Abstract
Background
Prompt primary percutaneous coronary intervention (pPCI) is crucial for the prognosis and reduction of myocardial damage in ST-segment elevation myocardial infarction (STEMI) patients. The Coronavirus Disease 2019 (COVID-19) pandemic had multifaceted impacts on healthcare. This study assessed the effects of the pandemic on pPCI procedures and clinical outcomes in emergency STEMI patients.
Methods
This retrospective, single-center study analyzed STEMI patients who underwent pPCI from February 2019 to January 2022. The COVID-19 pandemic was categorized into three periods: pre-COVID-19 (Period-I), early-pandemic (Period-II), and epidemic (Period-III). The impacts on Door-to-Device time, its segments, and clinical outcomes were analyzed using Statistical Package for the Social Sciences.
Results
A total of 404 STEMI patients were included, with a reduced number in Period-III. Compared to Period-I, the time intervals of Door-to-electrocardiogram (ECG), ECG-to-Cardiac Catheterization Laboratory Activation (CCLA), and CCLA-to-Cardiac Catheterization Laboratory Door in Period III were extended by 0.62 minutes (p = 0.006), 3.30 minutes (p = 0.009), and 9.65 minutes (p < 0.001), respectively. In contrast, the Angio-to-Device time was shorter in Period- II and III by 2.60 and 4.08 minutes (p < 0.001), respectively. Overall Door-to-Device time increased by 10.06 minutes (p < 0.001) in Period-III but decreased by 3.67 minutes in Period-II (p = 0.017). The odds of achieving a Door-to-Device time ≤ 90 minutes decreased by 70% in Period-III (p = 0.002). Clinical outcomes, including intensive care unit stay, hospital stay, in-hospital mortality, and 30-day readmission rate, remained stable across periods.
Conclusions
The COVID-19 pandemic had various effects on different segments of the Door-to-Device procedure, and they were influenced by the complex interplay between infection control measures and clinical workflow. The stability of clinical outcomes reflects the resilience and effective adaptations of the healthcare system during the pandemic.
Keywords: COVID-19, Door to Device, D2B, Percutaneous coronary intervention, ST-segment elevation myocardial infarction
Abbreviations
Angio-to-Device, Time interval from angiography to balloon dilation
CAD, Coronary artery disease
CCL, Cardiac catheterization laboratory
CCLA, Cardiac catheterization laboratory activation
CCLD, Cardiac catheterization laboratory door
CCLD-to-Device, From cardiac catheterization laboratory door to device (balloon)
COVID-19, Coronavirus disease 2019
D2B, Door-to-balloon
Door-to-Device, Interval from arrival at the ED to balloon dilation in the CCL
ECG, Electrocardiogram
ED, Emergency department
FEMH, Far Eastern Memorial Hospital
ICU, Intensive care unit
OR, Odds ratio
PCI, Percutaneous coronary intervention
PCR, Polymerase chain reaction
pPCI, Primary percutaneous coronary intervention
SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
SPSS, Statistical Package for the Social Sciences
STEMI, ST-segment elevation myocardial infarction
INTRODUCTION
ST-segment elevation myocardial infarction (STEMI) is severe blockage of a heart artery and considered a medical emergency. Prompt medical attention from symptom onset, rapid diagnosis, and timely early reperfusion techniques such as primary percutaneous coronary intervention (pPCI) are crucial for reducing heart tissue damage and preventing serious complications in STEMI patients.1,2
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), imposed considerable strains on the global healthcare system.3-5 Medical institutions were tasked with maintaining standard medical care for patients in need while also addressing COVID-19 cases.6,7 The SARS-CoV-2 virus is contagious through respiratory routes, direct contact with infected individuals, or indirect contact with droplet- or secretion-contaminated environmental surfaces.8,9 Healthcare facilities have necessarily implemented strengthened infection control measures to prevent virus transmission from the community and avoid nosocomial outbreaks, however these measures have also impacted emergency medical interventions. The Door-to-Device time, which measures the interval from a STEMI patient’s arrival at the emergency department (ED) to balloon dilation in the cardiac catheterization laboratory (CCL), is critical for patient management and prognosis, and serves as a key indicator of percutaneous coronary intervention (PCI) performance quality.10 It involves a tightly coordinated process, including initial triage, electrocardiogram, and cardiologist consultation at the ED, followed by transfer to the CCL for angiography, wire crossing, and balloon dilation procedures to address ischemic conditions.1,2,11 The impact of the COVID-19 pandemic on the healthcare system varied widely among regions and time periods.12-22 This study focused on a tertiary medical center in northern Taiwan located adjacent to the epicenter of the epidemic and which managed 10% of all COVID-19 intensive care services during the first peak of outbreak.6 We specifically assessed whether the patterns of Door-to-Device time intervals for the pPCI procedure differed during the COVID-19 pandemic. We also investigated the impact of anticipated longer Door-to-Device times due to stricter infection control measures during the COVID-19 outbreak on clinical outcomes.
