Abstract
Objective:
Leukemia is a group of hematologic malignancies in the bonemarrow that arise from the dysfunctional proliferation of developing leukocytes. It is classified as either acute or chronic based on the rapidity of proliferation and as myelocytic or lymphocytic based on the cell of origin. Newcastle Disease Virus (NDV) is an avian paramyxovirus, which has been demonstrated to possess significant oncolytic activity against mammalian cancers because its ability to kill tumor cells with limited toxicity to normal cells.
Methods:
In this study, the morphophical changes and apoptosis induction of WEHI 3B leukemia cell line treated with NDV strain AF2240 were studied by scanning electron microscopes and transmission electron microscopes techniques.
Result:
Electron microscopy indicated that NDV strain AF 2240 significantly altered cell morphology and reduced cell viability. Furthermore, early apoptosis was observed 6 h post- inoculation by fluorescence microscope.
Conclusion:
Our results suggest that NDV has ability to induce significant apoptoic structural changes in WEHI 3B leukemia cell line. These findings provide new insights into the mechanism of action of NDV virotherapy and could lead to the development of more effective treatments for leukemia.
Key Words: NDV strain AF 2240, apoptosis, scanning electron microscopes, transmission electron microscopes
Introduction
Leukemia is a type of cancer caused by the unregulated proliferation of immature blood cells derived from mutant hematopieotic stem cell. Leukemia is estimated to account for about 3% of incident cases of cancer worldwide [1]. The treatments for leukemia depend on type, severity of the leukemia, age, overall health, and other factors. Some possible treatments might be including are chemotherapy, radiation therapy and stem cell transplant. The goals of leukemia treatment are related to the type of leukemia. In acute leukemia, the therapeutic goal is to eradicate completely the leukemia cells and restore normal hematopoiesis. In chronic leukemia, the goal is somewhat different. In most instances, the intent is not cure but rather control of the disease with restoration of normal or near-normal hematopoiesis [2].
Virotherapy also called oncolytic virotherapy, is a treatment using an oncolytic virus (a virus that infects and breaks down cancer cells while leaving normal cells unharmed) [3]. Viral therapy for cancer has significantly been identified to show some promise in cancer therapy. Oncolytic virus therapy may make it easier to kill tumor cells with chemotherapy and radiation therapy. It is a type of targeted therapy. Some viruses such as adenovirus, herpes simplex virus (HSV), reovirus, rabies virus, poliovirus, measles virus, vesicular stomatitis virus, hepatitis A virus and Newcastle Disease Virus (NDV) are able to destroy cancer cells. One important attribute to consider virus as a good anti-cancer agent is that the virus must selectively infect, replicate in, and destroy human tumour cells, but should not initiate a symptom-free illness or only cause mild, well-characterised human disease [4]. The ability to infect and replicate in only particular types of cells is a natural characteristic of viruses and is known as cellular tropism of a virus [5].
Newcastle Disease Virus(NDV) is used as an antineoplastic and immunostimulatory agent in clinical tumor therapy [6]. NDV has been used as an anticancer agent for more than 30 years. The perception that NDV can replicate up to 10,000 times better in human cancer cells than in most normal cells, has prompted much interest in this virus as a potential anticancer agent. NDV has pleiotropic immune stimulatory properties in addition to good cell binding and selective proliferation in replicating cells. Multiple studies have demonstrated that NDV caries oncolytic potential due to its predilection for infection and replication in human cancer cells while sparing normal cells. Newcastle Disease Virus at the Forefront of Cancer Immunotherapy Most important for its use as an adjuvant in human cancer vaccines is its ability to introduce T- cell co-stimulatory activity and induce cytokines such as IFN-α, IFN-β and TNF-α that affect T-cell recruitment and activation [7]. The first intentional use of NDV to treat cancer in humans was documented in the early 1950s, where NDV and adenovirus were injected directly into uterine carcinoma, which underwent partial necrosis and sloughing followed by regrowth [8]. Many strains of NDV (73-T, MH68, Italian, Ulester, Rokin, PV701 and HUJ) have been shown to exhibit oncolytic activity [9, 10]. In addition, the oncolytic effects of six Malaysian strains of NDV (AF2240, 01/C, Ijuk, S, F, and V4) have also been studied on several tumor cell lines activity [9, 11, 12]. Furthermore NDV as a shuttle vector to deliver suicide genes into tumors of the xenographic mice for cancer therapy was reported by Zheng et al. [13] The CD/5-FC significantly improves the anti-tumor effect of the lengtogenic NDV.
