Abstract
Aims
Weight loss mediated by glucagon‐like peptide‐1 (GLP‐1) analogues is lower in patients with type 2 diabetes versus those without. Type 2 diabetes and obesity are risk factors for metabolic dysfunction‐associated steatotic liver disease (MASLD) and associated steatohepatitis (MASH). We evaluated weight changes in adults with MASLD/MASH with or without type 2 diabetes receiving the GLP‐1 analogue semaglutide.
Materials and Methods
This was a post hoc analysis of data from three 48–72‐week randomised trials investigating the effect of semaglutide versus placebo in adults with MASLD (NCT03357380) or biopsy‐confirmed MASH (NCT02970942 and NCT03987451). Pooled data for semaglutide (0.4 mg once daily and 2.4 mg once weekly [n = 163]) and placebo (n = 137) were analysed at 1 year. Weight changes were analysed by type 2 diabetes status (type 2 diabetes [n = 209], pre‐type 2 diabetes [n = 51] and no diabetes [n = 40]) and by other cardiometabolic risk parameters using analysis of covariance and Spearman's rank correlations.
Results
The overall mean weight change was −11.1 kg (−11.7%) and −0.7 kg (−0.6%) with semaglutide and placebo, respectively. While numerically higher for people without type 2 diabetes, estimated treatment differences with semaglutide versus placebo were similar overall for people with type 2 diabetes (−10.2 kg; −10.8%), pre‐type 2 diabetes (−9.8 kg; −10.2%) and no diabetes (−11.6 kg; −13.1%). Differences between groups were not statistically significant (p > 0.50 for all). Baseline fasting plasma glucose, glycated haemoglobin, insulin levels, insulin resistance and lipids did not correlate with weight change.
Conclusions
People with MASLD/MASH had similar semaglutide‐mediated weight loss regardless of type 2 diabetes status and other cardiometabolic risk parameters.
Keywords: GLP‐1 analogue, semaglutide, type 2 diabetes, weight control
1. INTRODUCTION
Metabolic dysfunction‐associated steatotic liver disease (MASLD, formerly known as non‐alcoholic fatty liver disease) 1 is the most common cause of chronic liver disease and liver‐related morbidity and mortality. 2 Risk factors for MASLD include other metabolic conditions such as obesity and type 2 diabetes. 2 , 3 Metabolic dysfunction‐associated steatohepatitis (MASH, formerly known as non‐alcoholic steatohepatitis) 1 is the more severe form of the spectrum of MASLD, characterised by the presence of steatosis, hepatocyte ballooning, inflammation and varying degrees of fibrosis. 2 People with MASLD and MASH are at increased risk of cardiovascular disease, partially mediated by their association with type 2 diabetes, hyperlipidaemia and obesity. 2
Given the historic lack of approved pharmacotherapies, lifestyle changes have been the mainstay of treatment of patients with MASLD/MASH. Accordingly, current MASLD guidelines primarily recommend a reduced calorie diet and habitual exercise, aiming for a weight reduction of 7%–10% in people with obesity and MASLD/MASH. 4 , 5 , 6 , 7 The benefits of weight loss are well established and include the reduction of steatosis and of plasma aminotransferase levels, lowering the risk of cardiovascular disease, and MASH resolution. 4 , 5 , 6 , 7 , 8 Resmetirom is the only pharmacotherapy provisionally approved in the United States by the FDA in March 2024 for the treatment of MASH with fibrosis. 9 Glucagon‐like peptide‐1 (GLP‐1) analogues, currently licensed for glycaemic control and weight loss in type 2 diabetes and overweight/obesity, 10 , 11 are now undergoing clinical trials on liver clinical outcomes (i.e., progression to cirrhosis and complications).
Semaglutide is a GLP‐1 analogue approved for the treatment of type 2 diabetes and obesity due to its effects in improving glycaemic control and reducing appetite and energy intake. 12 , 13 It is currently being investigated for the treatment of MASH in both people with and without type 2 diabetes. 14 In phase 2 trials, semaglutide and another GLP‐1 analogue, liraglutide, have shown significant improvements in MASH resolution on biopsy compared with placebo. 15 , 16 In phase 1 and 2 clinical trials, semaglutide reduced liver fat in people with MASLD (NCT03357380) 17 and led to histological liver improvements in people with MASH and fibrosis stage [F]1–3 (NCT02970942). 16 In people with MASH and F4 (compensated liver cirrhosis), semaglutide led to improvements in cardiometabolic risk parameters, non‐invasive markers of disease activity and reduction in liver fat (NCT03987451). 18
There appears to be a bidirectional association between MASLD/MASH and type 2 diabetes. 19 People with type 2 diabetes often display increased liver enzymes and accumulation of hepatic fat independently of body mass index (BMI), 4 , 7 with an increased risk of advanced fibrosis. 20 In people without diabetes, MASLD can lead to dyslipidaemia and hepatokine release and is associated specifically with dysfunction of visceral adipose tissue, which altogether increases the risk of developing insulin resistance and type 2 diabetes. 21 The challenges of weight reduction in people with type 2 diabetes are well known and may stem from several reasons, including the use of concomitant medications that promote weight gain, concerns regarding hypoglycaemia leading to non‐adherence with diet and exercise, and altered microbiota and/or genetic predisposition to weight gain. 22 , 23 , 24
Importantly, type 2 diabetes has been suggested to decrease weight‐loss response to GLP‐1 analogues 23 , 24 , 25 , 26 , 27 , 28 and the combined glucose‐dependent insulinotropic polypeptide (GIP) receptor and GLP‐1 receptor agonist tirzepatide. 29 , 30 Given the importance of weight loss for improving outcomes in patients with MASLD/MASH, it is critical to investigate how type 2 diabetes may impact GLP‐1 receptor agonist‐mediated weight loss. However, the weight‐loss effect of these agents has typically been investigated in people with and without type 2 diabetes in separate trials. Therefore, observed differences may be reflective of differences in trial design, highlighting the need for head‐to‐head comparisons of individuals with and without type 2 diabetes from within the same trial. To address this issue, this post hoc analysis of data from three randomised clinical trials in MASLD/MASH investigated whether the presence of type 2 diabetes and/or other cardiometabolic parameters (i.e., glycaemic control and lipid profile) affected weight loss in people with MASLD/MASH treated with semaglutide or placebo.
