Abstract
Background:
Oral squamous cell carcinoma is ranked as the predominant type of head and neck squamous cell carcinoma, comprising roughly 90% of all oral cancer cases. Natural products have proven to be highly valuable as complementary, or adjunctive in the treatment of cancer. Piperine, a natural compound derived from Piper nigrum, demonstrates anti-proliferative and anti-neoplastic effects across various types of cancer. This study focused on assessing the cytotoxic effect of piperine in conjunction with cisplatin within the OSCC cell line.
Methods:
In this in-vitro study, cultured OSCC cells were divided into four groups: a control group (untreated), a group exposed solely to piperine, a group exposed solely to cisplatin, and a group receiving both piperine and cisplatin. Cell viability was determined by the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) assay technique. Additionally, flow cytometric analysis was employed to examine cell cycle progression and apoptosis. Assessment of reactive oxygen species activity, morphological changes, and nuclear area factor measurements were carried out. Expression of the apoptotic regulator Bax was assessed through western blotting analysis.
Results:
Piperine has cytotoxic and apoptotic effects in a concentration-dependent manner. Piperine in combination with cisplatin exhibited a synergistic effect, resulting in more pronounced inhibition of cell viability in OSCC cells compared to using piperine and cisplatin alone. Piperine and cisplatin for 24 h induced apoptosis strongly by increasing Bax protein and ROS activity.
Conclusion:
Combining piperine with cisplatin demonstrated a greater effectiveness in triggering apoptosis in OSCC cells compared to using cisplatin alone, allowing for a reduction.
Key Words: Apoptosis, Piperine-, Bax, Cisplatin, ROS
Introduction
Oral cancer commonly manifests as oral squamous cell carcinoma (OSCC), which is linked to a significantly high mortality rate [1, 2]. Unfortunately, conventional cancer treatments like radiotherapy and chemotherapy have been linked to substantial morbidity due to their impact on vital structures, severe adverse reactions, and the emergence of therapeutic resistance. Hence, there is a critical demand for innovative alternative treatments for combating this disease [3]. Conventional chemotherapy is the standard approach for treating oral squamous cell carcinoma (OSCC). Platinum-based drugs, like cisplatin, have consistently been established as the primary gold standard systemic agent [4].
Cisplatin functions by disrupting the process of DNA replication, restraining the proliferation of cancer cells, and also interfering with DNA repair mechanisms, which results in DNA damage and triggering apoptosis in cancerous cells [5].
Despite the initial therapeutic effectiveness of cisplatin, its use can often be constrained by its toxicity to healthy cells and the development of resistance in tumor cells, ultimately leading to therapeutic failure. Cisplatin overdose is associated with several significant toxicities, including kidney damage (nephrotoxicity), nerve damage (neuropathy), suppression of bone marrow function, and hearing impairment (ototoxicity) [6].
Hence, considering the potential adverse side effects of conventional treatments such as cisplatin, exploring herbal medicine as an alternative and complementary approach in cancer treatment could be beneficial [7, 8]. Piperine, the primary phenolic compound extracted from Piper nigrum and Piper longum, has been extensively utilized as both a food enhancer and traditional remedy across Asian countries for many centuries [9]. Piperine is characterized by its simplicity, crystalline structure, yellow color, lack of odor, and pungent taste. It can be extracted from various plants within the Piperaceae family. It stands out as the most common dietary amide alkaloid, with recognized properties such as anti-inflammatory, immunosuppressive, anti-neoplastic, neuroprotective, and antioxidant properties [7, 10].
The effects of piperine have been reported in various cancer types, encompassing osteosarcoma [11], lung cancer [12], cervical cancer [13], breast cancer cell lines [9], adenocarcinoma [14] and melanoma [15], showing its promise as an anticancer treatment [7]. The potential of piperine to combat tumors are believed to be linked to its impact on the signaling pathways responsible for triggering the apoptosis of cancer cells. Regarding the anti-cancer drugs, apoptosis is mainly initiated through the mitochondrial pathway [16]. This pathway is activated by a series of intracellular events, which are finely regulated by the interplay between anti-apoptotic factors like Bcl-2 and pro-apoptotic factors like Bax [9].
Piperine inhibited tumor growth through multiple mechanisms, including the stimulation of reactive oxygen species (ROS) production, the initiation of apoptosis, and the arrest of the cell cycle [17]. The synergistic effect when combining cisplatin with piperine in various cancer types has been demonstrated in previous studies. This combination has been shown to enhance the bioavailability of cisplatin, allowing for a reduction in drug dosage and associated side effects [9].
In this paper, we evaluated piperine potential as an anticancer agent for oral squamous cell carcinoma by assessing apoptosis and expression of Bax protein. The antioxidant properties of piperine on oral squamous cell carcinoma by measuring ROS levels were evaluated. Furthermore, we investigated the potential synergistic outcomes of combining cisplatin with piperine on OSCC.
Materials and Methods
Material
Piperine (> 97% purity) and cisplatin were purchased from Sigma-Aldrich, USA. Dimethyl sulfoxide (DMSO), 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) assay kit, and Horseradish peroxidase (HRP)-conjugated secondary antibody were also procured from Sigma (Saint Louis, Missouri, USA). Piperine (C17H19NO3 with molecular weight of 285.34 g/ mol) was dissolved in DMSO. Additionally, antibiotics (penicillin and streptomycin), Fetal bovine serum (FBS) and Dulbecco Modified Eagle Medium (DMEM) were used. Primary antibodies for anti-Bax and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A). Human Reactive oxygen species (ROS) ELISA Kit from BIOVISION, California, USA, was used in the study.
Cell line and cell culture
Tongue carcinoma cell line (HNO-97) was acquired from NAWAH scientific, Cairo, Egypt. Tongue carcinoma cells were sourced in frozen vials from the “American Type Culture Collection”.
