Abstract
Background:
Aminolevulinic acid (ALA) mediated photodynamic therapy (PDT) is considered as an effective treatment option for oral premalignant lesions. ALA is a Food and Drug Administration (FDA) approved second- generation photosensitizer (PS) used both orally as well as topically.
Objective:
This systematic review aims to evaluate the efficacy of ALA-PDT for the treatment of oral premalignant lesions.
Methods:
The focused question was, “Is ALA-PDT effective in the treatment of oral premalignant lesions?”A literature search was made in PubMed/Medline and GoogleScholar using different combinations of the following keywords: photodynamic therapy, oral premalignant lesions, oral leukoplakia (OL), erythroplakia, oral erythroleukoplakia (OEL), oral verrucous hyperplasia (OVH); and oral lichen planus (OLP). Review articles, preclinical studies, case-reports, commentaries, letters to the Editor, unpublished articles, studies on photodynamic therapy used in areas other than the oral cavityand, articles published in languages other than English were excluded. The relevant information was summarized.
Results:
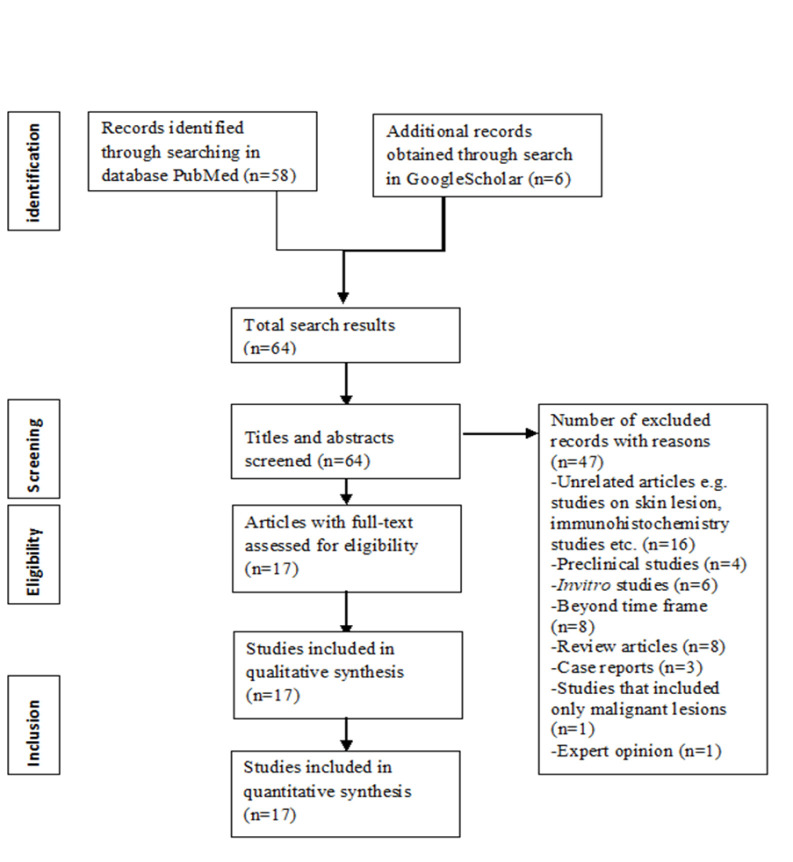
There were initially 64 results for the above parameters; 47 studies were excluded, leaving 17 studies for analysis. Characteristics of the included studies, PS, and PDT protocol were summarized.
Conclusion:
The outcome of the included studies suggested that ALA-PDT is an effective, easy to perform technique, well tolerated treatment with encouraging achievements in the treatment of oral premalignant lesions. No systemic side effects and skin photosensitivity were reported with topical ALA even within initial 48 hours after PDT, and patients were not required to avoid exposure to light following treatment. The clinical outcome of the ALA-PDT application, as reported in the studies, was also very promising, with either diminution in the size of the lesion or complete remission or improvement in signs and symptoms as well as reduced recurrence.
Key Words: Photodynamic therapy, photosensitizing agents, aminolevulinic acid, oral precancerous condition
Introduction
Topical PDT is minimally invasive, highly selective, easy to perform, repeatable with minimal scarring, and well tolerated by patients, and at present, no severe adverse reactions are reported [1, 2, 3]. ALA-PDT (with either systemic or topical administration) has been in use as a treatment modality for oral premalignant and malignant lesions. Kennedy et al. in the year 1990, first used PDT with topical ALA [4]. ALA-PDT can be utilized in patients where surgery is not feasible or in patients who are not willing for surgery; it can be performed in patients with pacemakers and in those patients who have bleeding tendency; it is well tolerated, and its repeated application does not have any cumulative toxicity. ALA is rapidly cleared from the tissues and body within 48 hours and patients usually do not face any problem of prolonged skin photosensitivity after treatment [5, 6]. The aim and objective of this systematic review is to evaluate the efficacy of ALA-PDT for the treatment of oral premalignant lesions. The rationale for this review is to compile the literature on ALA-PDT and attempt to draw a conclusion. Despite it being minimally invasive, its favorable adverse event profile, ability to enhance anti- tumor immune responses, it is not widely used. We have evaluated selected studies and discussed the efficacy of ALA-PDT as a treatment modality in the treatment and management of oral premalignant lesions.
