Abstract
Cardiac myxomas, the most common primary cardiac tumors, are believed to originate from multipotent mesenchymal cells. Approximately 75% of myxomas occur within the left atrium, increasing the risk of systemic thromboembolic events. While typically benign, atrial myxomas can rarely metastasize to the brain, with fewer than 60 cases reported. We present a case of a 56-year-old woman with a history of left atrial myxoma who developed headaches, right arm weakness, and blurry vision three months post-cardiac surgery. Imaging showed multiple hemorrhagic brain lesions, and she was treated with whole brain radiotherapy (20 Gy/5 fractions). Four years later, she remains stable with no new lesions and has fully regained function. Currently, there is no standard management for cardiac myxoma metastases. This case highlights the potential role of radiotherapy in managing brain metastases from left atrial myxomas, suggesting a possible treatment strategy based on this case and a review of the literature.
Keywords: Cardiac myxomas, Atrial myxomas, Brain metastasis, Radiotherapy
Introduction
Myxomas are predominantly benign growths, thought to originate from endocardial multipotent mesenchymal cells. These tumors most commonly manifest in patients around the age of 50 and exhibit a higher incidence in females (2:1). The risk of recurrence is increased in cases of incomplete surgical resection, multiple foci, and tumor embolism [1]. Approximately 75% of all cardiac myxomas develop in the left atrium, increasing the risk of systemic thromboembolic events associated with cerebral symptoms [2]. More than 90% of atrial myxomas are sporadic, while a minority are associated with the hereditary Carney complex, which is inherited in an autosomal dominant pattern and linked to PRKAR1A gene mutations. This complex presents with pigmented skin lesions, myxomas (cardiac and cutaneous), and multiple endocrine tumors [3].
Advancements in medical imaging have improved the detection rates of cardiac tumors. Cardiac myxomas, which are primary cardiac tumors, have been categorized into two main morphological subtypes: solid and villous types. The villous myxomas are characterized by their uneven, friable, and papillary surface. Immunohistochemical studies have shown that calretinin expression is uniformly observed in nearly all cardiac myxoma cases [4]. Cardiac complications mainly involve mitral valve obstruction by the tumor, leading to dyspnea, palpitations, and heart failure. Systemic symptoms such as fatigue, fever, weight loss, and muscle weakness may also occur [5]. Early diagnosis and complete surgical removal of the cardiac tumor are associated with a low mortality rate [6].
Neurologic and systemic embolization of atrial myxomas is a recognized complication, potentially affecting 30%–50% of patients [5]. The neurological complications stemming from embolization of left atrial myxomas are broad. Neurologic imaging studies have demonstrated that ischemic infarction events are the most frequent neurologic findings, occurring in 76.0%–88.8% of these patients [5]. Additionally, myxomatous aneurysms and hemorrhagic lesions have been described [7]. In very rare cases, metastatic spread with direct invasion into the central nervous system (CNS) parenchyma is observed [3]. To date, less than 60 cases of cardiac myxoma metastases to the brain have been reported, including the present case.
Case Report
A 56-year-old woman with a history of progressive headaches over 6 months, right arm weakness, and blurry vision presented to the emergency room. She had undergone surgery for a left atrial myxoma 3 months earlier (Fig. 1). Her past medical history included depression, osteoarthritis, and gastroesophageal reflux disease.
Fig. 1.
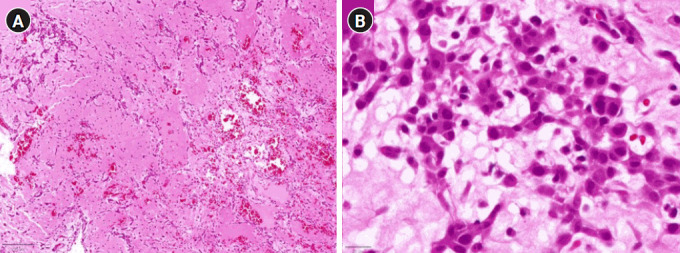
Histopathological analysis of cardiac myxoma tissue. (A) Low-power (5× objective) photomicrograph displaying stellate and polygonal tumor cells within a myxoid stroma, characteristic of a cardiac myxoma. (B) High-power (40× objective) photomicrograph depicting tumor cells with prominent eosinophilic cytoplasm, blurred cellular borders, and ovoid nuclei featuring open chromatin and subtle nucleoli, indicative of lesional cells within a cardiac myxoma.
