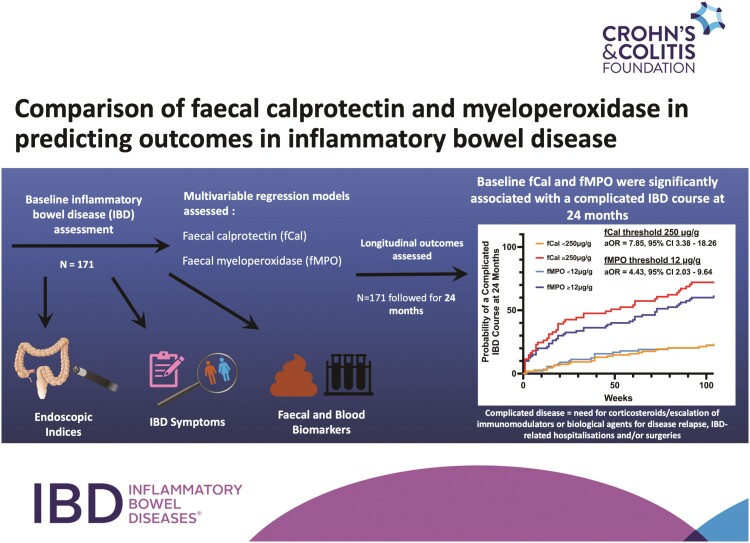
Abstract
Background
Biomarkers have been proposed as surrogate treatment targets for the management of inflammatory bowel disease (IBD); however, their relationship with IBD-related complications remains unclear. This study investigated the utility of neutrophil biomarkers fecal calprotectin (fCal) and fecal myeloperoxidase (fMPO) in predicting a complicated IBD course.
Methods
Participants with IBD were followed for 24 months to assess for a complicated IBD course (incident corticosteroid use, medication escalation for clinical disease relapse, IBD-related hospitalizations/surgeries). Clinically active IBD was defined as Harvey-Bradshaw index >4 for Crohn’s disease (CD) and simple clinical colitis activity index >5 for ulcerative colitis (UC). Area under the receiver-operating-characteristics curves (AUROC) and multivariable logistic regression assessed the performance of baseline symptom indices, fCal, and fMPO in predicting a complicated disease IBD course at 24 months.
Results
One hundred and seventy-one participants were included (CD, n = 99; female, n = 90; median disease duration 13 years [interquartile range, 5-22]). Baseline fCal (250 μg/g; AUROC = 0.77; 95% confidence interval [CI], 0.69-0.84) and fMPO (12 μg/g; AUROC = 0.77; 95% CI, 0.70-0.84) predicted a complicated IBD course. Fecal calprotectin (adjusted OR = 7.85; 95% CI, 3.38-18.26) and fMPO (adjusted OR = 4.43; 95% CI, 2.03-9.64) were associated with this end point after adjustment for other baseline variables including clinical disease activity. C-reactive protein (CRP) was inferior to fecal biomarkers and clinical symptoms (pdifference < .05) at predicting a complicated IBD course. A combination of baseline CRP, fCal/fMPO, and clinical symptoms provided the greatest precision at identifying a complicated IBD course.
Conclusions
Fecal biomarkers are independent predictors of IBD-related outcomes and are useful adjuncts to routine clinical care.
Keywords: biomarkers, IBD management, fecal biomarkers
Graphical abstract
Graphical Abstract.
Key Messages.
What is already known?
• Current models of care support the use of biomarkers as surrogate treatment targets for mucosal healing in inflammatory bowel disease (IBD); however, optimal targets for these biomarkers remain unclear.
What is new here?
• This study shows that fecal calprotectin or fecal myeloperoxidase (at identified thresholds) are independently associated with a complicated course of illness and may be appropriate targets to aim for to improve long-term IBD outcomes.
How can this study help patient care?
• Testing for fecal biomarkers helps to identify levels of gut inflammation and, in addition, may also prognosticate the longer-term disease course.
Introduction
Inflammatory bowel diseases (IBDs), comprised predominantly of Crohn’s disease (CD) and ulcerative colitis (UC), are chronic relapsing and remitting illnesses of the gastrointestinal (GI) tract. Inflammatory bowel disease results from aberrant stimulation of the immune system, including the innate immune response.1 Neutrophils play a vital role in the dysregulation of the immune system seen in IBD.2,3 These white blood cells release proteins such as calprotectin and myeloperoxidase (MPO) that have been implicated in sustaining the gut inflammatory response in IBD.2-5 Neutrophil-derived biomarkers such as fecal calprotectin (fCal) and fecal MPO (fMPO) have been shown to be effective biomarkers of intestinal inflammation.6
Management of IBD involves proactively assessing for GI inflammation with the aim of achieving mucosal healing as suggested in the STRIDE-II (selecting therapeutic targets in IBD) guidelines.7-9 Titrating therapies in response to disease activity results in improved rates of clinical, biochemical, and histological remission.8 Ileocolonoscopy has been the mainstay of objective disease assessment in IBD, but it is invasive and expensive. Fecal biomarkers have been proposed as surrogate treatment targets; however, the optimal “target” for these markers has not yet been established in widely accepted guidelines and often incorporates a wide range of values associated with outcomes including endoscopic healing, clinical, and mucosal remission.7 The association of these biomarkers with longitudinal outcomes such as disease progression, IBD-related surgeries, and/or hospitalizations has been shown in some studies10 but is still being established in multiple independent cohorts.7 This study investigated the utility of baseline fCal and fMPO in predicting a complicated IBD course in participants who had 24 months of prospective follow-up.
