Abstract
Objective:
The aim is to analyze the incidence of kidney cancer over a 15-year period, considering factors such as stage, age, sex, and morphological verification in the regional context in Kazakhstan.
Methods:
The retrospective study was done using descriptive and analytical methods of oncoepidemiology. The extensive, crude and age-specific incidence rates are determined according to the generally accepted methodology used in sanitary statistics. The data were used to calculate the average percentage change (APС) using the Joinpoint regression analysis to determine the trend over the study period.
Results:
Among the meticulously documented 15,277 cases, a conspicuous male predominance was noted, comprising 53.7% of cases compared to 46.3% in females, with peak incidences observed within the 50-69 age cohorts. The average age at diagnosis exhibited a progressive rise over the study period, with discernible variations observed in age-specific incidence rates, particularly pronounced within the 60-84 age brackets. Noteworthy temporal trends indicated a consistent uptick in crude incidence rates, with distinct regional disparities manifesting higher rates in northern regions relative to their southern and western counterparts. Stratification by cancer stage unveiled a significant surge in stages I-II cases alongside a concomitant decrement in stage III incidences, complemented by reductions in stage IV occurrences and instances of unspecified disease stages. Morphological verification rates displayed regional variations, with an overarching ascending trajectory across most regions, albeit exceptions noted, notably in the Kyzylorda region.
Conclusion:
Our study identified a rise in kidney cancer incidence in Kazakhstan, likely reflecting global trends driven by increased risk factor exposure and incidental imaging findings. Regional disparities and varied stage distributions highlight the complexity of kidney cancer epidemiology. Despite advancements in early detection, delayed diagnosis persists, necessitating improved surveillance and diagnostic practices.
Key Words: Renal cancer, cancer rate, dynamics, morphological verification, regions
Introduction
In 2022, approximately 434,840 new cases of kidney cancer were documented globally, ranking it 14th in the hierarchy of oncological pathologies. Additionally, it occupied the 16th position concerning mortality, with 155,953 deaths attributed to this malignant neoplasm [1]. The global incidence of kidney cancer is on the rise, with the most pronounced increases documented in developed nations [2]. Well-established risk determinants for kidney cancer comprise advancing age, male gender, tobacco usage, hypertension, and obesity [3].
Diagnosing kidney cancer poses a considerable challenge due to the elusive nature of renal cell carcinomas, which account for approximately 85% of kidney cancers [4]. Traditionally, the clinical presentation of kidney cancer was associated with a triad of symptoms, including hematuria, pain, and the presence of an abdominal mass [5]. However, contemporary understanding acknowledges that this classical triad is seldom encountered in clinical practice. Instead, symptoms, if manifested at all, tend to be nonspecific, vague, and often delayed in onset [6].
The paramount importance of early diagnosis in enhancing treatment outcomes is widely acknowledged in the medical community. Nevertheless, a significant proportion of patients still present with advanced-stage disease, underscoring the ongoing challenges in timely detection and diagnosis of kidney cancer. This underscores the critical need for heightened vigilance among healthcare providers and the general population alike to facilitate early recognition and prompt management of this malignancy.
Several prevalent malignancies, including colorectal, breast, prostate, lung, thyroid, uterine body, bladder, kidney/renal pelvis, skin melanoma, and non-Hodgkin’s lymphoma, have been linked to heightened susceptibility to kidney cancer within the initial five years post-diagnosis [7, 8]. Prior investigations into breast cancer [9-11], colorectal cancer [12], lung cancer [13], thyroid cancer [14], uterine body cancer [15], and non-Hodgkin’s lymphomas [16] have revealed escalating incidence rates across all except lung cancer. This trend warrants heightened concern regarding the potential development of primary kidney cancer in these cancer cohorts. While increased surveillance, effects of radiation therapy, or genetic predispositions to certain cancers may offer partial explanations, further exploration is imperative to elucidate the persistent elevation in risk beyond the five-year mark.
The 8th edition TNM system of the American Joint Committee on Cancer, published in 2018, is widely used for staging renal cancer [17, 18]. In this system, T1 and T2 renal cancer classification is primarily determined by tumor size, with specific size thresholds delineated. Conversely, T3 and T4 stages consider factors such as invasion of peripheral fat, fatty infiltration of the renal sinus, encroachment on the pyelonephric system, involvement of the renal veins, and invasion of Gerota’s fascia or the ipsilateral adrenal gland, regardless of tumor size [19]. While the prognosis for kidney cancer is generally favorable [20-22], certain subtypes exhibit a less favorable outlook [23]. Primary tumor size stands as a crucial clinical parameter [24, 25], serving as one of the key determinants in TNM staging [26]. The TNM staging system continues to be the universally acknowledged foundation for predicting kidney cancer prognosis. However, this system primarily relies on factors such as tumor size, lymph node metastases, and distant metastases. Recent studies have identified large tumor size and advanced age (>75 years old) as risk factors [27].
The cancer registry of Kazakhstan employs this classification system to document the staging of kidney cancer cases. In this research endeavor, we aim to conduct a comprehensive analysis focusing on the age and sex demographics of kidney cancer incidence, stage-specific incidence rates, regional variations, and morphological verification indicators over a specified time period.
Materials and Methods
Data
Incidences of novel cases of Kidney Cancer were derived from the Ministry of Health of the Republic of Kazakhstan’s reporting forms (form 7 and 35) spanning the years 2005 to 2019. The identification utilized the International Disease Code 10 with the code C64. The populace data was sourced from the Bureau of National Statistics, incorporating considerations of age and gender attributes along with administrative-territorial demarcations [28]. The report form provides data on the number of morphologically confirmed cases, the number of patients by stage. In oncological organizations, the stage of diagnosis was assigned based on clinical, imaging, and pathological information in accordance with the TNM classification.
However, in post-Soviet countries such as Kazakhstan, morphological verification of cancer does not always reach 100% due to various factors, including the availability of medical resources and equipment. In this regard, our study also took into account other methods of cancer diagnosis, including radiological and clinical data. These methods play an important role in the diagnostic process and are often used in combination with morphological verification to more accurately determine the presence and stage of cancer. We acknowledge that this may introduce a certain degree of uncertainty into the results, but we emphasize that this practice is common and necessary in this context.
Statistical analysis
The primary approach employed in this investigation encompassed a retrospective study employing descriptive and analytical techniques within the field of oncoepidemiology. Age-standardized rates (ASR) were computed for eighteen distinct age strata (0-4, 5-9, ..., 80-84, and 85+) by adopting the world standard population established by the World Health Organization [29], in accordance with guidelines provided by the National Cancer Institute [30].
The crude rate (CR) and age-specific incidence rates (ASIR) were computed employing the established methodology commonly utilized in sanitary statistics. The following statistical metrics were computed: annual averages (M, P), mean error (m), Student’s criterion, and a 95% confidence interval (95% CI). In statistical analysis, the mean error typically pertains to the average discrepancy between estimates and actual values [31]. Student’s criterion, often referred to as the t-test, serves the purpose of comparing means between two groups by utilizing the difference in means divided by an estimate of the standard error of the difference [31].
