Abstract
Ammonia oxidation plays a vital role in regulating soil nitrogen (N) cycle in agricultural soil, which is significantly influenced by different fertilizer regimes. However, there is still need to further investigate the effects of different fertilizer managements on rhizosphere soil ammonia-oxidizing archaea (AOA) and bacteria (AOB) community in the double-cropping rice field. Therefore, the effects of different long-term (37 years) fertilizer managements on rhizosphere soil potential nitrification activity (PNA), AOA and AOB community structure, and its relationship under the double-cropping rice system in southern of China were studied in the present paper. The field experiment included without fertilizer input as a control (CK), inorganic fertilizer (MF), rice straw and inorganic fertilizer (RF), 30% organic manure and 70% inorganic fertilizer (OM). This result indicated that rhizosphere soil organic carbon (SOC) (SOC), total N and ammonium N (NH4+-N) contents in paddy field with RF and OM treatments were increased. Rhizosphere soil PNA, potential nitrification rate (PNR) and abundance of AOB in paddy field with MF treatment were increased, and abundance of AOA in paddy field with RF and OM treatments were increased, respectively. The result also showed that rhizosphere soil diversity index of AOA and AOB with RF and OM treatments were enhanced, compared with CK treatment. Rhizosphere soil AOB and AOA community composition was dominated by Proteobacteria, Actinobacteria and Acidobacteria with all fertilizer treatments. There had significantly positively correlation between the abundance of AOA and SOC, total N, and NH4-N contents. However, there had significantly negatively correlation between soil pH and abundance of AOA, soil PNA, PNR. As a result, long-term application of rice straw and organic manure was benefit for increasing community structure of rhizosphere soil ammonia oxidizer under the double-cropping rice system in southern of China.
Keywords: Rice, Rhizosphere soil, Fertilizer regime, Ammonia-oxidizing archaea, Ammonia-oxidizing bacteria
Subject terms: Microbiology, Environmental sciences
Introduction
It is widely believed that microbial ammonia oxidation plays an important role in regulating nitrogen (N) cycle of agricultural soil, which were regulated nitrous oxide (N2O) and N2 gas emission in the global1. Therefore, the biogeochemical cycling of carbon (C), N, phosphorus (P), and sulfur (S) is primarily influenced by soil microbes2,3. The rhizosphere, which is the volume of soil adjacent to and affected by plant root4, plays a vital role in regulating plant growth and soil fertility3. Meanwhile, it was a benefit practices for increasing soil fertility and crop yield with fertilizer input, which strongly influence on soil biochemical and biological properties. Therefore, the effects of different fertilizer managements on activity and community structure of soil ammonia-oxidizing archaea (AOA) and bacteria (AOB) were focus on by more and more researcher in recent years5,6.
In the previous studies, these results indicated that composition and community structure of soil AOA and AOB were mainly affecting by different field managements, such as cropping system, tillage, fertilizer regime, irrigation and so on. Fertilizer regime was playing a vital role in regulating AOA and AOB community in paddy field. Previous results proved that soil ammonia oxidizer population, potential nitrification activity (PNA) and potential nitrification rate (PNR) were changed under applied with inorganic fertilizer and organic manure conditions7–9. Some results showed that AOB population and nitrification activity were enhanced with inorganic fertilizer practices8,10, whereas other result indicated that nitrification activity and abundances of AOA and AOB with organic manure management were higher than that of inorganic fertilizer management11. Rhizosphere is a zone of intense microbial activity that could profound influences on soil nutrient cycling and provides soil nutrient for plant growth12. Some results indicated that nitrifying population13,14 and PNA7,9 in rhizosphere soil with inorganic fertilizer, crop residue and organic manure input practices were increased. However, most researches were conducted on bulk soil or based on short-term experiment, there is still need to further investigate the change of rhizosphere soil ammonia oxidation in paddy field under long-term fertilization condition.
Rice (Oryza sativa L.) is the main agricultural land use in the tropical and subtropical monsoon regions15. It was a vital factor in maintaining or increasing soil quality and fertility of paddy field with different fertilizer regimes, including manure, crop residue and chemical fertilizer. In the previous studies, our result indicated that soil chemical properties (e.g., soil pH, total N, SOC contents) and rice yield were significantly changed under different long-term fertilizer conditions16,17. Furthermore, rhizosphere soil total N content, organic N fraction and N mineralization in the double-cropping rice field were obviously increased with manure and crop residue input managements, which in turn influence the function and community structure of AOA and AOB. However, it is still not known how about rhizosphere soil PNA and PNR, diversity and community of AOA and AOB responds to different fertilizer managements under the double-cropping rice system in southern of China. Therefore, the objectives of this study were: (1) to explore the effects of different fertilizer treatments on rhizosphere soil PNA and PNR in the double-cropping rice field, (2) to investigate the rhizosphere soil diversity and community of AOA and AOB in paddy field; (3) to analysis the relationship between soil chemical properties and community of ammonia oxidation under the double-cropping rice system in southern of China.
