Highlights
-
•
WT1 exhibits diverse roles and mechanisms of action across various malignancies.
-
•
Multiple therapeutic approaches target WT1, including synthetic drugs, natural products, and vaccines.
-
•
WT1-targeted immunotherapy is advancing through clinical trials, bringing it closer to potential clinical application in the near future.
Keywords: WT1, Malignant disease, Targeted therapies, Vaccines, Natural products
Abstract
Wilms tumor 1 (WT1) is a multifaceted protein with dual functions, acting both as a tumor suppressor and as a transcriptional activator of oncogenes. WT1 is highly expressed in various types of solid tumors and leukemia, and its elevated expression is associated with a poor prognosis for patients. High WT1 expression also indicates a greater risk of refractory disease or relapse. Consequently, targeting WT1 is an effective strategy for disease prevention and relapse mitigation. Substantial information is available on the pathogenesis of WT1 in various diseases, and several WT1-targeted therapies, including chemical drugs, natural products, and targeted vaccines, are available. We provide a comprehensive review of the mechanisms by which WT1 influences malignancies and summarize the resulting therapeutic approaches thoroughly. This article provides information on the roles of WT1 in the pathogenesis of different cancers and provides insights into drugs and immunotherapies targeting WT1. The goal of this work is to provide a systematic understanding of the current research landscape and of future directions for WT1-related studies.
Graphic abstract
Introduction
The WT1 gene, alternatively referred to as Wilms tumor 1, is located on chromosome 11p13 and encodes a zinc finger transcription factor. Its pivotal involvement lies in organogenesis and cellular differentiation, and it exhibits dynamic functions throughout various developmental phases [1,2]. During embryonic development, it orchestrates epithelial‒mesenchymal equilibrium, which is pivotal for proper organ formation. In adulthood, WT1 assumes a critical role in maintaining tissue homeostasis [3,4]
The WT1 protein has a structurally intricate composition. Its C-terminus hosts four zinc finger motifs, whereas its N-terminus contains a DNA-binding domain rich in proline and glutamine residues (Fig. 1). The WT1 gene spans 10 exons, producing a diverse array of mRNA variants through alternative splicing. This complexity results in the expression of distinct transcripts, with WT1 splice site mutations underscoring the importance of the +KTS and -KTS isoforms, both of which are prevalent in various cancers [5].
Fig. 1.
The complete structure of the WT1 protein. The structure was generated using AlphaFold. In the model, the putative RRM region is depicted in red (amino acids 11–72), the repression region in purple (amino acids 84–124), and the protein activity region in blue (amino acids 181–250). The exon region is marked in green (amino acids 250–266), the zinc finger domain is represented in orange (amino acids 323–449), and the light blue region indicates the isoform variation sites of WT1 (KTS).
WT1 is typically expressed in mature podocytes of the kidney and in a small subset of pluripotent progenitor cells. However, it is not expressed in mature leukocytes, and its expression levels are negatively correlated with the cellular differentiation status [6]. Aberrant expression or mutation of WT1 can precipitate the onset of various cancers, including leukemia, breast cancer, non-small cell lung cancer, and Wilms tumor, often serving as an indicator of a poor prognosis [[7], [8], [9]].
Research on mutations or aberrant expression of WT1 leading to malignant tumors has been extensive, with diverse therapeutic approaches targeting WT1. Currently, existing reviews on WT1 have focused primarily on its roles in specific diseases, such as breast cancer or leukemia, or they provide only a brief overview of its involvement in solid tumors without categorically detailing the underlying mechanisms involved. Recent studies have also explored the influence of WT1 on chromatin states in capsular cells, revealing that areas of high WT1 expression in Kaposi's sarcoma tissues are associated with T-cell infiltration and the creation of an immune-evasive tumor microenvironment [10,11]. However, comprehensive summaries of therapeutic approaches targeting WT1, particularly with respect to targeted drugs and immunotherapies, are lacking. This lack of systematic and thorough reviews hinders a holistic understanding of the potential of WT1 in future therapeutic strategies and research. Consequently, this review aims to comprehensively delineate the mechanisms of action and targeted therapeutic modalities of WT1 across various malignant diseases, offering a multitude of research methodologies and treatment alternatives for different diseases stemming from aberrant WT1 gene expression.
Mechanisms of WT1 in hematologic malignancies
Acute myeloid leukemia
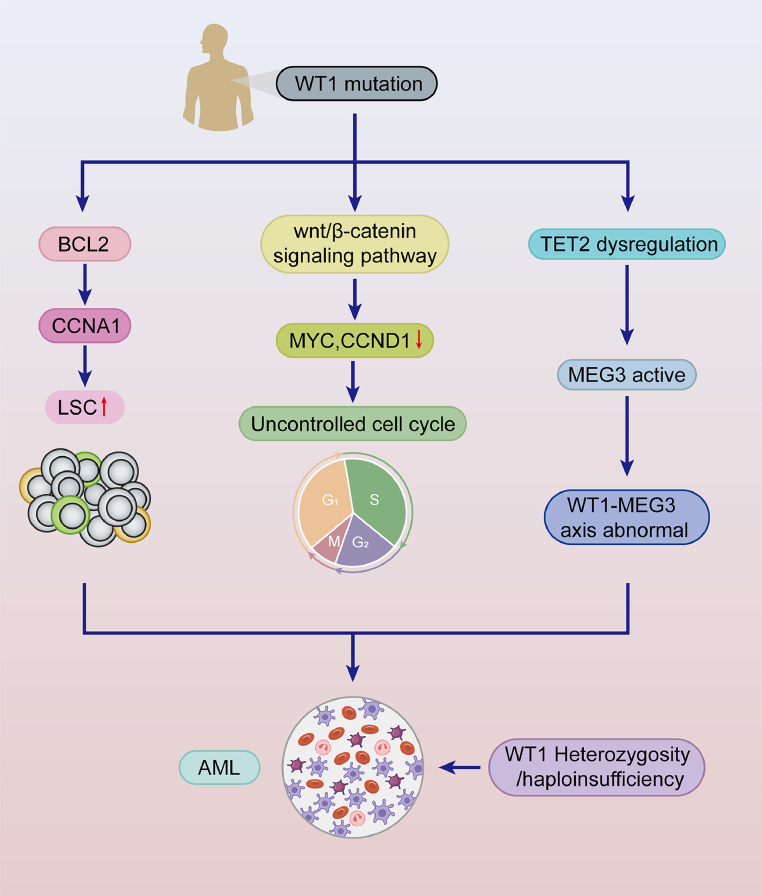
Acute myeloid leukemia (AML) is a heterogeneous disease characterized by extensive genomic changes and molecular mutations. It is the most common leukemia in adults and is characterized mainly by the abnormal proliferation and differentiation of myeloid precursors in the bone marrow [12]. WT1 mutations are present in approximately 15 % of AML patients [13]. Most studies support the oncogenic role of WT1 in AML and suggest that the oncogenic role of WT1 is closely related to the status of P53 and that WT1 affects AML cell proliferation in a p53-dependent manner [14]. WT1 regulates BCL2 and cell cycle protein A1 (CCNA1) expression, promotes leukemia stem cell (LSC) proliferation, alters human hematopoietic stem and progenitor cell (HSPC) homing, and accelerates AML development [15,16]. Furthermore, Li et al. reported that, in contrast to the typical targeting of Wnt-related pathways, WT1 is involved in regulating the Wnt/β-catenin signaling pathway by targeting Wnt11, resulting in decreased MYC and CCND1 levels and an uncontrolled cell cycle and cell proliferation, which promote the development of leukemia [17]. Genetic analyses have revealed that the loss of heterozygosity and haploinsufficiency of WT1 mutations are closely associated with epigenetic aberrations and somatic mutations. The heterozygous deletion of WT1 enhances stem cell self-renewal in an age-dependent manner, progressively strengthening stem cell function over time and leading to age-related leukemic transformation. Moreover, the loss of WT1 combined with the Flt3-ITD (Fms-like tyrosine kinase 3) mutation induces the development of AML. In addition, WT1 haploinsufficiency, rather than complete WT1 loss, can drive the differentiation of hematopoietic stem cells into leukemic cells [18]. TET2, IDH1, IDH2, and WT1 are methylation-related genes, and when mutations occur in TET2/IDH1/2/WT1 alongside NPM1, they activate a set of genes negatively correlated with RUNX1. These genes are closely linked to myeloid activation and can trigger the development of AML [19]. Fortunately, inhibitors targeting Enhancer of Zeste Homolog 2 (EZH2) have been shown to induce myeloid differentiation and produce therapeutic effects on AML cells harboring WT1 mutations [20]. Notably, MEG3 can be transcriptionally activated by WT1, with TET2 acting as a cofactor to increase MEG3 expression. Therefore, the dysregulation of TET2 leads to the aberrant expression of proteins in the WT1-MEG3 axis, which induces AML development (Table 1) (Fig. 2) [21]. These findings provide intriguing insights for further understanding the complex mechanisms of WT1.
Table 1.
WT1 related malignant diseases.
| Malignant diseases | Study model | Mechanism | Refs |
|---|---|---|---|
| AML | AML patients; K562 cells; MLL-AF9-induced murine; Wt1fl/+ mice; THP1 cell; Highly enriched human CD34+ cell |
Active BCL2/CCNA1; ↓MYC,CND1; WT1-MEG3 axis abnormal; WT1 with FIT3-ITD; WT1heterozygosity/haploinsufficiency |
13–19 |
| MDS | MDS patients; Mice model; | WT overexpression; WT1(R394 W) and FLT3/ITD mutation |
23–24 |
| T-ALL | T-ALL patients; MOLT4, PF382 and CCRF-HSB2 cells; | ↑IL7R-JAK-STAT; NOTCH1/FBXW7/FLT3 mutation |
26–29 |
| CML | CML patients; K562 cell; JURL-MK1\CD34+ cells | ↑WT1; Active BCR/ABL1,PI3K/Akt, ZNF224, Wt1/ZNF224/c-Myc |
31–33 |
| APL | APL patients | Non-coding mutation in the WT1 intron; MYB disruption |
36 |
| Lung Cancer | NSCLC patients; H1568/ H1650 NSCLC cell line; Wt1Lox/Lox mice |
↓CDH1;WT1-AS,PI3K/AKT; ↑Srpk1, Srsf1, VEGF isoforms |
40,41 |
| Breast cancer | BC patients; MDA-MB-231 cell | WT1 hypermethylation; ↑IGF 1R, IGF II, ↑EphA2, β-catenin |
46–48 |
| Neuroblastoma | NB69 cell; NB tissue; SH-SY5Y cell | ↑WT1 isoforms; Inhibite PI3K/Akt and MAPK/ERK pathways; |
51,52 |
| Prostate cancer | PCa/ PC3/DU145/LNCaP cell; | ↑WT1, VEGF, SRPK1; ↓E-calmodulin |
56–58 |
| Hepatocellular carcinoma |
MHCC97 L cell; Huh7, HLE, Huh7.5.1 cell |
↑WT1, LEF1, β-catenin, Wnt/ JAK2/STAT3 and MAPK signaling; Regulate cFLIP, FADD, and NF-κB |
66–67 |
| Pancreatic ductal adenocarcinoma | PC patients; AsPC-1/BxPC-3/PANC-1/MIA PaCa-2/ SW1990 cell lines; PANC-1/Capan-1/HDPE6C7 cell line |
Modulate miR-216a/KRT7 axis, USP5-E-calmodulin axis; ↑PI3K/AKT, STAT3 |
70,71 |
| Ovarian Cancer | OC patients; IOSE386/ A2780/HO8910//HO8910PM cell lines; HOSEpiC/IOSE80 cells | ↑ERK1/2; ↓E- calmodulin; Rap1/ Ras/MAPK signaling pathways |
76,77 |
| Astrocytomas | Astrocytomas patients; T98G/ LN18/ LNZ308/LN229 /U87MG/ VC95 G cell lines |
↑WT1; ↓IGF-1R | 81,82 |
| Malignant pleural mesothelioma | MPM patients | WT1 regulates MET and EMT | 87,88 |
Fig. 2.
The pathogenetic mechanisms of WT1 in AML. WT1 mutations regulate the expression of BCL2 and CCNA, promoting leukemic stem cell (LSC) proliferation and accelerating the progression of AML. Additionally, WT1 influences the Wnt/β-catenin signaling pathway, reducing MYC and CCND expression, which leads to cell cycle dysregulation and facilitates AML development. WT1 mutations also induce TET2 dysregulation, activating MEG3 and causing aberrant expression of the WT1-MEG3 axis, thereby promoting AML. Furthermore, heterozygous or haploinsufficient deletions of WT1 contribute to the pathogenesis of AML.
Myelodysplastic syndrome
Myelodysplastic syndrome (MDS) represents a heterogeneous group of clonal disorders originating from hematopoietic stem cells and characterized by dysplastic development of the myeloid lineage, resulting in ineffective hematopoiesis, refractory cytopenias, and a heightened propensity for progression to acute myeloid leukemia (AML) [22]. Among individuals diagnosed with MDS, aberrant expression of WT1 is observed in approximately 50 % [23]. WT1 is frequently overexpressed but less frequently mutated in patients with MDS and is considered an important prognostic and risk assessment marker for MDS [24]. The overexpression of WT1 is associated with increased blast counts and cytogenetic abnormalities. This dysregulation impacts chromosomal stability and stem cell differentiation, thereby increasing the risk of progression from MDS to AML [25,26]. Nonetheless, Colleen et al. reported that mutations in WT1 also play a significant role in the pathogenesis of MDS. Specifically, their study demonstrated that introduction of the Wt1 mutation (R394 W) in a murine model resulted in heightened susceptibility to MDS development, accompanied by a shortened latency period. Conversely, mutations in WT1 and FLT3/ITD act synergistically to predispose individuals with MDS to more aggressive progression, although an additional third strike may be required to develop AML (Table 1) [27]. Despite the association of WT1 with accelerated disease progression in patients with MDS, the precise mechanistic underpinnings of WT1 in this context necessitate further elucidation through additional experimental investigations.
T-cell acute lymphoblastic leukemia
T-cell acute lymphoblastic leukemia (T-ALL) is a rare and aggressive hematologic malignancy in which a deletion of the CDKN2A (p16) motif and aberrant NOTCH1 signaling constitute the predominant oncogenic lesions involved in the pathogenesis of T-ALL [28]. Mutations in the WT1 gene have been reported in approximately 10 % of T-ALL patients [29]. Vicente et al. [30] observed that WT1 mutations may stimulate leukemia cell proliferation through the activation of the IL7R-JAK-STAT pathway, thereby triggering T-ALL. Bordin et al. [29] showed that WT1 plays a crucial role in the DNA damage response in T-ALL, with WT1 loss attenuating the DNA damage response induced by TP53 in individuals with T-ALL. Bourkoula et al. uggested that the oncogenic role of WT1 is associated with its overexpression and ability to promote cell apoptosis via the CD95/CD95 L pathway [31]. Conversely, Heesch et al. proposed a markedly different perspective. They suggested that WT1 mutations result in truncation of the WT1 protein, which then exerts a dominant negative effect through DNA binding or protein interactions. The co-occurrence of WT1 mutations with mutations in NOTCH1/FBXW7/FLT3 exacerbates its transformation into an oncogenic factor, offering an intriguing perspective for understanding the specific roles of WT1 and its mutations (Table 1) [32]. In summary, the etiology of WT1 and T-ALL involves intricate mechanisms, providing valuable insights for devising targeted therapies against WT1.
Chronic myelogenous leukemia
Chronic myeloid leukemia (CML) is a disorder of hematopoietic stem cells (HSCs) characterized by the t (9;22) (q34; q11) translocation, which results in the fusion of the BCR and ABL1 genes to form the pathogenic BCR-ABL1 oncogene. Imatinib is employed as a first-line therapy in clinical management [33]. WT1 expression is significantly increased in patients with advanced-stage CML, and effective treatment with imatinib can attenuate WT1 expression in patients. In contrast to its role in AML, increased levels of the WT1 mRNA and protein in chronic myeloid leukemia (CML) are promoted by BCR/ABL1 tyrosine kinase signaling via the PI3 K and Akt pathways, thereby promoting the transcriptional activation of the WT1 gene. WT1, in turn, contributes to resistance to imatinib-induced apoptosis [34]. With respect to the regulatory interplay of WT1 in apoptotic processes in CML, Montano et al. [35] reported that ZNF224, a WT1-associated protein, acts as a coactivator in the regulation of the expression of proapoptotic genes by WT1 while concurrently inhibiting the WT1-mediated transactivation of antiapoptotic genes. This reciprocal modulation influences CML progression either positively or negatively by modulating the Wt1/ZNF224/c-Myc axis, highlighting its potential as a novel therapeutic target for drug resistance in CML (Table 1) [36].