Materials and Methods
Participant Recruitment and Study Procedures
The New Indicators of Disease Activity in IBD (NIDA-IBD) study was a cross-sectional and prospective cohort study performed in participants with established IBD who were undergoing objective disease assessment (ileocolonoscopy) at Christchurch Hospital, New Zealand between April 2019 and September 2020.6 All study participants had sociodemographic, phenotype, medical, and medication history recorded during baseline assessment. Study participants also provided stool and blood samples and completed questionnaires relating to IBD symptoms (Harvey-Bradshaw Index for CD, HBI; simple clinical colitis activity index for UC, SCCAI) during baseline assessment. Baseline biomarker assessment included full blood count, albumin, C-reactive protein (CRP), fCal, and fMPO using methods previously described.6 Samples were analyzed for full blood count, albumin and CRP using automated analyzers at Canterbury Health Laboratories, Christchurch, New Zealand as part of standard of care.6 Stool samples were independently assessed for fCal using a commercially available enzyme-linked immunosorbent assay (ELISA) in accordance with the manufacturer’s instructions (CALPRO ELISA, Calpro AS, Lysaker, Norway).6 Fecal myeloperoxidase was determined using an in-house ELISA that has previously been described.6 Subjectively active IBD was scored as HBI >4 and SCCAI >5.11-13 Baseline endoscopic disease activity was assessed using the simple endoscopic score for CD (SES-CD) and UC endoscopic index of severity (UCEIS).14,15 Inactive endoscopic disease was defined as an SES-CD ≤2 or UCEIS <2.14-16
All patients were prospectively followed for 24 months through access to their electronic health record that included clinic letters, medication prescriptions, hospital admissions, and surgical procedures. Patients unable to be followed for this time were excluded from analyses. The primary study end point was a complicated IBD course at 24 months (composite end point of the incident need for corticosteroids for flares of luminal IBD ≥4 weeks), incident escalation of/to immunomodulator/biological agents due to clinical disease relapse, IBD-related surgery and/or hospitalization). This end point is similar to those that have been previously reported and takes into account real-world outcomes.17-19
Statistical Analyses
Continuous variables were compared between groups using the Mann-Whitney U test. Optimal fecal biomarker thresholds predicting the primary study end point and each component of this composite end point were determined using the Youden’s index derived from the coordinates of the receiver-operating-characteristics (ROC) curve.20 Area under the ROC (AUROC) for CRP, fCal, and fMPO was compared using previously established methods by De Long et al.21 Internal cross-validation was performed using bootstrap bias corrected 95% confidence intervals (CIs) of AUROC.
Univariable logistic regression was used to determine baseline variables associated with a complicated IBD course. Only noninvasive variables were used in these analyses (ie, excluding endoscopic indices) to examine the potential for noninvasive disease assessment at predicting the longitudinal IBD course. Variables reaching a predetermined significance of P ≤ 0.01 on univariable analyses were included in subsequent multivariable models given the relatively small sample size in this study. Fecal biomarkers were included in multivariable regression models as categorical variables using the optimal thresholds identified in this study. Subgroup multivariable regression analyses were performed on individuals with CD and UC separately. These analyses investigated the utility of fecal biomarkers at predicting a complicated IBD course after adjusting for clinical disease activity and CRP as per “treat-to-target” paradigms suggested in the STRIDE-II guidelines.7 Log-rank testing compared the distributions of the time to the primary study end point at the optimal biomarker thresholds. The utility of multivariable regression models was compared with individual clinical variables (symptom indices and biomarkers) using AUROC and methods described by De Long et al.21
Decision curve analyses (DCAs) were also used to evaluate baseline variables associated with the primary study end point of a complicated IBD course. Decision analyses help ascertain the clinical utility of biomarkers and statistical models, as there is uncertainty over how the discriminative properties (sensitivity, specificity, AUROC) of these markers may translate into clinical utility.22 The variables assessed using DCA in this study included endoscopically active IBD at baseline, and baseline fCal and fMPO in isolation, and in models incorporating clinical symptoms and CRP. In this study, DCA calculated the clinical “net benefit” of a model or diagnostic test across a range of threshold probabilities (from 0% to 80% probability) for the end point of a complicated IBD course at 24 months.23,24 These analyses combine measures of sensitivity and specificity with the relative harms of a false positive (eg, over treatment and over investigation with invasive procedures such as ileocolonoscopy) and false negative (eg, under treatment and missed opportunities for proactive care) result to derive the “net benefit.”22,24
Statistical analyses were performed using SPSS 27 (IBM Corp., Armonk, New York) and STATA SE 17 (StataCorp., College Station, Texas). Graphs of study data were produced on GraphPad Prism 9 (GraphPad Software Inc., San Diego, California). This study was conducted in accordance with the World Medical Assembly Declaration of Helsinki and was approved by the New Zealand Health and Disability Ethics Committee (18/NTA/197).
Results
Description of Study Participants
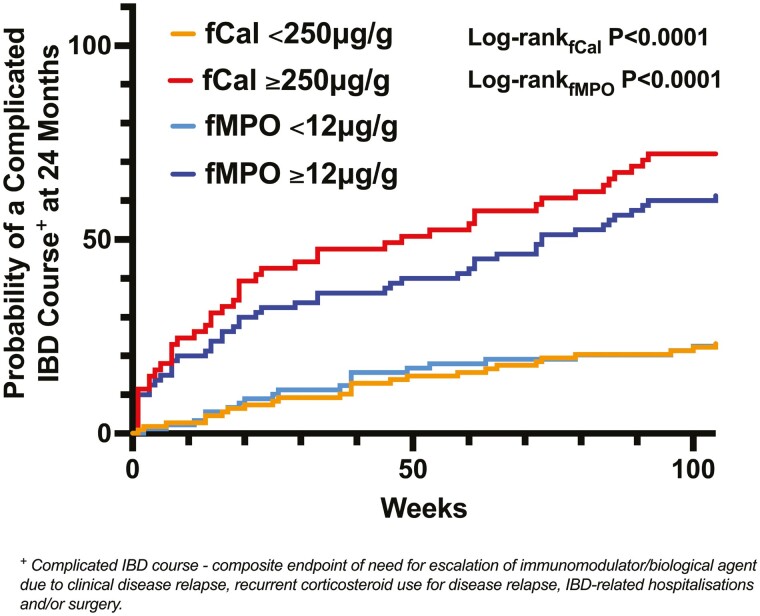
A total of 171 study participants (CD, n = 99; UC, n = 72; female, n = 90; median age 46 years; interquartile range [IQR], 36-59) were followed for 24 months (Table 1). The indication for disease assessment with ileocolonoscopy was for surveillance (CD, n = 57; UC, n = 41) or to assess current disease activity (CD, n = 42; UC, n = 31). There were no differences in baseline fCal (CD, median 120.0 μg/g; UC, median 146.1 μg/g; P = .45) and fMPO (CD, median 10.4 μg/g; UC, median 15.6 μg/g; P = .77) concentrations between individuals with CD and UC, respectively (Table 1). A complicated disease course was observed in 71 patients (CD, n = 45; UC, n = 26) by 24 months at a median time to first IBD complication of 23 weeks (IQR, 7-61; Figure 1). Of the 23 patients who were hospitalized during follow-up, 8 had recurrent corticosteroid use, and 8 had medication escalation for disease relapse prior to their hospitalization. Of the 17 patients who had IBD surgery during follow-up, 3 had corticosteroid use, and 3 had medication escalation prior to their surgery. Five of the patients who underwent IBD surgery during follow-up had an acute colectomy for severe colitis, 7 had an ileocolic resection for stricturing CD, and the remaining participants required operations for perianal disease (n = 2), stricturoplasty (n = 1), or small bowel exploration (n = 1) for stricturing CD and diverting stoma for active IBD (n = 1).
