Abstract
STUDY QUESTION
How accurately can artificial intelligence (AI) models predict sperm retrieval in non-obstructive azoospermia (NOA) patients undergoing micro-testicular sperm extraction (m-TESE) surgery?
SUMMARY ANSWER
AI predictive models hold significant promise in predicting successful sperm retrieval in NOA patients undergoing m-TESE, although limitations regarding variability of study designs, small sample sizes, and a lack of validation studies restrict the overall generalizability of studies in this area.
WHAT IS KNOWN ALREADY
Previous studies have explored various predictors of successful sperm retrieval in m-TESE, including clinical and hormonal factors. However, no consistent predictive model has yet been established.
STUDY DESIGN, SIZE, DURATION
A comprehensive literature search was conducted following PRISMA-ScR guidelines, covering PubMed and Scopus databases from 2013 to 15 May 2024. Relevant English-language studies were identified using Medical Subject Headings (MeSH) terms. We also used PubMed’s ‘similar articles’ and ‘cited by’ features for thorough bibliographic screening to ensure comprehensive coverage of relevant literature.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The review included studies on patients with NOA where AI-based models were used for predicting m-TESE outcomes, by incorporating clinical data, hormonal levels, histopathological evaluations, and genetic parameters. Various machine learning and deep learning techniques, including logistic regression, were employed. The Prediction Model Risk of Bias Assessment Tool (PROBAST) evaluated the bias in the studies, and their quality was assessed using the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines, ensuring robust reporting standards and methodological rigor.
MAIN RESULTS AND THE ROLE OF CHANCE
Out of 427 screened articles, 45 met the inclusion criteria, with most using logistic regression and machine learning to predict m-TESE outcomes. AI-based models demonstrated strong potential by integrating clinical, hormonal, and biological factors. However, limitations of the studies included small sample sizes, legal barriers, and challenges in generalizability and validation. While some studies featured larger, multicenter designs, many were constrained by sample size. Most studies had a low risk of bias in participant selection and outcome determination, and two-thirds were rated as low risk for predictor assessment, but the analysis methods varied.
LIMITATIONS, REASONS FOR CAUTION
The limitations of this review include the heterogeneity of the included research, potential publication bias and reliance on only two databases (PubMed and Scopus), which may limit the scope of the findings. Additionally, the absence of a meta-analysis prevents quantitative assessment of the consistency of models. Despite this, the review offers valuable insights into AI predictive models for m-TESE in NOA.
WIDER IMPLICATIONS OF THE FINDINGS
The review highlights the potential of advanced AI techniques in predicting successful sperm retrieval for NOA patients undergoing m-TESE. By integrating clinical, hormonal, histopathological, and genetic factors, AI models can enhance decision-making and improve patient outcomes, reducing the number of unsuccessful procedures. However, to further enhance the precision and reliability of AI predictions in reproductive medicine, future studies should address current limitations by incorporating larger sample sizes and conducting prospective validation trials. This continued research and development is crucial for strengthening the applicability of AI models and ensuring broader clinical adoption.
STUDY FUNDING/COMPETING INTEREST(S)
The authors would like to acknowledge Mashhad University of Medical Sciences, Mashhad, Iran, for financial support (Grant ID: 4020802). The authors declare no competing interests.
REGISTRATION NUMBER
N/A.
Keywords: male infertility, microdissection testicular sperm extraction, non-obstructive azoospermia, artificial intelligence, successful sperm retrieval
WHAT DOES THIS MEAN FOR PATIENTS?
Non-obstructive azoospermia is a severe form of male infertility where no sperm is present in the semen due to issues in the testes. This condition can be very challenging for couples wishing to have biological children. One of the most effective treatments is microdissection testicular sperm extraction (m-TESE), a surgical procedure that involves carefully searching the testes to find and extract sperm that can be used for in vitro fertilization. However, not all m-TESE procedures are successful, which can cause physical, emotional, and financial burdens for patients. Using artificial intelligence (AI) to predict the likelihood of successful sperm retrieval before m-TESE might significantly improve preoperative planning and patient counselling. This is where AI can help. AI can analyze various factors such as clinical data, hormone levels and genetic information to predict the success of sperm retrieval. Our review looked at studies from the past decade that have used AI to predict the outcomes of m-TESE in patients with non-obstructive azoospermia. We found that AI methods, including machine learning and deep learning, show great promise in improving these predictions. By considering a wide range of factors, AI can help doctors and patients make more informed decisions, potentially reducing the risks and improving the chances of successful treatment. The findings from this review highlight the importance of continuing research in this area. Better predictive models can lead to better patient outcomes, lessening the physical and emotional toll of infertility treatments and providing hope to many couples.
Introduction
Overview
Infertility is a significant global concern that impacts over 100 million individuals generally. It is believed that male-related factors contribute to as much as half of these instances, and this percentage has been on the rise in recent years (Cherouveim et al., 2023). Many clinical factors are linked to male infertility, and numerous studies have emphasized that lifestyle and environmental influences may affect sperm characteristics and diminish semen quality. These influences comprise tobacco, alcohol, drug use, stress, obesity, and sleep deprivation, as well as environmental factors like air pollutants and heavy metals. Additionally, prolonged periods of sitting (more than 4 h daily) are significantly connected with a higher percentage of immotile sperm (GhoshRoy et al., 2023). Male infertility can stem from various factors affecting the production, transport, or function of sperm (Kresch et al., 2021; GhoshRoy et al., 2023).
Non-obstructive azoospermia
Azoospermia, a condition characterized by the absence of sperm in ejaculated semen, affects about 1% of men. Azoospermic patients are broadly categorized into obstructive azoospermia (OA) and NOA. This differentiation impacts clinical management and reproductive outcomes as OA preserves spermatogenesis with mechanical obstruction along the reproductive tract (Lantsberg et al., 2022; Deng et al., 2023c). Approximately 40% of azoospermia cases are due to OA caused by a blockage in the vas deferens, while NOA constitutes roughly 60%, resulting from causes such as testicular spermatogenic failure. Successful retrieval of healthy sperm from the testes is essential for managing this condition and enabling patients to have biological offspring. NOA is caused by various factors, including acquired causes like cryptorchidism and exposure to gonadotoxic chemotherapy or pelvic radiation, as well as genetic causes such as Klinefelter’s syndrome and Y chromosome microdeletions (Brant and Schlegel, 2023).
Assisted reproductive technologies
ARTs consist of various medical procedures aimed at helping couples achieve pregnancy when natural conception is not successful. These may include methods such as intrauterine insemination, in vitro fertilization with intracytoplasmic sperm injection (ICSI), and surgical interventions to retrieve sperm for assisted reproduction purposes. However, it is important to note that the success of these interventions may vary depending on the underlying cause of male infertility. Microdissection testicular sperm extraction (m-TESE) has emerged as a significant advancement in the management of males with NOA. This innovative surgical technique allows for the precise identification and extraction of viable sperm from the testes, even in cases where sperm production is severely impaired. m-TESE has revolutionized the options available to couples facing male infertility, particularly those who were previously limited in their treatment choices (Marinaro and Goldstein, 2022). The collaboration between reproductive endocrinologists and reproductive urologists is crucial in optimizing the outcomes of assisted reproductive technologies, especially in cases of male factor infertility. By combining their expertise in hormonal manipulation, surgical interventions, and assisted reproductive procedures, these specialists can provide comprehensive and individualized treatment plans for couples struggling with male infertility.
m-TESE/sperm retrieval rate (SRR)
The treatment of NOA typically involves surgical interventions to retrieve mature sperm from dysfunctional testes, in combination with ICSI for assisted reproduction. There are three standard surgical procedures: testicular sperm aspiration (TESA), testicular sperm extraction (TESE), and m-TESE. Unlike TESA, which is performed blindly and may miss some spermatogenic regions, m-TESE fully exposes seminiferous tubules in the testicular lobules, allowing for enhanced examination and selective biopsy of thick and full tubules through microscopy. This improves the overall rate of successful sperm retrieval (Deng et al., 2023c).
m-TESE has resulted in an average pooled clinical pregnancy rate of 39% when used for ICSI, with some series reporting rates as high as 72.4%. It is endorsed by both the American Urological Association and the American Society for Reproductive Medicine as the premier approach for sperm retrieval in NOA cases (Brant and Schlegel, 2023).
Overview of SRR and predictors
m-TESE is widely recognized for its higher sperm retrieval rates compared to TESE, especially in patients with NOA. However, the overall success in achieving pregnancy with testicular sperm retrieved from m-TESE in NOA patients remains inadequately documented (Bachelot et al., 2023). Numerous studies have reported several preoperative predictors, including age, body mass index (BMI), testicular volume, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), anti-Müllerian hormone (AMH), inhibin B (InhB), and testosterone levels, which may help in predicting successful sperm retrieval using m-TESE. However, a tool for successful prediction for any single case has been elusive. Although these biomarkers provide valuable prognostic information, their predictive accuracy in unselected patient populations remains inconsistent. This demonstrates that male infertility is complex, and further research is needed in order to provide more reliable and universally applicable prognostic tools for m-TESE results (Lantsberg et al., 2022).
