Abstract
Although the clustered regularly interspaced short palindromic repeat (CRISPR)-Cas system has been extensively developed since its discovery for eukaryotic and prokaryotic genome editing and other genetic manipulations, there are still areas warranting improvement, especially regarding bacteria. In this study, BRD0539, a small-molecule inhibitor of Streptococcus pyogenes Cas9 (SpCas9), was used to suppress the activity of the nuclease during genetic modification of Lacticaseibacillus paracasei, as well as to regulate CRISPR interference (CRISPRi). First, we developed and validated a CRISPR-SpCas9 system targeting the sirA gene of L. paracasei. Then BRD0539 was used for CRISPR-dependent DNA cleavage in vivo. Our results suggested that the inhibitor worked partially in both Escherichia coli and L. paracasei. Next, we designed a CRISPRi system in a L. paracasei strain by inserting an inactive SpCas9 gene into the chromosome and introducing a plasmid encoding for a single guide RNA (sgRNA) targeting the sirA gene. Expression of sirA was successfully inhibited in the recombinant strains, and CRISPRi was abolished in an inhibitor-dependent manner. Our findings may help expand the CRISPR toolbox for research on lactic acid bacteria and other microbes.
Keywords: CRISPR/Cas9, Lactobacilli, BRD0539, Lacticaseibacillus paracasei, genetic modification, CRISPRi
INTRODUCTION
The clustered regularly interspaced short palindromic repeat (CRISPR)-Cas system is a powerful genetic tool for precise DNA editing [1]. In combination with a CRISPR-associated protein, such as Cas9, and an sgRNA, specific DNA sequences in the genome can be targeted [2]. CRISPR has many attractive applications in genetic research, including the ability to study a gene’s function by disrupting or modifying its activity, correcting genetic mutations, and regulating gene expression [3, 4].
CRISPR was originally discovered as a natural defense mechanism used by bacteria to protect themselves against viruses and other invading genetic elements [5, 6]. Interestingly, the existing CRISPR tools for genetic manipulation in bacteria are not as practical as those for eukaryotic cells owing to the double-strand breaks induced by Cas proteins often being lethal in prokaryotic cells [7, 8]. Moreover, as conventional genetic tools for bacteria are sufficient for most applications, a novel CRISPR-Cas system fine-tuned for bacteria is not regarded as a priority. Nonetheless, a wide range of potential CRISPR applications, such as gene silencing by CRISPR interference (CRISPRi), which could improve or complement existing methods, are attractive for bacterial research.
In recent decades, the application of CRISPR for lactic acid bacteria has been increasingly explored. In 2014, Oh and van Pijkeren demonstrated successful genome editing in Limosilactobacillus reuteri (former Lactobacillus reuteri) using CRISPR-Cas9 and RecT-assisted recombination [9]. To accomplish this, they utilized specific cleavage of chromosomal DNA by CRISPR-Cas9, which results in host cell death, as a selection marker to screen for mutated clones. Several studies have also applied CRISPR-Cas as a direct genome editing tool rather than a tool for screening mutated clones. For example, genetic manipulation using the Cas9D10A nickase in Lacticaseibacillus casei (former Lactobacillus casei) was reported by Song et al. [10]. Leenay et al. also showed that CRISPR-Cas9 could be available for genome editing in Lactiplantibacillus plantarum (former Lactobacillus plantarum) [11]. Other examples of CRISPR applications in lactic acid bacteria have been described by Goh and Barrangou [12].
Although CRISPR-Cas is a powerful tool for the genetic manipulation of lactic acid bacteria, there are certain limitations that require addressing. For example, the nuclease activity of Cas proteins must be strictly time-regulated, as even trace levels of activity may be enough to kill cells. Berlec et al. used a nisin-controlled inducible expression (NICE) system to control the expression of CRISPR-Cas9 [13]. This method may work sufficiently in Lactococcus lactis and other species/strains but could be challenging in other hosts because of interhost variations regarding the level of nisin-dependent gene expression [14].
As a simple method for the precise control of CRISPR-Cas9 activity, small-molecule inhibitors of Cas9 have been extensively studied by Maji et al. [15]. For example, BRD0539 is a cell-permeable, organic, and reversible inhibitor of Streptococcus pyogenes Cas9 (SpCas9), which effectively inhibits the DNA-binding ability of SpCas9 by disrupting the interaction between SpCas9 and the protospacer adjacent motif (PAM). BRD0539 has shown low toxicity in eukaryotic cells and is expected to resolve several issues associated with the use of CRISPR-Cas in bacteria. In this study, we developed several CRISPR-Cas tools and tested them in E. coli and Lacticaseibacillus paracasei in combination with BRD0539, so as to expand the usability of the CRISPR-Cas system in lactic acid bacteria. To our knowledge, this is the first study to validate the activity of BRD0539 on bacteria. As a target for this system, we selected the sirA gene of L. paracasei, owing to the availability of anti-SirA antibodies and the L. paracasei ΔsirA strain [16].
MATERIALS AND METHODS
Bacterial strains and culture conditions
The bacterial strains and plasmids used in this study are listed in Table 1. Lacticaseibacillus paracasei IGM394 exhibits high transformation efficiency and is a plasmid-cured derivative of L. paracasei IGM393 [17]. The L. paracasei strains were anaerobically grown in de Man, Rogosa, and Sharpe (MRS; BD, Franklin Lakes, NJ, USA) broth or on MRS agar (1.5% wt/vol) with or without 5 µg/mL of erythromycin at 37°C. Escherichia coli JM109 and its derivative strains were aerobically grown in LB (BD) broth or on LB agar (1.5% wt/vol) with or without 100 µg/mL of ampicillin at 37°C. E. coli MC1061 and its derivative strains were aerobically propagated in LB broth with or without 1,000 µg/mL of erythromycin at 37°C. Finally, E. coli EC101 and its derivative strains were aerobically amplified in Brain Heart Infusion (BHI; BD) broth or on BHI agar (1.5% wt/vol) with or without 200 µg/mL of erythromycin and 40 µg/mL of kanamycin at 37°C.
