Introduction
The Hospital Pharmacy Service Guideline 2015 of Nepal aims to promote accessible and quality pharmaceutical services from the hospital. 1 The Constitution of Nepal effectively grants all the right to information and health services by providing accessible and quality services. 2 Right to health (Part 3, Section 35) further explains that everyone has the right to get information about their medical treatments, including treatment therapy, choice, possible side effects, and unprecedented effects. 2
Pharmacists employed in drug information centers have a prominent role in enhancing the quality of health services by providing evidence-based information to patients, preventing possible medication errors (MEs), adverse drug reactions (ADRs) and drug-drug interactions (DDIs) which improves patient safety through rational use of medicine.3-5
Similarly, the Public Health Services Act 2075 (2018), Rule 10 explains that every health institution and health worker providing treatment shall provide the service recipient with information on health conditions, treatment, alternative treatment strategy, and risks/benefits of drug therapy. 6
Lack of accurate drug information in the service process poses problems in pharmacotherapy, failure of therapy, economic burden to patients, developing secondary illness as well as adverse drug effects. 7 In the context of multiple healthcare systems, there is a lack of reliable and appropriate information about the benefits and risks of drug therapy; hence, a system for authentic, accurate, unbiased and evidence-based drug information could contribute to the appropriate patient information. 7
The quality standards and regulation division of the Ministry of Health and Population, Nepal, has prepared a checklist or minimum service standards (MSS) to determine any gaps in the quality improvement of secondary hospitals with higher services. 8 As per the checklist, the pharmacy department needs a separate information and counseling unit with reference books or Information, education, and communication (IEC) materials (MSS Code 2.5.14) related to medicine and their appropriate use. Similarly, the MSS also mentions the need to initiate and operate a pharmacovigilance (PV) center for reporting ADRs (MSS Code 2.5.19.1). 8
PV in a hospital is essential for ensuring the safety of medications by monitoring, detecting, assessing, and preventing ADRs and other medication-related problems. 9 Lack of knowledge and awareness about PV and reporting ADRs among healthcare professionals may contribute to undetected ADRs, MEs, poor patient safety, and underreporting.9,10 PV aims to enhance patient care and patient safety concerning the use of medicinal products and to support public health programs by providing reliable, accurate, and evidence-based information to assess the risk-benefit profile of medicines. The existing limitation regarding information delivery and PV services within Nepal genuinely underscores the need for a coordinated department that provides evidence-based information and promotes drug safety along with the fulfillment of the MSS checklist.7,9
Implementation of Hospital Pharmacy Service Guideline 2015
Initiation of Drug Information Unit and Pharmacovigilance Cell
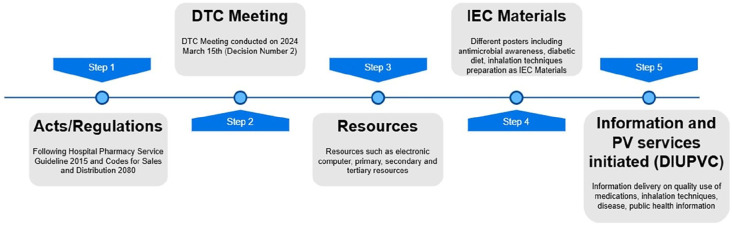
Hospital Pharmacy Service Guideline 2015 is essential for promoting hospital pharmacy practice in the country. 11 In response to the published literature 11 and to fulfill the objectives set by Hospital Pharmacy Service guideline 2015, 1 Codes for sales and distribution of drugs 2080, 12 and to promote pharmacy practice, various steps were followed. This ultimately led to the formation of the Drug Information Unit and Pharmacovigilance Cell (DIUPVC; Figure 1). Hence, in this editorial, we document how we initiated the first phase of hospital pharmacy practice along with the experience, work done, challenges, and future goals. This is a novel task in Nepal, and we believe this work will help promote hospital pharmacy practice in several hospitals in the coming days.
Figure 1.
Steps involved in the initiation of drug information unit and pharmacovigilance cell (DIUPVC).
Note. Cell, in this context, means the room designated for providing drug information and pharmacovigilance activities. DTC = drug and therapeutic committee; IEC = information, education, and communication; PV = pharmacovigilance.
Site and Aims of DIUPVC
The cell is established at Hetauda Hospital, Madan Bhandari Academy of Health Sciences. Hetauda Hospital is in the capital of Bagmati Province of Nepal, Makwanpur. It is a growing governmental hospital that provides service to an average of 800 to 1000 patients daily with more than 100 beds. Hence, to provide pharmaceutical services to patients, the cell which comes under the branch of Hospital Pharmacy aims:
To promote the rational use of medication and provide the maximum quality of health services to patients by providing accurate, unbiased, validated, and evidence-based information to healthcare professionals and patients.
