Abstract
Background:
Randomised comparative data on efficacy and safety of second-line antiretroviral therapy (ART) after failure of non-nucleoside reverse transcriptase inhibitors (NNRTIs) across diverse geographical settings are limited. The aim of this study was to evaluate optimal second ART for people with HIV.
Methods:
D2EFT is a completed international randomised phase III/IV trial evaluating three different second line ARV strategies in adults with HIV-1 whose first-line NNRTI therapy failed (NCT03017872).. Originally designed as a two-arm study comparing recommended standard of care (SOC: ritonavir boosted darunavir (DRV/r) +2 nucleoside reverse transcriptase inhibitors (NRTIs) to a novel nucleoside sparing arm of dolutegravir (DTG) with DRV/r, the study was adapted during the first year to add a third arm of DTG with fixed tenofovir and lamivudine or emtricitabine (TDF/XTC). D2EFT was conducted across 14 countries from Asia, Africa and Latin America and was designed to evaluate non-inferiority of either interventional arm against SOC for virological suppression as determined by HIV-RNA<50copies/mL at 48 weeks.
Results:
826 participants were enrolled between November 2017 and December 2021. Median age was 39 years and 54% were female. Baseline median CD4 was 206 cells/mm3 and median HIV-RNA was 15400 copies/ml. Proportion of participants with HIVRNA < 50 c/ml at 48 weeks was 75.5% (194/257) DRV/r +2NRTI, 84.1% (222/264) DRV/r + DTG and 78.0% (227/291) in DTG+TDF/XTC. Compared to DRV/r+2NRTIs the % difference in HIV RNA < 50c/ml was 8.6% (95% CI [1.7. 15.5], p=0.02) for DTG+DRV/r, and 6.7 [−1.2, 14.4, p=0.09] for DTG+TDF/XTC. 6 deaths (none treatment related) occurred. 35 pregnancies (24 deliveries) occurred with no congenital defects.
Conclusion:
In failing NNRTI-based first-line ARV, a switch to either DTG+DRV/r or DTG+TDF/XTC without universal access to genotyping, was non-inferior in achieving viral suppression compared to DRV/r+2NRTIs providing global data to support most recent WHO treatment guidelines.
Introduction
Transitioning the global millions living with HIV to simplified, well tolerated, highly efficacious and accessible antiretroviral therapy (ART) is a key public health objective supported by international agencies including the World Health Organization (WHO) (1). International guidelines support first-line ART comprising dolutegravir (DTG), an integrase strand inhibitor (INSTI), in combination with a nucleoside reverse transcriptase inhibitor (NRTI) backbone of tenofovir disoproxil fumarate (TDF) and lamivudine (3TC) in a co-formulated low cost fixed dose combination pill known as ‘TLD’ (1–3). Global roll-out of this regimen is rapidly occurring across most low- and middle-income countries (LMICs) (4). Optimal second line regimens are less well characterised. For individuals whose first line non-nucleoside reverse transcriptase inhibitor (NNRTI) regimens is failing, boosted protease inhibitor (b/PI) therapy, in combination with new NRTI chosen by genotyping or through defined algorithms, have traditionally been recommended. Downsides of this approach include the tablet burden, the frequent inclusion of zidovudine (ZDV) as one of the backbone NRTIs and the need for expensive HIV resistance testing in settings with limited resources.
Evidence supports the use of an INSTI based approach for second line therapy. In 2019 the DAWNING study found a INSTI based regimen to be superior to a b/PI regimen with virological suppression achieved in 84% versus 70% of participants at week 48 (5). Of note, DAWNING required resistance testing to guide NRTI selection and at least 1 active NRTI in the backbone. In 2021 the NADIA trial conducted in 464 patients in sub–Saharan Africa showed that DTG was non-inferior to ritonavir boosted darunavir (DRV/r) even when extensive resistance was present in the recycled NRTI backbone of TDF and 3TC (6). This study highlighted the potential for a simplified approach to second-line therapy, akin to that of first-line, whereby a pill such as TLD could be programmatically rolled out to people with HIV (PWH) whose first-line therapy is failing.
The D2EFT (Dolutegravir and Darunavir Evaluation in adults Failing Therapy) study was conceived in 2012, originally designed to compare a novel, simplified dual regimen of DRV/r in combination with DTG to the guideline recommended second-line regimen of DRV/r plus an NRTI-backbone based on resistance testing or rotation of NRTIs per WHO recommendations. After study commencement, the D2EFT protocol steering committee (PSC) amended the study to add a third arm, DTG with a backbone of fixed recycled NRTIs (TDF plus either 3TC or emtricitabine (FTC) : TDF/XTC) in light of a rapidly changing global therapeutic landscape including widespread TLD roll-out (7).
Methods
Trial design and adaptation
D2EFT is an international randomised open-label non-inferiority phase IIIB/IV trial comparing novel second-line ART regimens to standard of care (SOC) when first-line NNRTI based regimen is failing in people with HIV-1-infection (NCT03017872). In its original design D2EFT aimed to compare dual therapy of DTG + DRV/r to the WHO recommended SOC, a boosted protease inhibitor, (in this case darunavir), plus an NRTI backbone. This study design (subsequently referred to as Stage 1 of D2EFT) commenced in late 2017 with a target sample size of 610 participants.
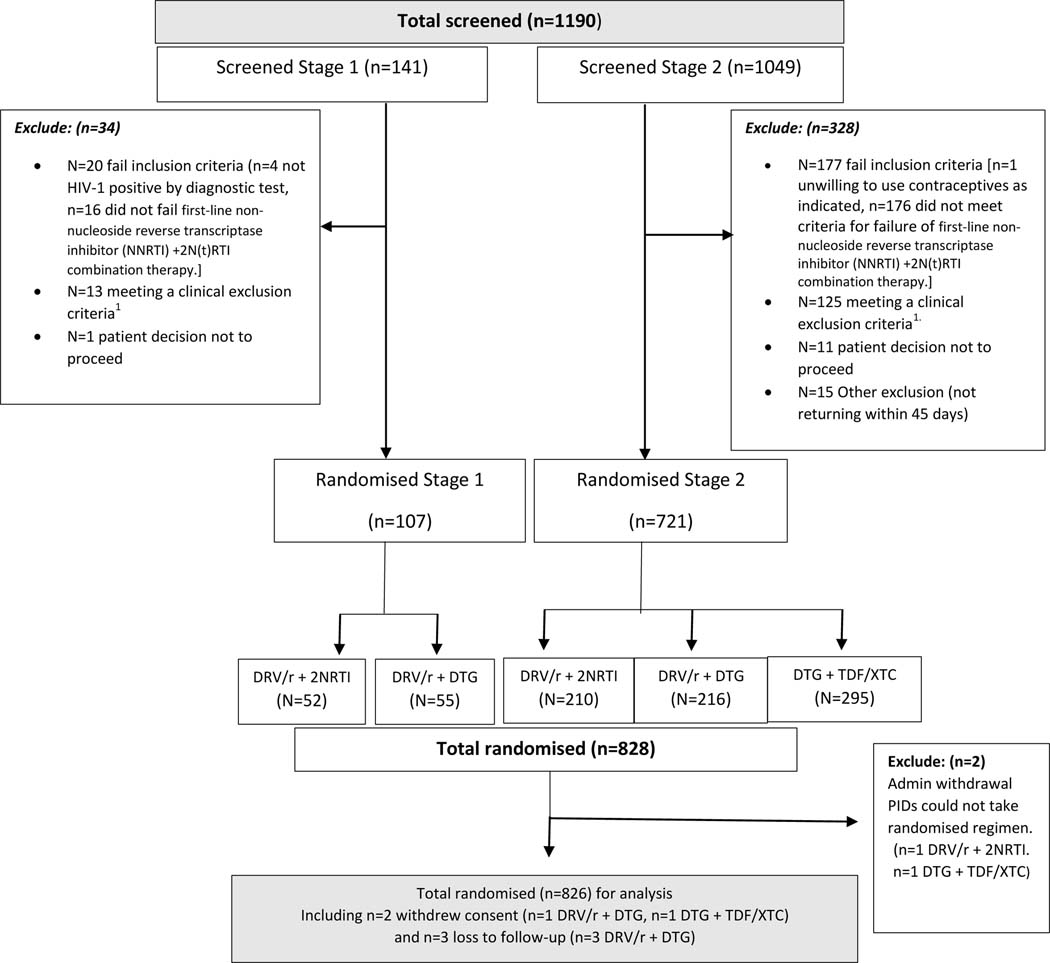
D2EFT study oversight is provided by a PSC, Community Advisory Board (CAB) and an independent Data Safety and Management Board (DSMB) as set out in Supplemental material. In early 2018 these agencies, in consultation with WHO, modified the study protocol through the addition of a third study arm. In the modified protocol eligible participants were randomly allocated to receive DTG +TDF/XTC. Consequently, D2EFT became a three-arm study (referred to as Stage 2) which commenced recruitment in September 2018 with a target total sample size of 1010. Full details of the D2EFT design adaptations including rationale and statistical considerations are described in (7) and shown schematically in Fig 1.
