Abstract
Menopausal users of hormone replacement therapy (HRT) are at increased breast cancer risk and decreased colorectal cancer (CRC) risk compared with individuals who have never used HRT, but these opposing associations may differ by familial risk of breast cancer and CRC. We harmonized data from 3 cohorts and generated separate breast cancer and CRC familial risk scores based on cancer family history. We defined moderate or strong family history as a risk score of 0.4 or higher, where 0.4 was equivalent to a 50-year-old woman with 1 parent diagnosed with either breast cancer or CRC at 55 years of age. Of 24 486 women assessed, 1243 and 405 were diagnosed with incident breast cancer and CRC, respectively. For breast cancer, menopausal HRT ever use versus never use hazard ratios were 1.27 (95% CI = 1.11 to 1.45) for a breast cancer familial risk score below 0.4 and 1.01 (95% CI = 0.82 to 1.25) for a breast cancer familial risk score of 0.4 or higher (Pdifference = .08). For CRC, menopausal HRT hazard ratios were 0.63 (95% CI = 0.50 to 0.78) for a CRC familial risk score below 0.4 and 1.21 (95% CI = 0.73 to 2.00) for a CRC familial risk score of 0.4 or higher (Pdifference = .03). Associations with menopausal HRT use that apply to the general population may not hold for women at moderate or strong familial risk of these cancers.
Epidemiological studies have found that women who have ever used menopausal hormone therapy, commonly refered to as hormone replacement therapy (HRT), are at approximately 20% increased risk of breast cancer but at approximately 20% decreased risk of colorectal cancer (CRC) compared with women of the same age who have never used HRT,1,2 although only a small proportion of women in these studies had a family history of these cancers. When counseling patients about risk, a common approach to estimate the overall risk from family history and menopausal HRT is to multiply the 2 relative risks, then multiply the product by the absolute risk for people without a family history and who do not use menopausal HRT.3 Studies using polygenic risk scores show that both breast cancer and CRC associations were strongest in the highest quintile of risk,4,5 but these polygenic risk scores explain less than 20% of the familial relative risk.6,7 As we observed differences in breast cancer risk for other exposures due to family history,8,9 we examined whether these menopausal HRT associations also applied to women at higher baseline risk because of their family history of cancer—a key clinical issue for risk management in such individuals seen in cancer genetic clinics because most of them are found not to carry high-risk genetic variations.10
