Abstract
Purpose
Protective ileostomy and colostomy are performed in patients undergoing low anterior resection with a high leakage risk. We aimed to compare surgical, medical, and daily care complications between these 2 ostomies in order to make individual choice.
Methods
Patients who underwent low anterior resection for rectal tumors with protective stomas between January 2011 and September 2018 were enrolled. Stoma-related complications were prospectively recorded by wound, ostomy, and continence nurses. The cancer stage and treatment data were obtained from the Taiwan Cancer Database of our Big Data Center. Other demographic data were collected retrospectively from medical notes. The complications after stoma creation and after the stoma reversal were compared.
Results
There were 176 patients with protective colostomy and 234 with protective ileostomy. Protective ileostomy had higher proportions of high output from the stoma for 2 consecutive days than protective colostomy (11.1% vs. 0%, P<0.001). Protective colostomy resulted in more stoma retraction than protective ileostomy (21.6% vs. 9.4%, P=0.001). Female, open operation, ileostomy, and carrying stoma more than 4 months were also significantly associated with a higher risk of stoma-related complications during diversion. For stoma retraction, the multivariate analysis revealed that female (odds ratio [OR], 4.00; 95% confidence interval [CI], 2.13–7.69; P<0.001) and long diversion duration (≥4 months; OR, 2.33; 95% CI, 1.22–4.43; P=0.010) were independent risk factors, but ileostomy was an independent favorable factor (OR, 0.40; 95% CI, 0.22–0.72; P=0.003). The incidence of complication after stoma reversal did not differ between colostomy group and ileostomy group (24.3% vs. 20.9%, P=0.542).
Conclusion
We suggest avoiding colostomy in patients who are female and potential prolonged diversion when stoma retraction is a concern. Otherwise, ileostomy should be avoided for patients with impaired renal function. Wise selection and flexibility are more important than using one type of stoma routinely.
Keywords: Colostomy, Ileostomy, Postoperative complications, Proctectomy
INTRODUCTION
A temporary diversion stoma is helpful to protect high risk anastomosis following sphincter-preserving low anterior resection with total mesorectal excision, especially for those who received neoadjuvant radiotherapy [1–4]. Although whether the diversion can reduce the risk of anastomosis leakage is controversial, the chance of emergency surgery following leakage is reduced [5–7]. Most patients refuse a stoma even temporarily because of its inconvenience in terms of sexual life, interpersonal relationships, and mental health [8, 9]. Creating a stoma with minimal complications is essential. Thus, every stoma-related complication should be taken seriously even in cases of peristomal dermatitis. The 2 common options for protective diversion stoma are loop colostomy and loop ileostomy. Some meta-analyses have compared the 2 stomas for different complications. However, the data lacked a comparison concerning about individual patient’s characteristics [10–12]. Until now, there has been no consensus or practice guideline for the selection of either colostomy or ileostomy as a protective stoma. Previous studies were limited by a small sample size less than 100 in both arms [13–16]. The only study that included more than 636 protective stomas was not originally designed to compare protective loop colostomy and loop ileostomy [5]. These studies were conducted before the laparoscopic era; they seldom mentioned the confounding factors of body mass index (BMI), concurrent chemoradiotherapy (CCRT), and neoadjuvant target therapy. In addition, stoma-related major complications which might need surgical intervention are easily identified and recorded, such as parastomal stomal hernia, intestinal obstruction, or stoma necrosis. But stoma-related mild complications such as stoma retraction, dermatitis, and stoma dehiscence are seldom mentioned. These stoma-related minor complications could lead to poor appliance of stoma bag and hence have impacts on patient’s quality of life. In our hospital, a consulting system provided by wound, ostomy, and continence nurses (WOCNs) has been established since 2010. The WOCNs have prospectively recorded every detailed stoma-related complication from the time of stoma creation to the time of stoma reversal for each patient who had problems about stoma care or was bothered by stoma-related complications. We retrospectively reviewed our records of protective stoma after rectal surgery and tried to analyze the risk factors of stoma-related complications. We aimed to compare these complications in order to provide useful information for personalized stoma creation by patients’ characteristics (such as sex, BMI) and their treatment (such as laparoscopic surgery or neoadjuvant CCRT).
METHODS
Ethics statement
The study design was approved by Institutional Review Board of Taipei Veterans General Hospital (No. 2021-02-008AC). The requirement for informed consent was waived.