Cell line was cultured in Dulbeco’s Modified Eagle’s Medium (DMEM) from Sigma-Aldrich with a pH=7.2containing 10% fetal bovine serum (FBS), 2mM glutamine and sodium bicarbonate from Invitrogen, USA. Cells were seeded into either 6-well or 96-well plates for subsequent experiments.
Cell treatment
Tongue carcinoma cells were divided into four groups:
Group I was control group (untreated group).
Group II was exposed to various concentrations of piperine.
Group III was exposed to various concentrations of cisplatin.
Group IV was exposed to a mixture of varying
concentrations of piperine and cisplatin.
Cell viability by MTT assay
Tongue carcinoma cells were first seeded in 96-well culture plates and exposed to varying concentrations of piperine (0.24-500 μM), cisplatin (0.24-500 μM), and combination of both for 24 hours. Following this incubation period, 10 μL of MTT solution (0.5 mg/ml) was introduced into each well and left to incubate at 37°C for 4 hours. Subsequently, the medium was carefully aspirated, and 100 μL of DMSO was gently introduced to dissolve the purple Formazan crystals. Spectrophotometric absorbance was quantified using Master Plex Reader spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 430 nm, with 630 nm as the reference wavelength.
Flow cytometric analysis
Cell Cycle Analysis
Tongue carcinoma cells were initially cultured in 25 cm2 cell culture flasks and subjected to IC50 concentrations of piperine (45.25 μM), cisplatin (0.43 μM) and their combination within a DMEM-based medium for 24 hours. The cells were detached through trypsinization, centrifuged at 2000 rpm for 10 minutes at 4°C, and subsequently rinsed twice with ice-cold PBS. Finally, they were treated with Annexin V-FITC and PI dye for 10 min in the absence of light at room temperature.
Assessment of Apoptosis using propidium iodide staining and Annexin V-FITC
After culturing with piperine, cisplatin and their combination for a 24-hours period, tongue carcinoma cells were harvested, centrifuged, rinsed, and then exposed to Annexin V-FITC and PI dye for 10 minutes in a dark room at room temperature. The differentiation between viable and apoptotic cells was subsequently assessed through flow cytometry.
Western blotting analysis
As mentioned before, tongue carcinoma cells exposed to cisplatin, piperine and their combination with the same concentrations, for 24 hours. Subsequently, the cells were gathered and lysed using a lysis buffer. The protein concentrations within the lysed samples were determined using the Bradford assay technique [18].
Proteins (50 μg/well) were isolated using 10%SDS-polyacrylamide gel electrophoresis. Following the separation process, the proteins were transferred from the gel onto a polyvinylidene difluoride (PVDF) membrane through a process called electroblotting. To prevent nonspecific binding of antibodies, an immunoblot was treated by incubating the PVDF membrane with 5% non-fat milk, which served as a blocking buffer, at room temperature for 1 hour. Following the blocking process, the PVDF membrane was subjected to an incubation with specific primary antibodies targeting Bax and β-actin at 4 ºC for overnight at a dilution of 1:1000. Protein bands were identified by utilizing an enhanced chemiluminescence (ECL) detection kit provided by Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A). Quantification of the protein bands was carried out using image J software provided by Bio-Rad (Hercules, CA, USA).
Reactive oxygen species (ROS) analysis
The assessment of ROS was conducted using Human ROS ELISA following the guidelines provided by the manufacturer.
The plates underwent two washing cycles before introducing the standard sample into the designated wells. Subsequently, 100 μL of standard sample was placed into each well and then allowed to incubate at 37°C for 90 minutes. Following this, 100 μL of a solution containing biotin-detection antibody was applied to each well and left to incubate at 37°C for 60 minutes. The solution was then removed, and the wells underwent three wash cycles. Additionally, 100 μL of a solution containing HRP-streptavidin conjugate (SABC) was introduced to each well and subjected to an incubation period of 30 minutes at 37°C. Following aspirating the solution, the wells were rinsed five times.
Subsequently, 90 μL of TMB substrate was placed in the wells and incubated at 37°C for 15–30 minutes. Subsequently, 50 μL of stop solution was introduced, and immediately reading the absorbance at 450 nm. For result determination, a standard curve was constructed by graphing the relative optical density (OD450) values
(Y) of each standard solution against their corresponding concentrations (X). Lastly, the human ROS concentration of the samples was droven by extrapolating from the standard curve.
Microscopic Examination
Slide Preparation
Cells were initially seeded in 6 well plate for 24 h then treated with the IC50 doses of piperine, cisplatin, and their combination for 24 h. Subsequently, the cells were detached using trypsin, centrifuged and formed into a pellet. Cells from each treatment group were then transferred onto a glass slide and immobilized by fixing them with 10% methanol.
The treated cells were subsequently stained using Hematoxylin and Eosin (H & E) staining.
Photomicrography and Cytological Evaluation
For each group’s ten microscopic fields were observed, examined, and captured in photomicrographs at a magnification of X1000 (oil immersion) using a digital video camera (C5060, Olympus, Japan) attached to a light microscope (BX60, Olympus, Japan). The selection of the fields was based on where the greatest number of apoptotic cells were present.
Concerning post treatment with various concentrations of combination of piperine and cisplatin, the apoptotic features such as shrunken apoptotic cells with condensed nuclei, blebbing of cell membrane, peripheral condensation of chromatin and the formation of apoptotic bodies were seen. Additionally, necrotic debris were also detected.
Nuclear Morphometric Analysis
The fields captured in the photomicrographs underwent analysis using image analysis software (Image J, 1.27z, NIH, USA).
The captured photomicrographs were subjected to automatic corrections for brightness and contrast and were subsequently converted into 8-bit grayscale images. A threshold was then utilized to identify the OSCC nuclei. From these thresholded images, ImageJ software was used to measure the surface area and circularity of these nuclei. The resulting data were organized in a Microsoft Excel sheet (Microsoft Office 365). The Nuclear Area Factor (NAF) was computed using the formula: NAF = Circularity × Surface area.