We harmonized data from 3 international cohorts: the Prospective Family Study Cohort (ProF-SC) baseline data from 1992 to 2011, the Colon Cancer Family Registry Cohort (CCFRC) baseline data from 1997 to 2012, and the Melbourne Collaborative Cohort Study (MCCS) follow-up visit 2 data from 2003 to 2007. The ProF-SC comprises baseline and follow-up data from the Breast Cancer Family Registry Cohort, formed by a collaboration among 6 centers in the United States, Canada, and Australia and the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer.11 The CCFRC was formed by a collaboration among 7 sites in the United States, Canada, and Australia.12 The MCCS is a prospective study of participants recruited in Melbourne, Australia.13 All participants provided written informed consent before enrollment, and the study protocols were approved by institutional review boards. We harmonized cancer risk factor data collected using questionnaires, which captured demographic characteristics; height and weight; reproductive history; lifestyle factors; and first-degree family history of breast cancer and CRC, including age at diagnosis. Information about vital status, with date or age of death (where applicable), was obtained from population registries and proxy reports. We sought confirmation of all reported invasive breast cancer and CRC diagnoses and ages at diagnosis for participants using pathology reports, medical records, cancer registry reports, and death certificates, where possible.11,13
Eligible participants included women aged 45 to 75 years at baseline without a personal history of any cancer and not known to have pathogenic (or likely pathogenic) variants in BRCA1, BRCA2, MLH1, MSH2, MSH6, or PMS2. For breast cancer and CRC, we generated the following:
familial risk score = log((individual 5-year risk calculated by Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm14 and CRISP15,16 risk tools, respectively) / population average risk for each age)
We modeled risk associations with the breast cancer and CRC familial risk scores as continua, and they did not diverge appreciably from linearity (P > .05). As the median familial risk score in these cohorts was 0.4 for women with a family history of either disease, we defined moderate to strong family history as a risk score of 0.4 or above, where 0.4 was equivalent to a 50-year-old woman with 1 parent diagnosed with either cancer before 55 years of age. Time at risk of breast cancer or CRC started at 2 months after the baseline questionnaire (to exclude undetected cancers at baseline) and continued to the first of date of diagnosis of invasive breast cancer or CRC, date last known to be undiagnosed with breast cancer or CRC, date of death, or (for breast cancer) date of bilateral mastectomy. We used Cox regression, with age as the time scale, to estimate hazard ratios and 95% confidence interval (CI) for menopausal HRT use, stratified by study and adjusted for body mass index, parity, education level, alcohol consumption, smoking status, oral contraceptive use, and country of residence. We evaluated menopausal HRT use by never or ever use; secondary analyses included never, former, or current use; age at baseline (<60 or ≥60 years); duration of menopausal HRT use (<5 or ≥5 years); and whether women had had a hysterectomy, all measured at baseline. We assumed that women on menopausal HRT were using a combined therapy unless they reported having had a hysterectomy, in which case