Summary
Positive affect promotes mental health and physical well-being, which may involve modifications in the autonomic nervous system activity. Here, we examine, using chemogenetic techniques, the effects of nucleus accumbens (NAc) activation on affect and body temperature regulation as a proxy of autonomic function. A conditioned place preference test revealed that nucleus accumbens activation induced positive affect. Chemogenetic and natural activations inhibited intruder stress-induced hyperthermia and prostaglandin E2-induced fever. Chemogenetic inhibition did not show a negative affect but canceled the positive affect induced by the natural stimulus of chocolate or sucrose. Counting of c-Fos expression confirmed chemogenetic and sucrose-induced activation of the NAc. Our findings indicate that nucleus accumbens activation modifies a component of autonomic nervous activity and that this mechanism may underscore the link between positive affect and physical well-being. Applying our observations to humans may reduce fever side reactions of vaccines by employing preventive treatments that induce positive affect.
Subject areas: Biological sciences, Neuroscience, Sensory neuroscience
Graphical abstract

Highlights
-
•
Chemogenetic activation of the nucleus accumbens induced favorable preference
-
•
The same treatment suppressed intruder- and prostaglandin-induced hyperthermia
-
•
Natural activation by palatable foods did the same
-
•
The nucleus accumbens modulates both psycho-behavioral and autonomic outputs
Biological sciences; Neuroscience; Sensory neuroscience
Introduction
The term “affect” encompasses mental events and physical changes in heart rate and skin temperature.1 Sensory inputs simultaneously induce psychological and physical changes via neural circuits to promote survival.2,3 Positive affect benefits mental health and physical well-being by modifying endocrine and autonomic nervous system activities.4 While several words express one’s state of mind, such as emotions, feelings, motivations, and moods, their critical definition is a matter of debate and out of the scope of this study. This paper uses the term “affect” as a hypernym of these concepts.3 Brain imaging studies in humans5 have identified several brain structures involved in happiness, including the nucleus accumbens (NAc), ventral pallidum, and anterior cingulate cortex. However, information on the neural pathways involved in mental and physical health interactions is lacking. In this regard, cataplexy in patients or animals with narcolepsy may provide critical insights given that cataplexy is triggered during behaviors associated with positive emotions, such as laughter in humans6,7 and palatable food intake in mice.8,9 Cataplexy is an abrupt and transient loss of muscle tone and a primary symptom of type 1 narcolepsy, a sleep disorder.6,7,8 We previously reported that ultrasound vocalizations during courtship behavior (also called “love song”) in male mice were strongly associated with cataplexy attacks.9 In addition, we identified the rostral part of the NAc shell as a critical region in eliciting cataplexy.10,11 Using a virus-mediated designer receptor exclusively activated by designer drugs (DREADD) system, chemogenetic activation of the NAc increased the number of cataplexy attacks in an animal model of narcolepsy.10 These findings suggest that the rostral part of the NAc shell elicits a positive affect, at least in animal models of narcolepsy.
NAc is a region in the basal forebrain rostral to the preoptic area of the hypothalamus. It is a component of mesocorticolimbic circuits involved in the reward pathway.12 Various neuronal subtypes within the NAc mediate different cognitive functions.13,14 Several roles are attributed to the NAc, such as the regulation of motivation to rewards, aversion, incentive salience, pleasure, positive reinforcement, drug abuse, response to stress, and regulation of depressive behavior.15,16,17,18,19,20 Furthermore, a previous study revealed rostrocaudal segregation of emotional behavior regulation in the NAc.21 Rostral NAc is implicated in positive emotions, such as feeding, whereas caudal NAc is involved in negative emotions, such as defensive treading in rats.21,22 These reports collectively highlight the critical role of NAc in mental health, although the contributions of NAc to bodily health have not been investigated in detail.
In this study, we examined the effects of NAc activation on emotion and body temperature regulation as a measure of autonomic nervous function in wild-type (WT) mice. We chemogenetically manipulated mice expressing DREADD-Gq or DREADD-Gi exclusively in the NAc to examine the effects of artificial activation or inhibition of the NAc on the magnitude of stress-induced hyperthermia induced by an intruder to the home cage. In addition, we examined the possible effect of perturbation of the NAc on another stress-induced body temperature regulation, namely, fever induced by intracerebroventricular (icv) injection of prostaglandin (PG) E2. We selected PGE2-induced fever since it would be a good model of the side reaction of vaccinations. Furthermore, we examined the impact of naturally induced positive emotions following sucrose ingestion on intruder-induced hyperthermia and PGE2-induced fever.
Results
NAc activation induced a positive affect
We first examined whether activation or inactivation of the NAc in WT mice would elicit or inhibit positive affect, similar to observations in orexin neuron-ablated (ORX-AB) mice, a mouse model of narcolepsy.10 WT mice do not typically exhibit cataplexy like ORX-AB mice, so we used the conditioned place preference paradigm. Adeno-associated virus (AAV)-Gi/Gq was injected into the NAc (Figure 1A). mCherry expression in NAc neurons confirmed the success of injections (Figure 1A, middle and right panels). The overall distribution of mCherry was centered on and overlapped with the rostral NAc shell region (Figure 1B). The maximum rostrocaudal distribution was ±0.8 mm from the center. Mice were subjected to a conditioned place preference test 2 weeks after AAV injection (Figure 1C). None of the mice demonstrated initial bias (Figure 1D, pre-values), indicating that the Gi/Gq expression in the NAc did not result in nonspecific effects on behavior. Administration of clozapine to Gq-expressing mice resulted in a significant preference for the clozapine-associated chamber (effect size [Cohen’s d] = 1.088, p = 0.0445, n = 6, paired t test) (Figure 1D, left panel), indicating that clozapine injection during the conditioning period induced positive affect. Gi-expressing mice did not exhibit constant avoidance or preference for the clozapine-associated chamber (effect size = 0.160, n = 6, Figure 1D, middle panel). Clozapine administration did not affect place preference in naive, non-AAV mice (effect size = 0.032, n = 6, Figure 1D, right panel), indicating negligible emotional effects of clozapine at the dose (0.1 mg/kg) used in this study.
Figure 1.
NAc activation induced positive affect as revealed by conditioned place preference test in AAV-Gi/Gq-expressing mice
(A) The left panel depicts the experimental protocol. AAV-Gi or AAV-Gq was stereotaxically injected bilaterally into the rostral part of the NAc.
Middle: Representative image of successful injection. The scale bar indicates 1 mm. The right panel shows an enlarged image of the area enclosed by the white rectangle in the middle panel. The scale bar indicates 100 μm.
(B) An expression marker, mCherry, in brain coronal sections from AAV-Gq (n = 6) and AAV-Gi (n = 6).
(C) Procedure for conditioned place preference test. On the 1st day, mice were placed in the chambers connected by a tunnel, and initial place preference was observed for 15 min to calculate pre-values. The two chambers were identical in size but different in floor texture and wall color. On days 2–5, mice received either clozapine or vehicle and were confined to one chamber with the tunnel closed for 30 min. Mice were randomly assigned to clozapine/vehicle and chambers. On day 6, the two chambers were connected, and place preference was observed for 15 min.
(D) Three groups of mice (AAV-Gq, AAV-Gi, and non-AAV, n = 6 per group) were subjected to the conditioned place preference test in which clozapine was used as a conditioning stimulus. Gray dots and lines indicate individual data and black ones are mean ± SEM. Preference scores at pre- and post-conditioning were compared using paired t tests and indicated by asterisks when statistically significant. Only AAV-Gq mice formed a preference for the clozapine-associated chamber.
(E) In the AAV-Gi group (n = 6), we also examined conditioning to chocolate/standard chow with pre-treatment with clozapine/vehicle. Pre-treatment with a vehicle induced a preference for the chocolate-associated chamber, but pre-treatment with clozapine failed. See also Figure S1.
We also examined whether the inactivation of the NAc by AAV-Gi inhibits chocolate-induced place preference (Figure 1E). Conditioning with vehicle injection and chocolate resulted in a significant preference for the chocolate-associated chamber over the standard chow-associated chamber (effect size [Cohen’s d] = 1.984, p = 0.0046, n = 6, paired t test) (Figure 1E, left panel). A similar preference for the chocolate-associated chamber was observed in the WT mice without preceding AAV injection (Figure S1, left panel), indicating the negligible emotional effect of Gi expression per se in the NAc. Inhibition of the NAc by clozapine injection canceled or even tended to decrease the preference for the chocolate-associated chamber (effect size [Cohen’s d] = 0.909, p = 0.0765, n = 6, paired t test) (Figure 1E, right panel), while the same conditioning procedure in the non-AAV WT did not (Figure S1, middle panel). As a positive control for the conditioned place preference test paradigm, we also confirmed conditioning with methamphetamine (2 mg/kg, ip) resulted in a significant preference for the methamphetamine-associated chamber in the non-AAV WT mice (Figure S1, right panel).
