Abstract
Background
The obesity paradox refers to lower mortality rates among overweight or obese individuals within certain populations. However, whether this paradox is applicable to patients undergoing percutaneous coronary intervention (PCI) remains unclear.
Methods
A total of 5,427 patients with coronary artery disease (CAD) who underwent successful PCI between 2005 and 2015 were enrolled. The association between body mass index (BMI) and future adverse cardiovascular events post PCI was analyzed. The study endpoints encompassed total cardiovascular (CV) events, including cardiac death, nonfatal myocardial infarction (MI), ischemic stroke, and hospitalization for congestive heart failure (CHF).
Results
Over an average follow-up period of 65.1 ± 32.1 months, 942 patients (17.4%) had CV events, including 200 CV deaths (3.7%), 294 acute MIs (5.4%), 111 ischemic strokes (2.0%), 469 CHF hospitalizations (8.6%), and 1,098 revascularizations (20.2%). A J-shaped relationship between BMI and future adverse events was observed, in which individuals with a BMI of 25.0-29.9 kg/m2 had significantly lower risks of total CV events [hazard ratio (HR) = 0.84, 95% confidence interval (CI) = 0.72-0.98], major adverse cardiovascular events (HR = 0.76, 95% CI = 0.63-0.93), acute MI (HR = 0.76, 95% CI = 0.58-1.00), and ischemic stroke (HR = 0.61, 95% CI = 0.39-0.95), compared to those with a BMI of 22.0-24.9 kg/m2.
Conclusions
We found a J-shaped relationship between baseline BMI and future adverse events in CAD patients undergoing PCI. Overweight individuals (BMI 25.0-29.9 kg/m2) had the lowest future risk of total CV events compared to those with a normal BMI (22.0-24.9 kg/m2).
Keywords: Body mass index (BMI), Coronary artery disease (CAD), Major adverse cardiovascular events (MACEs), Percutaneous coronary intervention (PCI)
Abbreviations
ACEi, Angiotensin-converting enzyme inhibitor
AF, Atrial fibrillation
AMI, Acute myocardial infarction
ARB, Angiotensinogen receptor blocker
BMI, Body mass index
BMS, Bare-metal stent
CAD, Coronary artery disease
CCB, Calcium channel blocker
CHF, Congestive heart failure
CI, Confidence interval
CKD, Chronic kidney disease
CV, Cardiovascular
CVD, Cardiovascular disease
DVD, Double vessel disease
ECG, Electrocardiography
eGFR, Estimated glomerular filtration rate
HDL-C, High-density lipoprotein cholesterol
HR, Hazard ratio
LDL-C, Low-density lipoprotein cholesterol
LVEF, Left ventricular ejection fraction
MACEs, Major adverse cardiovascular events
MI, Myocardial infarction
PCI, Percutaneous coronary intervention
PDAY, Pathobiological Determinants of Atherosclerosis in Youth
SVD, Single vessel disease
TVD, Triple vessel disease
WHO, World Health Organization
WHR, Waist-to-hip ratio
INTRODUCTION
Most of the world’s population lives in countries where overweight status and obesity pose greater risks of morbidity and mortality compared to underweight status. According to the World Health Organization (WHO), 39.0% of adults aged 18 and over are overweight, with 13.0% categorized as obese.1 Body mass index (BMI) is frequently used to assess excess body fat and obesity. According to the WHO’s definition, overweight is defined as a BMI of 25.0-29.9 kg/m2, and obesity as a BMI ≥ 30.0 kg/m2. Both overweight and obesity status are documented risk factors for chronic illnesses, including hypertension, dyslipidemia, fatty liver, sleep apnea, type 2 diabetes, symptomatic osteoarthritis, and coronary artery disease.2,3 Consequently, weight loss interventions are typically recommended for individuals with a BMI of 25.0 or higher to prevent or reverse complications associated with excess weight. According to the WHO, mortality increases as the BMI rises above 25.0 kg/m2, with the lowest mortality rates found among individuals in the ideal weight range (BMI of 18.5 to 24.9 kg/m2).4
Even though overweight and obesity are generally considered risk factors for premature mortality, some scientific evidence supports that overweight might be associated with reduced comorbidities and longer survival in patients with chronic kidney disease (CKD), cancer, and among the elderly.11 It is also not uncommon for overweight or obese individuals to have few metabolic abnormalities.5,6 One theory suggests that the adverse health effects of obesity often take many years to manifest.7 Additionally, individuals with the same BMI do not necessarily have the same body fat distribution. Pischon et al. noted that excessive abdominal fat accumulation is the primary contributor to cardiometabolic abnormalities, which can lead to cardiovascular disease (CVD).8 In addition, the "obesity paradox" has been observed among patients with congestive heart failure (CHF) and atrial fibrillation (AF).9,10 Studies have indicated a lower future risk of adverse events in overweight individuals compared to those with a normal BMI, suggesting a ‘J-shaped’ curve between BMI and mortality in patients with cardiovascular conditions.
Advances in percutaneous coronary intervention (PCI) techniques have significantly improved clinical outcomes in patients with coronary artery disease (CAD). However, whether the obesity paradox exists in CAD patients following successful coronary interventions remains unclear. In addition, there is limited information regarding the obesity paradox in relation to specific future cardiovascular events, such as ischemic stroke, myocardial ischemia, or cardiovascular death. Therefore, this cohort study aimed to investigate the association between baseline BMI and the risk of future adverse events in CAD patients following successful PCI.
METHODS
Study population
This single-center retrospective study recruited patients between 2005 and 2015 who had established CAD following PCI, including coronary stenting or balloon angioplasty. All CAD patients who underwent coronary interventions were enrolled for analysis. CAD was diagnosed if the patient met at least one of the following criteria: (1) ischemic change in 12-lead electrocardiography (ECG), elevated cardiac enzymes, and a diagnosis of myocardial infarction (MI) on medical records; or (2) symptoms of angina with ischemic change in 12-lead ECG or positive stress test. Patients were enrolled if they (1) had a history of at least one previously successful PCI with either coronary stenting or balloon angioplasty, and (2) were stable on medical treatment for at least 1 month before enrollment. The exclusion criteria were: (1) hospitalization for any CV event within the last 3 months, (2) significant malignancy requiring hospitalization or surgery, (3) other major systemic diseases necessitating hospitalization or surgery, (4) life expectancy of less than 6 months, (5) treatment with immunosuppressive agents, or (6) inability or unwillingness to be followed up for 1 year. The study adhered to the principles of the Declaration of Helsinki, and approval was obtained from the Ethics Committees and Independent Review Boards at Taipei Veterans General Hospital.
