Abstract
Introduction
Accurate identification of patients with pathologic complete response (pCR) following neoadjuvant radiochemotherapy (RCT) for locally advanced rectal cancer (LARC) is essential. 18-FDG PET/MRI provides metabolic information that complements the morphological assessment of standard MRI, potentially enhancing the differentiation between fibrotic and tumorous tissues post-treatment. This study aims to evaluate the performance of 18-FDG PET/MRI in assessing treatment response compared to standard MRI.
Materials and methods
A prospective study was conducted at HM Sanchinarro University Hospital, Madrid, from 2018 to 2021. Patients with LARC undergoing RCT were included and staged at diagnosis and restaged 8–12 weeks post-neoadjuvant treatment using 18-FDG PET/MRI. The primary outcome was to compare the performance of PET/MRI and standard MRI in detecting pCR and tumor regression grade (TRG) confirmed via histopathological examination. Quantitative analysis assessed the apparent diffusion coefficient (ADC) and standardized uptake value (SUV). A secondary outcome included survival analysis using the Kaplan–Meier method and Cox regression analysis for radiological and pathological prognostic markers.
Results
Among 33 patients, pCR was observed in 45% (14/33). PET/MRI demonstrated sensitivity, specificity, and accuracy values of 0.88, 0.80, and 0.84, respectively, for detecting pCR, compared to 0.82, 0.50, and 0.67 for standard MRI (p < 0.001). PET/MRI accurately identified TRG stages in 72% of cases, compared to 50% for standard MRI. Post-SUV, post-ADC, and delta-ADC were the most precise PET/MRI predictors for pCR, with AUC values of 0.81, 0.75, and 0.55, respectively. Patients with mrEMVI and mrTRG showed worse disease-free survival (DFS).
Conclusion
18-FDG PET/MRI emerges as a promising imaging tool for predicting response to neoadjuvant treatment in rectal cancer, with superior diagnostic accuracy compared to standard MRI. Radiological findings, such as EMVI, can identify high-risk patients, offering valuable prognostic insights.
Keywords: 18-FDG PET/MRI, Rectal cancer, Neoadjuvant radiochemotherapy
Introduction
With an estimated number of 106,590 new cases of colon cancer and 46,220 new cases of rectal cancer, colorectal cancer is the third-most commonly diagnosed cancer and, at the same time, the third-most common cause of cancer-related deaths [1, 2]. A proper and well-structured diagnostic work-up is essential to assess the clinical stage of the disease, especially for the locally advanced rectal cancer patient who could benefit from primary treatment with neoadjuvant radiochemotherapy (RCT) [3]. This involves curative intent surgery, preceded by neoadjuvant treatment and (traditionally) a low anterior resection (LAR) or abdominoperineal resection (APR). However, these types of surgery could lead to a drastic reduction in the quality of life for these patients [4].
Conversely, a major or complete clinical response following neoadjuvant radiochemotherapy is observed in 10–60% of patients depending on the neoadjuvant treatment regimen used and initial tumor stage [5–7]. In order to achieve this response rate, an accurate restaging after RCT with the assessment of the residual tumor and lymph node involvement is essential. Current guidelines suggest performing a pelvic MRI with diffusion (DW) protocol to assess the T-N parameter, plus a chest CT and an abdominal to assess the presence of distant metastasis [3], while more recent studies have shown how the FDG-PET/CT is proven to improve the accuracy of lymph node detection and distant metastasis [8]. PET can provide a more accurate assessment of nodal status and treatment response by combining anatomical and metabolic data, improving sensitivity and specificity in identifying lymph node metastases. Additionally, PET is particularly valuable for evaluating treatment response, as it monitors changes in tumor metabolism, which often precede morphologic alterations detectable by MRI or CT. Recent studies have shown that variations in metabolic parameters, such as delta-SUV, can predict pathologic complete response (pCR), helping to identify patients who might benefit from organ-preserving strategies [9].
The integration of PET with MRI (PET/MRI) allows for detailed analysis of lymph node characteristics, using parameters such as the maximum standardized uptake value (SUVmax) to differentiate metastatic from reactive nodes. However, the current data available, as well as the economic aspects involved, demonstrate that the PET/CT does not provide sufficient evidence to support its use in a clinical routine for the restaging of colorectal cancer.
The birth of a new technique, such as FDG-PET/MRI (which combines the information on an anatomical level from the MRI with the physiological and metabolic aspects supplied by the PET), could suggest the existence of a technique with the power to completely assess the TNM parameters. Recent studies have emerged showing promising results for the potential of this technique [10, 11].
This study aims to provide PET/MRI in our center’s clinical experience when assessing the response to neoadjuvant treatment in patients with rectal cancer, comparing it to the standard MRI, and investigating its role in predicting survival.
Material and methods
This is a prospective study carried out from September 2018 to September 2021. This study, which is part of a doctoral thesis, was approved by the institutional review board of HM Sanchinarro University Hospital, Madrid (protocol number 22.01.1950-GHM), and all subjects involved in the prospective study signed a written informed consent form.
