Abstract
Airag, a fermented mare’s milk in Mongolia, exhibits diverse flavors and microbiota due to distinct production processes and environments in nomadic households. Recently, there has been a shift from the traditional cow skin container, ‘khokhuur’, to a plastic container for airag production, potentially impacting the microbiota and quality. To address this notion, we aimed to elucidate the differences in the microbiota between airag samples from a plastic container and those from a khokhuur. We collected airag samples produced using either a plastic container or khokhuur from three houses in Mogod Sum (county) in Bulgan Aimag (province) and analyzed the chemical and microbiome properties of the obtained samples. Compared with the khokhuur, the plastic containers exhibited high heat retention at night and boosted lactate production, which sustained a lower pH level in airag. A series of alpha diversity indices of airag microbiota were significantly higher in airag produced in khokhuurs than in those produced in the plastic containers. In particular, Lactobacillus helveticus was the most dominant species, accounting for more than 90% of the total population in airags produced in the plastic containers and khokhuur, whereas some other lactic acid bacteria species and environmental bacteria more colonized airags produced in khokhuurs. The khokhuur itself is likely a source of these bacterial species and likely provides a niche, and the wider volatility of temperature may allow the growth of this wide range of bacteria while maintaining a lower level of lactic acid fermentation.
Keywords: airag, fermented milk, Mongolia, khokhuur, lactic acid bacteria, microbiome, Lactobacillus helveticus
INTRODUCTION
Airag is a traditional Mongolian beverage with a low alcohol content and acidity and is made through the lactic acid and alcohol fermentation of mare’s milk. It is a rich source of energy and vitamins [1]. A survey conducted in Uvurkhangai, the region of origin of airag, reported an average daily intake of four liters for herders in summer [1]. Airag is known for its health benefits. Over time, it has been known to have beneficial effects on health, including chronic disease management and the regulation of host immunity [2]. The Mongolian population has higher levels of Lactobacillus in their gut intestines than other Asian populations [3], and the high consumption of airag may potentially impact their health by modulating the intestinal microbiome.
Airag is produced mainly during the summer. A starter can be obtained from the local area or a market when creating airag for the first time in a given year. In addition, dried horse tendons soaked in airag obtained from the previous year are also used as a starter [4]. The mixture is stirred for several hours to achieve consistent fermentation. The fermentation process is conducted at ambient temperatures ranging from 20 to 30°C. As fermentation progresses, the alcohol content reaches 1–3%, and the pH drops to ≤4 [1, 5].
Lactic acid bacteria and yeasts are the predominant microorganisms in airag [6, 7]. Lactic acid bacteria can ferment the lactose present in mare’s milk directly. Yeast is responsible for alcohol fermentation from lactose, galactose, or glucose, depending on the species [8], and produces ethanol and carbon dioxide, resulting in a mildly alcoholic sour beverage.
Lactic acid bacterial strains have been actively isolated from airag [7, 9, 10], some of which have demonstrated immunomodulatory properties and have been documented as probiotics [11]. Research conducted on the microbial community in airag has shown that the predominant bacterium is Lactobacillus helveticus [6, 10, 12, 13].
There have been changes in the containers used for airag fermentation under accelerated modernization in Mongolia (Fig. 1A). Traditionally, a container called a ‘khokhuur’, made from cow skin, has been utilized. However, the use of plastic containers has been increasing. Compared with khokhuurs, the plastic containers retain more heat, resulting in higher temperatures during the day and a smaller decrease in temperature at night [14]. Therefore, the change in the container used is likely to impact the microbial community in the airag product.
Fig. 1.
Fermentation containers, sampling locations, characteristics, and production scheme of airags in this study.
(A) A plastic container (left) and khokhuur (right) used for airag production. (B) Sampling locations of airag samples used in this study. Airag samples were collected from three households in Mogod County in Mongolia. (C) The years of use of the khokhuurs and the location for fermentation of airag examined in this study. (D) Daily schedule for production and sampling of airags in this study. This 1-day schedule was cycled for up to six days. The images below depict the daily process of airag production.
In this study, we aimed to understand how the container type affects the airag microbiome in conjunction with the airag product quality. We compared the microbial and chemical compositions of airag samples produced in plastic containers and khokhuurs.
MATERIALS AND METHODS
Study design and sample collection
Airag samples were collected from Mogod County, Bulgan Province, a region in Mongolia known for its thriving airag production (Fig. 1B). The samples were collected from three households engaged in airag production using both the plastic containers and khokhuurs over a period of five to six days. The characteristics of the airags in this study are shown in Fig. 1C.