Statistical Analysis
The mean NAF values of tongue carcinoma cells exposed to piperine, cisplatin, and their combination in relation to the control cells were statistically assessed using the Statistical Package for Social Sciences (SPSS version 20).
Statistical significance among groups was assessed through a one-way ANOVA followed by post hoc Tukey B tests. Graphical representations were created using Microsoft Excel from Microsoft Office 365. The results were regarded as significant if the P-value was <0.05 and denoted by an asterisk (*).
Results
Cell viability by MTT assay
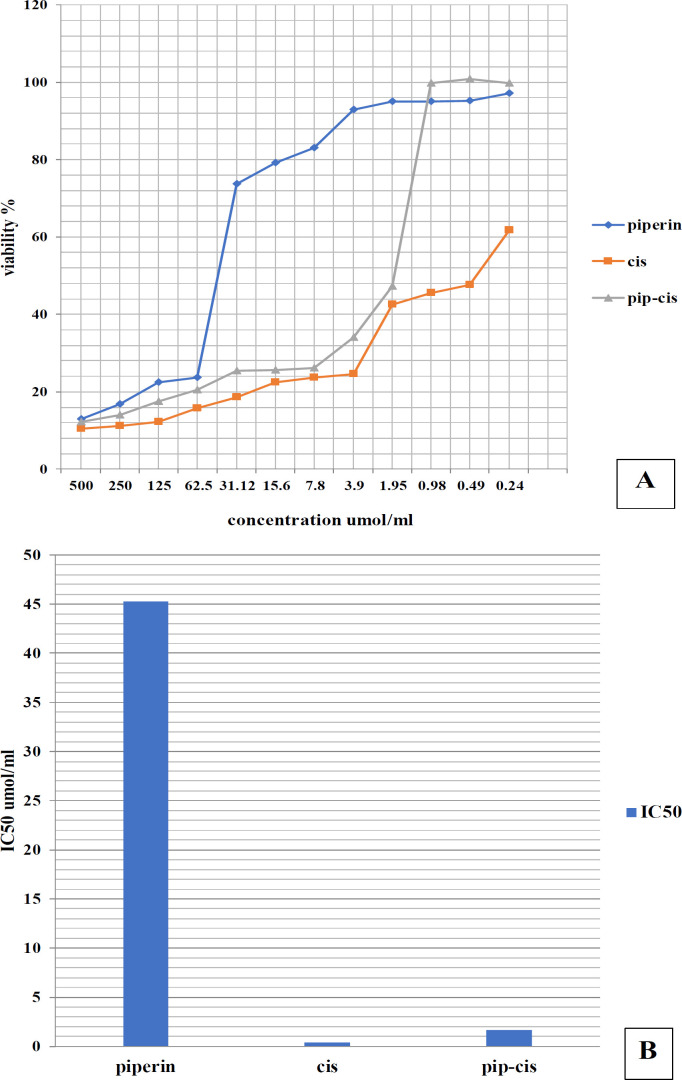
Treatment with combination of piperine and cisplatin revealed cytotoxic effects on tongue carcinoma cell line in a dose-dependent manner (Figure 1). The combined use of piperine and cisplatin demonstrated the highest cytotoxic impact on cell viability compared to piperine and cisplatin alone.
Figure 1.
(A) Graph showing the viability percentage of tongue carcinoma cells exposed to various concentrations of piperine, cisplatin, and their combination for 24 hours. (B) Bar chart showing comparison between the viability percentage of tongue carcinoma cells exposed to IC50 with piperine, cisplatin, and their combination.
Flow cytometric analysis
Cell Cycle Analysis
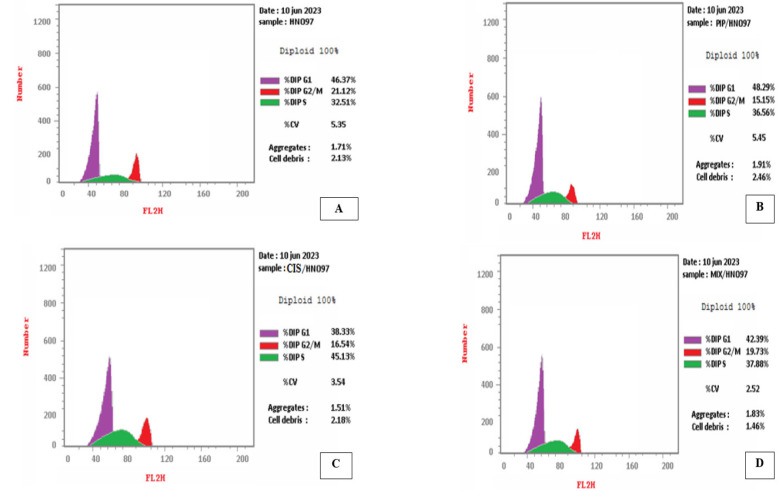
Regarding the control group, the cancer cells exhibited a high percentage of DNA content in both the G1 and S phases, reaching 46.37 % and 32.51%, respectively. Subsequently, there was a decline in the observed percentage in the G2-M phase, reaching 21.12%.
On the contrary, a reduction in the DNA content of cancer cells progressing through the cell cycle was noted in the piperine and cisplatin-treated group, particularly in the G1 phase (42.39%). In S phase, there was a rise in the number of cells compared to the control group (Figure 2).
Figure 2.
(A) DNA histogram of control group showing the highest peak of cells (46.37%) in the G1 phase, (B) DNA histogram of tongue carcinoma cells treated with piperine showing the highest peak of cells (48.29%) in the G1 phase, (C) DNA histogram of tongue carcinoma cells treated with cisplatin showing the highest peak of cells (38.33%) in the G1 phase, (D) DNA histogram of tongue carcinoma cells treated with combination of piperine and cisplatin showing the highest peak of cells (42.39%) in the G1 phase.