These results indicated that activation of the NAc induced a positive affect, and inactivation of the NAc suppressed a positive affect but did not induce an adverse affect.
Clozapine altered NAc neuronal activity
Next, we examined whether intraperitoneal injection of clozapine affected neuronal activity in the NAc using c-Fos immunohistochemistry. Brains were sampled 90 min after clozapine or vehicle injection. The number of c-Fos-positive cells in the NAc was quantified (Figure 2A). Significant differences were observed among the non-AAV-vehicle, non-AAV-clozapine, AAV-Gi-clozapine, and AAV-Gq clozapine groups (effect size [eta square] = 0.7376, F3, 36 = 33.73, p < 0.0001, n = 10 per group) (Figure 2B). The number of c-Fos-positive cells was significantly higher in clozapine-treated AAV-Gq mice (p < 0.0001, Holm-Sidak multiple comparison test) than in non-AAV mice and significantly lower (p = 0.0005) in AAV-Gi mice than in non-AAV mice (Figure 2B). We also confirmed chocolate-induced activation of the NAc (effect size [Cohen’s d] = 1.87, p = 0.046, Welch’s t test) (Figure S2).
Figure 2.
NAc activation in AAV-Gq mice and inhibition in AAV-Gi mice by clozapine
(A) Representative images of c-Fos expression in the NAc. Only a green channel was shown to compare AAV and non-AAV mice better.
(B) The number of c-Fos-expressing neurons in the rectangle area (400 × 1,000 μm, white dotted rectangle in A) in the NAc was quantified in vehicle-injected non-AAV mice (Non AAV-vehicle), clozapine-injected non-AAV mice (Non AAV-cloz), clozapine-injected AAV-Gi mice (Gi-cloz), and clozapine-injected AAV-Gq mice (Gq-cloz). Bars indicate mean ± SEM (n = 10 per group). p values were obtained using one-way ANOVA followed by Holm-Sidak’s multiple comparison test and indicated by asterisks when statistically significant. See also Figure S2.
Activation of the NAc prevented intruder-induced hyperthermia
Next, we examined the effects of artificial NAc activation on autonomic function using body temperature as a representative measure in a cohort of mice different from those in the conditioned place preference test. In addition to the AAV-Gq injection, mice were implanted with a transponder for body temperature measurement (Figure 3A). In total, 10 mice received AAV-Gq, and all the injections were successful (Figure 3B) based on mCherry expression examined after the experiment. On an experimental day, mice were moved to the experimental room and allowed to acclimatize for 60 min (Figure 3C).
Figure 3.
NAc activation prevents intruder-induced hyperthermia
(A) Mice underwent two operations under isoflurane anesthesia. Bilateral AAV-Gq injections and transponder implantation for body temperature measurement.
(B) Injection sites were identified by histological examination after the experiment. Only data from successful AAV injections were analyzed.
(C) Schedule of the experiment. The body temperature of mice was repeatedly measured using a transponder at 10-min intervals. Ten min after i.p. administration of clozapine or vehicle, a male intruder mouse was introduced into the resident animal’s home cage for 10 min.
(D and Ε) Time-related changes in body temperature (upper panels) and locomotor activity (lower panels) in (D) AAV-Gq mice (n = 10) and (E) non-AAV mice (n = 10). Lines indicate mean ± SEM, and symbols indicate individual values. Each animal was tested three (AAV-Gq) or two (non-AAV) times in random order with an interval of more than 3 days. The right panels show the area under the curve (AUC) values calculated between 0- and 20-min. Among treatments in the AAV-Gq mice, AUC values were significantly different for the body temperature (effect size [eta square] = 0.3372, F1.751, 15.76 = 6.591, p = 0.0102, repeated-measures one-way ANOVA) and locomotor activity (effect size = 0.7314, F1.695, 8.477 = 24.65, p = 0.0004). In non-AAV mice, AUC values were not different for the body temperature (effect size = 0.6156, p = 0.0834, paired t test) and locomotor activity (effect size = 0.115, p = 0.7241). p values between the treatments were obtained using Holm-Sidak’s multiple comparison test, and the only significant pairs were indicated in the figure with asterisks.
In control (non-AAV) mice (n = 10), body temperature increased by approximately 1.5°C–2 °C at the end of 10 min intruder introduction. It returned to the baseline value at approximately 30 min (Figure 3E, upper panel). Pre-treatment with clozapine did not affect intruder-induced hyperthermia. Locomotor activity of the mice increased by intraperitoneal injection of vehicle/clozapine and introduction of an intruder but soon returned to the baseline level after removal of the intruder (Figure 3E, lower panel). There was no difference between vehicle-injected and clozapine-injected groups as determined by the area under the curve (AUC) value 20 min after introducing an intruder (Figure 3E, right panels; see also Figure 3C). During the intruder test period, resident (experimental) mouse showed aggressive behavior, such as sniffing, chasing, fighting, and shaking tail.
In AAV-Gq mice pre-treated with vehicle (n = 10) (Figure 3D), an intruder increased body temperature with a magnitude and time course similar to that in non-AAV mice. Clozapine pre-treatment almost completely abolished intruder-induced hyperthermia as determined by the area under the curve (AUC) value 20 min after introducing an intruder (p = 0.0430). Without an intruder, clozapine alone did not affect body temperature in AAV-Gq mice. Locomotor activity of the mice increased by intraperitoneal injection of vehicle/clozapine and introduction of an intruder similar to the non-AAV mice (Figure 3D, lower panel). However, in the case of the clozapine pre-treated and intruder-introduced group, time spent in locomotion was more prolonged than that in the vehicle + intruder group, resulting in a higher AUC value (Figure 3D, lower right panel) (p = 0.0316). Clozapine pre-treated mice showed aggressive behavior as the vehicle-treated mice. Lower body temperature and higher locomotion in the clozapine-treated AAV-Gq mice indicate that suppression of hyperthermia was not caused by suppression of locomotor activity.
NAc inactivation canceled sucrose-induced prevention of intruder-induced hyperthermia
Since the results in the conditioned place preference test (Figure 1E) indicated that inactivation of the NAc suppresses a positive emotion rather than induces a negative emotion, we examined the possible effect of inactivation of the NAc on body temperature regulation in a similar context. Specifically, we gave sucrose (20%) water as a natural stimulant to activate the NAc after vehicle/clozapine treatment and compared the body temperature change in response to intruder stress (Figure 4C). In total, 7 mice received AAV-Gi (Figure 4A), but 1 mouse was excluded from later examination due to mistargeted injections based on unilateral mCherry expression (Figure 4B).
Figure 4.
NAc inactivation canceled sucrose-induced prevention of intruder-induced hyperthermia
(A) Mice underwent two operations under isoflurane anesthesia. Bilateral AAV-Gi injections and transponder implantation for body temperature measurement. They were acclimatized to a reverse light/dark cycle (lights off at 07:00 and lights on at 19:00) for more than 1 week before the experimental day.
(B) Injection sites were identified by histological examination after the experiment. Only data from successful AAV injections (n = 6) were analyzed.
(C) Schedule of the experiment. The body temperature of mice was repeatedly measured using a transponder at 10-min intervals. Ten min after i.p. administration of clozapine or vehicle, a bottle of 20% sucrose or tap water was supplied. An intruder mouse was introduced into the experimental animal’s home cage after another 10 min for 10 min. All the mice at least once licked the nozzle of a water bottle before introducing an intruder.
(D) Time-related changes in body temperature (upper left panel) and locomotor activity (lower left panel) for the five treatments. Lines indicate mean ± SEM, and symbols indicate individual values. Each animal was tested five times in random order with an interval of more than 3 days. The right panels show the area under the curve (AUC) values calculated between 0- and 20-min. AUC values were significantly different for the body temperature (effect size = 0.6523, F2.483, 12.42 = 12.21, p = 0.0008, repeated-measures one-way ANOVA) and locomotor activity (effect size = 0.4970, F1.605, 8.025 = 8.678, p = 0.0121). Holm-Sidak’s multiple comparison tests revealed a significant hyperthermia preventive effect of sucrose (p = 0.0377; vehicle + tap water vs. vehicle + sucrose) and a significant canceling impact of it by NAc inactivation (p = 0.0272; vehicle + sucrose vs. clozapine + sucrose). Inactivation of the NAc did not modify intruder-induced hyperthermia (p = 0.4249; vehicle + tap water vs. clozapine + tap water). Although sucrose ingestion suppressed intruder-induced hyperthermia, it was not associated with changes in locomotor activity.
