Abstract
Similar to other brain regions, the neurons in the lateral septum (LS) are of heterogeneous populations. However, their resting membrane potential (RMP) on average is not too far apart. Cells were characterized based on biological markers by using brain slices, as under these in vitro conditions, neurons retain their morphologies. Since the LS neurons are not spontaneously excitable at RMP, the action potentials (APs) were evoked via injections of currents of moderate magnitude during the patch-clamp recordings. In coronal brain slices of rats, a smaller portion of neurons generated a train of APs of complex nature. In order to define the types of neurons with similar phenotypes, we subsequently used the four lines of td-Tomato transgenic mice. The brains of these mice express the promoter fluorophore td-Tomato and enhanced green fluorescent protein (eGFP). Therefore, recordings were conducted in a targeted manner in neurons expressing glutamic acid decarboxylase (GAD), parvalbumin (PV), somatostatin (SOM), or vasoactive intestinal polypeptide (VIP). Similar spike phenotypes that we refer to as type III, in order to distinguish from AP in principal cells – type I and those in interneurons – type II, also exist in mice, substantiating a similitude among rodents. The type III AP is selectively triggered by Ca2+ in GAD and SOM-positive neurons. Conclusions are supported by established pharmacologic tools, nimodipine, TTX, and ZD7288, a selective HCN channel antagonist. Collectively, these observations revitalize our knowledge from pioneering studies with regard to the brain of mammals in general and septal structures in particular.
Keywords: Action potential, ADP, AHP, Double spiking, HCN channels, Spike
…firing may signal something not directly related to overt behavior, such as drive state, or some idea the rat has…
Introduction
Despite classical and pioneer studies and its connexion to the hippocampus – the major hub for memory – the septal structure of the brain has received sparse attention in recent decades (Carette, 1999; Freund and Antal, 1988; Joëls et al., 1987; López-Barneo et al., 1985; Racine et al., 1983; Stevens et al., 1984; van den Hooff et al., 1989; Vinogradova and Brazhnik, 1977). Although the hippocampus is the main comparator, the lateral septum (LS) is a crucial transmitter of information to other nuclei (Vinogradova, 2001). In response to 10 Hz sinusoidal photostimulation, patterns of local field potential in the cornu ammonis (CA1) area of the hippocampus and septum are identical (Bondar and Shubina, 2018). The septum is involved in learning and fear memory (Cisse et al., 2008; Habib and Dringenberg, 2009). The LS, via oxytocin, may modulate social fear conditioning (Zoicas et al., 2014). Importantly, the medial septum (MS), as a crucial element of a bigger neuronal network, enables spatial working memory with the required precision (Gemzik et al., 2021). Thus, the theta rhythms are important for memory and spatial processing, which are paced by the MS. There is a clear relationship not only between the MS but also the LS and θ-rhythm occurring in the CA1 area of the hippocampus (Pedemonte et al., 1998). Moreover, the θ-rhythm correlates with the oscillatory generation of spikes in some neurons of the LS. The LS is a key part of the reciprocal circuit between the medial septum and the hippocampus. Also, a crosstalk to the hypothalamus could be crucial since the LS neurons express type β estrogen receptors important for behaviors underlying the social anxiety and interest (Hasunuma et al., 2024).
The septal structure, or septum lucidum (Paget, 1846), in addition to LS and MS, also includes the dBB, diagonal band of Broca (Chédotal et al., 1994). Within the septum in general and in LS in particular, a diverse population of neurons is present, which are distinguishable based on ionic current, electrical, and synaptic properties (Armstrong and MacVicar, 2001). The hyperpolarization-activated cyclic nucleotide-gated non-selective cation (HCN, Ih, or If) channels may regulate neuronal excitability and contribute to CNS functions. The HCN channels are present in a subgroup of LS neurons, and nicotine exhibits dual effects on their excitability (Kodirov et al., 2016). They produce recurrent rhythmic activities in select types of cells, and the HCN is referred to as a ‘pace-maker’ channel (DiFrancesco and Ohba, 1978; Kodirov et al., 2014; Kodirov, 2022; Noma and Irisawa, 1976).
The HCN channels mediate the sag in membrane potential (MP). The sag and activation of underlying channels were also described in septal neurons since the ‘hyperpolarizing voltage jumps generated time-dependent current relaxations associated with a corresponding increase in conductance’ (Griffith, 1988). This study also documented a typical activation of channels upon hyperpolarization and subsequent inward tail currents (Kodirov et al., 2014), which are correlates of HCN-mediated sag and rebound tail potential (RTP). The presence of fast after-hyperpolarizing potential (fAHP) in MS/dBB was associated with the presence of sag in MP, while that of slow AHP was correlated with its absence (Griffith, 1988). The afterhyperpolarizing potential is also present in LS neurons (Joëls et al., 1987).
The main objective of this study was the peculiar features of spikes in LS. Targeted patch-clamp analyses helped to classify four major types of neurons, which was not previously performed in one setting. This is important since also recording conditions may contribute to heterogeneity in spike patterns and properties. The main knowledge gap addressed by the study was identifying specific neuronal populations that generate novel type III AP. The importance of discovering type III AP is its generation concurrently with Na+ spike and persistence after blockade of the latter, which may considerably advance the septal research.
Materials and methods
All handling and procedures related to experimental animals were conducted according to the guidelines of the local institutional committees in Poland and the USA. In this study, we have indeed reduced the number of animals by sharing among projects, as the other two parallel studies targeted the central amygdala and barrel cortex. Therefore, often the required slices were obtained from a single brain sequentially. In the case of coronal slices, cuttings were started first at Bregma 1.18 mm for septal preparations, then for the amygdala at Bregma − 0.94 mm.
Rodents
Mice and rats were housed with littermates of the same sex and kept under the most appropriate conditions, with food and water available ad libitum. The rooms were airconditioned, and the range of temperature was about 22–24 °C, while the humidity was 40–70 %. The number of animals did not exceed six in their home cage. This is important since rats grow significantly in body size in contrast to mice. Since rats were used gradually, one by one, the remaining animals were comfortable with the space in their home cage. They were exposed to a simulated diurnal cycle and have also received environmental enrichments to explore and play. Shelters in the form of small paper boxes within the home cages were included to hide and sleep in, though they were not always used appropriately by some animals. Only a single mouse or rat per day was subjected to preparation. We did our best to avoid the situation when the last solitary animal in a cage was alone for more than 24 h. Although in a room and in neighboring cages, other animals were always present. Before the dissection procedure, the animals underwent deep anesthesia with isoflurane.
Transgenic mice
In order to delineate the identity of neurons and features of type III AP, we made use of four lines of transgenic mice. The neurons in the CNS of these mice express a promoter fluorophore, td-Tomato, and enhanced green fluorescent protein (eGFP), targeted to respective mRNAs coding for glutamic acid decorboxylase 65, parvalbumin, somatostatin, or vasoactive intestinal polypeptide. The GAD, PV, and SOM neuropeptides represent primarily GABA-ergic neurons, while the VIP is present in cholin-ergic cells throughout the CNS of mammals (Chédotal et al., 1994).
The Jackson Laboratory provided the PV, SOM, and VIP transgenic mice (TG) mice with Cre-lox-dependent expression, which were later crossbred with the Ai14 line in the Nencki Institute’s animal facility. The Ai14 mouse crossbreeding results in Cre-lox-dependent expression of tdTomato in neurons with defined markers. Namely, in double heterozygous mice, e.g., the SOM interneurons express tdTomato that is inserted into the Gt(ROSA)26Sor locus. Since the breeding pairs were homozygotes, the offspring were not genotyped. The GAD65 TG animals were obtained by crossing heterozygous mice together. Since the offspring littermates were either wild-type or transgenic, they were genotyped using dedicated primers.
In regard to earlier generations of PV-Cre mice and depending on the brain regions, the specificity of the marker is approximately 90 %. Later, it decreases, although in some mice, the presence of PV in the CNS is still reliably specific. The non-specificity is not a crucial issue since, e.g., in the cerebral cortex, most PV+ cells are fast-spiking and can be substantiated by electrophysiology. The unspecific expression may be global, and then the whole brain glows with a Cre-dependent fluorescent marker. The proportion of unspecific Cre expression is sometimes minor and appears as glowing patches or columns throughout all cortical layers. There is information from the provider that parvalbumin is expressed in sperm, and when male PV-Cre mice are used, it may result in unwanted germline and global recombination.
Slice preparation
The brain of a rodent was sliced at either coronal (Fig. 1), conventional sagittal, or modified sagittal planes with a vibrotome, and their thicknesses were adjusted to 250, 300, and 450 μm (Kodirov et al., 2010; Kodirov et al., 2021; Psyrakis et al., 2024). Submerged in artificial cerebrospinal fluid (ACSF), we have made a coronal cut through the most ventral part of the hippocampus in order to exclude the cerebellum. At this stage, one also checks whether or not the angle is close to 90°. That is important for comparisons of coronal slices with those in the brain atlas (Paxinos and Franklin, 2001). Only then have we glued the brain on this side to the stage of the vibrotome and mounted it in its special metal chamber, which was cooled down in advance. Immediately after, the chamber was slowly filled with cold ACSF, which often contained ice slashes. The ASCF was based on (in mM) 119 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgSO4, 2.5 CaCl2, 26 NaHCO3, and 10 glucose and was equilibrated with 95 % O2 and 5 % CO2. Also, the vibrotome chamber containing the brain was always continually equilibrated in the same manner during the course of sectioning. Next, we started to slice the brain at the beginning of the septum at Bregma 1.18 mm.
Fig. 1. Coronal slice and morphology of septal neuron.
