Abstract
Background
Glucagon-like peptide-1 receptor agonist (GLP-1RA) treatment reduces cardiovascular events in type 2 diabetes. Yet, the impact of GLP-1RA treatment before ST-segment elevation myocardial infarction (STEMI) on long-term prognosis in patients with type 2 diabetes remains unclear. In patients with STEMI and type 2 diabetes, we aimed to investigate the association between long-term prognosis and GLP-1RA treatment before STEMI.
Methods
This nationwide cohort study included consecutive patients admitted with type 2 diabetes and STEMI in Denmark from 2010 to 2016. All data were retrieved from nationwide Danish registries. Type 2 diabetes was defined by prior hospital admission with type 2 diabetes or anti-diabetic prescriptions within one year before STEMI. Dispensed GLP-1RA medication was retrieved within one year before STEMI.
Results
Of 1421 patients with STEMI and diabetes, 7% were treated with GLP-1RA before STEMI and 93% were not. Patients treated with GLP-1RA were younger, had more comorbidities, and more often treated with other anti-diabetics. During 8.4 years, 36% patients treated with GLP-1RA died whereas 52% died in the no GLP-1RA group (p = 0.002). In adjusted Cox analysis, GLP-1RA was associated with lower long-term mortality (hazard ratio (HR) 0.60, 95% confidence interval (CI) 0.43–0.84). There was no association between GLP-1RA and ischemic stroke (adjusted HR 1.05, 95% CI 0.57–1.94), recurrent myocardial infarction (adjusted HR 0.74, 95% CI 0.48–1.15), or hospitalisation for heart failure (adjusted HR 0.71, 95% CI 0.48–1.05).
Conclusions
In patients with diabetes and STEMI, GLP-1RA treatment prior to STEMI admission was associated with significantly lower long-term mortality.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02548-w.
Keywords: Prognosis, GLP-1RA, STEMI, Therapeutic targets
Background
ST-segment elevation myocardial infarction (STEMI) is one of the most severe cardiovascular events [1]. Type 2 diabetes increases the risk of STEMI by two- to three-fold compared to the general population [2]. The elevated risk is attributed to factors such as endothelial dysfunction, insulin resistance, progressive atherosclerosis, and abnormal platelet function [2]. Consequently, patients with diabetes constitute up to 30% of patients with STEMI, and up to 50% of patients with STEMI have undiagnosed type 2 diabetes or pre-diabetes upon admission [2, 3]. Patients with both diabetes and STEMI have a worse prognosis compared to patients without diabetes [4]. Therefore, optimised therapeutic strategies are pivotal to improve prognosis in patients with STEMI and diabetes.
Glucagon-like peptide-1 (GLP-1) was initially identified as a gut-derived incretin hormone [5]. The actions of GLP-1 are pleotropic, encompassing glucose-dependent stimulation of insulin production, inhibition of glucagon secretion, and gut emptying [6]. Studies in GLP-1 receptor agonists (GLP-1RA) have shown to reduce the risk of major cardiovascular events (MACE) in patients with type 2 diabetes [7–11]. More recently, GLP-1RA have also shown to reduce the risk of MACE in obese individuals without diabetes [12]. GLP-1RA drive glycaemic reduction without increasing hypoglycaemic risk as well as reduce body weight, lower circulating lipoproteins, systolic blood pressure, and systemic and vascular inflammation [5]. These actions are collectively beneficial for the cardiovascular system and may explain the cardioprotective properties of GLP-1RA.
Most studies in GLP-1RA and type 2 diabetes have focused on prevention of MACE and not the potential protection by GLP-1RA during and after a cardiovascular event. In patients with STEMI with and without type 2 diabetes, GLP-1RA has been shown to reduce infarct size and increase myocardial salvage if infused intravenously prior to reperfusion [13, 14]. Whether these findings translate into an improved prognosis remain unknown. Thus, the impact of GLP-1RA treatment prior to STEMI on the clinical course in patients with type 2 diabetes warrants more evidence.
Therefore, we investigated the association between long-term prognosis and prior treatment with GLP1-RA in a consecutive cohort of patients with type 2 diabetes and STEMI treated with percutaneous coronary intervention (PCI).
Methods
Study design and population
This was a nationwide, retrospective cohort study of consecutive patients with type 2 diabetes and STEMI treated with PCI aged above 18 years. All patients were admitted to one of the four Heart Centres in Denmark between 2010 and 2016. Patients included in the present study were stratified according to GLP-1RA treatment prior to STEMI.
Data sources
All patients were identified through the Danish National Patient Registry, holding information on the date of admission, discharge, and diagnoses classified according to the International Classification of Disease, Tenth revision (ICD-10) [15]. All Danish citizens are provided with a unique civil registration number allowing for linkage to other Danish nationwide registries [16]. The distinct and personalised civil registration number is permanent and assigned to all Danish residents, allowing individual-level linkage between all national registries unambiguously [16]. Data on vital status, sex, and date of birth was retrieved from the Civil Registration Registry. All medical prescriptions were retrieved from the Danish National Prescription Registry including type of drug based on the international Anatomical Therapeutic Chemical (ATC) classification system and dispensing date [17]. Information was retrieved for every patient before admission with STEMI and within 30 days after STEMI.