Table 1. Bacterial strains and plasmids used in this study.
| Strain or plasmid | Description | Reference |
|---|---|---|
| Strain | ||
| L. paracasei IGM394 | Wild-type, derivative of L. paracasei IGM393 | [17] |
| L. paracasei BKN191 | sirA deleted, derived from IGM394 | [16] |
| E. coli JM109 | Cloning host for pGEM®-T Vector and pLP401t | Takara |
| E. coli MC1061 | Cloning host for pG+host5, hsdR, mcrB, araD139, Δ(araABC-leu)7679, ΔlacX74, galU, galK, rpsL, thi | Lucigen Co. |
| E. coli EC101 | Cloning host for pG+host5, Kmr, E. coli JM101 with repA from pWV01 integrated in chromosome | [25] |
| Plasmid | ||
| pG+host5 | Emr, thermosensitive E. coli-Lactic acid bacteria shuttle vector | [26] |
| pgRNA-bacteria | Plasmid for guide RNA transcription | [3] |
| pGCasRsirA | pG+host5 containing Spcas9 and sgRNA targeting sirA | This study |
| pGCasRslpA | pG+host5 containing Spcas9 and sgRNA targeting slpA | This study |
| pGdCas9 | pG+host5 containing Spcas9 with D10A and H840A mutations | This study |
| pGEM®-T Vector | Ampr, Cloning vector | Promega |
| pGEM::sirA | pGEM®-T Vector containing sirA | This study |
| pLP401-T | Ampr, Emr, Expression vector containing the pAmy promoter | [27] |
| pLPCasRsirA | pLP401-T containing Spcas9 and sgRNA targeting sirA | This study |
| pBTE | Ampr, Emr, thermosensitive E. coli- Lactic acid bacteria shuttle vector | [28] |
| pBTE::dcas9 | pBTE containing L. paracasei Cas locus with Ppgm promoter and dcas9 | This study |
| pGRsirA-up | pG+host5 containing sgRNA targeting sirA (up) | This study |
| pGRsirA-down | pG+host5 containing sgRNA targeting sirA (down) | This study |
Synthesis of BRD0539
BRD0539 was synthesized from 4-bromoaniline according to a previously reported method with several modifications [18, 19]. More detailed synthesis procedures will be published in due course.
In vitro cleavage assay
The SpCas9 and sgRNA complexes were formed by mixing 20 pmol of SpCas9 (TrueCut™ Cas9 Protein v2, Thermo Fisher Scientific, Waltham, MA, USA) and 50 pmol of synthesized sirA- targeting sgRNA (Integrated DNA Technologies, Coralville, IA, USA) and incubating for 10 min at 25°C. The SpCas9-sgRNA complexes were co-incubated for 30 min at 25°C with BRD0539 adjusted to a final concentration of 5 to 200 μM. The target DNA fragment containing sirA (600 ng) or a plasmid containing sirA (pGEM::sirA) was mixed with the SpCas9-sgRNA complex, incubated at 37°C for 3 hr, and then analyzed by agarose gel electrophoresis.
Cell toxicity of BRD0539
Overnight cultures of E. coli JM109 and L. paracasei IGM394 were inoculated (1% vol/vol) into 200 µL of their respective media containing BRD0539 (final concentration of 10 to 100 μM) in 96-well plates and incubated statically for 30 hr at 37°C. The optical density at 600 nm (OD600) was measured using a microplate reader at 0, 3, 6, 8, 24, and 30 hr of incubation.
Cloning of plasmids
The primer sets used are listed in Table 2. To construct pGCasRsirA, the cas9 gene was amplified from the chromosomal DNA of S. pyogenes JCM 5674 using the DOKJ251/DOKJ401 primer pair. The amplicon was digested with PstI and BglII, ligated into PstI/BamHI-digested pgRNA::sirA (derivative plasmid of pgRNA bacteria), and transformed into E. coli JM109. The Cas9 and sgRNA loci of the constructed plasmid were amplified by polymerase chain reaction (PCR) using the DOKJ751/DOKJ604 primer pair. The amplicon was digested with XhoI and XbaI, ligated into a similarly digested pG+host5 vector, and transformed into E. coli EC101 cells. The pgRNA-bacteria were a gift from Stanley Qi (Addgene plasmid #44251; http://n2t.net/addgene:44251; RRID: Addgene_44251) [3]. The thermosensitive plasmid pG+host5 was used because of its ease of curing.
Table 2. Primers used in this study.