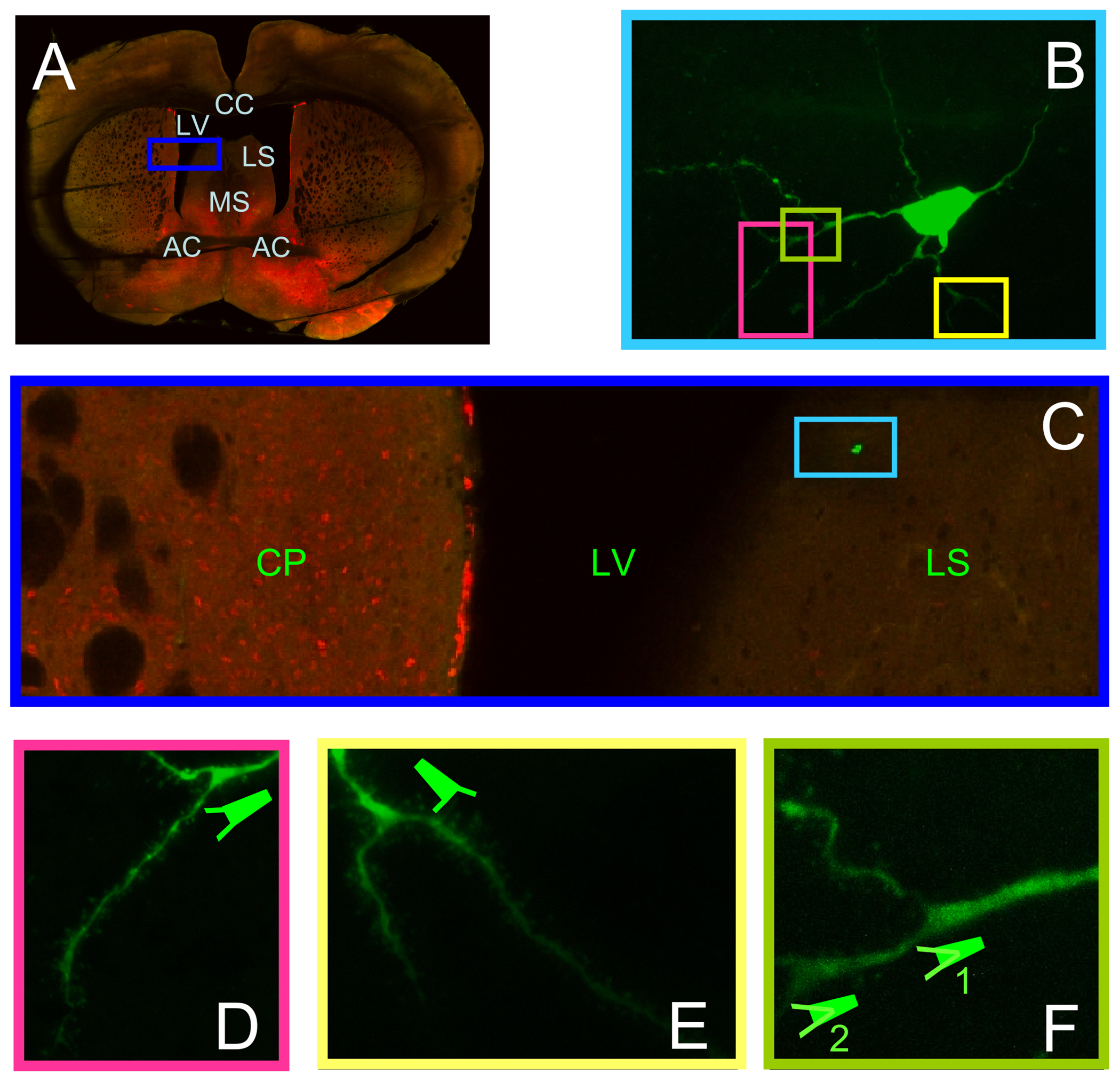
(A) Neurons were subjected to recordings in intact LS within the slice closely corresponding to Bregma 0.26 mm (Paxinos and Franklin, 2001). AC – anterior commissure, CC – corpus callossum, CP – caudate putamen, LS – lateral septum, LV – lateral ventricle, MS – medial septum. Note the horizontal traces left by the harp during recordings and detachment of LS from CC at some stage of imaging. (B) Lucifer yellow loaded cell reveals that its terminals were not cut during slicing. Clear staining occurred because of longer recordings and evoking trains of APs. (C) The location of this particular neuron in LS. This dorsal proximity was the recording site for majority of cells. (D – F) Part of B at higher magnification revealing dendrites and spines. Bifurcation of dendrites within the tree sometimes occurred with repeated patterns. Both bifurcation 1 and 2 in F substantiate that they occurred after thickening of dendrite and outbound from the main branch.
Coronal and conventional sagittal slices were maintained at room temperature immediately after cutting. The ACSF in the maintaining chamber was equilibrated with 95 % O2 and 5 % CO2 and adjusted to room temperature in advance. Thus, the isolation of the rat brain, its slicing, and maintenance were achieved in the latter solution, as CO2 facilitates O2 consumption (Lockwood, 1961). The ACSF can also be used for slicing the hippocampus, the most sensitive area of the CNS, during studies addressing interneurons and pyramidal cells (Bonni et al., 2008). However, modified sagittal brain slices of mice were first transferred to maintenance chamber containing ACSF equilibrated with 95 % O2 and 5 % CO2 at 30 °C. After 10 min of transfer, the heater was turned off so that the ACSF gradually cooled down to room temperature.
For GAD TG mice, we have used the NMDG-based slicing solution comprising (in mM) 135 NMDG, 1 KCl, 1.2 KH2PO4, 1.5 MgCl2, 0.5 CaCl2, 20 choline HCO3, and 10 glucose. The pH of dissolved NMDG is basic and may range up to 8.4 (Perrier and Hounsgaard, 1999). Therefore, first the pH of 7.4 for NMDG alone was equilibrated with 37 % HCl. Identical pH in a similar NMDG-containing solution is achieved by 8.5 mM HCl (Storozhevykh et al., 1998). It is known that the NMDG changes the electrical properties of cells and augments the glutamate-mediated Ca2+ transients (Khodorov et al., 1999; Perrier and Hounsgaard, 1999; Storozhevykh et al., 1998). However, we have used it in a cold and ice-slash form, so its effects are minimal. Besides, the cutting process was fast, and, thereby, the exposure time to NMDG was short since only 3 or 4 coronal slices were made.
Electrophysiology
Patch-clamp experiments were carried out in both current- and voltage-clamp modes. We have used the whole-cell configuration (Hamill et al., 1981; Kodirov et al., 2016; Kodirov et al., 2021; Kodirov, 2023a). Experiments were performed either at room temperature (~23 °C) or close to the bodily temperature of mammals (~32 °C). Also, in this study, we have focused on neurons within the dorsal part of the lateral septum adjacent to the ventricles (Fig. 1A and 2C). The forebrain slices contained the anterior commissure (AC), medial septum (MS), and nucleus of the diagonal band of Broca (nDBB).
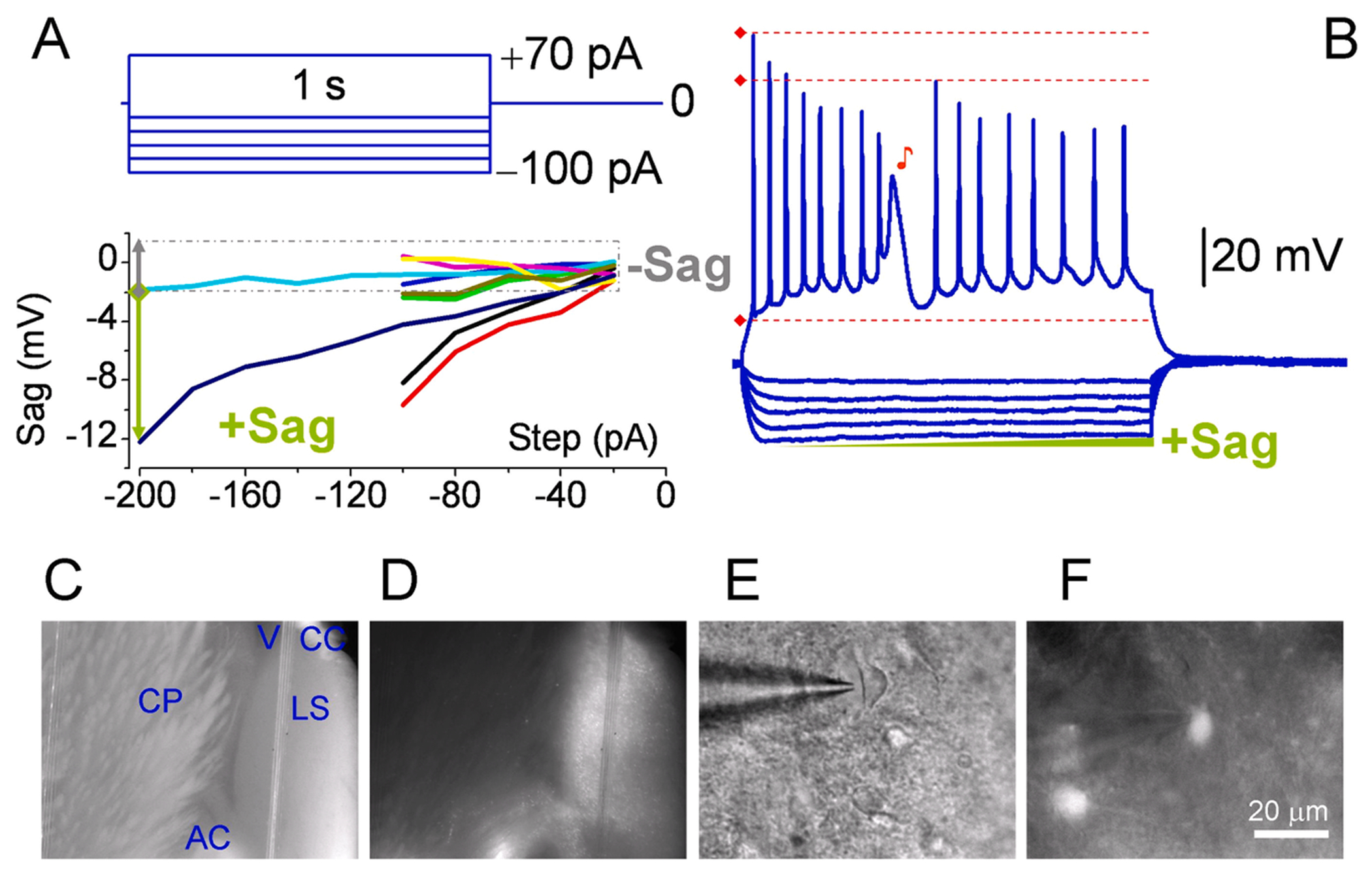
Fig. 2. Development of type III AP in GABA-ergic neurons.

(A) Using similar protocol and identical conditions MP properties were assessed in GAD expressing neurons in LS of td-Tomato mice. Significant sag was present in 4 out of 10 cells with mean values of 3.1 ± 0.5 mV. In 2 cells the MP slightly increased (not shown). However, the input resistance was not apart (288 ± 30 MΩ, n = 10). (B) Generation of type III AP is independent from temperature. Recording at 33.4 °C. The levels of fAHP and sAHP are indicated by dashed blue lines, while their peak by horizontal and vertical arrows, respectively. (C – F) Corresponding coronal slice and neuron. The RMP was – 58 mV. The absence of sag upon mild hyperpolarization correlated with that of RTP. Two type III APs ♪ were present during 1 s. Although the 1st type III AP was not as strong as 2nd one, nevertheless it significantly repolarized the MP and increased the subsequent Na+ spike’s amplitudes. Of note, the firing rates are slower after type III APs as compared to initial train of spikes.
In four lines of TG mice, we mainly targeted neurons expressing the fluorescent marker. The mean access and electrode resistance were 16 ± 1 and 3.1 ± 0.1 MΩ (n = 18) during experiments with rat LS neurons. Series resistance was not compensated for. The input resistance (Rin) was defined either by the recording software (Psyrakis et al., 2024) or was estimated in response to hyperpolarizing currents of − 40 pA (262 ± 17 MΩ, n = 19).
