Abstract
Multiple sham-controlled clinical trials have demonstrated significant reductions in both office and 24-h blood pressure (BP) following radiofrequency renal denervation (RDN) in the uncontrolled hypertension population. Notably, the blood pressure response varies widely within individual participants, thus showing a clinical need to identify potential RDN “responders” prior to the procedure. Despite multiple analytic efforts, no single parameter, aside from baseline blood pressure, has been consistently associated with BP reduction following RDN. However, this failure may be due to limitations in empiric definitions of responders. Indeed, commonly applied responder definitions based on the difference between two point-in-time BP measurements are fraught due to visit-to-visit variability in office and 24-h blood pressure endpoints. Several factors should be considered to develop a more clinically useful operational definition of procedural response including relative changes in office and 24-h BP, consideration of the temporal response to RDN, as well as adjustment for baseline BP. The current evidence may provide incentives for future expert consensus to precisely define responders to hypertension treatments.
Keywords: Renal denervation, Responders, Blood pressure variability
Catheter based renal denervation (RDN) is a treatment option for hypertension uncontrolled by lifestyle modifications and antihypertensive medication. Results from multiple sham-controlled clinical trials demonstrate significant reductions in both office and 24-h blood pressure (BP) following RDN [1–6]. However, the vast size of the uncontrolled hypertension population [7] has shifted clinical focus towards identification of populations that may benefit the most from RDN. Indeed, contemporary clinical consensus statements and position papers suggest that identification of so-called “responders” is one of the most pressing unmet clinical needs for RDN [8–11]. However, this clinically intuitive term is scientifically not well defined. Typically, responders are identified as treated subjects achieving a particular empirically defined threshold of reduction of office systolic BP reduction (for example 10 mmHg) at a single arbitrarily chosen follow-up time point. Multiple parameters associated with increased basal sympathetic activity, such as heart rate [12], plasma renin activity [13], and body mass index [14], as well as indices associated with lower arterial stiffness, including pulse wave velocity [15, 16], central pulse pressure [17], augmentation index [18] and aortic distensibility [19] have been identified as potential markers of procedural response. Likewise, investigation of genetic markers has not indicated that genetic variants or combinations of them could help to identify predictors of BP response after RDN [20]. Applying these definitions, the only clinical variable consistently associated with BP response to date has been severity of baseline BP [21].
Previously, we speculated that this lack of consistency in response predictors was due to in part to the impact of visit-to-visit BP variability on operational definitions of responders based on single-point-in-time measurements [22]. This review leverages available patient level data from recent radiofrequency RDN clinical trials in patients with uncontrolled hypertension, as well as other recently reported clinical data, to appraise the limitations of current definitions and suggest alternative approaches to more accurately designate treatment “responders”.
Limitations of operational definitions of procedural responders
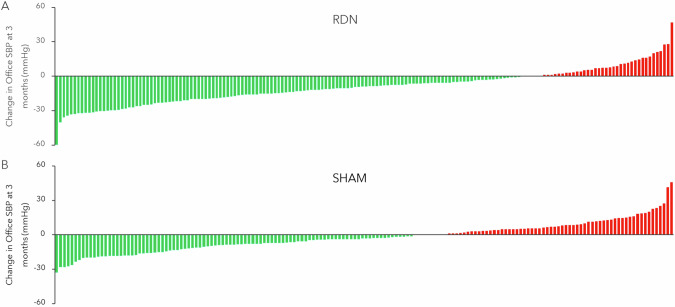
Prospectively powered sham controlled clinical trials for RDN were appropriately designed to detect a BP response within a specific population [23]. However, these methodologies were not necessarily intended to identify response on an individual level. Changes in office and 24-h mean BP between groups following RDN were invariably determined by the difference between two single-point-in-time measurements performed at baseline and at the primary follow up several months later. Responders have been retrospectively identified from these data sets by statistically comparing variables of interest between subgroups of RDN-treated patients with BP reduction above or below a certain threshold, as demonstrated for the SPYRAL HTN-OFF MED trial in Fig. 1A. However, this definition incorrectly assumes that each patient’s BP would have remained constant between measurements. Indeed, SBP did not remain constant in the untreated sham group as shown in Fig. 1B. Note that similar variability in individual BP response has also been shown in drug trials [24]. Hence, data from sham control groups in randomized control trials infer that single-point-in-time measurement cannot accurately identify an individual patient’s response in the RDN group.
Fig. 1.