Materials and Methods
Protocol
We constructed a focused question, “Is ALA-PDT safe and effective in the treatment of oral premalignant lesions?” following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).
Eligibility criteria
We included all the published clinical studies that evaluated the efficacy of ALA-PDT in the treatment of oral premalignant lesions January 2008 to February 2023. The inclusion criteria were i) original articles, clinical studies, case series ii) the study evaluated the efficacy of ALA-PDT in the management of oral premalignant lesions iii) lesion response was assessed and recorded iv) articles published in English language only v) clinical or histopathological diagnosis of oral premalignant lesions. The PICO questions applied were as follows:
Population (P): patients were diagnosed with oral premalignant lesions;
Intervention (I): patients were treated with ALA-PDT;
Comparison (C): condition of patients before PDT or any other treatment;
Outcome (O): lesion response and lesion size of patients with oral premalignant lesions.
We excluded the studies published in language other than English, letters to the Editor, abstracts, commentaries, preclinical studies, case reports, review articles, studies on photodynamic therapy used in areas other than oral cavity, unpublished articles, and meetings’ proceedings.
Information sources and search strategy
We conducted a comprehensive search in PubMed/ Medlineand GoogleScholar using different combinations of the different search keywords such as “PDT”, “oral premalignant lesions”, “oral leukoplakia” (OL), “erythroplakia”, “oral erythroleukoplakia” (OEL), “oral verrucous hyperplasia” (OVH) and “oral lichen planus” (OLP).
Selection process
The two authors (BB and AG) independently searched the databases. The search results were initially screened by titles and abstracts, and were independently examined by the authors. Scrutiny of the full texts of relevant studies was done adhering to the eligibility criteria. Following this, original studies’ reference lists were searched manually and checked for agreement via discussion between the authors. In case of disagreement between the authors, a third reviewer would review and resolve the discrepancies. Articles obtained from search in GoogleScholar were considered for inclusion if the journal was indexed in PubMed/Medline, EMBASE or Scopus. Search results are shown in the following figure (Figure 1).
Figure 1.
Flowchart of Literature Search According to Preferred Reporting Items for Systemic Reviews and Meta-Analysis Guidelines
Data collection process and data items
Data collection was done independently and manually by the two authors (BB and AG). The data which were sought from the studies included: first author’s name, publication year, type of the study, number of participants, their age range and gender, type of the lesion and their location in oral cavity, how long the follow-up was done, type of responses to the therapy; whether complete or partial or no response, recurrence, type of ALA and method of administration, light source, wavelength and energy of the light administered, duration (fractionated or continuous) and frequency of PDT. All extracted data was crosschecked by the reviewers (DR, PG and ID) and included after discussion and agreement.
In case of any missing and unclear information, its impact on the overall assessment was reviewed and discussed and final decision to include the study was taken if it was contributing important additional information that in turn might add value to this review without any risk.
Statistical approach and assessment of the risk of bias
Meta-analysis could not be performed because of nonuniformity in the methodology of the selected studies. This review was customized to mainly summarize the relevant information.
The assessment of the included studies was done by the two authors (BB & AG) according to the modified version of the Downs and Black checklist [7]. Concerning selection bias, Phase 1 studies were also included if they provided data not only on safety but also on efficacy of the treatment, case series were also included if the efficacy and follow-up data is valid. Concerning bias related to deviations from intended interventions and bias in outcome measurement, there was no blinding of participants, investigators and outcome assessors in the studies. All the studies provided valid evidence with no critical risk.
Synthesis methods
A total of 17 articles were included after excluding 47 articles. The authors assessed each study independently, screened the abstracts and main text followed by tabulation of the data according to the study parameters. The characteristics of the included studies are presented in Supplementary Table 1 and Table 2. For the outcome data, percentages were calculated and presented in the table for those studies where results were provided in absolute numbers.
Results
General characteristics of the studies
All the studies were prospective clinical studies. Clinically and histopathologically confirmed cases were included in the studies. The total number of participants recruited into the studies ranged between 5 to 147 participants. All participants were older than 18 years. All the studies included both males and females except two studies. In the study by Selvam et al., all the participants were male and in the study by Sulewska et al., all the participants were female [6, 8]. Premalignant lesions exposed to treatment included OL, erythroplakia, OEL, OVH, OLP. Except for 3 studies, ALA-PDT was the sole therapeutic strategy for the management of oral premalignant lesions. In the study by Kawczyk-Krupka et al., the efficacy of ALA-PDT was compared with that of cryotherapy [9]. Maloth et al. compared the efficacy of ALA-PDT with conventional therapy with medication [10]. Yao et al. comparFed ablative fractional laser- assisted photodynamic therapy (AFL-PDT) with ablative fractional laser (AFL) treatment [11].
PDT-related parameters of included studies
ALA was used either as gel, solution, emulsion or cream with a concentration ranging from 5% to 98%. All studies administered ALA topically except two studies. Wong et al. and Ahn et al. administered ALA orally at a dose of 30mg/kg and 60mg/kg respectively [12, 13] .