Table 1.
Description of new indicators of disease activity in inflammatory bowel disease (NIDA-IBD) cohort who were followed prospectively for 24 months.
| All IBD (n = 171) | CD (n = 99) | UC (n = 72) | |
|---|---|---|---|
| Median Age (IQR, years) | 46 (36-59) | 44 (34-57) | 48 (40-63) |
| Female participants (%) | 90 (52.6) | 52 (52.5) | 38 (52.8) |
| Median time since IBD diagnosis (range, years) | 13.0 (5.0-22.0) | 13.0 (6.0-21.0) | 11.5 (4.0-23.5) |
| European ethnicity (%) | 168 (98.2) | 99 (100) | 69 (95.8) |
| Montreal Classification of IBD(%) | |||
| A1 | 7 (7) | 0 (0) | |
| A2 | 74 (75) | 19 (26) | |
| A3 | 18 (18) | 53 (74) | |
| L1 | 17 (18) | ||
| L2 | 21 (21) | ||
| L3 | 61 (62) | ||
| B1 | 59 (60) | ||
| B2 | 20 (20) | ||
| B3 | 20 (20) | ||
| Perianal disease | 17 (17) | ||
| E1 | 6 (8.3) | ||
| E2 | 33 (45.8) | ||
| E3 | 33 (45.8) | ||
| Baseline corticosteroid use for IBD | 28 (16.4) | 12 (12.1) | 16 (22.2) |
| Corticosteroid use in the last year for IBD | 59 (34.5) | 33 (33.3) | 26 (36.1) |
| Baseline immunomodulator use for IBD | 67 (39.2) | 41 (41.4) | 26 (36.1) |
| Baseline use of biological agents for IBD | 37 (21.6) | 27 (27.3) | 10 (13.9) |
| History of depression | 17 (9.9) | 13 (13.1) | 4 (5.6) |
| History of anxiety | 13 (7.6) | 10 (10.1) | 3 (4.2) |
| History of concurrent irritable bowel syndrome | 11 (6.4) | 8 (8.1) | 3 (4.2) |
| Endoscopically active IBD (SES-CD >2, UCEIS ≥2, %) | 97 (56.7) | 62 (62.6) | 35 (48.6) |
| Endoscopically inactive/mildly active IBD (SES-CD <7, UCEIS<5, %) | 130 (76.0) | 71 (71.7) | 59 (81.9) |
| Active symptoms at baseline (HBI >4, SCCAI >5; %) |
96 (56.1) | 60 (60.6) | 36 (20.9) |
| Median fecal calprotectin (IQR, µg/g) | 132.7 (46.2-523.1) | 120.0 (46.2-353.5) | 146.1 (45.2-853.1) |
| Median fecal myeloperoxidase (IQR, µg/g) | 11.01 (2.43-61.12) | 10.40 (3.27-44.88) | 15.57 (1.66-107.46) |
| Median IBDQ-32 (IQR) | 169.0 (136.0-196.0) | 165.0 (132.0-195.0) | 177.0 (138.3-200.8) |
| Clinical relapse requiring biological/immunomodulator escalation at 24 months (%) | 45 (26.3) | 26 (26.3) | 19 (26.4) |
| Recurrent corticosteroid use at 24 months (%) | 25 (14.6) | 12 (12.1) | 13 (18.1) |
| IBD related hospitalization at 24 months (%) | 23 (13.5) | 16 (16.2) | 7 (9.7) |
| IBD related surgery at 24 months (%) | 17 (9.9) | 12 (12.1) | 5 (6.9) |
| Complicated IBD course 24 months (%) | 71 (41.5) | 45 (45.5) | 26 (36.1) |
Abbreviations: IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; Immunomodulator, thiopurine or methotrexate; SES-CD, simple endoscopic score for CD; UCEIS, ulcerative colitis endoscopic index of severity; HBI, Harvey-Bradshaw Index; CDAI, Crohn’s disease activity index; SCCAI, simple clinical colitis activity index; IBDQ-32, inflammatory bowel diseases questionnaire
Figure 1.
Elevations of baseline fecal calprotectin (fCal) above 250 µg/g and fecal myeloperoxidase (fMPO) above 12 µg/g were significantly associated with a complicated inflammatory bowel disease (IBD) course at 24 months of follow-up.
Biomarker Thresholds Associated with a Complicated IBD Course
C-reactive protein was associated with a complicated IBD course in all participants at 24 months (AUROC 0.63; 95% CI, 0.54-0.71; optimal threshold 3 μg/g; sensitivity 68%, specificity 56%). C-reactive protein was significantly associated with this study’s primary end point in individuals with UC (AUROC 0.69; 95% CI, 0.56-0.82; optimal threshold 3 μg/g; sensitivity 73%, specificity 59%) but not in those with CD (AUROC 0.58; 95% CI, 0.46-0.69).
