Abstract
Introduction
The rarest form of renal ectopia, the thoracic kidney, has been documented in only about 200 cases worldwide. There are four recognized causes of congenital thoracic renal ectopia: renal ectopia with an intact diaphragm, diaphragmatic eventration, diaphragmatic hernia, and traumatic diaphragmatic rupture. This condition often presents as an incidental finding in asymptomatic patients. The following is a report of mediastinal renal ectopia in a 52-year-old male patient.
Case description
The patient is a 52-year-old male who presented on admission with gastrointestinal bleeding, reporting melena and fatigue. On admission, laboratory workup revealed a hemoglobin level of 9.2 g/dL (normal: 13.5–17.5 g/dL) and a hematocrit of 28% (normal: 41–50%), indicating mild anemia. A stool guaiac test was positive for occult blood. Initial resuscitation with intravenous fluids stabilized the patient, and he did not require blood transfusion. Upper endoscopy (EGD) and colonoscopy were performed but did not identify a clear source of bleeding. Given the resolution of symptoms and stable laboratory values during hospitalization, the bleeding resolved spontaneously. However, a CT scan of the abdomen and pelvis incidentally showed a 4 × 4 cm soft tissue attenuation in the right paraspinous fat in the lower thoracic spine adjacent to the right hemidiaphragm. A follow-up MRI measured the mass to be 3 cm × 7.5 cm × 7.6 cm and showed the mass to resemble a kidney. The MRI also demonstrated two anatomically normal kidneys, as well. A previous CT angiogram of the chest and lower back 2 years prior showed a similar finding that measured 37 mm × 60 mm × 70 mm and is presumed to be the same mass. According to the radiology report, the findings are consistent with an ectopic thoracic kidney that is unchanged in size. Both Urology and Cardiothoracic Surgery were consulted. Due to the pathology being asymptomatic, both services have agreed to forego biopsy and to monitor the patient in the outpatient setting.
Discussion
A congenital ectopic thoracic kidney is the rarest form of kidney ectopia. IV urography used to be the diagnostic modality of choice; however, it has recently been replaced by less invasive imaging methods, such as ultrasonography or computed tomography. In normal urogenital development, the embryonic folds begin to form the urinary tract and urogenital ridge in week four. The urogenital ridge subsequently divides into the nephrogenic ridge and gonadal cord. The nephrogenic ridge then begins to form three kidneys: pronephros, mesonephros, and metanephros. Out of these three structures, only the metanephros progresses to fully developed human kidneys. The embryologic kidneys originally lie close together in the sacral region. But as the abdomen expands during weeks six through nine, the kidneys ascend and are drawn apart to their final location in the lumbar region. In some cases, the kidney may herniate into the hemithorax via a diaphragmatic defect known as a Bochdalek hernia. However, in our patient, the kidney metanephric cells migrated past the diaphragm prior to diaphragmatic closure, which resulted in a functional kidney located in the thorax without any associated diaphragmatic defect. Due to the patient’s asymptomatic nature, Cardiothoracic Surgery and Urology collectively decided not to proceed with surgical exploration and possible nephrectomy of the ectopic kidney due to the overwhelming risks, and have elected to observe and monitor the patient’s clinical course.
Conclusion
In conclusion, we have presented a rare case of a congenital renal ectopia that was incidentally discovered in 52-year-old male who presented with a gastrointestinal bleed, which was later confirmed with a CT scan and MRI. Given how rarity of this pathology, a multidisciplinary approach, involving medicine, urology, and cardiovascular surgery was needed. Given the pathologies asymptomatic nature, the decision to continue close observation and follow-up was chosen, especially considering the risk of invasive surgical intervention. Further research and documentation is essential not only to provide optimal care in this patient, but to better understand the pathophysiology and management of renal ectopia for future patients.
Introduction
The congenital intrathoracic kidney (CITK) is a rare anomaly in the already unique research realm of renal ectopia. Characterized by its atypical location within the thoracic cavity, CITK challenges conventional medical understanding and management approaches. This condition often emerges unexpectedly, discovered incidentally during evaluations for unrelated health concerns such as gastrointestinal issues. The rarity of CITK, coupled with its typically asymptomatic nature, underscores the importance of informed diagnostic and management strategies, particularly when surgical intervention may or may not be the most prudent course of action for asymptomatic individuals [8, 11].