Baseline data collection
All baseline characteristics were retrieved from the web-based electronic medical record system at Taipei Veterans General Hospital by physicians or trained assistants. These characteristics included age, sex, BMI, laboratory data, and comorbidities such as diabetes, hypertension, hyperlipidemia, heart failure, CKD, chronic obstructive pulmonary disease, stroke, CAD, or acute coronary syndrome at enrollment.
Body weight assessment
Baseline body weight was obtained from hospital medical records. BMI, calculated as the ratio of body weight to height squared, was used as an indicator of obesity. According to the WHO definition published in 2000,11,12 BMI values are classified as underweight (BMI < 18.5 kg/m2), normal weight (BMI = 18.5-24.9 kg/m2), overweight (BMI = 25.0-29.9 kg/m2), grade 1 obesity (BMI = 30.0-34.9 kg/m2), grade 2 obesity (BMI = 35.0-39.9 kg/m2), or grade 3 obesity (BMI ≥ 40.0 kg/m2). Given the low prevalence of underweight individuals in our population, we categorized BMI < 22.0 kg/m2 as low BMI and a BMI of 22.0-24.9 kg/m2 as the reference group for normal weight, consistent with previous studies.13 Although the WHO has recommended lower BMI thresholds for Asian populations (overweight: BMI = 23.0-24.9 kg/m2; obesity: BMI ≥ 25.0 kg/m2) due to the higher risk of cardiovascular diseases and metabolic disorders at lower BMI levels, we chose to use the global WHO definitions. This decision allows for easier comparison and integration of our findings with other studies, as the global WHO criteria are widely used in international research. Additionally, using the WHO criteria enabled us to assess the impact of overweight and obesity based on a more universally recognized standard. We also analyzed the association between BMI and future events. All BMI categories were analyzed, and the association was not affected by different cutoff values.
Outcomes
The primary outcome was the first occurrence of total cardiovascular (CV) events, defined as the combination of CV death, nonfatal MI, nonfatal stroke, and heart failure hospitalization. We recorded the time to the first occurrence of any of these events. Additionally, individual outcomes including CV death, nonfatal MI, nonfatal stroke, revascularization, heart failure hospitalization, and major adverse cardiovascular events (MACEs) were analyzed. MACEs encompassed CV death, nonfatal MI, and ischemic stroke. MI was defined as elevated serum cardiac enzyme levels accompanied by characteristic ECG changes, while ischemic stroke was defined as reduced blood flow to part or all of the brain, supported by evidence from brain imaging studies such as computed tomography or magnetic resonance imaging. CV death was defined as death from CV causes such as MI, heart failure, stroke, CV-related hemorrhage, procedure-related death, or sudden cardiac death. The last follow-up date for the study was December 31, 2019. Similar event definitions were also reported in our previous studies.13-15
Statistical analysis
Baseline patient characteristics were compared across BMI subgroups. Quantitative variables were expressed as means with standard deviations for normally distributed data, while qualitative variables were presented as absolute frequencies (number of patients) and relative frequencies (percentages). Continuous variables between groups were compared using ANOVA, while categorical variables were compared using the χ2 test or Fisher’s exact test. All outcomes were reported as the number of patients and corresponding percentages. Event-free survival rates for the BMI subgroups were calculated using the Kaplan-Meier method, with significance evaluated by log-rank tests. Hazard ratios (HRs) and 95% confidence intervals (CIs) for each outcome by BMI group were calculated using Cox proportional hazard regression analysis, adjusted for age, sex, and comorbidities, with a BMI of 22.0-24.9 kg/m2 serving as the reference group.
RESULTS
Baseline characteristics
A total of 5,427 patients who underwent PCI were enrolled in this study. The distribution of BMI in our study population is shown in Figure 1. In this cohort, 29.5% had a normal BMI (22.0-24.9 kg/m2), while the majority (41.1%) were overweight (BMI = 25.0-29.9 kg/m2). The baseline characteristics of the participants by BMI subgroups are presented in Table 1. The mean age was 69.2 ± 13.3 years, and 73.9% of the patients were male. The mean BMI was 25.5 ± 4.2 kg/m2. There were no significant differences in the angiographic severity of CAD across BMI groups. However, the patients with a higher BMI were younger than those with a lower BMI. The patients with a higher BMI had a higher prevalence of hypertension, hyperlipidemia, and diabetes, and were more frequently receiving medication therapy for these conditions. In contrast, the patients with a BMI < 22.0 kg/m2 had a higher prevalence of heart failure (17.6%), CKD (9.9%), stroke (6.9%), and were more likely to present with acute coronary syndrome (49.5%) at enrollment.
Figure 1.
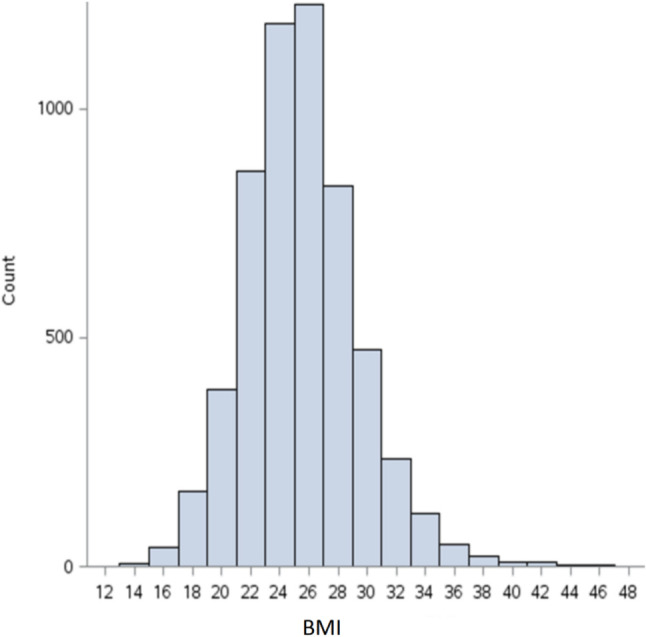
Distribution of body mass index in the study population. BMI, body mass index.