Apoptosis using propidium iodide staining and Annexin V-FITC
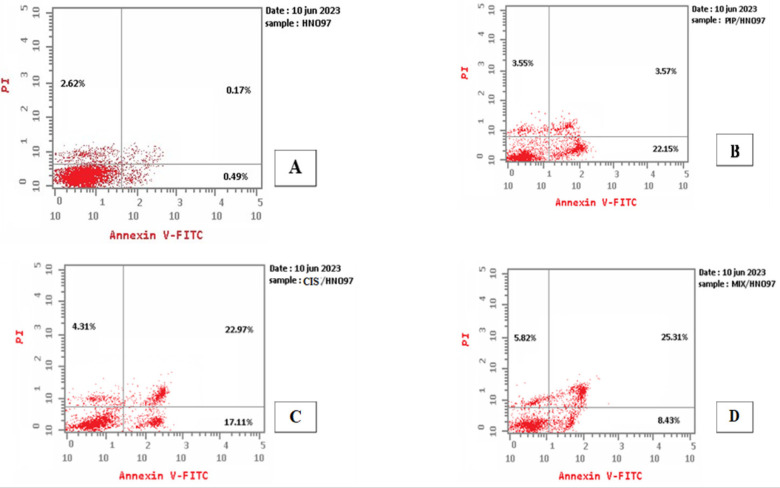
Concerning control cells, the highest fraction of cells is living cell (located in the lower left quadrant) with only few apoptotic cells (early apoptotic cells in the lower right quadrant and late apoptotic cells in the upper right quadrant). Additionally, there are necrotic cells present in the upper left quadrant.
In tongue carcinoma cells exposed to the combination of piperine and cisplatin, the early apoptosis rate was 8.43%, while the late apoptosis rate was 25.31%. Tongue carcinoma cells exposed to combination of piperine and cisplatin showing decrease in the number of living cells, and increase apoptotic and necrotic cells (39.56%) (Figure 3).
Figure 3.
Dot Plots Illustrating that the Majority of Cells are Viable in All Groups (Control, Piperine, Cisplatin and Their Combination). There was an elevation in the percentage of cells in early apoptotic phase in piperine, cisplatin and the combined groups. Late apoptotic phase has showed higher increase in percentage of cells in combined group followed by cisplatin group.
Western blotting analysis
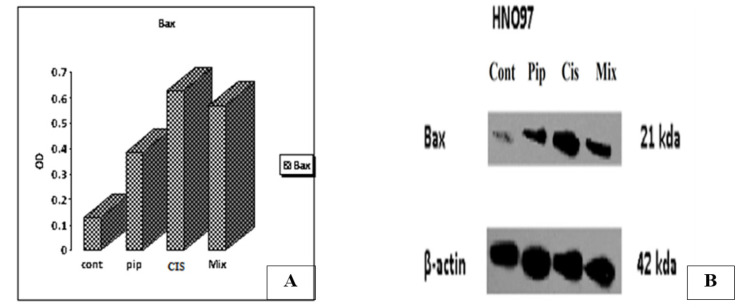
Decreased expression of Bax protein in the control group was observed. Our study demonstrated that piperine, cisplatin and their combination increased levels of Bax expression in comparison to the control group (Figure 4).
Figure 4.
(A) Bar Chart Showing the Effect of Piperine, Cisplatin and Their Combined Treatment on Bax Protein Expression Relative to the Control Group, (B) The effect of piperine, cisplatin and their combined treatment on Bax protein expression in comparison to the control group. β-actin was served as a gel loading control.
Reactive oxygen species (ROS) analysis
The findings showed that the level of ROS in tongue carcinoma cells increased following 48 hours of exposure to the combination of piperine and cisplatin in comparison to untreated control cells.
The concentration of ROS after treatment with piperine was 343.4 pg/ml, after treatment with cisplatin was 512.9 pg/ml, their combination was 490.1 pg/ml and 134.7 pg/ ml in untreated control cells (Figure 5).
Figure 5.
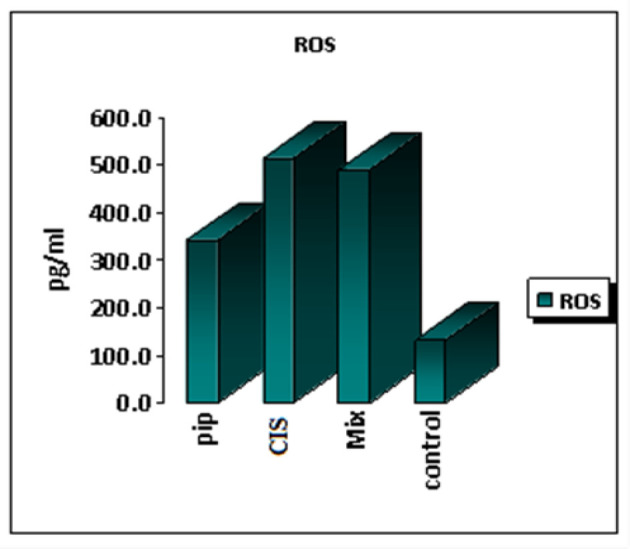
Bar Chart Showing Comparison of ROS Expression Values of Piperine, Cisplatin and Combination of Both Compared with Control Cells.
Microscopic Examination Nuclear Morphometric Analysis
The tongue carcinoma cells treated with varying concentrations of piperine and cisplatin for 24 hours showed a noticeable decrease in both the average surface area and circularity of their nuclei compared to the control cells. Consequently, there was a significant reduction in NAF (nuclear area factor). These findings provide supporting evidence for the presence of apoptotic and secondary necrotic alterations, as observed in cytological analysis (Figure 6,7 and 8).