Inactivation of the NAc did not suppress nor exaggerate the intruder-induced hyperthermia, as revealed by a comparison between the vehicle + tap water group and clozapine + tap water group (p = 0.4249, Figure 4D upper panel). Meanwhile, inactivation of the NAc canceled the suppressive effect of sucrose on the intruder-induced hyperthermia, as revealed by a comparison between vehicle + sucrose group and clozapine + sucrose group (p = 0.0272, Figure 4D). Canceling effect was almost complete since there was no difference between vehicle + tap water group and clozapine + sucrose group (p = 0.6656). Sucrose per se did not affect body temperature (vehicle + sucrose + no intruder group). Still, it successfully suppressed the intruder-induced hyperthermia, as revealed by comparing the vehicle + tap water group and the vehicle + sucrose group (p = 0.0377, Figure 4D).
Although sucrose ingestion successfully suppressed intruder-induced hyperthermia, it did not prevent intruder-induced increase in locomotion (p = 0.2198, Figure 4D lower panel). Inactivation of the NAc did not induce any changes in the rise of locomotor activity in response to the introduction of an intruder either when drinking tap water (p = 0.6985, vehicle + tap water group vs. clozapine + tap water group) or sucrose (p = 0.2470, vehicle + sucrose group vs. clozapine + sucrose group). Sucrose ingestion did not prevent aggressive behavior during the intruder test. Although sucrose groups showed tendency to drink more than tap water groups, no significant difference was noted in the amount of water ingested during the 70-min observation period among the 5 groups (vehicle + tap water group; 0.66 ± 0.09 mL, clozapine + tap water group; 0.62 ± 0.09 mL, vehicle + sucrose group; 0.95 ± 0.16 mL, clozapine + sucrose group; 1.02 ± 0.22 mL, vehicle + sucrose + no intruder group; 0.93 ± 0.17 mL, n = 6, F2.326, 11.63 = 1.470, p = 0.2718, repeated measures one-way ANOVA).
NAc activation prevented PGE2-induced fever
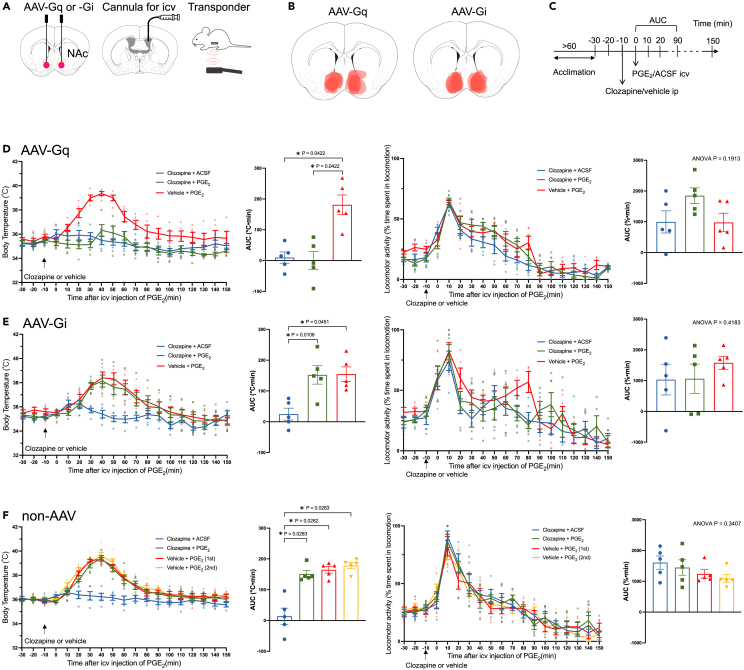
As chemogenetic (Figures 2 and 3) and natural (Figure 4) activation of the NAc prevented intruder-induced hyperthermia, we next examined whether the role of the NAc in the thermoregulatory system could be generalized to another form of stress-induced hyperthermia, precisely, inflammatory fever mimicking central injection of PGE2. For this purpose, mice were implanted with cannulas for intracerebroventricular injection and a transponder for body temperature measurement in addition to the AAV-Gi/Gq injection (Figure 5A). In total, 6 mice received AAV-Gq and 6 mice received AAV-Gi, but 1 mouse was excluded from each group due to mistargeted injections based on unilateral mCherry expression. Therefore, we analyzed the data of 5 AAV-Gq mice and 5 AAV-Gi mice that were successfully injected (Figure 5B). On an experimental day, mice were moved to the experimental room and allowed to acclimatize for 60 min (Figure 5C).
Figure 5.
NAc activation prevented PGE2-induced fever
(A) Mice underwent three operations under isoflurane anesthesia. Bilateral AAV injections, cannula implantation for intracerebroventricular (icv.) injection, and transponder implantation for body temperature measurement.
(B) Injection sites were identified by histological examination after the experiment. Only data from successful AAV injections were analyzed.
(C) Schedule of the experiment. The body temperature of mice was repeatedly measured using a transponder at 10-min intervals. Ten min after i.p. administration of clozapine or vehicle, PGE2 (2 μg) or ACSF was injected icv.
(D–F) Time-related changes in body temperature (left panels) and locomotor activity (right panels) in (D) AAV-Gq mice (n = 5), (E) AAV-Gi mice (n = 5), and (F) non-AAV mice (n = 5). Lines indicate mean ± SEM, and symbols indicate individual values. Each animal was tested three (AAV-Gi/Gq) or four (non-AAV) times in random order with an interval of more than 3 days. Bar graphs show the area under the curve (AUC) values calculated between 0 and 90 min. AUC values were significantly different among body temperature in AAV-Gq (effect size [eta square] = 0.7010, F1.811, 7.245 = 12.70, p = 0.0047, repeated-measures one-way ANOVA), AAV-Gi (effect size = 0.6012, F1.122, 4.490 = 7.553, p = 0.0438), and non-AAV (effect size = 0.8110, F1.867, 7.469 = 21.36, p = 0.0009) groups. AUC values in locomotor activity were not statistically different among the treatments in AAV-Gq (effect size = 0.3037, F1.627, 6.507 = 2.162, p = 0.1913), AAV-Gi (effect size = 0.083, F1.223, 4.891 = 0.8726, p = 0.4183), and non-AAV (effect size = 0.2086, F1.866, 7.463 = 1.234, p = 0.3407). p values between the treatments were obtained using Holm-Sidak’s multiple comparison test, and the only significant pairs were indicated in the figure with asterisks. Only in the AAV-Gq group did clozapine treatment successfully prevent PGE2-induced fever.
In control (non-AAV) mice (n = 5), body temperature increased by approximately 3 °C at 40 min post-intracerebroventricular injection of PGE2 and returned to the baseline value at approximately 90 min (Figure 5F). Repeated PGE2 injections with an interval of more than 3 days resulted in a similar increase in body temperature, indicating no adaptation in PGE2-induced fever. Pre-treatment with clozapine did not affect PGE2-induced fever, and clozapine alone did not affect body temperature.
In AAV-Gq mice (n = 5) (Figure 5D), when pre-treated with the vehicle, PGE2 increased body temperature with a magnitude and time course similar to that in non-AAV mice. Clozapine pre-treatment almost completely abolished PGE2-induced fever as determined by the area under the curve (AUC) value from 0 to 90 min after PGE2 injection (p = 0.0422). In AAV-Gi mice (n = 5) (Figure 5E), clozapine pre-treatment did not affect PGE2-induced fever. Locomotor activity increased by a similar magnitude for both artificial cerebrospinal fluid (ACSF) and PGE2 injections (Figures 5D–5F, right panels), indicating that the increase was caused by the intracerebroventricular injection procedure but not by the pharmacologic effect of PGE2. Changes in locomotor activity cannot explain fever suppression by activating the NAc, as was the case for intruder-induced hyperthermia.