Table 1. Baseline characteristics of study participants in body mass index categories.
| Characteristics | BMI < 22 (n = 934) | BMI = 22-24.9 (n = 1601) | BMI = 25-29.9 (n = 2263) | BMI = 30-34.9 (n = 530) | BMI > 35 (n = 99) | p value |
| Age (years) | 73.7 ± 11.67 | 70.2 ± 12.62 | 67.1 ± 12.95 | 62.7 ± 13.96 | 59.2 ± 14.21 | < .0001 |
| Weight (Kg) | 52.8 ± 6.82 | 62.5 ± 6.95 | 72.8 ± 8.66 | 84.3 ± 10.59 | 100.2 ± 20.16 | < .0001 |
| Height (cm) | 161.7 ± 8.35 | 162.6 ± 8.49 | 163.8 ± 8.77 | 162.6 ± 9.73 | 160 ± 11.70 | < .0001 |
| BMI (Kg/m2) | 20.1 ± 1.57 | 23.5 ± 0.85 | 27 ± 1.36 | 31.7 ± 1.36 | 38.9 ± 5.34 | < .0001 |
| Males | 633 (67.77) | 1188 (74.2) | 1757 (77.64) | 379 (71.51) | 56 (56.57) | < .0001 |
| Underlying diseases | ||||||
| Hypertension | 492 (52.68) | 960 (59.96) | 1484 (65.58) | 382 (72.08) | 80 (80.81) | < .0001 |
| Diabetes | 257 (27.52) | 553 (34.54) | 800 (35.35) | 241 (45.47) | 58 (58.59) | < .0001 |
| Hyperlipidemia | 167 (17.88) | 438 (27.36) | 785 (34.69) | 202 (38.11) | 38 (38.38) | < .0001 |
| Heart failure | 164 (17.56) | 228 (14.24) | 246 (10.87) | 70 (13.21) | 12 (12.12) | < .0001 |
| CKD | 92 (9.85) | 129 (8.06) | 101 (4.46) | 27 (5.09) | 4 (4.04) | < .0001 |
| Stroke | 64 (6.85) | 95 (5.93) | 121 (5.35) | 24 (4.53) | 1 (1.01) | 0.0765 |
| ACS at enrollment | 462 (49.46) | 665 (41.54) | 832 (36.77) | 196 (36.98) | 33 (33.33) | < .0001 |
| Lab data | ||||||
| HbA1c (%) | 6.7 ± 1.35 | 6.9 ± 1.42 | 7 ± 1.35 | 7.4 ± 1.38 | 7.6 ± 1.68 | < .0001 |
| LDL-C (mmol/L) | 99.2 ± 33.31 | 104.1 ± 33.82 | 108.4 ± 34.33 | 108.9 ± 37.25 | 108.5 ± 35.45 | < .0001 |
| HDL-C (mmol/L) | 45.4 ± 13.71 | 43.4 ± 12.36 | 41.4 ± 10.90 | 39.3 ± 10.17 | 40.5 ± 10.82 | < .0001 |
| eGFR (mL/min/1.73 m2) | 58 ± 29.34 | 62.3 ± 28.79 | 64.3 ± 25.57 | 64.6 ± 27.16 | 60.5 ± 27.75 | < .0001 |
| LVEF (%) | 48 ± 12.30 | 51.2 ± 11.78 | 52.6 ± 10.39 | 52.9 ± 9.59 | 52.7 ± 9.96 | < .0001 |
| Angiographic features | ||||||
| SVD | 254 (27.19) | 438 (27.36) | 688 (30.4) | 163 (30.75) | 34 (34.34) | 0.096 |
| DVD | 304 (32.55) | 492 (30.73) | 715 (31.6) | 175 (33.02) | 34 (34.34) | 0.7795 |
| TVD | 367 (39.29) | 666 (41.6) | 853 (37.69) | 187 (35.28) | 31 (31.31) | 0.0202 |
| Medication use | ||||||
| ACEi | 185 (19.81) | 312 (19.49) | 507 (22.4) | 109 (20.57) | 18 (18.18) | 0.186 |
| ARB | 271 (29.01) | 556 (34.73) | 913 (40.34) | 244 (46.04) | 44 (44.44) | < .0001 |
| Beta-blockers | 398 (42.61) | 742 (46.35) | 1132 (50.02) | 284 (53.58) | 54 (54.55) | < .0001 |
| CCB | 235 (25.16) | 533 (33.29) | 839 (37.07) | 219 (41.32) | 44 (44.44) | < .0001 |
| Statin | 461 (49.36) | 897 (56.03) | 1369 (60.49) | 336 (63.4) | 63 (63.64) | < .0001 |
| Thiazide diuretics | 69 (7.39) | 153 (9.56) | 244 (10.78) | 80 (15.09) | 8 (8.08) | < .0001 |
Data are mean ± SD or n (%).
ACEi, angiotensin converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensinogen receptor blocker; BMI, body mass index; CCB, calcium channel blocker; CKD, chronic kidney disease; DVD, double vessel disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; LVEF, left ventricle ejection fraction; SD, standard deviation; SVD, single vessel disease; TVD, triple vessel disease.
