Abstract
Introduction:
Liver disease is increasingly recognised in HIV-positive individuals , even among those without viral hepatitis, partly due to the recent availability of noninvasive methods of liver fibrosis assessment. The role of antiretroviral therapy (ART) on liver fibrosis pathogenesis is unclear.
Methods:
Sites in the Strategic Timing of AntiRetroviral Treatment (START) study with access to FibroScan® were invited to participate in the Liver Fibrosis Progression Substudy. All substudy participants underwent FibroScan® at baseline, and two noninvasive serum algorithms, APRI and FIB-4, were calculated. Demographic and liver-related information were collected for all START participants at baseline.
Results:
230 participants were enrolled (11.5% viral hepatitis [B or C] coinfection) of whom 221 had a valid transient elastography (TE) result. TE score was 4.9 kPa (IQR 4.3–6.0). 17 patients (7.8%) (95% CI: 5.1–12.1) had a TE score of >7.2kPa indicating significant liver fibrosis. Baseline factors associated with higher TE scores in multivariate analysis were higher ALT per 10 U/L (p=0.045), higher log10 HIV RNA (p<0.001) and Hispanic/Latino ethnicity (p=0.01). TE correlated weakly with noninvasive markers.
Discussion:
At baseline significant liver fibrosis was observed in approximately 8% of participants, with higher ALT and HIV RNA the only clinical factors associated with increasing TE score. TE will be used annually to monitor fibrosis and evaluate the role of ART in further fibrosis progression.
Keywords: HIV, viral hepatitis, liver fibrosis, FibroScan®, elastography
Introduction
Liver-related disease (LRD) is one of the most common causes of morbidity and mortality in HIV-positive individuals (1, 2). Rates of LRD show little evidence of decline since the introduction of widespread combination antiretroviral therapy (ART) (3, 4), although one recent study has suggested the rate may now be decreasing (5). Much of this burden is among individuals who are coinfected with either hepatitis B (HBV) or hepatitis C virus (HCV) and current guidelines recommend early initiation of ART in both HBV- and HCV-coinfected individuals (6–9); however, there is also concern for the potential for progressive liver disease in HIV-monoinfected individuals. Abnormal liver transaminases are commonly observed in HIV-monoinfected individuals, and the recent availability of noninvasive methods for detecting and monitoring liver fibrosis has resulted in the identification of asymptomatic liver damage in these individuals. The underlying causes have been variably linked to antiretroviral hepatotoxicity (including didanosine and abacavir use) (10, 11), nonalcoholic steatohepatitis (NASH) (12), protease inhibitor (PI)-induced metabolic syndrome, the effects of HIV infection itself (13) or immune activation resulting from microbial translocation (14, 15). The role of ART on liver enzyme elevation and the benefit of ART on limiting or preventing fibrosis progression or clinical liver disease is unclear (16).
The Strategic Timing of AntiRetroviral Treatment (START) study is the first randomised controlled trial to address the benefit of starting ART at CD4 cell counts >500 cells/μL. The aim of the Liver Fibrosis Progression Substudy is to compare the effect of early versus deferred ART on liver fibrosis progression in HIV-positive individuals. Transient elastography (TE) or FibroScan® technology was used at baseline and on an annual basis to determine both the prevalence of significant fibrosis within the START population and to evaluate factors associated with progression of fibrosis. TE is a relatively novel means of determining liver fibrosis through the use of ultrasound-based technology. A noninvasive probe is used to generate a shear wave that travels through liver tissue. The speed of this shear wave is translated into a value measured in kilopascals (kPa), which has been shown to correlate with liver fibrosis as determined by the comparator test, liver biopsy (17). This baseline analysis describes the substudy participants, the baseline distribution of fibrosis as determined by TE and noninvasive serum markers, as well as the factors associated with increased TE scores.