Study design and patients
Between January 2011 and September 2018, 484 consecutive patients underwent low anterior resection with protective diversion for rectal lesions at Taipei Veteran General Hospital (Taipei, Taiwan). The decision for a protective stoma was based on the surgeon’s discretion during the operation. The common indications for a protective stoma are neoadjuvant CCRT, worrisome perfusion on anastomosis, complex underlying disease, tumor obstruction, unpleasant anastomotic process, coloanal anastomosis, and a positive air-leak test during operation. The choice of loop transverse colostomy and loop ileostomy was made at the surgeon’s discretion. Sixty patients were excluded for the reasons shown in Fig. 1. Fourteen patients without stoma closure within 4 months after stoma creation were excluded. A total of 410 patients were enrolled for analysis with 176 patients receiving protective colostomy and 234 patients receiving protective ileostomy. Seventy patients were excluded from the analysis of complications following stoma closure because their follow-up days after closure were less than 6 months. The analysis for complications after stoma closure were performed in 340 patients with 144 patients in colostomy group and 196 patients in ileostomy group. The median follow-up time from stoma creation to the last hospital visit was 59.3 months in the colostomy group and 44.1 months in the ileostomy group.
Fig. 1.
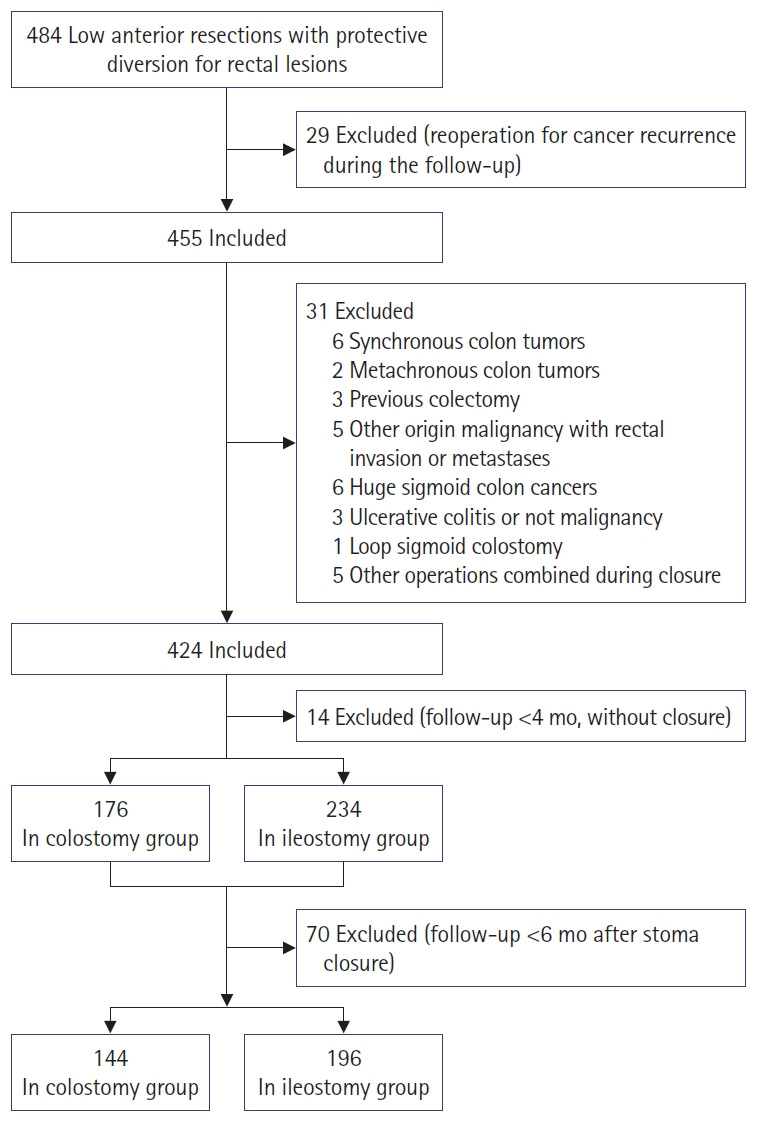
Flowchart of the study participants.
Stoma creation
Polyethylene glycol was used for bowel preparation prior to the primary tumor operation. Intravenous prophylactic antibiotics were administered before the incision was made. The operation was performed by open or laparoscopic-assisted technique, based on the surgeon’s discretion. The application of laparoscopic surgery for colorectal cancer has increased steadily in our hospital from 15% in 2007 to 80% in 2019. The stoma site could be selected by the WOCN preoperatively if possible. For patients receiving laparoscopic surgery, the 12-mm trocar wound in the right lower quadrant was the preferred site for ileostomy. Because the specimen was sometimes retrieved through the same wound, the fascia and skin of the wound were sutured to fit the size of the stoma if necessary. The stoma was created with mucocutaneous suture using 3-0 chromic catgut. An attempt was made to elevate the efferent limbs 2 to 4 cm for ileostomy and 1 to 2 cm for colostomy. Except the location and the height of stomas, there was no difference in surgical methods between ileostomy and colostomy. A plastic rod was placed under the stoma for 7 days to prevent early retraction of the stoma.