Materials and methods
Sites and cropping system
The field experiment was beginning from 1986. It was located in Ningxiang City (28°07′ N, 112°18′ E) of Hunan Province, China. Under a continental monsoon climate, the annual mean precipitation and potential evapotranspiration is 1553 mm and 1354 mm, respectively. The monthly mean temperature is 17.2 °C. Soil chemical characteristics at 0–20 cm layer beginning of this field experiment, cropping system were described as by Tang et al.16.
Experimental design
The field experiment included four treatments: without fertilizer input as a control (CK), inorganic fertilizer (MF), rice straw and inorganic fertilizer (RF), 30% organic manure and 70% inorganic fertilizer (OM). The experiment design ensured all fertilizer treatments received the same level of nitrogen (N), phosphorus pentoxide (P2O5), potassium oxide (K2O) (the amount of N, P2O5, K2O in chemical fertilizer plus that from rice residue or manure) during the early rice and late rice whole growing season, respectively. The kinds of N, P2O5, K2O chemical fertilizer included urea, ordinary superphosphate and potassium chloride, respectively. Other more detail information about the fertilizer managements and field practices were described as by Tang et al.16.
Soil sample collected
Soil samples were collected at tillering stage of late rice in 25 August 2022. Rhizosphere soil was operationally defined as soil adhering to rice root after gentle shaking. In order to obtain enough rhizosphere soil for analysis at laboratory, twenty rice plants were randomly selected from each plot, and these rhizosphere soils were pooled to form one composite sample. Thus, three composite soil samples of each fertilizer treatment were collected at sampling time, and the total number of 12 composite soil samples were collected at tillering stage of late rice. The fresh soil samples were placed immediately in ice box and transported to the laboratory. Plant root and small stone were removed, one part of soil samples were through a 2-mm sieve then stored at room temperature until chemical analysis, and another part of soil samples were stored at – 20 °C until molecular analysis.
Soil laboratory analysis
Soil chemical properties analysis
Soil pH was measured with a compound electrode (PE-10, Sartorious, Germany) using a soil to water ratio of 1: 2.5. Soil organic carbon (SOC) content was determined by dichromate oxidation, while total N content was measured using a vario MACRO cube element analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). Soil ammonium N (NH4+-N) and nitrate N (NO3−-N) contents were determined by extracting the soil with 0.01 M CaCl2 solution (1:10, w/v) for 30 min and then determining the NH4+ and NO3− concentrations using a flow injection autoanalyzer (FLA star 5000 Analyzer, Foss, Denmark).
Soil potential nitrification activity (PNA) and potential nitrification rate (PNR)
Soil PNA (µg NO3−-N g−1 h−1) was determined with the shaken slurry method described as by Hart et al.18, which evaluate the maximum nitrate production rate of a soil sample. Soil PNR was investigated with griess reagent colorimetry method described as by Kurola et al.19.
DNA extraction and PCR amplification
Soil total DNA was extracted using a Fast DNA SPIN Kit for soil (MP Biomedicals, Illkirch, France) according to the manufacturer’s instruction. DNA was finally eluted with 100 µL of the DNA elution solution included in the kit. Soil DNA extraction was characterized by using electrophoresis on 0.7% (wt/vol) agarose gels.
Polymerase chain reaction (PCR) reaction mixture included 12.5 µL 2 × EasyTaq PCR SuperMix (TransGen Biotech, Beijing, China), 0.5 µM of each primer, 1 µL of 10-fold diluted DNA template, and diluted to a final volume of 25 µL. PCR reaction was performed using the AOB-specific primers CTO654r and CTO189f20, AOA amoA genes were amplified using primers CrenamoA616r and CrenamoA23f21. The size and quality of soil PCR products were verified with 1.5% (wt/vol) agarose.