Association between BMI groups and CV outcomes after PCI
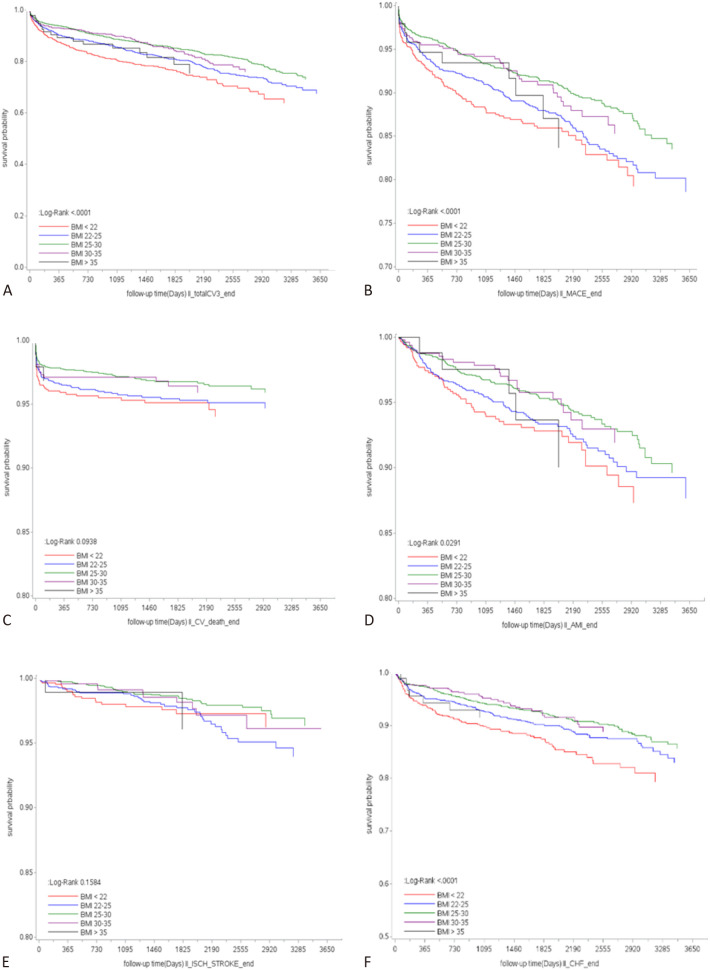
Clinical follow-up was conducted for a mean period of 65.6 ± 32.1 months. The incidence rates of outcomes in the BMI subgroups are shown in Table 2. During the entire cohort study, there were 942 patients (17.4%) having CV events: 569 MACEs (10.5%), 200 CV deaths (3.7%), 294 acute MIs (5.4%), 111 ischemic strokes (2.0%), 469 heart failure hospitalizations (8.6%), and 1,098 revascularizations (20.2%). During the follow-up period, the underweight group (BMI < 22.0 kg/m2) had the highest incidence of total CV events and MACEs. Specifically, this group had a higher rate of heart failure hospitalizations and revascularizations compared to the other groups. Conversely, in the highest BMI category (BMI ≥ 35.0 kg/m2), the risks of total CV events, heart failure hospitalizations, and revascularizations were significantly elevated, consistent with the increased risks associated with traditional obesity-related complications. Statistical analysis across the groups confirmed significant differences in outcomes such as total CV events, MACEs, heart failure hospitalizations, and revascularizations, demonstrating that both lower and higher BMI extremes were associated with increased adverse events, while the lowest incidence was observed in the overweight BMI category (25.0-29.9 kg/m2). Kaplan-Meier survival analysis of all outcomes by BMI subgroups is shown in Figure 2. The results revealed that patients in the BMI 25.0-29.9 kg/m2 group had lower rates of adverse clinical outcomes, while those in the BMI < 22 kg/m2 group tended to have the worst clinical outcomes. The log-rank test substantiated these differences, confirming statistical significance in total CV events (p < 0.001), MACEs (p < 0.001), acute MI (p = 0.029), and heart failure hospitalization (p < 0.001).
Table 2. Incidence of outcomes in BMI categories.
| Outcomes | BMI < 22 (n = 934) | BMI = 22-24.9 (n = 1601) | BMI = 25-29.9 (n = 2263) | BMI = 30-34.9 (n = 530) | BMI > 35 (n = 99) | p value |
| Total CV events* | 199 (21.31) | 300 (18.74) | 345 (15.25) | 81 (15.28) | 17 (17.17) | 0.0003 |
| MACE# | 116 (12.42) | 190 (11.87) | 205 (9.06) | 48 (9.06) | 10 (10.1) | 0.011 |
| CV death | 43 (4.6) | 66 (4.12) | 71 (3.14) | 17 (3.21) | 3 (3.03) | 0.2436 |
| Acute MI | 57 (6.1) | 98 (6.12) | 111 (4.9) | 23 (4.34) | 5 (5.05) | 0.3094 |
| Ischemic stroke | 19 (2.03) | 42 (2.62) | 37 (1.63) | 11 (2.08) | 2 (2.02) | 0.3337 |
| HF hospitalization | 103 (11.03) | 147 (9.18) | 175 (7.73) | 37 (6.98) | 7 (7.07) | 0.0185 |
| Revascularization† | 146 (15.63) | 316 (19.74) | 506 (22.36) | 113 (21.32) | 17 (17.17) | 0.0005 |
Data are n (%).
BMI, body mass index; CV, cardiovascular; HF, heart failure; MACE, major adverse cardiovascular event; MI, myocardial infarction.
* Include MACE + HF hospitalization; # Include acute myocardial infarction, ischemic stroke, cardiovascular death; † Include coronary stenting and bypass surgery.
Figure 2.
Time to event curves of individual outcomes in BMI categories. (A) Total CV events, (B) MACE, (C) CV mortality, (D) Acute myocardial infarction, (E) Ischemic stroke, (F) Acute decompensate heart failure hospitalization. BMI, body mass index; CV, cardiovascular; MACE, major adverse cardiovascular event.