we assumed the use of estrogen alone. For all analyses, the referent group was women who reported never on menopausal HRT. Tests of the proportional hazards assumption were based on Schoenfeld residuals. A robust variance estimator was used to account for multiple family members within the cohorts. We specified cross-product terms to test for multiplicative interactions of menopausal HRT with breast cancer and CRC familial risk scores. Statistical significance was determined as P less than .05 for a 2-sided hypothesis test. Analyses were conducted using Stata, version 16.1, statistical software (StataCorp LP).
There were 310 789 person-years of observation of 24 488 women (ProF-SC, N = 6181; CCFRC, N = 6726; MCCS, N = 11 581), of which 1243 individuals were diagnosed with incident breast cancer and 405 with incident CRC (Table S1). Hazard ratio estimates for ever use vs never use of menopausal HRT, not stratified by family history, were 1.20 (95% CI = 1.07 to 1.34) for breast cancer and 0.73 (95% CI = 0.59 to 0.90) for CRC. Menopausal HRT ever use vs never use hazard ratios by continuous variables of breast cancer–specific and CRC-specific familial risk scores show that the confidence intervals cross 1.0 at a familial risk score of approximately 0.4 for both breast cancer and CRC (Figure 1). For breast cancer, menopausal HRT hazard ratios were 1.28 (95% CI = 1.12 to 1.46) for a breast cancer–specific familial risk score less than 0.4 and 1.02 (95% CI = 0.83 to 1.27) for a score of 0.4 or higher (Pdifference = .08). For CRC, menopausal HRT hazard ratios were 0.66 (95% CI = 0.53 to 0.83) for a CRC-specific familial risk score below 0.4 and 1.21 (95% CI = 0.74 to 1.98) for a score of 0.4 or higher (Pdifference = .03).
Figure 1.
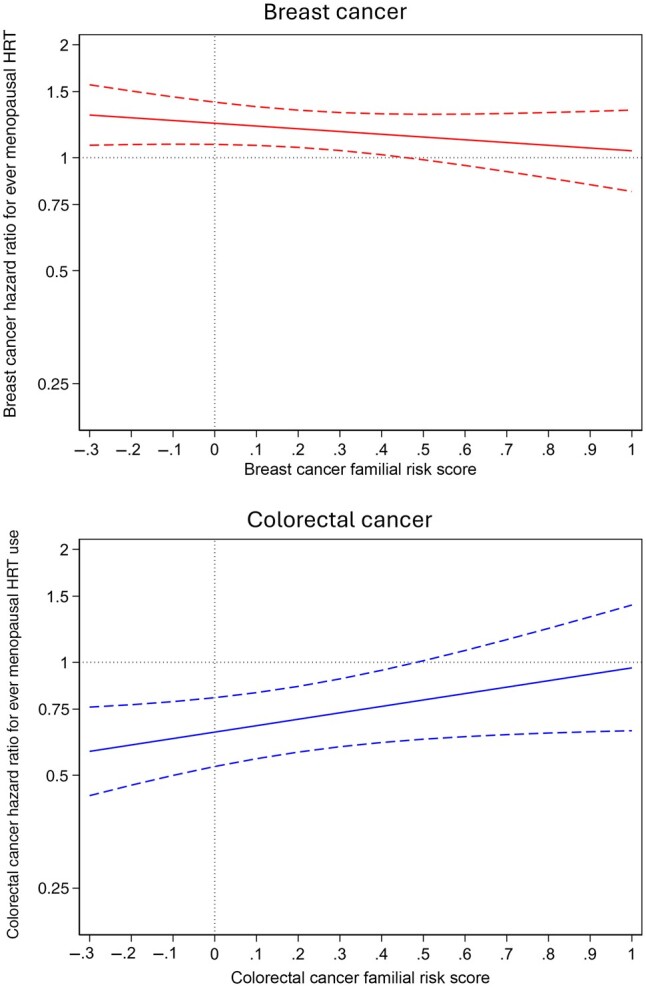
Hazard ratios (solid lines) and 95% confidence intervals (dashed lines) of breast and colorectal cancer risk in relation to ever vs never use of menopausal HRT by their cancer-specific familial risk score. A familial risk score of 0 denotes the population average. Analyses were stratified by study and adjusted for body mass index (continuous), parity (0, 1, 2, 3, ≥4 live births), education level (high school or less, some college or university, bachelor’s degree or higher), smoking status (never, former, current), oral contraceptive use (never, ever), and country of residence (Australia, Canada, United States). HRT = hormone replacement therapy.
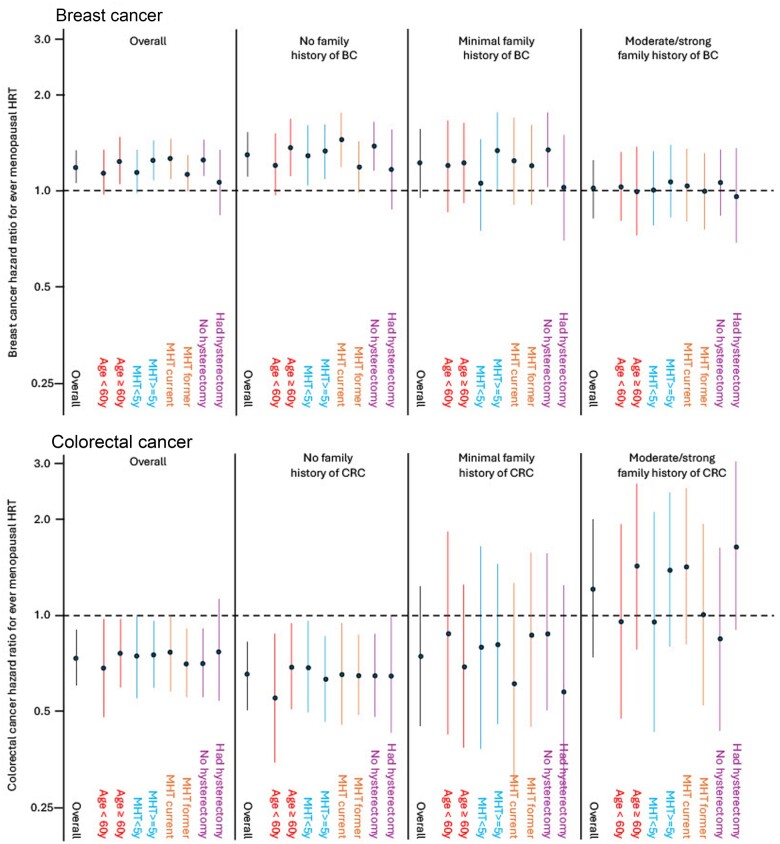
Breast cancer risk secondary analyses did not find any major differences (all Pinteraction > .05), except for family history of breast cancer (Figure 2)—that is, there was no evidence for multiplicative risk of menopausal HRT use by family history. For women without a family history of CRC, there was no evidence of differences in the negative risk associations by subgroups (all Pinteraction > .05). For women with a family history of CRC, there was no evidence of an association with decreased risk for any of the subgroups of menopausal HRT use, although hazard ratios were imprecise.
Figure 2.