Sucrose ingestion prevented PGE2-induced fever
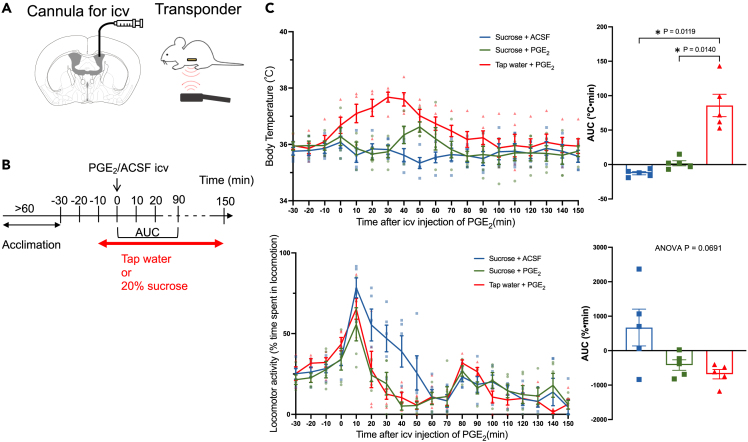
Next, we examined whether natural activation of the NAc by sucrose ingestion could prevent PGE2-induced fever, as was the case for chemogenetic (Figure 5) activation of the NAc. For this experiment, we used 5 animals with an indwelling icv cannula and a transponder (Figure 6A). After confirming stable body temperature, a bottle containing 20% sucrose or tap water (control) was introduced into the animal’s home cage. All the animals started licking sucrose/tap water within a minute and received an intracerebroventricular injection of PGE2 or ACSF (Figure 6B) after 10 min. Each animal was tested in random order thrice with an interval of more than 3 days.
Figure 6.
Sucrose ingestion prevents PGE2-induced fever
(A) Mice were implanted with a cannula for intracerebroventricular (icv.) injection and a transponder for body temperature measurement.
(B) Mice were acclimatized to a reverse light/dark cycle (lights off 07:00 and lights on 19:00) for more than 1 week. Experiments were conducted in a dark room between 10:00 and 16:00. Nocturnal mice hardly ingested sucrose during the light period. Mice were allowed to freely ingest 20% sucrose or tap water for an observation period of 160 min.
(C) Time-related changes in body temperature (upper panel) and locomotor activity (lower panel) (n = 5 animals). Lines indicate mean ± SEM. Symbols indicate individual values. Each animal was tested in random order thrice with an interval of more than 3 days. The right panel shows the area under the curve (AUC) values calculated between 0- and 90-min. AUC values in body temperature were significantly different among treatments (effect size [eta square] = 0.833, F1.177, 4.707 = 29.36, p = 0.0031, one-way repeated-measures ANOVA). AUC values in locomotor activity were not significantly different among treatments (effect size = 0.4410, F1.059, 4.234 = 5.823, p = 0.0691). p values between the treatments were obtained using Holm-Sidak’s multiple comparison test, and the only significant pairs were indicated in the figure with asterisks. See also Figure S3.
Body temperature in the tap water + PGE2 group increased by approximately 2°C and returned to baseline at approximately 90 min (Figure 6C upper left panel). Ingestion of sucrose almost completely prevented PGE2-induced fever (p = 0.0140, Holm-Sidak’s multiple comparison test) (Figure 6C upper right panel). Sucrose ingestion did not affect body temperature when ACSF was injected instead of PGE2. Locomotor activity increased, peaking at 10 min after the icv injection of the drugs, and then decreased in all the groups (Figure 6C lower panel). It decreased under the baseline value at 30 min after the injection of PGE2 and 60 min after the infusion of ACSF. In any way, the changes in locomotor activity did not parallel with the changes in body temperature, and there was no significant difference among the treatment groups as evaluated by AUC (Figure 6C lower right panel). The amount of sucrose ingested after PGE2 and ACSF treatment and the amount of tap water ingested after PGE2 treatment during the 160-min observation period were 1.64 ± 0.25 mL, 1.56 ± 0.17 mL, and 1.36 ± 0.10 mL (mean ± SEM), respectively, with no significant differences among the groups (effect size = 0.09, F1.137, 4.548 = 2.053, p = 0.2207).
We also examined the possible effect of honey and chocolate ingestion on PGE2-induced fever (Figure S3). As in sucrose water, honey and chocolate successfully suppressed PGE2-induced fever, indicating a positive emotion was the key.
Sucrose ingestion activated the NAc
Finally, we confirmed activation of the NAc by sucrose and examined the possible interaction of sucrose ingestion and stress exposures using c-Fos immunohistochemistry (Figure 7). Mice were divided into 8 groups (n = 6 per group) and allowed free access to tap water or sucrose water for 90 min (Figure 7A). Mice ingested more sucrose water than the tap water (Figure 7B). Still, no difference was observed among stressors or interaction. Without any stressor (no intruder group), sucrose ingestion significantly increased c-Fos-positive cells in the NAc (Figures 7C and 7D), as was the case in AAV-Gq injected with clozapine (Figure 2). Even when an intruder was introduced for 10 min, tap water and sucrose groups showed similar activation levels of the NAc to those corresponding controls in the no intruder groups. When ACSF was intracerebroventricularly administered, c-Fos levels were identical to the no-intruder groups, and sucrose significantly activated the NAc. When PGE2 was administered, c-Fos expression in the NAc of the sucrose group was considerably smaller than those in the no intruder group and ACSF group. However, within PGE2-administered groups, the sucrose group still showed greater c-Fos expression than the tap water group, indicating a limited activation by sucrose.
Figure 7.
Sucrose ingestion activates the NAc
(A) Experimental design. Mice were divided into 4 × 2 groups: no intruder, with an intruder, ACSF icv, or PGE2 icv x tap water or sucrose water.
(B) Amount of water ingestion during the observation period of 90 min. Mice ingested more sucrose water than the tap water (effect size [eta square] = 0.14, F1, 40 = 7.330, p = 0.0099, two-way ANOVA). Still, no difference was observed among stressors (effect size = 0.03, F3, 40 = 0.4977, p = 0.4977) or interaction (effect size = 0.04, F3, 40 = 0.6486, p = 0.5885).
(C) Representative images of c-Fos expression in the NAc.
(D) The number of c-Fos-expressing neurons in the rectangle area (400 × 1,000 μm, shown in a white dotted rectangle in (C) in the NAc was quantified. Bars indicate mean ± SEM (n = 6 per group). two-way ANOVA (water x stressor) revealed a significant difference between drinking water (effect size = 0.62, F1, 40 = 133.3, p < 0.0001), among stressors (effect size = 0.14, F3, 40 = 9.791, p < 0.0001) and interaction (effect size = 0.06, F3, 40 = 4.309, p = 0.01). p value was obtained using Fisher’s multiple comparison test, and the only significant pairs were indicated in the figure with asterisks.
Discussion
Positive affect prevents fever
Positive emotion and food hedonics reduce pain sensation by activating descending pain inhibitory pathways initiated in the brainstem.23 However, the effects of positive emotion on bodily health have yet to be examined in detail. Here, we demonstrate that the activation of the NAc by viral expression of DREADD-Gq induced conditioned place preference (Figures 1 and 2), indicating the generation of positive affect. This result agreed with our previous study,10,11 which demonstrated increased chocolate-induced cataplexy by NAc activation in a narcolepsy mouse model. The current study showed that NAc activation by the same method successfully suppressed intruder-induced hyperthermia (Figure 3) and PGE2-induced fever (Figure 5). An intruder-induced hyperthermia and PGE2-induced fever was also suppressed with naturally occurring positive affect induced by sucrose ingestion (Figures 4, 6, and 7). Even though chemogenetic activation of the NAc did not directly equal the generation of positive affect, sucrose experiments support our view.
Meanwhile, artificial NAc inactivation did not elicit negative affect (Figures 1 and 2) but canceled the naturally occurring positive affect-induced suppression of stress-induced hyperthermia (Figure 4). We measured locomotor activity in addition to the body temperature since body temperature would be affected by the animals’ movement. However, suppressing hyperthermia and fever was not associated with inhibition of locomotion, denying an indirect effect on thermoregulation by a change in locomotor activity. The current study demonstrates the possible modifying effects of positive affect elicited in the NAc on body temperature regulation.
Methodological considerations
Our previous study used clozapine-N-oxide (CNO, 0.45 mg/kg, i.p.) as an artificial agonist to stimulate DREADD-Gi/Gq.10 However, in the current study, we used clozapine (0.1 mg/kg, i.p.) rather than CNO based on a report demonstrating low penetration of CNO through the blood-brain barrier and low binding affinity of CNO to DREADD-Gi/Gq.24 Nevertheless, we exercised caution when administering clozapine, given that it is an antipsychotic drug and may affect place preference and body temperature.25,26 We thus established a clozapine control in the non-AAV group (Figures 1, 2, 3, and 5) and in the clozapine + no intruder and the clozapine + ACSF groups (Figures 3 and 5). In any of these experiments, clozapine did not affect preference score, c-Fos expression in the NAc, and body temperature at the administered dose. The clinical dose of clozapine is 12.5–600 mg/day,27 corresponding to 0.2–10 mg/kg when assuming a human body weight of 60 kg. When body surface area is considered in dose translation,28 it may be equivalent to 2.5–123 mg/kg in mice. We conclude that 0.1 mg/kg of clozapine was a subthreshold dose that affected the measured parameters in the control group. In line with our conclusion, clozapine does not affect conditioned place preference at 10 mg/kg, s.c.25 or body temperature at a dose of 0.5 mg/kg, s.c.26
The observations of naturally occurring positive affect concerning sucrose ingestion and body temperature (Figures 4, 6, and 7) were obtained during the dark phase when mice were most active. Circadian rhythms in autonomic activity and consciousness are well-established but tend to be overlooked due to convenience. In this regard, we employed a reverse light/dark cycle to minimize confounding effects.
