Abstract
Purpose:
To investigate the prevalence of myopia and high myopia in Olmsted County, Minnesota from 1966–2019.
Design:
Retrospective cross-sectional, trend study using a random population-based sample in Olmsted County, Minnesota from 1966–2019.
Methods:
Manifest refractions or lens prescription were converted to spherical equivalent (SE) to estimate prevalence of adult myopia and high myopia. Age, sex, race, and visual acuity were recorded. Subjects with SE ≤−0.5 diopters (D) were considered myopic; ≤−6.0 D were considered high myopia. Exclusion criteria included visually significant cataract, pseudophakia, prior refractive surgery, or age <18 years-old.
Results:
Among 81,706 sampled subjects, myopia prevalence increased from 33.9% (95% CI 31.1–36.8) in 1960s to 57.1% (95% CI, 56.6–57.6) in 2010s (p<0.001). High myopia prevalence increased from 2.8% (95% CI, 1.95–3.98) in 1960s to 8.3% (95% CI, 8.08–8.62) in 2010s (p<0.001). Both males (32.0%−55.1%, p<.001) and females (40.6%−58.5%, p<.001) experienced increasing myopia prevalence from 1960s-2010s while males (2.6%−7.4%, p<.001) and females (3.4%−9.1%, p<.001) also had higher high myopia prevalence rates from 1960s through 2010s. Increasing myopia and high myopia prevalence was detected by decade in nearly all age groups (excluding 18–24-year-old high myopia subjects). White and Asian subjects had the highest myopia prevalence while Black subjects had the lowest. From the 2000s-2010s, White (53.3%−57.0%, p<0.001) and Black (41.0%−47.0%, p=0.001) subjects had significant increases in myopia prevalence. Mean SE decreased from the 1960s (−0.42 D; 95% CI −0.59–+2.49) to 2010s (−1.85 D; 95% CI, −1.88–+2.96) (p<0.001).
Conclusions:
From 1966–2019 in Olmsted County, Minnesota, there was a 68% and 199% increase in myopia and high myopia prevalence, respectively.
Keywords: myopia, high myopia, prevalence, epidemiology, population-based
Table Of Contents Statement
This retrospective cross-sectional population-based study explores the prevalence of myopia and high myopia in Olmsted County, Minnesota, between 1966–2019. There was a 68% and 199% increase in myopia and high myopia prevalence, respectively. The increases are evident across genders, age groups, and specific racial categories. These trends underscore the need for targeted interventions, informed clinical practice, and further investigations into underlying causes and potential mitigations rising rates of myopia and high myopia.
Uncorrected myopia is a leading cause of visual impairment in the world with recent studies demonstrating progressively increasing prevalence of myopia in East Asia.1 In 2004, Lin et al. documented the prevalence of myopia for Taiwanese children approached 80%.2 Other studies reported that nearly 90% of young adults are myopic in Taiwan, Singapore, and China.3–5 In the United States (U.S.), the National Health and Nutrition Examination Surveys (NHANES) reported a 66% increase in myopia prevalence in a cross sectional study between the 1970’s (25.0%) to early 2000’s (41.6%) over approximately three decades.6
Increasing myopia prevalence is particularly concerning within a given population because of the association of increasing visual morbidity (e.g., retinal detachment, choroidal neovascularization, and macular atrophy)7 and the increasing socioeconomic factors necessary to address refractive correction and myopia related healthcare expenses.8 Vitale et al. estimated that increased myopia prevalence from 25% to 37% would increase associated annual U.S. healthcare costs from $2 billion to more than $3 billion.6 Furthermore, Holden et al. predict that 50% of the world’s population will be myopic by 2050, an increase from current estimates of 23%, and suggest prevention strategies to reduce myopia-related ocular complications.8
Our study examined trends of myopia and high myopia with data randomly sampled from a large, population database, the Rochester Epidemiology Project (REP) from a stable, U.S. population in Olmsted County, Minnesota from 1966 to 2019.
Methods
This retrospective population-based study was approved by both the Mayo Clinic Institutional Review Board (IRB) and the Olmsted Medical Center IRB and adhered to the tenets of the Declaration of Helsinki. The REP database was randomly sampled individuals living in Olmsted County, Minnesota from January 1, 1966, to December 31, 2019. The REP database is a regional population-based index with diagnostic information associated with subject encounters. The REP also links directly to medical records and contains nearly residents (participation approaches 98%) of Olmsted County, Minnesota. Diagnostic information is captured from Mayo Clinic and local community health care facilities.9–11 The REP has robust ophthalmology participation with one REP project reporting 87% of patients over a 50 year period had documented ocular examinations at a REP-affiliated institution.12
Randomly selected subjects and date of the specific healthcare or pre-employment visit were identified and refractive status data were obtained by identifying diagnoses and billing codes. Subjects were sampled from each decade and categorized from the 1960’s to 2010’s. Multiple subject visits during each decade were not re-sampled because each subject was assigned a unique record number. During random sampling in each decade, paper records were manually reviewed from 1966 to 1999 while electronic medical records were obtained from 2000 to 2020, and electronic access enabled larger sample sizes during the 2000’s and 2010’s. Eligibility criteria included age ≥18 years and living in Olmsted County. Exclusion criteria included pseudophakia, clinically significant cataract (2+ nuclear sclerosis or worse), or prior refractive surgery (e.g., radial keratotomy [RK], laser in situ keratomileusis [LASIK], and photorefractive keratectomy [PRK]).