Apoptosis is an active or programmed form of cell death [14], with participation of the cell along the pathway towards death through a process requiring energy. Apoptosis of mammalian cells is accompanied by various morphological characteristics including cell shrinkage, membrane blebbing, nuclear condensation and the emergence of apoptotic bodies [15, 16]. The oncolytic effects of NDV AF2240 have also been studied on several tumor cell lines. The Aim of this study was to characterize the nature of cell death mode (apoptosis and necrosis) caused by NDV strain AF 2240 in WEHI 3B cells line using Scanning electron microscope and Transmission Electron Microscopy.
Materials and Methods
Propagation and Purification of NDV Strain AF2240
NDV was propagated in allantoic fluid of 9–11 days-old embryonated chicken eggs at 37 °C for 48 h. The allantoic fluid was harvested and the presence of virus was confirmed by the haemaglutination test [17]. NDV purified as previously described by Chambers and Samson,1980 [18].
MTT Cytotoxicity Assay
WEHI-3B lines was cultured in DMEM (Sigma, USA) containing 10% fetal bovine serum (FBS), 100 U/ mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2 in air. The cells were grown to confluence and sub-cultured at three to four days’ interval before the experiments. WEHI-3B inhibition by NDV was measured using Microtitration cytotoxicity [19]. About 150 μL complete medium were added into of flat- bottom 96–well plate (Nunclon™, Denmark) and 50 μL of the 2-folded serial virus dilution were added into the wells. In the last well, 50 μL of PBS were added instead of the virus, which represented as control. Then 50 μL of 5 × 105 cells of WEHI-3B was added to top up the final volume to 200 μL and the plate was incubated at 37 °C in an atmosphere of 5% CO2. Seventy-two hours later, 20 μL of MTT (5 mg/mL) in PBS solution was added to each well then the plate was further incubated for 4 h. Most of the medium was removed and 100 μL of DMSO (dimethyl sulfoxide) was added into the wells to soluble the crystals. Finally, the OD was measured by (ELISA) reader at wavelength of 570 nm. Then graphs of percentage of viable cells versus virus titer HAU were plotted. The value of CD50 was determined from the graphs obtained at the concentration that cause 50% cell reduction as compared with controls.
Cell Proliferation and Growth Rate Inhibition using BrdU Cell Proliferation Assay
BrdU proliferation assay for treated and untreated WEHI-3B cells (1 × 106 cells/mL) was carried out using BrdU Cell Proliferation assay kit according to manufactures instructions (CHEMICON, USA). WEHI- 3B cells were treated at different virus titer HAU (CD 25, CD 50, and CD 75) of NDV. Then the plates were incubated in an atmosphere of 5% CO2 at 37 °C for 24, 48 and 72 h. After incubation periods, the cells were washed with PBS twice. The plates were read using ELISA reader. The OD of samples was plotted against time to determine the growth rates of cells in a given value.