Methods
Participants were enrolled into the START study as described elsewhere (18). Sites with access to FibroScan® technology were identified and invited to participate in the Liver Fibrosis Progression Substudy. At the time the trial began, FibroScan® devices were approved in the United States for research purposes only, so only one US-based site was included. Thirty-two sites in total from Asia (Thailand), Europe (Belgium, Denmark, Germany, Italy, Portugal, Spain, and Switzerland) and Australia had access to FibroScan® and agreed to participate. All participants gave written informed consent to enrol in this substudy. FibroScan® was performed by a trained operator at each site. Participants were only enrolled into the substudy if a valid FibroScan® was obtained at baseline (19). A participant’s FibroScan® measurements were considered valid and used in this analysis if 1) at least 10 readings were obtained; 2) the percent of successful readings was greater than 60%; and 3) the ratio of interquartile range and median value for the readings obtained was less than 0.3.
Demographic and clinical data obtained in START included serum markers, data on alcohol consumption, smoking, medical history and concomitant medication use.
Statistical Analysis
TE score was natural log transformed for all regression analyses due to the non-normal distribution. Univariate and multivariate linear regression models were built to examine the association between selected baseline variables and TE score. Variables examined included age, gender, race, region of enrolment, smoking history, body mass index (BMI), viral hepatitis B and C status, CD4 cell count, HIV RNA, alanine aminotransferase (ALT), alcohol use, glucose, diabetes mellitus and statin use. Variables with a p <0.20 in the univariate analysis were included in the multivariate model.
Two serum algorithms used to predict liver fibrosis (APRI and FIB-4) were calculated as follows: APRI: [(AST/ULN)/platelet counts (109 /L)] × 100,where a score of >1.5 is indicative of bridging fibrosis (F3) and a score of >2 is indicative of cirrhosis (F4), and FIB-4: (Age x AST ) / ( Platelets x (sqr ( ALT ) ) ) where a score of >3.35 is indicative of stage F3/4.Spearman correlations were calculated to assess the relationship between TE scores and both APRI and FIB-4.
Results
Two-hundred-thirty (230) START participants were enrolled into the Liver Fibrosis Progression Substudy, of whom 229 had a TE report obtained. Baseline characteristics of the substudy participants are given in Table 1. While substudy participants were generally comparable to the overall START population, key differences included a lower proportion of women (12% vs 27%), a greater proportion of Asians (33% vs 8%) and a higher proportion of viral hepatitis (B or C) coinfection (11.5% vs 6.3%) in substudy participants. Laboratory variables including ALT, AST, and platelet and CD4 cell count were similar.
Table 1: Baseline Characteristics of Liver Fibrosis Progression Substudy Participants.
| LFP Substudy | |
|---|---|
| No. participants enrolled | 230 |
| Demographics | |
| Age (years; median, IQR) | 35 [28, 42] |
| Gender (N, % female) | 28 (12.2) |
| Race | |
| Black (N, %) | 14 (6.1) |
| Hispanic/Latino (N, %) | 16 (7.0) |
| Asian (N, %) | 76 (33.0) |
| White (N, %) | 120 (52.2) |
| Other (N, %) | 4 (1.7) |
| Hepatitis Coinfection | |
| Hepatitis B1 (%) | 12 (5.4) |
| Hepatitis C2 (%) | 14 (6.1) |
| Laboratory Results | |
| CD4 (cells/μl; median, IQR) | 647 [583, 758] |
| Nadir CD4 (cells/ μl; median, IQR) | 554 [471, 654] |
| HIV RNA (log10copies/mL; median, IQR) | 4.4 [3.8, 4.8] |
| HIV RNA, undetectable (N, % with RNA ≤ 400 copies/mL) | 9 (3.9) |
| AST/SGOT (IU/L; median, IQR) | 24 [20, 31] |
| ALT/SGPT (IU/L; median, IQR) | 24 [18, 35] |
| Platelets (log10cells/mm3; median, IQR) | 5.4 [5.3, 5.4] |
Hepatitis B defined as HBsAg positive based on a test within one year before randomisation
Hepatitis C defined as HCV antibody positive from any time before randomisation
Distributions of characteristics known to be associated with chronic liver disease are given in Table 2 for substudy participants. Median BMI was in the “normal” range, the prevalence of diabetes mellitus was 2%. Current smoking was reported by 37% of substudy participants but the percent reporting heavy alcohol use was low, with the majority (75%) of participants drinking alcohol four or less drinks a day for three or less days per week. A history of alcoholism or substance dependence was reported in <1% and no participants had a known history of cirrhosis. Potentially hepatotoxic drugs (see Table 2 for list) were used in less than 3% of the cohort.
