Abstract
Objective:
Our study aimed to establish a standardized methodology for selecting “reference” and “evaluated” distributions in gamma analysis for Monte Carlo (MC) based intensity modulated treatment plans. Evaluation of importance of reference selection in MC based and non MC based treatment planning systems were analysed using a study classification.
Methods:
Three categories were utilized to analyzed gamma passing rates across using different treatment planning systems (TPS) and detectors for thirty five patients. Category 1 utilized MC-based Monaco TPS plans and a 2 dimensional(2D) I’mRTMatriXX detector. Category 2 employed non-MC-based Eclipse TPS plans, assessed with a 2D I’mRTMatriXX detector. In Category 3, MC-based Monaco TPS plans were validated using a Dolphin detector. All plans were subjected to analysis using gamma criteria, which considered a dose difference of 3% and a distance to agreement of 3mm. Additionally, another set of gamma criteria was employed, with a dose difference of 3% and a distance to agreement of 2mm. An introduced Asymmetric factors in both 2D and 3D analysis will quantify the asymmetric nature of gamma based on the choice of “reference” distribution.
Result:
For 2D Gamma analysis, MC-based Monaco TPS and I’mRTMatriXX showed a consistent positive Zk2D trend for all patients, with significant p-values below 0.01 for both gamma passing criteria. In contrast, non-MC based Eclipse TPS exhibited varied Zk2D results, with non-significant p-values. In 3D Gamma analysis, all patients exhibited positive Zk3D values with significant p-values below 0.01 when “references” were swapped. The Pearson correlation between asymmetricity and isodose volumes was notably high at 0.99 for both gamma criteria.
Conclusion:
Our study highlights the imperative of using MC-based TPS as the definitive “reference” in gamma analysis for patient specific quality assurance of intensity modulated radiation therapy, emphasizing that variations can mislead results, especially given gamma analysis’s sensitivity to MC calculation noise.
Key Words: Intensity Modulated Radiation Therapy, Monte Carlo, Patient specific QA, Gamma, Asymmetric factor
Introduction
The latest intensity-modulated radiation therapy (IMRT) modality has been demonstrated to provide superior target coverage while minimizing adverse effects on normal tissues compared with other treatment methods [1-9]. This is achieved by utilizing a computerized inverse planning technique that generates small subfields within each field, optimizing the dose to the tumor while minimizing the dose to the normal structures. The creation of a steep dose gradient in the vicinity of the critical organs was also achieved. The successful development of a pronounced dose gradient near critical organs involves rapidly varying the radiation dose levels around these normal structures, resulting in a shift from high to low doses. The optimizer then adjusts beam intensities to create an optimized desired dose distribution, resulting in a composite treatment plan. Given the inherently unpredictable nature of variable-intensity patterns, which are subject to dynamically adjusted radiation intensity across the diverse regions of the treatment area, it is of utmost importance to perform patient-specific quality assurance (PSQA) verification prior to treatment in order to guarantee accuracy [10, 11]. There are several methods for evaluating the quality of IMRT plans, including point dose comparison, isodose comparison, distance-to-agreement (DTA) analysis, dose difference analysis, and gamma analysis. Among these methods, gamma analysis is the most successful in pretreatment quality assurance, as it addresses the limitations of other existing methods. Gamma analysis involves calculating the minimum distance between two dose distributions in a Euclidean space of dose and distance, normalized by the dose difference and DTA. Thus gamma analysis providing a comprehensive assessment of treatment plan accuracy by considering both dose differences and spatial agreement. Here, we determined the percentage of points within the dose distribution with gamma values of less than one. Gamma analysis allows for the comparison of dose distributions in a dimensionless space, as reported in several studies [12-14 ].
The methods for assessing the planar dose measurements for the PSQA involve the use of detectors, including the Electronic Portal Imaging Device (EPID), Film, Map CHECK, Gel, I’mRTMatriXX, Dolphin, and customized devices developed at various centers [15-24]. Advancements in detector and software technology for gamma analysis make it possible to reconstruct doses in three-dimensional (3D) patient Computed Tomography (CT) images using the measured dose from a 2D detector array and compare it with the dose from the treatment planning system (TPS). Additionally, these techniques allow for the comparison of dose-volume parameters for normal structures and Planning Target Volumes (PTV) [25].
Gamma analysis was performed between two dose distributions: “reference” and “evaluated”. The selection of the “reference” and “evaluated” distributions in the gamma analysis is crucial because of the asymmetric nature of the gamma. Low et al. suggested that when comparing a TPS dose with a measured dose, the measured dose should be used as the “reference” and the TPS-calculated dose as the “evaluated” distributions. For the verification of Monte Carlo (MC) calculations, the MC-calculated dose distribution is recommended as the “reference” [26]. However in the modern TPS, treatment plans are created using MC-based algorithms. In this context, when utilizing the gamma tool to validate dose distributions computed with the Monte Carlo algorithm, it remains ambiguous as to which dose distribution should be designated as the “reference”. Additionally, the gamma analysis software like Omnipro and Compass from IBA dosymetry system provides the option to use either planned or measured doses as the “reference”. To address this concern, our study sought to define a standard methodology for selecting “reference” and “evaluated” distributions in PSQA for MC based treatment plans. We then assessed the impact of these discrepancies on gamma passing rate of 3%2mm gamma and a much tighter gamma criteria suggested by TG 218 [27].