Morphological changes Scanning Electron Microscopy
WEHI 3B cell at concentration of 1 x105 cell/ml in 2 ml culture medium containing 10% FBS was seeded into 6 well plates (Nunclon™, Nunc) and treated with NDV AF 2240 at CD50 concentrations (2 HA units). Then, the plates were incubated in an atmosphere of 5% CO2 at 37°C for 24, 48 and 72 hrs. After 24, 48 and 72 hrs incubation, the cells were spun down at 600 xg for 10 min. The supernatant was discarded and the pellet was washed with PBS twice. The cells were primarily fixed in 1.5% buffered glutaraldehyde at pH 7.4 for 12 to 24 hrs at 4°C. The cells were then washed with 0.1 M sodium cacodylate buffer for three changes of 10 min each. Subsequently, they were subjected to post-fixation of samples in 1% buffered osmium tetroxide for 2 hours at 4°C and washed again with 0.1 M sodium cacodylate buffer. The sample was dehydrated in a series concentration of acetone - 35%, 50%, 75%, 90% and 100%. Each step was left for 10 min at room temperature except for 100% acetone, which required three changes of 10 min each. Since the cells were in the form of suspension, they were centrifuged at every step of washing, fixation and dehydration. At the final stage of dehydration, the cells were resuspended in a small volume of absolute acetone (Merck, Germany). The cell suspension in absolute acetone was dropped slowly onto a 0.8 cm² aluminum foil coated with albumin (1 portion of egg white and 49 portion of distilled water) and left at room temperature until all the acetone had evaporated from the aluminum foil. Each specimen was then transferred into a separate specimen basket and put into a critical point dryer (HCP-2 Critical Dryer, Japan) for about 30 min. Then, the foil was mounted onto the stub using a double sided tape or colloidal silver followed by gold coating using a sputter coater (Palaron E5100 SEM Coating Unit, UK). The cells were examined under a scanning electron microscope.
Transmission Electron Microscopy
WEHI 3B leukemia cell at concentration of 1 x105 cell/ml were treated with NDV AF 2240 at CD50 concentrations (2 HA units) and incubated for 24, 48, and 72 h at 37C. The cultured cells were harvested using trypsin and centrifuged for 10 min at 1,500 rpm. The pellets were fixed in 4% (v/v) glutaraldehyde in 0.1 M coccadylate buffer (pH 7.4) for 4 h at 4C. The fixed cells were centrifuged, and the pellets were blocked in serum which was later fixed in glutaraldeyde overnight at 4C. The specimens were washed in three changes of sodium coccadylate buffer (pH 7.4) for 10 min each, postfixed in 1% osmium tetraoxide at 4C. The specimens were then washed in three changes of sodium coccadylate buffer (pH 7.4) for 10 min each and dehydrated with a graded series of acetone (35, 50, 75, 95, and 100%). The cells were then infiltrated with acetone and resin and embedded with 100% resin in beam capsule, and left to polymerize at 60 C for 48 h. The area of interest in the embedded cells resin block was chosen using the toluidine blue staining and later examined using light microscope. The selected area was cut in ulltrathin sections using ultramicrotome. The sections were placed into a grid and stained with uranyl acetate for 10 min followed by 50% filtered acetone, and finally stained using lead which was then washed twice with distilled water. The stained samples were then viewed under transmission electron microscopy (Phillips, Eindhoven, and The Netherlands).
Statistical Analysis
The data were presented as means + SEM and analyzed using one-way ANOVA and repeated measure one-way ANOVA in SPSS window program version 14.0 (Chicago, SPSS Inc). P value at 0.05 was considered as the level of significance. Tukey HSD post-hoc analysis was followed by ANOVA with p value of less than 0.05.
Results
MTT Cytotolytic assay of NDV Strain AF2240
In this study, the cytolytic effects of Newcastle disease virus strains AF2240 on mouse myelomonocytic leukemia (WEHI-3B) was determined by measuring the cytotoxic dose that kill 50% of the cell population as compared to the untreated control for various periods using colorimetric cytotoxicity assay (MTT). The assay was repeated three times. The results obtained showed cytolytic effect of NDV AF2240 on WEHI-3B cell and titer of virus that killed 50% (CD50) of WEHI-3B cells, compared to untreated cells after 72 h of treatment was 2
± 0.2 Haemagglutinating Units (HAU) (Table 1).