During her evaluation, multiple enhancing and hemorrhagic lesions within the brain parenchyma with surrounding vasogenic edema were identified, consistent with acute hemorrhagic metastasis. The largest lesion measured 17 mm. Other than mildly blurry bilateral vision, there was no specific neurological compromise; the clinical examination revealed no visual limitation, including normal visual fields. She exhibited full motor function bilaterally with normal reflexes.
A multidisciplinary review by neurosurgery, neurology, medical oncology, and radiation oncology teams was undertaken to assess the necessity of a biopsy, provide a prognosis, and consider therapeutic options. A review of the literature led the medical team to opt for palliative whole brain radiotherapy (WBRT) without surgical intervention. The patient was started on dexamethasone therapy at 2 mg twice daily and maintained a Glasgow Coma Scale score of 15.
A magnetic resonance imaging (MRI) prior to radiotherapy revealed multiple popcorn-like lesions with variable T2 signal intensity and high intrinsic T1 signal intensity, consistent with hemorrhage (Fig. 2A–2D). Some of the lesions demonstrated mild enhancement. The extent of surrounding edema and mass effect varied, with larger lesions causing more significant swelling and displacement of adjacent structures. Concerns for underlying aneurysms and subarachnoid hemorrhage were excluded. Radiotherapy began within 5 days following the consultation, with a tapering course of steroids prescribed after completion of a 20 Gy/5 fraction regimen. The patient underwent three-dimensional treatment using lateral opposing fields, with the planning target volume (PTV) defined as the whole brain parenchyma. Neither hippocampal sparing nor intensity-modulated radiotherapy was performed. A basic field-in-field technique was used to reduce hotspots to less than 110% to ensure 95% of the PTV received 100% of the dose.
Fig. 2.
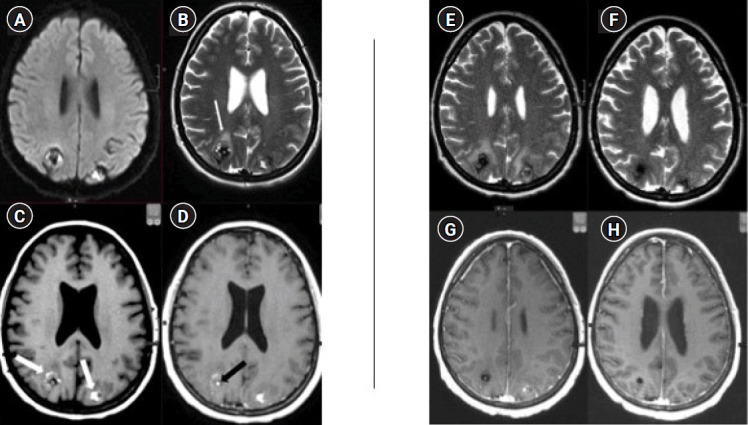
Serial magnetic resonance imaging (MRI) evaluation of cerebral metastases from cardiac myxoma. Pre-radiotherapy imaging: axial MRI scans including (A) diffusion-weighted imaging (DWI), (B) T2-weighted, and (C) pre- and (D) post-contrast T1-weighted sequences, performed prior to radiotherapy. These images display varied signal intensities on DWI and T2 sequences, with hyperintensities on T1 pre-contrast (indicated by white arrows in C), characteristic of subacute hemorrhage. Mild cerebral edema and mass effect are evident (white arrow in B), with localized contrast enhancement (black arrow in D), underscoring the active nature of the lesions. Post-treatment follow-up imaging: axial MRI scans taken 2 months post-treatment with (E) T2-weighted and (G) T1-weighted post-contrast sequences showing increased edema around both parietal lobe lesions, indicative of therapeutic effects rather than tumor progression. At a 2-year and 3-month follow-up, (F) and (H) demonstrate resolved edema and reduced signal intensity in the lesions, consistent with hemosiderin deposition and/or calcification.
