Abstract
Probiotics exert their beneficial effects by improving the intestinal environment. Heat-inactivated probiotics may show similar effects. However, whether multi-strain mixtures (MSM) are better than single strains, irrespective of whether the bacteria are alive or dead, is unknown. In this study, we examined the gut-improving efficacy of an MSM consisting of four dead bacterial strains using an in vitro model that simulates the human gut environment. In the in vitro model, human feces were inoculated with a single-strain or MSM, and the microbial composition and fermentation products were assessed. The 16S rRNA gene sequences showed that the MSM tended to change the microbial community structure more than single strains. Furthermore, the results of a microbial diversity analysis showed that despite differences among single strains, the effect of the MSM increased with the abundance of any single strain. A similar trend was observed for fermentation products. These results suggested that the MSM made from dead bacteria exerted additive effects that may provide new health benefits to more people.
Keywords: probiotics, dead bacteria, gut microbiota, multi-strain mixtures, additive effect
INTRODUCTION
According to the guidelines of the Food and Agriculture Organization, probiotics are “live microorganisms that, when administered in adequate quantities, confer health benefits on the host” [1]. Upon consumption, probiotics improve the microbial composition of the gut and interact with various immune cells, thereby enhancing immune function [2,3,4]. The health-promoting and immunomodulatory effects of probiotics have been widely accepted [5, 6]. Notably, probiotics may show beneficial effects even in their heat-inactivated form [7]. The immunomodulatory effects of dead bacteria have been reported for many bacterial genera and species, including Enterococcus faecalis EC-12 [8], Lacticaseibacillus paracasei MCC1849 [9], Lactococcus lactis JCM 5805 [10], Lactiplantibacillus pentosus S-PT84 [11], and Lactiplantibacillus plantarum L-137 [12]. Heat-killed probiotic bacteria are also effective at maintaining barrier integrity [13, 14]. Furthermore, the effects of heat-inactivated bacteria are similar to the gut-improving effects of probiotics [15, 16]; however, the mechanisms via which heat-inactivated bacteria affect the microbial composition and their metabolites remain unclear.
Whether probiotics comprising single strains are better than multi-strain mixtures (MSMs) is currently under investigation. The possible advantages of MSMs include the synergistic effects of the different strains in the mixtures [17, 18]. The mechanisms of action of different strains may vary, because of which MSMs may provide a wider range of effects [19, 20]. The additive, synergistic, and symbiotic effects of different strains with specific properties have been reported for multispecies probiotics [18]. Notably, symbiotic-enhanced immunomodulatory activity has been detected in a heat-killed MSM of lactic acid bacteria [21]. However, the same strain has not always been used in mixtures when comparing the effects of single strains and MSMs, and thus, the conclusions of the previous studies are potentially based on biased data [22]. Hence, whether synergistic or antagonistic effects have been assessed in MSMs remains unclear. To clarify this, single strains should be compared with MSMs containing the same single strain at similar doses. A potential issue with this approach is that the number of strains to be compared increases in proportion to the number of strains included in the MSM.
Human intervention testing is widely accepted as a highly accurate method for verifying the functions of lactic acid bacteria. However, implementation of such tests is difficult considering the expense and ethical restrictions. Therefore, when evaluating an MSM with a large number of comparators, pretesting is required to avoid unnecessary human intervention studies. In vitro models can be used to mimic the microbial processes of the human gut and predict the results of in vivo studies [23]. The Kobe University Human Intestinal Microbiota Model in Bottle (KUHIMMiB) [24] is a miniaturized in vitro human colonic microbiota model that can accurately imitate the human gut environment [25, 26]. This model uses vials under anaerobic conditions for culture, and the number of cells to be cultured simultaneously can be adjusted according to the number of flasks prepared.
This study aimed to use the KUHIMMiB to examine the gut-improving efficacy of a general MSM comprising four dead bacterial strains. To accurately assess the synergistic and antagonistic effects of the MSM, the MSM was prepared by mixing equal amounts of each single strain, the gut-improving efficacies of which were unknown, and adding equal amounts of the MSM and each single strain to the KUHIMMiB with feces. The findings of this study are expected to improve our understanding regarding the effects of an MSM comprising dead bacterial strains and ensure their correct functional examination in the future.
MATERIALS AND METHODS
Preparation of heat-killed bacterial strains
The bacterial strains used in this study were Lactiplantibacillus plantarum LOC1, Bifidobacterium lactis Bb12, Streptococcus thermophilus ST9618, and Lactobacillus delbrueckii subsp. bulgaricus LB9667. All bacterial strains were cultured in de Man-Rogosa-Sharpe broth (Oxoid, Basingstoke, UK) at 37°C for 24 hr under anaerobic conditions using an AnaeroPack system (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan). Aliquots of 10 µL suspension were inoculated in 10 mL de Man-Rogosa-Sharpe broth and cultured at 37°C for 12 hr under anaerobic conditions. The cells were collected via centrifugation at 2,000 × g for 30 min at 4°C, washed three times with phosphate-buffered saline (PBS), resuspended in 10 mL PBS, and heated at 121°C for 15 min to kill them. Direct microscopic counting of the cells was performed in a Helber bacteria counting chamber (System Helber, Saaringia, Germany) using a phase-contrast light microscope (400×), following which the cells were diluted in PBS to obtain a bacterial count of 109 cells/mL. Each of the four strains suspended in PBS was mixed in equal proportions for the MSM. The heat-killed bacterial strains were stored at 4°C until further use.
Preparation of human fecal inoculum
Fecal samples were collected from three healthy human volunteers (designated donors 1–3) ranging in age from 30 to 36 years. The donors had not been treated with antibiotics within, at least, the 3 weeks prior to collection. After collection, the fecal samples from the three donors were immediately placed under anaerobic conditions using an AnaeroPack system (Mitsubishi Gas Chemical Co.), diluted 10-fold with PreserWell (MPR, Miyagi, Japan) within 3 hr, and stored at −80°C until use. This study was approved by the Ethics Review Committee of the Graduate School of Agricultural Science, Kobe University (No. 202104-1; notification date, July 7, 2021). Written informed consent was obtained from each healthy donor.