Light sources used were either light-emitting diode (LED), or laser (diode, pulsed dye, argon pumped dye laser, or semiconductor laser), or xenon lamp. The wavelengths ranged between 420 nm and 660 nm, with the majority between 630 nm and 635 nm. Energy density and duration of irradiation ranged between 2-200 joules/ cm2 and 60-1000 sec respectively. The frequency of PDT application varied from single application, once a week, once every two weeks or 10 weekly application. Yao et al. administered PDT as 1 session in each treatment zone [11].
Outcomes of included studies
The outcomes in all studies were categorized as complete response (CR), partial response (PR), and no response (NR). One Phase 1 study reported no response in all the participants, where ALA was administered orally [12].
Discussion
Studies show promising results with ALA-PDT. It is observed that there is variation in the treatment results in different studies that could be due to several factors such as differences in the ALA formulations (different concentrations, different bases), the number of ALA-PDT sessions, the incubation period, the type of source of light (LED or laser), and the light administration protocol (fractionated delivery or continuous supply).
ALA formulation
5-ALA is a Food and Drug Administration (FDA)- approved second-generation PS [10]. In 2017, 5 ALA- induced PDT and diagnosis were approved by the FDA [14]. Structurally, 5-ALA is a modified version of hematoporphyrin,such as benzoporphyrin derivatives. ALA acts as an intrinsic PS, that is converted in situ to protoporphyrin IX. The introduction of exogenous ALA in vivo inhibits the first step of porphyrin synthesis, resulting in the accumulation of protoporphyrin IX in the tissue [15-17].
5-ALA has revolutionized the field of PDT. Its low molecular weight (131.13g/mol), facilitate easy penetration into the epithelium. An alternative to ALA is the methyl ester form, methyl 5-aminolevulinate (MAL). The methyl ester group makes the molecule more lipophilic enhancing its penetration into cells and tumor selectivity. However, MAL must be converted to ALA by intracellular enzymes. This may limit the availability of ALA, but MAL has been found to reach maximum intracellular concentrations of PpIX quickly, allowing a shorter incubation period [18, 19].
For topical use, ALA has been used as gel, emulsion,solution, or cream. Kvaal et al. used MAL in cream form for treatment of OLP [4], while most studies used ALA in gel form [5, 8, 11, 20-25].
Yu et al. reported that the 20% ALA they used was liquid at room temperature but converted to a gel at body temperature upon contact with the oral mucosa at the site of the lesion, owing to the thermoresponsive sol-gel transition property of the vehicle. This ALA gel was adhesive to the oral mucosa, which was partially resistant to the dilution by saliva [20]. A few studies administered ALA orally. Wong et al. used 30 mg/kg ALA dissolved in 50ml water for oral administration [12]. Ahn et al. used 60 mg/kg ALA for oral administration in patients with oral premalignant lesions and early-stage head and neck tumors [13].
Wang et al. developed a high-adhesion-strength dry polyacrylic acid (PAA)-chitosan (CHI)-ALA interpenetrating network hydrogel (PACA) patch. This patch can be applied topically to a moist surface and can easily work as a drug delivery model for 5-ALA. This may overcome many drawbacks of topical administration of ALA. Further research is necessary in this regard [26].
ALA concentration in formulations for topical use
The amount of ALA used topically ranges from 20 to 200 mg per treatment [5].While various concentration of ALA was used in the studies, it was observed that most of the studies used 20% ALA for topical administration [5, 9, 11, 20, 21, 23, 24, 27].
Solewska et al. used ALA in a form of 5% solution in gel with illumination from an LED light source (wavelength 630nm and energy density 150 J/cm2) for ten weekly session on OLP lesions [8]. Maloth et al.used 98% topical ALA with an LED light source with 420 nm wavelengths inmultiple sessions for OL and OLP lesions [10]. Kawczyk-Krupka et al. used 20% ALA with a 630nm laser in 30 patients with OL, and 10% ALA with a 635nm laser in 18 patients with OL. They found complete response in 67% (20 patients) in 20% ALA group and 83% (15 patients) in 10% ALA group. However, they observed that the recurrence was more in 20% ALA (11, 37%) group than in 10% ALA (2, 11%) group [9].
ALA incubation time
ALA was administered orally at least 3-6 hours before illumination [12, 13]. For topical administration, it was observed that, in most studies, ALA was applied for 1.5- 2 hours prior to illumination. Maloth et al. applied 98% ALA for 30 min [10].
Light source
Lasers, light-emitting diodes (LEDs), and lamps are the main types of light sources used in PDT. The choice of light source depends on factors such as the target location, PS used, and light dose to be delivered [28]. In the case of laser light, different lasers are used for different wavelengths, e.g., diode lasers (630-1,100nm) and dye lasers (390-1,000nm) [29, 30]. Near-infrared lasers with longer wavelengths (750-1,200nm) have deeper penetration, minimal thermal effects, and spatial selectivity than visible lasers and used for procedures which need deep penetration such as nano-gold mediated cancer therapy, whereas visible lasers (430-680nm) with strong absorption in blood and color dyes are used for phototherapy [30, 29, 31]. In the case of LED lights, even a low-cost, battery-operated LED can achieve irradiance, spectral, and output stability requirements for successful PDT results in vitro and in vivo [32, 33, 34]. Masuda et al. developed a flexible LED unit designed for multi-wavelength excitation of 5-ALA that can achieve more uniform irradiation of even areas, enhancing the therapeutic effects of PDT [35].