Additionally, the degree of approximation (R2) was ascertained. The level of approximation in linear regression assesses the proximity of the linear model to the original dataset. This metric gauges the extent to which the model aligns with the data and its capacity to predict dependent variable values with accuracy, predicated upon the independent variables.
In this study, we have refrained from presenting the fundamental calculation formulas, as these are extensively elucidated within methodological guidelines and textbooks dedicated to medical and biological statistics [32, 31, 33]. The assessment of the incidence trend spanned a period of 15 years. This trend’s determination was carried out utilizing the least squares methodology and facilitated by the employment of the Joinpoint program (https://surveillance.cancer.gov/joinpoint/). The dataset was harnessed for the computation of the average percentage change (APC) through the application of Joinpoint regression analysis.
Ethics approval
The study encompassed an examination of publicly accessible administrative data and did not necessitate interactions with individual subjects. The study’s conduct received approval from the Local Ethics Commission of the Central Asian Institute for Medical Research.
Results
Demographic Characteristics of Kidney Cancer Cases
In a comprehensive study spanning the years 2005 to 2019, a total of 15,277 cases of kidney cancer were meticulously documented. Among these cases, 8,202 (53.7%) were reported in males, while 7,075 (46.3%) were observed in females. The age cohorts of 50-54 years, 55-59 years, 60-64 years, and 65-69 years exhibited the highest numbers of reported cases of kidney cancer, with rates of 12.7%, 16.5%, 16.2%, and 13.9%, respectively.
Across the study period, a subtle yet discernible trend emerged in the average age of patients diagnosed with kidney cancer, depicting a gradual increase from 57.8±0.5 years (95% CI=56.7-58.9) in 2005 to 59.8±0.4 years (95% CI=59.1-60.5) in 2019. The overall average age stood at 58.5±0.3 years (95% CI=58.0-59.0), exhibiting a modest annual percentage change (APC) of +0.2. Further studies, stratified by gender, unveiled slight disparities in age trends. Among men, the average age was 58.2±0.2 years (95% CI=57.7-58.7) with an APC of +0.1, indicative of a relatively consistent pattern over time. Women exhibited a nearly identical average age of 58.8±0.4 years (95% CI=58.1-59.5), with an APC of +0.4 (Table 1).
Table 1.
Kidney Cancer in Kazakhstan, 2005-2019
| Age | All | Male | Female | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Crude Incidence Rate | Number | Crude Incidence Rate | Number | Crude Incidence Rate | ||||||||||
| % | per 100,000 | APC, % | R2 | % | per 100,000 | APC, % | R2 | % | per 100,000 | APC, % | R2 | ||||
| ˂ 30 | 425 | 4 | 0.5±0.03 | −2.76 | 0.2984 | 183 | 3.2 | 0.4±0.02 | −2.22 | 0.2965 | 242 | 4.9 | 0.5±0.04 | −2.92 | 0.2015 |
| 30-34 | 225 | 1.5 | 1.1±0.1 | 4.26 | 0.2013 | 117 | 1,4 | 1.2±0.2 | 6.09 | 0.2983 | 108 | 1,5 | 1.1±0.1 | 2.01 | 0.0522 |
| 35-39 | 423 | 2.8 | 2.4±0.1 | −0.25 | 0.0008 | 245 | 3,0 | 2.9±0.2 | −1.85 | 0.1324 | 178 | 2,5 | 2.0±0.1 | 1.71 | 0.1144 |
| 40-44 | 704 | 4.6 | 4.2±0.2 | −0.31 | 0.0093 | 397 | 4,8 | 5.0±0.4 | −0.01 | 0.0006 | 307 | 4,3 | 3.6±0.3 | −0.53 | 0.0133 |
| 45-49 | 1169 | 7.7 | 7.3±0.2 | 0.1 | 0.0024 | 698 | 8,5 | 9.2±0.2 | 0.51 | 0.0584 | 471 | 6,7 | 5.6±0.2 | −0.62 | 0.0267 |
| 50-54 | 1947 | 12.7 | 13.5±0.4 | −0.07 | 2.00E-05 | 1134 | 13,8 | 17.0±0.6 | −0.16 | 0.0019 | 813 | 11,5 | 10.5±0.4 | −0.24 | 0.0002 |
| 55-59 | 2518 | 16.5 | 21.1±0.4 | −0.48 | 0.0878 | 1393 | 17,0 | 26.3±0.7 | −1.31 | 0.329 | 1125 | 15,9 | 17.0±0.5 | 0.45 | 0.0253 |
| 60-64 | 2472 | 16.2 | 29.3±0.9 | 0.26 | 0.0499 | 1336 | 16,3 | 37.6±1.0 | 0.02 | 0.0231 | 1136 | 16,1 | 23.3±1.0 | 0.35 | 0.0395 |
| 65-69 | 2123 | 13.9 | 32.2±1.6 | 3.4 | 0.6887 | 1093 | 13,3 | 42.1±2.3 | 3.6 | 0.6289 | 1030 | 14,6 | 25.8±1.3 | 3.14 | 0.6169 |
| 70-74 | 1513 | 9.9 | 31.7±1.2 | 2.4 | 0.6047 | 786 | 9,6 | 44.7±2.3 | 2.5 | 0.3979 | 727 | 10,3 | 24.0±0.9 | 2.45 | 0.5049 |
| 75-79 | 1052 | 6.9 | 28.9±1.4 | 0.55 | 0.0319 | 499 | 6,1 | 41.4±1.9 | −0.70 | 0.0234 | 553 | 7,8 | 22.8±1.7 | 1.55 | 0.0919 |
| 80-84 | 407 | 2.7 | 20.6±1.3 | 2.07 | 0.2537 | 189 | 2,3 | 33.7±2.9 | 0.62 | 0.0515 | 218 | 3,1 | 15.3±1.1 | 2.66 | 0.2473 |
| 85+ | 113 | 0.7 | 11.0±1.0 | −1.97 | 0.0448 | 48 | 0,6 | 20.8±3.5 | −8.08 | 0.3721 | 65 | 0,9 | 8.1±1.2 | −0.65 | 0.0515 |
| Total | 15277 | 100 | 6.0±0.1 | 1.57 | 0.7424 | 8202 | 100,0 | 6.7±0.1 | 1.4 | 0.8104 | 7075 | 100,0 | 5.4±0.1 | 1.75 | 0.6069 |
APC, average percentage change. R2, the value of the approximation confidence.