The production methods for the three houses examined in this study were as follows (the daily schedule is shown in Fig. 1D). Milking began in the morning, and the collected milk was cooled to ambient temperature. Around noon, mare’s milk was mixed with recycled airag prepared on the previous day. The ratio of airag to mare’s milk was 4:3. The mixture was continuously stirred for approximately 3 hr in the plastic containers or khokhuurs. Stirring was conducted using a wooden stick in both the plastic containers or khokhuurs. After stirring, the milk was left to stand overnight and then served. The unconsumed airag was recycled as a starter culture and mixed with mare’s milk to initiate the next fermentation step, beginning in the afternoon.
To investigate the fermentation profile in the daily process, we collected airag samples for 5–6 days at the following time points: a) early morning, b) before mixing with fresh milk around noon, c) immediately after mixing with fresh milk, d) after stirring for 1 hr, and e) after stirring for 3 hr. In total, 77 samples from the plastic containers and 79 samples from khokhuurs were collected (Fig. 1D). Details for the sampling are shown in Supplementary Table 1.
Airag samples were collected in 50 mL falcon tubes (430291, Corning, NY, USA) and stored at −30°C. The samples were transported to Kyushu University, Japan, while being kept below 4°C and were immediately stored at −80°C until DNA or metabolite extraction.
Electrical conductivity (EC), pH, refractive index (RI), and temperature measurements
The following parameters were determined for each airag sample: EC using a conductivity meter (HORIBA LAQUAtwin B-771, HORIBA, Kyoto, Japan); pH using a pH meter (HORIBA LAQUAtwin B-712, HORIBA, Kyoto, Japan); RI using an RI meter (PAL-RI, ATAGO, Tokyo, Japan); and temperature using a temperature recorder (Ondotori TR-50U2, T&D, Nagano, Japan). The temperature was measured using sensors set up in the central section of each container over six days of sampling.
Quantification of lactate, ethanol, and lactose
The lactate, ethanol, and lactose levels were quantified as follows: 200 µL of the sample was mixed with 50 µL of 20% 5-sulfosalicylic acid dihydrate, and 800 µL of ultra-pure water was added. The mixture underwent centrifugation at 20,000 × g for 10 min, followed by filtration through a 0.45 µm filter (13CP045AS, Toyo Roshi Kaisha, Tokyo, Japan). The extract was subjected to high-performance liquid chromatography (HPLC; LC-2000Plus series, JASCO Corporation, Tokyo, Japan) under the following conditions: injection volume, 20 µL; column, Shodex SUGAR SH1011 (Shodex, Tokyo, Japan); column temperature, 50°C; solvent, 2.74 × 10−4 mM of sulfuric acid; flow rate, 0.5 mL/min; detector, RI-2031 (JASCO Corporation, Tokyo, Japan).
DNA extraction, 16S rRNA gene amplicon sequencing, and data processing
DNA was extracted according to a previously described method [12], except for the vortex temperature. Briefly, 1 mL of airag samples was centrifuged at 20,000 × g for 3 min at 4°C. The pellet was suspended in 500 µL of benzyl chloride and 250 µL of extraction buffer (100 mM Tris-HCl, 40 mM ethylenediaminetetraacetic acid (EDTA), pH 9.0). Then, 0.7 g of 0.1 mm diameter glass beads were added to this suspension, and the mixture was vigorously shaken for 30 sec using a FastPrep 24 Instrument (MP-Biomedicals, Irvine, CA, USA) at a speed of 6.5 m/sec. Following this, 50 µL of 10% sodium dodecyl sulfate (SDS) was introduced to the suspension, which was then vigorously vortexed at 4°C for 20 min using an M·BR-022UP (Taitec, Saitama, Japan). After adding 150 µL of 3 M sodium acetate (pH 5.2), the mixture was cooled at −20°C for 15 min. Following centrifugation at 20,000 × g for 15 min, the supernatant was collected, and DNA was obtained by precipitation using 300 µL of isopropanol. Ultimately, the DNA was diluted to a concentration of 10 µg/mL using TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 8.0) and stored at –80°C until further use.
High-throughput 16S rRNA gene sequencing and data processing were performed as described previously [15]. Briefly, the V3 to V4 region of the bacterial 16S rRNA gene was amplified using a two-step polymerase chain reaction (PCR) approach. In the first step, the bacterial universal primer set Bakt_341F (5′-CGCTCTTCCGATCTCTGCCTACGGGNGGCWGCAG-3′) and Bakt_805R (5′-TGCTCTTCCGATCTGACGACTACHVGGGTATCTAATCC-3′) was used. The second step involved using the same primers and index sequences. Amplicons containing index sequences were subjected to sequencing using MiSeq Reagent Kit v3 (MS-102-3003, Illumina, San Diego, CA, USA).