2. MATERIALS AND METHODS
2.1. Clinical studies
In this post hoc analysis, data were extracted from one phase 1 (NCT03357380) and two phase 2 trials (NCT02970942 and NCT03987451). 16 , 17 , 18 These randomised, placebo‐controlled trials of subcutaneous semaglutide were conducted on people with either MASLD (confirmed by magnetic resonance imaging) or biopsy‐proven MASH for 48–72 weeks. During the trials, people received standard advice on diet and general health but, otherwise, there were no mandated lifestyle interventions. In the phase 1 trial NCT03357380, people with MASLD were randomised to semaglutide 0.4 mg or placebo once daily for 72 weeks. 17 In the phase 2 trial NCT02970942, patients with MASH and F1–3 were randomised to semaglutide 0.1, 0.2 or 0.4 mg or placebo once daily for 72 weeks. 16 In the phase 2 trial NCT03987451, people with MASH and F4 received semaglutide 2.4 mg or placebo once weekly for 48 weeks. 18 The trial designs are summarised in Table S1 and the results have been previously published. 16 , 17 , 18
2.2. Post hoc analyses
Change in body weight was a secondary endpoint in NCT03357380 17 and NCT02970942. 16 Body weight changes were grouped by whether people had type 2 diabetes (diagnosed with type 2 diabetes or glycated haemoglobin [HbA1c] ≥6.5% [≥48 mmol/mol]), pre‐type 2 diabetes (HbA1c ≥5.7 to <6.5% [≥39 to <48 mmol/mol]) or no type 2 diabetes (HbA1c <5.7% [<39 mmol/mol]) at baseline, according to their status in the primary trial. Body weight was measured fasted, with an empty bladder, without shoes and only wearing light‐fitting clothing. The scale had to be calibrated according to the directions for use.
In the current analysis, only data for semaglutide 0.4 mg once daily, semaglutide 2.4 mg once weekly and placebo were included (based on pharmacokinetic modelling, which suggests that semaglutide 0.4 mg once daily has a maximum steady‐state concentration similar to semaglutide 2.4 mg once weekly). 28 The semaglutide 0.4 mg once‐daily arms in NCT03357380 and NCT03987451 were pooled with the semaglutide 2.4 mg once‐weekly arm in NCT02970942, and placebo groups were pooled across all trials. Change in body weight was based on in‐trial data, analysed as the estimated treatment difference (ETD) with 95% confidence intervals (CIs) from baseline. ETDs in body weight were evaluated at 1 year from randomisation to align across trials (week 48 for NCT03357380 and NCT03987451, and week 52 for NCT02970942). This was a complete case analysis and participants with any missing body weight data were excluded.
The treatment effect on body weight in people without type 2 diabetes at baseline compared with those with pre‐ and type 2 diabetes was analysed using analysis of covariance (ANCOVA) with change from baseline in body weight as the dependent variable; age and baseline body weight as covariates; and treatment, sex, age and trial as fixed factors; and included the interaction between treatment and diabetes. Analyses by diabetes group at baseline were performed by including the respective groups as a factor in the interaction with treatment and diabetes.
Additional analyses were conducted at the individual patient level to assess whether body weight change was affected by baseline levels of fasting plasma glucose, HbA1c, C‐peptide (NCT03987451 only), fasting insulin, diabetes duration and insulin resistance, as assessed by the Homeostatic Model Assessment of Insulin Resistance (HOMA‐IR). C‐peptide data were only reported in NCT03987451 and insulin and HOMA‐IR data were only reported in NCT03357380 and NCT02970942. The potential impact of baseline lipid concentrations (triglycerides, low‐density lipoprotein, high‐density lipoprotein) on weight loss was also examined. Lipid ratio data were analysed for the change from baseline to week 48. Correlations between changes in weight loss and different cardiometabolic risk parameters were analysed using Spearman correlations.
3. RESULTS
3.1. Study population and baseline characteristics
The overall study population included 300 participants with either MASLD on imaging (n = 67) or MASH on biopsy (n = 233 [77.7%]). The mean (standard deviation [SD]) age was 56.3 (10.2) years and there was a slight predominance of females (53.3%). The mean (SD) BMI was 35.2 (5.8) kg/m2 and mean (SD) body weight was 99.4 (19.9) kg (Table 1).
