Abstract
Background:
-positive male breast cancer (MBC) is a rare condition that has a poor prognosis. The purpose of this study was to establish a nomogram model for predicting the prognosis of HER2-positive MBC patients.
Methods:
240 HER2-positive MBC patients from 2004 to 2015 were retrieved from the surveillance, epidemiology, and end results (SEER) database. All HER2-positive MBC patients were divided randomly into training (n = 144) and validation cohorts (n = 96) according to a ratio of 6:4. Univariate and multivariate Cox regression analyses were used to determine the prognostic factors associated with HER2-positive MBC patients. A clinical prediction model was constructed to predict the overall survival of these patients. The nomogram model was assessed by using receiver operating characteristics (ROC) curves, calibration plots and decision curve analysis (DCA).
Results:
The Cox regression analysis showed that T-stage, M-stage, surgery and chemotherapy were independent risk factors for the prognosis of HER2-positive MBC patients. The model could also accurately predict the Overall survival (OS) of the patients. In the training and validation cohorts, the C indexes of the OS nomograms were 0.746 (0.677–0.815) and 0.754 (0.679–0.829), respectively. Calibration curves and DCA verified the reliability and accuracy of the clinical prediction model.
Conclusion:
In conclusion, the predictive model constructed had good clinical utility and can help the clinician to select appropriate treatment strategies for HER2-positive MBC patients.
Key Words: HER2-positive, male breast cancer, SEER database, nomogram, Cox regression
Introduction
The incidence and mortality of breast cancer rank first among women in the world [1]. However, male breast cancer (MBC) is a very rare malignancy, accounting for less than 1% of all breast cancer patients [2] and approximately 1% of male cancers worldwide [2]. Although the incidence of MBC is very low, its global incidence continues to increase annually [3]. Since, MBC patients have a relatively poorer prognosis, [4, 5] it is important to determine the prognostic factors with proper treatment strategies.
Human epidermal growth factor receptor-2 (HER2) is overexpressed in about 20-30% of breast cancer patients [6], and can affect the survival prognosis of these patients. Trastuzumab is one of the main targeted drugs for the treatment of HER2-positive breast cancer patients at present. Its mechanism of action is to inhibit the proliferation of breast cancer cells by targeting the synthesis of HER2 protein, to improve the survival prognosis of HER2-positive breast cancer patients [7-9].
The incidence of HER2-positive MBC is extremely low, and there have been only a few published studies on this condition. Only 15% of MBC patients are HER2-positive [10]. Due to the rare incidence and lack of prospective clinical data, there is no uniform standard for the treatment for HER2-positive MBC. Trastuzumab treatment has been used [11, 12], but due to the small number of cases, there is insufficient evidence to support its effectiveness. Studies have shown that the HER2 status can affect the survival prognosis of MBC patients, and those who were HER2-positive had a worse prognosis than female patients with breast cancer [13]. In addition, the incidence of MBC is increasing annually, and the prognosis for HER2-positive men is still poor. To date, the prognostic factors and treatment of HER2-positive MBC patients have been rarely reported. In this, we aimed to explore the prognostic factors and treatment methods of HER2-positive MBC patients. This study also aimed to establish a clinical prediction model for predicting the risk factors affecting the prognosis of HER2-positive MBC, in order to provide more evidence for the clinical treatment and prognosis evaluation of this condition.
Materials and Methods
Data source
Clinical data on HER2-positive MBC were retrieved by using the surveillance, epidemiology and end results (SEER)*Stat version 8.4.0.1 dataset (http://www.seer.cancer.gov/seerstat). Data was obtained on newly diagnosed HER2-positive MBC patients from the database from 2010 to 2015, and extracted age, race, marital status, tissue grade, tumor size, T stage, N stage, M stage, HER2 status, ER status, PR status, surgery, radiotherapy, chemotherapy, survival status, survival time as well as any other relevant information.