| DOKJ78 | GTAAAACGACGGCCAGT |
| DOKJ79 | CAGGAAACAGCTATGAC |
| DOKJ251 | ATATAGATCTTCAGTCACCTCCTAGCTG |
| DOKJ298 | AAAAGCACCGACTCGGT |
| DOKJ307 | ATATGAATTCCTGCTGGCGCGATTTGATA |
| DOKJ312 | ATATGGATCCAGACTAACTTGTCAGCAACCC |
| DOKJ401 | ATATCTGCAGTCAGCCATAAAACAATACTTAATAC |
| DOKJ583 | ATATAAGCTTCAAAAAAAGCACCGACTCGGTGCC |
| DOKJ604 | ATATTCTAGACCGTCACGGTCAAAGTGAATGG |
| DOKJ743 | ATTATCACTGTTTGTACGTTTTAGAGCTAGAAATAGCAAG |
| DOKJ744 | ACAAACAGTGATAATCAAACTAGTATTATACCTAGGACTGAGC |
| DOKJ751 | ATATCTCGAGCAGGATTCACAAGTCTTGCTATTGT |
| DOKJ1060 | TATCCGTCGTGCGTTCTGAC |
| DOKJ1061 | TAACAGCAATGCCAGCCATT |
| DOKJ1224 | ATATGAATTCGCAACAAAATTAGCTAAACGCAAAC |
| DOKJ1225 | ATATGGATCCCAGAACGGTTTTAAACTCATTCACC |
| DOKJ1264 | ATGGCGGTATGTATGGCCCAAC |
| DOKJ1265 | CCGGGAAGATACCGGGTGGC |
| DOKJ1292 | CCGGTATCTTCCCGGCAAAAGCTGGAGCTCCACCG |
| DOKJ1293 | CATACATACCGCCATATAGGGCGAATTGGGTACCG |
| DOKJ1346 | GTTTGATCTGCAAACCTTGTGAGTATAACACATTATCAATTCGC |
| DOKJ1347 | GTTTGCAGATCAAACATATAGTTTTAGAGCTAGAAATAGCAAGTTAAAA |
| DOKJ1646 | TAATAGGAGGCAAAAATGGATAAGAAATACTCAAT |
| DOKJ1647 | TTGTAAAACGACGGCCAGTGA |
| DOKJ1648 | GCCGTCGTTTTACAAAGAGTAAGTTCCAATGGCTGGCAT |
| DOKJ1649 | TTTTGCCTCCTATTAGCGGCCGCTAAAAAAAGAACAA |
| DOKJ1759 | GCACTGTCTATTGATAATTTACAAGCACA |
| DOKJ1760 | GACTACCAGCTTCGCAAATACG |
| DOKJ1761 | GCCGCGCTGAGTTTTAGA |
| SI608-F | TTCGGACTTTGTTGCTGACG |
| SI608-R | ATTTTGCCTTGGCGTAACCG |
The plasmid, pGCasRslpA, was constructed from pGCasRsirA using an In-Fusion® HD Cloning Kit (Takara Bio, Shiga, Japan). The linearized plasmid, in which the sirA sequence was replaced with that of slpA, was amplified by inverse PCR with the DOKJ743/DOKJ744 primer pair using pGCasRsirA as the template. The linearized plasmid was ligated using an In-Fusion® HD Cloning Kit and introduced into E. coli MC1061.
To construct the plasmid containing the target sirA gene, sirA was cloned into pGEM®-T Easy (Promega, Tokyo, Japan). The sirA gene of L. paracasei was amplified by PCR using Ex. Taq DNA polymerase (Takara-bio), chromosomal DNA from L. paracasei IGM394, and the primers DOKJ307 and DOKJ312. The amplicon was ligated with pGEM®-T Easy vector and transformed into E. coli JM109 competent cells (pGEM::sirA).
A plasmid carrying SpCas9 and sirA-specific sgRNA, pLPCasRsirA, was constructed using the Lactobacillus expression vector pLP401 for targeted DNA cleavage in L. paracasei. DNA fragments, including Cas9 and sgRNA targeting sirA, were amplified by PCR with the DOKJ1646/DOKJ1647 primer pair, using pGCasRsirA as the template. Linearized pLP401 was prepared by inverse PCR using the DOKJ1648 and DOKJ1649 primers. The linearized plasmid and the DNA fragment containing Cas9 and sgRNA targeting the sirA gene were ligated with an In-Fusion® HD Cloning Kit (Takara Bio) and cloned into E. coli JM109.
We designed two sgRNAs targeting the upstream and downstream sites of sirA. The DNA fragment of the sgRNA targeting downstream sirA was amplified by PCR with DOKJ583 and DOKJ751, using pGCasRsirA as the template. The amplicon was digested with HindIII and XhoI, ligated into a similarly digested pG+host5 vector, and transformed into E. coli MC1061 as a cloned host. The spacer sequence of the constructed plasmid, pGRsirA-D, was replaced with the sequence targeting the upstream of sirA using an In-Fusion® HD Cloning Kit (Takara Bio). The linearized plasmid, replacing sgRNA targeting the downstream of sirA crRNA with that targeting the upstream site, was amplified by inverse PCR with the DOKJ1346/ DOKJ1347 primer pair using pGRsirA-D as the template. The linearized plasmid was ligated using an In-Fusion® HD Cloning Kit (Takara Bio) and introduced into E. coli MC1061.
Construction of L. paracasei expressing dead Cas9
The gene encoding a catalytically dead Cas9 mutant (dCas9) derived from S. pyogenes Cas9 was inserted into the genome of L. paracasei IGM394 via double crossover, using the thermosensitive plasmid pBTE. The endogenous Cas locus of L. paracasei IGM394, which is the insertion site of the dCas9 gene, was amplified via PCR using the DOKJ1224/ DOKJ1225 primer pair. The amplicon was digested with BamHI and PstI, ligated into a similarly digested pBTE vector, and transformed into E. coli JM109 as the cloning host. Linearized pBTE harboring the Cas locus of L. paracasei IGM394 was prepared by inverse PCR with the DOKJ1264 and DOKJ1265 primers, using the constructed plasmid as the template. DNA fragments, including the dCas9 gene, were amplified by PCR with the DOKJ1292/DOKJ1293 primer pair, using pGdCas9, a derivative plasmid of pGCasR with D10A and H840A mutations in Cas9, as the template. The linearized plasmid and the DNA fragment containing the dCas9 gene were ligated with an In-Fusion® HD Cloning Kit (Takara Bio) and cloned into E. coli JM109. The plasmid constructed to insert the dCas9 gene, pBTE::dcas9, was introduced into L. paracasei IGM394 by electroporation, as described below. The dCas9 gene was then inserted into the IGM394 chromosome by double crossover.