METHODS
Study design and grouping
This single-center retrospective study used a before-and-after design. After the occurrence of the first nosocomial infection in Taiwan in February 2020, the Taiwan Centers for Disease Control promptly issued epidemic prevention guidance on February 26.23-27 Through a combination of non-pharmaceutical interventions and high levels of public adherence, Taiwan successfully contained the COVID-19 outbreak within hospitals and communities during the early stages of the COVID-19 pandemic until April to July 2021, which marked the first wave of the COVID-19 epidemic in Taiwan.5,23,24 To assess the impact of the COVID-19 pandemic on the Door-to-Device process, the epidemic period was classified into two experimental groups: February 2020 to January 2021 (Period-II, early-pandemic), and February 2021 to January 2022 (Period-III, epidemic). The control group (Period-I, pre-pandemic) was from February 2019 to January 2020 to avoid sampling bias resulting from visit variance throughout the year.
Patient and data collection
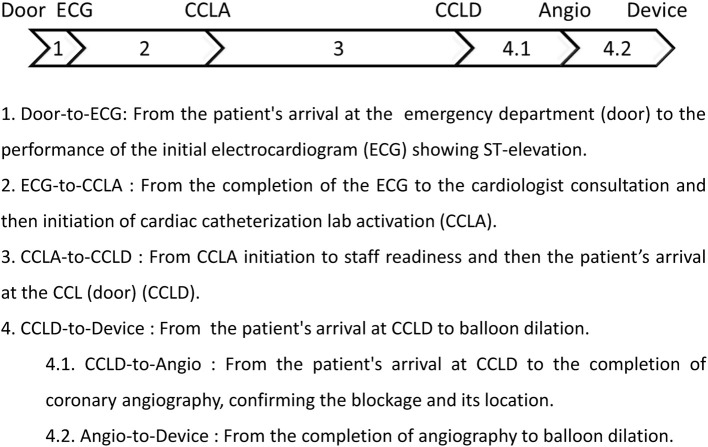
The inclusion criteria were patients diagnosed with STEMI in the ED and activated for pPCI at the CCL during the pre-pandemic and COVID-19 pandemic periods, as described above, as well as those who tested negative for the SARS-CoV-2 infection during the pandemic and were eligible for transfer to the CCL for pPCI. Patient and corresponding Door-to-Device time interval data were provided by the department of CCL of Far Eastern Memorial Hospital (FEMH). The total Door-to-Device time was converted into a categorical variable, with a cutoff of 90 minutes or less, as this is recommended in PCI-capable hospitals for new STEMI cases.2,28 The sequential intervals of Door-to-Device time are illustrated and described in Figure 1. The exclusion criteria were age below 20 years, atypical symptoms, non-STEMI on the first electrocardiogram (ECG), nosocomial infection, out-of-hospital cardiac arrest, execution of cardiopulmonary resuscitation, implementation of extracorporeal membrane oxygenation, defibrillation, and/or refusal of catheterization by the patients or relatives. Data were retrospectively collected from electronic medical records, including demographic and clinical characteristics, as well as the clinical outcomes. This study was approved by the Far Eastern Memorial Hospital Ethics Committee (FEMH No. 112028-E), and written informed consent was waived due to the retrospective study design.
Figure 1.
Flowchart and definitions of the sequential intervals of Door-to-Device time in the primary percutaneous coronary intervention (PCI) procedure.
Statistical analysis
Continuous data were presented as median (interquartile range). Categorical data were reported as frequency and percentage. The normality of numerical data was assessed using the Shapiro-Wilk test. Differences among the 3 periods (Period-I, II, and III) were tested by one-way analysis of variance, Kruskal-Wallis H or chi-square test, as appropriate, depending on whether the data were normally or non-normally distributed numerical or categorical. Multivariate regression analysis was conducted to evaluate the impact of the pandemic on Door-to-Device time and its intervals, adjusting for potential confounders. For data that did not follow a normal distribution, generalized linear models were employed, with the Tweedie or Gamma regression model selected based on goodness-of-fit measures for the independent variables, as shown in the Tables. Tweedie and Gamma regression models are suitable for analyzing non-normal and right-skewed numerical data distributions. For binary categorical data, logistic regression was performed. A p value < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS software.