In oral lichen planus (OLP), He et al. recommended the diode laser as the first option to relieve pain as it emits only one wavelength of light. However, to reduce size of the lesion, the efficacy of the semiconductor laser is better [36]. Different studies used different light sources. Yu-CH et al. found no significant difference in the clinical outcomes while treating OEL lesions with ALA-PDT using either LED or laser light [21]. Yao Y et al. compared the effects of ablative fractional laser (AFL) therapy and ALA-PDT with AFL in OL. All the patients in the AFL-PDT group achieved complete response or significant response (80.9%). The effective cure rate was higher in the AFL-PDT group. Recurrence was also less in the AFL-PDT group after 6-12 months of treatment. No systemic side effects or severe adverse events (AEs) were observed in either group [11].
Frequency of PDT
Kawczyk-Krupka et al. performed ALA-PDT on OL lesions upto 12 times, but in 27% of cases, there was recurrence over 6 months period [9]. On the other hand, Shafirstein et al. performed ALA-mediated PDT on OL lesions upto two times but recurrence was reported in only 4% (1 out of 23 cases) cases [27]. Yu et al.reported that treatment with topical ALA-PDT once a week can achieve complete regression of OVH lesions in less than seven treatment sessions [20]. Kvaal et al. reported that a single session of MAL-PDT (with 75 J/cm2 red light exposure at 600-660 nm wavelength using LED light) offers significant improvement in OLP after 6 months and during a 4-year follow-up period [4].
Duration of PDT
Regarding the duration of PDT, several studies have evaluated PDT usingthree-minute fractionated irradiations with either LED or laser light and found it to be effective as a successful treatment modality [5, 6, 10, 13, 20, 21, 27, 37] . Shafirstein et al. reported in their study that a short laser illumination time of 1.5ms contributed to moderate pain [27]. Han et al. reported that the use of high laser power density for short time can achieve high response rate in OL and it is well tolerated by patients, but it may cause more pain if the power exceeds 300 mW/cm2 [23].
Further studies are necessary to reach a consensus on the accurate duration of PDT and the number of PDT sessions for complete elimination of the oral premalignant lesions.
Light wavelength
Most studies used light wavelength of 630±5nm, that is recommended when 20% ALA is used, as light at 635 nm corresponds to the absorption peak of ALA [36, 38]. Shafirstein et al. found ALA PDT with 585 nm penetration depth (PDL) to be effective and can be used as an alternative to multiple treatments and long exposure times required with diode laser [27]. Maloth et al. used LED light wavelength of 420nm with 98% 5 ALA for 12 minute (with 3 minutes fractionization), achieving partial responses in majority of patients (8 out of 12 lesions, 66.7% among 13 patients) [10].
Efficacy of ALA-PDT
Complete resolution of OVH with ALA-PDT was observed in a few studies [5, 20]. Lin et al. also reported a significant outcome in their study using the same ALA preparation, and a similar PDT protocol but with a laser light source [5]. Yu et al. also used a similar study protocol and found complete response in all the 36 OVH lesions in 36 patients. They reported that the outcome of PDT treatment for OVH depends on several factors such as color and appearance of the lesion, size of the lesion, presence of dysplasia, and thickness of the keratin layer. Fewer number of PDT application is required in smaller OVH lesions than in larger lesions to achieve a complete response. The diffusion of ALA is facilitated by thinner surface keratin layer. They found that pink OVH lesions with dysplasia usually had thinner layers of keratin at the surface than the lesions which were white or had no dysplasia. Thus, the pink OVH lesions have more ability to retain an increased amount of ALA than white lesions. More ALA is absorbed by dysplastic OVH lesions. Permeability is also higher in the case of dysplastic epithelium because of wide intercellular spaces, and ALA can diffuse more easily in a dysplastic epithelium. There is also retention of more ALA in a dysplastic epithelium than in a hyperplastic epithelium, and epithelium with thinner keratin layers have minimal inhibitory effects in the light intensity. Better clinical outcome can be achieved for OVH lesions with pink color, dysplastic epithelium, and a thinner surface keratin layer with sufficient PS and light dose than for epithelium with white color, no dysplasia, and thicker surface keratin layer [20]. Han et al. observed ALA-PDT to be highly effective in patients with moderate or severe dysplasia. They achieved 55.2% complete remission and 31% partial remission of OL [23]. Yu et al. reported fewer mean number of PDT treatments in lesions with greatest diameter <1.5cm and in lesions with a surface keratin layer ≤30mm to achieve a CR than in the larger lesions and lesions with thicker keratin layers [21]. But several studies do not draw any association between the size and appearance of the lesion and PDT treatment outcome [9, 27, 37].