Table 1.
IC50 Values Obtained after NDV AF 2240 Virus Strain g Treatment on Mouse Myelomoncytic Leukemia (WEHI 3B) and Normal Cell Lines.
| Cell line | Duration (hours) | Cytotoxicity assay CD50 from MTT (HAU) |
|---|---|---|
| Normal mouse fibroblast cell line (3T3) | 72 | >150 |
| Mouse Myelomoncytic leukemia (WEHI 3B) | 24 | 10± 0.6 |
| Mouse Myelomoncytic leukemia (WEHI 3B) | 48 | 3.8± 0.1 |
| Mouse Myelomoncytic leukemia (WEHI 3B) | 72 | 2± 0.2 |
| Normal peripheral blood lymphocyte | 72 | >150 |
Cell Proliferation and Growth Rate Inhibition using BrdU Cell Proliferation Assay
Cell proliferation of WEHI 3B cells based on the DNA synthesis phase were investigated using BrdU cell proliferation assay. The results obtained showed that there was a decrease in optical density (OD) of WEHI 3B cells after treated with NDV in a time and concentration- dependent manner (Figure 1). As shown as in Figure 2 untreated WEHI 3B cells exhibited an increase in OD from 24 Hrs 1 to 72 h compared to WEHI-3B cells treated with CD 25, CD 50. The percentage of non-viable WEHI- 3B cells treated with CD25 value was 16% (24 h), 25% (48h) and 36% (72 h). But the percentage of non-viable WEHI-3B cells treated with CD50 values of were 28% (24 h), 44% (48h) and 55% (72 h).
Figure 1.
BrdU Proliferation assay of WEHI 3B Cells Treated with NDV at Different Virus titer HAU (CD 25 and CD 50). The viable and non-viable percentage of WEHI-3B cells in population at various time courses. (A) cells treated with CD25 value of (B) Cells treated with CD50 value). (C) Cells without treatment (control).
Figure 2.
Scanning Electron Microscope Photo-Micrographs Showing WEHI 3B Cells at Various Stages of Apoptosis and Necrosis after Treatment with NDV Strain AF 2240 at CD50 Value after 24, 48 and 72 hrs (Magnification x 4000).
(A) Untreated cells showing Viable cells (V) (B, and C) WEHI 3B cells treated with CD50 (2 HAU) of NDV AF 2240 showing Cell blebbing (P). (F) WEHI 3B cells treated with CD50 (2 HAU) of NDV AF 2240 showing Necrotic cell (N).
Morphological changes
This assay is the most straightforward method to determine the mode of cell death of WEHI 3B cells caused by NDV strain AF 2240 based on morphological changes. Two different kinds of microscopes were used for this observation: Scanning electron microscopy and Transmission electron microscopy.
Scanning Electron Microscopy
In this study, morphological changes of apoptosis and necrosis in WEHI 3B cells treated with AF 2240 at 24, 48, and 72 hrs compared to untreated WEHI 3B cells were determined. At 24 and 48 hrs post-inoculation, membrane blebbing on the treated cell surface as compared to untreated normal cells was observed. Furthermore, at 72 hrs post-inoculation, budding apoptotic cells together with cells that exhibit severe lesions on the membrane, which could be cells undergoing necrosis, were observed by using scanning microscopy (Figure 2).