Case description
The patient is a 52-year-old male with an incidentally discovered thoracic kidney during a gastrointestinal bleed evaluation, reporting melena and fatigue. On admission, laboratory workup revealed a hemoglobin level of 9.2 g/dL (normal: 13.5–17.5 g/dL) and a hematocrit of 28% (normal: 41–50%), indicating mild anemia. A stool guaiac test was positive for occult blood. Initial resuscitation with intravenous fluids stabilized the patient, and he did not require blood transfusion. Upper endoscopy (EGD) and colonoscopy were performed but did not identify a clear source of bleeding. Given the resolution of symptoms and stable laboratory values during hospitalization, the bleeding resolved spontaneously. A CT scan of the abdomen and pelvis incidentally showed a 4 × 4 cm soft tissue attenuation in the right paraspinous fat in the lower thoracic spine adjacent to the right hemidiaphragm (see Fig. 1). A follow-up MRI measured the mass to be 3 cm × 7.5 cm × 7.6 cm and showed the mass to resemble a kidney (Fig. 2). The MRI also demonstrated two anatomically normal kidneys, as well (Fig. 3).
Fig. 1.
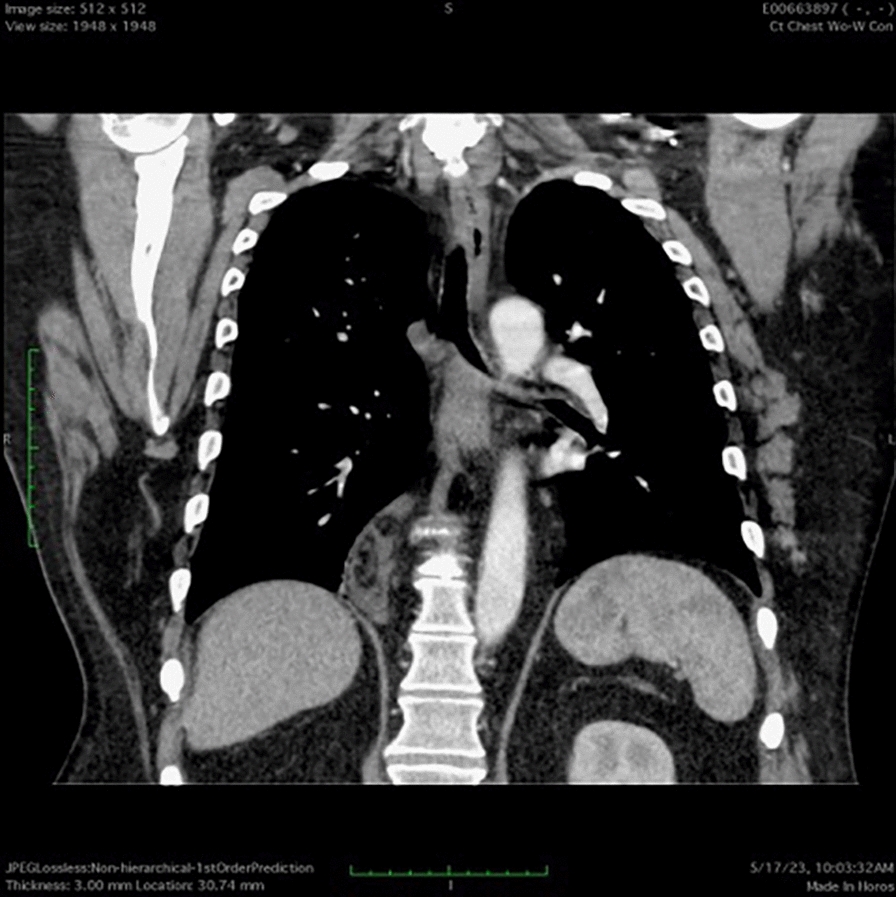
CT scan of the abdomen and pelvis demonstrating an ectopic kidney in the thoracic cavity
Fig. 2.
