Abstract
Background:
There is growing evidence that local recurrence after radiotherapy often occurs within the dominant intraprostatic lesions (DILs) in prostate cancer. This study aimed to evaluate the dose difference between DILs defined by Magnetic Resonance-guided and arc-based Intensity Modulated Radiation Therapy (IMRT) and to assess the association between the dose difference and biochemical recurrence-free survival.
Materials and Methods:
Between 2015 and 2019, 48 prostate cancer patients with DILs visible from multiparametric Magnetic Resonance Imaging (mpMRI) underwent arc-based IMRT with 70 Gy (2.5 Gy each fraction) to the prostate gland. Pretreatment mpMRI DILs contoured the prostate gland retrospectively.
Results:
Biochemical recurrence was 8.3%. There was a significant difference between the median dose of DILs from MRI-guided imaging, 69.22 Gy, and the median dose of the whole prostate from arc-based IMRT which was 67.09 Gy (p < 0.001*). The Kaplan-Meier survival curve compared by log-rank test showed an escalation dose of at least 2 Gy tends to improve biochemical recurrence-free survival. However, this tendency did not reach statistical significance (p = 0.2).
Conclusions:
The dose distribution within DILs defined by mpMRI is significantly higher than the whole prostate dose from arc-based IMRT. Escalation doses in DILs tend to improve biochemical recurrence-free survival, further validation in larger patient cohorts with extended follow-up is warranted.
Key Words: Prostate cancer, Dominant intraprostatic lesions, Radiation dose, MRI
Introduction
Prostate cancer is the third most common malignancy in the world, according to the World Health Organization (WHO), 2020 [1]. Based on the latest European Society for Medical Oncology (ESMO) clinical practice guidelines, radical radiotherapy is a treatment option for all risk groups [2]. Intensity-modulated radiotherapy (IMRT) is the standard technique for external beam therapy (EBRT) in localized prostate cancer. Currently, IMRT has been reclassified as a higher-level treatment involving an arc technique that supports the multiple directions of the beam in curved projections to the target called arc-based IMRT. This therapy ensures a high-level of intensity on the target while sparing the adjacent normal tissues [3].
The target of the radiotherapy used in prostate cancer treatment is the whole prostate gland because the cancer tends to present in the form of multifocal tumors [4-6]. A dose-response correlation between the homogenous radiation dose to the entire prostate gland and biochemical control rates has been reported in several randomized studies investigating the dose-escalation regimen [7-10]. Increasing the radiation dose to the whole prostate gland is associated with better results but has higher toxicity consequences including proctitis, cystitis, or impotence [11]. The dose escalation regimen to the whole prostate gland may not therefore be the best way forward and other ways of increasing the dose to the macroscopic lesion is potentially more beneficial. There is considerable evidence to show that local recurrence following radiotherapy occurs mainly at the site of the largest intraprostatic lesion, referred to as the dominant intraprostatic lesion (DIL) [12-15]. This most significant lesion can be identified using multiparametric MRI (mpMRI), which is the main and most effective diagnostic tool for giving precise anatomical information, tumor delineation, and imaging-guided accurate delivery of radiotherapy. A higher dose to the DIL may reduce prostate-specific antigen (PSA) levels and improve local tumor control [12, 16].
Another development in the use of radiotherapy for prostate cancer is hypofractionation. The radiobiologic characteristics of prostate cancer (α/β ratio = 1.5 Gy) support the use of a hypofractionation regimen (>2Gy per fraction) [17, 18]. Several randomized controlled studies have reported effective outcomes of the use of moderate hypo-fractionated regimens in prostate cancer [19-21]. Consequently, a total of 76-78Gy (dose-escalated conventional fractionation) or moderate hypofractionation (60Gy in 3Gy per fraction or 70Gy in 2.5Gy per fraction) is recommended, as described in the European Association of Urology (EAU) guidelines 2023 [22].
IMRT has been used to treat prostate cancer patients in our faculty since 2008, and a hypo-fractionated regimen (70Gy in 2.5Gy per fraction) was implemented in 2012. In 2020, we reported our experience in an international publication [23]. At that time, our treatment focused on the whole prostate without individual concern for the DIL. To build on our previous research we designed this study to evaluate the dose to DIL of prostate cancer patients who were treated with arc-based IMRT with a hypofractionation regimen to the whole prostate gland at that period.