Quantitative real-time PCR
In order to investigate of soil sample ammonia-oxidizing archaea community, bacteria community was analysis using the primers CrenamoA616r and CrenamoA23f, CTO654r and CTO189f, respectively22. Quantitative real-time PCR for AOB and AOA was conduct in triplicate using an ABI 7500 Real-Time PCR System (Applied Biosystems) under the following thermocycling conditions: 30 s at 95 °C, followed by 40 cycles of 5 s at 95 °C and 34 s at 60 °C. The amplification specificity of AOA was confirmed by generating a melting curve. Standard curves ranging from 1 × 102 to 1 × 107 copies were prepared by 10-fold serial dilution of known copy numbers of plasmid DNA possessing the genes of interest. In order to calculate abundances of AOA and AOB genes, it was assumed that AOA carry one copy of the amoA gene per cell23, and AOB contain one copy of 16 S rRNA per cell24. The sequences of AOA and AOB were then compared with those available in the National Center for Biotechnology Information (NCBI) GenBank database using the BLAST algorithm. Sequence analysis and operational taxonomic units (OTUs) identification were according to the method described as by Fagen et al.25. Matches were filtered at 80% length fraction and OTUs were classified at 97% identity level. Richness diversity, Shannon diversity and Chao 1 diversity index were used to calculate the diversity of ammonia-oxidizing archaea and bacteria community using the Mothur software26.
Statistical analysis
These results of every investigate items were presented as average value and standard error. The significance of differences between different fertilizer treatments was conducted using analysis of variance (ANOVA). The relationship between soil chemical properties, soil potential nitrification activity, soil potential nitrification rate and abundances of AOA and AOB were analyzed using the Pearson correlation test. All statistical analyses were calculated by using SAS 9.3 software package27 and a probability value of 0.05 was considered to indicate statistical significance.
Results
Soil chemical properties
This result indicated that rhizosphere soil chemical characteristics in paddy field were obvious changed under different long-term fertilizer conditions (Table 1). The result showed that soil pH value in paddy field with OM and RF treatments was significantly (p < 0.05) higher than that of CK treatment. SOC, soil NO3-N and NH4-N contents with MF, RF and OM treatments were significantly (p < 0.05) higher than that of CK treatment. The result showed that soil total N content in paddy field with OM and RF treatments was significantly (p < 0.05) higher than that of MF and CK treatments (Table 1).
Table 1.
Effects of different long-term fertilizer treatments on soil chemical characteristics in the double-cropping rice field.
| Treatments | pH | SOC (g kg−1) |
Total N (g kg−1) |
NO3-N (g kg−1) |
NH4-N (g kg−1) |
|---|---|---|---|---|---|
| MF | 6.32 ± 0.17ab | 21.46 ± 0.68c | 2.07 ± 0.06c | 0.17 ± 0.01c | 0.16 ± 0.01c |
| RF | 6.70 ± 0.17a | 24.82 ± 0.72b | 2.45 ± 0.07b | 0.21 ± 0.01b | 0.20 ± 0.01b |
| OM | 6.81 ± 0.18a | 29.62 ± 0.71a | 3.08 ± 0.08a | 0.27 ± 0.01a | 0.23 ± 0.01a |
| CK | 6.21 ± 0.16b | 19.68 ± 0.56d | 1.91 ± 0.06c | 0.13 ± 0.01d | 0.12 ± 0.01d |
Values were presented as mean ± standard error. Different lowercase letters in the same column were indicated significantly at difference in 0.05 levels. The same as below.
MF, inorganic fertilizer; RF, rice straw and inorganic fertilizer; OM, 30% organic manure and 70% inorganic fertilizer; CK, without fertilizer input as a control.
Soil potential nitrification rate and potential nitrification activity
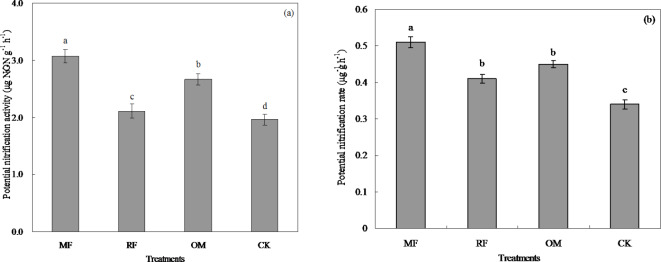
The effects of different fertilizer treatments on rhizosphere soil potential nitrification activity (PNA) and potential nitrification rate (PNR) in paddy field were showed as in Fig. 1. This result showed that rhizosphere soil PNA with all fertilizer treatments ranged from 1.96 to 3.11 µg NO3−-N g−1 h−1 (Fig. 1a). Soil PNA with MF treatment was significantly (p < 0.05) higher than that of RF, OM and CK treatments. Soil PNA with RF and OM treatments was significantly (p < 0.05) higher than that of CK treatment.
Fig. 1.