For patients consecutively admitted to Rigshospitalet, Denmark, data was linked via the civil registration number to the Eastern Danish Heart Registry holding detailed information on clinical, angiographic, and procedural data [18]. Rigshospitalet covers 2.8 million citizens of 5.9 Danish citizens [19].
Definitions
STEMI was defined according to ICD-10 codes (I210B, I211B, and I213). The positive predictive value of the ICD-10 codes for STEMI are previously validated with a positive predictive value of 96% [20]. To ensure the diagnosis of STEMI and treatment with PCI, patients were only included in the study if they had a PCI procedure performed on ± 1 day of STEMI admission (procedure code KFNG). The procedure codes for PCI are previously validated with a positive-predictive value of 98% [21].
To ensure a homogeneous cohort, type 2 diabetes was defined as a prior hospital admission with the primary diagnosis of type 2 diabetes (ICD-10: E11) from 1995 to admission with STEMI or dispensed anti-diabetic medication (ATC: A10) one year before STEMI. To ensure active treatment with GLP-1RA, prescriptions dispensed in the last year before STEMI were retrieved [18]. During the study period, GLP-1RA was not prescribed for weight loss. Time from onset of diabetes to STEMI was defined from dispensed anti-diabetic medication or date of diabetes diagnosis, whichever came first.
All ICD-10 and ATC codes for comorbidities and medication at baseline are given in Supplementary Table S1. Medication was retrieved one year before STEMI admission and within 30 days after STEMI. To avoid underestimation of hypertension and hyperlipidaemia, these comorbidities were identified according to both diagnosis and dispensed medication one year before STEMI admission (Supplementary Table S1) [18].
Clinical outcomes
The primary outcome of the study was all-cause mortality which was defined as death until end of the follow-up period (20th of January 2024). The secondary outcomes were ischemic stroke, recurrent myocardial infarction, and hospitalisation for heart failure. Follow-up started the day of STEMI admission and lasted until occurrence of an outcome of interest, date of emigration, or end of study. In accordance with guidelines for annotation of recurrent myocardial infarction in registries, the follow-up for myocardial infarction started 28 days after STEMI [22].
Statistical analyses
Baseline characteristics were presented as median and interquartile range (IQR) for continuous variables, and frequencies and percentage for categorical variables. Wilcoxon-Rank-Sum test was used to calculate differences between continuous variables. Chi-Square test or Fisher’s test were used to evaluate difference between categorical variables, appropriately. In variables with ≤ 3 observations, the variable was annotated ≤ 3 to ensure anonymity of the patients. A two-sided p-value ≤ 0.050 was considered statistically significant.
Event rates for each outcome were calculated and based on the adjusted cox proportional hazard model, confounder-adjusted survival curves standardised to the empirical distribution of the covariates were calculated [23]. For the secondary outcomes, cumulative incidence curves were calculated with death considered as a competing risk and tested for differences between groups using a Fine and Gray model [24].
Hazard ratios (HR) and 95% confidence intervals (CI) were calculated by unadjusted and adjusted Cox proportional hazard analyses to show the association between GLP-1RA treatment and the primary and secondary outcomes. Adjusted analyses were conducted for all-cause mortality, recurrent myocardial infarction, and hospitalisation for heart failure. The models were adjusted for all baseline variables that were significantly different between groups and clinically relevant for prognosis following STEMI [1, 25]: time from onset of diabetes to STEMI, age, sex, hyperlipidaemia, hypertension, complication to diabetes, metformin, insulin, statins, angiotensin converting enzyme/angiotensin-II inhibitor, loop diuretics, acetylsalicylic acid, and combinational diuretics. The assumptions for linearity for numeric values and proportional hazard were tested for all models and found valid. Interaction tests were performed for age, sex, treatment with metformin, and an ICD-10 code for complications to diabetes (See Supplementary Table S1). If an interaction was found, stratified analysis was performed.
Angiographic, procedural, and lesion characteristics may influence the prognosis following STEMI [1]. As a sensitivity analysis, procedural information for each group was conducted in a subgroup of patients. Unadjusted and adjusted hazard ratios were calculated for the primary outcome.
All statistical analyses were performed in SAS (version 9.4, SAS Institute, Cary NC, USA) and R Studio, version 4.3.2 (RStudio Team [2020]. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA; URL: http://www.rstudio.com/).