Hazard ratios (dots) and 95% confidence intervals (lines) of breast cancer and CRC risk in relation to subgroups of menopausal HRT use, by groups’ cancer-specific familial risk score. Minimal family history was defined as having a familial risk score greater than 0 and less than 0.4; moderate to strong family history was defined as having a familial risk score of 0.4 or higher. Analyses were stratified by study and adjusted for body mass index (continuous), parity (0, 1, 2, 3, ≥4 live births), education level (high school or less, some college or university, bachelor’s degree or higher), smoking status (never, former, current), oral contraceptive use (never, ever), and country of residence (Australia, Canada, United States). CRC = colorectal cancer; HRT = hormone replacement therapy.
Using a pooled analysis of 3 large prospective cohorts, we demonstrated the utility of examining the associations of menopausal HRT use across 2 common cancers, stratifying on cancer family history. Just as breast cancer and CRC have distinct etiologies, as reflected in the different directions of their associations with menopausal HRT use, these results suggest that they may also be the distinct etiologies within a given cancer for women with a family history of that cancer. Cancer risks for individuals with a moderate to strong family history may be influenced more by early-life exposures rather than hormone exposures such as menopausal HRT later in life.17 It is, however, currently unknown how menopausal HRT use could influence the risk of CRC.18
A key strength of this study was that we collected data to estimate a woman’s absolute risks of breast cancer and CRC based on the number of relatives with these cancers as well as the relatives’ ages at cancer diagnosis. Given our oversampling of cancer families, we were able to informatively study risk estimates for women across the continuum of familial risk scores as well as stratify the results based on no, minimal, or moderate to strong family history; there were at least 50 incident breast or colorectal cancer cases for most subanalyses. Further, we adjusted for several potential confounders measured in similar ways and data harmonized. Some women with moderate to strong family history may have chosen not to take menopausal HRT because of worries of their risk being further increased, but most of the participants were exposed before the landmark Women’s Health Initiative clinical trial results were published in 2002.19 Time-updated menopausal HRT use data were not available in our cohorts to explore this issue further.
Our results suggest that the potentially harmful and beneficial associations of menopausal HRT observed for breast cancer and CRC, respectively, for women in the general population may not apply to women with a first-degree family history of either cancer. Specifically, we found that use of menopausal HRT may not affect breast cancer or CRC risk for women with moderate to strong family histories. If replicated, these results support the need to understand mechanistically why the associations with menopausal HRT differ according to a woman’s family history of cancer.
Supplementary Material
Acknowledgments
We would like to thank all the investigators, staff, and participants of the Breast Cancer Family Registry. We wish to thank all the The Kathleen Cuningham Foundation Consortium for Research into Familial Aspects of Breast Cancer (kConFab) research nurses and staff, the heads and staff of the Family Cancer Clinics, and the Clinical Follow Up Study (which has received funding from the National Health and Medical Research Council, the National Breast Cancer Foundation, Cancer Australia, and the US National Institutes of Health [NIH]) for their contributions to this resource and the many families that contribute to kConFab. The CCFR graciously thanks the generous contributions of its study participants, the dedication of study staff, and the financial support from the US National Cancer Institute (NCI), without which this important registry would not exist. We thank the original MCCS investigators and the diligent team that recruited the participants and continue working on follow-up, for their contribution. We also express our gratitude to the many thousands of Melbourne residents who continue to participate in the study. The MCCS cohort recruitment was funded by Cancer Council Victoria (https://www.cancervic.org.au/) and VicHealth (https://www.vichealth.vic.gov.au/).
Contributor Information
Robert J MacInnis, Cancer Epidemiology Division, Cancer Council Victoria, East Melbourne, VIC 3002, Australia; Centre for Epidemiology and Biostatistics, The University of Melbourne, Parkville, VIC 3053, Australia.
Mark A Jenkins, Centre for Epidemiology and Biostatistics, The University of Melbourne, Parkville, VIC 3053, Australia; Cancer Research Centre, University of Melbourne, Parkville, VIC 3053, Australia.
Roger L Milne, Cancer Epidemiology Division, Cancer Council Victoria, East Melbourne, VIC 3002, Australia; Centre for Epidemiology and Biostatistics, The University of Melbourne, Parkville, VIC 3053, Australia; Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Clayton, VIC 3800, Australia.