Potential nitrification activity (a) and potential nitrification rate (b) in rhizosphere soil under different fertilizer treatments. MF: inorganic fertilizer; RF: rice straw and inorganic fertilizer; OM: 30% organic manure and 70% inorganic fertilizer; CK: without fertilizer input as a control. Vertical bars represent the standard error. Different lowercase letters were indicated significantly at difference among fertilizer treatments in 0.05 levels. The same as below.
This result showed that rhizosphere soil PNR with all fertilizer treatments ranged from 0.34 to 0.51 µg g−1 h−1 (Fig. 1b). Soil PNR with MF treatment was significantly (p < 0.05) higher than that of RF, OM and CK treatments. Soil PNR with RF and OM treatments was significantly (p < 0.05) higher than that of CK treatment, but there were no significantly difference (p > 0.05) in soil PNR between RF and OM treatments.
Abundances of AOA and AOB genes
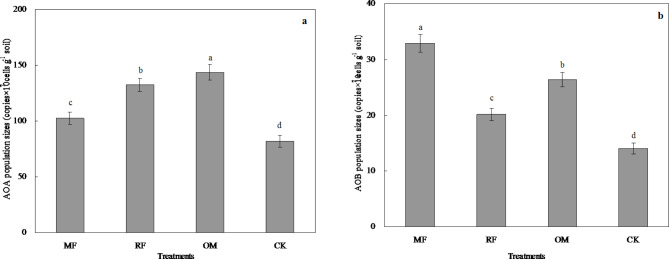
The effects of different fertilizer treatments on abundances of rhizosphere soil AOA and AOB genes in paddy field were showed as in Fig. 2. This result showed that abundance of AOA with all fertilizer treatments ranged from 81.79 to 143.75 copies × 107 cells g−1 soil (Fig. 2a). Abundance of AOA with RF and OM treatments was significantly (p < 0.05) higher than that of MF and CK treatments. Compared with CK treatment, abundance of AOA with MF, RF and OM treatments increased by 1.25, 1.62 and 1.76 times, respectively.
Fig. 2.
Effects of different long-term fertilizer treatments on abundances of AOA (a) and AOB (b) in rhizosphere soil under the double-cropping rice system.
This result showed that abundance of AOB with all fertilizer treatments ranged from 14.05 to 32.87 copies×107 cells g−1 soil (Fig. 2b). Abundance of AOB with MF treatment was significantly (p < 0.05) higher than that of RF, OM and CK treatments. Compared with CK treatment, abundance of AOB with MF, RF and OM treatments increased by 2.34, 1.44 and 1.88 times, respectively.
Diversity index of AOA and AOB
The effects of different fertilizer treatments on rhizosphere soil diversity index of AOA and AOB were showed as in Table 2. Soil Richness diversity index of AOA with RF and OM treatments was significantly (p < 0.05) higher than that of CK treatment, soil Shannon and Chao 1 diversity index of AOA with OM treatment was significantly (p < 0.05) higher than that of CK treatment, respectively.
Table 2.
Soil diversity index of AOA and AOB with different long-term fertilizer treatments.
| Bacteria and archaea | Diversity parameters | Treatments | |||
|---|---|---|---|---|---|
| MF | RF | OM | CK | ||
| AOA | Richness indices | 12.52 ± 0.37b | 12.96 ± 0.38a | 13.25 ± 0.38a | 12.27 ± 0.35b |
| Shannon indices | 2.59 ± 0.07ab | 2.63 ± 0.07ab | 2.79 ± 0.08a | 2.48 ± 0.06b | |
| Chao 1 indices | 5.26 ± 0.16b | 5.62 ± 0.16b | 6.29 ± 0.18a | 4.47 ± 0.13c | |
| AOB | Richness indices | 9.37 ± 0.27b | 9.82 ± 0.28b | 10.43 ± 0.31a | 8.98 ± 0.26c |
| Shannon indices | 2.68 ± 0.07b | 2.95 ± 0.08ab | 3.12 ± 0.09a | 2.74 ± 0.07b | |
| Chao 1 indices | 3.37 ± 0.10b | 3.72 ± 0.11a | 3.89 ± 0.11a | 2.94 ± 0.08c | |
AOA, soil ammonia-oxidizing archaea; AOB, soil ammonia-oxidizing bacterial. Different lowercase letters in the same line indicated significantly difference at p < 0.05. The same as below.
This result indicated that rhizosphere soil Richness and Shannon diversity index of AOB with OM treatment was significantly (p < 0.05) higher than that of MF and CK treatments. Soil Chao 1 diversity index of AOB with RF and OM treatments was significantly (p < 0.05) higher than that of MF and CK treatments, but there had no significantly (p > 0.05) difference in soil Chao 1 diversity index of AOB between OM and RF treatments (Table 2).