RESULTS
The study initially enrolled STEMI patients who visited the ED and received pPCI treatment at FEMH from February 2019 to January 2022. After applying the exclusion criteria, the study ultimately included 404 participants. These patients were divided into three groups based on the date of their ED visit. Notably, the number of STEMI patients in epidemic Period-III was 32% lower compared to pre-pandemic Period-I. The demographic and clinical characteristics of the enrolled patients among the study periods are summarized and compared in Table 1. In epidemic Period-III, there was a significant increase in the percentage of male patients, while the proportion of patients with a history of coronary artery disease (CAD) significantly decreased compared to both pre-pandemic Period-I and early-pandemic Period-II. Table 2 provides an overview of the time intervals for the Door-to-Device process and the clinical outcomes before and during the COVID-19 pandemic. There were significant differences among the three periods in the time intervals of Door-to-ECG, ECG-to-cardiac catheterization laboratory activation (CCLA), and CCLA-to-cardiac catheterization laboratory door (CCLD), with significant prolongation in Period-III. In contrast, while there were also significant differences in the intervals of CCLD-to-Device and CCLD-to-Angio, the times in Period-III were significantly reduced. The significant reduction in the CCLD-to-Device interval in Period-III was primarily due to the significant shortening of the Angio-to-Device interval. Meanwhile, the CCLD-to-Angio interval showed an increasing trend, although it did not reach statistical significance. The overall Door-to-Device time among the three periods showed significant differences, with an increase in Period-III and a notable shorter duration in Period-II. The percentage achieving a Door-to-Device time ≤ 90 minutes also exhibited significant differences among the three periods, with a decrease in the target rate in Period-III. The clinical outcomes, including intensive care unit (ICU) length of stay, total hospital days, in-hospital mortality and 30-day readmission rate, showed no significant differences between the periods. These findings indicated that while the COVID-19 epidemic extended the overall pPCI time, its effects on individual intervals varied, with some showing opposing trends, yet it did not adversely affect overall clinical outcomes.
Table 1. The demographic and clinical characteristics (N = 404).
| Characteristics | Period-I 2019/Feb-2020/Jan, n = 155 | Period-II 2020/Feb-2021/Jan, n = 144 | Period-III 2021/Feb-2022/Jan, n = 105 | p value |
| Age, year | 59 (15) | 61 (21) | 57 (17) | 0.135 |
| Biological sex, male | 129 (83.2%) | 120 (83.3%) | 100 (95.2%) | 0.003c* |
| Smoking | 106 (68.4%) | 91 (63.6%) | 75 (71.4%) | 0.413b |
| DBP, mmHg | 86 (31) | 82 (33) | 88 (25) | 0.392 |
| SBP, mmHg | 135 (38) | 128 (41) | 135 (40) | 0.203a |
| Heart rate, times/min | 74 (24) | 74.5 (26) | 80 (24) | 0.498a |
| Prehospital activation (for EMS) | 11 (7.1%) | 15 (10.4%) | 3 (2.9%) | 0.056c |
| CAD history | 18 (11.6%) | 13 (9.0%) | 3 (2.9%) | 0.024c* |
| Hypertension | 84 (54.2%) | 75 (52.1%) | 47 (44.8%) | 0.311b |
| Diabetes mellitus | 43 (27.7%) | 41 (28.5%) | 34 (32.4%) | 0.701b |
| Chronic kidney disease | 7 (4.5%) | 8 (5.6%) | 3 (2.9%) | 0.577c |
| Triage level | 0.723c | |||
| Level 1 | 18 (11.6%) | 19 (13.2%) | 9 (8.6%) | |
| Level 2 | 99 (63.9%) | 87 (60.4%) | 75 (71.4%) | |
| Level 3 | 37 (23.9%) | 37 (25.7%) | 20 (19.0%) | |
| Level 4 | 1 (0.6%) | 1 (0.7%) | 1 (1.0%) | |
| PCI of blocked coronary arteries RCA, LAD, and/or LCX | 0.058b | |||
| 1 vessel | 58 (37.4%) | 36 (25.0%) | 24 (22.9%) | |
| 2 vessels | 41 (26.5%) | 48 (33.3%) | 31 (29.5%) | |
| 3 vessels | 56 (36.1%) | 60 (41.7%) | 50 (46.7%) |
Numerical data was expressed as median (IQR) while categorical data was expressed as frequency and percentage. The differences among the three periods were tested by one-way ANOVA, Kruskal-Wallis Ha, or Chi-Square test appropriately depended on whether the data were normally distributed numerical, non-normally distributed numerical, or categorical, respectively.
b Pearson Chi-Square Test. c Likelihood Ratio Chi-Square test as more than one cell count was less than 5 or less than 20% of the group.