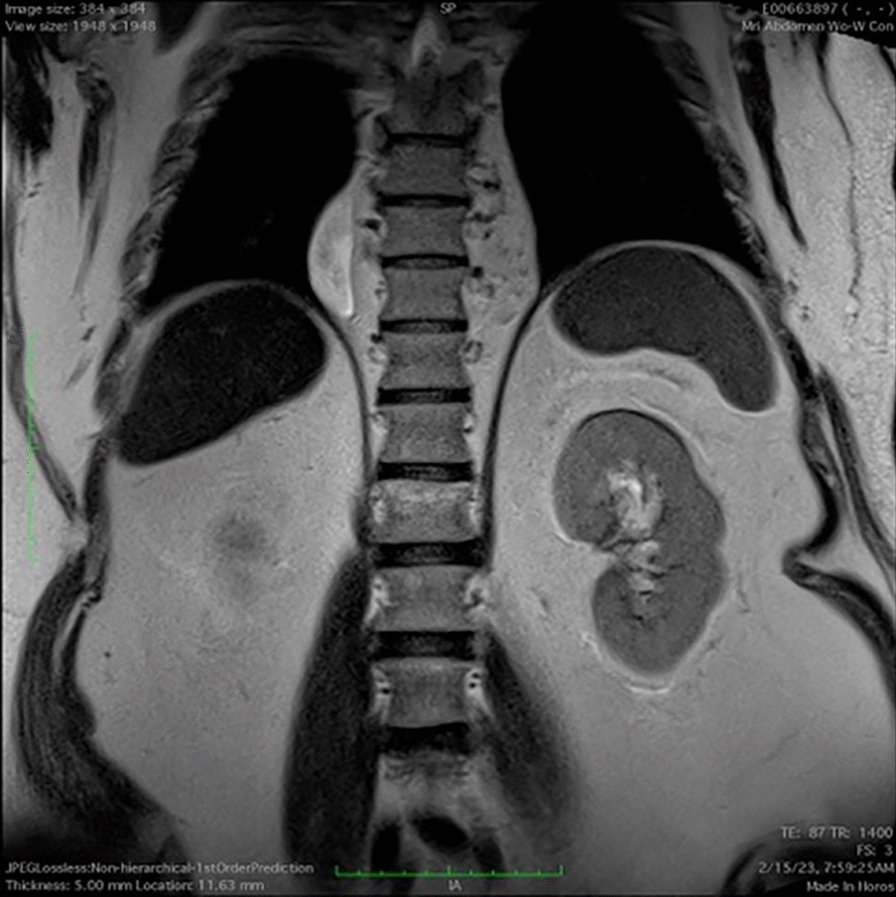
MRI of the chest and abdomen with and without contrast demonstrating an ectopic kidney in the thoracic cavity
Fig. 3.
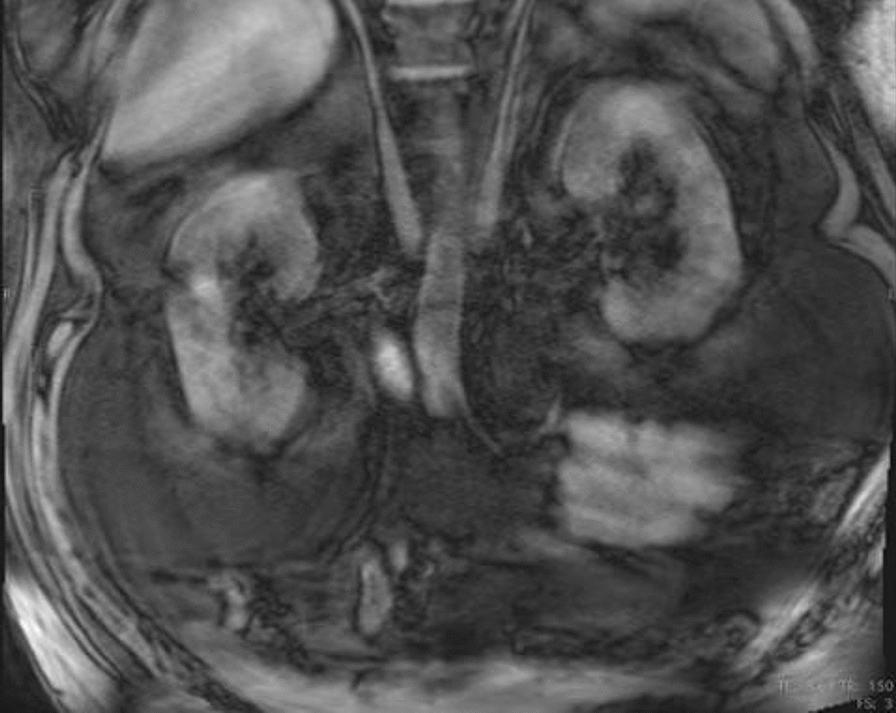
MRI of the chest and abdomen with contrast demonstrating anatomically normal kidneys
A previous CT angiogram of the chest and lower back 2 years prior showed a similar finding that measured 37 mm × 60 mm × 70 mm, and is presumed to be the same mass. According to the radiology report, the findings were consistent with an ectopic thoracic kidney that was unchanged in size. Both Urology and Cardiothoracic Surgery were consulted. Due to the pathology being asymptomatic, both services agreed to forego biopsy and to monitor the patient in the outpatient setting. The decision to monitor rather than intervene surgically aligned with current management strategies that prioritize patient safety and quality of life over aggressive treatment modalities. This case exemplifies the multidisciplinary, non-invasive approach recommended for CITK.