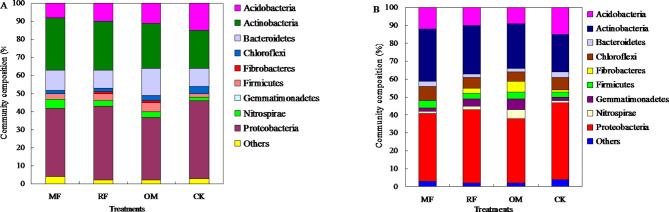
Community structure of AOA and AOB
This result indicated that rhizosphere soil ammonia-oxidizing archaea community composition was Proteobacteria, Actinobacteria,Bacteroidetes, Acidobacteria, and Nitrospirae. The Proteobacteria and Actinobacteria occupy absolute advantage in rhizosphere soil with all fertilizer treatments (Fig. 3A).
Fig. 3.
Community compositions of AOA (a) and AOB (b) in rhizosphere soil under different long-term fertilizer treatments.
The main community composition rhizosphere soil ammonia-oxidizing bacterial community composition was Proteobacteria, Actinobacteria, Acidobacteria, Chloroflexi, and Firmicutes with all fertilizer treatments. The Proteobacteria, Actinobacteria and Acidobacteria occupy absolute advantage in rhizosphere soil with MF, RF and OM treatments. This result showed that community composition of soil Actinobacteria with MF, RF, OM treatments were higher than that of CK treatment (Fig. 3B).
Correlations of soil properties with community structures of AOA and AOB
There had significantly positively (p < 0.01) correlation between abundance of AOA and SOC, total N, NH4-N contents (Table 3). Meanwhile, there had significantly negatively (p < 0.05) correlation between abundance of AOB and soil potential nitrification activity, potential nitrification rate. However, there had significantly negatively (p < 0.05) correlation between soil pH and abundance of AOA, soil potential nitrification activity, potential nitrification rate. There had not correlation between abundance of AOA and soil PNA, PNR. There had significantly positively (p < 0.01) correlation between abundance of AOB and soil PNA, PNR.
Table 3.
Correlation between soil chemical properties, potential nitrification activity (PNA), potential nitrification rate (PNR) and abundances of AOA and AOB.
| Items | pH | SOC | TN | NH4-N | NO3-N | AOA | AOB |
|---|---|---|---|---|---|---|---|
| AOB | − 0.425 | 0.301 | 0.327 | 0.287 | − 0.195 | 0.285 | — |
| AOA | − 0.702** | 0.706** | 0.715** | 0.734** | 0.406 | — | 0.284 |
| PNA | − 0.611* | 0.352 | 0.438 | 0.243 | − 0.275 | 0.347 | 0.872** |
| PNR | − 0.605* | 0.326 | 0.375 | 0.136 | − 0.231 | 0.284 | 0.836** |
TN: soil total nitrogen content; SOC: soil organic carbon. AOB: soil ammonia-oxidizing bacterial; AOA: soil ammonia-oxidizing archaea; PNA: soil potential nitrification activity; PNR: potential nitrification rate. *, ** were significantly at the 0.05 and 0.01 level, respectively.
Discussions
In the previous studies, these results indicated that rhizosphere soil microbial activity was affected during N transformation processes in paddy field7,14. In this study, our result showed that rhizosphere soil potential nitrification activity (PNA) and potential nitrification rate (PNR) in paddy field were obvious changed in different fertilizer treatments (Fig. 1), implying a profound fertilization effect on rhizosphere soil ammonia oxidation. Meanwhile, soil PNA and PNR in paddy field with MF treatment were significantly increased, compared with RF, OM and CK treatments. These results were consistent with previous results of paddy field in China28. The reason maybe attributed to that higher resource of rhizodeposition after mineralization may provide more substrate for ammonia oxidizers and stimulate microbial growth28,29. On the other hand, soil nitrification and ammonia oxidizers were frequently influenced by the rice varieties and climate factors, which were regarded as important environmental factors responsible for microbial nitrification30. Furthermore, there had significantly positively correlation between abundance of AOB and soil PNA, PNR. Meanwhile, there had significantly negatively correlation between soil pH and soil PNA, PNR. These results suggested that soil pH and abundance of AOB were important drivers in regulating soil PNA and PNR under the double-cropping rice system in southern of China.