Materials
The study was primarily divided into three categories by analyzing the gamma passing rate differences between treatment plans produced by various TPS and measurements taken with different detectors. Category 1 involved the analysis of gamma passing rate between plans created using MC based TPS with a 2D detector array. Category 2 pertained to the analysis of passing rate between the plans generated by using a non-MC-based TPS with a 2D detector array. Finally, Category 3 refers to the analysis of gamma passing rate between plans created by an MC-based TPS with a 2D detector array which enables reconstruction of dose in 3 dimensions (3D). The gamma passing rate was determined using a dose difference of 3% and a DTA of 3mm (3%, 3mm) criterion, with a passing threshold of 95% and , and also at a dose difference of 3% and a DTA of 2mm (3%, 2mm) criterion with a passing threshold of 90% for each patient.
For the PSQA analysis we retrospectively selected thirty five patient plans among which twenty patients where analysed using MC based TPS in 3D, fifteen patients each were analysed, using non MC and MC based TPS in 2D analysis. Out of these, fifteen patients were chosen commonly in both the MC based analysis in two and three dimensions. All plans based on Monte Carlo simulations were generated with a calculation uncertainty of 1%. All the patients underwent radiotherapy using intensity-modulated treatment technique for early stage head and neck squamous cell carcinoma of tongue with a prescribed simultaneous integrated boost dose of 60Gy to high risk and 54Gy to low risk regions in 30 fractions.
Category 1: (2D Gamma analysis of MC based TPS plans)
It included fifteen intensity modulated radiation treatment plans that were generated using MC-based Elekta Monaco TPS at version 5.51 and using 6MV photon energy. Dose computations were performed using the X-ray Voxel MC (XVMC) algorithm in Somatom go-up Computed Tomography (CT) slices of patients with a slice thickness of 2.5 mm. from Siemens Healthineers.
The dose calculation grid size was set to 2 mm in the TPS and PSQA plans corresponding to each patient were created using CT images of I’mRTMatriXX (IBA) with a slice width of 3 mm. I’mRTMatriXX is a planar detector array consisting of 1020 vented-to-air parallel plate ionization chambers arranged in a 32 cm x 32 cm matrix, which are spaced 7 mm apart and have an inherent buildup of 4 mm. Each detector had a size of 4.5 mm x 5 mm and a volume of 0.08 cm^3. The dose distributions in PSQA plans, generated using the Perpendicular Composite (PC) method with the treatment unit’s gantry, collimator, and couch rotation angles set to zero degrees, were exported to OmniPro software version 1.7.0021, and the plan was sent to an Elekta Versa HD linear accelerator for verification. The Elekta Versa HD Linear Accelerator has been fitted with 80 pairs of Agility MLC with a width of 0.5 cm at the isocenter for segment creation, utilizing 6MV photon energy for the delivery of IMRT treatment plans. To measure the dose distribution, I’mRTMatriXX was set up on the treatment couch with the help of lateral laser beams lined up along the lateral grooves of the device at the level of the active volume of the detector array. The crosshair in the sensor area was aligned with the shadow of the light field crosshair at a zero-degree gantry angle. Thus the array of chambers set up at the isocentre of the LINAC.Solid water slabs were placed above and below I’mRTMatriXX to achieve a total buildup and backscatter of 5 cm, providing full scatter conditions. The experimental setup is as shown in Figure 1.
Figure 1.

Measurement Setup with I’mRT MatrixXX with 5 cm of Buildup and Back Scatter. The lateral laser beams are lined up along the lateral grooves on I’mRT MatrixXX.
A radiation bath of 500 monitor units was delivered to the phantom with a field size of 24 cm x 24 cm, and correction for background was performed before data acquisition. The verification plans were executed, and the acquired dose distributions were saved. The resolution for both the measured doses and those from the TPS was standardized to a grid size of 2 mm. Notably, the software imposes no restrictions on choosing the “reference” distribution for gamma analyses. First, the gamma analyses were carried out by keeping the MC based TPS calculated dose as the “reference” and the measured dose as the “evaluated” distributions (γtps), and the gamma passing rate was obtained for each patient at dose passing criteria of 3%, 3 mm, and 3%, 2 mm. The gamma search area was confined to the area encompassing 10% isodose line. To check for asymmetry, a second set of matching gamma analyses (γm) was conducted by swapping the “reference” and “evaluated” distributions. To account for the inherent asymmetry of the gamma tool, we introduce a metric quantifying the difference in gamma in 2D, denoted as Δ γ(2D).