The optimal baseline fCal threshold associated with a complicated IBD course at 24 months was 250 μg/g (AUROC 0.77; 95% CI, 0.69-0.84; sensitivity 65%, specificity 83%). This cut-off had similar performance in individuals with CD (sensitivity 58%, specificity 85%) and UC (sensitivity 77%, specificity 80%). Optimal fCal thresholds calculated in CD and UC were similar to the cut-off value calculated for all patients with IBD: 244 μg/g in CD (sensitivity 60%, specificity 85%) and 270 μg/g in UC (sensitivity 77%, specificity 85%).
The optimal fMPO threshold associated with a complicated IBD course was 12 μg/g (AUROC 0.77; 95% CI, 0.70-0.84; sensitivity 72%, specificity 69%). Comparison of the AUROC for fCal and fMPO indicated that the performance of these biomarkers for predicting the primary study end point was not significantly different (P > .05). Optimal fMPO cut-offs were higher in individuals with UC (threshold = 30 μg/g, sensitivity 73%, specificity 83%) compared with those with CD (threshold = 12 μg/g, sensitivity 67%, specificity 70%). Internal cross-validation of these AUROC did not materially alter the observed trends (Table 2). Baseline fecal biomarkers also predicted components of the composite study end point (Table 2).
Table 2.
Area under the receiving operating curves (AUROC) for fecal biomarkers in predicting a complicated inflammatory bowel disease (IBD) course at 24 months of follow-up and for each component of this composite end point.
| Fecal calprotectin (fCal) | ||||
|---|---|---|---|---|
| End Point (N = 171) | AUROC (95% CI) |
Internal Cross-validated AUROC (Bootstrap bias corrected 95% CI)† |
Optimal Threshold | Sensitivity/ Specificity (%) |
| Complicated IBD course (N = 71) | 0.77 (0.69-0.84) |
0.77 (0.67-0.84) |
250 ug/g | 65/83 |
| Medication escalation (N = 45) | 0.83 (0.77-0.90) |
0.84 (0.67-0.87) |
270 ug/g | 73/80 |
| Recurrent corticosteroid use (N = 25) | 0.66 (0.55-0.78) |
0.66 (0.30-0.59) |
285 ug/g | 60/72 |
| IBD-hospitalization (N = 23) | 0.65 (0.51-0.79) |
0.65 (0.29-0.60) |
850 ug/g | 48/85 |
| IBD-surgery (N = 17) |
0.59 (0.43-0.75) |
0.50 (0.14-0.49) |
244 ug/g | 65/65 |
| Fecal myeloperoxidase (fMPO) | ||||
|---|---|---|---|---|
| End point (N = 171) | AUROC (95% CI) |
Internal cross-validated AUROC (Bootstrap bias corrected 95% CI)† |
Optimal threshold | Sensitivity/Specificity (%) |
| Complicated IBD course (N = 71) | 0.77 (0.70-0.84) |
0.77 (0.65-0.82) |
12 ug/g | 72/69 |
| Medication escalation (N = 45) | 0.83 (0.77-0.90) |
0.83 (0.67-0.87) |
10 ug/g | 91/62 |
| Recurrent corticosteroid use (N = 25) | 0.69 (0.60-0.78) |
0.69 (0.32-0.63) |
12 ug/g | 80/57 |
| IBD-hospitalization (N = 23) | 0.61 (0.49-0.74) |
0.62 (0.26-0.54) |
31 ug/g | 57/70 |
| IBD surgery (N = 17) |
0.53 (0.37-0.69) |
0.48 (0.16-0.51) |
31 ug/g | 53/69 |
†Kfold = 10 for complicated IBD; Kfold = 5 for each other end point.
No significant differences in AUROC between fCal and fMPO for a complicated IBD course (P = .95), medication escalation (P = .98), recurrent corticosteroid use (P = .45), IBD hospitalization (P = .40) and IBD-surgery (P = .14).
Exploratory subgroup analyses were carried out to investigate if fecal biomarkers predicted a complicated IBD course in individuals with objectively inactive disease at baseline (SES-CD ≤2, UCEIS <2). Fifteen individuals with inactive disease (CD = 11, UC = 4) at baseline reached the study composite end point at 24 months. Baseline fMPO predicted a complicated disease course in these individuals (AUROC 0.69; 95% CI, 0.56-0.82; optimal threshold 5 μg/g, sensitivity 80%, specificity 63%). However, fCal was not significantly associated with the primary study end point in this subgroup (AUROC 0.63; 95% CI, 0.46-0.79).
Utility of Fecal Biomarkers in Predicting a Complicated IBD Course
Amongst all patients with IBD in this study, univariable regression analyses identified that the presence of current or past corticosteroid use (in the last year), current biological agent use, serum albumin, elevated fecal biomarkers, and clinically active IBD were significantly associated with a complicated IBD course at 24 months (Supplementary Table 1). Multivariable regression models included these variables in evaluating the utility of fecal biomarkers in predicting the primary study end point (Table 3). These analyses showed that amongst all patients with IBD (after adjustment for these covariates), baseline fCal ≥ 250 μg/g (adjusted OR (aOR) = 7.85; 95% CI, 3.38-18.26) and baseline fMPO ≥12 μg/g (aOR = 4.43; 95% CI, 2.03-9.64) were associated with a complicated IBD course at 24 months (Figure 1).
Table 3.
Multivariable analyses for predicting a complicated inflammatory bowel disease (IBD) course using fecal biomarkers. Model 1 = using fecal calprotectin. Model 2 = using fecal myeloperoxidase.
| All IBD Patients (N = 171) | Multivariable Analysis (Model 1) | Multivariable Analysis (Model 2) | ||
|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P |
| Current steroids | 0.39 (0.10-1.61) | 0.19 | 0.57 (0.16-2.11) | .40 |
| Steroids in last year | 5.88 (2.05-16.88) | <0.001 | 5.35 (1.94-14.78) | .001 |
| Biological agent use | 1.43 (0.56-3.64) | 0.46 | 1.88 (0.75-4.67) | .18 |
| Albumin | 0.87 (0.76-0.98) | 0.02 | 0.89 (0.79-1.00) | .05 |
| fCal ≥ 250 ug/g | 7.85 (3.38-18.26) | <0.001 | ||
| fMPO ≥ 12 ug/g | 4.43 (2.03-9.64) | <.001 | ||
| Subjectively active IBD at baseline (HBI > 4, SCCAI > 5) |
2.95 (1.27-6.88) | 0.01 | 2.83 (1.25-6.43) | .01 |
Abbreviations: UC, ulcerative colitis; CD, Crohn’s disease; HBI, Harvey-Bradshaw index for CD; SCCAI, Simple clinical colitis activity index for UC.