CAD, coronary artery disease; DBP, diastolic blood pressure; LAD, left anterior descending artery; LCX, left circumflex artery; PCI, percutaneous coronary intervention; RCA, right coronary artery; SBP, systolic blood pressure.
Table 2. The time intervals of the Door-to-Device process and clinical outcomes before and during the COVID-19 pandemic.
| Variables | Period-I (2019/Feb-2020/Jan) | Period-II (2020/Feb-2021/Jan) | Period-III (2021/Feb-2022/Jan) | p value |
| Primary PCI process, min | ||||
| 1. Door-to-ECG | 3.98 (2.01) | 4.50 (1.42) | 4.93 (1.78) | 0.001* |
| 2. ECG-to-CCLA | 13.00 (10.02) | 13.58 (10.06) | 15.03 (15.42) | 0.049* |
| 3. CCLA-to-CCLD | 26.00 (11.00) | 26.00 (15.00) | 34.98 (16.00) | < 0.001* |
| 4. CCLD-to-Device | 24.98 (10.00) | 22.98 (8.62) | 22.11 (9.66) | 0.037* |
| 4.1. CCLD-to-Angio | 11.00 (4.98) | 11.98 (5.01) | 12.10 (5.81) | 0.058 |
| 4.2. Angio-to-Device | 12.00 (6.27) | 10.33 (5.02) | 8.52 (4.55) | < 0.001* |
| Door-to-Device time | 72.00 (21.00) | 68.47 (16.40) | 80.63 (17.13) | < 0.001* |
| Door-to-Device ≤ 90 min | 141 (91.0%) | 135 (95.7%) | 79 (78.2%) | < 0.001b* |
| Clinical outcomes | ||||
| ICU length of stay, days | 3 (1) | 3 (1) | 3 (1) | 0.472 |
| Hospitalization length, days | 5 (2) | 5 (1) | 5 (1) | 0.244 |
| In-hospital mortality | 3 (1.9%) | 4 (2.8%) | 1 (1.0%) | 0.569c |
| 30-day readmissiona | 3 (2.0%) | 3 (2.1%) | 0 (0%) | 0.157c |
Numerical data was expressed as median (IQR) while categorical data was expressed as frequency and percentage. The differences among the three periods were tested by Kruskal-Wallis H test for non-normally distributed numerical data or Chi-Square test for categorical data, as appropriate.
a 30-day hospital re-admission for discharged patients. b Pearson Chi-Square test. c Likelihood Ratio Chi-Square test as more than one cell count was less than 5 or less than 20% of the group.
CCLA, cardiac catheterization laboratory activation; CCLD, cardiac catheterization laboratory door; COVID-19, Coronavirus Disease 2019; ECG, electrocardiogram; ICU, intensive care unit; PCI, percutaneous coronary intervention.