Δ γ(2D) = γm - γtps (Equation 1)
However Δ γ(2D) will be a patient specific value as it is a function of degree of modulation of treatment plan and hence, to rule out its dependence in analyses we introduced an asymmetric factor (Zk2D) which is obtained by dividing Δ γ(2D) by the respective gamma obtained by keeping MC based TPS dose as “reference” distribution (γtps)
(Equation 2)
Hypothesis testing between the two analysis methods, performed by swapping the “reference”, was conducted separately for the passing criteria of 3%, 3mm and 3%, 2mm using the Student’s t-test assuming equal variance. The null hypothesis states that swapping the “reference” does not have any effect.
Category 2:(2D Gamma analysis of non MC based TPS plans)
Fifteen clinically acceptable intensity modulated radiation treatment plans utilizing 6MV photon energy were produced using the non-MC-based Eclipse at version 10 TPS. For each patient, PSQA dose computations were performed on the CT slices of the I’mRTMatriXX phantom using the Anisotropic Analytical Algorithm (AAA). These slices had a thickness of 2.5 mm, and the dose calculation within the TPS was set to a grid size of 2 mm. The PSQA plans were then sent to a Varian Clinac iX linear accelerator for measurements. The Clinac iX Linear Accelerator equipped with 60 pairs of Millennium MLCs, with the central 20 cm providing a width of 0.5 cm at the isocenter and the remaining 10 cm providing a width of 1 cm at the isocenter with 6MV photon energy for the delivery of intensity modulated radiation. The measurement setup used was identical to that in Category 1. Additionally, gamma analyses were conducted for all patients by comparing the Varian Eclipse-calculated dose and the corresponding measured dose from the Varian Clinac iX linac. In this analysis, the difference in gamma value obtained by keeping the TPS calculated dose the “reference” dose, and the measured dose as the “evaluated” distributions is defined as γtps, and the corresponding difference in vice versa analyses is defined as γm. Δγ(2D) and Zk2D were also determined here using equations 1 and 2 as outlined in Category 1. Just as in Category 1, hypothesis testing between the two analysis methods for two gamma passing criteria 3%, 3mm and 3%, 2mm, achieved by swapping the “reference”, was conducted using the Student’s t-test, assuming equal variance.
Category 3: (3D Gamma analysis of MC based TPS plans)
Twenty intensity modulated radiation treatment plans, produced using the MC-based Monaco TPS, were validated using a Dolphin detector array (IBA Dosimetry, Germany). The verification was done with Compass software at version 4.1 on a Versa-HD linear accelerator. The detector array comprises of a 2D transmission detector with 1513 parallel-plate ionization chambers and one diode on the side. Each chamber has a diameter of 3.2 mm, a height of 2 mm, and a volume of 16 mm3. The active area of the detector array is 24 cm x 24 cm. The spatial resolution of the detector is 5 mm for the field area within 14 cm x 14 cm and 10 mm for the field area greater than 14 cm x 14 cm. The dolphin detector system can be mounted on the LINAC head with a source-to-detector distance of 60 cm and is capable of online or offline measurements. In this category, measurements were taken by attaching the Dolphin detector assembly to the gantry head, as illustrated in Figure 2 and the analysis were conducted offline.
Figure 2.

Dolphin Detector Array Attached to the Gantry Head of Versa-HD for 3D Gamma Analysis.
The gantry and collimator angles adhered to those specified in the clinically approved treatment plan. Unlike the above two methods, the treatment plans, including the patient’s CT and corresponding structure set, were exported from the MC based Monaco TPS with a calculation grid size of 2 mm to the COMPASS system in the Digital Imaging and Communications in Medicine (DICOM) format. The software then “reconstructed” the 3D dose in the patient’s CT using measured dose distribution. It compares this reconstructed dose to the dose from the Monaco TPS, which uses MC based calculation, ensuring that the grid size is consistent with the one employed in the TPS. The COMPASS software allows users to determine the 3D gamma between the TPS dose and the reconstructed dose in the patient’s CT images [28,29]. The software also provides the option to select either the TPS-calculated dose or the measured dose as the “reference” distribution. Gamma analyses were performed by comparing the planned and reconstructed dose distributions for each patient.
The asymmetric nature of the gamma was evaluated by conducting gamma analyses in two ways: one with the TPS-calculated dose as the “reference” and the other with the 3D reconstructed dose as the “reference” [30]. This is similar to the analysis method performed in 2D. Gamma analyses were performed using dose difference criteria of 3% and DTA criteria of 3 mm, as well as that with 3% and 2 mm, within the 10% isodose volume (referred to for convenience as the “Z” patient) and encompassing the entire patient contour.
