Methyl prop-2-ynoate and dimethyl fumarate have been crystallized, the former for the first time. The protonated species of both were isolated for the first time and identified by single-crystal X-ray diffraction and Raman spectroscopy.
Keywords: crystal structure, superacidic system, ester, protonation, Raman spectroscopy
Abstract
Methyl prop-2-ynoate (C4H4O2) was investigated in the binary superacidic system HF/MF5 (M = Sb or As) and dimethyl fumarate (C6H8O4) in the superacidic system HF/SbF5, as well as HF/BF3. The starting materials methyl prop-2-ynoate and dimethyl fumarate were crystallized, the former for the first time. The protonated species of these esters, namely, (1-methoxyprop-2-yn-1-ylidene)oxidanium hexafluoroarsenate, C4H5O2+ AsF6−, 1,4-dimethoxy-4-oxobut-2-en-1-ylidene]oxidanium tetrafluoroborate bis(hydrogen fluoride), C6H9O4+ BF4− 2HF, and hemi{[1,4-dimethoxy-4-oxidaniumylidenebut-2-en-1-ylidene]oxidanium} undecafluorodiantimonate, 0.5C6H10O42+ Sb2F11−, were characterized by single-crystal X-ray diffraction and Raman spectroscopy. The protonated species were recrystallized from anhydrous hydrogen fluoride. In the solid state of the monoprotonated species of methyl prop-2-ynoate and the diprotonated species of dimethyl fumarate, strong intramolecular O—H⋯F hydrogen bonds build a three-dimensional network. The monoprotonated species of dimethyl fumarate builds chains by strong O—H⋯O hydrogen bonds between the cations.
Introduction
Protonated esters have occur in two conformations, namely, syn–anti and syn–syn (Hogeveen, 1967 ▸; Olah et al., 1967 ▸, 2009 ▸). The syn–anti conformation is more stable and is therefore consistent with the protonation of carboxylic acids (Olah et al., 2009 ▸; Hollenwäger et al., 2024b ▸; Hogeveen, 1968 ▸). The two conformers were observed in solution by NMR spectroscopy (Olah et al., 2009 ▸). It has not yet been possible to crystallize the syn–syn conformer of protonated esters in the solid state; the example of prop-2-ynoic acid (propiolic acid) shows that this could be achieved with a H/D exchange and solid-state effects through a larger anion (Hollenwäger et al., 2024b ▸). In magic acid (FSO3H/SbF5), the esters show the unimolecular cleavage of methanol by warming to 20 °C (Olah et al., 2009 ▸). An exception was observed with glycine methyl ester, which is still stable in magic acid even at 93 °C (Hollenwäger, et al., 2024a ▸).
The isolation of protonated esters enables the characterization of an important intermediate that is present in solution in every acid-catalyzed esterification process. The selected esters also offer the possibility of further functionalization steps due to the double and triple bonds present. This prompted us to investigate methyl prop-2-ynoate and dimethyl fumarate in the binary superacidic media HF/MF5.
Experimental
Synthesis and crystallization
[C4H5O4][MF6] (M = Sb or As)
The Lewis acids (SbF5: 433 mg, 2 mmol; AsF5: 340 mg, 2 mmol) were each condensed into a fluorine-passivated FEP reactor. Anhydrous hydrogen fluoride (0.5 l) was added as reactant and solvent at −196 °C. The mixture was homogenized at room temperature. Methyl prop-2-ynoate (83.6 µl, 84.1 mg, 1.0 mmol) was added at −196 °C under nitrogen. The mixture was allowed to warm to room temperature. The solvent was removed overnight at −78 °C. The protonated species II and III were obtained as white solids (Scheme 1).
[C6H9O4][MFy] (M = Sb or B; y = 6 or 4)
The Lewis acids (SbF5: 216 mg, 1 mmol; BF3: 67 mg, 1 mmol) were each condensed into a fluorine-passivated FEP reactor. Anhydrous hydrogen fluoride (0.5 l) was added as reactant and solvent at −196 °C. The mixture was homogenized at room temperature. Dimethyl fumarate (144 mg, 0.5 mmol) was added at −196 °C under nitrogen. The mixture was allowed to warm to room temperature. The solvent was removed overnight at −78 °C. The protonated species V and VI were obtained as white solids (Scheme 1). A clean Raman spectrum of monoprotonated species VI could not be obtained because it contains impurities of either IV or VII.
[C6H10O4][Sb2F11]2 and [C6H10O4][BF4]2
The Lewis acids (SbF5: 216 mg, 1 mmol; BF3: 67 mg, 1 mmol) were each condensed into a fluorine-passivated FEP reactor. Anhydrous hydrogen fluoride (0.5 l) was added as reactant and solvent at −196 °C. The mixture was homogenized at room temperature. Dimethyl fumarate (48 mg, 0.33 mmol) was added at −196 °C under nitrogen. The mixture was allowed to warm to room temperature. The solvent was removed overnight at −78 °C. The protonated species VII and VIII were obtained as white solids.