The inclusion criteria were biopsy-proven rectal adenocarcinoma, clinical stage II-III (cT3-4, N0-2, M0), finalized neoadjuvant treatment, and the ability to provide written informed consent. Exclusion criteria were prior surgical resection of rectal cancer (endoscopic or TME) and contraindication to PET/MRI or MRI examination. All participants underwent an 18 FDG PET/MRI scan at diagnosis and 10–12 weeks after completing neoadjuvant treatment.
Neoadjuvant chemoradiotherapy of 50.4 Gy was divided into 28 sessions (5 days/week) of 1.8 Gy. This regimen was associated with oral Capecitabine (625 to 825 mg/m/12 h). Selected patients were treated with total neoadjuvant therapy (TNT).
Patients with a disease-free survival (DFS) superior to 36 months were included.
Surgery was carried out on 8–12 weeks after RCT. The histopathological examination was performed by expert colorectal pathologists. Pathologic tumor staging (ypTNM) and scores of the surgical specimens were established in accordance with the eighth edition of the American Joint Committee on Cancer [3]. The TRG was assessed using the Mandard scale [12].
Circumferential resection margin (CRM) was considered negative if the distance between the tumor and CRM was more than 1 mm.
Follow-up consisted of periodic controls every 3 months during the first 2 years, every 6 months during the following 2 years, and annually thereafter. Physical examination, complete blood tests, serum carcinoembryonic antigen tests, and thoraco-abdominopelvic CT were performed. A colonoscopy was performed 1 year after surgery and once every 2 years thereafter. Local recurrence was defined as any local mucosal, intramural, or mesorectal recurrence. Metastatic recurrence was defined as any extra-pelvic distant recurrence and any pelvic recurrence confirmed by biopsy of the lesions.
Study procedure
All participants underwent an 18 FDG PET/MRI scan before and after neoadjuvant treatment. The sequences from the PET/MRI scan are analyzed and reported. From the same study, two independent radiologists evaluate only the MRI sequences and provide their findings in a separate, independent report.
Image protocol
The 18 FDG dose was established depending on body weight. Post-injection median uptake time was 40–50 min for the pelvic imaging study and 60–70 min for the whole body imaging study. The summarized protocol is shown in Table 1.
Table 1.
PET-MRI whole body and specific pelvic protocol
| Whole body |
1. Locator 2. Attenuation correction sequences MRAC (in each bed) 3. Axial T2 HASTE (in each bed) 4. PET acquisition (simultaneous 4 min/cama) 5. Axial diffusion with b0, 500 y 1000 values |
| Abdominal |
6. Sagittal TSE T2W 7. Coronal TSE T2W 8. Oblique Axial TSE T2W (section thickness 3.5 interslice gap 1) 9. DWI EPI 2D b50, 400, 1200 (section thickness 3.5 interslice gap 0) 10. Perfusion T1 twist axial dyn 11. PET acquisition (simultaneous 15 min) |
Image analysis
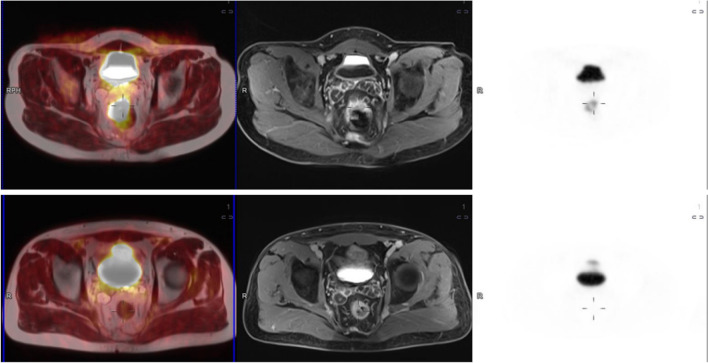
PET-MRI data sets were reviewed on a commercially available workstation (Fig. 1) (Syngo.Via, Siemens Healthcare, Erlangen, Germany) by a radiologist and a nuclear medicine physician, both with more than 10-years’ experience in MRI and PET/CT, respectively. For all tumors, the maximum SUV was analyzed in the PET dataset of the rectum plotting an isocontour volume of interest (VOI) around the tumor (SUVmax threshold 40%) by the nuclear medicine physician. ADC maps were automatically generated by the implemented software. After a qualitative evaluation of the ADC map, two regions of interest (ROI) in two different slices were manually drawn on this map in the area with the minimal signal intensity inside the tumor. In all lesions, two minimal ADC values (ADCmin) were registered. The response was assessed using the Mandard system’s mrTRG classification in MRI sequences (mrTRG) and in PET/MRI (petmriTRG), where TRG 1 indicates only low signal fibrosis without tumor signal, TRG 2 signifies more than 75% fibrosis with minimal tumor signal intensity, TRG 3 represents a balance of 50% tumor and fibrosis, TRG 4 suggests less than 25% fibrosis with predominant tumor signal, and TRG 5 indicates no fibrosis. The radiologist underwent specialized training in primary tumor assessment using TRG as part of a program run by leading experts in the field, which included reporting post-neoadjuvant treatment. The radiologists reviewed the images and were in consensus, while information on the histopathology results and patient outcomes was not provided to them.