PDT treatment in OLP leads to lesion reduction and improvement of the quality of life and induces local and systemic anti-inflammatory effects [39]. The topical use of 5-ALA had a higher efficacy than gargling methylene blue in terms of achieving partial response, and 5% ALA may be recommended as an optimal modality [36]. Kvaal et al. observed improvement in OLP after a single treatment session with MAL-PDT, which had a long-term effect compared to regular treatment with cortisone or tacrolimus application [4]. Suleswka et al. suggested that PDT can be an effective alternative and complementary treatment modality for treatment of erosive OLP (EOLP). They applied ten weekly sessions of ALA-PDT and found that there was continued healing and reduction in sizes of lesions during 12-month observation period and there was complete remissions of lesions on gingiva and tongue [8]. However, further research is needed for conclusive evidence that PDT affects the histological or immunological effects associated with OLP [40].
Ou et al. reported that ALA-PDT with Waterlase (that combines water, air, and laser energy) is very effective in treating OL, especially in lesions with mild to moderate dysplasia. They achieved an 82.3% CR with a recurrence rate of 14.6% in 71 patients, and the reason for recurrence was attributed to noncompliance of patients with continuation of habits of areca nut chewing and smoking. Their study findings suggest that ALA-PDT with Waterlase provides a new treatment modality for OL [24].
Lesion oxygenation
The mechanism of action of PDT is based on the interaction of light, PS, and oxygen. The PS reacts with the surrounding oxygen upon light excitation and generates either free radicals (type I process) or singlet oxygen (type II process), causing cell death [41]. Lin et al. reported that a fractionated PDT protocol results in successful clinical outcome. Multiple three-minute fractionated irradiations with multiple three-minute resting periods help in regeneration of PpIX by lesional epithelial cells and acquire new oxygen during the resting periods [5]. Ahn et al. reported in their Phase I study of premalignant lesions and early-stage head and neck tumors that better results were achieved with ALA-PDT in patients with better oxygenated lesions [42].
Safety of ALA-PDT
The commonly encountered adverse events (AEs) were pain during treatment, localized erythema and edema, and post-PDT pain. Sometimes secondary infection is reported with PDT [9]. Yu et al. carried out light treatments under local anesthesia using 2% lidocaine to alleviate the severe pain or burning sensation during PDT in 22 patients. In case of severe post-PDT pain experienced by 24 patients, analgesics were prescribed. Most patients experienced post-PDT pain one or two hours after PDT that lasted for 24 to 48 hours. Patients experiencing pain during treatment usually had post-PDT pain as well [20].
Jerjes et al.reported pain and swelling as commonly encountered AEs immediately after treatment. They reported that all patients experienced pain at some stage post-PDT, for which oral opiate analgesics were used. They found local analgesic and anti-inflammatory spray just before meals was also helpful. In most cases, pain commenced 24 to 48 hours post-PDT lasting for several days [37].
Shafirstein et al. evaluated efficacy of ALA-PDT in 23 patients with OL. Of 17 patients, 7 (41%) had more
than 75% regression (significant response) and 9 (53%) had more than 25% regression (partial response) at 90 days. They suggested that the ALA-PDT with 585nm light wavelength was safe in treating OL. They did not encounter any major AEs during the study; minor AEs encountered during treatment at the treatment site included sensitivity or loss of sensation, burning sensation or pain, taste alteration, swelling, and ulceration. During the follow-up period, most symptoms had resolved by 90th day, and all had resolved by 365 days [27].
Wong et al. evaluated oral ALA-PDT treatment in 11 participants with OL but no clinical responses (complete or partial) were observed in the treated participants. They observed no mucosal damage during safety assessment upto 3 months. They amended the initial dose of ALA of 60 mg to 30 mg after a participant experienced Grade 3 transaminase elevation [12] . Ahn et al. observed Grade 3 mucositis more commonly in the patients [13].
Selvam et al.reported mild burning sensation in two patients (40%) during light treatment which disappeared immediately post treatment. There was no need of local anesthesia or analgesics in their study [6]. Han et al. observed transient local ulcer and pain in 65.6% patients (19/29), which were typically resolved within 2 weeks and in 10.3% (3/29) patients who were all over 60 years of age exhibited ulcer and pain symptoms beyond a 2-week period [23]. Ou J et al. reported that the energy from waterlase was absorbed effectively by tissue and water causing minimal tissue damage [24].
Advantage and disadvantage of using topical ALA-PDT No systemic side effects and skin photosensitivity were reported with the use of topical ALA even within initial 48 hour after PDT and patients are not required to avoid exposure to light following treatment [37]. Field cancerization can be treated with ALA PDT [37, 11]. The major disadvantage of topical PS is the small treatment depth of only 1 2 mm. Therefore superficial lesions which are <1 mm thick can be treated successfully [37]. Light penetration depth of less than centimetre and heterogeneity of response from the variant light penetration depth is a significant limitation of PDT [43]. Significant disadvantages of ALA-PDT include low delivery efficiency, poor comfort, easy influence by saliva, treatment related pain, limited depth of penetration, pharmacokinetics of ALA with individual variation among patients influencing effective ALA concentrations in the treated area [26, 35, 37]. The nanotechnology provides new strategies to address the deficiencies associated with natural ALA such as weak lipophilicity, low stability and poor bioavalibility. The nanocarrier-assisted drug delivery offers high tumor selectivity, well-controlled drug release and good biocompatibility [44]. Chi et al. evaluated the effectiveness of 5-ALA combined with gold nanoparticles (18.2±1.4 nm) on cutaneous squamous cell carcinoma cells and achieved significant results [45]. Clinical trials are needed in this regard for future evidence-based treatment approach.