Single-crystal X-ray diffraction and Raman spectroscopic analysis
Compounds I, III, VI and VII were characterized by single-crystal X-ray diffraction. Complete data and devices for the X-ray measurements are listed in the CIF in the supporting information. Low-temperature Raman spectroscopic analysis was performed for I–VIII using a Bruker MultiRAM FT–Raman spectrometer with Nd:YAG laser excitation (λ = 1064 cm−1) under vacuum. For the measurements, the synthesized compound was transferred to a cooled glass cell.
Refinement
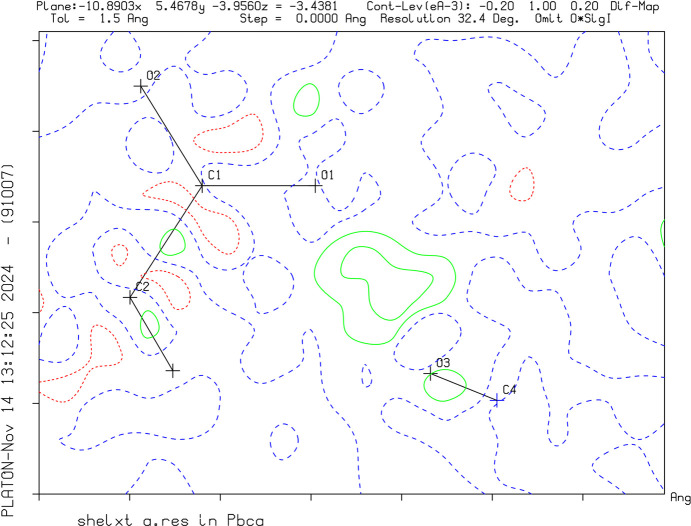
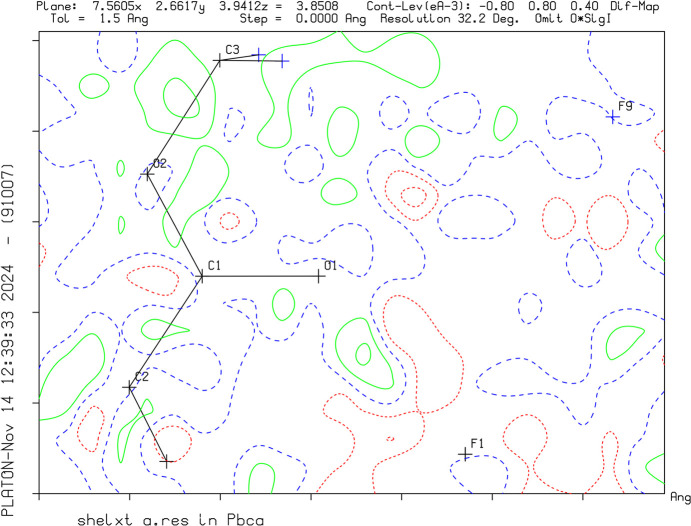
Crystal data, data collection and structure refinement details are summarized in Table 1 ▸. The successful protonation of the target molecules was confirmed by the charge of the asymmetric unit, as well as the interatomic distances. C—O distances have become nearly equal after protonation, as the charge can formally be localized on the C atom resulting in the loss of double-bond character. The positions of the H atoms were identified by Q-peaks on the difference Fourier map and by evaluation of the contacts (Figs. 1 ▸–3 ▸ ▸). Methyl, methylene and acetylenic H atoms were refined under restrictions and the proton positions were modulated.
Table 1. Experimental details.
Experiments were carried out with Mo Kα radiation using a Rigaku Xcalibur Sapphire3 diffractometer. Absorption was corrected for by multi-scan methods (CrysAlis PRO; Rigaku OD, 2020 ▸).
