Abstract
Background:
Spasticity is an upper motor neuron syndrome that exacerbates motor paralysis and is rarely associated with pain. This report elucidates the management of drug-resistant pain attributed to an adolescent brain tumor using botulinum therapy.
Case presentation:
A 15-year-old female patient experienced dizziness, developed muscle weakness in her upper extremities, and was diagnosed with diffuse glioblastoma of the pons. The tumor responded partially to radiation therapy. Three years later, at the time of recurrence, she had high muscle tone and pain in her extremities. On stimulation, her upper and lower extremities would bend and extend, respectively, causing excruciating pain. Despite experiencing pain-induced insomnia and restlessness, she was reluctant to use drugs, citing concerns about respiratory depression. She received botulinum therapy for her extremities 3 times (200, 300, and 500 U), with pain improvement after repeated treatments (Numerical rating scale from 7.5 to 1 and Short-form McGill Pain Questionnaire-2 score from 78 to 16). The effect lasted for more than three months after the final injection.
Conclusion:
Thus, botulinum therapy can potentially alleviate spasticity-associated pain in advanced stages of brain tumors in adolescents.
Keywords: Botulinum toxin, spasticity, brain tumor, adolescents, pain, numerical rating scale, advance care planning, botulinum neurotoxin type A
Introduction
Spasticity, an upper motor neuron syndrome caused by upper motor nervous system disorders, is defined as a speed-dependent increase in tonic stretch reflexes that worsen motor paralysis and abnormal limb position. Spasticity increases muscle tone, causes pain, and interferes with everyday life. Extensor-dominant spasticity occurs voluntarily when trying to move a paralyzed limb or because of skin irritation or owing to sensory stimuli such as loud noises. Hip and knee extensions trigger extension spasms,1,2 suggestive of proprioceptive receptor involvement.
Diffuse intrinsic pontine glioma (DIPG) develops in the brain stem and tends to progress rapidly, with a poor prognosis and an average survival time of less than 1 year after diagnosis. DIPGs account for approximately 10% to 20% of all pediatric central nervous system tumors, with the average age at diagnosis being approximately 6 to 7 years. DIPGs are classified as high-grade gliomas (WHO grade III or IV) and histologically present as astrocytomas that cannot be surgically removed due to diffuse infiltration into the brain stem.3-5 H3 KM27 gene mutations are known to be involved. 6 Given that surgical removal causes significant brain stem dysfunction, the standard initial treatment for DIPGs comprises fractionated local radiation therapy, which involves the delivery of a total dose of 54 to 60 Gy over approximately 6 weeks. Radiation therapy provides temporary relief of symptoms and usually increases survival by approximately 3 to 4 months. Although this has the effect of temporarily shrinking the tumor and alleviating symptoms, recurrence is inevitable, and chemotherapy, such as treatment with bevacizumab and temozolomide, has not been proven to be effective. At the time of recurrence, patients who responded well to initial radiation therapy can be considered for re-irradiation. However, this benefit is often short-lived. Approximately 75% to 85% of patients experience some improvement in the symptoms following radiation therapy. However, its long-term effectiveness is very low, and DIPGs almost always recur. Recurrence rates are close to 100%, and most patients experience tumor progression within months of completion of the initial treatment6,7 The estimated survival period is approximately 10 months to 1 year, and the cause of death is often respiratory depression.8-10
Symptoms begin with cranial nerve damage and ataxia and progress to motor paralysis. Few reports on spastic pain exist, possibly because the survival period after diagnosis is short and patients are less likely to complain of pain. Adolescent patients with brain tumors have a poor prognosis and limited lifespan; therefore, the choice of treatment method is important.