Brain slices of PV, SOM, and VIP TG mice were especially viable for longer, as several stepwise procedures were meticulously followed. The long-term maintenance was facilitated by using a voluminous closed chamber horizontally separated by a mash net structure. The quadratic chamber had four sources of inflow for 95 % O2 ± 5 % CO2 distributed through a small syringe needle. The recovery period was enabled at 30 °C with a higher concentration of MgSO4 (adjusted to 2 vs. 1 mM of ACSF). The external solution was identical to the ACSF as above, which was used for the preparation of brain slices in rats. The pipette solution contained (in mM) either 120 K+–gluconate, 5 NaCl, 1 MgCl2, 10 HEPES, 0.2 EGTA, 2 Mg2ATP, 0.2 NaGTP, or 125 K+–gluconate, 2 KCl, 10 HEPES, 0.5 EGTA, 4 Mg2ATP, 0.3 NaGTP (pH = 7.2 – 7.3 and 290 mOsm). Nimodipine and the majority of agents were obtained from Sigma. The final concentration of nimodipine may vary as it was applied by manual pipetting into the ACSF in a recording chamber that was constantly perfused at a rate of 1.2 ml ·min.
Depending on the age of the rodents or the type of neurons and their Rin, action potentials (AP) were either recorded at or triggered from resting membrane potential (RMP) by incremental injections of current of up to 380 pA. The average and fitted data are paired with the SEM and SD, respectively. Comparisons were made with a student’s t test, and P and n values are indicated.
Results
For imaging, we have used a whole septum of mouse brains in a coronal plane (Fig. 1A). In order to document the location of Lucifer Yellow-labeled cells (Fig. 1B), slices were subjected to two-photon laser scanning microscopy (2PLSM). Same morphology of neurons was also often observed in rats, consistent with those in pioneer studies (Kodirov et al., 2016; Twery et al., 1992). For these analyses, only a single neuron within the LS was subjected to a patch-clamp recording (Fig. 1B). The staining substantiates that all dendrites were intact, and one can appreciate their morphologies (Fig. 1d–f). The LS, especially the LSD, is one of the few relatively intact nuclei of rodents separated by the corpus callossum (CC) and lateral ventricles (LV).
Since we first observed an unusual type of AP in the subpopulation of cells in the LS of rats, we have aimed to explore whether or not the septal neurons in mice exhibit similar excitability. Comparable APs were also present in mice (Figs. 2–5 and Table 1). Next, we elucidated the cell specificity of type III AP, mechanisms, and correlation with the presence of HCN-mediated sag in transgenic mice with four different neuronal markers: GAD, PV, SOM, and VIP.
Fig. 5. Solitary cholinergic vasoactive intestinal polypeptide – VIP – expressing neurons in td-Tomato mice.

(A) Rheobase for neuron was defined by a sequential incremental 1 pA steps. (B) Since the IR was 1.3 GΩ the MP in the same neuron was hyperpolarized by steps only up to – 50 pA. Note the stable RMP at – 65 mV despite current steps applied every 3 s. (C) In these mice there are only few VIP+ cells in LS of one hemisphere. In this slice they were six and the location of cell 7th could not be determined with certainty in LS or CP. However, within the small area of Cx – cortex right above CC there are numerous VIP+ cells in close proximity. (D and E) The recoded neuron at different magnifications revealing dendrites. (F) Focus on patch pipette with the part of membrane inside of it after whole-cell configuration establishment.
Table 1.
Neuronal types in respective TG mice LS neurons based on markers.
| Parameters | GAD | Parvalbumin | Somatostatin | VIP |
|---|---|---|---|---|
| Input resistance (MΩ) | 287 ± 30 | 447 ± 161 | 329 ± 40 | 845 ± 217 |
| RMP (mV) | −63 ± 2 | −59 ± 3 | −58 ± 3 | −62 ± 5 |
| Rheobase (pA) | 54 ± 7 | 30 ± 6 | 26 ± 5 | 20 ± 0 |
| Overshoot (mV) | 39 ± 4 | 45 ± 8 | 44 ± 3 | 49 ± 5 |
| Hyperpolarization (mV) | −28 ± 3 | −26 ± 3 | −32 ± 4 | −38 ± 8 |
| Type III AP (n) | 6 | 0 | 4 | 0 |
| Temperature (°C) | 24 ± 0.4 | 24 ± 0.8 | 22 ± 0.2 | 23 ± 1 |
| Neuron n | 10 | 4 – 5 | 9 | 3 – 6 |
| Mice n | 8 | 2 | 8 | 5 |
| Female n | 2 | 0 | 0 | 1 |
| Age (Days) | 144 ± 15 | 31 ± 2 | 40 ± 3 | 43 ± 2 |
The overshoot is estimated during 80-pA steps for GAD+ and PV+ neurons, while by 100 and 60 pA pulses in SOM+ and VIP+ cells, respectively. The magnitude of hyperpolarization is estimated upon – 100 pA. Note differences in age spans. Based on one-way ANOVA the P < 0.01 for values in bold.
Gad65
Two isoforms of glutamic acid decorboxylase, GAD65 and GAD67, are concurrently present within the same brain nuclei (Bessières et al., 2019; Prestigio et al., 2019). We have studied the subpopulation of GABA-ergic neurons expressing GAD65 in coronal slices containing intact septal nuclei from both hemispheres (Fig. 2c). Moreover, connections to and from surrounding structures of the brain were also undisturbed (Fig. 2c–f). These types of neurons could also be subcategorized into the two groups based on the magnitude of sag with amplitude values of < 2 or > 2 mV and occurrences of type III AP (Fig. 2a and Table 2). The type III APs were triggered mostly upon relatively stronger step depolarization in GAD+ neurons (Fig. 2b), although the observed range was relatively wide and spanned from 20 to 240 pA (n = 6). In comparison, the current range in SOM+ neurons was narrow at 40–80 pA (n = 4). The reason for the observed difference is not clear since the Rin and RMP values are also comparable. Nevertheless, the main determinant could be the physiological properties, since in 2 out of 6 GAD+ neurons the rheobase current was 20 pA, while in 3 out of 4 in SOM+ ones. In these neurons, the respective rheobase current for generation of Na+ spikes was in a comparable range of 20 – 80 vs. 20 – 60 pA.
Table 2.
Comparisons among GAD+ and SOM+ neurons in regard to type III AP.
| Parameters Type III AP | GAD+ Absent | Present | SOM+ Absent | Present |
|---|---|---|---|---|
| Input resistance (MΩ) | 246 ± 47 | 315 ± 42 | 381 ± 64 | 264 ± 25 |
| RMP (mV) | −60 ± 4 | −66 ± 3 | −61 ± 2 | −55 ± 7 |
| Rheobase (pA) | 70 ± 6 | 43 ± 10 | 24 ± 4 | 30 ± 11 |
| Overshoot (mV) | 34 ± 10 | 43 ± 4 | 49 ± 2 | 37 ± 7 |
| Hyperpolarization (mV) | −24 ± 4 | −30 ± 4 | −38 ± 7 | −24 ± 1 |
| Sag present (mV) | −3.6 ± 0.2 | −2.4 ± 0.4 | −5.4 ± 1.8 | −1.6 ± 0.3 |
| Sag absent n | 1 | 3 | 1 | 2 |
| Temperature (°C) | 24 ± 0.6 | 24 ± 0.4 | 22 ± 0.3 | 22 ± 0.1 |
| Neuron n | 4 | 6 | 5 | 4 |
| Mice n | 2 | 5 | 5 | 3 |
| Female n | 1 | 1 | 0 | 0 |
| Age (Days) | 154 ± 23 | 137 ± 24 | 41 ± 5 | 39 ± 3 |
The overshoot is estimated during 80-pA pulses for GAD+ neurons, while by 100-pA steps in SOM+ cells, respectively. The magnitude of hyperpolarization and sag is estimated upon – 100 pA. Note differences in age spans. P < 0.05 for a pair of values in bold. All other differences among cells and between GAD+ SOM+ neurons are insignificant.
We have observed some degree of heterogeneity in terms of phenotypes occurring simultaneously in the same neuron. Although the two ♪ type III APs were distinct, their impacts on spike trains are comparable (Fig. 2b). The first ♪ one is not severe and comprises a vulnerability that enables a sudden switch in values of amplitude and rate within the train of spikes. That is, the amplitudes are slightly increased compared to the last spike immediately before the first type III AP. Next, there is no gradual decrease (adaptation) in the amplitudes of subsequent spikes, in contrast to that upon the onset of a pulse. The rate, in turn, has decreased. Nonetheless, the evoked intensity of 160 pA is not excessive, and the first ♪ event is similar to those occurring spontaneously during the in vivo observation (Pedemonte et al., 1998). Under these conditions, there were greater numbers of observations as to evoked type III APs in GAD+ neurons (n = 6 out of 10, Tables 1 and 2).
When the neuron is predisposed to type III AP, perhaps during the course of the protocol and gradual depolarization (priming), the intracellular Ca2+ was cumulatively increasing (Fig. 2b). The four lines of evidence may support this hypothesis. First, the depolarization of membrane potential (MP) is fast and gradual upon the onset of even moderate steps (Fig. 2b). Second, if type III AP is absent, then even a strong depolarization of 240 pA does not influence the new level of MP or lead to graded activities. Third, without priming, the type III APs extinguish gradually. The latter equally applies to the rate of depolarization, amplitude, and magnitude of slow after-hyperpolarizing potential (sAHP) of each type III AP in a row. Fourth, type III AP was always followed by effective repolarization because of its unusually high amplitude and long-lasting sAHP (Fig. 2b, ↑). In the absence of additional sources of Ca2+, the level of MP either should remain around that of the first fAHP (Fig. 2b, ↑) or equilibrate sooner. In either scenario, the AHP plays a crucial role, which typically results from the activation of Ca2+-dependent K currents (López-Barneo et al., 1985). The latter is also consistent with the idea that the more Ca2+ is available, the more Ca2+-dependent K currents are activated, resulting in a high-amplitude AHP.
Parvalbumin
Although the expression of parvalbumin (PV) in td-Tomato mice was perhaps not highly selective, we have recorded the APs and MP responses from several neurons (Fig. 3a). Also, in these cells, the magnitude of sag was heterogeneous. The sag was activated even by a – 50 pA step, and its amplitude sufficed to trigger the RTP (Fig. 3b). The firing pattern of these GABA-ergic neurons, if indeed they are, differs from those in the amygdala or cortex, even when the depolarization-dependent differences during each subsequent step are taken into account (Perrenoud et al., 2013).
Fig. 3. Properties of parvalbumin expressing neurons in LS of td-Tomato mice.

(A and B) When present, the sag could be revealed even at hyperpolarization by – 50 pA. Under these conditions, also RTP was recorded. The RMP was – 64 mV. Tables 1 – 2 are based on indicated parameters and estimation approaches. (C) Corresponding coronal LS slice with adjacent distinct nuclei. (D) The PV expression was unspecific and even non-neuronal cells on LS wall surface (arrow) within V – ventricle were fluorescent. Still some degree of specificity was present, as PV+ densities were higher close to MS compared to LSD. (E) Both recording electrode and site at higher magnification. (F and G) PV+ neuron from which the sag and spikes in B were registered. In this neuron the type III AP was absent.