The acute side effects of radiotherapy, including the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0 Grade II asthenia, anorexia, headache, nausea, and oral thrush, were more pronounced than typically seen in WBRT patients. Steroids were reintroduced after a follow-up MRI indicated a potential increase in vasogenic edema. Two months after treatment, the patient was weaned off steroids with the resolution of all symptoms except for persistent blurry vision and generalized fatigue. Palliative care commenced in response to the high progression rates reported in the literature. The patient experienced recurrent nausea, generalized weakness, and loss of appetite, which were managed with steroids, haloperidol, H2 antagonists, and ondansetron. These symptoms were unusual compared to other patients undergoing WBRT, but no specific etiology, such as Carney complex, was identified.
Serial imaging showed an initial increase in edema around the lesions 1 month after treatment, which then stabilized by the second month (Fig. 2E, 2G). This was felt to be radiotherapy effect. The size of the lesions improved by 50% by the fourth month. At the 1-year follow-up, the lesions were small and had no associated edema or mass effect. They had low signal intensity on T1 and T2 weighted images in keeping with hemosiderin deposition and/or calcification. These findings remained unchanged for 3 years with no new lesions emerging (Fig. 2F, 2H).
Long-term follow-up showed the patient’s return to full functionality and she was discharged by palliative care. She continued to experience dry eyes, photophobia, and blurry vision. At 4 years post-treatment, no significant clinical or imaging changes were noted.
Discussion
Atrial myxomas are rare, and their metastasis to the brain is even more uncommon, creating a clinical challenge due to the scarcity of cases and the resultant conflicting information regarding prognosis and treatment efficacy. We conducted a comprehensive search of the literature on cerebral myxomatous metastases published from 1973 to 2023, sourcing information from databases such as PubMed, Embase, and the Cochrane Library. Detailed information from these articles is organized in Supplementary Tables S1–S2. To date, less than 60 cases of cardiac myxomas metastasizing to the brain have been documented, including the present case. These metastatic brain lesions may present anywhere from 2 months to 8 years post-resection of the cardiac myxoma with most reports indicating recurrence within 2 years [8,9]. Embolic events, typically caused by tumor fragments or complete detachment of the tumor, represent a hazardous manifestation of the disease [10].
The mechanism of brain parenchymal seeding after complete local resection of cardiac tumors with benign histopathology remains unclear [3]. It is thought that embolic tumor cells may remain active and invade the wall of distant vessels [11-13]. Interleukin 6 (IL-6) expression produced by cardiac myxomas may contribute to the adhesiveness of myxoma cells through induction of intercellular adhesion molecule-1 during intracerebral embolization. Aguilar et al. [10] demonstrated that there is IL-6 expression in embolic tumor cells of a left atrial myxoma within brain parenchyma.
Given the potential for myxomatous metastases and aneurysm formation, timely detection and management are crucial. Although the development of intracerebral embolization associated with parenchymal seeding of myxoma emboli is an extremely rare complication, it can manifest in various neurological symptoms [14]. Seizures, headache, and hemiparesis are common clinical presentations, but unusual symptoms such as gait disorders and visual disturbances have also been reported [10].
Treatment protocols for cerebral metastases of cardiac myxomas lack standardization and have historically varied, encompassing surgical resection, chemotherapy, and radiotherapy [14-17]. A review of the literature suggests that surgical removal of metastases is common and generally associated with positive outcomes [1,8,18,19]. However, based on a comprehensive review and the multiplicity of lesions in our case, along with literature indicating a high risk of regional CNS recurrence after surgery, our multidisciplinary team decided against surgical intervention.
