Summary
Background
Pulmonary embolism causes a substantial burden of morbidity and mortality. Although there are several well-established risk factors for pulmonary embolism, a substantial proportion of cases cannot be attributed to provoked or known risk factors. Accumulating evidence has suggested an association of clonal hematopoiesis of indeterminate potential (CHIP) with the risk of arterial thromboembolism. However, the association between CHIP and the risk of pulmonary embolism remains unknown.
Methods
We performed a community-based cohort study (between 2006 and 2022) including 464,417 individuals with available whole exome sequencing (WES) data in the UK biobank (UKB) to examine the association between CHIP and pulmonary embolism. CHIP was ascertained by analyzing WES data. We used Cox regression models to calculate hazard ratios (HRs) with 95% confidence intervals (CIs) to assess the association between CHIP and pulmonary embolism. In addition, we performed analyses for several types of CHIP mutations, including DNMT3A, TET2, ASXL1, PPM1D, SRSF2, and JAK2.
Findings
The study included 14,418 individuals with CHIP and 449,999 individuals without CHIP. The median age at cohort entry was 58 and 63 years among individuals without and with CHIP, respectively. We observed an increased risk of pulmonary embolism (HR 1.17, 95% CI, 1.05–1.31) among individuals with CHIP. The increased risk was mainly noted for CHIP with TET2 (HR 1.42, 95% CI 1.16–1.74) or JAK2 (HR 4.17, 95% CI 2.09–8.35) mutation, but not for DNMT3A mutation (HR 1.01, 95% CI 0.86–1.19), ASXL1 mutation (HR 1.15, 95% CI 0.83–1.60), PPM1D mutation (HR 1.22, 95% CI 0.66–2.27), or SRSF2 mutation (HR 0.62, 95% CI 0.20–1.93).
Interpretation
Our results highlight the association of pulmonary embolism in individuals with CHIP, especially the TET2-mutant or JAK2-mutant CHIP. If further studies will identify a causal relationship between clonal hematopoiesis and pulmonary embolism, prioritizing early screening for pulmonary embolism in individuals with CHIP could be significantly beneficial.
Funding
Initial Founding for High Level Talented Scholars in Nanfang Hospital, Southern Medical University (No. 2023G001) and the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (Grant No. 2023J009).
Keywords: Clonal hematopoiesis, Pulmonary embolism, Epidemiology, Cohort study
Research in context.
Evidence before this study
We searched PubMed for relevant research up to Jan 10, 2024 using the search terms “clonal hematopoiesis of indeterminate potential” and “clonal hematopoiesis” in combination with “pulmonary embolism”, “embolism”, “thrombosis”, “thromboembolism”, and “thrombotic”. We found two original studies on a potential link between CHIP and pulmonary embolism. One study suggested an association of CHIP with risk of pulmonary embolism based on a small sample and retrospective design, while another study reported no association between CHIP and general venous thromboembolism among patients with cancer.
Added value of this study
We performed a community-based cohort study with a large sample size, providing novel evidence of the association between CHIP and pulmonary embolism. We also revealed that an association between CHIP and pulmonary embolism was predominantly noted for TET2-mutant CHIP and JAK2-mutant CHIP.
Implications of all the available evidence
Further studies are necessary to confirm a causal relationship between clonal hematopoiesis and pulmonary embolism and suggest early screening for pulmonary embolism among individuals with, especially TET2-mutant CHIP and JAK2-mutant CHIP.
Introduction
Pulmonary embolism is the most severe manifestation of venous thromboembolism and ranks among the leading causes of morbidity and mortality worldwide.1, 2, 3 The occurrence of pulmonary embolism involves embolus from venous thrombus traveling to and obstructing the arterial tree of the lungs, constituting a lethal risk particularly when not recognized.4 Although several risk factors (e.g., cancer, major trauma and immobility) have been well-documents for pulmonary embolism, a substantial proportion of cases cannot be attributed to provoked or known risk factors,3 limiting therefore the effectiveness of early prevention and screening in vulnerable populations.
Clonal hematopoiesis of indeterminate potential (CHIP) is an aging-related condition characterized by expansion of hematopoietic cell clones that possess specific somatic mutations, and is recognized as a precursor state of hematological malignancy.5 CHIP has been suggested to be associated with an increased risk of diverse somatic disorders, including cardiovascular disease, stroke, osteoporosis, gout, chronic obstructive pulmonary, chronic liver disease and solid tumors.6, 7, 8, 9, 10, 11, 12 In contrast to the accumulating evidence of a link between CHIP and thromboembolism in artery,6 research on pulmonary embolism, a type of venous thromboembolism, in relation to CHIP is limited. One previous study investigated the association between CHIP and general venous thromboembolism (including pulmonary embolism and deep vein thrombosis) among cancer patients, leaving the general population understudied.13 Another study demonstrated a plausible link between CHIP and pulmonary embolism, but the obvious methodological weaknesses of the study (i.e., retrospective design, very small sample size, and lack adjustment for important confounders) preclude robust conclusions.14 Therefore, we aimed to determine the relationship between CHIP and risk of pulmonary embolism in a cohort study of the UK Biobank (UKB).