Age-Specific Incidence Rates of Kidney Cancer
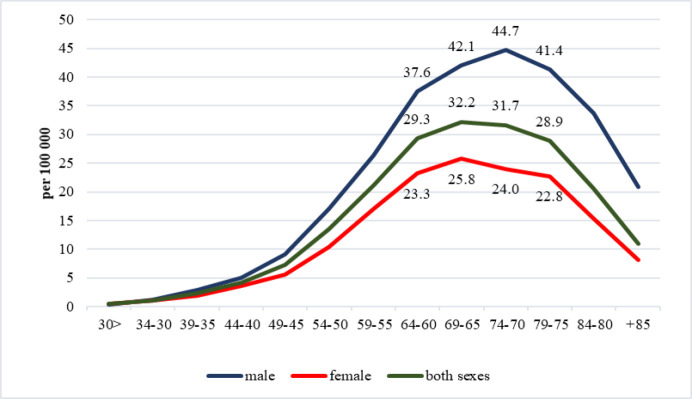
The highest age-specific incidence rates per 100,000 were observed within the age groups of 60-64 years (29.3±0.9), 65-69 years (32.2±1.6), 70-74 years (31.7±1.2), and 75-79 years (28.9±1.4) Figure 1. illustrates the gender-stratified age-related incidence rates.
Figure 1.
Age-Specific Incidence Rates of Kidney Cancer in Kazakhstan, 2005-2019
The incidence of kidney cancer exhibited varying trends across different age groups. Notably, between the ages of 60 and 84, there was a discernible increase in morbidity, with particularly pronounced growth observed in the age brackets of 65-69 years (APC=+3.40), 70-74 years (APC=+2.40), and 80-84 years (APC=+2.07). Moreover, notable approximations were observed primarily within the first two aforementioned age groups (R2=0.6887, R2=0.6047, respectively). These trends were consistently observed across gender lines. Of particular interest is the observation that males within the 30-34 age group experienced a notable increase in incidence rates (APC=+6.09). Conversely, in individuals aged 85 years and older, there was a marked decrease in incidence rates (APC=−8.08) (Table 1).
Temporal Trends in Kidney Cancer Incidence Rates
Throughout the entirety of the study period, there was a discernible trend towards an increase in the crude incidence rate of kidney cancer, rising from 5.8±0.2 (95% CI=5.4-6.2) cases per 100,000 in 2005 to 6.7±0.2 (95% CI=6.3-7.1) cases per 100,000 in 2019 (APC=+1.57). The average annual incidence rate over the 15-year period amounted to 6.0±0.1 (95% CI=5.8-6.3) cases per 100,000. Additionally, it was observed that both crude incidence rates in males and females displayed a tendency towards increase (APC=+1.40; R2=0.8104 and APC=+1.75; R2=0.6069, respectively), exhibiting a high level of approximation. The standardized indicators had some differences. The standardized incidence rate was 6.3±0.1 (95% CI=6.1-6.5) with APC=+0.8 in the entire study population, 8.1±0.1 (95% CI=7.9-8.3) with APC=+0.6 in men, and 5.0±0.1 (95% CI=4.8-5.3) with APC=+0.9 in women.
Regional Variations in Kidney Cancer Incidence and Stage-specific Analysis
The regional incidence rates varied significantly, with the South Kazakhstan region (2.7~0.1) exhibiting the lowest incidence and the northern regions, namely Pavlodar (9.7~0.5), Kostanay (9.9~0.3), and North Kazakhstan (11.8~0.8), demonstrating the highest rates. Standardized indicators closely mirrored this distribution, although Astana exhibited the highest incidence rate (9.3~0.4). Across most regions, there was a discernible trend towards an increase in both crude and standardized incidence rates, except for two areas: the Kyzylorda and West Kazakhstan regions, where a decline was observed. Astana and the Kostanay region showed low growth trends, whereas the North Kazakhstan and Atyrau regions displayed the highest growth rates, with the approximation level nearing 1 (Table 2).
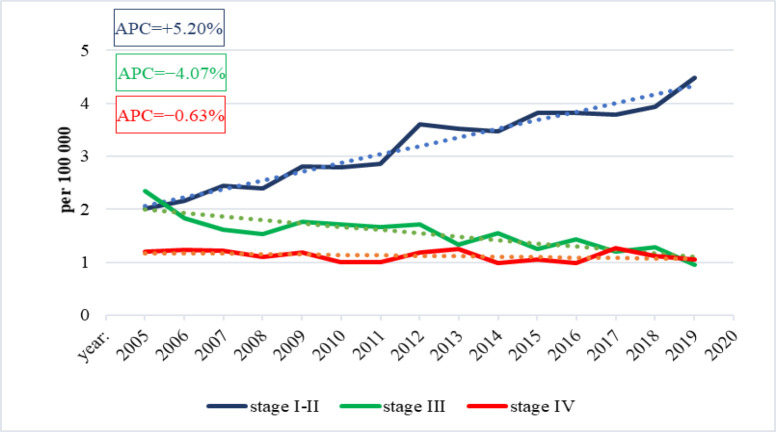
The empirical analysis delineates discernible trends in the incidence rates of kidney cancer across distinct stages. Specifically, there is a notable escalation in incidence rates from stage I–II (APC=+5.20). Conversely, a decrement in incidence rates is observed upon transition to stage III, indicated by an APC of −4.07. Moreover, the incidence rates exhibit a marginal decline in stage IV morbidity, denoted by an APC of −0.63. These observations, depicted in Figure 2, are indicative of discernible patterns in the epidemiological trajectory of kidney cancer, underlining the dynamic nature of its progression through distinct stages.
Figure 2.
