Abstract
Introduction:
A highly accurate diagnostic method is crucial to reduce mortality and increase hepatocellular carcinoma (HCC) survival. Current biomarkers have limited accuracy, and novel ones are needed. Fibroblast growth factor-19 (FGF-19) is overexpressed in HCC. This study aimed to assess FGF-19 as a potential novel diagnostic biomarker for HCC.
Methods:
This case-control study involved 114 individuals divided into three equal groups: HCC (n=38), Cirrhosis (n=38), and Control (n=38). HCC biomarkers included alpha-fetoprotein (AFP), Des-γ-carboxy prothrombin (DCP), and FGF-19.
Results:
The three markers, FGF-19, DCP, and AFP, were significantly different between the three groups, except that DCP was comparable between HCC and Cirrhosis groups (p=1.000). All individuals in the control group had FGF-19 levels below the minimum level in the HCC group. Thus, FGF-19 had 100% sensitivity and specificity in differentiating HCC from healthy controls. FGF-19 can discriminate between HCC and Cirrhosis groups at a 140.8 pg/mL cutoff with sensitivity and specificity of 81.8% and 87.9%, respectively. The sensitivity of FGF-19 was higher than AFP, trending toward statistical significance (p=0.095). Combining FGF-19 with AFP, DCP, or both improved sensitivity but decreased specificity.
Conclusion:
FGF-19 is a possible noninvasive serum biomarker for HCC. Its combination with AFP or DCP improves the sensitivity for detecting HCC.
Key Words: Alpha-Fetoprotein, carcinoma, liver neoplasms, liver cirrhosis, biomarkers, fibroblast growth factors
Introduction
Introduction
In 2020, hepatocellular carcinoma (HCC) was the sixth most prevalent cancer (4.7%) and the third leading cause of cancer-related death (8.3%) [1]. In Egypt, it is the leading cause of cancer-related mortality and morbidity [2], with the highest age-standardized incidence rate (ASIR) (34,1 per 100,000) in Africa and the Middle East [3]. HCV is the leading cause of head and neck cancer in Egypt, followed by HBV [4].
HCC is a highly malignant disease with aggressive invasion, rapid progression, and poor prognosis [5]. Symptoms are absent in the early stages, but the patients reach the middle and late stages at diagnosis [6]. Developing a high-accuracy diagnostic method is crucial to reduce disease mortality and increase patient survival time [5].
Abdominal US has an acceptable sensitivity of 84% for detecting HCC at any stage, but its sensitivity for early-stage disease is lower at 47% [7]. Its effectiveness is affected by operator expertise and patient-level factors like obesity and liver disease severity [8]. In clinical practice, utilization of alternative imaging modalities, such as CT and MRI, is increasing, but scant information on cross- sectional imaging for HCC surveillance [9].
Therefore, serum biomarkers have gained interest in early HCC detection. Many serum biomarkers are available, but they have low sensitivity and varying specificity, even when evaluated in combination with other serum biomarkers [10]. Serum alpha-fetoprotein (AFP) is the most commonly used biomarker. However, its specificity is weakened by its elevation in other benign and malignant conditions [10]. Thus far, there is ongoing debate surrounding the optimal threshold of AFP for diagnosing HCC [11]. Current guidelines recommend ultrasonography (US) with or without serum alpha-fetoprotein (AFP) for the early detection of HCC in high-risk populations [12]. A meta-analysis found that concomitant use of ultrasound and AFP improved early HCC detection, with 63% and 45% sensitivities, respectively [7]. Another biomarker with a high positive rate in the serum of HCC patients is des-γ-carboxy prothrombin (DCP). It is an abnormal form of prothrombin, also found in the serum of patients with vitamin K deficiency [13].
Several other tumor biomarkers, including insulin growth factor-1 (IGF-1), Serum dickkopf-1 (DKK1), and Golgi protein 73 (Gp-73), have been suggested. Lower levels of IGF-1 in cirrhotic patients with HCV were found to be linked to the development of HCC [14]. Serum dickkopf-1 (DKK1) was suggested to be a new biomarker for HCC, showing excellent diagnostic accuracy even in the early stages and in patients with normal AFP levels [15]. In a case-control study, the Gp- 73 was evaluated in HCC patients. However, its early detection had a high false-negative rate of 38%, which hindered its acceptance as a new diagnostic tool for HCC [16]. In recent years, there has been a growing interest in using genomic profiling and proteomics for the diagnosis of HCC [17]. There is a strong correlation between plasma micro-RNA and the development of cancer and the spread of tumors [18].