In modified sagittal slices, we observed a bundle of fibers originating from the CC into the medial septum (Fig. 3c). The patch-clamped PV+ neuron was located in close proximity to CC fibers (Fig. 3e–g). Since they are numerous and perhaps unspecific, the PV+ neurons are seen even under lower optical magnification in LS and elsewhere (Fig. 3d). However, in other brain regions, PV+ cells may represent up to 51 % of the subpopulation of interneurons, while SOM+ ones make up only 26 % (Geoffroy et al., 2013). Thus, the degree of non-selective labeling might be lower than expected for PV. Of note, the PV+ cells are very sparse in the hippocampus (Ivashkina et al., 2018). The latter were abundant in the neocortex and spread homogeneously within all laminae. Interestingly, none of the undergoing the experience-dependent Cre recombination cells were interneurons, as clearly deciphered by the eGFP expression. This was evidenced based on the absence of co-localization of eGFP with neuropeptide Y (NPY), PV, or SOM.
Somatostatin
The HCN-mediated sag was also present in somatostatin-expressing neurons located in close proximity to the lateral ventricle (LV) and CC (Fig. 4a and b). A mild depolarization elicited robust spikes intermediated by a single type III AP (Fig. 4b). This example of a complex spike in a SOM+ neuron hints that perhaps the type III AP resets and enables a firing mode with a more efficacy. The latter may also occur on demand, as the amplitude of the first spike right after the type III AP is again higher. That is, the neuron is empowering itself. As suggested in a previous study (López-Barneo et al., 1985), the latter empowering effect results primarily from the AHP of the type III AP, which, by repolarizing the membrane potential during a certain period of time, removes Na+ channel inactivation and enables the generation of fast spikes of larger amplitude.
Fig. 4. GABA-ergic somatostatin expressing neurons in the LS of td-Tomato mice.
(A and B) Also in SOM+ cells the type III AP was present and this phenomenon did not correlate with the absence or presence of sag. If the MP exhibited sag then it was revealed already at mild hyperpolarization. In neurons with lower IR (range of 216 — 582 MΩ, n = 9) the sag increased upon further hyperpolarization. RMP was – 66 mV. Small sag was not sufficient to induce RTP or RAP – rebound action potentials in this neuron. Top dashed red lines indicate the peak overshoot range before and after type III AP, while at bottom the level of fAHP after very first spike. (C) Modified sagittal slice. (D) Fluorescence lamp on. Note the higher densities of SOM positive neurons around AC and LS. (E) Non-fluorescence cell upon approaching with the patch pipette under pressure. (F) SOM+ neuron which exhibited typical type III AP in B.
The subsequent new firing mode resembles a spike amplitude adaptation (SAA), though it is not as gradual as it was during the initial response. Upon the termination of hyperpolarization, the rebound action potential (RAP) was absent, which is related to a lower magnitude of sag upon a mild 100-pA step. The latter was able to hyperpolarize the neuron to – 93 mV from a RMP of – 65 mV (Fig. 4b). Under these conditions, the amplitude of the rebound tail potential (RTP) was 2 mV and the MP gradually declined to the level of the RMP during 300 ms.
Thus, the 100-pA step was indeed mild since the identical intensity in another SOM+ neuron did not lead to a RAP, although a high amplitude sag in MP was preceded (see Fig. 7a). But a robust RAP was generated upon the cessation of the 200-pA pulse (Fig. 7b). The absence or presence of RAP can be accurately manifested only when the neuron is silent and the value of RMP is adequate. If there is spontaneous background activity, then the neuron will be excited by any small changes in MP that are not related to the presence of sag per se. It is also possible that some neurons, despite the presence of sag, may not trigger the RAP even upon termination of a strong hyperpolarization by a 280-pA step.
Fig. 7. Obliteration of type III AP by nimodipine in LS.

(A) MP responses to – 100 and + 20 pA current injections in SOM+ neuron. Note the presence of sag and a transition of regular spikes into two type III APs ♪ already close to threshold intensity. Even superimposed spikelets during type III APs overshooted the ± 0 mV range as indicated with upper dashed lines for all traces. (B) Blockade of type III APs by 3 μl bolus of 10 mM nimodipine after 5 min. The arrow indicates a depolarization of RMP (lower dashed lines) that prevailingly occurred independently. The cause perhaps is the excessive protocol used for continues recordings applied every 20 s in the following experiments. (C) Second control recordings 160 s apart from A. RMP was already depolarized to – 59 mV. Consistently, two type III APs were triggered, but the latency to 1st regular spike is shortened. (D) Nimodipine effects. (E) Three modes of rate for regular spiking before and after 1st and 2nd type III APs. (F) Amplitude adaptation – gradual decrease of overshoot peaks before each type III APs. (G and H) Behavior of frequency and overshoot range after nimodipine effects. Amplitude scale identically applies for all traces, while the time is reflected by pulse protocol for A, B and C, D.
The location of LS within the modified sagittal slice could be appreciated even by illumination with room light (Fig. 4c). The latter corresponds to LS positions with neighboring nuclei in one hemisphere. Note the massive fiber system within the caudate putamen (CP) and one of the threads of the harp appearing vertically. The harp was only gently securing the slice and was not too heavy, since no deformation in the tissue is visible. We have observed a much higher density of SOM+ neurons in LS compared to CP (Fig. 4d). A typical morphology of LS SOM− neurons in close proximity to fluorescence cells is shown in Fig. 4e. Only a solitary type III AP was recorded from this SOM+ neuron (Fig. 4b and d).
The SOM+ neurons are present not only in the LS and CP but also in the hippocampus. The Rin of SOM+ neurons in LS is in agreement with that of somatostatin-containing GABAergic interneurons in the hilus of the DG, dentate gyrus (Halabisky et al., 2010). The mean values in these two regions were 329 ± 40 vs. 321 ± 25 MΩ (n = 9, Table 2). However, the hilar interneurons do not exhibit sag, at least not upon hyperpolarization by a 100-pA step. In contrast, the SOM+ interneuron of stratum oriens (SO) with axons terminating within the lacunosum moleculare (SLM) of the hippocampus develops a sag during strong hyperpolarization by a 250-pA step (Griguoli et al., 2010). Finally, the estimated Rin value is always relative and also depends on particular electrophysiology methods. In closely related LS nuclei, the MS/nDBB, the 20–200 MΩ range is observed with a high impedance sharp electrode (Griffith, 1988). However, during the patch-clamp experiments, an adequate recording is not plausible if the Rin of neurons is < 100 MΩ.
VIP
In contrast to the other three subtypes, the number of neurons containing vasoactive intestinal polypeptide (VIP) was lower in LS (Fig. 5). In this slice, six out of seven VIP+ neurons were located in LS. However, often there were only a few solitary neurons within the recoding field under the microscope at X80 magnification (1.1 ± 0.2, n = 7). The latter is indeed significantly different when compared with, e.g., SOM+ neurons (5.3 ± 1.7, n = 8, P < 0.04) in LS or frontal cortex (Kawaguchi and Kubota, 1996). However, even in cortex, there were only two true fast-spiking (FS) VIP+ interneurons. The groups of 5 and 31 non-pyramidal VIP+ cells exhibited the burst and regular spiking phenotypes (BS and RS), respectively.
In most LS neurons, for the generation of AP, the current intensity is incremented by 20 or 40 pA since their Rin is lower. Therefore, the defined intensity cannot reflect a true rheobase (Psyrakis et al., 2024). However, the defined rheobase is valid for VIP+ neurons since the increment of 1 pA is the minimal possible gradual change in the intensity of subsequent depolarization. Thus, at rheobase current intensity, when a neuron fires 1 or 2 spikes (Fig. 5a), their properties across four types, i.e., GAD, PV, SOM, and VIP, are comparable. Thereafter, some neurons fire only upon the initial portion of depolarization, and depending on the magnitude of injected currents, it may occur during the first 518 ms (Fig. 5b). Even in this case, there was a clear SAA and moderate spike firing adaptation (SFA). Although the MP remained at the threshold level of the last spike, it might be a protective mechanism for those neurons that are not pre-programmed for sustained pacemaker firings. Upon stronger depolarization by a 100-pA pulse, this VIP-expressing neuron fired during the first 76 ms (data not shown). In the septum, the VIP-expressing cells were few and were located mainly within LSD in small clusters (Fig. 5c). Only solitary VIP-expressing neurons were found in other slices containing LS nuclei. The input resistance of VIP+ neurons was high, and their RMP was more positive. Upon hyperpolarization, no significant sag was observed. Thus, the hallmark of VIP+ neurons is their high Rin consistent with those in layer VI of the cerebral cortex (Perrenoud et al., 2013). However, this assumption is valid for mean values only, as an individual Rin may overlap among all types of neurons. The mean Rin in VIP+ neurons of the LS were the highest at 845 ± 217 MΩ (n = 6, Table 1) among the four types in the LS but lower compared to those of the cerebral cortex.
Another hallmark of VIP+ neurons in LS was the relative saturation of MP responses upon hyperpolarization. This can be learned by comparing the unproportional MP responses between – 10 and – 20 pA vs. – 20 and – 30 pA pulses (Fig. 5b). In the absence of HCN channels, the MP hyperpolarization is possibly mediated by the inward rectifier (IK1 or Kir) currents alone. Thus, at milder steps, a bigger hyperpolarization was observed, while upon subsequent low intensities, the MP responses gradually decreased. This property was accompanied by MP responses in PV+ and SOM+ but not in GAD+ neurons (Fig. 3b, 4b, and 2b, respectively). The aforementioned relative saturation was similar to that of silent sympathetic neurons (Gola and Niel, 1993), which do not exhibit sag. However, instead, there is a small event that we term an anti-sag (see Fig. 7b), i.e., the MP increases gradually as one would observe for HCN during the voltage-clamp experiments. Nonetheless, in current-clamp mode, the gradual increase in amplitude of MP, the anti-sag, during a hyperpolarizing pulse seen in cells lacking H-current most probably results from the slow closing of other ion channels that are usually open at RMP, foremost of all the Kir.
In VIP+ neurons, the sag was absent (Fig. 5b), but perhaps a – 50 pA pulse is not sufficient to activate the required number of HCN channels. In one of the VIP+ neurons, the sag amplitude was – 4.8 mV. We consider it sag with confidence when the MP spontaneously depolarizes by more than 2 mV (Fig. 4a), as there are often neurotransmission-related background fluctuations in the MP that are mediated by inputs into recorded neurons.
In summary, comparisons among all groups by one-way ANOVA revealed levels of significance at P < 0.01 and F > 6 only for Rin (Table 1). Levene’s test for equal variance yielded similar P and F values. At an alpha level of 0.05, the actual power was computed to be 0.94.
Type III AP is not influenced by HCN
These experiments substantiate that type III AP is generated upon depolarization and Ih currents cannot influence it since underlying HCN channels are activated by hyperpolarization and sometimes only upon high-amplitude steps (Kodirov et al., 2014; Morris et al., 2004).
Therefore, the behavior of type III AP was evaluated in the presence of 10 μM ZD7288 (Fig. 6a). The 300-μm coronal brain slice of a rat was exposed to this selective Ih inhibitor for 39 min since the concentration used was not excessive (BoSmith et al., 1993). The sag was absent during these experiments, but the ZD7288 did not alter the phenotype or preceding sequence of the Na+ spike and spikelet. However, during the additional 5 min of exposure, the number of spikes increased to 4 vs. 3 (Fig. 6b). The overall properties of type III AP, MP, and SAA remain unaltered along the RMP. A consistent increase in MP response was observed during hyperpolarization, as indicated by the dashed red line (Fig. 6b). The latter effects occur because of an increase in the Rin of neurons and are similar to those of Cs+ (Dibattista et al., 2008). This change in Rin is significant compared to the level of hyperpolarization in the previous experiment (Fig. 6a).