Acute promyelocytic leukemia
Acute promyelocytic leukemia (APL) is characterized by the accumulation of promyeloblasts in the bone marrow and peripheral blood. The formation of PML-RARA fusions due to chromosome t (15;17) rearrangements mainly impairs neutrophil differentiation [37]. In patients with APL, recurrent WT1 mutations are observed, resulting in a variant allele frequency (VAF) lower than that of PML-RARA. Noncoding variants in the WT1 gene disrupt the binding of MYB to chromatin, which in turn inhibits enhancer activity and WT1 expression by interfering with chromatin loop formation. This disruption results in a significant reduction in WT1 expression in APL patients harboring somatic and/or germline noncoding WT1 variants. Consequently, noncoding mutations within WT1 introns contribute to the pathogenesis of APL [38]. In contrast, after consolidation therapy in APL patients, the early emergence of high WT1 expression during maintenance therapy significantly predicts relapse. Patients with high WT1 expression at three months postmaintenance therapy have a higher relapse rate and lower survival rate (Table 1) [39]. Elevated WT1 levels serve as a reliable prognostic marker for predicting relapse. The early administration of anti-CD33 antibody therapy and WT1-specific treatments in patients with high WT1 expression can effectively prevent relapse [40].
WT1 in solid cancers
Lung cancer
Lung cancer (LC) is a heterogeneous cancer categorized into two main types, small cell LC (SCLC) and non-SCLC (NSCLC), and despite advances in diagnostic and therapeutic techniques, the survival rates of patients with lung cancer remain low, whereas the mortality rates are high [41]. WT1 is frequently overexpressed in lung cancer patients, promoting the proliferation of lung cancer cells. It contributes to oncogenesis through its interaction with the PI3K/Akt signaling pathway. A positive feedback loop exists between WT1 and AKT-1, with WT1 functioning as a transcriptional regulator of AKT-1. Moreover, WT1-AS, a long noncoding RNA (lncRNA), has been identified as a key modulator of the PI3K/Akt pathway, driving oncogenic processes in lung cancer by influencing gene expression at the transcriptional level without encoding proteins [42,43]. EGFR and KRAS are commonly mutated genes in lung cancer, with increased WT1 expression contributing to the upregulation of EGFR. Furthermore, WT1 acts as a critical downstream regulator of KRAS, influencing both cellular senescence and proliferation [44,45]. In LC cells harboring KRAS mutations, WT1 promotes cell proliferation and exhibits antiapoptotic functions by influencing the expression of multiple apoptosis-related genes and directly targeting the c-Myc promoter [46]. WT1 not only is associated with LC proliferation but also enhances the invasiveness of NSCLC by directly inhibiting the expression of E-calmodulin (CDH1) by binding to the CDH1 promoter [47]. Wagner et al. [48] observed that WT1 (-KTS) directly binds and activates the promoters of Srpk1 (serine/arginine-rich protein-specific kinase) and Srsf1 (serine/arginine-rich splicing factor 1) in endothelial cells, inducing the expression of angiogenic VEGF isoforms in the tumor endothelium, which may have significant implications for LC growth and metastasis (Table 1). In terms of treatment, tyrosine kinase inhibitors (TKIs), such as the first-line agent osimertinib, have shown significant early efficacy in the treatment of NSCLC. However, most patients eventually develop complex resistance mechanisms [49]. Recent findings indicate that miR-498–5p serves as a critical upstream, natural inhibitor of WT1 in NSCLC cells, suggesting that downregulating WT1 overexpression could be a therapeutic strategy for NSCLC [50]. Furthermore, the interaction of WT1 with major vault protein (MVP) increases MVP transcription, underscoring the potential advantage of combining WT1-targeted therapies with tyrosine kinase inhibitors (TKIs). This combined approach may improve clinical outcomes and reduce the development of drug resistance during advanced stages of treatment [51].
Breast cancer
Breast cancer (BC) is one of the three most common cancers worldwide with different molecular subtypes that may present different genetic and epigenetic susceptibilities. The presence or absence of the estrogen receptor, progesterone receptor, and human epidermal growth factor 2 receptor on BC cells is important in determining therapeutic options [52]. The prevalence of WT1 expression in breast cancer is relatively modest, ranging from 10 % to 30 %. WT1 expression is markedly elevated in ER-positive (luminal) tumors compared with that in ER-negative (basal) tumors [53]. The WT1 protein binds to the endogenous consensus site within the proximal promoter of ERα, suppressing its transcriptional activity in a sequence-specific manner. By downregulating ERα expression, WT1 serves as a mediator of antiestrogen resistance in breast cancer [54]. Recent studies have revealed that WT1 hypermethylation in blood leukocytes is closely associated with the risk of luminal subtypes of breast cancer, the mechanism of which may be related to the overexpression of IGF 1R and IGF II [55,56]. A meta-analysis revealed no significant associations between WT1 expression and overall survival (OS) or disease-free survival (DFS). However, WT1-targeted immunotherapy, when combined with endocrine treatments such as aromatase inhibitors, has shown favorable treatment tolerability in patients, alongside the induction of WT1-specific antibodies. Additionally, combining WT1 immunotherapy with taxane and/or anthracycline has been shown to result in a pathological complete response in some patients [57]. Furthermore, different isoforms of WT1 were found to have distinct effects on tumor proliferation and migration in triple-negative breast cancer (TNBC), including WT1 isoform B (+17AA/-KTS) and WT1 isoform C (−17AA/+KTS), which were found to have distinct effects on tumor proliferation and migration by increasing EphA2 and β-catenin expression, promoting the angiogenic mimicry (VM) formation capacity and enhancing cell migration (Table 1) [58]. The distinct functions of various isoforms of WT1 in BC hold significant promise for tailoring personalized treatment for patients with high-risk or refractory BC [59].
Neuroblastoma
Neuroblastoma (NB) is a neuroepithelial tumor of embryonic origin that is highly heterogeneous and is one of the most common and aggressive extracranial tumors in early childhood [60]. WT1 functions primarily as a tumor suppressor in NB. Wang et al. identified that neuroblastoma (NB) cells exhibiting the highest WT1 mRNA expression presented the least malignant behavior, whereas benign NB cells presented elevated levels of WT1 protein [61]. This observation suggests a potential role for WT1 in promoting the differentiation and maturation of NB cells while inhibiting cell proliferation. Mechanistically, this effect may be closely linked to the inhibition of the PI3K/Akt and MAPK/ERK signaling pathways, resulting in the overexpression of WT1 isoforms (Table 1) [62]. A separate investigation revealed that the binding of HIF-2α to the oxygen-sensitive enhancer located in intron 3 of WT1 stimulated its transcription in NB cells under hypoxic conditions, thereby promoting NB differentiation [63]. Additionally, MYCN amplification is indicative of a poor prognosis for patients with NB. However, an inverse correlation exists between WT1 expression and MYCN amplification. Notably, WT1 gene expression is associated with an adverse prognosis specifically for non-MYCN-amplified tumors [64]. Therefore, WT1 has emerged as a clinically relevant biomarker in NB, serving as a prognostic indicator for risk stratification and optimized therapeutic intervention in NB management.
Prostate cancer
Prostate cancer (PC) is the second most commonly diagnosed cancer in men worldwide and relies heavily on androgen production for early-stage growth. WT1 is a pivotal gene expressed and regulated within the epithelial cells of PC, exerting critical influences on tumor cell proliferation and metastasis within the PC milieu [65]. Brett et al. revealed that increased WT1 expression significantly downregulates the activity of the proximal E-cadherin promoter, resulting in a pronounced reduction in E-cadherin expression in prostate cancer (PC) cells. This downregulation facilitates PC cell migration and drives progression toward a more aggressive, metastatic phenotype [66]. In the context of PC metastasis, the upregulation of VEGF transcription by WT1 occurs in androgen receptor (AR)-intact PC cells, and the expression of WT1 enhances the hormone-induced activation of VEGF expression, which may be related to the activation of SRPK1 transcription by WT1 (Table 1) [67,68]. WT1 and EGR1 appear to affect STIM1 expression and subsequently Ca2+levels, leading to opposite changes in the PC androgen receptor and androgen-independent phenotypes [69]. Furthermore, prostate tumor cells (LNCaP) stably transfected with active wild-type WT1 (D) and the inactive mutant WT1 (D)R394 W underwent apoptosis. This process may be related to WT1 directly inhibiting the endogenous AR gene promoter, thereby downregulating the androgen response element (ARE) [70,71]. However, the precise mechanisms by which WT1 regulates androgen receptor signaling require further investigation. Additional studies are needed to explore the potential of WT1 as a therapeutic target for androgen-independent prostate cancer. The diverse and intricate effects of WT1 suggest that strategies targeting WT1 may yield favorable therapeutic outcomes for patients with PC.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is one of the most malignant cancers in humans, its mortality rate ranks second globally, and treatment outcomes are unsatisfactory. Overactivation of the Wnt signaling pathway through the β-catenin protein, which is encoded by the CTNNB1 gene, plays a crucial role in the initiation and progression of liver cancer [72]. The expression of WT1 is elevated in HCC and is associated with a poor prognosis. This overexpression of WT1 is a significant factor contributing to the progression of HCC [73]. Studies have indicated that aberrant expression of WT1 activates LEF1 and β-catenin to induce the activation of the Wnt signaling pathway and promote the proliferation and invasion of HCC. Moreover, WT1 modulates the expression of apoptosis-related genes such as cFLIP, FADD, and NF-κB, exerting an antiapoptotic effect on HCC, which facilitates its continuous proliferation [[74], [75], [76]]. In addition to promoting HCC proliferation, WT1 also promotes resistance to chemotherapy in HCC by activating JAK2/STAT3 and MAPK signaling (Table 1) [77]. These findings suggest that WT1-targeted therapy can not only inhibit the proliferation of HCC but also alleviate drug resistance.
Pancreatic ductal adenocarcinoma
The early diagnosis rate of pancreatic ductal adenocarcinoma (PC) is alarmingly low, which is attributed primarily to its inconspicuous clinical manifestations. This malignancy exhibits a rapid dissemination pattern, easily invading surrounding organs. Consequently, a significant proportion of patients presenting with symptoms of pancreatic cancer are often diagnosed with advanced stages of the disease, resulting in a considerably high mortality rate [78]. The WT1 protein is not expressed in normal pancreatic ductal tissues, whereas its expression is prevalent in the majority of pancreatic ductal adenocarcinomas [79]. WT1 exerts an oncogenic effect on PC by modulating the miR-216a/KRT7 axis, thereby activating the PI3K/AKT signaling pathway [80]. Furthermore, emerging evidence indicates that WT1, in conjunction with activated STAT3, promotes tumorigenesis in PC while concurrently modulating the USP5-E-calmodulin axis to facilitate tumor metastasis (Table 1) [81]. Conversely, the downregulation of WT1 expression via siRNA-mediated approaches inhibits the proliferation of pancreatic cancer cells and increases their responsiveness to curcumin therapy [82]. Moreover, investigations have revealed that the chemotherapeutic agent gemcitabine (GEM) alters WT1 expression in PC cells, consequently augmenting T-cell responses and bolstering the antitumor immunomodulatory effects of GEM against PC [83]. The multifaceted impact of WT1 on PC improves our comprehension of its pathogenesis and fosters the diversification of therapeutic strategies.
Ovarian cancer
Ovarian cancer (OC) is typified by the proliferation of aberrant cells within the ovaries, leading to tumor formation, with epithelial ovarian cancer (EOC) representing a prevalent subtype categorized into high-grade plasma, low-grade plasma, clear cell carcinoma, endometrioid carcinoma, and mucinous ovarian carcinoma [84]. WT1 is overexpressed in ovarian cancer and positively correlated with epithelial ovarian cancer. The expression of WT1 increases as ovarian cancer progresses to metastasis. WT1 expression can be used to distinguish between serous carcinoma and endometrioid carcinoma, with the former exhibiting high WT1 expression and the latter showing low WT1 expression [85]. In EOC, WT1 directly binds to the E-calmodulin promoter to negatively regulate its expression and indirectly downregulates E-calmodulin through the activation of the ERK1/2 pathway, thereby promoting the epithelial‒mesenchymal transition (EMT), invasion, and migration [86]. In high-grade EOC, both the (+KTS) and WT1(-KTS) isoforms facilitate the proliferation and migration of ovarian epithelial cells, with WT1 (+KTS) having a more pronounced effect. This differential impact is likely due to WT1 (-KTS) influencing the EMT through ECM‒receptor interactions and focal adhesion pathways, whereas WT1 (+KTS) directly affects the Rap1, Ras, and MAPK signaling pathways (Table 1) [87]. Furthermore, WT1, in conjunction with EZH2, induces the mesenchymal-to-epithelial transition (MET) of ovarian stromal cells, resulting in the formation of cancer-associated mesenchymal stem cells (CA-MSCs). Inhibiting EZH2 can reduce CA-MSC metastasis [88]. Interestingly, the degradation of WTAP, a nuclear protein that specifically binds WT1, leads to the release of WT1, resulting in tumor suppression [89]. These findings indicate that WT1 exhibits variability in its functions and that its characteristics are influenced by the tumor microenvironment.
Astrocytomas
Astrocytomas are observed in individuals across both adult and pediatric populations. High-grade astrocytomas, alternatively termed glioblastomas or glioblastoma multiforme (GBM), are the most malignant subtype among all types of brain tumors. GBM is characterized primarily by intense angiogenesis and high aggressiveness, leading to a reduced overall survival rate and limited therapeutic options [90]. In astrocytomas, WT1 has oncogenic properties, and WT1 expression is increased in astrocytes and exhibits high specificity and sensitivity elevated WT1 expression indicates a poor prognosis [91]. The oncogenic function of WT1 in glioblastomas is linked to insulin-like growth factor receptor 1 (IGF-1R). Silencing WT1 results in the upregulation of IGF-1R, resulting in a nonapoptotic, nonautophagic programmed cell death process termed "paraptosis" (Table 1) [92]. Clark et al. [93] observed that WT1 silencing enhances the radiosensitivity of glioblastoma cells, thereby increasing the efficacy of radiotherapy. Furthermore, investigations have revealed that the administration of WT1 peptide vaccines to GBM patients stimulates specific cytotoxic T lymphocytes (CTLs) and humoral immune responses, prolonging the survival of patients with recurrent GBM [94]. Targeted therapy for astrocytomas involving WT1 primarily focuses on cellular and immune-based approaches, with combined targeted drug and cellular treatments emerging as a promising avenue for future research in WT1-related diseases.
Malignant pleural mesothelioma
Malignant pleural mesothelioma (MPM) is a rare and aggressive tumor that is often associated with occupational exposure to asbestos. It is characterized by high local invasiveness and a poor clinical prognosis [95]. Positive expression of WT1 in the tissues of patients with malignant pleural mesothelioma is significantly associated with longer overall survival (OS), suggesting that WT1 is a useful prognostic factor for MPM [96]. Scattone et al. conducted immunohistochemical staining of samples from MPM patients and reported that those with low WT1 expression experienced significantly shorter survival times than those with high WT1 expression. The epithelial-to-mesenchymal transition (EMT) is a key process involved in the malignancy and metastasis of epithelial cancers. WT1 directly regulates the balance between the mesenchymal-to-epithelial transition (MET) and EMT, a mechanism that may also occur in MPM, considering its mesenchymal origin. Studies of MPM treatment have shown that knocking down WT1 can alleviate drug resistance in MPM cells and reduce the proliferation, chemotaxis, and invasiveness of mesothelioma cell lines. The positive expression of WT1 in MPM patients is correlated with prolonged survival, whereas the downregulation of WT1 in MPM cells can control drug resistance and cell growth (Table 1) [97,98]. This unique function of WT1 in MPM is worthy of in-depth study.