The causes of NOA are diverse, and the success rates of m-TESE can vary significantly based on the underlying etiology. Patients with Klinefelter’s syndrome have moderate success rates of about 50%, which is higher than those with idiopathic NOA but lower than patients with specific genetic deletions such as Y chromosome azoospermia factor C (AZFc) deletion, where success rates can reach up to 67%. In contrast, deletions in the AZFa and AZFb regions are associated with poor outcomes, often discouraging the use of TESE in these cases. Similarly, patients with a history of cryptorchidism have relatively high success rates of around 62%, while those with idiopathic NOA, the most common form, tend to have the lowest success rates, generally ranging between 30% to 40% (Lantsberg et al., 2022). In addition to genetic and clinical factors, histopathological evaluation of testicular tissue can provide valuable insights into the potential success of m-TESE. Histological patterns such as hypospermatogenesis or maturation arrest (MA), when present as the most advanced spermatogenic pattern, are associated with better outcomes compared to cases with Sertoli cell-only syndrome (SCOS) (Brant and Schlegel, 2023). Nonetheless, histopathological assessment involves invasive procedures that may be uncomfortable or distressing for patients (Deng et al., 2023c). Despite extensive examination, these factors have demonstrated variable effectiveness in predicting successful outcomes, making it challenging to develop predictive models that can better support clinical decision-making and improve patient counseling.
Artificial intelligence
AI has the potential to revolutionize the field of male infertility by assisting clinicians in developing personalized and precise treatment plans based on individual patient characteristics and predicting treatment outcomes (Guahmich et al., 2023). This can lead to more successful sperm retrievals and improved chances of achieving pregnancy for couples dealing with male infertility.
Machine learning uses computer programs to learn from a training dataset and make predictions within a predefined task. By supplying the computer with datasets and desired outputs, machine learning algorithms are created to predict future outcomes within specific tasks. This integration has been notably successful in medicine due to its ability to handle large amounts of complex medical data with which traditional algorithms struggle (Guahmich et al., 2023). Furthermore, the use of AI and machine learning algorithms in predicting the success of sperm retrieval in men with NOA has shown promising results. Machine learning has emerged as a technology that may potentially expedite and enhance the accuracy of sperm identification (Brant and Schlegel, 2023). Machine learning methods enable the prediction of outcomes by analyzing extensive datasets, benefiting from advancements in computational capabilities. Previous literature has highlighted the significance of mathematical modeling and machine learning strategies. In relation to testicular sperm extraction, various models have been created using standard clinical and biological data obtained during preoperative evaluations. The predominant models include logistic regression or artificial neural network models (Bachelot et al., 2023).
This study intends to investigate the evidence for predicting sperm retrieval in NOA patients undergoing m-TESE surgery using AI by answering “Which learning models are employed to predict the success of m-TESE for NOA patients, and which biomarkers have been highlighted as predictors of success in m-TESE?”.
Methods
Study design
This investigation was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) guidelines. This study was conducted to evaluate the predictive variables and machine learning models in determining the success of sperm retrieval in men with NOA undergoing an m-TESE procedure.
Search strategy
The research conducted involved a systematic search in PubMed and Scopus to gather all pertinent papers published from the last 11 years up to 15 May 2024. Various combinations of terms were utilized with language restrictions set to English, such as: ‘non-obstructive azoospermia’ OR ‘NOA’ AND ‘AI’ OR ‘machine learning’ OR ‘deep learning’ OR ‘logistic regression’ OR ‘predict*’ AND ‘sperm retrieval*’ OR ‘testicular sperm extraction*’ OR ‘micro-TESE’ OR ‘sperm extraction’ OR ‘testicular sperm extraction’ (Supplementary Table S1). The obtained records were imported into ZOTERO 6.0.30 (Corporation for Digital Scholarship, Fairfax, VA, USA), and duplicate entries were eliminated. Additionally, the references cited by these studies and those citing them were reviewed for supplementary relevant publications.
Study selection
The inclusion criteria encompassed studies involving individuals diagnosed with NOA and undergoing m-TESE. These studies could be non-randomized controlled trials, case-control studies, cohort studies, cross-sectional or analytical studies. Furthermore, the studies had to be original human research that involved factors such as clinical variables, histopathological parameters, genetic predictors, and hormone levels.
Based on the exclusion criteria developed for this review, several studies were excluded from the analysis. Studies that focused on populations with other types of male infertility or emphasized clinical outcomes such as ICSI, pregnancy, and live birth rather than successful sperm extraction by microdissection surgery were excluded. Additionally, studies that were based on statistical analysis instead of AI and machine learning methods for prediction were not considered.
Another crucial aspect of the exclusion criteria was the age of the study population. Therefore, studies that involved patients under 18 years old were excluded. In addition, studies published in venues that were not peer-reviewed scientific sources, such as books, book chapters, proceedings, editorials, PhD dissertations, MSc theses, commentaries, and others, were not considered in this review.
Two authors independently screened all potential literature by title and abstract. Disagreements were solved by discussion; if that failed, another author was called on to adjudicate the final judgment. The same authors performed the full-text screening to determine the final selection.
Data extraction and synthesis
The data extraction into an Excel spreadsheet was carried out and verified by the authors. Any inconsistencies were addressed through discussion and review of the original data. The extracted information included the first author, year of publication, title, single/multicenter(s), sample size, total SRR, machine learning methods, outcome summary, and conclusion drawn from the studies. Additionally noted were any potential research gaps identified in the literature as well as variables of interest such as clinical, hormonal, histopathological factors, and genetic/RNA-related elements along with their reported significance.
Risk of bias
To ensure the methodological soundness and applicability of the included studies, a thorough evaluation of bias was conducted.
The risk of bias and applicability of each AI model within the included studies were rigorously assessed using the Prediction Model Risk of Bias Assessment Tool (PROBAST).
The Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement is a comprehensive set of guidelines aimed at ensuring clear, transparent, and thorough reporting in studies that develop, validate or update prediction models.
PROBAST and TRIPOD, which were applied independently by two reviewers with any discrepancies resolved through discussion, are designed to evaluate the methodological integrity and relevance of multivariable prediction models in clinical research.
Results
Search results and characteristics of the included studies
A total of 45 articles (Fig. 1) were selected from the search on PubMed/Medline and Scopus for utilizing AI techniques in m-TESE for NOA patients. Recent studies have indicated a notable rise in application of machine learning for predicting successful sperm retrieval for m-TESE treatment of NOA. Our analysis, considering relevant inclusion criteria, demonstrates that the most prolific nation publishing research in this area has been China, followed by Italy (Supplementary Fig. S1).
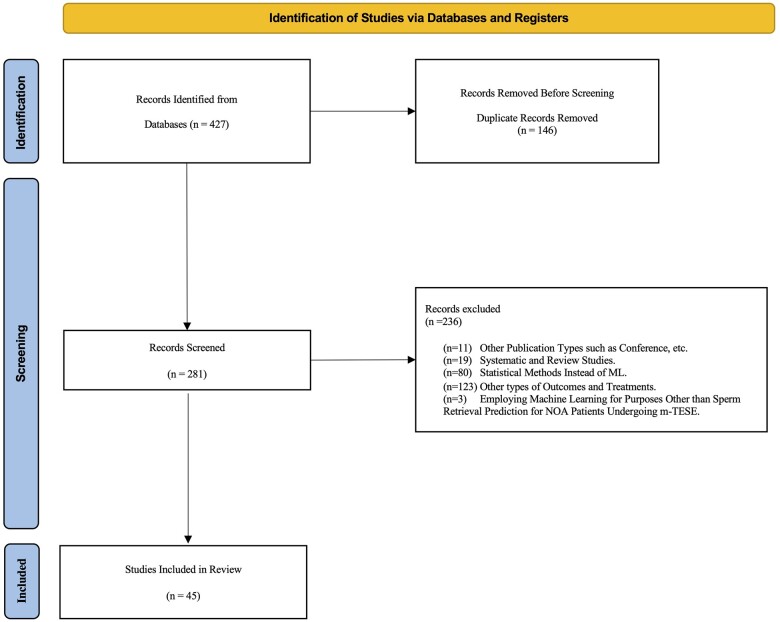
Figure 1.
Flowchart of the study selection process according to the PRISMA-ScR Guidelines. This flowchart outlines the process of identifying, screening, and selecting studies for inclusion in the systematic scoping review. It details the number of records identified through database searches, records screened, studies assessed for eligibility, and those included in the final review. The flowchart also highlights the number of studies excluded at each stage, with reasons provided for exclusions during the full-text review. PRISMA-ScR, preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews; ML, machine learning; m-TESE, microdissection testicular sperm extraction; NOA, non-obstructive azoospermia.
Patient characteristics
Out of 45 included articles, two specifically focused on the use of deep learning. Data extraction and synthesis were performed on a total of 38 articles, which together covered information from 11 636 patients with NOA who had undergone m-TESE.
AI and predictive models for m-TESE outcomes in patients with NOA
All the 45 selected articles used at least one AI technique, demonstrating a strong trend towards incorporating advanced technology in the prediction of sperm retrieval effectiveness for patients with NOA undergoing m-TESE. Of 45 articles, 40 focused on primary m-TESE, while five studies addressed salvage m-TESE empowered by AI (Tables 1 and 2). Furthermore, two articles incorporated deep learning methods, reflecting the increasing use of this advanced technique in predicting sperm identification in testicular biopsy samples. The use of these factors bolsters the depth and comprehensiveness of the predictive models, contributing to a more holistic and accurate assessment of sperm retrieval outcomes in patients with NOA. Also, it is important to consider that among the 45 total included articles, seven articles utilized from artificial neural networks (ANN), principal component analysis (PCA), gradient-boost, decision tree (DT), random forest (RF), support vector machine (SVM), extreme gradient-boosting (XGBoost), or deep neural networks (DNN), while the others used logistic regression.
Table 1.