Table 2: Baseline characteristics associated with liver fibrosis in Liver Fibrosis Substudy participants.
| BMI (kg/m 2 ; median, IQR) | 22.9 [20.9, 25.2] |
| Smoking Status (N,%) | |
| Current | 85 (37.0) |
| Former | 27 (11.7) |
| Never | 118 (51.3) |
| Alcohol Consumption - Days Per Week (N,%) | |
| 0 | 77 (34.7) |
| < 1 | 78 (35.6) |
| 1 – 3 | 44 (19.2) |
| 4 – 7 | 23 (10.5) |
| Alcohol Consumption - Drinks at a Time (N,%) | |
| 0 | 51 (22.8) |
| 1 | 41 (18.3) |
| 2 – 4 | 93 (42.0) |
| 5 – 7 | 24 (11.0) |
| >8 | 13 (5.9) |
| Medical History (N,%) | |
| Cirrhosis | 0 (0.0) |
| Chronic liver disease (excluding infectious hepatitis) | 1 (0.4) |
| Hepatic steatosis | 0 (0.0) |
| Diabetes mellitus | 5 (2.2) |
| Alcoholism or substance dependence | 2 (0.9) |
| Concomitant Treatments at Baseline (N,%) | |
| Fibric acid | 1 (0.4) |
| Statins | 2 (0.9) |
| Anti-tuberculous drugs | 0 (0.0) |
| Drug treatment for hepatitis C | 0 (0.0) |
| Anabolic steroids | 0 (0.0) |
| Systemic corticosteroids | 1 (0.4) |
| Systemic antifungal drugs | 1 (0.4) |
Transient elastography results
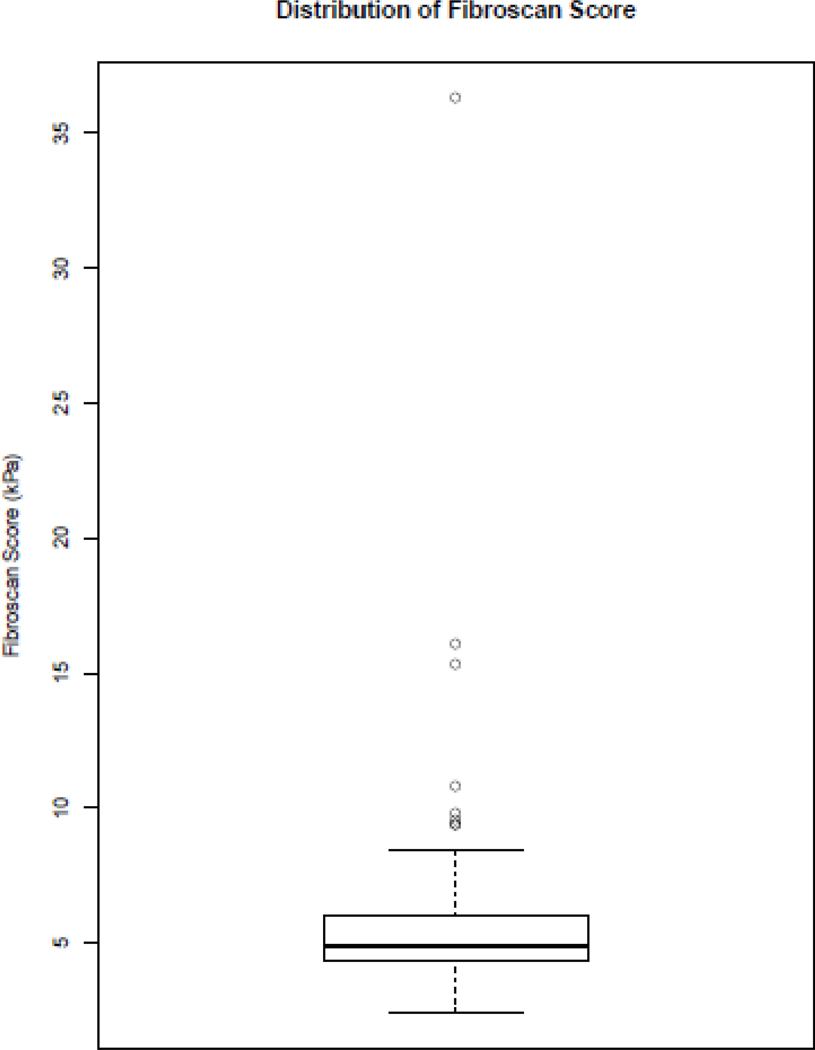
TE results were obtained for 229 participants, however, 8 (3.5 %) participants did not have scans that met the criteria due to an IQR/median ratio of >0.3. Therefore 221 results were used to calculate fibrosis scores. Median TE score was 4.9 kPa (IQR 4.3–6.0) (Figure 1). Breakdown of TE score by category is given in Table 3. The majority had TE scores consistent with Metavir (20) stage F0-F1 (92.3%). In total 17 participants (7.8%) had TE of >7.2kPa indicating significant liver fibrosis (95% CI 5.1–12.1). Of these, 3 (1.4%) participants had TE scores consistent with stage F3 fibrosis (TE 9.6–10.8kPa) and 3 (1.4%) had scores consistent with cirrhosis (15kPa, 16kPa and 36 kPa).