Results
Of 12,504 patients with STEMI treated with PCI, 1,421 patients with type 2 diabetes were included in the present study (Fig. 1). Of these patients, 105 (7%) were treated with GLP-1RA prior to STEMI and 1,316 (93%) were not. All baseline characteristics are presented in Table 1. Patients in the GLP-1RA group were younger, had more hyperlipidaemia and hypertension compared with the no GLP-1RA group. Patients treated with GLP-1RA were more often treated with metformin, insulin, and diuretics, and had had diabetes for a longer time before STEMI compared to patients with no GLP-1RA treatment. Medication within 30 days after STEMI is presented in Supplementary Table S2.
Fig. 1.
Flowchart of the Study Population. Legend: The figure presents a flowchart of the study population, GLP-1RA: glucagon-like peptide-1 receptor agonist; STEMI: ST-segment elevation myocardial infarction
Table 1.
Baseline characteristics of the Study Population
| Variables | GLP-1RA treatment (n = 105) |
No GLP-1RA treatment (n = 1316) |
P-value | |
|---|---|---|---|---|
| Age (years), median [IQR] | 63 [55; 68] | 67 [58; 75] | < 0.001 | |
| Male sex, n (%) | 68 (65) | 963 (73) | 0.06 | |
| Time from onset of diabetes to STEMI (years), median [IQR] | 2.0 [1.0; 8.2] | 1.0 [0.8; 4.3] | < 0.001 | |
| Comorbidities, n (%) | ||||
| Hyperlipidaemia | 79 (75) | 810 (62) | 0.005 | |
| Hypertension | 88 (84) | 844 (64) | < 0.001 | |
| Cardiac arrhythmia | 9 (9) | 125 (10) | 0.75 | |
| Peripheral vascular disease | 6 (6) | 116 (9) | 0.28 | |
| Congestive heart failure | 6 (6) | 110 (8) | 0.34 | |
| Ischemic stroke | 9 (9) | 136 (10) | 0.57 | |
| Previous myocardial infarction | 26 (25) | 279 (21) | 0.39 | |
| Chronic obstructive pulmonary disease | 6 (6) | 115 (9) | 0.29 | |
| Chronic ischemic heart disease | 37 (35) | 348 (26) | 0.05 | |
| Previous or active cancer | 12 (11) | 167 (13) | 0.71 | |
| Complication to diabetes | 39 (37) | 362 (28) | 0.035 | |
| Chronic renal failure | <=3 ( < = 3) | 79 (6) | 0.18 | |
| Pharmacotherapy prior to hospitalisation, n (%) | ||||
| Metformin | 86 (82) | 766 (58) | < 0.001 | |
| Insulin | 51 (49) | 399 (30) | < 0.001 | |
| SGLT-2 inhibitors | 9 (9) | 66 (5) | 0.12 | |
| Statins | 71 (68) | 748 (57) | 0.032 | |
| Angiotensin converting enzyme/angiotensin-II inhibitor | 86 (82) | 758 (58) | < 0.001 | |
| Beta-blockers | 39 (37) | 436 (33) | 0.40 | |
| Thiazides | 15 (14) | 235 (18) | 0.36 | |
| Acetylsalicylic acid | 60 (57) | 531 (40) | < 0.001 | |
| Calcium channel blockers | 35 (33) | 442 (34) | 0.96 | |
| Loop diuretics | 26 (25) | 224 (17) | 0.045 | |
| Spironolactone | 6 (6) | 63 (5) | 0.67 | |
| Combinational diuretics | 32 (30) | 250 (19) | 0.005 | |
GLP-1RA: Glucagon-like peptid-1 receptor agonist; IQR: interquartile range; SGLT-2: Sodium-glucose transporter 2; STEMI: ST-segment elevation myocardial infarction
Outcomes
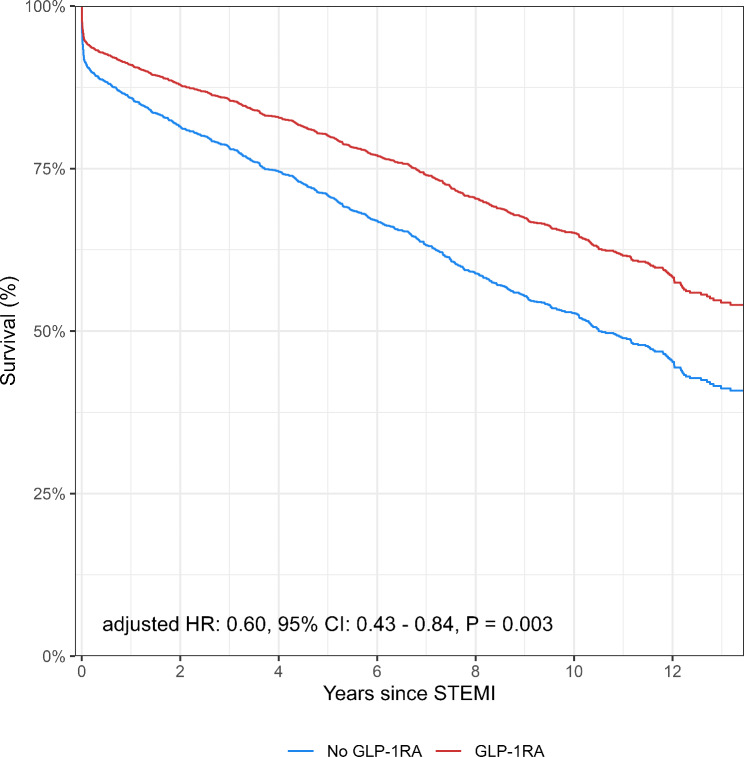
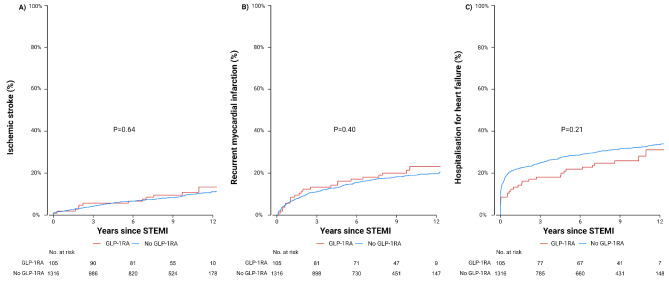