Changes in office SBP to 3 months in the SPYRAL HTN-OFF Med Pivotal trial for the RDN (A, N = 150) and sham control (B, N = 134) groups [4]. Green bars indicate patients with a decrease in office systolic blood pressure increase between single-point-in-time measurements at baseline and the 3-month primary endpoint, while red bars indicate patients with an increase
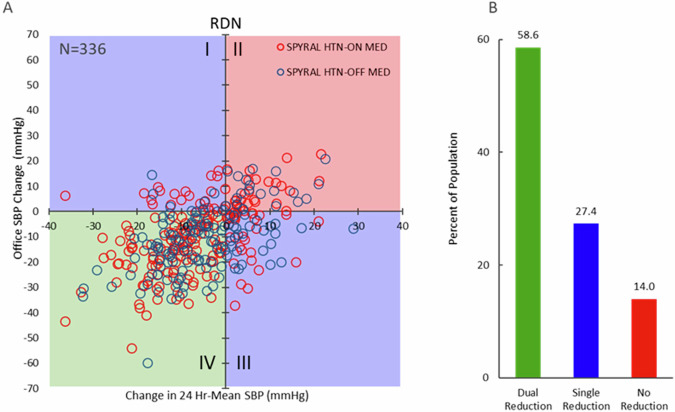
Responders are often classified based on office BP, but can also be independently identified via changes in 24-h mean systolic BP. However, office and 24-h BP are only very loosely correlated with each other within an individual patient [25]. Graphical comparison of the reduction in office SBP plotted against the reduction in 24-h SBP following RDN from the SPYRAL HTN-ON and -OFF MED trials at the primary follow up, as shown in Fig. 2, demonstrates incomplete agreement on responders for office vs 24-h SBP reduction. According to conventional descriptions, patients represented in quadrants II and IV would be classified as non-responders and responders, respectively, since their BP changes are consistent for office and 24-h SBP changes, although large numeric differences in the magnitude of BP changes can be observed within quadrants II and IV. However, the subset of patients (27% of all treated patients) represented in quadrants I and III defy consistent classification, since the BP changes for office and 24-h SBP occur in opposite directions making these patients simultaneously both responders and non-responders. These data suggest that a combined analysis including both changes in office and in 24-h SBP should be considered when defining “responders” to procedural interventions and, likewise, to antihypertensive drugs. Such a combination might increase specificity for accurately identifying a true response but would also reduce sensitivity given the variability in both office and 24-h BP, as further discussed below.
Fig. 2.
A Patient level relationship between the change from baseline in 24-h versus office systolic blood pressure in the SPYRAL HTN-OFF (N = 144, blue circles) and -ON MED (N = 192, red circles) trials at the primary follow up. Green box (quadrant IV) indicates patients with reductions in both office and 24-h SBP (dual reduction); red box (quadrant II) indicates patients with no reduction in either office or 24-h SBP; blue boxes (quadrants I and III) indication with a reduction in either office or 24-h SBP (single reduction). B Proportion of patients in both trials meeting the dual reduction, single reduction, or no reduction classifications (P < 0.01; Cochran–Mantel–Haenszel test)
In addition to this graphical approach, a generalized pairwise comparison approach, such as win ratio analysis [26] would further allow hierarchical consideration of multiple variables contributing to a clinically meaningful response [27, 28]. Considering the substantial and intriguing discrepancies between office and 24-hour BP changes, a primary criterion of at least 5 mmHg reduction in 24-h SBP, followed by a secondary criterion of at least 10 mmHg reduction in office SBP could define a “win” (success) for the treatment. A decrease of antihypertensive medication could further be applied as a third win criteria since drug changes also occur. Such a win ratio analysis has been applied in prospective randomized sham controlled RCTs [28]. Additional parameters besides blood pressure changes, such as changes in heart rate [12], arterial mechanical properties [15], or retinal capillary perfusion [29] could also be considered as part of a hierarchical construct.