Dynamics of Kidney Cancer Incidence by Stage in Kazakhstan, 2005-2019
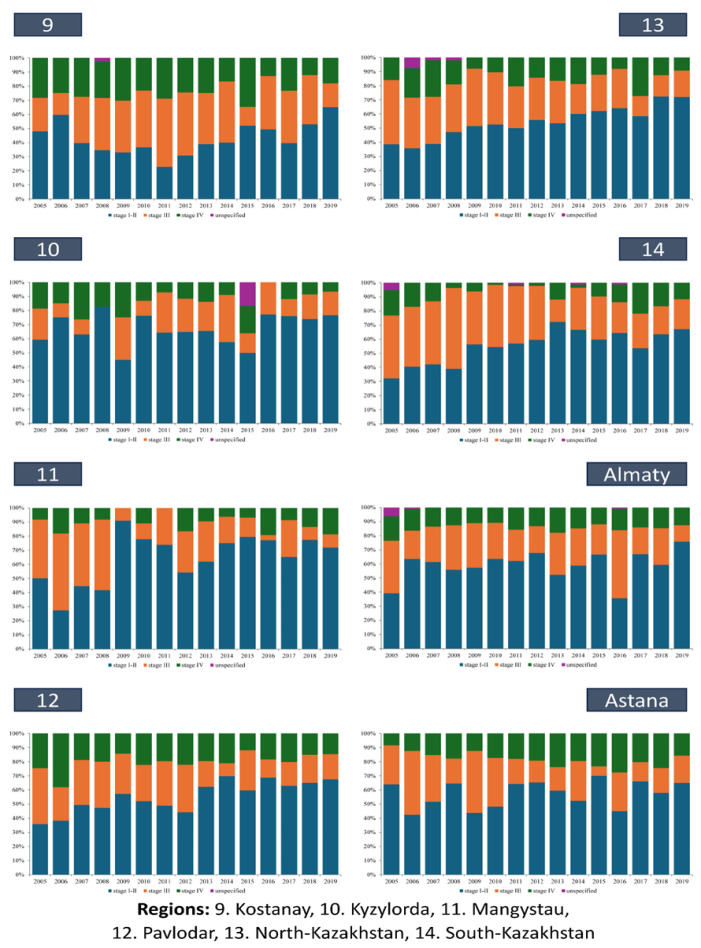
At the regional level, the incidence rates of stage І-II kidney cancer indicate lower rates in the southern and western regions (South-Kazakhstan, Almaty, Zhambyl, Aktobe, Atyrau, Mangistau, Kyzylorda regions) compared to the rest of Kazakhstan, with the highest rates observed in the Pavlodar (5.3~0.5) and North Kazakhstan (6.6~0.8) regions. Trends analysis revealed a decreasing trend in the Kyzylorda region (APC=−0.06) and the city of Almaty (APC=−0.23), while the East Kazakhstan (APC=+8.10), Karaganda (APC=+8.40), and North Kazakhstan (APC=+9.49) regions exhibited high growth rates, with an approximation level nearing 1. Similarly, the study of stage III kidney cancer incidence demonstrated lower rates in the southern and western regions and higher rates in the northern regions. Across all regions, there was a decreasing trend in the incidence of stage III kidney cancer, except for the Kyzylorda (APC=+0.51), Kostanay (APC=+1.32), and Atyrau (APC=+1.88) regions. Notably, the most significant decrease was observed in central Kazakhstan, particularly in the Karaganda region (APC=−9.84). Furthermore, the lowest incidence rates of stage IV kidney cancer were found in the southern and western regions (South-Kazakhstan, Mangistau, Kyzylorda, Almaty, Zhambyl, Atyrau regions). However, Almaty, North Kazakhstan, South Kazakhstan, Mangistau regions, and Astana showed growth rates over 15 years, with the highest growth detected in the Mangystau region (APC=+10.39) (Table 2).
Table 2.
Incidence of Kidney Cancer by Stage and Region, 2005-2019
| Region | Incidence Rate | Morphological verification | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR | ASR | Stage I-II | Stage III | Stage IV | ||||||||||||||
| per 100,000 | APC, % | R2 | per 100,000 | APC, % | R2 | per 100,000 | APC, % | R2 | per 100,000 | APC, % | R2 | per 100,000 | APC, % | R2 | % | APC, % | R2 | |
| Akmola | 8.5±0.5 | 3 | 0.3705 | 7.3±0.4 | 1.67 | 0.1503 | 4.2±0.4 | 6.59 | 0.7431 | 2.0±0.2 | −1.11 | 0.0644 | 2.1+0.2 | −0.24 | 0.0005 | 58.0+1.9 | 1.17 | 0.1734 |
| Aktobe | 4.6±0.3 | 2.36 | 0.2556 | 5.1±0.3 | 1.24 | 0.0925 | 2.2±0.3 | 6.11 | 0.5893 | 1.3±0.1 | −4.29 | 0.4184 | 1.0+0.1 | −0.28 | 0.0008 | 74.3+4.4 | 4.29 | 0.7222 |
| Almaty | 4.0±0.2 | 1.76 | 0.2471 | 4.3±0.2 | 0.96 | 0.0904 | 2.0±0.2 | 7.7 | 0.7046 | 1.3±0.1 | −7.59 | 0.7579 | 0.6+0.05 | 2.61 | 0.2156 | 77.5+3.8 | 3.41 | 0.6677 |
| Atyrau | 3.5±0.3 | 5.33 | 0.5276 | 4.3±0.3 | 4.11 | 0.4386 | 2.3±0.3 | 6.33 | 0.4771 | 0.7±0.1 | 1.88 | 0.1271 | 0.6+0.1 | −1.59 | 0.0027 | 76.3+3.2 | 2.03 | 0.3559 |
| East-Kazakhstan | 8.8±0.4 | 2.58 | 0.4845 | 7.3±0.3 | 1.32 | 0.2165 | 4.1±0.4 | 8.1 | 0.7924 | 2.2±0.2 | −4.05 | 0.3339 | 1.9+0.1 | −0.61 | 0.0324 | 72.5+4.6 | 3.1 | 0.4229 |
| Zhambyl | 3.8±0.2 | 2.02 | 0.2188 | 4.4±0.2 | 0.78 | 0.0412 | 2.2±0.2 | 7.04 | 0.6881 | 0.9±0.1 | −7.33 | 0.418 | 0.6+0.1 | −2.32 | 0.0613 | 76.4+4.3 | 3.59 | 0.592 |
| West-Kazakhstan | 5.7±0.2 | −0.67 | 0.0444 | 5.5±0.2 | −1.71 | 0.2411 | 3.3±0.2 | 0.55 | 0.0064 | 1.3±0.1 | −2.13 | 0.0791 | 0.9+0.1 | −3.80 | 0.2292 | 72.7+3.5 | 3.47 | 0.7307 |
| Karaganda | 8.3±0.4 | 2.69 | 0.5589 | 7.2±0.2 | 1.61 | 0.3047 | 4.5±0.5 | 8.4 | 0.8536 | 1.5±0.3 | −9.84 | 0.3236 | 2.0+0.1 | −0.24 | 0.0046 | 78.7+7.3 | 5.66 | 0.6681 |
| Kostanay | 9.9±0.3 | 1.59 | 0.4278 | 8.1±0.2 | 0.24 | 0.016 | 4.1±0.3 | 3.55 | 0.3425 | 3.2±0.3 | 1.32 | 0.0221 | 2.2+0.2 | −1.72 | 0.131 | 57.6+6.2 | 3.42 | 0.1025 |