TABLE 1.
Baseline characteristics by diabetes status.
| Pooled data | |||||||
|---|---|---|---|---|---|---|---|
| Treatment group | Semaglutide (n = 163) | Placebo (n = 137) | |||||
| Baseline T2D status | Non‐T2D (n = 21) | Pre‐T2D (n = 25) | T2D (n = 117) | Non‐T2D (n = 19) | Pre‐T2D (n = 26) | T2D (n = 92) | Total (n = 300) |
| Age, years | 57.1 (9.7) | 54.4 (9.6) | 57.5 (9.8) | 49.3 (10.2) | 53.8 (11.7) | 57.2 (10.0) | 56.3 (10.2) |
| Female sex | 10 (47.6) | 16 (64.0) | 63 (53.8) | 11 (13.9) | 10 (38.5) | 50 (54.3) | 160 (53.3) |
| Ethnicity | |||||||
| Hispanic or Latino | 2 (9.5) | 8 (32.0) | 15 (12.8) | 2 (10.5) | 2 (7.7) | 9 (9.8) | 38 (12.7) |
| Non‐Hispanic or Latino | 15 (71.4) | 17 (68.0) | 100 (85.5) | 16 (84.2) | 21 (80.8) | 81 (88.0) | 250 (83.3) |
| Body weight, kg | 94.7 (22.4) | 97.0 (20.0) | 98.8 (18.3) | 97.3 (19.6) | 104.1 (21.8) | 101.0 (21.1) | 99.4 (19.9) |
| BMI, kg/m2 | 33.9 (6.7) | 35.5 (6.3) | 35.0 (5.5) | 34.5 (5.9) | 34.4 (5.2) | 36.0 (6.0) | 35.2 (5.8) |
| Waist circumference, cm | 109.5 (14.7) | 112.2 (11.9) | 114.2 (11.9) | 110.0 (11.3) | 115.6 (12.7) | 117.0 (15.0) | 114.4 (13.3) |
| Biopsy‐confirmed MASH a | 19 (90.5) | 23 (92.0) | 87 (74.4) | 15 (78.9) | 18 (69.2) | 71 (77.2) | 233 (77.7) |
| Fibrosis stage a | |||||||
| Not assessed | 2 (9.5) | 2 (8.0) | 30 (25.6) | 4 (21.1) | 8 (30.8) | 21 (22.8) | 67 (22.3) |
| F1 | 4 (19.0) | 6 (24.0) | 16 (13.7) | 5 (26.3) | 5 (19.2) | 12 (13.0) | 48 (16.0) |
| F2 | 3 (14.3) | 4 (16.0) | 7 (6.0) | 2 (10.5) | 5 (19.2) | 15 (16.3) | 36 (12.0) |
| F3 | 7 (33.3) | 6 (24.0) | 29 (24.8) | 5 (26.3) | 6 (23.1) | 25 (26.3) | 78 (26.0) |
| F4 | 5 (23.8) | 7 (28.0) | 35 (30.0) | 3 (15.8) | 2 (7.7) | 19 (20.7) | 71 (23.7) |
| Known diabetes duration, years b | ‐ | ‐ | 8.4 (7.0) | ‐ | ‐ | 9.2 (7.2) | 8.8 (7.1) |
| HbA1c, % | 5.5 (0.2) | 5.9 (0.2) | 7.3 (1.1) | 5.3 (0.3) | 6.0 (0.2) | 7.4 (1.1) | 6.8 (1.2) |
| HbA1c, mmol/mol | 37 | 41 | 56 | 34 | 42 | 57 | 51 |
| HDL cholesterol, mmol/L | 1.1 (0.2) | 1.2 (0.3) | 1.1 (0.3) | 1.4 (0.3) | 1.1 (0.3) | 1.1 (0.3) | 1.14 (0.3) |
| LDL cholesterol, mmol/L | 3.1 (1.1) | 3.4 (1.1) | 2.7 (1.0) | 3.2 (0.8) | 3.1 (1.1) | 2.9 (1.0) | 2.91 (1.0) |
| Triglycerides, mmol/L | 1.7 (0.7) | 1.8 (1.1) | 2.2 (1.6) | 1.6 (0.8) | 2.3 (1.4) | 2.3 (1.5) | 2.12 (1.4) |
| Triglycerides/HDL | 1.6 (0.9) | 1.7 (1.2) | 2.3 (3.0) | 1.3 (0.8) | 2.2 (2.0) | 2.6 (3.1) | 2.2 (2.6) |
| Triglycerides/LDL | 0.6 (0.3) | 0.5 (0.3) | 1.3 (3.7) | 0.5 (0.2) | 1.0 (1.5) | 0.9 (0.5) | 1.0 (2.3) |
| ALT, U/L, Gmean (CV) | 48.0 (55.0) | 57.2 (53.4) | 47.5 (62.5) | 53.3 (61.5) | 48.2 (65.7) | 44.0 (67.5) | 47.6 (63.1) |
| AST, U/L, Gmean (CV) | 39.3 (40.3) | 48.8 (40.0) | 40.7 (53.0) | 40.8 (48.5) | 36.1 (50.5) | 37.7 (54.4) | 39.9 (51.4) |
Note: Data are mean (standard deviation) or n (%) unless otherwise stated.