Stoma closure
Routine bowel preparation was performed for the colostomy closure and occasional bowel preparation for the ileostomy closure. The ileostomy was closed with or without segmental resection, depending on the quality of the bowel wall following dissection. The colostomy was mostly closed without segmental resection. Sometimes, segmental resection was performed at the surgeon’s discretion. Some of the stomas were closed using the gastrointestinal anastomosis stapler with side-to-side anastomosis, but majority were hand-sewn end-to-end anastomoses or hand-sewn simple closures. Most wounds were approximated using 1 stitch of 3-0 nylon and a wet dressing. Sometimes, drainage with a Jackson-Pratt or Penrose drain was used at the surgeon’s discretion.
Primary endpoint and assessment
The primary endpoint was to compare the overall complications between protective loop ileostomy and loop colostomy. Stoma complications were recorded and assessed prospectively by 3 WOCNs. Assessment was performed during admission and at the outpatient department follow-up. Complications after stoma closure such as ileus, reoperation for obstruction, anastomotic leakage, management of leakage, surgical site infection, anastomotic stricture, incisional hernia, and hospital stay were retrospectively collected by reviewing operative and discharge notes. Acute kidney injury and high output of stoma were collected by reviewing admission records. A stoma output of more than 1,500 mL for 2 consecutive days was defined as high output. Perioperative mortality was recorded within 60 days postoperatively. A history of CCRT, chemotherapy, target therapy, and other demographics were based on data from the Taiwan Cancer Database of our Big Data Center at Taipei Veterans General Hospital. The tumor stage was defined as pathological stage based on 7th edition of the American Joint Committee on Cancer (AJCC) staging system [17]. Pathological complete response was defined as stage 0.
Statistical analysis
The means and the frequencies of the 2 groups were assessed using the Student t-test and the chi-square test, respectively. Risk factors for stoma-related complications were analyzed using univariate analysis, and factors with P-value less than 0.2 were included for multivariate logistic regression or linear regression with backward elimination method. Statistical significance was set at P<0.05. The analyses were performed using R ver. 3.2.2 (R Foundation for Statistical Computing).
RESULTS
Among 410 patients receiving protective stoma after rectal surgery, 176 patients had loop transverse colostomies and 234 patients had loop ileostomies. The demographic data between 2 groups showed no difference except a significant higher proportion of patients with diabetes mellitus and receiving open surgery in the colostomy group (Table 1). Four patients died following primary surgery due to leakage (1 ileostomy and 1 colostomy), pneumonia (1 ileostomy), and small bowel obstruction with sepsis (1 ileostomy) which was not related to stoma creation. Two mortalities occurred following closure due to acute myocardial infarction (1 ileostomy and 1 colostomy). These mortalities were not directly stoma creation or closure related.
Table 1.
Demographics of patients during diversion (n=410)
| Demographics | Colostomy group (n=176) | Ileostomy group (n=234) | P-value |
|---|---|---|---|
| Age (yr) | 63.0 (54.0–74.2) | 64.0 (54.0–74.0) | 0.737 |
| Sex | 0.996 | ||
| Male | 116 (65.9) | 153 (65.4) | |
| Female | 60 (34.1) | 81 (34.6) | |
| Body mass index (kg/m2) | 22.4 (20.2–24.6) | 23.1 (20.8–25.6) | 0.075 |
| Diabetes mellitus | 49 (27.8) | 43 (18.4) | 0.031 |
| Smoker | 28 (15.9) | 45 (19.2) | 0.459 |
| Alcohol drinker | 17 (9.7) | 30 (12.8) | 0.402 |
| Pathologic stage | 0.234 | ||
| 0 | 16 (9.1) | 32 (13.7) | |
| I | 54 (30.7) | 65 (27.8) | |
| II | 49 (27.8) | 63 (26.9) | |
| III | 41 (23.3) | 41 (17.5) | |
| IV | 16 (9.1) | 33 (14.1) | |
| Neoadjuvant CCRT | 92 (52.3) | 126 (53.8) | 0.829 |
| Target agent | 19 (10.8) | 39 (16.7) | 0.122 |
| Operative method | <0.001 | ||
| Open | 80 (45.5) | 55 (23.5) | |
| Laparoscopic | 96 (54.5) | 179 (76.5) | |
| Diversion duration (day) | 98.0 (79.0–134.2) | 98.0 (88.2–118.2) | 0.687 |
Values are presented as median (interquartile range) or number (%).