Fig. 6. Type III AP is not linked to HCN channels.

(A and B) Recordings from the same LS neuron of rat after respective 39 and 44 min of exposure to 10 μM ZD 7288, a selective Ih inhibitor. MP and AP properties are not apart during the course of experiments albeit the generation of 4 instead of 3 spikes after type III AP. Note the two traces during depolarizing and hyperpolarizing current injections are not aligned, but reflect the stability of resting membrane potential at – 69.1 vs. – 69.3 mV in A. Corresponding values in B are – 68.8 vs. – 69.0 mV. Identical amplitude and time scale bars apply for both conditions. (C) Initial portion of A in an expanded amplitude and time scales. This type III AP started sooner compared to one in Figs. 2 and 3. The transition here was enabled by spikelet that was absent in former neurons. Arrow points to a peak that occurred because of minor involvement of Na+ spike (see Fig. 2). The red line indicates the threshold of – 39 mV for the first Na+ spike in a train and MP during terminal repolarization of type III AP that was more negative and reached – 42 mV.
Note that the two traces during depolarizing and hyperpolarizing current injections are not aligned but reflect the stability of RMP with values of – 69.1 vs. – 69.3 mV (Fig. 6a). Corresponding values after 5 min were – 68.8 vs. – 69.0 mV (Fig. 6b). The transition to the type III AP was enabled by a spikelet, and the arrow points to a peak that occurred because of the background Na+ spike (Fig. 6c). This contrasts with the dynamic pattern of spikes in Figs. 2 and 4. However, this change might simply reflect the fact that, as the type III AP is generated at the dendritic compartment, and in the presence of ZD7288, neurons are more electrotonically compact, the depolarization of the soma more easily changes the RMP at these distant locations, reducing the time necessary to trigger the type III AP.
Nevertheless, the phenotype of an initial Na+ spike in a train is comparable at the threshold of – 39 mV (Fig. 6c). Consistently, also in the presence of ZD7288, the type III AP was able to repolarize the MP further than the Na+ spike, i.e., to – 42 vs. – 31 mV. However, this comparison might not be precise since, because of subsequently developed after-depolarizing potential (ADP), the repolarization of the Na+ spike is incomplete. Under these conditions, there is a transition from ADP to both spikelet and spike, depending on neurons and applied current magnitudes (Kodirov et al., 2016). Thus, type III AP is neither triggered nor influenced by HCN channels.
Collectively, HCN channels are activated by hyperpolarization and may contribute to rebound action potentials only immediately upon termination of steps. Also, based on the firing thresholds, the type III AP and the rebound spikes result from different mechanisms.
Ca2+ origin of type III AP
As expected from the MP values at which the type III AP occurs, the activation of L-type Ca2+ channels could be a trigger. This hypothesis was tested by using an antagonist, nimodipine, which also modulates memory (Izquierdo, 1990). After observing robust and recurrent occurrences of two ♪ type III APs in SOM+ neuron during the 1 s ·20-pA step, nimodipine was applied (Fig. 7a and b). Contrary to the aforementioned assumption, the type III APs occurred at threshold current intensity – rheobase, substantiating that the behaviors of cells, ion channels, or synapses do not always obey the doctrine. In this SOM+ neuron, upon hyperpolarization by – 100 pA, a sag in MP was revealed (Fig. 7a). However, its amplitude was not sufficient to lead to a RTP or RAP, which occurred when a 200-pA step was tested (Fig. 7c). Paradoxically, a 3 μl bolus of 10 mM nimodipine enabled RAPs after 5 min of application, although it abolished the sag (Fig. 7b and d). That is, there could be unrelated rebound activity and RAPs to HCN. The latter are triggered as a consequence of significant spontaneous and gradual depolarization of RMP. The same mechanism perhaps also underlies shorter latencies to the first spikes upon step depolarization (red arrows, Fig. 7c and d). Under these conditions and after application of nimodipine, the neuron was able to transit into the regular spiking mode (Fig. 7e–h).
The graded activity – the sudden change in amplitude and rate of spikes during a train – can be clearly observed at stimuli close to threshold intensity. At more depolarized potentials, e.g., when achieved by a 120-pA step, the type III AP ♪ occurs in-between the two grades in SOM+ neurons (Fig. 8a). Excessive depolarization can trigger recurrent type III APs interchanged with Na+ spikes (Fig. 2). Facilitated activation of type III AP may also occur under pathological conditions when the activity of Nav channels is diminished. Since 2 vs. 1 type III APs in the presence of TTX were triggered (Fig. 8B). In SOM+ LS neurons, type III AP can be evoked recurrently during long-term recordings. Moreover, there is fidelity during the train of complex action potentials, as substantiated by the superimposed traces recorded every 20 s (Fig. 8a, n = 5). The fidelity is specifically high with regard to the frequency of spikes preceding the type III AP. The trace in blue reveals the fidelity in the generation of eight Na+ spikes before type III AP (Fig. 8A). The fidelity also applies to overshoot amplitudes.
Fig. 8. Isolation and facilitation of type III AP by TTX.

(A) Train of complex action potentials are evoked every 20 s in SOM+ LS neuron and presented superimposed (n = 5). One of trace is in blue and reveals the generation fidelity of eight Na+ spikes before type III AP that also occurs with a close proximate of timing. Since a blue trace is seen on spikes triggered after type III AP. (B) Very high concentration of TTX at 3 μM not only prevented regular spikes, but also enabled generation of additional type III APs upon identical + 120 pA current injections (n = 5). (C and D) Clear reflections of complex spikes properties based on overshoot range and rate of firings. Note the two linear states before and after the type III AP. (E) Values upon continues registration of trains of spikes every 20 s for 20 min including TTX application time. (F) The same experiments revealing resistance of type III AP to TTX albeit a decrease in amplitude. Time scale is identically reflected by the duration of pulse protocol for A and B.
We have used a very high concentration of TTX at 3 μM in order to both completely and rapidly inhibit Nav channels. The TTX not only prevented regular spikes but also enabled the generation of additional type III APs upon + 120 pA current injections (Fig. 8b, n = 5). A clear reflection of the properties of complex spikes is substantiated by distinct linear behaviors before and after the type III AP based on overshoot range and rate of firings (Fig. 8c and d). Behaviors of amplitudes and instantaneous frequencies during the 1-s train correlated. The TTX effects occurred abruptly, as it obliterated the first AP upon the onset of depolarization; the subsequent spikes were not triggered. Therefore, the recorded excitability represents a train of APs. The values in each column of Fig. 8E represent those in 8C and are acquired every 20 s for 20 min, including the TTX application time. In the same LS neuron, the type III AP persisted in the presence of TTX, albeit the decrease in amplitude.
Spontaneous type III AP
Although most LS neurons do not exhibit spontaneous APs, we have always continuously observed the RMP for 1 min. When the RMP is more positive, the neuron may trigger spontaneous spikes (Fig. 9). These comparisons assure that a step depolarization is not physiological, as only the properties of first spikes within the train closely resemble those of spontaneous APs.
Fig. 9. Spontaneously occurring type III action potentials.

(A) Recording during 1 min at RMP of – 51 mV in GAD+ neuron. (B) Portion of trace revealing transition of a regular spike * to type III action potential. Note that type III AP did not overshoot. Start of overshoot potential and RMP are indicated. (C and D) Spontaneous regular spikes (n = 4) and spikelets preceding type III APs from A (n = 6). This neuron did not exhibit ADP, but a hyperpolarization resembled a slow AHP occurring during ~ 250 ms. (E) Phenotypes of spikes and spikelets were comparable. (F) Corresponding values of parameters. Amplitude scale applies equally to all traces, while time to C and D.
In this GAD+ neuron, four regular spikes within 60 s were observed since the value of RMP was relatively depolarized (Fig. 9a). The first spike was solitary; the second was followed by four spikelets, while the third and fourth had only one. The spikelets occurred at depolarized MP, which led to the generation of type III APs (Fig. 9b). The paired train of spikes, spikelets, and the type III AP resemble those during steps (Fig. 8a). This behavior of type III AP occurring after the Na+ spike (Fig. 9b and d), could be compared with an early afterdepolarization (EAD) that may occur if K channels are not functional (Kodirov et al., 2019; Kodirov, 2023b; Kodirov et al., 2024). This supports the notion that the main trigger for the type III AP is of Ca2+ nature.
Since the spontaneous spikes and spikelets are self-generated, in contrast to those after excessive steps, their parameters and phenotypes are not too different (Fig. 9c–f). Logically, the highest degree of difference was estimated for the amplitudes, area, and duration with a comparable standard deviation (72 ± 3 vs. 63 ± 2 mV, 514 ± 40 vs. 697 ± 61 mV ·ms, and 2.9 ±0.1 vs. 4.0 ± 0.6 ms). The two-sample independent t-test yielded a P < 0.01 for each pair (n = 4 vs. 6). In this neuron, not only do the regular spikes and type III AP alternate, but there are also recurrent background oscillations in MP. A lower RMP of – 51 mV is a trigger for oscillations, which in turn are prerequisite conditions for the generation of type III AP, whether evoked or occurring spontaneously. The latter value of RMP was significantly different compared with the mean of – 63 ± 2 mV among GAD+ cells (n = 10, Table 2).
Collectively, the type III AP in some LS neurons could be triggered by a single pair of Na+ spike and spikelet (Fig. 9b). However, this mild type III AP may not participate in the generation of the θ-rhythm occurring in the CA1 area of the hippocampus since the observed oscillations are intense in vivo (Pedemonte et al., 1998). Based on the literature (Pedemonte et al., 1998; Stevens et al., 1987) and phenotype of this action potential, it can be termed type III AP in order to decipher between two major types of excitability in both principal cells (type I) and those in interneurons (type II). Neurons exhibiting type I AP are not glutamatergic, in contrast to those in the amygdala and other nuclei (Psyrakis et al., 2024), since they do not release glutamate. However, they are principal neurons since they project to, e.g., the hippocampus (Vinogradova and Brazhnik, 1979). Based on previous studies, type III AP resembles the high-threshold Ca2+-mediated spike that most probably originates in the dendrites (Llinás and Yarom, 1981).
Discussion
Initial observations with regard to uncommon type III APs were made in rat brain slices. The latter exhibit phenotypic variation, as do all known spike types, but we will present them entirely under the umbrella term of type III AP. For simplicity, the typical firing of principal cells will be regarded as type I, while that of interneurons is type II.
The type III APs have occurred in a heterogeneous subpopulation of LS neurons, and the absence or presence of sag in MP was not a factor. Furthermore, the genus was not a determinant since identical events were recorded in mice. During the final revision of this study, it was determined that some variations of the type III AP have been observed in other areas of the CNS and were termed low-threshold Ca spikes (Llinás and Yarom, 1981).