During a median follow-up of 8.4 years, death occurred in 680 (52%) patients in the no GLP-1RA group, and in 36 (38%) patients in the GLP-1RA group (p = 0.002) (Table 2). The GLP-1RA group had significantly longer follow-up than the no GLP-1RA group (GLP-1RA: 10 years (IQR, 8–10), No GLP-1RA: 9 years (IQR, 4–11) p = 0.008). The long-term survival probability was significantly lower in patients who were not treated with GLP-1RA compared with patients who were (p = 0.003) (Fig. 2). There was no significant difference between groups in ischemic stroke (GLP-1RA: 12 (11) vs. no GLP-1RA: 134 (10), p = 0.69), heart failure (GLP-1RA: 29 (28) vs. no GLP-1RA: 433 (33), p = 0.27), or recurrent myocardial infarction (GLP-1RA: 24 (23) vs. no GLP-1RA 258 (20), p = 0.42) (Fig. 3). In unadjusted Cox analysis, GLP-1RA treatment was associated with significantly lower long-term all-cause mortality (HR 0.61, 95% CI 0.44–0.85, p = 0.003). There was no association between secondary outcomes and GLP-1RA treatment (Table 2). After adjusting for potential confounders, long-term all-cause mortality remained significant (HR 0.60, 95% CI 0.43–0.84, p = 0.003).
Table 2.
Primary and secondary outcomes
| Outcomes | No. of events, n (%) |
Unadjusted HR | Adjusted HR* | ||||
|---|---|---|---|---|---|---|---|
| GLP-1RA (n = 105) | No GLP-1RA (n = 1316) |
P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| All-cause mortality | 38 (36) | 680 (52) | 0.002 | 0.61 (0.44; 0.85) | 0.003 | 0.60 (0.43; 0.84) | 0.003 |
| Ischemic stroke | 12 (11) | 134 (10) | 0.69 | 0.99 (0.55; 1.79) | 0.97 | 1.05 (0.57; 1.94) | 0.88 |
| Recurrent MI | 24 (23) | 258 (20) | 0.42 | 1.05 (0.69; 1.59) | 0.83 | 0.74 (0.48; 1.15) | 0.18 |
| Heart Failure | 29 (28) | 433 (33) | 0.27 | 0.74 (0.51; 1.08) | 0.15 | 0.71 (0.48; 1.05) | 0.09 |
*Adjusted for age, sex, hyperlipidaemia, hypertension, complication to diabetes, metformin, insulin, statins, angiotensin converting enzyme/angiotensin-II inhibitor, loop diuretics, acetylsalicylic acid, combinational diuretics, and time from diabetes to ST-segment elevation myocardial infarction
CI: Confidence interval; GLP-1RA: Glucagon-like peptide-1 receptor agonist; HR: hazard ratio
Fig. 2.
Long-Term All-Cause Mortality Stratified By Prior Treatment With Glucagon-Like Peptid-1 Receptor Agonist Legend: The figure presents adjusted survival curves during the follow-up of all patients included in the study stratified by glucagon-like peptid-1 receptor agonist (GLP-1RA) treatment. Based on the adjusted cox proportional hazard model, the figure shows the confounder-adjusted survival probability standardised to the empirical distribution of the covariates. The adjusted hazard ratio including confidence interval are given at the bottom of the figure. The red line is the patients treated with GLP-1RA. The blue line presents patients with no GLP-1RA treatment. The x-axis is years since ST-segment elevation myocardial infarction (STEMI) and the y-axis is survival given in percentage. CI: confidence interval; GLP-1RA: glucagon-like peptide-1 receptor agonist; HR: hazard ratio; STEMI: ST-segment elevation myocardial infarction
Fig. 3.