Limitations
The limitations of the studies included in the review were nonuniformity in the methodology, variation in ALA formulations, incubation period, frequency and duration in PDTs, use of different light sources and variation in light administration protocol. Most of the studies were open label nonrandomized studies. Randomized controlled trials with large sample size are needed to be conducted in future. Another limitation in the review is authors’ bias that was tried to be limited by incorporating independent reviewers. However, this review throws light again upon a treatment strategy that can be adopted routinely in institutions and hospitals.
Despite having a favorable adverse event profile, the ability to enhance anti-tumor immune responses, and being minimally invasive, it is disappointing that PDT is not broadly utilized in the clinical setting for the treatment of malignant and/or non-malignant diseases because of the scarcity of randomized clinical trials [46]. Due to recent developments in photosensitizing drugs, light sources, and light deliveries, PDT merits further research as a therapy paradigm for several malignant and premalignant conditions [47].
In conclusion, reviewing the outcome of the studies, it can be suggested that ALA-PDT can be successfully used to treat oral premalignant lesions and topical ALA is much safer. Better clinical outcome can be achieved in lesions with dysplasia and thinner surface keratin layer. In case of large lesions, ALA-PDT can be an effective treatment with minimal tissue damage. It can also be used in patients with recurrent lesions. The main drawback, however, is the small treatment depth. Further research is required with intralesional injection of ALA, new drug delivery systems, combining with other nano agents, and in combination with other treatment modalities.
Acknowledgements
Registration and protocol
A protocol was not prepared for this review. The review was not registered in any registering database.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The template data collection forms are not publicly available. The data extracted from included studies are available freely online (PubMed, Google-Scholar).
Approval from any scientific body statement
The study did not have any approval from any scientific body.
Ethical issues and Institutional Review Board Statement No ethical issues were involved with this study.
Approval from ethics committee was not required.
Conflicts of interest
There are no conflicts of interest.
Author Contribution Statement
Authors BB and AG did the conceptualization, independent database search, study identification, screening and selection of studies, data extraction, tabulation and compilation and initial review. Author DR, PG, ID reviewed the selected studies and extracted data. BB did the original draft writing and preparation, BB, AG and DR did review and editing.
Supplementary materials
References
- 1.Kiss N, Farkas K, Tosti G, De Gado F, Bergler-Czop B, Fazia G, et al. Photodynamic therapy with 5-aminolevulinic acid patch for the treatment of actinic keratosis. J Clin Med. 2022;11:11. doi: 10.3390/jcm11113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin L, Song C, Wei Z, Zou H, Han S, Cao Z, et al. Multifunctional photodynamic/photothermal nano-agents for the treatment of oral leukoplakia. J Nanobiotechnology. 2022;20(1):106 . doi: 10.1186/s12951-022-01310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Stasio D, Romano A, Russo D, Fiori F, Laino L, Caponio VCA, et al. Photodynamic therapy using topical toluidine blue for the treatment of oral leukoplakia: A prospective case series. Photodiagnosis Photodyn Ther. 2020;31:101888. doi: 10.1016/j.pdpdt.2020.101888. [DOI] [PubMed] [Google Scholar]
- 4.Kvaal SI, Angell-Petersen E, Warloe T. Photodynamic treatment of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(1):62–70. doi: 10.1016/j.oooo.2012.08.448. [DOI] [PubMed] [Google Scholar]
- 5.Lin HP, Chen HM, Yu CH, Yang H, Wang YP, Chiang CP. Topical photodynamic therapy is very effective for oral verrucous hyperplasia and oral erythroleukoplakia. J Oral Pathol Med. 2010;39(8):624–30. doi: 10.1111/j.1600-0714.2010.00935.x. [DOI] [PubMed] [Google Scholar]
- 6.Selvam NP, Sadaksharam J, Singaravelu G, Ramu R. Treatment of oral leukoplakia with photodynamic therapy: A pilot study. J Cancer Res Ther. 2015;11(2):464–7. doi: 10.4103/0973-1482.147703. [DOI] [PubMed] [Google Scholar]
- 7.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sulewska M, Duraj E, Sobaniec S, Graczyk A, Milewski R, Wróblewska M, et al. A clinical evaluation of the efficacy of photodynamic therapy in the treatment of erosive oral lichen planus: A case series. Photodiagnosis Photodyn Ther. 2017;18:12–9. doi: 10.1016/j.pdpdt.2017.01.178. [DOI] [PubMed] [Google Scholar]