Among numerous well-known and newly discovered oncogenes associated with HCC, FGF-19 is overexpressed in HCC [19]. The fibroblast growth factors (FGFs) family consists of a sizable group of growth factors found in various multicellular organisms [20]. FGFR1, FGFR2, FGFR3, and FGFR4 are the four transmembrane fibroblast growth factor receptors (FGFRs) that transmit signals from FGFs. Numerous biological processes, including embryonic development, cell proliferation, differentiation, and tissue repair, are regulated by FGFs- FGFRs. The dysregulation of FGF-FGFR has also been extensively reported in numerous diseases, disorders, and malignancies [21]. Notably, aberrant FGF19/FGFR4 expression contributes to the progression of HCC [22].
This study aimed to assess FGF 19 as a potential novel diagnostic biomarker for HCC alone or combined with other biomarkers.
Materials and Methods
This cross-sectional study involved 114 individuals divided into three equal groups: HCC (n=38), Cirrhosis (n=38), and Control (n=38). They were recruited from Cairo University Hospitals from October 2021 to December 2022. The study was approved by the Scientific Ethical Committee of the Faculty of Medicine, Cairo University. Informed written consent was signed by all the patients before enrollment, with an explanation of all the study procedures.
The diagnosis of HCC was based on contrast-enhanced imaging findings using triphasic CT abdomen and pelvis or Dynamic MRI with or without elevated AFP as per the diagnostic criteria of the AASLD [23]. Cirrhosis was diagnosed based on clinical manifestations, laboratory data, and ultrasound findings. Control subjects were selected from patients presenting to the internal medicine clinic complaining of mild gastrointestinal symptoms, nausea, vomiting, or diarrhea. Inclusion criteria for all subjects were age between 18 and 80 of both sexes. HCC patients who received any therapeutic intervention like percutaneous ablation, transarterial embolization, or resection were excluded from the study. All participants were subjected to full detailed medical history and clinical examination. Laboratory investigations included routine CBC, liver biochemical profile, and viral markers: HCV Ab and HBsAg. The serum specimens were obtained after at least 8-10 hours of fasting and stored at -80°C before laboratory testing. HCC biomarkers included AFP (Chemiluminescence enzyme immune assay), DCP, and FGF-19 (Sandwich ELIZA technique). An ultrasound examination documented signs of liver cirrhosis, portal hypertension, or HCC [focal lesion(s), portal vein thrombosis, and lymph node enlargement]. Triphasic CT or Dynamic MRI was done to confirm the diagnosis of HCC based on contrast uptake and washout. Child-Turcotte- Pugh (CTP) score was calculated for cirrhosis and HCC groups [24]. Serum AFP levels were determined using a chemiluminescent immunoassay analyzer (UniCel DxI 800, Beckman Coulter, Brea, CA, USA). This AFP ELISA kit is an enzyme-linked immunosorbent assay (ELISA) for in vitro quantitative determination of α-fetoprotein (AFP) concentrations in the range of 2-400ng/mL in human serum or plasma samples (Figures 1, 2).
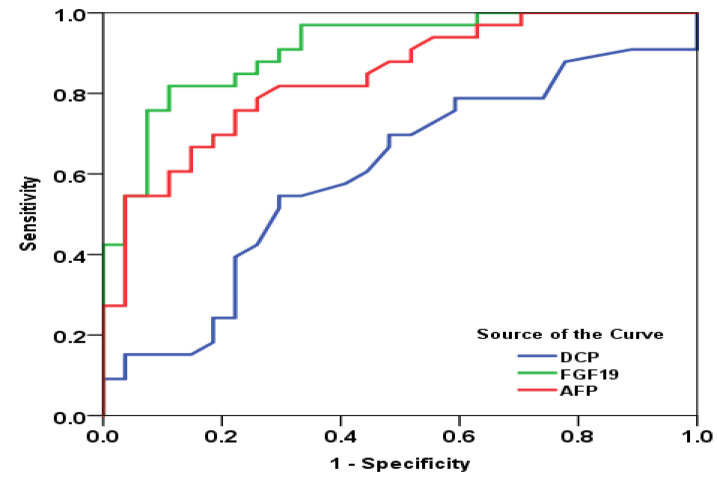
Figure 1.