The type III AP was observed in the LS but not in every neuron. Therefore, we used TG mice to delineate a possible correlation with neuronal markers (Table 1). The type III AP was present in the GAD+ and SOM+ neurons of the corresponding TG mouse lines (Table 2). Although the mean age of GAD TG mice was significantly different, the occurrence of type III AP is not age- or sex-dependent, as substantiated by experiments (Tables 1 and 2). The underlying mechanisms for the development of such unusual electrical activity are twofold. Although both require prior Na+ spikes, the majority of type III APs develop gradually after several spikes with a relatively stable rate, while the second type occurs faster and prevailingly in double-spiking neurons (Kodirov et al., 2016).
The VIP+ neurons did not trigger the type III AP or respond with a HCN-mediated sag in MP upon hyperpolarization, but instead saturation was observed. Similar saturation in MP as in LS VIP+ cells is also observed in MS/dBB neurons, which exhibit a fAHP immediately followed by a sAHP (Griffith, 1988). However, in LS VIP+ cells, the sAHP is absent. In LS neurons, we did not observe a typical bursting as in MS/dBB, though it was triggered by nicotine during spontaneous spiking (Kodirov et al., 2016). The AHP in LS neurons most probably results from the activation of a Ca2+-dependent K+ conductance (López-Barneo et al., 1985).
Although the mean RMP of dorsal LS neurons is comparable at about – 64 mV to previous studies, those of Rin are different (Joëls et al., 1987). The latter could be related to a classical approach in electrophysiology, i.e., impaling the neuron with a high-impedance electrode. Since for the patch-clamp recordings, neurons exhibiting Rin < 100 MΩ are not considered, as it correlates with bigger holding currents and leaks along the depolarized RMP.
Precise recordings in dendrites of CA1 pyramidal neurons also revealed type III AP, which occurred independent of distance from the perikaryon (Golding et al., 1999). This type III AP is distinct compared to the LS, but the behavior of Nav spikes – a gradual decrease in amplitude – is similar. The latter is also accompanied by a gradual depolarization of MP, while type III AP is terminated by stronger AHP. The differences in type III AP of CA1 and LS are obvious when one compares those recoded in the presence of TTX, albeit at a lower concentration. Importantly, the threshold intensity that triggered type III AP was 572 pA, which is very high compared to LS. Besides, in contrast to the perikaryon, the frequency of backpropagating spikes may reach 28 Hz or more when a heroic 2nA step is applied. During patch-clamp experiments, however, 2nA currents cannot be injected into the perikaryon of pyramidal cells or any neurons of the mammalian CNS (Kodirov, in preparation). Finally, the representative traces do not reflect the spiking behavior of pyramidal cells in CA1 (Golding et al., 1999).
Similar type III APs are also observed in inferior olivary neurons and referred to as Ca spikes (Llinás and Yarom, 1981). However, the high-threshold Ca spikes (HTS) do not closely resemble the type III AP in LS, as they have a clear-cut and overshooting upstroke mediated by Nav channels and are inhibited by TTX. Even the phenotype of low-threshold Ca spikes is clearly distinct in inferior olivary (a spikelet is still present during the depolarization phase of AP) and LS neurons, but the persistency to TTX is similar. Moreover, under near physiological conditions, in LS, the type III AP does not occur in isolation but is always followed by Nav spikes or Cav spikelets (Figs. 2, 4, 6–9). Although Nav spikes are prevented by TTX, which also consequently obliterates Cav spikelets, then the type III AP occurs independently and its frequency may increase (Fig. 8). Importantly, the spontaneous type III AP does not overshoot, and that is another similarity to those of inferior olivary neurons (Llinás and Yarom, 1981). It is possible that the type III AP in LS is also mediated by dendrites, but their simultaneous activation with that of the spike in the perikaryon does not occur. The latter clearly occurs in a tightly timed manner and is masked in inferior olivary neurons, which are unmasked by TTX. The most striking similarity was observed when comparing Fig. 6 (albeit subsequent type I APs) with those of LS in guinea pigs (López-Barneo et al., 1985). Also, the behavior of type III AP was similar when recorded after the application of TTX (Fig. 8).
Thus, in contrast to high-threshold spikes in inferior olivary neurons (Llinás and Yarom, 1981), the counterpart in guinea pig LS (López-Barneo et al., 1985) closely resembles the type III AP in mice and rats. Also, the descriptions of neuronal behaviors are of precise merit, as ‘with small pulses septal neurons fire repetitive Na+ spikes but upon larger depolarization they respond with a single full-Na+ action potential which is followed by a number of spikes of smaller amplitude. A further increase in the amplitude of the pulse evokes powerful Ca2+ spikes possibly generated in the dendrites’ (López-Barneo et al., 1985). Moreover, an AHP is much stronger after the type III AP vs. Na+ spike (Fig. 6c), which may enable partial recovery of Nav channels from inactivation leading to subsequent regular firing (Fig. 6a and b). It is possible that the underlying Cav channels for the generation of type III AP are located within the dendritic compartments of neurons (López-Barneo et al., 1985). This hypothesis is plausible since, in some cases, type III AP occurs after a short train of fast somatic Na+ spikes (Figs. 2 and 4). In fact, trains of somatic spikes may slowly depolarize the distant dendritic compartment until the threshold for type III AP is reached. However, based on the behavior of each Na+-spike during a single train, i.e., a correlated gradual decrease with a depolarization of MP preceding the type III AP (Fig. 4b), there is feedback into the perikaryon from dendrites. At least the gradual depolarization is controlled by dendrites, which subsequently decrease the amplitude of the Na+ spike since not all Nav channels transit into an inactivated state simultaneously. Of note, the very first Na+ spike causes a significant depolarization in MP.
A gradual depolarization leading to type III AP has been previously documented during spontaneous activities (Twery et al., 1992). Depending on RMP, a succession of fast spikes evokes a slower type III AP of higher amplitude, which in turn is followed by an AHP. However, despite the recordings being targeted to the dorsolateral septal nucleus (DSLN), Na+ spikes in LS decrease in amplitude as the threshold for type III AP is reached (Figs. 2, 4, and 8). The latter is consistent with another study in LS (Pedemonte et al., 1998).
In a previous study, there were no such dynamics, and perhaps Na+-spikes do not increase in amplitude and equally occur superimposed on gradually depolarizing MP, even with type III AP (Twery et al., 1992). Interestingly, during hyperpolarization vs. RMP, the type III AP requires fewer Na+ spikes to be triggered, which then may argue that it is a Ca2+-mediated active response. The most remarkable hallmark of type III AP is its phase of repolarization that terminates at a more negative MP compared to a prior or subsequent spike. The repolarization of type III AP is also slower than during Na+ spikes. Perhaps it is a compensatory intrinsic property of neurons to overcome a prolonged and intense depolarized state under pathological conditions. Alternatively, it may compensate for skipped Na+ spikes in order to enable an optimal opening of Nav channels from the most inactivated state during the train of APs.
However, the categorization of spikes based on whether they are produced by principal cells or interneurons is not always straightforward in this context. The typical firing of principal cells in the septum is regular or tonic, with a linear relationship between the injected current and the number of spikes and/or interspike frequency (input/output function). These cells are also regarded as integrators. In contrast, septal interneurons typically exhibit a burst-firing mode with a strongly nonlinear input/output function. In both cases, firing is supported by fast, Na+-mediated spikes. Principal neurons in other areas of the CNS, such as the amygdala, do not fire regularly, at least not in terms of stable frequency (Kodirov et al., 2006). Besides, not all interneurons exhibit a bursting mode, e.g., in the hippocampus (Kodirov, in preparation). However, interestingly, the principal neurons of the subiculum may burst (Kodirov, 2021).
An occurrence of type III AP could be predicted already during a relatively lower current magnitude, but not always close to threshold intensity (Fig. 4). In those neurons prone to type III AP, a double-spiking was also observed (Kodirov et al., 2016). The amplitude and frequency of subsequent spikes exhibited an adaptation at first, while transiting into a different mode later during the same step. An adaptation is a gradual process, while a transition is a sudden phenomenon. This transition resembles that of ‘persistent activity’ upon termination of 5 s depolarizing steps (Saada et al., 2009). Persistent activity is obliterated in the absence of extra-cellular Ca2+ or in the presence of Co2+ because of diminished autaptic excitation. In some neurons, graded persistent activity is evoked in the presence of 10 μM carbachol (Egorov et al., 2002). Even under these conditions, a 4 s depolarization is required in order to observe the subsequent persistent activity. In LS neurons, the two modes of activity occur spontaneously within only 1 s (Fig. 8). Nevertheless, the magnitudes of the depolarizing steps are not far apart at 120 vs. 200 pA (Egorov et al., 2002).
During a double-spiking, the anti-peak amplitude (lowest MP between spikes) after the 2nd AP is lower by up to 5.2 mV (data not shown). Thereafter, the MP levels between spikes adapt to this depolarization until they suddenly increase again, enabling a second state of firing within the train. The latter dynamic becomes more obvious if a type III AP occurs. It may occur even during a vulnerability state to type III AP (Fig. 2). A depolarization occurring after each spike could be considered a ‘summation’ – accumulation of Ca2+. When a 80-pA step triggered three spikes, the anti-peak MP differences were 8.4 vs. 7.9 mV between the 1st and 2nd spikes vs. the 2nd and 3rd spikes. The latter led to the generation of type III AP. It is more apparent in Fig. 4, as MP reaches the threshold for type III AP slowly. However, the dynamics of the three or more preceding spikes – a prerequisite condition for a transition into type III AP – are similar. That is, a single type III AP may switch the spiking pattern of a neuron. When present, type III AP occurs at a more depolarized MP earlier and requires fewer Na+ spikes (Fig. 6). However, during further depolarization, it may disappear. The vulnerability window at a different range of MP can be distinguished, which perhaps reflects an optimal range for the functioning of Ca2+ channels.
We have also observed differences in type III AP occurrences depending on how the pulse protocol was applied. Almost all our experiments start with the recording of membrane currents and synaptic activity at – 50 mV, as the whole-cell configuration of the patch-clamp technique is achieved at this level (Kodirov et al., 2021). Then the HP is gradually changed to – 70 mV (close to the expected RMP), and spontaneous activities are documented. Only after significant stabilization is the RMP recorded in a current clamp mode. Next, APs and sags in MP were somatically evoked. The intensity of depolarizing and hyperpolarizing steps was chosen depending on the response and passive property of each neuron, that is, input resistance. The increments were prevailingly 1, 10, or 20 pA, with the rare cases of depolarization by 380 pA and hyperpolarization by – 300 pA steps in neurons with low Rin.
If the neuron is predisposed for type III APs, this can be predicted by differences already at lower current magnitudes: 1st, longer APD at ± 0 mV (close to half-width); 2nd, lower AP amplitude; 3rd, higher threshold; 4th, increased sag/AHP. A different vulnerability of GAD+, PV+, SOM+, and VIP+ neurons to type III AP in LS is plausible. Since the generation of spikes by four types of interneurons in the neocortex correlates with the prevailing presence of neuropeptides (Porter et al., 1999) and all irregular-spiking ones express cholecystokinin (CCK) and VIP. Although morphologically distinct, in MS, not all HCN1-expressing neurons were PV+ (Hangya et al., 2009). Moreover, the local field potentials in the hippocampus are triggered by the spontaneous burst of spikes in PV+ and PV− neurons with a respective 79 vs. 11 ms delay.
