Abstract
STUDY QUESTION
What molecular mechanisms underlie the decline in ovarian reserve as the number and quality of oocytes decrease in patients with ovarian endometriomas (OEM)?
SUMMARY ANSWER
Elevated expression of the let-7 micro(mi)RNAs in the follicular microenvironment of OEM-affected ovaries targets the expression of type 1 insulin-like growth factor receptor (IGF1R) in granulosa cell (GC) and disrupts their proliferation, steroid hormone secretion levels, adenosine triphosphate (ATP) energy metabolism, and reactive oxygen species (ROS) oxidative stress levels.
WHAT IS KNOWN ALREADY
Patients with OEM exhibit diminished ovarian reserve, characterized by reduced oocyte quantity and quality. Fibrotic changes in the ovarian tissue surrounding the OEM create a disruptive microenvironment for follicular growth and development.
STUDY DESIGN, SIZE, DURATION
This is a cross-sectional study aimed to elucidate the molecular mechanisms underlying the impact of OEM on follicular development. Initially, miRNA expression profiles in follicular fluid (FF) samples were sequenced from patients with infertility related to OEM (N = 3) and male factor (MF) infertility (N = 3), with the latter serving as the control group. Differentially expressed miRNAs were validated in additional samples from each group (N = 55 in OEM group and N = 45 in MF group) to confirm candidate miRNAs. The study also investigated indicators associated with GCs dysfunction in vitro on rat GCs. Subsequently, rat models of OEM were established through endometrial allogeneic transplantation, and fertility experiments were conducted to assess the let-7/IGF1R axis response to OEM in vivo. Patient samples were collected between May 2018 and April 2019, and the mechanistic study was conducted over the subsequent three years.
PARTICIPANTS/MATERIALS, SETTING, METHODS
FF and GC samples were obtained from infertile patients undergoing IVF treatment for OEM and MF related infertility. miRNA expression profiles in FF samples were analyzed using second-generation high-throughput sequencing technology, and candidate miRNAs were validated through quantitative PCR (qPCR). In the in vitro experiments conducted with rat GCs, cell proliferation was assessed using the CCK-8 assay, while steroid hormone concentrations were measured using chemiluminescence. ATP content was determined with an ATP assay kit, and levels of ROS were quantified using flow cytometry. A dual luciferase reporter gene assay was employed to identify the target gene of let-7 based on the construction of a IGF1R reporter gene plasmid using 293T cells. Western blotting was utilized to evaluate the expression of IGF1R in GCs, as well as its downstream proteins, and changes in signaling pathways following let-7 agomir/antagomir transfection and/or Igf1r silencing. In the in vivo OEM rat models, alterations in ovarian structure and cyst morphology were observed using hematoxylin and eosin staining. The expressions of let-7 and Igf1r in GCs were evaluated through qPCR, while variations in IGF1R expression were investigated with immunohistochemistry.
MAIN RESULTS AND THE ROLE OF CHANCE
The cohort of patients with ovarian OEM in this study exhibited significantly decreased antral follicle counts, oocyte retrieval numbers, and normal fertilization rates compared to the control group with MF. The expression of the let-7 miRNA family was markedly upregulated in the FF and GCs of OEM patients. Transfection of rat GCs with let-7 agonists diminished the functions of GCs, including disrupted cell proliferation, mitochondrial oxidative phosphorylation, and steroid hormone secretion, while transfection of rat GCs with let-7 antagonists caused the opposite effects. Luciferase reporter gene experiments confirmed that let-7 complementarily bound to the 3′-untranslated regions of IGF1R. Stimulation of let-7 expression in rat GCs led to a significant decrease in IGF1R expression, while inhibition of let-7 increased IGF1R expression. The expression of IGF1R in the GCs of OEM patients was also significantly reduced compared to MF patients. Silencing of Igf1r led to the dysfunction of GCs, similar to the effects of let-7 agonization, as demonstrated by the downregulation of key proteins involved in cell proliferation (CCND2 and CCND3) and oestradiol synthesis, as well as an increase in progesterone synthesis (StAR), while implicating the PI3K-Akt and MAPK signaling pathways. The antagonistic effect of let-7 on GCs was ineffective when Igf1r was silenced. Conversely, the agonistic effect of let-7 on GCs could be reversed by stimulation with the IGF1R ligand IGF-1. These findings suggested that let-7 regulated the proliferation, differentiation, and ATP synthesis of GCs through targeting IGF1R. The OEM rat model demonstrated alterations in ovarian morphology and structure, along with reduced fertility. Let-7 expression was significantly upregulated in GCs of OEM rats compared to normal rats, while Igf1r and IGF1R expression in pre-ovulatory follicular GCs were notably downregulated, supporting the notion that elevated let-7 expression in the follicular microenvironment of OEM inhibited IGF1R, leading to abnormal GC function and impacting fertility at the molecular level.
LARGE SCALE DATA
N/A.
LIMITATIONS, REASONS FOR CAUTION
The synthesis and secretion mechanisms of steroid hormones are intricate and complex. Some enzymes that regulate oestrogen synthesis also play a role in progesterone synthesis. Moreover, certain receptors can respond to multiple hormone signals. Therefore, in this study, the expression patterns of key enzymes such as CYP17A, CYP11A1, HSD3B2, StAR, and receptors including AR, LHCGR, FSHR, ESR2, might be influenced by various factors and might not demonstrate complete consistency.
WIDER IMPLICATIONS OF THE FINDINGS
Future research will concentrate on investigating the potential impact of ovarian stromal cells on the external microenvironment of follicle growth. Additionally, screening for small molecule drugs that target let-7 and IGF1R actions can be conducted to intervene and modify the ovarian microenvironment, ultimately enhancing ovarian function.
STUDY FUNDING/COMPETING INTEREST(S)
This study received funding from the National Natural Science Foundation of China (grant number 82301851 to L.B.S., grant numbers U23A20403 and U20A20349 to S.Y.Z., and grant number 82371637 to Y.D.D.) and the Natural Science Foundation of Zhejiang Province (grant LTGY23H040010 to F.Z.). The authors have no conflicts of interest to declare.
Keywords: ovarian endometriomas (OEM), endometriosis, granulosa cells, follicular fluid, let-7, IGF1R, female infertility
Introduction
Endometriosis, also known as endometrial ectopia, affects ∼10% of women of reproductive age (Bulun et al., 2019; Chapron et al., 2019), leading to infertility in up to 50% of affected individuals (Taylor et al., 2021). Ovarian endometriosis (endometrioma, OEM) represents the predominant form of endometriosis, accounting for 17–44% of cases of pelvic endometriosis (Busacca and Vignali, 2003; Younis et al., 2019). Our initial research findings suggest that the layer of endometriotic cysts exhibits dense adhesion to the adjacent ovarian tissue, resulting in significant fibrosis in the surrounding ovarian tissue due to the secretion of TGF-β1 (Shi et al., 2017). Consequently, the normal ovarian tissue is damaged and follicles may develop within the fibrotic ovarian tissue, leading to a notable reduction in ovarian reserve.
Infertility resulting from OEM is recognized as one of the indications for IVF treatment (Chapron et al., 2019). Several meta-analyses investigating the impact of endometriosis on IVF outcomes have revealed that compared to women without endometriosis, those with the condition exhibit significantly lower numbers of oocytes retrieved and reduced implantation rates (Qu et al., 2022). Fertilization rates are diminished in stages I/II of endometriosis, while women with stages III/IV also experience decreased implantation and clinical pregnancy rates during IVF treatment (Harb et al., 2013). Additionally, individuals with endometriomas exhibit elevated basal FSH levels and reduced mean numbers of antral follicles, oocytes, MII oocytes retrieved, and total embryos formed, as well as increased cycle cancellation rates, in comparison to those without the disease (Hamdan et al., 2015; Yang et al., 2015; Alshehre et al., 2021). Consistent with these findings, our previous study unequivocally illustrates that IVF cycle outcomes in patients with endometriosis are markedly inferior to those without the condition, as evidenced by higher basal FSH levels and lower retrieved oocyte count and two-pronuclear (2PN) fertilization rate (Shi et al., 2022). The above meta-analyses, in conjunction with our research findings, validate that OEM contributes to a decline in ovarian reserve and compromised oocyte quality, although the precise molecular mechanisms driving this phenomenon remain unclear.
Antral follicles are primarily composed of oocytes, granulosa cells (GCs), follicular fluid (FF), and theca cells. Throughout the follicular growth process, GCs and FF constitute the internal microenvironment, which is crucial for the physiological processes such as oocyte maturation, ovulation, and follicular atresia. The main constituents of FF consist of serum and secretions from GCs, which contain a significant amount of hormones, kinases, growth factors, ionic compounds, and various metabolites (Poulsen et al., 2019). Among the regulatory factors present in FF, microRNA (miRNA) has garnered increasing attention in human reproduction (Eisenberg et al., 2015). miRNA is a non-coding, single-stranded small RNA molecule derived from eukaryotic nucleotides with a length typically ranging from 19 to 25 nucleotides. It has the ability to selectively recognize and bind to specific sites on the 3′-untranslated region (3′-UTR) of target mRNA, thereby exerting a post-transcriptional regulatory function (Lu and Rothenberg, 2018). Studies have indicated that miRNA plays a key role in processes such as regulating GCs proliferation and apoptosis (Li et al., 2022), steroid hormone synthesis and secretion (Zhang et al., 2020), follicle growth (Zhang et al., 2019), and embryo implantation (Paulson et al., 2022). A significant quantity of miRNAs can be found in human FF (Machtinger et al., 2016). The expression of miRNA in FF has the potential to serve as a preliminary indicator for assessing oocyte quality (Qasemi and Amidi, 2020; Zhang et al., 2021a) and predicting the clinical pregnancy outcome of IVF procedures (Khan et al., 2021a,b).
Nevertheless, there is limited research on the potential differences in miRNA expression in the FF of patients with OEM-related infertility, as well as its involvement in the regulation of ovarian reserve. Therefore, the aim of this study was to analyze the miRNA expression in the FF of patients with OEM-related infertility using high-throughput sequencing. The study identified differentially expressed miRNAs and further investigated one candidate miRNA, let-7 which impacts GCs function and follicular development, as well as the associated molecular mechanisms involved.
Materials and methods
Ethical approval
The utilization of human FF and GC samples was approved by the Institutional Review Board of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine (ethical approval No. 20200423-44), and signed informed consent was obtained from all participants before their inclusion. The inclusion of animals in this study was also authorized by the Ethics Review Committee for Laboratory Animal Welfare of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine.
Patient selection
FF and GCs were obtained from infertile women undergoing IVF either due to unilateral or bilateral OEM-related infertility or from women in couples with male factor (MF) infertility (referred to as the ‘MF group’) between May 2018 and April 2019. The inclusion criteria for both groups included individuals aged between 20 and 42 years, with regular menstrual cycles lasting 25–35 days, a body mass index (BMI) of 18–25 kg/m2, and normal levels of basal endocrine hormones (assessed on Days 2–5 of the menstrual cycle). Patients in the MF group did not exhibit signs of endometriosis or other female infertility issues; their infertility primarily resulted from reduced sperm quantity and/or quality in the male partner, chromosomal abnormalities, sexual dysfunction, among other factors. Patients in the OEM group had undergone surgical intervention for unilateral or bilateral OEM, received a definitive pathological diagnosis, and were classified as stage II–IV endometriosis (Johnson et al., 2017). Exclusion criteria for both groups included acute inflammation of the reproductive system, malignant tumors affecting the reproductive system, chromosomal abnormalities, ovulatory disorders due to endocrine conditions such as polycystic ovary syndrome, and a previous history of ovarian tumors or other abnormal ovarian conditions.
Follicular fluid and GCs collection
All patients enrolled in this study underwent ovarian stimulation using gonadotropins (Gn). The ovarian stimulation protocols were individualized based on monitoring of B-ultrasound and hormone levels. The dosage and duration of Gn usage were adjusted dynamically until the day of hCG trigger. The initial Gn dosage, total Gn dosage, duration of Gn stimulation, and daily Gn dosage for each participant in the study were documented, and their distribution between the two groups was compared, with the data presented in Table 1.
Table 1.
Clinical characteristics of infertile patients for let-7 validation.
| Parameters | Male factor | Endometriomas | P-value |
|---|---|---|---|
| (N = 45) | (N = 55) | ||
| Age (year) | 30.67 ± 3.94 | 32.15 ± 3.75 | 0.058 |
| BMI (kg/m2) | 20.61 ± 2.51 | 20.89 ± 2.34 | 0.572 |
| AMH (ng/ml) | 3.43 ± 2.25 | 2.30 ± 1.95 | 0.018* |
| Infertility duration (year) | 3.21 ± 2.44 | 2.94 ± 2.86 | 0.622 |
| Basal antral follicle count | 9.28 ± 3.69 | 5.75 ± 3.60 | <0.001*** |
| The initial Gn dosage (IU) | 207.5 ± 39.47 | 218.86 ± 61.33 | 0.286 |
| The total Gn dosage (IU) | 2056.06 ± 549.41 | 2274.77 ± 1039.42 | 0.181 |
| Duration of Gn stimulation (day) | 9.44 ± 1.47 | 9.45 ± 2.25 | 0.979 |
| Daily Gn dosage (IU) | 217.66 ± 42.35 | 232.85 ± 67.68 | 0.174 |
| Growing follicles no. | 8.98 ± 4.46 | 6.2 ± 3.89 | <0.001*** |
| Retrieved oocytes no. | 9.96 ± 6.45 | 5.84 ± 4.63 | <0.001*** |
| 2PN zygotes no. | 6.62 ± 5.07 | 3.65 ± 3.78 | <0.001*** |
| Cleavage stage embryos no. | 6.49 ± 4.93 | 3.56 ± 3.77 | <0.001*** |
| High-quality embryos no. | 2.07 ± 2.05 | 1.25 ± 1.83 | 0.039* |
| High-quality embryo rate | 31.85% (93/292) | 35.2% (69/196) | 0.44 |
| Fertilization no. | 7.22 ± 5.15 | 4.45 ± 4.23 | 0.004** |
| Fertilization rate | 79.27% (325/410) | 79.8% (245/307) | 0.86 |
| 2PN fertilization rate | 72.68% (298/410) | 65.47% (201/307) | 0.038* |
For continuous variables, the mean ± SD was provided. Student’s t-test was used to compare parameters for continuous data with a normal distribution, while the Mann–Whitney nonparametric U-test was employed for non-normally distributed parameters. Chi-squared test was utilized to analyze rates for comparison.
AMH, anti-Müllerian hormone; Gn, gonadotropins; No., number; 2PN, two-pronuclear.
P < 0.05,
P < 0.01,
P < 0.001.
When the diameter of the dominant follicle reached ≥18 mm or when there were three follicles with a diameter of ≥17 mm, the hCG (Livzon, Guangdong, China) trigger was administered. Oocyte retrieval was scheduled 36 h after the trigger. On the day of oocyte retrieval, oocyte–cumulus complexes (COCs) and FF were pulled from the follicular wall followed with pre-ovulatory follicle aspiration. FF from large follicles devoid of blood contamination was collected and subjected to centrifugation at 1000 g for 10 min at 4°C. Granulosa cells were isolated and collected from the COCs with syringe needles under a microscope for further experiments.
Based on previous meta-analysis and our research, the number of retrieved oocytes was identified as a significant indicator of difference between the two groups. Hence, the number of retrieved oocytes is utilized as the primary evaluation indicator for determining the sample size. It was anticipated that the average number of retrieved oocytes in the OEM group would be 6, while in the MF group, it was expected to be 10, with an SD of 5. With a one-sided α = 0.025 and a test power of 1 − β = 0.8, a minimum of 25 cases per group was determined to be necessary. Consequently, 45 patients from the MF group and 55 patients from the OEM group were included for let-7 validation from FF, and 32 patients from the MF group and 32 patients from the OEM group were included for let-7 validation from GCs.
miRNA extraction
Total RNA, including miRNA, was isolated from FF using the miRNeasy® Serum/Plasma Kit (Cat. no. 217184, QIAGEN, Germany) following the manufacturer’s instructions. Approximately 1 μg of total RNA was employed to generate a small RNA library as per the TruSeq Small RNA Sample Prep Kits protocol (Illumina, San Diego, USA). Subsequently, single-end sequencing (1 × 50 bp) was conducted on an Illumina Hiseq2500 at LC-BIO (Hangzhou, China) in accordance with the vendor’s recommended guidelines.
miRNA sequencing and analyzing
Raw reads underwent processing using an in-house software, ACGT101-miR (LC Sciences, Houston, TX, USA), for the removal of adapter dimers, junk, low complexity, common RNA families (rRNA, tRNA, snRNA, snoRNA), and repeats. Subsequently, unique sequences ranging from 18 to 26 nucleotides in length were aligned to specific species precursors in miRBase 22.0 (http://www.mirbase.org/) through a BLAST search to detect known miRNAs and novel 3p- and 5p-derived miRNAs. Length variations at both the 3′ and 5′ ends, as well as one mismatch within the sequence, were permitted in the alignment process. Unique sequences that aligned with mature miRNAs in the hairpin arms of specific species were recognized as known miRNAs. Those aligning with the opposite arm of the annotated mature miRNA-containing arm in specific species precursor hairpins were classified as novel 5p- or 3p-derived miRNA candidates. Differential expression of miRNAs based on normalized deep-sequencing counts was evaluated using the Student’s t-test. The significance threshold for the test was set at 0.05. Differentially expressed miRNAs were identified based on |log2(fold change (FC))| ≥ 1 and statistical significance (P < 0.05).
Cluster heatmap and volcano map analysis of differentially expressed miRNA
To analyze the distinct clustering patterns of miRNA from FF in the two groups, we conducted a differential miRNA clustering analysis. The normalized value of differential miRNA was represented by Z-value. The Z-value calculation formula is as follows: Zsample-i = [(normsample-i) − Mean(norm of all samples)]/[SD(norm of all samples)]. The overall distribution of miRNA expression was visualized with log2(FC) on the x-axis and −log10(Pvalue) on the y-axis. The x-axis indicated the FC of miRNA differential expression, while the y-axis indicated the statistical significance of the miRNA expression change.
Quantitative polymerase chain reaction
The bulge-loop™ let-7 quantitative polymerase chain reaction (qPCR) primer sets, consisting of one reverse transcription primer and a pair of qPCR primers specific for the let-7 family, were custom-designed and synthesized by RiboBio (MQP-0101, Guangzhou, China). The endogenous control for miRNAs was U6, while GAPDH served as the endogenous control for mRNA. Initially, miRNA extraction was carried out using the miRNeasy® Serum/Plasma Kit (QIAGEN) following the manufacturer’s protocol. Subsequently, the miRNA bulge-loop was synthesized through reverse transcription using M-MLV reverse transcriptase (Promega, Madison, WI, USA). Real-time qPCR was then conducted using the BeyoFast™ SYBR Green qPCR Mix (Beyotime, Shanghai, China) for monitoring amplification in real-time (40 cycles: 95°C/15s, 60°C/30s) with the primers from the bulge-loop™ let-7 qPCR primer set on a LightCycler® 480 system (Roche, Basel, Switzerland). The average cycle threshold (Ct) for each sample was calculated from triplicate wells, ensuring a variation of <0.5 Ct. All Ct values were normalized against the average Ct values from the MF group.
Oestradiol and progesterone concentration measurement
The concentrations of oestradiol (B84493, Beckman Coulter, Inc., USA) and progesterone (33550J2, Beckman Coulter, Inc., USA) were determined using the chemiluminescence method with the Beckman Coulter DXI 800 immunoassay system. Human FF was diluted 1000 times with 1% BSA, while the cell culture supernatant was directly subjected to detection.
Adenosine triphosphate detection
At 4°C, 80 μl of adenosine triphosphate (ATP) lysate was utilized to completely lyse the cells that were seeded into a 24-well plate. Subsequently, the lysate was centrifuged at 4°C, 12 000 g for 5 min, and the supernatant was collected for further analysis using the Enhanced ATP Assay Kit (S0027, Beyotime Biotechnology, China). Each sample was analyzed in triplicate wells, with 20 μl of lysate per well. The relative light unit (RLU) value was measured using a chemiluminescence instrument.
Reactive oxygen species detection
A reactive oxygen species (ROS) fluorometric assay kit (E-BC-K138-F, Elabscience, China) was employed to assess the ROS levels in GCs post-transfection. Adherent cells were detached using trypsin to obtain a cell suspension with a concentration ranging from 1.0 × 105 to 1.0 × 106 cells/ml. A positive control was established by treating cells with 50 μM t-butylhydroperoxide for 1 h. The GCs were then treated with 10 μM of the 2′,7′-dichlorofluorescin diacetate (DCFH-DA) probe at 37°C in the absence of light for 30 min. Any unabsorbed DCFH-DA was removed by washing the GCs two to three times with a washing solution. Subsequently, the fluorescence of 2′,7′-dichlorofluorescein was measured using flow cytometry with an excitation wavelength of 500 nm and a detection wavelength of 525 nm.
Isolation and culture of rat primary GCs
Primary GCs were isolated from the ovaries of 3-week-old female Sprague–Dawley rats. Following euthanasia of the rats via CO2 asphyxiation, the bilateral ovaries were promptly excised into phosphate-buffered saline (PBS) under sterile conditions. The surrounding adipose tissue and fallopian tubes were carefully removed in PBS. The ovaries were then transferred to L-15 Medium (21083-027, Gibco, USA) on ice for transportation. Under a stereoscope, the large follicles were punctured with two 1-ml syringe needles to fully release the GCs from the ovaries. The collected cell suspension containing GCs was filtered through a 40-μm filter, followed by centrifugation of the filtrate at 800 g for 5 min. The supernatant was discarded, and the GCs were resuspended in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12, MA0214-2, Meilunbio, China) supplemented with 10% fetal calf serum (35-079-CV, Corning, USA) and 1% Penicillin–Streptomycin solution (15140-122, Gibco, USA). The GCs were seeded onto culture dishes or plates and incubated at 37°C in a humidified atmosphere with 5% CO2. After 24 h of culturing, the culture medium was replaced to eliminate non-adherent cells and red blood cells. Adherent cells displaying robust growth were utilized for subsequent experiments.
Cell transfections
The GCs were transfected with let-7 agomir (miRNA mimic), antagomir (anti-miRNA), or siIgf1r using the riboFECT™ CP transfection kit (C10511, Ribobio, China) at a transfection concentration of 100 nM and a transfection duration of 48–96 h. For plasmid transfection into 293T cells, the Lipofectamine™ 3000 transfection kit (L3000001, Invitrogen, USA) was employed. The final transfection concentration of the plasmid was 2 μg per well in a 24-well plate, with a transfection duration of 48 h. Triplicate wells were utilized, and the experiments were conducted in triplicates or more.
Transcriptome sequencing of rat GCs transfected with let-7
Following transfection of let-7 into rat GCs at a concentration of 100 nM for 48 h, total RNA was extracted and purified using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA quality was assessed using the Bioanalyzer 2100 (Agilent, CA, USA), with a RIN number >7.0. Subsequently, the rat GCs RNA-Seq library was constructed according to the manufacturer’s instructions, with an average insert size of 300 ± 50 bp for the final cDNA library. Paired-end sequencing (2 × 150 bp) was conducted on an Illumina Novaseq™ 6000 at LC-Bio Technology Co. Ltd (Hangzhou, China). After removing reads containing adaptor contamination, low-quality bases, and undetermined bases, HISAT2 software (version: hisat2-2.0.4) was utilized for read mapping and StringTie was employed for read assembly. The transcriptomes from all samples were merged using gffcompare software to reconstruct a comprehensive transcriptome. Expression levels of mRNAs were estimated using StringTie by calculating FPKM values. Differentially expressed genes (DEGs) were identified based on |log2(FC)|≥1 and statistical significance (P < 0.05) using the R package edgeR. Subsequently, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment and gene set enrichment analysis (GSEA) analyses were performed on the DEGs. The R package Ggplot2 was used to visualize the data of enrichment analysis.
Reporter gene plasmid construction
Three potential complementary binding sites of let-7 on the 3′-UTR of IGF1R were predicted using the online prediction tools Targetscan and miRDB. The 3′-UTR sequence of IGF1R (gene number NM_000875.5) was retrieved from the GeneBank database. Forward and reverse primers containing the predicted let-7 binding sites on the 3′-UTR of IGF1R were designed using SnapGene. The forward primer was tagged with the restriction endonuclease NheI and its protective base at the 5′ end, while the reverse primer was tagged with the restriction endonuclease XhoI and its protective base. These primers were denoted as Wt-IGF1R-1#, Wt-IGF1R-2#, and Wt-IGF1R-3#. Corresponding site mutant primers were named Mut-IGF1R-1#, Mut-IGF1R-2#, and Mut-IGF1R-3#. The specific primer sequences were detailed in Supplementary Table S1, where capital letters signified the mutation site, and underlining indicated the NheI and XhoI restriction sites.
The reporter gene plasmid utilized in this study was pmirGLO, capable of expressing firefly luciferase and renilla luciferase. The three Wt-IGF1R 3′-UTR sequences were amplified and then ligated to the pmirGLO plasmid using NheI and XhoI restriction endonucleases to generate the wild-type pmirGLO-IGF1R 3′-UTR-WT constructs. Subsequently, the mutant vector pmirGLO-IGF1R 3′-UTR-MUT was created through site-directed mutagenesis PCR using the designed Mut-IGF1R primers. The plasmid construction methodology was in accordance with our previous study (Rong et al., 2019).
Dual luciferase reporter gene test
A dual luciferase reporter gene assay kit (RG027, Beyotime Biotechnology, China) was employed to determine the presence of a complementary binding site for the let-7 seed sequence within the 3′-UTR of IGF1R. The value of RLUfirefly luciferase/RLUrenilla luciferase served as an indicator of luciferase activity. A reduction in firefly luciferase activity signified binding of let-7 to the 3′-UTR of IGF1R, leading to a decrease in the RLU ratio, and vice versa.
Western blotting analysis
Proteins were extracted from primary or cultured GCs. The primary antibodies utilized in this study included GAPDH (10494-1-AP, Proteintech), IGF1R (ab182408), FSHR (22665-1-AP, Proteintech), ESR2 (14007-1-AP, Proteintech), LHCGR (19968-1-AP, Proteintech), AR (22089-1-AP, Proteintech), PR (ab32085, Abcam), CYP19A1 (ab124776, Abcam), CYP17A1 (ab125022, Abcam), CYP11A1 (14217s, Cell Signaling Technology), HSD3B2 (ab191515, Abcam), StAR (ab96637, Abcam), CCND2 (ab230883, Abcam), CCND3 (2936s, Cell Signaling Technology), Phospho-Erk1/2 (4370S, Cell Signaling Technology), Erk1/2 (4695S, Cell Signaling Technology), Phospho-Akt (Ser473) (4060S, Cell Signaling Technology), Phospho-Akt (Thr308) (13038S, Cell Signaling Technology), and Akt (pan) (4691S, Cell Signaling Technology). Following the incubation with primary antibodies, a horseradish peroxidase-conjugated secondary antibody was applied. The blots were developed using the ECL kit (Millipore, Billerica, MA, USA).
Animal experiment design
For the establishment of the OEM model, 24 female Sprague–Dawley rats were randomly allocated into three groups: Normal group (N = 6), Sham group (N = 6) where the ovarian capsule was incised, and OEM group (N = 12) where endometrial fragments were sutured onto bilateral ovaries following incision of the ovarian capsule. Additionally, 6 rats were designated as donors of uterine endometrial tissue. The presence of OEM cyst formation in the rat ovaries was evaluated at 4- and 8-week post-modeling.
For the fertility experiment, 32 female Sprague–Dawley rats were randomly divided into three groups: Normal group (N = 6), Sham group (N = 6), and OEM group (N = 20). Furthermore, 10 rats served as donors for endometrial fragments. At 8 weeks after establishing the OEM model, male and female mice were co-housed in a 1:2 ratio for 4 days. Female mice displaying vaginal plugs were considered impregnated and were housed individually. Between Days 8 to 12 post-co-housing, laparotomy was conducted on impregnated mice, with a 0.5% Evan’s Blue dye administered through the uterine vein to enumerate the embryos in each uterine horn.
Establishment of OEM model
The rats were anesthetized with 4% aqueous chloral hydrate (400 mg/kg) by intraperitoneal injection. The uterine artery of the donor rat in estrus was ligated to remove the uterus. Following excision, the uterus was cleansed with PBS, longitudinally opened, and cut into 0.5 × 1.0 cm fragments. These fragments were then preserved in L15 culture medium on ice for transplantation within 4 h. Subsequently, the endometrial fragments from the donor were then meticulously sutured onto the bilateral ovarian surface of a recipient rat using 6-0 absorbable sutures after incision of the ovarian capsule, ensuring proper orientation with the endometrial side facing the ovaries. Rats in the sham-surgery group underwent the same procedures, with the exception of endometrial fragment suturing.
Immunohistochemistry
Immunohistochemistry was performed as described previously (Shi et al., 2017) to detect the expression of IGF1R in the ovary of OEM rats. Briefly, a series of 5 μm sections was incubated with a rabbit monoclonal antibody [EPR19322] against IGF1 receptor (IGF1R; 1:200; ab182408, Abcam). Then goat anti-rabbit secondary antibodies (1:50; Beyotime Inc., Haimen, China) were employed for visualization.
Statistical analysis
Continuous variables were presented as mean ± SD (x ± S), and categorical variables were presented as percentages (%). The normality of all data was evaluated using the Shapiro–Wilk test. For continuous data following a normal distribution, comparisons of parameters were made using Student’s t-test, whereas non-normally distributed parameters were compared using the Mann–Whitney nonparametric U-test. Chi-squared tests were employed for the comparisons of rates. Linear regression and Pearson correlation coefficient were applied to assess the correlation between progesterone concentration in FF and the expression level of let-7. P < 0.05 was considered statistically significant. The statistical analysis was conducted using IBM SPSS Statistics v.20.0 for Mac software (IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics of infertile patients with OEM undergoing IVF treatment
The clinical characteristics of the patient cohort for validation are presented in Table 1. While there was no statistically significant difference in age between the two groups, the average AMH level, basal antral follicle count, growing follicle number, retrieved oocytes number, 2PN zygote number, cleavage stage embryo number, high-qualified embryo number, fertilization number, and 2PN fertilization rate of OEM group were significantly lower compared to those in MF group. These clinical findings were in line with the observation that OEM results in ovarian reserve dysfunction, resulting in a decrease in both the quantity and quality of oocytes. The FF and GC samples utilized in this study were all obtained from the cohort.
Differential miRNA expression profiling of FF associated with OEM
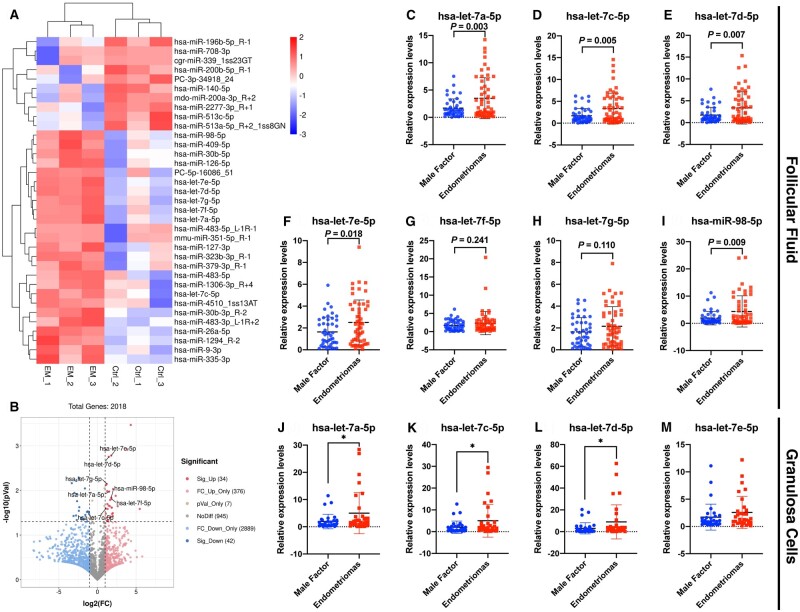
To elucidate the distinct miRNA expression profiles in FF of patients with OEM, we obtained FF samples from infertile patients with OEM (N = 3) and MF infertility (N = 3) for miRNA sequencing analysis. The cluster patterns for miRNA enrichment from FF between the OEM and MF groups are shown in Fig. 1A, demonstrating the similarity of miRNA expression profiles in different samples for each group. Except for infinite (Inf) values and duplicate miR_name, there were 4293 miRNAs detected in total, of which 76 significantly differentially expressed miRNAs (34 upregulated and 42 downregulated; Fig. 1B; Supplementary Table S2) were found in the FF by comparing the two groups. Interestingly, from the upregulated miRNA, we found that several miRNAs in let-7 family, including hsa-let-7a-5p, hsa-let-7c-5p, hsa-let-7d-5p, hsa-let-7e-5p, hsa-let-7f-5p, hsa-let-7g-5p, hsa-miR-98-5p, were all upregulated. We validated the upregulated miRNAs on expanded patient samples through qPCR (N = 55 in OEM group; N = 45 in MF group), and found that the expressions of hsa-let-7a-5p (Fig. 1C), hsa-let-7c-5p (Fig. 1D), hsa-let-7d-5p (Fig. 1E), hsa-let-7e-5p (Fig. 1F), and hsa-miR-98-5p (Fig. 1I) were significantly upregulated in FF from OEM patients, while hsa-let-7f-5p (Fig. 1G) and hsa-let-7g-5p (Fig. 1H) were not significantly upregulated. The above results validated the elevated expression of let-7 in the FF of infertile patients with OEM, indicating that aberrantly high let-7 expression was a potential target affecting follicle growth and development.
Figure 1.
miRNA sequencing of follicular fluid (FF) from patients with infertility associated with ovarian endometriomas (OEM) or male factor (MF), and validation of let-7 expression in FF and granulosa cells (GCs). (A) Cluster analysis of miRNA expression levels was shown, with the x-axis representing samples and the y-axis indicating the differentially expressed miRNAs (NMF = 3, NOEM = 3). (B) Volcano map analysis displayed differentially expressed miRNAs, with significantly upregulated miRNAs in red, downregulated miRNAs in blue, and non-significant miRNAs in gray. (C–I) Validation of hsa-let-7a-5p, hsa-let-7c-5p, hsa-let-7d-5p, hsa-let-7e-5p, hsa-let-7f-5p, hsa-let-7g-5p, and hsa-miR-98-5p in FF was conducted by expanding clinical samples through real-time quantitative PCR (qPCR) between the OEM and MF groups (NMF = 45, NOEM = 55). (J–M) Validation of hsa-let-7a-5p, hsa-let-7c-5p, hsa-let-7d-5p, and hsa-let-7e-5p in GCs was performed by expanding clinical samples through qPCR between the OEM and MF groups (NMF = 32, NOEM = 32). *P < 0.05.
Identification of let-7 family expression in human GCs
After confirming a notable increase in let-7 levels in the FF of patients with OEM, we proceeded to examine the variations in let-7 expression in GCs between the OEM and MF groups of infertile patients, considering that miRNAs primarily function intracellularly. From each group, 32 GC samples were collected, and the expressions of hsa-let-7a-5p, hsa-let-7c-5p, hsa-let-7d-5p, and hsa-let-7e-5p were assessed in GCs through qPCR analysis. Our results revealed a significant upregulation of hsa-let-7a-5p (Fig. 1J), hsa-let-7c-5p (Fig. 1K), and hsa-let-7d-5p (Fig. 1L) in the GCs of infertile patients in the OEM group compared to those in the MF group. The findings indicated that within the OEM patient follicular microenvironment, there was an upregulation in the expression levels of let-7 in both the FF and the GCs.
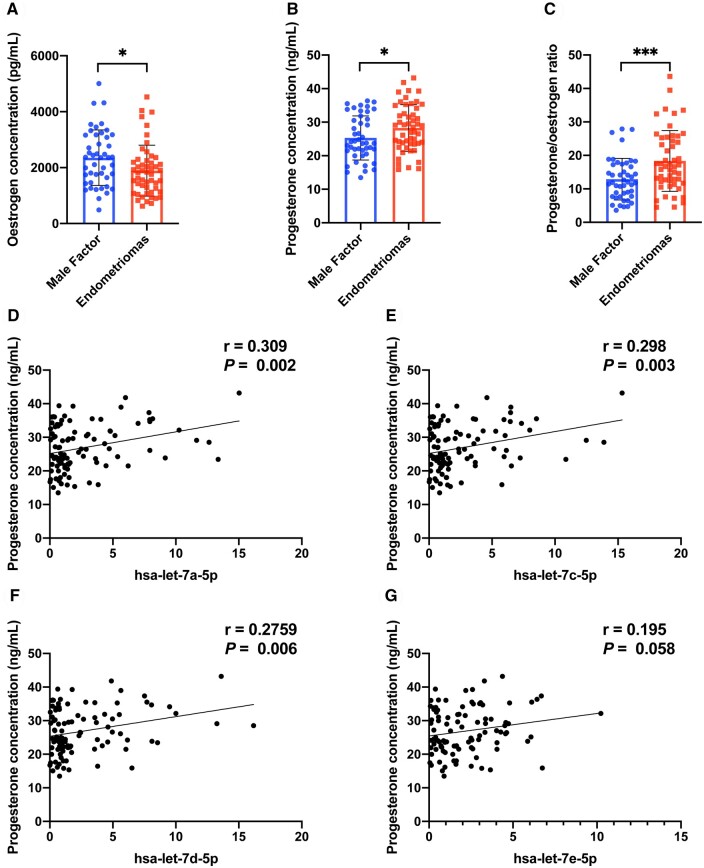
Comparison of hormone concentrations in FF between the OEM and MF groups
To further assess the differences in the microenvironment of FF between the two groups, we measured the concentrations of oestrogen and progesterone in FF on the day of oocyte retrieval. Table 1 shows that there were no significant differences in the initial Gn dosage, total Gn dosage, duration of Gn stimulation, and daily Gn dosage between the two groups during the ovarian stimulation process. However, the oestrogen concentration in the OEM group exhibited a significant decrease compared to the MF group (Fig. 2A), whereas the progesterone concentration showed a significant increase (Fig. 2B). Consequently, the progesterone/oestrogen ratio in the OEM group was notably higher (Fig. 2C). Intriguingly, the progesterone concentration displayed a significantly positive correlation with the expressions of hsa-let-7a-5p (P < 0.05, Fig. 2D), hsa-let-7c-5p (P < 0.05, Fig. 2E), and hsa-let-7d-5p (P < 0.05, Fig. 2F), while demonstrating a positive correlation trend with the expression of hsa-let-7e-5p (P = 0.058, Fig. 2G). These findings suggested that the elevated secretion of progesterone might be associated with the expression of let-7, which was implicated in compromised follicle development.
Figure 2.
Oestrogen and progesterone concentrations in follicular fluid (FF), and correlation analysis of let-7 expression and progesterone concentration. (A) Detection of oestrogen concentration in FF between the ovarian endometriomas (OEM) and male factor (MF) infertility groups. (B) Detection of progesterone concentration in FF between the OEM and MF groups. (C) Calculation of progesterone/oestrogen ratio in FF between the OEM and MF groups. (D) Correlation analysis of hsa-let-7a-5p expression and progesterone concentration. (E) Correlation analysis of hsa-let-7c-5p expression and progesterone concentration. (F) Correlation analysis of hsa-let-7d-5p expression and progesterone concentration. (G) Correlation analysis of hsa-let-7e-5p expression and progesterone concentration. NMF = 45, NOEM = 55, r, correlation coefficient, *P < 0.05, ***P < 0.001.
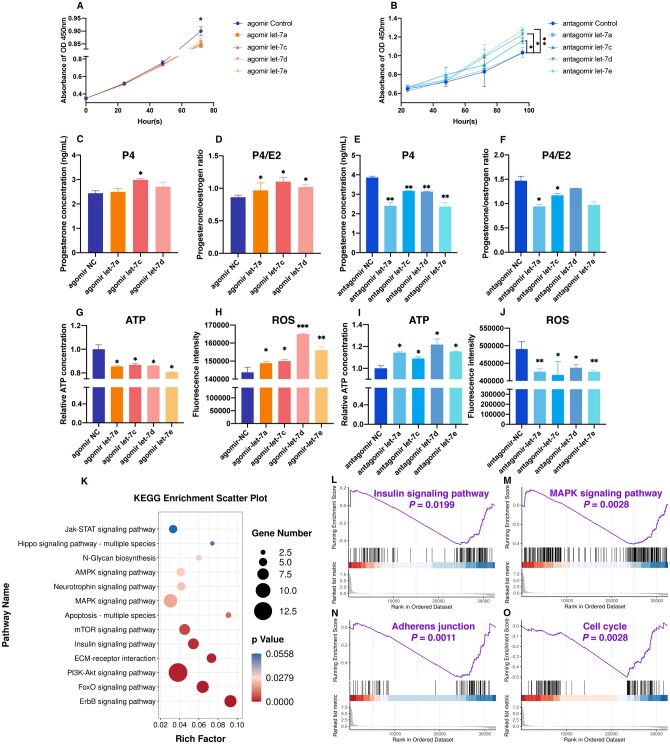
Transfection of let-7 into rat GCs leads to abnormal biological functions
Due to the increased levels of let-7 in FF and GCs of patients in the OEM group, we aimed to replicate this microenvironment in vitro in order to assess the impact of let-7 on the biological function of GCs. Rat GCs were isolated, cultured in vitro, and subsequently transfected by introducing let-7 agomir and antagomir into the culture medium. Subsequent to let-7 agomir transfection, the proliferation of rat GCs was suppressed (Fig. 3A), while it was increased after let-7 antagomir transfection (Fig. 3B). Progesterone secretion by GCs significantly increased following hsa-let-7c-5p agomir transfection (Fig. 3C), leading to a rise in the progesterone/oestrogen ratio (Fig. 3D). Conversely, inhibition of let-7 family members by let-7 antagomir led to a decrease in progesterone levels (Fig. 3E) and a reduction in the progesterone/oestrogen ratio (Fig. 3F). Particularly noteworthy is the pronounced inhibitory effect observed after transfection with hsa-let-7a-5p and hsa-let-7c-5p antagomir, demonstrating statistical significance. Furthermore, after let-7 agomir transfection, the oxidative stress in GCs was disrupted, as evidenced by a decrease in ATP levels (Fig. 3G) and an increase in ROS intensity (Fig. 3H). Conversely, ATP contents increased (Fig. 3I) and ROS levels decreased (Fig. 3J) after let-7 antagomir transfection. These findings collectively indicated that let-7 might be involved in impeding proliferation, modifying hormone secretion, and influencing the oxidative stress response in GCs.
Figure 3.
Effects of transfection of let-7 into rat primary granulosa cells (GCs). (A) Assessment of rat GCs proliferation within 72 h after transfection with let-7 agomir. (B) Assessment of rat GCs proliferation within 96 h after transfection with let-7 antagomir. (C) Measurement of progesterone concentration in rat GCs culture supernatant following let-7 agomir transfection. (D) Calculation of progesterone/oestrogen ratio in rat GCs culture supernatant post let-7 agomir transfection. (E) Evaluation of progesterone concentration in rat GCs culture supernatant after let-7 antagomir transfection. (F) Determination of progesterone/oestrogen ratio in rat GCs culture supernatant following let-7 antagomir transfection. (G) Adenosine triphosphate (ATP) detection in rat GCs post let-7 agomir transfection. (H) Reactive oxygen species (ROS) detection in rat GCs post let-7 agomir transfection via flow cytometry. (I) ATP detection in rat GCs post let-7 antagomir transfection. (J) ROS detection in rat GCs post let-7 antagomir transfection via flow cytometry. (K) Kyoto Encyclopedia of Genes and Genomes (KEGG) bubble chart illustrating the enrichment of signaling pathways post-transfection of let-7a agomir in rat GCs. Upregulated genes were depicted as red bars, while downregulated genes were represented by blue bars. (L–O) Gene set enrichment analysis (GSEA) demonstrating significant enrichment of signaling pathways post let-7a agomir transfection in rat GCs. *P < 0.05, **P < 0.01, ***P < 0.001.
Furthermore, we conducted the transcriptional profiling of rat GCs transfected with let-7. Functional enrichment analysis of the DEGs helped to identify the pathways affected by let-7. Through KEGG analysis, we discovered that these DEGs were implicated in various pathways, such as PI3K-Akt signaling, FoxO signaling, insulin signaling, and the mitogen-activated protein kinase (MAPK) signaling pathway (Fig. 3K). Through GSEA, we observed that the genes affected by let-7 agomir were predominantly enriched in insulin signaling pathways (Fig. 3L), MAPK signal pathways (Fig. 3M), adherens junctions (Fig. 3N), and the cell cycle (Fig. 3O). Notably, all of these pathways experienced downregulation.
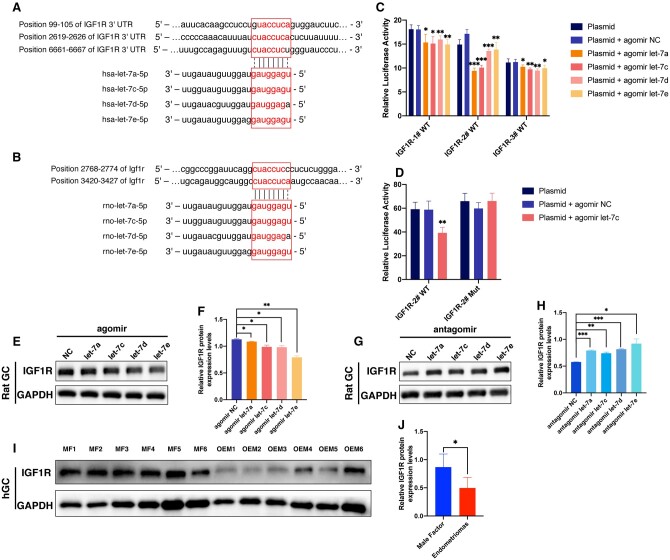
IGF1R identified as the target gene of let-7 in GCs
miRNAs have multiple target mRNAs. In order to predict the functional genes targeted by let-7 in GCs, we initially utilized public computational target prediction algorithms (TargetScan 7.1, miRDB 5.0 and miRTarBase Release 7.0) to compile a list of mRNAs with binding sites for let-7. Based on the literatures review, we selected the potential target mRNAs from this list including YAP1, IGF1R, CCND2, LIN28A/B, CYP19A1, and ESR2 as candidate target genes. Following let-7 agomir transfection, the protein expression levels of YAP1, IGF1R, ESR2, LIN28B, DUSP1, CYP19A1, and CCND2 were all reduced, while expressions of YAP1, IGF1R, ESR2, LIN28B, and CCND2 increased after let-7 antagomir transfection (Supplementary Fig. S1A–D). Similarly, mRNA expression levels of Igf1r, Esr2, Ccnd2, and DUSP1 decreased after let-7 agomir transfection in vitro, but increased following let-7 antagomir transfection (Supplementary Fig. S1E–L). The dual luciferase reporter assays conducted against these candidate target genes, including IGF1R, YAP1, ESR2, DUSP1, LIN28A, and LIN28B, revealed that only the 3′-UTR of IGF1R and LIN28B displayed potential complementary sites to let-7 in our system, resulting in a significant decrease in fluorescence intensity (Supplementary Fig. S1M–V).
Through a combination of previous KEGG and GSEA analyses of the insulin signaling pathway enrichment, as well as a literature review on the biological function of GCs, IGF1R was tentatively selected as the target gene of let-7 for further investigation. Subsequently, we performed a detailed analysis of the specific binding between let-7 and IGF1R.
Alignment analysis using SnapGene software revealed three binding sites for let-7 on the human IGF1R 3′-UTR sequence (NM_000875.5), located at positions 99-105, 2619-2626, and 6661-6667, respectively (Fig. 4A). Similarly, two binding sites for let-7 were identified on the rat Igf1r sequence (NM_052807.3), located at positions 2768-2774 and 3420-3427 (Fig. 4B). This suggested that IGF1R might be a downstream target gene through which let-7 exerted its effects.
Figure 4.
Identification of IGF1R as the target gene of let-7. (A) The let-7 family bound to three complementary sites in the 3′-untranslated region of the human IGF1R gene. (B) The let-7 family bound to two complementary sites in the rat Igf1r gene. (C) Validation of the interaction between let-7 and IGF1R was performed through a dual luciferase reporter assay, confirming the presence of the three complementary binding sites. (D) Further validation of the interaction between hsa-let-7c-5p and the IGF1R-2# site was conducted using the dual luciferase reporter gene. (E) Detection of IGF1R expression after transfection of let-7 agomir into rat granulosa cells (GCs) through western blotting. (G) Detection of IGF1R expression after transfection of let-7 antagomir into rat GCs via western blotting. (I) Evaluation of IGF1R expression in human GCs from the ovarian endometriomas (OEM) and male factor (MF) groups through western blotting (NMF = 6, NOEM = 6). (F, H, and J) represent the quantitative analysis of (E, G, and I), respectively. *P < 0.05, **P < 0.01, ***P < 0.001.
Subsequently, the validation of the target gene was performed using the dual luciferase reporter assay. Transfection of 293T cells with hsa-let-7a-5p, hsa-let-7c-5p, hsa-let-7d-5p, and hsa-let-7e-5p, along with three plasmids containing complementary sites of IGF1R 3′-UTR (pmirGLO-IGF1R-1#-WT, pmirGLO-IGF1R-2#-WT, and pmirGLO-IGF1R-3#-WT), resulted in significantly decreased fluorescence compared to the non-let-7 transfected group. Particularly, a pronounced reduction in fluorescence intensity was observed in the group, where hsa-let-7a-5p and hsa-let-7c-5p were co-transfected with pmirGLO-IGF1R-2#-WT (Fig. 4C). Furthermore, the IGF1R-2# site was mutated to construct a plasmid, pmirGLO-IGF1R-2#-Mut. Upon co-transfection with hsa-let-7c-5p, 293T cells with pmirGLO-IGF1R-2#-Mut showed no significant change in fluorescence, while cells with pmirGLO-IGF1R-2#-WT exhibited significantly decreased fluorescence intensity (Fig. 4D). These results demonstrated that hsa-let-7a-5p, hsa-let-7c-5p, hsa-let-7d-5p, and hsa-let-7e-5p could bind to the IGF1R 3′-UTR to exert post-transcriptional regulatory effects, with the most pronounced interaction effects observed for hsa-let-7a-5p and hsa-let-7c-5p at the IGF1R-2# site.
Furthermore, the post-transcriptional regulatory effects were validated in rat GCs. Transfection with hsa-let-7a-5p, hsa-let-7c-5p, hsa-let-7d-5p, and hsa-let-7e-5p agomir for 48 h resulted in a significant decrease in the expression of IGF1R protein in rat GCs as detected by western blotting (Fig. 4E and F). Conversely, transfection with hsa-let-7a-5p, hsa-let-7c-5p, hsa-let-7d-5p, and hsa-let-7e-5p antagomir for 48 h led to an increase in IGF1R expression (Fig. 4G and H). Additionally, human GC samples were collected from infertile patients with OEM and MF (six cases in each group) for IGF1R protein detection. The expression of IGF1R was found to be reduced in GCs from the OEM group compared to the MF group (Fig. 4I). Quantitative statistical analysis of the grayscale bars in Fig. 4I confirmed that the significantly decreased expression of IGF1R (Fig. 4J). These results demonstrated that let-7 bound to specific sites on IGF1R, leading to the degradation of IGF1R. Moreover, the expression of IGF1R in human GCs was indeed reduced in infertile patients with OEM, likely as a result of the increased levels of let-7.
Silencing of IGF1R affects the biological functions of GCs
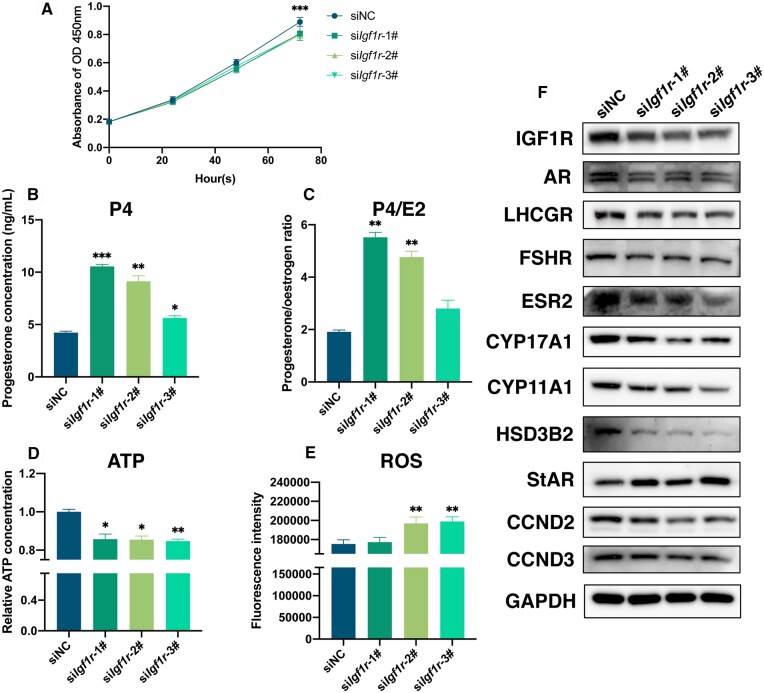
To investigate the importance of IGF1R in the biological function of GCs, the expression of Igf1r was silenced in rat GCs. Three siRNA sequences targeting Igf1r, namely siIgf1r-1#, siIgf1r-2#, and siIgf1r-3#, were designed and transfected into primary rat GCs. The effectiveness of Igf1r knockdown was demonstrated in Fig. 5F and Supplementary Fig. S2A, showing a decrease in the expression of IGF1R post-transfection. Additionally, a decrease in the number of proliferating GCs (Fig. 5A) was observed, accompanied by reduced expression of the proliferation-related proteins CCND2 and CCND3 (Fig. 5F and Supplementary Fig. S2J and K). These findings suggested that knockout of Igf1r resulted in the inhibition of GC proliferation.
Figure 5.
Evaluation of granulosa cell (GC) function after silencing Igf1r in rat GCs. (A) Assessment of rat GCs proliferation within 72-h post-Igf1r silencing. (B) Measurement of progesterone concentration in rat GCs culture supernatant following siIgf1r transfection. (C) Calculation of progesterone/oestrogen ratio in rat GCs culture supernatant post-siIgf1r transfection. (D) Adenosine triphosphate detection in rat GCs post-Igf1r silencing. (E) Reactive oxygen species detection in rat GCs post-siIgf1r transfection via flow cytometry. (F) Western-blotting detection of the transfection efficiency of IGF1R post-Igf1r silencing, and the expressions of AR, LHCGR, FSHR, ESR2, CYP17A1, CYP11A1, HSD3B2, StAR, CCND2, and CCND3 with GAPDH as the reference protein. *P < 0.05, **P < 0.01, ***P < 0.001.
Furthermore, the levels of oestradiol and progesterone in the cell culture supernatant of rat GCs were assessed post-transfection with siIgf1r. After 48 h of culturing rat GCs with siIgf1r-1#, siIgf1r-2#, and siIgf1r-3#, a significant increase in progesterone secretion by GCs was observed (Fig. 5B), along with an elevated progesterone/oestradiol ratio (Fig. 5C). The results indicated that knockout of Igf1r led to enhanced progesterone expression and GCs differentiation.
Subsequently, ATP levels and ROS content in rat GCs post-transfection with siIgf1r were measured. At 48 h after transfection with siIgf1r-1#, siIgf1r-2#, and siIgf1r-3#, rat GCs exhibited a notable reduction in ATP levels (Fig. 5D) and an increase in ROS intensity (Fig. 5E), suggesting that reduced Igf1r impacts the energy metabolism and oxidative phosphorylation levels of GCs mitochondria.
Moreover, the expression of downstream hormone related proteins was evaluated after siIgf1r transfection into rat GCs for 48 h. Western blotting results depicted in Fig. 5F revealed a significant downregulation in steroid hormone receptors protein, including AR, LHCGR, FSHR, and ESR2. Key enzymes involved in oestrogen synthesis, such as CYP17A1, CYP11A1, and HSD3B2, were downregulated, while the key enzyme involved in progesterone synthesis, StAR, was upregulated. The corresponding quantified graphs are presented in Supplementary Fig. S2. These results indicated that silencing of Igf1r impacted hormone synthesis and cellular responses.
MAPK and PI3K-Akt signaling pathways are involved in regulating GC function through IGF1R
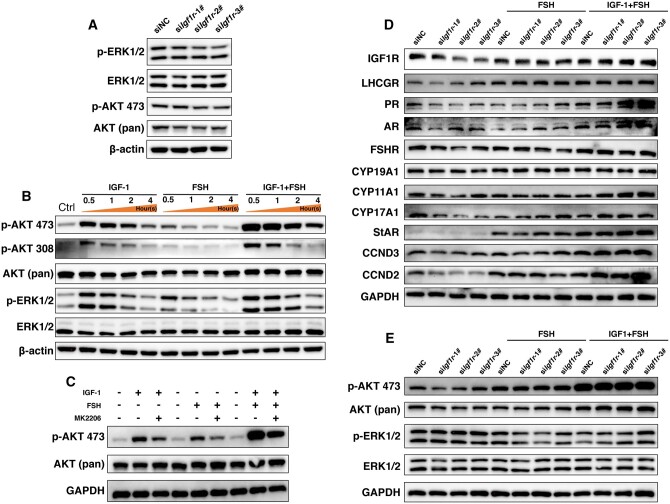
The sequencing results of let-7 transfected GCs in Fig. 3K suggested that MAPK and PI3K-Akt signaling pathways were likely involved in the let-7-induced dysfunction of GCs. Subsequently, key proteins in these two pathways were examined. Following the silencing of Igf1r by transfection with siIgf1r-1#, siIgf1r-2#, and siIgf1r-3#, the expression of phosphorylated ERK and AKT, involved in the MAPK and PI3K-Akt signaling pathways, was downregulated (Fig. 6A and Supplementary Fig. S3A–D), indicating that the impact of Igf1r on GCs dysfunction was primarily mediated through the MAPK and PI3K-Akt signaling pathways.
Figure 6.
Detection of changes in the MAPK and PI3K-Akt signaling pathways after silencing Igf1r in rat granulosa cells (GCs). (A) Detection of p-ERK1/2, ERK1/2, p-AKT 473, and AKT (pan) expression following siIgf1r transfection. (B) Detection of p-AKT 473, p-AKT 308, AKT (pan), p-ERK1/2, and ERK1/2 expression after stimulating with IGF-1/FSH within 4 h. (C) Detection of p-AKT 473 and AKT (pan) expression after co-culturing with a PI3K-Akt signaling pathway inhibitor (MK2206) prior to IGF-1/FSH stimulation. (D) Detection of the expressions of IGF1R, LHCGR, PR, AR, FSHR, CYP19A1, CYP11A1, CYP17A1, StAR, CCND3, and CCND2 post-Igf1r silencing, and the reverse effects with IGF-1/FSH stimulation. (E) Detection of the expressions of p-AKT 473, AKT (pan), p-ERK1/2, and ERK1/2 after siIgf1r transfection, and the reverse effects induced by IGF-1/FSH.
To investigate whether the PI3K-Akt and MAPK signaling pathways could be activated by IGF-1/FSH (as IGF-1 is the ligand of IGF1R and FSH has been reported to stimulate the expression of downstream signals of IGF1R; Francoeur et al., 2023), primary rat GCs were treated with 100 ng/ml IGF-1 and 1 IU/ml FSH. At 30-min post-IGF-1/FSH stimulation, key molecules AKT and ERK in the PI3K-Akt and MAPK signaling pathways exhibited significant phosphorylation, with p-AKT and p-ERK sustained for up to 4-h post-stimulation. Furthermore, the potency of the effect of IGF-1 was higher than that of FSH, and the combined effect of IGF-1+FSH on GCs surpassed the effects of IGF-1 and FSH individually (Fig. 6B and Supplementary Fig. S3F–H). To further explore the response of the PI3K-Akt pathway to IGF-1/FSH, a selective inhibitor of the PI3K-Akt signaling pathway, MK2206, was administered to rat GCs prior to IGF-1/FSH stimulation. The results revealed that MK2206 partially hindered the phosphorylation of AKT at the Ser473 site induced by IGF-1, FSH, and IGF-1+FSH (Fig. 6C and Supplementary Fig. S3E). These findings indicated that IGF-1 and FSH could stimulate the activation of the PI3K-Akt signaling pathway, with the most pronounced synergistical effect observed in their combined presence, and this activation could be selectively inhibited by MK2206.
Based on the aforementioned findings, the potential reversal effects of IGF-1/FSH on the silencing effects of siIgf1r on rat GCs were investigated. As depicted in Fig. 6D, the expression of IGF1R in rat GCs was significantly reduced post-transfection with siIgf1r. However, the addition of FSH led to an upregulation of IGF1R expression, with a more pronounced rescuing effect observed with FSH+IGF-1. Similar expression patterns were observed for steroid hormone receptors, including LHCGR, PR, AR, and FHSR, as well as key cell proliferation proteins such as CCND2 and CCND3. Notably, this induction pattern minimally affected the key enzyme CYP19A1 involved in oestradiol synthesis but had a more significant impact on progesterone synthesis key enzymes StAR and CYP11A1 (Fig. 6D and Supplementary Fig. S3I–S). Additionally, evaluation of changes in key molecules in the PI3K-Akt pathway and MAPK pathway revealed that compared to the MAPK pathway, the PI3K-Akt pathway exhibited a more pronounced response to stimulation by IGF-1+FSH (Fig. 6E and Supplementary Fig. S3T–U). These results suggested that the silencing effects on GCs induced by siIgf1r could be reversed with IGF-1+FSH, with the PI3K-Akt pathway playing a crucial role in this process.
let-7 causes dysfunction of GCs through targeted inhibition of IGF1R
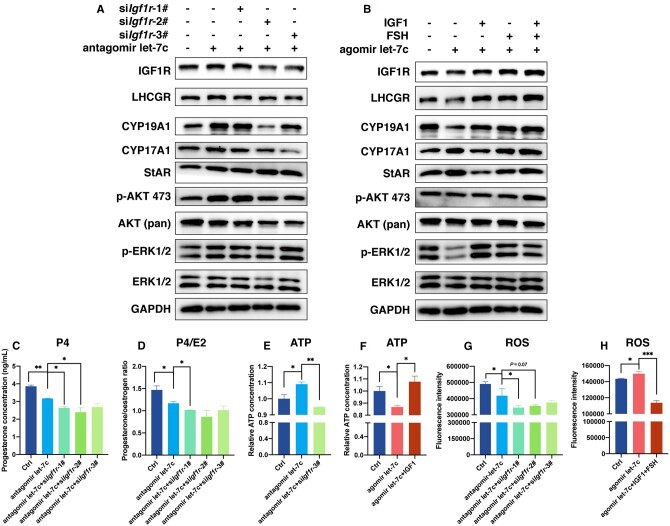
To investigate the regulatory role of let-7 on GCs via IGF1R, we conducted reversal experiments involving the antagonistic effect of let-7 reversed by siIgf1r, and the agonistic effect of let-7 reversed by IGF-1/FSH. Among the let-7 family members, let-7c was chosen as it exhibited the most significant impact on GC function.
In primary rat GCs, the expression of IGF1R was upregulated following the antagonism of let-7c. However, the antagonistic effect of let-7 on IGF1R expression was attenuated by silencing Igf1r, resulting in a downregulation of IGF1R expression, particularly evident when targeting the 2# and 3# sites of Igf1r. These findings suggested that let-7c exerted its antagonistic effect on GCs by directly targeting Igf1r. Key enzymes involved in the synthesis of oestradiol (CYP19A1 and CYP17A1), and luteal function supporting (LHCGR), exhibited similar expression patterns, indicating that these receptors and hormone synthesis enzymes were likely downstream targets of the let-7/Igf1r axis, with the PI3K-Akt pathway playing a more crucial role than the MAPK signaling pathway in this process (Fig. 7A and Supplementary Fig. S4A–I). Conversely, transfection of a let-7c agonist led to a reduction in IGF1R expression in GCs. However, the addition of IGF-1, FSH, or IGF-1+FSH reversed the let-7c-induced downregulation of IGF1R, resulting in an upregulation of IGF1R expression, with the synergized effect of IGF-1+FSH being the most pronounced. The key enzyme involved in oestradiol synthesis, CYP19A1, displayed a similar trend. Notably, the expression of key enzymes involved in progesterone synthesis, StAR, was upregulated following let-7c agonist treatment but downregulated after IGF-1 addition, indicating the ability of IGF-1 to reverse the inhibitory effect of let-7 on Igf1r. While changes in the PI3K-Akt pathway were minimal during this process, significant alterations were observed in the key proteins in the MAPK pathway, suggesting that the reversal effect primarily occurred through the MAPK pathway (Fig. 7B and Supplementary Fig. S4J–R).
Figure 7.
let-7 causes dysfunction of granulosa cells (GCs) through targeting Igf1r. (A) Detection of the expressions of IGF1R, LHCGR, CYP19A1, CYP17A1, StAR, p-AKT 473, AKT (pan), p-ERK1/2, and ERK1/2 following let-7c antagomir transfection, and let-7c antagomir plus Igf1r silencing, demonstrating that the antagonistic effects of let-7 on GC function could be interrupted by Igf1r silencing. (B) Detection of the expression of the same group of proteins after transfection with let-7c agomir, followed by stimulation with IGF-1/FSH, indicating that the agonistic effect of let-7 on GC function could be reversed by IGF-1/FSH stimulation. (C) Measurement of progesterone concentration in rat GCs after let-7c antagomir transfection plus Igf1r silencing. (D) Calculation of progesterone/oestrogen ratio after let-7c antagomir transfection plus Igf1r silencing. (E) Adenosine triphosphate (ATP) detection after let-7c antagomir transfection plus Igf1r silencing. (F) ATP detection following transfection with let-7c agomir, and subsequent stimulation with IGF-1/FSH. (G) Detection of reactive oxygen species (ROS) in rat GCs via flow cytometry after transfection with let-7c antagomir in conjunction with Igf1r silencing. (H) ROS detection in rat GCs using flow cytometry after transfection with let-7c agomir, and subsequent stimulation with IGF-1/FSH. *P < 0.05, **P < 0.01, ***P < 0.001.
By assessing the concentration of progesterone in the supernatant of primary rat GCs co-transfected with let-7c antagomir and siIgf1r, we observed a decrease in progesterone concentration following let-7 antagonism alone. Despite the expectation that silencing Igf1r would upregulate progesterone secretion (Fig. 5B), the expression of progesterone remained downregulated after silencing Igf1r in the presence of let-7 antagonism (Fig. 7C). The progesterone/oestradiol ratio exhibited a similar trend (Fig. 7D), indicating that the let-7/Igf1r axis influenced progesterone synthesis and secretion in GCs.
Furthermore, the addition of a let-7 antagonist resulted in elevated ATP contents (Fig. 3I) and decreased ROS levels (Fig. 3J), aligned with previous findings that silencing Igf1r led to decreased ATP contents (Fig. 5D) and increased ROS intensity (Fig. 5E). However, co-transfection with let-7c antagomir and siIgf1r resulted in a decrease in ATP levels (Fig. 7E) and also a decrease in ROS intensity (Fig. 7G), suggesting that the antagonistic effect of let-7 on ATP levels was abolished when Igf1r was silenced, and the effect of siIgf1r on ROS intensity was counteracted when let-7 was antagonized. Conversely, the agonistic effect of let-7 resulted in a targeted inhibition of Igf1r, leading to decreased ATP levels (Fig. 3G) and increased ROS intensity (Fig. 3H). Nonetheless, the addition of IGF-1 or IGF+FSH was able to rescue the decrease in ATP levels and increase in ROS intensity induced by the let-7 agonist (Fig. 7F and H). These results collectively indicated that let-7 regulates ATP levels and ROS intensity in GCs by targeting Igf1r.
Establishment of rat OEM models and validation of the let-7/Igf1r axis expression
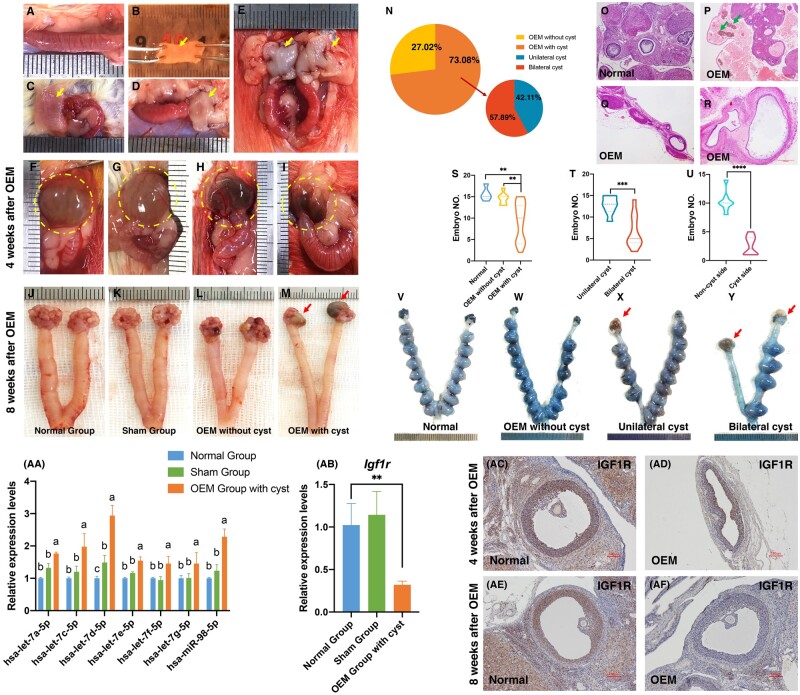
To explore the potential impact of OEM on the let-7 expression within the follicular microenvironment and its effect on the downregulation of IGF1R expression, rat models of OEM were established via endometrial allogeneic transplantation. The procedure involved the removal of the uterus from estrous rats, followed by washing with PBS, longitudinal cutting, unfolding, and horizontal cutting into 0.5×1.0 cm fragments (Fig. 8A–C). These fragments were then transplanted onto the bilateral ovarian capsules of recipient rats, with the endometrial side facing the ovaries (Fig. 8D–E). Ovarian cysts were observed 4-week post-transplantation, containing chocolate-colored fluid (Fig. 8F–I). By 8-week post-surgery, OEM cysts were evident, displaying distinct morphological differences from normal ovarian tissue and containing chocolate-colored substances (Fig. 8J–M). While not all transplanted rats developed OEM cysts, the overall cyst formation rate was 73.08%, with unilateral and bilateral cyst formation rates of 42.11% and 57.89%, respectively (Fig. 8N). Histological examination revealed disrupted ovarian morphology in OEM rats, with cysts compressing and deforming the ovaries, surrounded by irregular liquid-filled structures containing hemosiderin (Fig. 8O–R). These findings indicated a significant reduction in biologically active ovarian structures compared to normal rat ovaries.
Figure 8.
Establishment of the ovarian endometriomas (OEM) rat model and identification of the let-7/Igf1r axis in the follicular microenvironment. (A–E) Establishment of the OEM rat model through endometrial allogeneic transplantation. (F–I) Visualization of OEM cysts 4-week post-endometrium transplantation. (J–M) Visualization of OEM cysts 8-week post-endometrium transplantation. (N) Assessment of OEM cyst formation rate. (O–R) Histological examination of OEM cyst morphology using hematoxylin and eosin staining. (S) Comparison of embryo formation among normal rats, rats with and without OEM. (T) Comparison of embryo formation between rats with unilateral OEM cysts and rats with bilateral OEM cysts. (U) Comparison of embryo formation between the cystic and non-cystic sides in rats with unilateral OEM cysts. (V–Y) Illustration of embryo formation in normal rats (V), rats without OEM cysts (W), rats with unilateral OEM cysts (X), and rats with bilateral OEM cysts (Y). (Yellow arrows indicate fresh endometrium from donor rats, green arrows indicate the iron-containing heme in the fluid within the sac, and red arrows indicate OEM cysts.) (AA) Comparison of let-7 expression in granulosa cells (GCs) among rats in the normal group, sham group, and OEM group with ovarian cysts. Groups with different superscript letters above the columns indicate significant differences in the expression of let-7. (AB) Comparison of Igf1r expression in GCs among rats in the normal group, sham group, and OEM group with ovarian cysts. (AC–AF) Immunohistochemical analysis of IGF1R expression in pre-ovulation follicles of OEM cyst ovaries after 4 or 8 weeks of OEM model establishment. **P < 0.01, ***P < 0.001, ****P < 0.0001.
In line with the poor clinical outcomes observed in OEM patients undergoing IVF, fertility experiments were conducted in OEM rats. Embryo formation was significantly reduced in the OEM cystic group compared to both normal rats and non-cystic rats post-transplantation (Fig. 8S). Bilateral cyst rats exhibited a greater reduction in embryo formation compared to unilateral cyst rats (Fig. 8T). Additionally, the ability for embryonic formation on the cystic side was significantly lower than that on the non-cystic side (Fig. 8U). Images of uteri with embryos further illustrated the decreased embryo numbers in rats with OEM cysts (Fig. 8V–Y), confirming the adverse impact of OEM cysts on rat fertility.
GCs were collected from pre-ovulatory follicles of these rats under a stereomicroscope to assess let-7 family expression. The results demonstrated a significant upregulation of let-7 expression in rat GCs from the OEM group compared to those from the normal and sham groups (Fig. 8AA), supporting OEM as a driver of let-7 upregulation. Moreover, Igf1r expression was notably downregulated in OEM rat GCs (Fig. 8AB), suggesting a response to the elevated let-7 levels. Immunohistochemical analysis revealed decreased IGF1R expression in GCs of pre-ovulatory follicles in OEM rats compared to normal rats at both 4- (Fig. 8AC and AD) and 8-week post-surgery (Fig. 8AE and AF), indicating inhibition of IGF1R protein expression in the follicular microenvironment of OEM rats. These findings collectively confirmed that OEM induces upregulation of let-7 expression in the follicular microenvironment, leading to the downregulation of IGF1R expression at both the gene and protein levels.
Discussion
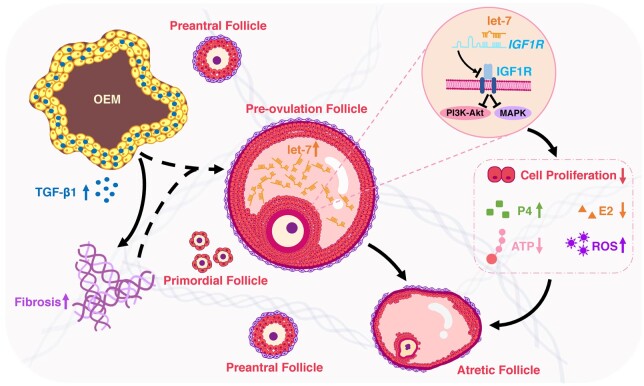
This study reveals that increased levels of let-7 in the FF of OEM patients can impede the functions of GCs by targeting IGF1R. This inhibition includes the suppression of GC proliferation, enhancement of GC differentiation (progesterone accumulation), and an impact on the mitochondrial oxidative phosphorylation system of GCs, leading to irregular follicular development (Fig. 9). These findings elucidate the molecular mechanisms underlying the ovarian dysfunction and potential infertility caused by OEM.
Figure 9.
Schematic diagram of this study. Ovarian endometriomas induce the upregulation of let-7 expression in follicular fluid, resulting in dysfunction of granulosa cells through targeting of IGF1R.
FF plays a crucial role as a microenvironment for GCs development and oocyte maturation. Various miRNAs are present in the FF and exert their influence on follicular development at different stages (Machtinger et al., 2016; Wang et al., 2021). In individuals with endometriosis, the expression of hsa-mir-451 is downregulated in the FF. Microinjection of an miR-451 inhibitor into MI oocytes leads to developmental arrest in 2PN zygotes, 2-cell, 8-10 cell, and blastocyst-stage embryos (Li et al., 2019b). Additionally, it has been reported that miR-122-5p is highly expressed in FF exosomes of patients with endometriosis, affecting the glucose metabolism function of cumulus cells and potentially compromising oocyte quality (Zhang et al., 2024).
In our investigation, we observed a significant upregulation of the let-7 family in the FF of infertile patients with OEM. The let-7 family was initially identified in the nematode Caenorhabditis elegans (C. elegans), where it plays a role in regulating the temporal development of the nematode and determining its morphological transition from larva to adult (Reinhart et al., 2000). Members of the let-7 family exhibit highly conserved nucleotide sequences at their 5′ ends, known as the ‘seed sequence’ (TGAGGTA) (Roush and Slack, 2008). By binding to the 3′-UTR region of target mRNA via the seed sequence, they can then utilize the induced silencing complex to degrade or inhibit the expression of target genes (Bagga et al., 2005). Previous studies have indicated the involvement of let-7 in regulating follicular atresia. In porcine atretic follicles, the expression of let-7g is upregulated (Cao et al., 2015a), with high expression targeting MAP3K1 to promote GCs apoptosis and follicular atresia (Cao et al., 2015a). Furthermore, the high expressions of let-7b and let-7i in bovine oocytes suggest that let-7 may play a role in regulating oocyte maternal gene expression and participate in oocyte development (Miles et al., 2012).
In patients with OEM, the high expression of let-7 in the FF can directly impact the GCs that are situated in the same follicular microenvironment, leading to disruptions in GC function. The proliferation and differentiation of GCs have direct implications on the recruitment, growth, development, ovulation, corpus luteum formation, and steroid hormone secretion of follicles (Llerena Cari et al., 2021). Increasing evidence indicates that miRNAs primarily regulate ovarian function by influencing GCs (Maalouf et al., 2016; Tu et al., 2019). The let-7 family is abundant in the ovary and is involved in follicular proliferation, apoptosis (Cao et al., 2015b), atresia (Zhang et al., 2019), autophagy (Li et al., 2021), and aging (Liu et al., 2021). In our study, it was observed that the let-7 agonist inhibited GC proliferation (Fig. 3A), whereas the let-7 antagonist promoted GC proliferation in vitro (Fig. 3B). The decreased proliferation function of GCs induced by the let-7 agonist directly leads to follicular atresia and apoptosis.
In atretic follicles, GCs show reduced expression of aromatase and FSHR mRNA (Dierschke et al., 1994), decreased synthesis of oestradiol, and increased synthesis of progesterone (Gross et al., 2001). The rise in progesterone levels during the late follicular phase has been associated with reduced reproductive outcomes (Friis Wang et al., 2019). Elevated progesterone levels on the day of oocyte maturation may impact the quality of embryos in IVF cycles (Huang et al., 2016). A higher progesterone/oestradiol ratio in FF is considered an objective indicator for follicular premature luteinization and atresia because it demonstrates a significant positive correlation with the activity of nucleases in GCs (Khandoker et al., 2001; Yu et al., 2004). In our study, patients with OEM demonstrated an upregulation of progesterone and a downregulation of oestradiol concentration in FF (Fig. 2A–C), suggesting a decline in follicular quality and a tendency toward atresia. Interestingly, the progesterone concentration in FF was positively correlated with the expression of let-7 (Fig. 2D–F), indicating that let-7 may impact the secretion of progesterone in GCs. Moreover, the administration of let-7 agomir led to a notable elevation in progesterone levels and the progesterone/oestradiol ratio in primary GCs (Fig. 3C and D), whereas the use of let-7 antagomir yielded the opposite outcome (Fig. 3E and F). These results suggest that let-7 is implicated in the premature luteinization of GCs. This finding aligns with a previous study demonstrating that the transfection of let-7 in vitro can enhance the secretion of progesterone in human GCs (Sirotkin et al., 2009).
The oxidative phosphorylation system, also known as the mitochondrial respiratory chain, serves as the primary cellular energy source. Disruption of this system results in reduced ATP production and excessive ROS generation, impacting cell growth and metabolism (Zotta et al., 2024). Normal mitochondrial function in GCs is essential for follicular development (Liu et al., 2017), as they can utilize glucose metabolic product pyruvate as an ATP synthesis substrate in oocytes (Wen et al., 2020), thereby safeguarding oocytes against oxidative stress damage (von Mengden et al., 2020). ATP, the direct cellular energy supply form, is pivotal for oocyte development and maturation (Roth, 2018), and is closely linked to the number of retrieved oocytes, mature oocyte proportion, implantation rate, and pregnancy outcomes (Hsu et al., 2015; Kansaku et al., 2017). Reduced ATP levels in GCs signify impaired or diminished mitochondrial function, directly impacting oocyte quality. ROS, as byproducts of mitochondria, are highly reactive oxygen free radicals from cellular metabolism, key markers of oxidative stress reactions. Excessive ROS accumulation in the follicular microenvironment and impaired clearance can disrupt the oxidation-antioxidant system balance, leading to oxidative stress damage (Yang and Lian, 2020). This process causes mitochondrial dysfunction and decreases ATP levels, ultimately triggering GCs apoptosis (Virant-Klun et al., 2018), negatively impacts follicular growth (Devine et al., 2012). This study indicated that following let-7 agomir transfection in GCs, ATP levels decreased (Fig. 3G) and ROS levels increased (Fig. 3H). Conversely, transfection with let-7 antagomir showed the opposite trend (Fig. 3I and J). These findings suggest that let-7 affects the mitochondrial oxidative phosphorylation level in GCs, thereby indirectly affecting oocytes. Our research results further provide evidence that let-7 can affect mitochondrial function by participating in energy metabolism (Jiang et al., 2018; Sun et al., 2019).
In this study, it was confirmed that IGF1R was a downstream target gene of let-7, involved in the regulation of GC function in patients with OEM. let-7 could specifically bind to 3′-UTR region of IGF1R, thereby inhibiting its translation and regulating its expression at the post-transcriptional level. The effects of IGF1R silencing on GC function resembled that after the transfection of let-7 agonist. Furthermore, the impact of let-7 on GC function was validated through two reverse experiments, indicating that its effects are achieved by targeting and inhibiting IGF1R, with the involvement of the PI3K-Akt and MAPK signaling pathways (Fig. 7).
IGF1R belongs to the insulin receptor subfamily, located on the cell membrane, and can be activated by insulin-like growth factors (IGF1 or IGF2), leading to phosphorylation of its own tyrosine kinase domain and initiation of intracellular signaling pathways that regulate cell growth, differentiation, development, aging, and other cellular processes (Li et al., 2019a). The targeted regulatory effects of let-7 on IGF1R have been reported in various systems. For instance, let-7 is implicated in the regulation of glucose metabolism through its interaction with IGF1R (Zhu et al., 2011). In colon cancer cells, let-7e and IGF1R mutually regulate each other, collectively inhibiting cell proliferation and migration (Li et al., 2018). Moreover, let-7 directly targets IGF1R to modulate the sensitivity of colon cancer cells to radiotherapy (Samadi et al., 2019). In endometrial stromal cells of endometriosis patients, let-7 can target IGF1R to reduce endometrial receptivity (Ghazal et al., 2015). Additionally, there are reports indicating that let-7 regulates autophagy in GCs by targeting IGF1R (Zhou et al., 2016).
It has been reported that the expression of IGF1R in GCs is essential for steroidogenesis, folliculogenesis, follicle survival, and the maintenance of fertility in female mice. Conditional knockout of Igf1r in mice antral follicles using an Amhr2-Cre resulted in increased apoptosis of GCs (Douglas et al., 2024). Conditional knockout of Igf1r in mice primary follicles using Esr2-Cre and Cyp19-Cre resulted in impaired pre-ovulatory follicle development, decreased expression of pre-ovulatory markers CYP19A1 and LHCGR in GCs, reduced ovarian volume, increased levels of follicle apoptosis, absence of pre-ovulatory and ovulatory follicles, and decreased serum oestradiol levels following gonadotropin stimulation, ultimately leading to female infertility (Baumgarten et al., 2017). Similarly, conditional knockout of Igf1r using Pgr-Cre in mice pre-ovulatory follicles led to reduced expression of key enzymes involved in steroid synthesis and lack of luteal support, resulting in decreased fertility in mice (Sekulovski et al., 2020). These findings underscore the significance of IGF1R in follicle development, ovulation, and luteinization processes. Our study further validated that the loss of IGF1R in GCs impairs functionality, including inhibition of proliferation, increased progesterone secretion, decreased ATP levels, and elevated ROS levels. These effects were mediated through the MAPK and PI3K-Akt signaling pathways, emphasizing the crucial role of IGF1R in maintaining GC function.
In the context of endometriosis pathogenesis, the current establishment of endometriosis models primarily involves spontaneous animal models of endometriosis, such as rhesus monkeys and baboons (Grummer, 2006; Yamanaka et al., 2012), induced models of endometriosis, which include monkeys, rabbits, and mice (Takahashi et al., 2007; Hayashi et al., 2020; Zhang et al., 2021b), and transplantation models of endometriosis, such as mice (Yuan et al., 2017; Jones et al., 2018), where uterine endometrial tissue is typically transplanted to various locations within the abdominal cavity or subcutaneously using diverse methods (Grummer, 2006). While most of these models are established to investigate the etiology, pathogenesis, and progression of endometriosis, only a few explore the impact of ectopic endometrial implantation on ovarian function. In our study, we developed an OEM rat model by allogeneic transplantation of endometrial fragments onto the ovaries (Fig. 8A–M). We confirmed the substantial effect of OEM cysts on the fertility of rats. The fertility of OEM rats was notably lower than that of normal rats, with a significant reduction in fertility observed on the cyst side compared to the non-cyst side (Fig. 8S–Y). This suggests that the presence of OEM cysts is the primary factor contributing to impaired fertility, mirroring clinical findings that demonstrate decreased AMH levels, reduced numbers of retrieved oocytes, and decreased 2PN fertilization rates in infertile patients with OEM (Table 1). This OEM rat model offers an in vivo experimental platform for further mechanistic exploration of the impact of OEM on ovarian reserve function. Based on this OEM model, we observed a significant upregulation of let-7 expression in OEM rat GCs, along with a significant decrease in both the gene and protein levels of IGF1R. These findings align with results from human samples, where upregulated let-7 suppressing IGF1R attenuates GC function in OEM patients. Therefore, the dysregulated let-7/IGF1R axis in the follicular internal microenvironment may be a contributing factor to the impaired fertility associated with OEM.
In conclusion, our study demonstrates the high expression of let-7 in the follicular microenvironment of infertile patients with OEM, which targets IGF1R to influence GC function and subsequently impact follicular development. This investigation unveils crucial molecular mechanisms underlying diminished ovarian reserve in OEM patients and identifies the miRNA let-7 as a key player in the regulation of follicle development and hormone synthesis. Future research will delve into the potential influence of ovarian stromal cells on the ovarian microenvironment where follicles mature. Furthermore, the screening of small molecule drugs targeting let-7 and IGF1R aims to intervene and modulate the ovarian microenvironment, ultimately enhancing ovarian reserve function.
Supplementary Material
Acknowledgements
The authors express their sincere gratitude to all the study participants who enabled this research, as well as the medical staff of the IVF unit at Sir Run Run Shaw Hospital for their collaboration and support in sample collection and clinical data acquisition.
Contributor Information
Libing Shi, Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, PR China; Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology, Hangzhou, PR China; Zhejiang Key Laboratory of Precise Protection and Promotion of Fertility, Hangzhou, PR China.
Hanqi Ying, Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, PR China; Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology, Hangzhou, PR China; Zhejiang Key Laboratory of Precise Protection and Promotion of Fertility, Hangzhou, PR China.
Yongdong Dai, Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, PR China; Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology, Hangzhou, PR China; Zhejiang Key Laboratory of Precise Protection and Promotion of Fertility, Hangzhou, PR China.
Yan Rong, Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, PR China; Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology, Hangzhou, PR China; Zhejiang Key Laboratory of Precise Protection and Promotion of Fertility, Hangzhou, PR China.
Jianmin Chen, Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, PR China; Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology, Hangzhou, PR China; Zhejiang Key Laboratory of Precise Protection and Promotion of Fertility, Hangzhou, PR China.
Feng Zhou, Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, PR China; Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology, Hangzhou, PR China; Zhejiang Key Laboratory of Precise Protection and Promotion of Fertility, Hangzhou, PR China.
Shasha Wang, Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, PR China; Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology, Hangzhou, PR China; Zhejiang Key Laboratory of Precise Protection and Promotion of Fertility, Hangzhou, PR China.
Shiqian Xu, Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, PR China; Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology, Hangzhou, PR China; Zhejiang Key Laboratory of Precise Protection and Promotion of Fertility, Hangzhou, PR China.
Xiaomei Tong, Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, PR China; Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology, Hangzhou, PR China; Zhejiang Key Laboratory of Precise Protection and Promotion of Fertility, Hangzhou, PR China.
Songying Zhang, Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, PR China; Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology, Hangzhou, PR China; Zhejiang Key Laboratory of Precise Protection and Promotion of Fertility, Hangzhou, PR China.
Data availability
The data underlying this article cannot be shared publicly due to the privacy and ethical restrictions of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
Authors’ roles
L.B.S. contributed significantly to the conception and design of the study, data interpretation, and writing and editing of the manuscript. H.Q.Y. conducted the western-blotting experiments. Y.D.D. performed the analysis of miRNA-seq data and validation, and provided critical revisions to the manuscript. Y.R. conducted the IGF1R silencing experiment. J.M.C., F.Z., and S.S.W. participated in sample collection and clinical data acquisition. S.Q.X. assisted in the execution of the animal experiments. X.M.T. and S.Y.Z. conceptualized and supervised the study, and gave final approval for the version to be published. All authors critically reviewed the manuscript, offered important feedback, and engaged in discussions.
Funding
National Natural Science Foundation of China (grant number 82301851 to L.B.S., grant numbers U23A20403 and U20A20349 to S.Y.Z., and grant number 82371637 to Y.D.D.); Natural Science Foundation of Zhejiang Province (grant number LTGY23H040010 to F.Z.).
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Alshehre SM, Narice BF, Fenwick MA, Metwally M. The impact of endometrioma on in vitro fertilisation/intra-cytoplasmic injection IVF/ICSI reproductive outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet 2021;303:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 2005;122:553–563. [DOI] [PubMed] [Google Scholar]
- Baumgarten SC, Armouti M, Ko C, Stocco C. IGF1R expression in ovarian granulosa cells is essential for steroidogenesis, follicle survival, and fertility in female mice. Endocrinology 2017;158:2309–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, Kohlmeier A, Yin P, Milad M, Wei J. Endometriosis. Endocr Rev 2019;40:1048–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busacca M, Vignali M. Ovarian endometriosis: from pathogenesis to surgical treatment. Curr Opin Obstet Gynecol 2003;15:321–326. [DOI] [PubMed] [Google Scholar]
- Cao R, Wu W, Zhou X, Liu K, Li B, Huang X, Zhang Y, Liu H. Let-7g induces granulosa cell apoptosis by targeting MAP3K1 in the porcine ovary. Int J Biochem Cell Biol 2015a;68:148–157. [DOI] [PubMed] [Google Scholar]
- Cao R, Wu WJ, Zhou XL, Xiao P, Wang Y, Liu HL. Expression and preliminary functional profiling of the let-7 family during porcine ovary follicle atresia. Mol Cells 2015b;38:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol 2019;15:666–682. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod 2012;86:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierschke DJ, Chaffin CL, Hutz RJ. Role and site of estrogen action in follicular atresia. Trends Endocrinol Metab 1994;5:215–219. [DOI] [PubMed] [Google Scholar]
- Douglas JC, Sekulovski N, Arreola MR, Oh Y, Hayashi K, MacLean JA II. Normal ovarian function in subfertile mouse with Amhr2-Cre-driven ablation of INSR and IGF1R. Genes (Basel) 2024;15:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg I, Kotaja N, Goldman-Wohl D, Imbar T. microRNA in human reproduction. Adv Exp Med Biol 2015;888:353–387. [DOI] [PubMed] [Google Scholar]
- Francoeur L, Scoville DM, Johnson PA. Effect of IGF1 and FSH on the function of granulosa cells from prehierarchal follicles in chickens. Biol Reprod 2023;109:498–506. [DOI] [PubMed] [Google Scholar]
- Friis Wang N, Skouby SO, Humaidan P, Andersen CY. Response to ovulation trigger is correlated to late follicular phase progesterone levels: a hypothesis explaining reduced reproductive outcomes caused by increased late follicular progesterone rise. Hum Reprod 2019;34:942–948. [DOI] [PubMed] [Google Scholar]
- Ghazal S, McKinnon B, Zhou J, Mueller M, Men Y, Yang L, Mueller M, Flannery C, Huang Y, Taylor HS. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol Med 2015;7:996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SA, Newton JM, Hughes FM Jr. Decreased intracellular potassium levels underlie increased progesterone synthesis during ovarian follicular atresia. Biol Reprod 2001;64:1755–1760. [DOI] [PubMed] [Google Scholar]
- Grummer R. Animal models in endometriosis research. Hum Reprod Update 2006;12:641–649. [DOI] [PubMed] [Google Scholar]
- Hamdan M, Dunselman G, Li TC, Cheong Y. The impact of endometrioma on IVF/ICSI outcomes: a systematic review and meta-analysis. Hum Reprod Update 2015;21:809–825. [DOI] [PubMed] [Google Scholar]
- Harb HM, Gallos ID, Chu J, Harb M, Coomarasamy A. The effect of endometriosis on in vitro fertilisation outcome: a systematic review and meta-analysis. BJOG 2013;120:1308–1320. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Nakamura T, Motooka Y, Ito F, Jiang L, Akatsuka S, Iwase A, Kajiyama H, Kikkawa F, Toyokuni S. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox Biol 2020;37:101726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AL, Townsend PM, Oehninger S, Castora FJ. Endometriosis may be associated with mitochondrial dysfunction in cumulus cells from subjects undergoing in vitro fertilization-intracytoplasmic sperm injection, as reflected by decreased adenosine triphosphate production. Fertil Steril 2015;103:347–352.e1. [DOI] [PubMed] [Google Scholar]
- Huang B, Ren X, Wu L, Zhu L, Xu B, Li Y, Ai J, Jin L. Elevated progesterone levels on the day of oocyte maturation may affect top quality embryo IVF cycles. PLoS One 2016;11:e0145895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Yan W, Wang SE, Baltimore D. Let-7 suppresses B cell activation through restricting the availability of necessary nutrients. Cell Metab 2018;27:393–403.e4. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, Bush D, Kiesel L, Tamimi R, Sharpe-Timms KL et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod 2017;32:315–324. [DOI] [PubMed] [Google Scholar]
- Jones RL, Lang SA, Kendziorski JA, Greene AD, Burns KA. Use of a mouse model of experimentally induced endometriosis to evaluate and compare the effects of bisphenol A and bisphenol AF exposure. Environ Health Perspect 2018;126:127004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansaku K, Itami N, Kawahara-Miki R, Shirasuna K, Kuwayama T, Iwata H. Differential effects of mitochondrial inhibitors on porcine granulosa cells and oocytes. Theriogenology 2017;103:98–103. [DOI] [PubMed] [Google Scholar]
- Khan HL, Bhatti S, Abbas S, Kaloglu C, Isa AM, Younas H, Ziders R, Khan YL, Hassan Z, Turhan BO et al. Extracellular microRNAs: key players to explore the outcomes of in vitro fertilization. Reprod Biol Endocrinol 2021a;19:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan HL, Bhatti S, Abbas S, Kaloglu C, Qurat-Ul-Ain Zahra S, Khan YL, Hassan Z, Turhan NO, Aydin HH. Melatonin levels and microRNA (miRNA) relative expression profile in the follicular ambient microenvironment in patients undergoing in vitro fertilization process. J Assist Reprod Genet 2021b;38:443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandoker MA, Imai K, Takahashi T, Hashizume K. Role of gelatinase on follicular atresia in the bovine ovary. Biol Reprod 2001;65:726–732. [DOI] [PubMed] [Google Scholar]
- Li H, Wang X, Mu H, Mei Q, Liu Y, Min Z, Zhang L, Su P, Xiang W. Mir-484 contributes to diminished ovarian reserve by regulating granulosa cell function via YAP1-mediated mitochondrial function and apoptosis. Int J Biol Sci 2022;18:1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Choi E, Yu H, Bai XC. Structural basis of the activation of type 1 insulin-like growth factor receptor. Nat Commun 2019a;10:4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang W, Fu J, Xu Y, Gu R, Qu R, Li L, Sun Y, Sun X. MicroRNA-451 is downregulated in the follicular fluid of women with endometriosis and influences mouse and human embryonic potential. Reprod Biol Endocrinol 2019b;17:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu YD, Zhou XY, Zhang J, Wu XM, Yang YZ, Chen YX, Zhang XF, Li X, Ma LZ et al. Let-7e modulates the proliferation and the autophagy of human granulosa cells by suppressing p21 signaling pathway in polycystic ovary syndrome without hyperandrogenism. Mol Cell Endocrinol 2021;535:111392. [DOI] [PubMed] [Google Scholar]
- Li Z, Pan W, Shen Y, Chen Z, Zhang L, Zhang Y, Luo Q, Ying X. IGF1/IGF1R and microRNA let-7e down-regulate each other and modulate proliferation and migration of colorectal cancer cells. Cell Cycle 2018;17:1212–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Jing F, Huang P, Geng Z, Xu J, Li J, Chen D, Zhu Y, Wang Z, Huang W et al. Thymopentin alleviates premature ovarian failure in mice by activating YY2/Lin28A and inhibiting the expression of let-7 family microRNAs. Cell Prolif 2021;54:e13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Han M, Li X, Wang H, Ma M, Zhang S, Guo Y, Wang S, Wang Y, Duan N et al. Age-related changes in the mitochondria of human mural granulosa cells. Hum Reprod 2017;32:2465–2473. [DOI] [PubMed] [Google Scholar]
- Llerena Cari E, Hagen-Lillevik S, Giornazi A, Post M, Komar AA, Appiah L, Bitler B, Polotsky AJ, Santoro N, Kieft J et al. Integrated stress response control of granulosa cell translation and proliferation during normal ovarian follicle development. Mol Hum Reprod 2021;27:gaab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol 2018;141:1202–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf SW, Liu WS, Pate JL. MicroRNA in ovarian function. Cell Tissue Res 2016;363:7–18. [DOI] [PubMed] [Google Scholar]
- Machtinger R, Laurent LC, Baccarelli AA. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update 2016;22:182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles JR, McDaneld TG, Wiedmann RT, Cushman RA, Echternkamp SE, Vallet JL, Smith TP. MicroRNA expression profile in bovine cumulus-oocyte complexes: possible role of let-7 and miR-106a in the development of bovine oocytes. Anim Reprod Sci 2012;130:16–26. [DOI] [PubMed] [Google Scholar]
- Paulson EE, Fishman EL, Schultz RM, Ross PJ. Embryonic microRNAs are essential for bovine preimplantation embryo development. Proc Natl Acad Sci USA 2022;119:e2212942119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen LC, Pla I, Sanchez A, Grondahl ML, Marko-Varga G, Yding Andersen C, Englund ALM, Malm J. Progressive changes in human follicular fluid composition over the course of ovulation: quantitative proteomic analyses. Mol Cell Endocrinol 2019;495:110522. [DOI] [PubMed] [Google Scholar]
- Qasemi M, Amidi F. Extracellular microRNA profiling in human follicular fluid: new biomarkers in female reproductive potential. J Assist Reprod Genet 2020;37:1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H, Du Y, Yu Y, Wang M, Han T, Yan L. The effect of endometriosis on IVF/ICSI and perinatal outcome: a systematic review and meta-analysis. J Gynecol Obstet Hum Reprod 2022;51:102446. [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 2000;403:901–906. [DOI] [PubMed] [Google Scholar]
- Rong Y, Ji SY, Zhu YZ, Wu YW, Shen L, Fan HY. ZAR1 and ZAR2 are required for oocyte meiotic maturation by regulating the maternal transcriptome and mRNA translational activation. Nucleic Acids Res 2019;47:11387–11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth Z. Symposium review: reduction in oocyte developmental competence by stress is associated with alterations in mitochondrial function. J Dairy Sci 2018;101:3642–3654. [DOI] [PubMed] [Google Scholar]
- Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol 2008;18:505–516. [DOI] [PubMed] [Google Scholar]
- Samadi P, Afshar S, Amini R, Najafi R, Mahdavinezhad A, Sedighi Pashaki A, Gholami MH, Saidijam M. Let-7e enhances the radiosensitivity of colorectal cancer cells by directly targeting insulin-like growth factor 1 receptor. J Cell Physiol 2019;234:10718–10725. [DOI] [PubMed] [Google Scholar]
- Sekulovski N, Whorton AE, Shi M, Hayashi K, MacLean JA II. Periovulatory insulin signaling is essential for ovulation, granulosa cell differentiation, and female fertility. FASEB J 2020;34:2376–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Wei X, Wu B, Yuan C, Li C, Dai Y, Chen J, Zhou F, Lin X, Zhang S. Molecular signatures correlated with poor IVF outcomes: insights from the mRNA and lncRNA expression of endometriotic granulosa cells. Front Endocrinol 2022;13:825934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LB, Zhou F, Zhu HY, Huang D, Jin XY, Li C, Dai Y, Pan YB, Zhang SY. Transforming growth factor beta1 from endometriomas promotes fibrosis in surrounding ovarian tissues via Smad2/3 signaling. Biol Reprod 2017;97:873–882. [DOI] [PubMed] [Google Scholar]
- Sirotkin AV, Ovcharenko D, Grossmann R, Laukova M, Mlyncek M. Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J Cell Physiol 2009;219:415–420. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhu M, Feng W, Lin Y, Yin J, Jin J, Wang Y. Exosomal miRNA Let-7 from menstrual blood-derived endometrial stem cells alleviates pulmonary fibrosis through regulating mitochondrial DNA damage. Oxid Med Cell Longev 2019;2019:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Yang DJ, Kurihara H, Borne A, Kohanim S, Oh CS, Mawlawi O, Kim EE. Functional imaging of estrogen receptors with radiolabeled-GAP-EDL in rabbit endometriosis model. Acad Radiol 2007;14:1050–1057. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet 2021;397:839–852. [DOI] [PubMed] [Google Scholar]
- Tu J, Cheung AH, Chan CL, Chan WY. The role of microRNAs in ovarian granulosa cells in health and disease. Front Endocrinol 2019;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virant-Klun I, Bauer C, Stahlberg A, Kubista M, Skutella T. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod Biomed Online 2018;36:508–523. [DOI] [PubMed] [Google Scholar]
- von Mengden L, Klamt F, Smitz J. Redox biology of human cumulus cells: basic concepts, impact on oocyte quality, and potential clinical use. Antioxid Redox Signal 2020;32:522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Meng K, Wang H, Wang Y, Zhao Y, Kang J, Zhang Y, Quan F. Identification of small extracellular vesicle subtypes in follicular fluid: Insights into the function and miRNA profiles. J Cell Physiol 2021;236:5633–5645. [DOI] [PubMed] [Google Scholar]
- Wen J, Wang GL, Yuan HJ, Zhang J, Xie HL, Gong S, Han X, Tan JH. Effects of glucose metabolism pathways on nuclear and cytoplasmic maturation of pig oocytes. Sci Rep 2020;10:2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Kimura F, Takebayashi A, Kita N, Takahashi K, Murakami T. Primate model research for endometriosis. Tohoku J Exp Med 2012;226:95–99. [DOI] [PubMed] [Google Scholar]
- Yang C, Geng Y, Li Y, Chen C, Gao Y. Impact of ovarian endometrioma on ovarian responsiveness and IVF: a systematic review and meta-analysis. Reprod Biomed Online 2015;31:9–19. [DOI] [PubMed] [Google Scholar]
- Yang S, Lian G. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem 2020;467:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis JS, Shapso N, Fleming R, Ben-Shlomo I, Izhaki I. Impact of unilateral versus bilateral ovarian endometriotic cystectomy on ovarian reserve: a systematic review and meta-analysis. Hum Reprod Update 2019;25:375–391. [DOI] [PubMed] [Google Scholar]
- Yu YS, Sui HS, Han ZB, Li W, Luo MJ, Tan JH. Apoptosis in granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors. Cell Res 2004;14:341–346. [DOI] [PubMed] [Google Scholar]
- Yuan M, Li D, An M, Li Q, Zhang L, Wang G. Rediscovering peritoneal macrophages in a murine endometriosis model. Hum Reprod 2017;32:94–102. [DOI] [PubMed] [Google Scholar]
- Zhang D, Lv J, Tang R, Feng Y, Zhao Y, Fei X, Chian R, Xie Q. Association of exosomal microRNAs in human ovarian follicular fluid with oocyte quality. Biochem Biophys Res Commun 2021a;534:468–473. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li K, Gao L, Zhu P, Shu L, Cai L, Diao F, Mao Y. Glucose metabolism disorder related to follicular fluid exosomal miR-122-5p in cumulus cells of endometriosis patients. Reproduction 2024;168:e240028. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu Y, Liu H, Pan Z. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod Biol Endocrinol 2019;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhuang L, Liu Q, Yu X, Min Q, Chen M, Chen Q. Rosiglitazone affects the progression of surgically induced endometriosis in a rat model. Mol Med Rep 2021b;23:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xiao H, Zhang X, E Q, Gong X, Li T, Han Y, Ying X, Cherrington BD, Xu B et al. Decreased microRNA-125b-5p disrupts follicle steroidogenesis through targeting PAK3/ERK1/2 signalling in mouse preantral follicles. Metabolism 2020;107:154241. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yao W, Liu K, Wen Q, Wu W, Liu H, Li Q. MicroRNA let-7g regulates mouse granulosa cell autophagy by targeting insulin-like growth factor 1 receptor. Int J Biochem Cell Biol 2016;78:130–140. [DOI] [PubMed] [Google Scholar]
- Zhu H, Shyh-Chang N, Segre AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 2011;147:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotta A, O’Neill LAJ, Yin M. Unlocking potential: the role of the electron transport chain in immunometabolism. Trends Immunol 2024;45:259–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy and ethical restrictions of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.