Predictive value of BMI for future CV risk after PCI
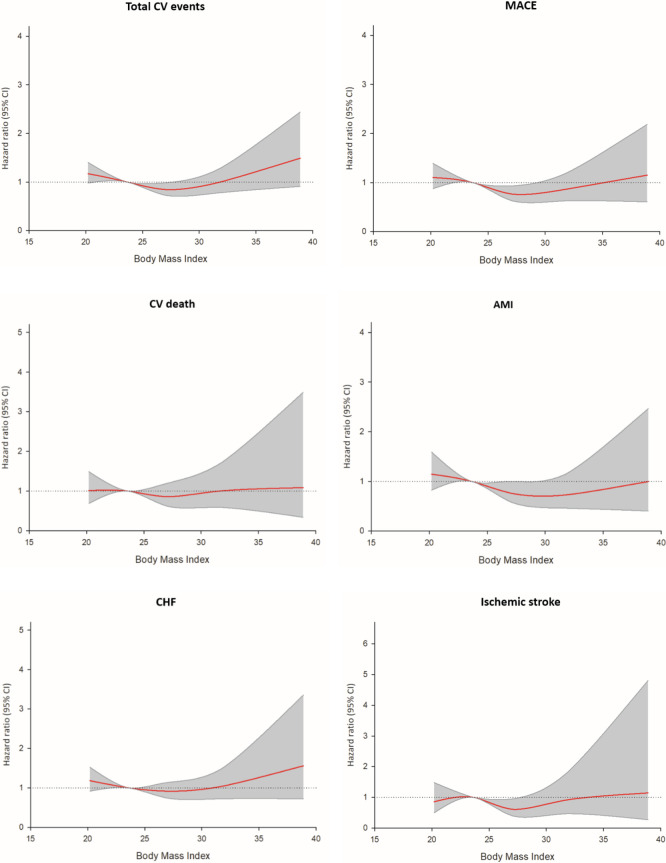
HRs for all outcomes by BMI subgroup are shown in Table 3. Using BMI 22.0-24.9 kg/m2 as the reference, the unadjusted risk model revealed that the overweight group (BMI = 25.0-29.9 kg/m2) had significantly lower HRs for total CV events, MACEs, acute MI, and ischemic stroke compared to the reference group. Conversely, the underweight group (BMI < 22.0 kg/m2) had significantly higher risks of total CV events and heart failure hospitalization. After adjusting for age, sex, and comorbidities, a "J-shaped" association between BMI categories and future adverse CV events remained. The overweight group (BMI = 25.0-29.9 kg/m2) was associated with a significantly lower risk of total CV events [hazard ratio (HR) = 0.84, 95% confidence interval (CI) = 0.72-0.98, p = 0.031], MACEs (HR = 0.76, 95% CI = 0.63-0.93, p = 0.008), acute MI (HR = 0.76, 95% CI = 0.58-1.00, p = 0.048), and ischemic stroke (HR = 0.61, 95% CI = 0.39-0.95, p = 0.030) (Table 3) (Figure 3). The underweight and obese groups continued to show a higher future risk, particularly for total CV events, MACEs, and heart failure.
Table 3. Association between BMI and future adverse events following PCI.
| Outcome | BMI category | Crude HR | Adjusted HR | ||
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Total CV events | BMI < 22 | 1.31 (1.09-1.57) | 0.003 | 1.17 (0.98-1.40) | 0.086 |
| BMI = 22-25 | 1.00 (1.00-1.00) | Reference | 1.00 (1.00-1.00) | Reference | |
| BMI = 25-30 | 0.77 (0.66-0.89) | 0.001 | 0.84 (0.72-0.98) | 0.031 | |
| BMI = 30-35 | 0.79 (0.62-1.01) | 0.064 | 1.00 (0.78-1.28) | 0.977 | |
| BMI > 35 | 1.01 (0.62-1.65) | 0.954 | 1.49 (0.91-2.44) | 0.114 | |
| MACE | BMI < 22 | 1.19 (0.94-1.50) | 0.145 | 1.10 (0.87-1.39) | 0.411 |
| BMI = 22-25 | 1.00 (1.00-1.00) | Reference | 1.00 (1.00-1.00) | Reference | |
| BMI = 25-30 | 0.72 (0.59-0.88) | 0.001 | 0.76 (0.63-0.93) | 0.008 | |
| BMI = 30-35 | 0.75 (0.54-1.02) | 0.069 | 0.86 (0.63-1.19) | 0.366 | |
| BMI > 35 | 0.92 (0.49-1.74) | 0.799 | 1.15 (0.61-2.19) | 0.669 | |
| CV death | BMI < 22 | 1.18 (0.80-1.73) | 0.402 | 1.01 (0.69-1.49) | 0.944 |
| BMI = 22-25 | 1.00 (1.00-1.00) | Reference | 1.00 (1.00-1.00) | Reference | |
| BMI = 25-30 | 0.75 (0.54-1.05) | 0.091 | 0.86 (0.61-1.20) | 0.378 | |
| BMI = 30-35 | 0.77 (0.45-1.32) | 0.342 | 1.01 (0.59-1.72) | 0.979 | |
| BMI > 35 | 0.76 (0.24-2.43) | 0.647 | 1.09 (0.34-3.49) | 0.887 | |
| Acute MI | BMI < 22 | 1.17 (0.85-1.63) | 0.339 | 1.14 (0.82-1.59) | 0.421 |
| BMI = 22-25 | 1.00 (1.00-1.00) | Reference | 1.00 (1.00-1.00) | Reference | |
| BMI = 25-30 | 0.74 (0.57-0.98) | 0.034 | 0.76 (0.58-1.00) | 0.048 | |
| BMI = 30-35 | 0.69 (0.44-1.09) | 0.110 | 0.73 (0.46-1.15) | 0.175 | |
| BMI > 35 | 0.91 (0.37-2.25) | 0.846 | 1.00 (0.40-2.47) | 0.995 | |
| Ischemic stroke | BMI < 22 | 0.92 (0.54-1.59) | 0.775 | 0.86 (0.50-1.48) | 0.585 |
| BMI = 22-25 | 1.00 (1.00-1.00) | Reference | 1.00 (1.00-1.00) | Reference | |
| BMI = 25-30 | 0.58 (0.37-0.90) | 0.015 | 0.61 (0.39-0.95) | 0.030 | |
| BMI = 30-35 | 0.78 (0.40-1.52) | 0.465 | 0.92 (0.47-1.80) | 0.805 | |
| BMI > 35 | 0.87 (0.21-3.59) | 0.846 | 1.15 (0.27-4.81) | 0.852 | |
| HF hospitalization | BMI < 22 | 1.39 (1.08-1.79) | 0.010 | 1.18 (0.92-1.52) | 0.191 |
| BMI = 22-25 | 1.00 (1.00-1.00) | Reference | 1.00 (1.00-1.00) | Reference | |
| BMI = 25-30 | 0.79 (0.64-0.99) | 0.040 | 0.92 (0.74-1.14) | 0.445 | |
| BMI = 30-35 | 0.75 (0.52-1.07) | 0.110 | 1.04 (0.73-1.50) | 0.816 | |
| BMI > 35 | 0.86 (0.40-1.83) | 0.695 | 1.56 (0.73-3.36) | 0.255 | |
| Revascularization | BMI < 22 | 0.88 (0.73-1.08) | 0.221 | 0.93 (0.76-1.13) | 0.479 |
| BMI = 22-25 | 1.00 (1.00-1.00) | Reference | 1.00 (1.00-1.00) | Reference | |
| BMI = 25-30 | 1.07 (0.93-1.23) | 0.335 | 1.03 (0.90-1.19) | 0.655 | |
| BMI = 30-35 | 1.06 (0.85-1.31) | 0.623 | 0.99 (0.80-1.23) | 0.949 | |
| BMI > 35 | 0.92 (0.56-1.49) | 0.723 | 0.86 (0.53-1.41) | 0.553 |
BMI, body mass index; CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; MACE, major adverse cardiovascular event; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Total CV event include MACE + HF hospitalization; MACE includes acute myocardial infarction, ischemic stroke, cardiovascular death; Revascularization includes coronary stenting and bypass surgery.