| Kyzylorda | 4.0±0.2 | −0.60 | 0.0045 | 5.0±0.3 | −1.86 | 0.0801 | 2.7±0.2 | −0.06 | 0.0024 | 0.7±0.1 | 0.51 | 0.013 | 0.5+0.1 | −7.09 | 0.3953 | 53.5+3.8 | −1.64 | 0.1111 |
| Mangystau | 3.8±0.2 | 2.18 | 0.2771 | 5.3±0.3 | 1.22 | 0.1291 | 2.5±0.3 | 3.34 | 0.3224 | 0.9±0.1 | −6.42 | 0.3915 | 0.4+0.1 | 10.39 | 0.2229 | 72.9+4.3 | 3.22 | 0.462 |
| Pavlodar | 9.7±0.5 | 3.14 | 0.538 | 8.1±0.3 | 1.58 | 0.2541 | 5.3±0.5 | 7.08 | 0.7949 | 2.3±0.2 | −2.42 | 0.1384 | 1.9+0.1 | −1.02 | 0.0126 | 58.4+2.9 | 2.28 | 0.3616 |
| North-Kazakhstan | 11.8±0.8 | 5.03 | 0.7741 | 9.2±0.4 | 3.11 | 0.5959 | 6.6±0.8 | 9.49 | 0.8683 | 3.2±0.2 | −1.60 | 0.0843 | 1.8+0.2 | 3.56 | 0.1152 | 66.9+2.4 | 1.72 | 0.3289 |
| South-Kazakhstan | 2.7±0.1 | 2.35 | 0.4403 | 3.7±0.2 | 1.47 | 0.2405 | 1.5±0.1 | 5.3 | 0.5974 | 0.9±0.1 | −4.24 | 0.3529 | 0.3+0.1 | 4.08 | 0.1175 | 77.2+4.2 | 2.73 | 0.457 |
| Almaty city | 7.2±0.2 | 1.76 | 0.3258 | 7.1±0.2 | 0.96 | 0.3514 | 4.1±0.2 | −0.23 | 0.0006 | 1.9±0.2 | −2.37 | 0.1439 | 1.0+0.1 | −1.75 | 0.1262 | 58.5+4.7 | 4.26 | 0.4028 |
| Astana city | 6.9±0.2 | 0.44 | 0.0083 | 9.3±0.4 | 0.32 | 0.0112 | 3.8±0.2 | 1.45 | 0.0689 | 1.6±0.2 | −5.62 | 0.28 | 1.2+0.1 | 4.71 | 0.4434 | 78.5+3.4 | 2.5 | 0.5057 |
| Kazakhstan | 6.0±0.1 | 1.57 | 0.7424 | 6.3±0.1 | 0.78 | 0.3813 | 3.2±0.2 | 5.2 | 0.9466 | 1.5±0.1 | −4.07 | 0.7282 | 1.1+0.03 | −0.63 | 0.1145 | 68.8+3.04 | 3.59 | 0.9043 |
CR, Crude Incidence Rate; ASR, Age-standardized Incidence Rate
Time Trends of Kidney Cancer Stage Distribution
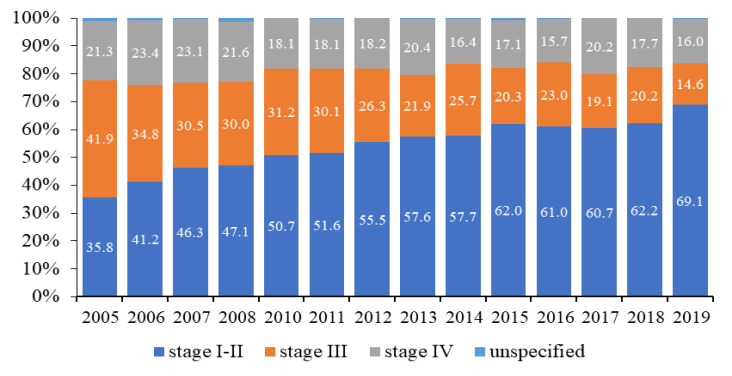
In the temporal trajectory, the mean proportion of kidney cancer patients in stages I–II exhibited a notable increase, rising from 35.8% in 2005 to 69.1% in 2019 (Figure 3), representing an annual average of 53.8%. Conversely, the proportion of patients diagnosed with stage III disease experienced a decline from 41.9% in 2005 to 14.6% in 2019 (Figure 3), with an annual mean of 26.7%. Over the same period, there was a decrease in the prevalence of patients with stage IV kidney cancer from 21.3% (2005) to 16.0% (2019), with an annual average of 19.2%. Additionally, instances where the disease stage was unspecified were observed, constituting 1.1% of cases in 2005 and decreasing to an average of 0.3% throughout subsequent years.
Figure 3.
Dynamics of Indicators of Early Diagnosis (stage I–II) and Neglect (stage III and IV) of Kidney Cancer in Kazakhstan in 2005-2019.
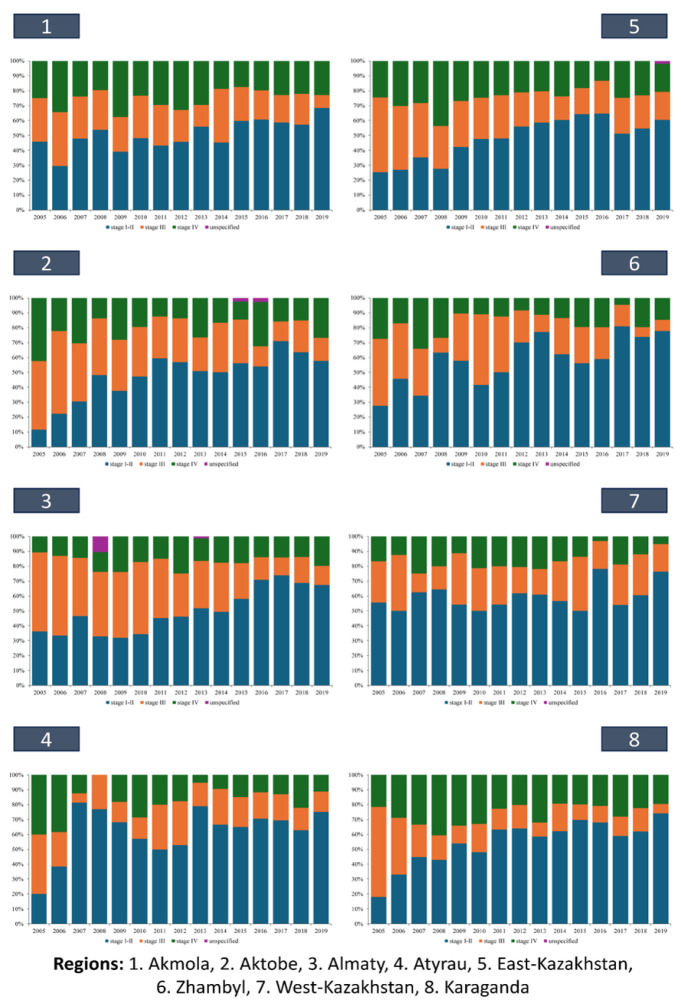
This trend was consistently mirrored across various regions of Kazakhstan (Figures 4A and 4B). Notably, in certain regions (Aktobe – 47.8%, Almaty – 49.8%, East Kazakhstan – 48.2%, Kostanay – 42.8%), the mean proportion of stage I-II cases remained below 50%. And only in Almaty (16.4%), Atyrau (17.6%), West Kazakhstan (16.0%), Zhambyl (16.8%), Kyzylorda (13.6%), Mangystau (10.4%), North Kazakhstan (15.8%), South Kazakhstan (10.0%) regions, and in the cities of Almaty (14.1%) and Astana (18.4%), did the proportion of stage IV cases was below 20% on average over the study period. Within specific regions such as Aktobe, Almaty, East Kazakhstan, Kostanay, Kyzylorda, North Kazakhstan, South Kazakhstan, as well as in the city of Almaty, instances of kidney cancer cases with unspecified stages were recorded (Figures 4A and 4B). Notably, in the Kyzylorda region, such cases constituted 16.7% of cases in 2015, marking a significant observation (Figures 4B).