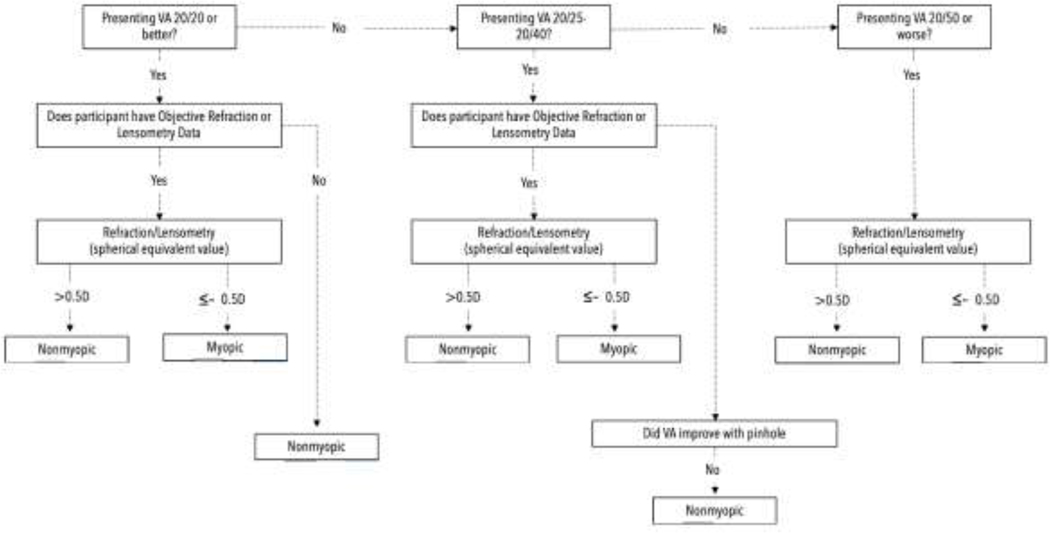
REP extracted data included: 1) age and birth year, 2) sex, 3) race and/or ethnicity, 4) visual acuity, and 5) refraction measurements in diopters (D). When available, spherical equivalent (SE) was calculated. Myopia was defined as a SE≤ (−)0.5 D in either eye while high myopia was a SE≤ (−)6.0 D in either eye per American Academy of Ophthalmology (AAO) definition.13 The World Health Organization (WHO) utilizes a slightly different definition of high myopia with a SE≤ (−5.0 D) and this data segmentation was included for study comparisons.13,14 To determine if a subject was myopic, we adapted Vitale et al.’s myopia classification algorithm.6 We modified the algorithm to include objective, refractive data when available as well as lensometry data (e.g., current glasses prescription measurement, SE). We also modified the algorithm to assign myopia as a SE≤ (−)0.5 D, rather than SE< 0 D (Figure 1). For a subject to be included in the 20/20 or better group, a presenting visual acuity (VA) of 20/20 or better was required in each eye. If one eye was 20/20 and the other was worse, then myopia status was documented. In all eligible subjects that met entry criteria, the more negative SE eye determined overall myopia status. For subjects with multiple visits in one decade, the most negative SE determined myopia status. We stratified randomly sampled subjects as: 1) missing data or did not meet inclusion, 2) met inclusion, 3) only with visual acuity data, and 4) both visual acuity and refractive status (lensometry/refraction) (Table 1). Prevalence rates for myopia and high myopia were calculated by decade for each group, then by age, sex, and racial group. Racial prevalence rates were only calculated from 2000 to 2020 due to a limited racial diversity in Olmsted County prior to 2000. Proportions were determined by dividing the total number of myopes and high myopes by all randomly sampled, eligible subjects in each decade. Prevalence rates were age- and sex-standardized using U.S. census figures from 1970–2020. The Cochran-Armitage trend test was used to compare proportions and two-sided, student t-test was used to compare SE. A significance value of p <0.05 was considered statistically significant. Data analysis and visualization were performed using SAS (version 9.4; SAS Institute Inc., Cary, North Carolina), Python (version 3.8), Pandas (version 1.3), Seaborn (version 0.11.2), and Excel (Microsoft, Redmond, Seattle.).
Figure 1.
Revised Myopia Classification Algorithm in the Present Study. D=Diopter
Table 1.
Sampling Breakdown of Subjects by Decade
| 1960s | 1970s | 1980s | 1990s | 2000s | 2010s | Total | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Subjects Randomly Sampled | 1400 | 1700 | 1400 | 1700 | 50653 | 55179 | 112032 |
| Missing Data or Excluded, (%) | 362 (25%) | 240 (14%) | 191 (14%) | 159 (9%) | 14558 (28%) | 14816 (27%) | 30326 |
| Subjects with VA or Refraction | 1038 | 1460 | 1209 | 1541 | 36095 | 40363 | 81706 |
| Subjects with VA and Refraction Data | 913 | 1378 | 937 | 1298 | 29704 | 35407 | 69637 |
| Subjects with Only VA Data | 125 | 82 | 272 | 243 | 6391 | 4956 | 12069 |
Results
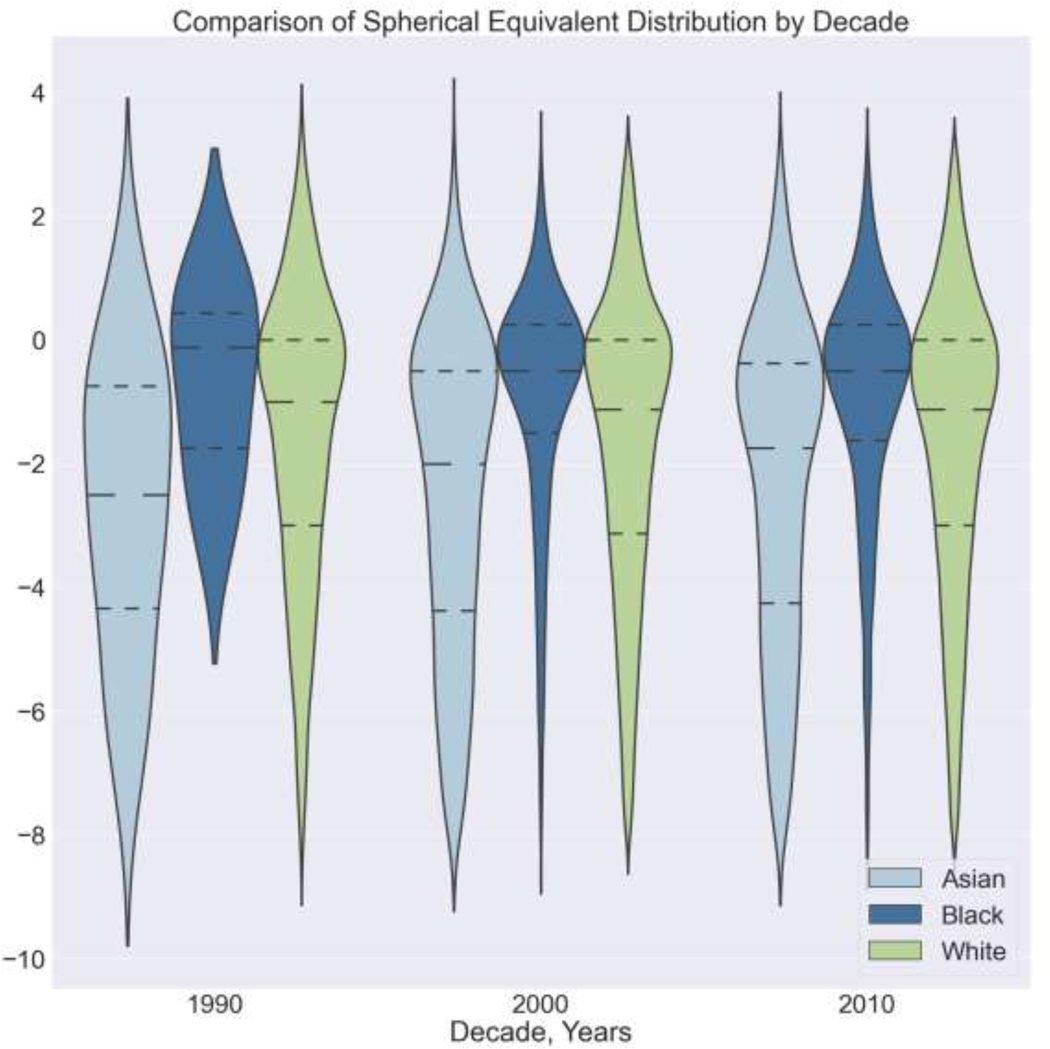