Fig. 1.
18F-FDG PET/MRI sequences before and after neoadjuvant radiochemotherapy
EMVI was identified based on criteria, such as vessel expansion or irregularity, loss of normal vascular flow void, and intraluminal intermediate tumor signal intensity, which could be contiguous with, or separate from, the primary tumor. EMVI was assessed using a 0 to 4 grading system, as described by Jhaveri et al.: where a score of 0 indicated no vessels near the extramural tumor penetration; a score of 1 indicated vessels of normal caliber and no definite tumor signal; a score of 2 indicated slightly expanded vessels without a definite tumor signal; a score of 3 indicated an intermediate tumor signal within expanded vessels; and a score of 4 indicated obvious irregular vessel contours or nodular expansion of the vessel due to a definite tumor signal. For purposes of analysis, scores of 0 to 2 were considered negative for EMVI, while scores of 3 and 4 were considered positive.
Outcomes
Primary outcomes were to compare the accuracy of PET/MRI versus MRI standard sequences in the identification of a complete pathologic response and pTRG. Furthermore, the performance of ADC and SUV and the variation of these values in pre- and post-neoadjuvant examinations were analyzed.
As secondary outcomes, a survival analysis was performed to correlate radiological features before neoadjuvant treatment (cT, cN, EMVI), after neoadjuvant treatment (mrTRG and petmriTRG), and at histopathological examination (pCR, ypN, lympho-vascular invasion) with disease-free survival.
Statistical analysis
Continuous data were reported as the mean and standard deviation and categorical data as a percentage. Also calculated were the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) in detection of a pathologic complete response derived from the PET/MRI model as well as the anatomical MRI sequences. The radiologists independently assessed whether patients exhibited pathological complete response (pCR) or no pCR. Cohen’s kappa analysis was conducted to measure the level of agreement. Diagnostic performance was compared using the McNemar test. Receiver operating characteristic (ROC) analysis was conducted using the following PET/MRI parameters: ADCmean, SUVmax, deltaADC (ADCpost-ADCpre)/ADCpost, deltaSUV (SUVpost-SUVpre)/SUVpost was calculated to identify pCR and AUC.
Survival analysis using a Cox-proportional hazard model with multivariate corrections, as well as the log-rank test, was performed. TRG 1–2 (good responders) versus TRG 3–5 (intermediate/poor responders) and EMVI 1–2 versus EMVI 3–4 were considered in the analysis.
All calculations were performed using SPSS, with a p-value of < 0.05 deemed statistically significant.
Results
Overall, 33 patients (24 men and 9 women) were prospectively included in the study. Patients’ characteristics are shown in Table 2. At the histopathological examination, 15 (45%) complete pathological responses were identified (Table 2).
Table 2.
Demographic characteristics according to pathologic response
| pCR0 = 15 | no-pCR = 18 | p | |
|---|---|---|---|
| Sex, n (%) | 0.210 | ||
| Male | 9 (60) | 15 (84) | |
| Female | 6 (40) | 3 (16) | |
| Age, years, median (± SD) | 60 (11) | 65 (12) | 0.308 |
| BMI Kg2/cm, median (+ / − SD) | 23 (6) | 22 (8) | |
| ASA n (%) | 0.455 | ||
| I | 5 (34) | 6 (33) | |
| II | 7 (46) | 10 (56) | |
| III | 3(22) | 2 (119 | |
| Distance anal verge, cm, mean (+ / − SD) | 0.67 | ||
| < 5 | 7 (46) | 2 (11) | |
| 5–10 | 4 (27) | 10 (56) | |
| > 10 | 4 (27) | 6 (33) | |
| T stage, n (%) | 0.316 | ||
| 2 | 4 (27) | 2 (11) | |
| 3 | 10 (67) | 15 (83) | |
| 4 | 1 (6) | 1 (5) | |
| N stage, n (%) | 0.762 | ||
| 0 | 9 (60) | 9 (50) | |
| 1 | 4 (27) | 5 (28) | |
| 2 | 2 (13) | 4 (22) | |
| Stage according to AJCC, n (%) |
9 (50) 9 (50) |
0.678 | |
| II | 9 (60) | ||
| III | 6 (40) | ||
| Surgery, n (%) | 0.083 | ||
| TME | 12 (80) | 17 (94) | |
| AAP | 3 (20) | 1 (6) |
TME, total mesorectal excision; AAP, abdomino-perineal amputation; CM, circumferential margin
Abdomino-perineal amputation was performed in four patients, while total mesolectal excision with primary anastomosis was performed in 29 patients. All patients underwent PET/MRI for staging and restaging. The mean time period between surgery and PET/MRI restaging was 72 days (range 42–96 days) (Table 3).