Radiotherapy has been administered to patients with numerous or progressive brain lesions, with promising results [3,17,20]. While our use of WBRT with a 20 Gy/5 fraction regimen was exploratory, its potential benefits for treating multiple cerebral metastases are evident in the effective disease control, as demonstrated by progressive imaging and sustained clinical stability of our patient. At the time of the consultation, WBRT was the standard of care for patients with widespread disease (>4 lesions) and poor survival prospects. However, updated literature regarding atrial myxomas now suggests longer survival than previous publications; this may suggest that these patients should be eligible for newer techniques to minimize neurocognitive toxicity, escalate dose and reduce recurrence. Today, stereotactic radiosurgery (SRS) would be considered "conditional" for this patient at our center. Extrapolating from other disease sites, SRS may better preserve cognitive function and improve patient-reported symptoms for atrial myxomas with CNS metastases. Additionally, this approach offers the opportunity to investigate dose escalation for this rare condition. Alternatively, WBRT with hippocampal avoidance could be offered. With advancements in radiotherapy technologies, future treatment options could include hippocampal avoidance and escalated SRS dosing for localized disease, with or without WBRT.
Atrial myxomas are associated with CNS metastases, typically diagnosed within the first 2 years after the initial cardiac disease presentation. Therefore, active screening protocols may be justified, although a standardized management approach is yet to be established. The potential of adjuvant chemotherapy to reduce the likelihood of extracardiac spread or recurrence has been used, but further research is necessary to determine its effectiveness. Definitive intervention is recommended, with surgery considered for minimal localized disease, often resulting in favorable outcomes. This case report supports the use of radiotherapy to provide long-term control of the disease.
Footnotes
Statement of Ethics
Ethics approval was not needed for this case study. Informed consent from the patient was obtained.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
We would like to thank the patient for her consent and contribution to this study. Her participation has been essential to our study.
Funding
None.
Author Contributions
Conceptualization, AB, ML, MC; Investigation and methodology, NG, RD,AL; Project administration, AB, ML, MC; Writing of the original draft, AB, ML, MC; Writing of the review and editing, AB, ML, MC, NG, RD,AL; Data curation, AB, ML, MC.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.3857/roj.2024.00395.
Cardiac myxomas metastasizing to the brain reported in literature
List of included studies in Table S1
References
- 1.Maas JA, Menes M, Siomin V. Cardiac myxoma with cerebral metastases and chronic lymphocytic leukemia/small lymphocytic lymphoma: a case report and review. J Neurol Surg Rep. 2020;81:e1–6. doi: 10.1055/s-0039-3399570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdow Iii VP, Breton JM, Nayar VV. Multiple craniotomies for the resection of symptomatic multifocal intracranial metastatic cardiac myxoma: a case report. Surg Neurol Int. 2023;14:322. doi: 10.25259/sni_593_2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roque A, Kimbrough T, Traner C, et al. Somatic PRKAR1A mutation in sporadic atrial myxoma with cerebral parenchymal metastases: a case report. J Med Case Rep. 2019;13:389. doi: 10.1186/s13256-019-2317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terracciano LM, Mhawech P, Suess K, et al. Calretinin as a marker for cardiac myxoma: diagnostic and histogenetic considerations. Am J Clin Pathol. 2000;114:754–9. doi: 10.1309/nr6g-t872-f090-lbrw. [DOI] [PubMed] [Google Scholar]