In total, 81,706 subjects living in Olmsted County, Minnesota met inclusion criteria from inclusive dates of 1966 to 2019. Demographics between the sampled population and Olmsted County were similar (Table 2). The estimated myopia prevalence by decade was 33.9% in 1960’s (95% confidence interval [CI], 31.1–36.8), 43.5% in 1970’s (95% CI, 41.0–46.0), 46.7% in 1980’s (95% CI, 43.9–49.6), 53.5% in 1990’s (95% CI, 51.0–56.0), 53.6% in 2000’s (95% CI, 53.1–54.1), and 57.1% in 2010’s (95% CI, 56.6–57.6) (Table 3). Across all decades, there was a statistically significant increase in myopia prevalence (p<0.001). The age- and sex-standardized myopia prevalence by decade was 37.0% in the 1960’s, 42.9% in the 1970s, 55.0% in the 1980s, 60.5% in the 1990s, 61.4% in the 2000s, and 63.0% in the 2010s (Table 3). The prevalence of high myopia nearly tripled from 2.8% in 1960’s (95% CI, 2.0–4.0), 4.0% in 1970’s (95% CI, 3.1–5.2), 5.1% in 1980’s (95% CI, 3.9–6.4), 5.8% in 1990’s (95% CI, 4.7–7.1), 7.9% in 2000’s (95% CI, 7.7–8.2), to 8.3% in 2010’s (95% CI, 8.1–8.6). Using both the AAO and WHO definition, statistically significant increases in high myopia prevalence occurred in all decades (p<0.001) (Table 3). The mean SE significantly decreased progressively from the 1960’s (−0.42 D; 95% CI −0.59-+2.49) to 2010’s (−1.85 D; 95% CI, −1.88-+2.96) (p<0.001; Table 3). The SE distribution (Table 4, Figure 2) from 1966 to 2019 demonstrated that myopia prevalence nearly doubled when comparing the 1960’s to 2010’s in the sampled population. Both myopia and high myopia increased from 1966 to 2019 in all groups except in the 18 to 24-year-old high myopia group (Table 3).
Table 2.
Characteristics of Sampled Population versus Olmsted County
| 1960s | 1970s | 1980s | 1990s | 2000s | 2010s | |
|---|---|---|---|---|---|---|
|
| ||||||
| Gender | ||||||
| Sampled Population | ||||||
| Females | 63.45 | 58.83 | 56.71 | 60.68 | 57.97 | 57.98 |
| Males | 36.55 | 41.17 | 43.29 | 39.32 | 42.03 | 42.01 |
|
| ||||||
| Olmsted Census | ||||||
| Females | 52.6 | 52.5 | 51.5 | 50.9 | 51.1 | 51.2 |
| Males | 47.4 | 47.5 | 48.5 | 49.1 | 48.9 | 48.8 |
| p-value | 0.158 | 0.448 | 0.545 | 0.211 | 0.404 | 0.411 |
|
| ||||||
| Racial Demographics | ||||||
| Sampled Population | ||||||
| White | 99.78 | 99.05 | 97.37 | 95.63 | 85.68 | 85.7 |
| Asian | 0.22 | 0.71 | 2.51 | 3.54 | 3.97 | 5.35 |
| Black | 0 | 0.238 | 0.13 | 0.82 | 2.99 | 3.99 |
| Other | 0 | 0 | 0 | 0 | 2.75 | 3.4 |
|
| ||||||
| Olmsted Census | ||||||
| White | 99.1 | 98 | 95.7 | 90.3 | 85.7 | 77.8 |
| Asian | 0.4 | 1.1 | 3 | 4.3 | 5.4 | 6.3 |
| Black | 0.3 | 0.4 | 0.7 | 2.7 | 4.8 | 6.8 |
| Other | 0.1 | 0.3 | 0.3 | 0.9 | 1.6 | 2.5 |
| p-value | 0.923 | 0.933 | 0.861 | 0.549 | 0.821 | 0.753 |
|
| ||||||
| Age | ||||||
| Sampled Population | ||||||
| Mean | 42.14 | 40.47 | 46.27 | 48.49 | 45.11 | 47.67 |
| Standard Deviation | 18.86 | 18.66 | 17.25 | 18.21 | 16.63 | 16.84 |
| 25th Percentile | 26 | 25 | 31 | 35 | 32 | 34 |
| 75th Percentile | 57 | 52 | 59 | 62 | 55 | 60 |
|
| ||||||
| Olmsted Census | ||||||
| Mean | 39.78 | 39.9 | 41.45 | 43.52 | 45.23 | 47.04 |
| Standard Deviation | 17.98 | 18.24 | 17.7 | 17.53 | 18.1 | 18.76 |
| 25th Percentile | 25 | 25 | 28 | 30 | 30 | 31 |
| 75th Percentile | 52 | 52 | 52 | 54 | 57 | 61 |
| p-value | 1 | 1 | 1 | 1 | 1 | 1 |
Table 3.