Methods
Study cohort
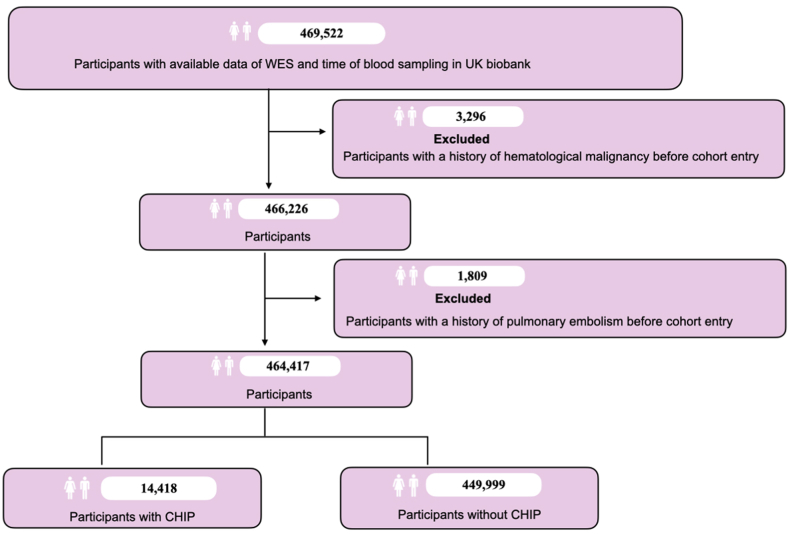
This study leveraged data from the UKB, a community-based cohort, including approximately 500,000 UK residents aged 37–73 years between 2006 and 2010.15 Recruitment of the UKB occurred at 22 centers distributed across the United Kingdom, gathering a comprehensive dataset encompassing a range of variables, including socioeconomic status, lifestyle factors, clinical diagnoses, and genetic sequencing.15 Initially, we included 469,522 participants with existing data on whole exome sequencing (WES) and time of blood sampling. We first excluded participants with pre-existing hematological malignancy (N = 3296) (the tenth revision of the International Classification of Diseases (ICD-10) codes for hematological malignancy were provided in Supplementary eTable S1) before the date of blood collection for WES. Then, we additionally excluded individuals with pulmonary embolism (N = 1809) before the date of blood sampling, resulting in a total of 464,417 individuals for the ultimate analysis (Fig. 1). Participants were followed from blood sampling until diagnosis of pulmonary embolism, death, or December 31, 2022, whichever occurred first.
Fig. 1.
Flowchart of the cohort and design in the study. WES, whole exome sequencing; CHIP, clonal hematopoiesis of indeterminate potential.
Ascertainment of CHIP
The original quality functionally equivalent (OQFE) protocol was used to generate CRAM files and the GRCh38 reference genome were used as the basis for germline variant calling.16 The identification of CHIP was based on 74 driver genes known to be associated with myeloid malignancies.17 Mutect2 was deployed in ‘tumor-only’ mode to detect potential CHIP somatic variants for the exonic regions of 73 out of the 74 genes, with the exception of U2AF1.16,17 For U2AF1, we used Rust-HTSLIB binary (available at https://github.com/weinstockj/pileup_region) to specifically identify variants, which could mitigate potential biases in the detection of genetic variants arising from segmental duplication errors in the GRCh38 reference genome.18 Gene annotation was conducted using ANNOVAR.19 The Genome Aggregation Database (gnomAD)20 was used as a reference to preliminarily distinguish potential germline variants from putative somatic mutations, under the threshold of a minor allele frequency (MAF) < 0.01.21 To filter out recurrent artifacts, we established a panel of normals (PON) through randomly selecting 200 of the youngest individuals (i.e., at the age of 40 or below) in the UKB who were free of hematological malignancy at blood sampling for WES. We filtered variants identified by Mutect2 by FilterMutectCalls for the probabilities generated by LearnReadOrientationModel.16,22 Only putative variants marked as ‘PASS’ by FilterMutectCalls were considered. Furthermore, variants labeled as ‘germline’ or ‘weak_evidence’ were eligible for inclusion under these conditions: they appeared more than five times with a ‘PASS’ flag, and they passed a one-sided exact binomial test with a variant allele frequency (VAF) of 0.5 (P < 0.001). The exclusion criteria were: (1) variants with a total read depth of less than 20; (2) a minimum read depth for the alternate allele less than 5; (3) a VAF below 2%; (4) a lack of support of both forward and reverse sequencing reads. Besides, variants did not associated with age or TERT promoter (rs7705526) were also excluded.17
Ascertainment of pulmonary embolism
Pulmonary embolism was identified through inpatient hospital records, using the ICD-10 codes (Supplementary eTable S1).23 The inpatient hospital records data incorporate admission and diagnosis information obtained from the Hospital Episode Statistics for England, Scottish Morbidity Record data for Scotland, and the Patient Episode Database for Wales. In addition, we included supplementary cases using death register data provided by the National Health Service Digital for England and Wales and the Information Services Division for Scotland, as well as the read coded from primary care data.15
Covariates
Information on sex, birth date, ethnicity, education level, Townsend deprivation index, and body mass index (BMI) determined at the time of recruitment to the UKB were included as covariates. Age was calculated as date of blood sampling minus birth date. Townsend deprivation index incorporates information on employment, car availability, and housing (none and overcrowding) to reflect socioeconomic status. BMI, calculated as weight in kilograms divided by the square of height in meters (kg/m2), was derived from measurements obtained from the baseline assessment at the assessment centers. Pre-existing cardiovascular disease (CVD) was defined as any diagnosis of CVD before cohort entry, identified through inpatient hospital records as well as primary healthcare data using ICD-10 codes (Supplementary eTable S1). Cancer was identified by ICD-10 codes (Supplementary eTable S1) from the Cancer Register, as a time-varying covariate during follow-up. Information of white blood cell levels, hemoglobin concentration, and platelet counts in serum were acquired through biochemical measures in UKB.