In vitro fermentation
The KUHIMMiB was used as described previously [24]. Briefly, 20 mL semi-solid Gifu anaerobic medium without dextrose (Nissui Pharmaceutical Co., Tokyo, Japan), the agar of which had been removed using a filter paper (Grade 2, GL Sciences, Tokyo, Japan), was added to each 50 mL vial (Nichiden-Rika Glass, Hyogo, Japan). Each vial was closed with a butyl rubber stopper (Nichiden-Rika Glass) and an open-top crimp cap (GL Sciences) and tightly sealed with a hand crimper (Osaka Chemical, Osaka, Japan). After degassing for 10 min using a vacuum pump (Ulvac Inc., Kanagawa, Japan), the medium was aerated three times using a mixture of N2 (80%), CO2 (18%), and H2 (2%) at 0.1 MPa for 15 sec to render the inside of the vial anaerobic. The medium was then sterilized in an autoclave at 115°C for 15 min. To initiate fermentation, 50 µL of the supernatant of thawed cryopreserved fecal inoculum centrifuged at 60 × g for 1 min was inoculated into the vial through a butyl rubber stopper using an injection syringe and a syringe needle. Immediately thereafter, 100 µL (109 cells/mL) of each of the four strains or the MSM (the cell concentration of each strain was 2.5 × 108 cells/mL) was inoculated in the same manner and then incubated at 37°C. After 30 hr, aliquots of the fermentation cultures were collected and stored at −20°C for future use.
DNA extraction
DNA was extracted according to the procedure described by Marmur [27]. Briefly, 200 µL culture medium was added to a screw-capped, free-standing microtube containing 300 mg glass beads (0.1 mm diameter), following which 500 µL TE (10 mM Tris-HCl, 1 mM ethylenediaminetetraacetic acid (EDTA), pH 8.0)-saturated phenol, 250 µL TE buffer, and 50 µL 10% sodium dodecyl sulfate were added to the microtube and mixed. The mixed solution was vortexed for 30 sec using a Fast Prep-24 instrument (MP Biomedicals SARL, Illkirch, France) and centrifuged at 17,120 × g for 5 min. The supernatant was extracted using 400 µL phenol-chloroform-isoamyl alcohol (25:24:1) mixture and again centrifuged at 17,120 × g for 5 min. Finally, the supernatant (250 µL) was collected in microtubes, mixed with 275 µL isopropanol and 25 µL 3 M sodium acetate, and allowed to stand at −20°C for 10–15 min. The extracted DNA precipitates were collected via centrifugation at 17,120 × g for 5 min, washed with 70% ethanol, and air-dried for 10–30 min. The extracted DNA was dissolved in TE buffer and stored at −20°C for future use.
Quantitative PCR
Quantitative polymerase chain reaction (qPCR) was performed in sealed 96-well microplates using a QuantStudio™ 5 system (Applied Biosystems Inc., Foster City, CA, USA) in a total volume of 20 µL, which contained 2 µL DNA, 10 µL SYBR Premix Ex Taq (Takara Bio Inc., Shiga, Japan), 0.4 µL of each primer (10 µM), 0.4 µL ROX reference dye, and sterile water. The primer sequences used to target the bacterial 16S rRNA gene and the conditions for PCR amplification are described in Table 1 [28,29,30]. The microorganisms used to generate standard curves for relating microbial counts to cycle threshold values for each of the microbial groups analyzed were grown overnight under anaerobic conditions in a Gifu anaerobic medium (Nissui Pharmaceutical). The standard strains were obtained from different culture collections. The number of bacteria in the feces or culture medium was quantified using the Crossing Point method. For this method, the cycle threshold fluorescence value was obtained from the intersection of the amplification curve, and the threshold value of a calibration curve was obtained using 102–107 copies of the PCR fragments of the 16S rRNA gene of a standard strain of each target bacterium. The number of bacteria per sample (culture medium, 1 mL; feces, 1 g) was obtained by converting the sample volume (culture medium, 100 ×; feces, 1,000 ×) to the number of bacteria.
Table 1. Primer sequences and conditions used in quantitative polymerase chain reaction (PCR).