| I | III | IV | |
|---|---|---|---|
| Crystal data | |||
| Chemical formula | C4H4O2 | C4H5O2+·AsF6− | C6H8O4 |
| M r | 84.07 | 274.00 | 144.12 |
| Crystal system, space group | Monoclinic, P21/n | Monoclinic, P21/n | Triclinic, P
|
| temperature (K) | 111 | 111 | 112 |
| a, b, c (Å) | 3.8409 (5), 15.593 (2), 7.6149 (10) | 6.9609 (5), 8.9319 (7), 13.7189 (9) | 3.8726 (11), 5.6546 (10), 8.3778 (18) |
| α, β, γ (°) | 90, 99.910 (12), 90 | 90, 91.664 (7), 90 | 100.642 (16), 100.42 (2), 105.73 (2) |
| V (Å3) | 449.27 (10) | 852.60 (11) | 168.30 (7) |
| Z | 4 | 4 | 1 |
| μ (mm−1) | 0.10 | 4.06 | 0.12 |
| Crystal size (mm) | 1.00 × 0.56 × 0.31 | 0.90 × 0.21 × 0.15 | 0.52 × 0.46 × 0.35 |
| Data collection | |||
| Tmin, Tmax | 0.457, 1.000 | 0.582, 1.000 | 0.579, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 3885, 1060, 891 | 4205, 2303, 1900 | 1403, 825, 703 |
| R int | 0.023 | 0.029 | 0.015 |
| θmax (°) | 27.9 | 29.1 | 28.3 |
| (sin θ/λ)max (Å−1) | 0.658 | 0.685 | 0.667 |
| Refinement | |||
| R[F2 > 2σ(F2)], wR(F2), S | 0.036, 0.099, 1.05 | 0.039, 0.099, 1.05 | 0.048, 0.144, 1.04 |
| No. of reflections | 1060 | 2303 | 825 |
| No. of parameters | 71 | 123 | 51 |
| No. of restraints | 0 | 0 | 0 |
| H-atom treatment | All H-atom parameters refined | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.18, −0.15 | 0.87, −0.92 | 0.52, −0.24 |
| VI | VII | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C6H9O4+·BF4−·2HF | 0.5C6H10O42+·Sb2F11− |
| M r | 271.96 | 525.57 |
| Crystal system, space group | Orthorhombic, Pbca | Orthorhombic, Pbca |
| temperature (K) | 101 | 101 |
| a, b, c (Å) | 12.8759 (5), 11.8899 (4), 14.6252 (7) | 7.8461 (6), 15.1531 (11), 19.5536 (17) |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 |
| V (Å3) | 2239.02 (16) | 2324.8 (3) |
| Z | 8 | 8 |
| μ (mm−1) | 0.19 | 4.79 |
| Crystal size (mm) | 0.28 × 0.18 × 0.11 | 0.25 × 0.11 × 0.05 |
| Data collection | ||
| Tmin, Tmax | 0.885, 1.000 | 0.807, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 7514, 1717, 1478 | 13316, 3784, 2878 |
| R int | 0.026 | 0.053 |
| θmax (°) | 23.8 | 32.2 |
| (sin θ/λ)max (Å−1) | 0.568 | 0.750 |
| Refinement | ||
| R[F2 > 2σ(F2)], wR(F2), S | 0.040, 0.100, 1.05 | 0.036, 0.071, 1.04 |
| No. of reflections | 1717 | 3784 |
| No. of parameters | 176 | 169 |
| No. of restraints | 1 | 0 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.77, −0.35 | 1.09, −1.01 |
Figure 1.
A difference Fourier map of III without the H atom between O1 and F1. The green solid lines and red dotted lines show positive and negative density distribution, respectively.
Figure 2.
A difference Fourier map of VI without the H atom between O1 and F1. The green solid lines and red dotted lines show positive and negative density distribution, respectively.
Figure 3.
A difference Fourier map of VII without the H atom between O1 and F1. The green solid lines and red dotted lines show positive and negative density distribution, respectively.
Results and discussion
Single-crystal X-ray diffraction
Crystal structure of methyl prop-2-ynoate (I)
Compound I crystallizes in the monoclinic space group P21/n with one formula unit per unit cell. Fig. 4 ▸ displays the asymmetric unit. The C1—C2 bond length [1.4466 (15) Å] is significantly elongated compared to an average Csp1—Csp2 hybridized bond (1.427 Å) determined by X-ray diffraction (Allen et al., 1987 ▸). The C2≡C3 triple bond [1.1780 (16) Å] is in the same range as average terminal C≡C bonds (1.181 Å; Allen et al., 1987 ▸). The C1=O1 bond [1.1972 (14) Å] is in the same range as other C=O bonds (1.196 Å) in esters (Allen et al., 1987 ▸). The C1—O2 bond [1.3195 (12) Å] is significantly shortened compared to other esters (1.337 Å; Allen et al., 1987 ▸). The C4—O2 bond [1.4463 (13) Å] is significantly elongated compared to average CH3—O bonds in esters (1.418 Å; Allen et al., 1987 ▸).
Figure 4.
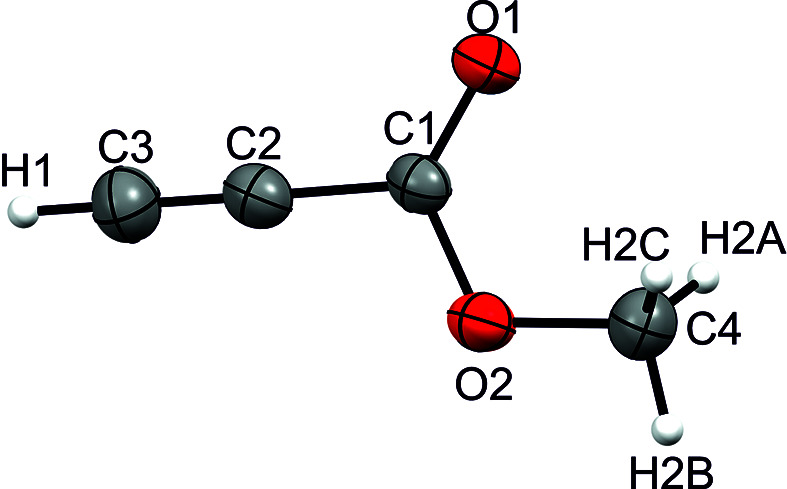
The asymmetric unit of I, with displacement ellipsoids drawn at the 50% probability level.