Table 3.
Anatomopathological characteristic
| ypT, n (%) | |
| 0 | 15 (45) |
| 1 | 8 (24) |
| 2 | 5 (15) |
| 3 | 5 (15) |
| ypN, n (%) | |
| 0 | 20 (60) |
| 1–2 | 13 (40) |
| Total lymph nodes, median (+ / − SD) | 12 (5.4) |
| Positive lymph nodes, median (+ / − SD) | 0.8 (0.3) |
| Affected CM, n (%) |
1 (3) 32 (97) |
| Yes | |
| No | |
Primary outcome: diagnostic performance
18-FDG PET/MRI demonstrated a superior performance in detecting pCR compared to standard MRI, with a sensitivity, specificity, and accuracy of 0.88, 0.80, and 0.84 for 18-FDG PET/MRI, respectively, and 0.82, 0.50, and 0.67 for standard MRI (p < 0.001) (Table 4). The inter-observed agreement between the radiologists for pCR detection was 90%, with a Cohen’s kappa value of 0.754.
Table 4.
Performance evaluation of PETMRI versus standard MRI in detecting pathologic complete response
| PETMRI | MRI | p | |
|---|---|---|---|
| Accuracy (%) (95% CI) | 84 (67.2–94.7) | 67 (48.6–83.3) | p < 0.01 |
| Sensitivity (%) (95% CI) | 88 (63.5–98.5) | 82 (56.6–96.2) | 0.06 |
| Specificity (%) (95% CI) | 80 (51.9–95.6) | 50 (53.1–72.6) | 0.12 |
| TPV (%) (95% CI) | 83 (64.1–93.3) | 66 (53.1–77.8) | - |
| TNV (%) (95% CI) | 85 (61.4–96.7) | 70 (48.6–83.3) | - |
TPV, true positive value; TNV, true negative value
18-FDG PET-MRI accurately identified pathologic TRG-stage in 24 out of 33 patients (72%), including TRG1 in 12 out of 15 (80%), TRG2 in five out of seven (71,4%), TRG3 in four out of seven patients (57.1%), and TRG4 in three out of four patients (75%), respectively (Table 5, p < 0.001). PET/MRI overestimated the TRG stage in five patients (pTRG0 was staged as mrTRG1 in three cases and two TRG 1 were staged as mrTRG2) and underestimated the TRG stage in four patients (three pTRG2 were staged as mrTRG0 and TRG1 and one patient’s pTRG3 was staged as mrTRG2).
Table 5.
Comparison of PETMRI and standard MRI in predicting pathologic TRG
| PathologicTRG | ||||||
|---|---|---|---|---|---|---|
| TRG1 | TRG2 | TRG3 | TRG4 | |||
| MRI | TRG1 | 8 | 1 | 2 | 0 | 14 |
| TRG2 | 5 | 6 | 3 | 1 | 10 | |
| TRG3 | 1 | 0 | 2 | 2 | 6 | |
| TRG4 | 1 | 0 | 0 | 1 | 2 | |
| n | 15 | 7 | 7 | 4 | 33 | |
| PET-MRI | TRG1 | 12 | 0 | 2 | 0 | 14 |
| TRG2 | 3 | 5 | 1 | 1 | 10 | |
| TRG3 | 0 | 2 | 4 | 0 | 6 | |
| TRG4 | 0 | 0 | 0 | 3 | 2 | |
| n | 15 | 7 | 7 | 4 | 33 | |
In the interpretation of MRI sequence alone, the pathologic tumor regression grade (pTRG) stage was accurately identified in 17 out of 33 patients (50.1%). This included correctly identifying TRG1 in eight out of 15 patients (53%), TRG2 in six out of seven patients (85.7%), TRG3 in two out of seven patients (28%), and TRG4 in one out of four patients (25%). MRI overestimated the TRG stage in seven patients; it underestimated the TRG stage in nine patients (Table 5, p = 0.321). The presence of mesorectal lymphadenopathy was accurately identified by PET/MRI, as confirmed by histopathology in 28 out of 33 patients (84.8%) with misinterpretations of five patients as node-positive and no patients as false-negative.
The median values of ADC, SUV pre- and post-neoadjuvant treatment, delta ADC, and delta SUV are reported in Table 6.
Table 6.