Our result showed that abundance of AOA was higher than that of AOB in rhizosphere soil, which were agree with previous reported by Chen et al.28. However, the community structure and abundance of AOB were significantly correlated with soil PNA and PNR, while those of AOA were not (Table 2; Fig. 2), suggesting that soil nitrification were primarily driven by AOB in the double-cropping rice paddy field. The predominance of bacterial nitrification were consistent with previous result31, but inconsistent with report for acidic soil in paddy field32, suggesting that soil texture, soil characteristics and soil pH were obvious difference from the present experimental field. Further results suggested that archaea can be well adapted to extreme conditions, such as soil type, soil pH, low ammonia availability, and climate conditions19,33, while the opposite response were detected for bacteria11. These soil texture and soil characteristics were main explain why AOB but not AOA play a vital role in regulating soil nitrification in this field experiment (pH from 6.21 to 6.81) (Table 1) under long-term fertilizer condition.
In the present study, our result indicated that abundance of AOA in rhizosphere soil were higher than that of AOB in paddy field, which were consistent with the previous result11. The reason maybe attributed to that soil pH of paddy field was greatly impacted by fertilizer managements (Table 1), possibly owing to strong carbonate buffering2. It had proved that soil pH had a considerable effect on activity of AOB and other microbial processes that they mediate6. In this study, the result indicated that abundance of AOB with MF treatment were significantly increased compared with RF and OM treatments, which were agree with previous study21, who found that N-induced stimulation of AOB under long-term inorganic fertilizer input condition. The reason was attributed to that dependence of monooxygenase on NH3, which would be ionized exponentially to NH4+ with decreasing pH in paddy field under long-term inorganic fertilizer input condition, but soil acid buffering capacity and soil pH were increased under long-term rice straw and organic manure input conditions11. Meanwhile, this result indicated that abundance of AOA with RF and OM treatments were higher than that of MF and CK treatments, which suggested there had higher soil organic carbon, total N contents and soil pH with organic manure and rice straw input practices, may stimulate growth of the AOA community. Compared with RF and OM treatments, abundance of AOA with MF treatment were decreased, which suggested there had lower soil organic carbon, total N contents and soil pH with long-term inorganic fertilizer input practice, may restricted growth of the AOA community28. Positively correlation between abundance of AOA and SOC, total N contents were also observed in the present study (Table 3), which suggested that AOA and AOB have alternative growth strategies for mixotrophic or heterotrophic growth10.
Previous studies results indicated that soil functional community involved in ammonia oxidizer respond different to fertilizer practices change7,9. In the present study, our result showed that diversity of ammonia oxidizer community (Richness index, Shannon index and Chao 1 diversity index) was significantly changed among the different fertilizer regimes. Our result indicated that long-term application of rice straw and organic manure was result in increased diversity of AOA and AOB community (Table 2). The reason maybe attributed to that soil texture and nutrient content were increased in the double-cropping rice paddy field under long-term application of rice straw and organic manure conditions16,17, which decreased in the competitive niche exclusion and selection mechanism between soil ammonia-oxidizing bacterial and archaea populations. Therefore, these consequences could lead to changes in ammonia-oxidizing bacterial and archaea diversity, and enhance in the Richness, Shannon and Chao 1 indices19. Meanwhile, this result indicated that diversity of ammonia oxidizer community with MF treatment were higher than that of CK treatment. The reason maybe attributed to that application of inorganic fertilizer practice, which caused promoted soil chemical properties and rhizosphere soil environment than the without fertilizer input practice did, could promoted in multiplying soil ammonia-oxidizing bacterial and archaea4,11. Therefore, this study supports the view that application of rice residue and manure treatments strongly influence on soil texture and altered soil chemical properties, causing changes in diversity and abundances of soil ammonia-oxidizing bacterial and archaea.
In the previous studies, these results indicated that soil AOB and AOA community composition was main influenced by different fertilizer treatments20,30. In this study, the result revealed that rhizosphere soil AOB and AOA community composition were dominated by Proteobacteria, Actinobacteria and Acidobacteria with fertilizer treatments (MF, RF and OM treatments), this findings were agree with the previous found7,9. The reason maybe attributed to that Proteobacteria, Actinobacteria and Acidobacteria were considered as the eutrophic bacteria in soil, which were using total N, NH4+-N and NO3−-N contents as main N source and growth rapidly under nutrient rich conditions. On the other hand, rhizosphere soil physical and chemical properties were important factors in influencing soil microbial abundance and community under application of fertilizer practice condition. In this study, the result revealed that ammonia oxidizer community were correlated with soil chemical properties (Table 3), implying that soil ammonia-oxidizing bacterial and archaea community in the double-cropping rice field were changed by combined effect of soil pH, SOC, total N, NH4+-N, NO3−-N contents, soil PNA and PNR. These results were supported by the observation that AOA and AOB abundances were negatively correlated with soil pH33,34, but positively correlated with SOC, total N and NH4+-N contents (Table 3). However, the controlling factor in influencing on ecological function of rhizosphere soil AOB and AOA in paddy field is still need to further study.