Figure 1. Consort diagram for all participants screened to randomisation.
1Clinical exclusion: Any participant checking any one of the following criterion were excluded: Haemoglobin <7.0g/dl, Platelet count <50,000 cells/μL, AST and/or ALT >5xULN or ALT >3xULN and bilirubin >1.5xULN, change in antiretroviral therapy within 12-weeks prior to randomisation, prior exposure to HIV protease inhibitors and/or HIV integrase inhibitors, participants with chronic viral hepatitis B infection, unstable liver disease, creatinine clearance of <50 mL/min, current use of rifampicin or rifabutin, intercurrent illness requiring hospitalization, use of contraindicated medication, an active opportunistic disease not under adequate control, pregnant or nursing mother, deemed unlikely to remain in the follow -up period.
Trial population
Participants were recruited from an international network involving 28 outpatient clinic sites across 14 mainly LMICs. Participating countries were Argentina, Chile, Colombia, India, Malaysia, Mexico, South Africa, Thailand, and Zimbabwe (in Stage 1) and Brazil, Guinea, Indonesia, Mali, and Nigeria (added in Stage 2). Eligible patients were adults aged 18 years or over, living with HIV-1, whose first line NNRTI + 2NRTI combination therapy had failed defined as a plasma HIV RNA load (pVL) >500 copies/ml on at least two consecutive occasions (≥7 days apart) after a minimum period of exposure to continuous NNRTI + 2NRTIs of 24 weeks. Full details of eligibility criteria are given in Supplementary Appendix.
Treatment
In Stage 1 participants were randomly assigned in a 1:1 ratio to DRV/r (800mg of darunavir plus 100mg of ritonavir) once daily plus DTG (50mg) once daily or DRV/r once daily plus 2 NRTIs (dosed once or twice daily). The choice of NRTIs was based on genotyping results if available (12 of 28 sites) or in the absence of genotyping on the WHO recommendations for rotating NRTIs in second line which included the use of ZDV when the first-line regimen contained TDF. In Stage 2, the additional third arm consisted of DTG (50 mg) once daily in combination with TDF (300mg) plus either 3TC (300mg) or FTC (200mg), all once daily. The fixed dose combination pill of TLD was allowed, as were fixed dose combinations of NRTIs. In Stage 2 the randomisation allocation recruited more to TDF/XTC to obtain equal power across comparisons.
Participants were randomised via the web based CRF using a computer generated, blocked randomisation scheme (block size 2) stratified by site, prior TDF use, and HIV viral load </>= 100,000 c/mL.
Participants in both NRTI-containing arms were allowed to switch NRTIs during the study for toxicity at the discretion of the treating clinician and depending upon the availability of local alternatives. In the case of intolerance attributed to DRV/r a switch to an alternative b/PI was advised. Participants who became pregnant during the study whilst on DTG were advised to switch to an alternative regimen, however this advice was lifted during the latter part of the study (from 12/2021) based on accumulating safety data on the use of DTG in pregnancy (8, 9).
Assessments and outcomes
D2EFT was a 96-week study with primary outcome assessment performed at week 48. Study visits were performed at weeks 0, 4, 24, 48 and 96. At each study visit a targeted medical history and examination was undertaken, and any Grade 3/4 adverse event, COVID-19 diagnosis, serious adverse events (SAE) or serious non-AIDS events (SNAE) recorded. Adherence was encouraged at each visit and formally assessed at week 4 using a self-reported 7-day recall adherence questionnaire (Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) Antiretroviral Medication Self-Report Form 065-BAS-2).
Statistical analysis
The primary outcome of the study was a noninferiority analysis comparing the proportion of participants with virological suppression defined as a pVL of less than or equal to 50 copies (c)/ml at week 48 in i) DRV/r +2NRTI (arm 1) to DRV/r plus DTG (arm 2), and ii) DRV/r +2NRTI (arm 1) to DTG+TDF/XTC (arm 3). Comparisons were made using a modified intention to treat (ITT) population, including all participants as randomised but excluding administrative withdrawals. The primary analysis set evaluated virological suppression in participants with data available at week 48. A secondary analysis was also performed with missing viral load assessment equal to failure.
The proportion of participants with virological suppression was assessed for non-inferiority using a two-sided 95% confidence interval from a simple, unadjusted 2-group comparison, and a pre-specified non-inferiority margin of 12%. Virological outcomes were also analysed by baseline viral load (≤100,000 and >100,000 copies/mL).
Secondary outcomes included virological efficacy using pVL cut-offs of 200 copies/ml and 400copies/ml at week 48, immunological recovery, and metabolic measures including fasting lipids, change in body mass index (BMI) and weight change. Safety was monitored through the reporting of adverse events grade 3 and above, SAEs, opportunistic diseases (AIDS events), SNAEs, pregnancies, and death.
Samples underwent resistance testing at the central laboratory at the Applied Centre for Medical Research (AMR) in Sydney for all samples at baseline and at week 48 where HIV VL >1000 copies/ml. The Stanford algorithm (version V9.5.0 August 22nd 2023) was used for interpretation of resistance sequences.
Efficacy outcomes were presented with risk difference and 95% confidence intervals. Difference in proportions were tested using a two-sided Fisher’s exact test; 95% confidence intervals around the difference in proportion were obtained using the standard formula for difference in two proportions:
Immunological recovery and metabolic measures were assessed using a two-sided, Satterthwaite t-test or Wilcoxon rank sum test when normality was not satisfied. All analyses were conducted in SAS (version 9.4) or Stata (version 16.1).
All participants provided written informed consent before study procedures. The study protocol was approved by University of New South Wales HREC (primary study committee), as well as through local ethics committees at all study sites, and was conducted according to the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice (ICH/GCP) guidelines. The study was registered with cinical trials.gov (NCT03017872)
Sample size
When the third arm (DTG+TDF/XTC) was added in stage 2 the sample size was re-evaluated. Under the hypothesis of no difference between randomised treatment arms a virological suppression rate of 75%, and 5% lost to follow-up, to have 90% power to demonstrate non-inferiority in the ITT analysis using a 12% margin, a total of 1010 patients were required, 305 in each of the original 2 arms, and 400 in the third arm, Further details are given in supplementary material and (6).