Median values of SUV and ADC according to pathologic complete response
| pCR | no PCR | p | |
|---|---|---|---|
| SUV pre | 21.52 (11.1) | 22.2 (13.6) | 0.875 |
| SUV post | 4.39 (1.7) | 6.39 (3.7) | 0.057 |
| Delta SUV | 0.72 (0.22) | 0.62 (0.28) | 0.279 |
| ADC pre | 0.65 (0.13) | 0.66 (0.15) | 0.831 |
| ADCpost | 1.15 (0.27) | 0.91 (0.24) | 0.019 |
| Delta ADC | 0.40 (0.22) | 0.22 (0.24) | 0.051 |
Using these values, the ROC curve was constructed. The AUC resulted in 0.403 (95% CI 0.183–0.621) for pre-ADC, 0.814 (95% CI 0.650–0.977) for post-ADC, and 0.755 (95% CI 0.570–0.940) for delta-ADC (Fig. 2). Regarding SUV values, the AUC was 0.483 (95% CI 0.272–0.694) for pre-SUV, 0.697 (95% CI 0.509–0.884) for post-SUV, and 0.551 (95% CI 0.342–0.761) for delta-SUV (Fig. 3). Post-SUVmax, post-ADC, and delta-ADC resulted in more accurate and precise PET/MRI values in predicting pCR.
Fig. 2.
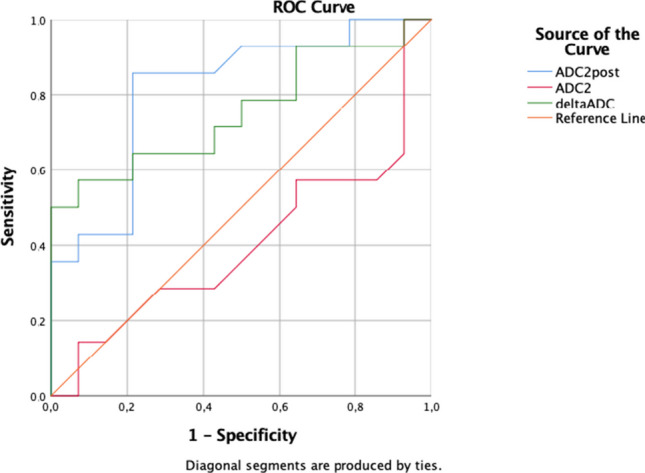
ROC curve for pre-ADC, post-ADC, and delta ADC
Fig. 3.

ROC curve for pre-SUV, post-SUV, and delta SUV
Secondary outcome
The median follow-up duration was 49.4 (+ / − 30.4) months, with no patient lost to follow-up. Throughout the observation period, five patients died for metastatic disease, and no 90-day mortality was observed.
Patients diagnosed with pre-treatment EMVI status 3–4 experienced worse DFS compared to those with EMVI status 1–2 (57.1% versus 88.9%, p < 0,05) with a mean survival of 35.8 + /19 months versus 61 + / − 28 months (Fig. 4). Furthermore, regarding preoperative staging, patients with positive nodes exhibited inferior DFS compared to those with negative nodes (57.1% vs. 88.9%, p < 0.05) (Fig. 5). Meanwhile, the T stage did not show a significant association with poor DFS.
Fig. 4.
Disease-free survival for N 0 vs N1-2 status
Fig. 5.
Disease-free survival for EMVI 1–2/EMVI 3–4
Patients with PET/MRI TRG 1–2 showed a better survival with respect to patients with PET/MRI TRG 3–5, but without statistical significance in the univariate analysis (65.1 + / − 21.3 months versus 47.9 + / − 31.4 months) (Table 7).
Table 7.
Univariate and multivariate analysis for DFS
| Univariate analysis | HR | 95%CI | p | |
|---|---|---|---|---|
| Pre-neoadjuvant staging | ||||
| T > 3 | 0.604 | |||
| N1-2 | 0.002 | 4.911 | 1.474–16.361 | 0.01 |
| EMVI | 0.003 | 4.849 | 1.249–18.823 | 0.023 |
| Post-neoadjuvant staging | ||||
| MRI TRG | 0.868 | |||
| PET MTI TRG | 0.030 | 1.488 | 0.162–13.629 | 0.725 |
| Histology | ||||
| pTRG | 0.056 | 2.295 | 0.276–19.057 | 0.442 |
| N | 0.392 | |||
| LV invasion | 0.322 | |||
Moreover, TRG in the MRI sequences did not show a statistically significant correlation with DFS, similar to the pathological complete response, lymphovascular invasion, and positive nodes in the histopathological examination. Finally, in the Cox regression analysis, EMVI and pre-neoadjuvant nodal status did not reach statistical significance for poor survival outcomes. However, the p-values were very close to the threshold of significance, suggesting that a larger sample size might yield significant results.
Discussion
This prospective study, involving 33 patients, investigated the efficacy of PET/MRI compared to standard MRI in staging and restaging tumors, with a specific focus on identifying responses to neoadjuvant treatment and predicting future oncological outcomes.
We included only stage II and III patients in the study because neoadjuvant treatment for stage I patients was limited to cases where the tumor was located less than 4 cm from the anal margin. Furthermore, most of these patients were enrolled in the “watch and wait” protocol, and therefore, pathological data was not available.