The sequence data were processed using QIIME 2 pipeline version 2021.2 [16]. Briefly, after demultiplexing the total reads based on the index sequences, paired-end sequences were merged and denoised. Primer sequences were removed using the q2-cutadapt plugin. The trimmed reads were further processed with DADA2 [17] using the recommended parameters to eliminate chimeric and low-quality reads and to cluster the reads into amplicon sequence variants (ASVs). Taxonomic classification of the representative sequence for each ASV was performed using the QIIME “classify-sklearn” feature from the SILVA 138 database [18].
We obtained 4,061,551 high-quality sequences corresponding to 26,035 ± 4,470 high-quality reads (minimum 16,348 reads) per sample clustered into 133 ASVs, which were assigned to four phyla, six classes, 14 orders, 14 families, 37 genera, and 46 species.
Quantitative real-time PCR
For the standard, genomic DNA was extracted from an exponential-phase culture of Lactobacillus helveticus JCM 1120T corresponding to 5.60 × 107 colony-forming units/mL by using the above benzyl-chloride beads-beating method. The extracted DNA was serially diluted and used to generate a standard curve.
Quantitative real-time polymerase chain reaction (qPCR) was performed by using the KOD SYBR qPCR Mix (QKD-201, Toyobo, Osaka, Japan) with a universal primer set of Bakt_341F and Bakt_805R, targeting the V3–V4 region of the bacterial 16S rRNA gene, to enumerate the total bacterial cells in the airag. The reactions were performed in triplicate using an Mx3000P instrument (Agilent Technologies, Santa Clara, CA, USA). The PCR cycle conditions comprised an initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 30 sec, annealing at 50°C for 30 sec, and extension at 72°C for 35 sec. Fluorescence was measured at the end of each extension step during the qPCR cycles. The copy number of the 16S rRNA gene was estimated based on the standard curve generated by using the genomic DNA of L. helveticus possessing four copies of 16S rRNA genes on the chromosome.
Bioinformatics analysis
Statistical analyses and data visualization were performed using R Studio 2022.07.2, R 4.2.1 [19], and Stata/SE 12.0 [20]. The Wilcoxon rank-sum test was performed in the R environment to find the tendencies toward differences between the two groups, and thereafter the Benjamini–Hochberg procedure was performed also in the R environment to evaluate statistically significance with the adjusted p-value. To overview the differences in the abundances of each taxonomic unit between the samples from the plastic containers and khokhuurs, linear discriminant analysis of effect size (LEfSe) was performed using the online Galaxy platform [21]. The Wilcoxon rank-sum test was used to calculate the linear discriminant analysis (LDA) scores.
Alpha and beta diversity (PCoA) analyses were performed in the QIIME 2 platform. The alpha and beta diversity indices were determined based on an even sampling depth of 16,348 reads of the 16S rRNA gene fragment for each sample. Permutational multivariate analysis of variance (PERMANOVA) was performed using the function for multilevel pairwise comparison using adonis2 in the R vegan package [22] to investigate the statistical differences in the beta diversity of airags from the plastic containers and khokhuurs.
The ‘envfit’ function in the R vegan package [23] was used to obtain the correlation of the bacterial beta diversity coordination (PCoA plot based on the Bray–Curtis distance of ASV composition) with the relative abundance of each genus as well as environmental and chemical parameters of each airag sample.
Accession codes
The sequence data for the airag presented in this article have been deposited in the DNA Data Bank of Japan (DDBJ) database under BioProject number PRJDB17651. This repository includes links and access to airag sampling data, accessible through BioSample IDs SAMD00751605 to SAMD00751760.
RESULTS
Comparison of pH, temperature, RI, and EC between the plastic containers and khokhuurs
The pH, temperature, RI, and EC were measured during the daily fermentation cycle of airag and compared between the plastic containers and khokhuurs (Fig. 2). The pH peaked at point c (immediately after adding milk to airag) and decreased thereafter as fermentation progressed. In the comparison of containers, airag produced in khokhuurs showed a significantly higher pH at time points a to c than that produced in the plastic containers. The temperature reached its lowest point in the morning (point a) in both containers, while it tended to be lower in the morning (point a) in khokhuurs than in the plastic containers (non-adjusted p-value <0.05). The RI reached its lowest at point b in airag produced in khokhuurs and at time point a in that produced in the plastic containers, suggesting a reduction of sugar content resulting from fermentation. The EC peaked at point a in the airag produced in khokhuurs and at point b in that produced in the plastic containers, suggesting the dissolution of casein-bound calcium and phosphorus salts under the acidification of mare’s milk. However, no significant differences were observed between airag produced in the containers for either the RI or EC.
Fig. 2.
Transition of the pH, temperature, electrical conductivity (EC), and refractive index (RI) of airag in the plastic containers and khokhuurs in the daily fermentation cycle.
The data were averaged within the samples from the plastic container or khokhuur at each sampling point and displayed as the mean ± SD. Single asterisks indicate a tendency toward a difference, with a non-adjusted p-value lower than 0.1, while double asterisks indicate a statistically significance, with an adjusted p-value lower than 0.05.