Figure 6.
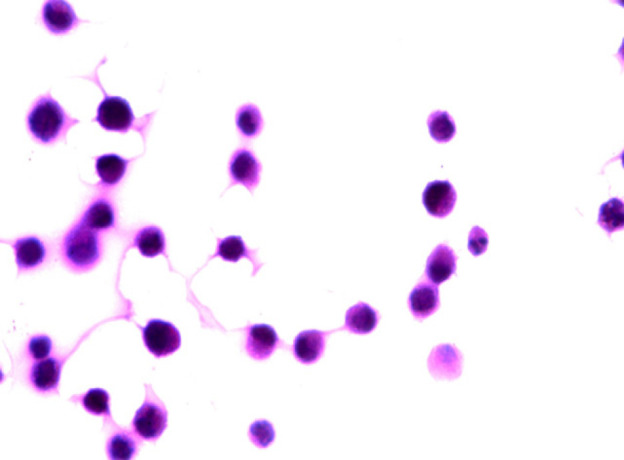
A Photomicrograph of Tongue Carcinoma Cells (Control Cells) Showing Pleomorphic Cells with Pleomorphic and Hyperchromatic Nuclei and Abnormal Mitotic Figures (H&E x 1000 oil).
Figure 7.
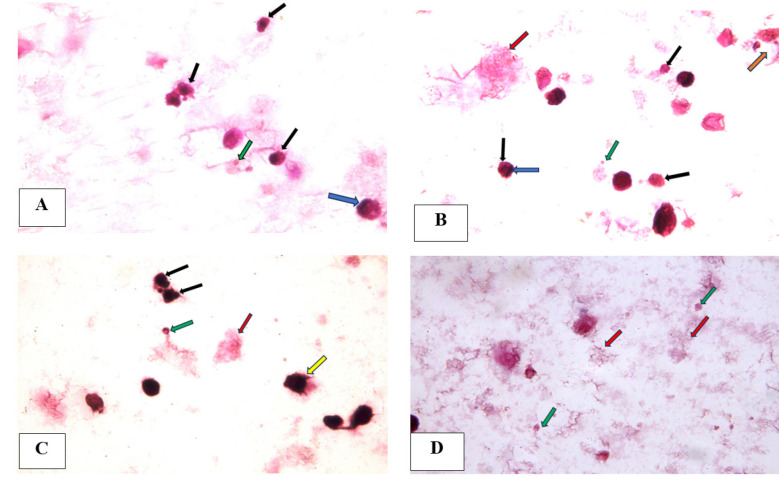
(A) A photomicrograph of tongue carcinoma cells after 24-hour treatment with piperine featuring apoptotic cells with apoptotic nuclei (black arrows), apoptotic body (green arrow) and peripheral condensation of chromatin (blue arrow), (B) A photomicrograph of tongue carcinoma cells after 24-hour treatment with cisplatin showing apoptotic cells (black arrows) with peripheral condensation of chromatin (blue arrow) , nuclear fragmentation (orange arrow) and apoptotic body (green arrow) and necrotic cell (red arrow), (C) (D) A photomicrograph of tongue carcinoma cells after 24-hour treatment with combination of piperine and cisplatin showing apoptotic cells with apoptotic nuclei (black arrows), apoptotic bodies (green arrows), membrane blebbing (yellow arrow) and necrotic debris (red arrow) (H & E, x1000 oil).
Figure 8.
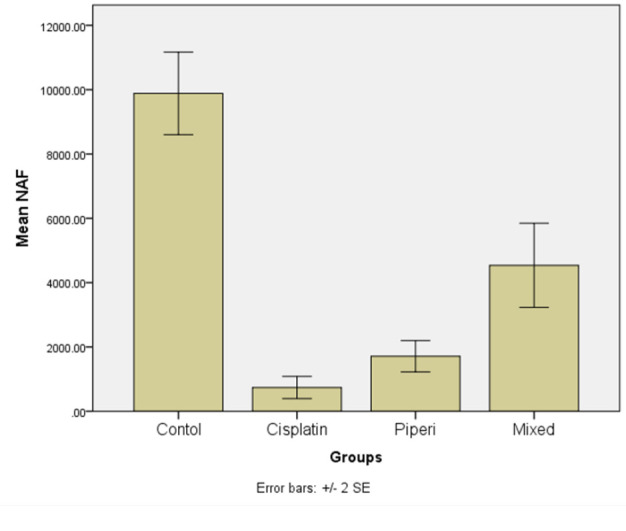
Error Bars of mean ± Stranded Deviation NAF of Control and Tongue Carcinoma Cells Treated by Cisplatin, Piperine, and Their Mix for 24 hours.
The ANOVA test showed a statistically significant difference between the mean values of NAF of tongue carcinoma cells exposed to cisplatin, piperine, and their combination and the control cells (P value < 0.0001) (Table 1).
Table 1.
ANOVA test for the Mean Values of NAF ± stranded deviation of Control and Tongue Carcinoma Cells Exposed to Cisplatin, Piperine, and Their Combination after 24 hours.
| Group | Number | NAF | F | P-value | |
|---|---|---|---|---|---|
| Mixed | 31 | 4538.30±3644.35 | 654.54 | ||
| Mean±SD | Standard error | ||||
| Control | 100 | 9883.40±6415.99 | 641.59 | 42.913 | <0.0001 |
| Cisplatin | 32 | 738.38±976.62 | 172.64 | ||
| Piperine | 33 | 1712.42±1394.08 | 242.67 | ||
The post hoc multiple comparisons test (Bonferroni) indicated no statistically significant difference among the mean NAF values of tongue carcinoma cells exposed to various concentrations of cisplatin, piperine, and their combination. Nevertheless, a significant difference was noted in the mean NAF values of these treated cells in comparison to the control cells after 24 hours. The combination group demonstrated the highest mean NAF value (4538.30) which is statistically significant (Table 2).