Studies which have utilized AI techniques to predict success in patients with non-obstructive azoospermia undergoing microdissection testicular sperm extraction.
| Author(s) and year | Country | No. of centers | Sample size | Success rate | Area under the curve (AUC)best model | Machine learning method | Calibration |
|---|---|---|---|---|---|---|---|
| Lv et al., 2024 | China | Single center | 190 |
|
AUCLR = 0.997 | Logistic regression | Absent |
| Tian et al., 2023 | China | Multicenter |
|
|
AUCRF = 0.75 | Logistic regression; LASSO, RF, and XG-boost | Absent |
| Kobayashi et al., 2023 | Japan | Single center | 430 |
|
AUCANN = 0.72 | A gradient-boosting tree and ANN | Absent |
| Bachelot et al., 2023 | France | Single center | 175 |
|
AUCRF = 0.9 |
|
Yes |
| Deng et al., 2023a | China | Single center | 168 |
|
AUCLR = 0.76 | Logistic regression | Absent |
| Shi et al., 2022 | China | Single center | 114 |
|
AUCLR = 0.83 | Logistic regression | Yes |
| Zhang et al., 2023 a | Canada | Multicenter | 122 | Not mentioned | 1–0.87 | Data mining | Absent |
| Pozzi et al., 2023 | Italy | Multicenter | 117 |
|
AUCLR = 0.703 | Logistic regression | Absent |
| Zhang et al., 2023b | China | Single center | 1030 |
|
AUCLR = 0.628 | Logistic regression | Absent |
| Kaltsas et al., 2023 | Greece | Single center | 50 |
|
AUCLR = 0.216 | Logistic regression | Absent |
| Willems et al., 2023 | Brussels | Single center | 38 |
|
Not mentioned | PCA | Absent |
| Cao et al., 2023 | China | Single center | 96 |
|
AUCLR = 0.81 | Logistic regression, LASSO | Absent |
| Deng et al., 2023b | China | Single center |
|
|
Not mentioned | Logistic regression | Absent |
| Zheng et al., 2024 | China | Single center |
|
|
0.81 | Logistic regression | Absent |
| Rachman et al., 2023 | Indonesia | Single center |
|
|
Not mentioned | Logistic regression | Absent |
| Kim and Koo, 2023 | Korea | Single center | 111 |
|
Not mentioned | Logistic regression | Absent |
| Zhang et al., 2022a | China | Single center | 116 |
|
AUCLR = 0.927 | Logistic regression | Absent |
| Falcone et al., 2022 | Italy | Single center | 80 |
|
Not mentioned | Logistic regression | Absent |
| Chen et al., 2022 | China | Single center | 162 |
|
AUCLR = 0.907 | Logistic regression | Absent |
| Aljubran et al., 2022 | Saudi Arabia | Single center | 108 |
|
Not mentioned | Logistic regression | Absent |
| Lantsberg et al., 2022 | Australia | Single center | 85 |
|
Not mentioned | Logistic regression | Absent |
| Zhang et al., 2022b | China | Single center |
|
|
AUCLR = 0.954 | Logistic regression, PCA | Absent |
| Ji et al., 2021 | China | Single center | 52 |
|
AUCLR = 0.958 | Logistic regression, LASSO | Absent |
| Zhou et al., 2021 | China | Single center | 62 | Not mentioned | AUCLR = 0.786 | Logistic regression | Absent |
| Lee et al., 2022 | Canada | Single center | 35 761 image patches | Not mentioned | Not mentioned | CNN based on the U-Net | Absent |
| Wu et al., 2021 | USA | Single center | 30 | Not mentioned | Not mentioned | MobileNetV2, SSD | Absent |
| Xie et al., 2020 | China | Multicenter | 96 |
|
AUCLR = 0.986 | Logistic regression | Absent |
| Chen et al., 2021 | China | Single center | 45 |
|
AUCLR = 0.82 | Logistic regression | Absent |
| Pavan-Jukic et al., 2020 | Slovenia and Croatia | Single center | 62 |
|
Not mentioned | Logistic regression | Absent |
| Maglia et al., 2018 | Italy | Single center |
|
|
Not mentioned | Logistic regression | Absent |
| Amer et al., 2019 | Egypt | Single center | 1395 |
|
AUCLR = 0.682 | Logistic regression | Absent |
| Caroppo et al., 2019 | Italy | Single center | 143 |
|
AUCLR = 0.93 | Logistic regression | Absent |
| Salehi et al., 2017 | Iran | Single center | 170 |
|
Not mentioned | Logistic regression | Absent |
| Klami et al., 2018 | Finland | Not mentioned | 100 |
|
Not mentioned | Logistic regression | Absent |
| Althakafi et al., 2017 | Saudi Arabia | Multicenter | 421 |
|
Not mentioned | Logistic regression | Absent |
| Cissen et al., 2016 | Netherland | Multicenter | 1371 |
|
AUCLR = 0.65 | Logistic regression | Absent |
| Franco et al., 2016 | Italy | Single center | 64 |
|
Not mentioned | Logistic regression | Absent |
| Enatsu et al., 2016 | Japan | Single center | 329 |
|
Not mentioned | Logistic regression | Absent |
| Modarresi et al., 2015 | Iran | Single center | 148 |
|
AUCLR = 0.68 | Logistic regression | Absent |
| Ramasamy et al., 2013 | USA | Single center | 1026 | Not mentioned | AUCANN = 0.59 |
|
Absent |
AI, artificial intelligence; m-TESE, microdissection testicular sperm extraction; NOA, non-obstructive azoospermia; OA, obstructive azoospermia; ANN, artificial neural network; AUC, area under the curve; CNN, convolutional neural network; LASSO, least absolute shrinkage and selection operator; PCA, principal component analysis; RF, random forest; RNN, recurrent neural network; SRF, sperm retrieval failure; SRR, sperm retrieval rate; SSR, successful sperm retrieval; SSD, single-shot detector.
Table 2.
Studies which have employed AI techniques to predict success in patients with non-obstructive azoospermia undergoing salvage microdissection testicular sperm extraction.
| Author(s) and year | Country | No. of centers | Sample size | Success rate | Best AUC | Machine learning method | Calibration |
|---|---|---|---|---|---|---|---|
| Boeri et al., 2022 | Italy | Single center | 61 |
|
Not mentioned | Logistic regression | Absent |
| Ghalayini et al., 2022 | Jordan | Single center |
|
|
Not mentioned | Logistic regression | Absent |
| Caroppo et al., 2021 | Italy | Single center | 79 |
|
AUCLR = 0.8104 | Logistic regression | Absent |
| Yücel et al., 2018 | Turkey | Single center | 49 |
|
Not mentioned | Logistic regression | Absent |
| Xu et al., 2017 | China | Single center | 52 |
|
Not mentioned | Logistic regression | Absent |
AI, artificial intelligence; m-TESE, microdissection testicular sperm extraction; AUC, area under the curve; SRF, sperm retrieval failure; SRR, sperm retrieval rate; SSR, successful sperm retrieval.
Machine learning techniques for predicting m-TESE success
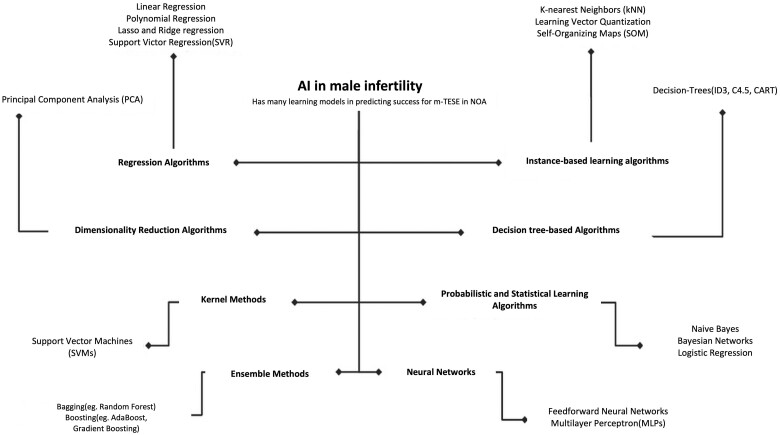
Machine learning models employed to predict sperm retrieval in patients with NOA before m-TESE are illustrated in Fig. 2. Logistic regression, a commonly utilized machine learning algorithm in medicine, enables researchers to assess the impact of multiple variables on an outcome. It is also valuable for comprehending variable relationships and identifying crucial factors in medical research (Boateng and Abaye, 2019).
Figure 2.
AI learning models for predicting success in microdissection testicular sperm extraction for non-obstructive azoospermia. This figure illustrates the application of various AI learning models used to predict the success of m-TESE in men with NOA based on data from male infertility studies. The figure highlights the performance of different models, such as neural networks, support vector machines, and random forests, in terms of accuracy and predictive power. The comparison aims to show which AI model provides the most reliable predictions for successful sperm retrieval in m-TESE. AI, artificial intelligence; m-TESE, microdissection testicular sperm extraction; NOA, non-obstructive azoospermia.
This approach has been extensively applied to evaluate male infertility and to identify influential factors regarding sperm recovery, as indicated by previous studies using logistic regression (Tables 1 and 2).
Two logistic regression studies on a large patient cohort reported differing outcomes (area under the curve (AUC) = 0.628, AUC = 0.69) compared to similar studies (Cissen et al., 2016; Zhang et al., 2023b). Another study with a sample size of 1395 identified significant associations between sperm recovery and only five parameters out of several including age, infertility duration, FSH level, occupation, residence, lifestyle habits, right testis volume, left testis volume, Sertoli cell only, tubular sclerosis, primary spermatocyte arrest, secondary spermatocyte arrest, spermatid arrest, hypospermatogenesis, hormonal therapy, and type of hormonal therapy (Amer et al., 2019). The complexity and non-linear nature of the data, coupled with the imbalance class in sperm retrieval outcomes, suggest the need for non-linear machine learning approaches like RF and SVM.