Therapeutic agents targeting WT1
Retinoic acid and derivatives
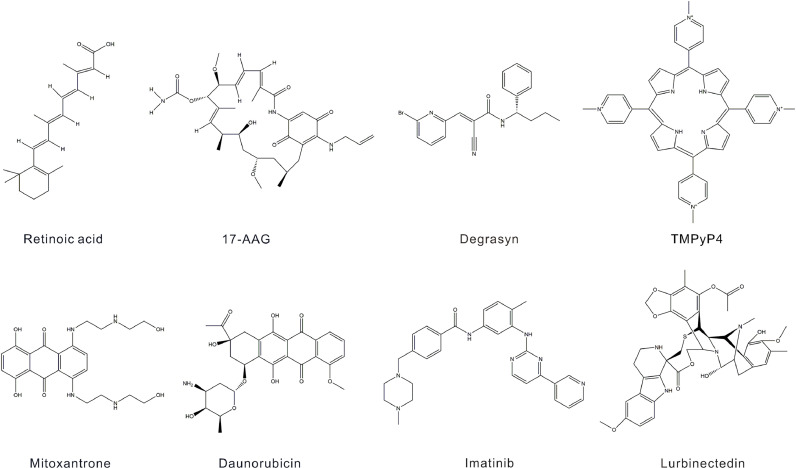
Retinoic acid (RA), also referred to as all-trans retinoic acid (ATRA), is the most biologically active metabolite of vitamin A and serves as a therapeutic agent in the management of acute promyelocytic leukemia (APL), stemming from the fusion of the retinoic acid receptor alpha (RARA) gene [99]. Its chemical structure is delineated as 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)−2E,4E,6E,8E, nonatetraenoic acid (Fig. 3) [100]. Investigations have shown that the conjunction of 5-azido-2-deoxycytidine (DAC) with RA induces the upregulation of CD11b expression and differentiation of leukemia cells with high WT1 expression, such as SHI-1 and U937 cells [101,102]. Furthermore, RA has been shown to impede the proliferation of nephroblastoma WiT49 and CCG99–11 cells lacking WT1 expression while simultaneously promoting epithelial differentiation in WiT49 cells (Table 2) [103]. Given the favorable clinical outcomes of RA treatment in APL patients, we hypothesize that RA may also have therapeutic potential in leukemias associated with elevated WT1 expression. Furthermore, RA inhibited tumor cell proliferation in nephroblastoma cells with WT1 deletion, potentially resulting in positive clinical effects.
Fig. 3.
Chemical structures of drugs targeting WT1.
Table 2.
Targeted WT1 drugs.
| Drug | Study model | Mechanism | Refs |
|---|---|---|---|
| Retinoic acid and derivatives |
SHI-1 / U937/ WiT49 / CCG99–11 cells |
↑CD11b; Induce leukemia cellular differentiation |
91–93 |
| 7-AAG | K562/KG-1/ MV4–11 / Kasumi-1 cell lines | ↓Hsp90, WT1, Myc, Bcl-2 | 94, 95 |
| Degrasyn | PANC-1/BxPC-3/AsPC-1/Capan-1/ HDPE6C7 cells | ↓WT1,USP5-WT1-E-calmodulin | 71, 96 |
| TMPyP4 | K562 cell | ↓WT1, stabilize G-quadruplexes; | 98 |
| Mitoxantrone and Daunorubicin | K562 cell | Interfere with DNA replication; Stabilize the G-quadruplex; ↓WT1, |
100 |
| Imatinib | K562 cell | ↑HtrA2, ↓WT1 | 103 |
| Lurbinectedin | Mice in a xenograft and PDX model of DSRCT; JN-DSRCT-1 cell | Cell cycle: ↓S-phase, ↑G2/M phase; ↓ EWS-WT1 |
105 |
17-AAG
Heat shock protein 90 (Hsp90) plays a critical role in regulating the folding and maturation processes of oncogenic proteins. In the context of myeloid leukemia, the formation of the WT1-HSP90 complex through stable binding between Hsp90 and the WT1 protein influences vesicular cell survival and contributes to the development of chemoresistance. The administration of the Hsp90 inhibitor 17-AAG (17-(allylamino)−17-demethoxygeldanamycin) significantly impedes the growth of myeloid leukemia xenografts in vivo and effectively suppresses the expression of WT1 and its downstream targets, namely, c-Myc and Bcl-2 (Table 2) (Fig. 3) [104,105]. This dual-targeted therapeutic approach represents a novel strategy for cancer treatment and provides valuable insights into the potential clinical application of Hsp90 inhibitors to mitigate the oncogenic effects of WT1. However, clinical studies of 17-AAG have documented its ability to induce clinical remission in a subset of lymphoma patients who experienced a relapse. In contrast, phase II clinical trials of patients with hormone-resistant metastatic prostate cancer have not shown promising results. This lack of efficacy may be attributed to drug toxicity and insufficient water solubility [106,107].
Degrasyn
Degrasyn is a small-molecule selective deubiquitinase inhibitor that also inhibits Bcr/Abl, JAK, and the transcriptional activator STAT, inducing cell apoptosis and blocking autophagy, and it has the chemical formula C19H18BrN3O (Fig. 3). In pancreatic ductal adenocarcinoma (PDAC), degrasyn has potent anticancer effects by inhibiting USP5-WT1-E-calmodulin signaling. Specifically, overexpression of USP5 (a ubiquitin-specific protease) leads to the deubiquitination of the WT1 protein and increased WT1 expression, suppressing E-calmodulin expression and promoting cancer metastasis. Degrasyn inhibits the activity of USP5, resulting in the ubiquitination and degradation of the WT1 protein. Compared with curcumin, low concentrations of degrasyn reduces WT1 protein expression, suggesting that this deubiquitinase inhibitor may serve as a therapeutic agent for PDAC with high expression of USP5 and WT1 (Table 2) [81,108]. However, the safety and bioavailability of degrasyn in humans must be further validated through well-designed clinical trials.
TMPyP4
TMPyP4 has emerged as a novel pharmacological agent that targets the transcriptional regulation of the WT1 gene. Functioning as a G-quadruplex ligand, its chemical structure is characterized by 5,10,15,20-tetra(N-methyl-4-pyridyl)-porphine (Fig. 3). G-quadruplex structures play pivotal roles in modulating the expression of oncogenes, with the G4 configuration within the WT1 promoter region serving as a critical determinant of transcriptional activity [109]. TMPyP4 predominantly acts to stabilize these G-quadruplex structures. In K562 cells, TMPyP4 exerts its regulatory effects by stabilizing G-quadruplexes within the WT1 promoter region, thereby diminishing WT1 transcription and subsequent expression. TMPyP4 neither exhibits cytotoxicity nor enhances the sensitivity of breast cancer cells to doxorubicin, but it significantly influences cell adhesion and migration, highlighting its specificity and potential as a promising therapeutic strategy for cancer treatment (Table 2) [110,111].
Mitoxantrone and daunorubicin
Mitoxantrone (MXR) is a topoisomerase II inhibitor that is primarily used to treat acute myeloid leukemia (AML). Its chemical structure is 1,4-dihydroxy-5,8-bis({2- [(2-hydroxyethyl) amino] ethyl} amino)−9,10-anthraquinone. Similarly, daunorubicin (DNR) is also a topoisomerase II inhibitor and an anthracycline antibiotic that inhibits cell proliferation by interfering with DNA replication, and its chemical structure is (7S,9S)−9-acetyl-7- [(2R,4S,5S,6S)−4-amino-5‑hydroxy-6-methyloxan-2-yl]oxy6,9,11-trihydroxy-4‑methoxy-8,10-dihydro-7H-tetracene-5,12‑dione (Fig. 3) [112]. In myeloid leukemia cells, both MXR and DNR have been shown to stabilize the G-quadruplex structures in the WT1 promoter, thereby inhibiting WT1 expression (Table 2). These drugs also downregulate the downstream signaling pathways mediated by WT1 as a transcription factor. Given that WT1 regulates the transcription of at least 137 target genes, further investigations are warranted to elucidate the specific mechanisms by which MXR and DNR influence WT1 downregulation and to assess their clinical efficacy [113].
Imatinib
Imatinib is a small-molecule tyrosine kinase inhibitor primarily used to treat Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL). It targets several tyrosine kinases, including FLT3, CSF1R, ABL, and c-KIT [114]. The chemical structure of imatinib is 4-(4-methyl-1-piperazinyl)methyl]-N-(4-methyl-3-{ [4-(3-pyridinyl)−2-pyrimidinyl] amino} phenyl) benzamide (Fig. 3) [115]. In the human K562 cell line, imatinib activates the HtrA2 gene at the transcriptional level, resulting in decreased WT1 protein levels in both the cytoplasm and nucleus (Table 2). The reduction in WT1 protein levels becomes more significant with prolonged imatinib exposure and occurs more rapidly in the nucleus than in the cytoplasm [116]. The clinical application of imatinib is well established, and its therapeutic efficacy warrants further exploration in cancer patients with elevated WT1 expression.
Lurbinectedin
Lurbinectedin is a synthetic tetrahydropyrrolo [4,3,2-de]quinolin-8(1H)-one alkaloid analog with potential antitumor activity that is used to treat small cell lung cancer (Fig. 3) [117]. Lurbinectedin covalently binds to residues in the DNA minor groove, leading to delayed S phase progression, cell cycle arrest at G2/M phase, and subsequent cell death. Lurbinectedin is an alternative treatment option for SCLC following the failure of platinum-based chemotherapy. Compared with other second-line therapies for SCLC, lurbinectedin has produced favorable therapeutic outcomes, making it a viable consideration for patients who fail first-line treatments. Ongoing research is exploring the potential of lurbinectedin in combination with other agents, including an ongoing phase III trial evaluating its efficacy in combination with doxorubicin compared with other second-line treatment regimens [118]. In research on desmoplastic small round cell tumors (DSRCTs), an aggressive form of soft tissue sarcoma, EWS-WT1 has been identified as the driver oncogene. Lurbinectedin effectively inhibits the EWS-WT1 transcription factor, inducing the redistribution of proteins from the nucleus to the nucleolus (Table 2) [119]. This mechanism significantly reduces tumor cell viability, underscoring the potential of lurbinectedin as an effective treatment for DSRCT.
Natural products targeting WT1
Bufalin
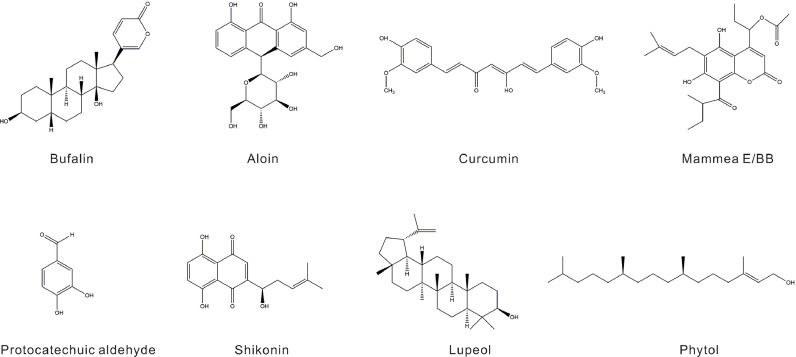
Bufalin, a steroidal toxin and cardiotonic agent derived from toad venom, is an active component in various traditional Chinese medicines. Its chemical structure is 3β,14-dihydroxy-5β-bufa-20,22-dienolide (Fig. 4). Bufalin exhibits potent antitumor activity against multiple malignancies, including hepatocellular carcinoma and lung cancer [120]. Bufalin has been shown to inhibit the proliferation of human erythroleukemia (HEL) cells by increasing DNMT3a and DNMT3b protein expression, which enhances WT1 methylation and downregulates its expression. This process leads to cell cycle arrest in G0/G1 phase and induces apoptosis (Table 3) [121]. Despite its potential, the clinical application of bufalin is hindered by its poor water solubility and low, variable oral bioavailability. To address these limitations, PEGylated bufalin-loaded PEGylated liposomes (BF/PEG-LP) have been developed to address these limitations, and they exhibit reduced hemolysis, lower cytotoxicity in various tumor cells, and improved pharmacokinetics and antitumor efficacy [122]. This advancement represents a promising direction for the targeted treatment of WT1-related malignancies.
Fig. 4.
Chemical structures of natural products targeting WT1.
Table 3.
Natural products targeting WT1.
| Natural product | Origin/Form | Study model | Mechanism | Refs |
|---|---|---|---|---|
| Bufalin | Toad venom | Human erythroleukemia cells | Induction of WT1 methylation; ↓WT1; Cells cycle: G0/G1 phase |
107 |
| Shikonin | Roots of Lithospermum erythrorhizon |
HL-60 cells | ↓WT1,CD34; ↑ CD11b; ↓Cells proliferation/Self-renewal; ↑Apoptosis |
109 |
| Aloin | Leaves of Aloe vera | HEK-293 T/HuH7/HCCLM3/ THLE-2 cell lines ; HCC patients |
circ_0011385/miR-149–5p/WT1 axis→WT1↓ | 111 |
| Curcumin | Rhizomes of turmeric | K562 / HL-60 cells | ↑miR-15a/16–1→WT1↓; ↓PKCα→JNK/ c-JUN→WT1↓; HOTAIR/miR-20a-5p/WT1→WT1↓ |
114–116 |
| Salvia chinensia Benth | Protocatechualdehyde | PLC/ PRF/5/ MHCC97 L cells | ↓Wnt/β-catenin→WT1↓ | 64 |
| Kaffir lime | Rutaceae family | K562 cells | Cells cycle:↓G2/M phase; ↓WT1 | 120 |
| Mammea E/BB | Seeds of Mammea siamensis | K562 cells | ↓WT1-DNA, c-Fos/AP-1; Effect ERK1/2 signaling pathway; Cells cycle:S-phase arrest |
122,123 |
| Yuping Feng San | Astragalus, Atractylodes macrocephala,Saposhnikoviae Radix |
A549 lung cancer cells |
mTORC2/AKT↓; WT1/MVP axis↓ |
125 |
Shikonin
Shikonin is a bioactive compound isolated from the roots of Lithospermum erythrorhizon that possesses the distinct chemical structure 5,8-dihydroxy-2-((1R)−1‑hydroxyl-4-methyl-3-pentenyl)−1,4-naphthoquinone and has diverse pharmacological properties, including anti-inflammatory, blood circulation-enhancing, and anticancer effects (Fig. 4) [123]. Research has shown that shikonin can effectively inhibit the expression of WT1 and CD34 in myeloid leukemia HL-60 cells while simultaneously inducing the expression of the mature granulocyte/monocyte surface antigen CD11b and increasing the counts of white blood cells and platelets. Additionally, shikonin has the ability to shift HL-60 cells to a state of reduced proliferation, weakened self-renewal, and increased apoptosis, suggesting its promising potential as a novel natural product extract for the effective treatment of myeloid leukemia (Table 3) [124]. Shikonin has low oral bioavailability, a low plasma protein binding rate, and enhances cytochrome P450 metabolism, limiting its pharmacokinetic potential. However, recently developed shikonin nanoparticles coated with saponin and sophorolipid have exhibited improved heat and light stability, prolonged durability, and enhanced in vitro bioavailability [125]. These advancements suggest the promising feasibility of the clinical application of shikonin.
Aloin
Aloin, an anthraquinone component extracted from the leaves of Aloe vera, has the chemical structure 10-glucopyranosyl-1,8-dihydroxy-3-(hydroxymethyl)−9 (10H)-anthracenone (Fig. 4) [126]. Aloin has anti-inflammatory, antibacterial, antioxidant, antiviral, and anticancer properties. It inhibits the proliferation, invasion, and tumor growth of hepatocellular carcinoma (HCC) cells while promoting apoptosis and autophagy by modulating the circ_0011385/miR-149–5p/WT1 axis. Specifically, circ_0011385 negatively regulates miR-149–5p, which directly targets and downregulates the expression of WT1 in HCC. Moreover, circ_0011385 can induce the overexpression of WT1 in HCC, whereas increased miR-149–5p expression in HCCLM3 cells reverses the expression of WT1 (Table 3) [127]. Aloin is prone to rapid degradation in aqueous solutions because of its inherent instability. However, when encapsulated within carbon dot nanoparticles, it exhibits significantly increased water stability and stronger antiproliferative activity [128].
Curcumin
Curcumin is a natural flavonoid compound extracted from the rhizomes of turmeric, and its chemical structure is (1E,6E)−1,7-bis(4‑hydroxy-3-methoxyphenyl)−1,6-heptadiene-3,5‑dione; it possesses antioxidant, anti-inflammatory, and plasma viscosity-reducing properties (Fig. 4) [129]. Curcumin downregulates NF-κB in human multiple myeloma cell lines by preventing the nuclear retention of p65. It inhibits sustained IκBα phosphorylation by suppressing IκB kinase (IKK) activity, leading to the downregulation of NF-κB-regulated proteins such as IκBα, Bcl-2, Bcl-xL, cyclin D1, and interleukin-6. The suppression of these proteins inhibits cell proliferation and causes cell cycle arrest at G1/S phase. Additionally, curcumin activates caspase-7 and caspase-9, inducing PARP cleavage. This NF-κB downregulation by curcumin also increases the sensitivity of cells to chemotherapeutic agents such as vincristine and melphalan [130]. As a DNA hypomethylating agent, curcumin can upregulate miR-15a/16–1 in leukemia cells, partially reducing WT1 expression. Studies of the inhibitory mechanisms of curcumin have revealed that inhibiting PKCα impacts downstream JNK and c-JUN signaling, thereby attenuating WT1 autoregulation, and that the depletion of c-JUN also suppresses WT1 gene expression [131,132]. Further research indicated that curcumin inhibits AML cell proliferation and migration by modulating the HOTAIR/miR-20a-5p/WT1 axis, which inhibits HOTAIR and WT1, thereby promoting miR-20a-5p expression and hindering AML cell growth [133]. Additionally, dimethoxycurcumin significantly suppresses WT1 and CD34 protein expression in LSCs (Table 3) [134]. Certain drug delivery systems have effectively increased the solubility of curcumin, prolonged its plasma retention time, and mitigated the challenges associated with its poor absorption, rapid metabolism, and chemical instability [135]. Collectively, these findings suggest that curcumin is a potent WT1 inhibitor with potential therapeutic applications for diseases characterized by elevated WT1 expression.