Cumulative Incidence of Secondary Outcomes Legend: Throughout the figure, the red line is the patients treated with GLP-1RA and the blue line presents patients with no GLP-1RA treatment. A presents cumulative incidence of ischemic stroke. B presents cumulative incidence of recurrent myocardial infarction. C presents cumulative incidence of hospitalisation for heart failure. The x-axes are years since ST-segment elevation myocardial infarction (STEMI) and the y-axes is the incidence of the outcome given in percentage. The p-value is calculated using Gray’s test. GLP-1RA: glucagon-like peptide-1 receptor agonist; STEMI: ST-segment elevation myocardial infarction
Sensitivity analysis
A total of 673/1,316 patients with STEMI (51%) had information on procedural characteristics, of whom 62 were treated with GLP-1RA and 611 were not. There was no difference between groups on procedural data (Supplementary Table S3). GLP-1RA was associated with significantly lower all-cause mortality (adjusted HR, 0.57, 95% 0.35–0.92, p = 0.023) (Supplementary Table S4).
Interaction analysis
In interaction analyses, all but one p-value interaction analyses were above 0.10 (Supplementary Table S5). There was an interaction between sex and GLP-1RA regarding all-cause mortality (p = 0.041). Baseline characteristics stratified by sex is presented in Supplementary Table S6. In women, GLP-1RA was associated with significantly lower risk of all-cause mortality (unadjusted HR 0.37, 95% CI 0.20–0.67, p = 0.001) The association remained significant after adjusting for potential clinical confounders (adjusted HR 0.40, 95% CI 0.21–0.73, p = 0.003) (Supplementary Table S7). There was no association between the primary outcome and GLP-1RA treatment in men (adjusted HR 0.79, 95% CI 0.53–1.20, p = 0.28).
Discussion
Over a median follow-up of 8.4 years in a consecutive cohort of patients with type 2 diabetes and STEMI treated with PCI, treatment with GLP-1RA prior to STEMI admission was associated with a 40% lower long-term all-cause mortality compared to patients not treated with GLP-1RA. The findings were consistent after including procedural characteristics. There was an interaction between sex and GLP-1RA regarding all-cause mortality. In women, GLP-1RA was associated with a 63% lower long-term all-cause mortality compared to women without GLP-1RA treatment. This association was not found in men. A vast number of randomised trials have investigated the impact of GLP-1RA in patients with diabetes type 2 and MACE, yet the present findings further expand the knowledge from clinical trials on the impact GLP-1RA in patients with type 2 diabetes after a severe cardiovascular event.
In an all-comer STEMI cohort, the long-term mortality is approximately 28% within a median follow-up of 7.4 years, whereas a subset of patients with diabetes have a significantly worse prognosis after STEMI [4, 26]. Endogenous GLP-1 serves to regulate key components that contribute to cardiovascular disease by positively influencing ischemia tolerance, plaque stability, vascular inflammation, arterial stiffness, and platelet aggregation [5]. Thus, GLP-1 levels are found to be predictive for future cardiovascular events after STEMI [27]. Hence, GLP-1RA treatment prior to STEMI may serve as a subsequent prevention for future fatal events in patients with diabetes surviving a STEMI by potentially improving the otherwise impaired protective GLP-1 system in the acute setting. In this study in patients with diabetes, the long-term mortality in the GLP-1RA group compared to the no GLP-1RA group is close to the mortality in an all-comer STEMI cohort. Thus, impairment of the endogenous GLP-1 system during STEMI in patients with type 2 diabetes may be improved by medical replacement [4, 27]. Having said this, the GLP-1RA group were younger which could impact the findings of an improved survival [25]. Nevertheless, GLP-1RA continued to be associated with lower risk of all-cause mortality after adjusting for potential confounders, and no interaction was found between GLP-1RA and age on all-cause mortality. Moreover, patients treated with GLP-1RA had a higher prevalence of hyperlipidaemia and hypertension and were more often treated with multiple anti-diabetics. These factors are well-established risk predictors of a poor prognosis [25, 28]. Hence, patients treated with GLP-1RA often have more dysregulated diabetes, unable to reach target glycated haemoglobin (HbA1c) on metformin monotherapy alone [29]. GLP-1RA is therefore often prescribed as an adjunct therapy in later stages of type 2 diabetes, and these patients may therefore inherently represent a higher risk diabetic population than patients treated without GLP-1RA [29]. Of note, the association between GLP-1RA and long-term mortality seemed more pronounced in women who are known to have a worse prognosis than men after STEMI [30]. Extending the current evidence from clinical trials, this study suggests that GLP-1RA also impacts prognosis after a severe cardiovascular event in patients with type 2 diabetes.
In line with previous studies in patients with diabetes treated with GLP-1RA [31, 32], the effect of GLP-1RA seemed more pronounced in women compared to men. However, the findings should be interpreted with caution as they are only hypothesis-generating. Thus, a clear underlying reason for the findings of a potential sex difference in response to GLP-1RA is unknown. Previous studies have highlighted that it may be attributed to either modifications by sex hormones and differences in platelet aggregation between men and women [33–35]. Still, no data on the exact mechanism exists and the findings of the present study may be random.