The crystal structure of I displays a layered structure built of weak C3—H1⋯O1 hydrogen bonds, according to the classification of Jeffrey (1997 ▸). The layered structure is connected via weak C4—H2A⋯O1 hydrogen bonds into a three-dimensional network (Fig. 5 ▸), according to the classification of Jeffrey (1997 ▸).
Figure 5.
Hydrogen bonds in the crystal structure of I, with displacement ellipsoids drawn at the 50% probability level. [Symmetry codes: (i) x −  , −y +
, −y +  , z +
, z +  ; (ii) −x + 1, −y + 1, −z + 2; (iii) x +
; (ii) −x + 1, −y + 1, −z + 2; (iii) x +  , −y +
, −y +  , z −
, z −  .]
.]
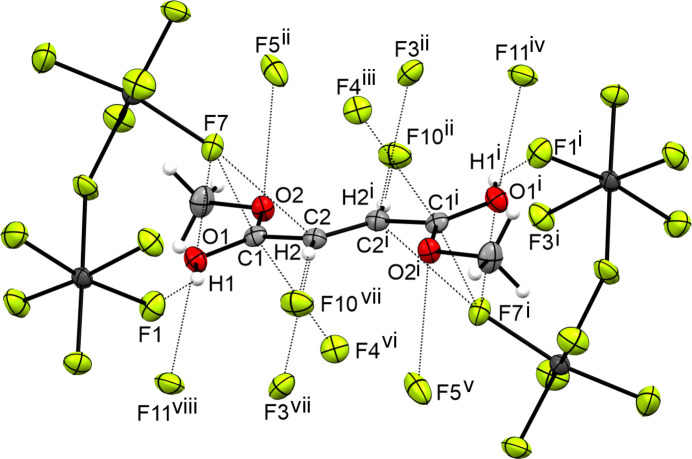
Crystal structure of (1-methoxyprop-2-yn-1-ylidene)oxidanium hexafluoroarsenate (III)
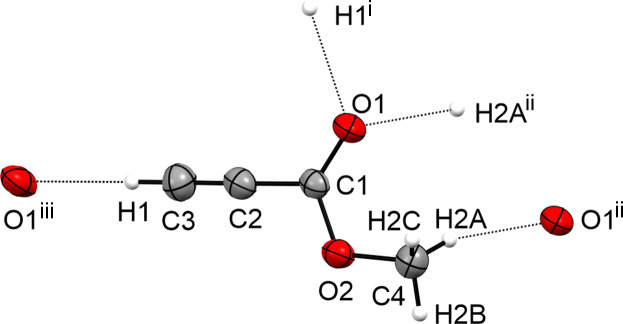
Salt III crystallizes in the monoclinic space group P21/n with four formula units per unit cell. Fig. 6 ▸ displays the asymmetric unit of III. The C1—O1 bond [1.261 (4) Å] is significantly elongated by 0.064 Å due to the protonation compared to I. The C1—O2 bond [1.270 (3) Å] is significantly shortened by 0.049 Å compared to the starting material I. Due to the protonation, the C4—O2 bond [1.484 (4) °] is elongated by 0.038 Å compared to the neutral compound I. The C2≡C3 triple bond is not significantly influenced by the protonation.
Figure 6.
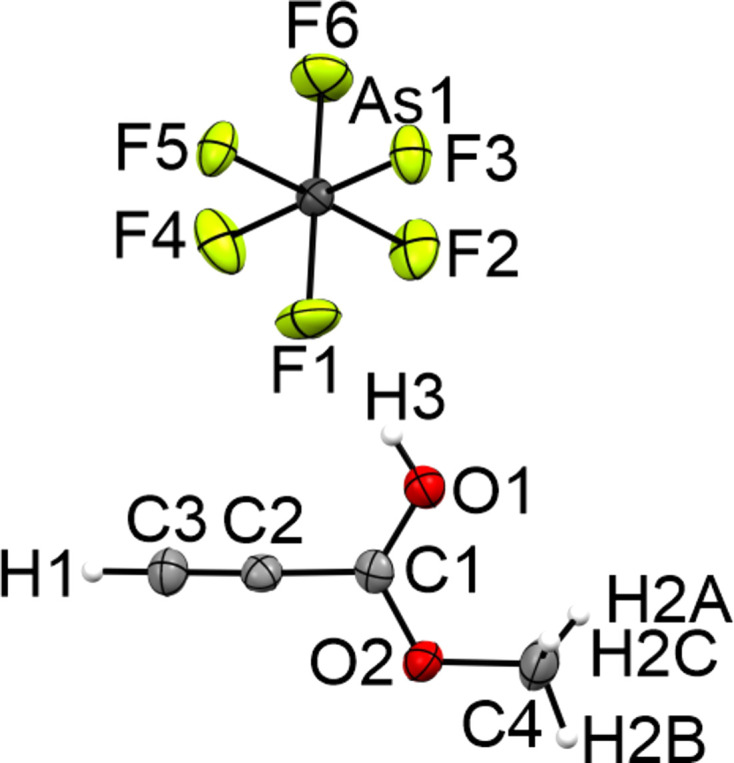
The asymmetric unit of III, with displacement ellipsoids drawn at the 50% probability level.