Transformation of bacteria
Overnight cultures of the L. paracasei strains were inoculated (1% vol/vol) into MRS broth containing 1% glycine and incubated at 37°C until the OD600 reached 0.4. The bacterial cells were harvested by centrifugation and washed twice with deionized water. The cells were rinsed with 50 mM EDTA (pH 8.0), then rinsed with 0.3 M sucrose, and suspended in 30% PEG8000. The plasmid DNA (500 ng) was mixed with 100 µL of the competent cells and transferred to a prechilled electroporation cuvette (Cell Projects Ltd., Kent, UK, 2 mm gap). Electroporation was performed using an Electro Cell ManipulatorTM (Harvard Bioscience, Canton, MA, USA) (2.5 kV, 50 μF, and 360 Ω). After electroporation, the cells were recovered in 900 µL of MRS broth with or without 100 μM of BRD0539 and incubated at 37°C for 3 hr. The cells were then plated on MRS plates with 5 µg/mL of erythromycin and incubated at 37°C for three to four days. Competent cells of E. coli were purchased from Takara (JM109) and Lucigen, Middleton, WI, USA (MC1061). Competent cells of recombinant E. coli were prepared using ZymoBroth (Zymo Research, Irvine, CA, USA), and transformation was performed in accordance with the manufacturer’s protocol. Plasmids in the transformants were detected by colony PCR. The DOKJ78 and DOKJ79 primers were used to detect pGCasRsirA, pGCasRslpA, pGEM, and pGEM::sirA, while DOKJ1060 and DOKJ1061 were used to detect pLPCasRsirA.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted and purified from E. coli or L. paracasei in the exponential phase using a NucleoSpin® RNA kit (Takara), following the manufacturer’s instructions. To digest the contaminated genomic DNA, RNA samples were treated with deoxyribonuclease (RT grade; Nippon Gene, Tokyo, Japan). The cDNA was synthesized from total RNA using a PrimeScriptTM 1st strand cDNA Synthesis Kit (Takara). PCR was performed with the cDNA as a template. The primer pairs DOKJ298/DOKJ1761, DOKJ1759/DOKJ1760, and SI608-F/SI608-R were used to detect sgRNAs sirA and gyrB, respectively. DNA contamination in the purified RNA was tested by PCR using the same primers as those used for RT-PCR.
Immunoblotting
The total protein content from E. coli and L. paracasei cells was extracted using 8 M urea and bead beating, respectively. The E. coli cells from 2 mL of overnight cultures were harvested via centrifugation and suspended in 100 µL of 8 M urea. The bacterial suspension was then centrifuged again, and the cleared supernatants were collected. The L. paracasei cells from 2 mL of overnight cultures were harvested via centrifugation and suspended in 100 µL of phosphate-buffered saline (PBS), followed by bead beating with a FastPrep Instrument (MP Biomedicals, Santa Ana, CA, USA). Cellular debris was removed by centrifugation, and cell extracts were subsequently obtained. The extracts of E. coli or L. paracasei were mixed with an equal volume of 2×Laemmli buffer (Bio-Rad, Hercules, CA, USA). Subsequently, 18 µL of the extracted proteins were separated on an 8% polyacrylamide gel by sodium dodecyl sulfate (SDS)- polyacrylamide gel electrophoresis (PAGE). The gels were electroblotted using a PVDF membrane (Millipore, Burlington, MA, USA). The membranes were then treated with 1% bovine serum albumin and 0.05% Tween20 in PBS containing the appropriate antibodies. The specific signals were developed with Luminata Forte Western HRP Substrate (Millipore) and visualized with a ChemiDoc system (Bio-Rad). Primary antibodies were obtained from Clontech (anti-Guide-it Cas9 polyclonal Abs) or prepared by immunizing mice (antiserum against SirA) [16]. An anti-rabbit/mouse IgG conjugated to horseradish peroxidase (HRP) (Sigma-Aldrich, St. Louis, MO, USA) was used as the secondary antibody.
RESULTS
Construction of CRISPR-Cas9 plasmid for targeted DNA cleavage in E. coli
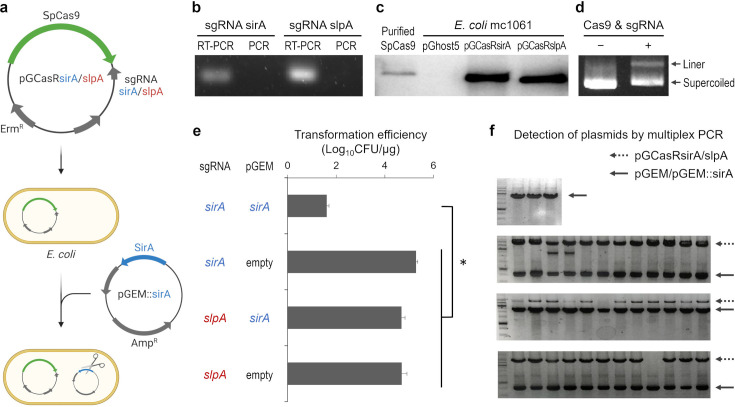
The CRISPR-Cas9 system for E. coli is composed of two plasmids: a plasmid carrying SpCas9, an sgRNA, and a plasmid containing the target gene (Fig. 1a). Two sgRNAs were designed, one targeting the sirA gene of L. paracasei and the other targeting the slpA gene of Lactobacillus acidophilus. An sgRNA targeting slpA was used as a control. Plasmids carrying SpCas9 and sgRNAs targeting sirA or slpA were constructed using the thermosensitive broad-host-range plasmid pG+host5. The constructed plasmids, pGCasRsirA and pGCasRslpA, were introduced into E. coli MC1061. RT-PCR using sgRNA-specific primers showed that the sgRNAs were expressed in E. coli harboring pGCasRsirA or pGCasRslpA (Fig. 1b). Expression of the Cas9 protein in E. coli strains was confirmed by western blotting using an anti-Cas9 antibody. As shown in Fig. 1c, a Cas9-specific band was detected in E. coli harboring pGCasRsirA or pGCasRslpA, but not in E. coli harboring the empty vector pG+host5. To construct a plasmid containing the target sirA gene, sirA was cloned into the pGEM-T Easy vector. The constructed plasmid, pGEM::sirA, was incubated with purified SpCas9 and synthesized sgRNA targeting sirA and analyzed by agarose gel electrophoresis. The results showed a linearized plasmid, indicating the cleavage of pGEM::sirA by SpCas9 (Fig. 1d). To determine the activity of SpCas9 in E. coli, E. coli cells harboring pGCasRsirA or pGCasRslpA were transformed with each of the plasmids, pGEM::sirA, or the empty vector, after which we calculated the transformation efficiency. When E. coli harboring pGCasRsirA was transformed with pGEM::sirA, the transformation efficiency was much lower than that of other transformations (Fig. 1e). This result indicates that SpCas9 cleaved pGEM::sirA in E. coli. We further detected plasmids in colonies by colony PCR using primers specific to both the pGCasR and pGEM plasmids. In colonies stemming from the transformation of E. coli harboring pGCasRsirA with pGEM::sirA, only the amplicon specific to pGEM::sirA was detected (Fig. 1f), albeit grown on plates containing ampicillin and erythromycin, the antibiotic markers for each plasmid. The reason for this inconsistency might be due to the mutations in the PCR detection site of pGCasRsirA or the integration of pGCasRsirA into the chromosome of E. coli. In contrast, amplicons specific to the pGCasR and pGEM plasmids were detected in most colonies from other transformations. These results suggest that both the pGCasRsirA and pGEM::sirA plasmids could not co-exist in E. coli due to SpCas9 activity.