Statistics
First, we profiled the demographic and characteristics of individuals with and without CHIP, including sex, age, ethnicity, educational level, Townsend deprivation index, BMI (<18.5, 18.5≤ and <25, 25≤ and <30, or ≥30), smoking and alcohol drinking. Chi-square test was used to test difference in the demographics and characteristics of individuals with or without CHIP. Second, we computed unadjusted incidence rates by dividing the number of pulmonary embolism cases by the accumulated person-years at risk among individuals with and without CHIP. Third, we used Cox regression model to calculate the hazard ratios (HRs) with 95% confidence intervals (CIs) for risk of pulmonary embolism in relation to CHIP. All analyses were conducted using time since follow-up initiation as the underlying time scale. In Model 1, we adjusted for sex, age at blood sampling (as natural cubic spline), ethnicity (white, others or unknown), educational level (college/university degree, other degree, or unknown), and the Townsend deprivation index (Q1, Q2, Q3, or Q4). In Model 2, we further adjusted for lifestyle factors, including BMI (as natural cubic spline), smoking (never, previous, current, or unknown) and alcohol drinking (never, previous, current, or unknown). Fourth, we explored potential effect modifiers of the investigated associations through subgroup analyses by sex, age at blood sampling (<60 or ≥ 60), ethnicity, Townsend deprivation index, educational level, BMI (<18.5, 18.5≤ and <25, 25≤ and <30, or ≥30), smoking, and alcohol drinking. Fifth, we performed additional analyses by type of CHIP mutations, including the most common CHIP mutations as DNMT3A, TET2, ASXL1, PPM1D, and SRSF2.
Supplementary and sensitivity analyses
(1) To reduce potential concerns regarding reverse causality, we performed a sensitivity analysis using different lag time (1-year, 3-year, and 5-year) before initiating follow-up after blood collection. (2) As previous studies have suggested a role of JAK2 in the pathogenesis of embolism and thrombosis,24,25 we further conducted stratified analysis by JAK2 mutation. (3) We also performed an additional analysis by the VAF of CHIP mutation (VAF < 0.1 or VAF ≥ 0.1) to determine whether the size of CHIP clone could influence the investigated association. (4) As some types of CVD have been suggested to be linked with both CHIP and pulmonary embolism,6,26 we conducted a sensitivity analysis stratified by pre-existing CVD. (5) As cancer status was associated with both CHIP and pulmonary embolism,3,11 to explore the effect of cancer on the studied association, we performed an additional analysis stratified by cancer (as time-varying variable). (6) To explore the impact of counts of blood cells on the observed association, we performed additional analyses by further adjusting for blood counts, including count of white blood cell, concentration of hemoglobin, or count of platelet, as natural cubic spline. (7) To investigate the robustness of time since cohort entry as the analytical time scale, we conducted a sensitivity analysis using age as the underlying time scale. (8) To account for the competing risk of death, we performed a sensitivity analysis using Fine–gray model to calculate the sub-distribution hazard ratio (sHR).27
The analyses were executed using SAS software (Version 9.4, SAS institute Inc). Wald test was used to test differences between the HRs of different groups. The significance level was determined using two-sided tests, with results considered statistically significant at a threshold of P < 0.05.
Ethics
All participants in the UKB signed informed consent before information collection and ethical approval has been obtained from the NHS National Research Ethics Service (REC reference: 21/NW/0157). This research has been approved by the Ethical Review Board in Nanfang Hospital, Southern Medical University in China (reference number: NFEC-2023-559).
Role of funding source
This study was partially supported by the Initial Founding for High Level Talented Scholars in Nanfang Hospital, Southern Medical University (No. 2023G001) and the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (Grant No. 2023J009). The funders for the present study had no role in study design, data collection, data analyses, interpretation, or writing of report.