NAc activation modulates body temperature regulation
We expected the opposite effect of DREADD-Gi and DREADD-Gq on conditioned place preference test, intruder-induced hyperthermia, and PGE2-induced fever based on our previous study using a narcolepsy mouse model10 that demonstrated the opposite effect on the number of cataplexy-like behaviors. In our earlier model, NAc activation increased while NAc inhibition decreased, cataplexy-like behavior. This study used WT mice, NAc activation, but not inhibition, modified conditioned place preference, intruder-induced hyperthermia, and PGE2-induced fever. There are at least two possible explanations for these findings. First, we used CNO in the previous study and clozapine in the current study, and CNO and clozapine have different potency for activating DREADDs. The clozapine dose required to induce behavioral changes in vivo through hM4Di is 10–100 times more potent than the CNO dose.24 The equivalent clozapine dose to our previously used CNO (0.45 mg/kg) is estimated to be 0.005–0.05 mg/kg, while we used 0.1 mg/kg of clozapine in this study. Therefore, insufficient clozapine dosage in the current study is unlikely to underscore the results. Clozapine significantly decreased c-Fos expression in the NAc of AAV-Gi-expressing mice (Figure 2), indicating sufficient dosage. Second, inhibiting positive affect may not necessarily induce negative affect. The place preference test can also be used as a place aversion test,29,30 but no such effect was observed in the current study. Inactivation of the NAc instead elicited suppression of positive affect induced by chocolate (Figure 1) or sucrose ingestion (Figure 4). Our previous study using narcolepsy model mice10 showed that inactivation of the NAc decreased cataplexy-like behavior in the presence of chocolate. We did not examine cataplexy-like behavior in the absence of chocolate at that time. Thus, the current result did not contradict the previous result. In this regard, the activation of negative affect-provoking brain sites is required to examine the effects of negative affect on body temperature regulation.
Psychostimulant drugs such as methamphetamine are known to increase the activity of the NAc, place preference, and body temperature.31,32,33 Other notable addictive drugs such as opioids and alcohol produce hypothermic responses while also increasing the activity of the NAc.34,35,36 These literatures apparently contradict our conclusion. However, those reports showed the coincidence but not the causative relationship between the activity of the NAc and the changes in body temperature. Although our sucrose experiment also showed coincidence, the results of chemogenetic activation indicated a more direct causative relationship between the activity of the NAc and body temperature regulation.
Interaction of sucrose ingestion and stress exposures on activities of the NAc
Sucrose ingestion activated NAc irrespective of the presence of stressors (Figure 7D). In a quantitative point of view, PGE2 injection significantly blunted the sucrose-induced increase of c-Fos expression in the NAc (Figure 7D). However, this group ingested a similar amount of sucrose water to the other groups (Figure 7B) and fever response was inhibited (Figure 6C). This observation may corroborate the close relationship between inflammatory sickness and depressive feeling.37 In the case of the intruder test, sucrose increased c-Fos expression in a similar magnitude to that without an intruder (Figure 7D). Apparent discrepancy between PGE2 and intruder may be explained by the different time course of the effect of the two stressors. Body temperature peaked at 10 min in the intruder test and returned to the baseline about 20 min (Figures 3 and 4). Whereas the PGE2 injection, body temperature peaked at 30–40 min after the injection and returned to the baseline about 100–150 min (Figures 5 and 6). Thus, the fixed brain sampling at 90 min (Figure 7A) might bring different results.
Downstream mechanisms of fever prevention
There is extensive literature on the neural mechanisms of PGE2- and stress-induced fever38,39,40,41; however, little is known of the neural mechanisms underlying body temperature reduction. Recently, body temperature-lowering neurons have been identified in the hypothalamic preoptic area (POA).42,43,44 Activation of these neurons induces a long-lasting (over several hours) decrease in body temperature and is considered to be responsible for hibernation and torpor. Although the POA (−0.2 to 0.6 mm anterior to bregma)45 lies caudal to the NAc (0.7–1.9 mm anterior to bregma), our AAV injections were centered on the rostral part of the NAc (approximately 1.5 mm anterior to bregma) and distributed ±0.8 mm rostrocaudally from the center. Therefore, it is unlikely that temperature-lowering neurons in the POA would have been activated in this study.
The NAc shell predominantly comprises two types of GABAergic output neurons or medium spiny neurons (MSNs) and, to a lesser extent, cholinergic interneurons. One population of the MSNs contains enkephalin and dopamine D2 receptors and projects to the pallidum; the other contains substance P and dopamine D1 receptors and projects to the substantia nigra.46 D1-type MSNs are involved in reward and drug addiction, whereas D2-type MSNs underscore avoidance and ambivalence.47 It remains unclear which neuronal types mediate a reduction in intruder-induced hyperthermia and PGE2-induced fever. The distribution of CaMKII is restricted to D1-type MSNs,48 indicating a possible involvement of this population. D1-type MSNs project predominantly to the substantia nigra, which is implicated in motor planning and reward seeking. In this regard, the substantia nigra is involved in body temperature regulation. Electrical or kainic acid-induced chemical stimulation of the substantia nigra produced hypothermia in rats when the ambient temperature was below 22°C and hyperthermia when the ambient temperature was 30°C.49 Recently, a paper showed the expression of CaMKII in the striatal cholinergic interneurons.50 Therefore, a possible contribution of the cholinergic interneurons in the current results cannot be excluded. Nevertheless, further studies are warranted to verify the mechanisms by which NAc outputs modify the thermoregulatory system.
Conclusion
Our findings indicate that NAc activation induces positive affect and modifies a component of the autonomic nervous activity. This mechanism may underscore the link between positive affect and physical well-being. Applying our observations to humans may reduce fever side reactions of vaccines by employing preventive treatments that induce positive affect.
Limitations of the study
Although the increased place preference due to NAc activation can be interpreted as indicating the occurrence of positive affection, it can also be interpreted as indicating an operational behavior due to an expectation of reward associated with that place. Whether a positive feeling accompanies this artificially induced reward expectation is still being determined from the current study using the place preference test. However, the sucrose experiment in this study lends support to our interpretation, and our previous report10 showing an increase of cataplexy in the animal model of narcolepsy also supports the occurrence of positive affection by artificial activation of the NAc. Nevertheless, we should be open to the possibility that while NAc activity correlates with positive affect and autonomic outputs, it does not cause them—it may indirectly modulate them. Therefore, further studies are warranted to clarify this issue.
In this study, we intended to inject AAV into the shell subregion of the NAc, but the results overlapped shell and core subregions (Figures 1B, 3B, 4B, and 5B). We cannot exclude the possible involvement of the NAc core in the present results. However, we counted activated cells in only the shell subregion (Figures 2 and 7; S2) and revealed consistent results to our conclusion. Therefore, we can say that at least the NAc shell neurons’ activity is related to the positive affect and body temperature regulation. Nevertheless, as mentioned above, we do not have direct evidence of whether D1-MSNs or cholinergic interneurons mediate a reduction in intruder-induced hyperthermia and PGE2-induced fever. Therefore, further studies are warranted to clarify this issue.
We only examined body temperature as an autonomic output in this study. However, cardiovascular and immune systems may also be modified by positive affect elicited by NAc activation, which should be discussed in future studies. We selected PGE2-induced fever as COVID-19 vaccinations can result in side effects such as fever. Extrapolation of our observations to humans needs a lot of caution since artificial excitation of NAc cannot be applicable in humans; moreover, in this study, the mice ingested sucrose for the first time in their lives, whereas humans are already aware of the fact that sucrose induces positive affect in most cases and negative affect in some cases, such as in those who want to reduce body weight. Such preconceptions may interfere with humans’ affective states. Nevertheless, testing whether positive affect reduces fever in the human population is worth testing.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Tomoyuki Kuwaki (kuwaki@m3.kufm.kagoshima-u.ac.jp).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Any data required to reanalyze the data reported in this paper are available from the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
We thank the members of the Department of Physiology for valuable discussions and technical advice, Mrs. Sakoda and Mrs. Sakurai for their skillful technical assistance, and the Joint Research Laboratory and Laboratory of Animal Science at Kagoshima University Graduate School of Medical and Dental Sciences for using their facilities. Funding: This study was supported by JSPS KAKENHI (22K07330, 16K13112 to TK).