PET/MRI demonstrated superior accuracy in detecting pCR (84%) compared to standard MRI (67%), showing that PET/MRI exhibits higher sensitivity and specificity in identifying residual disease post-neoadjuvant therapy compared to conventional imaging techniques. These findings are similar to a recent original research of Crime et al. [13], who reported better accuracy using PET/MRI compared with standard MRI for ypT staging (92% vs 82%) and for ypN (92% vs 86%). Similarly, a study by Catalano et al. reported a superior performance of PET/MRI versus standard MRI in evaluating tumor size, identifying N status, and in external sphincter infiltration [14].
The accuracy of mesorectal lymphadenopathy identification resulted in 84.8% in our study. These data are also confirmed in the recent meta-analysis of Crime et al. [15].
This paper did not explore the role of PET/MRI in diagnosing extra-mesorectal lymph nodes. However, studies like Ishihara et al. show that combining PET and imaging features (size ≥ 12 mm or SUVmax ≥ 1.6) can achieve 92.9% accuracy, 88.2% sensitivity, and 100% specificity for diagnosing lateral pelvic lymph node metastases [16]. PET/MRI, which integrates metabolic and anatomical imaging, could further enhance diagnostic precision, offering better localization and characterization of extra-mesorectal disease. It holds potential as the gold standard for these patients, especially for lateral pelvic lymph node evaluation. Future studies should confirm its utility in larger cohorts and assess its impact on treatment planning and decision-making.
Regarding post-treatment re-stratification, mrTRG is emerging as a promising prognostic radiological indicator in patients with locally advanced rectal cancer. This system has demonstrated reproducibility among radiologists, showing good interobserver agreement [17]. Furthermore, mrTRG has been shown to be a prognostic indicator for disease-free survival (hazard ratio [HR] 3.28; 95% confidence interval [CI] 1.22 to 8.80) and overall survival (HR 4.40; 95% CI 1.65 to 11.7) in a recent study [18]. In this study, PET/MRI accurately identified TRG stages in 72% of cases, significantly outperforming standard MRI, which had a 50.1% accuracy rate. In particular, PET/MRI showed a high level of accuracy in identifying TRG1 (80%) and TRG4 (75%) stages. This enhanced staging capability is critical for tailoring adjuvant therapies and optimizing patient outcomes.
Moreover, the qualitative analysis of parametric values ADC and SUV in detecting a pathological complete response, the ROC curve analysis, in this study highlights significant differences in the predictive capabilities of MRI-derived and PET-derived biomarkers. Our findings show that the post-treatment ADC, delta-ADC, and post-SUV resulted in better predictive accuracy markers. The AUC for post-treatment ADC (0.814; 95% CI 0.650–0.977) and delta ADC (0.755; 95% CI 0.570–0.940) is in accordance with previous studies that reported a high predictive value of diffusion-weighted MRI in assessing tumor response. These findings support the utility of ADC as a robust biomarker for treatment response, reflecting changes in tumor cellularity and microenvironment post-therapy.
Analyzing PET-derived values, the AUC for post-treatment SUV (0.697; 95% CI 0.509–0.884) was moderate, which is similar to a previous study [19]. However, our study indicates that SUV metrics, particularly pre-treatment and delta-SUV, are less reliable predictors compared to ADC measures. This discrepancy in predictive accuracy might be attributed to the inherent differences in the values that ADC and SUV represent: ADC reflects tissue cellularity and water diffusion, whereas SUV indicates metabolic activity, which may not directly correlate with the histopathological response.
In the secondary outcome, we investigate radiological prognostic factors, influencing disease-free survival (DFS). The median follow-up duration of 49.4 ± 30.4 months ensured a comprehensive assessment of patient outcomes, with no losses to follow-up and an absence of 90-day mortality.
The data indicates that patients with pre-treatment EMVI status 3–4 have significantly worse DFS compared to those with EMVI status 1–2, which is in line with the literature. EMVI status is emerging as a critical prognostic factor in colorectal cancer, and it may represent an important predictor of poor prognosis in patients with rectal cancer [20, 21]. Furthermore, our study confirms that patients with positive nodes have a poorer DFS compared to those with negative nodes, highlighting the importance of nodal status in predicting treatment response. Interestingly, our analysis does not reveal a significant association between T stage and DFS, suggesting that the depth of tumor invasion may not be as critical a factor in predicting treatment response as previously thought. In contrast, our analysis does not reveal a statistically significant correlation between TRG in MRI sequences and DFS. This finding may be due to the limited sample size or the variability in MRI sequences used in this study. Similarly, our analysis does not identify a significant association between pathological complete response, lymphovascular invasion, and positive nodes with DFS.
Finally, in the Cox regression analysis, EMVI and pre-neoadjuvant nodal status did not achieve statistical significance for poor survival outcomes. Nonetheless, the p-values were nearly significant, indicating that a larger sample size could potentially reveal significant results.
Despite its promising results, the clinical adoption of PET/MRI faces challenges, primarily due to its higher cost compared to standard imaging, which may limit accessibility in resource-limited settings. However, PET/MRI combines two diagnostic modalities into a single exam, reducing scheduling complexities, patient discomfort, and examination time. It also lowers radiation exposure, making it a safer option, especially for younger patients or those needing repeated scans. While PET/MRI offers superior diagnostic and prognostic insights, further studies are needed to assess its cost-effectiveness and impact on long-term outcomes. Research should also focus on how PET/MRI findings could guide treatment decisions, such as personalizing neoadjuvant therapy or selecting patients for organ-preserving strategies. Addressing these challenges could help integrate PET/MRI into routine care, improving treatment and outcomes for rectal cancer patients.
A promising area for future research is the integration of PET/MRI findings with genomic and molecular data. Combining imaging biomarkers with genetic markers such as microsatellite instability (MSI) or KRAS mutations could offer a more comprehensive understanding of tumor biology and enhance the ability to predict treatment response. Such integration could also improve the detection of minimal residual disease (MRD) after neoadjuvant therapy by correlating genetic alterations with imaging findings, providing a more sensitive method for identifying residual tumor activity. Composite models that combine PET/MRI and molecular data hold the potential to significantly enhance the accuracy of prognosis and therapeutic decision-making.
Future studies should validate these approaches in larger, multi-center cohorts to develop standardized protocols that incorporate genomic and imaging data into clinical practice. By bridging the gap between anatomical imaging and tumor biology, PET/MRI combined with molecular analysis could represent a key advancement in the personalized management of rectal cancer.
Conclusion
These results indicate that PET/MRI offers significantly higher accuracy in detecting pCR and TRG, suggesting its potential to improve clinical outcomes through more precise diagnostic capabilities with respect to standard MRI. PET/MRI provides additional and critical information on tumor biology for the improved disease-free survival of colorectal patients.
Acknowledgements
This study was supported by the Foundation for Development and Investigation in Surgical Oncology of Madrid and by the International Investigation Department in General and Digestive Surgery of the Catholic University of Murcia.
Author contribution
Emilio Vicente and Yolanda Quijano proposed the study. Valentina Ferri and Riccardo Caruso performed research and wrote the first draft. Hipolito Duran, Eduardo Diaz, Isabel Fabra and Luca Ballelli collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. Valentina Ferri is the guarantor.
Funding
No funding received.
Data availability
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to Valentina Ferri at valenpeglio@gmail.com.The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Sanchinarro Hospital. All experiments were performed in accordance with relevant guidelines and regulations (such as the Declaration of Helsinki). Informed consent was obtained from all participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Cancer Society - Key statistics for colorectal cancer. cancer.org | 1.800.227.2345
- 2.Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A (2023) Colorectal cancer statistics. 10.3322/caac.21772 [DOI] [PubMed]
- 3.NCCN Clinical practice guidelines in oncology – rectal cancer, version 2.2022. 10.6004/jnccn.2022.0051 [DOI] [PubMed]
- 4.Pucciarelli S, Giandomenico F, De Paoli A et al (2017) Bowel function and quality of life after local excision or total mesorectal excision following chemoradiotherapy for rectal cancer. Br J Surg 104:138–147 [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, Verheij FS, Omer DM, Lee M, Dunne RF, Marcet J, Cataldo P, Polite B, Herzig DO, Liska D, Oommen S, Friel CM, Ternent C, Coveler AL, Hunt S, Gregory A, Varma MG, Bello BL, Carmichael JC, Krauss J, Gleisner A, Paty PB, Weiser MR, Nash GM, Pappou E, Guillem JG, Temple L, Wei IH, Widmar M, Lin S, Segal NH, Cercek A, Yaeger R, Smith JJ, Goodman KA, Wu AJ, Saltz LB (2022) Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol 40(23):2546–2556. 10.1200/JCO.22.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erlandsson J, Lörinc E, Ahlberg M, Pettersson D, Holm T, Glimelius B, Martling A (2019) Tumour regression after radiotherapy for rectal cancer - results from the randomised Stockholm III trial. Radiother Oncol 135:178–186. 10.1016/j.radonc.2019.03.016 [DOI] [PubMed] [Google Scholar]