Transmission Electron Microscopy
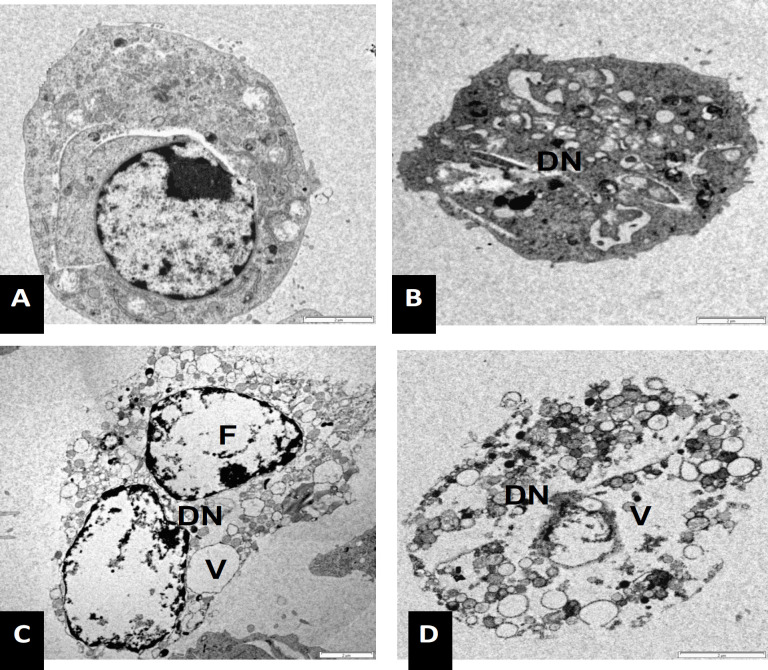
The ultra-structural examination of the WEHI 3B cells treated with NDV strain AF 2240 showed different stages of apoptosis and necrosis. In this study, at the early stage of apoptosis, chromatin began to condense, microvilli that were present originally disappeared and blebbing on the cell surface started to develop. In some cells, the protuberances, which were formed on the cell surface, had begun to separate producing a cluster of membrane bounded apoptotic bodies of roughly spherical or ovoid shapes of varying sizes and compositions. In some cells, cell membrane lyses and cell destruction were observed after 72 hours post-inoculation. Furthermore, WEHI 3B cells treated with NDV strain AF 2240 showed shrinkage compared to untreated WEHI 3B cells. Condensation of chromatin and disintegration of nucleus were clearly seen after 24, 48 and 72 hrs. At 24 hrs post-inoculation, cytoplasmic organelles such as intact mitochondria, membrane blebbing, cell shrinkage, chromatin condensation and intact rough endoplasmic reticulum were noted (Figure 4). The changes in the cells became more prominent at 48 hrs post-inoculation, where numerous mitochondria, membrane blebbing without disintegration of the cellular membrane, nucleus fragmentation and early stages of apoptotic bodies formation were observed (Figure 5). At 72 hrs post- inoculation, late stages of apoptotic cells formation, deep convolution of nuclear membrane, and necrosis cells were observed. Some apoptotic bodies eventually degenerated, when obvious damage in the cells with extensive vacuole was detected (Figure 5). On the other hand, Untreated WEHI 3B cells appeared in normal morphology of viable cells with intact nucleus and clear cytoplasm containing numerous mitochondria and endoplasmic reticulum, which were in their regular shape Figure 3, 4 and 5).
Figure 4.
Transmission electron microscope photo-micrographs showing WEHI 3B cells at various stages of apoptosis after treatment with NDV strain AF 2240 at CD50 value (2 HAU) for 48 hrs (Magnification x 7000). (A) Untreated cell (B, C, and D) treated cell at second stage of apoptosis. Nuclear fragmentation (F), crescent nucleolus (CN) and condensed chromatin (C)
Figure 5.
Transmission Electron Microscope Photo-Micrographs Showing WEHI 3B Cells at Various Stages of Apoptosis after Treatment with NDV Strain AF 2240 at CD50 Value (2 HAU) for 72 hrs (Magnification x 6000). (A) Untreated cell (B) treated cell showed condensation and intra-cytoplasmic vacuolation at the second stage of apoptosis. (C and D) cell undergo necrosis with swollen mitochondria containing flocculent densities, ruptured of cytoplasm and nuclear membrane. Nuclear fragmentation (F), disintegration of nucleus (DN) and cytoplasmic vacuoles (V)
Figure 3.