Subgroup analyses of participants with CD showed that fCal ≥250 μg/g (aOR = 7.60; 95% CI, 2.78-20.77) and fMPO ≥12 μg/g (aOR= = 3.55; 95% CI, 1.46-8.63) were associated with a complicated IBD course (these results were adjusted for the presence of clinical disease activity and CRP). Similarly, for participants with UC (when adjusting for clinical disease activity and CRP), fCal ≥250 μg/g (aOR = 6.65; 95% CI, 1.72-25.77) and fMPO ≥30 μg/g (aOR 7.75; 95% CI, 1.99-30.20) were associated with this end point.
Previous studies have shown reduced accuracy of fCal and fMPO in detecting active gut inflammation in individuals with isolated ileal CD.6,25 In this cohort, there were 17 individuals with isolated ileal CD, and 11 of these participants reached the end point of a complicated IBD course at 24 months of follow-up. Subgroup univariable logistic regression analyses showed that neither fCal (threshold ≥250 μg/g; OR = 1.76; 95% CI, 0.23-13.16) or fMPO (threshold ≥12 μg/g; OR = 1.33; 95% CI, 0.20-9.31) were associated with a complicated IBD course.
Comparison of Variables Associated With a Complicated IBD Course
Fecal biomarkers (fCal, fMPO) provided additional benefit to clinical disease indices alone in predicting a complicated IBD course at 24 months (Table 4). Fecal biomarkers (fCal and fMPO) had improved precision at predicting this study’s composite end point compared with CRP (pdifference < .05; Table 4). The addition of CRP to clinical disease activity did not provide additional benefit for predicting a complicated IBD course compared with assessing clinical activity alone (pdifference > .05; Table 4).
Table 4.
Area under the receiver operating characteristics curve (AUROC) of clinical disease indices, biomarkers, and statistical models incorporating multiple measures in predicting a complicated inflammatory bowel disease course at 24 months. The highest AUROC are shown in bold.
| Crohn’s Disease | Ulcerative Colitis | |
|---|---|---|
| AUROC (95% CI) | AUROC (95% CI) | |
| HBI | 0.67 (0.57-0.78) | |
| SCCAI | 0.83 (0.73-0.93)a | |
| CRP | 0.58 (0.47-0.69) | 0.69 (0.56-0.81) |
| fCal | 0.72 (0.61-0.83)a | 0.85 (0.76-0.95)a |
| fMPO | 0.71 (0.61-0.82)a | 0.86 (0.78-0.95)a |
| CRP + fCal | 0.75 (0.65-0.85)a | 0.85 (0.78-0.95)a |
| CRP + fMPO | 0.72 (0.61-0.82)a | 0.86 (0.78-0.95)a |
| fCal + fMPO | 0.72 (0.61-0.83)a | 0.86 (0.77-0.95)a |
| CRP > 3 mg/L + clinical disease activity | 0.65 (0.54-0.75)a | 0.80 (0.69-0.90)a |
| CRP > 3 mg/L + fCal ≥ 250 μg/g + clinical disease activity | 0.77 (0.68-0.87)a | 0.85 (0.75-0.95)a |
| CRP > 3 mg/L + fMPO ≥ 12 μg/g + clinical disease activity | 0.72 (0.62-0.82)a | 0.87 (0.77-0.96) a |
| fCal ≥ 250 μg/g + clinical disease activity | 0.78 (0.70-0.87) a , b | 0.84 (0.74-0.94)a |
| fMPO ≥ 12 μg/g + clinical disease activity | 0.73 (0.63-0.83)a | 0.85 (0.76-0.95)a |
HBI, Harvey-Bradshaw index; SCCAI, simple clinical colitis activity index; CRP, C-reactive protein; fCal, fecal calprotectin; fMPO, fecal myeloperoxidase.
Clinical disease activity = HBI > 4 or SCCAI > 5.
aAUROC significantly different from CRP (P < .05).
bAUROC significantly different from HBI (P < .05).