Figure 1. Distribution of TE scores.
Table 3: Distribution of fibrosis by elastography and noninvasive markers.
| TE Results | |
|---|---|
| Median score (kPa; median, IQR) | 4.9 [4.3, 6.0] |
| Median score (kPa; mean ± SD) | 5.4 ± 2.6 |
| Category 1: < 7.1 kPa (N,%) | 204 (92.3) |
| Category 2: 7.2–9.4 kPa (N,%) | 11 (5.0) |
| Category 3: 9.5–14.0 kPa (N,%) | 3 (1.4) |
| Category 4: > 14.0 kPa (N,%) | 3 (1.4 |
| Log median score (Log kPa; mean ± SD) | 1.6 ± 0.3 |
| IQR of TE score (kPa; median, IQR) | 0.7 [0.4, 1.0] |
| Noninvasive serum markers | |
| FIB-4 (median, IQR) | 0.72 [0.54, 1.00] |
| ≤ 1.45 (N,%) | 199 (89.6) |
| 1.55–3.25 (N,%) | 20 (9.0) |
| > 3.25 (N,%) | 3 (1.4) |
| APRI score (median, IQR) | 0.27 [0.20, 0.35] |
| ≤ 0.5 (%) | 204 (91.5) |
| 0.5–1.5 (%) | 17 (7.6) |
| > 1.5 (%) | 2 (0.9) |
Factors associated with higher TE score are displayed in Table 4. In univariate analysis, HCV coinfection, HIV RNA and ALT were associated with higher fibrosis score. In addition, Hispanic/Latino participants had on average higher TE scores than white participants. Age, known duration of HIV infection and trigylcerides were not associated with higher TE score. In multivariate analysis the only baseline factors associated with a higher fibrosis score were higher ALT per 10U/L (coefficient 0.01, p=0.045) a higher log10 HIV RNA (coefficient 0.09, p=0.002), and Hispanic/Latino race (coefficient 0.21, p=0.01). When the association between HIV RNA and TE score was examined using HIV RNA as a categorical rather than continuous variable (approximate quartiles of <5000 c/ml, 5000–19999 c/ml, 20000–74,999, > 750,000c/ml) there appeared to be a threshold effect with 3.9% and 5.3% of individuals in the lowest two quartiles having significant fibrosis compared to 12.7% and 10.0% of those in the higher two quartiles.