The three-dimensional network of III (Fig. 7 ▸) is built by a strong O1—H3⋯F1 and three weak C3—H1⋯F5, C3—H1⋯F6 and C4—H2B⋯F3 hydrogen bonds, according to the classification of Jeffrey (1997 ▸). Additionally, the crystal structure forms two interatomic contacts (C1⋯F2 and C1⋯F5) which are 8% shorter than the sum of the van der Waals radii.
Figure 7.

The intramolecular interactions in the crystal structure of III, with displacement ellipsoids drawn at the 50% probability level. [Symmetry codes: (i) −x +  , y −
, y −  , −z +
, −z +  ; (ii) −x, −y + 1, −z + 1; (iii) −x + 1, −y + 1, −z + 1; (iv) x, y − 1, z; (v) −x +
; (ii) −x, −y + 1, −z + 1; (iii) −x + 1, −y + 1, −z + 1; (iv) x, y − 1, z; (v) −x +  , y −
, y −  , −z +
, −z +  .]
.]
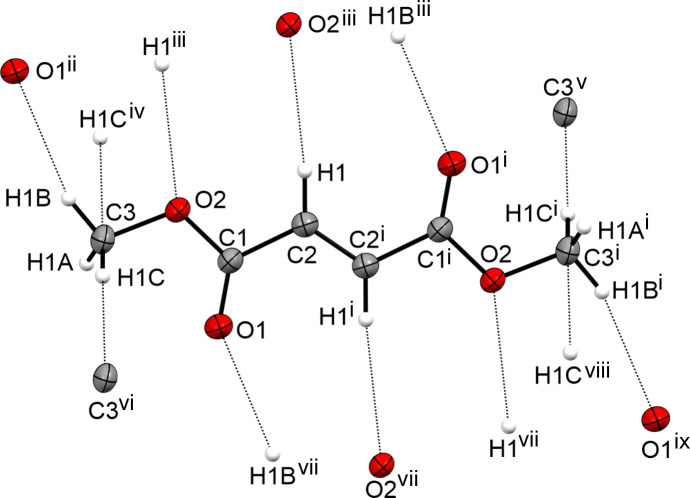
Crystal structure of dimethyl (E)-but-2-enedioate (IV)
The determined crystal structure is in the same range as that reported by Kooijman et al. (2004 ▸). The formula unit is shown in Fig. 8 ▸ and the crystal structure exhibits the same three-dimensional network (Fig. 9 ▸).
Figure 8.
The formula unit of IV, with displacement ellipsoids drawn at the 50% probability level. [Symmetry code: (i) −x + 1, −y + 2, −z + 1.]
Figure 9.
The intramolecular interactions in the crystal structure of IV, with displacement ellipsoids drawn at the 50% probability level. [Symmetry codes: (i) −x + 1, −y + 2, −z + 1; (ii) x − 1, y − 1, z; (iii) −x, −y + 1, −z + 1; (iv) x − 1, y, z; (v) −x, −y + 2, −z + 1; (vi) x + 1, y, z; (vii) x + 1, y + 1, z; (viii) −x + 2, −y + 2, −z + 1; (ix) −x + 2, −y + 3, −z + 1.]
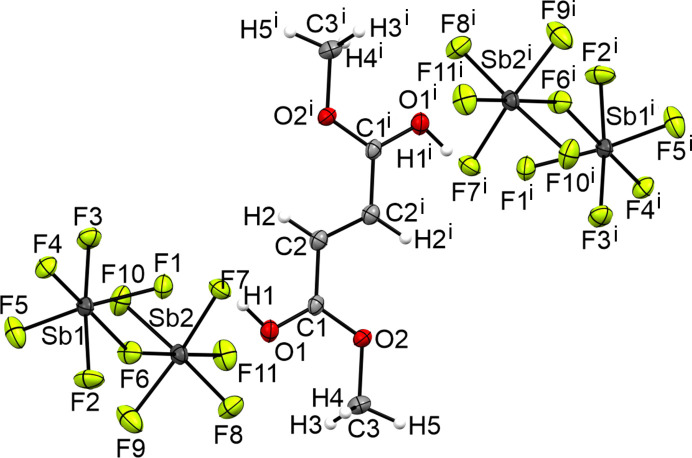
Crystal structure of 1,4-dimethoxy-4-oxobut-2-en-1-ylidene]oxidanium tetrafluoroborate–hydrogen fluoride (1/2) (VI)
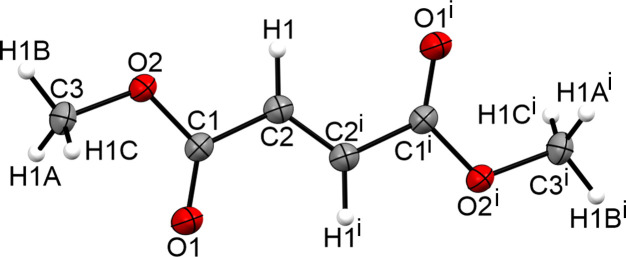
The crystal structure of the monoprotonated species VI of dimethyl fumarate crystallizes in the orthorhombic space group Pbca with eight formula units per unit cell. Fig. 10 ▸ displays the asymmetric unit. Similar to fumaric acid and acetylenedicarboxylic acid, the monoprotonated type forms extended chains of cations that are connected via strong O3—H9⋯O1 hydrogen bonds (Jessen & Kornath, 2022 ▸; Bayer et al., 2020 ▸; Jeffrey, 1997 ▸). Due to the protonation, the C1—O1 [1.248 (3) Å] and C4—O3 [1.247 (3) Å] bonds are significantly elongated compared to the neutral compound [1.205 (2) Å]. The C1—O2 and C4—O4 bonds [both 1.289 (3) Å] are shortened by 0.052 Å compared to the starting material IV. The CH3—O and C2=C3 bonds are not significantly influenced by the monoprotonation.