Transmission Electron Microscope Photo-Micrographs Showing WEHI 3B Cells at Various Stages of Apoptosis after Treatment with NDV Strain AF 2240 at CD50 Value (2 HAU) for 24 hrs (Magnification x 6800). (A) Untreated cell (B, C, and D) treated cell at early stage of apoptosis. Cell membrane blebbing (black arrow), nuclear fragmentation (F), crescent nucleolus (CN), apoptotic bodies (P) and condensed chromatin (C)
Discussion
Development of biologically targeted agents that exploit differences between cancerous and normal cells and permit greater specificity for cancer cells with less damage to normal cells is still the ultimate goal in the field of antineoplastic drug discovery [20]. The results obtained from this study showed that NDV AF2240 strain have cytolytic effect and the ability to induce apoptosis in (WEHI-3B) murine myelomoncytic leukemia cell line. By using MTT, NDV AF 2240 showed cytolyitic effects on the WEHI-3B cells. This finding was consistent with the results of many previous studies which stated that the effectiveness of NDV strains AF2240 and V4 UPM as an oncolytic agent was found on breast cancer cell lines (MCF-7 and MDA-231), leukemia cell lines (WEHI 3B, HL-60 and CEM-SS) and brain tumor cell line (DBTRG.5MG and U87MG) [9, 11].
Furthermore, apoptosis induction in WEHI-3B was determined using electron microscope and methods. To further confirm the potential of NDV strains AF2240 induced cell death on WEHI 3B cells, a BrdU cell proliferation assay was performed. The cell proliferation percentage was measured by the incorporation of the thymidine analogue bromodeoxyuridine into DNA. In treated WEHI 3B cells, the cell proliferation rate was reduced in a concentration dependent manner after the addition of NDV, compared to untreated control cells. The antiproliferative effects of NDV at CD50 concentrations against WEHI 3B were noticed at all the time points, percentage of viable cells had declined from 24 hours to 72 hours. Inhibition of DNA synthesis at the highest concentration is also time-dependent.
Moreover, the morphological changes of WEHI 3B cells especially at the plasma membrane could be readily detected using a scanning electron microscope. Convolution of nuclear and plasma membranes had occurred progressively with chromatin condensation, giving the cell a bubbling surface as seen by the scanning electron microscopy. Apoptotic cells were easily distinguishable from necrotic cells using scanning electron microscope in this approach. Surface blebbing is considered a hallmark pattern specific to apoptosis. It is due to a deep cytoskeleton rearrangement, causing progressive changes in cell shape and organelle distribution. The final stage of this process is the cell splitting in numerous cellular portions, termed apoptotic bodies, whose most common final fate in vivo is to be engulfed by phagocytes. Bleb is occasionally described on the surface of cells undergoing necrosis, but in this condition, they are followed by the rapid appearance of membrane discontinuities, causing water influx and strong ion distribution. No cell splitting appears in the course of necrosis, but a general cell hydration occurs, followed by cell swelling and disruption [21].
Ultra-structural morphology of mitochondria and nuclei in entire cells could be performed by TEM [21]. Briefly, after treatment of WEHI-3B cells with NDV strain AF2240, some cells showed typical morphologic changes of apoptosis, including shrinkage and membrane blebbing. Intracellular and plasma membrane structural modifications have been widely recognized as crucial factors involved in cell injury and death. Changes in nuclear morphology and in organelle structure as well as specific phenomena at the cell surface, namely surface smoothing and surface blebbing, are often considered as markers associated with cell pathology [22]. TEM analysis could reveal the changes of cell ultrastructure during the apoptotic process [23]. By means of TEM the results in this study showed that untreated cells showed no changes and exhibited intact nucleus with clear cytoplasm while after leukemia cell line treating with NDV strain AF2240 the typical morphological features of apoptotic cells appeared, including chromatin condensation in dense masses under the nuclear membrane, compaction of the cytoplasm, crowding of organelles and surface protuberances. This study agreed with a previous study, which showed that in the unaffected healthy cell, the cytoplasm of cells contained membranous organelles such as rod or round-shaped mitochondria, endoplasmic reticulum and golgi apparatus, that were embedded in a pool of ribonucleoprotein particles, which gave rise to the dark colour of the cytoplasm [24]. These findings also were consistent with previous study reported by Pesce and De Felici, [25] and Ali-Saeed et al. [26] which showed that the typical morphological features of apoptotic cells included protrusions known as blebs formed on the plasma membrane and the nucleus was often displaced to one edge of the cells; chromatin condensation in dense masses under the nuclear membrane; compaction of the cytoplasm; crowding of organelles and surface protuberances. The cells then broke up into discrete fragments (apoptotic bodies) which would eventually degenerate by secondary necrosis. Therefore, the results obtained from scanning and transmission microscopes confirmed apoptosis was the main mode of cell death induced by NDV strain AF 2240. Therefore, this result confirmed apoptosis was the main mode of cell death induced by NDV strain AF 2240.