Table 2.
Comparison of Mean Difference NAF ± Standard Error of Different Groups 24 hours Post Treatment (Post Hoc Multiple Comparisons Rest (Bonferroni)).
| Group | Mean Difference | Std. Error | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Piperine | Combination | -2825.88 | 1219.68 | 0.129 | -6077.4 | 425.63 |
| Lower Bound | Upper Bound | |||||
| Control | Cisplatin | 9145.02* | 990.39 | 0.0001* | 6504.76 | 11785.27 |
| Piperine | 8170.98* | 978.95 | 0.0001* | 5561.2 | 10780.75 | |
| Combination | 5345.09* | 1002.42 | 0.0001* | 2672.77 | 8017.41 | |
| Cisplatin | Piperine | -974.03 | 1209.81 | 1 | -4199.25 | 2251.17 |
| Combination | -3799.92* | 1228.87 | 0.014* | -7075.96 | -523.89 | |
*, The mean difference is significant at the 0.05 level.
Discussion
Despite the favorable outcomes observed in OSCC treatment with cisplatin, a significant challenge arises from cancer cells developing resistance to this compound, coupled with its harmful impact on healthy cells, particularly the kidneys, leading to dosage restrictions [19].
Hence, the exploration of alternative approaches to reduce the adverse impacts of cisplatin and other anti- cancer medications becomes imperative in combating OSCC. Natural compounds in conjunction with synthetic anti-cancer medications, like cisplatin, emerge as promising candidates for cancer treatment. This is attributed to their potential to enhance drug effectiveness while concurrently reducing undesirable side effects associated with chemotherapy [17].
In the current study, various assessments were conducted to elucidate the potential mechanisms behind the cytotoxic effects, growth inhibition, and induction of apoptosis by the examined drugs. These assessments included cell viability and cytotoxicity, morphological changes, NAF, cell cycle analysis, apoptosis, expression of Bax protein by western blotting, and ROS activity.
Piperine, a bioactive component found in black pepper, holds a place in traditional medicine and has garnered attention for its noted anti-proliferative and anti-cancer attributes against various human cancer cells, such as ovarian cancer, breast cancer and OSCC with minimal adverse effects [20, 21]. The current study used piperine due to its cytotoxic and anti-tumorigenic properties and the studies evaluating the combination of piperine and cisplatin effect on OSCC are so deficient.
The current study’s findings suggested a potential cytotoxic impact of piperine on OSCC cell lines, which appears to occur in a dose-dependent fashion. Piperine demonstrated a reduction in the percentage of viable and proliferating cells with increasing doses, while simultaneously elevating the percentage of apoptotic cells. This observed effect could be attributed to its known anti-proliferative, anti-inflammatory, and antioxidant properties. These findings were convergent with the research done by Siddiqui et al [22], who demonstrated the antineoplastic effect of piperine on human oral squamous carcinoma cell line, showing a concentration-dependent decrease in cell growth and viability.
Anitha et al. [11] concluded that piperine has a significant anti-proliferative activity on osteosarcoma cell line compared to the synthetic drug methotrexate. Moreover, they found that piperine inhibits the metastasis of osteosarcoma by suppressing the expression of Matrix metallo proteinases (MMP-2 and 9) [11]. To our knowledge, there haven’t been any previous investigations into how the combination of piperine and cisplatin affects the inhibition of the growth of cancer cells in OSCC. This study marks the first instance of using this combination. Therefore, the in vitro results from our current research aligned with and are consistent with studies conducted on various other types of cancer cells.
We demonstrated that the average of viability percentages decreased as the drug concentration of piperine, cisplatin, and their combination increased. The combination of piperine with cisplatin was more effective in decreasing tongue carcinoma cells viability (99.72%) compared to piperine (97.12%) or cisplatin (61.8%) alone. This observation matched with Anitha et al (2023) on osteosarcoma [11], Ahmadi et al (2023) on breast cancer
[23] and Fattah et al. [9] study on breast cancer [9]. Furthermore, Siddiqui et al. [22] found that exposure to varying concentrations (25–300 μM) of piperine resulted in decreased cell viability in OSCC cells [22].
FCM and Annexin-V/PI staining assays were utilized to confirm the presence of apoptosis. Analyzing cell cycle distribution and proliferation is of paramount importance in the examination of cell growth, differentiation and apoptosis. These assessments are crucial for investigating and assessing the therapeutic effectiveness of anticancer agents [24].
Piperine causes damage to cancer cells by triggering apoptosis [25]. In the current work, there was increased in the total cell death (apoptosis and necrosis) as the concentrations of piperine, cisplatin, and their combination increased to 29.27%, 44.39%, 39.56 % respectively when compared with untreated control cells 3.28%. These findings were also agreed with the research done by Siddiqui et al. [22], which suggested piperine ‘s effectiveness in inducing cell death by reducing ROS release, leading to caspase-3 activation and cell cycle arres. This in contrast to study done by Fattah et al. [9] who found that the highest apoptotic rate on breast cancer cells treated with the combination of piperine and cisplatin [9]. This disparity could be attributed to differences in tumor types.
Consistent with our findings, Singh et al. [26], Dixit et al. [27], and Chaudhari et al. [28], demonstrated that the anticancer properties of piperine appear to be primarily linked to triggering of apoptosis in OSCC. Our western blot analysis findings demonstrated that piperine and cisplatin elevated the levels of Bax expression in comparison to the control group. This suggested that piperine induces apoptosis by activating Bax proteins, consistent with previous research showing piperine’s ability to induce apoptosis through Bax activation [9]. However, cisplatin raised the protein expression of Bax more than piperine alone or their combination. Meanwhile, it is against a study done by Fattah et al. [9] which found that the combination of piperine with cisplatin elevated the protein expression of Bax in comparison to either compound alone.