Figure 3.
Age-and-sex-adjusted hazard ratio of individual outcomes in BMI categories (BMI = 22.0-24.9 Kg/m2 is used as reference category). AMI, acute myocardial infarction; BMI, body mass index; CHF, congestive heart failure; CV, cardiovascular; MACE, major adverse cardiovascular event.
DISCUSSION
The present study demonstrated a J-shape relationship between baseline BMI and future adverse CV risk in CAD patients after coronary intervention. Overweight status (BMI = 25.0-29.9 kg/m2) was associated with the lowest risk of total CV events, supporting the existence of the obesity paradox in CAD patients following coronary intervention.
Overweight status and obesity are independent risk factors for metabolic disorders and CVD. Most studies support the association between obesity with hypertension, hyperlipidemia, diabetes, insulin resistance and CAD, and lifestyle modification with weight control is strongly recommended as a treatment strategy to reduce future CV risk.16 However, it is not rare for overweight or obese people to have better clinical outcomes. Recently, the "BMI paradox" or "obesity paradox" has been widely discussed in cardiology, such as in studies concerning CHF and AF.10 These studies have shown that patients who are overweight or obese tend to have a lower mortality rate, and that this protective effect appears to be especially pronounced in older patients.17 Findings of the obesity paradox have been reported in several studies18-21 as not a statistical error but a meaningful result which requires more investigation to be used in clinical practice. Our findings are consistent with the obesity paradox phenomenon, in that the overweight group was associated with better clinical outcomes. A meta-analysis revealed that among patients undergoing PCI, those classified as overweight or obese had lower short-term (30 days) and long-term (1-5 years) mortality rates compared to those with a normal BMI.22 Furthermore, another meta-analysis confirmed the existence of a J-shaped relationship between BMI and total mortality in PCI patients with follow-up periods exceeding 5 years.23 Notably, underweight patients had the highest mortality risk, while overweight patients had the lowest risk. In addition, a cohort study conducted in Eastern Taiwan (ET-CHD) also reported a J-shaped relationship between BMI and mortality in patients with obstructive CAD.24 This study compared outcomes within and after a 5-year follow-up period. During the initial 5-year follow-up, underweight patients had elevated mortality risks, whereas obese patients had reduced mortality risks. However, after 5 years, the J-shaped pattern became more pronounced, with underweight and severely obese patients having higher mortality risks, and overweight patients having lower risks. In the present study, we analyzed data from over 5,000 patients from 2005 to 2015, with a mean follow-up period of approximately 7 years. The findings of our study further reinforce the notion that the obesity paradox is not merely a short-term phenomenon but also persists over the long term in patients undergoing PCI.
Regarding our results, the obesity paradox can be explained by several factors. First, catabolism is different between healthy people and diseased patients. Diseases such as CAD, HF, COPD, or CKD may increase catabolic state and decrease BMI levels, giving patients with overweight or grade 1 obesity more energy reserve to overcome this high catabolic state, along with a better prognosis during the post-PCI recovery phase. Second, there was a significant difference in age distribution in our study population (p < 0.001). The mean age of the BMI < 22.0 kg/m2 group was 73.7 ± 11.7 years, which was the oldest group in our study; on the other hand, the patients with higher BMIs were younger. Thus, age may be considered a confounding factor in the analysis of CV adverse events, in that older age may result in poorer CV outcomes. In the elderly, a lower BMI may indicate that the patient is involuntarily losing weight, is malnourished, or has sarcopenia,25 which may also lead to higher mortality. Thus, a higher BMI in the elderly may provide survival benefits and result in the obesity paradox. Third, there was a significant difference between subgroups using standard CAD medications (p < 0.001). There appeared to be a trend that more patients in the high BMI groups were taking ARBs, beta-blockers, statins, or anti-platelet medications than those in the low BMI group. These medications are not only used to control hypertension or hyperlipidemia, but also used for cardiac remodeling and providing survival benefits in CAD or heart failure patients. Thus, patients receiving more adequate medication therapy in the higher BMI groups may have resulted in the obesity paradox. Fourth, the influence of obesity on health status is slow. In patients with visceral obesity, the fatty tissue releases inflammatory adipocytokines (e.g., tumor necrosis factor-alpha, interleukin-6, monocyte chemoattractant protein-1, leptin) which may induce endothelial dysfunction and systemic inflammation, facilitating the atherosclerosis process.26 A compelling study, ‘Pathobiological Determinants of Atherosclerosis in Youth’ (PDAY),27 found that obesity in adolescents and young adults could accelerate atherosclerosis progression decades before the appearance of clinical manifestations. It is believed that at least two decades of obesity is likely to be an independent risk factor for CAD. After the patient manifests with hypertension, hyperlipidemia, diabetes, myocardial ischemia, or heart failure, the benefits of starting medication therapy to control the disease outweigh the benefits of losing weight. Our study focused on CAD patients treated with PCI, in whom the prevalence of hypertension was 91.8%, hyperlipidemia 45.1%, and diabetes 42.9%. With these comorbidities, the negative influence of being obese may be offset by the advantage of high energy reserve, which may result in the obesity paradox.