In summary, the induction of apoptosis by NDV AF 2240 strain was evident from the morphological examination of the treated WEHI 3B cells by using scanning, transmission electron microscopes and BrdU cell proliferation assay techniques. These results confirmed that NDV strain AF2240 has a potent antitumor agent and has ability to induce apoptosis pathway in WEHI 3B leukemia cell line.
Acknowledgements
This research was funded in part by the National Cancer Council (MAKNA), Malaysia. The authors would like to express their appreciation to Faculty of biotechnology and biomolecular sciences and institute of bioscience, Universiti Putra Malaysia (UPM), for providing facilities and materials for this study. This research was funded by Researchers Supporting Project number (RSPD2024R690), King Saud University, Riyadh, Saudi Arabia. The research project was conducted under the supervision of: Professor Abdul Manaf Ali, PhD, it was a part of an approved PhD student thesis. Aied M Alabsi and Rola Ali-Saeed conceived the study, carried out most experiments and performed the statistical analysis, All authors drafted and reviwed the manuscript. All authors have read and approved the final manuscript.
Conflict of Interests
All the authors declare no conflict of interests.
Author Contribution Statement
All authors contributed equally in this study.
References
- 1.Lecture notes on haematology. 6th ed. Paris: Blackwell science ltd.; 1996. Hughes-jones nc, wickramasinghe sn. Acute and chronic leukemia. Lecture notes on haematology. Acute and chronic leukemia; pp. 134–60. [Google Scholar]
- 2.Gale rp. Principle of leukemia treatment: In: Leukemia therapy. United states of america: Blackwell scientific publication, inc ; 1986. pp. 1–16. [Google Scholar]
- 3.You L, He B, Xu Z, McCormick F, Jablons DM. Future directions: Oncolytic viruses. Clin Lung Cancer. 2004;5(4):226–30. doi: 10.3816/CLC.2004.n.003. [DOI] [PubMed] [Google Scholar]
- 4.Bell JC, Lichty B, Stojdl D. Getting oncolytic virus therapies off the ground. Cancer Cell. 2003;4(1):7–11. doi: 10.1016/s1535-6108(03)00170-3. [DOI] [PubMed] [Google Scholar]
- 5.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5(12):965–76. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 6.Nelson NJ. Scientific interest in newcastle disease virus is reviving. J Natl Cancer Inst. 1999;91(20):1708–10. doi: 10.1093/jnci/91.20.1708. [DOI] [PubMed] [Google Scholar]
- 7.Schirrmacher V, Ahlert T, Pröbstle T, Steiner HH, Herold- Mende C, Gerhards R, et al. Immunization with virus- modified tumor cells. Semin Oncol. 1998;25(6):677–96. [PubMed] [Google Scholar]
- 8.Cassel WA, Garrett RE. Newcastle disease virus as an antineoplastic agent. Cancer. 1965;18:863 –8. doi: 10.1002/1097-0142(196507)18:7<863::aid-cncr2820180714>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Omar AR, Ideris A, Ali AM, Othman F, Yusoff K, Abdullah JM, et al. An overview on the development of newcastle disease virus as an anti-cancer therapy. Malays J Med Sci. 2003;10(1):4–12. [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman AI, Zakay-Rones Z, Gomori JM, Linetsky E, Rasooly L, Greenbaum E, et al. Phase i/ii trial of intravenous ndv-huj oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006;13(1):221–8. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad U, Ahmed I, Keong YY, Abd Manan N, Othman F. Inhibitory and apoptosis-inducing effects of newcastle disease virus strain af2240 on mammary carcinoma cell line. Biomed Res Int. 2015;2015:127828 . doi: 10.1155/2015/127828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assayaghi RM, Alabsi AM, Swethadri G, Ali AM. Liver pathology in rats treated with newcastle disease virus strains af2240 and v4-upm. Asian Pac J Cancer Prev. 2019;20(10):3071–5. doi: 10.31557/APJCP.2019.20.10.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv Z, Zhang TY, Yin JC, Wang H, Sun T, Chen LQ, et al. Enhancement of anti-tumor activity of newcastle disease virus by the synergistic effect of cytosine deaminase. Asian Pac J Cancer Prev. 2013;14(12):7489–96. doi: 10.7314/apjcp.2013.14.12.7489. [DOI] [PubMed] [Google Scholar]
- 14.Buja LM, Eigenbrodt ML, Eigenbrodt EH. Apoptosis and necrosis Basic types and mechanisms of cell death. Arch Pathol Lab Med. 1993;117(12):1208–14. [PubMed] [Google Scholar]
- 15.Wyllie AH, Beattie GJ, Hargreaves AD. Chromatin changes in apoptosis. Histochem J. 1981;13(4):681–92. doi: 10.1007/BF01002719. [DOI] [PubMed] [Google Scholar]
- 16.Maria SS, Vidal BD, mello ml. Image analysis of DNA fragmentation and loss in v79 cells under apoptosis. Genet Mol Biol. 2000;23:109–12. [Google Scholar]
- 17.Alexander Dj. Newcastle disease virus—an avian paramyxovirus. Innewcastle disease. 1988:11–22. [Google Scholar]
- 18.Yusoff K, Tan WS, Lau CH, Ng BK, Ibrahim AL. Sequence of the haemagglutinin-neuraminidase gene of the newcastle disease virus oral vaccine strain v4(upm) Avian Pathol. 1996;25(4):837–44. doi: 10.1080/03079459608419185. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Adams J. Proteasome inhibition in cancer: Development of ps-341. Semin Oncol. 2001;28(6):613–9. doi: 10.1016/s0093-7754(01)90034-x. [DOI] [PubMed] [Google Scholar]
- 21.KY Y. Cytotoxic of mahanimbine, murrayafoline a and s-benzyldithiocarbazate on human leukemic cell line cem- ss. Master thesis. universiti putra malaysia, malaysia; 2001. [Google Scholar]
- 23.Lin WL, Li DG, Chen Q, Lu HM. Clinical and experimental study of oxaliplatin in treating human gastric carcinoma. World J Gastroenterol. 2004;10(19):2911–5. doi: 10.3748/wjg.v10.i19.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pesce M, De Felici M. Apoptosis in mouse primordial germ cells: A study by transmission and scanning electron microscope. Anat Embryol (Berl) 1994;189(5):435–40. doi: 10.1007/BF00185438. [DOI] [PubMed] [Google Scholar]
- 26.Ali- Saeed R, Alabsi AM, Ideris A, Omar AR, Yusoff K, Ali AM. Evaluation of ultra-microscopic changes and proliferation of apoptotic glioblastoma multiforme cells induced by velogenic strain of newcastle disease virus af2240. Asian Pac J Cancer Prev. 2019;20(3):757–65. doi: 10.31557/APJCP.2019.20.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]