Salvia chinensia benth
Salvia chinensia Benth is referred to as SJC and is commonly used to treat various malignancies, enteritis, dysentery, skin abscesses and colorectal cancer [136,137]. Studies have shown that the Wnt/β-catenin pathway and SJC are associated with the inhibition of HCC cell proliferation and cell cycle progression. SJC inhibits β-catenin expression, and its transcriptional activity is linked to the WT1 protein. WT1 promotes HCC cell proliferation and invasion, as well as the transcriptional activation of β-catenin-dependent Wnt target genes, whereas WT1 knockdown has the opposite effect. A docking analysis identified protocatechualdehyde (PCA) as an active component of Salvia chinensia Benth (Fig. 4). PCA exerts its inhibitory effects on hepatocytes primarily by suppressing the transcriptional activity of the Wnt/β-catenin pathway in a WT1-dependent manner (Table 3) [74]. These findings suggest that PCA could serve as a lead compound that targets WT1, indicating its potential for HCC treatment. Salvia chinensia Benth has been widely used in clinical applications because of its strong safety profile, making it a potential adjunct therapy for cancer. This WT1-targeted treatment approach warrants further clinical research for a systematic and precise evaluation of its effects.
Kaffir lime
Kaffir lime (Citrus hystrix), a plant from the Rutaceae family, is known for its anti-inflammatory, antioxidant, and anticancer properties. Compounds extracted from kaffir lime leaves, namely, phytol and lupeol (Fig. 4), have been shown to inhibit WT1 gene expression in leukemia cells, significantly reducing WT1 protein expression in K562 cells. These compounds arrest cells at the G2/M phase of the cell cycle and decrease cell proliferation in a dose- and time-dependent manner, resulting in lower cytotoxicity than traditional chemotherapy drugs (Table 3) [138]. Owing to their favorable safety profile, these natural products show promise for incorporation into future leukemia treatment strategies.
Mammea E/BB
Mammea E/BB is an active compound extracted from the seeds of Mammea siamensis (Fig. 4) [139]. Studies have shown that Mammea E/BB reduces WT1 expression in K562 leukemia cells. This inhibitory effect on WT1 is achieved by disrupting WT1-DNA binding at the WT1 promoter region, thereby impairing WT1 gene autoregulation. Additionally, Mammea E/BB interferes with the ERK1/2 signaling pathway, disrupting c-Fos/AP-1 binding to the WT1 promoter, leading to S phase arrest in K562 cells and the inhibition of leukemia cell proliferation (Table 3) [140,141]. The impact of Mammea E/BB on kinase signaling pathways represents a novel mechanism for the therapeutic application of natural products in disease treatment and provides valuable insights for the development of natural product-based leukemia therapies. However, further research is needed to investigate the bioavailability and actual clinical efficacy of Mammea E/BB.
Yuping Feng San
Yuping Feng San (YPFS) is a traditional Chinese medicine composed of Astragalus, Atractylodes macrocephala, and Saposhnikoviae Radix. This formulation is known for its immunoregulatory effects, inhibition of inflammatory factors, hormone level modulation, and antioxidant effects [142]. Recent research highlights the enhanced effect of YPFS combined with Ginkgo biloba on cisplatin (DDP)-treated lung cancer cells. In cisplatin-treated A549 lung cancer cells, the combination of YPFS and Ginkgo biloba disrupts the MVP-mediated mTOR2/AKT signaling pathway. This disruption primarily involves the degradation of mTORC2 components and a decrease in the phosphorylation levels of the downstream protein AKT. By downregulating the WT1/MVP axis and interfering with the antiapoptotic mTORC2/AKT pathway, the combination therapy effectively overcomes DDP resistance (Table 3) [143]. By increasing cisplatin sensitivity and overcoming chemoresistance through the modulation of critical molecular pathways, this combination of Yuping Feng San and Ginkgo biloba represents a promising adjuvant therapy for NSCLC [144]. Clinical investigations into YPFS for the treatment of allergic rhinitis (AR) revealed that YPFS alone was not superior to pharmacological treatments. However, when combined with medication, it produced significantly improved outcomes [145]. These findings suggest that YPFS, in combination with other drugs or vaccines, is clinically relevant for the treatment of malignant diseases.
Immunotherapy targeting WT1
WT1 peptide vaccine
The WT1 peptide is recognized by the immune system through antigen-presenting cells (APCs) that present it to T cells. However, the immunogenicity of WT1 peptides might be too weak to elicit sufficient affinity through a single peptide alone [146]. Unlike cytomegalovirus (CMV)- or Epstein–Barr virus (EBV)-specific CTL formulations, WT1-specific products are challenging to produce in sufficient quantities, and their functionality relies on CD4+ T cells [147]. Brayer et al. designed a synthetic peptide that includes a mixture of native and xenogeneic WT1-derived class I peptides and extended peptides containing presumed class II epitopes. This synthetic peptide successfully induced stronger responses and increased the number of WT1-specific T cells, thereby enhancing the efficacy of the oligopeptide vaccine [148]. Other synthetic peptide vaccines, such as galinpepimut-S (GPS), have also improved oligopeptide vaccine efficacy. GPS, a WT1 peptide vaccine, consists of four peptides derived from WT1, including a synthetic heteroclitic short peptide (WT1-A1) to stimulate CD8+ T-cell responses, two native long peptides (331 and 427) to stimulate CD4+ T-cell responses, and a synthetic heteroclitic long peptide (122A1) to stimulate both CD4+ and CD8+ cells. In early clinical trials of GPS, the primary toxicities observed were fatigue, Grade 1/2 injection site reactions, and skin indentation. Although some AML patients who received GPS showed signs of relapse, compared with traditional treatments, the vaccine extended the median relapse-free survival following the first complete remission (CR1). GPS exhibited favorable tolerability, effectively stimulated a specific immune response, and contributed to an improved 5-year survival rate in patients [149]. GPS is currently undergoing a phase 3 clinical trial, showing promise as a maintenance therapy for AML patients in remission and demonstrating efficacy in elderly patients with relapsed and refractory AML. On the other hand, concurrent induction of tumor antigen-specific cytotoxic T lymphocytes (CTLs) and helper T lymphocytes (HTLs) can lead to a more robust antitumor immune response (Fig. 5) [150]. Research by Fujiki et al. revealed that patients vaccinated with a combination of WT1 CTL peptides and WT1332 helper peptides—a 16-oligomer WT1-derived peptide capable of inducing HTLs in an HLA class II-restricted manner—achieved significant WT1-specific CTL responses, with a subset of these patients presenting with initially low natural WT1 peptide-reactive CTLs but high expression of CD5 (a marker of resistance to activation-induced cell death) exhibiting a strongly amplified and persistent response for a long period [151].
Fig. 5.
WT1 Peptide Vaccine. GPS is a WT1 peptide vaccine composed of four peptides derived from the WT1 protein. These include a synthetic heteroclitic short peptide (WT1-A1) that stimulates CD8 responses, two native long peptides (331 and 427) that stimulate CD4 responses, and a synthetic heteroclitic long peptide (122A1) that stimulates both CD4 and CD8 cells. The GPS vaccine is designed to simultaneously induce tumor antigen-specific cytotoxic T lymphocytes (CTLs) and helper T lymphocytes (HTLs), thereby generating a robust antitumor immune response.
Dendritic cell vaccines
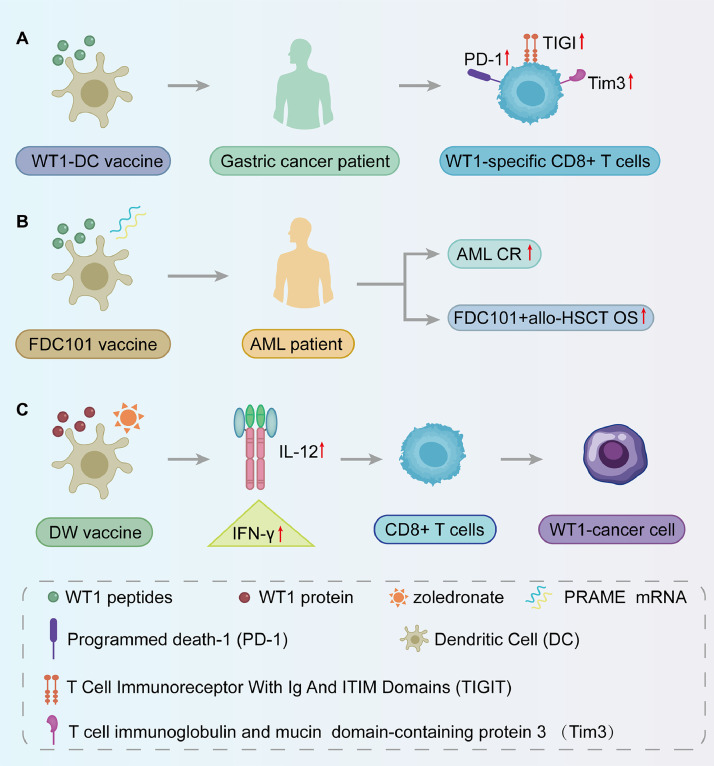
Dendritic cell (DC) vaccines are effective in stimulating tumor antigen-specific CD8+ T cells. In gastric cancer patients vaccinated with WT1-targeted DC vaccines, upregulation of the inhibitory molecules TIGIT, PD1, and Tim3 was observed. Coinhibition of these pathways increased the proliferation and cytokine production of WT1-specific CD8+ T cells (Fig. 6A). WT1-DC vaccines have shown promise in inducing antileukemic T-cell immunity in high-risk AML patients, reducing relapse rates and improving survival [152]. Fløisand et al. developed FDC101, a WT1 and PRAME RNA-loaded DC vaccine, which maintained complete remission (CR) in some AML patients who were ineligible for allogeneic hematopoietic stem cell transplantation (allo-HSCT), achieving a 5-year overall survival (OS) rate of approximately 75 % postvaccination and allo-HSCT (Fig. 6B) [153]. Additionally, the CellgramDC-WT1 (CDW) vaccine, which was pulsed with the WT1 protein and matured with zoledronic acid, induced high levels of IL-12 and IFN-γ secretion, activating CD8+ T cells and targeting WT1-expressing cancer cells (Fig. 6C). Phase II clinical trials of WT1 mRNA-electroporated DCs in AML patients have shown their efficacy in preventing or delaying relapse after standard chemotherapy, thereby improving OS rates [154]. Moreover, clinical observations of WT1-targeted dendritic cell (DC) vaccination in patients with AML, metastatic breast cancer (MBC), glioblastoma multiforme (GBM), and malignant pleural mesothelioma (MPM) revealed that most patients developed a positive delayed-type hypersensitivity response. Patients with GBM, MBC, or MPM exhibit partial response or stable disease following vaccination [155].
Fig. 6.
Dendritic cell vaccine. A: The WT1-DC vaccine induces increased expression of TIGIT, PD1, and Tim3 in gastric cancer patients, promoting the proliferation of WT1-specific CD8+ T cells;B: The FDC101 vaccine, containing WT1 and PRAME mRNA vectors, has been shown to increase complete remission rates (CR) in AML patients. When combined with hematopoietic stem cell transplantation, it can improve overall survival rates (OS) ;C: The CDW vaccine, utilizing WT1 protein and zoledronic acid for maturation, induces increased secretion of IL-12 and IFN-γ. This activation enhances CD8+ T cell targeting of WT1-expressing cancer cells.
Bifidobacterium-related vaccines
The Bifidobacterium longum (B. longum) strain can stimulate CD4+ T cells and promote the Th1 response [156]. Upon entering dendritic cells (DCs), these probiotics facilitate the activation of CD8+ T cells [157]. B. longum 420, an engineered strain expressing the WT1 gene, serves as an oral protein vaccine that increases the efficiency of DCs in presenting antigens to T cells. This vaccine enhances gut immunity by assisting CD4+ T cells in inducing WT1-specific CD8+ T-cell antitumor activity [158]. Moreover, the combination of B. longum 2656 with B. longum 420 accelerated WT1-specific CTL antitumor responses. A Bifidobacterium vector-based oral WT1 vaccine combined with PD-1 abolished tumor growth in a mouse model of bladder cancer [159,160]. In the TRAMP-C2 mouse tumor model, oral administration of the WT1 vaccine elicited a more robust antitumor response than did the peptide vaccine. This enhanced effect is likely due to the activation of both CD4+ and CD8+ T cells. Nevertheless, additional studies are necessary to evaluate the clinical feasibility of the oral WT1 cancer vaccine [161].
Modification of the WT1-TCR
T-cell receptor (TCR) therapy offers high sensitivity in targeting intracellular tumor antigens, increasing T-cell survival and inducing durable clinical responses in cancer patients [162]. Engineered T cells expressing TCRs (TCR-T) can recognize a broad range of intracellular antigens while maintaining the inherent affinity and signaling characteristics of native TCRs [163]. Chapuis et al. isolated a high-affinity WT1-specific TCR (TCRC4) from an HLA-A2+ normal donor gene pool and introduced it into Epstein–Barr virus-specific donor CD8+ T cells (TTCRC4). These modified cells were prophylactically infused into acute myeloid leukemia (AML) patients undergoing hematopoietic stem cell transplantation (HSCT), establishing sustained T-cell responses and safely preventing relapse in high-risk patients. Furthermore, researchers have utilized CRISPR-Cas9 genome editing to integrate the WT1-TCR gene into the TCRα-constant (TRAC) locus combined with TCRβ-constant (TRBC) knockout [164]. This approach created a high-affinity HLA-A*02:01-restricted WT137–45 epitope TCR, significantly enhancing its function and specificity. These modified TCRs produced antigen-specific responses in both CD4+ and CD8+ T cells, effectively killing AML cells, acute lymphoblastic leukemia (ALL) cells, and glioblastoma cells without off-target toxicity. A novel antibody-TCR (AbTCR) construct, which directs T cells to WT1 and utilizes CD33 as an input to activate a chimeric costimulatory receptor (CSR), has been proposed. These dual-receptor T cells (AbTCR + CSR) specifically recognize and kill WT1 RMFPNAPYL (RMF) epitope/HLA-A2 complexes on AML cells while sparing healthy hematopoietic cells [165]. In phase I/II clinical trials of autologous WT1-TCR gene-modified T-cell therapy for high-risk AML and MDS patients who are ineligible for allogeneic hematopoietic stem cell transplantation, WT1-TCR T cells demonstrated high safety and persistence. No on-target or off-tumor toxicity was observed in any of the treated patients, and no severe adverse reactions were reported [166].
Antibodies against WT1
To increase the efficacy of antibodies against WT1. Augsberger et al. designed a novel T-cell bispecific (TCB) antibody to treat patients with relapsed/refractory acute myeloid leukemia (AML). This TCB antibody features a bivalent T-cell receptor-like binding domain that recognizes the intracellular tumor antigen WT1-derived RMFPNAPYL peptide in the context of cell-surface HLA-A*02. WT1-TCB triggers antibody-mediated cytotoxicity against AML cell lines in a WT1- and HLA-restricted manner. When combined with the immunomodulatory drug lenalidomide, WT1-TCB amplified T-cell-mediated cytotoxicity in primary AML cells [167]. Further exploration of bispecific T-cell engager (BiTE) antibodies derived from T-cell receptor (TCR)-mimic monoclonal antibodies (mAbs), such as ESK1, revealed their potential in targeting WT1 presented on HLA-A*02:01. ESK1-BiTE effectively recruited and redirected T cells to WT1-expressing tumor cells, providing a promising therapeutic approach for epithelial ovarian cancer (EOC), which is characterized by antigen heterogeneity and low Muc16 expression. WT1-TCB and BiTE are often associated with off-target cytotoxicity and cytokine release syndrome (CRS). Kinase inhibitors such as dasatinib can be considered adjunct therapies that target WT1 to mitigate these adverse effects [[168], [169], [170]].