Spastic pain is often described as a burning or stabbing sensation; however, it can be perceived differently based on the severity and contributing factors of the neurological condition. Spastic pain can affect any muscle group, from the entire body to specific areas, and travels along nerve pathways. People with neurological conditions can also experience spasticity, which causes involuntary muscle contractions that make daily movements and activities challenging, causing discomfort, pain, and sleep disorders. Non-pharmacological therapies include physical therapy, occupational therapy, repositioning, splinting, and electrical stimulation. Pharmacological therapies include botulinum therapy, oral medications (painkillers, anticonvulsants, and muscle relaxants), intrathecal baclofen therapy, and in rare cases, opioids. Surgical interventions include selective dorsal rhizotomy. For best results, treatments should be appropriately selected based on the underlying condition.11,12
Case Presentation
We present a case of a 15-year-old female patient with a brain tumor. She presented with dizziness, diplopia, and upper limb weakness, and brain magnetic resonance imaging (MRI) revealed DIPG. Her symptoms worsened over several weeks, and she underwent radiotherapy (50.4 Gy/28 times, 10 MeV, 4-port irradiation), after which the tumors showed partial response. Two years after radiotherapy, no tumor growth was observed and the patient experienced only ataxia and diplopia, with no motor or pain symptoms. Three years after radiotherapy, she began experiencing new symptoms, such as spasms and pain in her upper and lower limbs, and MRI revealed an expanding brain tumor. The patient was restless and experienced insomnia due to pain. We considered pain as an effective descriptor and administered anti-anxiety drugs and non-steroidal anti-inflammatory drugs but without success. Pain was evaluated using the numerical rating scale (NRS) with a scale ruler; spasticity was evaluated using the modified Ashworth scale (MAS). 13 Average weekly pain score before admission was 5.5 (maximum, 8.0; minimum, 4.0). MAS score was 3, indicating severe spasticity. 14
Neurological examination at admission revealed right abducens nerve palsy, bilateral facial nerve palsy, insufficient elevation of the soft palates on both sides, and dysphagia (choking on fluids). The patient had no sensory deficits but exhibited decreased motor movements in her upper and lower extremities. Tendon reflexes generally increased, Babinski’s and Chaddock’s signs were positive, and the palmomental sign was positive on the right side. Cerebellar ataxia was observed in the upper and lower limbs and the trunk. Abnormal facial sweating was noticed; however, autonomic nerve disorders as associated with bladder and rectal diseases were not evident.
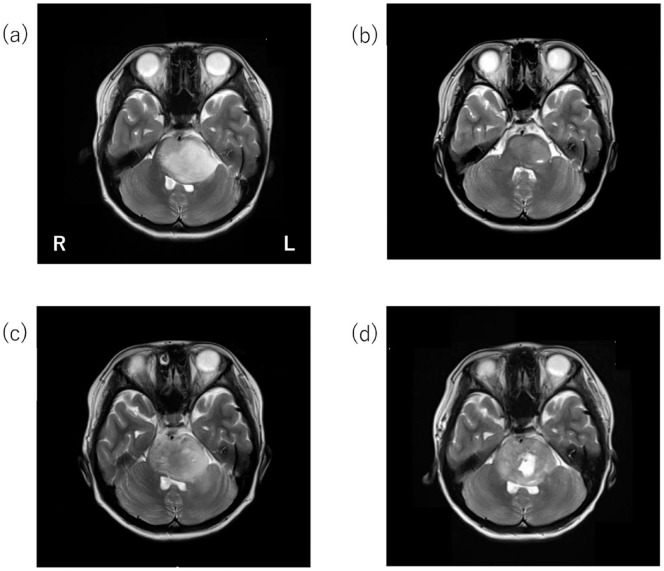
Plain brain MRI revealed a mass in the brainstem with high-signal intensity on T2-weighted images and a necrotic change (Figure 1).
Figure 1.
Plain brain magnetic resonance imaging (MRI) of brainstem tumor (T2-weighted images). Brain MRI at the initial examination revealed a mass in the brainstem with high-signal intensity on T2-weighted images (a). MRI after radiotherapy revealed that the tumor had shrunk (b). MRI in the year X − 1 showed that the necrotic changes below T2-hyperintensity increased. Extension of the left internal capsule toward the hindlimb increased (c). According to the MRI in the year X, the necrotic change at the bottom of the T2-hyperintense area further increased, and the tumor was enlarged (d).
Chemotherapy (bevacizumab 15 mg/kg, every 2 weeks) and radiotherapy (20 Gy/10 times, 10 MeV, Intensity Modulated Radiation Therapy (IMRT)/ Volumetric Modulated Arc Therapy (VMAT) were initiated, and the patient’s motor paralysis improved slightly. However, her upper and lower limbs had high muscle tone; when passive stimulation was applied, her upper limbs would bend and lower limbs would stretch, causing severe pain in her extremities.
The first rehabilitation treatment consisted of range of motion (ROM) training, basic movement training, and standing and walking training. The patient experienced spasticity and pain when changing positions, and had difficulty standing and sitting in a wheelchair. Before each training session, exercises to reduce muscle tension had to be performed, and rehabilitation treatment could only be performed for a short period of time. To reduce spasticity and pain and improve the efficiency of rehabilitation treatment, botulinum therapy was considered.