Figure 10.
The asymmetric unit of VI, with displacement ellipsoids drawn at the 50% probability level.
Besides the strong hydrogen bonding between the cations, the crystal structure forms a medium–strong C6—H6⋯F6 hydrogen bond [3.198 (3) Å] and two weak C5—H3⋯F4 [3.242 (3) Å] and C6—H7⋯F2 [3.261 (3) Å] hydrogen bonds. Furthermore, the crystal structure forms six interatomic C⋯F contacts, i.e. C1⋯F1 [2.900 (3) Å], C1⋯F6 [2.943 (3) Å], C2⋯F6 [2.987 (3) Å], C3⋯F6 [3.048 (3) Å], C4⋯F2 [3.020 (3) Å] and C4⋯F5 [2.974 (3) Å], which are shorter than the sum of the van der Waals radii (3.17 Å). The interactions in the crystal structure of VI are shown in Fig. 11 ▸.
Figure 11.
The intramolecular interactions in the crystal structure of VI, with displacement ellipsoids drawn at the 50% probability level. [Symmetry codes: (i) −x +  , y −
, y −  , z; (ii) −x +
, z; (ii) −x +  , y +
, y +  , z; (iii) x −
, z; (iii) x −  , y + 1, −z +
, y + 1, −z +  ; (iv) x, y + 1, z; (v) −x + 1, −y + 1, −z + 1; (vi) x, −y +
; (iv) x, y + 1, z; (v) −x + 1, −y + 1, −z + 1; (vi) x, −y +  , z +
, z +  ; (vii) x −
; (vii) x −  , −y +
, −y +  , −z + 1.]
, −z + 1.]
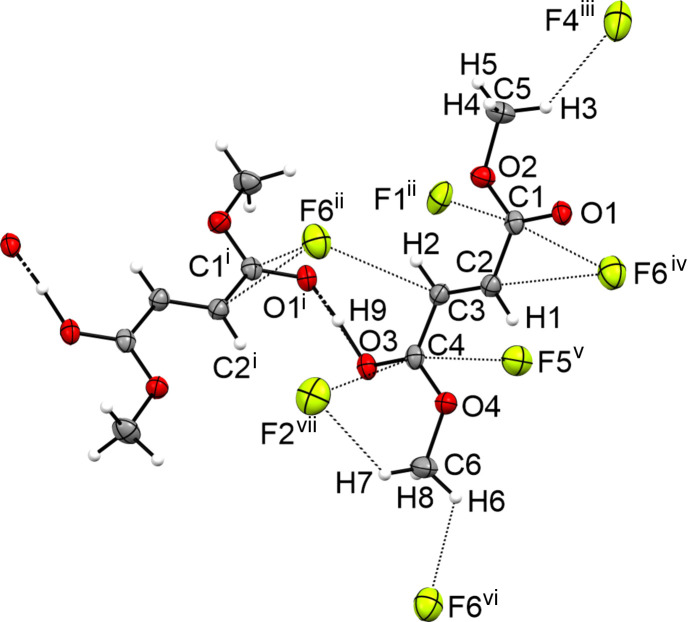
Crystal structure of hemi{[1,4-dimethoxy-4-oxidaniumylidenebut-2-en-1-ylidene]oxidanium} undecafluorodiarsenate (VII)
The crystal structure of the diprotonated species VII of dimethyl fumarate crystallizes in the orthorhombic space group Pbca with eight formula units per unit cell. Fig. 12 ▸ displays the formula unit. The crystal structure of the diprotonated species has a C1—O1 bond [1.277 (5) Å] significantly elongated by 0.072 Å compared to the starting material [1.205 (2) Å]. The C1—O2 bond [1.281 (5) Å] is shortened by 0.060 Å compared to the neutral compound [1.341 (1) Å]. The C3—O2 bond [1.489 (6) Å] is elongated by 0.034 Å compared to IV [1.455 (2) Å].
Figure 12.