Fig. 1.
Construction of CRISPR-Cas9 plasmids for targeted DNA cleavage in E. coli.
(a) The system components and procedure of CRISPR-Cas9 in E. coli. Plasmids carrying SpCas9 and sgRNAs, which target the sirA or slpA genes (pGCasRsirA/slpA), were introduced into E. coli MC1061. The E. coli strains were then transformed using plasmids with or without the target gene (sirA), and the transformation efficiencies were calculated. (b) Transcription of the sgRNAs in E. coli strains harboring pGCasRsirA or pGCasRslpA was detected by RT-PCR. PCR without reverse transcription was performed to detect contaminated DNA. (c) Expression of Cas9 protein in the E. coli strains was detected by western blotting using an anti-Cas9 antibody. (d) In vitro cleavage of a plasmid containing sirA (pGEM::sirA) by purified SpCas9 and synthesized sgRNA. The pGEM::sirA plasmid was incubated with purified SpCas9 and synthesized sgRNA targeting sirA at 37°C for 3 hr and then analyzed by agarose gel electrophoresis. (e) Transformation efficiency of the E. coli harboring pGCasRsirA or pGCasRslpA with pGEM::sirA or pGEM empty vector. Values are presented as the mean + standard deviation (SD) (n=4). Significant differences are indicated by an asterisk (*p<0.01; Welch’s t-test). (f) Detection of plasmids in the colonies from the transformations by colony PCR was performed via primers specific to both pGCasR and pGEM plasmids.
Partial inhibition of DNA cleavage of SpCas9 by BRD0539 in E. coli
The inhibitory activity of the synthesized BRD0539, a SpCas9 inhibitor, was first examined in an in vitro cleavage assay (Supplementary Fig. 1). The DNA fragment containing the sirA gene was mixed with purified SpCas9, synthesized sgRNA targeting sirA, and various concentrations of BRD0539. After incubation at 37°C for 3 hr, the DNA fragment was analyzed by agarose gel electrophoresis. As shown in Supplementary Fig. 1b, the synthesized BRD0539 showed dose-dependent inhibition of SpCas9. The inhibitory activity was high at concentrations above 50 μM. To confirm the cytotoxicity of BRD0539 to bacteria, E. coli and L. paracasei were propagated in broth containing 10 to 100 μM BRD0539. Using this approach, we did not observe significant growth inhibition of either E. coli or L. paracasei (Supplementary Fig. 2). The synthesized BRD0539 was used for the CRISPR-Cas9 system in E. coli, where E. coli cells harboring pGCasRsirA were transformed with pGEM::sirA or pGEM empty vector. The transformants were plated on LB plates with or without 100 μM BRD0539, and the transformation efficiency was calculated. As shown in Fig. 2a, the efficiency of transformation using the empty pGEM vector did not change in the presence or absence of BRD0539. In contrast, the transformation using pGEM::sirA increased in the presence of BRD0539, indicating that BRD0539 inhibited the cleavage of pGEM::sirA by SpCas9. However, the efficiency of transformation with pGEM::sirA was lower than that with the empty vector, even in the presence of BRD0539. This implies that BRD0539 did not completely inhibit the activity of SpCas9 in E. coli. Plasmids in colonies were detected by colony PCR using primers specific to both the pGCasR and pGEM plasmids. In the transformations using pGEM::sirA, only the amplicon specific to pGEM::sirA was detected in colonies grown on LB plates without BRD0539, whereas both plasmids were detected in a small number of colonies grown on LB plates containing BRD0539 (Fig. 2b). However, most of the colonies detected were either positive for pGCasRsirA or pGEM::sirA. Amplicons specific to the pGCasRsirA and pGEM plasmids were detected in all colonies from the transformations using the pGEM empty vector. Taken together, these results underscore that BRD0539 partially inhibits the activity of SpCas9 in E. coli.
Fig. 2.
Inhibition of DNA cleavage activity of SpCas9 by BRD0539 in E. coli.
(a) Transformation efficiency of E. coli harboring pGCasRsirA with pGEM::sirA or the pGEM empty vector. The transformants were grown on LB plates with or without 100 μM BRD0539. Values are presented as the mean + SD (n=2 to 4). Statistical significance was assessed by Welch’s t-test. (b) Detection of plasmids in the colonies from the transformations by colony PCR was performed via primers specific to both pGCasR and pGEM plasmids.