Gamma passing percentage obtained by keeping reconstructed dose as “reference” is defined as γm and that obtained by keeping MC based TPS dose as “reference” as γtps. The difference in gamma for 3D analysis denoted as Δγ(3D) is determined by the formula:
Δγ(3D) = γm - γtps Equation 3
Also, the corresponding asymmetric factor, Zk3D is calculated by:
Zk3D = Δ γ(3D) / γtps x 100 % Equation 4
The parameter Zk3D is affected by the inherent noise within the MC-based TPS, which correlates with the amount of patient volume under examination. In order to correlate Zk3D with the volume designated for gamma searching, we computed Zk3D for 20 patients using gamma passing criteria of 3%3 mm and 3% 2 mm. Gamma searching areas were delineated within the volume of isodose lines at increments of 5%, 10%, 20%, 30%, 40%, 50%, 60%, and 70% of the maximum prescription dose. Additionally, the entire patient volume was considered, which equates to a volume encompassing 0% of the prescribed dose. Pearson correlation coefficient is find out to understand the nature of correlation. In this part of the study, a total of 720 gamma values were analyzed.
In all three categories, the proposed study showed negligible or no impact related to the use of different Linear Accelerators. To aid clarity in perceiving the methods adopted in this study, the study stratification is demonstrated in the form of a flow chart in Figure 3.
Figure 3.
Flow Chart of Demonstrating the Stratification of the Study Primarily in to Three Categories.
Results
Category 1 and Category 2 (2D Gamma analysis)
The gamma passing rate 3%, 3mm and 3%, 2mm is measured with Monaco TPS versus I’mRTMatriXX and Eclipse TPS versus I’mRTMatriXX with “reference” swapping of TPS and measured dose. The Zk2D between the two gamma values shows a positive trend for all the patients in the case of MC based MONACO TPS and I’mRTMatriXX, and the corresponding value ranged from 0.2 to 5.69 and 0.2 to 10.4 for 3%3mm and 3%2mm respectively. In the case of non MC based Eclipse TPS and I’mRTMatriXX , Zk2D values shows a mix of positive and negative values which ranged from -3.04 to +3.14 and -10.02 to +7.93 for 3%3mm and 3%2mm respectively. P-values below 0.01 were observed when comparing the methods involving “reference” swapping for both gamma passing criteria of 3%, 3mm and 3%, 2mm, using MC-based Monaco TPS and I’mRTMatriXX measurements. Conversely, when comparing “reference” swapped gamma sets for non-MC based Eclipse TPS doses with I’mRTMatriXX, p-values of 0.264 and 0.061 were noted for the 3%3mm and 3%2mm gamma analysis criteria, respectively. The summary of the results are tabulated in Table 1.
Table 1.
Asymmetric Factor for Category 1 and Category 2 for 3%3mm and 3%2mm Gamma Passing Criteria
| Sr No | Zk2D | |||
|---|---|---|---|---|
| Monaco | Eclipse | |||
| 3%3mm | 3%2mm | 3%3mm | 3%2mm | |
| 1 | 5.69 | 7.4 | -2.09 | -1.34 |
| 2 | 3.88 | 7 | -0.46 | -4.31 |
| 3 | 3.87 | 5.5 | -1.91 | -5.99 |
| 4 | 3.34 | 5.1 | 0.78 | -0.17 |
| 5 | 2.17 | 3 | -1.41 | 0.96 |
| 6 | 0.31 | 4.1 | -3.04 | -8.98 |
| 7 | 2.37 | 4.5 | 3.14 | 1.42 |
| 8 | 1.63 | 3.5 | -2.57 | -6.9 |
| 9 | 0.2 | 0.2 | -2.88 | -10.02 |
| 10 | 0.81 | 2.3 | -1.75 | -6.57 |
| 11 | 5.25 | 7.2 | -0.15 | 1.03 |
| 12 | 4.97 | 10.4 | 1.23 | -0.8 |
| 13 | 2.38 | 4 | 1.89 | -0.1 |
| 14 | 0.7 | 2.1 | 1.17 | 7.93 |
| 15 | 0.91 | 4 | 0.01 | -2.63 |
| Avg ± Std deviation | 2.57±1.84 | 4.69±2.54 | -0.54±1.89 | -2.43±4.73 |
| p value | < 0.01 | < 0.01 | 0.264 | 0.061 |
Category 3: (3D Gamma analysis)
The Zk3D values calculated were positive for all patients. For the ‘Z’ patient volumes, they ranged from 0.76 to 3.47 for 3%3mm and from 0.87 to 4.15 for 3%2mm and in contrast, for the entire patient volumes, the values ranged from 0.25 to 1.09 for 3%, 3mm and from 0.33 to 1.24 for 3%, 2mm among the patient cohort. For both the ‘Z patient’ and the entire patient volume, and for the gamma passing criteria of 3%, 3mm and 3%, 2mm, the p-values were below 0.01 when “references” were swapped. The summary of the results are tabulated in Table 2.
Table 2.