- 7.Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, Morand C, de la Fouchardière C, Lamfichekh N, Juzyna B, Jouffroy-Zeller C, Rullier E, Marchal F, Gourgou S, Castan F, Borg C, Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group (2021) Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 22(5):702–715. 10.1016/S1470-2045(21)00079-6 [DOI] [PubMed] [Google Scholar]
- 8.Denecke T, Rau B, Hoffmann KT et al (2005) Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol 15:1658–1666 [DOI] [PubMed] [Google Scholar]
- 9.Maffione AM, Marzola MC, Capirci C, Colletti PM, Rubello D (2015) Value of (18)F-FDG PET for predicting response to neoadjuvant therapy in rectal cancer: systematic review and meta-analysis. AJR Am J Roentgenol 204:1261–1268 [DOI] [PubMed] [Google Scholar]
- 10.Crimì F, Spolverato G, Lacognata C, Gariri M, Cecchin D, Urso ED et al F-FDG PET/MRI for rectal cancer TNM restaging after preoperative chemoradiotherapy: initial experience. 10.1097/DCR.0000000000001568 [DOI] [PubMed]
- 11.Queiroz MA, Ortega CD, Ferreira FR, Nahas SC, Cerri GG, Buchpiguel CA Diagnostic accuracy of FDG-PET/MRI versus pelvic MRI and thoracic and abdominal CT for detecting synchronous distant metastases in rectal cancer patients. 10.1007/s00259-020-04911-x [DOI] [PubMed]
- 12.Ferri V, Vicente E, Quijano Y, Duran H, Diaz E, Fabra I, Malave L, Ruiz P, Costantini G, Pizzuti G, Cubillo A, Rubio MC, Cañamaque LG, Alfonsel JN, Caruso R (2023) Light and shadow of watch-and-wait strategy in rectal cancer: oncological result, clinical outcomes, and cost-effectiveness analysis. Int J Colorectal Dis 38(1):277. 10.1007/s00384-023-04573-9 [DOI] [PubMed] [Google Scholar]
- 13.Mandard AM, Dalibard F, Mandard JC et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73(11):2680–2686 [DOI] [PubMed] [Google Scholar]
- 14.Crimì F, Spolverato G, Lacognata C, Garieri M, Cecchin D, Urso ED, Zucchetta P, Pucciarelli S, Pomerri F (2020) 18F-FDG PET/MRI for rectal cancer TNM restaging after preoperative chemoradiotherapy: initial experience. Dis Colon Rectum 63(3):310–318. 10.1097/DCR.0000000000001568 [DOI] [PubMed] [Google Scholar]
- 15.Catalano OA, Lee SI, Parente C, Cauley C, Furtado FS, Striar R, Soricelli A, Salvatore M, Li Y, Umutlu L, Cañamaque LG, Groshar D, Mahmood U, Blaszkowsky LS, Ryan DP, Clark JW, Wo J, Hong TS, Kunitake H, Bordeianou L, Berger D, Ricciardi R, Rosen B (2021) Improving staging of rectal cancer in the pelvis: the role of PET/MRI. Eur J Nucl Med Mol Imaging 48(4):1235–1245. 10.1007/s00259-020-05036-x [DOI] [PubMed] [Google Scholar]
- 16.Ishihara S, Kawai K, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, Morikawa T, Watanabe T (2018) Diagnostic value of FDG-PET/CT for lateral pelvic lymph node metastasis in rectal cancer treated with preoperative chemoradiotherapy. Tech Coloproctol 22:347–354. 10.1007/s10151-018-1779-0 [DOI] [PubMed] [Google Scholar]
- 17.Crimì F, Valeggia S, Baffoni L, Stramare R, Lacognata C, Spolverato G, Albertoni L, Spimpolo A, Evangelista L, Zucchetta P, Cecchin D, Pucciarelli S (2021) [18F]FDG PET/MRI in rectal cancer. Ann Nucl Med 35(3):281–290. 10.1007/s12149-021-01580-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqui MRS, Gormly KL, Bhoday J, Balyansikova S, Battersby NJ, Chand M et al (2016) Interobserver agreement of radiologists assessing the response of rectal cancers to preoperative chemoradiation using the MRI tumour regression grading (mrTRG). Clin Radiol 71:854–862 [DOI] [PubMed] [Google Scholar]
- 19.Sorenson E, Lambreton F, Yu JQ, Li T, Denlinger CS, Meyer JE, Sigurdson ER, Farma JM (2019) Impact of PET/CT for restaging patients with locally advanced rectal cancer after neoadjuvant chemoradiation. J Surg Res 243:242–248. 10.1016/j.jss.2019.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferri V, Vicente Lopez E, QuijanoCollazo Y, Caruso R, Duran Gimenez Rico H, Ielpo B, Diaz Reques E, Fabra Cabrera I, Malavè Cardozo L, Isernia R, Pinna E, Plaza Hernandezv C, Garcerant M, Garcia Cañamaques L, PerezDueñas V (2019) Quantitative analysis of 18-FDG-PET/MRI to assess pathological complete response following neoadjuvant radiochemotherapy in locally advanced rectal cancer. A prospective preliminary study. Acta Oncol 58(9):1246–1249. 10.1080/0284186X.2019.1622774 [DOI] [PubMed] [Google Scholar]
- 21.Lord AC, D’Souza N, Shaw A, Rokan Z, Moran B, Abulafi M, Rasheed S, Chandramohan A, Corr A, Chau I, Brown G (2022) MRI-diagnosed tumor deposits and EMVI status have superior prognostic accuracy to current clinical TNM staging in rectal cancer. Ann Surg 276(2):334–344. 10.1097/SLA.0000000000004499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to Valentina Ferri at valenpeglio@gmail.com.The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.