Results
Primary analyses
The study included 464,417 individuals, among whom 14,418 (3.1%) had CHIP. The median age at cohort entry was 58 and 63 years among individuals without and with CHIP, respectively (Table 1). Compared to individuals without CHIP, individuals with CHIP were more likely to be male, of White ethnicity, and overweight, and have a lower educational level but a higher prevalence of previous or current smoking.
Table 1.
Characteristics of the cohort participants.
| Characteristics | Individuals without CHIP; No. (%) | Individuals with CHIP; No. (%) | P value |
|---|---|---|---|
| Number of individuals | 449,999 | 14,418 | |
| Sex | <0.001 | ||
| Female | 244,645 (54.4%) | 7538 (52.3%) | |
| Male | 205,354 (45.6%) | 6880 (47.7%) | |
| Median age at cohort entry (years) | 58 | 63 | |
| Age at cohort entry (years) | <0.001 | ||
| <60 | 249,710 (55.5%) | 4609 (32.0%) | |
| ≥60 | 200,289 (44.5%) | 9809 (68.0%) | |
| Ethnicity | <0.001 | ||
| White | 423,538 (94.1%) | 13,690 (95.0%) | |
| Other | 24,841 (5.5%) | 675 (4.7%) | |
| Unknown | 1620 (0.4%) | 53 (0.4%) | |
| Education level | <0.001 | ||
| College/University degree | 146,205 (32.5%) | 4177 (29.0%) | |
| Other degree | 222,718 (49.5%) | 6755 (46.9%) | |
| Unknown | 81,076 (18.0%) | 3486 (24.2%) | |
| Townsend deprivation index at recruitment | 0.59 | ||
| Q1 | 113,766 (25.3%) | 3601 (25.0%) | |
| Q2 | 112,654 (25.0%) | 3631 (25.2%) | |
| Q3 | 112,487 (25.0%) | 3568 (24.7%) | |
| Q4 | 111,092 (24.7%) | 3618 (25.1%) | |
| BMI | <0.001 | ||
| BMI < 18.5 | 3994 (0.9%) | 143 (1.0%) | |
| 18.5 ≤ BMI < 25 | 146,456 (32.5%) | 4398 (30.5%) | |
| 25 ≤ BMI < 30 | 190,652 (42.4%) | 6303 (43.7%) | |
| BMI ≥ 30 | 108,897 (24.2%) | 3574 (24.8%) | |
| Smoking | <0.001 | ||
| Never | 246,244 (54.7%) | 6907 (47.9%) | |
| Previous | 154,425 (34.3%) | 5672 (39.3%) | |
| Current | 47,076 (10.5%) | 1733 (12.0%) | |
| Unknown | 2254 (0.5%) | 106 (0.7%) | |
| Alcohol drinking | 0.17 | ||
| Never | 19,732 (4.4%) | 634 (4.4%) | |
| Previous | 15,929 (3.5%) | 561 (3.9%) | |
| Current | 413,208 (91.8%) | 13,188 (91.5%) | |
| Unknown | 1130 (0.3%) | 35 (0.2%) |
CHIP, clonal hematopoiesis of indeterminate potential; BMI, body mass index.
Individuals with CHIP were followed for a median duration of 13.6 years, whereas individuals without CHIP had a median follow-up of 13.8 years. Among individuals with CHIP, there were 339 incident cases of pulmonary embolism (incidence rate [IR], 180.0 per 100,000 person-years), while 7544 cases were identified among individuals without CHIP (IR, 124.6 per 100,000 person-years) (Table 2). Compared with individuals without CHIP, individuals with CHIP had an increased risk of pulmonary embolism in both model 1 (HR 1.18, 95% CI, 1.06–1.32) and model 2 (HR 1.17, 95% CI, 1.05–1.31) (Table 2). Given the similar estimates between model 1 and model 2, all results presented below are from model 2. The overall association did not demonstrate substantial variation across demographic, socioeconomic, or lifestyle factors (Table 2).
Table 2.
Risk of pulmonary embolism among individuals with CHIP by demographic factors, socioeconomic status and lifestyle factors, compared to the reference group.