To account for potential confounding factors such as sex, CAD history, and triage level that may have affected the impact of the COVID-19 pandemic on Door-to-Device intervals, a multivariate regression analysis was conducted. The results showed that after adjusting for these confounders, there was still a 0.62-minute mean delay in the Door-to-ECG interval in Period-III compared to Period-I (Table 3). Additionally, this initial assessment phase was found to be associated with male sex and CAD history, resulting in mean reductions of 0.68 minutes and 0.56 minutes, respectively. Similarly, the time intervals from ECG-to-CCLA and CCLA-to-CCLD remained significantly prolonged in Period-III after adjustment, with mean delays of 3.30 minutes and 9.65 minutes, respectively. On the other hand, the intervals from CCLD-to-Device and Angio-to-Device remained significantly shorter in Period-III, with mean reductions of 2.46 minutes and 4.08 minutes, respectively, even after accounting for confounders. This reduction in time was also observed in the early-pandemic Period-II, with mean reductions of 1.71 minutes and 2.60 minutes, respectively. These multivariate regression results highlighted the complex interplay between the COVID-19 pandemic, patient demographics, and clinical workflow in influencing the various intervals of the PCI procedure. For the total Door-to-Device time, the results presented in Table 4 show that compared to Period-I, the mean Door-to-Device time significantly decreased by 3.67 minutes in the early-pandemic Period-II. In contrast, during the epidemic Period-III, the mean Door-to-Device time significantly increased by 10.06 minutes. Additionally, Door-to-Device time was correlated with triage level, as each increase in the severity of the triage level was associated with a mean decrease of 2.31 minutes in the overall Door-to-Device time. For a Door-to-Device time ≤ 90 minutes, the odds ratio (OR) for the epidemic Period-III was 0.30, indicating a 70% reduction in the likelihood of achieving the target compared to the pre-pandemic Period-I. Moreover, the OR for each increase in the severity of the triage level was 2.18, suggesting a significantly higher likelihood of meeting the target of a Door-to-Device time ≤ 90 minutes when the patients were categorized as needing more urgent care.
Table 3. The effects of COVID-19 pandemic on the time intervals of the Door-to-Device procedure were examined using multivariate regression analysis, with adjustment for potential confounders.
| Variables | 1. Door-to-ECG | 2. ECG-to-CCLA | 3. CCLA-to-CCLD | 4.2. Angio-to-Device | ||||
| Betaa | pa | Betab | pb | Betaa | pa | Betab | pb | |
| COVID-19 periods | ||||||||
| Period-I | Ref | - | Ref | - | Ref | - | Ref | - |
| Period-II | 0.10 | 0.601 | -0.11 | 0.914 | -0.02 | 0.988 | -2.60 | < 0.001* |
| Period-III | 0.62 | 0.006* | 3.30 | 0.009* | 9.65 | < 0.001* | -4.08 | < 0.001* |
| Sex, male to female | -0.68 | 0.013* | -0.42 | 0.743 | -1.80 | 0.292 | 0.70 | 0.307 |
| CAD history | -0.56 | 0.042* | -1.39 | 0.319 | -0.41 | 0.845 | -0.43 | 0.616 |
| Triage level, from 4 to 1 | -0.28 | 0.072 | -2.41 | < 0.001* | 1.88 | 0.05 | -0.46 | 0.267 |
GLM Tweediea or Gammab regression was appropriately employed based on the goodness-of-fit test for non-normally distributed independent variables.
CAD, coronary artery disease; CCLA, cardiac catheterization laboratory activation; CCLD, cardiac catheterization laboratory door; COVID-19, Coronavirus Disease 2019; ECG, electrocardiogram; ICU, intensive care unit.
Table 4. The effect of COVID-19 pandemic on the Door-to-Device time was examined by multivariate regression analysis adjusted for the potential confounding factors.
| Variables | Door-to-Device time, min | Door-to-Device time, ≤ 90 min | ||
| Beta | p | OR | p | |
| COVID-19 periods | ||||
| Period-I | Ref | - | Ref | - |
| Period-II | -3.67 | 0.017* | 2.34 | 0.095 |
| Period-III | 10.06 | < 0.001* | 0.30 | 0.002* |
| Sex, male to female | -3.77 | 0.065 | 2.44 | 0.053 |
| CAD history | -3.49 | 0.138 | -a | -a |
| Triage level, from 4 to 1 | -2.31 | 0.042* | 2.18 | 0.007* |
GLM gamma regression was appropriately employed based on the goodness-of-fit test for non-normally distributed independent variables, while logistic regression analysis was performed for categorical data.
a The CAD history variable was excluded due to no CAD history cases in the group with Door-to-Device time exceeding 90 minutes, leading to perfect separation and unstable model estimates.
CAD, Coronary artery disease; COVID-19, coronavirus disease 2019; GLM, generalized linear model; OR, odds ratio.
DISCUSSION
This study explored the impact of the COVID-19 pandemic on the Door-to-Device time, its intervals, and clinical outcomes for STEMI patients undergoing pPCI at a medical center in northern Taiwan. The results showed that different segments of the Door-to-Device process were affected in different ways, with some segments experiencing delays and others being shortened during the pandemic. While the overall Door-to-Device time was extended during the epidemic surge, there was no significant impact on the clinical outcomes.