- 9.Kawczyk-Krupka A, Waśkowska J, Raczkowska-Siostrzonek A, Kościarz-Grzesiok A, Kwiatek S, Straszak D, et al. Comparison of cryotherapy and photodynamic therapy in treatment of oral leukoplakia. Photodiagnosis Photodyn Ther. 2012;9(2):148–55. doi: 10.1016/j.pdpdt.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Maloth KN, Velpula N, Kodangal S, Sangmesh M, Vellamchetla K, Ugrappa S, et al. Photodynamic therapy - a non-invasive treatment modality for precancerous lesions. J Lasers Med Sci. 2016;7(1):30–6. doi: 10.15171/jlms.2016.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Y, Shi L, Wang Y, Shen X, Ye S, Tang G, et al. Ablative fractional laser-assisted photodynamic therapy vs Ablative fractional laser for oral leukoplakia treatment: A randomized, controlled pilot study. Photodiagnosis Photodyn Ther. 2021;36:102523. doi: 10.1016/j.pdpdt.2021.102523. [DOI] [PubMed] [Google Scholar]
- 12.Wong SJ, Campbell B, Massey B, Lynch DP, Cohen EEW, Blair E, et al. A phase i trial of aminolevulinic acid- photodynamic therapy for treatment of oral leukoplakia. Oral Oncol. 2013;49(9):970–6. doi: 10.1016/j.oraloncology.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn PH, Quon H, O’Malley BW, Weinstein G, Chalian A, Malloy K, et al. Toxicities and early outcomes in a phase 1 trial of photodynamic therapy for premalignant and early stage head and neck tumors. Oral Oncol. 2016;55:37–42. doi: 10.1016/j.oraloncology.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YJ, Yi YC, Lin YC, Chen CC, Hung JH, Lin JY, et al. Purification and biofabrication of 5-aminolevulinic acid for photodynamic therapy against pathogens and cancer cells. Bioresour Bioprocess. 2022;9(1):68 . doi: 10.1186/s40643-022-00557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stájer A, Kajári S, Gajdács M, Musah-Eroje A, Baráth Z. Utility of photodynamic therapy in dentistry: Current concepts. Dent J (Basel) 2020;8:2. doi: 10.3390/dj8020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kou J, Dou D, Yang L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget. 2017;8(46):81591–603. doi: 10.18632/oncotarget.20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K, et al. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 18.Bapat P, Singh G, Nobile CJ. Visible lights combined with photosensitizing compounds are effective against candida albicans biofilms. Microorganisms. 2021;9:3. doi: 10.3390/microorganisms9030500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monfrecola G, Megna M, Rovati C, Arisi M, Rossi M, Calzavara-Pinton I, et al. A critical reappraisal of off-label use of photodynamic therapy for the treatment of non- neoplastic skin conditions. Dermatology. 2021;237(2):262–76. doi: 10.1159/000507926. [DOI] [PubMed] [Google Scholar]
- 20.Yu CH, Chen HM, Hung HY, Cheng SJ, Tsai T, Chiang CP. Photodynamic therapy outcome for oral verrucous hyperplasia depends on the clinical appearance, size, color, epithelial dysplasia, and surface keratin thickness of the lesion. Oral Oncol. 2008;44(6):595–600. doi: 10.1016/j.oraloncology.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Yu CH, Lin HP, Chen HM, Yang H, Wang YP, Chiang CP. Comparison of clinical outcomes of oral erythroleukoplakia treated with photodynamic therapy using either light-emitting diode or laser light. Lasers Surg Med. 2009;41(9):628–33. doi: 10.1002/lsm.20841. [DOI] [PubMed] [Google Scholar]
- 22.Sulewska M, Duraj E, Sobaniec S, Graczyk A, Milewski R, Wróblewska M, et al. A clinical evaluation of efficacy of photodynamic therapy in treatment of reticular oral lichen planus: A case series. Photodiagnosis Photodyn Ther. 2019;25:50–7. doi: 10.1016/j.pdpdt.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Han Y, Xu S, Jin J, Wang X, Liu X, Hua H, et al. Primary clinical evaluation of photodynamic therapy with oral leukoplakia in chinese patients. Front Physiol. 2018;9:1911. doi: 10.3389/fphys.2018.01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ou J, Gao Y, Li H, Ling T, Xie X. Application of 5-aminolevulinic acid-mediated waterlase-assisted photodynamic therapy in the treatment of oral leukoplakia. Sci Rep. 2022;12(1):9391 . doi: 10.1038/s41598-022-13497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sulewska ME, Tomaszuk J, Sajewicz E, Pietruski J, Starzyńska A, Pietruska M. Treatment of reticular oral lichen planus with photodynamic therapy: A case series. J Clin Med. 2023;12:3. doi: 10.3390/jcm12030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Yuan Z, Tao A, Wang P, Xie W, Yang S, et al. Hydrogel-based patient-friendly photodynamic therapy of oral potentially malignant disorders. Biomaterials. 2022;281:121377. doi: 10.1016/j.biomaterials.2022.121377. [DOI] [PubMed] [Google Scholar]
- 27.Shafirstein G, Friedman A, Siegel E, Moreno M, Bäumler W, Fan CY, et al. Using 5-aminolevulinic acid and pulsed dye laser for photodynamic treatment of oral leukoplakia. Arch Otolaryngol Head Neck Surg. 2011;137(11):1117–23. doi: 10.1001/archoto.2011.178. [DOI] [PubMed] [Google Scholar]
- 28.Kim MM, Darafsheh A. Light sources and dosimetry techniques for photodynamic therapy. Photochem Photobiol. 2020;96(2):280–94. doi: 10.1111/php.13219. [DOI] [PubMed] [Google Scholar]