Role of the funding source
The study was funded by UNITAID, National Institute of Allergy and Infectious Diseases, USA, National Health and Medical Research Council, Australia (APP1104610), ViiV Healthcare and Janssen. Study drug was provided by ViiV Healthcare and Janssen. The sponsor (The Kirby Institute, UNSW Sydney) collected the data, managed study samples, monitored study conduct, performed the statistical analysis, and drafted the manuscript. All authors had access to the study data and all agreed to submit for publication.
Results
Study management
Screening of participants commenced in November 2017 and completed in December 2021 with the last patient randomised in January 2022. During the study two screening pauses occurred; the first between June 2018 and September 2018 whilst the third arm adaptation was implemented, the second between April 2020 and November 2020 due to the impact of the COVID-19 pandemic. Of 1190 screened participants, 828 were randomised (107 in stage 1 and 721 in Stage 2). The PSC decided to halt recruitment in December 2021 before the target 1010 participants was achieved to ensure relevance of the results to rapidly changing ART programs. This decision was made without any knowledge of the trial results. Reasons for ineligibility are given in Suppl Table 1. Two participants were not able to receive their randomised regimen for administrative reasons, thus 826 participants commenced their randomised regimen and constituted the primary analysis population. (Fig 1).
Participant characteristics
Baseline characteristics of the participants were well balanced within Stage 1 and within Stage 2. Participants in Stage 2 tended to have higher BMI and were more commonly of Black African ethnicity (Table 1). Overall, 55% of the study population were female, median age was 39.0 years (IQR [33.0,46.0]) and median BMI was 23.0 (IQR [20.3,26.8]). Ethnicity was Black African in 69% and Asian in 25%. Median baseline CD4 count was 206 cells/mm3 (IQR 93–354) and 14% had a CD4 count < 50 cells/mm3. The majority (69%) had a baseline HIV RNA of less than 50,000 copies/mL with18% commencing with an HIV RNA > 100,000 copies/mL.
Table 1.
Baseline characteristics for n=826 randomised participants
| Characteristic1 | Stage 1 (n=107) | Stage 2 (n=719) | Total | |||
|---|---|---|---|---|---|---|
| DRV/r + 2NRTI (n=52) | DRV/r + DTG (n=55) | DRV/r + 2NRTI (n=209) | DRV/r + DTG (n=216) | DTG + TDF/XTC (n=294) | (n=826) | |
|
Age at randomisation (years):
Mean (SD) Median [IQR} |
39.2 (9.6) 39.0 [33.5, 44.5] |
40.0 (10.6) 42.0 [32.0, 50.0] |
39.7 (9.6) 40.0 [34.0, 46.0] |
39.2 (9.6) 39.0 [33.0, 44.5] |
39.0 (9.6) 39.0 [32.0, 46.0] |
39.4 (10.0) 39.0 [33.0, 46.0] |
| <40 | 27 (51.9) | 25 (45.5) | 100 (47.9) | 120 (55.6) | 152 (51.7) | 422 (51.3) |
| 40–44 | 12 (23.1) | 8 (14.6) | 46 (22.0) | 35 (16.2) | 52 (17.7) | 153 (18.5) |
| 45–54 | 9 (17.3) | 20 (36.4) | 53 (25.4) | 50 (23.2) | 68 (23.1) | 200 (24.2) |
| 55+ | 4 (7.7) | 2 (3.6) | 10 (4.8) | 11 (5.1) | 22 (7.5) | 49 (5.9) |
| Weight (kg) | 61.3 (12.6) 58.7 [52.9, 66.0] |
61.5 (14.5) 60.4 [49.6, 67.5] |
66.8 (15.9) 64.2 [57.0, 74.0] |
63.6 (15.7) 61.4 [52.2, 71.5] |
65.6 (14.9) 64.0 [54.8, 74.0] |
64.8 (15.3) 62.7 [54.1, 72.8] |
|
BMI (kg/m2): Mean (SD) Median [IQR] |
23.1 (4.4) 22.5 [20.1, 24.7] |
22.8 (5.2) 21.6 [19.3, 25.1] |
24.7 (5.4) 23.6 [21.0, 27.4] |
23.8 (5.8) 22.5 [19.8, 26.7] |
24.5 (5.7) 23.5 [20.4, 27.1] |
24.2 (5.6) 23.0 [20.3, 26.8] |
| Underweight [BMI <18.5] | 6 (11.5) | 8 (14.6) | 12 (17.1) | 32 (14.8) | 26 (8.9) | 84 (10.2) |
| Normal [18.5 ≤ BMI <25] | 34 (65.4) | 33 (60.0) | 116 (55.5) | 111 (51.4) | 155 (52.7) | 449 (54.4) |
| Overweight [25≤ BMI <30] | 8 (15.4) | 11 (20.0) | 47 (22.5) | 44 (20.4) | 73(24.8) | 183(22.1) |
| Obese [BMI ≥30] | 4 (7.7) | 3 (5.5) | 34 (16.3) | 29 (13.4) | 40 (13.6) | 110 (13.3) |
| Sex | ||||||
| Female | 29 (55.8) | 26 (47.3) | 113 (54.1) | 122 (56.5) | 160 (54.4) | 450 (54.5) |
| Male | 23 (44.2) | 29 (52.7) | 96 (45.9) | 93 (43.5) | 134 (45.7) | 376 (45.5) |
| Ethnicity | ||||||
| White | 0 (0.0) | 0 (0.0) | 2 (1.0) | 4 (1.9) | 7 (2.4) | 13 (1.6) |
| Asian | 19 (36.5) | 19 (34.6) | 51 (24.4) | 51 (23.6) | 67 (22.8) | 207 (25.1) |
| Black | 32 (61.5) | 36 (65.5) | 145 (69.4) | 149 (69.0) | 208 (70.8) | 570 (69.0) |
| Hispanic/Latino | 0 (0.0) | 0 (0.0) | 11 (5.3) | 12 (5.6) | 11 (3.7) | 34 (4.1) |
| Other | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 2 (0.2) |
|
CD4 T-cell count (cell/µL)
Mean (SD) Median [IQR] |
252.1 (221.1) 186.0 [92.0, 344.5] |
212.9 (182.2) 166.0 [61.0, 298.0] |
247.5 (213.5) 194.5 [84.5, 354.5] |
249.1 (202.6) 213.0 [93.0, 350.0] |
266.1 (218.8) 221.5 [110.5, 375.1] |
252.5 (211.4) 206.0 [93.0, 354.2] |
| <50 | 9 (17.3) | 10 (18.2) | 33 (15.8) | 28 (13.0) | 34 (11.6) | 114 (13.8) |
| 50 – 199 | 19 (36.5) | 21 (38.2) | 70 (33.5) | 71 (32.9) | 98 (33.3) | 279 (33.8) |
| 200 – 349 | 11 (21.2) | 12 (21.8) | 50 (23.9) | 59 (27.3) | 76 (25.9) | 208 (25.2) |
| ≥350 | 13 (25.0) | 12 (21.8) | 51 (24.4) | 53 (24.5) | 80 (27.2) | 209 (25.3) |
| Unknown | 0 (0.0) | 0 (0.0) | 5 (2.4) | 5 (2.3) | 6 (2.0) | 16 (1.9) |
|
HIV-RNA (copies/mL): median
[IQR] |
17000.0 [2200.0, 49561.0] |
21500.0 [5209.0, 87744.0] |
14195.0 [3109.0, 70923.0] |
13162.0 [3600.0, 72660.5] |
16000.0 [4100.0, 57891.0] |
15400.0 [3600.0, 65986.0] |