Table 4. Factors associated with increasing transient elastography score in the LFP substudy.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Baseline Predictor | Coef (SE) | p-value | Coef (SE) | p-value |
| Race* | 0.091 | 0.109 | ||
| White (reference) | --- | --- | --- | --- |
| Black | 0.046 (0.087) | 0.600 | 0.001 (0.094) | 0.994 |
| Hispanic/Latino | 0.209 (0.082) | 0.011 | 0.208 (0.084) | 0.014 |
| Asian | −0.013 (0.043) | 0.757 | −0.028 (0.046) | 0.544 |
| Other | −0.105 (0.147) | 0.474 | −0.026 (0.167) | 0.876 |
| BMI (per 5 kg/m2 increase) | 0.035 (0.025) | 0.168 | 0.017 (0.026) | 0.512 |
| Hepatitis C coinfection | 0.159 (0.080) | 0.048 | 0.084 (0.087) | 0.334 |
| HIV RNA (log10 copies/mL) | 0.072 (0.023) | 0.002 | 0.077 (0.025) | 0.002 |
| ALT SGPT (per 10 U/L increase) | 0.011 (0.005) | 0.024 | 0.010 (0.005) | 0.045 |
| Alcohol intake | ||||
| 4–7 days a week with 2+ drinks a day versus less consumption | −0.096 (0.072) | 0.187 | −0.066 (0.072) | 0.356 |
| Glucose (per 10 mg/dL increase) | 0.016 (0.009) | 0.086 | 0.010 (0.011) | 0.366 |
| Diabetes | 0.197 (0.131) | 0.136 | 0.065 (0.162) | 0.689 |
| Use of statins | 0.336 (0.206) | 0.104 | 0.274 (0.214) | 0.203 |
4df Chi-square p-value for race
Linear Regression of Natural log Transformed TE Score on baseline factors
Reduced Model - Including Predictors with p < 0.20 in Univariate Analysis
Serum fibrosis markers
Two serum algorithms commonly used to predict liver fibrosis (APRI and FIB-4) (21, 22) were calculated at baseline. As with TE, the median levels of both were in the normal range (median 0.27 APRI and median 0.72 FIB-4) and similar proportions of individuals were identified as having F2 stage fibrosis and above (8.5% by APRI and 10% by FIB-4). Concordance between the two serum algorithms was not always observed, 13.1% were identified as having ≥F2 by either APRI or FIB-4: 2.7% by APRI and not FIB4, 4.5% by FIB4 and not APRI and 5.9% by both. TE correlated weakly with APRI with a Spearman correlation of 0.139 (p=0.04, 95% CI 0.01, 0.27) but not with FIB-4, Spearman correlation 0.034 (p=0.63, 95% CI −0.11, 0.16). A number of significantly discordant outliers were observed (Figure 2). The participant with a TE score of 36 kPa was not characterised by either test (APRI 0.29, FIB4 0.56) as a cirrhotic. Removal of this outlier made no significant difference to the correlation (0.034, p=0.62 for FIB-4 and TE, 0.134, p=0.05 for APRI and TE). Additionally, in univariate analyses, HIV RNA was not associated with FIB-4 (p=0.37) or APRI (p=0.26).
Figure 2. A and B: Scatter plot of TE score with (A) APRI (B) FIB-4.
Discussion
The role and contribution of HIV viraemia in the pathogenesis of hepatic fibrosis has been postulated but remains unclear. HIV-positive individuals, particularly those on ART, may have multiple reasons to develop liver disease including comorbidities such as viral hepatitis coinfection and insulin resistance, lifestyle factors such as alcohol and drug use, and the use of potentially hepatotoxic medications including antiretroviral drugs themselves. In addition, liver fibrosis is usually insidious in onset and asymptomatic in nature and to date no simple test has been able to predict its presence accurately. Transaminase values are notoriously inaccurate for assessing presence of fibrosis, and liver biopsy (the previous gold standard) is invasive, unpopular with patients and often inaccurate due to sampling error. TE (using FibroScan®) is now increasingly accepted as a simple, noninvasive method for assessing liver fibrosis with high patient acceptability, allowing longitudinal measurements (17).