Conclusions
Our result indicated that activity and community structure of rhizosphere soil AOB and AOA in paddy field were obvious changed under different long-term of fertilization conditions. Rhizosphere soil potential nitrification activity and potential nitrification rate in paddy field were stimulated by application of inorganic fertilizer practice. Abundances of rhizosphere soil AOA and AOB genes in paddy field with MF, RF and OM treatments were significantly increased, compared with CK treatment. Long-term (37 years) application of chemical fertilizer increase soil PNA and abundance of AOB, whereas application of rice residue and organic manure significantly enhance abundance of AOA in the double-cropping rice field. Meanwhile, this result indicated that soil Richness, Shannon and Chao 1 diversity indices of AOB and AOA were improved under manure and crop residue input conditions. Rhizosphere soil AOB and AOA community composition were dominated by Proteobacteria, Actinobacteria and Acidobacteria with all fertilizer treatments. However, there is still need to further investigate effects of different fertilizer treatments on specific mechanistic of soil N-cycle ammonia-oxidizing bacterial and archaea based on long-term field experiment.
Acknowledgements
This study was supported by the National Key Research and Development Project of China (2023YFD2301403), the Hunan Provincial Natural Science Foundation of China (2022JJ30352), the Hunan Science and Technology Talent Lift Project (2022TJ-N07), the Innovative Research Groups of the Natural Science Foundation of Hunan Province (2024CX39), and the Special Funds for the Construction of Innovative Provinces in Hunan Province (2023NK2027).
Author contributions
Haiming Tang and Li Wen wrote the main manuscript text, Kaikai Cheng and Lihong Shi prepared Figs. 1 and 2, Geng Sun prepared Fig. 3, Mei Sun and Weiyan Li prepared Table 1, and 2. All authors reviewed the manuscript.
Data availability
Data is provided within the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang, Y. et al. Responses of potential ammonia oxidation and ammonia oxidizers community to arsenic stress in seven types of soil. J. Environ. Sci.127, 15–29 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Liu, R., Yang, Y. & Zhou, J. T. X. Mining activities accelerate the decomposition of organic matter from aquatic ecosystems through soil microbes. Land. Degrad. Dev.10, 2803–2811 (2026). [Google Scholar]
- 3.Ai, C., Liang, G., Sun, J., Wang, X. & Zhou, W. Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma173–174, 330–338 (2012). [Google Scholar]
- 4.Shi, L. H. et al. Effects of long-term fertilizer practices on rhizosphere soil nitrogen mineralization in the double-cropping rice field. J. Basic. Microb.63, 781–789 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Shen, J., Zhang, L., Zhu, Y., Zhang, J. & He, J. Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ. Microbiol.10, 1601–1611 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Verhamme, D. T., Prosser, J. I. & Nicol, G. W. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J.5, 1067–1071 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai, C. et al. Different roles of rhizosphere effect and long-term fertilization in the activity and community structure of ammonia oxidizers in a calcerous fluvo-aquic soil. Soil. Biol. Biochem.57, 30–42 (2013). [Google Scholar]
- 8.Chu, H., Fujii, T., Morimoto, S., Lin, X. & Yaki, K. Population size and specific nitrification potential of sol ammonia-oxidizing bacteria under long-term fertilization management. Soil. Biol. Biochem.40, 1960–1963 (2008). [Google Scholar]
- 9.He, Z. Y. et al. Fertilization has a greater effect than rhizosphere on community structures of comammox Nitrospira in an alkaline agricultural soil. Appl. Soil. Ecol.175, 104456 (2022). [Google Scholar]
- 10.Strauss, S. L., Reardon, C. L. & Mazzola, M. The response of ammonia-oxidizer activity and community structure to fertilizer amendment of orchard soils. Soil. Biol. Biochem.68, 410–418 (2014). [Google Scholar]
- 11.Meng, X. et al. Fertilization regimes and the nitrification process in paddy soils: lessons for agricultural sustainability from a meta-analysis. Appl. Soil. Ecol.186, 104844 (2023). [Google Scholar]
- 12.Berendsen, R., Pieterse, C. & Bakker, P. The rhizosphere microbiome and plant health. Trends Plant. Sci.17, 478–486 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Hussain, Q. et al. Temporal dynamics of ammonia oxidizer (amoA) and denitrifier (nirK) communities in the rhizosphere of a rice ecosystem from Tai Lake region, China. Appl. Soil. Ecol.48, 210–218 (2011). [Google Scholar]