The formula unit of VIII, with displacement ellipsoids drawn at the 50% probability level. [Symmetry code: (i) −x + 1, −y + 1, −z + 1.]
The three-dimensional network is formed by a strong O1—H1⋯F1 hydrogen bond and seven interatomic interactions (Jeffrey, 1997 ▸). The interatomic interactions are C1⋯F4 [3.061 (4) Å], C1⋯F7 [3.001 (4) Å], C2⋯F3 [2.958 (5) Å], C2⋯F7 [3.008 (5) Å], C3⋯F2 [3.050 (6) Å], O1⋯F7 [2.985 (4) Å] and O1⋯F11 [2.864 (4) Å]. Fig. 13 ▸ displays the interatomic distances in the crystal structure of VII.
Figure 13.
The intramolecular interactions in the crystal structure of VIII, with displacement ellipsoids drawn at the 50% probability level. [Symmetry codes: (i) −x + 1, −y + 1, −z + 1; (ii) −x +  , y +
, y +  , z; (iii) x +
, z; (iii) x +  , −y +
, −y +  , −z + 1; (iv) −x + 2, −y + 1, −z + 1; (v) x −
, −z + 1; (iv) −x + 2, −y + 1, −z + 1; (v) x −  , −y +
, −y +  , −z + 1; (vi) −x +
, −z + 1; (vi) −x +  , y +
, y +  , z; (vii) x −
, z; (vii) x −  , −y +
, −y +  , −z + 1; (viii) x − 1, y, z.]
, −z + 1; (viii) x − 1, y, z.]
Raman spectroscopy
Raman spectra of I, II and III
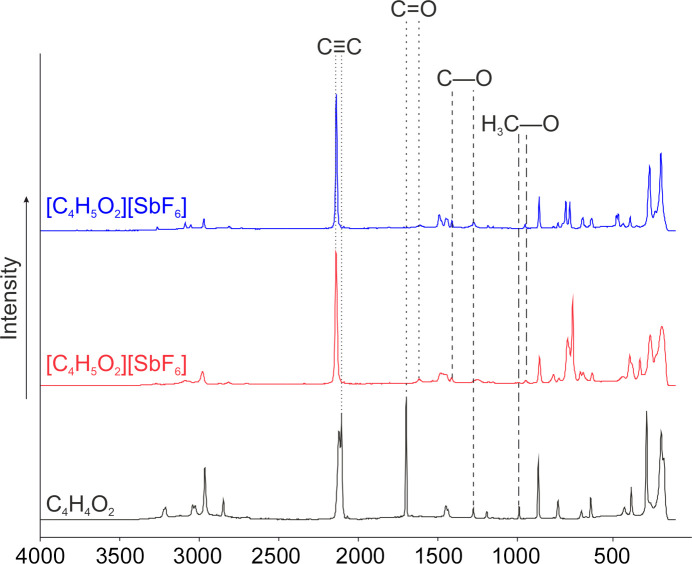
Fig. 14 ▸ displays the low-temperature Raman spectra of I, II and III. The first evidence for succesful protonation is the significantly red-shifted C=O oscillation from 1700 cm−1 in the starting material I to 1617 (in II) and 1615 cm−1 (in III). Due to the protonation, the C—O oscillation is blue-shifted in the Raman spectra to 1414 (in II) and 1413 cm−1 (in III) compared to the starting material (1278 cm−1). The H3C—O oscillation is red-shifted by 42 cm−1 to 948 (in II) and 951 cm−1 (in III) compared to I (990 cm−1). The oscillation of the triple bond is only slightly affected from 2107 cm−1 to 2141 (in II) and 2140 cm−1 (in III).
Figure 14.
The low-temperature Raman spectra of I (black), II (red) and III (blue).
Raman spectra of IV, V, VII and VIII
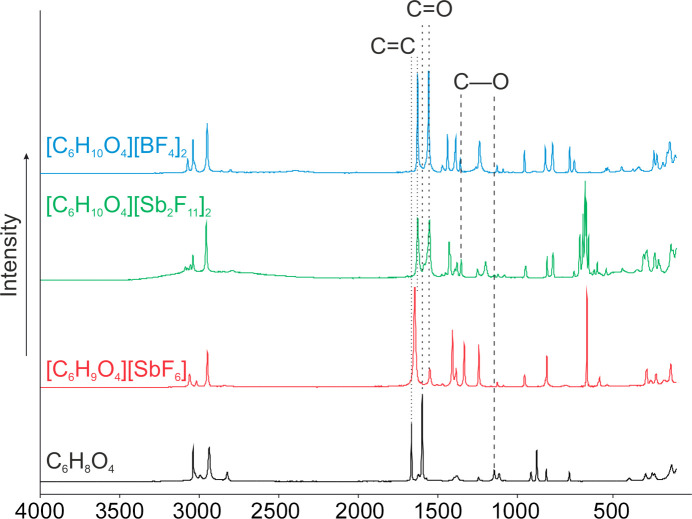
Fig. 15 ▸ shows the low-temperature Raman spectra of IV, V, VII and VIII. The C=O oscillation is red-shifted by 48 cm−1 to 1611 cm−1 compared to IV (1659 cm−1). Due to the protonation, the C—O oscillation is blue-shifted in the Raman spectra to 1401 cm−1 (V) compared to the neutral compound (1217 cm−1). The C=C oscillation is only slightly red shifted to 1705 cm−1 compared to the starting material (1725 cm−1).