Receiver Operating Characteristic Curves for Calculating the Diagnostic Performance of FGF-19, AFP, and DCP for the Differentiation of HCC from Cirrhosis. FGF-19: Fibroblast growth factor 19, AFP: Alpha fetoprotein, DCP: Des-γ-carboxy prothrombin
Figure 2.
Scatter Diagram for the Correlation between Fibroblast Growth Factor 19 (FGF-19) and Des-γ-carboxy Prothrombin (DCP) in Patients with HCC
An enzyme-linked immunosorbent assay (ELISA) kit (human AXL DuoSet ELISA, R&D Systems, Minneapolis, MN, USA; human abnormal prothrombin ELISA, Hotgen, Beijing, China) was used to detect DCP levels. The FGF-19 assay is a quantitative sandwich ELISA. The immunoplate was pre-coated with a rabbit polyclonal antibody specific for human FGF-19. Standards and samples were pipetted into the wells and any human FGF-19 present is bound by the immobilized antibody.
Statistical analysis
Statistical analysis was done using IBM© SPSS© Statistics version 23 (IBM© Corp., Armonk, NY, USA). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Chi- square test (Fisher’s exact test) was used to examine the relation between qualitative variables. The three groups were compared for quantitative data using ANOVA or Kruskal-Wallis test, followed by the appropriate post-Hoc test. Spearman-rho method was used to test the correlation between numerical variables. Sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), positive diagnostic likelihood ratio (PDLR), and negative diagnostic likelihood ratio (NDLR) were calculated for different index test cutoff values using the R package DTComPair.18 19. A p-value < 0.05 was considered significant.
Results
There was no significant difference between the three studied groups regarding age and sex (Table 1). Table 2 shows the baseline laboratory characteristics of the three studied groups. There was no statistically significant difference between the three groups regarding total leukocytic count and direct bilirubin. Significant intergroup differences existed between the three groups in hemoglobin concentration, platelet count, albumin, total bilirubin, ALT, AST, and INR. Hemoglobin concentration and albumin were significantly lower in the two patient groups than in the controls. Total bilirubin was significantly higher in the HCC group than in the Cirrhosis and Control groups. ALT and AST were also significantly higher in the HCC group. INR was significantly higher in the HCC and Cirrhosis groups compared to the Control group. Creatinine and urea were significantly higher in the HCC group compared to the Control group (Table 2). Table 3 shows no significant difference between the HCC and Cirrhosis groups in the Child score (p=0.136).
Table 1.
Demographic Characteristics among the Studied Groups
| HCC group n=38 | Cirrhosis group n=38 | Control group n=38 | p-value | |
|---|---|---|---|---|
| Male | 30 (80.6%) | 24 (63.9%) | 22 (58.3%) | 0.111 |
| Age (years) | 64.0±8.6 | 62.4±8.5 | 59.5±7.3 | 0.055 |
Data are presented as mean±SD or number (%)
Table 2.