Conclusion and prospect
Wilms tumor protein 1 (WT1) plays a unique dual role in tumor biology, functioning as both a tumor suppressor and, in specific cellular contexts, an oncogene. This duality is primarily mediated by the ability of WT1 to recognize and bind specific DNA sequences, regulating gene transcription [171]. Both the wild-type and mutant forms of WT1 are overexpressed in various hematologic malignancies and solid tumors, highlighting its pivotal role in oncogenesis [[172], [173], [174]]. Interestingly, research by Pons et al. revealed a novel role for WT1 in the chemotherapy-induced apoptosis of leukemic cells. Caspase-mediated cleavage of WT1 during replication stress serves as a marker of apoptosis [175]. Furthermore, WT1 expression is regulated by histone deacetylases (HDACs), and its downregulation has been proposed as a molecular indicator of the efficacy of HDAC inhibitors in the treatment of acute myeloid leukemia (AML) [176,177]. These findings underscore the dynamic regulatory mechanisms of WT1 and its potential as a therapeutic target.
WT1-targeted therapies, including synthetic drugs and natural products, have garnered increasing attention. Active compounds such as costunolide and parthenolide from Champi Sirindhorn have been shown to effectively reduce WT1 levels in K562 and Molt-4 cells [178]. Similarly, natural products, including berberine, resveratrol, and quercetin, have been shown to exert inhibitory effects on WT1 protein expression. Notably, resveratrol not only suppresses WT1 expression but also inhibits leukemic cell proliferation and cancer cell migration [178,179]. Marine-derived compounds, such as those from marine sponges, also exhibit anticancer activity against WT1, although their mechanisms and cytotoxicity require further investigation. Additionally, kinases such as glycogen synthase kinase 3β (GSK3β) target WT1 isoforms translated from the CUG initiation site (cugWT1). By mediating phosphorylation, ubiquitination, and degradation, GSK3β reduces WT1-associated oncoprotein levels, thereby inhibiting cancer cell growth [[180], [181], [182]]. Despite these advances, the dual nature of WT1 poses significant challenges to the development of therapeutic drugs. While WT1 inhibitors may be effective against tumors with high WT1 expression, metastatic tumors with heterogeneous WT1 levels may necessitate combination therapies. Currently, most WT1-targeted therapies remain in preclinical stages, with a focus on in vitro and animal model studies. Although a few drugs, such as imatinib, have reached clinical application, large-scale clinical trials targeting WT1 aberrations are still lacking.
In the context of immunotherapy, WT1 cancer vaccines are undergoing phase I/II clinical trials. Preliminary findings suggest that combining chemotherapy with WT1 vaccines can suppress tumor growth [183]. Furthermore, the combination of WT1 peptide vaccines with immune checkpoint inhibitors (ICIs) has shown potential in prolonging the survival of patients with solid tumors and Hodgkin lymphoma [184]. Advances in biotechnology have facilitated the development of various WT1-targeted vaccines, although only a subset is discussed here. Enhancing the efficacy of WT1 vaccines will likely rely on combination therapies with chemotherapeutic agents, ICIs, and PD-L1 inhibitors. Ongoing phase III clinical trials will provide critical insights into optimal therapeutic strategies and clinical efficacy [185].
In conclusion, WT1-targeted therapies, including small-molecule inhibitors and cancer vaccines, hold significant promise. However, achieving precise and effective clinical application requires further exploration and optimization of therapeutic strategies, dosing regimens, and combination approaches. Continued research in this field is essential to unlock the full potential of WT1-targeted treatments and advance them into routine clinical practice.
Availability of data and materials
Not applicable.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Funding
This work was supported by the Young Scientists Fund of the National Natural Science Foundation of China (Grant Nos. 82204858, 82302754, and 32200957), the Sichuan Science and Technology Program (Grant Nos. 2023NSFSC1761) and the Sichuan Provincial People's Hospital Foundation (Grant No. 2022QN09).
CRediT authorship contribution statement
Qing Nian: Writing – review & editing, Writing – original draft, Funding acquisition. Yan Lin: Writing – original draft. Jinhao Zeng: Writing – review & editing, Supervision. Yanna Zhang: Writing – review & editing, Funding acquisition. Rongxing Liu: Writing – review & editing, Software.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Contributor Information
Qing Nian, Email: young926@hotmail.com.
Rongxing Liu, Email: Joey@tmmu.edu.cn.
References
- 1.Hastie N.D. Wilms' tumour 1 (WT1) in development, homeostasis and disease. Development. 2017;144:2862–2872. doi: 10.1242/dev.153163. [DOI] [PubMed] [Google Scholar]
- 2.Madden S.L., Cook D.M., Morris J.F., Gashler A., Sukhatme V.P., Rauscher F.J. Transcriptional repression mediated by the WT1 Wilms Tumor gene product. Science (1979) 1991;253:1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- 3.Sampson V.B., David J.M., Puig I., Patil P.U., de Herreros A.G., Thomas G.V., Rajasekaran A.K. Wilms' tumor protein induces an epithelial-mesenchymal hybrid differentiation state in clear cell renal cell carcinoma. PLoS. One. 2014;9 doi: 10.1371/journal.pone.0102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.B. Wilm, R. Muñoz-Chapuli, the Role of WT1 in embryonic development and normal organ homeostasis, in: N. Hastie (Ed.) The Wilms' Tumor (WT1) Gene: Methods and Protocols, Springer New York, New York, NY, 2016, pp. 23–39. [DOI] [PubMed]
- 5.Wang D., Horton J.R., Zheng Y., Blumenthal R.M., Zhang X., Cheng X. Role for first zinc finger of WT1 in DNA sequence specificity: denys–Drash syndrome-associated WT1 mutant in ZF1 enhances affinity for a subset of WT1 binding sites. Nucleic Acids Res. 2017;46:3864–3877. doi: 10.1093/nar/gkx1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith C., Kitzman J.O. Benchmarking splice variant prediction algorithms using massively parallel splicing assays. Genome Biol. 2023;24:294. doi: 10.1186/s13059-023-03144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artibani M., Sims A.H., Slight J., Aitken S., Thornburn A., Muir M., Brunton V.G., Del-Pozo J., Morrison L.R., Katz E., Hastie N.D., Hohenstein P. WT1 expression in breast cancer disrupts the epithelial/mesenchymal balance of tumour cells and correlates with the metabolic response to docetaxel. Sci. Rep. 2017;7:45255. doi: 10.1038/srep45255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi X.-w., Zhang F., Wu H., Liu J.-l., Zong B.-g., Xu C., Jiang J. Wilms' tumor 1 (WT1) expression and prognosis in solid cancer patients: a systematic review and meta-analysis. Sci. Rep. 2015;5:8924. doi: 10.1038/srep08924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pronier E., Bowman R.L., Ahn J., Glass J., Kandoth C., Merlinsky T.R., Whitfield J.T., Durham B.H., Gruet A., Hanasoge Somasundara A.V., Rampal R., Melnick A., Koche R.P., Taylor B.S., Levine R.L. Genetic and epigenetic evolution as a contributor to WT1-mutant leukemogenesis. Blood. 2018;132:1265–1278. doi: 10.1182/blood-2018-03-837468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ettou S., Jung Y.L., Miyoshi T., Jain D., Hiratsuka K., Schumacher V., Taglienti M.E., Morizane R., Park P.J., Kreidberg J.A. Epigenetic transcriptional reprogramming by WT1 mediates a repair response during podocyte injury. Sci. Adv. 2020;6:eabb5460. doi: 10.1126/sciadv.abb5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morales A.E., Gumenick R., Genovese C.M., Jang Y.Y., Ouedraogo A., Ibáñez de Garayo M., Pannellini T., Patel S., Bott M.E., Alvarez J., Mun S.S., Totonchy J., Gautam A., Delgado de la Mora J., Chang S., Wirth D., Horenstein M., Dao T., Scheinberg D.A., Rubinstein P.G., Semeere A., Martin J., Godfrey C.C., Moser C.B., Matining R.M., Campbell T.B., Borok M.Z., Krown S.E., Cesarman E. Wilms' tumor 1 (WT1) antigen is overexpressed in Kaposi Sarcoma and is regulated by KSHV vFLIP. PLoS. Pathog. 2024;20 doi: 10.1371/journal.ppat.1011881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Kouchkovsky I., Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6 doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paschka P., Marcucci G., Ruppert A.S., Mrózek K., Whitman S.P., Maharry K., Langer C., Baldus C.D., Powell B.L., Baer M.R., Carroll A.J., Caligiuri M.A., Kolitz J.E., Larson R.A., Bloomfield C.D. Wilms Tumor 1 (WT1) gene mutations predict poor outcome in adults with cytogenetically normal (CN) Acute Myeloid Leukemia (AML): a cancer and leukemia Group B (CALGB) Study. Blood. 2007;110:362. doi: 10.1200/JCO.2007.15.2058. -362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y., Chai X., Gong C., Zou L. WT1 inhibits AML cell proliferation in a p53-dependent manner. Cell Cycle. 2021;20:1552–1560. doi: 10.1080/15384101.2021.1951938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey S., Moazam M., Snyder D., Kuerbitz S.J., Fraizer G.C. Abstract 773: WT1 regulation of Cyclin A1 in leukemia. Cancer Res. 2013;73:773. -773. [Google Scholar]
- 16.Zhou B., Jin X., Jin W., Huang X., Wu Y., Li H., Zhu W., Qin X., Ye H., Gao S. WT1 facilitates the self-renewal of leukemia-initiating cells through the upregulation of BCL2L2: WT1-BCL2L2 axis as a new acute myeloid leukemia therapy target. J. Transl. Med. 2020;18:254. doi: 10.1186/s12967-020-02384-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Wang J., Li X., Jia Y., Huai L., He K., Yu P., Wang M., Xing H., Rao Q., Tian Z., Tang K., Wang J., Mi Y. Role of the Wilms' tumor 1 gene in the aberrant biological behavior of leukemic cells and the related mechanisms. Oncol. Rep. 2014;32:2680–2686. doi: 10.3892/or.2014.3529. [DOI] [PubMed] [Google Scholar]
- 18.Pronier E., Bowman R.L., Ahn J., Glass J., Kandoth C., Merlinsky T.R., Whitfield J.T., Durham B.H., Gruet A., Hanasoge Somasundara A.V. Genetic and epigenetic evolution as a contributor to WT1-mutant leukemogenesis, Blood. J. Am. Soc. Hematol. 2018;132:1265–1278. doi: 10.1182/blood-2018-03-837468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasca S., Jurj A., Tomuleasa C., Zdrenghea M. TET2/IDH1/2/WT1 and NPM1 mutations influence the RUNX1 expression correlations in acute myeloid leukemia. Medicina (B Aires) 2020;56:637. doi: 10.3390/medicina56120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha S., Thomas D., Yu L., Gentles A.J., Jung N., Corces-Zimmerman M.R., Chan S.M., Reinisch A., Feinberg A.P., Dill D.L. Mutant WT1 is associated with DNA hypermethylation of PRC2 targets in AML and responds to EZH2 inhibition, Blood. J. Am. Soc. Hematol. 2015;125:316–326. doi: 10.1182/blood-2014-03-566018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyu Y., Lou J., Yang Y., Feng J., Hao Y., Huang S., Yin L., Xu J., Huang D., Ma B. Dysfunction of the WT1-MEG3 signaling promotes AML leukemogenesis via p53-dependent and-independent pathways. Leukemia. 2017;31:2543–2551. doi: 10.1038/leu.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperling A.S., Gibson C.J., Ebert B.L. The genetics of myelodysplastic syndrome: from clonal haematopoiesis to secondary leukaemia. Nature Rev. Cancer. 2017;17:5–19. doi: 10.1038/nrc.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hopfer O.J., Komor M., Hoelzer D., Thiel E., Hofmann W.-K. Identification of MDS-specific and methylation associated downregulation of survivine and WT1 in highly purified hematopoietic progenitor cells during in vitro lineage specific differentiation. Blood. 2005;106:3442. [Google Scholar]
- 24.Brieger J., Weidmann E., Maurer U., Hoelzer D., Mitrou P., Bergmann L. The Wilms' tumor gene is frequently expressed in acute myeloblastic leukemias and may provide a marker for residual blast cells detectable by PCR. Annal. Oncol. 1995;6:811–816. doi: 10.1093/oxfordjournals.annonc.a059321. [DOI] [PubMed] [Google Scholar]
- 25.Cilloni D., Gottardi E., Messa F., Fava M., Scaravaglio P., Bertini M., Girotto M., Marinone C., Ferrero D., Gallamini A. Significant correlation between the degree of WT1 expression and the International Prognostic Scoring System Score in patients with myelodysplastic syndromes. J. Clin. Oncol. 2003;21:1988–1995. doi: 10.1200/JCO.2003.10.503. [DOI] [PubMed] [Google Scholar]
- 26.Rampal R., Figueroa M.E. Wilms tumor 1 mutations in the pathogenesis of acute myeloid leukemia. Haematologica. 2016;101:672–679. doi: 10.3324/haematol.2015.141796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annesley C.E., Rabik C., Duffield A.S., Rau R.E., Magoon D., Li L., Huff V., Small D., Loeb D.M., Brown P. Knock-in of the Wt1 R394W mutation causes MDS and cooperates with Flt3/ITD to drive aggressive myeloid neoplasms in mice. Oncotarget. 2018;9:35313. doi: 10.18632/oncotarget.26238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiraz P., Jehangir W., Agrawal V. T-cell acute lymphoblastic leukemia—current concepts in molecular biology and management. Biomedicines. 2021;9:1621. doi: 10.3390/biomedicines9111621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bordin F., Piovan E., Masiero E., Ambesi-Impiombato A., Minuzzo S., Bertorelle R., Sacchetto V., Pilotto G., Basso G., Zanovello P. WT1 loss attenuates the TP53-induced DNA damage response in T-cell acute lymphoblastic leukemia. Haematologica. 2018;103:266. doi: 10.3324/haematol.2017.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vicente C., Schwab C., Broux M., Geerdens E., Degryse S., Demeyer S., Lahortiga I., Elliott A., Chilton L., Starza R.La. Targeted sequencing identifies associations between IL7R-JAK mutations and epigenetic modulators in T-cell acute lymphoblastic leukemia. Haematologica. 2015;100:1301. doi: 10.3324/haematol.2015.130179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourkoula K., Englert C., Giaisi M., Köhler R., Krammer P.H., Li-Weber M. The Wilms' tumor suppressor WT1 enhances CD95L expression and promotes activation-induced cell death in leukemic T cells. Int. J. Cancer. 2014;134:291–300. doi: 10.1002/ijc.28379. [DOI] [PubMed] [Google Scholar]
- 32.Heesch S., Goekbuget N., Stroux A., Tanchez J.O., Schlee C., Burmeister T., Schwartz S., Blau O., Keilholz U., Busse A. Prognostic implications of mutations and expression of the Wilms tumor 1 (WT1) gene in adult acute T-lymphoblastic leukemia. Haematologica. 2010;95:942. doi: 10.3324/haematol.2009.016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quintás-Cardama A., Cortes J. Molecular biology of bcr-abl1–positive chronic myeloid leukemia, Blood. J. Am. Soc. Hematol. 2009;113:1619–1630. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svensson E., Vidovic K., Lassen C., Richter J., Olofsson T., Fioretos T., Gullberg U. Deregulation of the Wilms' tumour gene 1 protein (WT1) by BCR/ABL1 mediates resistance to imatinib in human leukaemia cells. Leukemia. 2007;21:2485–2494. doi: 10.1038/sj.leu.2404924. [DOI] [PubMed] [Google Scholar]