Figure 4A.
Dynamics of Indicators of Stage I–II and Stage III and IV Kidney Cancer in Kazakhstan by Region, 2005-2019
Morphological Verification and Diagnostic Practices
Throughout Kazakhstan, the level of morphological verification for newly diagnosed diagnoses was generally low. On average, the morphological verification level across the country was 68.8+3.04%. Notably, in 5 (Akmola – 58.0+1.9%, Kyzylorda – 53.5+3.8%, Kostanay – 57.6+6.2%, Pavlodar – 58.4+2.9%) out of 14 regions and in the city of Almaty (58.5+4.7%), this indicator was even lower than 70%. In the remaining regions, the percentage slightly exceeded 70%, with the highest rates observed in the city of Astana (78.5%) and the Karaganda region (78.7%). Moreover, positive dynamics were observed in all regions during the study period, with an overall growth in the morphological verification indicator. The only exception was the Kyzylorda region, where a decline in the verification rate was noted (APC=−1.64) (Table 2).
Figure 4В.
Dynamics of Indicators of Stage I–II and Stage III and IV Kidney Cancer in Kazakhstan by Rregion, 2005-2019
Discussion
During the investigated timeframe, there was an increase in the incidence of kidney cancer in Kazakhstan. This trend likely mirrors global patterns attributed to heightened exposure to risk factors and the incidental discovery of malignancies during imaging evaluations for unrelated medical concerns [2].
Temporal analysis unveiled a consistent upward trajectory in crude incidence rates, albeit accompanied by regional disparities. Notably, northern regions exhibited elevated incidence rates compared to their southern and western counterparts. It is imperative to highlight that in certain regions, alongside the surge in early-stage kidney cancer incidence, there was also a concurrent rise in stage III and IV cases. Our interpretation suggests that these geographic variations may stem from unidentified environmental factors. Numerous studies have posited an augmented risk of kidney cancer linked to specific occupational and environmental exposures [34]. However, it is plausible that dietary practices within specific regions may contribute partially to the elevated incidence rates of kidney cancer. Therefore, concerted efforts are imperative to delve into the potential effects of these risk factors and their interplay. Establishing causal relationships necessitates dedicated investigations encompassing all plausible risk factors.
It is well-established that kidney cancer susceptibility is influenced by various factors including family history [35], male gender [2], age [2], hypertension [36], and a history of kidney disease [3]. Consistent with global trends, our analysis of kidney cancer cases in Kazakhstan revealed a higher prevalence among males compared to females. In kidney cancer, the incidence rates exhibit an age-dependent increase in both genders, with the highest rates typically observed among the elderly population [2]. The majority of cases were observed in individuals aged 50-69 years, indicating a potential age-related susceptibility to the disease. Furthermore, age-specific incidence rates exhibited peaks within the 60-79 age groups, with a discernible increase in incidence observed among individuals aged 60 to 84. These findings underscore the importance of age as a contributing factor to kidney cancer incidence, suggesting a potential age-related vulnerability to the disease within the population studied.
Renal malignancies in the younger population represent a rare occurrence, albeit exhibiting a progressive trajectory over time. Incidence rates of kidney cancer among younger individuals are notably lower compared to the broader demographic [37]. Despite the recognized significance, scant attention has been directed towards comprehensive investigations of cancer prevalence within the younger demographic, as evidenced by the limited scope of prior large-scale inquiries [38, 39]. Our study brings to light a noteworthy surge in the incidence of kidney cancer within the 30-34 age bracket, particularly pronounced among males, where the growth rate reached +6.09. This finding substantiates earlier research endeavors [39-41], which similarly highlighted a substantial uptick in kidney cancer incidence among younger individuals. The congruence between our results and previous findings underscores the persisting trend of escalating kidney cancer incidence rates among the younger population.
In our investigation spanning an average duration of 15 years, over 45% of individuals were diagnosed with diagnosed with stage III and stage IV kidney cancer upon initial evaluation. This phenomenon is primarily attributed to delayed detection of the condition. Consistent with international research, approximately sixty percent of all kidney cancer cases present asymptomatically [42], contributing to diagnosis at advanced stages, with over a quarter of patients exhibiting metastases upon initial diagnosis [43].
There has been a notable rise in the proportion of kidney cancer cases diagnosed at stages I-II over the study period, suggesting possible advancements in early detection methodologies. Our findings indicate that the upsurge in kidney cancer incidence primarily stems from heightened occurrences and quantities of localized tumors. These observations align with previous global studies [44-46]. which have also documented an escalation in localized disease prevalence. Such trends are attributed, in part, to the expanded utilization of imaging modalities facilitating early disease detection.
However, challenges remain in morphological verification practices, with overall low verification rates across the country, particularly in certain regions. While positive dynamics were observed in most regions, efforts are needed to enhance verification practices, particularly in regions with lower rates.
Kidney cancer stands as the most fatal urological malignancy, with a grim prognosis: approximately 50% of afflicted individuals succumb to the disease [47]. The five-year survival rate dramatically varies based on disease staging, with patients diagnosed at stage four facing a mere 12% chance of survival compared to an 87% survival rate for those diagnosed at stage one [43]. The rising incidence of kidney cancer, coupled with a significant proportion of asymptomatic cases at the time of diagnosis and elevated mortality rates, highlights kidney cancer’s potential suitability for screening interventions based on established criteria. Globally, there is considerable attention focused on delineating the most effective approach for the early detection of this “latent” malignancy [48], which holds significant potential for cure when diagnosed in its nascent stages. A recent investigation into the early identification and management of kidney cancer has been highlighted as a foremost research imperative by both patients affected by kidney cancer, their caregivers, and seasoned clinical practitioners [49, 50].
Thus, for the first time, our study provides a detailed analysis of the incidence of kidney cancer in Kazakhstan, taking into account the stage of the disease, age, gender, and morphological verification. The findings underscore the importance of ongoing surveillance and targeted interventions to address regional disparities and improve diagnostic accuracy, ultimately contributing to better management and outcomes for kidney cancer patients.
Limitations of the study
It should be noted that in our study, morphological verification of cancer was not always utilized in 100% of cases. In the post-Soviet region, other diagnostic methods, such as radiological studies and clinical data, often complement or replace morphological verification. This can lead to a certain degree of uncertainty in the accuracy of diagnosis and classification of cancer stages, representing an important limitation of our study.