| Target organism | Standard strain | Primer sequence (5′-3′) | PCR product siza (bp) | 3-step amplification | Reference | ||
|---|---|---|---|---|---|---|---|
| Denaturation | Annealing | Elongation | |||||
| Prevotella spp. | Faecalibacterium prausnitzii | F: CACRGTAAACGATGGATGCC | 527–529 | 94℃ for 20 s | 55℃ for 20 s | 72℃ for 30 s | [28] |
| JCM31915 | R: GGTCGGGTTGCAGACC | ||||||
| Lactobacillus spp. | Lactobacillus plantarum | F: AGCAGTAGGGAATCTTCCA | 341 | 95℃ for 15 s | 58℃ for 20 s | 72℃ for 30 s | [29] |
| 22A-3 | R: CACCGCTACACATGGAG | ||||||
| Bifidobacterium spp. | Bifidobacterium | F: TCGCGTC(C/T)GGTGTGAAAG | 549–563 | 95℃ for 15 s | 58℃ for 20 s | 72℃ for 30 s | [29] |
| longumJCM1217 | R: CCACATCCAGC(A/G)TCCAC | ||||||
| Fusobacterium spp | Fusobacterium nucleatum | F: CCCTTCAGTGCCGCAGT | 248 | 95℃ for 15 s | 61℃ for 15 s | 72℃ for 30 s | [29] |
| JCM8532 | R: GTCGCAGGATGTCAAGAC | ||||||
| Bacteroides spp. | Bacteroides thetaiotaomicron | F: GGTGTCGGCTTAAGTGCCAT | 495 | 95℃ for 15 s | 68℃ for 20 s | 72℃ for 30 s | [29] |
| DSMZ 2079 | R: CGGATGTAAGGGCCGTGC | ||||||
| Faecalibacterium | Faecalibacterium prausnitzii | F: GGAGGAAGAAGGTCTTCGG | 248 | 95℃ for 15 s | 60℃ for 15 s | 72℃ for 30 s | [30] |
| prausnitzii | DSMZ 17677 | R: AATTCCGCCTACCTCTGCACT | |||||
16S rRNA library preparation and sequencing
Genomic DNA was isolated from feces or culture medium using a DNeasy PowerSoil kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s protocol. The isolated DNA was assessed using NanoDrop and Qubit systems (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Genomic DNA was subjected to 16S rRNA analysis, and read counts were calculated. The V3–V4 region of the bacterial 16S rRNA gene was amplified using the S-D-Bact-0341-b-’-17 (5′-CCTACGGGNGGC’GCAG-3′) and S-D-Bact-0785-a-’-21 (5′-GACTACHVGGTATCT’ATCC-3′) primers [31]. The PCR conditions included an initial denaturation step at 95°C for 3 min, followed by 25 cycles at 95°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec and then a final extension step at 72°C for 5 min on a GeneAmp PCR system 9700 (Applied Biosystems). The amplicons were purified using a Monarch DNA gel extraction kit (New England Biolabs, Ipswich, MA, USA). Index PCR was performed using a Nextera XT index kit (Illumina Inc., San Diego, CA, USA) to attach a unique 8-bp barcode sequence to the adapters. The 25 µL reaction solution consisted of 12.5 µL KAPA HiFi HotStart ReadyMix (Kapa Biosystems, Inc., Wilmington, MA, USA), 2.5 µL of each index primer, and 1 µL 16S rRNA amplicon. The reaction conditions were as follows: 95°C for 3 min, 8 cycles at 95°C for 30 sec, 55°C for 30 sec, 72°C for 30 sec, and then 72°C for 5 min on a Mastercycler pro system (Eppendorf AG, Hamburg, Germany). The PCR products were purified using Agencourt AMPure XP beads (Beckman Coulter, Inc., Indianapolis, IN, USA), and the size and concentration were determined using a bioanalyzer with a DNA 1000 chip (Agilent Technologies Inc., Santa Clara, CA, USA) and a Qubit 2.0 fluorometer with a Qubit dsDNA HS assay kit (Thermo Fisher Scientific). The libraries were normalized and pooled to a final concentration of 10 pM. The amplicons were then sequenced on a MiSeq system using Illumina MiSeq reagent kit v3 (2 × 300 bp paired-end runs). A 16S rRNA metagenomic sequencing library was prepared according to the manufacturer’s instructions (Illumina). To normalize the DNA amplicons, the DNA concentrations of the PCR products were measured using a Qubit dsDNA HS assay kit (Thermo Fisher Scientific). Appropriate amounts of 16S rRNA gene products and internal controls (PhiX Control v3; Illumina, Tokyo, Japan) were subjected to paired-end sequencing on a MiSeq sequencer using a 600-cycle MiSeq reagent kit (Illumina).
Microbiota analysis
The results of 16S rRNA sequencing were analyzed using Quantitative Insights into Microbial Ecology 2 (QIIME 2) v2023.2 [32]. The demultiplexed sequences from each amplicon were quality-filtered, trimmed, de-noised, and merged, following which the chimeric sequences were identified and removed using the QIIME 2 DADA2 plugin to obtain the feature table of amplicon sequence variants [33]. The amplicon sequence variant feature counts of the 16S rRNA sequence were classified using the Naive Bayes method, and the Greengenes (v.13_8) database was used for aligning the assigned taxonomy IDs with the classification of different levels [34]. The alpha diversity indices (Chao1, observed species, Shannon diversity, Faith’s phylogenetic diversity, and Pielou’s evenness) were calculated and plotted using QIIME 2 and GraphPad Prism (version 9.5.1, GraphPad Software, San Diego, CA, USA). Beta diversity was calculated based on Bray–Curtis and weighted and unweighted UniFrac distances in QIIME 2 to compare the differences in the microbial composition between samples, whereas non-metric dimensional scaling was performed with the Bray–Curtis distance in the metaMDS function of the vegan R package [35]. To visualize the dispersion of short-chain fatty acid (SCFA) production data, a principal component analysis algorithm was applied using the standard principal component analysis tool of the Python package Sklearn based on SCFA molarities.
Measurement of fermentation products
The SCFAs (acetic acid, propionic acid, butyric acid, succinic acid, and lactic acid) in the samples were analyzed using high-performance liquid chromatography (HPLC; Shimadzu, Kyoto, Japan). All SCFAs used for the standard curve were purchased from Sigma-Aldrich, Japan. For fecal samples, 200–300 mg feces were homogenized with 1 mL of 0.15 mM sulfuric acid and then centrifuged (22,000 × g, 4°C, 20 min). The supernatant was extracted and filtered through a 0.22-μm organic filter into a 1.5 mL vial for HPLC or sealed and stored at 4°C for subsequent HPLC analysis. For the ex vivo culture medium, the samples were centrifuged (22,000 × g, 4°C, 20 min), and 1 mL of the supernatant was used for sulfuric acid treatment, as described above. The injection volume for HPLC analysis was 10 µL. Separation was performed using an Aminex HPX-87H column (300 mm × 7.8 mm, Bio-Rad Laboratories, Hercules, CA, USA) maintained at 40°C and 5 mM sulfuric acid as the fluid phase, with a flow rate of 0.6 mL/min. The UV detector was operated at 210 nm for a total operating time of 28 min. Ammonia concentrations were measured using a Sigma Ammonia Assay Kit (MilliporeSigma, St. Louis, MO, USA) according to the manufacturer’s instructions. The phenol and p-cresol concentrations in the samples were determined according to the method described by King et al. [36].