Nimodipine has depolarized the RMP, obliterated the type III AP, decreased the amplitude of regular spikes (type I AP), and increased their frequency, hinting that in LS there are also Ca2+-dependent APs occurring during the train (Fig. 7). Nimodipine did not pronouncedly affect the Na+ spikes but obliterated the sag. Possibly, the peak amplitude of MP upon hyperpolarization was decreased. That is, the latter effects occur via a different mechanism compared to those of ZD7288, a selective HCN antagonist.
Among these neurons, only the VIP+ ones are unique, as they do not concurrently express PV, SOM, or VGlut1 (Perrenoud et al., 2013). Moreover, while there are overlapping expressions of other neuropeptides in PV+ and SOM+ cells, they in turn do not express VIP. In the septum, there were a few VIP-expressing neurons despite the sufficient number of cholinergic cells (Fig. 5). This is perhaps not surprising, as the co-localization of the latter two has been observed in 15 % of post-ganglionic cells at most (Lundberg et al., 1979). Moreover, some compounds, including the addictive ones, exhibit selectivity toward subpopulation of neurons, as shown for the VIP+ ones (Hill et al., 2007; Porter et al., 1999). The presence of VIP+ neurons has been previously documented not only in dBB and MS but also in many cortical areas (Chédotal et al., 1994), including the prefrontal cortex (PFC). Furthermore, the VIP and choline acetyltransferase (ChAT) may colocalize in the same neuron in some cases. Importantly, there is a hint that the terminals of VIP+ neurons proximate the blood vessels in the cortex.
The type III APs are not only heterogeneous in terms of phenotype in LS, but their underlying mechanisms may also differ. As the GABAA receptor antagonist, bicuculline methiodide at 10 μM concentration triggers bursting activities (Stevens et al., 1987), which behave in such a way that type III AP is generated after Na+ spikes, often four of them. In this case, the AHP of type III AP is apparently followed by a single high-amplitude inhibitory postsynaptic potential (IPSP) that promotes effective depolarization and recurrent complex excitability. Therefore, in some cases, the type III AP in conjunction with preceding Na+ spikes could be considered a burst (Fig. 9). Previous and subsequent extensive studies, however, show that neither age nor genus play a role in the generation of bursts or type III AP (Tables 1 and 2).
The type III APs have two distinct properties, namely they depolarize the MP for a longer time and subsequently hyperpolarize the neuron much closer to RMP compared to the train of Na+ spikes. The depolarization is important for hippocampal recurrent θ-rhythm, and thereby, the type III APs could also contribute to memory formation. In turn, the stronger the hyperpolarization, the easier occurs proper rebound excitation. The hyperpolarization derived from type III APs could serve as a compensatory mechanism under certain conditions, e.g., when the expression of K channels is diminished. Also, there should be significant effects of temperature, based on literature, especially on Na+ channels and spikes. However, the type III AP could be less sensitive, or at least it should persist when the temperature is increased close to 36.7 °C in the recording chamber (Fig. 2). Finally, the spontaneous type III APs most plausibly will not directly contribute to the θ-rhythm in the hippocampus. However, they may depolarize other neurons within the LS local network or in MS, and thereby some indirect contributions could be expected. At least, the longer depolarization during the course of type III APs might serve as a trigger for interconnected neurons to fire at θ-rhythm frequency when required for learning and memory by the hippocampus in behaving animals.
Limitations
Despite its most closeness, it is still an in vitro condition, and the slice preparations may not fully capture the physiological behavior of neurons in vivo. Although the outcomes are consistent and robust, there is considerable variability in type III AP phenotype and sag amplitudes (Kodirov et al., 2014). This also constitutes a limitation because of the relatively small sample size of certain neuronal subtypes. Additionally, the use of pharmacological tools, nimodipine and TTX, for inhibition of Ca2+ and Na+ inward currents could have off-target effects, which should be considered when interpreting the results. Since the Ca2+ antagonist nifedipine, similarly from the dihydropyridine group, at 5 μM concentration also inhibits the IA and IK outward K currents (Nerbonne and Gurney, 1987). Interestingly, in MS/dBB neurons, the depolarization-induced spike is not obliterated in the concurrent presence of Ba2+ and TTX (Griffith, 1988). In contrast, the fAHP is blocked and instead an ADP emerges, which also leads to a spikelet, while the sAHP remains unaffected. Similarly, the Cav channel antagonist nimodipine obliterates also the HCN-mediated sag in MP (Fig. 7). Finally, the majority of experiments were conducted at room temperature.
Conclusions
Neurons in the LS of rodents, such as mice and rats, are prone to Ca2+-dependent type III AP. In the current study, type III AP was frequently observed in GAD+ cells. In addition to documenting type III AP, our multifaceted approaches and properties of HCN channels could enable more precise classifications of neurons and delineate their contributions to mammalian behaviors. Thus, the broader biological implications of these findings are the relevance of type III APs to neural circuit function since the prone neurons are well integrated based on the morphology of terminals after loading with Lucifer yellow. The implication in learning and memory along related behaviors is possible with interconnected brain regions such as the hippocampus.
The results are also of general interest, as some of the TG lines enable off-target labeling not restricted to interneurons despite using the GAD67 corresponding to the Gad1 gene as a promoter (Riedemann et al., 2019). Further experiments will determine whether or not type III AP, namely the ‘Ca spikes appear with larger amplitude in presumptive intradendritic recordings’ (López-Barneo et al., 1985), occurs exclusively in the perikaryon of LSD neurons. Finally, the next milestone would be to reveal this Ca2+-dependent type III AP in vivo. More precisely, whether or not they take place under a true physiological condition and are linked to minute behaviors, especially learning and memory. Since the type III AP in LS correlates with the oscillations at θ-frequency in the CA1 area of the hippocampus (Pedemonte et al., 1998).
Summa summarum, the study provides significant mechanistic and theoretical insights into the firing properties of LS neurons by identifying a novel type III AP in GAD+ and SOM+ neurons. This discovery expands the classification of neuronal firing in the LS, offering a refined understanding of its heterogeneity.
Acknowledgments
Kodirov is grateful to his mentor Vladimir Leonidovich Zhuravlev ’71 (Leningrad University, UdSSR) for inspiring fascination with regard to spikes, to Dr. Ewelina Knapska for her support, to Jennifer Bagley, who provided care for rats at Texas University, to the animal facility of the Nencki Institute for mouse husbandry, and to Raul Consunji (Boston, MA) for careful reading of the manuscript. We are also grateful to Herr Prof. Dr. Johannes Hirrlinger (University of Leipzig) for providing GAD65-tdTomato mice and to anonymous both the senior editor and four expert reviewers for constructive suggestions and overall evaluations. The outlined research was supported by grants from NIH 3SC1NS065386-02S1 and CoSI ERC (project 715148).
Abbreviations:
- ADP
after-depolarizing potential
- AHP
after-hyperpolarizing potential
- AP
action potential
- APD
action potential duration
- Cav
voltage-dependent calcium channel
- GAD65
glutamic acid deeorboxylase 65 kDa isoform
- HCN
hyperpolarization-activated cyclic nucleotide gated non-selective cation channel
- I f
funny current
- I h
currents via HCN channels
- R in
input resistance
- Kv
voltage-dependent K+ channel subfamily
- LS
lateral septum
- MP
membrane potential
- MS
medial septum
- Nav
voltage-dependent sodium channel
- PV
parvalbumin
- RAP
rebound action potential
- RMP
resting membrane potential
- RTP
rebound tail potential
- SAA
spike amplitude adaptation
- SFA
spike firing adaptation
- SOM
somatostatin
- TTX
tetrodotoxin
- VIP
vasoactive intestinal polypeptide
Footnotes
CRediT authorship contribution statement
Michael Trojan: Investigation. Dominik Kanigowski: Methodology, Investigation. Łukasz Bijoch: Investigation. Martyna Pękała: Investigation. Diana Legutko: Investigation. Anna Beroun: Resources. Marek Bekisz: Resources. Luis V. Colom: Funding acquisition. Sodikdjon A. Kodirov: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Armstrong JN, MacVicar BA, 2001. Theta-frequency facilitation of AMPA receptor-mediated synaptic currents in the principal cells of the medial septum. J Neurophysiol 85, 1709–1718. [DOI] [PubMed] [Google Scholar]
- Bessières B, Jia M, Travaglia A, Alberini CM, 2019. Developmental changes in plasticity, synaptic, glia, and connectivity protein levels in rat basolateral amygdala. Learn Mem 26, 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondar A, Shubina L, 2018. Nonlinear reactions of limbic structure electrical activity in response to rhythmical photostimulation in guinea pigs. Brain Res Bull 143, 73–82. [DOI] [PubMed] [Google Scholar]
- Bonni K, Psyrakis D, Kodirov SA. 2008. Whole cell patch clamp onto CA1 region of hippocampus. https://commons.wikimedia.org/wiki/File:WholeCellPatchClamp-03.jpg. [Google Scholar]
- BoSmith RE, Briggs I, Sturgess NC, 1993. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol 110, 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette B, 1999. Noradrenergic responses of neurones in the mediolateral part of the lateral septum: α 1-adrenergic depolarization and rhythmic bursting activities, and α 2-adrenergic hyperpolarization from guinea pig brain slices. Brain Res Bull 48, 263–276. [DOI] [PubMed] [Google Scholar]
- Chédotal A, Cozzani C, Faure M-P, Hartman BK, Hamel E, 1994. Distinct choline acetyltransferase (ChAT) and vasoactive intestinal polypeptide (VIP) bipolar neurons project to local blood vessels in the rat cerebral cortex. Brain Res 646, 181–193. [DOI] [PubMed] [Google Scholar]
- Cisse RS, Krebs-Kraft DL, Parent MB, 2008. Septal infusions of the hyperpolarization-activated cyclic nucleotide-gated channel (HCN-channel) blocker ZD7288 impair spontaneous alternation but not inhibitory avoidance. Behav Neurosci 122, 549–556. [DOI] [PubMed] [Google Scholar]
- Dibattista M, Mazzatenta A, Grassi F, Tirindelli R, Menini A, 2008. Hyperpolarization-activated cyclic nucleotide-gated channels in mouse vomeronasal sensory neurons. J Neurophysiol 100, 576–586. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Ohba M, 1978. Dependence of the apparent reversal potential for the pace-maker current iK2 on its degree of activation in cardiac Purkinje fibres. J Physiol 280, 73–74. [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransén E, Hasselmo ME, Alonso AA, 2002. Graded persistent activity in entorhinal cortex neurons. Nature 420, 173–178. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M, 1988. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 336, 170–173. [DOI] [PubMed] [Google Scholar]