Change in lactate, ethanol, and lactose concentrations in the daily fermentation cycle of airag
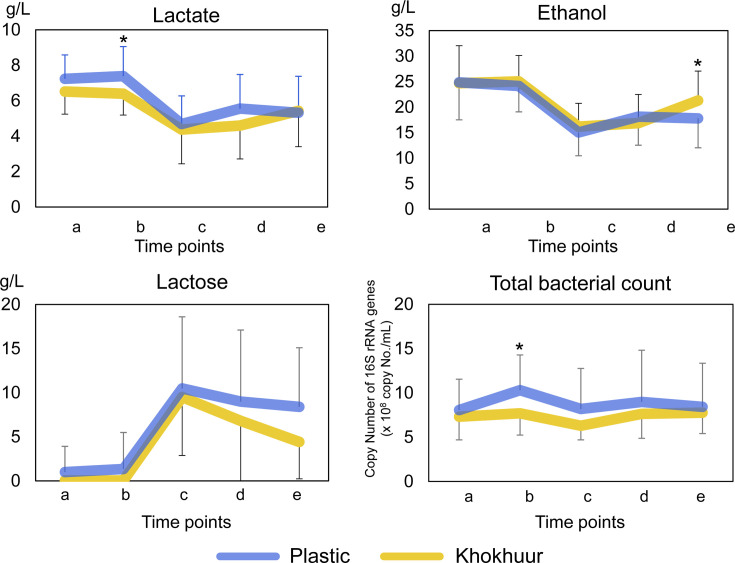
We monitored the changes in the concentrations of lactate, ethanol, and lactose during the daily fermentation cycle of airag (Fig. 3). The ethanol concentration ranged from 15 to 30 g/L, whereas concentration of lactate ranged from 5 to 7 g/L, suggesting that ethanol fermentation was higher than lactate fermentation. The kinetics of lactate and ethanol exhibited similar trends over the course of one day, in which their concentrations were lowest at point c (immediately after mixing with fresh milk) and gradually increased in the morning. The lactose concentration ranged from 0 to 10 g/L, and the kinetics of lactose exhibited an opposite trend to those of lactate and ethanol. This suggested that lactose was the main carbohydrate source for ethanol and lactate fermentation and that it was completely consumed in the morning, which was the end of daily fermentation.
Fig. 3.
Kinetics of lactate, ethanol, and lactose concentrations, and total bacteria count in airags in the plastic container and khokhuur in the daily fermentation cycle.
The data were averaged within the samples from the plastic container or khokhuur at each sampling point and displayed as the mean ± SD. Asterisks represent the same information as in Fig. 2.
Although no statistically significant differences were observed in its concentrations throughout the daily cycle, lactate showed a higher trend in the plastic containers at point b, the time just before mixing with fresh milk (non-adjusted p-value <0.1), suggesting that lactate fermentation tended to be more active in the plastic containers than in the khokhuurs. A higher total bacterial count was observed in the plastic containers than in the khokhuurs before adding the fresh milk (non-adjusted p-value <0.1), which was potentially attributable to the increased production of lactate in the plastic containers. This was consistent with the pH data, which showed a lower value in the plastic containers than in the khokhuurs at all points. Ethanol showed a higher trend in the khokhuurs after 3 hr of stirring (non-adjusted p-value <0.1), whereas lactose showed a lower trend in the khokhuurs after stirring. This suggested that ethanol fermentation from lactose tends to be more active in khokhuurs in the afternoon.
Differences in the microbial community composition between the plastic containers and khokhuurs
We assessed the alpha diversity of the airag microbiome based on the data from amplicon sequencing of the 16S rRNA gene. Alpha diversity was evaluated using the following indices: Pielou evenness, Faith’s phylogenetic diversity (PD), observed number of ASVs, and Shannon index (Fig. 4). Throughout the daily fermentation cycle, all alpha diversity indices were higher in airag produced in the khokhuurs than those in the plastic containers. Remarkably, the alpha diversities were highest in airag produced in khokhuurs immediately after the addition of fresh mare’s milk (point c). This phenomenon was not observed in the plastic containers, in which the alpha diversities were somewhat stable and remained lower than those in produced in the khokhuurs.
Fig. 4.
Transition of four different alpha diversity metrics of the airag microbiome in the plastic container and khokhuur in the daily fermentation cycle.
The data were averaged within the samples from the plastic container or khokhuur at each sampling point and displayed as the mean ± SD. Asterisks represent the same information as in Fig. 2. PD: phylogenetic diversity; ASVs: amplicon sequence variants.