Characteristics of Olmsted County, Minnesota, Residents’ Refractive Status, 1966–2019
| 1966–1969 (n=1038) | 1970–1979 (n=1460) | 1980–1989 (n=1209) | 1990–1999 (n=1541) | 2000–2009 (n=36095) | 2010–2019 (n=40363) | P Value | |
|---|---|---|---|---|---|---|---|
| Age- and Sex Standardized Prevalence, % (95% CI) | |||||||
| Myopia | 37.0 (37.0, 37.0) | 42.9 (42.9, 42.9) | 55.0 (55.0, 55.0) | 60.5 (60.5, 60.5) | 61.4 (61.4, 61.5) | 63.0 (62.9, 63.1) | <.0001 |
| High Myopia AAO | 3.21 (3.21, 3.21) | 3.81 (3.80, 3.81) | 7.35 (7.35, 7.36) | 6.89 (6.88, 6.89) | 8.51 (8.51, 8.52) | 8.83 (8.83, 8.84) | <.0001 |
| High Myopia WHO | 5.46 (5.46, 5.46) | 6.67 (6.67, 6.67) | 11.82 (11.81, 11.82) | 11.98 (11.98, 11.99) | 12.97 (12.96, 12.97) | 13.32 (13.31, 13.32) | <.0001 |
|
| |||||||
| Estimated Myopia Prevalence, % (95% CI) | |||||||
| Overall | 33.9 (31.1–36.8) | 43.5 (41.0–46.0) | 46.7 (43.9–49.6) | 53.5 (51.0–56.0) | 53.6 (53.1–54.1) | 57.1 (56.6–57.6) | <.0001 |
| Men | 32.0 (27.0–36.9) | 44.0 (39.9–48.0) | 53.0 (48.2–57.8) | 56.4 (52.2–60.6) | 51.3 (50.5–52.1) | 55.1 (54.3–55.8) | <.0001 |
| Women | 40.6 (36.6–46.6) | 44.5 (41.2–47.9) | 52.4 (48.2–56.6) | 61.6 (58.2–64.9) | 55.3 (54.6–56.0) | 58.5 (57.9–59.2) | <.0001 |
|
| |||||||
| Estimated High Myopia Prevalence AAO Definition (SE ≤ (−)6.0 D), % (95% CI) | |||||||
| Overall | 2.8 (2.0–4.0) | 4.0 (3.1–5.2) | 5.1 (3.9–6.4) | 5.8 (4.7–7.1) | 7.9 (7.7–8.2) | 8.3 (8.1–8.6) | <.0001 |
| Men | 2.6 (0.9–4.3) | 3.3 (2.0–5.1) | 8.3 (5.7–11.0) | 6.8 (4.6–8.9) | 6.9 (6.5–7.3) | 7.4 (7.0–7.6) | <.0001 |
| Women | 3.4 (2.0–4.9) | 4.7 (3.3–6.4) | 4.8 (3.0–6.6) | 6.5 (4.9–8.5) | 8.7 (8.3–9.1) | 9.1 (8.7–9.4) | <.0001 |
|
| |||||||
| Estimated High Myopia Prevalence WHO Definition (SE ≤ (−)5.0 D), % (95% CI) | |||||||
| Overall | 5.0 (3.8–6.5) | 7.0 (5.8–8.4) | 8.4 (7.0–10.1) | 10.3 (8.8–11.9) | 12.0 (11.7–12.3) | 12.6 (12.2–12.9) | <.0001 |
| Men | 4.7 (2.5–6.9) | 5.3 (5.3–7.2) | 12.9 (9.7–16.1) | 10.9 (8.3–13.6) | 10.6 (10.1–11.1) | 11.0 (10.6–11.5) | <.0001 |
| Women | 6.2 (4.2–8.2) | 8.4 (6.5–10.2) | 8.8 (6.4–11.2) | 12.4 (10.1–14.6) | 13.1 (12.6–13.5) | 13.7 (13.2–14.1) | <.0001 |
|
| |||||||
| Estimated Myopia Prevalence Race, % (95% CI) | |||||||
| White | 53.3 (52.7–53.8) | 57.0 (56.4–57.5) | <.0001 | ||||
| Black | 41.0 (38.0–43.9) | 47.0 (44.6–49.5) | 0.001 | ||||
| Asian | 65.4 (62.9–67.9) | 67.8 (66.0–69.8) | 0.7974 | ||||
| Other | 53.2 (50.2–56.2) | 55.3 (52.7–57.8) | 0.299 | ||||
|
| |||||||
| Estimated High Myopia Prevalence Race AAO Definition (SE ≤ (−)6.0 D), % (95% CI) | |||||||
| White | 7.6 (7.3–7.9) | 8.0 (7.7–8.3) | <.0001 | ||||
| Black | 3.5 (2.4–4.6) | 4.2 (3.2–5.2) | 0.2739 | ||||
| Asian | 15.8 (14.0–17.7) | 16.4 (14.8–18.0) | 0.3561 | ||||
| Other | 8.6 (6.9–10.3) | 8.5 (7.1–9.9) | 0.9384 | ||||
|
| |||||||
| Age Group Myopia Prevalence, % (95% CI) | |||||||
| 18–24 | 60.1 (52.7–67.5) | 61.8 (56.4–67.1) | 68.6 (55.9–81.4) | 79.0 (71.8–86.3) | 79.9 (78.6–81.2) | 79.2 (77.8–80.5) | <.0001 |
| 25–34 | 55.1 (47.8–62.5) | 69.5 (64.5–74.5) | 77.0 (71.8–82.2) | 74.2 (68.6–79.7) | 81.1 (80.1–82.2) | 81.6 (80.6–82.7) | <.0001 |
| 35–44 | 40.3 (31.9–48.8) | 41.0 (34.2–47.8) | 60.8 (53.7–67.9) | 73.2 (68.1–78.3) | 71.5 (70.3–72.7) | 72.3 (71.1–73.5) | <.0001 |
| 45–54 | 23.9 (16.7–31.1) | 32.1 (25.9–38.3) | 50.6 (42.9–58.4) | 64.0 (57.8–70.1) | 65.6 (64.6–66.7) | 65.4 (64.3–66.4) | <.0001 |
| 55–64 | 20.5 (16.0–25.1) | 15.2 (11.5–19) | 28.0 (23.0–33.0) | 36.0 (31.5–40.6) | 41.7 (40.6–42.9) | 50.1 (49.3–51.0) | <.0001 |
| Age Group High Myopia Prevalence AAO Definition (SE ≤ (−)6.0 D), % (95% CI) | |||||||
| 18–24 | 6.0 (2.4–9.5) | 6.9 (4.1–9.7) | 19.6 (8.7–30.5) | 5.9 (1.7–10.1) | 11.1 (10.1–12.2) | 9.3 (8.4–10.2) | 0.3547 |
| 25–34 | 4.6 (1.5–7.6) | 7.6 (4.8–10.5) | 10.7 (6.9–14.5) | 13.6 (9.2–17.9) | 15.5 (14.5–16.4) | 14.3 (13.4–15.3) | <.0001 |
| 35–44 | 3.9 (.6–7.2) | 3.0 (.6–5.4) | 6.6 (3.0–10.2) | 8.9 (5.7–12.2) | 12.2 (11.4–13.1) | 12.9 (12.0–13.8) | <.0001 |
| 45–54 | 2.2 (0–4.7) | 1.4 (0–2.9) | 5.0 (1.6–8.4) | 4.3 (1.7–6.9) | 9.2 (8.5–9.8) | 9.9 (9.2–10.5) | <.0001 |
| 55–64 | 1.0 (0–2.1) | .9 (0–1.8) | 1.3 (0–2.5) | 3.0 (1.4–4.6) | 3.4 (3.0–3.8) | 5.7 (5.3–6.1) | <.0001 |
Table 4.