- Gemzik ZM, Donahue MM, Griffin AL, 2021. Optogenetic suppression of the medial septum impairs working memory maintenance. Learn Mem 28, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy H, Rossier J, Perrenoud Q, Vitalis T, Gallopin T, 2013. Diversity of GABAergic interneurons in layer VIa and VIb of mouse barrel cortex. Cereb Cortex 23, 423–441. [DOI] [PubMed] [Google Scholar]
- Gola M, Niel JP, 1993. Electrical and integrative properties of rabbit sympathetic neurones re-evaluated by patch clamping non-dissociated cells. J Physiol 460, 327–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Jung HY, Mickus T, Spruston N, 1999. Dendritic calcium spike initiation and repolarization are controlled by distinct potassium channel subtypes in CA1 pyramidal neurons. J Neurosci 19, 8789–8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith WH, 1988. Membrane properties of cell types within guinea pig basal forebrain nuclei in vitro. J Neurophysiol 59, 1590–1612. [DOI] [PubMed] [Google Scholar]
- Griguoli M, Maul A, Nguyen C, Giorgetti A, Carloni P, Cherubini E, 2010.Nicotine blocks the hyperpolarization-activated current Ih and severely impairs the oscillatory behavior of oriens-lacunosum moleculare interneurons. J Neurosci 30, 10773–10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib D, Dringenberg HC, 2009. Alternating low frequency stimulation of medial septal and commissural fibers induces NMDA-dependent, long-lasting potentiation of hippocampal synapses in urethane-anesthetized rats. Hippocampus 19, 299–307. [DOI] [PubMed] [Google Scholar]
- Halabisky B, Parada I, Buckmaster PS, Prince DA, 2010. Excitatory input onto hilar somatostatin interneurons is increased in a chronic model of epilepsy. J Neurophysiol 104, 2214–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ, 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391, 85–100. [DOI] [PubMed] [Google Scholar]
- Hangya B, Borhegyi Z, Szilagyi N, Freund TF, Varga V, 2009. GABAergic neurons of the medial septum lead the hippocampal network during theta activity. J Neurosci 29, 8094–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasunuma K, Murakawa T, Takenawa S, Mitsui K, Hatsukano T, Sano K , Nakata M, Ogawa S, 2024. Estrogen receptor β in the lateral septum mediates estrogen regulation of social anxiety-like behavior in male mice. Neuroscience 537, 126–140. [DOI] [PubMed] [Google Scholar]
- Hill EL, Gallopin T, Ferezou I, Cauli B, Rossier J, Schweitzer P, Lambolez B, 2007. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol 97, 2580–2589. [DOI] [PubMed] [Google Scholar]
- Ivashkina OI, Vorobyeva NS, Gruzdeva AM, Roshchina MA, Toropova KA, Anokhin KV, 2018. Cognitive tagging of neurons: CRE-mediated genetic labeling and characterization of the cells involved in learning and memory. Acta Naturae 10, 37–47. [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, 1990. Nimodipine and the recovery of memory. Trends Pharmacol Sci 11, 309–310. [DOI] [PubMed] [Google Scholar]
- Joëls M, Shinnick-Gallagher P, Gallagher JP, 1987. Effect of serotonin and serotonin analogues on passive membrane properties of lateral septal neurons in vitro. Brain Res 417, 99–107. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y, 1996. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci 16, 2701–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorov B, Pinelis V, Vinskaya N, Sorokina E, Grigortsevich N, Storozhevykh T, 1999. Li+ protects nerve cells against destabilization of Ca2+ homeostasis and delayed death caused by removal of external Na+. FEBS Letters 448, 173–176. [DOI] [PubMed] [Google Scholar]
- Kodirov SA, 2021. HCN and nicotine. Acta Physiol 232, e13651. [DOI] [PubMed] [Google Scholar]
- Kodirov SA, 2022. Probability that there is a mammalian counterpart of cardiac clock in insects. Arch Insect Biochem Physiol 110, e21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodirov SA, 2023a. Whole-cell patch-clamp recording and parameters. Biophys Rev 15, 257–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodirov SA, 2023b. Adam, amigo, brain, and K channel. Biophys Rev 15, 1393–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodirov SA, Takizawa S, Joseph J, Kandel ER, Shumyatsky GP, Bolshakov VY, 2006. Synaptically released zinc gates long-term potentiation in fear conditioning pathways. Proc Natl Acad Sci USA 103, 15218–15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodirov SA, Jasiewicz J, Amirmahani P, Psyrakis D, Bonni K, Wehrmeister M, Lutz B, 2010. Endogenous cannabinoids trigger the depolarization-induced suppression of excitation in the lateral amygdala. Learn Mem 17, 43–49. [DOI] [PubMed] [Google Scholar]
- Kodirov SA, Wehrmeister M, Colom LV, 2014. Modulation of HCN channels in lateral septum by nicotine. Neuropharmacology 81, 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodirov SA, Wehrmeister M, Colom L, 2016. Nicotine-mediated ADP to spike transition: Double spiking in septal neurons. J Memb Biol 249, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodirov SA, Psyrakis D, Brachmann J, Zhuravlev VL, 2019. Limulus and heart rhythm. J Exp Zool A Ecol Genet Physiol 331, 61–79. [DOI] [PubMed] [Google Scholar]
- Kodirov SA, Bonni K, Wehrmeister M, Lutz B, 2021. Depolarization-initiated endogenous cannabinoids release and underlying retrograde neurotransmission in interneurons of amygdala. Learn Mem 28, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodirov SA, Herbinger T, Rohwedder A, 2024. Comparable properties of native K channels in the atrium and ventricle of snails. Comp Biochem Physiol Part C 282, 109938. [DOI] [PubMed] [Google Scholar]
- Llinás R, Yarom Y, 1981. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol 315, 569–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood APM, 1961. “Ringer” solutions and some notes on the physiological basis of their ionic composition. Comp Biochem Physiol 2, 241–289. [DOI] [PubMed] [Google Scholar]
- López-Barneo J, Alvarez de Toledo G, Yarom Y, 1985. Electrophysiological properties of guinea pig septal neurons in vitro. Brain Res 347, 358–362. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Hökfelt T, Schultzberg M, Uvnäs-Wallensten K, Köhler C, Said SI, 1979. Occurrence of vasoactive intestinal polypeptide (VIP)-like immunoreactivity in certain cholinergic neurons of the cat: Evidence from combined immunohistochemistry and acetylcholinesterase staining. Neuroscience 4, 1539–1559. [DOI] [PubMed] [Google Scholar]
- Morris NP, Fyffe RE, Robertson B, 2004. Characterisation of hyperpolarization-activated currents (Ih) in the medial septum/diagonal band complex in the mouse. Brain Res 1006, 74–86. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Gurney AM, 1987. Blockade of Ca2+ and K+ currents in bag cell neurons of Aplysia califomica by dihydropyridine Ca2+ antagonists. J Neurosci 7, 882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A, Irisawa H, 1976. Membrane currents in the rabbit sinoatrial node cell as studied by the double microelectrode method. Pflugers Archiv 364, 45–52. [DOI] [PubMed] [Google Scholar]
- Paget J, 1846. An account of a case in which the corpus callosum, fornix, and septum lucidum, were imperfectly formed. Med Chir Trans 29, 55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ, 2001. The mouse brain in stereotaxic coordinates. Academic Press, New York. [Google Scholar]
- Pedemonte M, Barrenechea C, Nunez A, Gambini JP, Garcia-Austt E, 1998. Membrane and circuit properties of lateral septum neurons: relationships with hippocampal rhythms. Brain Res 800, 145 153. [DOI] [PubMed] [Google Scholar]
- Perrenoud Q, Rossier J, Geoffroy H, Vitalis T, Gallopin T, 2013. Diversity of GABAergic interneurons in layer VIa and VIb of mouse barrel cortex. Cereb Cortex 23, 423–441. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J, 1999. Ca2+-activated nonselective cationic current (ICAN) in turtle motoneurons. J Neurophysiol 82, 730–735. [DOI] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Tsuzuki K, Lambolez B, Rossier J, Audinat E, 1999. Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J Neurosci 19, 5228–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestigio C, Ferrante D, Valente P, Casagrande S, Albanesi E, Yanagawa Y, Benfenati F, Baldelli P, 2019. Spike-related electrophysiological identification of cultured hippocampal excitatory and inhibitory neurons. Mol Neurobiol 56, 6276–6292. [DOI] [PubMed] [Google Scholar]
- Psyrakis D, Jasiewicz J, Wehrmeister M, Bonni K, Lutz B, Kodirov SA, 2024. Progressive long-term synaptic depression at cortical inputs into the amygdala. Neuroscience 556, 52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ, Milgram NW, Hafner S, 1983. Long-term potentiation phenomena in the rat limbic forebrain. Brain Res 260, 217–231. [DOI] [PubMed] [Google Scholar]
- Ranck JB Jr, 1973. Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats: Part I. Behavioral correlates and firing repertoires. Exp Neurol 41, 462–531. [DOI] [PubMed] [Google Scholar]
- Riedemann S, Sutor B, Bergami M, Riedemann T, 2019. Gad1-promotor-driven GFP expression in non-GABAergic neurons of the nucleus endopiriformis in a transgenic mouse line. J Comp Neurol 527, 2215–2232. [DOI] [PubMed] [Google Scholar]
- Saada R, Miller N, Hurwitz I, Susswein AJ, 2009. Autaptic excitation elicits persistent activity and a plateau potential in a neuron of known behavioral function. Curr Biol 19, 479–484. [DOI] [PubMed] [Google Scholar]
- Stevens DR, Gallagher JP, Shinnick-Gallagher P, 1984. Intracellular recordings from rat dorsolateral septal neurons, in vitro. Brain Res 305, 353–356. [DOI] [PubMed] [Google Scholar]
- Stevens DR, Gallagher JP, Shinnick-Gallagher P, 1987. In vitro studies of the role of γ-aminobutyric acid in inhibition in the lateral septum of the rat. Synapse 1, 184–190. [DOI] [PubMed] [Google Scholar]
- Storozhevykh T, Grigortsevich N, Sorokina E, Vinskaya N, Vergun O, Pinelis V, Khodorov B, 1998. Role of Na+/Ca2+ exchange in regulation of neuronal Ca2+ homeostasis requires re-evaluation. FEBS Letters 431, 215–218. [DOI] [PubMed] [Google Scholar]
- Twery MJ, Phelan KD, Gallagher JP, 1992. Spontaneous bursting and non-bursting activity in morphologically identified neurons of the rat dorsolateral septal nucleus, in vitro. Neuroscience 46, 669–679. [DOI] [PubMed] [Google Scholar]
- van den Hooff P, Urban IJ, de Wied D, 1989. Vasopressin maintains long-term potentiation in rat lateral septum slices. Brain Res 505, 181–186. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS, 2001. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus 11, 578–598. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS, Brazhnik ES, 1977. Neuronal aspects of septo-hippocampal relations. Ciba Found Symp 145–177. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS, Brazhnik ES, 1979. Theta-volleys of neurons of the hippocampus and septum. Neurosci Behav Physiol 9, 373–378. [DOI] [PubMed] [Google Scholar]
- Zoicas I, Slattery DA, Neumann ID, 2014. Brain oxytocin in social fear conditioning and its extinction: Involvement of the lateral septum. Neuropsychopharmacology 39, 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]