CCRT, concurrent chemoradiotherapy.
Complications and risk factors during diversion
The median diversion duration from stoma creation to stoma closure was 98 days in both groups (Table 1). The occurrence of stoma-related complications after stoma creation are shown in Table 2. The most common stoma-related complication was dermatitis (75 out of 410 patients, 18.3%). There was significantly more stoma retraction in the colostomy group than in the ileostomy group (21.6% vs. 9.4%, P=0.001), and there were higher proportions of high output in the ileostomy group than in the colostomy group (11.1% vs. 0%, P<0.001). Five patients (2.1%) had acute kidney injury due to high output of ileostomy. A low incidence of stoma prolapses and parastomal hernia was found in both groups. Only 4 patients (2.3%) in colostomy group and 1 patient (0.4%) in ileostomy group had stoma prolapse. All patients with prolapse were male. The open operation had higher risk of prolapse without significantly different (open, 3%; laparoscopy, 0.4%; P=0.059). No parastomal hernia occurred in patients with colostomy but occurred in 3 patients (1.3%) with ileostomy. Univariate analysis showed that female sex and diversion duration more than 4 months had significant higher risk to have stoma-related complications during diversion (Table 3). Multivariate logistic regression showed female, open operation, and the diversion duration more than 4 months were significantly associated with a higher risk of stoma-related complications during diversion. Because stoma retraction was the most common stoma-related complication in colostomy group, we further analyzed the risk factors for stoma retraction (Table 4). Female, colostomy, and diversion duration more than 4 months were independent risk factor for stoma retraction. In other words, loop ileostomy provided a protective effect to prevent stoma retraction (odds ratio [OR], 0.40; P=0.003).
Table 2.
Complications during diversion and outcomes
| Variable | No. of patients (%) | P-value | |
|---|---|---|---|
| Colostomy group (n = 176) | Ileostomy group (n = 234) | ||
| Stoma-related complication | |||
| Stoma retraction | 38 (21.6) | 22 (9.4) | 0.001 |
| Dermatitis | 26 (14.8) | 49 (20.9) | 0.142 |
| Stoma dehiscence | 29 (16.5) | 34 (14.5) | 0.687 |
| High output | 0 (0) | 26 (11.1) | <0.001 |
| Acute kidney injury | 0 (0) | 5/26 (19.2) | 0.134 |
| Mucosa hypertrophy | 6 (3.4) | 3 (1.3) | 0.265 |
| Parastomal infection | 4 (2.3) | 3 (1.3) | 0.703 |
| Readmission for parastomal cellulitis | 0 (0) | 1/3 (33.3) | >0.999 |
| Prolapse | 4 (2.3) | 1 (0.4) | 0.218 |
| Stoma bleeding | 1 (0.6) | 4 (1.7) | 0.557 |
| Parastomal hernia | 0 (0) | 3 (1.3) | 0.356 |
| Overall complication | 64 (36.4) | 102 (43.6) | 0.170 |
| Outcome (successful closure of stoma) | 160 (90.9) | 221 (94.4) | 0.235 |
Table 3.
Univariate and multivariate analysis for risk factors of overall complications during diversion
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age (≥70 yr) | 0.73 (0.48–1.10) | 0.136 | 0.69 (0.44–1.06) | 0.093 |
| Female sex | 2.13 (1.41–3.23) | <0.001 | 2.50 (1.61–3.85) | <0.001 |
| Body mass index (>27 kg/m2) | 1.63 (0.91–2.92) | 0.099 | 1.43 (0.78–2.63) | 0.243 |
| Diabetes mellitus | 0.93 (0.58–1.49) | 0.763 | - | - |
| Smoker | 0.90 (0.53–1.50) | 0.682 | - | - |
| Alcohol drinker | 1.21 (0.65–2.24) | 0.534 | - | - |
| Neoadjuvant chemotherapy (including CCRT) | 1.11 (0.74–1.67) | 0.627 | - | - |
| Target agent | 1.45 (0.83–2.53) | 0.194 | 1.20 (0.66–2.16) | 0.545 |
| Open surgery | 1.40 (0.92–2.12) | 0.117 | 1.60 (1.02–2.52) | 0.041 |
| Diversion duration (≥4 mo) | 1.64 (1.04–2.57) | 0.032 | 1.95 (1.20–3.16) | 0.007 |
| pT4 | 0.68 (0.27–1.56) | 0.375 | - | - |
| Stage IV disease | 1.35 (0.74–2.46) | 0.328 | - | - |
| Ileostomy | 1.35 (0.91–2.02) | 0.141 | 1.55 (1.01–2.42) | 0.047 |
OR, odds ratio; CI, confidence interval; CCRT, concurrent chemoradiotherapy.