Materials and Methods
Patients
This retrospective, single institution study included patients with histologically confirmed prostatic adenocarcinoma who received radically hypo fractionated arc-based IMRT with a dose of 70Gy in 2.5Gy per fraction to the whole prostate gland from January 2015 to December 2019. The availability of mpMRI images within six months before treatment was a requirement. Patients were excluded from the analysis if they had previously received pelvic EBRT, or had cN1 or cM1 disease, or had no detectable intraprostatic lesion on mpMRI. The Institutional Review Board gave approval to this study at the Faculty of Medicine, Chiang Mai University, with the study code RAD-2565-09083.
Co-registration of Images and Generation of Contours
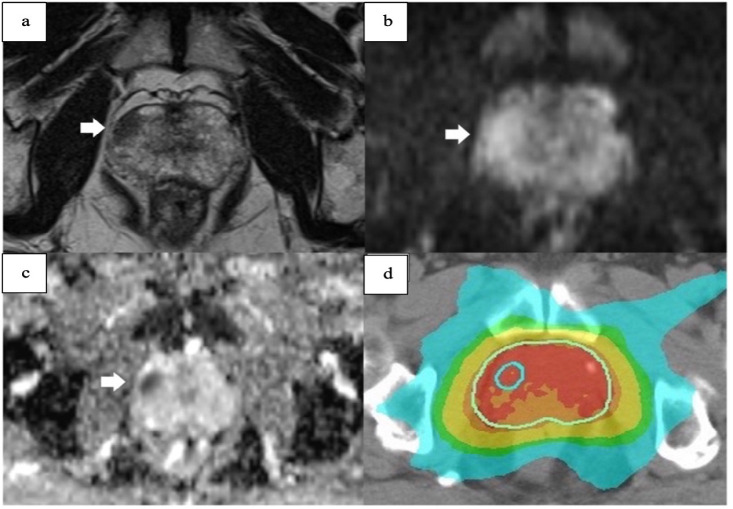
The DILs from mpMRI (T2W, Diffusion Weighted Imaging (DWI), and Dynamic contrast enhanced (DCE)-sequences) were contoured in the prostate gland via direct method (image fusion) or indirect method (image reference) on the Oncentra Contouring Workstation (Elekta AB, Sweden). The treatment plans and DILs for all patients were uploaded to MIM MAESTRO v.6.8.2 (MIM Software Inc., Cleveland, OH) to facilitate the dose evaluation of DILs and other relevant parameters. A summary of the study design and participants is shown as a flow diagram in Figure 1 and 2.
Figure 1.
Flow Diagram of Study Design and Participants. DIL, dominant intraprostatic lesion
Figure 2.
A DIL in a) T2 Weighted Imaging b) Diffusion Weighted Imaging (DWI) c) Apparent diffusion coefficient (ADC) and d) dose distribution of DIL when added to the treatment plan.
Data Analysis and Statistics
The statistical analysis was carried out using Stata 16 software (StataCorp LP, College Station, TX). Categorical variables were presented as frequency and percentage and continuous variables as median and inter-quartile ratio. The imaging dosages to the prostate gland and DIL of each patient were analyzed, and the median calculated doses were compared. Initial prostatic specific antigen (iPSA), prostate volume, PSA density, Gleason score, clinical tumor stage, volume DILs, risk group, age, usage of ADT, dose constraints, and total treatment time, were all recorded. The ASTRO-Phoenix criteria were used to define biochemical recurrence [24]. The Bland-Altman plot and the Kaplan-Meier survival curve were used for graphical representation of the data. A p-value of less than 0.05 was used to denote statistical significance.
Results
Patient Characteristics and Treatment Outcome
Forty-eight total cases were included in this investigation. Patients ranged in age from 59 to 86 years old, with 75 years being the median age. According to the D’Amico classification, the majority of patients (86.7%) had an illness that carried a high risk. Table 1 shows the characteristics of the patients enrolled onto the study.