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; CV, coefficient of variation; Gmean, geometric mean; HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MASH, metabolic dysfunction‐associated steatohepatitis; Non‐T2D, no type 2 diabetes; Pre‐T2D, pre‐type 2 diabetes; T2D, type 2 diabetes.
Data only available for studies NCT02970942 and NCT03987451.
People with diabetes only.
Across the three trials, 209/300 participants (70%) had type 2 diabetes (with a mean [SD] HbA1c of 7.3% [1.1] [56 mmol/mol]), 51/300 (17%) had pre‐type 2 diabetes and 40/300 (13%) had no type 2 diabetes. Compared with people without type 2 diabetes, those with pre‐type 2 diabetes and type 2 diabetes tended to have higher baseline body weight and BMI, but that was not uniformly the case (Table 1). Baseline data from the individual trials are presented in Supplementary Table S2.
3.2. Weight‐loss outcomes by type 2 diabetes status
At 1 year, 9/137 participants treated with semaglutide (6.6%) and 11/163 participants receiving placebo (6.7%) had missing body weight data and were excluded from the analysis.
Overall mean weight change was −11.1 kg (−11.7%) with semaglutide and −0.7 kg (−0.6%) for placebo: ETD –10.4 kg (95% CI –11.9, −8.9) (ETD –11.1% [95% CI –12.6, −9.5]) (Figure 1). Weight change with semaglutide ranged from −9.8 kg (−10.3%) in the pre‐type 2 diabetes group to −12.7 kg (−13.9%) in the no type 2 diabetes group and for placebo the range was 0.0 kg to −1.1 kg (0% to −0.8%). ETDs for semaglutide versus placebo were similar across the three groups. Numerically, people without type 2 diabetes had the greatest ETD (−11.6 kg; −13.1%), compared with those with type 2 diabetes (−10.2 kg; −10.8%) and pre‐type 2 diabetes groups (−9.8 kg; −10.2%), although differences were not statistically significant across groups (p > 0.50 for all, Figure 1).
FIGURE 1.
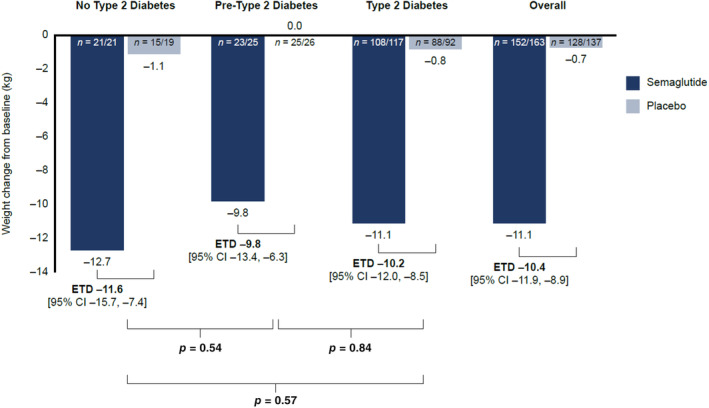
Weight change from baseline at 1 year in people treated with semaglutide or placebo by diabetes status and overall. n = number of participants with body weight data at 1 year included in the analysis/total number of study participants. CI, confidence interval; ETD, estimated treatment difference.
3.3. Weight‐loss outcomes by cardiometabolic risk parameters and known diabetes duration
The distribution of individual weight‐loss data at week 48 across the three studies according to other metabolic parameters is reported in Figures 2 and 3. Baseline fasting plasma glucose, HbA1c, diabetes duration, circulating C‐peptide, fasting insulin levels and insulin resistance (HOMA‐IR) showed no correlation with body weight change, either with semaglutide or placebo (Figures 2, S1, Table 2). Similarly, there was no pattern of weight change with either semaglutide or placebo when examining baseline levels of lipids (triglycerides, low‐density lipoprotein, high‐density lipoprotein) or changes from baseline to week 48 in lipid ratios (triglycerides/high‐density lipoprotein, triglycerides/low‐density lipoprotein) in relation to diabetes status (Figures 3, S2, Table 2).
FIGURE 2.
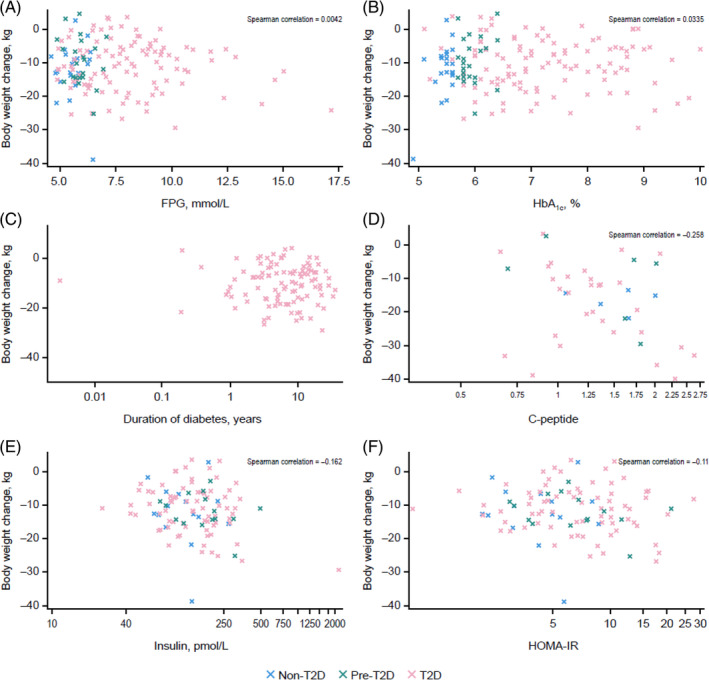
Individual patient weight‐loss data at 1 year by baseline FPG (A), HbA1c (B), diabetes duration (C), C‐peptide (D), insulin (E) and HOMA‐IR (F) with semaglutide. FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HOMA‐IR, Homeostatic Model Assessment of Insulin Resistance.