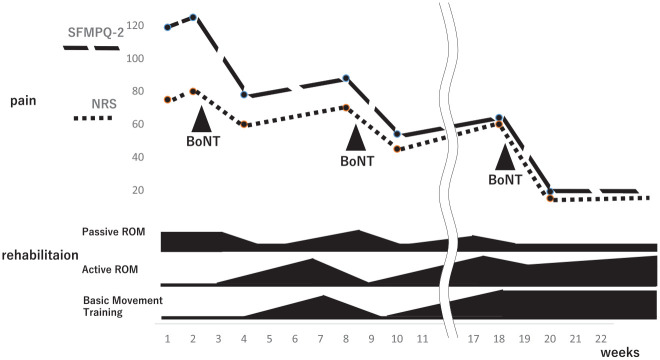
Immediately before botulinum administration, the NRS score was 7.5, the short-form McGill pain questionnaire (SFMPQ)-2 score was 119, and scores for intermittent and neuropathic pain were high (Figure 2). 15 Initial treatment with 200 units of botulinum intramuscularly administered in the upper arm and thigh, where pain was most severe, resulted in improvement in the NRS and SFMPQ-2 scores to 6.0 and 78, respectively, after a period of 2 weeks. However, after 1 month, the pain recurred; therefore, 400 U was additionally administered to the upper arm, forearm flexor, thigh, and lower leg extension groups. One week later, NRS and SFMPQ-2 scores were 4.5 and 54, respectively. Two months later, the pain recurred and worsened (NRS, 6.0; SFMPQ-2, 64); therefore, 500 U was administered, and NRS and SFMPQ-2 scores improved to 1.0 and 19, respectively. Thereafter, the patient was followed up for 3 months, and her condition did not worsen.
Figure 2.
The course of pain and rehabilitation treatment.
Abbreviations: NRS, numerical rating scale; ROM, range-of-motion; SFMPQ-2, short-form McGill pain questionnaire-2.
In rehabilitation treatment, by improving pain and spasticity after administering botulinum neurotoxin (BoNT), we were able to improve passive ROM in a shorter time and spend more time on basic movement training. The efficiency of rehabilitation treatment also improved, contributing to the improvement and maintenance of activities of daily living. She was followed up until 27 weeks after botulinum therapy (when she died of respiratory depression); no complaint of pain was reported during that time.
Discussion
Intramuscular BoNT injections have been used to treat movement disorders such as spasticity and dystonia. Recent clinical studies have reported that subcutaneous BoNT injection has analgesic effects on peripheral neuropathic pain.16,17 Furthermore, a randomized controlled study elucidated that BoNT may reduce chronic neuropathic pain in patients with spinal cord injuries. 18
BoNT also reduced and altered neuropathic pain in several animal models through various mechanisms: (i) inhibiting the secretion of pain mediators (substance P, glutamate, and calcitonin gene-related protein) from nerve terminals and dorsal root ganglia, (ii) reducing local inflammation around nerve terminals and inhibiting sodium channels, (iii) indicating and inactivating axonal inflammation, and (iv) inhibition of neurogenic inflammation and peripheral sensitization through attenuation of neuropeptide release from nociceptive sensory neurons via peripheral synaptosomal-associated protein (SNAP)-25 cleavage. The central effects of BoNT on pain modulation include axonal transport of BoNT from the periphery to the central nerve system (CNS). In a mouse model of sciatic nerve injury, the subcutaneous administration of BoNTs to the plantar surface of the hind paw revealed the presence of cleaved SNAP-25 in tissues along the nociceptive pathway from the peripheral nerve endings to the spinal cord. 19 Additionally, BoNTs hinder the delivery of vanilloid-1, a transient receptor potential, to neuronal cell membranes and may increase nociceptor excitability. Blocking peripheral sensitization reduces the transmission of nociceptive signals to the CNS and neuropeptide release within the spinal cord, thereby reducing central sensitization.20,21 Moreover, subcutaneous BoNT injection may directly inhibit primary sensory fibers, and BoNT is retrogradely transported to the CNS within the axonal compartment, reducing peripheral and central sensitization. 22
BoNT has been shown to be effective in reducing pain and improving the quality of life following the management of spasticity attributed to causes other than stroke, such as brain tumor.23,24 BoNT has also been reported to be used as analgesia to relieve pain attributed to cancerous lesions and muscle spasms. 12 The use of botulinum toxin for pain relief in cancer patients and its potential benefits have been discussed previously. 25 There have also been reports of BoNT being used as a post-radiotherapy pain reliever in cases where pain occurs after radiation therapy. 26
Patients with brainstem tumors are hesitant to receive opioids for pain because of concerns regarding respiratory depression. In this respect, BoNT has few irreversible medical side effects, excluding respiratory depression. BoNT used for neuropathic pain resulted in relatively mild complications, such as antibody formation and immune-related complications, when small amounts of BoNT entered circulation. 27 Notably, neutralizing antibodies against BoNT reduce its effectiveness. An evidence-based review by the American Academy of Neurology described the safety and effectiveness of botulinum neurotoxin in treating spasticity in both adults and children, 28 including those with limited life expectancy. Nevertheless, we believe that BoNT use does not need to be avoided when a short life is prognosticated. 29
Promising new approaches to spasticity have been proposed and implemented in recent years; combining BoNT with other solutions may further improve the quality of life.30-32
Conclusion
Botulinum therapy potentially alleviates spasticity-associated pain in advanced stages of brain tumors in adolescents.