Tian et al. (2023) aimed to create and assess a predictive model for the clinical outcome of m-TESE in a diverse population with NOA. The study involved 1292 patients as the development cohort and an additional 530 patients for external validation. m-TESE was used for sperm retrieval, and a random forest machine learning method was employed to build a model implemented as a web-based calculator. Results showed that the SRR was 38.1% (492/1292) in the development cohort and 48.5% (257/530) in the validation group. The AUC values indicated good performance; specifically, 0.76 (0.74–0.79) in the development cohort and 0.75 (0.71–0.79) in the external validation, suggesting robustness across cohorts.
Kobayashi et al. (2023), a retrospective study involving 430 patients, aimed to develop a model for predicting the likelihood of sperm recovery before m-TESE operation. Results indicated that the neural network model with an AUC of 0.7246 was identified as the most effective in this investigation.
Bachelot et al. emphasized the potential of machine learning models to enhance decision support systems in the context of azoospermia by providing more comprehensive information compared to individual variables. Despite exploring various innovative AI models, none were able to conclusively predict TESE results with absolute certainty among the eight different models that were developed, optimized, and assessed. The research involved testing ML algorithms like Bayesian naive classification, logistic regression, k-nearest neighbor classifier, support vector machines, random forest, gradient-boosted tree, XGBoost, as well as deep learning models utilizing different neural network architectures. Among all the models, random forest demonstrated superior performance with an AUC of 0.90 (Bachelot et al., 2023).
Ramasamy et al. (2013) developed an artificial neural network and nomogram to predict the probability of discovering sperm using m-TESE in men with NOA based on clinical characteristics. The receiver operating characteristics (ROC) area for the neural computational system was 0.641 in the test set and it accurately predicted results for 152 out of 256 patients (59.4%). Ensemble models based on DT, particularly RF, showed the best performance in predicting outcomes. By comparing various machine learning models, the study found that ensemble models based on decision trees, particularly the random forest model, showed the best performance in predicting.
Predictors
This research provides a concise overview of the biomarkers and parameters obtained from the study involving four categories encompassing genetics and hormones, as well as histopathological and clinical characteristics. Table 3 illustrates the specific variables that were collected and their corresponding significance.
Table 3.
Biomarkers of non-obstructive azoospermia patients derived from studies for success prediction in microdissection testicular sperm extraction treatment using AI methods.
| Author(s) and year | Variables |
||||
|---|---|---|---|---|---|
| Clinical | Hormonal | Genetics/micro-RNA/proteins | Histopathology | Importance variable | |
| Lv et al., 2024 |
|
|
|
Not mentioned |
|
| Tian et al., 2023 |
|
|
|
Not mentioned |
|
| Kobayashi et al., 2023 |
|
|
|
Not mentioned | T/E2 |
| Bachelot et al., 2023 |
|
|
|
Not mentioned |
|
| Deng et al., 2023a |
|
|
Not mentioned | Not mentioned | InhB/AMH |
| Shi et al., 2022 |
|
|
circ_MGLL |
|
|
| Zhang et al., 2023 a | Not mentioned | Not mentioned | Germ cell-specific proteins | Not mentioned | AKAP4+/ASPX+/Hoechst+ assay |
| Pozzi et al., 2023 |
|
|
Not mentioned |
|
AMH |
| Zhang et al., 2023b |
|
|
Etiology (Y microdeletion AZFc) | Not mentioned | AMH |
| Kaltsas et al., 2023 |
|
|
Not mentioned | Diagnostic testicular biopsy (DTB) | DTB |
| Willems et al., 2023 | Not mentioned | Not mentioned | microRNA in seminal plasma and urine | Not mentioned | Non-significant |
| Cao et al., 2023 |
|
|
|
Not mentioned |
|
| Deng et al., 2023b |
|
|
Y microdeletion AZFc | Not mentioned | Non-significant |
| Zheng et al., 2024 |
|
|
|
Not mentioned | AMH |
| Rachman et al., 2023 |
|
|
Not mentioned | Not mentioned | Varicocele |
| Kim and Koo, 2023 |
|
|
Not mentioned |
|
|
| Zhang et al., 2022a |
|
|
Circulating microRNAs | Not mentioned | hsa-miR-34b-3p, hsa-miR-34c-3p, hsa-miR-3065-3p, and hsa-miR-4446-3p |
| Falcone et al., 2022 |
|
FSH | Not mentioned |
|
|
| Chen et al., 2022 |
|
|
Not mentioned | Not mentioned |
|
| Aljubran et al., 2022 |
|
|
Not mentioned | JS |
|
| Lantsberg et al., 2022 |
|
|
|
|
|
| Zhang et al., 2022b | Not mentioned | Not mentioned | Circulating plasma exosomal tRFs | Not mentioned | Plasma circulating exosomal tRF-Gly-GCC-002 and tRF-Glu-CTC-005 |
| Ji et al., 2021 |
|
|
Circulating RNAs |
|
hsa_circ_0000277, hsa_circ_0060394 and hsa_circ_0007773 |
| Zhou et al., 2021 |
|
|
Expression of Beclin-1 | JS |
|
| Xie et al., 2020 | Not mentioned | Not mentioned | Long non-coding RNAs | Not mentioned |
|
| Chen et al., 2021 | Not mentioned | Not mentioned | EV-piRNAs in seminal plasma | Not mentioned |
|
| Pavan-Jukic et al., 2020 |
|
|
|
Not mentioned | Smoking |
| Maglia et al., 2018 |
|
|
Not mentioned |
|
|
| Amer et al., 2019 |
|
FSH | Not mentioned |
|
|
| Caroppo et al., 2019 |
|
|
Not mentioned |
|
|
| Salehi et al., 2017 |
|
|
Normal karyotyping |
|
|
| Klami et al., 2018 |
|
|
|
|
Testicular size |
| Althakafi et al., 2017 |
|
|
Chromosomal analysis (XXY47) |
|
|
| Cissen et al., 2016 |
|
|
|
Not mentioned |
|
| Franco et al., 2016 | Age |
|
Not mentioned |
|
Non-significant |
| Enatsu et al., 2016 |
|
|
|
|
Etiology |
| Modarresi et al., 2015 | Age |
|
Not mentioned | SCOS | Non-significant |
| Ramasamy et al., 2013 |
|
FSH | KS | Not mentioned |
|
AI, artificial intelligence; AMH, anti-Müllerian Hormone; AZF, azoospermia factor; BMI, body mass index; CC, clomiphene citrate; CCI, Charlson comorbidity index; cFT, circulating free testosterone; circ_MGLL, circular RNA monoglyceride lipase; DTB, diagnostic testicular biopsy; E2, estradiol; EMA, early maturation arrest; EVs, extracellular vesicles; FSH, follicle-stimulating hormone; HL, hyalinosis; HS, hypo spermatogenesis; IN, intraepithelial neoplasia; iNOA, idiopathic non-obstructive azoospermia; InhB, Inhibin B; JS, Johnsen score; KS, Klinefelter syndrome; LH, Luteinizing Hormone; LMA, late maturation arrest; MA, maturation arrest; MER, monocyte-to-eosinophil ratio; m-TESE, microdissection testicular sperm extraction, NLR, neutrophil-to-lymphocyte ratio; NS, normal spermatogenesis; NOA, non-obstructive azoospermia; PLR, platelet-to-lymphocyte ratio; PRL, prolactin; SA, spermatogenic arrest; SCOS, Sertoli cell-only syndrome; SHBG, sex hormone-binding globulin; ST, seminiferous tubules; T, testosterone; tRFs, transfer RNA-derived fragments; TSH, thyroid-stimulating hormone; tT, total testosterone; TTB, therapeutic testicular biopsy; TV, testicular volume.
Clinical factors
Clinical factors like infertility cause, age, testicular size, BMI, and medical history are evaluated in NOA. Studies have conflicting results on the impact of age on m-TESE success. Some found no link, while others suggest orchidopexy age and testicular volume may affect sperm retrieval rates. Research indicates male age does not significantly impact m-TESE results, with highest success in men over 40 years. Obesity does not affect m-TESE sperm retrieval but lowers pregnancy rates. The correlation of success with testicular volume remains inconsistent. Etiology is a key predictor; idiopathic NOA is associated with a poor prognosis, while patients with conditions like cryptorchidism have higher sperm retrieval rates (Qi et al., 2021).
Histopathology
Histopathological evaluation also plays an essential role in predicting m-TESE success in NOA patients. Certain patterns, such as hypospermatogenesis, where spermatogenesis is present but reduced, are associated with higher sperm retrieval rates. Conversely, conditions like Sertoli cell-only syndrome (SCOS) and maturation arrest (MA) are linked to lower success rates. While biopsies can provide predictive information, they are not always recommended due to risks like pain, infection, and hematoma. Recent advancements in machine learning have improved the accuracy of predictions by analyzing complex clinical, hormonal, and histopathological data. Integrating histopathological evaluation with hormonal assessment enhances the ability to predict and manage sperm retrieval outcomes, offering a more comprehensive approach to treating NOA patients undergoing m-TESE (Abdullah and Bondagji, 2011; Weedin et al., 2011; Ghanami Gashti et al., 2021).
Hormones
Hormone levels are crucial in predicting the success of m-TESE in patients with NOA. FSH and LH are primary indicators, with elevated FSH levels often signaling impaired spermatogenesis and testicular failure. Testosterone is essential for spermatogenesis and sperm maturation, while high prolactin levels can lead to hypogonadism and spermatogenic failure. Inhibin B, produced by Sertoli cells, inversely regulates FSH and correlates with spermatogenic activity, although its predictive efficacy for sperm retrieval remains debated. AMH, involved in spermatogenic cell differentiation, shows potential as a spermatogenesis marker, but further research is needed to establish its role definitively. Collectively, these hormones provide valuable insights into the likelihood of successful sperm retrieval in NOA patients undergoing m-TESE (Qi et al., 2021; Sengupta et al., 2021; Li et al., 2023).