| Characteristics | Individuals without CHIP |
Individuals with CHIP |
Hazard ratio (95% confidence interval) |
|||
|---|---|---|---|---|---|---|
| No. of cases | IR | No. of cases | IR | Model 1a | Model 2b | |
| Overall | 7544 | 124.6 | 339 | 180.0 | 1.18 (1.06–1.32) | 1.17 (1.05–1.31) |
| Sex | ||||||
| Female | 3630 | 109.3 | 171 | 169.9 | 1.25 (1.07–1.46) | 1.26 (1.08–1.47) |
| Male | 3914 | 143.0 | 168 | 191.5 | 1.12 (0.96–1.30) | 1.10 (0.94–1.29) |
| Age at cohort entry (years) | ||||||
| <60 | 2761 | 80.8 | 62 | 99.5 | 1.10 (0.86–1.42) | 1.12 (0.87–1.43) |
| ≥60 | 4783 | 181.3 | 277 | 219.7 | 1.20 (1.06–1.35) | 1.19 (1.05–1.34) |
| Ethnicity | ||||||
| White | 7218 | 126.5 | 326 | 182.3 | 1.18 (1.06–1.32) | 1.17 (1.05–1.31) |
| Other | 301 | 91.4 | 12 | 134.4 | 1.17 (0.66–2.09) | 1.10 (0.60–2.01) |
| Unknown | 25 | 117.7 | <5 | 153.2 | 1.19 (0.16–8.87) | 1.63 (0.21–12.76) |
| Education level | ||||||
| College/University degree | 1706 | 110.3 | 67 | 140.0 | 1.05 (0.82–1.34) | 1.04 (0.82–1.33) |
| Other degree | 1837 | 120.6 | 83 | 173.4 | 1.17 (0.93–1.45) | 1.17 (0.94–1.45) |
| Unknown | 1829 | 120.9 | 84 | 181.4 | 1.23 (0.99–1.54) | 1.21 (0.97–1.51) |
| Townsend deprivation index at recruitment | ||||||
| Q1 | 2172 | 147.3 | 105 | 226.5 | 1.25 (1.03–1.52) | 1.25 (1.02–1.52) |
| Q2 | 1870 | 94.3 | 73 | 131.8 | 1.11 (0.88–1.41) | 1.10 (0.87–1.39) |
| Q3 | 3631 | 120.9 | 147 | 165.8 | 1.13 (0.96–1.33) | 1.11 (0.94–1.32) |
| Q4 | 2043 | 190.9 | 119 | 268.2 | 1.30 (1.08–1.56) | 1.30 (1.08–1.57) |
| BMI | ||||||
| BMI < 18.5 | 74 | 145.6 | <5 | 115.2 | 0.68 (0.17–2.77) | – |
| 18.5 ≤ BMI < 25 | 1548 | 78.1 | 69 | 119.0 | 1.19 (0.93–1.52) | 1.16 (0.91–1.48) |
| 25 ≤ BMI < 30 | 3157 | 122.8 | 145 | 175.4 | 1.18 (1.00–1.39) | 1.16 (0.98–1.37) |
| BMI ≥ 30 | 2765 | 190.2 | 123 | 267.4 | 1.20 (1.00–1.43) | 1.20 (1.00–1.44) |
| Smoking | ||||||
| Never | 3605 | 107.7 | 139 | 150.7 | 1.14 (0.96–1.35) | 1.13 (0.95–1.34) |
| Previous | 2939 | 142.4 | 146 | 199.6 | 1.17 (0.99–1.38) | 1.18 (1.00–1.39) |
| Current | 946 | 153.2 | 52 | 239.8 | 1.27 (0.96–1.68) | 1.29 (0.98–1.71) |
| Unknown | 54 | 184.1 | <5 | 152.6 | 0.73 (0.18–3.04) | 0.74 (0.18–3.06) |
| Alcohol drinking | ||||||
| Never | 379 | 144.0 | 12 | 144.1 | 0.86 (0.49–1.53) | 0.86 (0.49–1.54) |
| Previous | 377 | 181.6 | 20 | 285.2 | 1.36 (0.87–2.14) | 1.39 (0.89–2.19) |
| Current | 6759 | 121.3 | 306 | 177.3 | 1.19 (1.06–1.33) | 1.18 (1.05–1.32) |
| Unknown | 29 | 197.6 | <5 | 229.3 | 1.02 (0.13–7.69) | 1.13 (0.15–8.75) |
CHIP, clonal hematopoiesis of indeterminate potential; BMI, body mass index; IR, incidence rate.
Adjusted for sex (female or male), age at cohort entry (as natural cubic spline), ethnicity (white, other, or unknown), educational level (college or university degree, other degree, or unknown), Townsend deprivation index (Q1, Q2, Q3, or Q4).
Adjusted for sex (female or male), age at cohort entry (as natural cubic spline), ethnicity (white, other, or unknown), educational level (college or university degree, other degree, or unknown), Townsend deprivation index (Q1, Q2, Q3, or Q4), BMI (as natural cubic spline), smoking (never, previous, current, or unknown) and alcohol drinking (never, previous, current, or unknown).