To improve medication safety and treatment outcomes via prescription monitoring, detection, and reporting of ADR, as well as patient-based ADR reporting and active surveillance systems. This section aims to promote patient safety, quality medication use, and identification and resolution of drug-related problems (Figure 2).
Figure 2.
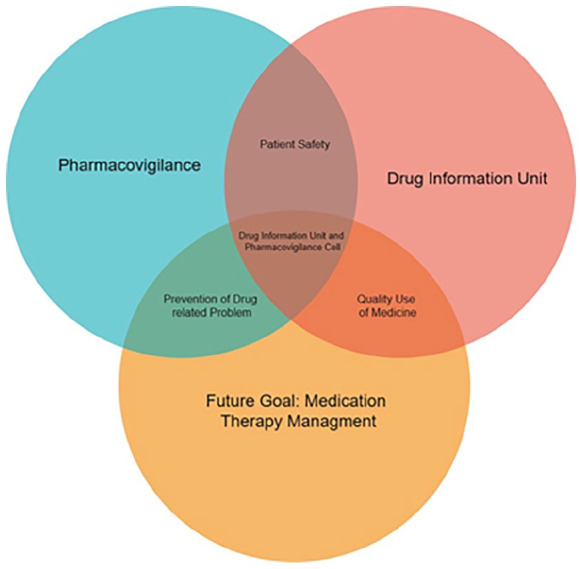
Current and future aims of the drug information unit and pharmacovigilance cell.
Experience and Works Done To Date
After the initiation, the cell employed itself to deliver drug information to patients and health care professionals by conducting various activities (Table 1). Posters have been prepared as IEC materials in the Nepalese language to convey critical information. The posters were created using literature, converted to Nepalese language, and published/printed within the hospital pharmacy section on every pharmacist consensus.
Table 1.
Activities Conducted by the Drug Information Unit and Pharmacovigilance Cell.
| Activities | Sub domain | Contents dealt with | References/link/section used while preparing the materials | Materials present |
|---|---|---|---|---|
| Counseling | Medication counseling, information delivery | Inhalation medication counseling for patients diagnosed with COPD and asthma for drugs with fixed-dose combinations and Rotacaps (R/Cs) such as (R/Cs formoterol + Budesonide, R/Cs formoterol 6 µg + budesonide 400 µg, MDI salbutamol 100 µg | GOLD guidelines and quality use of medicines for inhalers 13 | N/A |
| Query on drug allergies, smoking history | ||||
| Query on substance history | ||||
| General information on OTC drugs and diseases | ||||
| Presentations | Insurance benefit package | Insurance, history of health insurance in Nepal | https://hib.gov.np/en/pages/health-care-packages | N/A |
| Information on risk pooling | ||||
| Health insurance act and directive | ||||
| What medicines are included in the insurance benefits package? | ||||
| Pharmacovigilance | Introduction of pharmacovigilance, the importance of detection, assessment, understanding, and reporting of ADR | Hospital pharmacy service guideline 2015 rule 4 (sub-rule 5), 1 Codes for sales and distribution 2080 section 5 subsection 15, 12 minimum service standards clause 2.5.19 8 | ADR reporting form | |
| Concept of drug safety | ||||
| Pharmacovigilance is a part of MSS | ||||
| ADR reporting form | ||||
| Pharmacovigilance network in Nepal | ||||
| Medication errors | Introduction to medication errors, causes, incidence, common types of medication errors, ways to tackle errors | National coordinating council for medication error reporting and prevention, 14 Key facts about medication errors (MEs) in the World Health Organization (WHO) European Region, 15 American Society of Health-System Pharmacy standard definition of a medication error 16 | ||
| Preparation of IEC materials | Diabetic diet counseling | Concept of diabetic diet with diagrammatic figures and what kind of foods are included within the 9-inch plate (0 calorie drink, 1/4th carbohydrates, 1/4th protein, and 1/2 non-starchy vegetables) | Healthy living with diabetes, National Institute of Diabetes and Digestive and Kidney Disease 17 | Counseling leaflet and poster |
| Inhalation device (inhaler counseling) | Steps to consider while using it for the first time | Inhalation technique using several brands of leaflets | Inhaler, inhalation use leaflet, placebo | |
| Step-by-step method for using the inhaler | ||||
| Inhalation device for dry powers (eg, Rotahaler handling) | A step-by-step method for using the inhalation device | Prepared from literature 13 | Inhalation device, inhalation use leaflet, placebo | |
| Antibiotic awareness | Antimicrobial resistance and awareness | WHO resource materials 18 | Posters | |
| Hospital Formulary | Development of hospital formulary | Presentation on formulary, its contents, and importance, and process for its development via formulary committee | Hospital Pharmacy Service Guideline 2015 rule 19, 1 minimum service standards 2.5.3 8 | Hospital formulary draft |
Note. This table summarizes the activities conducted within the drug information unit and pharmacovigilance cell since its initiation. COPD = chronic obstructive pulmonary disease; MDI = meter dose inhaler; GOLD = global initiative for chronic obstructive lung disease; OTC = over the counter; ADR = adverse drug reaction; MSS = minimum service standards; IEC = information, education and communication; N/A = not available.