Table 4.
Risk factors of stoma retraction by univariate and multivariate analysis
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age (≥70 yr) | 0.89 (0.49–1.57) | 0.690 | - | - |
| Female sex | 3.23 (1.85–5.56) | <0.001 | 4.00 (2.13–7.69) | <0.001 |
| Body mass index (>27 kg/m2) | 1.43 (0.64–2.93) | 0.352 | - | - |
| Diabetes mellitus | 0.95 (0.47–1.80) | 0.877 | - | - |
| Smoker | 0.47 (0.17–1.06) | 0.094 | 0.90 (0.31–2.22) | 0.821 |
| Alcohol drinker | 0.51 (0.15–1.32) | 0.214 | - | - |
| Neoadjuvant chemotherapy (including CCRT) | 1.40 (0.78–2.58) | 0.271 | - | - |
| Target agent | 1.08 (0.47–2.25) | 0.837 | - | - |
| Open surgery | 1.69 (0.96–2.95) | 0.065 | 1.48 (0.80–2.71) | 0.202 |
| Diversion duration (≥4 mo) | 1.76 (0.97–3.13) | 0.058 | 2.33 (1.22–4.43) | 0.010 |
| pT4 | 0.78 (0.18–2.36) | 0.701 | - | - |
| Stage IV disease | 0.97 (0.38–2.15) | 0.941 | - | - |
| Ileostomy | 0.38 (0.21–0.66) | 0.001 | 0.40 (0.22–0.72) | 0.003 |
OR, odds ratio; CI, confidence interval; CCRT, concurrent chemoradiotherapy.
In univariate analysis, we found female (OR, 3.05; P=0.044) and longer diversion duration (OR, 2.33; P=0.042) were also risk factors of high output. However, these 2 risks were not significant in multivariate analysis. Besides, old age (OR, 0.95; P=0.913) was not a related issue of high output. The other complications during diversion had no correlation with the type of stoma. Concerning about stoma site infection, we found that a neoadjuvant target therapy had a significant risk for stoma site infection in logistic regression (OR, 8.62; P=0.006).
Stoma closure
Successful reversal of stoma was performed in 160 patients (90.9%) carrying colostomies, and in 221 patients (94.4%) carrying ileostomies (P=0.235). The reasons for failure of stoma reversal were poor anastomotic healing, disease progression, or poor sphincter function. Operation time for stoma closure was significant longer for reversal of ileostomy than that for reversal of colostomy (102.5±40.2 minutes vs. 91.5±38.0 minutes, P=0.023). The analysis for complications after stoma closure were performed in 144 patients with colostomy and 196 patients with ileostomy after excluding patients with follow-up less than 6 months after stoma closure. Complications that occurred after stoma reversal included wound infection, incisional hernia, obstruction or ileus, and the occurrence of these complications had no correlation with the type of stoma (Table 5). The incidence of stoma-related complication after reversal of stoma did not differ between colostomy group and ileostomy group (24.3% vs. 20.9%, P=0.542) (Table 5). Reoperations were needed for 12 patients in colostomy group and 17 patients in ileostomy group (8.3% vs. 8.7%, P>0.999) either due to incisional hernia or obstruction. The total complication rates during stoma creation were not different between colostomy group and ileostomy group (51.4% vs. 55.6%, P=0.508). We found that obese patients with BMI of >27 kg/m2 had a significant risk of wound infection after stoma closure in our univariate analysis (OR, 4.76; P=0.006). Age of ≥60 years (OR, 5.99; P=0.025) and BMI of >27 kg/m2 (OR, 12.05; P<0.001) were 2 risk factors for incisional hernia.
Table 5.