Limitations
Our study has some limitations. First, the data were obtained from a single-center medical records system, with data collection spanning from October 2005 to July 2015, and more than 5,000 patients were enrolled. Due to the low percentage of underweight patients (BMI < 18.5 kg/m2) and those with grade 3 obesity (BMI ≥ 35.0 kg/m2), sampling bias may be present. As a result, the findings should not be generalized to populations in countries with a higher prevalence of underweight or extremely obese individuals, given the epidemiological differences.
Second, BMI may not be the optimal indicator for assessing obesity and CVD risk. While BMI is simple to use, it does not differentiate between muscle mass and visceral fat distribution. Research has shown that excess abdominal visceral adipose tissue, regardless of BMI, is diabetogenic and atherogenic, increasing triglyceride and low-density lipoprotein levels while decreasing high-density lipoprotein levels, thereby elevating CVD risk.28,29 According to the INTERHEART study, waist-to-hip ratio (WHR) and waist circumference (WC) are stronger predictors of MI than BMI alone.30 A WHR above 0.9 in men or 0.85 in women, and a WC above 102 cm in men or 88 cm in women indicate central obesity, which carries a higher CV risk. Future studies could further investigate the obesity paradox using WHR or WC as alternative indicators of obesity.
Third, our study lacks data on weight loss interventions. Clinically, providing nutritional support to underweight patients and encouraging weight loss in overweight patients are logical strategies. Randomized trials have shown that reducing body weight through lifestyle changes lowers inflammatory biomarkers and insulin resistance, thereby decreasing the risk of adverse CV events.31,32 In addition, small trials involving exercise and diet control in post-PCI cardiac rehabilitation programs have demonstrated reductions in revascularization rates.33,34 However, less weight fluctuation in patients with CAD has also been associated with better CV outcomes.35,36 Therefore, the impact of weight loss interventions on CAD patients post-PCI requires further investigation.
Fourth, several confounding factors may influence clinical outcomes in the post-PCI population. These include achieving functionally complete revascularization, using intravascular imaging guidance rather than angiography alone, and adhering to global consensus guidelines for pre- and post-stenting procedures: (1) selecting suitable patients, (2) pre-stenting balloon sizing, (3) stent sizing, (4) post-stenting balloon sizing, (5) ensuring complete apposition and adequate expansion (avoiding underexpansion or malapposition), and (6) preventing edge dissection. All of these factors could impact clinical outcomes.
As a retrospective study, our findings are subject to inherent biases including recall bias, selection bias, and the potential for incomplete data recording. Although we applied robust statistical methods to mitigate these biases, prospective, randomized controlled trials are necessary to validate our results. Given these limitations, our findings should be interpreted with caution. We emphasize the need for further research, including large-scale, multicenter, prospective studies with long-term follow-up.
CONCLUSION
Our study suggests a J-shaped relationship between baseline BMI and future CV risk in CAD patients following coronary intervention. Among the BMI categories, overweight individuals (BMI 25.0-29.9 kg/m2) exhibited the lowest risk of total cardiovascular events.
DECLARATION OF CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Acknowledgments
We thank the technical services provided by the Health and Welfare Data Science Center. The abstract was presented as both an e-poster and an oral presentation at ESC Asia 2021 with APSC & AFC. It is also published in the online abstract supplement of the European Heart Journal, Volume 43, Issue Supplement_1, February 2022, ehab849.064.
DATA AVAILABILITY
Raw data were generated at Taipei Veterans General Hospital and obtained through Taiwan’s National Health Insurance Research Database. The data supporting the findings of this study are available upon request from the corresponding author, Hsin-Bang Leu. These data are not publicly available due to privacy concerns regarding research participants.