Challenges for Initiation and Implementation of DIUPVC
The major challenge resides with the continuation of the cell and the underreporting of ADR and patient engagement. It is challenging to inform patients about the importance of the cell and help them handle their medication. The majority of patients tend to be unaware and ignore their medication-taking behavior. Hence, this opens room for the initiation of social media to bring out the purpose of drug information even more widely. However, the major challenge with ADR reporting is its underreporting by nurses and healthcare professionals.9,10 Thus, specific actions, such as planned awareness campaigns or additional training for healthcare staff, would help overcome these challenges.
Future Goals and Directions of DIUPVC
In the coming days, DIUPVC aims to form a liaison with a non-governmental organization in the hospital’s permission to provide information about rational drug use as outlined by the National Drug Policy 1995. 19 Providing information about complementary and alternative medicines in the coming days is also a priority because of its vast consumption and use. The cell aims to conduct research activities and provide evidence-based information for healthcare professionals (Table 2).
Table 2.
Future Goals and Direction of Drug Information Unit and Pharmacovigilance Cell.
| Future goals | Term | Comments |
|---|---|---|
| Drug utilization studies | Short-term objective | To promote and assess the rationality of therapy 20 and adherence to MSS Clause 2.5.19.2 |
| Continuous professional development programs | Short-term objective | To enhance the skills of pharmacy and health care professionals on regular updates |
| Medication therapy management | Medium-term objective | To promote the idea of clinical pharmacy services |
| Newsletter/drug bulletin | Medium-term objective | To share ADR and pertinent information on recent drugs and hazard |
| Liaison with non-governmental organizations | Long-term objective | To promote the idea of rational use of drug |
| Academia-hospital sessions | Long-term objective | To promote and enhance the skills and learning of students through a practical approach |
Note. Short-term: goals we aim to achieve within 1-month; medium-term: goals to achieve within 1.5 to 2.5 months; long-term: goals to achieve within 3-6 months. MSS = minimum service standards; ADR = adverse drug reaction.
Conclusion
Intending to promote pharmacy practice in hospital settings and implement hospital pharmacy service guidelines in 2015, DIUPVC helped provide pharmaceutical services with a major focus on the quality use of medicines, rational use of drugs, and evidence-based information delivery. With this implementation, the cell aims to promote the practice further, thus improving the quality of the hospital itself.
Acknowledgments
The authors thank the online flowchart maker and online diagram software Draw.io (https://app.diagrams.net/) for helping with the diagram. The authors are incredibly thankful to the entire hospital administration and Dr. Ramchandra Sapkota, the Acting Hospital Director, for helping initiate this purpose.
Footnotes
Author Contribution: NP conceptualized the idea and wrote the initial version of the manuscript. AR, SB, DK, AR, DRG, and PS added contents to the manuscript later and helped in the management of the drug information unit and pharmacovigilance cell. SD and SS are experts in pharmacovigilance, pharmacy practice and research, provided valuable feedback in starting the drug information unit and pharmacovigilance cell, and critically reviewed the manuscript. All authors agreed on the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Nabin Pathak  https://orcid.org/0000-0002-1952-642X
https://orcid.org/0000-0002-1952-642X
Amod Rasaili  https://orcid.org/0009-0000-6980-7222
https://orcid.org/0009-0000-6980-7222
Sachita Barma  https://orcid.org/0009-0009-2014-4339
https://orcid.org/0009-0009-2014-4339
Dibyata Khatiwada  https://orcid.org/0009-0002-7050-0652
https://orcid.org/0009-0002-7050-0652
Anusha Raila  https://orcid.org/0009-0007-4034-8959
https://orcid.org/0009-0007-4034-8959
Devendra Raj Gautam  https://orcid.org/0009-0005-1266-4861
https://orcid.org/0009-0005-1266-4861
Prerana Shrestha  https://orcid.org/0009-0008-7016-2028
https://orcid.org/0009-0008-7016-2028
Shreya Dhungana  https://orcid.org/0000-0001-6176-0255
https://orcid.org/0000-0001-6176-0255
Sunil Shrestha  https://orcid.org/0000-0002-9174-7120
https://orcid.org/0000-0002-9174-7120
References