- 35.Montano G., Cesaro E., Fattore L., Vidovic K., Palladino C., Crescitelli R., Izzo P., Turco M.C., Costanzo P. Role of WT1–ZNF224 interaction in the expression of apoptosis-regulating genes. Hum. Mol. Genet. 2013;22:1771–1782. doi: 10.1093/hmg/ddt027. [DOI] [PubMed] [Google Scholar]
- 36.Sodaro G., Cesaro E., Montano G., Blasio G., Fiorentino F., Romano S., Jacquel A., Aurberger P., Costanzo P. Role of ZNF224 in c-Myc repression and imatinib responsiveness in chronic myeloid leukemia. Oncotarget. 2017;9 doi: 10.18632/oncotarget.23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madan V., Shyamsunder P., Han L., Mayakonda A., Nagata Y., Sundaresan J., Kanojia D., Yoshida K., Ganesan S., Hattori N. Comprehensive mutational analysis of primary and relapse acute promyelocytic leukemia. Leukemia. 2016;30:1672–1681. doi: 10.1038/leu.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song H., Liu Y., Tan Y., Zhang Y., Jin W., Chen L., Wu S., Yan J., Li J., Chen Z. Recurrent noncoding somatic and germline WT1 variants converge to disrupt MYB binding in acute promyelocytic leukemia, Blood. J. Am. Soc. Hematol. 2022;140:1132–1144. doi: 10.1182/blood.2021014945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nejatifar F., Rostami S., Kasaeian A., Vaezi M., Kamranzadeh H., Mousavi S.A., Farbod A., Alimoghaddam K., Ghavamzadeh A. Incidence and prognostic impact of WT-1 Gene Exon7 and 9 mutations in acute promyelocytic leukemia. Int. J. Hematol. Oncol. Stem Cell Res. 2022;16:74. doi: 10.18502/ijhoscr.v16i2.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon J.-H., Kim H.-J., Kwak D.-H., Park S.-S., Jeon Y.-W., Lee S.-E., Cho B.-S., Eom K.-S., Kim Y.-J., Lee S. High WT1 expression is an early predictor for relapse in patients with acute promyelocytic leukemia in first remission with negative PML-RARa after anthracycline-based chemotherapy: a single-center cohort study. J. Hematol. Oncol. 2017;10:1–4. doi: 10.1186/s13045-017-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schabath M.B., Cote M.L. Cancer progress and priorities: lung cancer. Cancer Epidemiol. Biomarker Prevent. 2019;28:1563–1579. doi: 10.1158/1055-9965.EPI-19-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X., Gao P., Lin F., Long M., Weng Y., Ouyang Y., Liu L., Wei J., Chen X., He T., Zhang H., Dong K. Wilms' tumour suppressor gene 1 (WT1) is involved in the carcinogenesis of Lung cancer through interaction with PI3K/Akt pathway. Cancer Cell Int. 2013;13:114. doi: 10.1186/1475-2867-13-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almalki W.H. Beyond the genome: lncRNAs as regulators of the PI3K/AKT pathway in lung cancer. Pathol. - Res. Practice. 2023;251 doi: 10.1016/j.prp.2023.154852. [DOI] [PubMed] [Google Scholar]
- 44.Vicent S., Chen R., Sayles L.C., Lin C., Walker R.G., Gillespie A.K., Subramanian A., Hinkle G., Yang X., Saif S., Root D.E., Huff V., Hahn W.C., Sweet-Cordero E.A. Wilms tumor 1 (WT1) regulates KRAS-driven oncogenesis and senescence in mouse and human models. J. Clin. Invest. 2010;120:3940–3952. doi: 10.1172/JCI44165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L., Zhang X., Wang Z.Y. The Wilms' tumor suppressor WT1 regulates expression of members of the epidermal growth factor receptor (EGFR) and estrogen receptor in acquired tamoxifen resistance. Anticancer Res. 2010;30:3637–3642. [PMC free article] [PubMed] [Google Scholar]
- 46.Wu C., Wang S., Xu C., Tyler A., Li X., Andersson C., Oji Y., Sugiyama H., Chen Y., Li A. WT1 enhances proliferation and impedes apoptosis in KRAS mutant NSCLC via targeting cMyc. Cellular Physiol. Biochem. 2015;35:647–662. doi: 10.1159/000369726. [DOI] [PubMed] [Google Scholar]
- 47.Wu C., Zhu W., Qian J., He S., Wu C., Chen Y., Shu Y. WT1 promotes invasion of NSCLC via suppression of CDH1. J. Thoracic Oncol. 2013;8:1163–1169. doi: 10.1097/JTO.0b013e31829f6a5f. [DOI] [PubMed] [Google Scholar]
- 48.Wagner K.-D., El Maï M., Ladomery M., Belali T., Leccia N., Michiels J.-F., Wagner N. Altered VEGF splicing isoform balance in tumor endothelium involves activation of splicing factors Srpk1 and Srsf1 by the Wilms’ tumor suppressor Wt1. Cells. 2019;8:41. doi: 10.3390/cells8010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hondelink L.M., Jebbink M., von der Thüsen J.H., Cohen D., Dubbink H.J., Paats M.S., Dingemans A.-M.C., de Langen A.J., Boelens M.C., Smit E.F., Postmus P.E., van Wezel T., Monkhorst K. Real-world approach for molecular analysis of acquired EGFR tyrosine kinase inhibitor resistance mechanisms in NSCLC. JTo Clin. Res. Rep. 2021;2 doi: 10.1016/j.jtocrr.2021.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X., An W., Pan H., Fan Y., Huang H., Wang Y., Shen W., Zu L., Meng F., Zhou X. Wilms’ tumour gene 1 (WT1) enhances non-small cell lung cancer malignancy and is inhibited by microRNA-498-5p. BMC. Cancer. 2023;23:824. doi: 10.1186/s12885-023-11295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu C., Wang Y., Xia Y., He S., Wang Z., Chen Y., Wu C., Shu Y., Jiang J. Wilms’ tumor 1 enhances Cisplatin-resistance of advanced NSCLC. FEBS Lett. 2014;588:4566–4572. doi: 10.1016/j.febslet.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 52.Katsura C., Ogunmwonyi I., Kankam H.K., Saha S. Breast cancer: presentation, investigation and management. Br. J. Hosp. Med. 2022;83:1–7. doi: 10.12968/hmed.2021.0459. [DOI] [PubMed] [Google Scholar]
- 53.Artibani M., Sims A.H., Slight J., Aitken S., Thornburn A., Muir M., Brunton V.G., Del-Pozo J., Morrison L.R., Katz E. WT1 expression in breast cancer disrupts the epithelial/mesenchymal balance of tumour cells and correlates with the metabolic response to docetaxel. Sci. Rep. 2017;7:45255. doi: 10.1038/srep45255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han Y., Yang L., Suarez-Saiz F., San-Marina S., Cui J., Minden M.D. Wilms' Tumor 1 suppressor gene mediates antiestrogen resistance via down-regulation of estrogen receptor-α expression in breast cancer cells. Mol. Cancer Res. 2008;6:1347–1355. doi: 10.1158/1541-7786.MCR-07-2179. [DOI] [PubMed] [Google Scholar]
- 55.Ge A., Gao S., Liu Y., Zhang H., Wang X., Zhang L., Pang D., Zhao Y. Methylation of WT1, CA10 in peripheral blood leukocyte is associated with breast cancer risk: a case-control study. BMC. Cancer. 2020;20:1–9. doi: 10.1186/s12885-020-07183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Werner H., Re G.G., Drummond I.A., Sukhatme V.P., Rauscher F., 3rd, Sens D.A., Garvin A.J., LeRoith D., Roberts C.T., Jr Increased expression of the insulin-like growth factor I receptor gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R promoter activity by the WT1 Wilms tumor gene product. Proc. Natl. Acad. Sci. 1993;90:5828–5832. doi: 10.1073/pnas.90.12.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y., Yan W.T., Yang Z.Y., Li Y.L., Tan X.N., Jiang J., Zhang Y., Qi X.W. The role of WT1 in breast cancer: clinical implications, biological effects and molecular mechanism. Int. J. Biol. Sci. 2020;16:1474–1480. doi: 10.7150/ijbs.39958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bissanum R., Lirdprapamongkol K., Svasti J., Navakanitworakul R., Kanokwiroon K. The role of WT1 isoforms in vasculogenic mimicry and metastatic potential of human triple negative breast cancer cells. Biochem. Biophys. Res. Commun. 2017;494:256–262. doi: 10.1016/j.bbrc.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 59.Ben Haj Othmen H., Othman H., Khamessi O., Bettaieb I., Gara S., Kharrat M. Overexpression of WT1 in all molecular subtypes of breast cancer and its impact on survival: exploring oncogenic and tumor suppressor roles of distinct WT1 isoforms. Mol. Biol. Rep. 2024;51:544. doi: 10.1007/s11033-024-09450-4. [DOI] [PubMed] [Google Scholar]
- 60.Ponzoni M., Bachetti T., Corrias M.V., Brignole C., Pastorino F., Calarco E., Bensa V., Giusto E., Ceccherini I., Perri P. Recent advances in the developmental origin of neuroblastoma: an overview. J. Exp. Clin. Cancer Res. 2022;41:92. doi: 10.1186/s13046-022-02281-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J., Oue T., Uehara S., Yamanaka H., Oji Y., Fukuzawa M. The role of WT1 gene in neuroblastoma. J. Pediatr. Surg. 2011;46:326–331. doi: 10.1016/j.jpedsurg.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 62.Maugeri G., D'Amico A.G., Rasà D.M., Reitano R., Saccone S., Federico C., Parenti R., Magro G., D'Agata V. Expression profile of Wilms Tumor 1 (WT1) isoforms in undifferentiated and all-trans retinoic acid differentiated neuroblastoma cells. Genes. Cancer. 2016;7:47. doi: 10.18632/genesandcancer.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krueger K., Catanese L., Sciesielski L.K., Kirschner K.M., Scholz H. Deletion of an intronic HIF-2α binding site suppresses hypoxia-induced WT1 expression. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms. 2019;1862:71–83. doi: 10.1016/j.bbagrm.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Masserot C., Liu Q., Nguyen E., Gattolliat C.-H., Valteau-Couanet D., Bénard J., Huber C., Ségal-Bendirdjian E. WT1 expression is inversely correlated with MYCN amplification or expression and associated with poor survival in non-MYCN-amplified neuroblastoma. Mol. Oncol. 2016;10:240–252. doi: 10.1016/j.molonc.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asadi F., Lazennec G., Jorgensen C. Importance of stromal stem cells in prostate carcinogenesis process. Cancer Stem Cells Theorie Practice. 2014 [Google Scholar]
- 66.Brett A., Pandey S., Fraizer G. The Wilms’ tumor gene (WT1) regulates E-cadherin expression and migration of prostate cancer cells. Mol. Cancer. 2013;12:1–13. doi: 10.1186/1476-4598-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fraizer G.C., Eisermann K., Pandey S., Brett-Morris A., Bazarov A., Nock S., Ghimirey N., Kuerbitz S.J. Exon Publications; 2016. Functional Role of WT1 in Prostate Cancer; pp. 235–259. [PubMed] [Google Scholar]
- 68.Belali T., Wodi C., Clark B., Cheung M.-K., Craig T.J., Wheway G., Wagner N., Wagner K.-D., Roberts S., Porazinski S. WT1 activates transcription of the splice factor kinase SRPK1 gene in PC3 and K562 cancer cells in the absence of corepressor BASP1. Biochimica et Biophysica Acta (BBA)-Gene Regulat. Mech. 2020;1863 doi: 10.1016/j.bbagrm.2020.194642. [DOI] [PubMed] [Google Scholar]
- 69.Ritchie M.F., Zhou Y., Soboloff J. WT1/EGR1-mediated control of STIM1 expression and function in cancer cells. Front. Biosci.: J. Virtual Lib. 2011;16:2402. doi: 10.2741/3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fraizer G., Leahy R., Priyadarshini S., Graham K., Delacerda J., Diaz M. Suppression of prostate tumor cell growth in vivo by WT1, the Wilms' tumor suppressor gene. Int. J. Oncol. 2004;24:461–471. [PubMed] [Google Scholar]
- 71.Zaia A., Fraizer G.C., Piantanelli L., Saunders G.F. Transcriptional regulation of the androgen signaling pathway by the Wilms' tumor suppressor gene WT1. Anticancer Res. 2001;21:1–10. [PubMed] [Google Scholar]
- 72.Monga S.P. β-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology. 2015;148:1294–1310. doi: 10.1053/j.gastro.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qiu L., Tang Q., Li G., Chen K. Long non-coding RNAs as biomarkers and therapeutic targets: recent insights into hepatocellular carcinoma. Life Sci. 2017;191:273–282. doi: 10.1016/j.lfs.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 74.Wang N., Tan H.-Y., Chan Y.-T., Guo W., Li S., Feng Y. Identification of WT1 as determinant of heptatocellular carcinoma and its inhibition by Chinese herbal medicine Salvia chinensis Benth and its active ingredient protocatechualdehyde. Oncotarget. 2017;8 doi: 10.18632/oncotarget.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan H.-Y., Wang N., Li S., Hong M., Guo W., Man K., Cheng C.-s., Chen Z., Feng Y. Repression of WT1-mediated LEF1 transcription by mangiferin governs β-catenin-independent Wnt signalling inactivation in hepatocellular carcinoma. Cellul. Physiol. Biochem. 2018;47:1819–1834. doi: 10.1159/000491063. [DOI] [PubMed] [Google Scholar]
- 76.Uesugi K., Hiasa Y., Tokumoto Y., Mashiba T., Koizumi Y., Hirooka M., Abe M., Matsuura B., Onji M. Wilms’ tumor 1 gene modulates Fas-related death signals and anti-apoptotic functions in hepatocellular carcinoma. J. Gastroenterol. 2013;48:1069–1080. doi: 10.1007/s00535-012-0708-7. [DOI] [PubMed] [Google Scholar]
- 77.Lv L., Chen G., Zhou J., Li J., Gong J. WT1-AS promotes cell apoptosis in hepatocellular carcinoma through down-regulating of WT1. J. Exp. Clin. Cancer Res. 2015;34:1–9. doi: 10.1186/s13046-015-0233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamisawa T., Wood L.D., Itoi T., Takaori K. Pancreatic cancer. The Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 79.Kanai T., Ito Z., Oji Y., Suka M., Nishida S., Takakura K., Kajihara M., Saruta M., Fujioka S., Misawa T. Prognostic significance of Wilms' tumor 1 expression in patients with pancreatic ductal adenocarcinoma. Oncol. Lett. 2018;16:2682–2692. doi: 10.3892/ol.2018.8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang W., Wang J., Yang C., Wang J. MicroRNA-216a targets WT1 expression and regulates KRT7 transcription to mediate the progression of pancreatic cancer—a transcriptome analysis. IUBMB Life. 2021;73:866–882. doi: 10.1002/iub.2468. [DOI] [PubMed] [Google Scholar]
- 81.Li J., Li H., Zhu W., Zhou B., Ying J., Wu J., Zhang H., Sun H., Gao S. Deubiquitinase inhibitor degrasyn suppresses metastasis by targeting USP5-WT1-E-cadherin signalling pathway in pancreatic ductal adenocarcinoma. J. Cell. Mol. Med. 2020;24:1370–1382. doi: 10.1111/jcmm.14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glienke W., Maute L., Wicht J., Bergmann L. Wilms’ tumour gene 1 (WT1) as a target in curcumin treatment of pancreatic cancer cells. Eur. J. Cancer. 2009;45:874–880. doi: 10.1016/j.ejca.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 83.Takahara A., Koido S., Ito M., Nagasaki E., Sagawa Y., Iwamoto T., Komita H., Ochi T., Fujiwara H., Yasukawa M. Gemcitabine enhances Wilms’ tumor gene WT1 expression and sensitizes human pancreatic cancer cells with WT1-specific T-cell-mediated antitumor immune response. Cancer Immunol. Immunother. 2011;60:1289–1297. doi: 10.1007/s00262-011-1033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lheureux S., Braunstein M., Oza A.M. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019;69:280–304. doi: 10.3322/caac.21559. [DOI] [PubMed] [Google Scholar]
- 85.Mondal S.K., Basak B., Bhattacharya S., Panda U.K. Role of WT1, B-cell lymphoma 2, Ki-67 (Mib1), and Her2/Neu as diagnostic and prognostic immunomarkers in ovarian serous and endometroid carcinoma. J. Cancer Res. Ther. 2021;17:164–169. doi: 10.4103/jcrt.JCRT_311_19. [DOI] [PubMed] [Google Scholar]