Statistical analysis
All experiments were conducted at least three times, with three replicates per trial. When comparing treatments, a one-way ANOVA followed by Dunnett’s test was employed. Results were deemed statistically significant if the p-values were <0.05.
RESULTS
Influences of the single strains and MSM on microbial composition
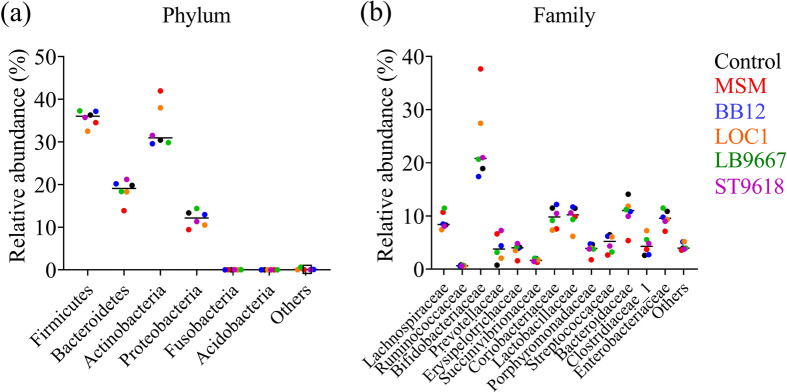
We analyzed the taxonomic profiles of fecal and culture medium samples using 16S rRNA amplicons (Fig. 1). Slightly higher relative abundances of Proteobacteria at the phylum level (Fig. 1a) and Enterobacteriaceae at the family level (Fig. 1b) were observed when feces were cultured. Overall, the microbial composition of the feces did not differ significantly from that of the culture medium. This indicated that the culture system used in this study was capable of culturing feces while maintaining the microbial composition. Therefore, the average relative abundances of bacteria were compared at the phylum (Fig. 2a) and family levels (Fig. 2b) among the culture medium samples. The MSM tended to plot the farthest away from the controls compared with all the single bacteria. The results indicated that the MSM tended to alter the microbial composition the most.
Fig. 1.
Taxonomic profiles of fecal and culture medium samples were analyzed using 16S rRNA amplicons.
Each bar represents the relative abundance of bacterial taxa. The top 6 and 13 most abundant taxa are shown at the phylum (a) and family (b) levels, respectively. BB12: Bifidobacterium lactis Bb12, LOC: Lactiplantibacillus plantarum LOC1, LB9667: Lactobacillus delbrueckii subsp. bulgaricus LB9667, ST9618: Streptococcus thermophilus ST9618.
Fig. 2.
Dot plot graph showing the average relative abundances of bacteria at the phylum (a) and family (b) levels among the culture medium samples.
MSM: multi-strain mixtures.
Three principal coordinate analyses and non-metric dimensional scaling were performed to compare the differences in microbial composition among the culture medium samples (Fig. 3). The principal coordinate analyses of Bray–Curtis dissimilarity (Fig. 3a), unweighted UniFrac distance (Fig. 3b), and non-metric dimensional scaling (Fig. 3d) showed that the three donors clustered differently. This suggested that the differences between the donors affected the microbial composition more than the addition of bacterial cells. For each donor, the MSM tended to cluster further away from the controls compared with all the single bacteria, suggesting that the MSM affected the microbial composition the most. In the principal coordinate analyses of the weighted UniFrac distance, cultures with BB12, LOC1, and ST9618 were plotted in similar positions for donors 2 and 3, indicating an overlap in their effect on the microbial compositions of donors 2 and 3 (Fig. 3c).
Fig. 3.
Beta diversity of gut microbiota based on principal coordinate analysis and non-metric dimensional scaling among the culture medium samples.
(a) Principal coordinate analyses of Bray–Curtis dissimilarity, (b) Principal coordinate analyses of unweighted UniFrac distance. (c) Principal coordinate analyses of weighted UniFrac distance, (d) Non-metric dimensional scaling of Bray–Curtis dissimilarity. The percentage of variability explained by each axis is shown. MSM: multi-strain mixtures.
To compare the differences in microbial diversity among the culture medium samples, the alpha diversity indices—Chao1, observed species, Shannon diversity, Faith’s phylogenetic diversity, and Pielou’s evenness—were calculated (Fig. 4). The Chao1 index increased significantly for the MSM (p=0.043), BB12 (p=0.042), and LB9667 (p=0.007) cultures compared with that of the controls (Fig. 4a). The Shannon index increased significantly in the MSM (p=0.026) and LOC1 (p=0.018) cultures (Fig. 4c). Pielou’s evenness increased significantly in the MSM (p=0.028) and BB12 (p=0.046) cultures (Fig. 4e). No significant increase or decrease was observed in the observed species (Fig. 4b) and Faith’s phylogenetic diversity indices (Fig. 4d). The results for the Chao1 index, Shannon index, and Pielou’s evenness indicated that the MSM had the characteristics of BB12, LB9667, and LOC1.
Fig. 4.
Alpha diversity indices among the culture medium samples.
(a) Chao1 index, (b) Observed species index, (c) Shannon diversity index, (d) Faith’s phylogenetic diversity, and (e) Pielou’s evenness. P-values were determined using one-way ANOVA followed by Dunnett’s test. *p<0.05, **p<0.01. MSM: multi-strain mixtures.
The microbial population in the culture medium for the six different bacterial groups was analyzed using qPCR (Fig. 5). The abundance of Bifidobacterium increased significantly in the MSM (p=0.023) and BB12 (p=0.046) cultures (Fig. 5a), whereas that of Prevotella increased significantly in the MSM (p=0.017), BB12 (p=0.007), and ST9618 (p=0.010) cultures (Fig. 5d). In contrast, a decrease in Bacteroides abundance was only observed in the LOC1 culture (p=0.028) (Fig. 5e), whereas an increase in the abundance of Faecalibacterium and a decrease in that of the harmful bacterium (Fig. 5c) Fusobacterium were observed only in the LB9667 culture (Faecalibacterium, p=0.015; Fusobacterium, p=0.033; Fig. 5f). No significant increase or decrease in Lactobacillus was observed (Fig. 5b). The results for Bifidobacterium and Prevotella indicated that the characteristics of the MSM were identical to those of BB12 and ST9618. These results indicated that the MSM maximally affected the microbial composition by combining the effects of each of the single strains, which was indicative of an additive effect.