Genetic factors
Genetic factors are pivotal in predicting the success of m-TESE in patients with NOA. Key genetic analyses include tests for sex chromosome anomalies like Klinefelter syndrome (KS), XYY syndrome, and Y chromosome microdeletions. Among these, KS and Y chromosome microdeletions are the most common in infertile patients. The Y chromosome contains AZF regions, crucial for spermatogenesis. Microdeletions in the AZFc region are the most prevalent and offer a higher chance of successful sperm retrieval compared to deletions in AZFa or AZFb regions (Glina and Vieira, 2013; Flannigan et al., 2017; Li et al., 2023; Şen et al., 2023).
Recent research has highlighted the significance of microRNAs (miRNAs) as potential non-invasive biomarkers for predicting m-TESE outcomes. miRNAs are small RNA molecules that regulate gene expression and play roles in germ cell development and spermatogenesis. Abnormal miRNA expression in NOA patients suggests their potential for predicting sperm retrieval success. Moreover, miRNAs in seminal plasma are protected from degradation, making them stable markers. Additionally, long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), and tRNA-derived small RNAs (tsRNAs) in seminal plasma are also being explored as biomarkers. These RNAs contribute to spermatogenesis and offer insights into the spermatogenic status, providing valuable non-invasive predictors for m-TESE success (Li et al., 2023; Şen et al., 2023).
Machine learning techniques for salvage m-TESE
Salvage m-TESE is a surgical technique increasingly used to extract viable sperm from the testes of men with NOA after unsuccessful prior attempts at sperm retrieval procedures. This advancement in male infertility offers potential reproductive outcomes for individuals with NOA. Subsequent attempts at salvage m-TESE have shown relatively satisfactory rates of retrieving sperm but are not guaranteed. Machine learning methods successfully identified variables linked to SSR with high precision, offering insights into how specific intra-surgical parameters can predict salvage m-TESE outcomes for NOA patients who have previously faced unsuccessful surgical retrieval attempts. The current research leverages machine learning to gain significant benefits, especially in predicting and projecting elements that strongly correlate with the SRR outcome in individuals with NOA (Table 2).
Boeri et al. (2022) developed a study proposing m-TESE as a possible remedy for men who have previously undergone unsuccessful classic TESE (c-TESE). While information on this topic is limited, we sought to assess the outcomes and potential factors that could indicate successful salvage m-TESE in a cohort of individuals with previously unsuccessful c-TESE. The analysis encompassed data from 61 men who underwent m-TESE after failed c-TESE between January 2014 and October 2020 at six tertiary-referral centers in Italy. Various assessments were conducted on all participants before the procedures and comprehensive diagnoses from prior TESE were compiled. Descriptive statistics and logistic regression models were utilized to investigate potential predictors of positive sperm retrieval following salvage m-TESE. The research revealed that hypospermatogenesis was independently linked with favorable results during salvage m-TESE, even after adjusting for age and FSH levels. In conclusion, it can be inferred that salvage m-TESE presents as a safe option for NOA patients with previous negative c-TESE findings and may result in an approximately 50% success rate in achieving a sperm retrieval positive status.
Caroppo et al. (2021), by utilizing machine learning methods like predictive models, successfully identified variables linked to SSR with a high level of precision. This approach yielded valuable insights into how specific intra-surgical parameters can predict the result of salvage m-TESE, even for NOA patients who have previously faced unsuccessful surgical retrieval attempts. Additionally, employing machine learning facilitated an elucidation and understanding of variability within the results. The capacity of the model to accurately classify 88.3% of cases and clarify 29.7% of variability highlights its effectiveness in analyzing intricate histopathological findings and recognizing key factors contributing to successful SSR outcomes. Notably, exponential moving average (EMA) emerged as a significant factor within this predictive model, demonstrating how machine learning not only enhances comprehension but also provides practical value by highlighting vital histological categories influencing m-TESE outcomes for NOA patients undergoing salvage procedures following failed conventional approaches.
The research of Yücel et al. (2018) was to investigate the practical application of salvage m-TESE in individuals diagnosed with NOA, and to determine the factors that affect the presence of spermatozoa during preoperative salvage m-TESE. The study used multivariate analysis to identify and predict factors linked to successful sperm retrieval during a salvage m-TESE procedure. A model incorporating parameters such as age, BMI, history of varicocele, history of cryptorchidism, duration of infertility, results of genetic analysis, and hormone profiles was developed using multiple logistic regression analysis for comparison between patient groups. The results showed that among predictors influencing successful sperm retrieval in salvaging m-TESE within NOA patients who had previously undergone unsuccessful attempts at an m-TESE operation, only pre-operative FSH levels displayed a significant correlation with success rates in salvage m-TESE procedures.
The aims of the research by Xu et al. (2017) were to evaluate the practical application and factors for predicting sperm retrieval through salvage m-TESE in NOA patients who have had unsuccessful c-TESE attempts. A total of 52 NOA males underwent salvage m-TESE, and data on various factors such as age, BMI, presence of Klinefelter’s syndrome, varicocele, or cryptorchidism, mean testicular volume, hormonal profile (total testosterone, FSH, LH, inhibin B levels), testicular histology, and surgical duration were gathered and analyzed. Multivariate logistic regression indicated that total testosterone levels and testicular histology were significant predictors for the likelihood of sperm retrieval during salvage m-TESE. In addition, a predictive model was created using multivariate regression analysis to estimate the probability of sperm retrieval. ROC analysis established a cutoff value at 71% predicted probability with a sensitivity of 78.0% and specificity of 72.4%. These results underscore the benefit of utilizing advanced techniques like multivariate regression models to identify important predictors associated with outcome variables in order to inform clinical decisions regarding potential candidates for salvage m-TESE procedures in NOA patients following failed c-TESE attempts.
Ghalayini et al. (2022) showed that repeated m-TESE demonstrates a high success rate in retrieving sperm from patients with NOA, particularly following initial successful procedures. Their findings suggest that when cryopreserved testicular sperm for ICSI yields no viable spermatozoa, scheduling a repeat TESE procedure may be warranted. The use of machine learning provides significant advantages in this study for modeling and predicting factors that are closely related to the outcome variable, such as testicular volume and FSH levels. Specifically, our data indicate a correlation between the success rate of repeated m-TESE and testicular volume as well as FSH concentration. Furthermore, preoperative prognostic data can accurately predict cases where m-TESE is most beneficial, especially in instances involving atrophied testicles or SCOS with elevated FSH concentrations.
Deep learning in sperm detection and identification for optimizing m-TESE procedures
The accurate detection of sperm within m-TESE samples post-procedurally remains a critical component of the overall treatment process. There is evidence in some studies that deep learning can identify sperm from testicular biopsies with near-human performance. Deep learning improves sperm identification by enhancing image analysis capabilities, increasing accuracy and efficiency, reducing manual effort, and continuously adapting to new data. These advantages make deep learning a powerful tool in the field of male infertility treatment, particularly for identifying sperm in challenging cases such as NOA. Although these methods are typically used during or post-procedurally to find sperm, it is worth noting that these technologies not only support the precise execution of assisted reproductive techniques like ICSI but also contribute to the broader goal of improving outcomes for patients undergoing m-TESE. Advanced deep learning techniques, as demonstrated in studies by Lee et al. (2022) and Wu et al. (2021), offer significant potential to enhance the identification of rare sperm in testicular biopsy samples. This information is provided for researchers interested in this aspect, but as it is not directly related to the aim of our study, further exploration of this topic is beyond our current scope.
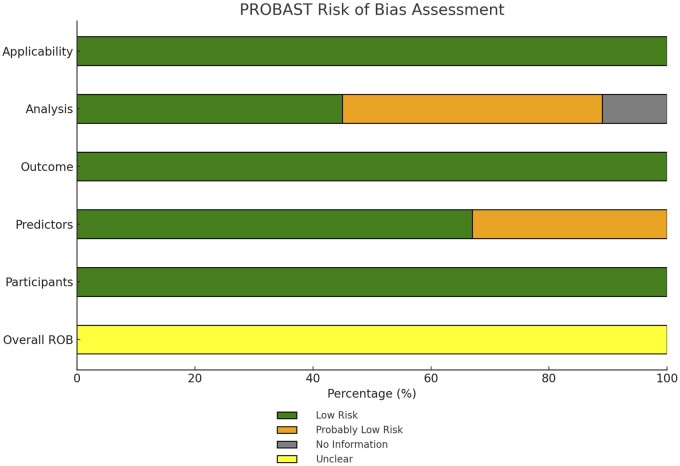
Risk of bias in included studies
Our review revealed that all included studies demonstrated a low risk of bias and minimal concerns regarding applicability, emphasizing their strong methodological foundation and relevance to clinical practice. Using PROBAST, we evaluated key domains such as participant selection, predictor assessment, outcome determination, and analysis methods. The results showed that 100% of the studies had a low risk of bias in participant selection and outcome determination. For predictor assessment, 33% of studies had a ‘probably low’ risk, while 67% showed a ‘low’ risk of bias. The analysis methods presented more variability, with 44% of studies rated as ‘probably low’, 45% as ‘low’, and 11% categorized under ‘no information’, reflecting varying levels of analytical rigor. Figure 3 and Supplementary Fig. S2 provide a detailed breakdown of these assessments, underscoring the reliability and clinical significance of the reviewed studies. The Cohen’s kappa for interrater agreement was 0.87.
Figure 3.
Overall PROBAST risk of bias assessment for entire included studies predicting sperm retrieval in non-obstructive azoospermia patients undergoing microdissection testicular sperm extraction. This figure presents the PROBAST assessment of bias risk across key domains in studies focused on predicting sperm retrieval outcomes for NOA patients undergoing m-TESE. The evaluation covers participant selection, predictors, outcomes, analysis, and applicability, with the risk levels indicated by color-coded bars: green (low risk), orange (probably low risk), yellow (unclear), and grey (no information). m-TESE, microdissection testicular sperm extraction; NOA, non-obstructive azoospermia; PROBAST, prediction model study risk of bias assessment tool.
In addition, the transparent reporting of TRIPOD guidelines were used to ensure thorough and transparent reporting across all studies. The TRIPOD checklist, covering 27 essential items from title to discussion, was applied to evaluate the reporting quality. By adhering to these guidelines, the studies ensured clarity, reproducibility, and critical appraisal. Each study’s TRIPOD score is detailed in Supplementary Fig. S3, offering an overview of how well each article met the reporting standards. This comprehensive evaluation of both bias and reporting quality highlights the overall robustness, transparency and reliability of the multivariable prediction model studies reviewed.
Discussion
Treating patients with NOA often involves a procedure known as m-TESE, which has proven to be highly successful in retrieving sperm, with success rates reaching up to 64% for eligible cases (Glina and Vieira, 2013).
Accurately predicting the success of m-TESE plays a vital role in the effective management of NOA. This research underscores the value of machine learning models in refining preoperative predictions of sperm retrieval, thereby aiding in better patient counseling and surgical decision-making. By incorporating clinical, hormonal, and genetic factors, these models enable a more precise evaluation of which NOA patients are most likely to benefit from m-TESE. Our findings indicate a significant reliance on machine learning techniques across the studies reviewed, highlighting their ability to synthesize complex datasets and deliver predictive insights. This approach has the potential to minimize unnecessary surgeries and enhance treatment outcomes.
Notably, 45 of these studies employed machine learning methods, showcasing a predominant reliance on this type of AI technique in their analyses, emphasizing the multifaceted approach adopted in the prediction models. In predicting successful sperm retrieval prior to m-TESE, studies have reported varied outcomes when employing AI and machine learning algorithms. Logistic regression emerges as the most prevalent algorithm, particularly favored for its simplicity and efficiency in handling big datasets, especially within single-center studies. Consequently, models developed using preoperative clinical, biological, and histopathological data exhibit considerable variability in predictive accuracy. Reported AUC values range widely, from a low of 0.216 (Kaltsas et al., 2023), reflecting significant challenges in smaller, more diverse populations, to a high of 0.997 (Lv et al., 2024), indicative of near-perfect performance in more controlled single-center environments. In more complex multicenter datasets, advanced machine learning techniques, such as random forests, support vector machines, and artificial neural networks, have been employed to enhance model generalizability and predictive performance. While some studies have reported promising results with AUCs reaching up to 0.90, these advanced predictive models still encounter difficulties in maintaining consistent accuracy across diverse patient populations, with AUCs ranging from 0.59 to 0.9. This variability is influenced by factors such as sample size, population diversity, and study design. These findings strongly advocate for rigorous study designs, careful model selection, and comprehensive validation processes to develop AI models that are not only accurate but also broadly applicable for predicting m-TESE outcomes. High AUC scores signify robust model performance but do not guarantee flawless or absolute outcomes due to the intrinsic uncertainty inherent in medical predictions. Information is presented based on the data summarized in Table 1 (Bachelot et al., 2023; Kobayashi et al., 2023; Tian et al., 2023).
Deep learning techniques, particularly convolutional neural networks, have shown potential in automated sperm identification and selection in testicular biopsy samples (Wu et al., 2021; Lee et al., 2022).
The research underlines that the prediction of SRR should consider several combined parameters because no single parameter has been found to be strongly predictive of the success of m-TESE. It has been identified that the most important predictors include testicular histology, genetic markers, hormone levels, and clinical characteristics. As summarized by Table 3, some studies have noted the importance of etiology, age, and testicular volume, while others found those biomarkers to be less predictive. In addition, histopathological patterns also show predictive potential for success in sperm retrieval; hypospermatogenesis shows more chance of sperm retrieval than MA and SCOS. However, the risks associated with diagnostic biopsies limit the spread of use.
The contribution of hormonal factors in predicting SRR is controversial. Whereas some studies have shown that FSH, LH, testosterone, and AMH were correlated with SRR status, others have demonstrated no such significant associations. Furthermore, AMH has been suggested as a potential marker for spermatogenesis in azoospermic men; however, results are diverse. Genetic factors are especially important in predicting the success of m-TESE in NOA patients, including sex chromosome abnormalities such as Klinefelter syndrome and Y chromosome microdeletions in the AZF regions. Genetic studies have promising potential, but they are yet to be incorporated into day-to-day practice due to issues such as complexity, the cost of genetic testing, and the extent of validation in different populations. The emerging advances in non-invasive biomarkers, including microRNAs and other non-coding RNAs, offer some promise for enhanced predictive capabilities but must first pass through clinical testing and standardization procedures before being introduced in practice to improve routine infertility diagnostics and treatment planning. These insights are detailed in Table 3.
Therefore, further research and progress in the field are necessary to enhance patient selection through clinical, hormonal, histopathological, and genetic factors. This includes improving retrieval rates and streamlining the process of sperm identification and analysis. Considering all these variables for predicting outcomes emphasizes the significance of complexity and precision in determining the most suitable treatment approach for patients with NOA. The utilization of advanced AI methods, such as machine learning and deep learning, to predict the effectiveness of sperm retrieval underscores how technology is increasingly influencing clinical decision-making and patient care within male infertility management. It is worth noting that the use of calibration information in predictive modeling within original research articles helps to ensure that the model’s predictions are not only accurate but also interpretable and reliable for practical applications. Our review identifies that only two (Bachelot et al., 2023, Shi et al., 2022) articles have included calibration data in their assessment of predictive model performance. The remaining articles, however, did not discuss the absence of calibration data nor provided any evaluation concerning calibration. This represents a significant gap in the literature, as calibration is crucial for determining how well predicted probabilities align with actual outcomes in clinical settings. Without evaluating calibration, the generalizability of these predictive models is questionable. Consequently, future research should focus on incorporating both discrimination and calibration measures to ensure robust model validation, ultimately improving their utility and reliability in clinical practice. Moreover, the studies acknowledged several limitations, including the need for validation studies, modeling-related constraints, legal barriers in certain countries, calls for prospective randomized control trials, the consideration of additional predictors, and sample size limitations. Addressing these limitations could enhance the accuracy and generalizability of AI-based prediction models in male infertility management.
Limitations of SRR prediction studies
While this study provides a comprehensive overview of AI predictive models in m-TESE for NOA, it is important to acknowledge certain limitations. The heterogeneity of the included studies, varying in population characteristics, methodologies, and machine learning models, may affect the comparability and generalizability of the findings. Additionally, as the review focused solely on two databases, PubMed and Scopus, this could limit the scope of included studies, potentially overlooking relevant research from other databases. Moreover, the potential for publication bias remains, as studies with inconclusive or negative results may be underrepresented. Finally, the absence of a meta-analysis in this scoping review means that while a broad overview is provided, it lacks the statistical synthesis to quantitatively assess the consistency of the predictive models across studies.
Despite advancements in sperm recovery prediction models and the identification of influential factors using AI to assist doctors in determining whether to initiate or continue m-TESE treatment, this remains a complex issue with limitations within existing studies. The articles covered in this review outline six main categories of limitations and suggestions for further research (Supplementary Table S2).
First, there is a recommendation for validation studies to explore the generalizability and assess the effectiveness of the proposed models for predicting successful sperm recovery.
Second, modeling-related constraints include conducting retrospective studies, challenges associated with data collection, the presence of confounding factors and biases (such as patient selection), along with clinical and laboratory techniques impacting sperm recovery rates.
Third, limitations due to legal or regulatory barriers exist in certain countries, regarding investigation into infertility among men. It is also suggested that sample preparation processes should be simplified and standardized.
Fourth, there are calls for prospective randomized controlled studies intended to validate the current findings; these trials must be carefully designed to adhere to ethical principles. Rather than denying m-TESE treatment based on AI predictions, such RCTs could focus on comparing different approaches where AI-based predictions are used to guide supplementary diagnostic testing or preparatory interventions, aiming to improve patient outcomes without compromising access to care.
Fifth, the inclusion of emerging and less commonly utilized predictors such as microRNAs, Y chromosome microdeletions, and specific histopathological patterns, in conjunction with traditional markers like hormonal levels and genetic factors, can offer better insights into identifying individuals who would benefit most from m-TESE surgery.
Finally, there have been sample size limitation issues, such as small numbers or a lack of samples collected from multiple centers. By addressing these limitations and filling research gaps, future AI-based approaches toward male infertility could significantly enhance diagnostic accuracy, personalized treatment strategies and overall patient outcomes.
Conclusion
In conclusion, the best use of advanced AI techniques holds a lot of promise in improving the prediction of sperm retrieval success in individuals with NOA. New algorithms based on random forests and support vector machines have great potential for the identification of predictive biomarkers, selection of treatment at an optimal level, and assessment of therapeutic outcomes. Some critical remaining challenges include the need for proper validation studies, overcoming some intrinsic limitations of actual modeling approaches, legal or regulatory barriers in some jurisdictions, and conducting prospective randomized trials. In addition, emerging predictors and the challenge of small sample sizes need to be considered. Overcoming these challenges represents an enormous opportunity for future research to advance diagnostic accuracy, personalize strategies in treating cases, and ultimately improve patient outcomes in the management of male infertility.