After a median follow-up period of 41 months (range: 32–52 months), 8.3% (4/48) of the 48 patients met the Phoenix criteria for biochemical failure. In three out of the four patients (75%) with biochemical recurrence, the location of the recurrence was detected using MRI, and in one of these patients (25%), the MRI was not available. Visual analysis of the imaging data revealed that in three of these four patients, the prostate cancer lesion before arc-based IMRT and the advent of biochemical recurrence had a high degree of special overlap. At the time of the last evaluation, 47 out of 48 prostate cancer patients were alive, while one had died.
From the univariate and multivariate analyses, none of the patient-related characteristics recorded such as initial prostatic specific antigen (iPSA), prostate volume, PSA density, Gleason score, clinical tumor stage, volume DILs, risk group, age, usage of ADT, dose constraints, and total treatment time were shown to be clinically significant (Table 2).
Table 2.
Cox-Regression Analyses Considering Biochemical Recurrence of Prostate Cancer
| Patient and treatment-related parameters |
HR (95% CI) |
p-value |
|---|---|---|
| iPSA in ng/ml (< 10, 10–20, > 20) | 3.257 (0.480, 22.121) |
0.227 |
| Gleason score in biopsy (< 7, 7, or > 7) |
0.811 (0.300, 2.194) |
0.68 |
| clinical T stage (2, 3, 4) | 1.468 (0.389, 5.545) |
0.571 |
| Volume DIL-imaging (continuous) | 1.021 (0.897, 1.162) |
0.755 |
| Age in years (continuous) | 1.143 (0.945, 1.383) |
0.169 |
| Usage of ADT (0, 1, 2) | 0.738 (0.240, 2.270) |
0.596 |
| Prescription dose in Gy (continuous) | 1.08 (0.280, 4.165) |
0.911 |
| Arc-based IMRT technique (TOMO, VMAT) |
0.028 (0.000, 85.116) |
0.382 |
Abbreviations: ADT, Androgen Deprivation; DIL, Dominant Intraprostatic Lesion; Gy, Gray; IMRT, Intensity Modulated Radiation Therapy; iPSA, initial Prostate Specific Antigen; VMAT, Volumetric Modulated Arc Therapy
Regarding the side effects of arc-based IMRT in all patients, Grade 2-4 proctitis was recorded in four patients (8.3%) and Grade 0–1 proctitis in 44 patients (91.6%). Grade 2-4 cystitis presented in only one patient (2.1%), and Grade 0–1 cystitis in 47 patients (97.9%).
Impact of Dose Difference
The dose parameters for the targets and normal tissues are shown in Additional Table 1. The data shows that the dose received by patients from the DILs is higher than that received by the whole prostate gland for all individuals. The median dose of DILs was 69.22 (Q1 67.81, Q3 71.66), and the median dose of the whole prostate gland was 67.09 (Q1 66.54, Q3 69.68) indicating that the dose difference was statistically significant (p < 0.001*) according to Wilcoxon Signed-Rank Test (Table 3). The Bland-Altman plot shows a cut-point of 2Gy between DILs and the whole prostate gland (PTV_D98%) (Figure 3).
Table 1.
Patient Characteristics
| Characteristics | |
|---|---|
| Median age in years (range) | 75 (59-86) |
| Median initial PSA in ng/ml (range) | 21.70 (6.86-406.00) |
| Median prostate volume (ml) (range) | 34.79 (11.00-137.20) |
| Median PSAD (ng/ml2) (range) | 0.76 (0.10-16.69) |
| PIRADS, n (%) | |
| 4 | 17 (35.4) |
| 5 | 31 (64.5) |
| Hormonal treatment, n (%) | |
| No | 3 (6.3) |
| STADT | 5 (10.4) |
| LTADT | 40 (83.3) |
| Biopsy Gleason score, n (%) | |
| Up to 6 | 11 (22.9) |
| 7 | 11 (22.9) |
| 8-10 | 26 (54.2) |
| Clinical T stage, n (%) | |
| 2 | 18 (37.5) |
| 3 | 24 (50.0) |
| 4 | 6 (12.5) |
| D’Amico classification, n (%) | |
| Intermediate risk | 7 (14.6) |
| High risk | 41 (85.4) |
| Median, mean volume, ml (range) | |
| Whole Prostate Gland | 49.26 (25.53-181.83) |
| DIL | 3.67 (0.77-27.63) |
| Median total treatment time (months) (range) | 41 (32-52) |
Abbreviations: DIL, Dominant Intraprostatic Lesion; LTADT, Long-term Androgen Deprivation Therapy; PSA, Prostate Specific Antigen; PSAD: Prostate Specific Antigen Density; The prostate volume was measured on mpMRI; STADT, Short-term Androgen Deprivation Therapy.