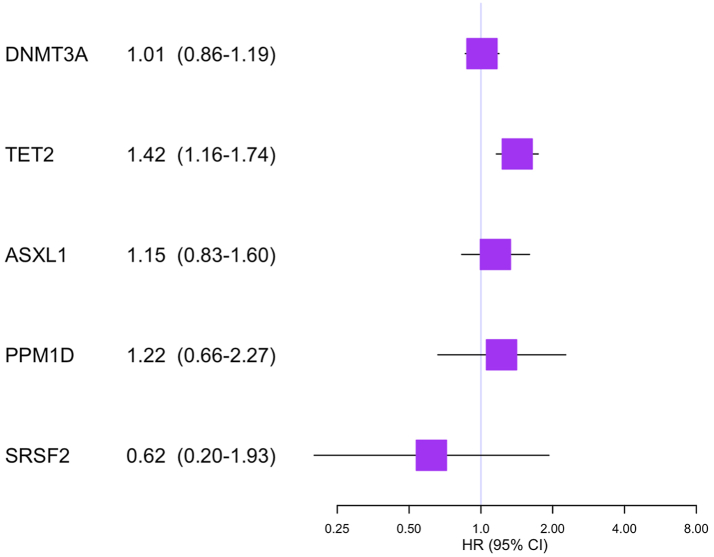
The analyses stratified by mutation type of CHIP showed an increased risk of pulmonary embolism in relation to TET2 mutation (HR 1.42, 95% CI 1.16–1.74), but not to DNMT3A mutation (HR 1.01, 95% CI 0.86–1.19), ASXL1 mutation (HR 1.15, 95% CI 0.83–1.60), PPM1D mutation (HR 1.22, 95% CI 0.66–2.27), or SRSF2 mutation (HR 0.62, 95% CI 0.20–1.93) (Fig. 2).
Fig. 2.
Hazard ratios of pulmonary embolism among individuals of CHIP by different gene mutations. Adjusted for sex (female or male), age at cohort entry (as natural cubic spline), ethnicity (white, other, or unknown), educational level (college or university degree, other degree, or unknown), Townsend deprivation index (Q1, Q2, Q3, or Q4), BMI (as natural cubic spline), smoking (never, previous, current, or unknown) and alcohol drinking (never, previous, current, or unknown). CHIP, clonal hematopoiesis of indeterminate potential; BMI, body mass index; HR, hazard ratio; CI, confidence interval.
Supplementary and sensitivity analysis
(1) Largely comparable results were found when using a 1-year (HR 1.17, 95% CI 1.05–1.31), 3-year (HR 1.19, 95% CI 1.06–1.34), or 5-year (HR 1.16, 95% CI 1.03–1.32) lag time in the analyses. (2) We observed increased risks among individuals with (HR 4.17, 95% CI 2.09–8.35) or without JAK2 mutation (HR 1.15, 95% CI 1.03–1.29), although the risk increase was much stronger for the former. (3) The observed association was more noticeable for individuals with a high level of VAF (≥0.1) of any CHIP mutation (HR 1.24, 95% CI 1.09–1.40), compared with those with VAF <0.1 (HR 1.03, 95% CI 0.83–1.26), though the difference of HRs between the two groups was not statistically significant (P for difference = 0.14) (Supplementary eTable S2). (4) The association did not vary greatly by the history of pre-existing CVD (HR 1.13, 95% CI 0.99–1.30 for presence of pre-existing CVD vs. HR 1.24, 95% CI 1.04–1.48 for absence of pre-existing CVD, P for difference = 0.41). (5) We observed comparable results among individuals with (HR 1.17, 95% CI 1.01–1.35) or without (HR 1.09, 95% CI 0.93–1.28) cancer (P for difference = 0.52) during follow-up. (6) The overall association did not vary after adjusting for count of white blood cell, concentration of hemoglobin, or count of platelet (Supplementary eTable S3). (7) Using age as underlying time-scale yielded very similar results (HR 1.16, 95% CI, 1.04–1.29). (8) Largely similar risk estimates (sHR 1.15, 95% CI, 1.04–1.29) were observed when accounting for the competing risk of death.
Discussion
Leveraging a large community-based cohort study from UKB, we found that individuals with CHIP, especially individuals with TET2 or JAK2 mutation, had an increased risk of pulmonary embolism. The association did not vary greatly across demographic, socioeconomic, or lifestyle factors, pre-existing CVD, or cancer, and was independent of other biomarkers such as count of blood cell, concentration of hemoglobin, or count of platelet.
Consistent with our research, a prior study also observed a positive association between CHIP and pulmonary embolism.14 However, this study was limited by a retrospective design, small sample size (N = 61), and little control for confounding factors.14 In the present study, we applied a prospective design with a large sample size and comprehensive adjustment for potential confounders, therefore providing robust evidence for the studied association. Additionally, we observed an association between CHIP and pulmonary embolism among individuals with cancer, in contrast with a previous study that found no association between CHIP and general venous thromboembolism (including pulmonary embolism and deep vein thrombosis) among cancer patients.13 This discrepancy might be due to different study design and analytical approaches. For instance, we excluded the first year of follow-up in the present study to mitigate concern of reverse causality, whereas the previous study focused solely on outcome events within the first year after accession.