Construction of a CRISPR-Cas9 plasmid for targeted DNA cleavage in L. paracasei, and partial inhibition of CRISPR-induced cell death by BRD0539
A plasmid carrying SpCas9 and sirA-specific sgRNAs was constructed using the Lactobacillus expression vector pLP401 (Fig. 3a). The constructed plasmid, pLPCasRsirA, was introduced into the sirA deletion mutant of L. paracasei (ΔsirA). Subsequently, RT-PCR using the sgRNA-specific primers showed that the sgRNA was expressed in L. paracasei ΔsirA harboring pLPCasRsirA (Fig. 3b). The expression of the Cas9 protein in the L. paracasei strain was detected by western blotting using an anti-Cas9 antibody. As depicted in Fig. 3c, a Cas9-specific band was detected in the L. paracasei strain but not in L. paracasei harboring the pLP401empty vector. To test the activity of SpCas9 in L. paracasei, the wild-type (WT) and ΔsirA strains of L. paracasei were transformed with pLPCasRsirA, and the transformation efficiency was calculated. The transformation efficiency varied between strains, with that of L. paracasei WT being markedly decreased, with no detectable colonies (Fig. 3d). This result indicated that SpCas9 cleaved the sirA gene in the chromosome of L. paracasei WT and induced cell death. This lethality was alleviated with the use of BRD0539, whereas the transformation efficiency of the WT strain remained lower than that of ΔsirA even in the presence of BRD0539 (Fig. 3e). Colony PCR using primers specific for pLPCasRsirA showed that all colonies from the transformation contained pLPCasRsirA (Fig. 3f). Collectively, these findings highlight that BRD0539 partially inhibits the activity of SpCas9 in L. paracasei, similar to E. coli.
Fig. 3.
Construction of a CRISPR-Cas9 plasmid for targeted DNA cleavage in L. paracasei, and partial inhibition of CRISPR-induced cell death by BRD0539.
(a) The system components and procedure of CRISPR-Cas9 in L. paracasei. An SpCas9 and sgRNA, which targets the sirA-carrying plasmid (pLPCasRsirA), was introduced into the wild-type (WT) and ΔsirA strains of L. paracasei. The transformants were grown on MRS plates with or without BRD0539, and the transformation efficiencies were calculated. (b) Transcription of sgRNA in L. paracasei ΔsirA harboring pLPCasRsirA was detected by RT-PCR. PCR without reverse transcription was performed to detect contaminated DNA. (c) Expression of Cas9 protein in the recombinant L. paracasei was detected by western blotting using an anti-Cas9 antibody. (d) Transformation efficiency of the ΔsirA and WT L. paracasei strains with pLPCasRsirA. (e) Inhibition of SpCas9 nuclease activity by BRD0539 in the CRISPR-Cas9 system for L. paracasei. The transformants were grown on MRS plates with or without 100 μM BRD0539. Values are presented as the mean + SD (n=3). Significant differences are indicated by an asterisk (*p<0.05; Welch’s t-test or Tukey’s multiple comparison test). (f) Detection of the plasmid in colonies from the transformations by colony PCR was performed via primers specific to pLPCasRsirA.
Construction of a CRISPRi system in L. paracasei
Next, we developed a CRISPRi system for L. paracasei. The gene encoding for dCas9 derived from S. pyogenes Cas9 was inserted into the chromosome of L. paracasei IGM394 (WT) via double crossover, using the thermosensitive plasmid pBTE (Fig. 4a). Western blotting using an anti-Cas9 antibody revealed that dCas9 was expressed in the L. paracasei mutant (Fig. 4b). As shown in Fig. 4c, two sgRNAs targeting the non-template DNA strand upstream or downstream of the sirA gene were designed. The sgRNAs were cloned into the pG+host5, and the constructed plasmids, pGRsirA-up or pGRsirA-down, were then introduced into the WT or dCas9-expressing strains of L. paracasei. The transcription of sgRNAs in L. paracasei strains harboring pGRsirA-up or pGRsirA-down was confirmed by RT-PCR (Fig. 4d). To examine whether dCas9 and sgRNAs could repress the sirA gene expression, RT-PCR using sirA-specific primers and western blotting using an anti-SirA antibody were performed with the recombinant L. paracasei strains. As expected, the co-expression of dCas9 and sgRNAs repressed the transcription of sirA, and the repression effect was higher with the sgRNA targeting the upstream region of sirA than that with the other sgRNA (Fig. 4e).
Fig. 4.
Construction of a CRISPRi system in L. paracasei.
(a) Genetic maps of the Cas locus in the L. paracasei wild type (WT) and the dCas9-expressing strain (dCas9). The genome accession number and position of the Cas locus in L. paracasei BL23 are shown above the genetic maps. (b) Expression of dCas9 protein in the recombinant L. paracasei was detected by western blotting using an anti-Cas9 antibody. (c) The target site and sequence of two sgRNAs, sirA-up and sirA-down. (d) Transcription of the sgRNAs in L. paracasei strains harboring a plasmid with sirA-up or sirA-down was detected by reverse transcription polymerase chain reaction (RT-PCR). PCR without reverse transcription was performed to detect contaminated DNA. (e) Repression of sirA expression by the CRISPRi system in L. paracasei. Two sgRNA-expression plasmids (pGRsirA-up/down) and an empty vector (pG+host5) were individually introduced into L. paracasei WT and dCas9. The expression of sirA and SirA protein in the L. paracasei mutants was detected by RT-PCR and western blotting using an anti-SirA antibody. Transcription of gyrB, a housekeeping gene, in the L. paracasei mutants was also detected by RT-PCR as a control. sirA-D, sirA-down; sirA-U, sirA-up.
BRD0539 dose-dependent inhibition of CRISPRi in L. paracasei
We used BRD0539 as the CRISPRi system for L. paracasei. Recombinant L. paracasei strains were cultured in MRS broth containing various concentrations of BRD0539, and SirA expression in the L. paracasei strains was analyzed by western blotting using an anti-SirA antibody. In the strains expressing dCas9 and sgRNAs, BRD0539 inhibited the SirA protein expression in a dose-dependent manner (Fig. 5). In the L. paracasei strain expressing dCas9 and the sgRNA targeting the upstream region of sirA, the use of 10 μM BRD0539 yielded only marginal expression of the SirA protein, and its expression was more clearly detected with 100 μM BRD0539. Together, these results indicate that BRD0539 can also function as an inhibitor of the CRISPRi system in L. paracasei.