Table 3.
Wilcoxon Signed-Rank Test of Dose WPG from arc-based IMRT with Dose DIL from MRI-Guidance
| WPG from arc-based IMRT Median |
DIL from MRI-Guidance Median |
p value | |
|---|---|---|---|
| Dose (Gy) | 67.09 | 69.22 | p < 0.001* |
| (66.54, 69.68) | (67.81, 71.66) |
Figure 3.
Bland-Altman Plot of Difference Dose between Prostate Gland from arc-based IMRT and DIL from MRI-Guided Treatment
The Kaplan-Meier survival curve comparison of the log-rank test results showed an escalation dose of at least 2 Gy tends to improve biochemical recurrence-free survival. However, the results did not reach statistical significance (p = 0.2) (Figure 4).
Figure 4.
Kaplan-Meier Curves for Biochemical Recurrence-Free Survival
Discussion
In our study, the median dose to the DILs was 69.22Gy and the median dose to the whole prostate gland was 67.09Gy. Although, we did not intend to treat the DILs at that time, the dose to the DILs was significantly higher than the dose to the whole prostate gland. Comparing the Kaplan-Meier survival curves using the log-rank test revealed that an escalation dose of at least 2Gy at the DILs in comparison to the whole prostate gland dose tended to enhance better biochemical recurrence-free survival. However, the data did not reach statistical significance (p = 0.2) in our study. Similar to the results of a study conducted by Zamboglou and colleagues, it was found that the dose received by the DILs is significantly higher than the dose received by the whole prostate gland [12]. However, when comparing the mean dose received by the DILs with that of the whole prostate gland in terms of its impact on biochemical recurrence-free survival, no statistically significant effect was observed [12]. This might be because of an insufficient number of patients enrolled on the study and also that there was a stronger focus on the whole prostate gland in comparison to the DILs. Further study into the differences in DIL dose is warranted and the dose escalation by simultaneous integrated boost (SIB) to increase the dose to DILs may be the best way to improve the clinical outcome.
Increasing the dose of radiation delivered to the prostate during radical radiotherapy (RT) has been shown to enhance biochemical control [8, 12, 25]. Many studies have reported that an increased dose of up to 80 Gy can be safe and effective [8, 26]. A meta-analysis demonstrated that increasing the total RT dose on the whole prostate by 1 Gy reduces the risk of biochemical failure in patients with primary prostate cancer by approximately 1.8% [25]. On the other hand, however, increasing the dose to the entire prostate is related to an increase in the toxicity to the bladder and the rectum [8, 26, 27]. Although prostate cancer is typically multifocal, the histopathology of prostate cancer typically presents with a larger focus or intraprostatic lesion (DIL). Local recurrence following radical RT occurs predominantly at the DILs, and this leads us to focus on the DILs in the IMRT era.
A randomized study in DIL-focusing treatment has been published. The long-term results of the FLAME trial revealed a 7% increase in biochemical recurrence-free survival (bRFS) for dose escalation at DILs based on mpMRI [28]. A higher dose to the DILs may reduce prostate-specific antigen (PSA) levels and improve local tumor control [12, 16]. However, the precise value of the specific DIL boost varies and is dependent on imaging factors, including technical factors and tumor characteristics. To minimize the variation which may lead to errors and toxic impact, our study involved a diagnostic radiologist who delineated the DILs using 3D matching tools and subsequently performed manual readjustments. To ensure accuracy in measurement multiple steps were identified to ensure the best patient outcome, including optimal imaging, accurate transmission of this information to the planning CT, accurate identification, fiducial placement, and delineation of the lesion, and then delivery of the RT with target recognition. However, our findings must be validated by future studies that include more patients and extended follow-up. In order to prevent underdosing and possibly escalating the RT dose in these areas, routine delineation of the intraprostatic tumor using mpMRI information should be performed before radiotherapy for prostate cancer.