Asymmetric Factor for “Z”Patient (10% Isodose volume) and Entire Patient (0% Isodose Volume) for 3%3mm and 3%2mm Gamma Passing Criteria.
| Sr No | "Z"patient(Zk3D) | Patient(Zk3D) | ||
|---|---|---|---|---|
| 3%3mm | 3%2mm | 3%3mm | 3%2mm | |
| 1 | 1.63 | 2.1 | 0.54 | 0.68 |
| 2 | 3.26 | 4.15 | 0.73 | 0.9 |
| 3 | 2.25 | 2.8 | 0.49 | 0.59 |
| 4 | 3.12 | 3.75 | 1.04 | 1.24 |
| 5 | 1.35 | 1.94 | 0.29 | 0.41 |
| 6 | 2.54 | 3.45 | 0.58 | 0.78 |
| 7 | 2.33 | 3.2 | 0.76 | 1.02 |
| 8 | 1.57 | 2.2 | 0.47 | 0.66 |
| 9 | 1.75 | 2.12 | 0.54 | 0.65 |
| 10 | 1.89 | 2.5 | 0.7 | 0.93 |
| 11 | 1.23 | 1.57 | 0.27 | 0.36 |
| 12 | 3.33 | 3.86 | 0.96 | 1.13 |
| 13 | 3.01 | 3.3 | 1.09 | 1.18 |
| 14 | 2.49 | 3.28 | 0.73 | 0.94 |
| 15 | 1.63 | 1.98 | 0.68 | 0.81 |
| 16 | 1.34 | 1.73 | 0.39 | 0.49 |
| 17 | 0.76 | 0.87 | 0.25 | 0.33 |
| 18 | 2.58 | 2.79 | 0.79 | 0.91 |
| 19 | 2.82 | 3.1 | 0.82 | 0.9 |
| 20 | 3.47 | 3.52 | 1.06 | 1.08 |
| Avg ± Std deviation | 2.22±0.79 | 2.71±0.87 | 0.66±0.25 | 0.80±0.27 |
| p value | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
Quantification of asymmetricity of gamma: The average Zk3D values for each volume are detailed in Table 3, and they are also visually represented in Figure 4. Additionally, the absolute Pearson correlation coefficient between the asymmetric factor and isodose volumes was calculated, yielding a correlation coefficient of 0.99 for both 3%, 3mm and 3%, 2mm criteria.
Table 3.
Presenting Data Comparing Two Zk3D Methods (3%3mm and 3%2mm) at Different Search Volume Percentages
| Search Volume | Zk3D (3%3mm) | Zk3D(3%2mm) |
|---|---|---|
| Entire Patient | 0.66 | 0.8 |
| 5% | 1.8 | 2.21 |
| 10% | 2.22 | 2.71 |
| 20% | 2.84 | 3.44 |
| 30% | 3.39 | 4.08 |
| 40% | 3.91 | 4.65 |
| 50% | 4.29 | 5.17 |
| 60% | 4.69 | 5.64 |
| 70% | 5.29 | 6.22 |
Figure 4.
Qualitative Variation of Average Asymmetric Factor in 3D with Response to Changes in the Gamma Search Volume.
Discussion
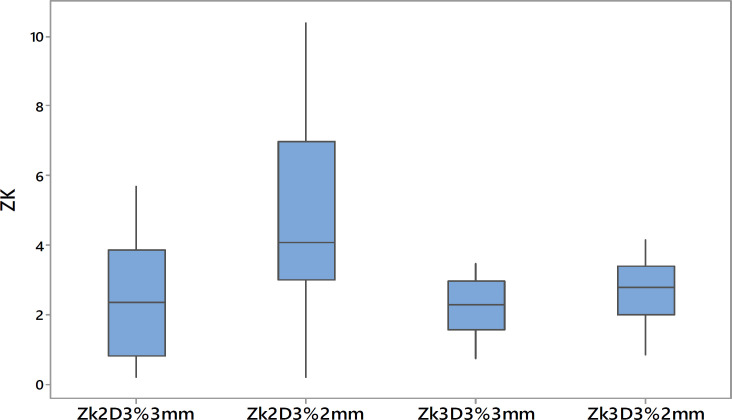
The Gamma analysis tool, which is used to compare “reference” and “evaluated” dose distributions in clinical settings, often overlooks the impact of asymmetric selection between these distributions. Typically, the “reference” distribution, which should ideally be the measured dose as recommended by Low et al., is smaller or equal in dimension to the “evaluated” distributions [26]. However, many commercial software programs do not enforce this, leading to inconsistencies in gamma analysis. The impact is often overlooked in clinical settings due to prevailing institutional protocols for gamma analysis, where a threshold gamma value of 95% is typically set for the 3%3mm passing criteria. Our study underscores the importance of proper “reference” selection, especially in MC based TPS, like the Monaco planning system. We used I’mRTMatriXX and dolphin detector arrays for measurements and introduced an asymmetric factor to refine gamma analysis by minimizing the influence of plan-specific uncertainties. When the measured dose was set as the “reference,” the asymmetric factor consistently showed positive values, indicating the impact of noise, especially under tighter gamma criteria like 3%, 2mm. Furthermore, the Student’s t-test results confirmed the difference between the two approaches, as evidenced by the gamma values derived from both “reference” selections having p-values less than 0.01. The average asymmetric factor obtained are 2.57 for 3%3mm and 4.69 for 3%2mm and indicates the impact of noise in gamma analysis and is well highlighted in tighter gamma criteria of 3%, 2mm.Comparatively, in non-MC-based TPS like Eclipse, which uses the AAA, the asymmetric factor varied, suggesting that the choice of “reference” in gamma analysis is less critical. The presence of both positive and negative values for the asymmetric factor, Zk2D, implies that gamma analysis with this combination remains unaffected by the choice of “reference”. Furthermore, the p-values from the Student’s t-test value 0.26 for 3%3mm and 0.06 for 3%2mm which suggest that the methods of “reference” selection have no significant influence on the gamma analysis of plans produced by the non-MC based TPS. This finding highlights the sensitivity of gamma function to inherent noise in MC-calculated dose distributions. Our results also show that for MC-based TPS, using the TPS calculated dose as the “reference” is crucial to avoid underestimating gamma values, a point corroborated by Low et al.