Visit-to-visit blood pressure variability and responder identification
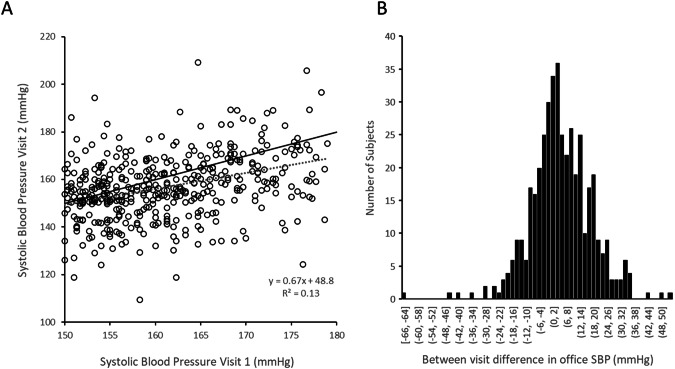
Both office and 24-h BP exhibit substantial visit-to-visit within patient variability, thus complicating identification of individual therapy responders using definitions based on single- point-in-time measurements. A sub analysis of 434 patients enrolled but not randomized in the SPYRAL HTN-ON MED trial compared office SBP between 2 consecutive trial screening visits with no change in medication regimen prior to randomization and RDN treatment (Fig. 3). The mean difference between visits was ±10.7 mmHg the data points were widely scattered relative to the line of identity and demonstrated a low correlation coefficient (r2 = 0.13). Interestingly this analysis in RDN patients agrees closely with a recent report of consecutive office BP values in over 500,000 patients from a large clinical drug trial data set showing that the average magnitude of BP variation between 2 consecutive office visits was ±11.6 mmHg [30]. Another recent report of data from drug trials demonstrated that 24-h mean BP also exhibits visit to visit BP variability that is similar in magnitude to that for office BP. Importantly, office visit to visit variability was not correlated to 24 h BP visit to visit variability [31] which further complicates the definition of therapy responder. Furthermore, the variability in SBP is similar in magnitude to the actual population treatment effect reported in the sham controlled clinical trials. Therefore, the actual treatment effect within an individual cannot be distinguished from the variability effect when only a single type of measurement is used to define a responder. For example, even when the pretreatment BP is relying on two or more BP measurements performed at least 2–4 weeks apart, the individual BP response of less than 10.7 mmHg in the SPYRAL OFF and ON trial does not reliably identify a patient to be a responder to the specific intervention. Note that other factors such as antihypertensive drug non-adherence, psychosocial factors, and environmental influences [32], might also impact BP variability and hence the identification of true responders.
Fig. 3.
Sub analysis of office SBP in patients enrolled but not randomized in the SPYRAL HTN-ON MED trial (n = 434). A The mean magnitude of the difference in blood pressure between screening visits 1 and 2 was ±10.7 mmHg. Black solid line: identity; dashed line: linear regression fit. B Histogram of between visit difference in office systolic blood pressure
Time response
Besides visit-to-visit BP variability, the changing response to the RDN procedure over time also complicates identification of procedural responders. Multiple recent reports have shown sequentially increased BP reductions over time that were not associated with increased medication burden. For example, 24-h blood pressure (Fig. 4) progressively decreased to 3 years post RDN in the Global SYMPLICITY Registry-DEFINE [33], SPYRAL HTN-ON MED pilot trial [34], and the SYMPLICITY HTN-3 trial [35] with minimal increase in prescribed antihypertensive medications. The mechanism for this progressive improvement in BP reduction is not yet clarified but may be related to recalibration of the neurohormonal cascade and reduction of vascular remodeling in the microcirculation known to need months and years for complete reversal [36]. Regardless of the mechanism, this increasing response to RDN over time suggests that any definition of “responders” must consider the timing of the assertion as well as the possibility that patients may change responder status over time when using traditional responder definitions.
Fig. 4.

24-h systolic BP to 3 years in the Global SYMPLICITY Registry (blue, N = 2452), SPYRAL HTN-ON MED trial (red, N = 38); and SYMPLICITY HTN-3 trial (black, N = 360): (P < 0.05 vs. baseline for all time points using ANCOVA). Error bars are SD
One possible way to account for both visit to visit BP variability and increased response over time is to define responders using multiple time points. For example, “time in therapeutic target range” (TTR) is an estimate of the proportion of time spent within a specified BP range and has been shown to be an independent predictor of cardiovascular risk in hypertensive patients [37, 38]. Higher TTR within 6 months of RDN in the Global Symplicity-DEFINE registry was associated with fewer CV events, including stroke and death [39]. Additional analogous metrics of BP reduction that may also appropriately account for time include “hypertension burden” [40], time updated blood pressure [41], and “cumulative blood pressure load” [42].