This retrospective investigation has some limitations. The systemic modes of treatment (e.g., ADT duration) are not identical for all patients. Persistent testosterone suppression after ADT may affect PSA levels [29]. According to our study, forty patients (83.3%) had long-term ADT, five patients (10.4%) had short-term ADT and three patients (6.3%) had no ADT. Thus, our findings require validation, preferably through a prospective study. Another drawback is that there was only a very limited number of patients and the arc-based IMRT plan at that time did not focus on DILs.
In conclusions, the median dose to the DILs was 69.22 Gy and the median dose to the whole prostate was 67.09 Gy. Escalation doses in DIL >2Gy (in comparison to the whole gland) showed an increasing trend regarding improvement in biochemical recurrence-free survival. Further investigation into dose-escalated DIL treatment in larger patient cohorts with extended follow-up is warranted.
Acknowledgements
This research is a part of Amonlaya’s PhD thesis. We would like to express our gratitude to all those who supported and guided us throughout this project.
Ethical Standards:
This protocol is exempt from review by the Research Ethics.
Availability of Data
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Committee of Faculty of Medicine, Chiang Mai University. Date of issue: 22 August 2022. This Ethics Committee is organized and operates in accordance with GCPs and relevant international ethical guidelines, the applicable laws and regulations. (Study code: RAD-2565-09083)
Conflicts of interest
We confirm that there are no conflicts of interest associated with this work.
Author Contribution Statement
Amonlaya Amantakul (First author): Conceptualization, methodology, data collection, formal analysis, writing-original draft. Ekkasit Tharavichitkul (Corresponding author): Supervision, writing-review and editing, project administration. Thanat Kanthawang: Participated in the concept and design of the study, data interpretation, supervision. Apichat Tantraworasin: Participated in the concept and design of the study, data interpretation, supervision.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Kanesvaran R, Castro E, Wong A, Fizazi K, Chua MLK, Zhu Y, et al. Pan-asian adapted esmo clinical practice guidelines for the diagnosis, treatment and follow-up of patients with prostate cancer. ESMO Open. 2022;7(4):100518. doi: 10.1016/j.esmoop.2022.100518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehman J, Syed Z, Ahmad N, Khalid M, Noor H, Asghar H, et al. Intensity modulated radiation therapy: A review of current practice and future outlooks. 2020. [Google Scholar]
- 4.Pathmanathan AU, Alexander EJ, Huddart RA, Tree AC. The delineation of intraprostatic boost regions for radiotherapy using multimodality imaging. Future Oncol. 2016;12(21):2495–511. doi: 10.2217/fon-2016-0129. [DOI] [PubMed] [Google Scholar]
- 5.von Eyben FE, Kiljunen T, Kangasmaki A, Kairemo K, von Eyben R, Joensuu T. Radiotherapy boost for the dominant intraprostatic cancer lesion-a systematic review and meta-analysis. Clin Genitourin Cancer. 2016;14(3):189–97. doi: 10.1016/j.clgc.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Cooper CS, Eeles R, Wedge DC, Van Loo P, Gundem G, Alexandrov LB, et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat Genet. 2015;47(4):367–72. doi: 10.1038/ng.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, et al. Long-term results of the m D Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 8.Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, Graham JD, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the mrc rt01 randomised controlled trial. Lancet Oncol. 2014;15(4):464–73. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 9.Zietman AL, Bae K, Slater JD, Shipley WU, Efstathiou JA, Coen JJ, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol. 2010;28(7):1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heemsbergen WD, Al-Mamgani A, Slot A, Dielwart MF, Lebesque JV. Long-term results of the dutch randomized prostate cancer trial: Impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol. 2014;110(1):104–9. doi: 10.1016/j.radonc.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Hatano K, Tohyama N, Kodama T, Okabe N, Sakai M, Konoeda K. Current status of intensity-modulated radiation therapy for prostate cancer: History, clinical results and future directions. Int J Urol. 2019;26(8):775–84. doi: 10.1111/iju.14011. [DOI] [PubMed] [Google Scholar]