| Log10(HIV-RNA): Median [IQR] | 4.2 [3.3, 4.7] | 4.3 [3.7, 4.9] | 4.2 [3.5, 4.9] | 4.1 [3.6, 4.9] | 4.2 [3.6, 4.8] | 4.2[3.6, 4.8] |
|
HIV-RNA (copies/mL)
<200 |
2 (3.9) | 1 (1.8) | 4 (1.9) | 3 (1.4) | 5 (1.7) | 15 (1.8) |
| 200 – 499 | 2 (3.9) | 3 (5.5) | 8 (3.8) | 4 (1.9) | (3.1) | 26 (3.2) |
| 500 – 49,000 | 35 (67.3) | 30 (54.6) | 135 (64.6) | 143 (66.2) | 199 (67.7) | 542(65.6) |
| 50, 000 – 100, 000 | 6 (11.5) | 8 (14.6) | 24 (11.5) | 23 (10.7) | 32 (10.9) | 93 (11.3) |
| >100, 000 | 7 (13.5) | 13 (23.6) | 38 (18.2) | 43 (19.9) | 48 (16.3) | 149 (18.0) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 1 (0.1) |
| Mode of HIV transmission | ||||||
| Homosexual contact | 4 (7.7) | 9 (16.4) | 31 (14.8) | 21 (9.7) | 27 (9.2) | 92 (11.2) |
| Heterosexual contact | 40 (76.9) | 42 (76.4) | 156 (74.6) | 179 (82.9) | 239 (81.3) | 656 (79.4) |
| Injecting drug use | 1 (1.9) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 2 (0.7) | 4 (0.5) |
| Blood transfusion | 0 (0.0) | 0 (0.0) | 3 (1.4) | 3 (1.4) | 2 (0.7) | 8 (1.0) |
| Other | 7 (13.5) | 4 (7.3) | 18 (8.6) | 13 (6.0) | 24 (8.2) | 66 (8.0) |
| Absolute neutrophil count (cells/µL) | 2.4 (1.0) 2.1 [1.7, 3.0] |
2.4 (1.4) 2.0 [1.2, 3.1] |
2.4 (1.2) 2.2 [1.6, 3.0] |
2.4 (1.9) 2.1 [1.5, 2.8] |
2.3 (1.2) 2.0 [1.5, 3.0] |
2.4 (1.4) 2.1 [1.5, 2.9] |
| Haemoglobin (g/dL) |
12.9 (1.9) 13.0 [11.9, 14.2] |
12.8 (1.7) 12.9 [11.9, 14.1] |
12.7 (2.0) 12.8 [11.4, 14.2] |
12.3 (2.1) 12.4 [11.0, 13.9] |
12.6 (2.0) 12.7 [11.3, 13.9] |
12.6 (2.0) 12.7 [11.3, 14.0] |
| Platelet count (cells/µl) |
245.3 (87.3) 236.0 [193.0, 288.5] |
248.3 (79.3) 237.0 [203.0, 300.0] |
241.2 (79.8) 235.0 [194.0, 238.0] |
253.2 (113.0) 240.0 [198.0, 294.0] |
247.6 (80.2) 238.5 [193,0, 293.5] |
247.5 (90.1) 237.0 [196.0, 290.0] |
| Serum ALT (U/L) | 32.3 (46.0) 24.0 [16.0, 32.0] |
31.5 (18.7) 26.0 [18.0, 36.0] |
28.6 (22.6) 23.0 [16.1, 33.0] |
28.3 (17.4) 23.8 [17.0, 34.5] |
28.5 (17.2) 25.0 [17.0, 36.0] |
28.9 (21.7) 24.0 [17.0, 34.0] |
| eGFR (mL/min/1.73m2) | 117.9 (23.5) 119.7 [104.7, 132.0] |
118.1 (24.4) 118.2 [104.1, 130.4] |
121.7 (21.2) 121.4 [107.9, 136.6] |
121.4 (23.5) 123.2 [110.7, 136.9] |
122.8 (21.5) 123.6 [109.4, 136.8] |
121.5 (22.3) 122.3 [108.5, 136.4] |
|
Duration ART: years mean (SD) median [IQR] |
5.1 (3.1) 5.1 [2.9, 7.5] |
6.1 (4.1) 5.8 [2.7, 8.0] |
5.5 (3.8) 4.7 [2.5, 7.9] |
5.4 (4.4) 4.4 [1.8, 8.2] |
6.1 (4.4) 5.4 [2.3, 8.9] |
5.7 (4.2) 5.0 [2.2, 8.3] |
.All categorical characteristics are presented with n (%) of total, all clinical continuous characteristics are presented with mean (SD) and median [IQR]. SD=standard deviation, IQR = interquartile range.
The NNRTIs in use at time of first-line failure were efavirenz (EFV) in 84.1% (81.4% TDF/XTC/EFV, 2.7% ZDV/XTC/EFV) with a limited number of other regimens used (Suppl Table 2a). NRTIs included in the backbone of the second line regimens in arms 1 and 3 differed by study arm (Suppl Table 2b). In arm 1 which mandated choice of NRTI based on genotyping or WHO algorithm, the most common backbone was ZDV/3TC (76.4%) followed by TDF/XTC (19.0%). In arm 3, with fixed NRTIs as backbone, 96.2% were correctly prescribed TDF/XTC. 9 participants were inadvertently enrolled whilst on an alternative NRTI-backbone all of whom but one were switched on to TDF/XTC within a median of 8 days after randomisation.
Baseline resistance data was performed centrally and available following study completion for 87%. In those with sequence data available, (675/720) 93% had high level resistance to NRTI and (711/726) 98% had high level resistance to NNRTI comparable across the arms. No patient had any DRV specific mutations and one patient had potentially low level DTG resistance (S147SG, randomised to DRV/r + DTG). Listing of resistance mutations by class is given in Supplemental tables 6 and 7.
Study retention was extremely high with only 6 participant withdrawals and 4 lost to follow-up through week 48. Over 99% of all study scheduled visits were attended. 94% of participants remained on their randomised regimen at week 48 (93% DRV/r +2NRTI, 95.6% DRV/r + DTG and 93.5% DTG+TDF/XTC). Reasons for discontinuing randomised regimen are given in Suppl Table 3. Six participants in the DRV/r + DTG arm and five participants in the DTG+TDF/XTC arm switched their DTG containing regimens due to pregnancy.
Efficacy
At week 48 in the modified ITT analysis based on all available data the proportion of participants with a pVL of < 50 copies/ml was 75.5% (194/257) DRV/r +2NRTI, 84.1% (222/264) DRV/r + DTG and 78.0% (227/291) in DTG+TDF/XTC. In non-inferiority analysis comparing the proportion with VL<50 copies/mL in each arm to the SOC (DRV/r +2NRTI), the efficacy difference was 8.6% (95%CI 1.7,15.5), p=0.004 when comparing DRV/r + DTG to DRV/r +2NRTI (Stage 1 and 2), and 6.7% (95% CI −1.2,14.4), p=0.09 comparing DTG+TDF/XTC to DRV/r +2NRTI (Stage 2 only). Both intervention arms met the criteria for non-inferiority against the SOC DRV/r +2NRTI with the dual arm of DRV/r + DTG also meeting superiority criteria against SOC (Figure 2). These results were consistent when considering different viral load thresholds (< 200 and < 400 copies/ml) and at different baseline HIV RNA levels (< or >100,000 copies/ml) (Table 2). Further, in additional sensitivity analysis in which missing data was considered failure, the results were unchanged (Suppl Table 4, Suppl Fig 1).