Fig. 5.
Inhibition of CRISPR interference by BRD0539 in L. paracasei.
The L. paracasei wild type (WT) and dCas9-expressing strain (dCas9) harboring an sgRNA-expression plasmid (pGRsirA-up/down) or an empty vector (pG+host5) were grown in MRS containing 10 to 100 μM BRD0539. Expression of the SirA protein was detected by western blotting using an anti-SirA antibody. sirA-D, sirA-down; sirA-U, sirA-up. The data without BRD0539 are reproduced from Fig. 4e.
DISCUSSION
The CRISPR-Cas9 system is a revolutionary genome editing tool for eukaryotes. In prokaryotes, however, the double-strand break in chromosomal DNA often results in lethality. Therefore, CRISPR-Cas9 has been used in bacteria as a sequence-dependent selection pressure to screen for recombinants and mutants generated by existing methods instead of direct genome editing [9]. In this process, the action of CRISPR-Cas9 needs to work after the desired mutation/recombination has occurred in the target population. The aim of this study was to apply an SpCas9 inhibitor for several CRISPR-Cas tools in E. coli and L. paracasei, so as to expand the usability of the CRISPR-Cas system in bacteria.
A plasmid-based CRISPR-Cas9 system was established and applied to both E. coli and L. paracasei. In E. coli expressing CRISPR-Cas9 against the sirA gene, the transformation efficiency decreased significantly only when the target sequence was also present. The limited surviving colonies failed to maintain the introduced plasmid; thus, the CRISPR-Cas9 system was considered effective. We then introduced BRD0539 to the transformation step and observed that the transformation efficiency improved by comparison. Although only a small portion of the transformants possessed the two expected plasmids, our results indicate that the inhibitor likely contributed to the suppression of Cas9 activity. Similarly, we observed a CRISPR-dependent deficiency in the transformation of L. paracasei, which improved when BRD0539 was added to the culture. The effect of the inhibitor on this system was not as significant as initially expected. Nonetheless, a proof of concept for the suppression of the CRISPR-Cas9 system for bacteria using BRD0539 was achieved through these experiments. In general, an efficient selection pressure is required to select mutants and recombinants that are present in only a very small fraction of the total cell population. While often this does not pose an issue when a selection marker such as drug resistance or auxotrophy is an option, such selection pressures are largely unavailable in most screening processes. This can in turn limit the ability to screen for specific mutants with the desired gene deletions or point mutations. CRISPR-Cas9 can overcome this issue by setting the DNA sequence under selective pressure. In this application, CRISPR-Cas9 acts only after the target cells have arisen in the cell population. Transformation of a cell population containing the mutant with a CRISPR-Cas9-containing plasmid is a possible strategy; however, this approach highly depends on the transformation efficiency of the host because only the cells that have incorporated the CRISPR-Cas9 vector are subject to screening. To include the entire cell population for screening, repressed CRISPR-Cas9 was pre-installed before the desired mutation occurred, and then nuclease activity was activated. The suppression of CRISPR-Cas9 using inducible promoters such as Pnis is an option [13], although many inducible promoters exhibit basal-level expression even before induction [20, 21], which could be critical in this application. An alternative approach using inhibitors, such as BRD0539 described in this study, may provide a simple solution to this challenge.
As BRD0539 could interfere with SpCas9 (D10A and H840A), the dCas9 CRISPRi system could also be controlled using the inhibitor. To test this hypothesis, a CRISPRi system was established for L. paracasei. Since plasmids including the SpCas9 gene appeared to be unstable, a recombinant L. paracasei in which dCas9 gene was inserted into the genome was constructed. Targeted sequences complemented by sgRNA were chosen from the promoter region and end of the sirA gene because the binding site of sgRNA affects the efficiency of CRISPRi [3]. No gene expression was observed when the promoter was targeted, whereas the suppression of gene expression was limited when the target was the downstream site. As expected, gene silencing by CRISPRi could be abolished by the addition of BRD0539 to the medium. These results indicated the effectiveness of this inhibitor in regulating CRISPRi. Gene silencing is a useful technique, especially in instances where gene knockout or gene deletion is not applicable in eukaryotic or prokaryotic cells. Recently, CRISPRi has become an important tool for the genetic analysis of bacteria [13, 22,23,24]. The ability to regulate gene expression in an inhibitor-dependent manner demonstrated in this study may be useful for the detailed analysis of gene function.
In summary, this study demonstrated the utility of a small-molecule Cas9 inhibitor as an auxiliary tool upon genetic modification and CRISPRi. One of the remaining obstacles is that complete inhibition of Cas9 requires the addition of BRD0539 at high concentrations. In addition, this inhibitor may affect the expression of other genes. Nevertheless, the ability to control Cas9 activity by simply adding an inhibitor to the culture medium is a convenient and versatile method. This technique in the CRISPRi system may allow for the detailed analysis of essential genes that cannot be deleted or knocked out by regulating their expression levels and timing. It may also be applied for biological containment in biotherapeutics using lactic acid bacteria. For example, recombinants designed to cleave chromosomal DNA or deplete essential gene expression by CRISPR tools can only grow in the presence of the inhibitor, and such recombinants may not be viable in the environment. In conclusion, this study expands the toolbox of CRISPR-Cas technology for lactic acid bacteria by the application of the inhibitor compound BRD0539.
FUNDING
This work was supported by the Tokyo University of Agriculture. The funding body played no role in the study design, article preparation, or publication.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
Supplementary Material
Acknowledgments
This work was supported by the Tokyo University of Agriculture. Graphical illustrations were made with Biorender.com.