Author contributions
Conceptualization, T.K.; methodology, H.S., I.K.-Y., H.K., and T.K.; investigation, H.S., S.O., M.Y., S.K., and T.K.; writing-original draft, H.S. and T.K.; writing-review and editing, I.K.-Y., H.K., and T.K., funding acquisition, T.K.; supervision, T.K. All authors approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors used Grammarly to improve the manuscript’s readability and language. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the content of the published article.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-c-Fos rabbit antibody | Millipore Corp. | ABE457, RRID: AB_2631318 |
| Anti-rabbit IgG conjugated with CF488 fluorescent dye | Biotium | Catalog #20015, RRID: AB_10559669 |
| Bacterial and virus strains | ||
| AAV5-CaMKIIα-hM4Di-mCherry | Addgene | 50477-AAV5, Lot v4493 |
| AAV5-CaMKIIα-hM3Dq-mCherry | Addgene | 50476-AAV5, Lot v9235 |
| Chemicals, peptides, and recombinant proteins | ||
| Clozapine | Tocris | 0444, Cas: 5786-21-0 |
| Prostaglandin E2 | Nakalai | 29334, Cas: 363-24-6 |
| Experimental models: Organisms/strains | ||
| Mouse: CBA/C57BL6 hybrid | Kagoshima University Experimental Animal Facility | N/A |
| Software and algorithms | ||
| Prism | GraphPad | v.10, RRID: SCR_002798 |
| EthoVision XT | Noldus Information Technology | RRID: SCR_000441 |
| Other | ||
| Place preference test chambers | This paper, Figure 1C | N/A, home made |
| Guide cannula | Plastics One Inc. | C315GS-5/2.5 |
| Wireless passive transponder system | Bio Medic Data Systems, Inc. | DAS7007R, Electric Laboratory Animal Monitoring System |
| Video camera with an infrared lamp | Ailipu Technology | ELP-USBFHD06MT-KL36IR |
Experimental model and study participant details
We used WT male CBA/C57BL6 hybrid mice (24−31 g, bred in Kagoshima University’s animal facility), as we considered a hybrid strain a better representation of “wild type” than an inbred strain. Only male mice were used because aggressive behavior in the resident-intruder test dramatically differs between the sexes,51 and therefore, intruder-induced hyperthermia may be different between the sexes. All mice were housed in a room maintained at 22°C–24°C with lights on at 07:00 and off at 19:00 unless otherwise stated. Mice were provided food and water ad libitum. We used mice individually housed after the surgery to minimize initial differences in body temperature. In experiments using sucrose as a natural stimulant to evoke positive affect, mice were housed in a room with lights on at 19:00 and off at 07:00 at least 2 weeks before experimentation commenced. We selected a reversed light/dark cycle to enable experimenters to observe mice in their active nocturnal behavior during the daytime. Our pilot experiments revealed that mice did not consume sucrose during the light phase without prior water restriction; hence, we selected a reversed light/dark cycle rather than water restriction since the latter may influence the affect.
When an animal received more than two treatments (such as vehicle and the drug), the order of the treatments was randomized. Whenever possible, the investigators were blinded during the experiments. Every effort was made to minimize the number of animals suffering.
All experiments were conducted at Kagoshima University following the guiding principles for the care and use of animals in the field of physiological sciences published by the Physiological Society of Japan (2015) and were approved by the Experimental Animal Research Committee of Kagoshima University (MD17105, MD19102).
Method details
Intra-NAc stereotaxic injection of AAV
Surgeries for injections were performed under isoflurane anesthesia (3% inhalation) using a stereotaxic instrument (SR-8N, Narishige, Tokyo, Japan). A 30-G needle was filled with recombinant AAV (serotype 5)-CaMKIIα-hM4Di-mCherry (50477-AAV5, Addgene, Cambridge, MA, USA, Lot v4493, 4.4 × 1012 GC/mL, 300 nL/side over 3 min) or AAV(5)-CaMKIIα-hM3Dq-mCherry (50476-AAV5, Addgene, Lot v9235, 3.1 × 1012 GC/mL, 300 nL/side over 3 min). AAVs were stereotaxically injected bilaterally into the NAc. The needle was connected with polyethylene tubing (PE20, Braintree Scientific, Braintree, MA) to a micro syringe (model 7001 for 1 μL, Hamilton, Reno, NV) that was set on a syringe pump (11Elite, Harvard Apparatus, Holliston, MA). Injection coordinates were anterior 1.50 mm, lateral ±0.8 mm, and ventral 4.80 mm from bregma. Before and after injection, the needle tip was left in place for 5 min. After surgery, mice were administered antibiotics (penicillin G, 40,000 U/kg) and analgesics (buprenorphine, 0.05 mg/kg). They were individually housed to avoid scratching by mates and potential social ranking effects on male behavior.52
Most neurons in the NAc are GABAergic.53 Seminal reports demonstrated that CaMKII is almost exclusively expressed in excitatory neurons, except in the olfactory bulb.54,55 Nevertheless, we selected the CaMKIIα promoter as CaMKII expression has been reported in NAc GABAergic neurons.48,56,57 We previously demonstrated that AAV injections into the NAc of narcolepsy model mice and DREADD activation/inhibition successfully modulated the number of cataplexy attacks, indicating the emotional modulatory effects of CaMKII-expressing neurons in the NAc.10
Conditioned place preference test
The conditioned place preference test was performed as described previously.58,59 A custom-made place preference apparatus was used to examine the effects of artificial activation/inactivation on place preference (Figure 1C). Two plastic chambers (12 × 15 × 11 cm) were connected via a tube that allowed animals to move freely between chambers. The floor of one chamber was filled with wooden mesh and the other with a plastic board, while the wall of one chamber contained black stripes. Thus, the two chambers contained distinct tactile and visual cues. The test chambers were thoroughly cleaned after each test to avoid any odorous cues.
Mice were first allowed to explore the apparatus for acclimatization freely. The next day, we tested whether mice preferred a specific side chamber or floor material without administration of clozapine/vehicle for 15 min. The time spent in each chamber was recorded to assess basal preference (pre-value). The preference score was calculated by dividing the time spent in the treatment-paired chamber by the total time spent in both chambers. None of the animals exhibited an initial bias (preference score >0.7 or <0.3) for either chamber. Conditioning sessions were conducted for 4 consecutive days. In the first conditioning session in the morning, mice were intraperitoneally injected (10 mL/kg) with clozapine (Tocris 0444) and confined to one chamber for 30 min. In the afternoon, the mice were administered with the vehicle (1% DMSO in phosphate-buffered saline [PBS]) and confined to the other chamber. The interval between injections was more than 4 h. On the second day, mice received vehicle treatment in the morning session and clozapine treatment in the afternoon session. The same treatments as those on conditioning days 1 and 2 were repeated. A combination of floor texture and treatment was randomly assigned to the mice. The day after the final conditioning session, a preference test was conducted as the basal preference assessment. In both basal and test sessions, preference score was calculated and compared.
The clozapine solution was prepared by dissolving 1 mg of clozapine in 1 mL of DMSO (Sigma-Aldrich 472301) and stored in a deep freezer (−70°C). On the day of use, the solution was diluted 100 times with PBS to make 10 μg/mL in 1% DMSO.
Body temperature and locomotor activity measurements
The abdominal temperature was measured using a wireless passive transponder system (Electric Laboratory Animal Monitoring System, Bio Medic Data Systems, Inc., Seaford, DE, USA). At least 7 days before experimentation, the transponder was implanted in the abdominal cavities of mice under isoflurane anesthesia (3% inhalation). On the measurement day, the animals’ home cage was moved onto the shelf in the experimental room. The system’s scanner (DAS7007R) advanced toward the mouse underneath the shelf to minimize visual stimulation as a potential stressor. The experiment started once 3 consecutive body temperature measurements exhibited stable values within 0.5°C.
During the experiment, the mice’s behavior was continuously video-recorded using a video camera with an infrared lamp (ELP-USBFHD06MT-KL36IR, Ailipu Technology, Shenzhen, China) and stored in a computer. After the experiment, locomotor activity was determined by video tracking software (EthoVision XT, Noldus Information Technology, Wageningen, The Netherlands; RRID: SCR_000441).
Intruder test
The mice were implanted with a transponder for body temperature measurement and acclimatized to a reverse light/dark cycle (lights off at 07:00 and on at 19:00) for more than 1 week before the experimental day. Experiments were conducted between 10:00 and 16:00 in a dark room with dim red lights. After confirming the stabilization of body temperature (<0.5°C during three consecutive measurements), the mouse received an intraperitoneal injection of clozapine or vehicle. Ten minutes later, an intruder male mouse was introduced into the resident mouse’s home cage for 10 min. Each animal was tested more than twice in a random order (vehicle + intruder/clozapine + intruder/clozapine + none) with an interval of more than 3 days. The intruder mouse was changed between the sessions to avoid possible acclimatization to each other.