Figure 2:
Undetectable viral load at week 48 by study arm – available data
Table 2.
Efficacy endpoints for week 48: with available data1
| HIV RNA at week 48 | ||||||
|---|---|---|---|---|---|---|
| Study Arm | N with data | N miss | <50 copies/mL (n, % N with data) | <200 copies/mL (n, % N with data) | <400 copies/mL (n, % N with data) | |
| DRV/r + 2NRTI: stage 1+2 | 257 | 4 | 194 (75.5) | 222 (86.4) | 227 (88.3) | |
| DRV/r + DTG: stage 1+2 | 264 | 7 | 222 (84.1) | 246 (93.2) | 250 (94.7) | |
| DRV/r + 2NRTI: stage 2 |
206 | 3 | 147 (71.4) | 174 (84.5) | 179 (86.9) | |
| DRV/r + DTG: stage 2 | 209 | 7 | 177 (84.7) | 194 (92.8) | 198 (94.7) | |
| DTG + TDF/XTC: stage2 |
291 | 3 | 227 (78.0) | 252 (86.6) | 262 (90.0) | |
| Difference % [95% CI], P-value 2 | ||||||
| DRV/r + DTG vs DRV/r + 2NRTI | Stage 1 +2 | 8.6 [1.7, 15.5], P=0.004 | 6.8 [1.6, 12.0], P=0.01 | 6.4 [1.6, 11.4], P=0.01 | ||
| DTG + TDF/XTC vs DRV/r + 2NRTI: | Stage 2 | 6.7 [−1.2, 14.4], P=0.09 | 2.1 [−4.2, 8.4], P=0.52 | 3.1 [−2.6, 8.9], P=0.31 | ||
| Baseline HIV RNA <= 100,000 copies/mL | ||||||
| DRV/r + 2NRTI: stage 1+2 | 213 | 3 | 169 (79.3) | 188 (88.3) | 193 (90.6) | |
| DRV/r+DTG stage 1+2 | 210 | 5 | 181 (86.2) | 195 (92.9) | 198 (94.3) | |
| DRV/r + 2NRTI: stage 2 |
169 | 2 | 128 (75.7) | 146 (86.4) | 151 (89.4) | |
| DRV/r + DTG: stage 2 | 168 | 5 | 146 (86.9) | 156 (92.9) | 159 (94.6) | |
| DTG + TDF/XTC: stage 2 |
244 | 1 | 200 (82.0) | 217 (88.9) | 224 (91.8) | |
| Difference % [95% CI], P-value 2 | ||||||
| DRV/r + DTG vs DRV/r + 2NRTI: | Stage 1+2 | 6.9 [−0.3, 14.0], P=0.07 | 4.6 [−0.1, 10.2], P=0.13 | 3.7 [−1.3, 8.7], P=0.20 | ||
| DTG + TDF/XTC vs DRV/r + 2NRTI | Stage 2 | 6.2 [−1.8, 14.3], P=0.14 | 2.5 [−4.0, 9.0], P=0.45 | 2.5 [−3.3, 8.2], P=0.40 | ||
| Baseline HIV RNA >100,000 copies/mL | ||||||
| DRV/r + 2NRTI: stage 1+2 | 44 | 1 | 25 (56.8) | 34 (77.3) | 34 (77.3) | |
| DRV/r + DTG: stage 1+2 | 54 | 2 | 41 (75.9) | 51 (94.4) | 52 (96.3) | |
| DRV/r + 2NRTI: stage 2 |
34 | 1 | 19 (51.4) | 28 (75.7) | 28 (75.7) | |
| DRV/r + DTG: stage 2 | 41 | 2 | 31 (75.6) | 38 (92.7) | 39 (95.1) | |
| DTG + TDF/XTC: stage2 |
47 | 1 | 27 (57.5) | 35 (74.5) | 38 (80.9) | |
| Difference % [95% CI], P-value 2 | ||||||
| DRV/r + DTG vs DRV/r + 2NRTI: | Stage 1+2 | 19.1 [0.6, 37.7], P=0.05 | 17.2 [3.4, 31.0], P=0.02 | 19.0 [5.7, 33.3], P=0.01 | ||
| DTG + TDF/XTC vs DRV/r + 2NRTI | Stage 2 | 6.1 [−15.3, 27.5], P=0.66 | −1.2 [−19.8, 17.4], P=1.00 | 5.2 [−12.7, 23.0], P=0.60 | ||
Efficacy percentage excludes participants with missing RNA at week 48, efficacy endpoints were assessed following a modified intention to treat approach, excluding any participant randomised who did not commence ART.
P-value calculated using Fisher’s exact (two-sided) test.
DRV/r + 2NRTI (ritonavir boosted darunavir plus two nucleoside reverse transcriptase inhibitors); DRV/r + DTG (ritonavir boosted darunavir plus dolutegravir); DTG + TDF/XTC (dolutegravir plus tenofovir with either emtrictabine or lamivudine (XTC))
An additional snapshot analysis was performed in which response was defined as both participants who remained on randomised ART regimen and had viral load <50 copies/mL at week 48. The proportion with response was 81.2% (220/271) in DRV/r + DTG compared to 70.5% (184/261) for DRV/r +2NRTI, difference 10.7% (95%CI [[3.5, 17.9], P=0.005); and 75.2% (221/294) in DTG+TDF/XTC compared to 66.5% (139/209) for DRV/r +2NRTI, difference 8.7% (95%CI [5.8,16.8], p=0.04), Stage 2 only. (Suppl Table 5). In 169 participants who did not achieve virological suppression at week 48, 143 (84.6%) had viral suppression to < 50 copies/ml prior to week 48 with subsequent rebound at week 48, and 26 (15.3%) failed to suppress during 48 weeks of follow-up. Of those with detectable HIV RNA at week 48, 65% (110/169) had an HIV viral load of <=1,000 copies/ml.
Resistance testing was performed on all samples where HIV VL > 1000 copies/ml with available sequence data for n=53 for INSTI and n=47 for PR_RT gene types, respectively. No DRV mutations were observed. DTG mutations were observed in 1 patient in the DRV/r + DTG arm at week 48 (G118R) and 3 patients in the DTG+TDF/XTC arm (1x G118R and 2 x R263K).
Immunological recovery
Recovery in CD4 count at week 48 was observed across all three study arms. Median (IQR) CD4 rise was 130.0 cells/mm3 [56, 214.0] in SOC DRV/r +2NRTI, 174.0 cells/ mm3 [90.0, 282.0] in DRV/r + DTG and 160.5 cells/ mm3 [81.5, 272.0] in DTG+TDF/XTC (Stage 2 only). Rise in CD4 count was significantly greater in both DRV/r + DTG (difference 55.8 cells/ mm3 [26.4,85.2], p<0.001) and DTG+TDF/XTC (difference 41.7 cells/µl [11.2,72.1], p=0.01 when compared to DRV/r +2NRTI. (Supplementary Fig 2)
Weight and BMI changes
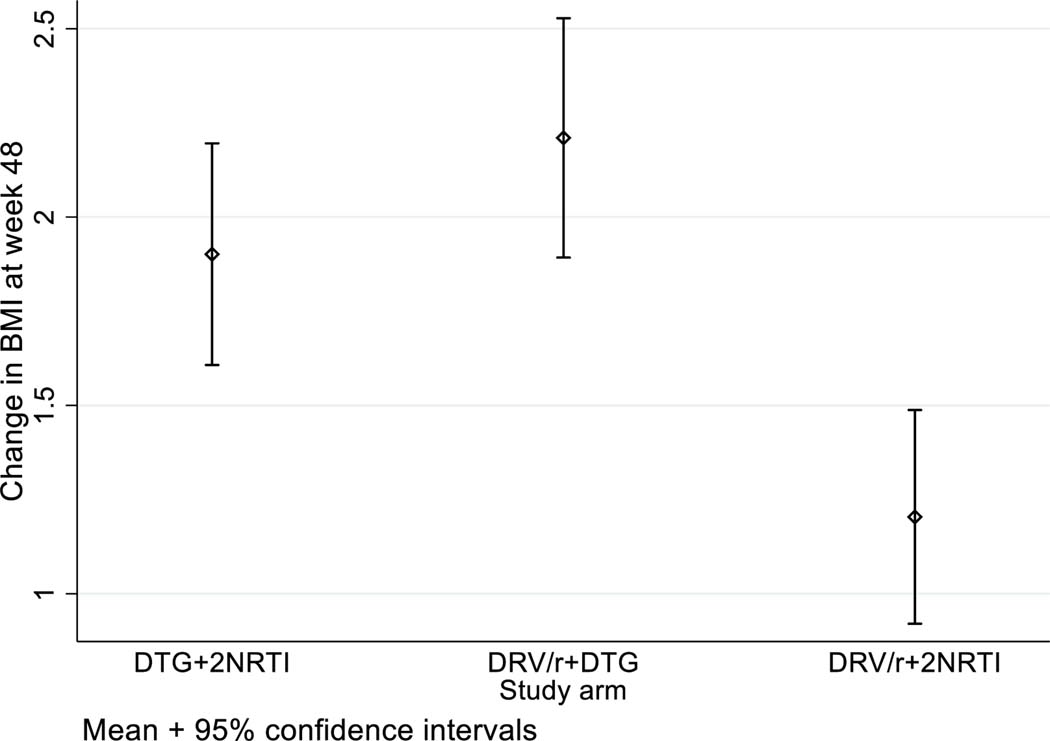
Increases in weight and BMI were observed across all three arms but were greatest in the arms containing DTG. Mean weight gain was 3.2kg (SD 6.3kg) in SOC DRV/r +2NRTI arm, 5.9kg (SD 7.0kg) in DRV/r + DTG arm and 5.0kg (SD6.8kg) in DTG+TDF/XTC. In comparison against the SOC arm of DRV/r +2NRTI, BMI change was significantly greater in both DRV/r + DTG arm (1.01 [0.6,1.4], p<0.001, Stage 1 and 2) and DTG+TDF/XTC arms (0.66 [0.2,1.1], p=0.004, Stage 2 only) Figure 3.
Figure 3.
Change in BMI at week 48 by Study arm
Of the 532 participants that were not overweight or obese at baseline, there was no significant difference between arms in the proportions who moved into one of these categories: 44/177 (24.9%) in DRV/r+DTG, and 42/178 (23.6%) DTG+TDF/XTC arms and 36/162 (22.2%) in DRV/r+2NRTI arm (p=0.56)
Safety
Overall, 59 serious adverse events occurred in 47 individuals of which 6 resulted in death (Suppl Table 8.2). None of the deaths were considered related to study drug (3 related to infection including one from COVID-19 infection, one progressive multifocal leukoencephalopathy (PML), one sudden cardiac death and one trauma related). Grade 3/4 ALT elevations were observed in six participants (four in SOC and one each in DTG containing arms). Grade 3/4 anaemia developed in three participants in DTG+TDF/XTC, one in r=DRV/r + DTG and 7 participants in DRV/r +2NRTI arm. 15 participants discontinued drug for toxicity reasons (6 in DRV/r +2NRTI, 4 in DRV/r + DTG and 5 in DTG+TDF/XTC), Supplementary Table 3. One case of acute hepatitis B infection occurred in a participant on the DRV/r + DTG arm.
An AIDS defining condition occurred in 12 participants, of which 7 were tuberculosis related. Of note, 34 pregnancies occurred (26 in participants on DTG-containing regimens) with no documented birth defects.
Discussion
The D2EFT study, originally designed to test a novel NRTI-sparing second line strategy of two highly potent agents (DRV/r plus DTG) against the WHO preferred standard of care (DRV/r + 2NRTIs), required an innovative adaptation to study design during implementation to address rapidly changing practice in global policy. Our results clearly demonstrate that both the original dual DRV/r +DTG arm, and the subsequently added DTG+TDF/XTC arm, are non-inferior to DRV/r+2NRTIs with a margin well below the 10% pre-set criteria and at all viral load cut-offs, and that further, the DRV/r+DTG arm demonstrated statistical superiority. The implications of these findings must be viewed through a lens that broadly encompasses aspects of current HIV policy and public health intervention.
Firstly, reassurance on the efficacy of the DTG+TDF/XTC arm as an effective second line therapy supports current WHO and global guidelines and modelling (1, 2, 9, 10) which advocate this regimen as a preferred choice in this setting due to lower cost, pill simplification and programmatic delivery. Despite an extremely high rate of background NRTI resistance the virological suppression rate of 78% to < 50 copies/ml at 48 weeks (90% to < 400copies/ml) confirms that this is not only a highly effective option but also very well tolerated. Indeed, in the 48-week snapshot analysis (Suppl Table 5) which evaluated both virological efficacy and regimen switches, the DTG+TDF/XTC arm was significantly better than the SOC DRV/r + 2NRTI arm (8.7 % difference [5.8, 16.8], p=0.04). Notably only six participants switched off DTG+TDF/XTC for toxicity, three of which were related to renal dysfunction. This data is in strong support of other recent second line switch studies such as NADIA (6), and VISEND (11). NADIA importantly demonstrated in the Sub-Saharan African (SSA) setting that recycling of first-line TDF/3TC when used in combination with a high genetic barrier to resistance such as DTG was overall non-inferior (although not superior) to a PI based regimen including DRV and that this benefit extended out to 96 weeks (12). Confirmation of this data in a broader global setting encompassing not only SSA but also Latin America and Asia is critical to support global HIV policy and provides reassurance to community and policy makers worldwide, supporting a pragmatic public health approach of abandoning costly genotyping for first line NNRTI failures. Reassuringly no evidence of significant resistance substitutions in the PI class and minimal evidence in the INSTI class were observed at week 48 although 4 of 53 patients did have evidence of mutations that may confer intermediate resistance to DTG. More detailed subsequent analyses of the longer term D2EFT data will determine what proportion of the 48-week failures maintained or gained further resistance substitutions and to which agents, and whether these had subsequent impact on outcomes at week 96.
D2EFT is the first study to comprehensively evaluate a novel strategy of DRV/r plus DTG as second line therapy in LMIC settings. This dual regimen offers a combination that is highly attractive in terms of potency, barrier to resistance and tolerability, but is not co-formulated as a single pill and comes at significantly higher cost to other options (currently 4–5 x higher than DTC+TDF/XTC) - albeit price reductions are occurring (13). Within D2EFT this arm demonstrated statistical superiority to the SOC DRV/r + 2NRTI arm at all levels of virological suppression both in the non-inferiority analysis and also when analysed in the 48-week snapshot analysis (10.7% difference [3.5, 17.9], P=0.005). Rates of 48-week response in the latter were 81% in DRV/r + DTG versus 71% in DRV/r + 2NRTI suggesting clear benefit. Indeed, when switches for pregnancy or TB management were excluded, only three participants switched off the DRV/r plus DTG regimen for toxicity over 48 weeks, compared to six for DRV/r + 2NRTI. Of note, however, the occurrence of a case of acute hepatitis B occurring in the DRV/r+DTG arm highlights one of the major benefits to including NRTIs in ART is the anti-HBV activity of TDF (and to a lesser extent 3TC and FTC) which is critical for control of HBV viraemia in people with HIV/HBV co-infection (14). NRTI-sparing regimens are inadequate for people living with HIV and HBV and for this reason, people who were HBsAg positive at baseline were excluded from the study. The case of newly acquired HBV within the D2EFT study is a reminder that this regimen would have limitations above cost and formulation for widespread roll-out, particularly in LMIC with high background prevalence of HBV co-infection. Nevertheless, despite the drawbacks of this novel regimen in terms of cost, lack of co-formulation and lack of anti-HBV activity, it clearly is highly potent and, at week 48 at least, no evidence of emergent resistance mutations. Whilst from a programmatic perspective it is unlikely to currently replace DTG+XTC/FTC, its role and performance against other regimens over the longer term should continue to be evaluated.
Prior studies of other dual b/PI +INSTI second line combinations such as lopinavir/r + raltegravir have not been shown to be superior to SOC b/PI + 2 NRTIs (15). Our findings suggest that the dual PI/INSTI regimen of DRV/r+DTG is one of the most effective and well tolerated second line combinations available.
Although D2EFT demonstrated some differences between the three study regimens it is notable that the overall rate of HIV RNA suppression at 48 week was high across all therapies ranging from 76–84% at the 50 copies/ml cut-off to 88–95% at 400 copies/ml. This is encouraging data on treatment success in second line after first line NNRTI failures and is reflected in the overall mean CD4 gain of 175 cells/mm3. Six deaths occurred across the 826 participants in the study and only a small number of ADI occurred despite 14% of the participants commencing study with a CD4 count <50/cells mm3 and 48% < 200 cells/mm3. Unsurprisingly, the majority (58%) of AIDS Defining Illnesses were related to tuberculosis, both pulmonary and extrapulmonary, and tuberculosis remains a major source of morbidity and mortality across the regions in which D2EFT was conducted (16, 17). Nevertheless, the low rate of adverse events, deaths and loss-to follow-up is remarkable, particularly as the study was conducted throughout the years of the COVID-19 pandemic. Key reasons for this may include not only improved therapeutic options but also the successful integration of HIV care packages into services, community led initiatives to enhance engagement and education, and individual clinic-based strategies to ensure continuum of care during the COVID-19 crisis (18, 19).
The phenomenon of ART related weight gain has been reported extensively in diverse global populations and linked repeatedly, although not exclusively, to the use of integrase-based regimens (20–25). The relative contribution of agents included in the NRTI backbone has also been widely debated as well as the possible underlying pathophysiology. Certain agents including TDF, ZDV and EFV may exert a weight loss effect, largely through their effect on appetite and nausea; conversely the NRTI tenofovir alafenamide (TAF) and agents within the integrase class, particularly DTG and bictegravir, appear to be most strongly linked to weight gain (26). Disentangling the relative contributions of opposing agents, as well as separating a healthy weight gain following return of appetite with better tolerated ART, from the negative consequences of unhealthy weight gain and progression to obesity, is challenging.
In D2EFT an average weight gain of between 3.1 and 5.9kg was observed in all three study arms and at least some component of this is likely to be healthy weight return consistent with re-suppression of HIV viremia and CD4 count restoration. Nevertheless only 10% of the study population had a BMI <=18.5kg/m2 at baseline and overall, the mean baseline BMI was 24.2 kg/m2 indicating that this was not a largely underweight population. Further exploration of the differential weight gain by drug exposure and baseline BMI, as well as by different sub populations, requires deeper analysis and is ongoing.
Our study has several important limitations the most significant of which was the challenge of adding a third arm after the study had commenced. This resulted in an imbalanced allocation of study subjects over time and the need to consider the study results in two stages avoiding comparisons between non-contemporaneously enrolled subjects. The later inclusion of additional sites to enhance recruitment and meet the larger sample size also led to some imbalance in baseline characteristics in participants recruited in stage 1 and stage 2. Aware of these potential limitations the study team consulted extensively with stakeholders including WHO, funders and community prior to making this decision, and have been careful to present the data by Stage 1 and Stage 2 throughout. A further potential limitation of D2EFT was the decision to halt recruitment before the target sample size was reached. This decision was taken primarily for two reasons, namely the prolonged timeframe of enrolment into the study for stage 2 due to COVID-19 pauses and the increasingly small pool of individuals with first-line NNRTI failure due to TLD transition. A recalculation of sample size was performed, including the known low rates of loss to follow-up within the study, providing reassurance that terminating enrolment at between 800–850 participants would provide at least 80% power to show non-inferiority for the primary endpoint.
Extremely high rates of study engagement, and low discontinuations are a major strength of the study, especially given the challenges the sites and participants faced during multiple lockdowns in 2020–21. Continuation of study and study drug procurement throughout this period presented major logistical issues that required significant commitment at all levels including the site investigators, central study team and funding bodies. The addition of a third arm whilst raising challenges was also a strength - allowing the comparison of two intervention arms - the widely available pragmatic option of TLD plus a more novel NA-sparing strategy of DRV/r + DTG.
In summary D2EFT is one of the first studies to generate truly global data on the safety and efficacy of second line regimens including people living with HIV from Sub Saharan Africa, Latin America, East and Southeast Asia. It provides critical data supporting the current WHO recommendation of recycling TDF/XTC without genotyping in combination with DTG as an effective and well tolerated second line strategy across multiple settings. It also provides novel data on a highly potent second line strategy combination of DRV/r boosted protease with DTG. Whilst not suggested for broad roll-out due to cost and pill burden this combination presents an attractive option for salvage with minimal toxicity and extremely high efficacy, including at the highest viral loads. Future analyses will shed additional light on the complex issue of weight gain with different ART in different settings and populations, as well as the role of treatment emergent resistance in those without virological suppression at week 48.
Supplementary Material
Acknowledgements
We thank the D2EFT participants without whom this work would not have been possible. We extend our thanks to the members of our D2EFT community advisory boards who have provided essential input to the study (chair: Leonardo Perelis). We also recognize the significant contribution to this study of Prof. David Cooper (Sydney, NSW, Australia) who was instrumental in D2EFT’s design and implementation, and country leads Prof James Gita Hakim (Zimbabwe) and Dr Ernest Ekong (Nigeria). All of them began the journey of D2EFT but sadly did not live to see its conclusion. Their wisdom and insight are missed.
The authors also gratefully acknowledge the support of the other academic collaborators (Dr. H. Clifford Lane; NIH) and institutional collaborators (WHO and CHAI).
Funding:
The study was funded by UNITAID, National Institute of Allergy and Infectious Diseases, USA, National Health and Medical Research Council, Australia, ViiV Healthcare and Janssen.
Funding acknowledgement
The study was funded by UNITAID, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA, National Health and Medical Research Council, Australia (APP1104610), ViiV Healthcare and Janssen. ViiV Healthcare and Janssen provided study drug.