In this analysis of liver fibrosis in untreated HIV-positive individuals we demonstrate firstly a low but not insignificant (approximately 8%) prevalence of significant fibrosis. Secondly, we demonstrate in multivariate analysis that the only baseline factors associated with higher TE score are higher ALT and higher HIV RNA level, along with Hispanic/Latino race. Finally we show a poor correlation between TE score and APRI and FIB-4, the two most commonly used serum noninvasive markers of liver fibrosis.
The prevalence of liver fibrosis in untreated HIV individuals has been infrequently assessed, particularly in the absence of viral hepatitis coinfection. However, with widespread availability of highly effective ART and extended life expectancy, attention is now shifting to the contribution of non-AIDS-related conditions to morbidity and mortality in HIV-positive individuals. Recently a number of studies have attempted to quantify the presence of liver disease in HIV-positive individuals, generally using noninvasive serum algorithms such as APRI or FIB-4 (23–25). In a cross-sectional study of 432 HIV-positive individuals (without HBV or HCV), significant fibrosis as determined by APRI was found in 8% of patients (24). In another study, again in HIV-monoinfected individuals but this time using FIB-4, a score of >1.45 (consistent with significant fibrosis) was found in 15.5% of individuals (23). In our study based on elastography assessment the prevalence of significant fibrosis (TE > 7.2 kPa indicating F2 and above) in untreated HIV-positive individuals was 7.8%.
In a general population study from France of 1358 “healthy” individuals significant fibrosis was identified in 8% (26). Many factors predisposing to liver disease exist within an otherwise healthy general population including alcohol excess and obesity linked to steatohepatitis. In fact, these two mechanisms were identified as the underlying cause of liver disease in 80% of the cases of fibrosis in the French study (26). In the START cohort, traditional factors associated with liver disease were generally infrequent; BMI was within normal range in the vast majority, rates of diabetes, alcohol use and substance dependence were very low as was the use of hepatotoxic drugs. Rates of coinfection with hepatitis B or C were higher than in the general population but much lower than would be expected in a typical HIV-positive population. This likely reflects the reluctance of clinicians to enrol coinfected participants in a randomised trial of deferral of ART when guidelines recommend early initiation for both HBV- and HCV-coinfected individuals.
The only two clinical factors in this substudy that were associated with a higher TE score were baseline ALT and baseline HIV RNA. The association with ALT is expected, but not causal as elevated transaminases are known to be a marker for liver injury and are commonly, although not always, abnormal in the setting of liver fibrosis. The association with HIV RNA is important as it underpins the biological notion that HIV itself may be associated with liver inflammation and that control of HIV viraemia may be beneficial for liver health. Various biological theories support a role for HIV-infection in liver injury including the role of HIV-related microbial translocation in promoting fibrogenesis via toll like receptor signalling (27), HIV activation of hepatic stellate cells that could play a primary role in fibrogenesis, direct invasion of the hepatocyte by HIV and the role of monocyte activation and cell death (14, 15, 28). Additionally, a number of studies have supported indirectly the role of HIV in promoting hepatic damage. In a recent large matched cohort study of over 20,000 HIV-positive individuals and 215,000 HIV-negative individuals, HIV-positive individuals had a significantly greater risk of both hepatic dysfunction (as measured by serious hepatic event or laboratory markers of dysfunction) and hepatic-related death (29). Factors associated with a higher risk were not only the predicted factors of viral hepatitis coinfection, alcohol and diabetes but also lower CD4 and higher HIV RNA. Other studies have confirmed the association between greater HIV RNA and increased hepatic fibrosis as measured using the serum algorithms of APRI and FIB-4 (24, 25, 30) but none have confirmed this association using elastography.