Spherical Equivalent by Decade
| Decade | Total Subjects | Mean (D) | Std Dev | 95% CI (D) |
|---|---|---|---|---|
| 1960s | 913 | −0.416 | 2.61 | [−0.59, 2.49] |
| 1970s | 1378 | −0.889 | 2.6 | [−1.03, 2.51] |
| 1980s | 937 | −1.33 | 2.88 | [−1.52, 2.75] |
| 1990s | 1298 | −1.592 | 2.76 | [−1.74, 2.66] |
| 2000s | 29704 | −1.88 | 2.95 | [−1.91, 2.92] |
| 2010s | 35407 | −1.85 | 2.98 | [−1.88, 2.96] |
Figure 2.
Comparison of Spherical Equivalent Distribution by Decade. The dashed lines in each plot represent the mean, 25th percentile, and 75th percentile spherical equivalent, respectively, for each decade (viewing top to bottom). D = Diopters
Both males and females had significant increases in myopia (p<0.001) and high myopia (p<.001) prevalence from the 1960s to 2010s (Table 3), while the SE became more negative in both sexes over time (Figure 2, Table 4). Females had a higher myopia prevalence except in the 1980’s (Table 3).
Racial comparisons of myopia prevalence were performed from 2000 to 2019 as the prior sampled years had extremely small non-White populations. In both 2000’s and 2010’s, the prevalence and mean SE of myopia (p<.001) and high myopia (p<.001) was highest in Asians followed by White and then Black subjects (Tables 3, 5, Figure 3). Between the 2000’s and 2010’s, there was a statistically significant increase in myopia prevalence among White (53.3% to 57.0, p<0.001) and Black subjects (41.0% to 47.0, p<0.001) (Table 3) with White subjects having a significant increase in high myopia prevalence (7.6% to 8.0, p<0.001) between the 2000s and 2010s (Table 3).
Table 5.
Spherical Equivalent Stratified by Sex and Decade
| Year | Sex | Mean (D) | Standard Deviation (D) | Median (D) | Minimum (D) | Maximum (D) |
|---|---|---|---|---|---|---|
| 1960s | Female | −0.47 | 2.58 | 0 | −13.5 | 11.875 |
| Male | −0.31 | 2.66 | 0 | −21 | 14.5 | |
| 1970s | Female | −0.87 | 2.78 | −0.25 | −29 | 11.5 |
| Male | −0.92 | 2.31 | −0.25 | −9.75 | 12 | |
| 1980s | Female | −1.23 | 2.91 | −0.75 | −31.25 | 9.25 |
| Male | −1.46 | 2.84 | −0.75 | −11.375 | 13.25 | |
| 1990s | Female | −1.64 | 2.80 | −1.125 | −11.5 | 13 |
| Male | −1.52 | 2.68 | −0.875 | −14.375 | 8.125 | |
| 2000s | Female | −1.98 | 2.98 | −1.25 | −22.625 | 15.5 |
| Male | −1.74 | 2.90 | −1.125 | −30 | 38.5 | |
| 2010s | Female | −1.93 | 3.00 | −1.25 | −26 | 15.5 |
| Male | −1.73 | 2.94 | −1.125 | −33 | 15.5 |
D= Diopters
Figure 3.
Comparison of Spherical Equivalent Distribution by Race from 1990’s to 2010’s. The dashed lines in each plot represent the mean, 25th percentile, and 75th percentile spherical equivalent, respectively, by race by decade (viewing top to bottom). D = Diopter
We did not detect meaningful interaction terms using stepwise logistic regression models. Multivariate analysis did not reveal any significant predictors. For final logistic regression models, Birth year (odds Ratio [OR]: 1.044, 95% CI: 1.04–1.047, p<0.001) and female gender (OR: 1.238, 95% CI: 1.09–1.41, p<.001) were both significant univariate predictors for myopia.
Discussion
We report progressively increasing, long-term prevalence rates of both myopia and high myopia from 1966 to 2019 with data derived randomly from a large, population database (REP) with high participation rates of individuals living in Olmsted County, Minnesota, a Midwestern U.S. population where multiple population based studies have been concordant with national prevalence data15,16. Myopia prevalence increased by 68% from the 1960’s to 2010’s (33.9% to 57.1%, respectively; p<0.001) while the prevalence of high myopia increased by 196% (2.8% to 8.3%, respectively; p<0.001). These data indicate a concerning public health trend that predicts both increasing ocular morbidity as well as increasing healthcare costs to society.7,8,13 This study is the first to provide six decades of U.S. myopia prevalence data and U.S. myopia prevalence data from 2000–2019. Furthermore, our data expands upon key, primary data from the NHANES study, by providing information in all major definitions of myopia and high myopia stratified by race, gender and age groups. From our sampled population, we agree that the U.S. is developing a similar, increasing prevalence of myopia and high myopia, yet delayed when compared to Asian populations. 3–5
The definitions of myopia and high myopia differ in the literature, limiting direct study comparisons.13 In 2015, the WHO defined myopia as a SE ≤ −0.5 D and high myopia as SE ≤ −5.0 D in either eye.14 The International Myopia Institute (IMI) in 2019 reviewed 138 myopia epidemiologic/survey studies and reported that most studies (88%) defined myopia as a SE ≤ −0.5 D.13 The definition of high myopia has less consensus: 61% studies used ≤ −6.0 D and 36% used ≤ −5.0 D.13 Both the AAO and IMI defined high myopia as a SE ≤ −6.0 D and encouraged this definition for meta-analysis. 13,17,18 Herein, we prioritized the SE ≤ −6.0 D, yet also provide the WHO high myopia definition (Table 2).
Long-term myopia prevalence trends offer insight into associated factors that influence myopia progression such as educational exposure, outside activities, and others.19 Increasing high myopia prevalence is associated with increasing ocular morbidity, more retinal detachments, myopic degeneration, vision loss, and other associated disorders.7,8 Should this trend continue, we can expect further ocular morbidity and increasing socioeconomic costs from managing myopia.
Prior to 2000, few U.S. studies examined myopia prevalence. The Beaver Dam Study defined myopia as SE <−0.5 D and found an age-adjusted rate of 26% in adults aged 43 to 84 from 1988 to 1990.20 During the same timeframe, age range, and myopia definition from our population in an adjacent state (approximately 200 miles apart), we found a myopia prevalence of 20%. The Baltimore Eye Study performed subset analysis from NHANES (1971–1972) with myopia defined more broadly as a SE <0.0 D in adults from Baltimore (1985 from 1988) found a 48% prevalence rate in White persons aged 45 to 54.21 Using the more narrow definition (SE ≤−0.5 D) in Olmsted County, we found a higher myopia prevalence rate of 53% among White persons 45 to 54-years-old during this same time period. The Baltimore Eye Study found a high myopia rate (SE< −6.0 D) of 1.8% in White persons21 while in Olmsted County, using the same definition and time-frame, we found a rate of 2.3%. Overall prevalence in the Baltimore Eye Study had a higher Black population than in Olmsted County during the 1980s. The NHANES data represent the sole U.S. population based, multi-decade study prior to 2000 that compared the increasing myopia prevalence between 1971–1972 and 1999–2004 (25% to 42%).6 During a similar timespan, the Olmsted County myopia prevalence rate increased from 34% to 54% (1970s to 2000s). NHANES defined myopia with a SE<0.0 D and included younger subjects 12 to 54-years-old.6 We limited our analysis to adults since myopia tends to stabilize in adulthood. Furthermore, NHANES reported a larger proportion of Black subjects as compared to Olmsted County. Unlike the NHANES study, we elected to use a modified myopia algorithm that included manifest refractions when available. In the 2000s, we found that 21% of subjects who were non-myopic (based on VA 20/20 sans correction) also obtained a manifest refraction within the decade. Of these subjects, the majority (74%) had myopia SE (≤ −0.5 D). Assuming 21% of subjects in the NHANES study who had a visual acuity of 20/20 sans correction were also myopic, then the NHANES study may have underestimated myopia prevalence by up to 2.5% (1970’s) and 2.8% (2000’s).
Other observed racial variations include that Black subjects in Olmsted County (2000;s), had a lower myopia prevalence when compared to White subjects (41% vs. 53%, respectively), similar to data from NHANES data (34% vs. 43%, respectively),6 and this trend has been documented in other populations.8,21,22 In Olmsted County, the prevalence of myopia/high myopia (2010s) in Asian subjects was 68% and 16%; respectively, lower than East Asian studies also in the 2010’s.3–5,23–25 Differences in Asian subjects is likely environment-realted as studies have shown myopic progression were less for East Asians in Australia versus East Asia.26
The etiology for an increase in myopia and high myopia prevalence may be multifactorial. Factors such as increased education exposure, reduced time outdoors, and degree of urbanization have been implicated as environmental risk factors for myopia development and progression, especially in East Asian populations.26,27 Other important risk factors include parental history of high myopia, which is one of the strongest predictors of myopia progression in children.26 Socioeconomic factors in Olmsted County have changed from 1966 to 2019 with an increase in education demands, a shift from an agrarian/industrial economy to a technical (computer science industry) and healthcare, service-based economy that may also explain the increasing myopia trend locally.28
Myopia and high myopia prevalence rates in both males and females increased in Olmsted County from 1966 to 2019. Many studies report higher myopia and high myopia prevalence rates in females compared to males.6,22 We only found significant differences in female versus males during the 2000’s and 2010’s decades. While this may represent statistical variation, societal changes in educational and professional-related visual demands between the 1960’s and 2000’s may also influence sex-dependent myopia. A societal trend that shifted the balance during the 1980’s from male to majority female college-educated adults has developed.29 This trend coincides with increasing female myopia prevalence in Olmsted County during the past two decades. While labor statistics support a transition in the U.S. labor force from an industrial/agrarian to a service/technology-based, college educated workforce, this trend was initially noted in male subjects and only more recently by females.28 Similar factors may also explain the increasing myopia prevalence in Black subjects from the 2000’s to 2010’s.30
Olmsted County’s increase in both myopia and high myopia prevalence adds to the growing body of evidence that the myopia epidemic in affecting the U.S.6,8 Holden et al. estimated the 2020 rate of myopia in North America at 42%, similar to NHANES estimate in 2004 at 42%, both lower than our data at 57% in Olmsted County.6,8 Our data predicts a myopia prevalence rate of 72% in 2050.