- 29.Lin JT. Design aspects of medical laser devices. Medical Device Diagn Eng. 2017;2:75–7. [Google Scholar]
- 30.Lin J, Ni G, Ding T, Lei S, Zhong L, Liu N, et al. Photodynamic therapy for oral squamous cell carcinoma: A systematic review and meta‐analysis. Int J Photoenergy. 2021;2021(1):6641358. [Google Scholar]
- 31.Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Reviews Clin Oncol. 2020;17(11):657–74. doi: 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Rudd G, Daly L, Hempstead J, Liu Y, Khan AP, et al. Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy. SPIE; 2016. Development of low-cost devices for image-guided photodynamic therapy treatment of oral cancer in global health settings; pp. 48–53. [Google Scholar]
- 33.Daly L, Rudd G, Liu H, Leon P, Celli J, Čučkov F. 2nd International Conference on Bio-engineering for Smart Technologies (BioSMART) Paris, France: IEEE; 2017 . Embedded system for a battery-operated LED-based photodynamic therapy device for treatment of early-stage oral cancers in resource-limited settings; pp. 1–4. [Google Scholar]
- 34.Liu H, Daly L, Rudd G, Khan AP, Mallidi S, Liu Y, et al. Development and evaluation of a low-cost, portable, led- based device for pdt treatment of early-stage oral cancer in resource-limited settings. Lasers Surg Med. 2019;51(4):345–51. doi: 10.1002/lsm.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuda H, Kimura M, Nishioka A, Kato H, Morita A. Dual wavelength 5-aminolevulinic acid photodynamic therapy using a novel flexible light-emitting diode unit. J Dermatol Sci. 2019;93(2):109–15. doi: 10.1016/j.jdermsci.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 36.He Y, Deng J, Zhao Y, Tao H, Dan H, Xu H, et al. Efficacy evaluation of photodynamic therapy for oral lichen planus: A systematic review and meta-analysis. BMC Oral Health. 2020;20:1–10. doi: 10.1186/s12903-020-01260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jerjes W, Upile T, Hamdoon Z, Mosse CA, Akram S, Hopper C. Photodynamic therapy outcome for oral dysplasia. Lasers Surg Med. 2011;43(3):192–9. doi: 10.1002/lsm.21036. [DOI] [PubMed] [Google Scholar]
- 38.Jin X, Xu H, Deng J, Dan H, Ji P, Chen Q, et al. Photodynamic therapy for oral potentially malignant disorders. Photodiagn Photodyn Ther. 2019;28:146–52. doi: 10.1016/j.pdpdt.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Cosgarea R, Pollmann R, Sharif J, Schmidt T, Stein R, Bodea A, et al. Photodynamic therapy in oral lichen planus: A prospective case-controlled pilot study. Sci Rep. 2020;10(1):1667. doi: 10.1038/s41598-020-58548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waingade M, Medikeri RS, Rathod P. Effectiveness of methylene blue photosensitizers compared to that of corticosteroids in the management of oral lichen planus: A systematic review and meta-analysis. J Dent Anesth Pain Med. 2022;22(3):175. doi: 10.17245/jdapm.2022.22.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Algorri JF, Ochoa M, Roldan-Varona P, Rodriguez-Cobo L, López-Higuera JM. Light technology for efficient and effective photodynamic therapy: A critical review. Cancers. 2021;13(14):3484. doi: 10.3390/cancers13143484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahn PH, Finlay JC, Gallagher-Colombo SM, Quon H, O’Malley Jr BW, Weinstein GS, et al. Lesion oxygenation associates with clinical outcomes in premalignant and early stage head and neck tumors treated on a phase 1 trial of photodynamic therapy. Photodiagn Photodyn Ther. 2018;21:28–35. doi: 10.1016/j.pdpdt.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baskaran R, Lee J, Yang S-G. Clinical development of photodynamic agents and therapeutic applications. Biomaterials Res. 2018;22(1):25. doi: 10.1186/s40824-018-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lou L, Zhou S, Tan S, Xiang M, Wang W, Yuan C, et al. Amplifying the efficacy of ala-based prodrugs for photodynamic therapy using nanotechnology. Front Pharmacol. 2023;14:1137707. doi: 10.3389/fphar.2023.1137707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chi Y-f, Qin J-j, Li Z, Ge Q, Zeng W-h. Enhanced anti- tumor efficacy of 5-aminolevulinic acid-gold nanoparticles- mediated photodynamic therapy in cutaneous squamous cell carcinoma cells. Braz J Med Biol Res. 2020;53(5):e8457. doi: 10.1590/1414-431X20208457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunaydin G, Gedik ME, Ayan S. Photodynamic therapy— current limitations and novel approaches. Front Chem. 2021;9:691697. doi: 10.3389/fchem.2021.691697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pignatelli P, Umme S, D’Antonio DL, Piattelli A, Curia MC. Reactive oxygen species produced by 5-aminolevulinic acid photodynamic therapy in the treatment of cancer. Int J Mol Sci. 2023;24(10):8964. doi: 10.3390/ijms24108964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The template data collection forms are not publicly available. The data extracted from included studies are available freely online (PubMed, Google-Scholar).