REFERENCES
- 1.Doudna JA, Charpentier E. 2014. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346: 1258096. [DOI] [PubMed] [Google Scholar]
- 2.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152: 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JY, Doudna JA. 2023. CRISPR technology: a decade of genome editing is only the beginning. Science 379: eadd8643. [DOI] [PubMed] [Google Scholar]
- 5.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315: 1709–1712. [DOI] [PubMed] [Google Scholar]
- 6.Garneau JE, Dupuis MÈ, Villion M, Romero DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadán AH, Moineau S. 2010. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468: 67–71. [DOI] [PubMed] [Google Scholar]
- 7.Gomaa AA, Klumpe HE, Luo ML, Selle K, Barrangou R, Beisel CL. 2014. Programmable removal of bacterial strains by use of genome-targeting CRISPR-Cas systems. MBio 5: e00928–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vercoe RB, Chang JT, Dy RL, Taylor C, Gristwood T, Clulow JS, Richter C, Przybilski R, Pitman AR, Fineran PC. 2013. Cytotoxic chromosomal targeting by CRISPR/Cas systems can reshape bacterial genomes and expel or remodel pathogenicity islands. PLoS Genet 9: e1003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh JH, van Pijkeren JP. 2014. CRISPR-Cas9-assisted recombineering in Lactobacillus reuteri. Nucleic Acids Res 42: e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song X, Huang H, Xiong Z, Ai L, Yang S. 2017. CRISPR-Cas9D10A Nickase-assisted genome editing in Lactobacillus casei. Appl Environ Microbiol 83: e01259–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leenay RT, Vento JM, Shah M, Martino ME, Leulier F, Beisel CL. 2019. Genome editing with CRISPR-Cas9 in Lactobacillus plantarum revealed that editing outcomes can vary across strains and between methods. Biotechnol J 14: e1700583. [DOI] [PubMed] [Google Scholar]
- 12.Goh YJ, Barrangou R. 2019. Harnessing CRISPR-Cas systems for precision engineering of designer probiotic lactobacilli. Curr Opin Biotechnol 56: 163–171. [DOI] [PubMed] [Google Scholar]
- 13.Berlec A, Škrlec K, Kocjan J, Olenic M, Štrukelj B. 2018. Single plasmid systems for inducible dual protein expression and for CRISPR-Cas9/CRISPRi gene regulation in lactic acid bacterium Lactococcus lactis. Sci Rep 8: 1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleerebezem M, Beerthuyzen MM, Vaughan EE, de Vos WM, Kuipers OP. 1997. Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl Environ Microbiol 63: 4581–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maji B, Gangopadhyay SA, Lee M, Shi M, Wu P, Heler R, Mok B, Lim D, Siriwardena SU, Paul B, et al. 2019. A high-throughput platform to identify small-molecule inhibitors of CRISPR-Cas9. Cell 177: 1067–1079.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres-Barredo GA, Atarashi H, Kajikawa A, Hirata A, Endo A, Nakagawa J. 2018. Intracellular localization of sirtuin and cell length analysis of Lactobacillus paracasei suggest possible role of sirtuin in cell division and cell shape regulation. Biosci Biotechnol Biochem 82: 1–10. [DOI] [PubMed] [Google Scholar]
- 17.Naraki S, Igimi S, Sasaki Y. 2020. NADH peroxidase plays a crucial role in consuming H2O2 in Lactobacillus casei IGM394. Biosci Microbiota Food Health 39: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerard B, O’Shea MW, Donckele E, Kesavan S, Akella LB, Xu H, Jacobsen EN, Marcaurelle LA. 2012. Application of a catalytic asymmetric Povarov reaction using chiral ureas to the synthesis of a tetrahydroquinoline library. ACS Comb Sci 14: 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerard B, O’Shea MW, Donckele E, Kesavan S, Akella LB, Xu H, Jacobsen EN, Marcaurelle LA. 2014. Erratum: Application of a catalytic asymmetric povarov reaction using Chiral ureas to the synthesis of a tetrahydroquinoline library (ACS Combinatorial Science (2012) 14 (621–630) DOI: 10.1021/co300098v). ACS Comb Sci 16: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryan EM, Bae T, Kleerebezem M, Dunny GM. 2000. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid 44: 183–190. [DOI] [PubMed] [Google Scholar]
- 21.Duong T, Miller MJ, Barrangou R, Azcarate-Peril MA, Klaenhammer TR. 2011. Construction of vectors for inducible and constitutive gene expression in Lactobacillus. Microb Biotechnol 4: 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis NA, Kim B, Tung J, Machner MP. 2021. A multiplex CRISPR interference tool for virulence gene interrogation in Legionella pneumophila. Commun Biol 4: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhary E, Thakur P, Pareek M, Agarwal N. 2015. Gene silencing by CRISPR interference in mycobacteria. Nat Commun 6: 6267. [DOI] [PubMed] [Google Scholar]
- 24.Peters JM, Koo BM, Patino R, Heussler GE, Hearne CC, Qu J, Inclan YF, Hawkins JS, Lu CHS, Silvis MR, et al. 2019. Enabling genetic analysis of diverse bacteria with Mobile-CRISPRi. Nat Microbiol 4: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law J, Leenhouts K, Venema G, Kok J, Haandrikman A, Buist G. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fLaw, J., Leenhouts, K., Venema, G., Kok, J., Haandrikman, A., & Buist, G. (1995). A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of. J Bacteriol 177: 7011–7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas I, Gruss A, Ehrlich SD, Maguin E. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J Bacteriol 175: 3628–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pouwels PH, Vriesema A, Martinez B, Tielen FJ, Seegers JFML, Leer RJ, Jore J, Smit E. 2001. Lactobacilli as vehicles for targeting antigens to mucosal tissues by surface exposition of foreign antigens. Methods Enzymol 336: 369–389. [DOI] [PubMed] [Google Scholar]
- 28.Satoh E, Kajikawa A, Okada S, Tsuji A. 2013. Metabolic analysis of a phosphotransferase system-deficient strain of Lactobacillus plantarum NCIMB 8826. Nihon Shokuhin Hozo Kagakkaishi 39: 319–324 (in Japanese). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.