Early trials on intensive glycaemic control in patients with type 2 diabetes indicated increased risk of both all-cause and cardiovascular mortality [36, 37]. Therefore, concerns were raised on safety of newer anti-diabetic drugs. Yet, subsequent trials in patients with type 2 diabetes showed a significant reduction in MACE if treated with GLP-1RA [8–11, 38]. In line with clinical trials, a previous observational study in patients with diabetes and myocardial infarction found an association between GLP-1RA and a lower risk of overall MACE [39]. However, GLP-1RA was not associated with a lower risk of the individual components of MACE [39]. Interestingly, this study did not find an association between GLP-1RA and lower risk of ischemic stroke, recurrent myocardial infarction, or hospitalisation for heart failure despite a lower long-term all-cause mortality. The mechanism behind the lower mortality in the GLP-1RA group is therefore not clear. A key component in this study may be the long-term follow-up, as cardiovascular mortality after STEMI significantly decreases beyond 30 days [40]. Whether the association between GLP-1RA and lower long-term all-cause mortality was driven by cardiovascular mortality is unclear. However, as the potential mortality benefit of GLP-1RA may persist on long-term after STEMI, it is possible that the protective effects of GLP-1RA prior to STEMI in patients with type 2 diabetes is driven more by long-term factors rather than short-term cardiovascular effects.
Few observational studies have investigated the prognostic impact of GLP-1RA in patients with type 2 diabetes and myocardial infarction [39, 41]. However, the heterogeneity in study populations encompassing all subtypes of acute myocardial infarction, varying definitions of diabetes, differences in exposure to GLP-1RA, and different follow-up durations challenge the comparative value of the studies. Nevertheless, previous and the present study consistently find a positive prognostic impact of GLP-1 treatment prior to myocardial infarction in patients with type 2 diabetes [39, 41]. The present study is the first to show data on the long-term prognostic impact of GLP-1RA treatment in patients with type 2 diabetes and an a priori increased risk defined by the presence of STEMI, while previous studies have evaluated the impact of GLP-1RA on a median follow-up of 2.98 years or in-hospital. Overall, previous and present studies indicate that GLP-1RA prior to admission with STEMI has a significant prognostic impact during admission, as well as on short- and long-term. Due to the observational nature of this study, the impact of GLP-1RA in patients with type 2 diabetes suffering an adverse cardiovascular event warrants future prospective evidence. However, the findings are intriguing and may be a key component to reducing the prognostic gap between patients with and without diabetes admitted with STEMI. Thus, treatment with GLP-1RA in patients with type 2 diabetes may have the potential to reduce mortality after STEMI. However, clinical studies are needed to proof causality.
Strengths and limitations
The present nationwide study links data with Danish nationwide registries which enables completeness of the data. The registries have been extensively validated resulting in a reliable data source with minimal demographic sampling bias [15, 20, 21]. Moreover, the diagnosis and procedural codes have previously been validated with a high positive predictive value of STEMI and PCI. Finally, the completeness of the data allowed for evaluation of long-term follow-up with a median of 8.4 years.
This study is an observational cohort study, and the findings does therefore not prove causality but are considered hypothesis-generating and limitations exist. The definition of type 2 diabetes was based on prior hospital admission with the ICD-10 code for type 2 diabetes or dispensed anti-diabetic medication prior to STEMI. Thus, the study may have excluded patients with undiagnosed diabetes or misclassified patients who were treated with anti-diabetics due to lifestyle and diet modification. Moreover, there was no information on blood tests that could have an influence on prognosis such as estimated glomerular filtration rate, glycated haemoglobin, and left ventricular ejection fraction. Therefore, we cannot exclude the risk of residual confounding of variables not included in the adjusted models. In addition, patients treated with GLP-1RA were more often treated with more anti-diabetics than patients without GLP-1RA indicating a closer diabetic control in the GLP-1RA group. We can therefore not exclude that this may have confounded the results. Treatment with insulin and metformin was, however, included in the adjusted cox model. Lastly, the cause of death would have been valuable to include. However, there may be a lack of specificity in registries regarding causes of death, as they are obtained from death certificates completed by medical doctors and not based on autopsies. Of note, cardiovascular mortality after STEMI is drastically declining 30 days after admission [40], therefore all-cause mortality is encouraged when evaluating long-term outcomes. Nevertheless, the lack of information of cause-specific mortality restricts the ability to determine whether the observed survival benefit of GLP-1 is cardiovascular.
Conclusion
In patients with type 2 diabetes and STEMI treated with PCI, GLP-1RA treatment before STEMI admission was associated with a significantly lower long-term all-cause mortality. This effect of GLP-1RA seemed to be more pronounced in women compared to men.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- ATC
Anatomical Therapeutic Chemical
- CI
Confidence interval
- GLP-1RA
Glucagon-like peptid-1 receptor agonist
- HR
Hazard ratio
- ICD-10
The International Classification of Disease, Tenth revision
- IQR
Interquartile range
- MACE
Major cardiovascular events
- PCI
Percutaneous coronary intervention
- STEMI
ST-segment elevation myocardial infarction
Author contributions
JMM and TE were involved with the conception and design of the work. All authors were involved with the acquisition, analysis, and interpretation of data for the work. JMM wrote the first draft of the manuscript. All authors edited, critically revised the manuscript, and ap-proved the final version of the manuscript. JMM is the guarantor of this work and, as such, had full access to all the data and takes responsibility for the integrity of the data and accuracy of the data analysis.
Funding
Open access funding provided by Copenhagen University
This work was supported by the Alfred Benzon Foundation and the Novo Nordisk Foundation. The funders were not involved in any aspects of the study or the decision to submit for publication. No extramural funding was used to support this work.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The salary of JM. Madsen was supported by a grant from the Research Foundation of Rigshospitalet (E-22670–08). T. Engstrøm has received speaker fees from Abbott Vascular, Boston Scientific, and Bayer, an advisory board fee from Abbott, Novo Nordisk and partici-pated in Data Safety Monitoring of the INFINITY Trial, unrelated to this topic. J. Lønborg has received an advisory board fee, an unrestricted grant, and speakers fee from Boston Sci-entific and speakers fee from Abbott, unrelated to this topic. All other authors have no con-flicts of interest to disclose. L. Køber has received speaker’s honorarium from Novo Nordisk, Astra-Zeneca, Boehringer and Novartis. All other authors have nothing to declare.
Ethics approval and consent for publication
All civil registration numbers were encrypted which enabled anonymity in all included patients. All register-based studies in an anonymous setup do not require ethical approval in Denmark. The authors are solely responsible for the design and conduct of this study, all analyses, drafting and editing of the paper and its final contents.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720–826. [DOI] [PubMed] [Google Scholar]
- 2.Babes EE, Bustea C, Behl T, Abdel-Daim MM, Nechifor AC, Stoicescu M, et al. Acute coronary syndromes in diabetic patients, outcome, revascularization, and antithrombotic therapy. Biomed Pharmacother. 2022;148:112772. [DOI] [PubMed] [Google Scholar]
- 3.Lankisch M, Füth R, Gülker H, Lapp H, Bufe A, Haastert B, et al. Screening for undiagnosed diabetes in patients with acute myocardial infarction. Clin Res Cardiol off J Ger Card Soc. 2008;97:753–9. [DOI] [PubMed] [Google Scholar]
- 4.Skoda R, Nemes A, Bárczi G, Vágó H, Ruzsa Z, Édes IF, et al. Survival of myocardial infarction patients with diabetes Mellitus at the invasive era (results from the Városmajor Myocardial Infarction Registry). J Clin Med. 2023;12:917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with Glucagon-Like Peptide-1 receptor agonists and dipeptidyl Peptidase-4 inhibitors. Circulation. 2017;136:849–70. [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet Lond Engl. 2006;368:1696–705. [DOI] [PubMed] [Google Scholar]
- 7.Sattar N, Lee MMY, Kristensen SL, Branch KRH, Prato SD, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653–62. [DOI] [PubMed] [Google Scholar]
- 8.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and Cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- 9.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and Cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–29. [DOI] [PubMed] [Google Scholar]
- 11.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet Lond Engl. 2019;394:121–30. [DOI] [PubMed] [Google Scholar]
- 12.Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and Cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389:2221–32. [DOI] [PubMed] [Google Scholar]
- 13.Lønborg J, Kelbæk H, Vejlstrup N, Bøtker HE, Kim WY, Holmvang L, et al. Exenatide reduces final infarct size in patients with ST-segment-elevation myocardial infarction and short-duration of ischemia. Circ Cardiovasc Interv. 2012;5:288–95. [DOI] [PubMed] [Google Scholar]
- 14.Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–9. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Özcan C, Juel K, Flensted Lassen J, von Kappelgaard LM, Mortensen PE, Gislason G. The Danish Heart Registry. Clin Epidemiol. 2016;8:503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saj AP, H W-K S, Ht S. J H, M S. Data Resource Profile: The Danish National Prescription Registry. Int J Epidemiol [Internet]. 2017 [cited 2024 Jul 23];46. https://pubmed.ncbi.nlm.nih.gov/27789670/ [DOI] [PMC free article] [PubMed]