The beta diversity of the bacterial community among the collected airag samples was estimated using four distance matrices (Bray–Curtis, Jaccard, Weighted UniFrac distance, and Unweighted UniFrac distance), and the distinctiveness of the community structure between that of the airag produced in the plastic containers and that of the airag produced in the khokhuurs was evaluated using permutation analysis (Table 1). All four distances showed that the microbial community composition differed significantly between the plastic containers and khokhuurs.
Table 1. Permutational multivariate analysis of variance (PERMANOVA) test for statistical differences of the microbial community between the plastic containers and khokhuurs.
| Distance used for beta diversity | p-value |
|---|---|
| Bray–Curtis | 0.002 |
| Jaccard | 0.0032 |
| Weighted UniFrac distance | 0.0028 |
| Unweighted UniFrac distance | 0.0074 |
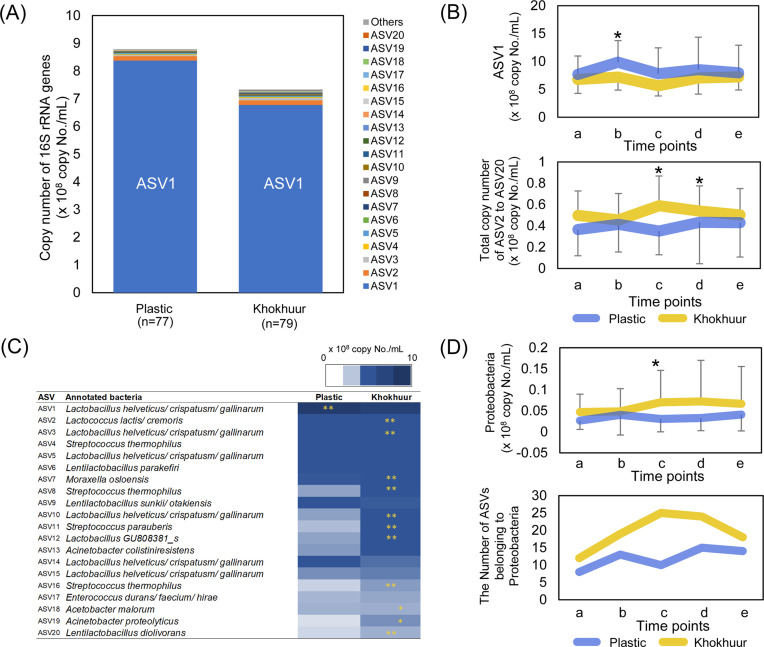
LEfSe analysis was conducted to assess the differences in microbiota composition at different taxonomic levels between the airags produced in the plastic containers and khokhuurs (Fig. 5). The genus Lactobacillus was notably abundant in the airag produced in the plastic containers, whereas a wide variety of taxonomic groups across the phyla Firmicutes, Actinobacteria, and Proteobacteria (six bacterial families, and nine bacterial genera) were enriched in airag produced in khokhuurs. At the genus level, airag in khokhuurs was enriched with Acetobacter, Rothia, uncl_Enterobacteriaceae, Citrobacter, and Enhydrobacter, all of which are known to be present in the environment and intestine [24,25,26,27,28], in addition to Lentilactobacillus, Streptococcus, Lactococcus, and Macrococcus, all of which are known lactic acid producers.
Fig. 5.
Linear discriminant analysis of effect size (LEfSe) analysis to compare the bacterial composition at each taxonomic rank between airags produced in the plastic container and khokhuur.
Taxonomic groups with a linear discriminant analysis (LDA) >2.0 and p<0.05 are highlighted by the indicated color on the circular and rectangular cladograms.
The absolute quantities of all ASVs were determined using the total bacterial count of each sample and the relative abundance of each ASV (Supplementary Table 2). The abundances of the 20 most abundant ASVs are displayed in a stacked bar graph (Fig. 6A). ASV1, closely related to Lactobacillus helveticus/crispatus/gallinarum, was most abundant and accounted for more than 90% of total bacteria abundance. In the series of airag samples examined in this study, ASV1 ranged from 1.04 × 108 to 2.50 × 109 copies/mL. The counts are close to the data of a previous report showing viable cell numbers of lactic acid bacteria in airags ranging from 8.07 to 8.76 log CFU/mL [10]. Figure 6B shows the kinetics of the abundances of ASV1 and ASV2 to ASV20. The plastic containers maintained a higher level of ASV1 that fluctuated more than that in khokhuurs, similar to the total bacterial count. In contrast, non-ASV1 bacteria were statistically more abundant in khokhuurs than in the plastic containers (Fig. 6C). Notably, the abundance of Proteobacteria was higher in khokhuurs with a larger number of ASVs, particularly at point c immediately after the fresh milk was mixed with the previous batch (Fig. 6D).
Fig. 6.
Differences in the 20 most abundant and Proteobacteria amplicon sequence variants (ASVs) between airags fermented in the plastic container or khokhuur.