Concerning ROS, they play complex and multifaceted roles as a diverse biochemical entity in the progression of cancer. Hence, modulating intracellular ROS levels, either by reducing them through antioxidation or increasing them, could represent a potential strategy for cancer prevention or treatment [29]. Piperine, as a polyphenol, has the potential to disrupt normal energy metabolism and resulting in the accumulation of reactive oxygen species (ROS) in cells [22]. Consequently, our findings exhibited a significant increase in ROS production in cells exposed to piperine, cisplatin, and their combination compared to control cells. This observation is consistent with our finding that cells exposed to piperine, cisplatin, and their combination showed reduced viability compared to control cells. This in agreement with the research done by Siddiqui et al. [22], who reported that piperine induced a dose-dependent elevation in ROS production and nuclear condensation.
There exist a variety of biochemical and image-based assays for detecting apoptosis that vary significantly in terms of their complexity, precision, and expense [30].
The determination of NAF is a relatively simple process and can be readily employed alongside a nuclear dye like hematoxylin, as utilized in this study, which can serve as a marker for apoptosis [30, 31]. In the current research, the mean values of NAF of tongue carcinoma treated cells with piperine, cisplatin, and their combination exhibited a significant decrease compared to untreated control cells. This was further corroborated by the presence of apoptotic changes and subsequent necrotic alterations observed during cytological analysis, indicating that apoptosis rather than necrosis is the primary mechanism of cell death. Evaluating the morphology of treated cells can offer a qualitative assessment of apoptotic cells [30]. Piperine has cytotoxic effects, and its combination with cisplatin demonstrates synergistic effects that are more potent in reducing cell viability in the OSCC cell line than piperine or cisplatin alone. The combination of piperine and cisplatin cause oxidative stress by triggering ROS-controlled activation, subsequently initiating both intrinsic and extrinsic apoptotic signaling pathways within OSCC cells. This study suggested that combining piperine with cisplatin may offer a more efficient, potent, and lower-dose anticancer treatment option for cancer therapy.
Acknowledgements
Approval
The research is a part of a doctoral thesis that will be submitted to Faculty of Dentistry, Minia University. Publishing a paper is a prerequisite for thesis defense. It is approved by supervisors, prof. Dr. Sherif Farouk Elgayar and Ass.prof. Dr. Enas Alaa Eldin Abd Elaziz.
Ethical Declaration
The research ethical committee of the Faculty of Dentistry, Minia University approved the protocol of the study (approval number: 89/2022).
Conflict of Interest
The authors declare that they have no potential conflicts of interest to disclose.
Author Contribution Statement
Conceptualization: MAM, SFE, EAA; Data curation: MAM, SFE, EAA; Formal analysis: MAM; Methodology: MAM, SFE, EAA; Resources: MAM, EAA; Writing – original draft: MAM. All authors revised the manuscript and approved it.
References
- 1.He S, Chakraborty R, Ranganathan S. Proliferation and apoptosis pathways and factors in oral squamous cell carcinoma. Int J Mol Sci. 2022;23(3):1562. doi: 10.3390/ijms23031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edirisinghe ST, Devmini T, Pathmaperuma S, Weerasekera M, De Silva K, Liyanage I, et al. Risk assessment of alcohol consumption for oral cancer: A case-control study in patients attending the national cancer institute (apeksha hospital, maharagama) of sri lanka. Asian Pac J Cancer Prev. 2023;24(4):1181–5. doi: 10.31557/APJCP.2023.24.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Ghani SF, Amr EM. A comparative study on anticancer effect of crude venoms of the egyptian naja-haje and viper cerastescerastes on head and neck squamous cell carcinoma (in vitro study) Egypt Dent J. 2020;66:237 –46. [Google Scholar]
- 4.Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (nrg oncology rtog 1016): A randomised, multicentre, non- inferiority trial. Lancet. 2019;393(10166):40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CM, Jeong YI, Kook MS, Kim BH. Combinatorial effect of cold atmosphere plasma (cap) and the anticancer drug cisplatin on oral squamous cell cancer therapy. Int J Mol Sci. 2020;21(20):7646. doi: 10.3390/ijms21207646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oun R, Moussa YE, Wheate NJ. The side effects of platinum- based chemotherapy drugs: A review for chemists. Dalton Trans. 2018;47(19):6645–53. doi: 10.1039/c8dt00838h. [DOI] [PubMed] [Google Scholar]
- 7.de Almeida GC, Oliveira LF, Predes D, Fokoue HH, Kuster RM, Oliveira FL, et al. Piperine suppresses the wnt/β-catenin pathway and has anti-cancer effects on colorectal cancer cells. Sci Rep. 2020;10(1):11681 . doi: 10.1038/s41598-020-68574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nazhvani AD, Sarafraz N, Askari F, Heidari F, Razmkhah M. Anti-cancer effects of traditional medicinal herbs on oral squamous cell carcinoma. Asian Pac J Cancer Prev. 2020;21(2):479. doi: 10.31557/APJCP.2020.21.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fattah A, Morovati A, Niknam Z, Mashouri L, Asadi A, Rizi ST, et al. The synergistic combination of cisplatin and piperine induces apoptosis in mcf-7 cell line. Iran J Public Health. 2021;50(5):1037–47. doi: 10.18502/ijph.v50i5.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mekkawy SA, Abdalla MS, Omran MM, Hassan NM, Abdelfattah R, Abdel-Salam IM. Cancer stem cells as a prognostic biomarker and therapeutic target using curcumin/ piperine extract for multiple myeloma. Asian Pac J Cancer Prev. 2022;23(10):3507. doi: 10.31557/APJCP.2022.23.10.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anitha E, Neevedha K, Isswariya A, Gokul T, Vivekkumar SP. Synergistic effects of methotrexate and piperine on human osteosarcoma cell lines–an in-vitro study. Natl J Physiol Pharm Pharmacol. 2023;13(5):909–13. [Google Scholar]