Intracerebroventricular injection of PGE2
For intracerebroventricular injection of the pyrogen PGE2, a guide cannula (C315GS-5/2.5; Plastics One Inc., Roanoke, VA, USA) was implanted in the lateral ventricle (0.8 mm lateral to bregma, 2.5 mm beneath the skull) under isoflurane anesthesia (3% inhalation). The guide cannula was closed with a cannula dummy cap and firmly fixed to the skull with dental cement. Antibiotics and analgesics were administered as described above. PGE2 was dissolved in artificial CSF (1 mg/mL) and injected at 2 μL.41
Immunohistochemistry
Mice were deeply anesthetized with urethane (1.8 g/kg, i.p.) and transcardially perfused with 25 mL of PBS (0.01 M, pH 7.4), followed by 25 mL of 4% paraformaldehyde (PFA) solution. The brain was removed, post-fixed in 4% PFA solution at 4°C overnight, and immersed in 30% sucrose in PBS at 4°C for 2 days. A series of 40-μm sections were obtained with a vibratome (SuperMicroSlicer Zero1; DOSAKA EM, Kyoto, Japan), and every fourth section was used for immunostaining. The brain sections were immersed in a blocking solution (1% normal horse serum and 0.3% Triton X- in 0.01 M PBS) for 1 h at room temperature (20°C–24°C). The sections were incubated overnight with anti-c-Fos rabbit antibody (ABE457, Millipore Corp., RRID: AB_2631318, at 1/1000 in blocking solution). The sections were washed with PBS and then incubated with anti-rabbit IgG conjugated with CF488 fluorescent dye (raised in donkey, Biotium 20015, RRID: AB_10559669, 1/500) for 90 min. The number of c-Fos-positive cells in the NAc was quantified in a manner blinded to treatment.
Quantification and statistical analysis
Statistical analyses were performed using Prism software v.10 (GraphPad, RRID: SCR_002798). We used repeated-measures one-way ANOVA with Geisser-Greenhouse correction to avoid assuming sphericity or one-way ANOVA for comparing three or more groups. When ANOVA revealed a significant main effect, pairwise comparisons were followed by Holm-Sidak’s multiple comparison test. A paired or unpaired t-test (two-tailed) was used according to the data structure. Kolmogorov-Smirnov tests tested the normality of residuals, and all the data passed the tests (α = 0.05). In the AAV-Gq/Gi manipulation test on body temperature, only the data from the successful injection of AAV into NAc were analyzed. In other experiments, no attempt was made to exclude the possible outliers. No statistical methods were used to predetermine the sample size. P-values <0.05 were considered statistically significant. Data are presented as mean ± SEM. The effect size was reported as eta square for ANOVA and Cohen’s d for pairwise comparison.
Published: October 18, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.111197.
Supplemental information
References
- 1.Ekman P., Levenson R.W., Friesen W.V. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221:1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- 2.LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dukes D., Abrams K., Adolphs R., Ahmed M.E., Beatty A., Berridge K.C., Broomhall S., Brosch T., Campos J.J., Clay Z., et al. The rise of affectivism. Nat. Hum. Behav. 2021;5:816–820. doi: 10.1038/s41562-021-01130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eccles J.A., Owens A.P., Mathias C.J., Umeda S., Critchley H.D. Neurovisceral phenotypes in the expression of psychiatric symptoms. Front. Neurosci. 2015;9:4. doi: 10.3389/fnins.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suardi A., Sotgiu I., Costa T., Cauda F., Rusconi M. The neural correlates of happiness: A review of PET and fMRI studies using autobiographical recall methods. Cogn. Affect. Behav. Neurosci. 2016;16:383–392. doi: 10.3758/s13415-016-0414-7. [DOI] [PubMed] [Google Scholar]
- 6.Krahn L.E., Lymp J.F., Moore W.R., Slocumb N., Silber M.H. Characterizing the emotions that trigger cataplexy. J Neuropsychiat Clin Neurosci. 2005;17:45–50. doi: 10.1176/jnp.17.1.45. [DOI] [PubMed] [Google Scholar]
- 7.Overeem S., van Nues S.J., van der Zande W.L., Donjacour C.E., van Mierlo P., Lammers G.J. The clinical features of cataplexy: A questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med. 2011;12:12–18. doi: 10.1016/j.sleep.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Burgess C.R., Oishi Y., Mochizuki T., Peever J.H., Scammell T.E. Amygdala lesions reduce cataplexy in orexin knock-out mice. J. Neurosci. 2013;33:9734–9742. doi: 10.1523/JNEUROSCI.5632-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuwaki T., Kanno K. Sexual excitation induces courtship ultrasonic vocalizations and cataplexy-like behavior in orexin neuron-ablated male mice. Comm Biol. 2021;4:165. doi: 10.1038/s42003-021-01696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su J., Li Z., Yamashita A., Kusumoto-Yoshida I., Isomichi T., Hao L., Kuwaki T. Involvement of the nucleus accumbens in chocolate-induced cataplexy. Sci. Rep. 2020;10:4958. doi: 10.1038/s41598-020-61823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawashima S., Lou F., Kusumoto-Yoshida I., Hao L., Kuwaki T. Activation of the rostral nucleus accumbens shell by optogenetics induces cataplexy-like behavior in orexin neuron-ablated mice. Sci. Rep. 2023;13:2546. doi: 10.1038/s41598-023-29488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro D.C., Bruchas M.R. A Motivational and Neuropeptidergic Hub: Anatomical and Functional Diversity within the Nucleus Accumbens Shell. Neuron. 2019;102:529–552. doi: 10.1016/j.neuron.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saddoris M.P., Cacciapaglia F., Wightman R.M., Carelli R.M. Differential dopamine release dynamics in the nucleus accumbens core and shell reveal complementary signals for error prediction and incentive motivation. J. Neurosci. 2015;35:11572–11582. doi: 10.1523/JNEUROSCI.2344-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calipari E.S., Bagot R.C., Purushothaman I., Davidson T.J., Yorgason J.T., Peña C.J., Walker D.M., Pirpinias S.T., Guise K.G., Ramakrishnan C., et al. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc. Natl. Acad. Sci. USA. 2016;113:2726–2731. doi: 10.1073/pnas.1521238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenzel J.M., Rauscher N.A., Cheer J.F., Oleson E.B. A role for phasic dopamine release within the nucleus accumbens in encoding aversion: A review of the neurochemical literature. ACS Chem. Neurosci. 2015;6:16–26. doi: 10.1021/cn500255p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salamone J.D., Pardo M., Yohn S.E., López-Cruz L., SanMiguel N., Correa M. In: Simpson E., Balsam P., editors. Springer; 2015. Mesolimbic Dopamine and the Regulation of Motivated Behavior; pp. 231–257. (Behavioral Neuroscience of Motivation. Current Topics in Behavioral Neurosciences). [DOI] [PubMed] [Google Scholar]
- 17.Gold P.W. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiat. 2015;20:32–47. doi: 10.1038/mp.2014.163. [DOI] [PubMed] [Google Scholar]
- 18.Francis T.C., Lobo M.K. Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biol Psychiat. 2017;81:645–653. doi: 10.1016/j.biopsych.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbit L.H., Balleine B.W. In: Simpson E., Balsam P., editors. Springer; 2015. Learning and Motivational Processes Contributing to Pavlovian–Instrumental Transfer and Their Neural Bases: Dopamine and Beyond; pp. 259–289. (Behavioral Neuroscience of Motivation. Current Topics in Behavioral Neurosciences). [DOI] [PubMed] [Google Scholar]
