Abstract
Beer contains a variety of bioactive ingredients and trace elements that can regulate bodily functions, and moderate consumption of beer can enhance immune responses. This study aimed to investigate the potential benefits of moderate consumption of alcoholic or non-alcoholic beer on the gut microbiome, immunity, and intestinal barrier function in immunosuppressed BALB/c mice induced by cyclophosphamide (CTX). Model mice with CTX-induced immunosuppression were administered alcoholic or non-alcoholic beer or galacto-oligosaccharides (GOS) for 28 consecutive days. The GOS and beer intervention groups all showed alleviation of spleen tissue damage, an increased immune organ index, decreased gut inflammation, and reduced serum concentrations of D-lactic acid, lipopolysaccharide, and tumor necrosis factor α. High-throughput 16S rRNA gene sequencing revealed higher relative abundances of Firmicutes and Actinobacteriota, and lower relative abundances of Bacteroidota, Lactobacillus, and Bifidobacterium, in CTX mice than in normal control mice. In addition, Firmicutes showed lower abundance, while Desulfobacterota showed higher abundance in CTX mice with non-alcoholic beer intake than without it. Spearman correlation analysis indicated that Bacteroidota was negatively correlated with propionic acid and butyric acid, while Desulfobacterota was positively correlated with butyric acid. Proteobacteria was negatively correlated with acetic acid, propionic acid, isobutyric acid, and valeric acid. Helicobacter was positively correlated with valeric acid. In conclusion, this is one of the first studies to examine the modulatory effects of moderate alcohol consumption in immunocompromised mice. Our findings indicate that beer consumption can alter the gut microbiome and metabolites, enhancing immunity in mice.
Keywords: moderate drinking, alcoholic beer, non-alcoholic beer, immunosuppression, gut microbiome, cyclophosphamide
INTRODUCTION
Beer is one of the most commonly consumed fermented drinks worldwide [1, 2]. Heavy alcohol consumption, such as that in alcoholism, can suppress the immune function of the body, thereby weakening its ability to fight bacteria and viruses [3, 4]. Studies have shown that alcohol can disrupt the migration of lymphocytes to damaged areas, leading to dysfunctions in natural killer cells, T cells, B cells, monocytes, and macrophages and affecting the secretion of inflammatory factors [5, 6]. However, research on humans and experimental animals has shown that low-dose alcohol intake enhances the immune system [7]. There are different definitions of non-alcoholic beer in different regions. For example, in some regions of Europe, beer with an alcohol content below 0.5% is considered non-alcoholic beer, whereas in the UK, the alcohol concentration of non-alcoholic beer cannot exceed 0.05% [8]. Furthermore, such beverages may positively impact the immune system, not only because of the low alcohol content, but also because of the antioxidants and other beneficial substances [9].
Starting in the late 1950s, the public began to take an interest in research suggesting that moderate drinking could be beneficial to health [10]. However, multiple epidemiological studies subsequently demonstrated negative connections between moderate drinking and the incidence and mortality of cardiovascular disease [11]. Moderate drinking refers to alcohol consumption that does not pose health problems for the drinker or society in general [12]. According to the literature, moderate drinking equates to not more than one drink per day for women and two per day for men. A drink is defined as 1.5 ounces of 80-proof distilled spirits, 5 ounces of wine, or 12 ounces of normal beer [13]. This standard can also be defined as 14 units per week for women and 21 units of alcohol per week for men, with one unit being equivalent to 10 grams of alcohol [14]. However, moderate drinking benefits the immune system more than abstinence or excessive drinking [12, 15]. For example, moderate alcohol consumption can reduce the likelihood of common cold infection in individuals with rhinovirus, indicating that moderate drinking can enhance immune responses and provide a more effective defense against pathogens [16]. Furthermore, a study has shown that one important mechanism by which moderate drinking reduces inflammation involves inhibiting the inflammatory response of monocytes and upregulating the anti-inflammatory cytokine interleukin (IL)-10 [6]. An in vitro study found that moderate drinking may exert certain health benefits through modulation of cellular hormone levels and reduction of inflammatory markers in the immune system [17]. Drinking beer in moderation helps improve immune system performance and blood circulation and prevents atherosclerosis [2, 18]. Additionally, beer has demonstrated antioxidant and anti-aging effects that can help prevent the occurrence of cardiovascular disease [11, 19]. Moderate or low levels of drinking can improve immunity and return the compromised immune system of the human body return to normal [20].
A popular medication with significant promise for the treatment of cancer is cyclophosphamide (CTX) [21]. However, it has adverse consequences that include DNA damage, immune cell destruction, inhibition of B- and T-lymphocyte proliferation and differentiation, and immunosuppression [22, 23]. Our team’s preliminary studies in mice have shown that injection of CTX for 4 days can lead to spleen damage, thymus index decrease, immune function decline, intestinal microbiota imbalance, and intestinal barrier damage [24]. In addition, Tang et al. reported that intraperitoneal injection of CTX leads to dysbiosis of the intestinal microbiota in C57BL/6 mice [25]. In this study, we aimed to investigate the impacts of moderate consumption of two types of beer (alcoholic and non-alcoholic) on the immune function and gut microbiota in CTX-induced immunosuppressive mice.
MATERIALS AND METHODS
Animals and experimental design
Forty male BALB/c mice (age, 6−7 weeks; body weight, 22 ± 2 g; specific-pathogen-free grade) obtained from Dalian Medical University were randomly divided into the following five groups (n=8 per group) after acclimatization for 1 week: normal control (N), CTX-induced immunosuppressive model (M), galacto-oligosaccharides (GOS), alcoholic beer (P), and non-alcoholic beer (AFP).
The basic compositional components of the alcoholic and non-alcoholic beers are detailed in Table 1. In this study, the concentrations of alcohol in the alcoholic and non-alcoholic beers were 4.30% v/v and 0.01% v/v, respectively (Tsingtao Brewery Co., Ltd., Qingdao, China). GOS, which are functional oligosaccharides with natural prebiotic properties, exhibit a structure of 1 to 7 galactose units attached to lactose or glucose molecules. The GOS formulation used here was 90% pure, with 8% lactose and 2% glucose and galactose (Quantum Hi-Tech (Guangdong) Biological Co., Ltd., Jiangmen, China) [26, 27].
Table 1. Composition of the alcoholic and non-alcoholic beers.
| Composition | Alcoholic beer | Non-alcoholic beer | |||
|---|---|---|---|---|---|
| (mg/L) | (mg/L) | ||||
| Total polyphenols | 138.6 | 120.5 | |||
| Polyphenolic compounds | Phenolic acid | 4-Hydroxybenzoic acid | 4-Hydroxy-3-methoxy benzoic acid | 0.36 | 0.50 |
| Salicylic acid | 0.23 | 0.37 | |||
| Syringic acid | 0.62 | 0.43 | |||
| 4-Hydroxybenzoic acid | 0.60 | 0.84 | |||
| Gallic acid | <0.2 | <0.2 | |||
| Protocatechuic acid | 4.25 | 3.85 | |||
| P-Hydroxy-cinnamic acid | Ferulic acid | 1.78 | 1.62 | ||
| P-Coumaric acid | 0.90 | 0.98 | |||
| Sinapicacid | 1.07 | 0.99 | |||
| 3,4-Dihydroxycinnamic acid | 0.25 | 0.55 | |||
| 4-Hydroxyphenylacetic acid | <0.2 | 0.45 | |||
| Flavonoid | Chalcone | Xanthohumol | <0.2 | <0.2 | |
| Flavanol | Catechin | 1.37 | 2.33 | ||
| Epicatechin | 0.50 | 0.49 | |||
| Epigallocatechin (EGC) | <0.2 | <0.2 | |||
| Flavanone | Isoxanthohumol | <0.2 | <0.2 | ||
| Others | Epigallocatechin gallate (EGCG) | <0.2 | <0.2 | ||
| Epigallocatechin gallate (ECG) | <0.2 | <0.2 | |||
| Others | 4-Vinyl guaiacol | 4-VG | <0.2 | 0.26 | |
| Alkylphenol | 4-VP | <0.2 | <0.2 | ||
| Benzaldehyde | Vanillin | <0.2 | <0.2 | ||
| 4-Hydroxyphenylethanol | 9.73 | 11.14 | |||
During the 28-day experimental period, the mice were fed ad libitum with a standard diet and distilled water. For 28 days, the groups were administered the indicated liquids daily by gavage: 0.2 mL/day saline solution in the N and M groups; 8.33 g/kg/day GOS solution in the GOS group; 0.2 mL/day alcoholic beer in the P group; and 0.2 mL/day non-alcoholic beer in the AFP group. On the 24th day, mice in the M, GOS, P, and AFP groups were intraperitoneally injected with 60 mg/kg/day of CTX (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China) for 4 consecutive days. On the 28th day, all mice underwent carbon dioxide asphyxiation to obtain blood from the eyeballs and collect tissue samples. Blood samples were centrifuged at 3,000 rpm at 4°C for 15 min to obtain serum. Samples were immediately frozen in liquid nitrogen and then stored at −80°C. Body weight was measured and recorded weekly, with a final fasting body weight measurement on the final day of the experiment. Spleen weight was also measured. Organ indices were calculated using the following formula: Organ index (mg/g) = organ weight/body weight. All experiments were approved by the Committee for Animal Ethics of Dalian Medical University (AEE23076).
Histopathological staining
Fresh spleen, ileum, colon, and liver tissues were fixed in 4% neutral formaldehyde at room temperature overnight. The tissues were then rinsed with distilled water, dehydrated with ethanol, embedded in paraffin, and sliced (thickness, 3–5 μm). Staining was performed using hematoxylin-eosin (HE) and Alcian blue-periodic acid-Schiff (AB-PAS). Imaging was conducted at 100×, 200×, and 400× magnification using an optical microscope (Nikon Instruments Inc., Tochigi, Japan).
Cytokine and intestinal permeability assays
Using the enzyme-linked immunosorbent assay (ELISA) method (Jiangsu Enzyme-labeled Biotechnology Co., Ltd., Jiangsu, China), the serum concentrations of tumor necrosis factor α (TNF-α), lipopolysaccharide (LPS), and D-lactic acid (D-LA) were detected. Absorbance values measured at 450 nm using an ELISA reader were used to calculate the mass concentration.
Short-chain fatty acid (SCFA) detection in feces
Mouse feces were collected and weighed. Each sample of ~50 mg was submerged in water, shaken, and centrifuged, and the supernatant was collected. For the derivatization reaction, we sequentially added to the supernatant 200 mmol/L 3-nitrophenylhydrazine hydrochloride (NPH·HCL) solution, 120 mmol/L 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCL) solution, and 6% pyridine solution and mixed well. A mixture of 15% KOH solution and methanol was added to stop the derivatization reaction. The solution was acidified, the ether extracted, and the residue was dissolved with methanol. The obtained solution was filtered, and each sample was subjected to analysis on a U3000 HPLC (Thermo Fisher Scientific Inc., Shanghai, China).
High-throughput sequencing of 16S rRNA genes in the gut microbiota
Genomic DNA was extracted from the contents of the cecum using a fecal DNA isolation kit (Foregene, Chengdu, China), in accordance with the manufacturer’s instructions. PCR amplification of the V3–V4 hypervariable region of the 16S rRNA gene was carried using the universal primers 338F (5′-ACTCCTACGGGAGGCAGAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Polymerase chain reaction (PCR) products were purified and quantified. The data were optimized using the QIIME 2 platform followed by taxonomic analysis of the representative sequences using the USEARCH11-uparse algorithm. Raw data from the obtained bacterial community were compared with the SILVA v.138 16S rRNA database by clustering the sequences into amplicon sequence variants (ASVs) with a similarity of 97%. Alpha diversity of the samples was calculated using the Chao1 and Shannon indices, and the coverage index of the ASVs was analyzed. Beta diversity was calculated using the unweighted pair group method with arithmetic mean (UPGMA) clustering method to conduct principal component analysis (PCA) and principal coordinate analysis (PCoA).
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM). The results were compared using one-way analysis of variance (ANOVA) and a post hoc least significant difference test. A p-value <0.05 was considered to indicate statistical significance.
RESULTS
Effects of alcoholic and non-alcoholic beer on body weight and immune function in immunosuppressed mice
Before induction with CTX, the body weights of the mice in all five groups steadily increased, with no significant differences in among the groups. After injecting the M, GOS, P, and AFP groups with CTX on the 24th day, body weight was significantly decreased compared with the pre-injection weight in all mice (Fig. 1A and 1B). The spleen index was significantly lower in the M group compared with that in the N group (uninduced control) but was significantly higher in the GOS, P, and AFP intervention groups compared with that in the M group (p<0.05; Fig. 1D). In addition, based on the appearance of the spleen (Fig. 1C), the spleens were obviously smaller in the M group compared with those in the N group. The GOS intervention group showed a certain degree of spleen enlargement compared with the M group, but the difference was not visibly apparent. The spleens of the GOS, P, and AFP groups were similar in size. Furthermore, HE staining showed that the spleen follicles of the N group mice were normal in shape, with high numbers of closely arranged spleen cells. By contrast, the M group showed a disrupted spleen structure, with significant decreases in the numbers of spleen follicles and spleen cells, sparse and disordered arrangement, and chaotic medullary tissue. Compared with the M group, the spleen follicles in the GOS, P, and AFP groups showed increased numbers of closely arranged spleen cells and visible edges (Fig. 1E). Taken together, these findings indicated that intake of prebiotic GOS or beer, alcoholic or non-alcoholic, boosted immunological function in immunosuppressed mice.
Fig. 1.
Effect of alcoholic and non-alcoholic beer on body weight and immunity in immunosuppressed mice. (A) Body weight change. (B) Mouse weight was recorded both before and after receiving a cyclophosphamide injection. (C) Morphological observations of the spleen. (D) Spleen index. (E) H&E staining of spleen tissue with 100×, 200×, and 400× magnification. Data are presented as the mean ± SEM. #p<0.05 compared with the N group. ##p<0.01 compared with the N group. *p<0.05 compared with the M group. **p<0.01 compared with the M group. GOS: galacto-oligosaccharides; AFP: non-alcoholic beer; SEM: standard error of the mean.
Effects of alcoholic and non-alcoholic beer on inflammatory factors and intestinal permeability in immunosuppressed mice
The serum concentration of TNF-α was noticeably increased in the M group compared with that in the N group (p<0.05). After intervention in the CTX-immunosuppressed mice with alcoholic or non-alcoholic beer, the serum TNF-α concentration significantly decreased (p<0.05; Fig. 2A). Similarly, serum LPS and D-LA concentrations and intestinal permeability were significantly decreased in the GOS, P, and AFP intervention groups compared with those in the M group (p<0.05; Fig. 2B and 2C).
Fig. 2.
Effect of alcoholic and non-alcoholic beer on inflammatory cytokine and intestinal permeability in immunosuppressed mice. (A) Levels of TNF-α in serum. (B) Levels of LPS in serum. (C) Levels of D-LA in serum. Data are presented as the mean ± SEM. #p<0.05 compared with the N group. ##p<0.01 compared with the N group. *p<0.05 compared with the M group. **p<0.01 compared with the M group. GOS: galacto-oligosaccharides; AFP: non-alcoholic beer; TNF-α: tumor necrosis factor α; LPS: lipopolysaccharide; D-LA: D-lactic acid; SEM: standard error of the mean.
Effects of alcoholic and non-alcoholic beer on the structure of the small intestine, colon, and liver of immunosuppressed mice
In the N group, HE-stained intestinal tissue (Fig. 3A) showed clear structures and normal thickness of the mucosal layer, with well-arranged and closely packed intestinal villi. In contrast, the M group exhibited extensive intestinal damage, with thinning of the intestinal wall, shortened and sparse villi, villous detachment, and numerous inflammatory cells in the mucosa. Compared with the M group, the GOS, P, and AFP groups showed an increase in the lengths of intestinal villi, tendency for the mucosal epithelium to recover integrity, and regular and clear arrangement of villi, with less villous detachment.
Fig. 3.
Effect of alcoholic and non-alcoholic beer on the histopathological structures of immunosuppressed mice. (A) H&E staining of ileum tissue with 100× and 400× magnification. (B) H&E staining of colonic tissue with 100× and 400× magnification. (C) AB-PAS staining of colonic tissue with 100×, 200×, and 400× magnification. (D) H&E staining of liver tissue with 200× magnification. GOS: galacto-oligosaccharides; AFP: non-alcoholic beer.
HE staining of colonic tissues (Fig. 3B) in the N group showed intact colonic structure, whereas that in the M group showed structural damage, with a decreased number of goblet cells and a large number of infiltrated inflammatory cells. Intervention in the P, AFP, and GOS groups led to consistent recovery in the goblet cell count, a normal mucosal layer thickness, relatively normal colonic fold structure, and reductions in inflammatory cell infiltration.
AB-PAS staining of colonic tissues (Fig. 3C) showed that, compared with the N group, the M group had decreased expression of acidic mucins, increased secretion of glycogen and neutral mucins, and significant infiltration of inflammatory cells. Compared with the M group, the GOS, P, and AFP groups had increased levels of acidic mucins and notable decreases in inflammatory cell infiltration.
The HE staining results of liver tissues are shown in Fig. 3D. The liver tissue structure of the N group was intact, with hepatocytes that exhibited tight arrangement, normal nuclear and cytoplasmic morphology, and normal morphology overall. The liver tissue structure in the M group showed no significant abnormalities or inflammatory cell infiltration. Therefore, moderate intake of alcoholic or non-alcoholic beer did not appear to cause significant changes in the liver structures of the mice.
Effects of alcoholic and non-alcoholic beer on fecal short-chain fatty acids in immunosuppressed mice
In the M group, the concentrations of acetic acid, propionic acid, isobutyric acid, butyric acid, valeric acid, and total SCFAs were decreased compared with the N group. Intervention in the GOS, P, and AFP groups effectively increased the concentrations of acetic acid, propionic acid, isobutyric acid, butyric acid, valeric acid, and total SCFAs compared with those in the M group. Among the intervention groups, the GOS group showed comparatively more pronounced increases in acetic acid and isobutyric acid compared with the other two groups; on the other hand, the AFP group showed an increased concentration of isobutyric acid compared with the M group, and the P group showed an increased concentration of valeric acid compared with the M group (p<0.05; Fig. 4A). Spearman correlation analysis (Fig. 4B) revealed that butyric acid was negatively correlated with DL-A and TNF-α, while the total SCFA concentration was positively correlated with LPS and TNF-α.
Fig. 4.
Effect of alcoholic and non-alcoholic beer on short-chain fatty acids in the feces of immunosuppressed mice. (A) Content of short-chain fatty acids in feces. (B) Heatmap of correlation analysis of serum factors and short-chain fatty acids. Positive correlation is indicated by the color green; negative correlation is indicated by the color blue. Data are presented as the mean ± SEM. #p<0.05 compared with the N group. *p<0.05 compared with the M group. LPS: lipopolysaccharide; D-LA: D-lactic acid; SCFAs: short-chain fatty acids; TNF-α: tumor necrosis factor α; SEM: standard error of the mean.
Effects of alcoholic and non-alcoholic beer on the gut microbiome of immunosuppressed mice
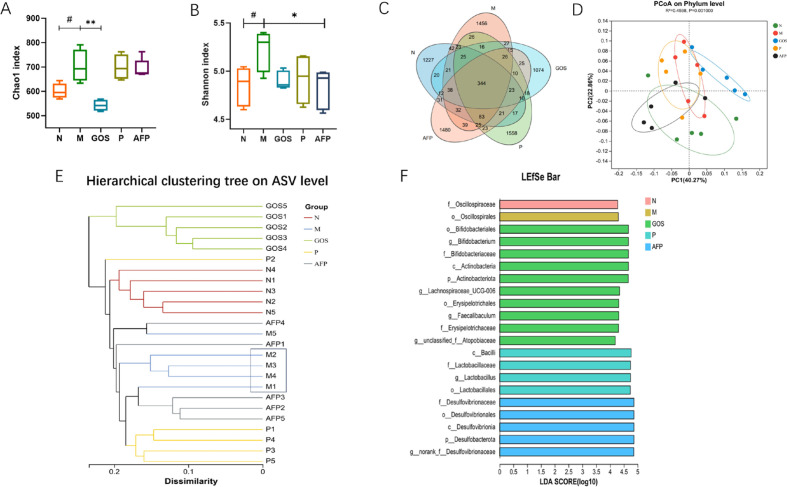
Our 16S rRNA gene sequencing data revealed notable alterations in the mouse gut microbiome between the N and M groups, with the M group showing more diversity and richness, as evidenced by enhancements in the Chao1 and Shannon α-diversity indices (Fig. 5A and 5B). Compared with the M group, microbial diversity and richness were generally lower in the GOS, P, and AFP groups, with a significantly lower Chao1 index in the GOS group (p<0.05) and a significantly lower Shannon index in the AFP group (p<0.05). For beta-diversity analysis, we performed PCoA at the phylum level and hierarchical clustering analysis. Additionally, we constructed a Venn diagram of the ratios of shared and unique ASVs, revealing a total of 344 ASVs shared among all five groups, with 1,227, 1,456, 1,074, 1,558, and 1,480 unique ASVs in the N, M, GOS, P, and AFP groups, respectively (Fig. 5C). The PCoA revealed that the gut microbiota of the N and GOS groups diverged, falling into two different categories at the phylum level, whereas those of the N, M, P, and AFP groups overlapped, indicating some similarity in the microbiota (Fig. 5D). The hierarchical clustering analysis (Fig. 5E) revealed distinct patterns between the M and N groups, forming two major clusters. The GOS group was closest to the N group, while the P and AFP groups were closer to the M group. Furthermore, substantial differences in the microbial communities were identified by linear discriminant analysis effect size (LEfSe) analysis of inter-group samples (Fig. 5F). The characteristic microbial taxa were Bifidobacteriales, Actinobacteria, and Erysipelotrichaceae in the GOS group, Bacilli and Lactobacillales in the P group, and Desulfovibrionales in the AFP group. Diversity analysis of the gut microbiota revealed that intake of alcoholic or non-alcoholic beer in immunosuppressed mice had differential impacts on the microbial communities and led to the formation of distinct microbiota compositions.
Fig. 5.
Effect of alcoholic and non-alcoholic beer on the gut microbiota of immunosuppressed mice. Alpha diversity indices of the gut microbiota: (A) Chao 1 index and (B) Shannon’s index. β-diversity analysis of intestinal microbiota: (C)Venn diagram and (D) principal co-ordinate analysis (PCoA) at the phylum level. (E) Microbial composition with clusters at the ASV level. (F) LDA distribution. Data are presented as the mean ± SEM. #p<0.05 compared with the N group. *p<0.05 compared with the M group. **p<0.01 compared with the M group. GOS: galacto-oligosaccharides; AFP: non-alcoholic beer; ASV: amplicon sequence variants.
Next, we conducted an analysis of the abundances of the top six microbial communities at the phylum level (Fig. 6A). The dominant phyla in these communities were Bacteroidota and Firmicutes, with a significance difference between the gut microbiota of the M group and those of the other four groups. Specifically, the M group showed much higher relative abundances of Firmicutes and Actinobacteriota and significantly lower relative abundances of Bacteroidota and Desulfobacterota, compared with the N group. Compared with the M group, the GOS group showed decreased and increased relative abundances of Firmicutes and Bacteroidota, respectively, while the AFP group showed a decreased relative abundance of Firmicutes and a noteworthy rise in Desulfobacterota (p<0.05; Fig. 6B).
Fig. 6.
Relative abundance (%) of major microbial communities at the phylum (A) and genus levels (C). (B) Phylum-level microbial distributions of distinct groups. (D) Genus-level microbial distributions of distinct groups. Data are presented as the mean ± SEM. #p<0.05 compared with the N group. ##p<0.01 compared with the N group. ###p<0.001 compared with the N group; *p<0.05 compared with the M group. **p<0.01 compared with the M group. ***p<0.001 compared with the M group. GOS: galacto-oligosaccharides; AFP: non-alcoholic beer; SEM: standard error of the mean.
To identify specific alterations in the gut microbiota, we evaluated the relative abundances of the top 28 microbial communities at the genus level (Fig. 6C and 6D). Compared with the N group, the M group showed significant decreases in the relative abundances of norank_f_Muribaculaceae, Lachnospiraceae_NK4A136_group, Lactobacillus, Bifidobacterium, and Bacteroides. The GOS, P, and AFP intervention groups showed higher relative abundances of norank_f_Muribaculaceae, Lachnospiraceae_NK4A136_group, and Lactobacillus compared with the M group. The GOS group exhibited significantly higher relative abundances of Bifidobacterium and Bacteroides, as well as Lachnospiraceae_NK4A136_group. There were no significant differences in the relative abundances of Bifidobacterium and Bacteroides between the P and AFP groups. The relative abundance of norank_f_Desulfovibrionaceae was significantly increased in the AFP group compared with that in the M group (p<0.05).
Correlation analyses among the gut microbiota, cytokines, and short-chain fatty acids
In the heatmap of the Spearman correlation analyses of serum immune parameters and the gut microbiota (Fig. 7A), orange indicates a positive correlation between a bacterial community and a parameter, while blue indicates a negative correlation. Firmicutes was positively correlated with DL-A and LPS and negatively correlated with TNF-α. In the case of Bacteroidota, DL-A and LPS were negatively correlated, while TNF-α was positively correlated. LPS and TNF-α was negatively correlated with Campilobacterota but positively correlated with Proteobacteria. TNF-α was positively correlated with Bacteroides, while DL-A was positively correlated with norank_f_Lachnospiraceae and negatively correlated with Faecalibaculum and Lachnoclostridium.
Fig. 7.
Analysis of the correlation between gut microbiota, serum cytokines, and short-chain fatty acids. (A) Heatmap of the correlation analysis of gut microbiota and serum cytokines. (B) Heatmap of the correlation analysis of gut microbiota and short-chain fatty acids. Data are presented as the mean ± SEM. *p<0.05 compared with the M group. **p<0.01 compared with the M group. LPS: lipopolysaccharide; D-LA: D-lactic acid; TNF-α: tumor necrosis factor α.
Figure 7B presents the Spearman correlation analysis of relationships among gut microbial communities and SCFAs. Positive correlations are indicated in pink, and negative correlations are indicated in green. We identified negative correlations between Bacteroidota and propionic acid as well as butyric acid, and a positive correlation between Desulfobacterota and butyric acid. Acetic acid, propionic acid, isobutyric acid, valeric acid, and total SCFAs were negatively correlated with Proteobacteria and Patescibacteria. There were also positive correlations between norank_f_Desulfovibrionaceae and butyric acid, Bifidobacterium and total SCFAs, and Helicobacter and valeric acid. Finally, acetic acid and total SCFAs were both negatively correlated with Lachnospiraceae_NK4A136_group and norank_f_Lachnospiraceae.
DISCUSSION
The body’s defense against infectious diseases and tumor cells is mostly provided by the immune system, a complex network of molecules, cells, tissues, and organs [28, 29]. The immune systems of individuals who consume alcohol are influenced by various factors, including age, race, sex, physique, environment, drinking duration, drinking frequency, and type of alcohol consumed [30]. Alcohol abuse is known to cause immune system dysfunction, leading to decreases in both humoral and cellular immune responses [31, 32]. However, there is also evidence to suggest that moderate alcohol consumption is beneficial to physical health [7, 12, 18, 33]. One study revealed that healthy adults who consumed moderate amounts of beer (330 mL for women, 660 mL for men) experienced within 30 days a significant increase in CD3+ T cell count and enhanced cellular production of factors such as IL-2, IL-4, IL-10, and interferon (IFN)-γ, with no change in IL-6. These results indicate that moderate alcohol consumption has immunomodulatory effects [15]. There is ample evidence that alcoholic beverages rich in polyphenols, such as wine and beer, contain components that can prevent immune system suppression and trigger protective effects [12, 34]. Beneficial components in beer, such as polyphenols, act as prebiotics and thus participate in immune regulation mechanisms [33]. Non-alcoholic beer also contains polyphenols, which have antioxidant, anti-disease, and anti-inflammatory effects [10]. This study used CTX to construct an immunosuppressive mouse model, inducing significant decreases in body weight and spleen index. Our findings led to the discovery that intake of alcoholic or non-alcoholic beer reversed the immunosuppressive impact of CTX by considerably increasing the spleen and thymus indices. Previous studies have shown that alcohol consumption impacts both natural and acquired immunity in humans and animals, but there has been no systematic evaluation of the amount and duration of alcohol consumption associated with such effects [6, 35]. In this study, we found that moderate consumption of non-alcoholic or alcoholic beer for 28 days improved immunity in immunosuppressed mice.
The main components of beer are water, malt, hops, carbohydrates, and polyphenols (derived from the malt and hops). The alcohol content depends on the type of beer [32]. When alcohol intake is controlled at a healthy level, the nutritional components in beer can reduce biomarkers of white blood cell adhesion and inflammation and can increase plasma antioxidant capacity [36]. Wu et al. claimed that hop extract reduced the levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α [37]. Research has demonstrated that drinking beer in moderation can raise the levels of immunoglobulin (Ig)G, IgM, and IgA, as well as the production of IL-2, IL-4, IL-10, and IFN-γ, suggesting that moderate alcohol consumption has certain immune regulatory functions in the body [12]. Additionally, moderate drinking regulates cardiovascular function by reducing the activity of TNF-α [11]. Almost all immune system cells, as well as many other cell types, produce TNF-α, triggering the release of numerous immune regulatory and inflammatory mediators [38]. In this study, we discovered that moderate drinking reduces the release of TNF-α and improves inflammatory infiltration in the spleen. In other words, moderate alcohol consumption can enhance the immune response, thus improving the defense against pathogens.
The intestinal barrier, an essential immunological organ, protects against dangerous macromolecules and foreign infections [39]. However, exposure to external stimuli can damage the structure and barrier of intestinal tissues, leading to an increase in intestinal permeability [40]. Chemotherapeutic medications typically cause damage to the intestinal wall and interfere with the tight junctions that connect intestinal epithelial cells [41]. In this study, CTX induction was found to increase the concentrations of LPS and D-LA in mice. Moderate alcohol consumption reduced the concentrations of LPS and D-LA in these immunosuppressed mice, restoring the integrity of the mucosal epithelium and repairing defects in the villi. While it is known that excessive alcohol consumption and obesity can lead to fat accumulation in liver tissue, the effects of moderate alcohol consumption on the liver remain controversial [42]. Most of the water, vitamins, and other low-molecular-weight substances in beer are absorbed in the small intestine [20]. The gut microbiome and intestinal barrier work in synergy to protect the body against diseases and activate the immune system [39].
There are a variety of research perspectives on the effects of ingredients in beer, with or without alcohol, on the gut microbiome [33]. In the small intestine, the amino acids, vitamins, minerals, and low-molecular-weight carbohydrates found in beer are quickly absorbed [43, 44]. Dysbiosis of the gut microbiota affects the distribution of dominant bacterial groups, such as Bacteroidetes, Firmicutes, and Proteobacteria [2]. Moderate beer consumption influences the gut microbiome, with researchers discovering a positive relationship between the polyphenols in beer and the gut microbiome that is partially attributable to the bioactivity of polyphenols and phenolic acids [33, 45]. In a study by Hernández-Quiroz et al., healthy volunteers consumed 355 mL of alcoholic or non-alcoholic beer every day for 30 consecutive days. The study observed an increase in Bacteroidetes relative to Firmicutes, with no significant change in alpha diversity of the microbial community, indicating a positive impact on the gut microbiota and human health [45]. This is consistent with the results of the present study in mice, in which the abundance of Firmicutes decreased while that of Bacteroidetes increased after 28 days of gavage administration of beer, with or without alcohol. In this study, beer (alcoholic or non-alcoholic) intake did not increase the diversity of the microbiota. However, notable variations in the relative abundances of specific taxa were noted for both types of beer. Both non-alcoholic and low-alcohol forms of beer, consumed in moderation, greatly enhance the intake of soluble fibers, many of which have prebiotic effects [12]. Polyphenolic compounds and their metabolites interact with and are transformed by the gut microbiome, regulating biological activity. The gut microbiome is also influenced by polyphenolic compounds [46]. Following polyphenol intake, the production of SCFAs increases, thereby inducing local anti-inflammatory effects [47]. In animal models, abundant polyphenols in beer, such as ferulic acid, can increase the richness and diversity of bacteria and stimulate the growth of strains that produce butyrate and propionate [48]. Beer can regulate the level of butyric acid, which directly interacts with the host immune system, reducing the levels of inflammation markers [49]. This study has shown that moderate beer consumption increases butyrate levels in mice. In terms of the gut microbiome structure, the addition of red wine polyphenols to the diet has been shown to alter the gut microbiome structure in mice, leading to the dominance of Bacteroidetes, Lactobacillus, and Bifidobacterium [13, 50]. Additionally, Faecalibacterium utilizes lactate produced by Bifidobacterium to generate butyrate [51]. An increase in Desulfobacterota was shown to be positively correlated with increased butyrate levels, whereas an increase in Lachnospiraceae_NK4A136_group and Bacteroidota was negatively correlated with increased butyrate levels. The mechanisms underlying the effects of alcohol consumption, alcoholic and non-alcoholic beer, immune function, and beer components on the gut microbiome are not fully understood.
The results of this study showed that moderate intake of alcoholic or non-alcoholic beer improved immune suppression, restored intestinal barrier structure and function, effectively regulated changes in gut microorganisms and their metabolic products, and did not damage the mouse liver. Furthermore, our investigation of the correlations among cytokines, SCFAs, and the structure of the gut microbiome revealed that beer can regulate the gut microbiome to exert immunomodulatory functions. However, further comprehensive research is needed on the immunomodulatory mechanism underlying the interaction between beer and the gut microbiome.
AUTHOR CONTRIBUTIONS
Shumin Hu: writing – original draft, methodology. Hua Yin: conceptualization. Xiaxia Li: conducted the experiments, writing – review & editing, designed the experiments. Minghao Fan: data curation. Huajun Li: supervision.
DATA AVAILABILITY
Data will be made available on request.
FUNDING
This work was supported by the Open Research Fund of the State Key Laboratory of Biological Fermentation Engineering of Beer.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this article.
REFERENCES
- 1.Meister KA, Whelan EM, Kava R. 2000. The health effects of moderate alcohol intake in humans: an epidemiologic review. Crit Rev Clin Lab Sci 37: 261–296. [DOI] [PubMed] [Google Scholar]
- 2.Osorio-Paz I, Brunauer R, Alavez S. 2020. Beer and its non-alcoholic compounds in health and disease. Crit Rev Food Sci Nutr 60: 3492–3505. [DOI] [PubMed] [Google Scholar]
- 3.MacGregor RR. 1986. Alcohol and immune defense. JAMA 256: 1474–1479. [PubMed] [Google Scholar]
- 4.Barr T, Helms C, Grant K, Messaoudi I. 2016. Opposing effects of alcohol on the immune system. Prog Neuropsychopharmacol Biol Psychiatry 65: 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muralidharan S, Ambade A, Fulham MA, Deshpande J, Catalano D, Mandrekar P. 2014. Moderate alcohol induces stress proteins HSF1 and hsp70 and inhibits proinflammatory cytokines resulting in endotoxin tolerance. J Immunol 193: 1975–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romeo J, Wärnberg J, Marcos A. 2010. Drinking pattern and socio-cultural aspects on immune response: an overview. Proc Nutr Soc 69: 341–346. [DOI] [PubMed] [Google Scholar]
- 7.Nova E, Baccan GC, Veses A, Zapatera B, Marcos A. 2012. Potential health benefits of moderate alcohol consumption: current perspectives in research. Proc Nutr Soc 71: 307–315. [DOI] [PubMed] [Google Scholar]
- 8.Okaru AO, Lachenmeier DW. 2022. Defining No and Low (NoLo) alcohol products. Nutrients 14: 3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendenhall CL, Theus SA, Roselle GA, Grossman CJ, Rouster SD. 1997. Biphasic in vivo immune function after low- versus high-dose alcohol consumption. Alcohol 14: 255–260. [DOI] [PubMed] [Google Scholar]
- 10.Rehm J, Rovira P, Manthey J, Anderson P. 2023. Reduction of alcoholic strength: does it matter for public health? Nutrients 15: 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oppenheimer GM, Bayer R. 2020. Is moderate drinking protective against heart disease? The science, politics and history of a public health conundrum. Milbank Q 98: 39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton R, Sheron N. 2022. Complex relationship between health and moderate alcohol use. Lancet 400: 141–143. [DOI] [PubMed] [Google Scholar]
- 13.Watzl B, Bub A, Pretzer G, Roser S, Barth SW, Rechkemmer G. 2004. Daily moderate amounts of red wine or alcohol have no effect on the immune system of healthy men. Eur J Clin Nutr 58: 40–45. [DOI] [PubMed] [Google Scholar]
- 14.Dufour MC. 1999. What is moderate drinking? Defining “drinks” and drinking levels. Alcohol Res Health 23: 5–14. [PMC free article] [PubMed] [Google Scholar]
- 15.Romeo J, Wärnberg J, Nova E, Díaz LE, Gómez-Martinez S, Marcos A. 2007. Moderate alcohol consumption and the immune system: a review. Br J Nutr 98 Suppl 1: S111–S115. [DOI] [PubMed] [Google Scholar]
- 16.Díaz LE, Montero A, González-Gross M, Vallejo AI, Romeo J, Marcos A. 2002. Influence of alcohol consumption on immunological status: a review. Eur J Clin Nutr 56 Suppl 3: S50–S53. [DOI] [PubMed] [Google Scholar]
- 17.Feng Y, Sun H, Zhu R, Tao J, Su R, Sun Y, Wang D. 2023. Effects of alcohol on the symptoms of gouty arthritis and taxonomic structure of gut microbiota in C57BL/6 mice. Front Microbiol 14: 1257701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trius-Soler M, Martínez-Carrasco P, Tresserra-Rimbau A, Moreno JJ, Estruch R, Lamuela-Raventós RM. 2023. Effect of moderate beer consumption (with and without ethanol) on cardiovascular health in postmenopausal women. J Sci Food Agric 103: 7506–7516. [DOI] [PubMed] [Google Scholar]
- 19.Cecarini V, Gogoi O, Bonfili L, Veneruso I, Pacinelli G, De Carlo S, Benvenuti F, D’Argenio V, Angeletti M, Cannella N, et al. 2022. Modulation of gut microbiota and neuroprotective effect of a yeast-enriched beer. Nutrients 14: 2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Jin S, Zhang C, Hu S, Li H. 2023. Beer-gut microbiome alliance: a discussion of beer-mediated immunomodulation via the gut microbiome. Front Nutr 10: 1186927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houston S. 2022. Immunosuppression. Nat Immunol 23: 1520–1520. [DOI] [PubMed] [Google Scholar]
- 22.Haskell CM. 1977. Immunologic aspects of cancer chemotherapy. Annu Rev Pharmacol Toxicol 17: 179–195. [DOI] [PubMed] [Google Scholar]
- 23.Ehrke MJ. 2003. Immunomodulation in cancer therapeutics. Int Immunopharmacol 3: 1105–1119. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Huang J, Li Y, Wang Y, Wang F, Qiu X, Liu X, Li H. 2021. Sodium alginate modulates immunity, intestinal mucosal barrier function, and gut microbiota in cyclophosphamide-induced immunosuppressed BALB/c Mice. J Agric Food Chem 69: 7064–7073. [DOI] [PubMed] [Google Scholar]
- 25.Tang Y, Pu Q, Zhao Q, Zhou Y, Jiang X, Han T. 2022. Effects of fucoidan isolated from Laminaria japonica on immune response and gut microbiota in cyclophosphamide-treated mice. Front Immunol 13: 916618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Huang J, Zhang S, Yang F, Zhou H, Song Y, Wang B, Li H. 2022. Sodium alginate and galactooligosaccharides ameliorate metabolic disorders and alter the composition of the gut microbiota in mice with high-fat diet-induced obesity. Int J Biol Macromol 215: 113–122. [DOI] [PubMed] [Google Scholar]
- 27.van Leeuwen SS, Kuipers BJH, Dijkhuizen L, Kamerling JP. 2016. Comparative structural characterization of 7 commercial galacto-oligosaccharide (GOS) products. Carbohydr Res 425: 48–58. [DOI] [PubMed] [Google Scholar]
- 28.Donald K, Finlay BB. 2023. Early-life interactions between the microbiota and immune system: impact on immune system development and atopic disease. Nat Rev Immunol 23: 735–748. [DOI] [PubMed] [Google Scholar]
- 29.Turk JL, Parker D. 1982. Effect of cyclophosphamide on immunological control mechanisms. Immunol Rev 65: 99–113. [DOI] [PubMed] [Google Scholar]
- 30.Marques C, Dinis L, Barreiros Mota I, Morais J, Ismael S, Pereira-Leal JB, Cardoso J, Ribeiro P, Beato H, Resende M, et al. 2022. Impact of beer and nonalcoholic beer consumption on the gut microbiota: a randomized, double-blind, controlled trial. J Agric Food Chem 70: 13062–13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avitabile E, Díaz A, Montironi C, Pérez-Guasch M, Gratacós-Ginès J, Hernández-Évole H, Moreira RK, Sehrawat TS, Malhi H, Olivas P, et al. 2023. Adding inflammatory markers and refining national institute on alcohol abuse and alcoholism criteria improve diagnostic accuracy for alcohol-associated hepatitis. Clin Gastroenterol Hepatol 21: 3080–3088.e9. [DOI] [PubMed] [Google Scholar]
- 32.de Gaetano G, Costanzo S, Di Castelnuovo A, Badimon L, Bejko D, Alkerwi A, Chiva-Blanch G, Estruch R, La Vecchia C, Panico S, et al. 2016. Effects of moderate beer consumption on health and disease: a consensus document. Nutr Metab Cardiovasc Dis 26: 443–467. [DOI] [PubMed] [Google Scholar]
- 33.Quesada-Molina M, Muñoz-Garach A, Tinahones FJ, Moreno-Indias I. 2019. A new perspective on the health benefits of moderate beer consumption: involvement of the gut microbiota. Metabolites 9: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kivimäki M, Batty GD. 2022. Alcohol and health. Lancet 400: 1763–1764. [DOI] [PubMed] [Google Scholar]
- 35.Gano A, Pautassi RM, Doremus-Fitzwater TL, Deak T. 2017. Conditioned effects of ethanol on the immune system. Exp Biol Med (Maywood) 242: 718–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arranz S, Chiva-Blanch G, Valderas-Martínez P, Medina-Remón A, Lamuela-Raventós RM, Estruch R. 2012. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 4: 759–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu CN, Sun LC, Chu YL, Yu RC, Hsieh CW, Hsu HY, Hsu FC, Cheng KC. 2020. Bioactive compounds with anti-oxidative and anti-inflammatory activities of hop extracts. Food Chem 330: 127244. [DOI] [PubMed] [Google Scholar]
- 38.Nakayama T. 2016. An inflammatory response is essential for the development of adaptive immunity-immunogenicity and immunotoxicity. Vaccine 34: 5815–5818. [DOI] [PubMed] [Google Scholar]
- 39.Sylvestre M, Di Carlo SE, Peduto L. 2023. Stromal regulation of the intestinal barrier. Mucosal Immunol 16: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gustafsson JK, Johansson MEV. 2022. The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol 19: 785–803. [DOI] [PubMed] [Google Scholar]
- 41.Ahlmann M, Hempel G. 2016. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol 78: 661–671. [DOI] [PubMed] [Google Scholar]
- 42.Oh H, Sohn W, Cho YK. 2023. The effects of moderate alcohol consumption on non-alcoholic fatty liver disease. Clin Mol Hepatol 29 Suppl: S261–S267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbería-Latasa M, Gea A, Martínez-González MA. 2022. Alcohol, drinking pattern, and chronic disease. Nutrients 14: 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zugravu CA, Medar C, Manolescu LSC, Constantin C. 2023. Beer and microbiota: pathways for a positive and healthy interaction. Nutrients 15: 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, Murugesan S, Cruz-Narváez Y, Rico-Arzate E, Hoyo-Vadillo C, Chavez-Carbajal A, Pizano-Zárate ML, García-Mena J. 2020. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and β-cell function. Alcohol 85: 77–94. [DOI] [PubMed] [Google Scholar]
- 46.Ozdal T, Sela DA, Xiao J, Boyacioglu D, Chen F, Capanoglu E. 2016. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 8: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. 2013. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem 24: 1415–1422. [DOI] [PubMed] [Google Scholar]
- 48.Ou JY, Huang JQ, Song Y, Yao SW, Peng XC, Wang MF, Ou SY. 2016. Feruloylated oligosaccharides from maize bran modulated the gut microbiota in rats. Plant Foods Hum Nutr 71: 123–128. [DOI] [PubMed] [Google Scholar]
- 49.González-Zancada N, Redondo-Useros N, Díaz LE, Gómez-Martínez S, Marcos A, Nova E. 2020. Association of moderate beer consumption with the gut microbiota and SCFA of healthy adults. Molecules 25: 4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriquez-Saavedra M, Tamargo A, Molinero N, Relaño de la Guía E, Jiménez-Arroyo C, Bartolomé B, González de Llano D, Victoria Moreno-Arribas M. 2023. Simulated gastrointestinal digestion of beer using the simgi® model. Investigation of colonic phenolic metabolism and impact on human gut microbiota. Food Res Int 173: 113228. [DOI] [PubMed] [Google Scholar]
- 51.Yao Y, Cai X, Fei W, Ye Y, Zhao M, Zheng C. 2022. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr 62: 1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.