Wilder’s law of initial values
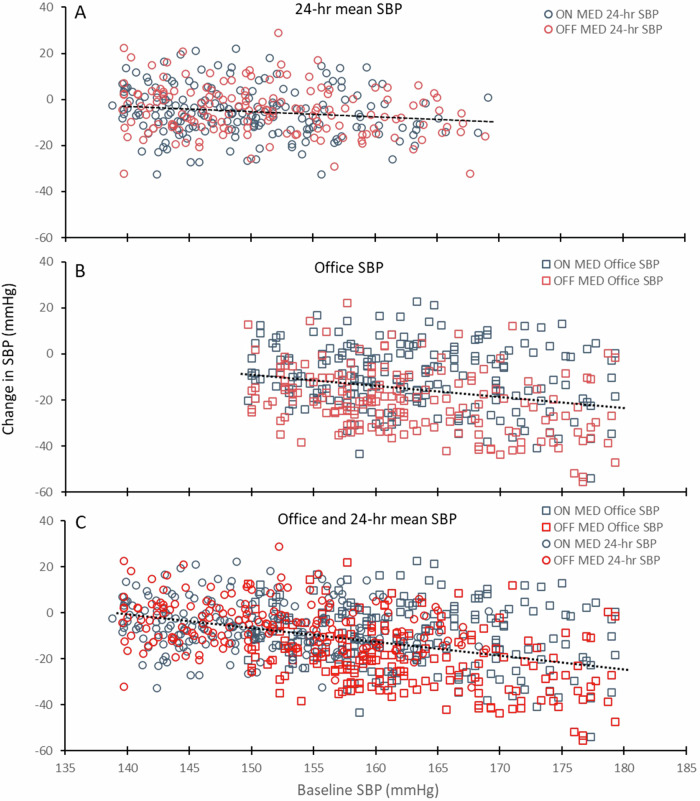
Wilder’s principle of initial value states that the response to a stimulus is related to the pre-stimulus level of the responding system [43, 44]. Considered from a practical perspective, those individuals with the greatest disease severity experience the greatest absolute and relative treatment benefit. This phenomenon is independent of other statistical phenomena such as regression to the mean and the Hawthorne effect, has been observed for several biomarkers including heart rate, LDL cholesterol or HBA1c, and has been applied to interventions for hypertension [45]. Analyses of office BP compared to 24-h BP showed a statistically significant linear relationship between the drop in office BP and the drop in ABPM from baseline following drug therapy [46, 47]. Accordingly, the change in BP following RDN is determined by the change in BP related to the baseline BP and the additional BP reduction related to RDN. The relationship between baseline BP and the subsequent change in BP at follow up for both office and 24-h SBP for all subjects randomized in the SPYRAL HTN -ON and -OFF MED trials is shown in Fig. 5. Note that the relationship between baseline SBP and SBP drop is comparable between 24-h (A) and office (B), when both parameters are plotted together (C). Interestingly, the increase in BP reduction at higher baseline BP was not observed in the sham group of the SPYRAL HTN-OFF MED trial as the average BP decrease was very low in the sham group.
Fig. 5.
Reductions from baseline in 24-h mean and office SBP according to baseline BP following RDN in the SPYRAL HTN -OFF MED (blue, N = 182) and ON MED (red, N = 206) trials at the time of the primary endpoint (3 and 6 months, respectively). Dashed lines = linear regression fit. A 24 h-mean SBP (regression slope = −0.26; intercept = 33.6, r2 = 0.03; p = 0.001). B Office SBP (slope = −0.45; intercept = 57.5, r2 = 0.05; p < 0.001). C Both office (squares) and 24-h SBP (circles) SBP appear to follow a similar relationship (slope: −0.54; intercept = 73.9, r2 = 0.14; p < 0.001). Despite a significant correlation for all three regression fits, the relationship is weakly predictive
Patients represented in the regression line may have no detectable treatment effect beyond the BP reduction related to the pretreatment BP level, or an apparent reverse effect, potentially due to changes in drug adherence behavior or to BP variability. Conversely, those patients represented below the regression line have a true BP lowering effect specifically related to the RDN intervention, independent from the nonspecific effect related to the baseline BP. Therefore, any definition of responder to a procedural intervention should also consider baseline BP and focus on the specific, baseline-independent BP decrease. For example, a recent single-center study of RDN in patients with and without CKD reported BP reductions in relation to baseline blood pressure that helped to identify similar responses in both groups [47].
Conclusions and future directions
A useful definition of responders to interventions for BP must account for multiple factors including the method of BP measurement, visit to visit BP variability, increased treatment response over time and the baseline BP. Notably, these principles, exemplified here using data available from recent RDN trials, also fully apply to pharmacologic therapy, which also demonstrates variable response [46]. Combining office and 24-h BP parameters, including integration of multiple measurements, as well as assessing the true BP lowering effect of an intervention independent of unspecific response phenomenon, pairwise comparisons that consider drug changes as well as BP changes (generalized pairwise comparisons), may add insight into clinical trial results beyond those available when observing individual BP parameters in isolation. Future RDN trials should minimally consider longer term monitoring of home BP in order to allow for some of the suggested analyses. The current evidence may provide incentives for expert consensus statements [9, 48, 49] to accurately identify hypertension therapy responders.
Compliance with ethical standards
Conflict of interest
RES reports grants and personal fees from Medtronic, Recor, and Ablative Solutions. DAH is a full-time employee of Medtronic, Inc. MB reports support from Astra-Zeneca, Bayer, BMS, Boehringer-Ingelheim, Glaxo, Medtronic, Novartis, ReCor, Servier and Vifor. MB is funded by the German Research Foundation (DFG, TTR 219, S-01, M-03, project number 322900939). DEK receives institutional research/grant support from Biotronik, Boston Scientific,. Orbus Neich, Teleflex, Medtronic, and Ablative Solutions; he also receives personal consulting honoraria from Medtronic and HyperQure. KK receives personal fees from Medtronic, receives grants from A&D Company, JIMRO, OmronHealthcare, CureApp, Terumo, and Fukuda Denshi, receives honoraria from Otsuka Pharmaceuticals and Omron Healthcare, and participates on the advisory board of Fukuda Denshi. FM is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219, Project-ID 322900939), and Deutsche Herzstiftung. Saarland University has received scientific support from Ablative Solutions, Medtronic and ReCor Medical. Until May 2024, FM has received speaker honoraria/consulting fees from Ablative Solutions, Amgen, Astra-Zeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, ReCor Medical, Servier, and Terumo. KT receives payments from Medtronic for work as centre principal investigator. MAW is a consultant for Medtronic, ReCor Medical, Ablative Solutions, Johnson & Johnson, and Urovant. MDE has received consulting fees and travel and research support from Medtronic. RET is a consultant for Medtronic, Axio, Regeneron, Bard, OBIO and AstraZeneca. Royalties from UpToDate.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Roland E. Schmieder, Email: roland.schmieder@uk-erlangen.de
Douglas A. Hettrick, Email: doug.hettrick@medtronic.com
References
- 1.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–70. [DOI] [PubMed] [Google Scholar]
- 2.Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–55. [DOI] [PubMed] [Google Scholar]
- 3.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–45. [DOI] [PubMed] [Google Scholar]
- 4.Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–51. [DOI] [PubMed] [Google Scholar]
- 5.Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476–86. [DOI] [PubMed] [Google Scholar]
- 6.Azizi M, Saxena M, Wang Y, Jenkins JS, Devireddy C, Rader F, et al. Endovascular ultrasound renal denervation to treat hypertension: the RADIANCE II randomized clinical trial. JAMA. 2023;329:651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCDRF Collaboration. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbato E, Azizi M, Schmieder RE, Lauder L, Böhm M, Brouwers S, et al. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2023;44:1313–30. [DOI] [PubMed] [Google Scholar]
- 9.Kandzari DE, Mahfoud F, Weber MA, Townsend R, Parati G, Fisher NDL, et al. Clinical trial design principles and outcomes definitions for device-based therapies for hypertension: a consensus document from the Hypertension Academic Research Consortium. Circulation. 2022;145:847–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swaminathan RV. SCAI Position Statement on Renal Denervation for Hypertension: patient selection, operator competence, training and techniques, and organizational recommendations. J Soc Cardiovasc Angiograpy Interventions. 2023;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmieder RE, Mahfoud F, Mancia G, Azizi M, Böhm M, Dimitriadis K, et al. European Society of Hypertension position paper on renal denervation 2021. J Hypertens. 2021;39:1733–41. [DOI] [PubMed] [Google Scholar]
- 12.Bohm M, Tsioufis K, Kandzari DE, Kario K, Weber MA, Schmieder RE, et al. Effect of heart rate on the outcome of renal denervation in patients with uncontrolled hypertension. J Am Coll Cardiol. 2021;78:1028–38. [DOI] [PubMed] [Google Scholar]
- 13.Mahfoud F, Townsend RR, Kandzari DE, Kario K, Schmieder RE, Tsioufis K, et al. Changes in plasma renin activity after renal artery sympathetic denervation. J Am Coll Cardiol. 2021;77:2909–19. [DOI] [PubMed] [Google Scholar]
- 14.Eikelis N, Hering D, Marusic P, Duval J, Hammond LJ, Walton AS, et al. The effect of renal denervation on plasma adipokine profile in patients with treatment resistant hypertension. Front Physiol. 2017;8:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fengler K, Rommel KP, Kriese W, Kresoja KP, Blazek S, Obradovic D, et al. Assessment of arterial stiffness to predict blood pressure response to renal sympathetic denervation. EuroIntervention. 2022;18:e686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fengler K, Rommel KP, Hoellriegel R, Blazek S, Besler C, Desch S, et al. Pulse wave velocity predicts response to renal denervation in isolated systolic hypertension. J Am Heart Assoc. 2017;6:e005879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ott C, Schmid A, Toennes SW, Ditting T, Veelken R, Uder M, et al. Central pulse pressure predicts BP reduction after renal denervation in patients with treatment-resistant hypertension. EuroIntervention. 2015;11:110–6. [DOI] [PubMed] [Google Scholar]
- 18.Weber T, Wassertheurer S, Mayer CC, Hametner B, Danninger K, Townsend RR, et al. Twenty-four-hour pulsatile hemodynamics predict brachial blood pressure response to renal denervation in the SPYRAL HTN-OFF MED trial. Hypertension. 2022;79:1506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoiber L, Mahfoud F, Zamani SM, Hametner B, Danninger K, Townsend RR, et al. Renal sympathetic denervation restores aortic distensibility in patients with resistant hypertension: data from a multi-center trial. Clin Res Cardiol. 2018;107:642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delles C, Schmieder RE, Daly R, Hamilton G, Schmid A, Herzy P, et al. Response of blood pressure to renal denervation is not associated with genetic variants. J Hypertens. 2023;41:e52–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauder L, Azizi M, Kirtane AJ, Bohm M, Mahfoud F. Device-based therapies for arterial hypertension. Nat Rev Cardiol. 2020;17:614–28. [DOI] [PubMed] [Google Scholar]
- 22.Kandzari DE, Mahfoud F, Bhatt DL, Böhm M, Weber MA, Townsend RR, et al. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension. 2020;76:1410–7. [DOI] [PubMed] [Google Scholar]
- 23.Kandzari DE, Kario K, Mahfoud F, Cohen SA, Pilcher G, Pocock S, et al. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J. 2016;171:82–91. [DOI] [PubMed] [Google Scholar]
- 24.Persu A, Jin Y, Azizi M, Baelen M, Völz S, Elvan A, et al. Blood pressure changes after renal denervation at 10 European expert centers. J Hum Hypertens. 2014;28:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kario K, Tomitani N, Hoshide S, Nishizawa M, Yoshida T, Kabutoya T, et al. Agreement between guideline thresholds using an “all-in-one” device to measure office, home, and ambulatory blood pressures. J Am Heart Assoc. 2023;12:e030992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbeeck J, De Backer M, Verwerft J, Salvaggio S, Valgimigli M, Vranckx P, et al. Generalized pairwise comparisons to assess treatment effects: JACC Review Topic of the Week. J Am Coll Cardiol. 2023;82:1360–72. [DOI] [PubMed] [Google Scholar]
- 27.Redfors B, Gregson J, Crowley A, McAndrew T, Ben-Yehuda O, Stone GW, et al. The win ratio approach for composite endpoints: practical guidance based on previous experience. Eur Heart J. 2020;41:4391–9. [DOI] [PubMed] [Google Scholar]
- 28.Kandzari DE, Hickey GL, Pocock SJ, Kandzari DE, Hickey GL, Pocock SJ, et al. Prioritised endpoints for device-based hypertension trials: the win ratio methodology. EuroIntervention. 2021;16:e1496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ott C, Harazny JM, Schmid A, Ditting T, Veelken R, Bladowski M, et al. Retinal microperfusion after renal denervation in treatment-resistant hypertensive patients. Clin Res Cardiol. 2015;104:782–9. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Linderman GC, Mahajan S, Liu Y, Huang C, Khera R, et al. Quantifying blood pressure visit-to-visit variability in the real-world setting: a retrospective cohort study. Circ Cardiovasc Qual Outcomes. 2023;16:e009258. [DOI] [PubMed] [Google Scholar]
- 31.Mancia G, Facchetti R, Quarti-Trevano F, Dell’Oro R, Cuspidi C, Grassi G. Comparison between visit-to-visit office and 24-h blood pressure variability in treated hypertensive patients. J Hypertens. 2024;42:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James GD, Pickering TG. The influence of behavioral factors on the daily variation of blood pressure. Am J Hypertens. 1993;6:170S–3S. [DOI] [PubMed] [Google Scholar]
- 33.Mahfoud F, Mancia G, Schmieder RE, Ruilope L, Narkiewicz K, Schlaich M, et al. Outcomes following radiofrequency renal denervation according to antihypertensive medications: subgroup analysis of the Global SYMPLICITY Registry DEFINE. Hypertension. 2023;80:1759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahfoud F, Kandzari DE, Kario K, Townsend RR, Weber MA, Schmieder RE, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet. 2022;399:1401–10. [DOI] [PubMed] [Google Scholar]
- 35.Bhatt DL, Vaduganathan M, Kandzari DE, Leon MB, Rocha-Singh K, Townsend RR, et al. Long-term outcomes after catheter-based renal artery denervation for resistant hypertension: final follow-up of the randomised SYMPLICITY HTN-3 Trial. Lancet. 2022;400:1405–16. [DOI] [PubMed] [Google Scholar]
- 36.Bosch A. Why happens a progressive decrease of blood pressure after renal denervation. J Hypertens. 2024;42:2. [Google Scholar]
- 37.Fatani N, Dixon DL, Van Tassell BW, Fanikos J, Buckley LF. Systolic blood pressure time in target range and cardiovascular outcomes in patients with hypertension. J Am Coll Cardiol. 2021;77:1290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doumas M, Tsioufis C, Fletcher R, Amdur R, Faselis C, Papademetriou V. Time in therapeutic range, as a determinant of all-cause mortality in patients with hypertension. J Am Heart Assoc. 2017;6:e007131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahfoud F, Mancia G, Schmieder RE, Ruilope L, Narkiewicz K, Schlaich M, et al. Cardiovascular risk reduction after renal denervation according to time in therapeutic systolic blood pressure range. J Am Coll Cardiol. 2022;80:1871–80. [DOI] [PubMed] [Google Scholar]
- 40.Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243–51. [DOI] [PubMed] [Google Scholar]
- 41.Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, et al. Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med. 2015;162:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S, Zhao D, Wang M, Xu J, Zhao M, Jin Y, et al. Association between cumulative blood pressure and long-term risk of cardiovascular disease: findings from the 26-year Chinese Multi-provincial Cohort Study-Beijing Project. Chin Med J. 2021;134:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oken D. Health HA. The law of initial values: some further considerations. Psychosom Med. 1963;25:3–12. [DOI] [PubMed] [Google Scholar]
- 44.Flume JW. Zeitschrift für die gesamte. Neurol Psychiatrie. 1931;137:317–38. [Google Scholar]
- 45.Messerli FH, Bangalore S, Schmieder RE. Wilder’s principle: pre-treatment value determines post-treatment response. Eur Heart J. 2015;36:576–9. [DOI] [PubMed] [Google Scholar]
- 46.Schmieder RE, Schmidt ST, Riemer T, Dechend R, Hagedorn I, Senges J, et al. Disproportional decrease in office blood pressure compared with 24-hour ambulatory blood pressure with antihypertensive treatment: dependency on pretreatment blood pressure levels. Hypertension. 2014;64:1067–72. [DOI] [PubMed] [Google Scholar]
- 47.Gunes-Altan M, Schmid A, Ott C, Bosch A, Pietschner R, Schiffer M, et al. Blood pressure reduction after renal denervation in patients with or without chronic kidney disease. Clin Kidney J. 2024;17:sfad237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kario K, Kim BK, Akoi J, Wong AY, Lee YH, Wongpraparut N, et al. Renal denervation in Asia: Consensus statement of the Asia Renal Denervation Consortium (ARDeC). Hypertension. 2020;75:590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahfoud F, Azizi M, Ewen S, Pathak A, Ukena C, Blankestijn PJ, et al. Proceedings from the 3rd European Clinical Consensus Conference for clinical trials in device-based hypertension therapies. Eur Heart J. 2020;41:1588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]