Myopia prevention is key to address the myopia epidemic. By intervening during childhood, perhaps these trends could be reduced or stopped. Proposed therapies include increased time outdoors, optical devices, pharmacologic intervention (e.g. low-dose atropine), and possibly orthokeratology.31,32 Increased daily outdoor playtime for children has been shown to reduce myopia.19,33–35 A study from Taiwan reported myopia incidence reduction from 17% to 8% with at least 80 minutes of outdoor time in 6–7 year old children.36 The ATOM-1, ATOM-2, and LAMP studies reduced myopia progression using low dose atropine eye drops.37–39 Finally, orthokeratology has demonstrated both a reduction in myopia as well as reduced axial length using specially designed contact lenses to reshape the cornea.19 Both the ROMIO study and HM-PRO study demonstrated reduced axial elongation using orthokeratology, particularly helpful in children with progressive myopia.40,41 Further research is needed to explore the risks and benefits of orthokeratology interventions due to some reports of increasing risks of infectious keratitis associated with overnight lens wear.42
Strengths of this study include the random sampling from a large, stable population-based database in a well-defined community from a US population in Southeast Minnesota. This strategy reduces bias and increases the accuracy of the myopia prevalence estimation in the county. Olmsted County, Minnesota has high participation rates in pre-employment health screens that include a visual acuity assessment and has been supported by most local businesses during the six decades reported. Study limitations included: 1) a predominantly White population, 2) selection bias resulting from data obtained from those individuals who seek health care options and 3) an increase in private optical shops from 2005 to present and thus data would not be included. Thus, errors of omission may occur from individuals who 1) seek vision care outside the REP, 2) have insurance coverage changes, or 3) use alternative technological (online refractions). A predominantly White population may limit the generalizability of the results to other studies with larger minority populations and the study’s ability to derive trends about non-white groups. This is particularly important as the study population does not necessarily represent the racial and ethnic diversity of the United States. Moreover, this study lacks the statistical power to assess potential disparities in the impact of myopia among Asian Americans and other ethnic groups who may disproportionally experience the impact of myopia. Selection bias and errors of omission could serve as sources of bias in our estimated prevalence estimates.
In conclusion, our findings demonstrate an increasing myopia and high myopia prevalence in Olmsted County, Minnesota over the past six decades. Estimated myopia rates in this representative U.S. population were higher than those previously predicted in other North American studies and raises concern for the epidemic progressing in the U.S.8 Furthermore, since the rate of myopia appears to be increasing faster than anticipated, more urgent prevention strategies are needed, particularly in younger individuals. We also need to consider the increasing socioeconomic costs associated with both the refractive management demands as well as the healthcare costs, especially associated with high myopia. Healthcare policy decisions directed toward prevention seem logical, rationale, and we speculate that these would be highly cost-effective. Policy delays would lead to progressive acceleration of myopia. A proactive and organized strategy is critical for developing effective local, regional, and national strategies to reduce myopia in the U.S.
Table 6.
Spherical Equivalent Stratified by Race and Decade
| Year | Race | Mean (D) | Standard Deviation (D) | Median (D) | Minimum (D) | Maximum (D) |
|---|---|---|---|---|---|---|
| 1990s | Asian | −3.19 | 3.14 | −2.75 | −11.5 | 1.625 |
| Black | −0.70 | 1.55 | −0.125 | −3.125 | 1 | |
| White | −1.58 | 2.73 | −1 | −14.375 | 13 | |
| 2000s | Asian | −3.04 | 3.32 | −2.25 | −24.625 | 5.125 |
| Black | −0.98 | 2.44 | −0.5 | −19.5 | 10.5 | |
| White | −1.83 | 2.93 | −1.25 | −30 | 38.5 | |
| 2010s | Asian | −2.92 | 3.45 | −2 | −24.75 | 6.75 |
| Black | −1.18 | 2.68 | −0.5 | −21.25 | 12 | |
| White | −1.81 | 2.94 | −1.125 | −33 | 15.5 |
Acknowledgements
a. Financial Support: 1) Mayo Clinic, Department of Ophthalmology, 200 First Street SW, Rochester, MN 55905, 2) Partial support and travel provided by the VitreoRetinal Surgery Foundation. 3601 W 76th Street, Suite 300 Edina, MN 55435. VitreoRetinal Surgery (VRS) Foundation Research Award. The sponsors/funding organization had no role in the design or conduct of this research. 3) National Institutes of Health through National Institute of Aging R33 AG0587386
Footnotes
b. Conflict of Interest: No direct conflicting relationship exists for any author. Dr. Olsen has equity in iMacular Regeneration, LLC with no conflict of interest related to data presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spillmann L. Stopping the rise of myopia in Asia. Graefes Arch Clin Exp Ophthalmol. May 2020;258(5):943–959. doi: 10.1007/s00417-019-04555-0 [DOI] [PubMed] [Google Scholar]
- 2.Lin LL, Shih YF, Hsiao CK, Chen CJ. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acad Med Singap. Jan 2004;33(1):27–33. [PubMed] [Google Scholar]
- 3.Chen M, Wu A, Zhang L, et al. The increasing prevalence of myopia and high myopia among high school students in Fenghua city, eastern China: a 15-year population-based survey. BMC Ophthalmol. Jul 3 2018;18(1):159. doi: 10.1186/s12886-018-0829-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh V, Yang A, Saw SM, et al. Differences in prevalence of refractive errors in young Asian males in Singapore between 1996–1997 and 2009–2010. Ophthalmic Epidemiol. Aug 2014;21(4):247–55. doi: 10.3109/09286586.2014.928824 [DOI] [PubMed] [Google Scholar]
- 5.Tsai TH, Liu YL, Ma IH, et al. Evolution of the Prevalence of Myopia among Taiwanese Schoolchildren: A Review of Survey Data from 1983 through 2017. Ophthalmology. Feb 2021;128(2):290–301. doi: 10.1016/j.ophtha.2020.07.017 [DOI] [PubMed] [Google Scholar]
- 6.Vitale S, Sperduto RD, Ferris FL, 3rd. Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. Dec 2009;127(12):1632–9. doi: 10.1001/archophthalmol.2009.303 [DOI] [PubMed] [Google Scholar]
- 7.Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. Jan 2014;157(1):9–25 e12. doi: 10.1016/j.ajo.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 8.Holden BA, Fricke TR, Wilson DA, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. May 2016;123(5):1036–42. doi: 10.1016/j.ophtha.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 9.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. Oct 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54 [DOI] [PubMed] [Google Scholar]