Laboratory Characteristics of the Three Studied Groups
| HCC group n=38 | Cirrhosis group n=38 | Control group n=38 | p-value | ||
|---|---|---|---|---|---|
| p3=0.095 | |||||
| Hemoglobin (gm/dL) | 11.4±1.3 | 11.7±2.1 | 13.2±0.9 | <0.001 | p1=0.659 |
| p2<0.001 | |||||
| p3=0.010 | |||||
| TLC x103 | 6.6±3.8 | 6.7±2.9 | 7.1±2.2 | 0.693 | |
| Platelet count x103 | 151±61 | 174±82 | 250±54 | <0.001 | p1=0.467 |
| p2<0.001 | |||||
| p3=0.001 | |||||
| Albumin (gm/dL) | 3.23±0.49 | 3.51±0.53 | 4.54±0.62 | <0.001 | p1=0.325 |
| p2<0.001 | |||||
| p3=0.012 | |||||
| T. Bilirubin (mg/dL) | 1.5 (0.8-4.3) | 1.1 (0.1-3.3) | 0.7 (0.1-1.8) | 0.002 | p1=0.039 |
| p2=0.004 | |||||
| p3=0.633 | |||||
| D. Bilirubin (mg/dL) | 0.7 (0.1-2.2) | 0.4 (0.1-1.6) | 0.3 (0.2-0.7) | 0.057 | |
| ALT (IU/mL) | 48 (12-198) | 28 (12-139) | 20 (12-28) | 0.005 | p1=0.241 |
| p2=0.005 | |||||
| p3=0.183 | |||||
| AST (IU/mL) | 55 (18-271) | 35 (1-78) | 17 (10-30) | <0.001 | p1<0.001 |
| p2<0.001 | |||||
| p3=0.078 | |||||
| INR | 1.53±0.37 | 1.33±0.21 | 1.02±0.09 | <0.001 | p1=0.161 |
| p2<0.001 | |||||
| p3=0.001 | |||||
| S. creatinine (mg/dL) | 1.16±0.39 | 0.99±0.33 | 0.83±0.27 | 0.003 | p1=0.341 |
| p2=0.002 | |||||
| p3=0.300 | |||||
| B. urea (mg/dL) | 32.5 (15-98) | 24 (12-45) | 18 (6-23) | 0.001 | p1=0.148 |
| p2=0.001 |
Data are presented as mean±SD or median (range); TLC,Total leucocytic count; ALT, Alanine transaminase; AST, Aspartate aminotransferase; INR, International normalized ratio; p1, HCC vs. Cirrhosis; p2, HCC vs. Control; p3, Cirrhosis vs. Control
Table 3.
Child-Pugh Class of HCC and Cirrhosis Groups
| HCC group n=38 | Cirrhosis group n=38 | p-value | ||
|---|---|---|---|---|
| C | 8 (19.4%) | 4 (8.3%) | ||
| Child class | A | 19 (52.8%) | 27 (75.0%) | 0.136 |
| B | 11 (27.8%) | 7 (16.7%) |
Data are presented as number (%)
The three markers, FGF-19, DCP, and AFP, were significantly different between the three groups, except that DCP was comparable between HCC and Cirrhosis groups (p=1.000) (Table 4). All individuals in the control group had FGF-19 levels below the minimum level in the HCC group. Thus, FGF-19 had 100% sensitivity and specificity in differentiating HCC from healthy controls. The diagnostic performance of the three markers for differentiating HCC from liver cirrhosis and differentiating cirrhosis from healthy controls using ROC curve analysis is shown in Table 5.
Table 4.
The Levels of Fibroblast Growth Factor 19, Des-γ-carboxy Prothrombin (DCP), and alpha-fetoprotein in the Three Studied Groups
| HCC group n=38 | Cirrhosis group n=38 | Control group n=38 | p-value | ||
|---|---|---|---|---|---|
| Range | 2.8- 2766 | 0.6-267 | 0.5-5.8 | ||
| FGF-19 (pg/mL) | Median | 187 | 86.8 | 49.3 | < 0.001 |
| Range | 70.1-371.6 | 28.9-191.8 | 30.1-66.3 | ||
| DCP (ng/mL) | Median | 0.77 | 0.73 | 0.49 | < 0.001 |
| Range | 0.33-2.03 | 0.55-1.12 | 0.43-0.95 | ||
| AFP (ng/mL) | Median | 113 | 5 | 0.5 | < 0.001 |
FGF-19, Fibroblast Growth Factor-19; DCP, Des-γ-carboxy prothrombin; AFP, Alpha-fetoprotein
Table 5.