FIGURE 3.
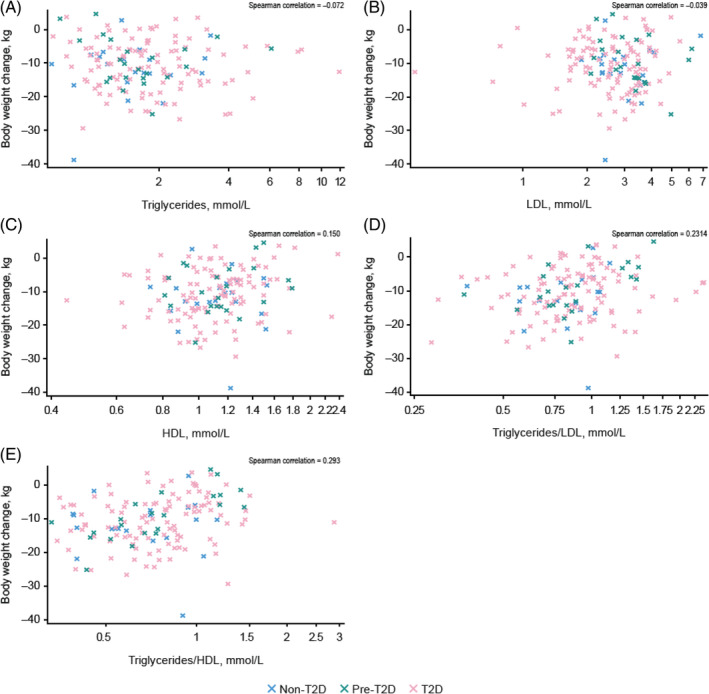
Individual patient weight‐loss data at 1 year by baseline triglycerides (A), LDL (B), HDL (C) and by change from baseline to week 48 in ratios for triglycerides/LDL (D) and triglycerides/HDL (E) with semaglutide. HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
TABLE 2.
Spearman correlation coefficients for individual weight‐loss data by cardiometabolic parameters in baseline at 1 year.
| Semaglutide | Placebo | |
|---|---|---|
| Fasting plasma glucose | 0.004 | −0.253 |
| HbA1c | 0.033 | −0.236 |
| Fasting insulin | −0.162 | 0.061 |
| HOMA‐IR | −0.110 | −0.010 |
| HDL cholesterol | 0.150 | 0.138 |
| LDL cholesterol | −0.039 | 0.132 |
| Triglycerides | −0.072 | 0.050 |
| Triglycerides/HDL | 0.293 | 0.113 |
| Triglycerides/LDL | 0.231 | 0.087 |
| C‐peptide | −0.258 | 0.070 |
Abbreviations: HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; HOMA‐IR, Homeostatic Model Assessment for Insulin Resistance; LDL, low‐density lipoprotein.
4. DISCUSSION
In this post hoc analysis of three placebo‐controlled randomised trials of semaglutide in people with MASLD/MASH that demonstrated improvements in steatosis, 16 , 17 , 18 the magnitude of semaglutide‐mediated weight loss was slightly numerically greater in people without diabetes (approximately 14% reduction) compared with type 2 diabetes. However, ETDs with semaglutide versus placebo were similar for people with type 2 diabetes, with pre‐type 2 diabetes and without type 2 diabetes, and differences between the groups were not statistically significant. Furthermore, changes in body weight were not influenced by baseline glycaemic, insulin resistance or lipid levels.