- 1. Hospital Pharmacy Guideline 2072. Department of Drug Administration. Updated 2015. Accessed October 18, 2024. https://dda.gov.np/content/hospital-pharmacy-guideline-2072 [Google Scholar]
- 2. Constitution of Nepal 2072. Updated 2015. Accessed October 18, 2024. https://ag.gov.np/files/Constitution-of-Nepal_2072_Eng_www.moljpa.gov_.npDate-72_11_16.pdf
- 3. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med (Milton). 2019;2(1):42-49. doi: 10.1002/agm2.12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Almuqbil M, Alrojaie L, Alturki H, et al. The role of drug information centers to improve medication safety in Saudi Arabia—a study from healthcare professionals’ perspective. Saudi Pharmac J. 2022;30(4):377-381. doi: 10.1016/j.jsps.2022.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kunwor P, Basyal B, Pathak N, Vaidya P, Shrestha S. Study to evaluate awareness about medication errors and impact of an educational intervention among healthcare personnel in a cancer hospital. J Oncol Pharm Pract. Published online February 25, 2024:10781552241235898. doi: 10.1177/10781552241235898 [DOI] [PubMed] [Google Scholar]
- 6. The Public Health Service Act 2075. Ministry of Health and Population. Updated 2018. Accessed October 18, 2024. https://fwd.gov.np/wp-content/uploads/2023/12/The-Public-Health-Service-Act-2075-2018.pdf [Google Scholar]
- 7. Shrestha S, Khatiwada AP, Gyawali S, Shankar PR, Palaian S. Overview, challenges and future prospects of drug information services in Nepal: a reflective commentary. JMDH. 2020;13:287-295. doi: 10.2147/JMDH.S238262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minimum Service Standards, Secondary Hospitals with Higher Services, Ministry of Health and Population. 2018. Accessed October 18, 2024. https://www.nhssp.org.np/Resources/HPP/MSS_Secondary_Hospitals_with_Higher_Services.pdf [Google Scholar]
- 9. Danekhu K, Shrestha S, Aryal S, Shankar PR. Health-care professionals’ knowledge and perception of adverse drug reaction reporting and pharmacovigilance in a tertiary care teaching hospital of Nepal. Hosp Pharm. 2021;56(3):178-186. doi: 10.1177/0018578719883796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shrestha S, Sharma S, Bhasima R, Kunwor P, Adhikari B, Sapkota B. Impact of an educational intervention on pharmacovigilance knowledge and attitudes among health professionals in a Nepal cancer hospital. BMC Med Educ. 2020;20(1):179. doi: 10.1186/s12909-020-02084-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khadka S, Dc M, Maleku K, Thapa P. Implementing hospital pharmacy service guideline in Nepal: a critical analysis. Hosp Pharm. 2023;58(6):527-529. doi: 10.1177/00185787231172383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Codes on Sales and Distribution of Drugs, Department of Drug Administration. 2024. Accessed October 18, 2024. https://dda.gov.np/content/codes-on-sales-and-distribution-of-drugs [Google Scholar]
- 13. Poudel RS, Piryani RM, Shrestha S, Prajapati A. Benefit of hospital pharmacy intervention on the current status of dry powder inhaler technique in patients with asthma and COPD: a study from the Central Development Region, Nepal. IPRP. 2016;6:7-13. doi: 10.2147/IPRP.S119202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Medication Error Definition, National Coordinating Council for Medication Error Reporting and Prevention. 2024. Accessed October 18, 2024. http://www.nccmerp.org/about-medication-errors
- 15. Key facts about medication errors (MEs) in the WHO European Region. 2022. Accessed October 18, 2024. https://www.who.int/andorra/publications/m/item/key-facts-about-medication-errors-mes-in-the-who-european-region
- 16. ASHP standard definition of a medication error. Am J Hosp Pharm. 1982;39(2):321. doi: 10.1093/ajhp/39.2.321 [DOI] [PubMed] [Google Scholar]
- 17. Healthy Living with Diabetes, National Institute of Diabetes and Digestive and Kidney Diseases. National Institute of Diabetes and Digestive and Kidney Diseases. 2023. Accessed October 18, 2024. https://www.niddk.nih.gov/health-information/diabetes/overview/healthy-living-with-diabetes [Google Scholar]
- 18. Antimicrobial Resistance, World Health Organization. Accessed October 18, 2024. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance [Google Scholar]
- 19. National Drug Policy 1995, Ministry of Health and Population. 1995. Accessed October 18, 2024. https://www.opmcm.gov.np/wp-content/uploads/npolicy/Health/National-Drug-Policy-2005.pdf [Google Scholar]
- 20. Introduction to drug utilization research, World Health Organization. Accessed October 19, 2024. https://www.who.int/publications/i/item/8280820396 [Google Scholar]