- 20.Heidbreder C., Feldon J. Amphetamine-induced neurochemical and locomotor responses are expressed differentially across the anteroposterior axis of the core and shell subterritories of the nucleus accumbens. Synapse. 1998;29:310–322. doi: 10.1002/(SICI)1098-2396(199808)29:4<310::AID-SYN3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds S.M., Berridge K.C. Fear and feeding in the nucleus accumbens shell: Rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J. Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berridge K.C., Kringelbach M.L. Pleasure systems in the brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foo H., Mason P. Analgesia accompanying food consumption requires ingestion of hedonic foods. J. Neurosci. 2009;29:13053–13062. doi: 10.1523/JNEUROSCI.3514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez J.L., Bonaventura J., Lesniak W., Mathews W.B., Sysa-Shah P., Rodriguez L.A., Ellis R.J., Richie C.T., Harvey B.K., Dannals R.F., et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosten T.A., Nestler E.J. Clozapine attenuates cocaine conditioned place preference. Life Sci. 1994;55:9–14. doi: 10.1016/0024-3205(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 26.Blessing W.W., Blessing E.M., Mohammed M., Ootsuka Y. Clozapine, chlorpromazine and risperidone dose-dependently reduce emotional hyperthermia, a biological marker of salience. Psychopharmacology. 2017;234:3259–3269. doi: 10.1007/s00213-017-4710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian S., Völlm B.A., Huband N. Clozapine dose for schizophrenia. Cochrane Database Syst. Rev. 2017;6:CD009555. doi: 10.1002/14651858.CD009555.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 29.Yonemitsu T., Kuroki C., Takahashi N., Mori Y., Kanmura Y., Kashiwadani H., Ootsuka Y., Kuwaki T. TRPA1 detects environmental chemicals and induces avoidance behavior and arousal from sleep. Sci. Rep. 2013;3:3100. doi: 10.1038/srep03100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S., Takahashi N., Chen C., Pauli J.L., Kuroki C., Kaminosono J., Kashiwadani H., Kanmura Y., Mori Y., Ou S., et al. Transient receptor potential ankyrin 1 mediates hypoxic responses in mice. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.576209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown P.L., Wise R.A., Kiyatkin E.A. Brain Hyperthermia Is Induced by Methamphetamine and Exacerbated by Social Interaction. J. Neurosci. 2003;23:3924–3929. doi: 10.1523/JNEUROSCI.23-09-03924.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantsch J.R., Baker D.A., Funk D., Lê A.D., Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacol. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyata K., Ikoma Y., Murata K., Kusumoto-Yoshida I., Kobayashi K., Kuwaki T., Ootsuka Y. Multifaceted roles of orexin neurons in mediating methamphetamine-induced changes in body temperature and heart rate. IBRO Neurosci. Rep. 2022;12:108–120. doi: 10.1016/j.ibneur.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orts A., Alcaraz C., Goldfrank L., Turndorf H., Puig M.M. Morphine-ethanol interaction on body temperature. Gen. Pharmacol. 1991;22:111–116. doi: 10.1016/0306-3623(91)90319-2. [DOI] [PubMed] [Google Scholar]
- 35.Avegno E.M., Gilpin N.W. Reciprocal midbrain-extended amygdala circuit activity in preclinical models of alcohol use and misuse. Neuropharmacol. 2022;202 doi: 10.1016/j.neuropharm.2021.108856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fields H.L., Margolis E.B. Understanding opioid reward. Trends Neurosci. 2015;38:217–225. doi: 10.1016/j.tins.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maes M., Berk M., Goehler L., Song C., Anderson G., Gałecki P., Leonard B. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kataoka N., Shima Y., Nakajima K., Nakamura K. A central master driver of psychosocial stress responses in the rat. Science. 2020;367:1105–1112. doi: 10.1126/science.aaz4639. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R1207–R12281228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- 40.Morrison S.F., Madden C.J., Tupone D. Central control of brown adipose tissue thermogenesis. Front. Endocrinol. 2012;3:5. doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi Y., Zhang W., Sameshima K., Kuroki C., Matsumoto A., Sunanaga J., Kono Y., Sakurai T., Kanmura Y., Kuwaki T. Orexin neurons are indispensable for prostaglandin E2-induced fever and defence against environmental cooling in mice. J. Physiol. 2013;591:5623–5643. doi: 10.1113/jphysiol.2013.261271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hrvatin S., Sun S., Wilcox O.F., Yao H., Lavin-Peter A.J., Cicconet M., Assad E.G., Palmer M.E., Aronson S., Banks A.S., et al. Neurons that regulate mouse torpor. Nature. 2020;583:115–121. doi: 10.1038/s41586-020-2387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi T.M., Sunagawa G.A., Soya S., Abe M., Sakurai K., Ishikawa K., Yanagisawa M., Hama H., Hasegawa E., Miyawaki A., et al. A discrete neuronal circuit induces a hibernation-like state in rodents. Nature. 2020;583:109–114. doi: 10.1038/s41586-020-2163-6. [DOI] [PubMed] [Google Scholar]
- 44.Uchino E., Kusumoto-Yoshida I., Kashiwadani H., Kanmura Y., Matsunaga A., Kuwaki T. Identification of hypothermia-inducing neurons in the preoptic area and activation of them by isoflurane anesthesia and central injection of adenosine. J. Physiol. Sci. 2024;74:33. doi: 10.1186/s12576-024-00927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paxinos G., Franklin K.B.J. Academic Press; 2001. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- 46.Kaneko S., Hikida T., Watanabe D., Ichinose H., Nagatsu T., Kreitman R.J., Pastan I., Nakanishi S. Synaptic Integration mediated by striatal cholinergic interneurons in basal ganglia function. Science. 2000;289:633–637. doi: 10.1126/science.289.5479.633. [DOI] [PubMed] [Google Scholar]
- 47.Engeln M., Francis T.C., Lobo M.K. In: Neural Mechanism of Addiction. Torregrossa M., editor. Academic Press; 2018. Striatal Cell-Type Specific Plasticity in Addiction; pp. 259–269. [DOI] [Google Scholar]
- 48.Robison A.J., Vialou V., Mazei-Robison M., Feng J., Kourrich S., Collins M., Wee S., Koob G., Turecki G., Neve R., et al. Behavioral and structural responses to chronic cocaine require a feedforward loop involving FosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J. Neurosci. 2013;33:4295–4307. doi: 10.1523/JNEUROSCI.5192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin M.T., Ho M.T., Young M.S. Stimulation of the nigrostriatal dopamine system inhibits both heat production and heat loss mechanisms in rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 1992;346:504–510. doi: 10.1007/BF00169004. [DOI] [PubMed] [Google Scholar]
- 50.Caubit X., Arbeille E., Chabbert D., Desprez F., Messak I., Fatmi A., Habermann B., Gubellini P., Fasano L. Camk2a-Cre and Tshz3 Expression in Mouse Striatal Cholinergic Interneurons: Implications for Autism Spectrum Disorder. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.683959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aubry A.V., Joseph Burnett C., Goodwin N.L., Li L., Navarrete J., Zhang Y., Tsai V., Durand-de Cuttoli R., Golden S.A., Russo S.J. Sex differences in appetitive and reactive aggression. Neuropsychopharmacology. 2022;47:1746–1754. doi: 10.1038/s41386-022-01375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shansky R.M. Are hormones a ''female problem'' for animal research? Science. 2019;364:825–826. doi: 10.1126/science.aaw7570. [DOI] [PubMed] [Google Scholar]
- 53.Tappaz M.L., Brownstein M.J., Palkovits M. Distribution of glutamate decarboxylase in discrete brain nuclei. Brain Res. 1976;108:371–379. doi: 10.1016/0006-8993(76)90193-1. [DOI] [PubMed] [Google Scholar]
- 54.Benson D.L., Isackson P.J., Hendry S.H., Jones E.G. Differential gene expression for glutamic acid decarboxylase and type II calcium-calmodulin dependent protein kinase in basal ganglia, thalamus, and hypothalamus of the monkey. J. Neurosci. 1991;11:1540–1564. doi: 10.1523/JNEUROSCI.11-06-01540.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X., Zhang C., Szábo G., Sun Q.-Q. Distribution of CaMKIIα expression in the brain in vivo, studied by CaMKIIα-GFP mice. Brain Res. 2013;1518:9–25. doi: 10.1016/j.brainres.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortiz J., Harris H.W., Guitart X., Terwilliger R.Z., Haycock J.W., Nestler E.J. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: Regional distribution and regulation by chronic morphine. J. Neurosci. 1995;15:1285–1297. doi: 10.1523/JNEUROSCI.15-02-01285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papa M., Sergeant J.A., Sadile A.G. Reduced transduction mechanisms in the anterior accumbal interface of an animal model of attention-deficit hyperactivity disorder. Behav. Brain Res. 1998;94:187–195. doi: 10.1016/s0166-4328(97)00179-4. [DOI] [PubMed] [Google Scholar]
- 58.Hillhouse T., Prus A. In: The Brain Reward System, Neuromethods 165. Fakhoury M., editor. Springer Nature); 2021. Conditioned Place Preference Test for Assessing the Rewarding Effects of Drugs of Abuse. [DOI] [Google Scholar]
- 59.Yoshida M., Yamamoto K., Kuwaki T. Positive memory increases cataplexy-like behaviors in narcolepsy mice as revealed using conditioned place preference test. BMC Neurosci. 2022;23:82. doi: 10.1186/s12868-022-00772-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Any data required to reanalyze the data reported in this paper are available from the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.