IV urography used to be the diagnostic modality of choice; however, it has recently been replaced by less invasive imaging methods, such as ultrasonography or computed tomography. In normal urogenital development, the embryonic folds begin to form the urinary tract and urogenital ridge in week four. The urogenital ridge subsequently divides into the nephrogenic ridge and gonadal cord. The nephrogenic ridge will then begin to form three kidneys: pronephros, mesonephros, and metanephros. Out of these three structures, only the metanephros progresses to fully developed human kidneys. The embryologic kidneys originally lie close together in the sacral region. However, as the abdomen expands during weeks six through nine, the kidneys ascend and are drawn apart to their final location in the lumbar region [12]. In some cases, the kidney may herniate into the hemithorax via a diaphragmatic defect known as a Bochdalek hernia [15]. In this patient, the kidney metanephric cells migrated past the diaphragm prior to diaphragmatic closure, which resulted in a functional kidney located in the thorax without any associated diaphragmatic defect.
Historical perspective, epidemiology, and embryogenesis
CITK’s epidemiological data point primarily toward congenital factors like diaphragmatic hernias and, less frequently, traumatic events leading to its occurrence. The scarcity of cases, with less than 200 documented instances, emphasizes the need for meticulous examination and reporting to enhance the medical literature surrounding this condition [8]. There are four recognized causes of congenital thoracic renal ectopia: “renal ectopia with an intact diaphragm, diaphragmatic eventration, diaphragmatic hernia, and traumatic diaphragmatic rupture” [11]. CITK is mediated by defects in the complex process of kidney migration to the lumbar region during development. If the kidneys have disruptions along the way, ectopic placements often result. These misplacements are caused by both environmental and genetic factors.
In a case mentioned above, we learn that CITK can occur even without diaphragmatic defects. The case involved metanephric cells migrating past the diaphragm before its closure during embryogenesis, leading to a functional kidney located in the thorax and emphasizes the variability in the embryologic processes that can lead to renal ectopia [14].
Embryogenesis in congenital intrathoracic kidney (CITK)
The development of ectopic kidneys, particularly CITK, is a result of intricate genetic and cellular processes during embryogenesis. Numerous studies provide deeper insight into these mechanisms:
Gene involved in kidney development
Research by Mugford et al. [10] highlights the vital role of Hox11 paralogs in kidney development. These genes help position metanephric structures within the intermediate mesoderm. This genetic pathway emphasizes the pre-determined nature of kidney location and how genetic factors can predispose to anomalies like CITK.
Embryonic kidney processes of induction and differentiation
The stages of nephrogenesis are classified by Horster et al. [6]. The study focuses especially on mesenchymal and epithelial tissue.
Functional capability of ectopic kidneys
The study by Dekel et al. [4] provides an intriguing look at the potential of human metanephri to differentiate into fully functional nephrons after transplantation into mice. This ability to develop and function outside the typical anatomical context not only underscores the adaptability of renal tissues but also suggests that ectopic kidneys, while misplaced, may still achieve functional maturation.
Clinical implications of ectopic kidney positioning
The case study by Sözübir¸ et al. [17] further explores the clinical implications of an ectopic thoracic kidney in a newborn.
Genetic and molecular control over kidney development
Gdnf expression from the metanephric blastema is very important for the formation of the ureteric bud and positioning of the kidney. Mutations or alterations in this signaling pathway could contribute to the anomalous positioning of kidneys, including cases of CITK [12].
Disorders of diaphragmatic development and ectopic kidney positioning
The case reported by Shah et al. [15] demonstrates a rare occurrence where the disorderly ascent of the kidney during embryogenesis, likely due to delayed closure of the diaphragm, results in an ectopic intrathoracic kidney associated with Bochdalek hernia.