[26]. In 3D analysis, the trend is similar, with the magnitude of the average Zk3D value being lower than Zk2D, which aligns with previous studies showing better passing rates in 3D gamma analysis [31, 32]. A box plot in Figure 5 visually represents the spread of the asymmetric factor in both 2D and 3D analyses, highlighting these findings.
Figure 5.
Boxplots Show Spread of 2D and 3D Asymmetry Factors for 3% 3mm and 3% 2mm Gamma Criteria
In 2D and 3D gamma analysis the impact of MC based TPS on “reference” selection becomes more pronounced when tighter gamma criteria of 3% 2mm is set as the gamma passing criteria. This aligns with the gamma passing criteria recommended in TG 218 protocol [27]. From the study it is recommended to use MC based TPS as the “reference” for 3D gamma analysis. From the able 2 results shows that the calculated distribution as the “reference “ against MC based TPS positive Zk3D shows the under estimation of gamma value when MC based TPS is used as “evaluated” distribution. The Low wt.al [26] recommendation is valid for 3D gamma analysis also. Furthermore, it ought to be noted that MC-based Monaco calculations typically account for the fine structure and non-homogeneity of the dose distribution [32]. This suggests that the TPS dose distribution may possess more intricate features than the measured one. Therefore, it is appropriate to employ the dose distribution that captures such minute details as the “reference” in gamma analysis.
Having provision to measure the extent of variation of Zk3D with respect to the search volume in COMPASS software an additional sets of Δγ3D and Zk3D was calculated within the entire patient volume and in incremental volumes of patient covered within 70%, 60%, 50%, 40%, 30%, 20%, 10% and 5% of the prescription dose. Within the entire patient volume, the results exhibit a similar trend to that observed in the “Z” patient volume, however presenting lower Zk3D values of 0.66 and 0.8 for the 3%, 3mm and 3%, 2mm criteria, respectively. The results suggest that as the search volume for gamma analysis increases in MC-based plans, there’s a noticeable reduction in noise and its associated impact. The findings are consistent with those presented by Low et al., suggesting that the reduction in gamma value evaluation is linearly proportional to noise [14]. The decrease in gamma search volume led to a higher average Zk3D, highlighting the significant noise impact when the analysis volume is reduced. The present findings are supported by prior research, which has demonstrated that considering a volume of interest rather than a point dose in Monaco TPS can result in a reduction of the MC noise impact [33]. This suggests that smaller volumes intensify the noise effects inherent in MC-based dose distributions when designated as the “evaluated” distribution. This was substantiated by a Pearson correlation coefficient value of 0.99 for both 3% 3mm and 3% 2mm gamma criteria.
Thus gamma passing percentage has implicit dependence on the choice of “reference”, and extent of gamma search volumes. With the implementation of TG-218 protocol and popularity of MC based TPS the choice of “reference” for gamma analysis becomes crucial and excellent results can be derived by placing TPS dose generated using MC algorithm as “reference” and measured dose as “evaluated” distributions. However, the use of conventional criteria of 3%, 3mm will not have much effect on the gamma passing percentage in clinics when the “reference” selections are done randomly, even if the approaches are statistically significant. The variation can also go unnoticed because of the implementation of exceedingly liberal institutional thresholds for gamma passing percentages.