Acknowledgments
We thank the staff of the Department of Rehabilitation at Nara Prefectural General Medical Center for their contribution.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: TMano and HM: conceptualization; TMano, TMurakami and HM: validation; TMasuda: formal analysis; TMurakami: investigation and resources; TMano: data curation and visualization; TMano: writing original draft preparation, project administration, and writing – review and editing; and TMasuda: supervision. All authors have read and agreed to the published version of the manuscript.
Data Availability: The data supporting this study’s findings are available on request from the corresponding author.
Ethics Approval Statement: The study protocol was reviewed and approved by the Ethics Committee of Nara Prefectural General Medical Center (approval number: 903).
Consent to Participate and for Publication: Written informed consent was obtained from the patient and her mother, who is the legally authorized representative of the minor participant, for their anonymized information to be published in this article.
ORCID iD: Tomoo Mano  https://orcid.org/0000-0002-4788-3344
https://orcid.org/0000-0002-4788-3344
References
- 1. Schmit BD, Benz EN. Extensor reflexes in human spinal cord injury: activation by hip proprioceptors. Exp Brain Res. 2002;145:520-527. [DOI] [PubMed] [Google Scholar]
- 2. Wu M, Hornby TG, Hilb J, Schmit BD. Extensor spasms triggered by imposed knee extension in chronic human spinal cord injury. Exp Brain Res. 2005;162:239-249. [DOI] [PubMed] [Google Scholar]
- 3. Bartlett AL, Lane A, Chaney B, et al. Characteristics of children ⩽36 months of age with DIPG: a report from the international DIPG registry. Neurooncol. 2022;24:2190-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janjua MB, Ban VS, El Ahmadieh TY, et al. Diffuse intrinsic pontine gliomas: diagnostic approach and treatment strategies. J Clin Neurosci. 2020;72:15-19. [DOI] [PubMed] [Google Scholar]
- 5. Perrone MG, Ruggiero A, Centonze A, et al. Diffuse intrinsic pontine glioma (DIPG): breakthrough and clinical perspective. Curr Med Chem. 2021;28:3287-3317. [DOI] [PubMed] [Google Scholar]
- 6. Yamasaki F, Nishibuchi I, Karakawa S, et al. T2-FLAIR mismatch sign and response to radiotherapy in diffuse intrinsic pontine glioma. Pediatr Neurosurg. 2021;56:1-9. [DOI] [PubMed] [Google Scholar]
- 7. Gupta N, Goumnerova L, Manley P, et al. Prospective feasibility and safety assessment of surgical biopsy for patients with newly diagnosed diffuse intrinsic pontine glioma. Neurooncol. 2018;20:1547-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JH, Holste KG, Bah MG, et al. Influence of socioeconomic status on clinical outcomes of diffuse midline glioma and diffuse intrinsic pontine glioma. J Neurosurg Pediatr. 2024;33:507-515. [DOI] [PubMed] [Google Scholar]
- 9. Tetens AR, Martin AM, Arnold A, et al. DNA methylation landscapes in DIPG reveal methylome variability that can be modified pharmacologically. Neurooncol Adv. 2024;6:vdae023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Veldhuijzen van Zanten SEM, Baugh J, Chaney B, et al. Development of the SIOPE DIPG network, registry and imaging repository: a collaborative effort to optimize research into a rare and lethal disease. J Neurooncol. 2017;132:255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ward AB, Kadies M. The management of pain in spasticity. Disabil Rehabil. 2002;24:443-453. [DOI] [PubMed] [Google Scholar]