Acknowledgements
The authors greatly appreciate the contribution of the Ministry of Healthcare of the Republic of Kazakhstan to the current research by providing the data.
This study was not funded, it was performed within the framework of Sergey Dyakov’s dissertation, the topic of the dissertation was approved at the University Council of Akhunbaev Kyrgyz State Medical Academy.
Conflict of interest
The authors declare that there is no conflict of interest.
Author Contribution Statement
SD, ZhT, Ish, ASh – Collection and preparation of data, primary processing of the material and their verification. AJ, ZB, SD, SA, SM – Statistical processing and analysis of the material, writing the text of the article (material and methods, results). SD, GI, DT, NS, VG – Writing the text of the article (introduction, discussion). NI, ZB, IK, VL– Concept, design and control of the research, approval of the final version of the article. All authors approved the final version of the manuscript.
References
- 1.Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L. Global cancer observatory: Cancer today (version 1 1) Lyon: france: International agency for research on cancer; [Google Scholar]
- 2.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67(3):519–30. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Rossi SH, Klatte T, Usher-Smith J, Stewart GD. Epidemiology and screening for renal cancer. World J Urol. 2018;36(9):1341–53. doi: 10.1007/s00345-018-2286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, van Melle M, Singh H, Hamilton W, Lyratzopoulos G, Walter FM. Quality of the diagnostic process in patients presenting with symptoms suggestive of bladder or kidney cancer: A systematic review. BMJ Open. 2019;9(10):e029143 . doi: 10.1136/bmjopen-2019-029143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capitanio U, Montorsi F. Renal cancer. Lancet. 2016;387(10021):894–906. doi: 10.1016/S0140-6736(15)00046-X. [DOI] [PubMed] [Google Scholar]
- 6.Vasudev NS, Wilson M, Stewart GD, Adeyoju A, Cartledge J, Kimuli M, et al. Challenges of early renal cancer detection: Symptom patterns and incidental diagnosis rate in a multicentre prospective uk cohort of patients presenting with suspected renal cancer. BMJ Open. 2020;10(5):e035938 . doi: 10.1136/bmjopen-2019-035938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Rahman O. Risk of subsequent primary kidney cancer after another malignancy: A population-based study. Clin Genitourin Cancer. 2017;15(5):e747–e54. doi: 10.1016/j.clgc.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Jazieh K, Baidoun F, Torrejon N, Merjaneh Z, Saad A, Gad M, et al. Studying the association between breast cancer and renal cell carcinoma. Breast Cancer Res Treat. 2022;191(3):643–52. doi: 10.1007/s10549-021-06465-4. [DOI] [PubMed] [Google Scholar]
- 9.Igissinov N, Kozhakhmetov S, Zhantubetova M, Igissinova G, Bilyalova Z, Akpolatova G, et al. Thyroid cancer in kazakhstan: Component analysis of incidence dynamics. Asian Pac J Cancer Prev. 2019;20(9):2875–80. doi: 10.31557/APJCP.2019.20.9.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toguzbayeva A, Telmanova Z, Khozhayev A, Jakipbayeva A, Aimbetova G, Zhantureyeva A, et al. Impact of screening on breast cancer incidence in kazakhstan: Results of component analysis. Asian Pac J Cancer Prev. 2021;22(9):2807–17. doi: 10.31557/APJCP.2021.22.9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igissinov N, Toguzbayeva A, Telmanova Z, Baibusunova A, Abdykalikova A, Igissinova G, et al. Evaluation of breast cancer incidence trends in the karaganda region of kazakhstan. Iran J Public Health. 2022;51(10):2308–16. doi: 10.18502/ijph.v51i10.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauyenova D, Zhadykova Y, Khozhayev A, Turebayev D, Kulmirzayeva D, Urazova S, et al. Trends of colorectal cancer incidence in kazakhstan. Asian Pac J Cancer Prev. 2021;22(10):3405. doi: 10.31557/APJCP.2021.22.10.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yessenbayev D, Khamidullina Z, Tarzhanova D, Orazova G, Zhakupova T, Kassenova D, et al. Epidemiology of lung cancer in kazakhstan: Trends and geographic distribution. Asian Pac J Cancer Prev. 2023;24(5):1521–32. doi: 10.31557/APJCP.2023.24.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igissinov N, Toguzbayeva A, Turdaliyeva B, Igissinova G, Bilyalova Z, Akpolatova G, et al. Breast cancer in megapolises of kazakhstan: Epidemiological assessment of incidence and mortality. Iran J Public Health. 2019;48(7):1257–64. [PMC free article] [PubMed] [Google Scholar]
- 15.Azhetova Z, Khamidullina Z, Telmanova Z, Assylbek A, Bilyalova Z, Igissinova G, et al. Corpus uteri cancer in kazakhstan: Recent incidence trends. Asian Pac J Cancer Prev. 2023;24(3):849–57. doi: 10.31557/APJCP.2023.24.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayembayev F, Rakhimzhanova R, Dautov T, Saduakassova A, Telmanova Z, Bilyalova Z, et al. Lymphosarcoma incidence in kazakhstan: A retrospective survey (2010-2019) Asian Pac J Cancer Prev. 2023;24(6):1885–96. doi: 10.31557/APJCP.2023.24.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edition s, edge s, byrd d. Ajcc cancer staging manual. 8th ed. Springer-verlag; 2017. [Google Scholar]
- 18.Attalla K, Weng S, Voss MH, Hakimi AA. Epidemiology, risk assessment, and biomarkers for patients with advanced renal cell carcinoma. Urol Clin North Am. 2020;47(3):293–303. doi: 10.1016/j.ucl.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhindi B, Lohse CM, Mason RJ, Westerman ME, Cheville JC, Tollefson MK, et al. Are we using the best tumor size cut-points for renal cell carcinoma staging? Urology. 2017;109:121–6. doi: 10.1016/j.urology.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi M, Becker A, Trinh QD, Abdollah F, Tian Z, Shariat SF, et al. An analysis of patients with t2 renal cell carcinoma (rcc) according to tumour size: A population-based analysis. BJU Int. 2013;111(8):1184–90. doi: 10.1111/bju.12084. [DOI] [PubMed] [Google Scholar]
- 21.Patel HD, Kates M, Pierorazio PM, Hyams ES, Gorin MA, Ball MW, et al. Survival after diagnosis of localized t1a kidney cancer: Current population-based practice of surgery and nonsurgical management. Urology. 2014;83(1):126–32. doi: 10.1016/j.urology.2013.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laird A, Choy KC, Delaney H, Cutress ML, O’Connor KM, Tolley DA, et al. Matched pair analysis of laparoscopic versus open radical nephrectomy for the treatment of t3 renal cell carcinoma. World J Urol. 2015;33(1):25–32. doi: 10.1007/s00345-014-1280-y. [DOI] [PubMed] [Google Scholar]
- 23.Ornellas AA, Andrade DM, Ornellas P, Wisnescky A, Schwindt AB. Prognostic factors in renal cell carcinoma: Analysis of 227 patients treated at the brazilian national cancer institute. Int Braz J Urol. 2012;38(2):185–94. doi: 10.1590/s1677-55382012000200006. [DOI] [PubMed] [Google Scholar]
- 24.Schiavina R, Borghesi M, Chessa F, Dababneh H, Bianchi L, Della Mora L, et al. The prognostic impact of tumor size on cancer-specific and overall survival among patients with pathologic t3a renal cell carcinoma. Clin Genitourin Cancer. 2015;13(4):e235–e41. doi: 10.1016/j.clgc.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Brookman-May SD, May M, Wolff I, Zigeuner R, Hutterer GC, Cindolo L, et al. Evaluation of the prognostic significance of perirenal fat invasion and tumor size in patients with pt1-pt3a localized renal cell carcinoma in a comprehensive multicenter study of the corona project Can we improve prognostic discrimination for patients with stage pt3a tumors? Eur Urol. 2015;67(5):943–51. doi: 10.1016/j.eururo.2014.11.055. [DOI] [PubMed] [Google Scholar]
- 26.Dunnick NR. Renal cell carcinoma: Staging and surveillance. Abdom Radiol (NY) 2016;41(6):1079–85. doi: 10.1007/s00261-016-0692-0. [DOI] [PubMed] [Google Scholar]
- 27.Bai J, Lu Q, Wen Y, Shangguan T, Ye Y, Lin J, et al. Development and validation of a nomogram for predicting the impact of tumor size on cancer-specific survival of locally advanced renal cell carcinoma: A seer-based study. Aging (Albany NY) 2024;16(4):3823–36. doi: 10.18632/aging.205562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bureau of national statistics of the agency for strategic planning and reforms of the republic of kazakhstan (2023) [cited 2024 april 01] Available from: https://www.Cancerresearchuk.Org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer.