Esther M John, Department of Epidemiology & Population Health, Stanford University School of Medicine, Stanford, CA 94305, United States; Department of Medicine (Oncology), Stanford University School of Medicine, Stanford, CA 94305, United States; Stanford Cancer Institute, Stanford University School of Medicine, Stanford, CA 94305, United States.
Mary B Daly, Department of Clinical Genetics, Fox Chase Cancer Center, Philadelphia, PA 19111, United States.
Irene L Andrulis, Lunenfeld-Tanenbaum Research Institute, Sinai Health System, Department of Molecular Genetics, University of Toronto, Toronto, ON M5G 1X5, Canada.
Sarah V Colonna, Department of Internal Medicine and Huntsman Cancer Institute, University of Utah, Salt Lake City, UT 84112, United States.
Kelly-Anne Phillips, Centre for Epidemiology and Biostatistics, The University of Melbourne, Parkville, VIC 3053, Australia; Department of Medical Oncology, Peter MacCallum Cancer Centre, Melbourne, VIC 3052, Australia; Sir Peter MacCallum Department of Oncology, The University of Melbourne, VIC 3010, Australia.
kConFab Investigators, Sir Peter MacCallum Department of Oncology, The University of Melbourne, VIC 3010, Australia; The Kathleen Cuningham Foundation Consortium for Research into Familial Aspects of Breast Cancer (kConFab), Research Department, Peter MacCallum Cancer Centre, Melbourne, VIC 3052, Australia.
Loic Le Marchand, Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI 96813, United States.
Polly A Newcomb, Epidemiology Department, University of Washington, Seattle, WA 98195, United States; Public Health Sciences Division, Fred Hutchinson Cancer Center, Seattle, WA 98109, United States.
Amanda I Phipps, Epidemiology Department, University of Washington, Seattle, WA 98195, United States; Public Health Sciences Division, Fred Hutchinson Cancer Center, Seattle, WA 98109, United States.
Stephanie L Schmit, Genomic Medicine Institute, Cleveland Clinic, Cleveland, OH 44196, United States; Population and Cancer Prevention Program, Case Comprehensive Cancer Center, Cleveland, OH 44106, United States.
Finlay A Macrae, Department of Colorectal Medicine and Genetics, Royal Melbourne Hospital, VIC 3052, Australia.
Daniel D Buchanan, Cancer Research Centre, University of Melbourne, Parkville, VIC 3053, Australia; Colorectal Oncogenomics Group, Department of Clinical Pathology, The University of Melbourne, Parkville, VIC 3010, Australia; Genomic Medicine and Family Cancer Clinic, The Royal Melbourne Hospital, Parkville, VIC 3052, Australia.
Steven Gallinger, Lunenfeld-Tanenbaum Research Institute, Sinai Health System, Department of Molecular Genetics, University of Toronto, Toronto, ON M5G 1X5, Canada; PanCuRx Translational Research Initiative, Ontario Institute for Cancer Research, Toronto, ON M5G 1M1, Canada; Hepatobiliary/Pancreatic Surgical Oncology Program, University Health Network, Toronto, ON M5G 2C4, Canada.
Rish K Pai, Department of Laboratory Medicine and Pathology, Mayo Clinic, Scottsdale, AZ 85259, United States.
Niloy J Samadder, Division of Gastroenterology and Hepatology, Department of Medicine, Mayo Clinic, Phoenix, AZ 85259, United States.
Graham G Giles, Cancer Epidemiology Division, Cancer Council Victoria, East Melbourne, VIC 3002, Australia; Centre for Epidemiology and Biostatistics, The University of Melbourne, Parkville, VIC 3053, Australia; Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC 3800, Australia.
Melissa C Southey, Cancer Epidemiology Division, Cancer Council Victoria, East Melbourne, VIC 3002, Australia; Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Clayton, VIC 3800, Australia; Genetic Epidemiology Laboratory, Department of Pathology, The University of Melbourne, Parkville, VIC 3010, Australia.
John L Hopper, Centre for Epidemiology and Biostatistics, The University of Melbourne, Parkville, VIC 3053, Australia.
Mary Beth Terry, Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, NY 10032, United States; Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY 10032, United States.