Diagnostic Performance of Fibroblast Growth Factor 19, Des-γ-carboxy Prothrombin (DCP), and Alpha-Fetoprotein for Diagnosis of HCC and Liver Cirrhosis
| Differentiating HCC from Cirrhosis | Differentiating Cirrhosis from Controls | |||
|---|---|---|---|---|
| NDLR | 0.31 | 0.17-0.59 | 0.12 | 0.04-0.36 |
| Estimate | 95%CI | Estimate | 95%CI | |
| FGF-19 (pg/mL) | ≥ 140.8 | ≥ 58.8 | ||
| Sensitivity | 81.80% | 68.7%-95.0% | 84.80% | 72.6%-97.1% |
| Specificity | 87.90% | 76.7%-99.0% | 90.90% | 78.9%-100.0% |
| PPV | 87.10% | 75.3%-98.9% | 93.30% | 84.4%-100.0% |
| NPV | 82.90% | 70.4%-95.3% | 80.00% | 64.3%-95.7% |
| PDLR | 6.75 | 2.66-17.15 | 9.33 | 2.47-35.26 |
| NDLR | 0.21 | 0.10-0.43 | 0.17 | 0.07-0.38 |
| DCP (ng/mL) | ≥ 0.725 | ≥ 0.585 | ||
| Sensitivity | 57.60% | 40.7%-74.4% | 97.00% | 91.1%-100.0% |
| Specificity | 51.50% | 34.5%-68.6% | 90.90% | 78.9%-100.0% |
| PPV | 54.30% | 37.8%-70.8% | 94.10% | 86.2%-100.0% |
| NPV | 54.80% | 37.3%-72.4% | 95.20% | 86.1%-100.0% |
| PDLR | 1.19 | 0.75-1.88 | 10.67 | 2.84-40.04 |
| NDLR | 0.82 | 0.49-1.38 | 0.03 | 0.01-0.23 |
| AFP (ng/mL) | ≥ 13.1 | ≥ 1.77 | ||
| Sensitivity | 75.80% | 61.1%-90.4% | 88.90% | 77.0%-100.0% |
| Specificity | 77.70% | 62.1%-93.5% | 90.90% | 78.9%-100.0% |
| PPV | 80.60% | 66.7%-94.5% | 92.30% | 82.1%-100.0% |
| NPV | 72.40% | 56.1%-88.7% | 87.00% | 73.2%-100.0% |
| PDLR | 3.4 | 1.64-7.09 | 9.78 | 2.59-36.90 |
FGF-19, Fibroblast Growth Factor-19; DCP, Des-gamma-carboxy prothrombin; AFP, Alpha-fetoprotein; PPV, Positive predictive value; NPV, Negative predictive value; PDLR, Positive diagnostic likelihood ratio; NDLR, Negative diagnostic likelihood ratio
FGF-19 can discriminate between HCC and Cirrhosis groups at a 140.8 pg/mL cutoff with sensitivity and specificity of 81.8% and 87.9%, respectively. It discriminated cirrhosis from healthy Controls at a cutoff of 58.5 with sensitivity and specificity of 84.8% and 90.9%, respectively. DCP has poor diagnostic value for differentiating HCC from Cirrhosis. FGF-19 had significantly higher sensitivity and specificity than DCP. The sensitivity and specificity of FGF-19 were higher than AFP, trending toward statistical significance (p=0.095) (supplementary Table 6). On the other hand, DCP had significantly higher sensitivity than FGF-19 for differentiating cirrhosis from healthy controls. Also, FGF-19 had comparable sensitivity and specificity for differentiating cirrhosis from healthy controls (supplementary Table 7). In the HCC group, FGF-19 was negatively correlated with serum creatinine and positively correlated with DCP. In the cirrhotic group, FGF-19 was positively correlated with platelet count and serum albumin and negatively correlated with INR and total and direct bilirubin (supplementary Table 8). There was no statistically significant relation between FGF-19 and Child score (p=0.627, supplementary Table 9).
A combined marker of FGF-19 ≥ 140.8 pg/mL or AFP ≥ 13.1 ng/mL improved the sensitivity of differentiating HCC from Cirrhosis, but it decreased specificity to 66.7%. A combined marker of FGF-19 ≥ 140.8 pg/mL or DCP ≥ 0.725 ng/mL improved the sensitivity of differentiating HCC from Cirrhosis, but it decreased specificity to 45.5%. A combination of the three markers when anyone is positive upgraded sensitivity to 93.9%, but the specificity was further dropped to 27.3% (supplementary Table 10).