- 12.Zamboglou C, Klein CM, Thomann B, Fassbender TF, Rischke HC, Kirste S, et al. The dose distribution in dominant intraprostatic tumour lesions defined by multiparametric mri and psma pet/ct correlates with the outcome in patients treated with primary radiation therapy for prostate cancer. Radiat Oncol. 2018;13(1):65 . doi: 10.1186/s13014-018-1014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arrayeh E, Westphalen AC, Kurhanewicz J, Roach M, Jung AJ, Carroll PR, et al. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal mri and mrsi study. Int J Radiat Oncol Biol Phys. 2012;82(5):e787–93. doi: 10.1016/j.ijrobp.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pucar D, Hricak H, Shukla-Dave A, Kuroiwa K, Drobnjak M, Eastham J, et al. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: Magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007;69(1):62–9. doi: 10.1016/j.ijrobp.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 15.Mendez LC, Ravi A, Chung H, Tseng CL, Wronski M, Paudel M, et al. Pattern of relapse and dose received by the recurrent intraprostatic nodule in low- to intermediate-risk prostate cancer treated with single fraction 19 gy high-dose-rate brachytherapy. Brachytherapy. 2018;17(2):291–7. doi: 10.1016/j.brachy.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Nicholls L, Suh YE, Chapman E, Henderson D, Jones C, Morrison K, et al. Stereotactic radiotherapy with focal boost for intermediate and high-risk prostate cancer: Initial results of the sparc trial. Clin Transl Radiat Oncol. 2020;25:88–93 . doi: 10.1016/j.ctro.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44(3):265–76. doi: 10.1080/02841860410002824. [DOI] [PubMed] [Google Scholar]
- 18.Henry A, Pieters BR, André Siebert F, Hoskin P. Gec-estro acrop prostate brachytherapy guidelines. Radiother Oncol. 2022;167:244–51. doi: 10.1016/j.radonc.2021.12.047. [DOI] [PubMed] [Google Scholar]
- 19.Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized phase iii noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20):2325–32. doi: 10.1200/JCO.2016.67.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aluwini S, Pos F, Schimmel E, Krol S, van der Toorn PP, de Jager H, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (hypro): Late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464–74. doi: 10.1016/S1470-2045(15)00567-7. [DOI] [PubMed] [Google Scholar]
- 21.Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 chhip trial. Lancet Oncol. 2016;17(8):1047–60. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tharavichitkul E, Chakrabandhu S, Klunklin P, Onchan W, Jia-Mahasap B, Meungwong P, et al. Early results of localised, high-risk prostate cancer treated by moderate hypo-fractionation (70 gy at 2· 5 gy per fraction): 5-year experiences of a moderate hypo-fractionation regimen. Journal of radiotherapy in practice. 2020 ;19(3):233–6. [Google Scholar]
- 24.Roach M, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the rtog-astro phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: A meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74(5):1405–18. doi: 10.1016/j.ijrobp.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 26.Beckendorf V, Guerif S, Le Prisé E, Cosset JM, Bougnoux A, Chauvet B, et al. 70 gy versus 80 gy in localized prostate cancer: 5-year results of getug 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80(4):1056–63. doi: 10.1016/j.ijrobp.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 27.Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: First results from the mrc rt01 randomised controlled trial. Lancet Oncol. 2007;8(6):475–87. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 28.Kerkmeijer LGW, Groen VH, Pos FJ, Haustermans K, Monninkhof EM, Smeenk RJ, et al. Focal boost to the intraprostatic tumor in external beam radiotherapy for patients with localized prostate cancer: Results from the flame randomized phase iii trial. J Clin Oncol. 2021;39(7):787–96. doi: 10.1200/JCO.20.02873. [DOI] [PubMed] [Google Scholar]
- 29.D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Interval to testosterone recovery after hormonal therapy for prostate cancer and risk of death. Int J Radiat Oncol Biol Phys. 2009;75(1):10–5. doi: 10.1016/j.ijrobp.2008.10.082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Committee of Faculty of Medicine, Chiang Mai University. Date of issue: 22 August 2022. This Ethics Committee is organized and operates in accordance with GCPs and relevant international ethical guidelines, the applicable laws and regulations. (Study code: RAD-2565-09083)