- 14.Kleineidam, K. et al. Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on ammonia-oxidizing bacteria and archaea in rhizosphere and bulk soil. Chemosphere84, 182–186 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Yang, X. Y., Ren, W. D., Sun, B. H. & Zhang, S. L. Effects of contrasting soil management regimes on total and labile soil organic carbon fractions in a loess soil in China. Geoderma177–178, 49–56 (2012). [Google Scholar]
- 16.Tang, H. M. et al. Long-term effects of NPK fertilizers and organic manures on soil organic carbon and carbon management index under a double-cropping rice system in Southern China. Commun. Soil. Sci. Plan.49, 1976–1989 (2018). [Google Scholar]
- 17.Tang, H. M. et al. Effects of long-term fertiliser regime on soil organic carbon and its labile fractions under double cropping rice system of southern China. Acta Agr Scand. B-S P70, 409–418 (2020). [Google Scholar]
- 18.Hart, S. C., Stark, J. M., Davidson, E. A. & Firestone, M. K. Nitrogen mineralization, immobilisation and nitrification. In Methods of Soil Analysis. Part 2. Microbial and Biogeochemical Properties (eds. Weaver, R. W., Angle, J. S. & Bottomley, P. S.) 985–1018 (Soil Science Society of America, 1994). [Google Scholar]
- 19.Kurola, J., Salkinoja-Salonen, M., Aarnio, T., Hultman, J. & Romantschuk, M. Activity, diversity and population size of ammonia-oxidising bacteria in oil-contaminated landfarming soil. FEMS Microbiol. Lett.250, 33–38 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Kowalchuk, G. A. et al. Analysis of ammonia-oxidizing bacteria of the beta subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl. Environ. Microb.63, 1489–1497 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tourna, M., Freitag, T. E., Nicol, G. W. & Prosser, J. I. Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ. Microbiol.10, 1357–1364 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Hermansson, A. & Lindgren, P. E. Quantification of ammonia-oxidizing bacteria in arable soil by real-time PCR. Appl. Environ. Microb.67, 972–976 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agogué, H., Brink, M., Dinasquet, J. & Herndl, G. J. Major gradients in putatively nitrifying and non-nitrifying Archaea in the deep North Atlantic. Nature456, 788–791 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Coci, M. et al. Quantitative assessment of ammonia-oxidizing bacterial communities in the epiphyton of submerged macrophytes in shallow lakes. Appl. Environ. Microb.76, 1813–1821 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fagen, J. R. et al. Characterization of the relative abundance of the citrus pathogen ca. Liberibacter asiaticus in the microbiome of its insect 525vecor, Diaphorina citri, using high throughput 16S rRNA sequencing. Open. Microbiol. J.6, 29–33 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgar, R. C. UPARSE: highly accurate OUT sequences from microbial amplicon reads. J. Nat. Methods. 10, 996 (2013). [DOI] [PubMed] [Google Scholar]
- 27.SAS. SAS Software of the SAS System for Windows (SAS Institute Inc, 2008).
- 28.Chen, X. P., Zhu, Y. G., Xia, Y., Shen, J. P. & He, J. Z. Ammonia-oxidizing archaea: important players in paddy rhizosphere soil? Environ. Microbiol.10, 1978–1987 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Malchair, S. et al. Do climate warming and plant species richness affect potential nitrification, basal respiration and ammonia-oxidizing bacteria in experimental grasslands? Soil. Biol. Biochem.42, 1944–1951 (2010). [Google Scholar]
- 30.Hei, Z. et al. Full substitution of chemical fertilizer by organic manure decreases soil N2O emissions driven by ammonia oxidizers and gross nitrogen transformations. Global Change Biol.29, 7117–7130 (2023). [DOI] [PubMed] [Google Scholar]
- 31.Jia, Z. & Conrad, R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol.11, 1658–1671 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Yao, H. et al. Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl. Environ. Microb.77, 4618–4625 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicol, G. W., Leininger, S., Schleper, C. & Prosser, J. I. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol.10, 2966–2978 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Xie, J. et al. Manure combined with biochar reduces rhizosphere nitrification potential and amoA gene abundance of ammonia-oxidizing microorganisms in acid purple soil. Appl. Soil. Ecol.181, 104660 (2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript.