of the multidrug resistance gene MDR1 in cancer cells. Biochim. Biophys. Acta. 2007;1771(12):1407–1417. doi: 10.1016/j.bbalip.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pavitra E., Kancharla J., Gupta V.K., Prasad K., Sung J.Y., Kim J., et al. The role of NF-kappaB in breast cancer initiation, growth, metastasis, and resistance to chemotherapy. Biomed. Pharmacother. 2023;163 doi: 10.1016/j.biopha.2023.114822. [DOI] [PubMed] [Google Scholar]
- 56.Yin J., Ding F., Cheng Z., Ge X., Li Y., Zeng A., et al. METTL3-mediated m6A modification of LINC00839 maintains glioma stem cells and radiation resistance by activating Wnt/beta-catenin signaling. Cell Death. Dis. 2023;14(7):417. doi: 10.1038/s41419-023-05933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cong Y., Cui Y., Zhu S., Cao J., Zou H., Martin T.A., et al. Tim-3 promotes cell aggressiveness and paclitaxel resistance through NF-kappaB/STAT3 signalling pathway in breast cancer cells. Chin. J. Cancer Res. 2020;32(5):564–579. doi: 10.21147/j.issn.1000-9604.2020.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu L., Ding W., Wang X., Li X., Yang J. Interference KRT17 reverses doxorubicin resistance in triple-negative breast cancer cells by Wnt/beta-catenin signaling pathway. Genes Genom. 2023;45(10):1329–1338. doi: 10.1007/s13258-023-01437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou M., Li Z., Han Z., Tian N. Paclitaxel-sensitization enhanced by curcumin involves down-regulation of nuclear factor-kappaB and Lin28 in Hep3B cells. J. Recept. Signal. Transduct. Res. 2015;35(6):618–625. doi: 10.3109/10799893.2015.1041644. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X., Yu X. Crosstalk between Wnt/beta-catenin signaling pathway and DNA damage response in cancer: a new direction for overcoming therapy resistance. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1230822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krishnamurthy K., Vedam K., Kanagasabai R., Druhan L.J., Ilangovan G. Heat shock factor-1 knockout induces multidrug resistance gene, MDR1b, and enhances P-glycoprotein (ABCB1)-based drug extrusion in the heart. Proc. Natl. Acad. Sci. U. S. A. 2012;109(23):9023–9028. doi: 10.1073/pnas.1200731109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Correa S., Binato R., Du Rocher B., Castelo-Branco M.T., Pizzatti L., Abdelhay E. Wnt/beta-catenin pathway regulates ABCB1 transcription in chronic myeloid leukemia. BMC Cancer. 2012;12:303. doi: 10.1186/1471-2407-12-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nasrollahzadeh A., Momeny M., Bashash D., Yousefi H., Mousavi S.A., Ghaffari S.H. Blockade of Nuclear Factor-Kappab (NF-Kappab) Pathway Using Bay 11-7082 Enhances Arsenic Trioxide-Induced Antiproliferative Activity in U87 Glioblastoma Cells. Rep. Biochem. Mol. Biol. 2022;10(4):602–613. doi: 10.52547/rbmb.10.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lv Z., Xu H., Si X., Xu S., Li X., Li N., et al. XAV-939 inhibits epithelial-mesenchymal transformation in pulmonary fibrosis induced by crystalline silica via the Wnt signaling pathway. Environ. Toxicol. 2023;38(2):460–471. doi: 10.1002/tox.23693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available from the corresponding author upon reasonable request.