- 86.Han Y., Song C., Zhang T., Zhou Q., Zhang X., Wang J., Xu B., Zhang X., Liu X., Ying X. Wilms’ tumor 1 (WT1) promotes ovarian cancer progression by regulating E-cadherin and ERK1/2 signaling. Cell Cycle. 2020;19:2662–2675. doi: 10.1080/15384101.2020.1817666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X., Zhao J., Zhang Y., Liu Y., Wang J., Shi R., Yuan J., Meng K. Molecular mechanism of Wilms’ tumor (Wt1)(+/− KTS) variants promoting proliferation and migration of ovarian epithelial cells by bioinformatics analysis. J. Ovarian. Res. 2023;16:46. doi: 10.1186/s13048-023-01124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fan H., Atiya H.I., Wang Y., Pisanic T.R., Wang T.-H., Shih I.-M., Foy K.K., Frisbie L., Buckanovich R.J., Chomiak A.A. Epigenomic reprogramming toward mesenchymal-epithelial transition in ovarian-cancer-associated mesenchymal stem cells drives metastasis. Cell Rep. 2020:33. doi: 10.1016/j.celrep.2020.108473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu H.-L., Ma X.-D., Tong J.-F., Li J.-Q., Guan X.-J., Yang J.-H. WTAP is a prognostic marker of high-grade serous ovarian cancer and regulates the progression of ovarian cancer cells. Onco Targets. Ther. 2019:6191–6201. doi: 10.2147/OTT.S205730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fernando D., Ahmed A.U., Williams B.R. Therapeutically targeting the unique disease landscape of pediatric high-grade gliomas. Front. Oncol. 2024;14 doi: 10.3389/fonc.2024.1347694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schittenhelm J., Mittelbronn M., Nguyen T.D., Meyermann R., Beschorner R. WT1 expression distinguishes astrocytic tumor cells from normal and reactive astrocytes. Brain Pathol. 2008;18:344–353. doi: 10.1111/j.1750-3639.2008.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen M.Y., Clark A.J., Chan D.C., Ware J.L., Holt S.E., Chidambaram A., Fillmore H.L., Broaddus W.C. Wilms’ tumor 1 silencing decreases the viability and chemoresistance of glioblastoma cells in vitro: a potential role for IGF-1R de-repression. J. Neurooncol. 2011;103:87–102. doi: 10.1007/s11060-010-0374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clark A.J., Ware J.L., Chen M.Y., Graf M.R., Van Meter T.E., Dos Santos W.G., Fillmore H.L., Broaddus W.C. Effect of WT1 gene silencing on the tumorigenicity of human glioblastoma multiforme cells. J. Neurosurg. 2010;112:18–25. doi: 10.3171/2008.11.JNS08368. [DOI] [PubMed] [Google Scholar]
- 94.Oji Y., Hashimoto N., Tsuboi A., Murakami Y., Iwai M., Kagawa N., Chiba Y., Izumoto S., Elisseeva O., Ichinohasama R. Association of WT1 IgG antibody against WT1 peptide with prolonged survival in glioblastoma multiforme patients vaccinated with WT1 peptide. Int. J. Cancer. 2016;139:1391–1401. doi: 10.1002/ijc.30182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Panou V., Vyberg M., Weinreich U., Meristoudis C., Falkmer U., Røe O. The established and future biomarkers of malignant pleural mesothelioma. Cancer Treat. Rev. 2015;41:486–495. doi: 10.1016/j.ctrv.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 96.Scattone A., Serio G., Marzullo A., Nazzaro P., Corsi F., Cocca M.P., Mattoni M., Punzi A., Gentile M., Buonadonna A.L. High Wilms’ tumour gene (WT1) expression and low mitotic count are independent predictors of survival in diffuse peritoneal mesothelioma. Histopathology. 2012;60:472–481. doi: 10.1111/j.1365-2559.2011.04108.x. [DOI] [PubMed] [Google Scholar]
- 97.Plönes T., Fischer M., Höhne K., Sato H., Müller-Quernheim J., Zissel G. Turning back the wheel: inducing mesenchymal to epithelial transition via Wilms tumor 1 knockdown in human mesothelioma cell lines to influence proliferation, invasiveness, and chemotaxis. Pathol. Oncol. Res. 2017;23:723–730. doi: 10.1007/s12253-016-0181-3. [DOI] [PubMed] [Google Scholar]
- 98.Miller-Hodges E., Hohenstein P. WT1 in disease: shifting the epithelial–mesenchymal balance. J. Pathol. 2012;226:229–240. doi: 10.1002/path.2977. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X., Zhang H., Chen L., Wang M., Xi J., Liu X., Xie M., Li D., Gulati E.S., Gong S. Arsenic trioxide and all-trans retinoic acid (ATRA) treatment for acute promyelocytic leukemia in all risk groups: study protocol for a randomized controlled trial. Trials. 2018;19:1–7. doi: 10.1186/s13063-018-2812-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buschner G., Feuerecker B., Spinner S., Seidl C., Essler M. Differentiation of acute myeloid leukemia (AML) cells with ATRA reduces 18F-FDG uptake and increases sensitivity towards ABT-737-induced apoptosis. Leuk. Lymphoma. 2021;62:630–639. doi: 10.1080/10428194.2020.1839648. [DOI] [PubMed] [Google Scholar]
- 101.Fenaux P., Mufti G.J., Hellstrom-Lindberg E., Santini V., Finelli C., Giagounidis A., Schoch R., Gattermann N., Sanz G., List A. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiang L., Zhou J., Gu W., Wang R., Wei J., Qiu G., Cen J., Xie X., Chen Z. Changes in expression of WT1 during induced differentiation of the acute myeloid leukemia cell lines by treatment with 5-aza-2′-deoxycytidine and all-trans retinoic acid. Oncol. Lett. 2016;11:1521–1526. doi: 10.3892/ol.2015.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jansson C., Mengelbier L.H. Retinoic acid promotes differentiation of WiT49-but not of CCG99-11 Wilms tumour cells. Cancer Rep. 2023;6:e1819. doi: 10.1002/cnr2.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu W., Neckers L. Targeting the molecular chaperone heat shock protein 90 provides a multifaceted effect on diverse cell signaling pathways of cancer cells. Clin. Cancer Res. 2007;13:1625–1629. doi: 10.1158/1078-0432.CCR-06-2966. [DOI] [PubMed] [Google Scholar]
- 105.Bansal H., Bansal S., Rao M., Foley K.P., Sang J., Proia D.A., Blackman R.K., Ying W., Barsoum J., Baer M.R. Heat shock protein 90 regulates the expression of Wilms tumor 1 protein in myeloid leukemias, Blood. J. Am. Soc. Hematol. 2010;116:4591–4599. doi: 10.1182/blood-2009-10-247239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heath E.I., Hillman D.W., Vaishampayan U., Sheng S., Sarkar F., Harper F., Gaskins M., Pitot H.C., Tan W., Ivy S.P., Pili R., Carducci M.A., Erlichman C., Liu G. A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin. Cancer Res. 2008;14:7940–7946. doi: 10.1158/1078-0432.CCR-08-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oki Y., Copeland A., Romaguera J., Fayad L., Fanale M., Faria Sde C., Medeiros L.J., Ivy P., Younes A. Clinical experience with the heat shock protein-90 inhibitor, tanespimycin, in patients with relapsed lymphoma. Leuk. Lymphoma. 2012;53:990–992. doi: 10.3109/10428194.2011.631236. [DOI] [PubMed] [Google Scholar]
- 108.Yuan T., Yan F., Ying M., Cao J., He Q., Zhu H., Yang B. Inhibition of ubiquitin-specific proteases as a novel anticancer therapeutic strategy. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.01080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.S.G. Zidanloo, Z. Bagherian, Stabilization of G-quadruplexes in Intronic Hematopoietic-specific Enhancer of WT1 gene with G-quadruplex targeting ligands: in silico and in vitro techniques, (2024).
- 110.Zidanloo S.G., Hosseinzadeh Colagar A., Ayatollahi H., Raoof J.-B. Downregulation of the WT 1 gene expression via TMPyP4 stabilization of promoter G-quadruplexes in leukemia cells. Tumor Biol. 2016;37:9967–9977. doi: 10.1007/s13277-016-4881-9. [DOI] [PubMed] [Google Scholar]
- 111.Konieczna N., Romaniuk-Drapała A., Lisiak N., Totoń E., Paszel-Jaworska A., Kaczmarek M., Rubiś B. Telomerase inhibitor TMPyP4 Alters adhesion and migration of breast-cancer cells MCF7 and MDA-MB-231. Int. J. Mol. Sci. 2019:20. doi: 10.3390/ijms20112670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Evison B.J., Sleebs B.E., Watson K.G., Phillips D.R., Cutts S.M. Mitoxantrone, more than just another topoisomerase II poison. Med. Res. Rev. 2016;36:248–299. doi: 10.1002/med.21364. [DOI] [PubMed] [Google Scholar]
- 113.Ghazaey Zidanloo S., Hosseinzadeh Colagar A., Ayatollahi H., Bagheryan Z. G-quadruplex forming region within WT1 promoter is selectively targeted by daunorubicin and mitoxantrone: a possible mechanism for anti-leukemic effect of drugs. J. Biosci. 2019;44:1–9. [PubMed] [Google Scholar]
- 114.Hunger S.P. Tyrosine kinase inhibitor use in pediatric Philadelphia chromosome–positive acute lymphoblastic anemia. Hematology. 2010;2011(2011):361–365. doi: 10.1182/asheducation-2011.1.361. the American Society of Hematology Education Program Book. [DOI] [PubMed] [Google Scholar]
- 115.Wang C., Bai X., Wang R., Zheng X., Ma X., Chen H., Ai Y., Bai Y., Liu Y. Synthesis of imatinib by C–N coupling reaction of primary amide and bromo-substituted pyrimidine amine. Org. Process. Res. Dev. 2019;23:1918–1925. [Google Scholar]
- 116.Zhang L., Li Y., Li X., Zhang Q., Qiu S., Zhang Q., Wang M., Xing H., Rao Q., Tian Z. Regulation of HtrA2 on WT1 gene expression under imatinib stimulation and its effects on the cell biology of K562 cells. Oncol. Lett. 2017;14:3862–3868. doi: 10.3892/ol.2017.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barreca M., Spanò V., Montalbano A., Cueto M., Díaz Marrero A.R., Deniz I., Erdoğan A., Lukić Bilela L., Moulin C., Taffin-de-Givenchy E. Marine anticancer agents: an overview with a particular focus on their chemical classes. Mar. Drugs. 2020;18:619. doi: 10.3390/md18120619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shinn L.T., Vo K.A., Reeves D.J. Lurbinectedin: a new treatment option for relapsed/refractory small-cell lung cancer. Annal. Pharmacother. 2020;55:1172–1179. doi: 10.1177/1060028020983014. [DOI] [PubMed] [Google Scholar]
- 119.Gedminas J.M., Kaufman R., Boguslawski E.A., Gross A.C., Adams M., Beddows I., Kitchen-Goosen S.M., Roberts R.D., Grohar P.J. Lurbinectedin inhibits the EWS–WT1 transcription factor in desmoplastic small round cell tumor. Mol. Cancer Ther. 2022;21:1296–1305. doi: 10.1158/1535-7163.MCT-21-1003. [DOI] [PubMed] [Google Scholar]
- 120.Soumoy L., Ghanem G.E., Saussez S., Journe F. Bufalin for an innovative therapeutic approach against cancer. Pharmacol. Res. 2022;184 doi: 10.1016/j.phrs.2022.106442. [DOI] [PubMed] [Google Scholar]
- 121.Wang L.-p., Zhao Y.-n., Sun X., Gao R.-l. Effects of bufalin on up-regulating methylation of Wilm's tumor 1 gene in human erythroid leukemic cells. Chin. J. Integr. Med. 2017;23:288–294. doi: 10.1007/s11655-017-2404-1. [DOI] [PubMed] [Google Scholar]
- 122.Yuan J., Zeng C., Cao W., Zhou X., Pan Y., Xie Y., Zhang Y., Yang Q., Wang S. Bufalin-Loaded PEGylated Liposomes: antitumor Efficacy, Acute Toxicity, and Tissue Distribution. Nanoscale Res. Lett. 2019;14:223. doi: 10.1186/s11671-019-3057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guo C., He J., Song X., Tan L., Wang M., Jiang P., Li Y., Cao Z., Peng C. Pharmacological properties and derivatives of shikonin—a review in recent years. Pharmacol. Res. 2019;149 doi: 10.1016/j.phrs.2019.104463. [DOI] [PubMed] [Google Scholar]
- 124.Guo Z., Sun L., Xia H., Tian S., Liu M., Hou J., Li J., Lin H., Du G. Shikonin as a WT1 inhibitor promotes promyeloid leukemia cell differentiation. Molecules. 2022;27:8264. doi: 10.3390/molecules27238264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He J., Xie Y., Zhong J., Chen W., Fang S., Chen X., Peng S., Liu W., Liu C. Improving shikonin solubility and stability by encapsulation in natural surfactant-coated shikonin nanoparticles. J. Food Sci. 2023;88:825–836. doi: 10.1111/1750-3841.16445. [DOI] [PubMed] [Google Scholar]
- 126.Egbuna C., Gupta E., Ezzat S.M., Jeevanandam J., Mishra N., Akram M., Sudharani N., Adetunji C.O., Singh P., Ifemeje J.C. Aloe species as valuable sources of functional bioactives. Funct. Foods Nutraceuticals: Bioact. Component. Formulat. Innovat. 2020:337–387. [Google Scholar]
- 127.Fu D., Ji Q., Wang C., Yu L., Yu R. Aloin decelerates the progression of hepatocellular carcinoma through circ_0011385/miR-149-5p/WT1 axis. Cell Cycle. 2021;20:2476–2493. doi: 10.1080/15384101.2021.1988227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zimbone S., Romanucci V., Zarrelli A., Giuffrida M.L., Sciacca M.F.M., Lanza V., Campagna T., Maugeri L., Petralia S., Consoli G.M.L., Di Fabio G., Milardi D. Exploring the therapeutic potential of Aloin: unraveling neuroprotective and anticancer mechanisms, and strategies for enhanced stability and delivery. Sci. Rep. 2024;14:16731. doi: 10.1038/s41598-024-67397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.G.Y. Savcun, E. Özkan, E. Dulundu, Ü. Topaloğlu, A.Ö. Şehirli, O.E. Tok, F. Ercan, G. Şener, Antioxidant and anti-inflammatory effects of curcumin against hepatorenal oxidative injury in the experimental sepsis model created in rats, (2013). [DOI] [PubMed]
- 130.Bharti A.C., Donato N., Singh S., Aggarwal B.B. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor–κB and IκBα kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis, Blood. J. Am. Soc. Hematol. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 131.Gao S.-m., Yang J.-j., Chen C.-q., Chen J.-j., Ye L.-p., Wang L.-y., Wu J.-b., Xing C.-y., Yu K. Pure curcumin decreases the expression of WT1 by upregulation of miR-15a and miR-16-1 in leukemic cells. J. Exp. Clin. Cancer Res. 2012;31:1–9. doi: 10.1186/1756-9966-31-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Semsri S., Krig S.R., Kotelawala L., Sweeney C.A., Anuchapreeda S. Inhibitory mechanism of pure curcumin on Wilms’ tumor 1 (WT1) gene expression through the PKCα signaling pathway in leukemic K562 cells. FEBS Lett. 2011;585:2235–2242. doi: 10.1016/j.febslet.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 133.Liu J.-M., Li M., Luo W., Sun H.-B. Curcumin attenuates Adriamycin-resistance of acute myeloid leukemia by inhibiting the lncRNA HOTAIR/miR-20a-5p/WT1 axis. Lab. Investigat. 2021;101:1308–1317. doi: 10.1038/s41374-021-00640-3. [DOI] [PubMed] [Google Scholar]
- 134.Panyajai P., Tima S., Chiampanichayakul S., Anuchapreeda S. Dietary turmeric bisdemethoxycurcumin suppresses wilms’ tumor 1 and CD34 protein expressions in KG-1a leukemic stem cells. Nutr. Cancer. 2019;71:1189–1200. doi: 10.1080/01635581.2019.1598565. [DOI] [PubMed] [Google Scholar]
- 135.Dei Cas M., Ghidoni R. Dietary Curcumin: correlation between bioavailability and health potential. Nutrients. 2019;11 doi: 10.3390/nu11092147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang K.-n., Hu Y., Han L.-l., Zhao S.-s., Song C., Sun S.-w., Lv H.-y., Jiang N.-n., Xv L.-z., Zhao Z.-w. Salvia chinensis benth inhibits triple-negative breast cancer progression by inducing the DNA damage pathway. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.882784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zheng Q., Wang X., Gao T., Zhang B., Zhao N., Du R., Zhao Z. Exploring the pharmacological and molecular mechanisms of Salvia chinensis Benth in colorectal cancer: a network pharmacology and molecular docking study. Medicine (Baltimore) 2023;102:e36602. doi: 10.1097/MD.0000000000036602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Anuchapreeda S., Chueahongthong F., Viriyaadhammaa N., Panyajai P., Anzawa R., Tima S., Ampasavate C., Saiai A., Rungrojsakul M., Usuki T. Antileukemic cell proliferation of active compounds from kaffir lime (Citrus hystrix) leaves. Molecules. 2020;25:1300. doi: 10.3390/molecules25061300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mahidol C., Kaweetripob W., Prawat H., Ruchirawat S. Mammea coumarins from the flowers of Mammea s iamensis. J. Nat. Prod. 2002;65:757–760. doi: 10.1021/np010579u. [DOI] [PubMed] [Google Scholar]