Serum algorithms based on biochemical and clinical markers have been widely used as markers of liver fibrosis as they are easily calculated in large cohorts with clinically available data. APRI utilises AST and platelet counts, whereas FIB-4 relies on age, ALT, AST and platelet count. Both have been validated in many liver disease settings and correlate moderately well with liver biopsy and other proprietary noninvasive markers such as Fibrotest® (31). Both also correlate reasonably well in large studies with elastography although TE outperforms serum markers against the gold standard of histology (32, 33), and the relatively poor performance of APRI and FIB-4 against histology have been noted in a previous large meta-analysis (34). In our study APRI correlated weakly with TE whereas there was no significant correlation with FIB-4. It is not clear why this should be although it is likely related to the relatively small number of participants in the study and the ability of a few outliers to distort results (Figure 1). It is also likely that HIV infection impacts some of these laboratory data, such as platelet count, skewing the results for reasons unrelated to liver injury or fibrogenesis (35). The relatively small numbers of participants in the study not only limited the potential for an association between serum markers and TE score to be observed but is also the reason why some of the other expected predictors of fibrosis, in particular the presence of viral hepatitis coinfection, were not associated with liver fibrosis.
In summary, this baseline cross-sectional analysis of untreated HIV-positive participants undergoing elastography assessment demonstrates that overall rates of significant liver fibrosis are low in this generally liver-healthy population. However an association between TE scores and HIV RNA suggests that untreated HIV viraemia may promote liver fibrogenesis and may support a rationale for earlier ART initiation. Ongoing follow-up using TE assessment annually after initiation or during deferral of ART within the randomised arms of START may help answer this question.
The START study is registered at clinicaltrials.gov (NCT00867048).
Acknowledgements
We would like to thank the START participants without whom this work would not be possible. See INSIGHT START Study Group, 2015, this supplement for a complete list of START investigators.
Funding
The START study is primarily funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1-AI068641, the Department of Bioethics at the NIH Clinical Center and five NIH institutes: the National Cancer Institute, the National Heart, Lung, and Blood Institute, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal disorders. Financial support is also provided by the French Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS), the German Ministry of Education and Research, the European AIDS Treatment Network (NEAT), the Australian National Health and Medical Research Council, and the UK Medical Research Council and National Institute for Heath Research. Six pharmaceutical companies (AbbVie, Inc., Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, LLC, and Merck Sharp and Dohme Corp.) donate antiretroviral drugs to START.
Footnotes
Disclosures
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The University of Minnesota, the sponsor of START, receives royalties from the use of abacavir, one of the HIV medicines that can be used in START.
References
- 1.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Int Med 2006;166(15):1632–1641. [DOI] [PubMed] [Google Scholar]
- 2.Salmon-Ceron D, Lewden C, Morlat P, et al. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J Hepatol 2005;42(6):799–805. [DOI] [PubMed] [Google Scholar]
- 3.Thio CL, Seaberg EC, Skolasky R Jr., et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360(9349):1921–1926. [DOI] [PubMed] [Google Scholar]
- 4.Weber R, Ruppik M, Rickenbach M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med 2013;14(4):195–207. [DOI] [PubMed] [Google Scholar]
- 5.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014;384(9939):241–248. [DOI] [PubMed] [Google Scholar]
- 6.Wilkins E, Nelson M, Agarwal K, et al. British HIV association guidelines for the management of hepatitus viruses in adults infected with HIV 2013. HIV Med 2013;14(Suppl 4):1–71. [DOI] [PubMed] [Google Scholar]
- 7.Quirishi N, Kreuzberg C, Luchters G, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIv and hepatitis C virus coinfection. Lancet 2003;362:1708–1713. [DOI] [PubMed] [Google Scholar]