Patient Selection
Patients were selected with confirmed HER2-positive MBC as the research subjects. The included patients met the following criteria: (1)Their gender was “male”, (2) their lesions were defined as “Breast” according to ICD-O-3/WHO 2008, (2) ICD-O-3 Hist/behav, malignant: ICD-O-3 Hist/ Behav, Malignant: 8500/3-8504/3,8507/3-8510/3,8512/3-8514/3, 8520/3-8525/3,8530/3,8540/3-8543/3 and (3) the year of diagnosis was “2010-2015”. The exclusion criteria were as follows: (1) the type of source of diagnostic report were missing from either the autopsy or death certificate and (2) their age, race, marital status, tissue grade, tumor size, T stage, N stage, M stage, HER2 status, ER status, PR status, Surgery, radiotherapy, chemotherapy, survival status, survival time were not known. The procedure used for screening the research subjects is shown in Figure 1.
Figure 1.
A Diagrammatic Representation of the Patient Selection Process. AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis; SEER, surveillance, epidemiology and end results.
Nomogram Construction and Validation
All HER2-positive MBC patients were divided into training (n=144) and validation (n=96) cohorts at a ratio of 6 : 4. Check the frequency distribution of the training and validation cohorts and the results are presented as a KDE (kernel density estimation) chart (Figure 2). All variables were tested for Pearson correlations and the results are presented as a heat map (Figure 3). Univariate COX analysis was performed to screen for potential prognostic factors. Variables with a P value < 0.05 in univariate analysis were further incorporated into multivariate COX regression analysis to identify the prognostic factors of HER2-positive MBC patients. In addition, we established a predictive model based on prognostic factors in order to predict the prognosis of HER2-positive MBC patients. The area under the curve (AUC) was used to evaluate the accuracy of the model. The calibration and clinical decision analysis (DCA) curves were used to evaluate and assess the models.
Figure 2.
The KDE Chart Shows that the Individual variables (Race, Age, Marital status,Grade, Laterality, T stage, N stage, M stage, Tumor size, ER status, PR status, Surgery, Radiotherapy, Chemotherapy, survival status) in the training and test queues have the same distribution
Figure 3.
The Results of Pearson Correlation Analysis between All the Variables. The heat map shows the correlation between the variables.
Statistical Analysis
All statistical calculations were performed using R (version 4.2.0) and Python (version 3.8.1, Python Software Foundation). Categorical data were compared using the chi-square and Fisher’s exact tests. Kaplan–Meier analysis was used to calculate the overall survival (OS) of HER2-positive MBC patients. A two-sided P value < 0.05 was considered to be statistically significant.
Results
Demographic and clinical characteristics of HER2-positive MBC patients
Based on the inclusion criteria, between 2010 and 2015, 240 patients were diagnosed with HER2-positive MBC. The median survival was 60 months. All the patients were randomly divided into training (n = 144) and verification cohorts (n = 96) according to the ratio of 6:4. In the training cohort, white and married patients accounted for 72.9 and 66.0% of the total, respectively. 64 (44.4%) of the patients were less than 65 years old and the tumor grade they presented with was mainly grade III (55.6%). According to the AJCC guidelines, there were 67 cases (46.5%) at T2 and 56 cases (52.8%) at N0. With respect to the M stage, there were 130 cases (90.3%) at stage M0. Most of the patients had a tumor size < 50 (92.4%). The ER and PR status of most patients were positive, accounting for 92.4 and 83.3%, respectively. In terms of treatment, 129 patients (89.6%) received surgical treatment, 108 (75.0%) received no radiation therapy and 98 (68.1%) received chemotherapy. The chi-square and Fisher’s exact tests showed no significant differences in any of the variables when the training and verification cohorts were compared (Table 1).
Table 1.