In conclusions, with the rising adoption of commercial MC-based TPS for dose calculations in clinics and the TG-218 protocol’s application to intensity modulated radiotherapy, selecting the most trustworthy methods and appropriate analyzing tools is paramount. Our study on gamma analysis emphasizes that when using MC-based TPS for dose calculations, the “reference” distribution should strictly be the MC-based TPS dose. Failing to do so might lead to underestimations of gamma values. This discrepancy can erroneously classify a failing plan as passing in IMRT QA results. Therefore, when crafting IMRT QA protocols, it’s crucial to recognize the sensitivity of gamma analysis. Specifically, its results are directly influenced by the inherent noise present in MC calculations.
Author Contribution Statement
All authors contributed equally in this study.
Acknowledgements
Ethical approval
Informed consent was obtained from the patient as per the institution (MVR Cancer Centre and Research Institute, Kozhikode 693601, India) protocol.
Conflict of interest
Authors declare that they have no conflict of interest.
References
- 1.Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (parsport): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du T, Xiao J, Qiu Z, Wu K. The effectiveness of intensity-modulated radiation therapy versus 2d-rt for the treatment of nasopharyngeal carcinoma: A systematic review and meta-analysis. PLoS One. 2019;14(7):e0219611. doi: 10.1371/journal.pone.0219611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne M, Archibald-Heeren B, Hu Y, Fong A, Chong L, Teh A. Comparison of semiautomated tangential vmat with 3dcrt for breast or chest wall and regional nodes. J Appl Clin Med Phys. 2018;19(5):684–93. doi: 10.1002/acm2.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moningi S, Ajani JA, Badgwell BD, Murphy MB, Ikoma N, Mansfield PF, et al. Imrt reduces acute toxicity in patients treated with preoperative chemoradiation for gastric cancer. Adv Radiat Oncol. 2020;5(3):369–76. doi: 10.1016/j.adro.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ismael Dk, Hassan Ff. 3d-conformal radiation therapy and intensity-modulated radiation therapy techniques for laryngeal cancer taking parotid glands as organ at risk. Hospital practices and research. 2020;5(1):10–6. [Google Scholar]
- 6.Kaur H, Thakur N, Sharma R, Sudan M, Jain N, Kaur S, et al. Dosimetric comparison between carotid-sparing imrt and 3dcrt in early glottic cancer patients treated with definitive radiation therapy. J Cancer Res Ther. 2024;20(1):327–32. doi: 10.4103/jcrt.jcrt_1912_22. [DOI] [PubMed] [Google Scholar]
- 7.Prunaretty J, Bourgier C, Gourgou S, Lemanski C, Azria D, Fenoglietto P. Different meaning of the mean heart dose between 3d-crt and imrt for breast cancer radiotherapy. Front Oncol. 2022;12:1066915. doi: 10.3389/fonc.2022.1066915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnapriya P, Sivanandan CD, Roshni S, Sarin B, Geethi MH, Jagathnath Krishna KM. Dosimetric comparison of 3dcrt and imrt in radical chemoradiotherapy of squamous cell carcinoma esophagus. J Cancer Res Ther. 2023;19(7):1844–51. doi: 10.4103/jcrt.jcrt_1664_21. [DOI] [PubMed] [Google Scholar]
- 9.Salim N, Popodko A, Tumanova K, Stolbovoy A, Lagkueva I, Ragimov V. Cardiac dose in the treatment of synchronous bilateral breast cancer patients between three different radiotherapy techniques (vmat, imrt, and 3d crt) Discov Oncol. 2023;14(1):29 . doi: 10.1007/s12672-023-00636-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho B. Intensity-modulated radiation therapy: A review with a physics perspective. Radiat Oncol J. 2018;36(1):1–10. doi: 10.3857/roj.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syed Z, Rehman J, Khan M, Ahmad N, Gilani Z, Nasar G, et al. Quality assurance of intensity modulated radiation therapy treatment planning using head and neck phantom. Journal of Radiotherapy in Practice. 2019;18:1–7. [Google Scholar]
- 12.Low DA. Quality assurance of intensity-modulated radiotherapy. Semin Radiat Oncol. 2002;12(3):219–28. doi: 10.1053/srao.2002.33700. [DOI] [PubMed] [Google Scholar]
- 13.Low DA, Harms WB, Mutic S, Purdy JA. A technique for the quantitative evaluation of dose distributions. Med Phys. 1998;25(5):656–61. doi: 10.1118/1.598248. [DOI] [PubMed] [Google Scholar]
- 14.Low DA. Gamma dose distribution evaluation tool. InJournal of Physics: Conference Series. 2010 Nov;:250 . [Google Scholar]