Discussion
In recent years, novel biomarkers have been suggested to complement AFP and enhance the accuracy of HCC diagnosis [25]. In the current study, we aimed to evaluate the potential of FGF-19 as a new biomarker for HCC. The sensitivity and specificity of FGF-19 to discriminate between HCC and cirrhosis were 81.8% and 87.9%, respectively, at a cutoff of 140.8 pg/mL. At a cutoff of 58.5 pg/mL, its sensitivity and specificity to discriminate cirrhosis from healthy controls were 84.8% and 90.9%, respectively. FGF-19 had a higher sensitivity and specificity to AFP (75.8%, 77.7%, respectively) for diagnosis of HCC and cirrhosis, but the difference was not significant (p=0.527). DCP has poor diagnostic value for differentiating HCC from cirrhosis, significantly lower than FGF-19. Conversely, DCP had significantly higher sensitivity than FGF-19 for differentiating cirrhosis from healthy controls. A combined marker of FGF-19 ≥ 140.8 pg/mL or AFP ≥ 13.1 ng/mL improved the sensitivity of differentiating HCC from cirrhosis to 93.9%, but it decreased the specificity to 66.7%. A combined marker of positive FGF-19 or DCP had a better sensitivity but markedly low specificity for differentiating HCC from Cirrhosis. Notably, FGF-19 had 100% sensitivity and specificity in differentiating HCC from healthy controls. It worth noting the lowered specificity of combined markers is attributed to the number of cirrhotic patients having levels of AFP and DCP higher the selected cut-off values. We found significantly higher FGF-19 levels in HCC patients than in cirrhotic patients and healthy controls. Also, the levels in cirrhosis patients were higher than controls, concordant with many previous studies [26–30]. Mohamed et al. [27] reported the same findings, while Maeda et al. [26] found no significant difference between the cirrhotic cases and controls. Li et al. [28] detected higher FGF-19 levels in serum and tissues in the HCC group compared to the control group. These results are consistent with Sun et al. [29] findings, who found higher FGF-19 levels in the diabetic HCC and HCC groups than in the diabetes and control groups. Sweed et al. showed overexpression of FGF19 protein in HCC patients compared to cirrhosis and healthy groups, irrespective of etiology, previous HCV treatment, or morphological variants of the disease [31]. These results suggest that FGF-19 may have a role in the pathogenesis of HCC.
FGF19 is secreted from the ileum and modulated its endocrine functions by binding to FGFR4 on hepatocytes [32]. FGF19 regulates liver BA and lipid metabolism and plays a crucial role in liver regeneration after partial hepatectomy [33]. FGF19 increase stimulates hepatocellular protein synthesis and proliferation. However, the signaling pathways for FGF19-dependent tumorigenesis are not yet clear. Extracellular signal- regulated protein kinase (ERK) and β-catenin have been linked to hepatocyte proliferation, survival, migration, invasion, and angiogenesis [34].
FGF19 regulates β-catenin during epithelial- mesenchymal transition (EMT) in human colon cancer cells. FGF19 is significantly increased and negatively associated with E-cadherin expression in HCC tissues [22]. It also activates the stress-regulated transcription factor called nuclear factor (erythroid-derived 2)-like 2 in HCC cells. However, in a subtype of HCC patients, β-catenin can play a role in HCC without FGF19 amplification [35]. FGF19 overexpression regulates endoplasmic reticulum (ER) stress, which promotes tumor cell proliferation via activation of the FGFR4- GS3Kβ-Nrf2 signaling pathway [36]. FGF19 increases IL-6 production in a mouse model, followed by STAT3 activation in hepatocytes [37].
FGF19 can activate Ras and ERK, which play critical roles in HCC development [38]. Latasa et al. found that FGF19 induced amphiregulin expression by activating β-catenin signaling in HCC. Amphiregulin is a ligand of the epidermal growth factor receptor, which is crucial for the proliferation, survival, and resistance of HCC [39]. Additionally, FGF19/FGFR4 binding contributed to the regulation of various features of cancer [19].
Therefore, FGF19 could be used as a serum biomarker of HCC. The results of the present study support this notion, as its sensitivity and specificity for detecting HCC in cirrhotic patients were 81.8% and 87.9%, respectively. The sensitivity was higher than that of AFP (p=0.527), and their combination had 93.3% sensitivity.
Previous studies reported various diagnostic accuracy values with different cutoffs. For example, Mohamed et al. [27] found a sensitivity of 100% and specificity of 90% at a cutoff > 180 pg/mL. A markedly lower sensitivity of 53.2% and higher specificity of 95.1% were reported by Maeda et al. [26] at a more conservative cutoff point of 200 pg/mL. We can conclude that FGF-19 appears to be a possible noninvasive serum biomarker for HCC. Its combination with AFP improves the sensitivity for the detection of HCC.
Acknowledgements
Declaration of interest statement
The authors report there are no competing interests to declare
Data availability statement
The data that support the findings of this study are available from the corresponding author, [AR], upon reasonable request.