The COVID-19 pandemic had multifaceted impacts on acute myocardial infarction admissions. In the early wave of the pandemic, individuals experiencing health issues, including STEMI patients, were deterred from seeking medical care due to fear of contracting the virus, leading to a reduction in cases and prolonging the interval from symptom onset to hospital admission in most regions of the world.21,22,29-33 This study also showed a decrease in STEMI patients during the epidemic period (Table 1). This reduction may be attributed to concerns about increased infection risk when receiving medical care at our hospital, which was located in a hotspot, experienced a short-term nosocomial outbreak, and also treated COVID-19 cases from the community.6 However, following the global outbreak of the COVID-19 pandemic, the course of reperfusion time and clinical outcomes for STEMI varied among healthcare institutions, regions, and periods, influenced by factors such as local outbreak severity, preventive policies, healthcare resources, and national income levels.12-22 In the present study, there were significant delays in the initial phases of the pPCI process from Door to CCLD intervals during the epidemic period (Period-III), while there were no significant differences during the early-pandemic period (Period-II). In the early stages of the pandemic, Taiwan successfully controlled COVID-19 cases until a community outbreak occurred in May 2021 in the northern region.5,23-25,27,34,35 Following this, strengthened infection control measures were implemented, which included requiring a negative polymerase chain reaction (PCR) test result to access the hospital premises with the aim of preventing transmission from the community to hospitals.6,36 The time waiting for PCR results was considered to be the major cause of delays in the intervals before entering the CCL for PCI procedures during the epidemic period. On the other hand, after arriving at the CCL, the time required to execute the PCI procedure was considerably shortened during the epidemic period, especially in the Angio-to-Device step. This improvement was also observed during the early-pandemic period, even after accounting for confounders. This phenomenon suggests that certain workflows were expedited during the pandemic. Elective medical services declined markedly in Taiwan after the COVID-19 pandemic.37 The reduction in patient volume may have temporarily eased the usual burden on tertiary medical services in Taiwan,18 such as reducing the likelihood of consecutive patients for PCI, which requires preparation time between procedures. This, in turn, resulted in shorter times in the CCL and can also explain why the overall Door-to-Device time was significantly shorter during the early-pandemic period. However, the high-intensity infection control measures implemented in response to the community outbreak, including the Travel, Occupation, Contact and Cluster surveys, wearing personal protective equipment, enhanced disinfection, and waiting for PCR test results5,7,38 presumably contributed to the eventual prolongation of the overall Door-to-Device time in Period-III and decrease in the proportion of Door-to-Device time ≤ 90 minutes in the present study. Our data showed that the clinical outcomes including ICU days, total hospital days, in-hospital mortality and 30-day readmission rate remained stable across periods. This highlights the efforts of the PCI medical care team in adapting to the challenges posed by the epidemic, supported by the Taiwanese Ministry of Health and Welfare and the institution’s leadership in providing guidelines and medical resources.5-7,39
Limitations
Our study has several limitations. First, as a single-center study conducted at a tertiary medical center located adjacent to the epicenter of the epidemic and dealing with a significant volume of COVID-19 cases during peak periods, the results may not be generalizable to other regions or healthcare settings. Second, this study did not analyze which specific pandemic-related variables, such as patient volume, staff adjustments, or changes in infection control measures and healthcare policies, contributed to the variations in the intervals of the pPCI procedure across the study periods. Third, this study did not include patients who tested positive for SARS-CoV-2 infection, who may have experienced more complex and severe cardiovascular complications, particularly in the context of STEMI.40
CONCLUSIONS
This study provides critical insights into how the COVID-19 pandemic impacted the time of the Door-to-Device process for STEMI patients who underwent pPCI at a tertiary medical center in northern Taiwan. Of note, many COVID-19 patients were admitted to our hospital during the pandemic.41-43 Although the overall Door-to-Device time was delayed during the epidemic period, the clinical outcomes were not compromised. These results reflect the resilience and effective adaptation of healthcare professionals in managing acute and critical care, highlighting the ability to maintain high-quality medical care despite the challenges posed by the epidemic.
DECLARATION OF CONFLICT OF INTEREST
The authors declare no conflict of interest.
Acknowledgments
We pay tribute to all dedicated frontline healthcare professionals during the COVID-19 pandemic and the cardiovascular care team of FEMH. Thanks to Nurse Yin-Chen Yeh for assisting with the collection of patient medical records.
FUNDING
This study was supported by grants from Far Eastern Memorial Hospital (FEMH-2023-C043, FEMH-2024-C-040).