Author contributions
Robert J. MacInnis, PhD (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Writing—original draft), Melissa C. Southey, PhD (Funding acquisition; Resources; Writing—review & editing), Graham G. Giles, PhD (Funding acquisition; Resources; Writing—review & editing), Niloy J. Samadder, MD (Resources; Writing—review & editing), Rish K. Pai, MD, PhD (Funding acquisition; Resources; Writing—review & editing), Steven Gallinger, MD (Funding acquisition; Resources; Writing—review & editing), Daniel D. Buchanan, PhD (Funding acquisition; Resources; Writing—review & editing), Finlay A. Macrae, MD (Resources; Writing—review & editing), Stephanie L. Schmit, PhD (Funding acquisition; Resources; Writing—review & editing), Amanda I. Phipps, PhD (Funding acquisition; Resources; Writing—review & editing), Polly A. Newcomb, PhD (Funding acquisition; Resources; Writing—review & editing), Loic Le Marchand, MD, PhD (Funding acquisition; Resources; Writing—review & editing), kConFab Investigators, N/A (Resources; Writing—review & editing), Kelly-Anne Phillips, MD (Funding acquisition; Resources; Writing—review & editing), Sarah V. Colonna, MD (Funding acquisition; Resources; Writing—review & editing), Irene L. Andrulis, PhD (Funding acquisition; Resources; Writing—review & editing), Mary B. Daly, MD (Funding acquisition; Resources; Writing—review & editing), Esther M. John, PhD (Funding acquisition; Resources; Writing—review & editing), Roger L. Milne, PhD (Funding acquisition; Project administration; Resources; Writing—review & editing), Mark A. Jenkins, PhD (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Writing—review & editing), John L. Hopper, PhD (Conceptualization; Funding acquisition; Methodology; Resources; Writing—review & editing), Mary Beth Terry, PhD (Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Supervision; Writing—original draft).
Supplementary material
Supplementary material is available at JNCI Cancer Spectrum online.
Funding
The 6 sites of the Breast Cancer Family Registry were supported by UM1 CA164920 from the US NCI. The content of this manuscript does not necessarily reflect the views or policies of the NCI or any of the collaborating centers in the Breast Cancer Family Registry, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government or the Breast Cancer Family Registry.
kConFab and the kConFab Follow-Up Study have received funding support from the National Breast Cancer Foundation, the Australian National Health and Medical Research Council; the National Institute of Health U.S.A.; the Queensland Cancer Fund; the Cancer Councils of New South Wales, Victoria, Tasmania, and South Australia; and the Cancer Foundation of Western Australia.
The CCFR (www.coloncfr.org) is supported in part by funding from the NCI and NIH (award No. U01 CA167551). Additional support for the Seattle PMH-CCFR was through NCI and NIH awards U01/U24 CA074794 and R01 CA076366 (to P.A.N.). Support for case ascertainment was provided in part by the Surveillance, Epidemiology, and End Results Program Arizona, Colorado, Minnesota, New Hampshire, and North Carolina cancer registries; and by the Victoria Cancer Registry (Australia) and Ontario Cancer Registry (Canada). The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the views or policies of the NIH or any of the collaborating centers in the CCFR, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government, any cancer registry, or the CCFR.
The MCCS was further supported by Australian National Health and Medical Research Council (https://www.nhmrc.gov.au/) grants 209057, 396414, and 1074383, and ongoing follow-up and data management have been funded by Cancer Council Victoria since 1995. Cases and their vital status were ascertained through the Victorian Cancer Registry and the Australian Institute of Health and Welfare, including the National Death Index and the Australian Cancer Database.
Kelly-Anne Phillips is an Australian National Health and Medical Research Council leadership fellow (1195294). Mary Beth Terry was supported by the Breast Cancer Research Foundation.
Conflicts of interest
Kelly-Anne Phillips has an unpaid advisory role with AstraZeneca and research funding (to Kelly-Anne Phillips’s institution) from AstraZeneca. The other authors declared no conflicts of interest during the conduct of this study outside the grant funding listed in the “Funding” section.
Data availability
For information about how to collaborate with the ProF-SC cohort in making further use of the data and resources and with the Breast Cancer Family Registry, please see http://www.bcfamilyregistry.org. For access to kConFab resources, see www.kconfab.org. The data generated in this study can be accessed by request to the CCFR (https://www.coloncfr.org/collaboration). The MCCS data can be made available on request to pedigree@cancervic.org.au.