- 12. Mittal SO, Jabbari B. Botulinum neurotoxins and cancer-a review of the literature. Toxins. 2020;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He S, Renne A, Argandykov D, Convissar D, Lee J. Comparison of an emoji-based visual analog scale with a numeric rating scale for pain assessment. JAMA. 2022;328:208-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dimitrova R, Kim H, Meilahn J, et al. Efficacy and safety of onabotulinumtoxinA with standardized physiotherapy for the treatment of pediatric lower limb spasticity: a randomized, placebo-controlled, phase III clinical trial. NeuroRehabilitation. 2022;50:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shigetoh H, Koga M, Tanaka Y, Morioka S. Central sensitivity is associated with poor recovery of pain: prediction, cluster, and decision tree analyses. Pain Res Manag. 2020;2020:8844219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu HT, Tsai SK, Kao MC, Hu JS. Botulinum toxin A relieved neuropathic pain in a case of post-herpetic neuralgia. Pain Med. 2006;7:89-91. [DOI] [PubMed] [Google Scholar]
- 17. Oh HM, Chung ME. Botulinum toxin for neuropathic pain: a review of the literature. Toxins. 2015;7:3127-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han ZA, Song DH, Oh HM, Chung ME. Botulinum toxin type A for neuropathic pain in patients with spinal cord injury. Ann Neurol. 2016;79:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marinelli S, Vacca V, Ricordy R, et al. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS One. 2012;7:e47977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicol. 2005;26:785-793. [DOI] [PubMed] [Google Scholar]
- 21. Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004;107:125-133. [DOI] [PubMed] [Google Scholar]
- 22. Favre-Guilmard C, Auguet M, Chabrier PE. Different antinociceptive effects of botulinum toxin type A in inflammatory and peripheral polyneuropathic rat models. Eur J Pharmacol. 2009;617:48-53. [DOI] [PubMed] [Google Scholar]
- 23. Otero-Luis I, Martinez-Rodrigo A, Cavero-Redondo I, et al. Effect of botulinum toxin injections in the treatment of spasticity of different etiologies: an umbrella review. Pharmaceuticals. 2024;17:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baricich A, Battaglia M, Cuneo D, et al. Clinical efficacy of botulinum toxin type A in patients with traumatic brain injury, spinal cord injury, or multiple sclerosis: an observational longitudinal study. Front Neurol. 2023;14:1133390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lippi L, de Sire A, Turco A, et al. Botulinum toxin for pain relief in cancer patients: a systematic review of randomized controlled trials. Toxins. 2024;16:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Daele DJ, Finnegan EM, Rodnitzky RL, et al. Head and neck muscle spasm after radiotherapy: management with botulinum toxin A injection. Arch Otolaryngol Head Neck Surg. 2002;128:956-959. [DOI] [PubMed] [Google Scholar]
- 27. Sławek J, Madaliński MH, Maciag-Tymecka I, Duzyński W. [Frequency of side effects after botulinum toxin A injections in neurology, rehabilitation and gastroenterology]. Pol Merkur Lekarski. 2005;18:298-302. [PubMed] [Google Scholar]
- 28. Simpson DM, Gracies JM, Graham K, et al. Assessment: botulinum neurotoxin for the treatment of spasticity (an evidence-based review). Neurology. 2009;73:736-737. [PubMed] [Google Scholar]
- 29. Kessler KR, Skutta M, Benecke R. Long-term treatment of cervical dystonia with botulinum toxin A: efficacy, safety, and antibody frequency. J Neurol. 1999;246:265-274. [DOI] [PubMed] [Google Scholar]
- 30. Farì G, Ranieri M, Marvulli R, et al. Is there a new road to spinal cord injury rehabilitation? A case report about the effects of driving a Go-Kart on muscle spasticity. Diseases. 2023;11:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marvulli R, Lagioia G, Ianieri G, et al. Intrathecal baclofen infusion-botulinum toxin combined treatment efficacy in the management of spasticity due to cerebral palsy. CNS Neurol Disord Drug Targets. 2024;23:917-926. [DOI] [PubMed] [Google Scholar]
- 32. Megna M, Marvulli R, Farì G, et al. Pain and muscles properties modifications after botulinum toxin type A (BTX-A) and radial extracorporeal shock wave (rESWT) combined treatment. Endocr Metab Immune Disord Drug Targets. 2019;19:1127-1133. [DOI] [PubMed] [Google Scholar]