Presentation, differential diagnosis, and resolution
Patients with CITK typically present with either vague symptoms such as slight respiratory discomfort or poor findings on abdominal or CT scans. There is frequently a misdiagnosis between CITK and other thoracic anomalies, so comprehensive scanning is recommended. CITK management is largely dependent on the patient’s symptoms and the likelihood of adverse outcomes. Surgery ought to be considered in severe symptomatic cases only. Emerging data suggest better patient selection and development of management strategies may improve outcomes in CITK to preserve renal function and reduce morbidity. This case underscores the often-incidental nature of such findings and supports the notion that CITK can remain asymptomatic and stable over time, as earlier imaging had shown a similar mass [14].
Intravenous pyelography (IVP) and excretory urography are outdated imaging modalities and no longer have a role in evaluating mediastinal masses or ectopic kidneys in modern practice. Instead, CT with intravenous contrast is critical for diagnosing this rare condition, as it provides detailed imaging at appropriate phases for differential diagnosis. Static renal scintigraphy (DMSA) is also important to assess the functional capacity of the ectopic kidney and aids in treatment planning.
Differential diagnosis and functional considerations
Accurately differentiating CITK from other posterior mediastinal masses is essential for appropriate management. Conditions such as congenital diaphragmatic hernias, neurogenic tumors, and other mediastinal anomalies must be considered in the differential. Ultrasonography, computed tomography (CT) scan, urography, and magnetic resonance imaging (MRI) have developed as an indispensable means of correct localization and characterization of CITK. These help distinguish the mediastinal or diaphragmatic abnormalities from those of other diseases [8, 11].
The diagnosis of CITK requires a comprehensive approach. The following imaging modalities further help to confirm its presence and evaluate anatomic and functional status:
Radiography and ultrasound
Initial chest radiography may frequently reveal a smooth cylindrical mass with obvious suggestions of CITK. Ultrasound, on the other hand, may have to be secondarily confirmed with more definitive imaging modalities due to the limited penetration of the lung by the ultrasound.
Computed tomography (CT)
CT imaging provides much detail about renal size, shape, and volume and thus allows differentiation of CITK from all other pulmonary structures and with thickened, soft flesh, characteristic of renal tissue [2, 17].
Magnetic resonance imaging (MRI)
Because MRI has high resolution, it is useful for observing mediastinal or diaphragmatic abnormalities of CITK. It complements CT and ultrasonography, especially in situations where these may be limited in the information.
Static renal scintigraphy (DMSA)
Static renal scintigraphy with technetium-99 m dimercaptosuccinic acid (DMSA) plays a vital role in assessing the functional capacity of the ectopic kidney. This modality details differential renal function, helping to determine whether the ectopic kidney is contributing effectively to overall renal activity. This especially is valuable for planning treatment strategies. Functional compromise in the ectopic kidney may require surgical intervention or other targeted management approaches.
Integration of these diagnostic tools enables health care providers to make informed patient management decisions. The following sections now will delve deeper into strategies tailored for CITK based on documented case studies and clinical outcomes.
Management strategies
The management of congenital intrathoracic kidney (CITK) is nuanced, often influenced by the asymptomatic nature of the condition and the functional normalcy of the ectopic kidney.
Because of embryologic comorbidities, understanding the development of CITK is pivotal for accurate diagnosis and the formulation of appropriate management plans (Shah et al., 2012). Our understanding of CITK has evolved over time. Generally, the treatment paradigm has shifted from an interventional to a monitoring-focused, non-invasive approach.
The conservative strategy is supported by the understanding that CITK, despite its unusual location, typically retains normal functionality and does not inherently carry a higher risk of renal complications such as infections or nephrolithiasis [2, 3, 9, 13].
In the case above, we observe a practical application of this conservative strategy, where the asymptomatic nature and stable imaging findings led to a decision against invasive procedures. The team’s approach exemplifies a patient-centered strategy that minimizes potential surgical risks while maintaining patient quality of life.
Conservative management
Simple monitoring is often the preferred approach for asymptomatic CITK, as suggested by Rouanne et al. [13] and Aydın et al. [3]. This includes non-invasive imaging techniques as described above, to determine kidney status and long-term function. Reasons for such an approach are:
To avoid surgical hazards, mostly in patients who could be at higher risk because of age or comorbidities.