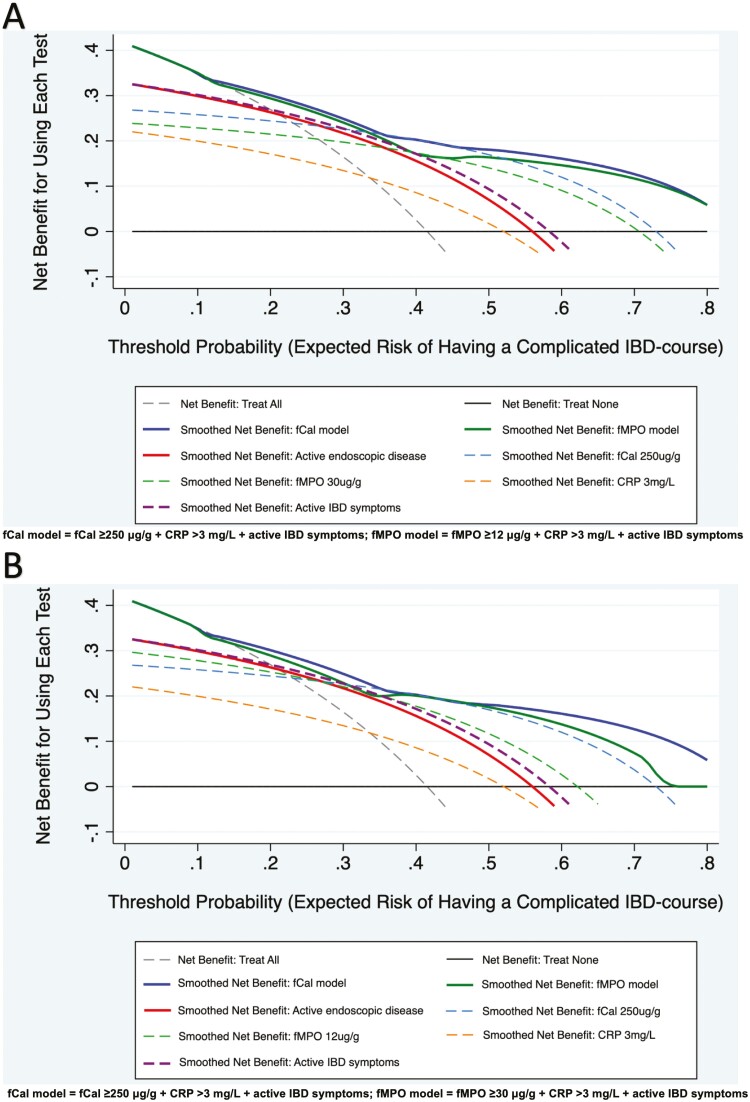
Decision curve analyses assessed the clinical benefit of individual biomarkers, disease assessment indices (symptoms and endoscopy), and models combining these measures in predicting a complicated IBD course. Clinical models using either fecal biomarker (fCal ≥250 μg/g in all patients with IBD (fCal model) or fMPO ≥12 μg/g in CD and fMPO ≥30 μg/g in UC (fMPO model)), in conjunction with abnormal CRP (>3 mg/L, >upper limit of normal) and active IBD symptoms (HBI >4, SCCAI >5) at baseline provided the greatest benefits in predicting this study’s primary end point (Figure 2). This benefit was superior to each of these variables in isolation (CRP, fCal, or fMPO at chosen thresholds, clinical disease activity) and superior to the presence of endoscopically active IBD at baseline. The benefit of the fCal and fMPO models remained consistent across a range of threshold probabilities (0%-80%) for reaching a complicated IBD course at 24 months of follow-up in patients with CD (Figure 2A) and UC (Figure 2B). The benefit of these clinical models (which utilized noninvasive methods of disease assessment) compared with endoscopic disease activity was most pronounced when the probability for reaching a complicated IBD course was <20% or >50%.
Figure 2.
Decision curve analyses show the greatest benefit for predicting a complicated inflammatory bowel disease (IBD) course at 24 months of follow-up is with a multimodal disease assessment (combining the use of fecal and blood markers with clinical disease activity). This is consistent in patients with Crohn’s disease (CD; A) and ulcerative colitis (UC; B), and across a range of expected probabilities for reaching a complicated IBD course. Abbreviations: fCal, fecal calprotectin; fMPO, fecal myeloperoxidase; active endoscopic disease = simple endoscopic score for CD >2 or UC endoscopic index of severity ≥2; active IBD symptoms = Harvey Bradshaw index for CD >4 or simple clinical colitis activity index >5.
Discussion
Current IBD treatment paradigms rely on the proactive assessment of disease activity with the goal of achieving mucosal healing of the gut.7-9 Biomarkers have been proposed as surrogate treatment targets; however, the “target” that should be aimed for remains unclear. This study demonstrates that the fecal biomarkers fCal or fMPO (at concentrations of fCal ≥250 μg/g and fMPO >12 μg/g) are independently associated with longitudinal IBD outcomes, irrespective of clinical disease activity. Given that the median time to IBD-related complications is 23 weeks after baseline, this study emphasizes the value of biomarker measurement at 6 monthly intervals in a “treat-to-target” management algorithm. Utilization of such algorithms (as incorporated in the clinical models shown in Figure 2) appears to provide the greatest benefit in predicting a complicated IBD course compared with clinical disease indices or biomarker assessments used in isolation.
The findings of the current study are in line with previous observations, which describe an fCal threshold of 250 μg/g being associated with active endoscopic disease,26 disease progression in CD,10 and normalization of fCal levels below this threshold being associated with a reduced risk for disease progression.27 Previous investigation of fMPO in IBD identified 7 μg/g as the optimal threshold for identifying endoscopic disease activity.6 Taken together, these findings suggest that neutrophil-derived biomarkers such as fCal and fMPO reflect the gut inflammatory burden, which ultimately drives the long-term progression of IBD.1 Activation of neutrophils has been implicated as playing a key role in sustaining the abnormal innate immune responses observed in IBD.3 Thus, these fecal biomarkers may be appropriate surrogate measures of disease activity at a snapshot in time and may help stratify overall disease prognosis.
Although CRP has previously been associated with IBD activity and long-term risk of complications,28,29 fecal biomarkers had significantly improved diagnostic precision at identifying a complicated disease course in this cohort. This may be due in part to the lack of specificity of CRP for gastrointestinal inflammation in IBD.30 The STRIDE-II guidelines support the use of measuring the IBD symptom burden and for normalization of CRP and fCal during clinical assessments.7 This study reiterates the value of clinical symptom indices (especially in UC), which are simple measures that can be used to guide treatment changes and have an influence on longer-term outcomes in IBD. However, the performance of these indices in prognosticating the longitudinal disease course is significantly improved and augmented by the use of biomarkers. “Normalization” of fCal may be achieved at a target of 250 μg/g given the association of this threshold with disease related complications.
Furthermore, the value of fecal biomarkers in treat-to-target approaches may be significantly greater than serum biomarkers such as CRP. The trade-off in clinical situations, however, is the ease and patient acceptability of obtaining blood sampling compared with fecal analyses and the rapidity of acquiring biomarker results from serum samples.31 Regardless of these limitations, this study highlights the powerful potential of fCal and fMPO as adjuncts to disease assessment during routine clinical care. Using a combination of fecal biomarkers (either fCal or fMPO), CRP and clinical disease activity provided significant benefit in identifying individuals at risk for developing a more complicated IBD course (Table 4 and Figure 2). This trend remained consistent across a range of risk thresholds for reaching this outcome (Figure 2). Utilizing a combination of clinical disease indices and biomarkers during disease assessment is likely to be helpful in predicting the disease course in individuals who are at low (<20%) and high (>50%) risk of achieving disease complications in a 24-month interval (Figure 2). Thus, using the models of care as described in this study may help identify individuals requiring a closer degree of outpatient follow-up and those who may benefit most from escalation of IBD therapies.