- 29.Ahmad ob, boschi-pinto c, lopez ad, murray cj, lozano r, inoue m. Age standardization of rates: A new who standard. Vol. 9. Geneva: World health organization; 2001. pp. 1–4. [Google Scholar]
- 30.National cancer institute. Recommendations on the use of the world standard (who 2000-2025) [cited 2024 april 02] 2013. Available from: http://seer.Cancer.Gov/stdpopulations/world.Who.html.
- 31.Glanz s. Biomedical statistics. Moscow: Practice, russian: 1998. p. 459. [Google Scholar]
- 32.Merkov am. Health statistics. Leningrad: Medicine, russian. p. 384 .
- 33.Dos santos silva i. 1999. p. 441. [Google Scholar]
- 34.Li P, Znaor A, Holcatova I, Fabianova E, Mates D, Wozniak MB, et al. Regional geographic variations in kidney cancer incidence rates in european countries. Eur Urol. 2015;67(6):1134–41. doi: 10.1016/j.eururo.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Clague J, Lin J, Cassidy A, Matin S, Tannir NM, Tamboli P, et al. Family history and risk of renal cell carcinoma: Results from a case-control study and systematic meta-analysis. Cancer Epidemiol Biomarkers Prev. 2009;18(3):801–7. doi: 10.1158/1055-9965.EPI-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macleod LC, Hotaling JM, Wright JL, Davenport MT, Gore JL, Harper J, et al. Risk factors for renal cell carcinoma in the vital study. J Urol. 2013;190(5):1657–61. doi: 10.1016/j.juro.2013.04.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74–84. doi: 10.1016/j.eururo.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barr RD, Ries LA, Lewis DR, Harlan LC, Keegan TH, Pollock BH, et al. Incidence and incidence trends of the most frequent cancers in adolescent and young adult americans, including “nonmalignant/noninvasive” tumors. Cancer. 2016;122(7):1000–8. doi: 10.1002/cncr.29867. [DOI] [PubMed] [Google Scholar]
- 39.Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: A population-based study. Lancet Oncol. 2017;18(12):1579–89. doi: 10.1016/S1470-2045(17)30677-0. [DOI] [PubMed] [Google Scholar]
- 40.Kehm RD, Yang W, Tehranifar P, Terry MB. 40 years of change in age- and stage-specific cancer incidence rates in us women and men. JNCI Cancer Spectr. 2019;3(3):pkz038. doi: 10.1093/jncics/pkz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palumbo C, Pecoraro A, Rosiello G, Luzzago S, Deuker M, Stolzenbach F, et al. Renal cell carcinoma incidence rates and trends in young adults aged 20-39 years. Cancer Epidemiol. 2020;67:101762. doi: 10.1016/j.canep.2020.101762. [DOI] [PubMed] [Google Scholar]
- 42.Selby PJ, Banks RE, Gregory W, Hewison J, Rosenberg W, Altman DG, et al. Programme grants for applied research Methods for the evaluation of biomarkers in patients with kidney and liver diseases: Multicentre research programme including elucidate rct. Southampton (UK): [PubMed] [Google Scholar]
- 43.Cancer research uk. 2024. [[cited 2024 april 10]]. Available from: https://www.Cancerresearchuk.Org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer.
- 44.King SC, Pollack LA, Li J, King JB, Master VA. Continued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease: United states 2001 to 2010. J Urol. 2014;191(6):1665–70. doi: 10.1016/j.juro.2013.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan HJ, Filson CP, Litwin MS. Contemporary, age-based trends in the incidence and management of patients with early-stage kidney cancer. Urol Oncol. 2015;33(1):21.e19–21. doi: 10.1016/j.urolonc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Palumbo C, Pecoraro A, Knipper S, Rosiello G, Luzzago S, Deuker M, et al. Contemporary age-adjusted incidence and mortality rates of renal cell carcinoma: Analysis according to gender, race, stage, grade, and histology. Eur Urol Focus. 2021;7(3):644–52. doi: 10.1016/j.euf.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Jones J, Bhatt J, Avery J, Laupacis A, Cowan K, Basappa N, et al. The kidney cancer research priority-setting partnership: Identifying the top 10 research priorities as defined by patients, caregivers, and expert clinicians. Can Urol Assoc J. 2017;11(12):379–87. doi: 10.5489/cuaj.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossi SH, Fielding A, Blick C, Handforth C, Brown JE, Stewart GD. Setting research priorities in partnership with patients to provide patient-centred urological cancer care. Eur Urol. 2019;75(6):891–3. doi: 10.1016/j.eururo.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Rossi SH, Blick C, Handforth C, Brown JE, Stewart GD. Essential research priorities in renal cancer: A modified delphi consensus statement. Eur Urol Focus. 2020;6(5):991–8. doi: 10.1016/j.euf.2019.01.014. [DOI] [PubMed] [Google Scholar]