Complications and outcomes after closure of stoma (n=340)
| Variable | No. of patients (%) | P-value | |
|---|---|---|---|
| Colostomy group (n=144) | Ileostomy group (n=196) | ||
| Stoma-related complication | |||
| Stoma wound infection | 17 (11.8) | 14 (7.1) | 0.199 |
| Incisional hernia of stoma site | 8 (5.6) | 7 (3.6) | 0.540 |
| Obstruction or ileus | 14 (9.7) | 22 (11.2) | 0.790 |
| Operation for obstruction | 4 (2.8) | 10 (5.1) | 0.430 |
| Ileus (conservative treatment) | 10 (6.9) | 12 (6.1) | 0.935 |
| Outcome | |||
| Reoperation after stoma closure | 12 (8.3) | 17 (8.7) | >0.999 |
| Complication after stoma closure | 35 (24.3) | 41 (20.9) | 0.542 |
DISCUSSION
Rectal cancer accounts for approximately 37.8% of all colorectal cancers in Taiwan [18]. Protective stoma is sometimes necessary for high-risk anastomosis to reduce the risk of severe sepsis and emergent exploratory laparotomy [5]. However, unwilling to receive a stoma surgery may result in delayed treatments since patients have a poor understanding of the stoma. The present study collected a consecutive number of patients to assess the details of stoma-related complications before and following closure between colostomy and ileostomy. Overall total complication rate during stoma creation to stoma reversal were not different between colostomy group and ileostomy group. However, unique complications were found in either group, such as stoma retraction in colostomy group and high output in ileostomy group. Proper choices between protective colostomy or ileostomy for patients receiving rectal surgery could further reduced the occurrence of stoma-related complications.
Our result showed that after multivariate logistic regression, ileostomy was significantly associated with a higher risk of stoma-related complication during diversion. Two systematic reviews comparing the morbidity of loop ileostomy and loop colostomy creation after rectal surgery also showed no difference in overall morbidity after stoma creation and closure [10, 11]. One systematic review reported significantly lower morbidity in the ileostomy group (18.2% vs. 30.6%, P=0.001) [10], while another found no significant difference (23% vs. 24%, P=0.440) [11]. Our series had a higher proportion of high output and ileus without operation in the ileostomy group, and at the same time with fewer proportion of stoma prolapses, stoma site infections, and parastomal hernias in colostomy group, leading to a result favoring colostomy during diversion.
High output was seldom observed in loop colostomy in previous studies. Systematic reviews observed 0% incidence of high output or dehydration in colostomy group, which is similar to ours. The incidence of high output or dehydration in ileostomy group was 3.1% and 4%, respectively, which is lower than those of our series (11.1%) [10, 11]. These studies lacked the definition of dehydration or high output. On the other hand, we used a restrictive definition of high output with a stoma output of more than 1,500 mL for 2 consecutive days. In another study, Klink et al. [19] showed a significant higher rate of renal insufficiency in the ileostomy group than in the colostomy group (10% vs. 1%, P=0.005). However, the authors recommended loop ileostomy as protective stoma since wound infection rate was lower and hospital stay was shorter during stoma reversal. In our study, 5 patients (2.1%) had acute kidney injury and 1 patient was admitted for dehydration in the ileostomy group. In univariate analysis, we found female (OR, 3.05; P =0.044) and longer diversion duration (OR, 2.33; P=0.042) were also risk factors of high output.
Stoma retraction occurred more frequently in colostomy group (21.6%) than in ileostomy group (9.4%) in our study. The incidence was much higher than other studies with stoma retraction rates of 0.37% in ileostomy and 2.69% in colostomy [13–15, 19]. The high incidence might be explained by the discrepant definition of stoma retraction. We retrieved our data via WOCN care system. Any skin dimpling or inadequate protruding of stoma resulting in poor appliance of stoma bag would be recorded as stoma retraction. Clinically, the occurrence of stoma retraction was frequently seen in female patients with a protective colostomy because the appearance of a skin fold at waist over upper abdomen due to a loose and floppy abdomen. We found that colostomy, female sex, and longer follow-up were risk factors to have stoma retraction. Based on our result, protective ileostomy might be a better choice for these patients in order to prevent the occurrence of stoma retraction if a protective stoma is necessary.
The stoma prolapse rate in the colostomy varies from 8.2% to 42.1% in previous studies, and the incidence of parastomal hernia was not higher in colostomy group in 2 randomized studies [13, 20] and 1 case-matched study [14], but a contradictory result in 1 systematic review [10]. We know that the occurrence of stoma prolapse and parastomal hernia increased as the duration of carrying a stoma increased. Since most of our patients received stoma reversal operation about 3 months after stoma creation, the incidence of stoma prolapses and parastomal hernia were predictably low with such a short duration of carrying a stoma in our series. Due to the low incidence, we could not find risk factors for its occurrence.