These pooled results contrast with previous studies in people with overweight/obesity, in which those with diabetes lost considerably less weight than those without diabetes when results were compared across studies. For instance, in phase 3 clinical trials with semaglutide, the magnitude of weight loss achieved at week 68 in people with type 2 diabetes was 9.6% (ETD of −6.2% for semaglutide vs. placebo), compared with 14.9% (ETD of −12.4% for semaglutide vs. placebo) in people without type 2 diabetes in similar settings. 25 , 28 In trials with liraglutide 3.0 mg, weight losses of 6.0% (ETD of −4.0% for liraglutide vs. placebo) and 8.0% (ETD of −5.4% for liraglutide vs. placebo) were seen in people with and without type 2 diabetes, respectively. 26 , 27 In trials with tirzepatide 10 mg, weight losses of 12.8% (ETD of −9.6% for tirzepatide vs. placebo) and 19.5% (ETD of −16.4% for tirzepatide vs. placebo), respectively, were achieved. 29 , 30 However, these prior trials did not include in‐depth liver disease characterisation with histology, and therefore, it is not known whether MASLD was present in the trial populations, meaning comparisons to the current analysis should be made with caution. Moreover, other differences in trial length and sample size are also apparent. 27 , 28 , 29 , 30 While we cannot exclude that the lack of statistically significant weight loss between people with and without type 2 diabetes may be a result of the low sample size in the non‐type 2 diabetes group, it is worth noting that the observed weight loss in people with type 2 diabetes and MASLD/MASH was greater than in previous trials in people with type 2 diabetes and obesity 25 but lower than in those with obesity without type 2 diabetes. 28 It should also be noted that the trials included in this post hoc analysis were not weight‐loss studies, which may also explain why the magnitude of weight loss in the MASLD/MASH populations was lower than in people with obesity without type 2 diabetes in weight‐loss trials. For instance, the baseline body weight and BMI of people in the current analysis was lower than several previous studies that have shown differences in weight loss across those with and without diabetes. 26 , 27 , 28 , 29
Weight loss with semaglutide is mediated by reduced appetite and energy intake, with increased feelings of satiety and fullness and changes in food choice and preference. 31 Semaglutide directly and indirectly activates brain regions in the hypothalamus and hindbrain involved in appetite regulation. 31 , 32 , 33 Investigation of the underlying mechanisms in GLP‐1 receptor analogue‐mediated weight loss and the interaction with type 2 diabetes, MASLD and MASH was beyond the scope of the current analysis and has been further discussed in a recent review. 21 Chronic metabolic diseases, such as type 2 diabetes and MASH, may impair weight loss with GLP‐1 analogues, but the coexisting impact of the two diseases does not appear to be additive. It is likely the presence of MASLD/MASH features, including hepatic steatosis, tissue‐specific differences in insulin resistance (hepatic vs. adipose vs. muscle), as well as degree of lipotoxicity, low‐grade inflammation and/or gut dysbiosis, 34 , 35 , 36 may all play a relevant role in weight loss in people with MASLD/MASH and type 2 diabetes. Interestingly, in a study of 519 people with type 2 diabetes who underwent gastric bypass and concurrent liver biopsy (of whom 407 had histologically confirmed liver steatosis), multivariate analysis indicated an independent association between liver steatosis and postoperative long‐term diabetes remission. However, no such association was seen for other features of MASLD at baseline, including hepatocyte ballooning, fibrosis and lobular inflammation. 37 The authors concluded that distinct variants of type 2 diabetes may exist, with different metabolic responses to, in this case, surgical weight loss, including a subgroup of people with diabetes characterised by hepatic steatosis (presumably associated with insulin resistance). 37 Given that another study identified a severely insulin‐resistant diabetes endotype with higher risk of MASLD, 38 the association between type 2 diabetes subgroups, MASLD and metabolic regulation (and its impact on body weight) warrants further scrutiny.
Diabetes duration is often linked with duration of obesity, which may influence a person's adherence to exercise and lifestyle modifications and, consequently, make achieving and sustaining weight loss more challenging. 22 In the current analysis, weight loss was not affected by diabetes duration, or baseline glycaemic and cardiometabolic parameters such as HbA1c, fasting plasma glucose, HOMA‐IR or low‐density lipoprotein in both semaglutide and placebo groups. No correlations between these parameters and weight loss were found, independently of type 2 diabetes status. These results further support the observations that type 2 diabetes status did not influence efficacious weight loss with semaglutide in people with MASLD or MASH and overweight or obesity, indicating that the use of semaglutide in this population may be beneficial to achieve guideline‐recommended weight loss. 4 , 5 , 7 However, it is important to note that there are likely additional factors that were not assessed in the current analysis, such as genetics that may affect the observed relationships. For example, the PNPLA3‐rs738409 risk genotype may influence the correlation between HOMA‐IR and intrahepatic lipid content. 39 Further, in a recent study of 220 patients receiving semaglutide, PNPLA3 genotype was shown to significantly impact treatment response to semaglutide, with greater increases in alanine transaminase in people carrying at least one PNPLA3 risk allele. 40 Further studies are required to assess how these genetic factors may influence weight loss in people with MASLD/MASH treated with semaglutide.
The presence of type 2 diabetes in people with MASH predicts advanced fibrosis. 41 In a study of 1562 individuals aged 36–64 years with MASLD and Fibrosis‐4 index (FIB‐4) score of <1.30, 186 progressed to advanced fibrosis (FIB‐4 score of >2.67) over a median follow‐up of 7.5 years. 42 The presence of type 2 diabetes was independently associated with progression to advanced fibrosis (hazard ratio, 1.879; 95% CI, 1.401, 2.520; p < 0.001). The mechanism behind this remains unclear and the current post hoc analysis was not designed to investigate this association. However, the present study highlights that the degree of weight loss (i.e., >10%) required to halt progression and potentially reverse fibrosis 8 is possible with semaglutide irrespective of the presence or absence of type 2 diabetes.