Demographic and Clinical Characteristics of HER2-Positive MBC Patients.
| Characteristics | Overall N (%) |
Train N (%) |
Test N (%) |
P |
|---|---|---|---|---|
| Race | ||||
| Black | 41 (17.1) | 28 (19.4) | 13 (13.5) | 0.136 |
| Other | 14 (5.8) | 11 (7.6) | 3 (3.1) | |
| White | 185 (77.1) | 105 (72.9) | 80 (83.3) | |
| Age | ||||
| <65 | 117 (48.8) | 64 (44.4) | 53 (55.2) | 0.102 |
| ≥65 | 123 (51.2) | 80 (55.6) | 43 (44.8) | |
| Marital status | ||||
| Married | 160 (66.7) | 95 (66.0) | 65 (67.7) | 0.78 |
| Unmarried | 80 (33.3) | 49 (34.0) | 31 (32.3) | |
| Grade | ||||
| I | 3 (1.2) | 2 (1.4) | 1 (1.0) | 0.751 |
| II | 109 (45.4) | 62 (43.1) | 47 (49.0) | |
| III | 128 (53.3) | 80 (55.6) | 48 (50.0) | |
| Laterality | ||||
| Left | 130 (54.2) | 68 (47.2) | 62 (64.6) | 0.008 |
| Right | 110 (45.8) | 76 (52.8) | 34 (35.4) | |
| T stage | ||||
| T1 | 84 (35.0) | 53 (36.8) | 31 (32.3) | 0.617 |
| T2 | 120 (50.0) | 67 (46.5) | 53 (55.2) | |
| T3 | 9 (3.8) | 6 (4.2) | 3 (3.1) | |
| T4 | 27 (11.2) | 18 (12.5) | 9 (9.4) | |
| N stage | ||||
| N0 | 113 (47.1) | 76 (52.8) | 37 (38.5) | 0.023 |
| N1 | 89 (37.1) | 46 (31.9) | 43 (44.8) | |
| N2 | 21 (8.8) | 9 (6.2) | 12 (12.5) | |
| N3 | 17 (7.1) | 13 (9.0) | 4 (4.2) | |
| M stage | ||||
| M0 | 218 (90.8) | 130 (90.3) | 88 (91.7) | 0.715 |
| M1 | 22 (9.02) | 14 (9.7) | 8 (8.3) | |
| Tumor size | ||||
| <50 | 223 (92.9) | 133 (92.4) | 90 (93.8) | 0.681 |
| ≥50 | 17 (7.1) | 11 (7.6) | 6 (6.2) | |
| ER | ||||
| Negative | 15 (6.2) | 11 (7.6) | 4 (4.2) | 0.276 |
| Positive | 225 (93.8) | 133 (92.4) | 92 (95.8) | |
| PR | ||||
| Negative | 39 (16.2) | 24 (16.7) | 15 (15.6) | 0.83 |
| Positive | 201 (83.8) | 120 (83.3) | 81 (84.4) | |
| Surgery | ||||
| No | 22 (9.2) | 15 (10.4) | 7 (7.3) | 0.411 |
| Yes | 218 (90.8) | 129 (89.6) | 89 (92.7) | |
| Radiation | ||||
| None/Unknown | 176 (73.3) | 108 (75.0) | 68 (70.8) | 0.475 |
| Yes | 64 (26.7) | 36 (25.0) | 28 (29.2) | |
| Chemotherapy | ||||
| No/Unknown | 78 (32.5) | 46 (31.9) | 32 (33.3) | 0.822 |
| Yes | 162 (67.5) | 98 (68.1) | 64 (66.7) |
Analysis of survival prognostic factors in HER2-positive MBC patients
It was found that the variables that related to the survival prognosis of HER2-positive MBC patients were grade, T stage, N stage, M stage, tumor size, surgery and chemotherapy by using univariate COX regression analysis. Multivariate COX regression analysis showed that T stage, M stage, surgery and chemotherapy were independent risk factors for the prognosis of HER2-positive MBC patients (P < 0.05), (Table 2).
Table 2.
COX Regression Analysis of the Prognostic Factors Associated with HER2-Positive MBC Patients.
| Characteristics | Univariable OR (95% CI)" |
P | Multivariable OR (95% CI)" |
P |
|---|---|---|---|---|
| Race | ||||
| Black | 1 (reference) | |||
| Other | 0.19 (0.02-1.44) | 0.108 | ||
| White | 0.90 (0.46-1.75) | 0.75 | ||
| Age | ||||
| <65 | 1 (reference) | |||
| ≥65 | 1.44 (0.82-2.50) | 0.202 | ||
| Marital status | ||||
| Married | 1 (reference) | |||
| Unmarried | 1.57 (0.91-2.72) | 0.104 | ||
| Grade | ||||
| I | 1 (reference) | |||
| II | 0.18 (0.04-0.76) | 0.02 | 1.08 (0.15 - 7.93) | 0.94 |
| III | 0.14 (0.03-0.61) | 0.009 | 1.05 (0.14 - 7.98) | 0.962 |
| Laterality | ||||
| Left | 1 (reference) | |||
| Right | 0.63 (0.37-1.09) | 0.1 | ||
| T stage | ||||
| T1 | 1 (reference) | |||
| T2 | 2.03 (1.00-4.14) | 0.051 | 2.03 (0.96 - 4.33) | 0.066 |
| T3 | 6.41 (2.03-20.2) | 0.002 | 2.29 (0.34 - 15.61) | 0.398 |
| T4 | 4.81 (2.15-10.74) | <0.001 | 4.19 (1.54 - 11.4) | 0.005 |
| N stage | ||||
| N0 | 1 (reference) | |||
| N1 | 1.98 (1.08-3.62) | 0.028 | 1.21 (0.59 - 2.49) | 0.61 |
| N2 | 1.93 (0.66-5.63) | 0.231 | 2.10 (0.69 - 6.37) | 0.19 |
| N3 | 2.12 (0.9-5.01) | 0.085 | 0.50 (0.14 - 1.78) | 0.283 |
| M stage | ||||
| M0 | 1 (reference) | |||
| M1 | 6.57 (3.48-12.43) | <0.001 | 3.90 (1.27 - 11.92) | 0.017 |
| Tumor size | ||||
| <50 | 1 (reference) | |||
| ≥50 | 3.82 (1.79-8.15) | <0.001 | 1.25 (0.34 - 4.67) | 0.738 |
| ER | ||||
| Negative | 1 (reference) | |||
| Positive | 1.95 (0.47-8.01) | 0.355 | ||
| PR | ||||
| Negative | 1 (reference) | |||
| Positive | 1.49 (0.64-3.48) | 0.36 | ||
| Surgery | ||||
| No | 1 (reference) | |||
| Yes | 0.19 (0.09-0.39) | <0.001 | 0.31 (0.12 - 0.84) | 0.021 |
| Radiation | ||||
| No | 1 (reference) | |||
| Yes | 0.94 (0.5-1.75) | 0.841 | ||
| Chemotherapy | ||||
| No | 1 (reference) | |||
| Yes | 0.52 (0.3-0.89) | 0.018 | 0.39 (0.21 - 0.71) | <0.002 |
Performance and validation of a nomogram for predicting the prognosis of HER2-positive MBC patients
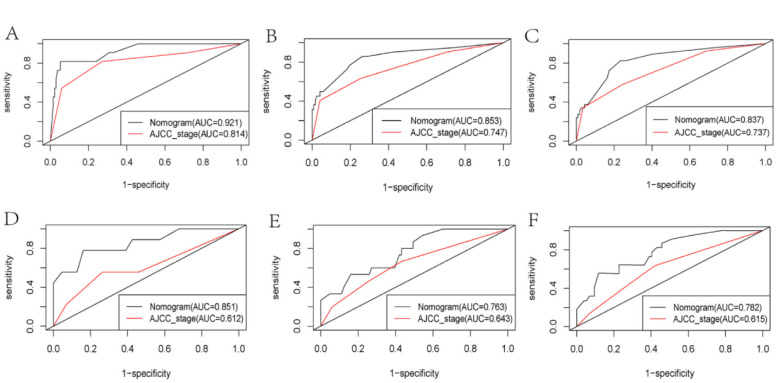
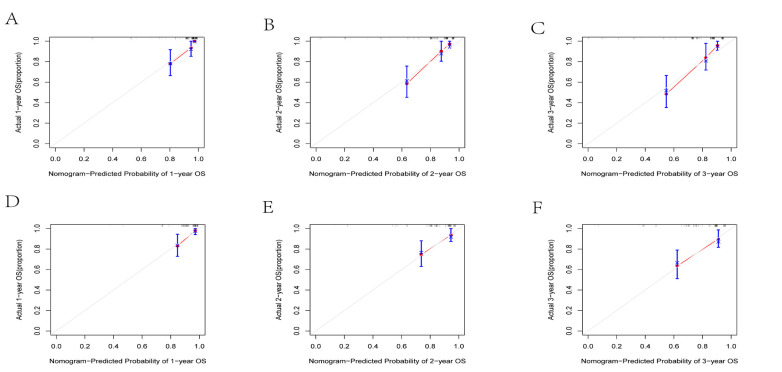
Based on the results of multivariate Cox risk regression analysis, we constructed a prognostic nomogram, which can demonstrate the impact of independent prognostic factors on OS (Figure 4). ROC curve analysis showed that in the training cohort, the AUCs of OS at 1, 2 and 3 years were 0.921, 0.853 and 0.837, respectively (Figure 5A-C). In the validation cohort, the AUCs were 0.851, 0.763 and 0.782, respectively (Figure 5D-F). In both the training and validation cohorts, the area under the ROC curves for OS were greater than those predicted by the AJCC_stage. This demonstrated a good discriminative ability of the nomogram prediction model. The C indexes of the training and validation cohorts were 0.746 (95%CI: 0.677 -- 0.815) and 0.754 (95%CI: 0.679 -0.829) (Table 3), respectively, indicating that the nomogram model constructed by the training cohort had a good overall performance. DCA showed that the nomogram model would have a good net benefit in clinical practice and that it showed a greater clinical predictive value than the AJCC_stage (Figure 6A-F). The calibration curves showed a high degree of agreement between the predicted and actual observations (Figure 7A-F), suggesting that the nomogram had a good calibration capability.
Figure 4.
A Nomogram Prediction Model for the Prognostic Factors in HER2-Positive MBC Patients.To use this nomogram, the specific point for each variable of the patient lies on each variable axis. Draw a vertical line upward to determine the point at which each variable accepts; the sum of these points is located on the Total Points axis, and draw a vertical line down to the survival axis to determine the probability of 1-, 2- and 3- years overall survival.
Figure 5.
ROC Curves of the Nomograms and AJCC_Stage to Predict Overall Survival of Patients at 1, 2 and 3 Years in the Training (A-C) and Validation Cohorts (D-F). In both the training and validation cohorts, the area under the ROC curves for OS were greater than those predicted by the AJCC_stage.
Table 3.
The C-indexes for Predictions of Overall Survival in the Training and Validation Cohorts
| Training cohorts HR (95% CI)" |
Validation cohorts HR (95% CI)" |
|
|---|---|---|
| C-index | 0.746 (0.677–0.815) | 0.754 (0.679–0.829) |
Figure 6.
Clinical Decision Curves Comparing the 1, 2 and 3 Year Overall Survival of Patients in the Nomogram and AJCC_Stage Models for the Training (A-C) and Validation (D-F) Cohorts.
Figure 7.
Calibration Curves for the Column line Plots. Calibration curves for 1, 2 and 3 year overall survival in the (A-C) training and (D-F) validation cohorts.
Discussion
The incidence of HER2-positive MBC is extremely low, and it only accounts for a small percentage of breast cancer patients. However, as the incidence of MBC continues to increase [14], HER2-positive MBC has attracted more attention in recent years. In addition, studies have found that HER2-positive patients had a worse prognosis when compared with HER2-negative male patients [15]. Therefore, it is necessary to develop a model to predict the prognosis of HER2-positive male patients to help formulate optimal treatment strategies for those with this condition. At present, clinically, the AJCC staging system is mainly used to determine the patients’ treatment plan and evaluate their survival prognosis. However, this assessment ignores the impact of some important risk factors on the patients’ survival prognosis, such as the ER and PR status, age, race and marriage. In this study, we developed a novel clinical prediction model to better predict the prognosis of HER2-positive MBC patients. ROC curves, calibration plots and DCA showed this model to have a high predictive accuracy and that the nomogram has a net benefit. When compared with the assessment from the traditional AJCC staging system, this nomogram enabled more accurate assessment and prediction of HER2-positive MBC patients in both the training and validation cohorts.
Our survey found that the median follow-up time of HER2-positive MBC patients was 60 months, which was inconsistent with the results of Arslan et al. [16]. These authors showed that the median follow-up time of HER2-positive male patients reached 85 months. However, their study only included non-metastatic HER2-positive male patients, which may be one of the reasons for the inconsistent results. Studies have shown that tumor staging is an important factor affecting the survival and prognosis of tumor patients [17-19], especially with respect to distant metastasis, which is the main cause of death of these cancer patients. The study of Wang et al. [20] showed that the T, N and M stages were prognostic factors for MBC patients. However, our study suggested that only the T and M stages affected the prognosis of these patients. One of the reasons for this discrepancy in the results could have been due to only triple-positive MBC patients being included this study. Our findings suggest that surgery and chemotherapy are protective factors for prognosis in men with HER2-positive breast cancer. As mentioned above, due to its low incidence, there is no unified treatment standard for MBC and it is usually managed in a similar way to menopausal female breast cancer patients [21].At present, the treatment of early breast cancer is mainly surgical treatment, which is supplemented by chemotherapy, radiotherapy, endocrine therapy and other therapeutic means [22]. However, there is minimal evidence to support the hypothesis that men with HER2-positive breast cancer benefit from surgery. However, studies have shown that surgery brings longer survival for HER2-positive breast cancer patients [23]as well as men with breast cancer [24]. It is well-known that chemotherapy is an important treatment for malignant tumors. Hayashi et al. showed that this procedure combined with trastuzumab can achieved a very good effective treatment for HER2-positive breast cancer patients, but this was only from a case report and a general conclusion cannot be made [12]. However, many studies have shown that chemotherapy can achieve good results in other types of breast cancer. Adjuvant chemotherapy was shown to significantly improve OS in a study of MBC [25]. Another study has shown that chemotherapy is a protective factor for prognosis in patients with HER2-positive breast cancer who also have bone metastases [23]. Additionally, the conclusion that chemotherapy can improve the survival prognosis of patients with malignant tumors has also been confirmed in other tumor studies [26-28]. In general, chemotherapy is a protective factor for the survival and prognosis of most patients with malignant tumors. This is consistent with our findings. Some studies have found that a positive PR is regarded as a protective factor for the prognosis of HER2-positive breast cancer [29], but our study did not reach this conclusion. This may be due to different research subjects leading to different conclusions, and there are major differences in the PR status of male and female breast cancer patients. Our study showed that there was no significant correlation between age, race, marriage and tumor size and the prognosis of MBC patients. As HER2-positive MBC is relatively rare, there is no report on the relationship between these factors and the prognosis of HER2-positive MBC patients.
In future studies, we will continue to collect additional clinical data and analyze these in order to obtain external verification of our results. Our study showed that in addition to the TNM stage of tumor as a prognostic factor for HER2-positive MBC patients, the treatment methods were also important prognostic factors. Therefore, the traditional AJCC staging system may not accurately predict the survival of patients with HER2-positive breast cancer. To this end, we constructed a column graph for predicting survival in patients with HER2-positive MBC. In comparison with the traditional AJCC stage, we found that the line graph model could accurately predicted the 1-, 2- and 3-year survival of HER2-positive MBC patients. In addition, calibration curves and DCA also demonstrated the reliability and accuracy of our clinical prediction model.
The study had some limitations. Firstly, the HER2 status in the SEER database was not registered until 2010. We only included patients diagnosed from 2010 to 2015, and only included 240 HER2-positive MBC patients, and this may have led to a potential bias. Secondly, the information and results we extracted from the SEER database were only based on data from the USA, and our conclusions may only apply to patients from that country. Finally, our column graph could not been verified externally. In the future, we will collect the data of HER2-positive MBC patients in the Chinese population and conduct external verification of our results.
In conclusion, our study constructed a prognostic model for HER2-positive MBC patients. Our results suggest that the T and M stages are prognostic risk factors for this disease. Surgery at the primary tumor site and systemic chemotherapy improved survival of the patients. The predictive model we constructed can accurately predict the survival prognosis of HER2-positive MBC patients and this can help clinicians make better treatment decisions for their wellbeing.
Acknowledgements
General
The authors thank Dr. Dev Sooranna, Imperial College London, for editing the manuscript. This article has been submitted for research square preprint on December 24, 2022, that could be removed upon acceptance in this journal.
Funding Statement
The present study was supported by the Affiliated Hospital of Youjiang Medical University for Nationalities Young and Middle-aged Talent Fund project (grant no. Y202210316).
Data Availability Statement
All raw data for this study were obtained from the SEER database (https://seer.cancer.gov/) which is a public database. Further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. All authors followed the SEER database regulations throughout the study.
Competing interests
The authors declare that they have no competing interests.
Author Contribution Statement
Concept and Design, Lifeng Zhao, Ziren Lin, Sher Zaman Safi, Shiqing Huang; Methodology, Shitang Nong, Caixin Li, Statistic Analysis, Junnan Li, ChengLin; Writing Original Draft, Lifeng Zhao, Ziren Lin, Review and Editing, Ikram Shah Bin Ismail; Supervision, Sher Zaman Safi, Shiqing Huang.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Abdelwahab Yousef AJ. Male breast cancer: Epidemiology and risk factors. Semin Oncol. 2017;44(4):267–72. doi: 10.1053/j.seminoncol.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: A population-based study. Cancer. 2004;101(1):51–7. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 4.Liu N, Johnson KJ, Ma CX. Male breast cancer: An updated surveillance, epidemiology, and end results data analysis. Clin Breast Cancer. 2018;18(5):e997–e1002. doi: 10.1016/j.clbc.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Chen K, Yang Y, Tan L, Chen L, Zhu L, et al. Incidence and survival outcomes of early male breast cancer: A population-based comparison with early female breast cancer. Ann Transl Med. 2019;7(20) doi: 10.21037/atm.2019.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu JL, Hung MC. The role of her2, egfr, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016;35(4):575–88. doi: 10.1007/s10555-016-9649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goddard KA, Weinmann S, Richert-Boe K, Chen C, Bulkley J, Wax C. Her2 evaluation and its impact on breast cancer treatment decisions. Public Health Genomics. 2012;15(1):1–10. doi: 10.1159/000325746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in her2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 9.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in her2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudlowski C, Friedrichs N, Faridi A, Füzesi L, Moll R, Bastert G, et al. Her-2/neu gene amplification and protein expression in primary male breast cancer. Breast Cancer Res Treat. 2004;84(3):215–23. doi: 10.1023/B:BREA.0000019953.92921.7e. [DOI] [PubMed] [Google Scholar]
- 11.Carmona-Bayonas A. Potential benefit of maintenance trastuzumab and anastrozole therapy in male advanced breast cancer. Breast. 2007;16(3):323–5. doi: 10.1016/j.breast.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi H, Kimura M, Yoshimoto N, Tsuzuki M, Tsunoda N, Fujita T, et al. A case of her2-positive male breast cancer with lung metastases showing a good response to trastuzumab and paclitaxel treatment. Breast Cancer. 2009;16(2):136–40. doi: 10.1007/s12282-008-0060-1. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Weng YM, Hu MX, Peng M, Song QB. Effects of her2 status on the prognosis of male breast cancer: A population-based study. Onco Targets Ther. 2019;12:7251–60. doi: 10.2147/OTT.S209949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddington R, Galer M, Hagedorn A, Liu P, Barrack S, Husain E, et al. Incidence of male breast cancer in scotland over a twenty-five-year period (1992-2017) Eur J Surg Oncol. 2020;46(8):1546–50. doi: 10.1016/j.ejso.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Leone JP, Leone J, Zwenger AO, Iturbe J, Vallejo CT, Leone BA. Prognostic significance of tumor subtypes in male breast cancer: A population-based study. Breast Cancer Res Treat. 2015;152(3):601–9. doi: 10.1007/s10549-015-3488-y. [DOI] [PubMed] [Google Scholar]
- 16.Arslan UY, Oksüzoğlu B, Ozdemir N, Aksoy S, Alkış N, Gök A, et al. Outcome of non-metastatic male breast cancer: 118 patients. Med Oncol. 2012;29(2):554–60. doi: 10.1007/s12032-011-9978-9. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Lin Y, Cheng B, Zhang Q, Cai Y. Prognostic model for predicting overall and cancer-specific survival among patients with cervical squamous cell carcinoma: A seer based study. Front Oncol. 2021;11:651975. doi: 10.3389/fonc.2021.651975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi M, Zhou B, Yang SP. Nomograms for predicting overall survival and cancer-specific survival in young patients with pancreatic cancer in the us based on the seer database. PeerJ. 2020;8:e8958. doi: 10.7717/peerj.8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Yu W, Petersen RH, Sheng H, Wang Y, Lv W, et al. A competing risk nomogram predicting cause-specific mortality in patients with lung adenosquamous carcinoma. BMC Cancer. 2020;20(1):429 . doi: 10.1186/s12885-020-06927-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang B, Wang H, Zhao A, Zhang M, Yang J. Poor prognosis of male triple-positive breast cancer patients: A propensity score matched seer analysis and molecular portraits. BMC Cancer. 2021;21(1):523 . doi: 10.1186/s12885-021-08267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.English JC, Middleton C, Patterson JW, Slingluff CL. Cancer of the male breast. Int J Dermatol. 2000;39(12):881–6. doi: 10.1046/j.1365-4362.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 22.Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, et al. Multidisciplinary meeting on male breast cancer: Summary and research recommendations. J Clin Oncol. 2010;28(12):2114–22. doi: 10.1200/JCO.2009.25.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyu X, Luo B. Prognostic factors and survival prediction in her2-positive breast cancer with bone metastases: A retrospective cohort study. Cancer Med. 2021;10(22):8114–26. doi: 10.1002/cam4.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X, Zhang J, Wang Y, Cao Z. Survival analysis in male breast cancer with bone metastasis based on the seer database. Front Oncol. 2022;12:659812. doi: 10.3389/fonc.2022.659812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li WP, Gao HF, Ji F, Zhu T, Cheng MY, Yang M, et al. The role of adjuvant chemotherapy in stage i-iii male breast cancer: A seer-based analysis. Ther Adv Med Oncol. 2020;12:1758835920958358. doi: 10.1177/1758835920958358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan H, Zhang K, Wang M, Ling L, Wang S, Zhou W. The effect of chemotherapy on survival in patients with nonmetastatic male breast cancer: A population-based observational study. Cancer. 2020;126 Suppl 16:3830–6. doi: 10.1002/cncr.32829. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Ji L, Wang X, Zhu S, Luo J, Zhang Y, et al. Nomogram predicts risk and prognostic factors for bone metastasis of pancreatic cancer: A population-based analysis. Front Endocrinol (Lausanne). 2021;12:752176. doi: 10.3389/fendo.2021.752176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu C, Yang J, Huang Z, Liu C, Lin Y, Tong Y, et al. Diagnostic and prognostic nomograms for bone metastasis in hepatocellular carcinoma. BMC Cancer. 2020;20(1):494 . doi: 10.1186/s12885-020-06995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan Y, Wang Y, He L, Imani S, Wen Q. Clinical features of patients with her2-positive breast cancer and development of a nomogram for predicting survival. ESMO Open. 2021;6(4):100232. doi: 10.1016/j.esmoop.2021.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All raw data for this study were obtained from the SEER database (https://seer.cancer.gov/) which is a public database. Further inquiries can be directed to the corresponding author.