The mechanisms underlying the observed association might be multifactorial. CHIP has been suggested to be associated with risk of cancer, which might further cause pulmonary embolism. However, our findings of comparable results among individuals with or without cancer suggested altered functions of somatic CHIP mutations, beyond oncogenesis, might also contributed. Corroborating with the well-documented mechanisms between JAK2 mutation and thrombosis,24,25 we observed a strong association between JAK2-CHIP and pulmonary embolism. Potential mechanisms for such a link might include biological changes of platelet caused by JAK2 mutation, such as hypersensitivity of thrombopoietin (TPO)/MPL (TPO receptor) pathway, increased megakaryocyte activity, and enhanced platelet functions.25 An alternative explanation for the observed association between JAK2-CHIP and increased risk of pulmonary embolism might be the alterations of endothelial cells.28 In current study, we also noted an increased risk of pulmonary embolism among individuals without JAK2 mutation, suggesting that other mutations beyond JAK2 might also play a role in the development of pulmonary embolism. We observed a pronounced association of TET2 with risk of pulmonary embolism, which might be mechanistically attributed to the upregulation of proinflammatory cytokines, such as interleukin-8 and interleukin-1β,6,29 subsequently contributing to the pathogenesis of pulmonary embolism.30,31
Our study exhibits a number of strengths. One major advantage is the application of prospective design with a large sample size. In addition, independent collection of WES data and diagnosis of pulmonary embolism reduce potential information bias. Moreover, the availability of detailed information on demographic, socioeconomic, and lifestyle factors provided us with an ability to meticulously control for potential confounders. Nevertheless, this study also has some limitations. First, our study lack extensive repeated WES data to monitor time-varying exposures. Consequently, individuals who were initially without CHIP at cohort recruitment but acquired CHIP during the follow-up period remained unidentified, which might have led to a lower estimation of the association we noted. Second, we cannot fully exclude reverse causality, although largely similar results were observed in the analyses with a 1-, 3-, or 5-year lag time. Third, the sample size on different CHIP mutations is limited, leading to inadequate statistical power in this analysis. Future studies with larger sample sizes are needed to validate these findings. Fourth, this study is limited by using a single cohort without validation from external cohort. Particularly, participants of the present study were predominantly white British and were not representative of the whole UK population.32 Future studies, ideally incorporating multiple cohorts from diverse backgrounds, are essential to corroborate our initial findings and examine the generalizability of these findings in broader contexts. Fifth, given that individuals with CHIP might have a higher mortality than individuals without CHIP, the observed association might have been affected by the competing risk of death from other causes. Sixth, the present study may be subject to residual confounding. Although we made efforts to control for the effect of some potential confounders, through multivariable adjustment or stratified analysis, the observational nature of this study could not exclude residual confounding due to unmeasured or unknown confounders. Seventh, despite the considerable sample size of the present study, the statistical power remained insufficient to detect modest risk differences or to perform analyses in small subgroups (e.g., certain CHIP mutations with a limited number of cases).
In conclusion, we observed a higher risk of pulmonary embolism among individuals with CHIP, especially those with TET2 or JAK2 mutations. These findings suggest potential shared mechanisms between CHIP and pulmonary embolism, and the value of early screening for pulmonary embolism among individuals with CHIP should be evaluated. Future studies are warranted to verify these findings.
Contributors
QL and XL conceived the idea for the study. QL, KS, FF, and XL designed the study. QL and HX performed the analysis. XL prepared the manuscript draft. QL and HX have verified the underlying data. QL, KS, TW, HX, JL, FF, and XL interpreted data, and revised the manuscript for submission. All authors read and approved the final version of the manuscript.
Data sharing statement
This study utilized the UK Biobank Resource, under Application Number 106912. Researchers can access data from the UK Biobank by submitting an application at www.ukbiobank.ac.uk.
Declaration of interests
The authors declare no conflict of interest.
Acknowledgements
This research has been conducted using the UK Biobank Resource under application number [106912]. This study was partially supported by the Initial Founding for High Level Talented Scholars in Nanfang Hospital, Southern Medical University (No. 2023G001) and the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (Grant No. 2023J009).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102753.
Contributor Information
Qianwei Liu, Email: qianweiliu@smu.edu.cn.
Xinyuan Liu, Email: liuxinyuancsu@outlook.com.