References
- 1. Zhang GQ, Chen JL, Luo Y, et al. Menopausal hormone therapy and women's health: An umbrella review. PLoS Med. 2021;18:e1003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chlebowski RT, Aragaki AK. The Women's Health Initiative randomized trials of menopausal hormone therapy and breast cancer: Findings in context. Menopause. 2023;30:454-461. [DOI] [PubMed] [Google Scholar]
- 3. Huntley C, Torr B, Kavanaugh G, et al. Breast cancer risk assessment for prescription of Menopausal Hormone Therapy in women who have a family history of breast cancer. Br J Gen Pract. 2024;74:e610-e618. 10.3399/BJGP.2023.0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rudolph A, Song M, Brook MN, et al. Joint associations of a polygenic risk score and environmental risk factors for breast cancer in the Breast Cancer Association Consortium. Int J Epidemiol. 2018;47:526-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tian Y, Lin Y, Qu C, et al. Genetic risk impacts the association of menopausal hormone therapy with colorectal cancer risk. Br J Cancer. 2024;130:1687-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernandez-Rozadilla C, Timofeeva M, Chen Z, et al. Deciphering colorectal cancer genetics through multi-omic analysis of 100,204 cases and 154,587 controls of European and east Asian ancestries. Nat Genet. 2023;55:89-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mavaddat N, Michailidou K, Dennis J, et al. ; NBCS Collaborators. Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am J Hum Genet. 2019;104:21-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kehm RD, Genkinger JM, MacInnis RJ, et al. Recreational physical activity is associated with reduced breast cancer risk in adult women at high risk for breast cancer: a cohort study of women selected for familial and genetic risk. Cancer Res. 2020;80:116-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeinomar N, Knight JA, Genkinger JM, et al. ; kConFab Investigators. Alcohol consumption, cigarette smoking, and familial breast cancer risk: Findings from the Prospective Family Study Cohort (ProF-SC). Breast Cancer Res. 2019;21:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hopper JL, Dite GS, MacInnis RJ, et al. ; kConFab Investigators. Age-specific breast cancer risk by body mass index and familial risk: Prospective family study cohort (ProF-SC). Breast Cancer Res. 2018;20:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terry MB, Phillips KA, Daly MB, et al. Cohort Profile: The Breast Cancer Prospective Family Study Cohort (ProF-SC). Int J Epidemiol. 2016;45:683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newcomb PA, Baron J, Cotterchio M, et al. ; Colon Cancer Family Registry. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331-2343. [DOI] [PubMed] [Google Scholar]
- 13. Milne RL, Fletcher AS, MacInnis RJ, et al. Cohort Profile: The Melbourne Collaborative Cohort Study (Health 2020). Int J Epidemiol. 2017;46:1757-1757i. [DOI] [PubMed] [Google Scholar]
- 14. Lee A, Mavaddat N, Wilcox AN, et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med. 2019;21:1708-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Win AK, Jenkins MA, Dowty JG, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:404-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng Y, Hua X, Win AK, et al. A new comprehensive colorectal cancer risk prediction model incorporating family history, personal characteristics, and environmental factors. Cancer Epidemiol Biomarkers Prev. 2020;29:549-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamilton AS, Mack TM. Puberty and genetic susceptibility to breast cancer in a case-control study in twins. N Engl J Med. 2003;348:2313-2322. [DOI] [PubMed] [Google Scholar]
- 18. Amitay EL, Carr PR, Jansen L, et al. Postmenopausal hormone replacement therapy and colorectal cancer risk by molecular subtypes and pathways. Int J Cancer. 2020;147:1018-1026. [DOI] [PubMed] [Google Scholar]
- 19. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321-333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For information about how to collaborate with the ProF-SC cohort in making further use of the data and resources and with the Breast Cancer Family Registry, please see http://www.bcfamilyregistry.org. For access to kConFab resources, see www.kconfab.org. The data generated in this study can be accessed by request to the CCFR (https://www.coloncfr.org/collaboration). The MCCS data can be made available on request to pedigree@cancervic.org.au.