Figure 15.
The low-temperature Raman spectra of IV (black), V (red), VII (green) and VIII (blue).
The diprotonation is characterized by a red-shifted C=O oscillation by 46 cm−1 to 1613 cm−1 in the spectra of VII and VIII compared to the Raman spectrum of IV (1659 cm−1). The C=C oscillation is significantly red-shifted by 38 cm−1 to 1686 cm−1 in the spectra of VII and VIII compared to the Raman spectrum of IV (1724 cm−1). Due to the protonation, the C—O oscillation is blue-shifted in the Raman spectra to 1419 (in VII) and 1425 cm−1 (in VIII) compared to the neutral compound (1217 cm−1).
Conclusion
We present herein the first single-crystal X-ray diffraction and Raman spectroscopy study of the monoprotonated species of methyl prop-2-ynoate and mono- and diprotonated species of dimethyl fumarate. All three protonated species crystallize in the more stable syn–anti conformation. Furthermore, the first single-crystal structure of methyl prop-2-ynoate is reported. The protonated species are important intermediates of acid-catalyzed reactions.
Supplementary Material
Crystal structure: contains datablock(s) I, III, IV, VI, VII, global. DOI: 10.1107/S2053229624011653/oj3025sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2053229624011653/oj3025Isup2.hkl
Structure factors: contains datablock(s) III. DOI: 10.1107/S2053229624011653/oj3025IIIsup3.hkl
Structure factors: contains datablock(s) IV. DOI: 10.1107/S2053229624011653/oj3025IVsup4.hkl
Structure factors: contains datablock(s) VI. DOI: 10.1107/S2053229624011653/oj3025VIsup5.hkl
Structure factors: contains datablock(s) VII. DOI: 10.1107/S2053229624011653/oj3025VIIsup6.hkl
Acknowledgments
We are grateful to the Department of Chemistry at the Ludwig Maximilian University of Munich, the Deutsche Forschungsgemeinschaft (DFG), the F-Select GmbH and Professor Dr Konstantin Karaghiosoff and Dr Constantin Hoch for their support. Open access funding enabled and organized by Projekt DEAL.
Funding Statement
Funding for this research was provided by: Ludwig-Maximilians-University; F-Select GmbH; Deutsche Forschungsgemeinschaft.
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
- Bayer, M. C., Jessen, C. & Kornath, A. J. (2020). Z. Anorg. Allg. Chem.646, 333–339.
- Farrugia, L. J. (2012). J. Appl. Cryst.45, 849–854.
- Hogeveen, H. (1967). Recl Trav. Chim. Pays Bas, 86, 816–820.
- Hogeveen, H. (1968). Recl Trav. Chim. Pays Bas, 87, 1313–1317.
- Hollenwäger, D., Morgenstern, Y., Daumer, L., Bockmair, V. & Kornath, A. J. (2024a). ACS Earth Space Chem.8, 2101–2109.
- Hollenwäger, D., Thamm, S., Bockmair, V., Nitzer, A. & Kornath, A. J. (2024b). J. Org. Chem.89, 11421–11428. [DOI] [PubMed]
- Jeffrey, G. A. (1997). In An Introduction to hydrogen Bonding. New York, Oxford: Oxford University Press.
- Jessen, C. & Kornath, A. J. (2022). Eur. J. Inorg. Chem.2022, e202100965,
- Kooijman, H., Sprengers, J. W., Agerbeek, M. J., Elsevier, C. J. & Spek, A. L. (2004). Acta Cryst. E60, o917–o918.
- Olah, G. A., O’Brien, D. H. & White, A. M. (1967). J. Am. Chem. Soc.89, 5694–5700.
- Olah, G. A., Prakash, G. K. S., Molnar, A. & Sommer, J. (2009). In Superacid Chemistry, 2nd ed. Hoboken, NJ: Wiley.
- Rigaku OD (2020). CrysAlis PRO. Rigaku Oxford Diffraction Ltd, Yarnton, Oxfordshire, England.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spek, A. L. (2020). Acta Cryst. E76, 1–11. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, III, IV, VI, VII, global. DOI: 10.1107/S2053229624011653/oj3025sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2053229624011653/oj3025Isup2.hkl
Structure factors: contains datablock(s) III. DOI: 10.1107/S2053229624011653/oj3025IIIsup3.hkl
Structure factors: contains datablock(s) IV. DOI: 10.1107/S2053229624011653/oj3025IVsup4.hkl
Structure factors: contains datablock(s) VI. DOI: 10.1107/S2053229624011653/oj3025VIsup5.hkl
Structure factors: contains datablock(s) VII. DOI: 10.1107/S2053229624011653/oj3025VIIsup6.hkl