- 140.Rungrojsakul M., Katekunlaphan T., Saiai A., Ampasavate C., Okonogi S., Sweeney C.A., Anuchapreeda S. Down-regulatory mechanism of mammea E/BB from Mammea siamensis seed extract on Wilms’ Tumor 1 expression in K562 cells. BMC. Complement. Altern. Med. 2016;16:1–11. doi: 10.1186/s12906-016-1107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rungrojsakul M., Saiai A., Ampasavate C., Anuchapreeda S., Okonogi S. Inhibitory effect of mammea E/BB from Mammea siamensis seed extract on Wilms' tumour 1 protein expression in a K562 leukaemic cell line. Nat. Prod. Res. 2016;30:443–447. doi: 10.1080/14786419.2015.1017491. [DOI] [PubMed] [Google Scholar]
- 142.Li Y., Zheng B., Tian H., Xu X., Sun Y., Mei Q., Lin X., Liu L. Yupingfeng Powder relieves the immune suppression induced by dexamethasone in mice. J. Ethnopharmacol. 2017;200:117–123. doi: 10.1016/j.jep.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 143.Lou J.-S., Xia Y.-T., Wang H.-Y., Kong X.-P., Yao P., Dong T.T., Zhou Z.-Y., Tsim K.W. The WT1/MVP-mediated stabilization on mTOR/AKT axis enhances the effects of cisplatin in non-small cell lung cancer by a reformulated Yu Ping Feng San herbal preparation. Front. Pharmacol. 2018;9:853. doi: 10.3389/fphar.2018.00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Du Y., Zheng Y., Yu C.X., Zhong L., Li Y., Wu B., Hu W., Zhu E.W., Xie V.W., Xu Q. The mechanisms of Yu Ping Feng San in tracking the cisplatin-resistance by regulating ATP-binding cassette transporter and glutathione S-transferase in lung cancer cells. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.678126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luo Q., Zhang C.S., Yang L., Zhang A.L., Guo X., Xue C.C., Lu C. Potential effectiveness of Chinese herbal medicine Yu ping feng san for adult allergic rhinitis: a systematic review and meta-analysis of randomized controlled trials. BMC. Complement. Altern. Med. 2017;17:485. doi: 10.1186/s12906-017-1988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sawada A., Inoue M., Kondo O., Yamada-Nakata K., Ishihara T., Kuwae Y., Nishikawa M., Ammori Y., Tsuboi A., Oji Y. Feasibility of cancer immunotherapy with WT1 peptide vaccination for solid and hematological malignancies in children. Pediatr. Blood Cancer. 2016;63:234–241. doi: 10.1002/pbc.25792. [DOI] [PubMed] [Google Scholar]
- 147.Krishnadas D.K., Stamer M.M., Dunham K., Bao L., Lucas K.G. Wilms’ tumor 1-specific cytotoxic T lymphocytes can be expanded from adult donors and cord blood. Leuk. Res. 2011;35:1520–1526. doi: 10.1016/j.leukres.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 148.Brayer J., Lancet J.E., Powers J., List A., Balducci L., Komrokji R., Pinilla-Ibarz J. WT 1 vaccination in AML and MDS: a pilot trial with synthetic analog peptides. Am. J. Hematol. 2015;90:602–607. doi: 10.1002/ajh.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maslak P.G., Dao T., Bernal Y., Chanel S.M., Zhang R., Frattini M., Rosenblat T., Jurcic J.G., Brentjens R.J., Arcila M.E., Rampal R., Park J.H., Douer D., Katz L., Sarlis N., Tallman M.S., Scheinberg D.A. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia. Blood Adv. 2018;2:224–234. doi: 10.1182/bloodadvances.2017014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jain A.G., Talati C., Pinilla-Ibarz J. Galinpepimut-S (GPS): an investigational agent for the treatment of acute myeloid leukemia. Expert. Opin. Investig. Drugs. 2021;30:595–601. doi: 10.1080/13543784.2021.1928635. [DOI] [PubMed] [Google Scholar]
- 151.Fujiki F., Tsuboi A., Morimoto S., Hashimoto N., Inatome M., Nakajima H., Nakata J., Nishida S., Hasegawa K., Hosen N. Identification of two distinct populations of WT1-specific cytotoxic T lymphocytes in co-vaccination of WT1 killer and helper peptides. Cancer Immunol. Immunother. 2021;70:253–263. doi: 10.1007/s00262-020-02675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Anguille S., Van de Velde A.L., Smits E.L., Van Tendeloo V.F., Juliusson G., Cools N., Nijs G., Stein B., Lion E., Van Driessche A. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia, Blood. J. Am. Soc. Hematol. 2017;130:1713–1721. doi: 10.1182/blood-2017-04-780155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fløisand Y., Remberger M., Bigalke I., Josefsen D., Vålerhaugen H., Inderberg E.M., Olaussen R.W., Gjertsen B.T., Goedkoop R., Geiger C. WT1 and PRAME RNA-loaded dendritic cell vaccine as maintenance therapy in de novo AML after intensive induction chemotherapy. Leukemia. 2023;37:1842–1849. doi: 10.1038/s41375-023-01980-3. [DOI] [PubMed] [Google Scholar]
- 154.Cho S.Y., Jeong S.M., Jeon Y.J., Yang S.J., Hwang J.E., Yoo B.M., Kim H.S. WT1 Pulsed Human CD141+ Dendritic Cell Vaccine Has High Potential in Solid Tumor-Targeted Immunotherapy. Int. J. Mol. Sci. 2023;24:1501. doi: 10.3390/ijms24021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Berneman Z.N., Anguille S., Willemen Y., de Velde A.V., Germonpre P., Huizing M., Van Tendeloo V., Saevels K., Rutsaert L., Vermeulen K., Snoeckx A., de Beeck B.O., Cools N., Nijs G., Stein B., Lion E., Van Driessche A., Peeters M., Smits E. Vaccination of cancer patients with dendritic cells electroporated with mRNA encoding the wilms' tumor 1 protein (WT1): correlation of clinical effect and overall survival with T-cell response. Cytotherapy. 2019;21:S10. [Google Scholar]
- 156.Ruiz L., Delgado S., Ruas-Madiedo P., Sánchez B., Margolles A. Bifidobacteria and their molecular communication with the immune system. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kitagawa K., Oda T., Saito H., Araki A., Gonoi R., Shigemura K., Hashii Y., Katayama T., Fujisawa M., Shirakawa T. Development of oral cancer vaccine using recombinant Bifidobacterium displaying Wilms’ tumor 1 protein. Cancer Immunol. Immunother. 2017;66:787–798. doi: 10.1007/s00262-017-1984-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nakagawa N., Hashii Y., Kayama H., Okumura R., Nakajima H., Minagawa H., Morimoto S., Fujiki F., Nakata J., Shirakawa T. An oral WT1 protein vaccine composed of WT1-anchored, genetically engineered Bifidobacterium longum allows for intestinal immunity in mice with acute myeloid leukemia. Cancer Immunol. Immunother. 2023;72:39–53. doi: 10.1007/s00262-022-03214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Minagawa H., Hashii Y., Nakajima H., Fujiki F., Morimoto S., Nakata J., Shirakawa T., Katayama T., Tsuboi A., Ozono K. Enhanced antitumor activity of a novel, oral, helper epitope-containing WT1 protein vaccine in a model of murine leukemia. BMC. Cancer. 2023;23:167. doi: 10.1186/s12885-023-10547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kitagawa K., Tatsumi M., Kato M., Komai S., Doi H., Hashii Y., Katayama T., Fujisawa M., Shirakawa T. An oral cancer vaccine using a Bifidobacterium vector suppresses tumor growth in a syngeneic mouse bladder cancer model. Mol. Therapy-Oncolytics. 2021;22:592–603. doi: 10.1016/j.omto.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shirakawa T., Kitagawa K. Antitumor effect of oral cancer vaccine with Bifidobacterium delivering WT1 protein to gut immune system is superior to WT1 peptide vaccine. Hum. Vaccin. ImmunOther. 2018;14:159–162. doi: 10.1080/21645515.2017.1382787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Baulu E., Gardet C., Chuvin N., Depil S. TCR-engineered T cell therapy in solid tumors: state of the art and perspectives. Sci. Adv. 2023;9:eadf3700. doi: 10.1126/sciadv.adf3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shao W., Yao Y., Yang L., Li X., Ge T., Zheng Y., Zhu Q., Ge S., Gu X., Jia R. Novel insights into TCR-T cell therapy in solid neoplasms: optimizing adoptive immunotherapy. Exp. Hematol. Oncol. 2024;13:37. doi: 10.1186/s40164-024-00504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chapuis A.G., Egan D.N., Bar M., Schmitt T.M., McAfee M.S., Paulson K.G., Voillet V., Gottardo R., Ragnarsson G.B., Bleakley M. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med. 2019;25:1064–1072. doi: 10.1038/s41591-019-0472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ruggiero E., Carnevale E., Prodeus A., Magnani Z.I., Camisa B., Merelli I., Politano C., Stasi L., Potenza A., Cianciotti B.C. CRISPR-based gene disruption and integration of high-avidity, WT1-specific T cell receptors improve antitumor T cell function. Sci. Transl. Med. 2022;14:eabg8027. doi: 10.1126/scitranslmed.abg8027. [DOI] [PubMed] [Google Scholar]
- 166.Morris E.C., Tendeiro-Rego R., Richardson R., Fox T.A., Sillito F., Holler A., Thomas S., Xue S.-A., Martínez-Dávila I.A., Nicholson E., Ahmadi M., King J., Kottaridis P., Chakraverty R., Thomson K., Robinson S., Virchis A.E., Khwaja A., Quaye M., Zhan H., Qasim W., Himoudi N., Barry J., Mount N., Gaskell T., Sharpe M., Inman S.M., Gunter K.C., Stauss H. A phase I study evaluating the safety and persistence of Allorestricted WT1-TCR gene modified autologous T cells in patients with high-risk myeloid malignancies unsuitable for allogeneic stem cell transplantation. Blood. 2019;134:1367. -1367. [Google Scholar]
- 167.Augsberger C., Hänel G., Xu W., Pulko V., Hanisch L.J., Augustin A., Challier J., Hunt K., Vick B., Rovatti P.E. Targeting intracellular WT1 in AML with a novel RMF-peptide-MHC-specific T-cell bispecific antibody, Blood. J. Am. Soc. Hematol. 2021;138:2655–2669. doi: 10.1182/blood.2020010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dao T., Pankov D., Scott A., Korontsvit T., Zakhaleva V., Xu Y., Xiang J., Yan S., de Morais Guerreiro M.D., Veomett N. Therapeutic bispecific T-cell engager antibody targeting the intracellular oncoprotein WT1. Nat. Biotechnol. 2015;33:1079–1086. doi: 10.1038/nbt.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mun S.S., Meyerberg J., Peraro L., Korontsvit T., Gardner T., Malviya M., Kyi C., O'Cearbhaill R.E., Liu C., Dao T. Dual targeting ovarian cancer by Muc16 CAR T cells secreting a bispecific T cell engager antibody for an intracellular tumor antigen WT1. Cancer Immunol. Immunother. 2023;72:3773–3786. doi: 10.1007/s00262-023-03529-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leclercq G., Haegel H., Schneider A., Giusti A.M., Marrer-Berger E., Boetsch C., Walz A.-C., Pulko V., Sam J., Challier J. Src/lck inhibitor dasatinib reversibly switches off cytokine release and T cell cytotoxicity following stimulation with T cell bispecific antibodies. J. ImmunOther Cancer. 2021;9 doi: 10.1136/jitc-2021-002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Torban E., Goodyer P. Wilms Tumor Gene 1: lessons from kidney development and cancer. Am. J. Physiol. Renal Physiol. 2023 doi: 10.1152/ajprenal.00248.2023. [DOI] [PubMed] [Google Scholar]
- 172.Lee W.-C., Chiu C.-H., Chu T.-H., Chien Y.-S. WT1: the hinge between anemia correction and cancer development in chronic kidney disease. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.876723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rampal R., Figueroa M.E. Wilms tumor 1 mutations in the pathogenesis of acute myeloid leukemia. Haematologica. 2016;101:672. doi: 10.3324/haematol.2015.141796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Das S., Dey M.K., Devireddy R., Gartia M.R. Biomarkers in cancer detection. Diagnosis Prognosis, Sensors. 2023;24:37. doi: 10.3390/s24010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pons M., Reichardt C.M., Hennig D., Nathan A., Kiweler N., Stocking C., Wichmann C., Christmann M., Butter F., Reichardt S., Schneider G., Heinzel T., Englert C., Hartkamp J., Krämer O.H., Mahendrarajah N. Loss of Wilms tumor 1 protein is a marker for apoptosis in response to replicative stress in leukemic cells. Arch. Toxicol. 2018;92:2119–2135. doi: 10.1007/s00204-018-2202-3. [DOI] [PubMed] [Google Scholar]
- 176.Pons M., Nagel G., Zeyn Y., Beyer M., Laguna T., Brachetti T., Sellmer A., Mahboobi S., Conradi R., Butter F., Krämer O.H. Human platelet lysate as validated replacement for animal serum to assess chemosensitivity. ALTEX. 2019;36:277–288. doi: 10.14573/altex.1809211. [DOI] [PubMed] [Google Scholar]
- 177.Beyer M., Romanski A., Mustafa A.M., Pons M., Büchler I., Vogel A., Pautz A., Sellmer A., Schneider G., Bug G., Krämer O.H. HDAC3 Activity is Essential for Human Leukemic Cell Growth and the Expression of β-catenin, MYC, and WT1. Cancers. (Basel) 2019:11. doi: 10.3390/cancers11101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Saosathan S., Khounvong J., Rungrojsakul M., Katekunlaphan T., Tima S., Chiampanichayakul S., Berkland C., Anuchapreeda S. Costunolide and parthenolide from Champi Sirindhorn (Magnolia sirindhorniae) inhibit leukemic cell proliferation in K562 and molt-4 cell lines. Nat. Prod. Res. 2021;35:988–992. doi: 10.1080/14786419.2019.1610752. [DOI] [PubMed] [Google Scholar]
- 179.Rueankham L., Panyajai P., Saiai A., Rungrojsakul M., Tima S., Chiampanichayakul S., Yeerong K., Somwongin S., Chaiyana W., Dejkriengkraikul P. Biological activities of extracts and compounds from Thai Kae-Lae (Maclura cochinchinensis (Lour.) Corner) BMC. Complement. Med. Ther. 2023;23:191. doi: 10.1186/s12906-023-03979-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Garvin A.J., Khalaf A.H., Rettino A., Xicluna J., Butler L., Morris J.R., Heery D.M., Clarke N.M. GSK3β-SCFFBXW7α mediated phosphorylation and ubiquitination of IRF1 are required for its transcription-dependent turnover. Nucleic Acids Res. 2019;47:4476–4494. doi: 10.1093/nar/gkz163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Okabe H., Lee S.-H., Phuchareon J., Albertson D.G., McCormick F., Tetsu O. A critical role for FBXW8 and MAPK in cyclin D1 degradation and cancer cell proliferation. PLoS. One. 2006;1:e128. doi: 10.1371/journal.pone.0000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yoshitomi H., Lee K.Y., Yao K., Shin S.H., Zhang T., Wang Q., Paul S., Roh E., Ryu J., Chen H. GSK3β-Mediated Expression of CUG-Translated WT1 Is Critical for Tumor Progression. Cancer Res. 2021;81:945–955. doi: 10.1158/0008-5472.CAN-20-1880. [DOI] [PubMed] [Google Scholar]
- 183.Ogasawara M., Miyashita M., Yamagishi Y., Ota S. Phase I/II pilot study of Wilms' tumor 1 peptide-pulsed dendritic cell vaccination combined with conventional chemotherapy in patients with head and neck cancer. Therapeutic Apheresis Dialysis. 2019;23:279–288. doi: 10.1111/1744-9987.12831. [DOI] [PubMed] [Google Scholar]
- 184.Oka Y., Tsuboi A., Oji Y., Kawase I., Sugiyama H. WT1 peptide vaccine for the treatment of cancer. Curr. Opin. Immunol. 2008;20:211–220. doi: 10.1016/j.coi.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 185.Ogasawara M. Wilms’ tumor 1-targeting cancer vaccine: recent advancements and future perspectives. Hum. Vaccin. ImmunOther. 2024;20 doi: 10.1080/21645515.2023.2296735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.