- 10.Melton LJ 3rd. History of the Rochester Epidemiology Project. Mayo Clin Proc. Mar 1996;71(3):266–74. doi: 10.4065/71.3.266 [DOI] [PubMed] [Google Scholar]
- 11.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. Dec 2012;41(6):1614–24. doi: 10.1093/ije/dys195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai P, Barkmeier AJ, Hodge DO, Mohney BG. Ocular Sequelae in a Population-Based Cohort of Youth Diagnosed With Diabetes During a 50-Year Period. JAMA Ophthalmol. Jan 1 2022;140(1):51–57. doi: 10.1001/jamaophthalmol.2021.5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flitcroft DI, He M, Jonas JB, et al. IMI - Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Invest Ophthalmol Vis Sci. Feb 28 2019;60(3):M20–M30. doi: 10.1167/iovs.18-25957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute WHO-BHV. The impact of Myopia. 2016. The Impact of Myopia and High Myopia Report of the Joint World Health Organization–Brien Holden Vision Institute Global Scientific Meeting on Myopia. https://www.visionuk.org.uk/download/WHO_Report_Myopia_2016.pdf
- 15.Project RE. Olmsted County comapred to Upper Midwest and Total US (1970–2020 US Decennial Census Data). https://rochesterproject.org/wp-content/uploads/2023/03/Tables-for-website_olmsted_vs_midwest_vs_us-populations.pdf [Google Scholar]
- 16.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. Feb 2012;87(2):151–60. doi: 10.1016/j.mayocp.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory I. Ostrow MD LKC. Myopia. American Academy of Ophthalmology. Accessed 9/27/2021, 2021. https://eyewiki.aao.org/Myopia#Classification [Google Scholar]
- 18.Ophthalmology AAo. Clinical Optics. American Academy of Ophthalmology; 2020. [Google Scholar]
- 19.Jonas JB, Ang M, Cho P, et al. IMI Prevention of Myopia and Its Progression. Invest Ophthalmol Vis Sci. Apr 28 2021;62(5):6. doi: 10.1167/iovs.62.5.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Klein BE, Klein R, Moss SE. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. Dec 1994;35(13):4344–7. [PubMed] [Google Scholar]
- 21.Katz J, Tielsch JM, Sommer A. Prevalence and risk factors for refractive errors in an adult inner city population. Invest Ophthalmol Vis Sci. Feb 1997;38(2):334–40. [PubMed] [Google Scholar]
- 22.Pan CW, Klein BE, Cotch MF, et al. Racial variations in the prevalence of refractive errors in the United States: the multi-ethnic study of atherosclerosis. Am J Ophthalmol. Jun 2013;155(6):1129–1138 e1. doi: 10.1016/j.ajo.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Zhou J, Zhao P, et al. High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai. Invest Ophthalmol Vis Sci. Nov 1 2012;53(12):7504–9. doi: 10.1167/iovs.11-8343 [DOI] [PubMed] [Google Scholar]
- 24.Jung SK, Lee JH, Kakizaki H, Jee D. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in seoul, South Korea. Invest Ophthalmol Vis Sci. Aug 15 2012;53(9):5579–83. doi: 10.1167/iovs.12-10106 [DOI] [PubMed] [Google Scholar]
- 25.Lee YY, Lo CT, Sheu SJ, Lin JL. What factors are associated with myopia in young adults? A survey study in Taiwan Military Conscripts. Invest Ophthalmol Vis Sci. Feb 5 2013;54(2):1026–33. doi: 10.1167/iovs.12-10480 [DOI] [PubMed] [Google Scholar]
- 26.Modjtahedi BS, Abbott RL, Fong DS, Lum F, Tan D, Task Force on M. Reducing the Global Burden of Myopia by Delaying the Onset of Myopia and Reducing Myopic Progression in Children: The Academy’s Task Force on Myopia. Ophthalmology. Jun 2021;128(6):816–826. doi: 10.1016/j.ophtha.2020.10.040 [DOI] [PubMed] [Google Scholar]
- 27.Morgan IG, Wu PC, Ostrin LA, et al. IMI Risk Factors for Myopia. Invest Ophthalmol Vis Sci. Apr 28 2021;62(5):3. doi: 10.1167/iovs.62.5.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toossi M. A century of change: the U.S. labor force, 1950–2050. 2002. Monthly Labor Review. https://www.bls.gov/opub/mlr/2002/05/art2full.pdf [Google Scholar]
- 29.Fry R. U.S. women near milestone in the college-educated labor force. Pew Research Center. 2021. https://www.pewresearch.org/fact-tank/2019/06/20/u-s-women-near-milestone-in-the-college-educated-labor-force/ [Google Scholar]
- 30.Synder TD. 120 Years of American Education: A Statistical Portrait. 1993. https://nces.ed.gov/pubs93/93442.pdf [Google Scholar]
- 31.Walline JJ, Lindsley KB, Vedula SS, et al. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. Jan 13 2020;1(1):Cd004916. doi: 10.1002/14651858.CD004916.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mak CY, Yam JC, Chen LJ, Lee SM, Young AL. Epidemiology of myopia and prevention of myopia progression in children in East Asia: a review. Hong Kong Med J. Dec 2018;24(6):602–609. doi: 10.12809/hkmj187513 [DOI] [PubMed] [Google Scholar]
- 33.Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. Aug 2007;48(8):3524–32. doi: 10.1167/iovs.06-1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose KA, Morgan IG, Smith W, Burlutsky G, Mitchell P, Saw SM. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol. Apr 2008;126(4):527–30. doi: 10.1001/archopht.126.4.527 [DOI] [PubMed] [Google Scholar]
- 35.Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. Aug 2008;115(8):1279–85. doi: 10.1016/j.ophtha.2007.12.019 [DOI] [PubMed] [Google Scholar]
- 36.Wu PC, Chen CT, Lin KK, et al. Myopia Prevention and Outdoor Light Intensity in a School-Based Cluster Randomized Trial. Ophthalmology. Aug 2018;125(8):1239–1250. doi: 10.1016/j.ophtha.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 37.Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology. Jan 2019;126(1):113–124. doi: 10.1016/j.ophtha.2018.05.029 [DOI] [PubMed] [Google Scholar]
- 38.Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. Feb 2014;157(2):451–457 e1. doi: 10.1016/j.ajo.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 39.Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. Dec 2006;113(12):2285–91. doi: 10.1016/j.ophtha.2006.05.062 [DOI] [PubMed] [Google Scholar]
- 40.Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. Oct 11 2012;53(11):7077–85. doi: 10.1167/iovs.12-10565 [DOI] [PubMed] [Google Scholar]
- 41.Charm J, Cho P. High myopia-partial reduction ortho-k: a 2-year randomized study. Optom Vis Sci. Jun 2013;90(6):530–9. doi: 10.1097/OPX.0b013e318293657d [DOI] [PubMed] [Google Scholar]
- 42.Bullimore MA, Ritchey ER, Shah S, Leveziel N, Bourne RRA, Flitcroft DI. The Risks and Benefits of Myopia Control. Ophthalmology. Nov 2021;128(11):1561–1579. doi: 10.1016/j.ophtha.2021.04.032 [DOI] [PubMed] [Google Scholar]