(A) Bar graphs stacking the 16S rRNA gene copy numbers of the top 20 ASVs in the airags produced in the plastic container and khokhuur. (B) Population dynamics of ASV1 and ASV2-20 in the daily fermentation cycle. The abundance of ASV2-20 was calculated by summing the 16S rRNA gene copies from ASV2 to ASV20. The data were averaged within the samples from the plastic container or khokhuur at each sampling point and displayed as the mean ± SD. Asterisks represent the same information as in Fig. 2. (C) Heat map for the abundances of the top 20 ASVs in the airags produced in the plastic container and khokhuur. Asterisks indicate the group with significantly higher abundance (*0.05 <p<0.1, **p≤0.05). (D) Population dynamics of ASVs belonging to Proteobacteria in the daily fermentation cycle. Upper panel: Abundance was calculated by summing the 16S rRNA gene copies of ASVs belonging to phylum Proteobacteria. The data were averaged within the samples from the plastic container or khokhuur at each sampling point and displayed as the mean ± SD. Asterisks represent the same information as in Fig. 2. Lower panel: The number of the Proteobacteria ASVs observed in the airag samples in either the plastic container or khokhuur was summed for all the samples at each sampling point and graphed.
Microbiological properties of airag produced in the plastic containers and khokhuurs
The diversity of the airag bacterial community structures was ordinated in the principal coordinate plot calculated based on the Bray–Curtis distances of the ASV compositions between samples (Fig. 7). Significant differences were observed between the airags produced in the plastic container and khokhuur, based on the PERANOVA analysis (p<0.01; Table 1). The correlations of the bacterial communities to the environmental and chemical properties were calculated and displayed as vectors in the PCoA plot. The bacterial communities of airag in the plastic containers, which were highly driven by the high abundance of Lactobacillus (G1), tended to positively correlate with lactate and temperature and negatively correlated with pH, whereas samples from the khokhuurs showed a positive correlation with pH and lactose. These results suggest that lactate fermentation by lactic acid bacteria tends to be suppressed by the lower temperature in khokhuurs, particularly at night.
Fig. 7.
Variance in bacterial beta diversity of airag samples produced in the plastic containers and khokhuur and its correlation with environmental and chemical properties.
Principal coordinate analysis (PCoA) was performed based on the Bray–Curtis distances of amplicon sequence variant (ASV) compositions between samples and plotted in two dimensions. The blue and yellow arrows indicate the centroids of samples from the plastic containers and khokhuurs, respectively. The correlation of the PCoA ordination to the relative abundance of each genus is indicated by the position of letters beginning with “G”, each representing a certain genus. The correlations of the bacterial community variance with environmental and chemical factors are indicated by the position of the names of factors in the plot. Box plots beside the PCoA plot show the distributions of PC1 and PC2 of airag samples in the plastic containers and khokhuurs. PERMANOVA: permutational multivariate analysis of variance. Double asterisks in the box plots indicated p<0.01 in the Wilcoxon rank-sum test between two groups.
The box plots beside the PCoA plot further indicate the significant difference in bacterial communities between the plastic container and khokhuur. Notably, PC1 was distributed broadly toward the negative range in association with vectors of a number of non-Lactobacillus bacteria in the khokhuur samples, suggesting a diverse microbial community containing higher numbers of non-Lactobacillus species in khokhuurs.
DISCUSSION
Traditionally, the khokhuur has been used as a container for airag production in Mongolia; however, using plastic containers has become prevalent with modernization. The transition of the fermentation container is anticipated to significantly affect the microbial communities and the metabolites generated by the microorganisms in airag, affecting both its nutritional profile and flavor. In this study, we addressed how the transition of the container type affects the microbial community and its fermentation properties.
First, we monitored the temperature, components, and properties of airag during fermentation. The temperature in the plastic containers was stable enough to keep the airag temperature sustainable for lactic acid fermentation even during the night (Fig. 2). This observation agrees with a previous study, which showed that, in contrast to khokhuurs, the plastic containers exhibited a tendency to maintain heat inside. The temperature in the plastic containers was higher during the day, and the drop in temperature at night was smaller than that in khokhuurs [14]. Chemical component measurements revealed that airag produced in the plastic containers tended to contain higher lactate levels, particularly in the morning (Fig. 3). Furthermore, a lower pH was maintained in the plastic containers throughout the daily cycle (Fig. 2). This suggested the occurrence of more active lactate fermentation, most likely owing to the heat-retaining capacity of the plastic containers.
Second, a higher diversity of the bacterial community in airag produced in khokhuurs was observed in the alpha diversity metrics (Fig. 4). Immediately after the addition of fresh milk (point c), significant increases in alpha diversity were observed, specifically in khokhuurs. This phenomenon is probably due to the release of a cluster of bacteria, notably Proteobacteria species, which appeared to be adhered to the skin of the khokhuurs, likely as a biofilm. This non-ASV1 bacteria group includes environmental and animal intestinal bacteria, and these bacteria were sustained throughout the airag production process, as higher alpha diversity was observed over time in the khokhuurs.