To maintain the functional capacity of the ectopic kidney. Often the organ remains uncompromised in spite of its unusual location.
Conservative management in CITK often involves regular non-invasive monitoring, echoing the principles described by Rouanne et al. [13] and Aydın et al. [3]. This strategy, highlighted in the case of the 52-year-old male, involved multidisciplinary consultations and follow-ups to ensure the stability and functionality of the thoracic kidney, thereby avoiding unnecessary surgical risks and preserving the organ’s integrity [14].
Surgical interventions: considerations and implications
Deciding which type of surgical intervention to take involves an even more thorough evaluation of the patient’s potential risks and benefits. For these decisions, it is helpful to have a multidisciplinary team of an anesthesiologist, nephrologist, urologist, and cardiothoracic surgeon, each playing a role in the decision and patient-counseling [1]. This ensures that any decision made is well-informed and tailored to the patient’s individual circumstances [14].
Risk–benefit analysis
The decision process usually involves a detailed detection of the CITK and its effect. Since CITK is rare, the evidence may be limited which ultimately means the patient and physician will need to rely on broader principles of oncologic and surgical management.
Case studies and clinical outcomes
Patient involvement in the decision-making process is also key. Communication and education help the patient make informed decisions that align with their values, preferences, and risk tolerance [16].
These documented cases offer valuable insights into the diagnosis and management of CITK: they illustrate the complexities of patient management in real-world settings and the importance of tailored approaches based on individual patient presentations. They also reinforce the utility of conservative management and the pivotal role of ongoing monitoring in managing this rare condition [14].
Congenital thoracic kidney with intact diaphragms
Many patients that have a congenital thoracic kidney (CTK) and an intact diaphragm do not have any readily apparent symptoms, and therefore remained unnoticed. Few cases are noted, though, when the patient’s condition is discovered under clinical examinations. Keleş et al. [7] treated a 7-month-old male child with a diagnosis of late-presenting congenital diaphragmatic hernia (CDH), associated with thoracic ectopic kidney. This case highlights the necessity for each physician to have the acumen of diagnosing an intact-diaphragm ectopic kidney, specifically when a child presents with respiratory distress and there is no apparent logical explanation. CT plays a very important role in the accurate diagnosis of such complex cases [7].
Fukuhara et al. [5] describes thoracoscopic repair in a 7-year-old boy with late-diagnosed CDH and thoracic kidney. This approach not only improved the visibility and safety during the surgical correction, but also ensured a successful outcome, further demonstrating the potential benefits of modern surgical techniques in treating congenital thoracic kidneys [5].
The case from Keleş et al. [7] and recent advances like the thoracoscopic techniques described by Yin et al. [18] and Fukuhara et al. [5] highlight the crucial roles of early detection and modern surgical interventions in improving outcomes for such complex ectopic conditions.
Future directions: gaps in current literature
This specific case report also follows the pattern of similar studies emphasizing the need for further research and documentation to refine the management of CITK. Enhancing our collective understanding of its pathophysiology and optimal care strategies is the shared goal.
Conclusion
Management of CITK, particularly in the event of an incidental diagnosis, requires a well-informed approach that prioritizes patient safety and psychological functioning. Through careful consideration, multidisciplinary collaboration, and an emphasis on patient involvement and education, healthcare providers can navigate the complexities of CITK management. Future research endeavors are essential to fill the existing knowledge gaps and further refine the management strategies for this rare and complex condition.
Acknowledgements
None to declare
Author contributions
Sahil Sabharwal and Brandyn Young wrote the main manuscript. Sahil Sabharwal and Sarat Sabharwal prepared the figures. Sahil Sabharwal wrote the abstract. All authors reviewed the manuscript.
Funding
None of the authors have any funding to declare.
Availability of data and materials
This manuscript does not report data generation or analysis.
Declarations
Consent for publication
All of the material is owned by the authors and/or no permissions are required.
Dual publication
The results/data/figures in this manuscript have not been published elsewhere, nor are they under consideration by another publisher.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Al Eraky M, Hassan L, Abou Zaid A, Arabi H. Congenital intrathoracic ectopic kidney in association with Bochdalek hernia. Cureus. 2022. 10.7759/cureus.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arslan H, Aydogan C, Orcen C, GonIllu E. A rare case: congenital thoracic ectopic kidney with diaphragmatic eventration. J Pak Med Assoc. 2016;66:339. [PubMed] [Google Scholar]
- 3.Aydin HI, Sarici SU, Alpay F, Gökçay E. Thoracic ectopic kidney in a child: a case report. Turk J Pediatr. 2000;42:253. [PubMed] [Google Scholar]
- 4.Dekel B, Amariglio N, Kaminski N, Schwartz A, Goshen E, Arditti F, Tsarfaty I, Passwell JH, Reisner Y, Rechavi G. Engraftment and differentiation of human metanephroi into functional mature nephrons after transplantation into mice. J Am Soc Nephrol. 2002. 10.1681/ASN.V134977. [DOI] [PubMed] [Google Scholar]
- 5.Fukuhara M, Kaisyakuji Y, Sato T, Izaki T. Thoracoscopic repair for late-presenting congenital diaphragmatic hernia with thoracic kidney in a child. Asian J Endosc Surg. 2023;16:640–3. 10.1111/ases.13214. [DOI] [PubMed] [Google Scholar]
- 6.Horster M, Braun GS, Huber SM. Embryonic renal epithelia: induction, nephrogenesis, and cell differentiation. Physiol Rev. 1999. 10.1152/physrev.1999.79.4.1157. [DOI] [PubMed] [Google Scholar]
- 7.Keleş S, Artaç H, Elmaci M, Reisli I, Dilsiz A. Late-presenting congenital diaphragmatic hernia associated with ectopic thoracic kidney. Eur J Pediatr. 2006;165:571–2. 10.1007/s00431-006-0119-y. [DOI] [PubMed] [Google Scholar]
- 8.Kian B, Kayedi M, Teimouri A. Intrathoracic kidney: a rare presentation of ectopic kidney. Radiol Case Rep. 2022. 10.1016/j.radcr.2022.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langer FW, Dos Santos D, Dartora EG, Alves GR, Haygert CJ. Ectopic intrathoracic kidney presenting as recurrent pneumonias in a 1-year-old infant: a case report. Lung. 2015;193:839. 10.1007/s00408-015-9760-4. [DOI] [PubMed] [Google Scholar]
- 10.Mugford JW, Sipilä P, Kobayashi A, Behringer RR, McMahon AP. Hoxd11 specifies a program of metanephric kidney development within the intermediate mesoderm of the mouse embryo. Dev Biol. 2008. 10.1016/j.ydbio.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlandi G, Toscano P, Gabrielli O, Di Lella E, Lettieri A, Manzo L, Mazzarelli LL, Sica C, Di Meglio L, Di Meglio L, Gulino FA, Incognito GG, Tuscano A, Cianci S, Di Meglio A. Prenatal diagnosis of an intrathoracic left kidney associated with congenital diaphragmatic hernia: case report and systematic review. J Clin Med. 2023. 10.3390/jcm12113608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehman S, Ahmed D. Embryology, kidney, bladder, and ureter. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547747/. [PubMed]
- 13.Rouanne M, Le Mandat A, Dorgeret S, Philippe-Chomette P, El Ghoneimi A. A rare case of ectopic intrathoracic kidney in a 1-year-old child. Urology. 2010. 10.1016/j.urology.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Sabharwal S, Ali S, Sabharwal S and Brandon K. A functional, congenital intrathoracic kidney: an incidental finding from gastrointestinal bleeding. 2024.
- 15.Shah AD, Ajay S, Adalia M, Rathi A. Bochdalek hernia with intrathoracic kidney. Lung India. 2012;29(4):373–5. 10.4103/0970-2113.102837.PMID:23243354;PMCID:PMC3519026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stivers T, Tate A. The role of health care communication in treatment outcomes. Ann Rev Linguist. 2023. 10.1146/annurev-linguistics-030521-054400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sözübir S, Demir H, Ekingen G, Güvenç BH. Ectopic thoracic kidney in a child with congenital diaphragmatic hernia. Eur J Pediatr Surg. 2005. 10.1055/s-2005-837608. [DOI] [PubMed] [Google Scholar]
- 18.Yin F-R, Wang J, Gao X, Yi L. Right thoracic ectopic kidney with congenital diaphragmatic hernia. J Pediatr Surg Case Rep. 2021;71:101865. 10.1016/J.EPSC.2021.101865. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This manuscript does not report data generation or analysis.