- 15.Zhang J, Li X, Lu M, Zhang Q, Zhang X, Yang R, et al. A method for in vivo treatment verification of imrt and vmat based on electronic portal imaging device. Radiat Oncol. 2021;16(1):232 . doi: 10.1186/s13014-021-01953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.M M, V J, O PG. Clinical experience of intensity modulated radiotherapy pre-treatment quality assurance for carcinoma head and neck patients with epid and imatrixx in rural center. J Biomed Phys Eng. 2020;10(6):691–8. doi: 10.31661/jbpe.v0i0.2004-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan Y, Yang R, Zhang S, Li J, Dai J, Wang J, et al. National survey of patient specific imrt quality assurance in china. Radiat Oncol. 2019;14(1):69 . doi: 10.1186/s13014-019-1273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bramoulle C, Cortina S, Romain B, Husson F. Epid-based pretreatment quality assurance: Dosimetric evaluation of application software. Physica Medica. 2017;44:34. [Google Scholar]
- 19.Silveira MA, Pavoni JF, Baffa O. Three-dimensional quality assurance of imrt prostate plans using gel dosimetry. Phys Med. 2017;34:1–6. doi: 10.1016/j.ejmp.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Chung JB, Kang SW, Eom KY, Song C, Choi KS, Suh TS. Comparison of dosimetric performance among commercial quality assurance systems for verifying pretreatment plans of stereotactic body radiotherapy using flattening-filter-free beams. J Korean Med Sci. 2016;31(11):1742–8. doi: 10.3346/jkms.2016.31.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaros D, Kolarevic G, Kostovski A, Savanovic M, Cazic D, Marosevic G, et al. Evaluation of patient specific quality assurance of gated field in field radiation therapy technique using two-dimensional detector array. J Health Sci. 2020;10(2):109–14. [Google Scholar]
- 22.Kadoya N, Abe K, Nemoto H, Sato K, Ieko Y, Ito K, et al. Evaluation of a 3d-printed heterogeneous anthropomorphic head and neck phantom for patient-specific quality assurance in intensity-modulated radiation therapy. Radiol Phys Technol. 2019;12(3):351–6. doi: 10.1007/s12194-019-00527-5. [DOI] [PubMed] [Google Scholar]
- 23.Alexandrian AN, Mavroidis P, Narayanasamy G, McConnell KA, Kabat CN, George RB, et al. Incorporating biological modeling into patient-specific plan verification. J Appl Clin Med Phys. 2020;21(3):94–107. doi: 10.1002/acm2.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SY, Park JW, Park J, Yea JW, Oh SA. Fabrication of 3d printed head phantom using plaster mixed with polylactic acid powder for patient-specific qa in intensity-modulated radiotherapy. Sci Rep. 2022;12(1):17500 . doi: 10.1038/s41598-022-22520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puzhakkal N, Kochunny AK, Makuny D, Krishnan MPA, Poyil RC, Raveendran V. Validation of dolphin dosimetry in three dimensional patient-specific quality assurance programme. Rep Pract Oncol Radiother. 2019;24(5):481–90. doi: 10.1016/j.rpor.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low DA, Dempsey JF. Evaluation of the gamma dose distribution comparison method. Med Phys. 2003;30(9):2455–64. doi: 10.1118/1.1598711. [DOI] [PubMed] [Google Scholar]
- 27.Miften M, Olch A, Mihailidis D, Moran J, Pawlicki T, Molineu A, et al. Tolerance limits and methodologies for imrt measurement-based verification qa: Recommendations of aapm task group no 218. Med Phys. 2018;45(4):e53–e83. doi: 10.1002/mp.12810. [DOI] [PubMed] [Google Scholar]
- 28.Park SY, Park JM, Kim JI, Lee S, Choi CH. Validation of new transmission detector transmission factors for online dosimetry: An experimental study. Radiat Oncol. 2018;13(1):156 . doi: 10.1186/s13014-018-1106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong Y, Oh JG, Kang JK, Moon SR, Lee KK. Three-dimensional dose reconstruction-based pretreatment dosimetric verification in volumetric modulated arc therapy for prostate cancer. Radiat Oncol J. 2020;38(1):60–7. doi: 10.3857/roj.2020.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shukla ak, bhamra hk, rathore nk, patidar ak, verma a, rajpurohit vs, et al. Comparison of 2d and 3d gamma evaluation method in patient specific intensity-modulated radiotherapy quality assurance. Int j res med sci. 2023;11(4):1160–4. [Google Scholar]
- 31.Pal B, Pal A, Bag S, Ali MA, Das S, Palit S, et al. Comparative performance analysis of 2d and 3d gamma metrics for patient specific qa in vmat using octavius 4d with 2d-array 1500. Phys Med. 2021;91:18–27. doi: 10.1016/j.ejmp.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Bosse C, Narayanasamy G, Saenz D, Myers P, Kirby N, Rasmussen K, et al. Dose calculation comparisons between three modern treatment planning systems. J Med Phys. 2020;45(3):143–7. doi: 10.4103/jmp.JMP_111_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodall SK, Rowshanfarzad P, Ebert MA. Correction factors for commissioning and patient specific quality assurance of stereotactic fields in a monte carlo based treatment planning system : Tps correction factors. Phys Eng Sci Med. 2023;46(2):735–45. doi: 10.1007/s13246-023-01246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]