Author Contribution Statement
All authors have substantially contributed to the conception and design, acquisition of data, data analysis, and interpretation. All authors have agreed on the content of the manuscript. Ahmed Hamed Rashad, MD: manuscript writing. Mohamed Oraby, MSc: Patient recruitment and follow-up. Amaal A. Abdelaal, MD: Performed the laboratory studies and revised the data. Amel E Salem, MD: Revised and prepared data for manuscript writing. Rabab Mohamed Maher, MD: Patient recruitment and follow-up. Mahmoud Abdo Abdelalem, MD: Study design, conception, and manuscript revision. I declare that no financial or editorial assistance has been received by any of the authors to support the research project and/or preparation of the article.
Supplementary materials
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Rashed WM, Kandeil MAM, Mahmoud MO, Ezzat S. Hepatocellular carcinoma (hcc) in egypt: A comprehensive overview. J Egypt Natl Canc Inst. 2020;32(1):5. doi: 10.1186/s43046-020-0016-x. [DOI] [PubMed] [Google Scholar]
- 3.Cancer today [internet] [[cited 2023 aug 24]]. Available from: http://gco.Iarc.Fr/today/home.
- 4.Ezzat R, Eltabbakh M, El Kassas M. Unique situation of hepatocellular carcinoma in egypt: A review of epidemiology and control measures. World J Gastrointest Oncol. 2021;13(12):1919–38. doi: 10.4251/wjgo.v13.i12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zong J, Fan Z, Zhang Y. Serum tumor markers for early diagnosis of primary hepatocellular carcinoma. J Hepatocell Carcinoma. 2020;7:413–22. doi: 10.2147/JHC.S272762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farvardin S, Patel J, Khambaty M, Yerokun OA, Mok H, Tiro JA, et al. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65(3):875–84. doi: 10.1002/hep.28770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: A meta-analysis. Gastroenterology. 2018;154(6):1706–18. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons O, Fetzer DT, Yokoo T, Marrero JA, Yopp A, Kono Y, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45(1):169–77. doi: 10.1111/apt.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72(2):250–61. doi: 10.1016/j.jhep.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in hepatocellular carcinoma: Diagnosis, prognosis and treatment response assessment. Cells. 2020;9:6. doi: 10.3390/cells9061370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Chen G, Zhang P, Zhang J, Li X, Gan D, et al. The threshold of alpha-fetoprotein (afp) for the diagnosis of hepatocellular carcinoma: A systematic review and meta- analysis. PLoS One. 2020;15(2):e0228857. doi: 10.1371/journal.pone.0228857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–80. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 13.Ji J, Wang H, Li Y, Zheng L, Yin Y, Zou Z, et al. Diagnostic evaluation of des-gamma-carboxy prothrombin versus α-fetoprotein for hepatitis b virus-related hepatocellular carcinoma in china: A large-scale, multicentre study. PLoS One. 2016;11(4):e0153227. doi: 10.1371/journal.pone.0153227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazziotti G, Sorvillo F, Morisco F, Carbone A, Rotondi M, Stornaiuolo G, et al. Serum insulin-like growth factor i evaluation as a useful tool for predicting the risk of developing hepatocellular carcinoma in patients with hepatitis c virus-related cirrhosis: A prospective study. Cancer. 2002;95(12):2539–45. doi: 10.1002/cncr.11002. [DOI] [PubMed] [Google Scholar]
- 15.Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, et al. Serum dkk1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: A large-scale, multicentre study. Lancet Oncol. 2012;13(8):817–26. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- 16.Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, et al. Gp73, a resident golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43(6):1007–12. doi: 10.1016/j.jhep.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shademan B, Karamad V, Nourazarian A, Masjedi S, Isazadeh A, Sogutlu F, et al. Micrornas as targets for cancer diagnosis: Interests and limitations. Adv Pharm Bull. 2023;13(3):435–45. doi: 10.34172/apb.2023.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raja A, Park I, Haq F, Ahn SM. Fgf19-fgfr4 signaling in hepatocellular carcinoma. Cells. 2019;8:6). doi: 10.3390/cells8060536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2(3):Reviews300 . doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh N, Ornitz DM. Fibroblast growth factors: From molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149(2):121–30. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao H, Lv F, Liang G, Huang X, Wu G, Zhang W, et al. Fgf19 promotes epithelial-mesenchymal transition in hepatocellular carcinoma cells by modulating the gsk3β/β- catenin signaling cascade via fgfr4 activation. Oncotarget. 2016;7(12):13575–86. doi: 10.18632/oncotarget.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137(1):110–8. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsoris A, Marlar CA. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing : 2023. Use of the Child Pugh Score in Liver Disease. [PubMed] [Google Scholar]
- 25.Wang T, Zhang KH. New blood biomarkers for the diagnosis of afp-negative hepatocellular carcinoma. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda T, Kanzaki H, Chiba T, Ao J, Kanayama K, Maruta S, et al. Serum fibroblast growth factor 19 serves as a potential novel biomarker for hepatocellular carcinoma. BMC Cancer. 2019;19(1):1088 . doi: 10.1186/s12885-019-6322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamed GA, Nashaat EH, Fawzy HM, ElGhandour AM. Assessment of fibroblast growth factor 19 as a non-invasive serum marker for hepatocellular carcinoma. World J Hepatol. 2022;14(3):623–33. doi: 10.4254/wjh.v14.i3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Zhang W, Doughtie A, Cui G, Li X, Pandit H, et al. Up-regulation of fibroblast growth factor 19 and its receptor associates with progression from fatty liver to hepatocellular carcinoma. Oncotarget. 2016;7(32):52329–39. doi: 10.18632/oncotarget.10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y, Zhu M, Zhao H, Ni X, Chang R, Su J, et al. Serum fibroblast growth factor 19 and total bile acid concentrations are potential biomarkers of hepatocellular carcinoma in patients with type 2 diabetes mellitus. Biomed Res Int. 2020;2020 doi: 10.1155/2020/1751989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gad S, Elagrody A. Serum fibroblast growth factor 19 as a predictor and follow up of hepatocellular carcinoma. Egypt J Hosp Med. 2020;81:2217–21. [Google Scholar]
- 31.Sweed D, Kilany S, Taha M, Sweed E, Abdelsattar S. Mosbeh Fibroblast growth factor 19 is an independent prognostic parameter for hepatocellular carcinoma recurrence. Int J Cancer Biomed Res. 2021 [Google Scholar]
- 32.Wunsch E, Milkiewicz M, Wasik U, Trottier J, Kempińska- Podhorodecka A, Elias E, et al. Expression of hepatic fibroblast growth factor 19 is enhanced in primary biliary cirrhosis and correlates with severity of the disease. Sci Rep. 2015;5:13462. doi: 10.1038/srep13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez-Sola G, Uriarte I, Latasa MU, Jimenez M, Barcena- Varela M, Santamaría E, et al. Engineered fibroblast growth factor 19 protects from acetaminophen-induced liver injury and stimulates aged liver regeneration in mice. Cell Death Dis. 2017;8(10):e3083. doi: 10.1038/cddis.2017.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to fgf signaling. Cytokine Growth Factor Rev. 2005;16(2):233–47. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Ahn SM, Jang SJ, Shim JH, Kim D, Hong SM, Sung CO, et al. Genomic portrait of resectable hepatocellular carcinomas: Implications of rb1 and fgf19 aberrations for patient stratification. Hepatology. 2014;60(6):1972–82. doi: 10.1002/hep.27198. [DOI] [PubMed] [Google Scholar]
- 36.Teng Y, Zhao H, Gao L, Zhang W, Shull AY, Shay C. Fgf19 protects hepatocellular carcinoma cells against endoplasmic reticulum stress via activation of fgfr4-gsk3β- nrf2 signaling. Cancer Res. 2017;77(22):6215–25. doi: 10.1158/0008-5472.CAN-17-2039. [DOI] [PubMed] [Google Scholar]
- 37.Zhou M, Yang H, Learned RM, Tian H, Ling L. Non-cell- autonomous activation of il-6/stat3 signaling mediates fgf19- driven hepatocarcinogenesis. Nat Commun. 2017;8:15433. doi: 10.1038/ncomms15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, et al. Ubiquitous activation of ras and jak/stat pathways in human hcc. Gastroenterology. 2006;130(4):1117–28. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Latasa MU, Salis F, Urtasun R, Garcia-Irigoyen O, Elizalde M, Uriarte I, et al. Regulation of amphiregulin gene expression by β-catenin signaling in human hepatocellular carcinoma cells: A novel crosstalk between fgf19 and the egfr system. PLoS One. 2012;7(12):e52711. doi: 10.1371/journal.pone.0052711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [AR], upon reasonable request.