REFERENCES
- 1.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2016;133:1135–1147. doi: 10.1161/CIR.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 2.Li YH, Lee CH, Huang WC, et al. 2020 focused update of the 2012 guidelines of the Taiwan Society of Cardiology for the management of ST-segment elevation myocardial infarction. Acta Cardiol Sin. 2020;36:285–307. doi: 10.6515/ACS.202007_36(4).20200619A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haldane V, De Foo C, Abdalla SM, et al. Health systems resilience in managing the COVID-19 pandemic: lessons from 28 countries. Nat Med. 2021;27:964–980. doi: 10.1038/s41591-021-01381-y. [DOI] [PubMed] [Google Scholar]
- 4.Filip R, Gheorghita Puscaselu R, Anchidin-Norocel L, et al. Global challenges to public health care systems during the COVID-19 pandemic: a review of pandemic measures and problems. J Pers Med. 2022;12:1295. doi: 10.3390/jpm12081295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai CC, Lee PI, Hsueh PR. How Taiwan has responded to COVID-19 and how COVID-19 has affected Taiwan, 2020-2022. J Microbiol Immunol Infect. 2023;56:433–441. doi: 10.1016/j.jmii.2023.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang JH, Chang HT, Liao CH, Chiu KM. Rapid response of a medical center upon the surge of COVID-19 epidemic in Taiwan. J Microbiol Immunol Infect. 2022;55:1–5. doi: 10.1016/j.jmii.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin KY, Pan SC, Wang JT, et al. Preventing and controlling intra-hospital spread of COVID-19 in Taiwan - looking back and moving forward. J Formos Med Assoc. 2024;123(Suppl 1):S27–S38. doi: 10.1016/j.jfma.2023.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–14-8. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nallamothu BK, Normand SL, Wang Y, et al. Relation between door-to-balloon times and mortality after primary percutaneous coronary intervention over time: a retrospective study. Lancet. 2015;385:1114–1122. doi: 10.1016/S0140-6736(14)61932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YH, Li AH, Chen TC, et al. Compared with physician overread, computer is less accurate but helpful in interpretation of electrocardiography for ST-segment elevation myocardial infarction. J Electrocardiol. 2023;81:60–65. doi: 10.1016/j.jelectrocard.2023.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Pessoa-Amorim G, Camm CF, Gajendragadkar P, et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: a survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes. 2020;6:210–216. doi: 10.1093/ehjqcco/qcaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chew NWS, Ow ZGW, Teo VXY, et al. The global effect of the COVID-19 pandemic on STEMI care: a systematic review and meta-analysis. Can J Cardiol. 2021;37:1450–1459. doi: 10.1016/j.cjca.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YH, Huang WC, Hwang JJ. No reduction of ST-segment elevation myocardial infarction admission in Taiwan during coronavirus pandemic. Am J Cardiol. 2020;131:133–134. doi: 10.1016/j.amjcard.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannan EL, Wu Y, Cozzens K, et al. Percutaneous coronary intervention for ST-elevation myocardial infarction before and during COVID in New York. Am J Cardiol. 2021;142:25–34. doi: 10.1016/j.amjcard.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erol MK, Kayıkçıoğlu M, Kılıçkap M, et al. Treatment delays and in-hospital outcomes in acute myocardial infarction during the COVID-19 pandemic: a nationwide study. Anatol J Cardiol. 2020;24:334–342. doi: 10.14744/AnatolJCardiol.2020.98607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao K, Takahashi J, Sato K, et al. The influence of COVID-19 pandemic on management of acute myocardial infarction in Japan; insight from the Miyagi AMI Registry Study. Int J Cardiol Heart Vasc. 2022;43:101116. doi: 10.1016/j.ijcha.2022.101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su YH, Wu KH, Su CM, et al. Influence of the coronavirus disease 2019 pandemic on patients with ST-segment elevation myocardial infarction in Taiwan. Emerg Med Int. 2021;2021:5576220. doi: 10.1155/2021/5576220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu SA, Wu CL, Chou IJ, et al. The impacts of COVID-19 on healthcare quality in tertiary medical centers-a retrospective study on data from Taiwan clinical performance indicators system. Int J Environ Res Public Health. 2022;19:2278. doi: 10.3390/ijerph19042278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altobelli E, Angeletti PM, Marzi F, et al. Impact of SARS-CoV-2 outbreak on emergency department presentation and prognosis of patients with acute myocardial infarction: a systematic review and updated meta-analysis. J Clin Med. 2022;11:2323. doi: 10.3390/jcm11092323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang D, Xiang X, Zhang W, et al. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol. 2020;76:1318–1324. doi: 10.1016/j.jacc.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Luca G, Verdoia M, Cercek M, et al. Impact of COVID-19 pandemic on mechanical reperfusion for patients with STEMI. J Am Coll Cardiol. 2020;76:2321–2330. doi: 10.1016/j.jacc.2020.09.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan TW, Tan HL, Chang MN, et al. Effectiveness of epidemic preventive policies and hospital strategies in combating COVID-19 outbreak in Taiwan. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18073456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng HY, Chueh YN, Chen CM, et al. Taiwan’s COVID-19 response: timely case detection and quarantine, January to June 2020. J Formos Med Assoc. 2021;120:1400–1404. doi: 10.1016/j.jfma.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng HY, Li SY, Yang CH. Initial rapid and proactive response for the COVID-19 outbreak – Taiwan’s experience. J Formos Med Assoc. 2020;119:771–773. doi: 10.1016/j.jfma.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin C, Braund WE, Auerbach J, et al. Policy decisions and use of information technology to fight COVID-19, Taiwan. Emerg Infect Dis. 2020;26:1506–1512. doi: 10.3201/eid2607.200574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang CJ, Ng CY, Brook RH. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020;323:1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- 28.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 29.Lin YT, Chen HA, Wu HY, et al. Influence of the door-to-ECG time on the prognosis of patients with acute coronary syndrome. Acta Cardiol Sin. 2023;39:127–134. doi: 10.6515/ACS.202301_39(1).20220602B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mafham MM, Spata E, Goldacre R, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41:2083–2088. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammad TA, Parikh M, Tashtish N, et al. Impact of COVID-19 pandemic on ST-elevation myocardial infarction in a non-COVID-19 epicenter. Catheter Cardiovasc Interv. 2021;97:208–214. doi: 10.1002/ccd.28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YY, Yang MH, Lai JZ, et al. Seroprevalence of anti-SARS-CoV-2 remained extremely low in Taiwan until the vaccination program was implemented. Open Forum Infect Dis. 2024;11:ofad614. doi: 10.1093/ofid/ofad614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng TC, Cheng HY, Chang HH, et al. Comparison of estimated effectiveness of case-based and population-based interventions on COVID-19 containment in Taiwan. JAMA Intern Med. 2021;181:913–921. doi: 10.1001/jamainternmed.2021.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang JY, Liao CH, Hung FM, et al. Transformation from zero tolerance to living with COVID-19 in New Taipei City, Taiwan. Experience of the FEMH "home-hotel-hospital" care model. J Formos Med Assoc. 2024;123(Suppl 1):S39–S46. doi: 10.1016/j.jfma.2023.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang YT, Chiang SC, Lee WC, et al. Varied impacts on outpatient services among departments and divisions in the early phase of the COVID-19 pandemic: implications for personnel mobilization and preparatory training. J Chin Med Assoc. 2021;84:951–955. doi: 10.1097/JCMA.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 38.Hsu FF, Yang CJ, Tsai MS, et al. Control of an outbreak of COVID-19 at a tertiary hospital in Taiwan. J Microbiol Immunol Infect. 2022;55:1052–1059. doi: 10.1016/j.jmii.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao TH, Yeh HI, Shyu KG, et al. Rationale and study design of the TSOC-fully organized registry for the management of symptomatic ACS study (T-FORMOSA Study). Acta Cardiol Sin. 2023;39:561–571. doi: 10.6515/ACS.202307_39(4).20230306D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Leor O, Cid Alvarez AB, Pérez de Prado A, et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention. 2021;16:1426–1433. doi: 10.4244/EIJ-D-20-00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh CC, Liu CY, Tsai KC, et al. The hypoxia-age-shock index at triage to predict the outcomes of Covid-19 patients. Am J Emerg Med. 2023;65:65–70. doi: 10.1016/j.ajem.2022.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu CY, Chou SF, Chiang PY, et al. The FIB-4 scores in the emergency department to predict the outcomes of COVID-19 patients in Taiwan. Heliyon. 2024;10:e25649. doi: 10.1016/j.heliyon.2024.e25649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh CC, Ting MJ, Chang CT, et al. Is the shock index associated with adverse outcomes among geriatric patients with COVID-19 in the emergency department triage? Int J Gerontol. 2023;17:177–182. [Google Scholar]