The main strength of this post hoc analysis was the inclusion of data from clinical trials in MASLD/MASH that included people with and without diabetes in the same study population, thereby avoiding potential confounding due to differences in trial design. This is in contrast to trials in overweight/obesity, such as those in the STEP programme, which investigated the effect of semaglutide in people with and without diabetes in separate trials. We also report weight loss both in kilograms and percent reduction and observed equivalent results across these outcomes, which adds to the robustness of the findings. This analysis also had certain limitations. The primary semaglutide trials were not designed to investigate weight loss nor to specifically investigate the effect of treatment on weight loss in diabetes and other subgroups, as has been done here post hoc. 16 , 17 , 18 Therefore, people were not mandated to receive strict lifestyle and dietary interventions that are standard of care in weight‐loss studies. Furthermore, these were phase 1 and 2 studies recruiting a relatively low number of people, and number of participants were not balanced across groups. Consequently, results should be generalised with caution and further studies are needed to support the current findings. Additionally, a large, randomised phase 3 trial of semaglutide for treatment of MASH (ESSENCE; NCT04822181) is ongoing and will provide further information on semaglutide‐related body weight change in individuals with MASH and type 2 diabetes and/or obesity.
In conclusion, in this post hoc analysis, people with MASLD or MASH treated with semaglutide had similar weight loss regardless of type 2 diabetes status, suggesting that the previously observed attenuation of semaglutide‐induced weight loss in individuals with overweight/obesity and type 2 diabetes is not pronounced in the context of MASLD/MASH. Cardiometabolic factors, including glucose parameters, insulin resistance and lipid levels at baseline, did not affect the magnitude of body weight loss with semaglutide. These results contrast with previous studies in people with overweight/obesity without diagnosed MASLD/MASH, in which the presence of type 2 diabetes affected weight loss with semaglutide. Detailed further studies are needed to examine whether presence or severity of MASLD/MASH could impair weight loss effects in type 2 diabetes, and, if so, which are the underlying metabolic and genetic factors that may mediate this effect.
AUTHOR CONTRIBUTIONS
All authors were involved in the data curation, investigation, validation, visualisation, writing, review and editing. MJA and A‐SS were involved in the conceptualisation and methodology. MSP performed the formal analysis and software writing. All authors approved the final version of the manuscript. MJA is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
FUNDING INFORMATION
The trials were sponsored by Novo Nordisk A/S and are registered with ClinicalTrials.gov (NCT03357380, NCT02970942, NCT03987451).
CONFLICT OF INTEREST STATEMENT
MJA: consultancy and sponsored lectures for Norgine and Novo Nordisk. TO: no conflicts of interest to declare. MSP: employee and shareholder of Novo Nordisk. A‐SS: employee and shareholder of Novo Nordisk. MT: employee and shareholder of Novo Nordisk. MR: research grants from Boehringer Ingelheim and Novo Nordisk; consulting or advisory boards for AstraZeneca, Boehringer Ingelheim, Echosens, Eli Lilly, MSD and Novo Nordisk; speaker fees from AstraZeneca, Madrigal, MSD and Novo Nordisk.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1111/dom.16065.
ETHICS STATEMENT
This study was a post hoc analysis of data from three clinical trials (NCT03357380, NCT02970942 and NCT03987451). The trials were conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice guidelines. All trial participants provided written informed consent before data collection.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGEMENTS
The authors thank the trial participants, investigators and trial site staff who conducted the trial. Medical writing support was provided by Diana Marouco, PhD, and Liam Gillies, PhD, of Apollo, OPEN Health Communications, and funded by Novo Nordisk A/S, in accordance with Good Publication Practice (GPP) guidelines (www.ismpp.org/gpp-2022).
Armstrong MJ, Okanoue T, Sundby Palle M, Sejling A‐S, Tawfik M, Roden M. Similar weight loss with semaglutide regardless of diabetes and cardiometabolic risk parameters in individuals with metabolic dysfunction‐associated steatotic liver disease: Post hoc analysis of three randomised controlled trials. Diabetes Obes Metab. 2025;27(2):710‐718. doi: 10.1111/dom.16065
DATA AVAILABILITY STATEMENT
Datasets will be shared with bona fide researchers who submit a research proposal approved by an independent review board after research completion and approval of the product and product use in the EU and the USA. Information about data access request proposals can be found at novonordisk‐trials.com.
REFERENCES
- 1. Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542‐1556. [DOI] [PubMed] [Google Scholar]
- 2. Chan WK, Chuah KH, Rajaram RB, Lim LL, Ratnasingam J, Vethakkan SR. Metabolic dysfunction‐associated steatotic liver disease (MASLD): a state‐of‐the‐art review. J Obes Metab Syndr. 2023;32(3):197‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong VW, Ekstedt M, Wong GL, Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023;79(3):842‐852. [DOI] [PubMed] [Google Scholar]
- 4. Rinella ME, Neuschwander‐Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77(5):1797‐1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cusi K, Isaacs S, Barb D, et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co‐sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28(5):528‐562. [DOI] [PubMed] [Google Scholar]
- 6. Tacke F, Horn P, Wai‐Sun Wong V, et al. EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction‐associated steatotic liver disease (MASLD). J Hepatol. 2024;81(3):492‐542. [DOI] [PubMed] [Google Scholar]
- 7. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388‐1402. [DOI] [PubMed] [Google Scholar]
- 8. Vilar‐Gomez E, Martinez‐Perez Y, Calzadilla‐Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367‐378.e5. quiz e14‐15. [DOI] [PubMed] [Google Scholar]
- 9. FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease [press release]. 2024.