Supplementary Material
Contributor Information
Hossein Jamalirad, Department of Medical Informatics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Mahdie Jajroudi, Department of Medical Informatics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; Pharmaceutical Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
Bahareh Khajehpour, Midwifery Department, Faculty of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran.
Mohammad Ali Sadighi Gilani, Department of Andrology, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran; Department of Urology, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran.
Saeid Eslami, Department of Medical Informatics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; Pharmaceutical Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
Marjan Sabbaghian, Department of Andrology, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran.
Hassan Vakili Arki, Department of Medical Informatics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Data availability
The data presented in this article were obtained solely from the original published sources. No additional data were generated or analyzed to substantiate the content of this article. Correspondence and requests for materials should be addressed to M.S. or H.V.A.
Authors’ roles
H.J. was responsible for the conceptualization, investigation, data extraction and analysis, and development of the methodology. He also contributed to the interpretation of results and was the primary author of the original draft, with significant involvement in reviewing and editing the manuscript. M.J. played a key role in the investigation, data extraction and analysis, and methodology. She also contributed to the interpretation of the data and participated in drafting, reviewing, and editing the manuscript. B.K. was involved in the acquisition and extraction of data. M.A.S.G. made substantial contributions to the interpretation of the results. S.E. contributed to the conceptualization of the study. M.S. provided oversight for the study, contributing to the conceptualization, interpretation of the findings, and project administration while critically reviewing and revising the manuscript for intellectual content. H.V.A. supervised the study in its entirety, contributing to the conceptualization, interpretation, and project administration, and also critically reviewed and revised the manuscript for significant intellectual content. All authors have reviewed and approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Funding
The authors would like to acknowledge Mashhad University of Medical Sciences, Mashhad, Iran, for financial support (4020802).
Conflict of interest
The authors declare no conflicts of interest.
References
- Abdullah L, Bondagji N. Histopathological patterns of testicular biopsy in male infertility: a retrospective study from a tertiary care center in the western part of Saudi Arabia. Urol Ann 2011;3:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljubran A, Safar O, Elatreisy A, Alwadai R, Shalkamy O, Assiri HM, Eskander M, Arezki A, Ibrahim A. Factors predicting successful sperm retrieval in men with nonobstructive azoospermia: a single center perspective. Health Sci Rep 2022;5:e727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althakafi SA, Mustafa OM, Seyam RM, Al-Hathal N, Kattan S. Serum testosterone levels and other determinants of sperm retrieval in microdissection testicular sperm extraction. Transl Androl Urol 2017;6:282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer MK, Ahmed AR, Abdel Hamid AA, GamalEl Din SF. Can spermatozoa be retrieved in non-obstructive azoospermic patients with high FSH level?: a retrospective cohort study. Andrologia 2019;51:e13176–13184. [DOI] [PubMed] [Google Scholar]
- Bachelot G, Dhombres F, Sermondade N, Haj Hamid R, Berthaut I, Frydman V, Prades M, Kolanska K, Selleret L, Mathieu-D’Argent E et al. A machine learning approach for the prediction of testicular sperm extraction in nonobstructive azoospermia: algorithm development and validation study. J Med Internet Res 2023;25:e44047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boateng EY, Abaye DA. A review of the logistic regression model with emphasis on medical research. J Data Anal Inform Proess 2019;07:190–207. [Google Scholar]
- Boeri L, Bebi C, Dente D, Greco E, Turetti M, Capece M, Cocci A, Cito G, Preto M, Pescatori E et al. Outcomes and predictive factors of successful salvage microdissection testicular sperm extraction (mTESE) after failed classic TESE: results from a multicenter cross-sectional study. Int J Impot Res 2022;34:795–799. [DOI] [PubMed] [Google Scholar]
- Brant A, Schlegel PN. Modern surgical treatment of azoospermia. Curr Opin Urol 2023;33:39–44. [DOI] [PubMed] [Google Scholar]
- Cao H, Wang C, Cai R, Wan Z, Ma L. Seminal plasma ExLncRNA pairs: updating perspectives in the search for testicular spermatozoa retrieval biomarkers in nonobstructive azoospermia patients with mTESE by WGCNA. Urol J 2023;20:246–254. [DOI] [PubMed] [Google Scholar]
- Caroppo E, Castiglioni F, Campagna C, Colpi EM, Piatti E, Gazzano G, Colpi GM. Intrasurgical parameters associated with successful sperm retrieval in patients with non‐obstructive azoospermia undergoing salvage microdissection testicular sperm extraction. Andrology 2021;9:1864–1871. [DOI] [PubMed] [Google Scholar]
- Caroppo E, Colpi EM, Gazzano G, Vaccalluzzo L, Piatti E, D’Amato G, Colpi GM. The seminiferous tubule caliber pattern as evaluated at high magnification during microdissection testicular sperm extraction predicts sperm retrieval in patients with non-obstructive azoospermia. Andrology 2019;7:8–14. [DOI] [PubMed] [Google Scholar]
- Chen H, Xie Y, Li Y, Zhang C, Lv L, Yao J, Deng C, Sun X, Zou X, Liu G. Outcome prediction of microdissection testicular sperm extraction based on extracellular vesicles piRNAs. J Assist Reprod Genet 2021;38:1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XL, Wei YA, Ren XH, Zhang X, Li GY, Lu ZW, Zhang D, Qin C, Su SF. Predictive factors for successful sperm retrieval by microdissection testicular sperm extraction in men with nonobstructive azoospermia and a history of cryptorchidism. Asian J Androl 2022;24:503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherouveim P, Velmahos C, Bormann CL. Artificial intelligence for sperm selection-a systematic review. Fertil Steril 2023;120:24–31. [DOI] [PubMed] [Google Scholar]
- Cissen M, Meijerink AM, D’Hauwers KW, Meissner A, van der Weide N, Mochtar MH, de Melker AA, Ramos L, Repping S, Braat DDM et al. Prediction model for obtaining spermatozoa with testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod 2016;31:1934–1941. [DOI] [PubMed] [Google Scholar]
- Deng C, Liu D, Zhao L, Lin H, Mao J, Zhang Z, Yang Y, Zhang H, Xu H, Hong K et al. Inhibin B-to-Anti-Mullerian Hormone Ratio as Noninvasive Predictors of Positive Sperm Retrieval in Idiopathic Non-Obstructive Azoospermia. J Clin Med 2023a;12:500. Doi: 10.3390/jcm12020500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Mao J, Zhao L, Liu D, Lin H, Zhang Z, Yang Y, Zhang H, Hong K, Jiang H. Testicular sperm aspiration has a poor effect in predicting micro-TESE outcomes in NOA patients with AZFc deletion. Basic Clin Androl 2023b;33:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C-Y, Liu D-F, Zhao L-M, Lin H-C, Mao J-M, Zhang Z, Yang Y-Z, Zhang H-T, Hong K, Xu H-Y et al. Development of a predictive model for increasing sperm retrieval success by microdissection testicular sperm extraction in patients with nonobstructive azoospermia. Asian J Androl 2023c;25:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enatsu N, Miyake H, Chiba K, Fujisawa M. Predictive factors of successful sperm retrieval on microdissection testicular sperm extraction in Japanese men. Reprod Med Biol 2016;15:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Boeri L, Timpano M, Cirigliano L, Preto M, Russo GI, Peretti F, Ferro I, Plamadeala N, Gontero P. Combined Trifocal and Microsurgical Testicular Sperm Extraction Enhances Sperm Retrieval Rate in Low-Chance Retrieval Non-Obstructive Azoospermia. J Clin Med 2022;11:4058. Doi: 10.3390/jcm11144058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannigan R, Bach PV, Schlegel PN. Microdissection testicular sperm extraction. Transl Androl Urol 2017;6:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco G, Scarselli F, Casciani V, De Nunzio C, Dente D, Leonardo C, Greco PF, Greco A, Minasi MG, Greco E. A novel stepwise micro-TESE approach in non obstructive azoospermia. BMC Urol 2016;16:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalayini IF, Alazab R, Halalsheh O, Al-Mohtaseb AH, Al-Ghazo MA. Repeated microdissection testicular sperm extraction in patients with non-obstructive azoospermia: outcome and predictive factors. Arab J Urol 2022;20:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanami Gashti N, Sadighi Gilani MA, Abbasi M. Sertoli cell-only syndrome: etiology and clinical management. J Assist Reprod Genet 2021;38:559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GhoshRoy D, Alvi PA, Santosh K. Unboxing industry-standard AI models for male fertility prediction with SHAP. Healthcare (Basel) 2023;11:929–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glina S, Vieira M. Prognostic factors for sperm retrieval in non-obstructive azoospermia. Clinics (Sao Paulo) 2013;68 Suppl 1:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guahmich NL, Borini E, Zaninovic N. Improving outcomes of assisted reproductive technologies using artificial intelligence for sperm selection. Fertil Steril 2023;120:729–734. [DOI] [PubMed] [Google Scholar]