Appendix ASupplementary data
References
- 1.Goldhaber S.Z., Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379(9828):1835–1846. doi: 10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- 2.Di Nisio M., van Es N., Büller H.R. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388(10063):3060–3073. doi: 10.1016/S0140-6736(16)30514-1. [DOI] [PubMed] [Google Scholar]
- 3.Khan F., Tritschler T., Kahn S.R., Rodger M.A. Venous thromboembolism. Lancet. 2021;398(10294):64–77. doi: 10.1016/S0140-6736(20)32658-1. [DOI] [PubMed] [Google Scholar]
- 4.Pulmonary embolism. Nat Rev Dis Prim. 2018;4:18031. doi: 10.1038/nrdp.2018.31. [DOI] [PubMed] [Google Scholar]
- 5.Steensma D.P., Bejar R., Jaiswal S., et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaiswal S., Natarajan P., Silver A.J., et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim P.G., Niroula A., Shkolnik V., et al. Dnmt3a-mutated clonal hematopoiesis promotes osteoporosis. J Exp Med. 2021;218(12) doi: 10.1084/jem.20211872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agrawal M., Niroula A., Cunin P., et al. TET2-mutant clonal hematopoiesis and risk of gout. Blood. 2022;140(10):1094–1103. doi: 10.1182/blood.2022015384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharya R., Zekavat S.M., Haessler J., et al. Clonal hematopoiesis is associated with higher risk of stroke. Stroke. 2022;53(3):788–797. doi: 10.1161/STROKEAHA.121.037388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller P.G., Qiao D., Rojas-Quintero J., et al. Association of clonal hematopoiesis with chronic obstructive pulmonary disease. Blood. 2022;139(3):357–368. doi: 10.1182/blood.2021013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed S.C., Croessmann S., Park B.H. CHIP happens: clonal hematopoiesis of indeterminate potential and its relationship to solid tumors. Clin Cancer Res. 2023;29(8):1403–1411. doi: 10.1158/1078-0432.CCR-22-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong W.J., Emdin C., Bick A.G., et al. Clonal haematopoiesis and risk of chronic liver disease. Nature. 2023;616(7958):747–754. doi: 10.1038/s41586-023-05857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunbar A., Bolton K.L., Devlin S.M., et al. Genomic profiling identifies somatic mutations predicting thromboembolic risk in patients with solid tumors. Blood. 2021;137(15):2103–2113. doi: 10.1182/blood.2020007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soudet S., Jedraszak G., Evrard O., Marolleau J.P., Garcon L., Pietri M.A.S. Is hematopoietic clonality of indetermined potential a risk factor for pulmonary embolism? TH Open. 2021;5(3):e338–e342. doi: 10.1055/s-0041-1733856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bycroft C., Freeman C., Petkova D., et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David B., Takuto S., Kristian C., Gad G., Chip S., Lee L. Calling somatic SNVs and indels with Mutect2. bioRxiv. 2019 doi: 10.1101/861054. [DOI] [Google Scholar]
- 17.Vlasschaert C., Mack T., Heimlich J.B., et al. A practical approach to curate clonal hematopoiesis of indeterminate potential in human genetic data sets. Blood. 2023;141(18):2214–2223. doi: 10.1182/blood.2022018825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller C.A., Walker J.R., Jensen T.L., et al. Failure to detect mutations in U2AF1 due to changes in the GRCh38 reference sequence. J Mol Diagn. 2022;24(3):219–223. doi: 10.1016/j.jmoldx.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16) doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karczewski K.J., Francioli L.C., Tiao G., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawoud A.A.Z., Tapper W.J., Cross N.C.P. Age-related loss of chromosome Y is associated with levels of sex hormone binding globulin and clonal hematopoiesis defined by TET2, TP53, and CBL mutations. Sci Adv. 2023;9(16) doi: 10.1126/sciadv.ade9746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu M., Kovilakam S.C., Dunn W.G., et al. Multiparameter prediction of myeloid neoplasia risk. Nat Genet. 2023;55(9):1523–1530. doi: 10.1038/s41588-023-01472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casez P., Labarère J., Sevestre M.A., et al. ICD-10 hospital discharge diagnosis codes were sensitive for identifying pulmonary embolism but not deep vein thrombosis. J Clin Epidemiol. 2010;63(7):790–797. doi: 10.1016/j.jclinepi.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Arellano-Rodrigo E., Alvarez-Larrán A., Reverter J.C., Villamor N., Colomer D., Cervantes F. Increased platelet and leukocyte activation as contributing mechanisms for thrombosis in essential thrombocythemia and correlation with the JAK2 mutational status. Haematologica. 2006;91(2):169–175. [PubMed] [Google Scholar]
- 25.Hobbs C.M., Manning H., Bennett C., et al. JAK2V617F leads to intrinsic changes in platelet formation and reactivity in a knock-in mouse model of essential thrombocythemia. Blood. 2013;122(23):3787–3797. doi: 10.1182/blood-2013-06-501452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becattini C., Agnelli G., Prandoni P., et al. A prospective study on cardiovascular events after acute pulmonary embolism. Eur Heart J. 2005;26(1):77–83. doi: 10.1093/eurheartj/ehi018. [DOI] [PubMed] [Google Scholar]
- 27.Austin P.C., Fine J.P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391–4400. doi: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sozer S., Fiel M.I., Schiano T., Xu M., Mascarenhas J., Hoffman R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood. 2009;113(21):5246–5249. doi: 10.1182/blood-2008-11-191544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuster J.J., MacLauchlan S., Zuriaga M.A., et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355(6327):842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christiansen S.C., Naess I.A., Cannegieter S.C., Hammerstrøm J., Rosendaal F.R., Reitsma P.H. Inflammatory cytokines as risk factors for a first venous thrombosis: a prospective population-based study. PLoS Med. 2006;3(8) doi: 10.1371/journal.pmed.0030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bontekoe E., Brailovsky Y., Hoppensteadt D., et al. Upregulation of inflammatory cytokines in pulmonary embolism using biochip-array profiling. Clin Appl Thromb Hemost. 2021;27 doi: 10.1177/10760296211013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fry A., Littlejohns T.J., Sudlow C., et al. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.