- 5.Stefanou MI, Rath D, Stadler V, et al. Cardiac myxoma and cerebrovascular events: a retrospective cohort study. Front Neurol. 2018;9:823. doi: 10.3389/fneur.2018.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amemiya K, Yonemoto Y, Ishibashi-Ueda H, et al. Morphological characteristics of cardiac myxoma causing embolism: a series of 40 years of experience at a single institute. Virchows Arch. 2023;482:377–84. doi: 10.1007/s00428-022-03461-x. [DOI] [PubMed] [Google Scholar]
- 7.Kierdaszuk B, Gogol P, Kolasa A, et al. Multiple metastatic intracranial lesions associated with left atrial myxoma. Pol J Radiol. 2014;79:262–7. doi: 10.12659/pjr.890332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desousa AL, Muller J, Campbell R, Batnitzky S, Rankin L. Atrial myxoma: a review of the neurological complications, metastases, and recurrences. J Neurol Neurosurg Psychiatry. 1978;41:1119–24. doi: 10.1136/jnnp.41.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernet F, Stulz PM, Carrel TP. Long-term remission after resection, chemotherapy, and irradiation of a metastatic myxoma. Ann Thorac Surg. 1998;66:1791–2. doi: 10.1016/s0003-4975(98)00917-5. [DOI] [PubMed] [Google Scholar]
- 10.Aguilar C, Carbajal T, Beltran BE, Segura P, Muhammad S, Choque-Velasquez J. Cerebral embolization associated with parenchymal seeding of the left atrial myxoma: potential role of interleukin-6 and matrix metalloproteinases. Neuropathology. 2021;41:49–57. doi: 10.1111/neup.12697. [DOI] [PubMed] [Google Scholar]
- 11.Samaratunga H, Searle J, Cominos D, Le Fevre I. Cerebral metastasis of an atrial myxoma mimicking an epithelioid hemangioendothelioma. Am J Surg Pathol. 1994;18:107–11. doi: 10.1097/00000478-199401000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez FJ, Brown RD, Mohr JP, et al. Embolic atrial myxoma with neoplastic aneurysm formation and haemorrhage: a diagnostic challenge. Neuropathol Appl Neurobiol. 2006;32:213–6. doi: 10.1111/j.1365-2990.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- 13.Castano-Leon AM, Hernandez-Lain A, Maronas L, et al. Pathology-confirmed cerebral arterial invasion and recurrent multiple brain metastasis from cardiac myxoma without evidence of disease after surgery and radiotherapy. Clin Neuropathol. 2016;35:84–8. doi: 10.5414/np300900. [DOI] [PubMed] [Google Scholar]
- 14.Moiyadi AV, Moiyadi AA, Sampath S, et al. Intracranial metastasis from a glandular variant of atrial myxoma. Acta Neurochir (Wien) 2007;149:1157–62. doi: 10.1007/s00701-007-1291-1. [DOI] [PubMed] [Google Scholar]
- 15.Altundag MB, Ertas G, Ucer AR, et al. Brain metastasis of cardiac myxoma: case report and review of the literature. J Neurooncol. 2005;75:181–4. doi: 10.1007/s11060-005-1859-7. [DOI] [PubMed] [Google Scholar]
- 16.Cote I, Sinclair J, Woulfe J, Glikstein R, Veinot J. Cerebral metastasis presenting after complete primary resection of atrial myxoma: case report. Can J Neurol Sci. 2015;42:457–60. doi: 10.1017/cjn.2015.293. [DOI] [PubMed] [Google Scholar]
- 17.Panos LD, Brunel C, Berezowska S, et al. Early and delayed neurological manifestations of cardiac myxomas. Clin Neurol Neurosurg. 2020;190:105673. doi: 10.1016/j.clineuro.2020.105673. [DOI] [PubMed] [Google Scholar]
- 18.Budzilovich G, Aleksic S, Greco A, Fernandez J, Harris J, Finegold M. Malignant cardiac myxoma with cerebral metastases. Surg Neurol. 1979;11:461–9. [PubMed] [Google Scholar]
- 19.Kanda T, Sakamaki T, Murata K. A cardiac myxoma with interleukin-6 production and cerebral metastasis. Int J Cardiol. 1994;45:144–6. doi: 10.1016/0167-5273(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 20.Ma K, Zhao D, Li X, et al. Case report: Multiple brain metastases of atrial myxoma: clinical experience and literature review. Front Neurol. 2023;13:1046441. doi: 10.3389/fneur.2022.1046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cardiac myxomas metastasizing to the brain reported in literature
List of included studies in Table S1