- 10. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925‐1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. ElSayed NA, Aleppo G, Aroda VR, et al. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of care in diabetes‐2023. Diabetes Care. 2023;46(Suppl 1):S49‐S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FDA . OZEMPIC prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf. Accessed 22 January 2024
- 13. FDA . WEGOVY prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215256s000lbl.pdf. Accessed 22 January 2024
- 14. ClinicalTrials.gov . Research study on whether semaglutide works in people with non‐alcoholic steatohepatitis (NASH) (ESSENCE). 2024. https://classic.clinicaltrials.gov/ct2/show/NCT04822181. Accessed 27 March 2024
- 15. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet. 2016;387(10019):679‐690. [DOI] [PubMed] [Google Scholar]
- 16. Newsome PN, Buchholtz K, Cusi K, et al. A placebo‐controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113‐1124. [DOI] [PubMed] [Google Scholar]
- 17. Flint A, Andersen G, Hockings P, et al. Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non‐alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2021;54(9):1150‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loomba R, Abdelmalek MF, Armstrong MJ, et al. Semaglutide 2·4 mg once weekly in patients with non‐alcoholic steatohepatitis‐related cirrhosis: a randomised, placebo‐controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8(6):511‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep. 2019;1(4):312‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care. 2021;44(2):399‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gancheva S, Roden M, Castera L. Diabetes as a risk factor for MASH progression. Diabetes Res Clin Pract. 2024;217:111846. [DOI] [PubMed] [Google Scholar]
- 22. Jensterle M, Rizzo M, Haluzík M, Janež A. Efficacy of GLP‐1 RA approved for weight management in patients with or without diabetes: a narrative review. Adv Ther. 2022;39(6):2452‐2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bergmann NC, Davies MJ, Lingvay I, Knop FK. Semaglutide for the treatment of overweight and obesity: a review. Diabetes Obes Metab. 2023;25(1):18‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pi‐Sunyer FX. Weight loss in type 2 diabetic patients. Diabetes Care. 2005;28(6):1526‐1527. [DOI] [PubMed] [Google Scholar]
- 25. Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double‐blind, double‐dummy, placebo‐controlled, phase 3 trial. Lancet. 2021;397(10278):971‐984. [DOI] [PubMed] [Google Scholar]
- 26. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687‐699. [DOI] [PubMed] [Google Scholar]
- 27. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11‐22. [DOI] [PubMed] [Google Scholar]
- 28. Wilding JPH, Batterham RL, Calanna S, et al. Once‐weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989‐1002. [DOI] [PubMed] [Google Scholar]
- 29. Garvey WT, Frias JP, Jastreboff AM, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT‐2): a double‐blind, randomised, multicentre, placebo‐controlled, phase 3 trial. Lancet. 2023;402(10402):613‐626. [DOI] [PubMed] [Google Scholar]
- 30. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205‐216. [DOI] [PubMed] [Google Scholar]
- 31. Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blundell J, Finlayson G, Axelsen M, et al. Effects of once‐weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23(3):754‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bril F, Sanyal A, Cusi K. Metabolic syndrome and its association with nonalcoholic steatohepatitis. Clin Liver Dis. 2023;27(2):187‐210. [DOI] [PubMed] [Google Scholar]
- 35. Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537‐2564. [DOI] [PubMed] [Google Scholar]
- 36. Pafili K, Roden M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol Metab. 2021;50:101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vangoitsenhoven R, Wilson RL, Cherla DV, et al. Presence of liver steatosis is associated with greater diabetes remission after gastric bypass surgery. Diabetes Care. 2021;44(2):321‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zaharia OP, Strassburger K, Strom A, et al. Risk of diabetes‐associated diseases in subgroups of patients with recent‐onset diabetes: a 5‐year follow‐up study. Lancet Diabetes Endocrinol. 2019;7(9):684‐694. [DOI] [PubMed] [Google Scholar]
- 39. Nádasdi Á, Gál V, Masszi T, Somogyi A, Firneisz G. PNPLA3 rs738409 risk genotype decouples TyG index from HOMA2‐IR and intrahepatic lipid content. Cardiovasc Diabetol. 2023;22(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Urias E, Tedesco NR, Oliveri A, Raut C, Speliotes EK, Chen VL. PNPLA3 risk allele association with ALT response to semaglutide treatment. Gastroenterology. 2024;166(3):515‐517.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ekstedt M, Nasr P, Kechagias S. Natural history of NAFLD/NASH. Curr Hepatol Rep. 2017;16(4):391‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tada T, Toyoda H, Sone Y, et al. Type 2 diabetes mellitus: a risk factor for progression of liver fibrosis in middle‐aged patients with non‐alcoholic fatty liver disease. J Gastroenterol Hepatol. 2019;34(11):2011‐2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
Datasets will be shared with bona fide researchers who submit a research proposal approved by an independent review board after research completion and approval of the product and product use in the EU and the USA. Information about data access request proposals can be found at novonordisk‐trials.com.