The neutrophil-derived fecal biomarkers studied in this cohort were fCal and fMPO. There were no significant differences in the diagnostic precision of either marker in predicting a complicated IBD course at 24 months of follow-up. However, the utility of fCal and fMPO in predicting IBD complications in individuals with isolated ileal disease requires further exploration and was limited in this study by the small sample size of this subgroup. A previous study highlighted that fMPO is equivalent to fCal at detecting moderate to severe endoscopic disease activity in IBD, and the majority of fMPO detected is physiologically active.6 Optimal thresholds of fCal predicting a complicated IBD course remained consistent between the 12 months of follow-up reported in the previous study and the current 24-month findings (250 μg/g). However, the threshold for fMPO was significantly lower for 24-month analyses (12 μg/g) compared with those reported for complications at 12 months (26 μg/g).6 Furthermore, baseline fMPO accurately predicted a complicated IBD course at 24 months in individuals with objectively inactive disease at baseline. These results suggest the potential for fMPO to be a sensitive marker to prognosticate the longitudinal disease course; however, these findings require validation in external cohorts. Additional benefits of fMPO compared with fCal lie in the potential for detecting the active MPO component using methodologies that allow for a more rapid turnover of results compared with routine fCal enzyme linked immunoassays.
The key limitations of this study are the relatively small sample size and low frequency of disease complications, which will have an impact on the generalizability of the presented results. This may have largely contributed to the relatively wide confidence intervals for the adjusted odds ratios of fecal biomarkers and a complicated IBD course on multivariable regression analyses. Thus, the optimal biomarker thresholds identified in this study require ongoing external validation before being widely implemented as the goal of treat-to-target management. Additionally, the utility of fecal biomarkers in predicting IBD-related hospitalizations and surgeries was reduced compared with other components of the study’s primary composite end point. This may be partly due to the proportion of patients requiring hospitalization and surgery first reaching the end points of medication escalation and/or corticosteroid use but could also reflect the small sample size. The effect of this sample size may have also influenced the variability in optimal fMPO thresholds between individuals with CD and UC, as there were almost double the number of participants with CD with a complicated disease course compared with those with UC. The cumulative dose of incident corticosteroid use for study participants and its effect on IBD outcomes were unable to be studied in this cohort because the time to the first episode of needing steroids (≥4 weeks) for IBD relapse was recorded as a dichotomous outcome.
This study included a largely European population, and the utility of fecal biomarkers in predicting IBD-related outcomes in ethnically diverse populations requires further investigation. The utility of fCal and fMPO at identifying complicated disease in those with newly diagnosed IBD compared with those with established IBD (such as the participants included in this cohort) requires further examination in inception cohorts. Additionally, the utility of fCal and fMPO at identifying disease complications such as disease extension, stricture, and intestinal fistulae requires exploration in cohorts with follow-up for longer than the 24-month period presented in this study.
In summary, fecal biomarkers such as fCal or fMPO are useful and independent predictors of longitudinal IBD-related complications such as the need for medication escalation and healthcare utilization (including the need for hospitalization and surgery). These markers are likely to be useful aides to clinical care in the era of “treat-to-target” management in IBD.
Supplementary Data
Supplementary data is available at Inflammatory Bowel Diseases online.
Acknowledgments
Authors wish to thank Dr. Thomas C. Mules and Dr. Kate Kilpatrick for their assistance with acquisition of study data in relation to this manuscript.
Glossary
Abbreviations
- IBD
inflammatory bowel disease
- CD
Crohn’s disease
- UC
ulcerative colitis
- MPO
myeloperoxidase
- fCal
fecal calprotectin
- fMPO
fecal myeloperoxidase
- STRIDE
selecting therapeutic targets in inflammatory bowel disease
- NIDA-IBD
new indicators of disease activity in inflammatory bowel disease
- HBI
Harvey-Bradshaw index
- SCCAI
simple clinical colitis activity index
- ROC
receiver operating characteristics curve
- AUROC
area under the receiver operating characteristics curve
- aOR
adjusted odds ratio
- CRP
C-reactive protein
- DCA
decision curve analyses
Contributor Information
A Swaminathan, Department of Medicine, University of Otago Christchurch, Christchurch, New Zealand; Department of Gastroenterology, Christchurch Hospital, New Zealand.
G M Borichevsky, Mātai Hāora, Centre for Redox Biology and Medicine, Department of Pathology and Biomedical Science, University of Otago Christchurch, Christchurch, New Zealand.
C M Frampton, Department of Medicine, University of Otago Christchurch, Christchurch, New Zealand.
A S Day, Department of Paediatrics, University of Otago Christchurch, Christchurch, New Zealand.
M B Hampton, Mātai Hāora, Centre for Redox Biology and Medicine, Department of Pathology and Biomedical Science, University of Otago Christchurch, Christchurch, New Zealand.
A J Kettle, Mātai Hāora, Centre for Redox Biology and Medicine, Department of Pathology and Biomedical Science, University of Otago Christchurch, Christchurch, New Zealand.
R B Gearry, Department of Medicine, University of Otago Christchurch, Christchurch, New Zealand; Department of Gastroenterology, Christchurch Hospital, New Zealand.
Author Contributions
A.S., A.S.D., M.A.H., A.J.K., and R.G.B. conceived the study design. A.S. and G.M.B. acquired the study data and performed the laboratory analyses. A.S. and C.M.F. performed the statistical analyses, and A.S.D., G.M.B., C.M.F., M.A.H., A.J.K., and R.G.B. assisted in the interpretation of the study results. A.S. prepared the initial article draft. All authors contributed to the critical revision of this manuscript and approved the final submitted version.
Funding
This research was supported by the Royal Australasian College of Physicians Research Entry Grant and the New Zealand Society of Gastroenterology Research Fellowship Grant.
Conflicts of Interest
A.S. has received honoraria for educational activities for Janssen (unrelated to this study). A.S.D. has served on advisory boards for Janssen, Abbvie, and Nestle (all unrelated to this manuscript). A.S.D.’s research is supported by Cure Kids. R.B.G. has received research grants, served on advisory boards, and received honoraria for educational activities for Janssen, AbbVie, and Zespri (unrelated to this study).
Data Availability
Data is available upon request to the corresponding author.
References
- 1. Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med. 2020;383(27):2652-2664. [DOI] [PubMed] [Google Scholar]
- 2. Fournier BM, Parkos CA.. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5(4):354-366. [DOI] [PubMed] [Google Scholar]
- 3. Wéra O, Lancellotti P, Oury C.. The dual role of neutrophils in inflammatory bowel diseases. J Clin Med. 2016;5(12):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Renz M, Ward M, Eastwood MA, Harkness RA.. Neutrophil function and myeloperoxidase activity in inflammatory bowel disease. Lancet. 1976;308(7985):584. [DOI] [PubMed] [Google Scholar]
- 5. Jukic A, Bakiri L, Wagner EF, Tilg H, Adolph TE.. Calprotectin: from biomarker to biological function. Gut. 2021;70(10):1978-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swaminathan A, Borichevsky GM, Edwards TS, et al. Fecal myeloperoxidase as a biomarker of endoscopic activity in inflammatory bowel disease. J Crohns Colitis. 2022;16(12):jjac098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turner D, Ricciuto A, Lewis A, et al. ; International Organization for the Study of IBD. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583. [DOI] [PubMed] [Google Scholar]
- 8. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet (London, England) 2017;390(10114):2779-2789. [DOI] [PubMed] [Google Scholar]
- 9. Yoon H, Jangi S, Dulai PS, et al. Incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: a systematic review and meta-analysis. Gastroenterology. 2020;159(4):1262-1275.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kennedy NA, Jones GR, Plevris N, et al. Association between level of fecal calprotectin and progression of Crohn’s disease. Clin Gastroenterol Hepatol. 2019;17(11):2269-2276.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P.. Correlation between the Crohn’s disease activity and Harvey–Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8(4):357-363. [DOI] [PubMed] [Google Scholar]
- 12. Jowett SL, Seal CJ, Barton JR, Welfare MR.. 1. Use of the simple clinical colitis activity index (sccai) to define relapse of ulcerative colitis (uc). Gut. 2001;48(suppl 1):A1-A5.11286195 [Google Scholar]
- 13. Walmsley R, Ayres R, Pounder R, Allan R.. A simple clinical colitis activity index. Gut. 1998;43(1):29-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sipponen T, Nuutinen H, Turunen U, Färkkilä M.. Endoscopic evaluation of Crohn’s disease activity: comparison of the CDEIS and the SES-CD. Inflamm Bowel Dis. 2010;16(12):2131-2136. [DOI] [PubMed] [Google Scholar]
- 15. Travis SPL, Schnell D, Krzeski P, et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013;145(5):987-995. [DOI] [PubMed] [Google Scholar]
- 16. Xie T, Zhang T, Ding C, et al. Ulcerative colitis endoscopic index of severity (UCEIS) versus Mayo Endoscopic Score (MES) in guiding the need for colectomy in patients with acute severe colitis. Gastroenterol Rep. 2018;6(1):38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rath T, Atreya R, Bodenschatz J, et al. Intestinal barrier healing is superior to endoscopic and histologic remission for predicting major adverse outcomes in inflammatory bowel disease: the prospective ERIca trial. Gastroenterology. 2023;164(2):241-255. [DOI] [PubMed] [Google Scholar]
- 18. Dias CC, Rodrigues PP, Coelho R, et al. ; on behalf GEDII. Development and validation of risk matrices for Crohn’s disease outcomes in patients who underwent early therapeutic interventions. J Crohns Colitis. 2017;11(4):445-453. [DOI] [PubMed] [Google Scholar]
- 19. Magro F, Dias CC, Portela F, et al. ; GEDII [Portuguese IBD Group]. Development and validation of risk matrices concerning ulcerative colitis outcomes—Bayesian network analysis. J Crohns Colitis. 2019;13(4):401-409. [DOI] [PubMed] [Google Scholar]
- 20. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32-35. [DOI] [PubMed] [Google Scholar]
- 21. DeLong ER, DeLong DM, Clarke-Pearson DL.. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. [PubMed] [Google Scholar]
- 22. Vickers AJ, Calster BV, Steyerberg EW.. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6. doi: https://doi.org/ 10.1136/bmj.i6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vickers AJ, Elkin EB.. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vickers AJ, van Calster B, Steyerberg EW.. A simple, step-by-step guide to interpreting decision curve analysis. Diagn Progn Res. 2019;3:18. doi: https://doi.org/ 10.1186/s41512-019-0064-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith LA, Gaya DR.. Utility of fecal calprotectin analysis in adult inflammatory bowel disease. World J Gastroenterol. 2012;18(46):6782-6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin JF, Chen JM, Zuo JH, et al. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014;20(8):1407-1415. [DOI] [PubMed] [Google Scholar]
- 27. Plevris N, Fulforth J, Lyons M, et al. Normalization of fecal calprotectin within 12 months of diagnosis is associated with reduced risk of disease progression in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2021;19(9):1835-1844.e6. [DOI] [PubMed] [Google Scholar]
- 28. Solem CA, Loftus EV Jr, Tremaine WJ, et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):707-712. [DOI] [PubMed] [Google Scholar]
- 29. Click B, Vargas EJ, Anderson AM, et al. Silent Crohn’s disease: asymptomatic patients with elevated C-reactive protein are at risk for subsequent hospitalization. Inflamm Bowel Dis. 2015;21(10):2254-2261. [DOI] [PubMed] [Google Scholar]
- 30. Sandor Kiss L, Papp M, Dorottya Lovasz B, et al. High-sensitivity C-reactive protein for identification of disease phenotype, active disease, and clinical relapses in Crohnʼs disease: a marker for patient classification? Inflamm Bowel Dis. 2012;18(9):1647-1654. [DOI] [PubMed] [Google Scholar]
- 31. Buisson A, Gonzalez F, Poullenot F, et al. ; ACCEPT study group. Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(8):1425-1433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon request to the corresponding author.