- 18.Madsen JM, Jacobsen MR, Sabbah M, Topal DG, Jabbari R, Glinge C, et al. Long-term prognostic outcomes and implication of oral anticoagulants in patients with new-onset atrial fibrillation following St-segment elevation myocardial infarction. Am Heart J. 2021;238:89–99. [DOI] [PubMed] [Google Scholar]
- 19.Statistics Denmark - Annual Report. (2023) [Internet]. https://www.statistikbanken.dk/20021
- 20.Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adelborg K, Sundbøll J, Munch T, Frøslev T, Sørensen HT, Bøtker HE, et al. Positive predictive value of cardiac examination, procedure and surgery codes in the Danish National Patient Registry: a population-based validation study. BMJ Open. 2016;6:e012817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231–64. [DOI] [PubMed] [Google Scholar]
- 23.Lundgreen C, Larson DR, Atkinson EJ, Devick KL, Lewallen DG, Berry DJ, et al. Adjusted survival curves improve understanding of Multivariable Cox Model results. J Arthroplasty. 2021;36:3367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 25.Vermeulen MJ, Tu JV, Schull MJ. ICD-10 adaptations of the Ontario acute myocardial infarction mortality prediction rules performed as well as the original versions. J Clin Epidemiol. 2007;60:971–4. [DOI] [PubMed] [Google Scholar]
- 26.Thrane PG, Olesen KKW, Thim T, Gyldenkerne C, Hansen MK, St ødkilde-JN, et al. 10-Year Mortality after ST-Segment Elevation myocardial infarction compared to the General Population. J Am Coll Cardiol. 2024;83:2615–25. [DOI] [PubMed]
- 27.Kahles F, Rückbeil MV, Mertens RW, Foldenauer AC, Arrivas MC, Moellmann J, et al. Glucagon-like peptide 1 levels predict cardiovascular risk in patients with acute myocardial infarction. Eur Heart J. 2020;41:882–9. [DOI] [PubMed] [Google Scholar]
- 28.Radovanovic D, Maurer L, Bertel O, Witassek F, Urban P, Stauffer J-C, et al. Treatment and outcomes of patients with recurrent myocardial infarction: a prospective observational cohort study. J Cardiol. 2016;68:498–503. [DOI] [PubMed] [Google Scholar]
- 29.Meece J. The role of the pharmacist in managing type 2 diabetes with Glucagon-Like Peptide-1 receptor agonists as Add-On therapy. Adv Ther. 2017;34:638–57. [DOI] [PubMed] [Google Scholar]
- 30.Kosmidou I, Redfors B, Selker HP, Thiele H, Patel MR, Udelson JE, et al. Infarct size, left ventricular function, and prognosis in women compared to men after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: results from an individual patient-level pooled analysis of 10 randomized trials. Eur Heart J. 2017;38:1656–63. [DOI] [PubMed] [Google Scholar]
- 31.Raparelli V, Elharram M, Moura CS, Abrahamowicz M, Bernatsky S, Behlouli H, et al. Sex differences in Cardiovascular effectiveness of newer glucose-lowering drugs added to Metformin in type 2 diabetes Mellitus. J Am Heart Assoc. 2020;9:e012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rentzeperi E, Pegiou S, Koufakis T, Grammatiki M, Kotsa K. Sex differences in response to treatment with glucagon-like peptide 1 receptor agonists: opportunities for a tailored Approach to diabetes and Obesity Care. J Pers Med. 2022;12:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang TY, Angiolillo DJ, Cushman M, Sabatine MS, Bray PF, Smyth SS, et al. Platelet biology and response to antiplatelet therapy in women: implications for the development and use of antiplatelet pharmacotherapies for cardiovascular disease. J Am Coll Cardiol. 2012;59:891–900. [DOI] [PubMed] [Google Scholar]
- 34.Richard JE, Anderberg RH, López-Ferreras L, Olandersson K, Skibicka KP. Sex and estrogens alter the action of glucagon-like peptide-1 on reward. Biol Sex Differ. 2016;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cameron-Vendrig A, Reheman A, Siraj MA, Xu XR, Wang Y, Lei X, et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes. 2016;65:1714–23. [DOI] [PubMed] [Google Scholar]
- 36.The ACCORD Study Group. Nine-Year effects of 3.7 years of Intensive Glycemic Control on Cardiovascular outcomes. Diabetes Care. 2016;39:701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral Semaglutide and Cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–51. [DOI] [PubMed] [Google Scholar]
- 39.Trevisan M, Fu EL, Szummer K, Norhammar A, Lundman P, Wanner C, et al. Glucagon-like peptide-1 receptor agonists and the risk of cardiovascular events in diabetes patients surviving an acute myocardial infarction. Eur Heart J Cardiovasc Pharmacother. 2021;7:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedersen F, Butrymovich V, Kelbæk H, Wachtell K, Helqvist S, Kastrup J, et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol. 2014;64:2101–8. [DOI] [PubMed] [Google Scholar]
- 41.Trombara F, Cosentino N, Bonomi A, Ludergnani M, Poggio P, Gionti L, et al. Impact of chronic GLP-1 RA and SGLT-2I therapy on in-hospital outcome of diabetic patients with acute myocardial infarction. Cardiovasc Diabetol. 2023;22:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.