Footnotes
D2EFT Writing group (alphabetical order for each sub-groups)
Dona Arlinda, Iskandar Azwa, Jacyln Ann Bennet, Margaret Borok, Dannae Brown, Ploenchan Chetchotisakd, Mohamed Cisse, Sandra Wagner Cardoso, Sounkalo Dao, Sean Emery, Nnakelu Eriobu, Jolie Hutchison, Simone Jacoby, Richard Kaplan, Muhammad Karyana, Anthony Kelleher, Nagalingeswaran Kumarasamy, H. Clifford Lane, Matthew Law, Marcelo H. Losso, Gail V. Matthews, Emmanuelle Papot, Mark N. Polizzotto.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing
The data in this study cannot be made publicly available due to ethical restrictions in the study’s informed consent documents and in countries in which D2EFT was conducted; public data availability could compromise participant confidentiality. However, de-identified study data may be available upon request and submission and approval from the D2EFT PSC of a concept sheet summarising the analyses to be done. The data archive will be held at the Kirby Institute and kept on a secure network drive accessible to authorised personnel only. Data generated from the study results may be used in future reports, or used in scientific presentations at both national and international conferences. Further inquiries can be directed to D2EFT@kirby.unsw.edu.au
References
- 1.World Health Organisation. Consolidated guidelines on HIV, viral hepatitis and STI prevention, diagnosis, treatment and care for key populations. 2022. [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. 2023. [Available from: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv..
- 3.European AIDS Society EACS Guidelines. 2022. [Available from: https://www.eacsociety.org/media/guidelines-11.1_final_09-10.pdf.
- 4.World Health Organization. WHO HIV policy adoption and implementation status in countries. 2023. Fact sheet WHO/UCN/HHS/SIA/2023.02.2023 [Available from: In: https://cdn.who.int/media/docs/default-source/hq-hiv-hepatitis-and-stis-library/who-hiv-policy-adoption-incountries_2023.pdf?sfvrsn=e2720212_1#:~:text=A%20total%20of%2099%20countries,of%2099)%20report%20countrywide%20implementation.
- 5.Aboud M, Kaplan R, Lombaard J, Zhang F, Hidalgo JA, Mamedova E, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis. 2019;19(3):253–64. [DOI] [PubMed] [Google Scholar]
- 6.Paton NI, Musaazi J, Kityo C, Walimbwa S, Hoppe A, Balyegisawa A, et al. Dolutegravir or Darunavir in Combination with Zidovudine or Tenofovir to Treat HIV. N Engl J Med. 2021;385(4):330–41. [DOI] [PubMed] [Google Scholar]
- 7.Papot E, Jacoby S, Arlinda D, Avihingsanon A, Azwa I, Borok M, et al. Adaption of an ongoing clinical trial to quickly respond to gaps in changing international recommendations: the experience of D(2)EFT. HIV Res Clin Pract. 2022;23(1):37–46. [PMC free article] [PubMed] [Google Scholar]
- 8.Bollen P, Freriksen J, Konopnicki D, Weizsacker K, Hidalgo Tenorio C, Molto J, et al. The Effect of Pregnancy on the Pharmacokinetics of Total and Unbound Dolutegravir and Its Main Metabolite in Women Living With Human Immunodeficiency Virus. Clin Infect Dis. 2021;72(1):121–7. [DOI] [PubMed] [Google Scholar]
- 9.Phillips AN, Bansi-Matharu L, Venter F, Havlir D, Pozniak A, Kuritzkes DR, et al. Updated assessment of risks and benefits of dolutegravir versus efavirenz in new antiretroviral treatment initiators in sub-Saharan Africa: modelling to inform treatment guidelines. Lancet HIV. 2020;7(3):e193–e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.British HIV Association. BHIVA guidelines on antiretroviral treatment for adults living with HIV-1 2022 (2023 interim update). 2022. https://www.bhiva.org/HIV-1-treatment-guidelines
- 11.Mulenga L, Fwoloshi S, Mweemba A, al. e. Dolutegravir with recycled NRTIs is non-inferior to PI-based ART: VISEND trial.. 29th Conference on Retroviruses and Opportunistic Infections; Feb 13–16 and 22–24, 2022.; Virtual2022. [Google Scholar]
- 12.Paton NI, Musaazi J, Kityo C, Walimbwa S, Hoppe A, Balyegisawa A, et al. Efficacy and safety of dolutegravir or darunavir in combination with lamivudine plus either zidovudine or tenofovir for second-line treatment of HIV infection (NADIA): week 96 results from a prospective, multicentre, open-label, factorial, randomised, non-inferiority trial. Lancet HIV. 2022;9(6):e381–e93. [DOI] [PubMed] [Google Scholar]
- 13.CHAI UNITAID Innovative agreement launches affordable, optimal second-line HIV treatment in low- and middle-income countries. 2021. [press release]. https://unitaid.org/news-blog/innovative-agreement-affordable-optimal-second-line-hiv-treatment/#en2021.
- 14.Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS. 2017;31(15):2035–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Second Line Group, Boyd MA, Kumarasamy N, Moore CL, Nwizu C, Losso MH, et al. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet. 2013;381(9883):2091–9. [DOI] [PubMed] [Google Scholar]
- 16.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163(9):1009–21. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. https://www.who.int/teams/global-tuberculosis-programme/tbreports. Global tuberculosis report. 2023.
- 18.Dorward J, Khubone T, Gate K, Ngobese H, Sookrajh Y, Mkhize S, et al. The impact of the COVID-19 lockdown on HIV care in 65 South African primary care clinics: an interrupted time series analysis. Lancet HIV. 2021;8(3):e158–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jardim CGR, Zamani R, Akrami M. Evaluating the Impact of the COVID-19 Pandemic on Accessing HIV Services in South Africa: A Systematic Review. Int J Environ Res Public Health. 2022;19(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calza L, Colangeli V, Borderi M, Bon I, Borioni A, Volpato F, et al. Weight gain in antiretroviral therapy-naive HIV-1-infected patients starting a regimen including an integrase strand transfer inhibitor or darunavir/ritonavir. Infection. 2020;48(2):213–21. [DOI] [PubMed] [Google Scholar]
- 21.Esber AL, Chang D, Iroezindu M, Bahemana E, Kibuuka H, Owuoth J, et al. Weight gain during the dolutegravir transition in the African Cohort Study. J Int AIDS Soc. 2022;25(4):e25899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickey MD, Wafula E, Ogachi SM, Ojwando H, Orori G, Adede RO, et al. Weight Change Following Switch to Dolutegravir for HIV Treatment in Rural Kenya During Country Roll-Out. J Acquir Immune Defic Syndr. 2023;93(2):154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calmy A, Tovar Sanchez T, Kouanfack C, Mpoudi-Etame M, Leroy S, Perrineau S, et al. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 noninferiority trial in Cameroon. Lancet HIV. 2020;7(10):e677–e87. [DOI] [PubMed] [Google Scholar]
- 24.Sax PE, Erlandson KM, Lake JE, McComsey GA, Orkin C, Esser S, et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin Infect Dis. 2020;71(6):1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venter WDF, Sokhela S, Simmons B, Moorhouse M, Fairlie L, Mashabane N, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. 2020;7(10):e666–e76. [DOI] [PubMed] [Google Scholar]
- 26.Venter WDF, Hill A. Weighing considerations with newer antiretrovirals. Lancet HIV. 2020;7(6):e374–e5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this study cannot be made publicly available due to ethical restrictions in the study’s informed consent documents and in countries in which D2EFT was conducted; public data availability could compromise participant confidentiality. However, de-identified study data may be available upon request and submission and approval from the D2EFT PSC of a concept sheet summarising the analyses to be done. The data archive will be held at the Kirby Institute and kept on a secure network drive accessible to authorised personnel only. Data generated from the study results may be used in future reports, or used in scientific presentations at both national and international conferences. Further inquiries can be directed to D2EFT@kirby.unsw.edu.au