- 12.Mitra S, Anand U, Jha NK, Shekhawat MS, Saha SC, Nongdam P, et al. Anticancer applications and pharmacological properties of piperidine and piperine: A comprehensive review on molecular mechanisms and therapeutic perspectives. Front Pharmacol. 2021;12:772418 . doi: 10.3389/fphar.2021.772418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardoso LP, de Sousa SO, Gusson-Zanetoni JP, de Melo Moreira Silva LL, Frigieri BM, Henrique T, et al. Piperine reduces neoplastic progression in cervical cancer cells by downregulating the cyclooxygenase 2 pathway. Pharmaceuticals (Basel) 2023;16(1):103. doi: 10.3390/ph16010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuksel B, Hizli Deniz AA, Sahin F, Sahin K, Turkel N. Cannabinoid compounds in combination with curcumin and piperine display an anti-tumorigenic effect against colon cancer cells. Front Pharmacol. 2023;14:1145666 . doi: 10.3389/fphar.2023.1145666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pressete CG, Viegas FPD, Campos TG, Caixeta ES, Hanemann JAC, Ferreira-Silva GA, et al. Piperine- chlorogenic acid hybrid inhibits the proliferation of the sk-mel-147 melanoma cells by modulating mitotic kinases. Pharmaceuticals (Basel) 2023;16(2):145. doi: 10.3390/ph16020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Y, Sriwiriyajan S, Tedasen A, Hiransai P, Graidist P. Anti-cancer effects of piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J Ethnopharmacol. 2016;188:87 –95. doi: 10.1016/j.jep.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 17.Thao DT, Minh LN, Anh TTM, Thi Nga N, Hue PTK, Van Kiem P. The improved anticancer activities of piperine nanoliposome conjugated cd133 monoclonal antibody against ntera-2 cancer stem cells. Nat Prod Commun. 2021;16(2):1934578X21998184. [Google Scholar]
- 18.Kruger NJ. The Bradford method for protein quantitation. The protein protocols handbook. 2009:17–24. [Google Scholar]
- 19.Tchounwou PB, Dasari S, Noubissi FK, Ray P, Kumar S. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J Exp Pharmacol. 2021:303 –28. doi: 10.2147/JEP.S267383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Lv M, Xu H. Overview of piperine: Bioactivities, total synthesis, structural modification, and structure-activity relationships. Mini Rev Med Chem. 2023;23(8):917–40. doi: 10.2174/1389557522666220726121012. [DOI] [PubMed] [Google Scholar]
- 21.Lwamba C, Aboushanab SA, Ambati RR, Kovaleva EG. Innovative green approach for extraction of piperine from black pepper based on response surface methodology. Sustain Chem. 2023;4(1):40–53. [Google Scholar]
- 22.Siddiqui S, Ahamad MS, Jafri A, Afzal M, Arshad M. Piperine triggers apoptosis of human oral squamous carcinoma through cell cycle arrest and mitochondrial oxidative stress. Nutr cancer. 2017;69(5):791–9. doi: 10.1080/01635581.2017.1310260. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadi F, Akbari J, Saeedi M, Seyedabadi M, Ebrahimnejad P, Ghasemi S, et al. Efficient synergistic combination effect of curcumin with piperine by polymeric magnetic nanoparticles for breast cancer treatment. J Drug Deliv Technol. 2023:104624v. [Google Scholar]
- 24.Alzahrani B, Elderdery AY, Alsrhani A, Alzerwi NAN, Althobiti MM, Elkhalifa AME, et al. Sodium alginate encapsulated iron oxide decorated with thymoquinone nanocomposite induces apoptosis in human breast cancer cells via pi3k-akt-mtor pathway. Int J Biol Macromol. 2023:125054v. doi: 10.1016/j.ijbiomac.2023.125054. [DOI] [PubMed] [Google Scholar]
- 25.Qi Y, Yao L, Liu J, Wang W. Piperine improves the sensitivity of osteosarcoma cells to doxorubicin by inducing apoptosis and inhibiting the pi3k/akt/gsk-3beta pathway. J Orthop Surg Res. 2023;18(1):180 . doi: 10.1186/s13018-023-03642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh K, Anurag JKG, Kumar S, Shrivastava A, Kumar K, Mukherjee S. Pharmacological approaches: A review of piperine anticancer activities in oral cancer. YMER Digital. 2023;21(12):2726–39. [Google Scholar]
- 28.Chaudhari VS, Gawali B, Saha P, Naidu VGM, Murty US, Banerjee S. Quercetin and piperine enriched nanostructured lipid carriers (nlcs) to improve apoptosis in oral squamous cellular carcinoma (fadu cells) with improved biodistribution profile. Eur J Pharmacol. 2021;909:174400 . doi: 10.1016/j.ejphar.2021.174400. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Sun D, Huang L, Wang S, Jin Y. Targeting reactive oxygen species capacity of tumor cells with repurposed drug as an anticancer therapy. Oxid Med Cell Longev. 2021;2021:8532940. doi: 10.1155/2021/8532940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afifi N, Abdel-Hamid E, Baghdadi H, Mohamed A. Nuclear area factor as a novel estimate for apoptosis in oral squamous cell carcinoma-treated cell line: A comparative in-vitro study with DNA fragmentation assay. J Clinic Experiment Pathol. 2012;2(107):2161–0681. [Google Scholar]
- 31.Mostafa RG, Abd-El-Hamid ES, El-Bolok AH, ELdin EA, Tohamy SM. Combined Effect of Doxorubicin and Pyrogallol on Tongue Squamous Cell Carcinoma SCC-25 Cells, an in vitro Study. Systematic Reviews in Pharmacy. 2020 ;11:10. [Google Scholar]