- Ji C, Wang Y, Wei X, Zhang X, Cong R, Yao L, Qin C, Song N. Potential of testis-derived circular RNAs in seminal plasma to predict the outcome of microdissection testicular sperm extraction in patients with idiopathic non-obstructive azoospermia. Hum Reprod 2021;36:2649–2660. [DOI] [PubMed] [Google Scholar]
- Kaltsas A, Markou E, Zachariou A, Dimitriadis F, Symeonidis EN, Zikopoulos A, Mamoulakis C, Tien DMB, Takenaka A, Sofikitis N. Evaluating the Predictive Value of Diagnostic Testicular Biopsy for Sperm Retrieval Outcomes in Men with Non-Obstructive Azoospermia. J Pers Med 2023;13:1362. Doi: 10.3390/jpm13091362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TJ, Koo KC. Testosterone to luteinizing hormone ratio as a potential predictor of sperm retrieval in non-obstructive azoospermia patients. Yonsei Med J 2023;64:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klami R, Mankonen H, Perheentupa A. Microdissection testicular sperm extraction in Finland—results of the first 100 patients. Acta Obstet Gynecol Scand 2018;97:53–58. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Uetani M, Yamabe F, Mitsui Y, Nakajima K, Nagao K. AI model developed using machine learning for predicting sperm retrieval in micro-TESE for nonobstructive azoospermia patients. Andrologia 2022 2023;2023:1. [Google Scholar]
- Kresch E, Efimenko I, Gonzalez D, Rizk PJ, Ramasamy R. Novel methods to enhance surgical sperm retrieval: a systematic review. Arab J Urol 2021;19:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantsberg D, Mizrachi Y, Katz DJ. Micro‐testicular sperm extraction outcomes for non‐obstructive azoospermia in a single large clinic in Victoria. Aust N Z J Obstetr Gynaecol 2022;62:300–305. [DOI] [PubMed] [Google Scholar]
- Lee R, Witherspoon L, Robinson M, Lee JH, Duffy SP, Flannigan R, Ma H. Automated rare sperm identification from low-magnification microscopy images of dissociated microsurgical testicular sperm extraction samples using deep learning. Fertil Steril 2022;118:90–99. [DOI] [PubMed] [Google Scholar]
- Li J, Yang F, Dong L, Chang D, Yu X. Seminal plasma biomarkers for predicting successful sperm retrieval in patients with nonobstructive azoospermia: a narrative review of human studies. Basic Clin Androl 2023;33:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv M-Q, Yang Y-Q, Li Y-X, Zhou L, Ge P, Sun R-F, Zhang J, Gao J-C, Qu L-Q, Jing Q-Y et al. A detection model of testis‐derived circular RNAs in serum for predicting testicular sperm retrieval rate in non‐obstructive azoospermia patients. Andrology 2024;12:1751–1763. [DOI] [PubMed] [Google Scholar]
- Maglia E, Boeri L, Fontana M, Gallioli A, De Lorenzis E, Palmisano F, Zanetti S, Sampogna G, Restelli L, Somigliana E et al. Clinical comparison between conventional and microdissection testicular sperm extraction for non-obstructive azoospermia: understanding which treatment works for which patient. Arch Ital Urol Androl 2018;90:130–135. [DOI] [PubMed] [Google Scholar]
- Marinaro J, Goldstein M. Microsurgical management of male infertility: compelling evidence that collaboration with qualified male reproductive urologists enhances assisted reproductive technology (ART) outcomes. J Clin Med 2022;11:4593–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modarresi T, Hosseinifar H, Daliri Hampa A, Chehrazi M, Hosseini J, Farrahi F, Dadkhah F, Sabbaghian M, Sadighi Gilani MA. Predictive factors of successful microdissection testicular sperm extraction in patients with presumed Sertoli cell-only syndrome. Int J Fertil Steril 2015;9:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan-Jukic D, Stubljar D, Jukic T, Starc A. Predictive factors for sperm retrieval from males with azoospermia who are eligible for testicular sperm extraction (TESE). Syst Biol Reprod Med 2020;66:70–75. [DOI] [PubMed] [Google Scholar]
- Pozzi E, Raffo M, Negri F, Boeri L, Saccà A, Belladelli F, Cilio S, Ventimiglia E, d’Arma A, Pagliardini L et al. Anti-Müllerian hormone predicts positive sperm retrieval in men with idiopathic non-obstructive azoospermia—findings from a multi-centric cross-sectional study. Human Reprod 2023;38:dead125–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Liu YP, Zhang NN, Su YC. Predictors of testicular sperm retrieval in patients with non-obstructive azoospermia: a review. J Int Med Res 2021;49:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachman RI, Nurullah G, Atmoko W, Rasyid N, Cho SY, Birowo P. Clinical parameters as predictors for sperm retrieval success in azoospermia: experience from Indonesia. F1000Res 2023;12:1567–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R, Padilla WO, Osterberg EC, Srivastava A, Reifsnyder JE, Niederberger C, Schlegel PN. A comparison of models for predicting sperm retrieval before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol 2013;189:638–642. [DOI] [PubMed] [Google Scholar]
- Salehi P, Derakhshan-Horeh M, Nadeali Z, Hosseinzadeh M, Sadeghi E, Izadpanahi MH, Salehi M. Factors influencing sperm retrieval following testicular sperm extraction in nonobstructive azoospermia patients. Clin Exp Reprod Med 2017;44:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şen E, Kızılkan Y, Duran MB, Turunc T, Şahin Fİ, Özkardeş H. Evaluation of the genetic analysis results in infertile patients with non-obstructive azoospermia. jus 2023;10:233–237. [Google Scholar]
- Sengupta P, Dutta S, Karkada IR, Chinni SV. Endocrinopathies and male infertility. Life (Basel) 2021;12:10–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Wang T, Wang L, Wang M. Nomogram based on a circular RNA biomarker for predicting the likelihood of successful sperm retrieval via microdissection testicular sperm extraction in patients with idiopathic non-obstructive azoospermia. Front Endocrinol (Lausanne) 2022;13:1109807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R, Zhang J, Xu Y, Liu S, Deng C, Chen H, Li P, Huang Y, Zhi E, Liu G et al. Predicting Micro-TESE among Heterogeneous Nonobstructive Azoospermic Patients: The Impact on Surgical Decision and ICSI. Andrologia 2023;2023:4825062. [Google Scholar]
- Weedin JW, Bennett RC, Fenig DM, Lamb DJ, Lipshultz LI. Early versus late maturation arrest: reproductive outcomes of testicular failure. J Urol 2011;186:621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems M, Devriendt C, Olsen C, Caljon BEN, Janssen T, Gies I, Vloeberghs V, Tournaye H, Van Saen D, Goossens E. Micro RNA in Semen/Urine from Non-Obstructive Azoospermia Patients as Biomarkers to Predict the Presence of Testicular Spermatozoa and Spermatogonia. Life (Basel) 2023;13:616. Doi: 10.3390/life13030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DJ, Badamjav O, Reddy VV, Eisenberg M, Behr B. A preliminary study of sperm identification in microdissection testicular sperm extraction samples with deep convolutional neural networks. Asian J Androl 2021;23:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Yao J, Zhang X, Chen J, Gao Y, Zhang C, Chen H, Wang Z, Zhao Z, Chen W et al. A panel of extracellular vesicle long noncoding RNAs in seminal plasma for predicting testicular spermatozoa in nonobstructive azoospermia patients. Hum Reprod 2020;35:2413–2427. [DOI] [PubMed] [Google Scholar]
- Xu T, Peng L, Lin X, Li J, Xu W. Predictors for successful sperm retrieval of salvage microdissection testicular sperm extraction (TESE) following failed TESE in nonobstructive azoospermia patients. Andrologia 2017;49:e12642. Doi: 10.1111/and.12642. [DOI] [PubMed] [Google Scholar]
- Yücel C, Budak S, Keskin MZ, Kisa E, Kozacioglu Z. Predictive factors of successful salvage microdissection testicular sperm extraction (mTESE) after failed mTESE in patients with non-obstructive azoospermia: long-term experience at a single institute. Arch Ital Urol Androl 2018;90:136–140. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tang Y, Huang J, Liu H, Liu X, Zhou Y, Ma C, Wang Q, Yang J, Sun F et al. Circulating microRNAs in seminal plasma as predictors of sperm retrieval in microdissection testicular sperm extraction. Ann Transl Med 2022a;10:392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Liu Z, Han X, Li Y, Xia T, Zhu Y, Li Z, Wang L, Hao L, Hu F et al Circulatory exosomal tRF-Glu-CTC-005 and tRF-Gly-GCC-002 serve as predictive factors of successful microdissection testicular sperm extraction in patients with nonobstructive azoospermia. Fertil Steril 2022b;117:512–521. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kanoatov M, Jarvi K, Gauthier-Fisher A, Moskovtsev SI, Librach C, Drabovich AP. Germ cell–specific proteins AKAP4 and ASPX facilitate identification of rare spermatozoa in non-obstructive azoospermia. Mol Cell Proteomics 2023a;22:100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-X, Yao C-C, Huang Y-H, Li P, Zhi E-L, Zhu Z-J, Zhang J-X, Zhao F-J, Li Z, Tian R-H. Efficacy of stepwise mini-incision microdissection testicular sperm extraction for nonobstructive azoospermia with varied etiologies. Asian J Androl 2023b;25:621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Li D-M, Jiang X-H, Bai H-Z, Zhao G-C. A prediction model of sperm retrieval in males with idiopathic non-obstructive azoospermia for microdissection testicular sperm extraction. Reprod Sci 2024;31:366–374. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lv M-Q, Ge P, Yang Y-Q, He D-L, Wang H-X, Zhou D-X. The expression of Beclin-1 in testicular tissues of non-obstructive azoospermia patients and its predictive value in sperm retrieval rate. Transl Androl Urol 2021;10:3267–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this article were obtained solely from the original published sources. No additional data were generated or analyzed to substantiate the content of this article. Correspondence and requests for materials should be addressed to M.S. or H.V.A.