- 8.Brau N, Salvatore M, Rios-Bedoya CF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol 2006;44(1):47–55. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JP, Tchetgen Tchetgen EJ, Lo Re V 3rd, et al. Antiretroviral therapy reduces the rate of hepatic decompensation among HIV- and hepatitis C virus-coinfected veterans. Clin Infect Dis 2014; 58(5):719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta AS, Long RE, Comunale MA, et al. Increased levels of galactose-deficient anti-Gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis. J Virol 2008;82(3):1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vispo E, Moreno A, Maida I, et al. Noncirrhotic portal hypertension in HIV-infected patients: unique clinical and pathological findings. AIDS 2010;24(8):1171–1176. [DOI] [PubMed] [Google Scholar]
- 12.Ingiliz P, Valantin MA, Duvivier C, et al. Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology 2009;49(2):436–442. [DOI] [PubMed] [Google Scholar]
- 13.Blackard JT, Sherman KE. HCV/ HIV co-infection: time to re-evaluate the role of HIV in the liver? J Viral Hepat 2008;15(5):323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology 2008;135(1):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12(12):1365–1371. [DOI] [PubMed] [Google Scholar]
- 16.Lo Re V 3rd, Kallan MJ, Tate JP, et al. Hepatic Decompensation in Antiretroviral-Treated Patients Co-Infected With HIV and Hepatitis C Virus Compared With Hepatitis C Virus-Monoinfected Patients: A Cohort Study. Ann Intern Med 2014;160(6):369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008;134(4):960–974. [DOI] [PubMed] [Google Scholar]
- 18.Babiker AG, Emery S, Fatkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials 2013;10(1 Suppl):S5–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48(5):835–847. [DOI] [PubMed] [Google Scholar]
- 20.Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 2007;47(4):598–607. [DOI] [PubMed] [Google Scholar]
- 21.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- 22.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38(2):518–526. [DOI] [PubMed] [Google Scholar]
- 23.Tahiri M, Sodqi M, Lahdami FE, et al. Risk factors for liver fibrosis among human immunodeficiency virus monoinfected patients using the FIB4 index in Morocco. World J Hepatol 2013; 5(10):584–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V, 3rd. Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis 2010;10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price JC, Seaberg EC, Badri S, Witt MD, D’Acunto K, Thio CL. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. J Infect Dis 2012;205(6):1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roulot D, Costes JL, Buyck JF, et al. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011;60(7):977–984. [DOI] [PubMed] [Google Scholar]
- 27.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 2003;37(5):1043–1055. [DOI] [PubMed] [Google Scholar]
- 28.Redd AD, Wendel SK, Grabowski MK, et al. Liver stiffness is associated with monocyte activation in HIV-infected Ugandans without viral hepatitis. AIDS Res Hum Retroviruses 2013; 29(7):1026–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Towner WJ, Xu L, Leyden WA, et al. The effect of HIV infection, immunodeficiency, and antiretroviral therapy on the risk of hepatic dysfunction. J Acquir Immune Defic Syndr 2012; 60(3):321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendeni M, Foca E, Gotti D, et al. Evaluation of liver fibrosis: concordance analysis between noninvasive scores (APRI and FIB-4) evolution and predictors in a cohort of HIV-infected patients without hepatitis C and B infection. Clin Infect Dis 2011;52(9):1164–1173. [DOI] [PubMed] [Google Scholar]
- 31.Imbert-Bismut FRV, Peironi L, for the MULTIVIRC Group. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001;357:1069–1075. [DOI] [PubMed] [Google Scholar]
- 32.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005; 128(2):343–350. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Conde M, Montes-Ramirez ML, Miralles P, et al. Comparison of transient elastography and liver biopsy for the assessment of liver fibrosis in HIV/hepatitis C virus-coinfected patients and correlation with noninvasive serum markers. J Viral Hepat 2010;17(4):280–286. [DOI] [PubMed] [Google Scholar]
- 34.Poynard T, Ngo Y, Perazzo H, et al. Prognostic value of liver fibrosis biomarkers: a meta-analysis. Gastroenterol Hepatol 2011; 7(7):445–454. [PMC free article] [PubMed] [Google Scholar]
- 35.Gibellini D, Clo A, Morini S, Miserocchi A, Ponti C, Re MC. Effects of human immunodeficiency virus on the erythrocyte and megakaryocyte lineages. World J Virol 2013; 2(2):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]