FUNDING STATEMENT
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.WHO. Obesity and overweight. 2021, June 9. [Google Scholar]
- 2.Krauss RM, Winston M, Fletcher BJ, Grundy SM. Obesity: impact on cardiovascular disease. Circulation. 1998;98:1472–1476. [PubMed] [Google Scholar]
- 3.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 4.Bhaskaran K, Dos-Santos-Silva I, Leon DA, et al. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6:944–953. doi: 10.1016/S2213-8587(18)30288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefan N, Haring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 6.Iacobini C, Pugliese G, Blasetti Fantauzzi C, et al. Metabolically healthy versus metabolically unhealthy obesity. Metabolism. 2019;92:51–60. doi: 10.1016/j.metabol.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Deedwania P, Lavie CJ. Dangers and long-term outcomes in metabolically healthy obesity: the impact of the missing fitness component. J Am Coll Cardiol. 2018;71:1866–1868. doi: 10.1016/j.jacc.2018.02.057. [DOI] [PubMed] [Google Scholar]
- 8.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 9.Chandramouli C, Tay WT, Bamadhaj NS, et al. Association of obesity with heart failure outcomes in 11 Asian regions: a cohort study. PLoS Med. 2019;16:e1002916. doi: 10.1371/journal.pmed.1002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavie CJ, Pandey A, Lau DH, et al. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70:2022–2035. doi: 10.1016/j.jacc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1-253. [PubMed] [Google Scholar]
- 12.National Institutes of Health, National Heart, Lung, and Blood Institute; NHLBI Obesity Education Initiative; North American Association for the Study of Obesity. The practical guide: Identification, evaluation, and treatment of overweight and obesity in adults. Bethesda, MD: National Institutes of Health; 2000. p. p. 1, Table 1. [Google Scholar]
- 13.Chen SC, Yang YL, Wu CH, et al. Association between preoperative nutritional status and clinical outcomes of patients with coronary artery disease undergoing percutaneous coronary intervention. Nutrients. 2020;12:1295. doi: 10.3390/nu12051295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YL, Wu CH, Hsu PF, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50:e13230. doi: 10.1111/eci.13230. [DOI] [PubMed] [Google Scholar]
- 15.Lim SS, Yang YL, Chen SC, et al. Association of variability in uric acid and future clinical outcomes of patient with coronary artery disease undergoing percutaneous coronary intervention. Atherosclerosis. 2020;297:40–46. doi: 10.1016/j.atherosclerosis.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Dwivedi AK, Dubey P, Cistola DP, Reddy SY. Association between obesity and cardiovascular outcomes: updated evidence from meta-analysis studies. Curr Cardiol Rep. 2020;22:25. doi: 10.1007/s11886-020-1273-y. [DOI] [PubMed] [Google Scholar]
- 18.Doehner W, Gerstein HC, Ried J, et al. Obesity and weight loss are inversely related to mortality and cardiovascular outcome in prediabetes and type 2 diabetes: data from the ORIGIN trial. Eur Heart J. 2020;41:2668–2677. doi: 10.1093/eurheartj/ehaa293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forgie K, Bozso SJ, Hong Y, et al. The effects of body mass index on outcomes for patients undergoing surgical aortic valve replacement. BMC Cardiovasc Disord. 2020;20:255. doi: 10.1186/s12872-020-01528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faggioni M, Baber U, Afshar AE, et al. Effects of body mass index on clinical outcomes in female patients undergoing percutaneous coronary intervention with drug-eluting stents: results from a patient-level pooled analysis of randomized controlled trials. JACC Cardiovasc Interv. 2018;11:68–76. doi: 10.1016/j.jcin.2017.06.060. [DOI] [PubMed] [Google Scholar]
- 21.Elagizi A, Kachur S, Lavie CJ, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–150. doi: 10.1016/j.pcad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Oreopoulos A, Padwal R, Norris CM, et al. Effect of obesity on short- and long-term mortality postcoronary revascularization: a meta-analysis. Obesity (Silver Spring) 2008;16:442–450. doi: 10.1038/oby.2007.36. [DOI] [PubMed] [Google Scholar]
- 23.Li YH, Lin GM, Lin CL, et al. Relation of body mass index to mortality among patients with percutaneous coronary intervention longer than 5 years follow-up: a meta-analysis. Int J Cardiol. 2013;168:4315–4318. doi: 10.1016/j.ijcard.2013.04.174. [DOI] [PubMed] [Google Scholar]
- 24.Lin GM, Li YH, Lin CL, et al. Relation of body mass index to mortality among Asian patients with obstructive coronary artery disease during a 10-year follow-up: a report from the ET-CHD registry. Int J Cardiol. 2013;168:616–620. doi: 10.1016/j.ijcard.2013.01.204. [DOI] [PubMed] [Google Scholar]
- 25.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 26.Vachharajani V, Granger DN. Adipose tissue: a motor for the inflammation associated with obesity. IUBMB Life. 2009;61:424–430. doi: 10.1002/iub.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahan CA, Gidding SS, Malcom GT, et al. Pathobiological determinants of atherosclerosis in youth risk scores are associated with early and advanced atherosclerosis. Pediatrics. 2006;118:1447–1455. doi: 10.1542/peds.2006-0970. [DOI] [PubMed] [Google Scholar]
- 28.Hwang YC, Fujimoto WY, Hayashi T, et al. Increased visceral adipose tissue is an independent predictor for future development of atherogenic dyslipidemia. J Clin Endocrinol Metab. 2016;101:678–685. doi: 10.1210/jc.2015-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aparecida Silveira E, Vaseghi G, de Carvalho Santos AS, et al. Visceral obesity and its shared role in cancer and cardiovascular disease: a scoping review of the pathophysiology and pharmacological treatments. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21239042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosengren A. Overweight, obesity... and BMI. The INTERHEART study shows that the BMI should probably be abolished--the waist-hip ratio is better. Lakartidningen. 2006;103:628. [PubMed] [Google Scholar]
- 31.Porter Starr KN, Orenduff M, McDonald SR, et al. Influence of weight reduction and enhanced protein intake on biomarkers of inflammation in older adults with obesity. J Nutr Gerontol Geriatr. 2019;38:33–49. doi: 10.1080/21551197.2018.1564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garanty-Bogacka B, Syrenicz M, Goral J, et al. Changes in inflammatory biomarkers after successful lifestyle intervention in obese children. Endokrynol Pol. 2011;62:499–505. [PubMed] [Google Scholar]
- 33.Pothineni NV, Gondi S, Kovelamudi S. Cardiac rehabilitation after percutaneous coronary intervention – evidence and barriers. Heart and Mind. 2018;2:1–4. [Google Scholar]
- 34.Wallner S, Watzinger N, Lindschinger M, et al. Effects of intensified lifestyle modification on the need for further revascularization after coronary angioplasty. Eur J Clin Invest. 1999;29:372–379. doi: 10.1046/j.1365-2362.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 35.Bangalore S, Fayyad R, Laskey R, et al. Body-weight fluctuations and outcomes in coronary disease. N Engl J Med. 2017;376:1332–1340. doi: 10.1056/NEJMoa1606148. [DOI] [PubMed] [Google Scholar]
- 36.Lissner L, Odell PM, D'Agostino RB, et al. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324:1839–1844. doi: 10.1056/NEJM199106273242602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were generated at Taipei Veterans General Hospital and obtained through Taiwan’s National Health Insurance Research Database. The data supporting the findings of this study are available upon request from the corresponding author, Hsin-Bang Leu. These data are not publicly available due to privacy concerns regarding research participants.