Concerning about stoma site infection, we found that a neoadjuvant therapy or neoadjuvant chemotherapy had a significant risk for stoma site infection in logistic regression. Only 7 patients had parastomal infection in our study and every patient with infection received neoadjuvant chemotherapy and none of them had diabetes mellitus. Whether neoadjuvant chemotherapy had impact on the occurrence of stomal infection needs further observation in a larger series.
For the complications after stoma closure, the occurrence of ileus, surgical site infection, incisional hernia and reoperation did not differ between colostomy group and ileostomy group in our study. A meta-analysis showed more incidence of ileus after ileostomy closure (5.2% vs. 1.7%, P=0.020) [10], but no difference in the incidence was found in another 2 studies [13, 16] and 1 metaanalysis [11]. Significantly more surgical site infections following colostomy closure was mentioned in 1 meta-analysis [12], but not in our series. We found that obese patients with BMI of >27 kg/m2 had a significant risk of wound infection after stoma closure in our analysis. Rullier et al. [15] observed a higher incidence of incisional hernias after colostomy closure (16.0% vs. 4.2%), but Mala and Nesbakken [21] found no difference, which is similar to ours. In our study, age of ≥60 years and BMI of >27 kg/m2 were 2 risk factors for incisional hernia. There may be some correlation between wound infection, wound dehiscence, and incisional hernia. More powerful evidence is necessary to elucidate this issue.
The conclusion is limited due to single center retrospective design. The quality of life and severity of complication were not available in our data. We did not include the duration of complications to avoid overwhelming information. The choice of stoma type was not randomized so it may have bias. However, the complications record was prospectively collected by WOCNs with their own data table which making this data valuable.
This is the first study with detailed records of complications analyzed with confounding factors such as BMI, laparoscopic surgery, and target therapy in ample case numbers. Although the overall complication rate did not differ between protective colostomy and ileostomy, unique complication in each type of stoma was found. We suggest avoiding colostomy in patients who are female and potential prolong diversion when stoma retraction is a concern. Otherwise, ileostomy should be avoided for patients with impaired renal function. Wise selection and flexibility are more important than using single type of stoma routinely.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Acknowledgments
The authors thank the wound, ostomy, and continence nurses (WOCNs) of Taipei Veterans General Hospital (Taipei, Taiwan) for their contributions to the study.
Author contributions
Conceptualization: YWY, SCH; Investigation: HHC; Methodology: SCC; Project administration: YTL, JKJ; Resources: JKJ, SCC, YTL, HSW, CCL, HHL; Software: YWY; Supervision: YTL; Validation: YTL, HSW; Visualization: YTL, HHL; Writing–original draft: YWY, SCH, HHC; Writing–review & editing: YTL, SCC, JKJ, HSW, CCL, HHL. All authors read and approved the final manuscript.
REFERENCES
- 1.Bax TW, McNevin MS. The value of diverting loop ileostomy on the high-risk colon and rectal anastomosis. Am J Surg. 2007;193:585–8. doi: 10.1016/j.amjsurg.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Carlsen E, Schlichting E, Guldvog I, Johnson E, Heald RJ. Effect of the introduction of total mesorectal excision for the treatment of rectal cancer. Br J Surg. 1998;85:526–9. doi: 10.1046/j.1365-2168.1998.00601.x. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Ramírez SE, Uribe A, Ruiz-García EB, Labastida S, Luna-Pérez P. Risk factors for anastomotic leakage after preoperative chemoradiation therapy and low anterior resection with total mesorectal excision for locally advanced rectal cancer. Rev Invest Clin. 2006;58:204–10. [PubMed] [Google Scholar]
- 4.Zhou S, Zhou H, Zheng Z, Liang J, Zhou Z, Wang X. Predictive risk factors for anastomotic leakage after anterior resection of rectal cancer in elderly patients over 80 years old: an analysis of 288 consecutive patients. World J Surg Oncol. 2019;17:112. doi: 10.1186/s12957-019-1655-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gastinger I, Marusch F, Steinert R, Wolff S, Koeckerling F, Lippert H, et al. Protective defunctioning stoma in low anterior resection for rectal carcinoma. Br J Surg. 2005;92:1137–42. doi: 10.1002/bjs.5045. [DOI] [PubMed] [Google Scholar]
- 6.Marusch F, Koch A, Schmidt U, Geibetaler S, Dralle H, Saeger HD, et al. Value of a protective stoma in low anterior resections for rectal cancer. Dis Colon Rectum. 2002;45:1164–71. doi: 10.1007/s10350-004-6384-9. [DOI] [PubMed] [Google Scholar]
- 7.Peeters KC, Tollenaar RA, Marijnen CA, Klein Kranenbarg E, Steup WH, Wiggers T, et al. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg. 2005;92:211–6. doi: 10.1002/bjs.4806. [DOI] [PubMed] [Google Scholar]
- 8.Ayaz-Alkaya S. Overview of psychosocial problems in individuals with stoma: a review of literature. Int Wound J. 2019;16:243–9. doi: 10.1111/iwj.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vonk-Klaassen SM, de Vocht HM, den Ouden ME, Eddes EH, Schuurmans MJ. Ostomy-related problems and their impact on quality of life of colorectal cancer ostomates: a systematic review. Qual Life Res. 2016;25:125–33. doi: 10.1007/s11136-015-1050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chudner A, Gachabayov M, Dyatlov A, Lee H, Essani R, Bergamaschi R. The influence of diverting loop ileostomy vs. colostomy on postoperative morbidity in restorative anterior resection for rectal cancer: a systematic review and meta-analysis. Langenbecks Arch Surg. 2019;404:129–39. doi: 10.1007/s00423-019-01758-1. [DOI] [PubMed] [Google Scholar]
- 11.Gavriilidis P, Azoulay D, Taflampas P. Loop transverse colostomy versus loop ileostomy for defunctioning of colorectal anastomosis: a systematic review, updated conventional meta-analysis, and cumulative meta-analysis. Surg Today. 2019;49:108–17. doi: 10.1007/s00595-018-1708-x. [DOI] [PubMed] [Google Scholar]
- 12.Tilney HS, Sains PS, Lovegrove RE, Reese GE, Heriot AG, Tekkis PP. Comparison of outcomes following ileostomy versus colostomy for defunctioning colorectal anastomoses. World J Surg. 2007;31:1142–51. doi: 10.1007/s00268-006-0218-y. [DOI] [PubMed] [Google Scholar]
- 13.Gooszen AW, Geelkerken RH, Hermans J, Lagaay MB, Gooszen HG. Temporary decompression after colorectal surgery: randomized comparison of loop ileostomy and loop colostomy. Br J Surg. 1998;85:76–9. doi: 10.1046/j.1365-2168.1998.00526.x. [DOI] [PubMed] [Google Scholar]
- 14.Sakai Y, Nelson H, Larson D, Maidl L, Young-Fadok T, Ilstrup D. Temporary transverse colostomy vs loop ileostomy in diversion: a case-matched study. Arch Surg. 2001;136:338–42. doi: 10.1001/archsurg.136.3.338. [DOI] [PubMed] [Google Scholar]
- 15.Rullier E, Le Toux N, Laurent C, Garrelon JL, Parneix M, Saric J. Loop ileostomy versus loop colostomy for defunctioning low anastomoses during rectal cancer surgery. World J Surg. 2001;25:274–8. doi: 10.1007/s002680020091. [DOI] [PubMed] [Google Scholar]
- 16.Edwards DP, Leppington-Clarke A, Sexton R, Heald RJ, Moran BJ. Stoma-related complications are more frequent after transverse colostomy than loop ileostomy: a prospective randomized clinical trial. Br J Surg. 2001;88:360–3. doi: 10.1046/j.1365-2168.2001.01727.x. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 18.Taiwan Health Promotion Administration (HPA) Ministry of Health and Welfare . HPA; 2020. [106 Cancer registry report: cancer registry annual report, 2017] Taiwanese. [Google Scholar]
- 19.Klink CD, Lioupis K, Binnebösel M, Kaemmer D, Kozubek I, Grommes J, et al. Diversion stoma after colorectal surgery: loop colostomy or ileostomy? Int J Colorectal Dis. 2011;26:431–6. doi: 10.1007/s00384-010-1123-2. [DOI] [PubMed] [Google Scholar]
- 20.Law WL, Chu KW, Choi HK. Randomized clinical trial comparing loop ileostomy and loop transverse colostomy for faecal diversion following total mesorectal excision. Br J Surg. 2002;89:704–8. doi: 10.1046/j.1365-2168.2002.02082.x. [DOI] [PubMed] [Google Scholar]
- 21.Mala T, Nesbakken A. Morbidity related to the use of a protective stoma in anterior resection for rectal cancer. Colorectal Dis. 2008;10:785–8. doi: 10.1111/j.1463-1318.2007.01456.x. [DOI] [PubMed] [Google Scholar]