Fig. 5.
The microbial populations in the culture medium were analyzed using qPCR for the six different bacterial groups among the culture medium samples.
(a) Bifidobacterium, (b) Lactobacillus, (c) Faecalibacterium, (d) Prevotella, (e) Bacteroides, and (f) Fusobacterium. P-values were determined using one-way ANOVA followed by Dunnett’s test. *p<0.05, **p<0.01. MSM: multi-strain mixtures.
Influence of the single strains or MSM on fermentation products
The concentrations of SCFAs in the feces and culture medium were analyzed using HPLC (Fig. 6). Acetic, propionic, and butyric acids were the major SCFAs, with slight levels of succinic and lactic acids. The SCFA compositions of the feces and culture medium were similar (Fig. 6a, 6b), suggesting that the culture system used in this study maintained the fermentative potential of the fecal microbial flora. The SCFA concentrations in the culture medium samples were subsequently analyzed using HPLC and compared (Fig. 7). The acetic acid level increased significantly in the MSM (p=0.036) and BB12 (p=0.033) cultures (Fig. 7a). Furthermore, the butyric acid level increased significantly in the MSM (p=0.011), BB12 (p=0.010), and ST9618 (p=0.014) cultures (Fig. 7c), and the level of succinic acid increased significantly in the MSM (p=0.049) and BB12 (p=0.036) cultures (Fig. 7e). No significant increase or decrease was observed in propionic and lactic acids (Fig. 7b, 7d).
Fig. 6.
Short-chain fatty acid (SCFA) composition of fecal and culture medium samples.
(a) Feces and (b) Culture medium.
Fig. 7.
Concentrations of short-chain fatty acids (SCFAs) among the culture medium samples.
(a) Acetic acid, (b) Propionic acid, (c) Butyric acid, (d) Lactic acid, and (e) Succinic acid. P-values were determined using one-way ANOVA followed by Dunnett’s test. *p<0.05, **p<0.01. MSM: multi-strain mixtures.
The concentrations of the putrefactive products in the culture medium samples were measured (Fig. 8). The ammonia concentration decreased significantly in the LB9667 culture (p=0.037; Fig. 8a), whereas the phenol concentration decreased significantly in the MSM (p=0.036) and ST9618 (p=0.040) cultures (Fig. 8c). No significant increase or decrease in p-crezol was observed (Fig. 8b). The results for acetic acid, butyric acid, and phenol suggested that the MSM exerted an additive effect that combined the influences of BB12 and ST9618 on fermentation products.
Fig. 8.
Concentrations of putrefactive products in the culture medium samples.
(a) Ammonia, (b) p-cresol, and (c) Phenol. P-values were determined using one-way ANOVA followed by Dunnett’s test. *p<0.05. MSM: multi-strain mixtures.
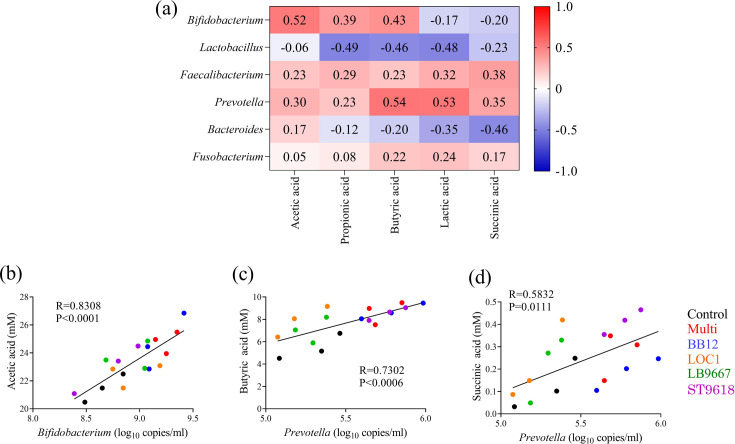
Correlations between bacteria and SCFAs
To investigate the effects of the single strains or MSM on the gut environment in detail, the correlations between bacteria, measured using qPCR, and SCFAs, measured using HPLC, were investigated (Fig. 9a). The results showed positive correlations between the number of Bifidobacterium and the acetic acid concentration (p<0.0001; Fig. 9b) and between the number of Prevotella and the butyric (p=0.0006) and succinic acid (p=0.011) concentrations (Fig. 9c, 9d). Significant correlations were found in the linear regression analysis. The MSM and BB12 significantly increased Bifidobacterium abundance and acetic acid production. In contrast, the MSM, BB12, and ST9618 significantly increased Prevotella abundance and the butyric acid level, whereas the MSM and ST9618 significantly increased Prevotella abundance and succinic acid production. These results indicated that the MSM had a combination of metabolites derived from BB12 and ST9618.
Fig. 9.
(a) Heatmap showing Spearman’s correlations between bacteria, measured using real-time PCR, and short-chain fatty acids, measured using HPLC. Red and blue tiles indicate positive and negative correlations, respectively. (b) Pairwise scatterplots illustrating the relationships between Bifidobacterium and acetic acid, (c) Bifidobacterium and acetic acid, (d) and Prevotella and succinic acid. MSM: multi-strain mixtures.
Effects of the single strains or MSM on the improvement of donor parameters
Coverage is expected to increase when multiple strains with specific characteristics are mixed. The number of donors with an improved microbial composition when the single stains or MSM were used was investigated. Variations between the single strains were observed in the number of parameters showing high improvement (three donors), suggesting that their effects differed (Fig. 10). In particular, for putrefactive products, a large variation was observed among the single strains; however, the MSM showed the highest number of improvements for all putrefactive products. This suggested that the MSM provided an additive effect that combined the effects of the single strains in improving the gut environment.
Fig. 10.
Heatmap showing the number of donors with improved parameters related to the microbial composition among the culture medium samples.
Arrows indicate increases or decreases considered to be improvements for each parameter. PCR: polymerase chain reaction.
DISCUSSION
In this study, to examine the gut-improving efficacy of an MSM consisting of four dead bacterial strains, human feces were inoculated with either a single-strain or an MSM using a KUHIMMiB, and the microbial composition and fermentation products were assessed. Donor feces were cultured in the in vitro model, which showed comparable microbial and SCFA compositions for the feces and culture medium. This suggests that a KUHIMMiB can be used to culture feces while maintaining the fecal microbial flora and its fermentative potential. It is also a reliable model for the functional evaluation of food ingredients. Therefore, this model was used to investigate the effect of the MSM composed of dead bacteria on the gut microbial composition. To the best of our knowledge, reports directly comparing the effects of an MSM composed of dead bacteria on the improvement of the gut microbial composition with those of the single strains that make up the MSM are lacking. To date, continuous culture models are the most widely accepted models of the human colonic microbiota for studying the gut-improving efficacy of food compositions [37, 38]. These models are complex because they consist of multistage setups and have a limited number of cultures. In contrast, the KUHIMMiB, which has no limitation on the number of cultures, is a human colonic microbiota model that solves the abovementioned problems; therefore, it can be used for evaluating the functionality of an MSM.
The results of taxonomic profiling, SCFA analysis, and measurements of putrefactive products showed that the effective parameters differed between the strains. This indicated that the mechanism of action of each strain differed. Several parameters of the MSM were similar to those of the respective single strains, which suggested that the MSM exerted an additive effect by combining the effects of the single strains. Moreover, the correlations between bacteria and SCFAs demonstrated the mechanism via which the MSM and BB12 increased Bifidobacterium and Prevotella abundance and promoted acetic acid and butyric acid production, respectively. Similarly, the mechanism via which the MSM and ST9618 increased Prevotella abundance and promoted succinic acid production was revealed. Bifidobacteria can increase acetic acid levels, which improves the epithelial cell-mediated intestinal defense and thereby protects the host against lethal infections [39]. Prevotella produces butyric and succinic acids [40, 41]. Butyric acid promotes the differentiation of regulatory T cells, which play a key role in the regulation of immunity via the intestinal mucosa [42]. Succinic acid improves glucose homeostasis via intestinal gluconeogenesis [43]. Therefore, the MSM provided maximum human health benefits considering the combination of metabolites derived from the constituent strains. These results indicated that strains with different beneficial effects should be mixed when investigating the effects of an MSM. However, we did not identify any parameter for which the MSM was significantly more effective than the single strains. This suggested that a synergistic effect of the MSM was unlikely. The reasons for this are unclear, mainly because the mechanisms of action of dead bacteria on the gut microbial composition and their metabolites remain unknown. However, in this study, dead bacteria maintained their morphology as bacterial cells. Therefore, the cell wall of the bacteria may be the component involved in the change in the gut microbiota. Gram-positive bacteria, such as lactic acid bacteria and bifidobacteria, have different cell wall structures depending on the strain [44, 45]. Thus, an MSM with multiple types of cell walls may have an additive effect by combining the effects of the single strains because the gut microbiota, which utilizes the cell wall structures of dead bacteria, also differs among strains. Conversely, we assumed that dead bacteria interfere with the gut microbiota in a simple manner and do not produce metabolites, which renders the demonstration of a synergistic effect difficult.
The inactivation of probiotics can be achieved using different methods, such as those utilizing heat, chemicals (e.g., formalin), gamma or ultraviolet radiation, and sonication [7, 46]. The effects of different inactivation methods on the structural components of the cell may vary, which may affect the biological activity of the cell [47]. Therefore, the results obtained using different inactivation methods may vary, and verification using inactivation methods other than heat treatment is required. In the present study, several parameters were effective only for a single strain. This may be because the amount of each single strain in the MSM was a quarter of the total amount of each single strain alone. This could be demonstrated more clearly if the experiments were performed simultaneously with a quarter of the amount of a single strain added to the model. This suggests that if the number of bacteria to be mixed is increased excessively, the characteristics of the individual single strains in the MSM could possibly be lost. Therefore, the number of strains to be mixed should be carefully considered when investigating the effects of an MSM.
The proposed additive effect of mixing several single strains is expected to provide health benefits to a large number of people. This is because the individual human gut microbiota varies widely [48], and the effects of lactic acid bacteria supplementation may vary among individuals. To verify this additive effect, the number of people with improvement in parameters related to the gut environment was examined. The results showed that the parameters for which the highest number of people showed improvement were common in the MSM group. In particular, for putrefactive products, we observed a large variation in the number of people who showed improvement with a single strain; however, the number of people who showed improvement was the highest for all putrefactive products in the MSM group. High concentrations of putrefactive products, such as colonic ammonia, can damage the colonic epithelial cells and increase intestinal permeability [49]. p-Cresol is a uremic toxin involved in the development of renal and cardiovascular complications in patients with chronic kidney disease [50]. Phenol is detrimental to the intestinal epithelium, as it stimulates cancer development by increasing the potency of carcinogens [51, 52]. Putrefactive products considerably affect health; therefore, an MSM may contribute more to human health than single-strain probiotics.
It should be noted, however, that the sample size of the study was small (including only three donors) and that this was a limitation of this study. In addition, donor age was limited. In the future, a larger number of people from a wider age range should be recruited and examined. Nevertheless, the results of this study, which focused on the number of people who showed improved health, are informative because they show the effects of an MSM. In most previous studies, the effects of MSMs were judged based on significant increases or decreases after including the parameters of the whole sample. We suggest that when evaluating the effectiveness of MSMs in the future, the percentage of people who showed improvement should be considered as one of the criteria. Therefore, a trial with a large number of people should be set up when investigating the effects of an MSM.
In conclusion, this study showed that an MSM of dead bacteria can exert an additive effect and improve the gut environment. These findings will help ensure correct functional examination of MSMs of dead bacteria in the future. Furthermore, dead bacteria are commercially valuable, as they are sterilized and consequently can be used in a wide variety of food products regardless of storage and transport conditions or form. In addition, significant changes in flavor do not occur, as they do not produce acid. Finally, the sterilization process preserves the components that are useful for the body and maintains them in the best condition for maximum effect. Our findings on the functionality of an MSM comprising dead bacteria will considerably facilitate their commercial use and provide new health benefits.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
AUTHORS’ CONTRIBUTIONS
YT drafted and edited the manuscript. MS, JY, and SI conceived the study and collected information. FI revised the manuscript. All authors contributed to the article and approved the submitted version.
FUNDING
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Morelli L, Capurso L. 2012. FAO/WHO guidelines on probiotics: 10 years later. J Clin Gastroenterol 46 Suppl: S1–S2. [DOI] [PubMed] [Google Scholar]
- 2.Adel M, El-Sayed AM, Yeganeh S, Dadar M, Giri SS. 2017. Effect of potential probiotic Lactococcus lactis subsp. lactis on growth performance, intestinal microbiota, digestive enzyme activities, and disease resistance of Litopenaeus vannamei. Probiotics Antimicrob Proteins 9: 150–156. [DOI] [PubMed] [Google Scholar]
- 3.Kałużna-Czaplińska J, Gątarek P, Chartrand MS, Dadar M, Bjørklund G. 2017. Is there a relationship between intestinal microbiota, dietary compounds, and obesity? Trends Food Sci Technol 70: 105–113. [Google Scholar]
- 4.Umair M, Jabbar S, Zhaoxin L, Jianhao Z, Abid M, Khan KR, Korma SA, Alghamdi MA, El-Saadony MT, Abd El-Hack ME, et al. 2022. Probiotic-based bacteriocin: immunity supplementation against viruses. An updated review. Front Microbiol 13: 876058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashraf R, Shah NP. 2014. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr 54: 938–956. [DOI] [PubMed] [Google Scholar]
- 6.Peng X, Ed-Dra A, Song Y, Elbediwi M, Nambiar RB, Zhou X, Yue M. 2022. Lacticaseibacillus rhamnosus alleviates intestinal inflammation and promotes microbiota-mediated protection against Salmonella fatal infections. Front Immunol 13: 973224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshpande G, Athalye-Jape G, Patole S. 2018. Para-probiotics for preterm neonates—the next frontier. Nutrients 10: 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto S, Komiya M, Fujii G, Hamoya T, Nakanishi R, Fujimoto K, Tamura S, Kurokawa Y, Takahashi M, Ijichi T, et al. 2017. Preventive effects of heat-killed Enterococcus faecalis strain EC-12 on mouse intestinal tumor development. Int J Mol Sci 18: 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murata M, Kondo J, Iwabuchi N, Takahashi S, Yamauchi K, Abe F, Miura K. 2018. Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Benef Microbes 9: 855–864. [DOI] [PubMed] [Google Scholar]
- 10.Komano Y, Shimada K, Naito H, Fukao K, Ishihara Y, Fujii T, Kokubo T, Daida H. 2018. Efficacy of heat-killed Lactococcus lactis JCM 5805 on immunity and fatigue during consecutive high intensity exercise in male athletes: a randomized, placebo-controlled, double-blinded trial. J Int Soc Sports Nutr 15: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maekawa T, Ishijima AS, Ida M, Izumo T, Ono Y, Shibata H, Abe S. 2016. Prophylactic effect of lactobacillus pentosus strain s-pt84 on candida infection and gastric inflammation in a murine gastrointestinal candidiasis model [Errata]. Med Mycol J 57: E81–E92. [DOI] [PubMed] [Google Scholar]
- 12.Hirose Y, Yamamoto Y, Yoshikai Y, Murosaki S. 2013. Oral intake of heat-killed Lactobacillus plantarum L-137 decreases the incidence of upper respiratory tract infection in healthy subjects with high levels of psychological stress. J Nutr Sci 2: e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liévin-Le Moal V, Sarrazin-Davila LE, Servin AL. 2007. An experimental study and a randomized, double-blind, placebo-controlled clinical trial to evaluate the antisecretory activity of Lactobacillus acidophilus strain LB against nonrotavirus diarrhea. Pediatrics 120: e795–e803. [DOI] [PubMed] [Google Scholar]
- 14.Miyauchi E, Morita H, Tanabe S. 2009. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J Dairy Sci 92: 2400–2408. [DOI] [PubMed] [Google Scholar]
- 15.Sugawara T, Sawada D, Ishida Y, Aihara K, Aoki Y, Takehara I, Takano K, Fujiwara S. 2016. Regulatory effect of paraprobiotic Lactobacillus gasseri CP2305 on gut environment and function. Microb Ecol Health Dis 27: 30259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terada A, Bukawa W, Kan T, Mitsuoka T. 2004. Effects of the consumption of heat-killed Enterococcus faecalis EC-12 preparation on microbiota and metabolic activity of the faeces in healthy adults. Microb Ecol Health Dis 16: 188–194. [Google Scholar]
- 17.Ouwehand AC, Isolauri E, Kirjavainen PV, Tölkko S, Salminen SJ. 2000. The mucus binding of Bifidobacterium lactis Bb12 is enhanced in the presence of Lactobacillus GG and Lact. delbrueckii subsp. bulgaricus. Lett Appl Microbiol 30: 10–13. [DOI] [PubMed] [Google Scholar]
- 18.Timmerman HM, Koning CJ, Mulder L, Rombouts FM, Beynen AC. 2004. Monostrain, multistrain and multispecies probiotics—a comparison of functionality and efficacy. Int J Food Microbiol 96: 219–233. [DOI] [PubMed] [Google Scholar]
- 19.Medina M, Izquierdo E, Ennahar S, Sanz Y. 2007. Differential immunomodulatory properties of Bifidobacterium logum strains: relevance to probiotic selection and clinical applications. Clin Exp Immunol 150: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mileti E, Matteoli G, Iliev ID, Rescigno M. 2009. Comparison of the immunomodulatory properties of three probiotic strains of lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS One 4: e7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CY, Tsen HY, Lin CL, Lin CK, Chuang LT, Chen CS, Chiang YC. 2013. Enhancement of the immune response against Salmonella infection of mice by heat-killed multispecies combinations of lactic acid bacteria. J Med Microbiol 62: 1657–1664. [DOI] [PubMed] [Google Scholar]
- 22.Sniffen JC, McFarland LV, Evans CT, Goldstein EJC. 2018. Choosing an appropriate probiotic product for your patient: an evidence-based practical guide. PLoS One 13: e0209205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venema K, van den Abbeele P. 2013. Experimental models of the gut microbiome. Best Pract Res Clin Gastroenterol 27: 115–126. [DOI] [PubMed] [Google Scholar]
- 24.Akazawa H, Fukuda I, Kaneda H, Yoda S, Kimura M, Nomoto R, Ueda S, Shirai Y, Osawa R. 2023. Isolation and identification of hyaluronan-degrading bacteria from Japanese fecal microbiota. PLoS One 18: e0284517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki K, Sasaki D, Okai N, Tanaka K, Nomoto R, Fukuda I, Yoshida KI, Kondo A, Osawa R. 2017. Taurine does not affect the composition, diversity, or metabolism of human colonic microbiota simulated in a single-batch fermentation system. PLoS One 12: e0180991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki D, Sasaki K, Ikuta N, Yasuda T, Fukuda I, Kondo A, Osawa R. 2018. Low amounts of dietary fibre increase in vitro production of short-chain fatty acids without changing human colonic microbiota structure. Sci Rep 8: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marmur J. 1961. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol 3, 208–218, IN1. [Google Scholar]
- 28.Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R. 2004. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol 70: 7220–7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. 2009. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr 101: 541–550. [DOI] [PubMed] [Google Scholar]
- 30.Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A. 2004. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol 97: 1166–1177. [DOI] [PubMed] [Google Scholar]
- 31.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO. 2013. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, Huttley GA, Gregory Caporaso J. 2018. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. 2016. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13: 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2007. The vegan package. Community Ecology Package 10: 719. [Google Scholar]
- 36.King RA, May BL, Davies DA, Bird AR. 2009. Measurement of phenol and p-cresol in urine and feces using vacuum microdistillation and high-performance liquid chromatography. Anal Biochem 384: 27–33. [DOI] [PubMed] [Google Scholar]
- 37.Minekus M, Smeets-Peeters M, Bernalier A, Marol-Bonnin S, Havenaar R, Marteau P, Alric M, Fonty G, Huis in’t Veld JH. 1999. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Appl Microbiol Biotechnol 53: 108–114. [DOI] [PubMed] [Google Scholar]
- 38.Molly K, Woestyne MV, Smet ID, Verstraete W. 1994. Validation of the simulator of the human intestinal microbial ecosystem (SHIME) reactor using microorganism-associated activities. Microb Ecol Health Dis 7: 191–200. [Google Scholar]
- 39.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, et al. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469: 543–547. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Liu Y, Wang Y, Chen X, Wang C, Chen X, Yuan X, Liu L, Yang J, Zhou X. 2022. Prevotellaceae produces butyrate to alleviate PD-1/PD-L1 inhibitor-related cardiotoxicity via PPARα-CYP4X1 axis in colonic macrophages. J Exp Clin Cancer Res 41: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pybus V, Onderdonk AB. 1996. The effect of pH on growth and succinate production by Prevotella bivia. Microb Ecol Health Dis 9: 19–25. [Google Scholar]
- 42.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450. [DOI] [PubMed] [Google Scholar]
- 43.De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. 2016. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab 24: 151–157. [DOI] [PubMed] [Google Scholar]
- 44.Chapot-Chartier MP, Kulakauskas S. 2014. Cell wall structure and function in lactic acid bacteria. Microb Cell Fact 13 Suppl 1: S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pyclik M, Srutkova D, Schwarzer M, Górska S. 2020. Bifidobacteria cell wall-derived exo-polysaccharides, lipoteichoic acids, peptidoglycans, polar lipids and proteins—their chemical structure and biological attributes. Int J Biol Macromol 147: 333–349. [DOI] [PubMed] [Google Scholar]
- 46.Zorzela L, Ardestani SK, McFarland LV, Vohra S. 2017. Is there a role for modified probiotics as beneficial microbes: a systematic review of the literature. Benef Microbes 8: 739–754. [DOI] [PubMed] [Google Scholar]
- 47.Taverniti V, Guglielmetti S. 2011. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr 6: 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, et al. 2013. The long-term stability of the human gut microbiota. Science 341: 1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meletis CD, Zabriskie N. 2008. Supporting gastrointestinal health with nutritional therapy. Altern Complement Ther 14: 132–138. [Google Scholar]
- 50.Six I, Flissi N, Lenglet G, Louvet L, Kamel S, Gallet M, Massy ZA, Liabeuf S. 2020. Uremic toxins and vascular dysfunction. Toxins (Basel) 12: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma N, Tian Y, Wu Y, Ma X. 2017. Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Curr Protein Pept Sci 18: 795–808. [DOI] [PubMed] [Google Scholar]
- 52.Zhao J, Zhang X, Liu H, Brown MA, Qiao S. 2019. Dietary protein and gut microbiota composition and function. Curr Protein Pept Sci 20: 145–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.