In the PCoA biplot showing the variance of the airag microbiome in association with environmental and chemical properties (Fig. 7), the plastic containers showed a negative correlation with pH and a positive correlation with Lactobacillus, lactate, and temperature. This agrees with the above discussion of fermentation being more active in the plastic containers, notably at higher temperatures from night to morning, resulting in higher production of lactate with a drop in pH. There was also a noticeable trend of predominant growth of a single species of homofermentative lactic acid bacterium, L. helveticus, which was associated with higher lactic acid production in the plastic containers.
A previous study compared the ethanol tolerance of L. helveticus strains isolated from whiskey and dairy products. The results demonstrated that while the L. helveticus strains from dairy products did not show the same level of ethanol tolerance as those from whiskey, growth was still observed up to an ethanol concentration of 5% [29]. This may explain why L. helveticus dominates the bacterial community of airag as an ethanol fermented beverage as well as lactic acid fermented one. It was also noted that the second most dominant species in our airag samples was Lactococcus lactis, which has also been reported to show alcohol tolerance [30]. Alcohol and lactic acid co-fermentation is most likely to stabilize the microbiome of Mongolian airag.
In contrast to the plastic containers, airag produced in khokhuurs contains a greater variety of bacteria comprising a larger number of lactic acid bacterial and environment bacterial species. There are several possible explanations for these results. (1) Khokhuur is a likely source of these bacteria. (2) The wider temperature volatility in khokhuurs may allow the growth of a wide range of bacteria. (3) Weaker acidic pH due to lower lactic acid fermentation levels may enable the survival of other lactic acid and environmental bacteria. (4) A high level of alcohol fermentation in the early milk-homogenizing stage (point e) may hamper the growth of the dominant L. helveticus.
It is still not known whether using a plastic container that is easy to use and maintain and enables easier control during fermentation or khokhuur with the opposite characteristics is better for airag production. The lower level of lactate produced in the khokhuur may be favorable, considering that airag produced in the khokhuur is renowned as tastier, although other components may contribute to its rich flavor. Further analysis is warranted to examine the correlation between the container and flavor of airag, as well as the impact of airag consumptionon the health maintenance of Mongolian people living under severe climate conditions.
CONFLICT OF INTEREST
None.
Supplementary Material
Acknowledgments
This work was supported by Grants-in-Aids for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI grant numbers 22KJ2447, 20H01391 and 20KK0130), the JSPS Core-to-Core program on “Establishment of Gut Microbiome Research Core linking Asian Foods and Health”, and an ANRI fellowship, and Kyushu University Institute for Asian and Oceanian Studies. We also would like to express our gratitude for the technical support provided by the Center for Advanced Instrumental and Educational Support, Faculty of Agriculture, Kyushu University, in operating the MiSeq.
REFERENCES
- 1.Bat-Oyun T, Erdenetsetseg B, Shinoda M, Ozaki T, Morinaga Y. 2015. Who is making airag (fermented mare’s milk)? A nationwide survey of traditional food in Mongolia. Nomad People 19: 7–29. [Google Scholar]
- 2.Xue W, Yuan X, Ji Z, Li H, Yao Y. 2023. Nutritional ingredients and prevention of chronic diseases by fermented koumiss: a comprehensive review. Front Nutr 10: 1270920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shinoda A, Shirchin D, Jamiyan D, Lkhagvajav T, Purevdorj C, Sonomtseren S, Battogtokh C, Bira N, Therdtatha P, Nakayama J. 2021. Comparative study of gut microbiota Mongolian and Asian people. Mongolian Journal of Agricultural Sciences 33: 1–7. [Google Scholar]
- 4.Azam M, Mohsin M, Ijaz H, Tulain UR, Ashraf MA, Fayyaz A, Abadeen Z, Kamran Q. 2017. Review—Lactic acid bacteria in traditional fermented Asian foods. Pak J Pharm Sci 30: 1803–1814. [PubMed] [Google Scholar]
- 5.Liu S, Han Y, Zhou Z jiang. 2011. Lactic acid bacteria in traditional fermented Chinese foods. Food Res Int 44: 643–651. [Google Scholar]
- 6.Uchida K, Hirata M, Motoshima H, Urashima T, Arai I. 2007. Microbiota of “airag”, “tarag” and other kinds of fermented dairy products from nomad in Mongolia. Anim Sci J 78: 650–658. [Google Scholar]
- 7.Ganzorig O, Sumisa F, Batdorj B, Yoshida T. 2016. Isolation and identification of new lactic acid bacteria with potent biological activity and yeasts in airag, a traditional Mongolian fermented beverage. Food Sci Technol Res 22: 575–582. [Google Scholar]
- 8.Sudun W, Wulijideligen, Arakawa K, Miyamoto M, Miyamoto T. 2013. Interaction between lactic acid bacteria and yeasts in airag, an alcoholic fermented milk. Anim Sci J 84: 66–74. [DOI] [PubMed] [Google Scholar]
- 9.Takeda S, Yamasaki K, Takeshita M, Kikuchi Y, Tsend-Ayush C, Dashnyam B, Ahhmed AM, Kawahara S, Muguruma M. 2011. The investigation of probiotic potential of lactic acid bacteria isolated from traditional Mongolian dairy products. Anim Sci J 82: 571–579. [DOI] [PubMed] [Google Scholar]
- 10.Choi SH. 2016. Characterization of airag collected in Ulaanbaatar, Mongolia with emphasis on isolated lactic acid bacteria. J Anim Sci Technol 58: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura M, Danno K, Yasui H. 2006. Immunomodulatory function and probiotic properties of lactic acid bacteria isolated from Mongolian fermented milk. Biosci Microflora 25: 147–155. [Google Scholar]
- 12.Oki K, Dugersuren J, Demberel S, Watanabe K. 2014. Pyrosequencing analysis of the microbial diversity of airag, khoormog and tarag, traditional fermented dairy products of mongolia. Biosci Microbiota Food Health 33: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Dang N, Ren D, Zhao F, Lv R, Ma T, Bao Q, Menghe B, Liu W. 2020. Comparison of bacterial microbiota in raw mare’s milk and koumiss using PacBio single molecule real-time sequencing technology. Front Microbiol 11: 581610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoyama S. 2022. Doing Fieldwork on Fermented Foods in the World. Rural Culture Association Japan, Saitama, p. 190 (in Japanese). [Google Scholar]
- 15.Kisuse J, La-Ongkham O, Nakphaichit M, Therdtatha P, Momoda R, Tanaka M, Fukuda S, Popluechai S, Kespechara K, Sonomoto K, et al. 2018. Urban diets linked to gut microbiome and metabolome alterations in children: a comparative cross-sectional study in Thailand. Front Microbiol 9: 1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37: 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13: 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41: D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R core Team. The R project for statistical computing. Available at: https://www.r-project.org/ (accessed 2023-11-17)
- 20.Stata Technical Support. Citing stata software, documentation, and FAQs. Available at: https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/ (accessed 2023-11-17)
- 21.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol 12: R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez Arbizu P. pairwiseAdonis: Pairwise multilevel comparison using adonis. 2020. Available at: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed 2024-8-4)
- 23.Oksanen J. Multivariate analysis of ecological communities in R: VeganTutorial. 2015. Available at: https://www.mooreecology.com/uploads/2/4/2/1/24213970/vegantutor.pdf (accessed 2023-12-21)
- 24.Hommel R, Ahnert P. 1999. Acetobacter In Encyclopedia of Food Microbiology, Academic Press, Cambridge, pp. 1–7. [Google Scholar]
- 25.Tsuzukibashi O, Uchibori S, Kobayashi T, Umezawa K, Mashimo C, Nambu T, Saito M, Hashizume-Takizawa T, Ochiai T. 2017. Isolation and identification methods of Rothia species in oral cavities. J Microbiol Methods 134: 21–26. [DOI] [PubMed] [Google Scholar]
- 26.Berhe G, Wasihun AG, Kassaye E, Gebreselasie K. 2020. Milk-borne bacterial health hazards in milk produced for commercial purpose in Tigray, northern Ethiopia. BMC Public Health 20: 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabla R, Gómez A, Simancas A, Rebollo JE, Molina F, Roa I. 2016. Enterobacteriaceae species during manufacturing and ripening of semi–hard and soft raw ewe’s milk cheese: gas production capacity. Small Rumin Res 145: 123–129. [DOI] [PubMed] [Google Scholar]
- 28.Guo X, Yu Z, Zhao F, Sun Z, Kwok LY, Li S. 2021. Both sampling seasonality and geographic origin contribute significantly to variations in raw milk microbiota, but sampling seasonality is the more determining factor. J Dairy Sci 104: 10609–10627. [DOI] [PubMed] [Google Scholar]
- 29.Kido Y, Maeno S, Tanno H, Kichise Y, Shiwa Y, Endo A. 2021. Niche-specific adaptation of Lactobacillus helveticus strains isolated from malt whisky and dairy fermentations. Microb Genom 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hviid AMM, Ruhdal-Jensen P, Kilstrup M. 2017. Butanol is cytotoxic to Lactococcus lactis while ethanol and hexanol are cytostatic. Microbiology (Reading) 163: 453–461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.